Abstract
Background
Several microorganisms inhabit the mammalian gastrointestinal tract and are associated with the pathogenesis of various diseases, including cancer. Recent studies have indicated that several probiotics produce antitumor molecules and inhibit host tumor progression. We demonstrated that heptelidic acid (HA), a sesquiterpene lactone derived from the probiotic Aspergillus oryzae, exerts antitumor effects against pancreatic cancer in vitro and in vivo. In this study, the antitumor effects of HA against extraintestinal melanoma were assessed in vitro and in vivo.
Results
Sulforhodamine B (SRB) assay revealed that the growth of B16F10 cells was significantly inhibited by HA in a concentration-dependent manner. The enzymatic activity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) decreased in proportion with the growth inhibition effect of HA. Moreover, oral HA administration significantly suppressed the growth of transplanted B16F10 tumors without any significant changes in biochemical test values. Moreover, GAPDH activity in the transplanted tumor tissues in the HA group significantly decreased compared with that in the PBS group.
Conclusion
This study suggests that orally administered HA was absorbed in the gastrointestinal tract, reached the cancer cells transplanted in the skin, and inhibited GAPDH activity, thereby inhibiting the growth of extraintestinal melanoma cells. Thus, this study proposes a novel system for extraintestinal tumor regulation via gut bacteria-derived bioactive mediators.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-022-02530-0.
Keywords: Glyceraldehyde-3-phosphate dehydrogenase, Heptelidic acid, Melanoma
Background
Several microorganisms inhabit the gastrointestinal tract and have a symbiotic relationship with their mammalian hosts. Previous studies have demonstrated that the disruption of intestinal microflora is closely associated with the pathogenesis of various diseases, such as atopic dermatitis, inflammatory bowel disease, and cancer [1–3]. Several studies have indicated that oral probiotic administration and fecal microbiota transplantation (FMT) are potentially effective methods to prevent and treat such diseases [4–6].
Recently, some bacterial molecules produced by probiotics have been identified to possess antitumor properties. Tsai et al. revealed that antimicrobial peptides such as m2163 and m2386 induced colorectal cancer cell apoptosis in vitro [7]. Hatakeyama et al. determined that a Natto peptide can reduce the number of uterine and cervical cancer cells in vitro [8]. Ferrichrome derived from Lactobacillus casei also induced the apoptosis in gastrointestinal cancer cells, including colorectal, gastric, and pancreatic cancer cells [9–12]. These studies suggest that bacterial molecules directly inhibit tumor cell progression, thus providing health benefits to mammalian hosts.
Aspergillus oryzae is an imperfect fungus that is used in the production of Japanese fermented food, including soybean paste and soy sauce, since ancient times. Recent studies have presented the beneficial effects of foods fermented by A. oryzae, e.g., conventional intake of soybean paste fermented by A. oryzae have a preventive effect on lifestyle-related diseases such as hypertension and type II diabetes [13–15]. Interestingly, fermented soybean paste also shows antitumor activity against various cancers, including extraintestinal tumors [16–18]. Recently, we demonstrated that heptelidic acid (HA) from A. oryzae shows antitumor effects against pancreatic cancer cells [19]. In this previous study, a bacterial culture supernatant was collected and dissociated using HPLC, and HA was identified as an antitumor mediator derived from A. oryzae. We also performed an ex vivo study on mice intestinal loop and determined that HA in a resected mouse intestine passes through the intestinal wall and inhibits the progression on pancreatic cancer cells. This indicates that some probiotic molecules are absorbed by the host, circulate in the whole body, and directly affect digestive lesions. However, it has not been clearly understood whether such probiotic molecules exert antitumor effects on tumors other than those in the digestive tract when administered in the gastrointestinal tract in vivo.
HA was first identified from Trichoderma koningii as a specific and irreversible inhibitor of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [20]. GAPDH is an important enzyme involved in cellular metabolism. In the glycolytic/gluconeogenic metabolic pathways, GAPDH converts glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate using NAD(P)+ as a coenzyme. Moreover, numerous moonlighting activities of GAPDH have been reported in the literature. Majority of these activities are irrelevant to its main function in energy metabolism; it is involved in apoptotic–autophagic cell death, DNA repair, tRNA export, as well as membrane fusion and transport [21–24]. GADPH was also highlighted as a therapeutic target for inhibiting the abnormal tumor glycolysis pathway called the Warburg effect in malignant tumors [25, 26]. However, whether orally administered HA affects the GAPDH activity of extraintestinal tumors, including melanoma, and exhibits antitumor functions has not been elucidated.
This study assessed the growth and GAPDH inhibitory effects of orally administered HA on extraintestinal melanoma using in vitro cell proliferation assay and in vivo homograft melanoma mouse model.
Methods
Materials
B16F10 cells were obtained from Dr. Takayuki Ohkuri (Department of Pathology, Asahikawa Medical University, Asahikawa, Japan.). High-glucose Dulbecco’s modified Eagle’s medium was purchased from FUJIFILM Wako Pure Chemical, Osaka, Japan. Fetal bovine serum, L-glutamine, penicillin, and streptomycin were purchased from Thermo Fisher Scientific, MA, USA. Sulforhodamine B was purchased from Merck KGaA, Darmstadt, Germany. Heptelidic acid was purchased from Adipogen Life Sciences, CA, USA. A GAPDH activity assay kit was purchased from Abcam, Cambridge, UK.
Cell culture
B16F10 cells are murine melanoma cells that originated from a C57BL/6 J mouse. These cells were cultured in a high-glucose Dulbecco’s modified Eagle’s medium (FUJIFILM Wako Pure Chemical) supplemented with 10% (vol/vol) fetal bovine serum, 2 mM L-glutamine, 50 U/mL penicillin, and 50 mg/mL streptomycin (Thermo Fisher Scientific, MA, USA).
Sulforhodamine B assay
B16F10 cells were seeded onto 96-well microplates at 1 × 104 cells/well and cultured for 24 h. After 24 h of HA treatment (n = 5) at different concentrations (0, 10, 102, 103, and 104 ng/mL), the cells were fixed in 10% trichloroacetic acid for 1 h at 4 °C and washed four times in distilled water. The microplates were then dehydrated at room temperature, stained with 0.057% (wt/vol) Sulforhodamine B (SRB) in 1% (vol/vol) acetic acid at 100 μL per well, washed four times with 0.1% acetic acid, and re-dehydrated at room temperature. The stained cells were lysed in 10 mM unbuffered Tris base solution, and the optical density was measured at 510 nm [27].
Cell migratsion assay
B16F10 cells were seeded on 12-well microplates at 1 × 105 cells per well and cultured for 24 h. Scratches were made using a sterile 200-μL pipette tip; subsequently, HA was added to the HA group (final concentration of HA: 1 μg/mL) and the cells were cultured for 24 h (n = 3). The areas of the scratches were recorded using a digital camera, and the distance of scratches was measured using an ImageJ software program [28].
GAPDH activity assay
The activity of GAPDH was determined using a GAPDH Activity Assay Kit (Abcam) following the manufacturer’s instructions. B16F10 cells were seeded onto 12-well microplates at 1 × 105 cells/well and cultured for 24 h. After 24 h of HA treatment (n = 3) at different HA concentrations (0.025, 0.05, 0.25, 0.5, and 2.5 μg/mL), the cells were lysed using GAPDH assay buffer and the its activity was determined (mU/mL). The GAPDH activity in tumors from B16F10 graft mice model was also determined following the manufacturer’s protocol.
Study animals
This study was approved by the Institutional Animal Care and Use Committee of the Asahikawa Medical University (Approval number: R3-113). C57BL/6 mice were purchased from Charles River Laboratories Japan Inc. (Yokohama, Japan).
Homografts
B16F10 cells (2 × 106 cells/35 μL phosphate buffer saline [PBS]/tumor) were mixed with Matrigel (15 μL/tumor). After removing all hair, 50 μL of cell suspension was subcutaneously injected into the backs of the 6-week-old mice. The mice were then randomly divided into groups based on whether they received phosphate buffer saline (PBS) or HA, with five mice per group. Subsequently, 100 μL of PBS or 10 μg/100 μL PBS of HA was orally administered daily, starting with the day after the injection of B16F10 cells. The tumor size was calculated using the following formula: tumor size (mm2) = (major diameter) × (minor diameter). Whole blood was collected from the inferior vena cava and subjected to centrifugation at 2,500 × g for 10 min at room temperature, and the serum was obtained for mice in both groups. The serum samples were stored at − 80 °C and sent for biochemical examination to Oriental Yeast Co., Ltd. (Tokyo, Japan).
Statistical analysis
The assay data were analyzed using Student’s unpaired t-test. A p-value of < 0.05 was considered statistically significant.
Results
HA exhibited cytostatic activity and GAPDH inhibition in melanoma-derived B16F10 cells
To assess whether HA exerts an antiproliferative effect on melanoma-derived B16F10 cells, an SRB assay was performed. The growth of B16F10 cells was significantly suppressed by HA in a concentration-dependent manner on Days 2 and 3 (Fig. 1A). To investigate whether HA suppresses the migration of melanoma cells, a cell-scratch assay was performed. Cell migration significantly reduced after treatment with 1 μg/mL HA for 24 h (Fig. 1B), indicating that HA exerts an antiproliferative effect by suppressing the cell growth and migration of melanoma cells.
Fig. 1.
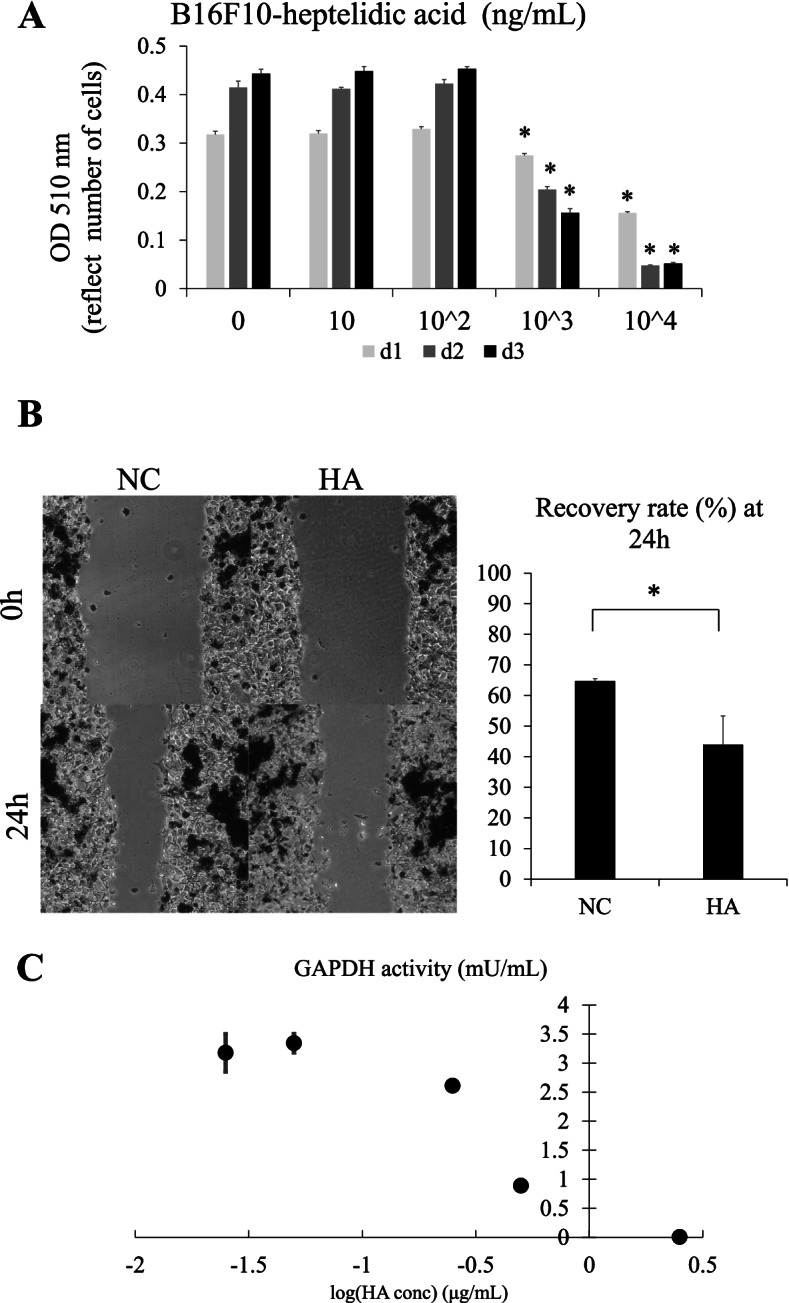
Heptelidic acid (HA) exerts an antiproliferative effect via inhibiting GAPDH in vitro. A sulforhodamine B assay revealed that HA exerted growth-suppressing effects on B16F10 cells in a concentration-dependent manner (A). A cell scratch assay indicted that HA inhibited the migration of B16F10 cells (B). GAPDH activity assay revealed that HA inhibited GAPDH activation in a concentration-dependent manner (C). The error bars represent the standard deviation (SD). * p < 0.05 by Student’s t-test
HA inhibits GAPDH activity in melanoma cells
Previous studies have suggested that HA irreversibly inhibits GAPDH activity, thereby exerting a growth-suppressive effect, particularly on cancer cells that depend on the Warburg effect [29]. Therefore, GAPDH activity in HA melanoma cells was assessed, and it was observed that HA inhibited the GAPDH activity in a concentration-dependent manner (Fig. 1C). Notably, the reduction in GAPDH enzymatic activity and cell density was parallelly shifted, indicating that the growth-suppressive function of HA was mediated by the inhibition of GAPDH activity in melanoma cells.
Orally administered HA exerted an antiproliferative effect on in vivo B16F10 homograft mice model
To confirm whether HA exerts tumor-suppressive effects in vivo, B16F10 cells were transplanted into C57BL/6 mice and 10 μg of HA was orally administered daily. The tumor size significantly decreased after HA administration (Fig. 2A).
Fig. 2.
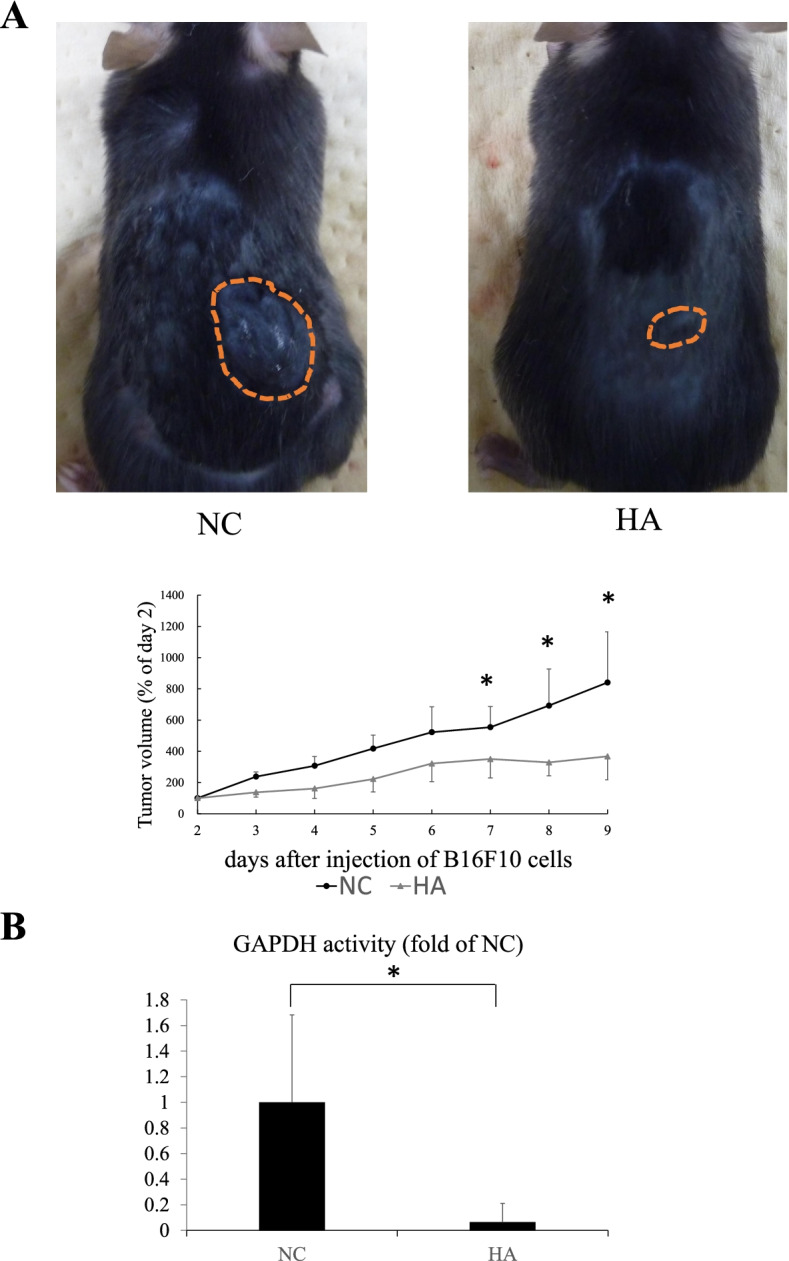
Heptelidic acid (HA) inhibited the tumor growth in an in vivo homograft model. B16F10 cells were transplanted into C57BL/6 mice and 10 μg of HA was orally administered daily. Tumor growth was significantly inhibited after HA treatment (A). GAPDH activity assay showed that the tumor GAPDH activity in the HA group was significantly decreased compared with that in the negative control group (B). The error bars represent the standard deviation (SD). * p < 0.05 by Student’s t-test
HA inhibited GAPDH activity in the grafted tumor
GAPDH activity assay was performed using isolated tumors to assess whether the activity in the transplanted tumor cells decreased after HA administration. The tumor GAPDH activity in the HA group significantly decreased by 0.07 times compared with that in the PBS group (Fig. 2B), suggesting that orally administered HA was absorbed through the intestinal lumen, reached the location of the transplanted tumor, and inhibited the GAPDH activity, thereby exerting antitumor effects.
HA exerted tumor-suppressive effects with less adverse effects
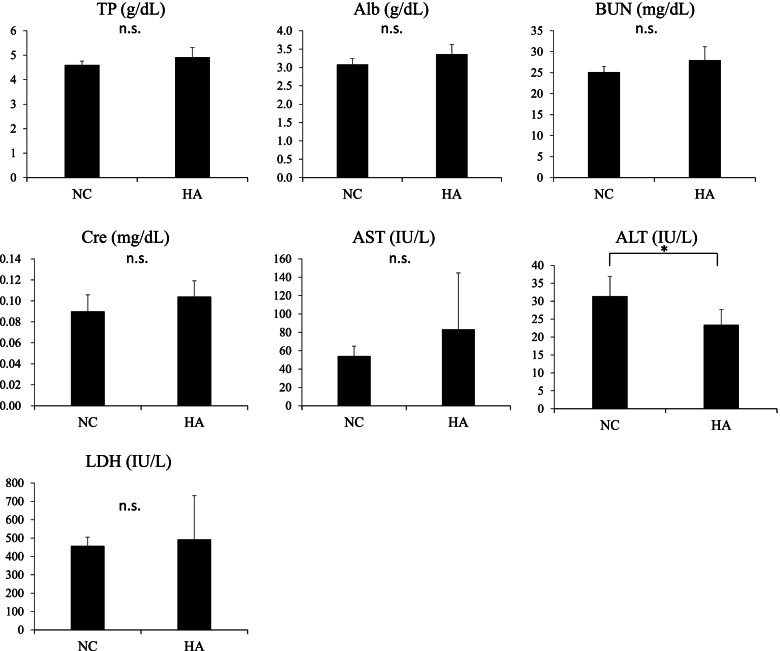
To assess whether HA induced tissue damage in vivo, biochemical testing was performed, the results of which revealed that the values of alanine transaminase in the HA group were significantly lower than those in the PBS group. The levels of total protein, albumin, blood urea nitrogen, creatinine, aspartate aminotransferase, and lactate dehydrogenase were not significantly different in the HA and PBS groups (Fig. 3).
Fig. 3.
Heptelidic acid (HA) treatment did not affect the results of serum biochemistry tests. Biochemical analysis showed no significant differences between the HA and negative control group in terms of TP, Alb, BUN, Cre, AST, and LDH values. The ALT level was significantly decreased after HA treatment. The error bars represent the standard deviation (SD). * p < 0.05 by Student’s t-test. TP, total protein; Alb, albumin; BUN, blood urea nitrogen; Cre, creatinine; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase
Discussion
This study determined that HA derived from A. oryzae exerts a strong tumor-suppressive effect on melanoma-derived B16F10 cells in an in vitro and in vivo homograft model. Oral HA treatment suppressed the growth of the extraintestinal tumors that were grafted under the skin of the back. A GAPDH activity assay indicated that HA treatment significantly decreased the GAPDH activity in directly treated B1610 cells in vitro and orally treated in vivo homograft mouse models. Therefore, HA may be one of the key molecules of the probiotic A. oryzae that inhibit extraintestinal tumor growth. This study proposes a novel extraintestinal tumor regulation system using bacteria-derived bioactive mediators.
In the past several decades, numerous investigations have demonstrated the efficacy of probiotics in the treatment of gastrointestinal cancer in preclinical tests. For instance, several Lactobacillus strains inhibited cell proliferation by inducing cell-cycle arrest in vitro [30] and inhibited the growth of colon cancer in dimethyl hydrazine-induced Sprague Dawley rats by inducing apoptosis in vivo [31]. Interestingly, probiotics and foods fermented by probiotics have therapeutic effects on various cancer types in vitro and in vivo. For instance, Kaga et al. showed that breast cancer progression is inhibited by the administration of L. casei Shirota in a rat chemical carcinogenesis model [32]. Fatahi et al. revealed that probiotic-fermented kefir exhibits antitumor activity against glioblastoma cells in vitro [33]. Greathouse et al. indicated that replenishing Lactobacillus and Bacteroides in the gut, which were decreased due to chemotherapy, can improve the efficacy of therapies for lung cancer [34]. Another clinical study revealed that FMT from a patient with melanoma who responded to immune checkpoint inhibitor (ICI) therapy improved the therapeutic response of an ICI-refractory patient by altering the intestinal flora [6]. These results indicate that probiotics and intestinal microbes can modulate the homeostasis of extraintestinal organs. However, the underlying mechanism of how microbiome affects the extraintestinal tumor progression and its mediators was poorly understood.
Bioactive natural products from microorganisms have been investigated to understand the symbiotic relationship between microbes and mammalian hosts. Some bacterial molecules, including lipopolysaccharides and flagellin, stimulate the toll-like receptor pathway in host epithelial and immune cells and are associated with the maintenance of host homeostasis. Septic shock and multiple organ failure occur when these molecules infiltrate the host’s body under conditions such as intestinal disorders [35, 36]. In this study, we demonstrated that HA, a probiotic molecule derived from A. oryzae, passes through the gastrointestinal tract and reaches the extragastrointestinal malignancy without losing its activity and suppresses tumor progression. This suggests that mammals have unique systems that would benefit them by mediating the function of bacterial molecules under an appropriate organic environment.
Previously, it was demonstrated that HA passes through the intestinal tract and exerts its antiproliferative effects on pancreatic cancer cells in an ex vivo study on mice intestinal loop [19]. However, whether orally administered HA reaches extra-gastrointestinal tumors was not clarified in that study. In the present study, the GAPDH inhibitory effect, which were assumed to be due to HA, was confirmed in transplanted tumors by HA administration in vivo, as seen in Fig. 2B. This strongly suggests that orally administered HA passes through the gut, is circulated in the bloodstream, reaches the transplanted tumors, and inhibits tumor growth in vivo.
The Warburg effect was first reported by Otto Warburg as a characteristic of glucose metabolization in cancer cells [37]. This report stated that glycolysis is activated even in the presence of sufficient oxygen and produces large amounts of lactate, thereby supporting tumor cell progression. Over the past decades, compounds that inhibit the Warburg effect were identified and their potential in cancer therapeutics was tested in in vitro clinical studies [38–40]. GAPDH is a rate-determining enzyme associated with the Warburg effect and it converts glyceraldehyde 3-phosphate (G3P) to D-1,3-bisphosphoglycerate (1,3-BPG). Tumor cells are highly dependent on the GAPDH-mediated glycolysis pathway for ATP production [29]. In this study, the in vitro and in vivo HA treatments downregulated GAPDH activity in melanoma cells, suggesting that the extraintestinal tumor-suppressive effects of HA were mediated by GAPDH inactivation. Regarding the observed adverse events, HA did not result in abnormal biochemical test results in vivo, indicating the little effect of the effective dose of HA as part of cancer therapy on hepatic and renal functions. Meanwhile, a high HA dose was reported to suppress the growth of non-cancerous cells, such as endothelial cells and fibroblasts, in vitro [41]. This might suggest that HA has a high affinity to tumor-associated antigens compared with non-cancerous cells; thus, an appropriate HA dose has a therapeutic effect without cytotoxicity.
Conclusion
We demonstrated that HA derived from A. oryzae HA exerts antiproliferative effects by inhibiting GAPDH in melanoma-derived B16F10 cells. Furthermore, orally administered HA exerted antitumor effects on subcutaneously grafted tumor cells via GAPDH inhibition in vivo, indicating that novel interactions occur between mutualistic bacteria and host tumors located in the distant gastrointestinal tract.
Supplementary Information
Additional file 1: Supplementary Table 1.
Acknowledgements
We thank Professor Satoshi Ichikawa and Dr. Takayuki Ohkuri for their great support in this study. B16-F10 cells were gifted by Dr. Takayuki Ohkuri. We thank Anzu Ushijima and Nobue Tamamura for their technical support.
Authors’ contributions
S.I., H.K., and M.F. provided major input regarding the conceptual development of the studies, wrote the manuscript, and supervised all the investigations. S.I. and C.Y. performed the biochemical experiments. H.T., K.M., and N.O. helped design the studies, interpret the data, and prepare and review the manuscript. All of the authors read and approved the final manuscript.
Funding
This study was partially supported by a grant-in-aid of The Ishidsu Shun Memorial Scholarship (S. Isozaki), Grants-in-Aid for Scientific Research, No. 19K19477 (S. Isozaki), 19K16484 (H. Konishi), 25460923 (K. Moriichi), 26460956 (M. Fujiya), Intractable Disease Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare (M. Fujiya).
Availability of data and materials
All data generated or analyzed in this study are included in this published article and its supplementary Table 1.
Declarations
Ethics approval and consent to participate
Approval (R3-113) was obtained from the Institutional Animal Care and Use Committee of Asahikawa Medical University. All animal experiments were performed in compliance with the ARRIVE guidelines. All methods were performed in accordance to relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
Dr. Fujiya acknowledges grants from Japanese Grants-in-Aid for Scientific Research, grants from Translational Research Network Program of Japan Agency for Medical Research and Development (21K07929), non-financial support from Development and Intractable Disease Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare.
Dr. Fujiya reports grants and personal fees from EA Pharma Co., Ltd., AYUMI Pharmaceutical Corporation, AbbVie Inc, Otsuka Pharmaceutical Co., Ltd., ZERIA Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nobelpharma Co., Ltd., Pfizer Inc, Janssen Pharmaceutical K.K., KYORIN Pharmaceutical Co., Ltd., MOCHIDA PHARMACEUTICAL CO.,LTD., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Company Limited, Yakult Honsha Co., Ltd., SHIONOGI & Co., Ltd. and ONO PHARMACEUTICAL CO., LTD., personal fees from AstraZeneca, OLYNPUS Co., Ltd., Sanofi K.K., celltrionhealthcare.jp, Medical Review Co., Ltd., NIHON PHARMACEUTICAL CO., LTD., Horii Pharmaceutical Ind.,Ltd, Alfresa Pharma Corporation, Merck Biopharma Co., Ltd., Mylan Inc., Bayer Yakuhin, Ltd., Boston Scientific Corporation, TEIJIN PHARMA LIMITED and Covidien Japan, Inc., personal fees and non-financial support from FUJIFILM Corporation, and grants from GlaxoSmithKline K.K., Bristol-Myers Company, Eli Lilly Japan K.K., Kanamic Network Co., Ltd., Fuji Chemical Industries Co., Ltd., JIMRO Co., Ltd., Chugai Pharmaceutical Co., Ltd., SHIN NIPPON BIOMEDICAL LABORATORIES, LTD. and Kamui Pharma. Inc.
Financial source
Dr. Fujiya reports grants from Japanese Grants-in-Aid for Scientific Research, grants from Translational Research Network Program of Japan Agency for Medical Research and Development (21K07929), non-financial support from Development and Intractable Disease Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare.
All the other authors have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pascal M, Perez-Gordo M, Caballero T, Escribese MM, Lopez Longo MN, Luengo O, et al. Microbiome and allergic diseases. Front Immunol. 2018;9:1584. doi: 10.3389/fimmu.2018.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 3.Gagnière J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016;22:501–518. doi: 10.3748/wjg.v22.i2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y, Dunaway S, Champer J, Kim J, Alikhan A. Changing our microbiome: probiotics in dermatology. Br J Dermatol. 2020;182:39–46. doi: 10.1111/bjd.18088. [DOI] [PubMed] [Google Scholar]
- 5.Smits LP, Bouter KEC, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 6.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 7.Tsai T-L, Li A-C, Chen Y-C, Liao Y-S, Lin T-H. Antimicrobial peptide m2163 or m2386 identified from Lactobacillus casei ATCC 334 can trigger apoptosis in the human colorectal cancer cell line SW480. Tumour Biol. 2015;36:3775–3789. doi: 10.1007/s13277-014-3018-2. [DOI] [PubMed] [Google Scholar]
- 8.Hatakeyama S, Kafuku M, Okamoto T, Kakizaki Y, Shimasaki N, Fujie N, et al. Studies on the anticancer mechanisms of the natto extract. J Soc Mater Eng Resour Jpn. 2016;27:15–19. doi: 10.5188/jsmerj.27.15. [DOI] [Google Scholar]
- 9.Konishi H, Fujiya M, Tanaka H, Ueno N, Moriichi K, Sasajima J, et al. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat Commun. 2016;7:12365. doi: 10.1038/ncomms12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ijiri M, Fujiya M, Konishi H, Tanaka H, Ueno N, Kashima S, et al. Ferrichrome identified from Lactobacillus casei ATCC334 induces apoptosis through its iron-binding site in gastric cancer cells. Tumour Biol. 2017;39:1010428317711311. doi: 10.1177/1010428317711311. [DOI] [PubMed] [Google Scholar]
- 11.Kita A, Fujiya M, Konishi H, Tanaka H, Kashima S, Iwama T, et al. Probiotic-derived ferrichrome inhibits the growth of refractory pancreatic cancer cells. Int J Oncol. 2020;57:721–732. doi: 10.3892/ijo.2020.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwama T, Fujiya M, Konishi H, Tanaka H, Murakami Y, Kunogi T, et al. Bacteria-derived ferrichrome inhibits tumor progression in sporadic colorectal neoplasms and colitis-associated cancer. Cancer Cell Int. 2021;21:21. doi: 10.1186/s12935-020-01723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo H, Sakuyama Tomari H, Yamakawa S, Kitagawa M, Yamada M, Itou S, et al. Long-term intake of miso soup decreases nighttime blood pressure in subjects with high-normal blood pressure or stage I hypertension. Hypertens Res. 2019;42:1757–1767. doi: 10.1038/s41440-019-0304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda K, Sato T, Nakayama T, Tanaka D, Nagashima K, Mano F, et al. Dietary habits associated with reduced insulin resistance: the Nagahama study. Diabetes Res Clin Pract. 2018;141:26–34. doi: 10.1016/j.diabres.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Nakamoto M, Uemura H, Sakai T, Katsuura-Kamano S, Yamaguchi M, Hiyoshi M, Arisawa K. Inverse association between soya food consumption and insulin resistance in Japanese adults. Public Health Nutr. 2015;18:2031–2040. doi: 10.1017/S136898001400247X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohara M, Lu H, Shiraki K, Ishimura Y, Uesaka T, Katoh O, Watanabe H. Prevention by long-term fermented miso of induction of colonic aberrant crypt foci by azoxymethane in F344 rats. Oncol Rep. 2002;9:69–73. [PubMed] [Google Scholar]
- 17.Watanabe H. Beneficial biological effects of miso with reference to radiation injury, cancer and hypertension. J Toxicol Pathol. 2013;26:91–103. doi: 10.1293/tox.26.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95:906–913. doi: 10.1093/jnci/95.12.906. [DOI] [PubMed] [Google Scholar]
- 19.Konishi H, Isozaki S, Kashima S, Moriichi K, Ichikawa S, Yamamoto K, et al. Probiotic Aspergillus oryzae produces antitumor mediator and exerts antitumor effects in pancreatic cancer through the p38 MAPK signaling pathway. Sci Rep. 2021;11:11070. doi: 10.1038/s41598-021-90707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endo A, Hasumi K, Sakai K, Kanbe T. Specific inhibition of glyceraldehyde-3-phosphate dehydrogenase by koningic acid (heptelidic acid) J Antibiot (Tokyo) 1985;38:920–925. doi: 10.7164/antibiotics.38.920. [DOI] [PubMed] [Google Scholar]
- 21.Kosova AA, Khodyreva SN, Lavrik OI. Role of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) in DNA Repair. Biochemistry (Mosc) 2017;82:643–654. doi: 10.1134/S0006297917060013. [DOI] [PubMed] [Google Scholar]
- 22.Nicholls C, Li H, Liu J-P. GAPDH: a common enzyme with uncommon functions. Clin Exp Pharmacol Physiol. 2012;39:674–679. doi: 10.1111/j.1440-1681.2011.05599.x. [DOI] [PubMed] [Google Scholar]
- 23.Tristan C, Shahani N, Sedlak TW, Sawa A. The diverse functions of GAPDH: views from different subcellular compartments. Cell Signal. 2011;23:317–323. doi: 10.1016/j.cellsig.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayna A, Moody PC. Crystal structures of a dual coenzyme specific glyceraldehyde-3-phosphate dehydrogenase from the enteric pathogen Campylobacter jejuni. J Mol Struct. 2021;1242:130820. doi: 10.1016/J.MOLSTRUC.2021.130820. [DOI] [Google Scholar]
- 25.Ganapathy-Kanniappan S. Evolution of GAPDH as a druggable target of tumor glycolysis? Expert Opin Ther Targets. 2018;22:295–298. doi: 10.1080/14728222.2018.1449834. [DOI] [PubMed] [Google Scholar]
- 26.Shestov AA, Liu X, Ser Z, Cluntun AA, Hung YP, Huang L, et al. Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. Elife. 2014 doi: 10.7554/eLife.03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 28.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberti MV, Dai Z, Wardell SE, Baccile JA, Liu X, Gao X, et al. A Predictive model for selective targeting of the Warburg effect through GAPDH inhibition with a natural product. Cell Metab. 2017;26:648–659.e8. doi: 10.1016/j.cmet.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxami G, Karapetsas A, Lamprianidou E, Kotsianidis I, Chlichlia A, Tassou C, et al. Two potential probiotic lactobacillus strains isolated from olive microbiota exhibit adhesion and anti-proliferative effects in cancer cell lines. J Funct Food. 2016;24:461–471. doi: 10.1016/j.jff.2016.04.036. [DOI] [Google Scholar]
- 31.Gamallat Y, Meyiah A, Kuugbee ED, Hago AM, Chiwala G, Awadasseid A, et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed Pharmacother. 2016;83:536–541. doi: 10.1016/j.biopha.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Kaga C, Takagi A, Kano M, Kado S, Kato I, Sakai M, et al. Lactobacillus casei Shirota enhances the preventive efficacy of soymilk in chemically induced breast cancer. Cancer Sci. 2013;104:1508–1514. doi: 10.1111/cas.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fatahi A, Soleimani N, Afrough P. Anticancer activity of kefir on glioblastoma cancer cell as a new treatment. Int J Food Sci. 2021;2021:8180742. doi: 10.1155/2021/8180742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greathouse KL, White JR, Vargas AJ, Bliskovsky VV, Beck JA, von Muhlinen N, et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018;19:123. doi: 10.1186/s13059-018-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Burgueño JF, Abreu MT. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17:263–278. doi: 10.1038/s41575-019-0261-4. [DOI] [PubMed] [Google Scholar]
- 37.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. doi: 10.1126/science.124.3215.269. [DOI] [PubMed] [Google Scholar]
- 38.Amadori D, Frassineti GL, de Matteis A, Mustacchi G, Santoro A, Cariello S, et al. Modulating effect of lonidamine on response to doxorubicin in metastatic breast cancer patients: results from a multicenter prospective randomized trial. Breast Cancer Res Treat. 1998;49:209–217. doi: 10.1023/a:1006063412726. [DOI] [PubMed] [Google Scholar]
- 39.Chen X-S, Li L-Y, Guan Y, Yang J-M, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37:1013–1019. doi: 10.1038/aps.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nath K, Nelson DS, Heitjan DF, Leeper DB, Zhou R, Glickson JD. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed. 2015;28:281–290. doi: 10.1002/nbm.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumagai S, Narasaki R, Hasumi K. Glucose-dependent active ATP depletion by koningic acid kills high-glycolytic cells. Biochem Biophys Res Commun. 2008;365:362–368. doi: 10.1016/j.bbrc.2007.10.199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1.
Data Availability Statement
All data generated or analyzed in this study are included in this published article and its supplementary Table 1.