Abstract
The anxiolytic and antidepressant properties of cannabidiol (CBD) have been evaluated in several studies. However, the molecular mechanisms involved in these actions remain unclear. A total of 130 male mice were used. CBD’s ability to modulate emotional disturbances (anxiety and depressive-like behaviors) was evaluated at different doses in wild-type (CD1; 10, 20 and 30 mg/kg; i.p.) and knockout (CB1KO, CB2KO; GPR55KO; 20 mg/kg) mice. Moreover, CBD effects (20 mg/kg; i.p.) were evaluated in mice previously treated with the CB1r-antagonist SR141716A (2mg/kg; i.p.). Relative gene expression analyses of Cnr1 and Cnr2, Gpr55 and GABA(A)α2 and γ2 receptor subunits were performed in the amygdala (AMY) and hippocampus (HIPP) of CD1 mice. CBD (10 and 20 mg/kg) showed anxiolytic and antidepressant actions in CD1 mice, being more effective at 20 mg/kg. Its administration did not induce anxiolytic actions in CB1KO mice, contrary to CB2KO and GPR55KO. In all of them, the lack of cannabinoid receptors did not modify the antidepressant activity of CBD. Interestingly, the administration of the CB1r antagonist SR141716A blocked the anxiolytic-like activity of CBD. Real-time PCR studies revealed a significant reduction in Cnr1 and GABA(A)α2 and γ2 gene expression in the HIPP and AMY of CD1 mice treated with CBD. Opposite changes were observed in the Cnr2. Indeed, Gpr55 was increased in the AMY and reduced in the HIPP. CB1r appears to play a relevant role in modulating the anxiolytic actions of CBD. Moreover, this study revealed that CBD also modified the gene expression of GABA(A) subunits α2 and γ2 and CB1r, CB2r and GPR55, in a dose- and brain-region-dependent manner, supporting a multimodal mechanism of action for CBD.
Keywords: cannabidiol, anxiety, depression, cannabinoid receptor 1, cannabinoid receptor 2, G-protein-coupled receptor 55, GABA(A) receptor
1. Introduction
Mood disorders are considered one of the most prevalent psychiatric disorders with a high socioeconomic and health impact. Anxiety and depression are the most common, with an estimated 280 million people suffering from each [1,2,3,4,5,6]. The treatment of both includes complex pharmacological strategies combined with cognitive-behavioral therapies. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used antidepressant drugs, with significant limitations regarding their therapeutic effectiveness. Indeed, up to 30% of patients with major depression develop treatment resistance to the first-line selected drugs [7,8,9,10,11]. On the other hand, benzodiazepines, the most commonly prescribed anxiolytic drugs, are limited because of their high risk of abuse and adverse effects [12,13]. Thus, the therapeutic limitations in treating these disorders highlight the need to develop new, more effective and safer pharmacological strategies.
In recent years, the endocannabinoid system (ECS) has attracted interest because of its implication in the pathophysiology of neuropsychiatric disorders, including mood disorders [14,15,16,17,18,19]. Thus, the modulation of this system could be an exciting tool for their treatment. Cannabidiol (CBD) is one of the main compounds of the Cannabis sativa plant without properties as a drug of abuse [20]. This drug can interact with more than 65 different targets, such as G-protein-coupled receptor 55 (GPR55), vanilloid receptors (TRPV1), serotonergic receptor 5-HT1A, mu and delta-opioid receptors and peroxisome proliferator-activated receptor gamma (PPAR-γ) [21,22,23,24,25,26,27]. Notably, CBD acts as a non-competitive allosteric modulator of cannabinoid receptor 1 (CB1r) [21,28] and as an inverse agonist of cannabinoid receptor 2 (CB2r) [25]. Several clinical and preclinical studies showed that CBD presents antidepressant, anxiolytic, antipsychotic and neuroprotective actions, presenting an attractive potential therapeutic strategy for treating mood disorders [29,30,31]. In this respect, its possible utility in anxiety and depression has been evaluated in several animal models, with promising results [32,33,34,35,36,37]. The involvement of 5-HT1A receptors in CBD’s mechanism of action has been proposed [32,33,36]. Additional studies revealed the involvement of CB1r in the anxiolytic activity of CBD [38,39,40].
Cumulative evidence supports the role of cannabinoid receptors (CB1 and CB2) in regulating the response to stress, anxiety, depression, schizophrenia and in cognition [41,42,43,44,45,46,47]. Interestingly, both cannabinoid receptors are associated with significant alterations in the expression of anxiolytic-mediated subunits of the GABA(A) receptor [42,48,49] and with the anxiolytic action of benzodiazepines [41,42]. More recently, additional receptors on which endocannabinoids also act, such as the GRP55 receptor, have been associated with the regulation of emotional reactivity [50,51,52] and hippocampal plasticity [53]. Interestingly, previous studies have demonstrated that CBD modifies the gene expression of these targets in animal models of PTSD [54], alcohol consumption [55,56] and spontaneous cannabinoid withdrawal [57], in which CBD showed efficacy.
The present study aimed to characterize the mechanisms by which CBD exhibits its anxiolytic and antidepressant actions, emphasizing CB1r, CB2r and GPR55. In the first part, we evaluated dose-response acute CBD effects in wild-type animals (WT) in a battery of tests for assessing anxiety and coping-like behavior. In the second part, CBD effects were evaluated in genetically modified mice lacking CB1r (CB1KO), CB2r (CB2KO), and GPR55 (GPR55KO) exposed to representative behavioral tests for measuring anxiogenic- and coping-like behaviors. In addition, pharmacological studies using the CB1r-antagonist, SR141716A, were carried out to further clarify the role of CB1r in CBD effects. Finally, gene expression studies were conducted to analyze potential changes in Cnr1, Cnr2, Gpr55 and GABA(A) genes induced by acute CBD administration in WT animals using real-time PCR.
2. Results
2.1. Behavioral Evaluation of CBD Actions in WT Mice
We first wanted to evaluate the acute anxiolytic and antidepressant-like effects of CBD. For this purpose, we chose well-accepted animal models for assessing anxiety-like behaviors, using the light-dark box (LDB), the elevated plus maze (EPM) test and novelty suppressed feeding (NSFT) test, coping behavior, and tail suspension test (TST) in rodents. These studies were designed to help further characterize the acute effects of CBD in the modulation of anxiety and depressive-like behaviors in mice.
2.1.1. Light-Dark Box Test (LDB)
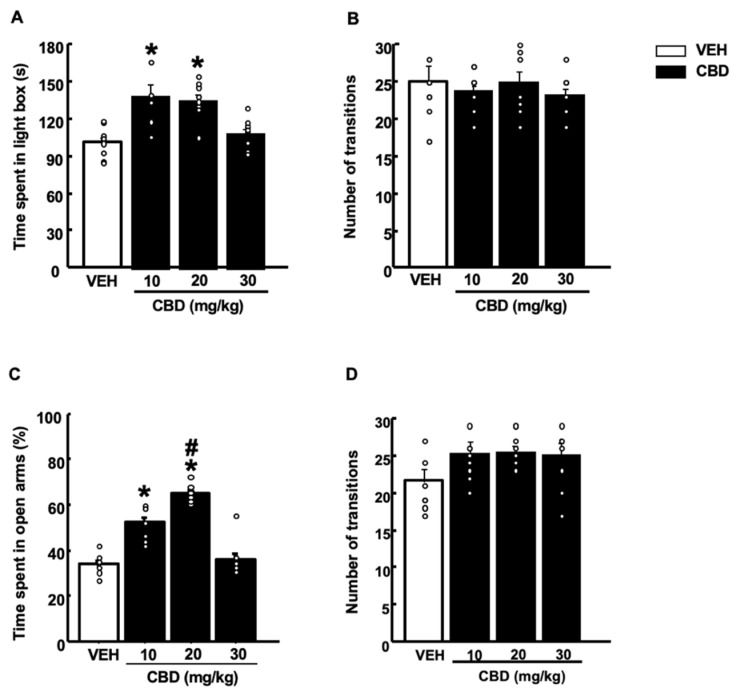
Mice treated with CBD at 10 and 20 mg/kg doses spent more time in the lighted box than vehicle (VEH)-treated mice. Interestingly, these anxiolytic actions were not observed at a dose of 30 mg/kg (Figure 1A, one-way ANOVA followed by Student–Newman–Keuls test, F(3,39) = 10.124, p < 0.001) (n = 9–10/group). In addition, no changes were observed in the number of transitions between groups (Figure 1B, one-way ANOVA followed by Student–Newman–Keuls test, F(3,39) = 0.421, p = 0.739) (n = 9–10/group).
Figure 1.
Effects of a single administration of cannabidiol (CBD) (10, 20 and 30 mg/kg, i.p.) on anxiety-like behaviors in the light-dark box (A,B) and elevated plus maze (C,D) paradigms. The behavioral evaluation was developed 1 h and 30 min after the administration of CBD (or vehicle (VEH)). Columns represent the means and vertical lines ± SEM of (A) the time in the lighted box (s); (B) the number of transitions in the light-dark box test; (C) the percentage of time in the open arms (%); and (D) the number of transitions in the elevated plus-maze test. * Values from CBD-treated mice that were different (p < 0.05) from VEH-treated mice and # values from CBD-20 mg/kg-treated mice that were different from CBD-10 mg/kg-treated mice (one-way ANOVA followed by Student–Newman–Keuls test, p < 0.05).
2.1.2. Elevated Plus Maze Test (EPM)
CBD exerted an anxiolytic-like effect after a dose of 10 mg/kg, increasing the percentage of time spent in the open arms compared to controls. Interestingly, a dose of 20 mg/kg induced a more pronounced anxiolytic action than for CBD-10 mg/kg-treated mice (Figure 1C, one-way ANOVA followed by Student–Newman–Keuls test, F(3,38) = 66,908, p < 0.001) (n = 9–10/group). In contrast, no effect was observed at the highest dose of CBD (30 mg/kg) compared to the VEH group. No differences were observed in the number of transitions between all four groups (Figure 1D, one-way ANOVA followed by Student–Newman–Keuls test, F(3,35) = 2.056, p = 0.126) (n = 9–10/group).
2.1.3. Tail Suspension Test (TST)
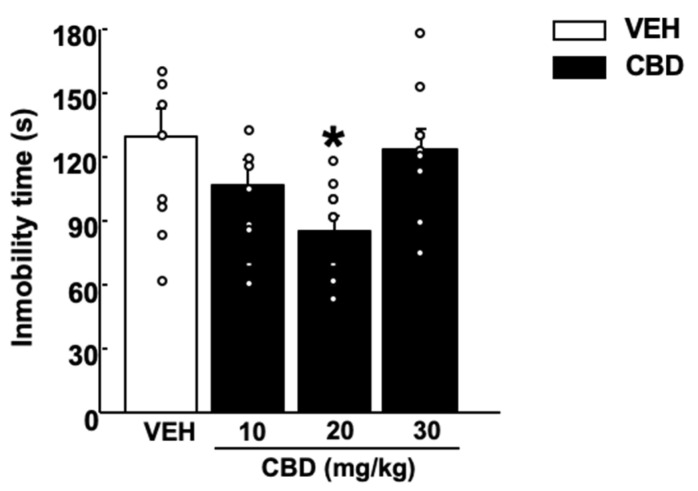
Treatment with CBD significantly reduced the immobility time at a dose of 20 mg/kg. Interestingly, the lower and the higher doses of CBD (10 and 30 mg/kg) did not induce any effects (Figure 2, One-way ANOVA followed by Student–Newman–Keuls test, F(3,38) = 3.364, p = 0.029) (n = 9–10/group).
Figure 2.
Effects of a single administration of cannabidiol (CBD) (10, 20 and 30 mg/kg, i.p.) on coping behaviors in the tail suspension test paradigm. The behavioral evaluation was developed 1 h and 30 min after the administration of CBD (or vehicle (VEH)). Columns represent the means and vertical lines ± SEM of immobility time (s). * Values from CBD-20 mg/kg-treated mice that were different (p < 0.05) from VEH-treated group (one-way ANOVA followed by Student–Newman–Keuls test, p < 0.05).
2.1.4. Novelty Suppressed Feeding Test (NSFT)
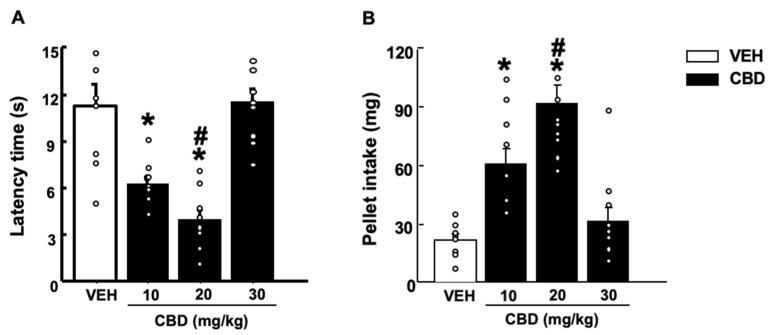
Mice showed significantly shorter latency time and increased consumption of food pellets (mg) with a dose of 10 mg/kg of CBD compared with the control group. The 20 mg/kg intermediate dose revealed major anxiolytic and hedonic actions. In contrast, the dose of 30 mg/kg did not induce any differences compared with VEH-treated mice (latency time: Figure 3A, one-way ANOVA followed by Student–Newman–Keuls test, F(3,36) = 19.411, p < 0.001; Food consumption: Figure 3B, one-way ANOVA followed by Student–Newman–Keuls test, F(3,37) = 16.840, p < 0.001) (n = 9–10/group).
Figure 3.
Effect of a single administration of cannabidiol (CBD) (10, 20 and 30 mg/kg, i.p.) on anxiety-like behaviors in the novelty suppressed feeding test. The behavioral evaluation was developed 1 h and 30 min after the administration of CBD (or vehicle (VEH)). Columns represent the means and vertical lines ± SEM of (A) latency time (s) and (B) pellet intake (mg). * Values from CBD-treated mice that were different (p < 0.05) from VEH-treated mice, and # values from 20 mg/kg of CBD-treated mice that were different from mice treated with the lower dose of CBD (10 mg/kg) (one-way ANOVA followed by Student–Newman–Keuls test, p < 0.05).
2.2. Effects of CBD on Anxiety and Coping-like Behaviors in Mice Lacking CB1r, CB2r and GPR55
Considering the role of CB1r, CB2r and GPR55 in modulating emotional reactivity, and that they are proposed targets on which CBD directly or indirectly acts, we aimed to explore their involvement in the anxiolytic and antidepressant-like effects of CBD. For this purpose, we evaluated the effects of CBD in the LDB and the TST in mice lacking the CB1r (CB1KO), CB2r (CB2KO) and GPR55 (GPR55KO) receptors. These results enable an improved understanding of the mechanism of action of CBD.
2.2.1. Light-Dark Box (LDB)
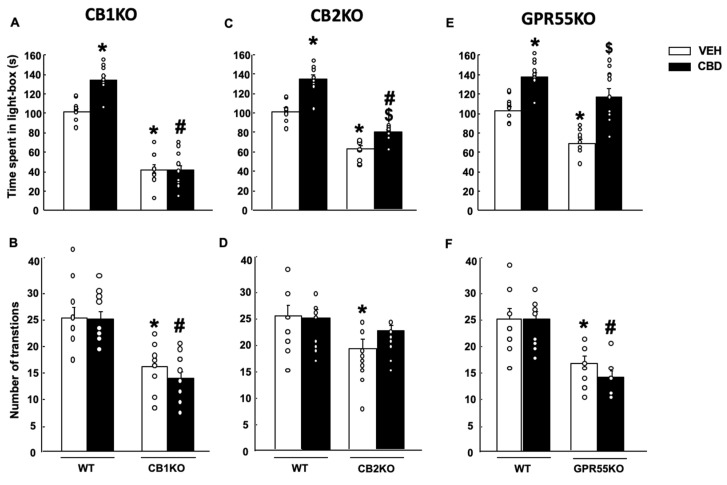
Acute CBD (20 mg/kg) administration did not modify anxiety-like behaviors in CB1KO mice compared to the control group (Figure 4A, two-way ANOVA followed by Student–Newman–Keuls test: genotype F(1,40) = 263.42, p < 0.001; treatment F(1,40) = 12.386, p = 0.001; genotype − treatment: F(1,40) = 13.242, p < 0.001) (n = 10–11). In contrast, in CB2KO and GPR55KO mice an anxiolytic effect was observed after CBD administration (CB2KO: Figure 4C, two-way ANOVA followed by Student–Newman–Keuls test: genotype F(1,38) = 180.23, p < 0.001; treatment F(1,40) = 56.126, p < 0.001; genotype − treatment: F(1,40) = 4.829, p = 0.035) (n = 9–10/group); GPR55KO: Figure 4E, two-way ANOVA followed by Student–Newman–Keuls test: genotype F(1,37) = 23.265, p < 0.001; treatment F(1,37) = 53.020, p < 0.001; genotype − treatment: F(1,37) = 1.555, p = 0.221) (n = 8–10/group).
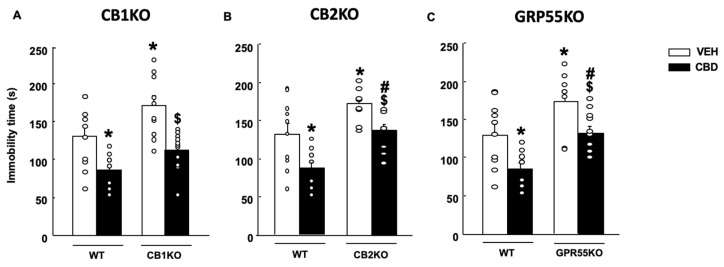
Figure 4.
Effects of a single administration of cannabidiol (CBD) at a dose of 20 mg/kg (i.p.) on anxiety-like behaviors in the light-dark box in mice lacking the cannabinoid receptor 1 (CB1KO) (A,B), lacking the cannabinoid receptor 2 (CB2KO) (C,D), and lacking the G-protein-coupled receptor 55 (GPR55KO) (E,F) mice. The behavioral evaluation was developed 1 h and 30 min after the administration of CBD (or vehicle (VEH)). Columns represent the means and vertical lines ± SEM of the time in the lighted box (s) (A,C,E) and the number of transitions (B,D,F). Results from CD1 VEH and CBD (20 mg/kg) groups have been included for comparative purposes. * Values from groups that were different from wild-type (WT)-VEH treated mice (two-way ANOVA followed by Student–Newman–Keuls test, p < 0.05). $ Values from groups that were different from KO-VEH treated mice (two-way ANOVA followed by Student–Newman–Keuls test, p < 0.05). # Values from groups that were different from WT-CBD-treated mice (two-way ANOVA followed by Student–Newman–Keuls test, p < 0.05).
No changes were observed in the number of transitions between CBD- and VEH-treated mice (CB1KO: Figure 4B, two-way ANOVA followed by Student–Newman–Keuls test: genotype F(1,40) = 39.216, p < 0.001; treatment F(1,40) = 0.580, p = 0.451; genotype x treatment F(1,40) = 0.407, p = 0.528) (CB2KO: Figure 4D, two-way ANOVA followed by Student–Newman–Keuls test: genotype F(1,38) = 6.762, p = 0.014; treatment F(1,38) = 0.899, p = 0.349; genotype x treatment F(1,38) = 1.146, p = 0.292) (GPR55KO: Figure 4E, two-way ANOVA followed by Student–Newman–Keuls test: genotype F(1,37) = 24.433, p < 0.001; treatment F(1,37) = 0.429, p = 0.517; genotype x treatment F(1,37) = 0.306, p = 0.584).
2.2.2. Tail Suspension Test (TST)
CBD at the dose of 20 mg/kg elicited antidepressant-like effects among all CB1KO, CB2KO and GPR55KO mice (CB1KO: Figure 5A, two-way ANOVA followed by Student–Newman–Keuls test: genotype F(1,40) = 9.878, p = 0.003; treatment F(1,40) = 23.176, p < 0.001; genotype x treatment F(1,40) = 0.390, p = 0.536; n = 10–11) (CB2KO: Figure 5B, two-way ANOVA followed by Student–Newman–Keuls test: genotype F(1,38) = 22.938, p < 0.001; treatment F(1,38) = 17.379, p < 0.001; genotype x treatment F(1,38) = 0.278, p = 0.601; n = 9–10) (GPR55KO: Figure 5C, two-way ANOVA followed by Student–Newman–Keuls test: genotype F(1,37) = 16.233, p < 0.001; treatment F(1,37) = 14.633, p < 0.001; genotype x treatment F(1,37) = 0.0389, p = 0.845; n = 8–9).
Figure 5.
Effects of a single administration of cannabidiol (CBD) at 20 mg/kg (i.p.) on coping behaviors in the tail suspension test in mice lacking the cannabinoid receptor 1 (CB1KO) (A), lacking the cannabinoid receptor 2 (CB2KO) (B) and lacking the G-protein-coupled receptor 55 (GPR55KO) (C). The behavioral evaluation was developed 1 h and 30 min after administration of CBD (or vehicle (VEH)). Columns represent the means and vertical lines ± SEM of immobility time (s). Results from CD1-VEH and CBD (20 mg/kg) groups have been included for comparative purposes. * Values from groups that were different from wild-type (WT)-VEH-treated mice (two-way ANOVA followed by Student–Newman–Keuls test, p < 0.05). $ Values from groups that were different from KO-VEH treated mice (two-way ANOVA followed by Student–Newman–Keuls test, p < 0.05). # Values from groups that were different from WT CBD-treated mice (two-way ANOVA followed by Student–Newman–Keuls test, p < 0.05).
2.3. Effects of CBD in Combination with a Selective CB1r Antagonist on Anxiety-like Behaviors in WT Mice
Considering that CBD did not show any anxiolytic-like effect in CB1KO mice, we thoroughly explored the role of CB1r in CBD properties by administering the CB1r-antagonist SR141716A before CBD administration in CD1 mice and evaluated its effects in the LBD test. The results would demonstrate the involvement of CB1r in CBD anxiolytic properties.
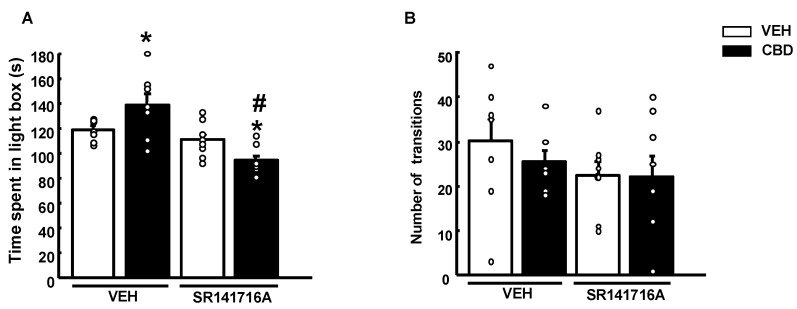
The administration of SR141716A did not modify the time spent in the lighted box in the LDB paradigm. In contrast, CBD showed an anxiolytic action at 20 mg/kg. Interestingly, this effect was completely blocked when combined with the CB1r-antagonist, inducing even a mild anxiogenic effect (Figure 6A, two-way ANOVA followed by Student–Newman–Keuls test, SR: (1,31) F = 22,002, p < 0.001; CBD: (1,31) F = 0.0427, p = 0.838; SR × CBD: (1,31) F = 10,226, p = 0.003) (n = 8/group). No differences were observed in the number of transitions between groups (Figure 6B, two-way ANOVA followed by Student–Newman–Keuls test, SR: F(1,31) = 2.080, p = 0.160; CBD: F(1,31) = 0.382, p = 0.542; SR × CBD: (1,31) F = 0.308, p = 0.584) (n = 8/group).
Figure 6.
Effects of a single administration of cannabidiol (CBD) (20 mg/kg, i.p.) in mice pre-treated with the cannabinoid receptor 1 (CB1r)-antagonist SR141716A (2 mg/kg, i.p.) on the light-dark box test. The behavioral evaluation was developed 2 h after SR141716A (or vehicle) and 1 h and 30 min after the administration of CBD (or vehicle (VEH)). Columns represent the means and vertical lines ± SEM of the time in the lightbox (s) (A) and the number of transitions (B). * Values from CBD-treated mice that were different (p < 0.05) from vehicle (VEH)-treated mice, and # values from SR141716A + CBD-treated mice that were different from VEH + CBD and SR141716A + VEH-treated animals (two-way ANOVA followed by Student–Newman–Keuls test, p < 0.05).
2.4. Gene Expression Studies of Cnr1, Cnr2, Gpr55 and GABA (A)α2 and γ2 Subunits in Wt Mice Treated with CBD
Additionally, we carried out gene expression studies to identify alterations in key targets closely related to emotional reactivity and anxiety, such as Cnr1, Cnr2, Gpr55 and the α2 and γ2 subunits of GABA(A) receptors in the amygdala (AMY) and hippocampus (HIPP) of mice treated with CBD, and two brain corticolimbic regions involved in a broad range of behavioral and cognitive functions, including emotional regulation. We chose real-time PCR to measure these targets’ relative gene expression.
2.4.1. Cannabinoid Receptors
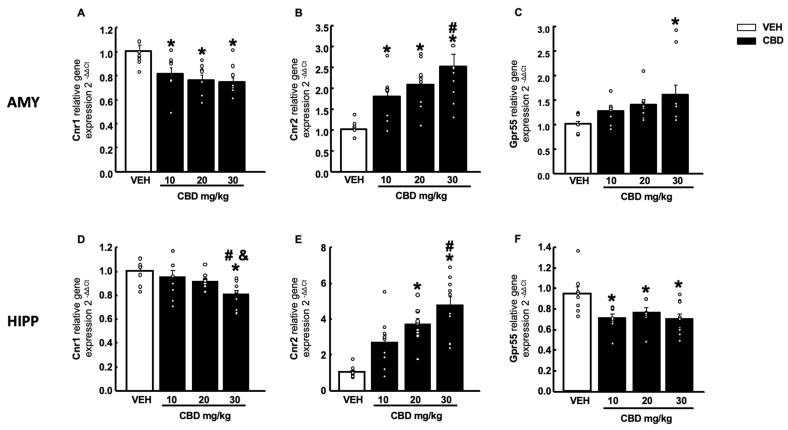
CBD administration induced a dose-dependent decrease in Cnr1 gene expression in the AMY at all doses (Figure 7A, one-way ANOVA followed by Student–Newman–Keuls test, F(3,35) = 6.699, p < 0.001) (n = 9–10/group). The same effect was observed in the HIPP but only at the highest dose of CBD (Figure 7D, one-way ANOVA followed by Student–Newman–Keuls test, F(3,36) = 4.692, p = 0.008) (n = 9–10/group). This reduction was accompanied by an increase in gene expression of Cnr2 (at all 3 doses) in the AMY (Figure 7B, one-way ANOVA followed by Student–Newman–Keuls test, F(3,34) = 8.910, p < 0.001) (n = 9–10/group) and HIPP (at 20 and 30 mg/kg) (Figure 7E, one-way ANOVA followed by Student–Newman–Keuls test, F(3,36) = 7.178, p < 0.001) (n = 9–10/group). Gpr55 only increased at a dose of 30 mg/kg (Figure 7C, one-way ANOVA followed by Student–Newman–Keuls test, F(3,35) = 3.521, p = 0.026) (n = 9–10/group) in the AMY, whereas in the HIPP, a significant reduction was observed at all doses tested (Figure 7F, one-way ANOVA followed by Student–Newman–Keuls test, F(3,36) = 4.948, p = 0.006) (n = 9–10/group).
Figure 7.
Effects of a single administration of cannabidiol (CBD) (10, 20 and 30 mg/kg, i.p.) on the relative gene expression of cannabinoid receptor 1 (Cnr1), cannabinoid receptor 2 (Cnr2) and G-protein-coupled receptor 55 (GPR55) in the amygdala (AMY) (A–C) and hippocampus (HIPP) (D–F). Columns represent the means and vertical lines ± SEM of the relative gene expression (2-ΔΔCt). * Values from CBD-treated mice that were different from vehicle (VEH)-treated mice, and # values from 30 mg/kg of CBD-treated mice that were different from the lower dose of CBD (10 mg/kg) treated animals. & Values from 30 mg/kg of CBD-treated mice that were different from the CBD (20 mg/kg) treated mice (one-way ANOVA followed by Student–Newman–Keuls test, p < 0.05).
2.4.2. GABA (A) Receptor Subunits
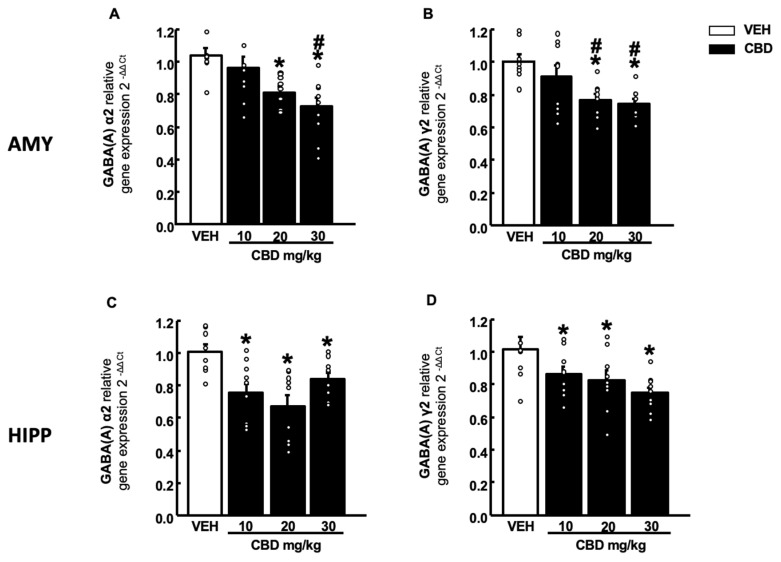
CBD administration at doses of 20 and 30 mg/kg decreased GABA(A)α2 gene expression in the AMY, this reduction being more pronounced with the highest dose of 30 mg/kg (Figure 8A, one-way ANOVA followed by Student–Newman–Keuls test, F(3,35) = 7.048, p < 0.001) (n= 9–10/group). Similarly, in the HIPP, a reduction of GABA(A)α2 was observed at all doses of CBD tested (Figure 8C, one-way ANOVA followed by Student–Newman–Keuls test, F(3,36) = 6.759, p < 0.001) (n = 9–10/group). In addition, for GABA(A)γ2 gene a dose-dependent decrease was observed in both regions, AMY (Figure 8B, one-way ANOVA followed by Student–Newman–Keuls test, F(3,35) = 6.899, p < 0.001) (n = 9–10/group) and HIPP (Figure 8D, one-way ANOVA followed by Student–Newman–Keuls test, F(3,36) = 4.460, p = 0.010) (n = 9–10/group).
Figure 8.
Effects of a single administration of CBD (10, 20 and 30 mg/kg, i.p.) on the relative gene expression of GABA(A) α2 and γ2 in the amygdala (AMY) (A,C) and hippocampus (HIPP) (B,D). Columns represent the means and vertical lines ± SEM of the relative gene expression (2-ΔΔCt). * Values from CBD-treated mice that were different from vehicle (VEH)-treated mice and # values from CBD (20 or 30 mg/kg)-treated mice that were different from the lower dose of CBD (10 mg/kg) treated animals (one-way ANOVA followed by Student–Newman–Keuls test, p < 0.05).
3. Discussion
The present study results confirm that CBD may significantly promote anxiolytic- and antidepressant-like effects in mice in a dose-dependent manner, effects that are mediated, at least in part, by CB1r. This statement is based on the following observations: (1) Low and intermediate acute doses of CBD (10 and 20 mg/kg) induced anxiolytic- and antidepressant-like effects in the behavioral tests assessed in WT mice; (2) Acute CBD administration (20 mg/kg) failed to induce any anxiolytic-like effects in CB1KO mice, whereas it was observed in CB2KO and GPR55KO mice; (3) the administration of the CB1r-antagonist, SR141716A, blocked the anxiolytic action of CBD; (4) CBD presented an antidepressant-like effect in all the knockout mice used; and (4) the administration of CBD reduced Cnr1, GABA(A)α2 and GABA(A)γ2 gene expression in the AMY and HIPP, whereas it increased Cnr2 in both regions. In contrast, Gpr55 gene expression increased in the AMY but decreased in the HIPP after administration of CBD.
Previous studies have shown that CBD induced anxiolytic- and antidepressant-like effects following an inverted U-shape curve, being effective at intermediate but not at very low or high doses [31,58,59,60]. In agreement with these studies, we found that low (10 mg/kg) and intermediate doses (20 mg/kg) induced an anxiolytic-like effect, since both doses increased the exploration time in anxiogenic environments in the LDB and EPM test. Moreover, CBD at these doses also reduced the latency in the NSFT, increasing the food intake. The intermediate CBD dose presented a more robust antidepressant-like effect than the lower dose since it significantly reduced coping behavior, indirectly measured by the immobility time in the TST.
In none of the behavioral tests performed, did CBD at the highest dose (30 mg/kg) show any anxiolytic or antidepressant-like effect, as described previously [61]. However, opposite results were found in other studies in which the same dose induced an anxiolytic- [34,62] or antidepressant-like effect [35,36,63,64]. These discrepancies may be due to differences in methodological procedures, such as different animal species (rats and mice), strains (Wistar rats, C57Bl6J, ICR), behavioral tests applied, and/or the pattern of administration (for more details, see [30]).
Despite evidence supporting the anxiolytic and antidepressant properties of CBD under certain experimental conditions, the complete characterization of the underlying mechanisms of action is still pending. In this respect, 5-HT1A is one of the main targets studied among the more than 65 targets on which CBD acts [29,65,66], demonstrating the involvement of this receptor in its anxiolytic [32,67,68,69,70] and antidepressant-like effects [33,36,40]. Here, we aimed to explore further the implication of additional CBD proposed targets, such as CB1r, CB2r and GPR55, given the critical role these receptors play in emotional reactivity, anxiety and mood disorders [14,42,44,48,51,52,71,72,73]. Studies on genetically modified mice have provided evidence, since CB1KO [47,74,75,76] and CB2KO mice [45,77] showed increased anxiety and depressive-like behaviors. Moreover, recent studies carried out by our group demonstrated that GPR55KO mice also displayed anxiogenic-like responses (to be published).
Genetic (CB1KO) and pharmacological (SR141716A) approaches show CB1r as an undoubtedly active receptor mediating the anxiolytic properties of CBD. Thus, we evaluated the effects of the effective CBD dose (20 mg/kg) in CB1KO, CB2KO and GPR55KO mice. CBD induced anxiolytic-like effects in CB2KO and GRP55KO but did not affect CB1KO mice. Moreover, a pharmacological study using the CB1r-antagonist SR141716A demonstrated that the blockade of CB1r avoids CBD-induced anxiolytic-like effects in the LBD test. Importantly, SR141716A did not induce any effect when it was given alone. These results agree with previous studies demonstrating the involvement of CB1r in the anxiolytic actions of CBD [40,78,79,80,81]. However, when evaluating the effects of CBD on coping behaviors in the tail suspension test in the different knockout mice, the lack of these receptors did not prevent CBD antidepressant effects. Therefore, based on these results, it is tempting to speculate that other receptors, such as the 5-HT1A receptor described above, may be even more critical in understanding the antidepressant action of CBD.
Real-time PCR analyses revealed that acute CBD administration modified gene expression of Cnr1, Cnr2 and Gpr55 in a dose- and brain-region-dependent manner. AMY and HIPP analyses showed that CBD downregulated Cnr1 and increased Cnr2 gene expression dose-dependently, with the most pronounced effects occurring with the highest dose (30 mg/kg). These results agree with previous studies of our group and others demonstrating that CBD treatment reduced Cnr1 [56,82] and increased Cnr2 gene expression in different brain areas [56]. These alterations are compatible with CBD acting as a CB1r-agonist (directly or indirectly) and as a CB2r -antagonist.
Regarding Gpr55, opposite results were observed in the AMY and HIPP. On the one hand, CBD significantly upregulated Gpr55 at the highest dose in the AMY. On the other hand, Gpr55 expression was significantly reduced in the HIPP at all doses tested, with no differences between them. Similarly, our previous studies revealed that CBD reduced Gpr55 in the NAcc of mice exposed to the oral ethanol self-administration paradigm [56]. The exact mechanism by which CBD induced these opposite changes in Gpr55 gene expression between the two brain regions needs to be further explored.
The GABAergic system plays an essential role in the regulation of emotional responses. It is a crucial therapeutic target for controlling anxiety and mood disorders and the critical target by which benzodiazepines (BZD) exert their anxiolytic properties [82]. The anxiolytic effect of BZD is mediated by GABA(A) receptors containing α2 and γ2 subunits, with high expression in the limbic system and cortex [83,84,85,86]. The pentameric GABA(A) receptors are formed by the assembly of different subunits containing α1, α2, α3 or α5, in combination with β and γ2 subunits. Despite less information about the involvement of GABA(A) receptors in depression, studies carried out in patients with major depressive disorder revealed reduced GABA levels, which were normalized after chronic treatment with antidepressants [83,84]. In addition, heterozygous γ2 [85] and α2 [86] knockout mice exhibited more vulnerability to developing anxiety and depressive-like behaviors.
Furthermore, a close interaction between cannabinoid receptors and the GABA system has been demonstrated. Alterations in GABA subunits have been observed in CB1KO knockout mice [49] and mice overexpressing the CB2r (CB2xP) [42]. Interestingly, these mice also showed an impaired anxiolytic action of BZD [41,42]. Pharmacological studies using drugs acting on CB1r and CB2r also showed modified response to stress, anxiety and behavioral despair, and gene expression of GABA(A) subunits, including α2 and γ2 [48,74,87,88]. Thus, considering the role of GABA(A) in anxiety and mood disorders and the crosstalk between GABAergic and cannabinoid systems, we analyzed changes in GABA(A)α2 and GABA(A)γ2 gene expression in the AMY and HIPP of WT mice treated with CBD.
Acute administration of CBD downregulated the gene expression of both GABAergic subunits in the AMY and HIPP at all doses tested. Despite studies of CBD effects in mice lacking α2 and γ2, it would be of great interest to elucidate the exact role of these GABA(A) subunits in CBD anxiolytic properties; it is tempting to speculate that CBD regulates, directly or indirectly, GABA(A) neurotransmission. In line with these findings, previous studies have revealed that CBD inhibited GABA uptake in rat brain synaptosomes at 0.1mM [89]. More recently, an electrophysiological study comparing the actions of CBD and 2-AG on human recombinant GABA(A) receptors expressed on Xenopus oocytes showed that CBD acts as a positive allosteric modulator at GABA(A) receptors containing α2 subunits. This study supported the fact that the site of action of CBD is different from the classic BZD site [90]. Altogether the results obtained suggest that the effects of CBD on GABAergic neurotransmission may be a potential target for its anxiolytic and antidepressant properties that deserve to be explored in future studies.
Overall, the gene expression studies undertaken here further support the complex network through which CBD acts. Behavioral studies revealed that CBD induced anxiolytic- and antidepressant-like effects in WT mice at low and intermediate doses (10 and 20 mg/kg), whereas the highest dose (30 mg/kg) did not induce any behavioral effect. Gene expression studies showed that CBD modified the gene expression of Cnr1, Cnr2 and Gpr55 depending on the doses and the brain region analyzed. Curiously, the highest dose induced the most pronounced changes. Consequently, the study’s main limitation is that gene expression alterations in almost all the targets analyzed were induced by different doses of CBD, making it difficult to correlate some of these biological alterations with the anxiolytic or antidepressant-like effects of CBD. Although future studies are necessary to understand the role of each receptor on CBD actions, based on our results, it is tempting to speculate that the anxiolytic and antidepressant-like effects of CBD may be due to a multimodal mechanism involving different key targets and brain regions, as has been proposed recently [91].
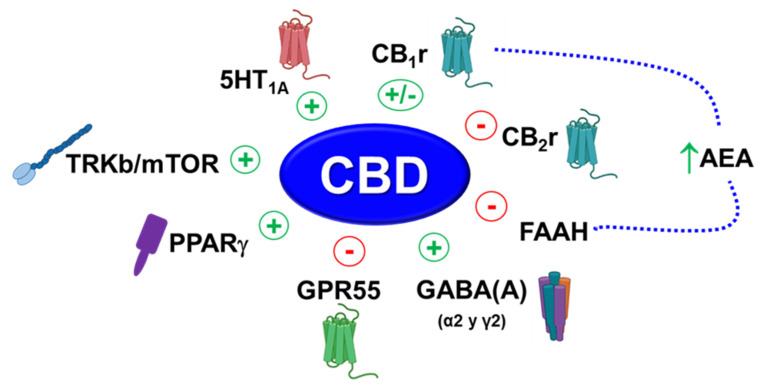
In conclusion, the present study demonstrated that acute administration of CBD produced anxiolytic and antidepressant-like effects in a dose-dependent manner, suggesting that CB1r is one of the crucial targets involved in its anxiolytic properties. Moreover, this study revealed that CBD also modified the gene expression of GABA(A) subunits α2 and γ2 and Cnr1, Cnr2 and Gpr55, in a dose and brain region-dependent manner, indicating that CBD presents a multimodal mechanism of action (Figure 9).
Figure 9.
Main targets involved in the anxiolytic and antidepressant actions of CBD. A summary of previously published data and those supported by the present work. 5-HT1A: serotonin 1A receptor; CB1r: cannabinoid 1 receptor; CB2r: cannabinoid 2 receptor; AEA: anandamide; FAAH: fatty acid amide hydrolase; GPR55: G-protein-coupled receptor 55; PPARγ: Peroxisome proliferator-activated receptor gamma; TRKb/mTOR: Tropomyosin receptor kinase B/mammalian target of rapamycin.
4. Materials and Methods
4.1. Animals
A total of 130 mice were used in the present study. Forty Swiss CD1 mice were purchased from Charles River Laboratories (Lodi, Italy) to develop the dose-response study with CBD. An additional set of 32 CD1 male mice was used to conduct the pharmacological study with the CB1 receptor antagonist (SR141716A) and CBD. We used twenty-one CB1KO [41,92] and nineteen CB2KO mice [43,44] generated in our laboratory. Dr. Andrei Kolovko kindly provided GPR55KO mice at the Institute of Genomic Medicine. Eighteen of them, bred at our animal vivarium, were used in the present study (TIGM, Houston, TX, USA) [92]. All mice were males and between 2–3 months of age. At the beginning of the experiments, mice were five weeks old and weighed 25–30 g. All animals were maintained under controlled temperature (23 ± 2 °C) and with a light-dark cycle from 0800 to 2000 h, with free access to food (commercial diet for rodents A04 Panlab, Barcelona, Spain) and water. All animal care and experimental studies complied with the Spanish Royal Decree 53/2013, the Spanish Law 32/2007, and the European Union Directive of 22 September 2010 (2010/63/UE), regulating the care of experimental animals and were approved by the Ethics Committee of Miguel Hernández University (ref. UMH.IN.JM.02.17).
4.2. Treatment
CBD was obtained from Jazz Pharmaceuticals (Dublin, Ireland) and dissolved in ethanol: cremophor: saline (1:1:18) to obtain the required doses of 10, 20 and 30 mg/kg for wild-type (WT) animals, and the dose of 20 mg/kg for knockout mice (CB1KO, CB2KO and GPR55KO). The drug was prepared immediately before its intraperitoneal (i.p.) administration at a volume of 10 mL/kg of weight (0.3 mL for each mouse). According to its pharmacokinetic properties, CBD was administered 1h and a half before the behavioral evaluation [54,93].
The CB1r-antagonist SR141716A was purchased from Sigma-Aldrich (Madrid, Spain) and dissolved in ethanol, cremophor and saline (1:1:18) to obtain the required dose of 2 mg/kg for its i.p. administration 30 min before CBD administration and 2 h before the behavioral evaluation. The dose of SR141716A was selected based on previous studies demonstrating that this dose does not produce any effects by itself [94,95].
4.3. Behavioral Analyses
Mice were randomly divided into groups and subjected to different experimental paradigms to evaluate the anxiolytic and antidepressant actions of the acute administration of CBD. Before every behavioral test, mice were brought to the experimental room in their home cages and were given 60 min to adapt to the environmental conditions of the testing room. The same conditions were maintained for all the behavioral tests. Each test was assessed during the light cycle between 0900 and 1200 h. After each evaluation, mice were undisturbed for 2 to 3 days to allow pharmacokinetic clearance of CBD [93]. WT mice were subjected to a wide range of behavioral evaluations, including the light-dark box (LDB), elevated plus maze (EPM), tail suspension test (TST) and novelty suppressed feeding test (NSFT). According to the results obtained in these studies, only two (LDB and TST) were selected to analyze the emotional behavior in knockout animals (CB1KO, CB2KO and GPR55KO) and CBD’s ability to modulate it. The selective CB1r-antagonist (SR141716A) was used to further evaluate the implication of CB1r in CBD anxiolytic-like effects (20 mg/kg) in the LDB paradigm. For this, WT mice were randomly assigned into four groups: control group (VEH + VEH), CBD group (VEH + CBD), antagonist group (SR-141716A +VEH) or the combination of both drugs (SR-141716A + CBD). The antagonist SR141716 was administered 30 min before CBD, and the behavioral evaluation was carried out 1 h and 30 min after CBD administration.
4.3.1. Light-Dark Box (LDB)
This test uses the natural aversion of rodents to bright areas compared with darker ones [48,96]. The apparatus consisted of two methacrylate boxes (20 × 20 × 15 cm), one transparent and one black and opaque, separated by an opaque tunnel (4 cm). Light from a 60 W desk lamp placed 25 cm above the lightbox provided room illumination. Mice were individually tested in 5 min sessions. At the beginning of the session, mice were placed in the lightbox facing the tunnel that connects to the dark box. The time spent in the lightbox and the number of transitions between the two compartments were recorded in this period. A mouse whose four paws were in the new box was considered to have changed boxes. The apparatus was cleaned between sessions with ethanol 70%.
4.3.2. Elevated Plus Maze Test (EPM)
The EPM consists of two open arms and two enclosed horizontal perpendicular arms 50 cm above the floor [48,97]. The junction of four arms formed a central squared platform (5 × 5 cm). The test began with the animal being placed in the center of the apparatus facing one of the enclosed arms and allowed to explore freely for 5 min. During this period, the time spent in the open arms (as a percentage of total test time) and the number of entries from open arms to closed arms (and vice versa) were recorded. An arm entry was considered an entry of four paws into the arm. The apparatus was cleaned between sessions with ethanol 70%.
4.3.3. Novelty Suppressed Feeding Test (NSFT)
The NSFT was used to measure anxiety-induced hyponeophagia, which is the inhibition of ingestion and approach to food pellets when exposed to an anxiety-provoking novel environment. The testing apparatus consisted of a square, transparent methacrylate cage 40 × 40 × 50 cm, with a food pellet on the white platform in the center of the cage [44,98]. Before the experiment, mice were deprived of food for 24 h, and then each mouse was placed in the corner of the apparatus. The latency time before the mouse started to eat the pellet was recorded up to 5 min. Once the mice began to eat, the total amount of pellets was measured over 5 min. The decrease or increase in the latency time indicates anxiolytic or anxiogenic actions of different drugs, respectively. The anhedonia was measured by calculating the food pellet intake (mg), which increased when mice presented more motivation or ability to experience pleasure.
4.3.4. Tail Suspension Test (TST)
TST is a widely accepted test to evaluate depressive-like behaviors by measuring the immobility time [44,99]. Mice were individually suspended by the tail at the edge of a lever above the tabletop (the distance to the table surface was 35 cm), affixed with the adhesive tape placed approximately 1–2 cm from the tip of the tail. In this situation, mice develop escape-orientated behaviors interspersed with temporally increasing bouts of immobility. The immobility time was measured for 6 min.
4.4. Relative Gene Expression Analyses by Real-Time PCR
Relative gene expression analyses of GABA(A)α2 and γ2 subunits, Cnr1, Cnr2 and Gpr55 in the AMY and HIPP, were carried out in WT mice to assess changes in these targets under anxiety or depressive-like conditions and the ability of CBD to modulate them. Briefly, mice were sacrificed 150 min after the administration of CBD (or vehicle) and brain samples were removed from the skull and frozen at −80 °C. These samples were used to obtain coronal sections (500 μm) of regions of interest in a cryostat (−10 °C) according to Paxinos and Franklin’s atlas [100]. Brain nuclei of interest were microdissected following Palkovit’s method as previously modified by our group [101,102]. Total RNA was extracted from brain micropunches with TRI Reagent (Applied Biosystems, Madrid, Spain) and reverse transcription was carried out to obtain the complementary DNA (cDNA) (4374966, High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor, Applied Biosystems, Madrid, Spain). To perform the real-time PCR, 6.25 μL of water with DEPC (diethylpyrocarbonate, RNAase inhibitor), 5 μL of the cDNA, 11.25 μL of the TaqmanTM Master Mix (4369514, Applied Biosystems, Madrid, Spain), and 1.25 μL of the corresponding Taqman assay were added in each well (4346907, Applied Biosystems, Madrid, Spain). Quantitative analyses of the relative expression of GABA(A) α2 (Mm00433435_m1) and γ2 subunits (Mm00433489_m1), Cnr1 (Mn00432621_s1), Cnr2 (Mm00438286_m1) and Gpr55 (Mm02621622_s1) genes were performed on the StepOne Sequence Detector System (Applied Biosystems, Madrid, Spain). All reagents were used following the manufacturer’s instructions. The reference gene used was 18S rRNA (Mm03928990_g1), and data for each target was normalized to the endogenous reference gene. The fold change in target gene expression was calculated using the 2ΔΔ−Ct method [103].
4.5. Data and Statistical Analysis
Statistical analyses were performed using one-way or two-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls post hoc test for comparing four groups affected by the treatment with CBD (and vehicle). Moreover, for the behavioral assay with the antagonist SR141716A, a two-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls post hoc test was assessed for comparing four groups affected by the individual treatment with CBD, SR141716A or its combination. Differences were considered significant if the probability of error was less than 5%. SigmaPlot 11 software (Systat Software Inc., Chicago, IL, USA) was used.
5. Conclusions
CBD induced anxiolytic- and antidepressant-like effects in a dose-dependent manner, the intermediate dose (20 mg/kg) being the one that produced these effects most robustly. CB1r appears to be an essential key target for CBD anxiolytic properties.
Changes in Cnr1, Cnr2, Gpr55, GABA(A) subunits α2 and γ2 in limbic areas, including the AMY and HIPP, also suggest that these targets may contribute to CBD effects. Further studies are necessary to understand the specific role of each target and brain region on CBD anxiolytic and antidepressant properties.
Acknowledgments
The authors would like to thank the PFIS fellowship “Contrato Predoctoral de Formación en Inestigación en Salud” (PFIS) granted to AAO (FI19/00170).
Author Contributions
All named authors made an active contribution to the study. Conceptualization: J.M.; methodology, investigation and analyses: A.A.-O., M.S.G.-G., L.I. and A.G.; writing—original draft preparation: A.A.-O., M.S.G.-G., L.I. and A.G.; writing—review and editing, supervision, funding acquisition: J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “Instituto de Salud Carlos III” (RD. PI18/00576, Spanish Ministerio de Ciencia e Inovación) and RD21/0009/0008 “Red de Investigación en Atención Primaria de Adicciones”) to J.M.
Institutional Review Board Statement
All animal care and experimental studies complied with the Spanish Royal Decree 53/2013, the Spanish Law 32/2007, and the European Union Directive of 22 September 2010 (2010/63/UE), regulating the care of experimental animals and approved by the Ethics Committee of Miguel Hernández University (ref. UMH.IN.JM.02.17).
Informed Consent Statement
Not applicable.
Data Availability Statement
The date is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang P.S., Aguilar-Gaxiola S., Alonso J., Angermeyer M.C., Borges G., Bromet E.J., Bruffaerts R., de Girolamo G., de Graaf R., Gureje O., et al. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet. 2007;370:841–850. doi: 10.1016/S0140-6736(07)61414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansseau M., Fischler B., Dierick M., Albert A., Leyman S., Mignon A. Socioeconomic correlates of generalized anxiety disorder and major depression in primary care: The GADIS II study (Generalized Anxiety and Depression Impact Survey II) Depress. Anxiety. 2008;25:506–513. doi: 10.1002/da.20306. [DOI] [PubMed] [Google Scholar]
- 4.Auerbach R.P., Mortier P., Bruffaerts R., Alonso J., Benjet C., Cuijpers P., Demyttenaere K., Ebert D.D., Green J.G., Hasking P., et al. WHO World Mental Health Surveys International College Student Project: Prevalence and distribution of mental disorders. J. Abnorm. Psychol. 2018;127:623–638. doi: 10.1037/abn0000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans-Lacko S., Aguilar-Gaxiola S., Al-Hamzawi A., Alonso J., Benjet C., Bruffaerts R., Chiu W.T., Florescu S., de Girolamo G., Gureje O., et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO World Mental Health (WMH) surveys. Psychol. Med. 2018;48:1560–1571. doi: 10.1017/S0033291717003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson F., van Ommeren M., Flaxman A., Cornett J., Whiteford H., Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet. 2019;394:240–248. doi: 10.1016/S0140-6736(19)30934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S.M., Han C., Bahk W.M., Lee S.J., Patkar A.A., Masand P.S., Pae C.U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med. J. 2018;54:101–112. doi: 10.4068/cmj.2018.54.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandarakalam J.P. Challenges of Treatment-resistant Depression. Psychiatr. Danub. 2018;30:273–284. doi: 10.24869/psyd.2018.273. [DOI] [PubMed] [Google Scholar]
- 9.Fava M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry. 2003;53:649–659. doi: 10.1016/S0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 10.Gaynes B. Assessing the risk factors for difficult-to-treat depression and treatment-resistant depression. J. Clin. Psychiatry. 2016;77((Suppl. 1)):4–8. doi: 10.4088/JCP.14077su1c.01. [DOI] [PubMed] [Google Scholar]
- 11.Ionescu D.F., Rosenbaum J.F., Alpert J.E. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin. Neurosci. 2015;17:111–126. doi: 10.31887/DCNS.2015.17.2/dionescu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uzun S., Kozumplik O., Jakovljevic M., Sedic B. Side effects of treatment with benzodiazepines. Psychiatr. Danub. 2010;22:90–93. [PubMed] [Google Scholar]
- 13.Stewart S.A. The effects of benzodiazepines on cognition. J. Clin. Psychiatry. 2005;66((Suppl. 2)):9–13. [PubMed] [Google Scholar]
- 14.Yin A.Q., Wang F., Zhang X. Integrating endocannabinoid signaling in the regulation of anxiety and depression. Acta Pharmacol. Sin. 2019;40:336–341. doi: 10.1038/s41401-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marco E.M., Garcia-Gutierrez M.S., Bermudez-Silva F.J., Moreira F.A., Guimaraes F., Manzanares J., Viveros M.P. Endocannabinoid system and psychiatry: In search of a neurobiological basis for detrimental and potential therapeutic effects. Front. Behav. Neurosci. 2011;5:63. doi: 10.3389/fnbeh.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadwick V.L., Rohleder C., Koethe D., Leweke F.M. Cannabinoids and the endocannabinoid system in anxiety, depression, and dysregulation of emotion in humans. Curr. Opin. Psychiatry. 2020;33:20–42. doi: 10.1097/YCO.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 17.Navarrete F., Garcia-Gutierrez M.S., Jurado-Barba R., Rubio G., Gasparyan A., Austrich-Olivares A., Manzanares J. Endocannabinoid System Components as Potential Biomarkers in Psychiatry. Front. Psychiatry. 2020;11:315. doi: 10.3389/fpsyt.2020.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisboa S.F., Gomes F.V., Terzian A.L., Aguiar D.C., Moreira F.A., Resstel L.B., Guimaraes F.S. The Endocannabinoid System and Anxiety. Vitam. Horm. 2017;103:193–279. doi: 10.1016/bs.vh.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Huang W.J., Chen W.W., Zhang X. Endocannabinoid system: Role in depression, reward and pain control (Review) Mol. Med. Rep. 2016;14:2899–2903. doi: 10.3892/mmr.2016.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viudez-Martinez A., Garcia-Gutierrez M.S., Medrano-Relinque J., Navarron C.M., Navarrete F., Manzanares J. Cannabidiol does not display drug abuse potential in mice behavior. Acta Pharmacol. Sin. 2019;40:358–364. doi: 10.1038/s41401-018-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., Moriello A.S., Davis J.B., Mechoulam R., Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo E.B., Burnett A., Hall B., Parker K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 23.Kathmann M., Flau K., Redmer A., Trankle C., Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn-Schmiedeberg‘s Arch. Pharmacol. 2006;372:354–361. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- 24.Ryberg E., Larsson N., Sjogren S., Hjorth S., Hermansson N.O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas A., Baillie G.L., Phillips A.M., Razdan R.K., Ross R.A., Pertwee R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos A.C., Moreira F.A., Gomes F.V., Del Bel E.A., Guimaraes F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:3364–3378. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izzo A.A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Laprairie R.B., Bagher A.M., Kelly M.E., Denovan-Wright E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015;172:4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elsaid S., Kloiber S., Le Foll B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: A review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 2019;167:25–75. doi: 10.1016/bs.pmbts.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Gutierrez M.S., Navarrete F., Gasparyan A., Austrich-Olivares A., Sala F., Manzanares J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules. 2020;10:1575. doi: 10.3390/biom10111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blessing E.M., Steenkamp M.M., Manzanares J., Marmar C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics. 2015;12:825–836. doi: 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fogaca M.V., Reis F.M., Campos A.C., Guimaraes F.S. Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior: Involvement of 5HT1A receptors and previous stressful experience. Eur. Neuropsychopharmacol. 2014;24:410–419. doi: 10.1016/j.euroneuro.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Linge R., Jimenez-Sanchez L., Campa L., Pilar-Cuellar F., Vidal R., Pazos A., Adell A., Diaz A. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology. 2016;103:16–26. doi: 10.1016/j.neuropharm.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Moreira F.A., Aguiar D.C., Guimaraes F.S. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:1466–1471. doi: 10.1016/j.pnpbp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Sales A.J., Fogaca M.V., Sartim A.G., Pereira V.S., Wegener G., Guimaraes F.S., Joca S.R.L. Cannabidiol Induces Rapid and Sustained Antidepressant-Like Effects Through Increased BDNF Signaling and Synaptogenesis in the Prefrontal Cortex. Mol. Neurobiol. 2019;56:1070–1081. doi: 10.1007/s12035-018-1143-4. [DOI] [PubMed] [Google Scholar]
- 36.Zanelati T.V., Biojone C., Moreira F.A., Guimaraes F.S., Joca S.R. Antidepressant-like effects of cannabidiol in mice: Possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 2010;159:122–128. doi: 10.1111/j.1476-5381.2009.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zieba J., Sinclair D., Sebree T., Bonn-Miller M., Gutterman D., Siegel S., Karl T. Cannabidiol (CBD) reduces anxiety-related behavior in mice via an FMRP-independent mechanism. Pharmacol. Biochem. Behav. 2019;181:93–100. doi: 10.1016/j.pbb.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Malvestio R.B., Medeiros P., Negrini-Ferrari S.E., Oliveira-Silva M., Medeiros A.C., Padovan C.M., Luongo L., Maione S., Coimbra N.C., de Freitas R.L. Cannabidiol in the prelimbic cortex modulates the comorbid condition between the chronic neuropathic pain and depression-like behaviour in rats: The role of medial prefrontal cortex 5-HT1A and CB1 receptors. Brain Res. Bull. 2021;174:323–338. doi: 10.1016/j.brainresbull.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann A., Lisboa S.F., Sonego A.B., Coutinho D., Gomes F.V., Guimaraes F.S. Cannabidiol attenuates aggressive behavior induced by social isolation in mice: Involvement of 5-HT1A and CB1 receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;94:109637. doi: 10.1016/j.pnpbp.2019.109637. [DOI] [PubMed] [Google Scholar]
- 40.Sartim A.G., Guimaraes F.S., Joca S.R. Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex-Possible involvement of 5-HT1A and CB1 receptors. Behav. Brain Res. 2016;303:218–227. doi: 10.1016/j.bbr.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 41.Uriguen L., Perez-Rial S., Ledent C., Palomo T., Manzanares J. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology. 2004;46:966–973. doi: 10.1016/j.neuropharm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Gutierrez M.S., Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J. Psychopharmacol. 2011;25:111–120. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Gutierrez M.S., Ortega-Alvaro A., Busquets-Garcia A., Perez-Ortiz J.M., Caltana L., Ricatti M.J., Brusco A., Maldonado R., Manzanares J. Synaptic plasticity alterations associated with memory impairment induced by deletion of CB2 cannabinoid receptors. Neuropharmacology. 2013;73:388–396. doi: 10.1016/j.neuropharm.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Gutierrez M.S., Perez-Ortiz J.M., Gutierrez-Adan A., Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br. J. Pharmacol. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortega-Alvaro A., Aracil-Fernandez A., Garcia-Gutierrez M.S., Navarrete F., Manzanares J. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology. 2011;36:1489–1504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacob W., Marsch R., Marsicano G., Lutz B., Wotjak C.T. Cannabinoid CB1 receptor deficiency increases contextual fear memory under highly aversive conditions and long-term potentiation in vivo. Neurobiol. Learn. Mem. 2012;98:47–55. doi: 10.1016/j.nlm.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Haller J., Varga B., Ledent C., Barna I., Freund T.F. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur. J. Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Gutierrez M.S., Garcia-Bueno B., Zoppi S., Leza J.C., Manzanares J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br. J. Pharmacol. 2012;165:951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uriguen L., Garcia-Gutierrez M.S., Manzanares J. Decreased GABAA and GABAB receptor functional activity in cannabinoid CB1 receptor knockout mice. J. Psychopharmacol. 2011;25:105–110. doi: 10.1177/0269881109358204. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez-Leon P., Miranda-Paez A., Calvillo-Robledo A., Marichal-Cancino B.A. Blockade of GPR55 in dorsal periaqueductal gray produces anxiety-like behaviors and evocates defensive aggressive responses in alcohol-pre-exposed rats. Neurosci. Lett. 2021;764:136218. doi: 10.1016/j.neulet.2021.136218. [DOI] [PubMed] [Google Scholar]
- 51.Shi Q.X., Yang L.K., Shi W.L., Wang L., Zhou S.M., Guan S.Y., Zhao M.G., Yang Q. The novel cannabinoid receptor GPR55 mediates anxiolytic-like effects in the medial orbital cortex of mice with acute stress. Mol. Brain. 2017;10:38. doi: 10.1186/s13041-017-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahimi A., Hajizadeh Moghaddam A., Roohbakhsh A. Central administration of GPR55 receptor agonist and antagonist modulates anxiety-related behaviors in rats. Fundam. Clin. Pharmacol. 2015;29:185–190. doi: 10.1111/fcp.12099. [DOI] [PubMed] [Google Scholar]
- 53.Hurst K., Badgley C., Ellsworth T., Bell S., Friend L., Prince B., Welch J., Cowan Z., Williamson R., Lyon C., et al. A putative lysophosphatidylinositol receptor GPR55 modulates hippocampal synaptic plasticity. Hippocampus. 2017;27:985–998. doi: 10.1002/hipo.22747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gasparyan A., Navarrete F., Manzanares J. Cannabidiol and Sertraline Regulate Behavioral and Brain Gene Expression Alterations in an Animal Model of PTSD. Front. Pharmacol. 2021;12:694510. doi: 10.3389/fphar.2021.694510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viudez-Martinez A., Garcia-Gutierrez M.S., Manzanares J. Gender differences in the effects of cannabidiol on ethanol binge drinking in mice. Addict. Biol. 2020;25:e12765. doi: 10.1111/adb.12765. [DOI] [PubMed] [Google Scholar]
- 56.Viudez-Martinez A., Garcia-Gutierrez M.S., Navarron C.M., Morales-Calero M.I., Navarrete F., Torres-Suarez A.I., Manzanares J. Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict. Biol. 2018;23:154–164. doi: 10.1111/adb.12495. [DOI] [PubMed] [Google Scholar]
- 57.Navarrete F., Aracil-Fernandez A., Manzanares J. Cannabidiol regulates behavioural alterations and gene expression changes induced by spontaneous cannabinoid withdrawal. Br. J. Pharmacol. 2018;175:2676–2688. doi: 10.1111/bph.14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guimaraes F.S., Chiaretti T.M., Graeff F.G., Zuardi A.W. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology. 1990;100:558–559. doi: 10.1007/BF02244012. [DOI] [PubMed] [Google Scholar]
- 59.Onaivi E.S., Green M.R., Martin B.R. Pharmacological characterization of cannabinoids in the elevated plus maze. J. Pharmacol. Exp. Ther. 1990;253:1002–1009. [PubMed] [Google Scholar]
- 60.Almeida V., Levin R., Peres F.F., Niigaki S.T., Calzavara M.B., Zuardi A.W., Hallak J.E., Crippa J.A., Abilio V.C. Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;41:30–35. doi: 10.1016/j.pnpbp.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 61.Schiavon A.P., Bonato J.M., Milani H., Guimaraes F.S., Weffort de Oliveira R.M. Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:27–34. doi: 10.1016/j.pnpbp.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 62.Assareh N., Gururajan A., Zhou C., Luo J.L., Kevin R.C., Arnold J.C. Cannabidiol disrupts conditioned fear expression and cannabidiolic acid reduces trauma-induced anxiety-related behaviour in mice. Behav. Pharmacol. 2020;31:591–596. doi: 10.1097/FBP.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 63.Shbiro L., Hen-Shoval D., Hazut N., Rapps K., Dar S., Zalsman G., Mechoulam R., Weller A., Shoval G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019;201:59–63. doi: 10.1016/j.physbeh.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 64.Shoval G., Shbiro L., Hershkovitz L., Hazut N., Zalsman G., Mechoulam R., Weller A. Prohedonic Effect of Cannabidiol in a Rat Model of Depression. Neuropsychobiology. 2016;73:123–129. doi: 10.1159/000443890. [DOI] [PubMed] [Google Scholar]
- 65.Pisanti S., Malfitano A.M., Ciaglia E., Lamberti A., Ranieri R., Cuomo G., Abate M., Faggiana G., Proto M.C., Fiore D., et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017;175:133–150. doi: 10.1016/j.pharmthera.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 66.Ibeas Bih C., Chen T., Nunn A.V., Bazelot M., Dallas M., Whalley B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics. 2015;12:699–730. doi: 10.1007/s13311-015-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marinho A.L., Vila-Verde C., Fogaca M.V., Guimaraes F.S. Effects of intra-infralimbic prefrontal cortex injections of cannabidiol in the modulation of emotional behaviors in rats: Contribution of 5HT(1)A receptors and stressful experiences. Behav. Brain Res. 2015;286:49–56. doi: 10.1016/j.bbr.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 68.Campos A.C., Ferreira F.R., Guimaraes F.S. Cannabidiol blocks long-lasting behavioral consequences of predator threat stress: Possible involvement of 5HT1A receptors. J. Psychiatr. Res. 2012;46:1501–1510. doi: 10.1016/j.jpsychires.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 69.Gomes F.V., Resstel L.B., Guimaraes F.S. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology. 2011;213:465–473. doi: 10.1007/s00213-010-2036-z. [DOI] [PubMed] [Google Scholar]
- 70.Campos A.C., Guimaraes F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology. 2008;199:223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Gutierrez M.S., Navarrete F., Navarro G., Reyes-Resina I., Franco R., Lanciego J.L., Giner S., Manzanares J. Alterations in Gene and Protein Expression of Cannabinoid CB2 and GPR55 Receptors in the Dorsolateral Prefrontal Cortex of Suicide Victims. Neurotherapeutics. 2018;15:796–806. doi: 10.1007/s13311-018-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomes-de-Souza L., Bianchi P.C., Costa-Ferreira W., Tomeo R.A., Cruz F.C., Crestani C.C. CB1 and CB2 receptors in the bed nucleus of the stria terminalis differently modulate anxiety-like behaviors in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;110:110284. doi: 10.1016/j.pnpbp.2021.110284. [DOI] [PubMed] [Google Scholar]
- 73.Ivy D., Palese F., Vozella V., Fotio Y., Yalcin A., Ramirez G., Mears D., Wynn G., Piomelli D. Cannabinoid CB2 receptors mediate the anxiolytic-like effects of monoacylglycerol lipase inhibition in a rat model of predator-induced fear. Neuropsychopharmacology. 2020;45:1330–1338. doi: 10.1038/s41386-020-0696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haller J., Bakos N., Szirmay M., Ledent C., Freund T.F. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- 75.Martin M., Ledent C., Parmentier M., Maldonado R., Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 76.Burokas A., Martin-Garcia E., Gutierrez-Cuesta J., Rojas S., Herance J.R., Gispert J.D., Serra M.A., Maldonado R. Relationships between serotonergic and cannabinoid system in depressive-like behavior: A PET study with [11C]-DASB. J. Neurochem. 2014;130:126–135. doi: 10.1111/jnc.12716. [DOI] [PubMed] [Google Scholar]
- 77.Zoppi S., Madrigal J.L., Caso J.R., Garcia-Gutierrez M.S., Manzanares J., Leza J.C., Garcia-Bueno B. Regulatory role of the cannabinoid CB2 receptor in stress-induced neuroinflammation in mice. Br. J. Pharmacol. 2014;171:2814–2826. doi: 10.1111/bph.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Casarotto P.C., Gomes F.V., Resstel L.B., Guimaraes F.S. Cannabidiol inhibitory effect on marble-burying behaviour: Involvement of CB1 receptors. Behav. Pharmacol. 2010;21:353–358. doi: 10.1097/FBP.0b013e32833b33c5. [DOI] [PubMed] [Google Scholar]
- 79.Silva-Cardoso G.K., Lazarini-Lopes W., Hallak J.E., Crippa J.A., Zuardi A.W., Garcia-Cairasco N., Leite-Panissi C.R.A. Cannabidiol effectively reverses mechanical and thermal allodynia, hyperalgesia, and anxious behaviors in a neuropathic pain model: Possible role of CB1 and TRPV1 receptors. Neuropharmacology. 2021;197:108712. doi: 10.1016/j.neuropharm.2021.108712. [DOI] [PubMed] [Google Scholar]
- 80.Fogaca M.V., Campos A.C., Coelho L.D., Duman R.S., Guimaraes F.S. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology. 2018;135:22–33. doi: 10.1016/j.neuropharm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Do Monte F.H., Souza R.R., Bitencourt R.M., Kroon J.A., Takahashi R.N. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav. Brain Res. 2013;250:23–27. doi: 10.1016/j.bbr.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 82.Fogaca M.V., Duman R.S. Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Front. Cell. Neurosci. 2019;13:87. doi: 10.3389/fncel.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanacora G., Mason G.F., Rothman D.L., Krystal J.H. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 84.Duman R.S., Sanacora G., Krystal J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron. 2019;102:75–90. doi: 10.1016/j.neuron.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Earnheart J.C., Schweizer C., Crestani F., Iwasato T., Itohara S., Mohler H., Luscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J. Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vollenweider I., Smith K.S., Keist R., Rudolph U. Antidepressant-like properties of alpha2-containing GABA(A) receptors. Behav. Brain Res. 2011;217:77–80. doi: 10.1016/j.bbr.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moreira F.A., Kaiser N., Monory K., Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Gaetani S., Cuomo V., Piomelli D. Anandamide hydrolysis: A new target for anti-anxiety drugs? Trends Mol. Med. 2003;9:474–478. doi: 10.1016/j.molmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Banerjee S.P., Snyder S.H., Mechoulam R. Cannabinoids: Influence on neurotransmitter uptake in rat brain synaptosomes. J. Pharmacol. Exp. Ther. 1975;194:74–81. [PubMed] [Google Scholar]
- 90.Bakas T., van Nieuwenhuijzen P.S., Devenish S.O., McGregor I.S., Arnold J.C., Chebib M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res. 2017;119:358–370. doi: 10.1016/j.phrs.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 91.Alexander C., Vasefi M. Cannabidiol and the corticoraphe circuit in post-traumatic stress disorder. IBRO Neurosci. Rep. 2021;11:88–102. doi: 10.1016/j.ibneur.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sisay S., Pryce G., Jackson S.J., Tanner C., Ross R.A., Michael G.J., Selwood D.L., Giovannoni G., Baker D. Genetic background can result in a marked or minimal effect of gene knockout (GPR55 and CB2 receptor) in experimental autoimmune encephalomyelitis models of multiple sclerosis. PLoS ONE. 2013;8:e76907. doi: 10.1371/journal.pone.0076907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deiana S., Watanabe A., Yamasaki Y., Amada N., Arthur M., Fleming S., Woodcock H., Dorward P., Pigliacampo B., Close S., et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Delta(9)-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology. 2012;219:859–873. doi: 10.1007/s00213-011-2415-0. [DOI] [PubMed] [Google Scholar]
- 94.Biala G., Budzynska B., Staniak N. Effects of rimonabant on the reinstatement of nicotine-conditioned place preference by drug priming in rats. Behav. Brain Res. 2009;202:260–265. doi: 10.1016/j.bbr.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 95.Ray A.P., Griggs L., Darmani N.A. Delta 9-tetrahydrocannabinol suppresses vomiting behavior and Fos expression in both acute and delayed phases of cisplatin-induced emesis in the least shrew. Behav. Brain Res. 2009;196:30–36. doi: 10.1016/j.bbr.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crawley J., Goodwin F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 97.Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 98.Surget A., Saxe M., Leman S., Ibarguen-Vargas Y., Chalon S., Griebel G., Hen R., Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 99.Vaugeois J.M., Passera G., Zuccaro F., Costentin J. Individual differences in response to imipramine in the mouse tail suspension test. Psychopharmacology. 1997;134:387–391. doi: 10.1007/s002130050475. [DOI] [PubMed] [Google Scholar]
- 100.Paxinos G., Franklin K.B.J., Franklin K.B.J. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. Academic Press; San Diego, CA, USA: 2001. [Google Scholar]
- 101.Palkovits M. Punch sampling biopsy technique. Methods Enzymol. 1983;103:368–376. doi: 10.1016/s0076-6879(83)03025-6. [DOI] [PubMed] [Google Scholar]
- 102.Navarrete F., Perez-Ortiz J.M., Manzanares J. Pregabalin- and topiramate-mediated regulation of cognitive and motor impulsivity in DBA/2 mice. Br. J. Pharmacol. 2012;167:183–195. doi: 10.1111/j.1476-5381.2012.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The date is contained within the article.