Abstract
The post-translational modification of serine and threonine residues of proteins by O-linked N-acetylglucosamine (O-GlcNAc) regulates diverse cellular processes in the cardiovascular system. UDP-GlcNAc is a substrate for O-GlcNAc transferase, which catalyzes the attachment of O-GlcNAc to proteins. O-GlcNAcase catalyzes the removal of O-GlcNAc from proteins. UDP-GlcNAc is the end product of the hexosamine biosynthesis pathway, which is regulated primarily by glucose-6-phosphate-Glutamine:fructose-6-phosphate amidotransferase (GFAT). GFAT catalyzes the formation of glucosamine-6-phosphate from fructose-6-phosphate and glutamine. Whereas O-GlcNAc is essential for cell viability, sustained increases in O-GlcNAc levels have been implicated in the etiology of many chronic diseases and is associated with glucose toxicity and diabetic complications in various organs including the cardiovascular system. This review provides an overview of the regulation of protein O-GlcNAcylation followed by a discussion of potential mechanisms by which dysregulation in O-GlcNAc cycling contributes to the adverse effects of diabetes on the cardiovascular system.
Keywords: O-GlcNAc, diabetes, heart, muscle, glucose, metabolism
Introduction
Protein glycosylation was once believed to occur exclusively in the ER, Golgi and secretory pathway consisting of relatively stable extended glycan structures [1]. Then in the mid-1980s Hart and colleagues characterized the modification of nuclear and cytoplasmic proteins by a single N-acetylglucosamine (GlcNAc) via an O-linkage (O-GlcNAc) on serine and threonine residues [2]*. Protein O-GlcNAcylation is not modified beyond a single sugar moiety, and is highly dynamic and readily reversible, more akin to phosphorylation than traditional protein glycosylation [3]. Similar to phosphorylation, O-GlcNAcylation regulates the activity, sub-cellular localization and stability of target proteins [3]. Studies quickly linked O-GlcNAc levels to glucose availability, and increases in O-GlcNAc modifications were associated with the adverse effects of hyperglycemia, including insulin resistance, glucose toxicity, and pancreatic β-cell death [3].
Diabetes is a major risk factor for cardiovascular disease increasing all cause cardiovascular mortality at least 3-fold as well as reducing tolerance of the heart to adverse events, such as myocardial infarction [4]. Moreover, both type 1 and type 2 diabetes markedly increase the risk of developing heart failure independent of ischemic disease [4,5]. Diabetes leads to endothelial cell, vascular smooth muscle cell and cardiomyocyte dysfunction although the specific molecular mechanisms remain poorly understood [5]. Chronic hyperglycemia secondary to poorly controlled blood glucose levels, is one of many factors that contribute to the adverse effects of glucose on the cardiovascular system, leading to activation of a number of different pathways including increased glucose metabolism via the hexosamine biosynthesis pathway resulting in higher protein O-GlcNAc levels [6,7]. The goal of this review is to provide a brief overview of the regulation of protein O-GlcNAcylation followed by an examination of the contributions of aberrant protein O-GlcNAcylation to diabetic complications of the cardiovascular system.
Hexosamine Biosynthesis Pathway and O-GlcNAc Synthesis
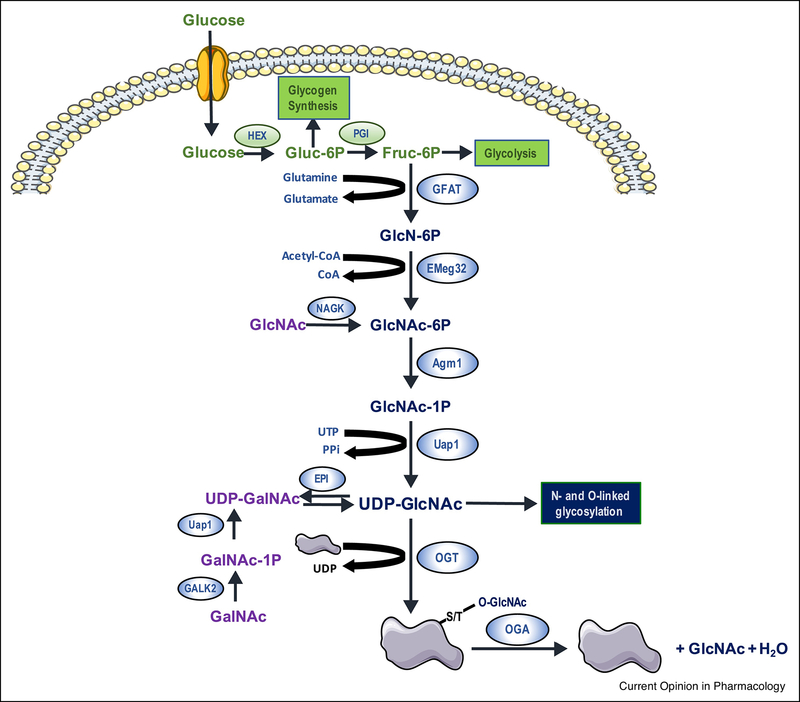
UDP-GlcNAc, the primary substrate for traditional protein glycosylation, is the end product of the hexosamine biosynthesis pathway (HBP, Figure 1), a widely recognized nutrient sensing pathway [8]. L-Glutamine: D-fructose-6-phosphate amidotransferase (GFAT; EC 2.6.1.16) catalyzes the first and rate limiting step of the HBP resulting in the conversion of fructose 6-phosphate to glucosamine 6-phosphate (Fig 1) [9]. Emeg32 (EC: 2.3.1.4), also known as glucosamine 6-phosphate N-acetyltransferase, converts glucosamine 6-phosphate to N-acetylglucosamine 6-phosphate, which is subsequently converted to N-acetylglucosamine-1-phosphate by phosphoglucomutase (EC 5.4.2.2). UDP-N-acetylglucosamine pyrophosphorylase (EC 2.7.7.23), also known as UDP-N-acetylhexosamine pyrophosphorylase, converts N acetylglucosamine-1-phosphate to UDP-GlcNAc. GFAT is considered the primary regulatory step in the HBP, controlling influx of glucose into the pathway. GFAT activity is dependent on the availability of fructose-6-phosphate, generated primarily from glucose and glutamine. Both UDP-GlcNAc and glucosamine-6-phosphate are feedback inhibitors of GFAT. Acetyl-CoA and UTP are also required for synthesis of UDP-GlcNAc (Fig 1). GFAT has numerous phosphorylation sites, but as discussed in greater detail below, kinases have been identified only for Ser-205, Ser-235 and Ser-243 phosphorylation. At the transcriptional level, GFAT is regulated by Activating Transcription Factor 4 (ATF4), Specificity protein 1 (Sp1) and X-Box Binding protein-s (XBP1s). For example, ischemic stress increases GFAT1 expression in the heart via Xbp1s [10]. Similarly, GFAT1 expression is increased in response to pro-hypertrophic signaling both in vitro with phenylephrine (PE), Insulin Growth Factor-1 (IGF-1), Endothelin-1 (ET1), or angiotensin II (AngII) and in vivo via transaortic constriction (TAC) [11].
Figure 1:
Schematic illustrating the hexosamine biosynthesis pathway (HBP) and protein O-GlcNAc turnover. Following its entry into the cell glucose is metabolized to fructose-6-phosphate, which is the branch point between the HBP and glycolysis. L-Glutamine: D-fructose-6-phosphate amidotransferase (GFAT), which regulates glucose entry into the HBP, converts fructose-6-phosphate to glucosamine-6-phosphate. Subsequent metabolism by glucosamine 6-phosphate N-acetyltransferase (Emeg32), Phosphoacetylglucosamine mutase (Agm1) and UDP-N-acetylglucosamine pyrophosphorylase (Uap1) leads to the synthesis of uridine-diphosphate-N-acetylglucosamine (UDP-GlcNAc). UDP-GlcNAc is the obligate sugar donor for (O-GlcNAc transferase (OGT) which catalyzes the formation of O-linked β-N-acetylglucosamine (O-GlcNAc)-modification on ser/thr residues of proteins. The removal of O-GlcNAc from the proteins is catalyzed by β-N-acetylglucosaminidase (OGA) resulting in unmodified proteins and free GlcNAc, which can re-enter the HBP following its phosphorylation to GlcNAc-6-Phosphate by N-acetylglucosamine kinase (NAGK). There is also a multi-step process where GalNAc is metabolized to UDP-GalNAc before subsequent conversion to UDP-GlcNAc by an epimerase.
A major difficulty in understanding the mechanisms regulating HBP has been the absence of methods to reliably measure flux through the pathway. Changes in HBP flux are frequently assessed by alterations in the steady state levels of UDP-GlcNAc. However, this is can lead to erroneous conclusions because changes in steady state levels can occur due to differences in synthesis, utilization or both. It is widely stated that 3–5% of glucose entering cells is metabolized by the HBP; however this figure based on an estimate in cultured adipocytes [12]* has largely gone unchallenged. A study using cultured neonatal cardiomyocytes suggested that HBP flux could be substantially higher than previously recognized [13]. On the other hand, Olsen et al. recently reported an HBP flux of ~2.5 nmol/g protein/min in the isolated perfused working mouse heart, representing only 0.003–0.006% of the glycolytic flux [14]**.
UDP-GlcNAc is a substrate for O-GlcNAc transferase (OGT; EC 2.4.1.255), which catalyzes the attachment of O-GlcNAc to serine and threonine residues of proteins [3]. OGT is a soluble glycosyltransferase, encoded by a highly conserved gene located on the X-chromosome in mammals. Alternative splicing results in 3 mammalian isoforms of OGT; nucleocytoplasmic OGT (ncOGT; 110kDa), short OGT (sOGT; 78kD) and mitochondrial OGT (mOGT; 90kD). Although ubiquitously expressed, these isoforms differ in subcellular localization; ncOGT is found in both the nucleus and cytosol, sOGT is primarily localized to the cytosol, while the mOGT isoform contains a mitochondrial targeting sequence at its N-terminus (and is therefore located in the mitochondria); It is noteworthy that the specific function of mOGT remains open to debate [15]. The differences between the isoforms are in the number of tetratricopeptide repeats (TPRs) in the N-terminal domain, which are responsible for binding target proteins and not the catalytic domain [16]. Thus, primary differences in function between the isoforms are likely reflected in their protein targets rather than their catalytic activity.
In addition to UDP-GlcNAc and OGT, protein O-GlcNAc levels are also regulated by O-GlcNAcase (OGA; EC 3.2.1.169), a hexosaminidase that catalyzes the removal of O-GlcNAc from proteins [3]. OGA is encoded by a single gene on chromosome 10 in humans, and alternative splicing leads to a full length OGA (103kD) and a short OGA (sOGA; 76kD), which are ubiquitously expressed [3]. The full length OGA is predominantly found in the cytosol but is also found in the nucleus. sOGA, which exhibits significantly lower hexosaminidase activity, is both found in the nucleus and is also associated with lipid droplets; however, its function is largely unknown [15].
Regulation of O-GlcNAc homeostasis
As highlighted above, the three key regulatory steps involved in protein O-GlcNAcylation homeostasis are GFAT, OGT, and OGA [3]. To date, more is known about the regulation of GFAT and OGT, relative to OGA. GFAT and OGA are phosphorylated by several kinases, which have the potential to alter their activities [3,17]. For example, GFAT is phosphorylated by protein kinase A (PKA), calmodulin-dependent protein kinase II (CAMKII) and 5’ AMP-activated protein kinase (AMPK) [17], while OGT is phosphorylated by glycogen synthase kinase (GSK)-3β, CaMKII, checkpoint kinase 1 (Chk1), and AMPK [3]. The effects of phosphorylation on GFAT and OGT activities remain poorly understood. For example, PKA phosphorylation of GFAT has been reported to both increase activity in GFAT from rat liver and Drosophila, whereas PKA decreased activity of recombinant human GFAT1 [17]. Studies in neonatal rat cardiomyocytes and in vivo in the mouse heart found that activation of AMPK decreases GFAT activity and O-GlcNAc levels [18]. In the same study it was shown that genetic deletion of AMPKα2, the main cardiac isoform of AMPK, increased basal O-GlcNAc levels. Interestingly, however, this was not associated with any changes in GFAT or OGT protein levels, rather a decrease in OGA protein was observed [18]. Phosphorylation of OGT leads to changes in activity, stability, subcellular localization and substrate affinity; for example, AMPK phosphorylation of OGT leads to changes in subcellular localization and substrate affinity, whereas GSK-3β phosphorylation increase OGT activity [3]. A number of different stimuli, including insulin, leptin, ET1 and PE, increase O-GlcNAc levels through alterations in GFAT/OGT activity and/or expression; however, the mechanisms by which these agonists regulated OGT or GFAT are not known [19]. Glucagon increases CaMKII phosphorylation of OGT, which has been reported to alter its specificity towards target proteins involved in autophagy [20]. Chk1 phosphorylation of OGT appears to attenuate its proteasomal degradation [15]. To date little is known about how phosphorylation regulates OGA activity or function.
A widely accepted concept is that under normal conditions, OGT and OGA keep O-GlcNAc levels within a hypothetical “optimal” range, facilitating rapid changes in response to physiological stimuli. Conversely, pathophysiological conditions lead to chronic increases or decreases in O-GlcNAc levels outside that range, resulting in impaired cellular function and increased susceptibility to cell death [19]. This model is supported by the observations that low O-GlcNAc levels are associated with reduced tolerance of neonatal rat cardiomyocytes to ischemia/reperfusion or acute oxidative stress [21]. Similarly, sustained increases in cardiac O-GlcNAc levels, as observed in rodent models of type 1 and type 2 diabetes, are associated with cardiac dysfunction [22,23]. Chronic perturbations in O-GlcNAc levels outside the “optimal range” can occur following fluctuations in glucose availability as well as changes in GFAT, OGT and/or OGA activities. In the following sections we will highlight studies demonstrating the contributions of aberrant protein O-GlcNAcylation to diabetic complications of the cardiovascular system.
O-GlcNAc and vascular function/dysfunction
Vascular dysfunction is a common feature of both type 1 and type 2 diabetes, resulting in impaired vasodilation, increased atherosclerosis, hypertension and vascular calcification. Han and Kudlow demonstrated that in vascular smooth muscle cells, excess HBP flux from either hyperglycemia or glucosamine resulted in increased O-GlcNAcylation of the transcription factor Sp1, which attenuated Sp1 degradation [24]*. In rat aortic smooth muscle cells, hyperglycemia resulted in an increase of both OGT protein and its activity, leading to increased O-GlcNAc levels; it was proposed that this increase of O-GlcNAc could represent a mechanism mediating glucose toxicity in vasculature [25]. Phosphorylation of endothelial nitric oxide synthase (eNOS) at Ser1177 is required for its activation by AKT; O-GlcNAcylation of this same site in response to hyperglycemia attenuates its activation [26]**. This O-GlcNAc mediated impairment of eNOS has been implicated in the development macrovascular dysfunction, increased atherosclerosis and impaired angiogenesis [27], all of which are associated with type 1 and type 2 diabetes. In support of this endothelial O-GlcNAcylation in carotid plaques were higher in type 2 diabetic patients compared to non-diabetics [28]. Increased O-GlcNAcylation of AKT has also been reported to promote vascular calcification [29]*, thereby providing another mechanism by which O-GlcNAc contributes to the adverse effects of diabetes on vascular function. For example, in a mouse model of type 1 diabetes, chronically increasing O-GlcNAc levels by inhibiting OGA accelerated the development of vascular calcification [29]. Elevated vascular O-GlcNAc levels have also been associated with increased vascular reactivity and hypertension in an ET-1 dependent manner [30]. The hyperglycemia-mediated increase in OGT and O-GlcNAc was associated with downregulation of miR-200a/200b, further contributing to the adverse effects of diabetes on endothelial function [31]. In addition to macrovascular disease, increased O-GlcNAc levels have also been implicated in microvascular disease and the increased development of renal dysfunction and retinopathy associated with diabetes [32]. In type 2 diabetic mice, endothelial cell-specific overexpression of OGA improved coronary microvascular function by reducing the O-GlcNAc mediated increase in p53 in coronary endothelial cells [33]. Thus, in summary, sustained increases in O-GlcNAcylation in endothelial and vascular smooth muscle cells, are strongly associated with vascular dysfunction associated with type 1 and type 2 diabetes.
O-GlcNAc, contractility and calcium handling
Diabetes is known to cause cardiac dysfunction, observed at both whole heart and cardiomyocyte levels. In the latter case, impaired relation is observed in cardiomyocytes isolated from animal models of type 1 diabetes. Davidoff and colleagues were the first to show that diabetes-induced relaxation impairment is mimicked by incubation of normal cardiomyocytes with high glucose [34]. They also linked this response to increased HBP flux, as exogenous glucosamine had similar effects to hyperglycemia on both relaxation and cytosolic Ca2+ levels [34]*. Subsequent studies in neonatal cardiomyocytes demonstrated that hyperglycemia and glucosamine impaired relaxation, slowed Ca2+ decay and reduced sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) proteins levels; the effects of hyperglycemia could be reversed by overexpression of OGA [35]*. The same group replicated these findings in adult cardiomyocytes from a mouse model of type 2 diabetes and suggested a role for O-GlcNAcylation of Sp1 in the decrease in SERCA protein [36]**. Yokoe et al. subsequently reported that phospholamban (PLB) was an O-GlcNAc target, and that diabetes increased PLB O-GlcNAcylation; the latter was associated with decreased PLB phosphorylation, consistent with reduced SERCA activity and slower Ca2+ reuptake [37]. In a high fat diet induced mouse model of diabetes, an increase in overall O-GlcNAc levels was associated with increased in O-GlcNAcylation of PLB but not of SERCA [38]. Collectively these findings support the notion that increased O-GlcNAcylation, contributes to impaired cardiomyocyte relaxation and diastolic dysfunction that occurs with diabetes.
Contractile proteins such as actin, myosin, troponin and tropomyosin all have multiple O-GlcNAc modification sites [39]. The functional significance of these modifications is beginning to emerge. In skinned rat trabeculae, increased O-GlcNAc levels significantly reduced myofilament Ca2+ sensitivity while maximal contractile force was unchanged. The study reported that OGT was localized predominantly at the Z-line and OGA at the A-band in the normal heart; however, in a rat model of type 1 diabetes, this sub-cellular localization was lost, while overall O-GlcNAc levels were increased and myofilament Ca2+ sensitivity was impaired [39]**. Similar changes also occur in the myocardium of human diabetic patients compared to non-diabetics [39]**. Lowering O-GlcNAc levels in skinned fibers from type 1 diabetic rat hearts restored Ca2+ sensitivity to control levels. In skeletal muscle, myosin light chain (MLC) 2 is O-GlcNAcylated on its sole phosphorylation site [40]. It is not yet known whether this occurs in the heart; however, given the importance of MLC phosphorylation in maintaining optimal myocardial contractility [41], an increase in O-GlcNAcylation could be a contributing factor to impaired contractility seen in both type 1 and type 2 diabetes.
CaMKII plays a key role in both physiological and pathological Ca2+ signaling in the heart. Erickson and colleagues demonstrated that hyperglycemia elevated CaMKII activity in cardiomyocytes and this change in CaMKII activity was augmented by increasing O-GlcNAc levels via OGA inhibition [42]**. The effect of hyperglycemia on CaMKII was attenuated by inhibiting HBP flux. In the same study hyperglycemia increased O-GlcNAcylation of S279 on CaMKII resulting in higher levels of autophosphorylation [42]. Hyperglycemia and type 2 diabetes were both arrhythmogenic in an HBP dependent manner; consequently, O-GlcNAc mediated activation of CaMKII was proposed to be an important contributor to the adverse effects of diabetes on the heart, including decreased contractility and increased cardiac arrhythmias [42]. Of note, O-GlcNAcylation of CaMKII was significantly increased in the myocardium of heart failure patients compared to non-failing hears and was increased further in heart failure patients with diabetes [42]. Interestingly, Kronlage et al. demonstrated that in mouse models of both type 1 and type 2 diabetes O-GlcNAcylation of HDAC4 at serine (Ser)-642 protected against the consequences of increased CaMKII signaling in hearts [43]. Given the diverse roles of CaMKII in the heart, its complex regulation by O-GlcNAcylation has important implications with respect to the adverse effects of diabetes on the heart.
A number of other Ca2+ signaling pathways have been shown to be regulated by O-GlcNAcylation, which could have implications for the effects of diabetes on the heart. For example, in C2C12 cells, GPCR-induced Ca2+ mobilization was attenuated by increased O-GlcNAcylation of PLC-β1 [44]. In neonatal cardiomyocytes hyperglycemia attenuated hypertrophic signaling and angiotensin-II (AngII) induced increase in cytosolic Ca2+ in an HBP and O-GlcNAc dependent manner [45]. Stromal Interacting Molecule-1 (STIM1), which regulates store operated Ca2+ entry (SOCE) pathways, has been linked to hypertrophic signaling, is O-GlcNAcylated in cardiomyocytes, where its function impaired by increased O-GlcNAc levels [46]. Ca2+ plays a key role in regulating numerous processes essential for normal cardiomyocyte function. Therefore, a better understanding of how protein O-GlcNAcylation influences myocardial Ca2+ signaling will be important in elucidating its role in normal cardiac physiology as well as in disease such as diabetes.
Mitochondrial function
Impaired mitochondrial function is one of the hallmarks of the adverse effects of diabetes on the heart; moreover, hyperglycemia induced increases in O-GlcNAcylation have adverse effects on mitochondrial function [5]. In neonatal cardiomyocytes hyperglycemia increased O-GlcNAc levels on several subunits of mitochondrial respiratory complexes leading to decreased complex activity [47]. Increasing overall O-GlcNAcylation by hyperglycemia or inhibition of OGA resulted in O-GlcNAc modification of proteins involved in regulating mitochondrial dynamics such as optical atrophy 1 (OPA1) and dynamin-related protein 1 (DRP1) leading to an imbalance between fission and fusion, which could be reversed by overexpression of OGA [48,49]. While these studies linked hyperglycemia and O-GlcNAcylation to mitochondrial function, the lack of key control experiments raised the possibility that factors independent of O-GlcNAc could contribute to impaired mitochondrial function [50].
In a rat model of type 1 diabetes cardiac mitochondrial O-GlcNAc levels and OGT protein were increased, although OGT activity was unchanged compared to controls [51]**. In control mitochondria OGT was primarily localized to the inner membrane. Following diabetes, OGT was predominantly localized in the mitochondrial matrix. There was also a decrease in interaction between OGT and complex IV following diabetes and this decreased activation was associated with lower complex IV activity [51]. A proteomics study identified almost 90 different O-GlcNAc modified mitochondrial proteins in control and type 1 diabetic hearts from numerous metabolic pathways, including those in the TCA cycle, oxidative phosphorylation and fatty acid oxidation [52]*. Diabetes had a marked effect on O-GlcNAc levels of specific proteins, with 45 sites exhibiting an increase in O-GlcNAc levels compared to a 68 exhibiting decreased O-GlcNAcylation [52]. Proteins involved in pyruvate carboxylation, TCA cycle and fatty acid oxidation were among those that were differentially O-GlcNAcylated in response to diabetes [52]. It is commonly proposed that diabetes results in increased protein O-GlcNAcylation; however, these mitochondrial studies suggest that the effects of diabetes on O-GlcNAc modification of individual proteins is much more complex.
It is clear that numerous mitochondrial proteins are targets for O-GlcNAcylation and that diabetes leads to changes in the mitochondrial O-GlcNAcome. There are reports demonstrating that increases in O-GlcNAc levels decrease [53], increase [54] or have no effect on mitochondrial bioenergetics [50]. In addition to directly regulating function of proteins in major metabolic pathways, O-GlcNAc has also been implicated in regulating mitochondrial protein turnover via changes in the mitochondrial Lon protease homolog 1 (LonP1) [53], as well as mtDNA damage by decreasing the activity of 8-oxoguanine DNA glycosylase (Ogg1) which repairs mtDNA mutations [55]. In hearts from type 1 diabetic mice, Ogg1 O-GlcNAcylation was increased, thereby decreasing its activity and this was reversed by inhibiting OGT using a dominant negative OGT mutant [55]. While these changes could contribute to the adverse effects of diabetes on the heart, our understanding of how changes in O-GlcNAcylation of proteins affects mitochondrial function is still lacking. For example, the role of mOGT remains poorly understood, with some reports indicating that it is not required for O-GlcNAcylation of mitochondrial proteins [56]. Others have suggested that mOGT might be responsible for regulating mitochondrial structure and stress responses whereas ncOGT is important for the regulation of bioenergetics and metabolism [57].
Autophagy
Autophagy plays a crucial role in maintaining cellular quality control by degrading damaged proteins and organelles such as mitochondria. In models of both type 1 and type 2 diabetes cardiac autophagy is inhibited at several different levels and has been implicated in adverse remodeling and cardiac dysfunction [58]. There is increasing appreciation for a role of O-GlcNAcylation in regulating autophagy, with several key proteins including BECN-1, ULK1, mTOR, SNAP29, TPPP and GRASP55 all identified as O-GlcNAc targets [19]. Adult cardiomyocytes isolated from type 2 diabetic mice exhibited impaired autophagic signaling compared to control cardiomyocytes, which could be partially reversed by inhibiting the HBP [59]. Moreover, increasing O-GlcNAc levels in control cardiomyocytes impaired autophagic signaling in a similar fashion to that seen in diabetic cardiomyocytes [59]*. In a type 1 model of diabetes, basal autophagic flux was found to be inhibited and this could be replicated in normal cardiomyocytes with either hyperglycemia or directly increasing O-GlcNAc levels [60]. Potential mechanism by which diabetes impairs autophagy may involve increased SNAP29 O-GlcNAcylation which inhibited SNAP29/STX17/VAMP8 complex formation thus preventing autophagasome degradation [60].
Transcription and epigenetics
Sp1 is a ubiquitously expressed transcription factor that regulates thousands of genes, was one of the first transcription factors shown to be O-GlcNAcylated [61]. It was soon recognized that this represented a potential molecular mechanism in the glucose dependent regulation of transcription and thus a mediator of the adverse effects of hyperglycemia and diabetes on cellular function [62]. In neonatal cardiomyocytes, hyperglycemia increased Sp1 O-GlcNAc levels and this was associated with reduced mRNA and protein levels of SERCA2a [35]. Overexpression of OGA reversed the effects of hyperglycemia on Sp1 O-GlcNAcylation, restored SERCA2a levels and normalized contractility [35]. An increase in Sp1 O-GlcNAcylation by hyperglycemia leads to upregulation of intercellular adhesion molecule-1 (ICAM-1) [63], increased vascular endothelial growth factor (VEGF)-A [64] and increased collagen synthesis [65]. These changes are implicated in diabetes-related endothelial dysfunction, diabetic retinopathy and increased cardiac fibrosis that occurs in response to type 1 and type 2 diabetes.
Numerous transcription factors are now known to be O-GlcNAcylated and it has been estimated that over a quarter of all known O-GlcNAcylated proteins contribute to transcriptional regulation [3]. In addition to Sp1, transcription factors forkhead Box O1 (FOXO1) [66,67] and peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α [68] may also be of particular relevance to the adverse effects of hyperglycemia and diabetes on heart. In a rat model of Type 1 diabetes there was increased O-GlcNAcylation of FOXO1, providing a mechanism by which hyperglycemia increases FOXO1 activity [66]. PGC-1α O-GlcNAcylation increases its stabilization, in addition PGC-1α a FOXO1 co-activator recruits OGT to FOXO1 further increasing FOXO1 O-GlcNAcylation and activity [68]. While these studies were not in the heart, increased FOXO1 activation has been shown to be a key mediator of the adverse effects of type 2 diabetes on the heart [69]. Increased PGC-1α is also implicated in the increased myocardial fatty acid metabolism and lipotoxicity seen with type 2 diabetes and obesity [70].
The circadian clock is a key transcriptional mechanism that ensures metabolic and physiological rhythms occur at the appropriate time of day. In the cardiovascular system intrinsic circadian clocks are essential for regulating the time of day-dependent changes in heart rate, blood pressure and metabolism [71]. Core components of the mammalian circadian clock, BMAL1, PER2 and CLOCK are all O-GlcNAcylated as are key regulators of clock genes such as PGC-1α, GSK3β, AKT and AMPK [71]. Moreover, changes in O-GlcNAc levels directly altered the length of circadian rhythms [71]. In the heart overall O-GlcNAc levels were shown to exhibit time of day dependent rhythms that were abolished in cardiomyocyte-specific ablation of the circadian clock [72]**. REV-ERBα, which is a key component of a negative feedback loop is not directly modified by O-GlcNAc, but directly interacts with and stabilizes OGT providing a mechanism for the clock regulation of O-GlcNAc levels. In type 1 diabetes, the cardiomyocyte clock is phase shifted by approximately 3 hours [73]. In type 2 diabetes, the circadian rhythms of clock genes and genes of contraction related proteins in vascular smooth muscle were suppressed [74]. Thus, the resulting O-GlcNAc-mediated circadian misalignment in the cardiovascular system could be an important factor in the increased risk of heart disease associated with diabetes. Thus, it is clear that O-GlcNAcylation is a highly regulated process sensitive to nutrient and stress conditions that contribute to the adverse effects of diabetes on the cardiovascular system via mechanisms including but not limited to regulation of mitochondrial function, autophagy and transcription activities. In the following section we will discuss studies that target O-GlcNAc regulation in development of treatment strategies for cardiovascular diseases.
Therapeutic potential of pharmacological modulation of O-GlcNAc levels
The adverse effects of sustained increases in O-GlcNAc levels on the heart raises the question as to whether decreasing O-GlcNAc levels in such a setting could reverse or prevent these effects. There are two overall approaches to achieving this, the first is to inhibit O-GlcNAc synthesis, the second is to increase its removal. In type 1 diabetic hearts, increasing the rate of O-GlcNAc removal by overexpression of OGA improved contractile function, normalized SERCA levels and PLB phosphorylation levels [36]. Inhibiting O-GlcNAc synthesis by overexpression of a dominant negative OGT reduced cardiac O-GlcNAc levels in a model of type 1 diabetes, and this was associated with a reduction in apoptosis and mtDNA damage [55].
Whether sustained increases in myocardial O-GlcNAc levels is sufficient to be a major driver of the adverse effects of diabetes on the heart remains unclear. However, chronic overexpression of OGT in the heart increases O-GlcNAc levels and leads to progressive decline in cardiac function resulting in a dilated cardiomyopathy [75]. Conversely, prolonged overexpression of OGA exhibited no phenotype at baseline, but was protective in response to a pathological stress [75]. Cardiomyocyte specific overexpression of GFAT1 accelerated the response to pressure overload hypertrophy whereas GFAT1 deletion attenuated this response [11], further supporting a role for increased HBP in contributing to adverse cardiac remodeling. While neither of these studies were in the setting of diabetes it is consistent with the cardioprotective effects of acute adenovirus mediated OGA overexpression in a model of type 2 diabetes [36]. Additional evidence for a causal role of O-GlcNAc on the adverse effects of diabetes was demonstrated by Prakoso et al., who reported that sustained AAV-mediated OGT overexpression in normal mice resulted in cardiac remodeling similar to that seen in type 1 diabetes, whereas AAV-mediated overexpression of OGA attenuated diabetes related adverse remodeling [76]. GFAT inhibitors azaserine and DON have been used in vitro to reverse the effects of type 2 diabetes on isolated cardiomyocytes [77]; however, their lack of specificity prevents their use in vivo. Effective small molecule inhibitors of OGT have been described only very recently [78]* and their effects on O-GlcNAc levels in the diabetic heart and cardiomyocytes have yet to be studied. A number of microRNAs have been shown to regulate OGA and OGT levels and could represent future targets for modulating O-GlcNAc levels [19].
Conclusions
Our understanding of the role of O-GlcNAc in the regulation of the cardiovascular system is improving, nevertheless, its role in the etiology of cardiac pathophysiology continues to be under appreciated. There is a general consensus that diabetes leads to a sustained increase in O-GlcNAc levels in cardiomyocytes, vascular smooth muscle and endothelial cells, which lead to wide ranging effects on protein function and transcriptional regulation (Fig 2). These effects in turn contribute to the adverse effects of diabetes on vascular and cardiomyocyte function (Fig 2). An overview of some of the key O-GlcNAcylated proteins implicated in the adverse effects of diabetes on the cardiovascular system are listed in Table 1. A significant caveat is that virtually all of these data are based on animal models of diabetes; consequently, our knowledge of how O-GlcNAc contributes to cardiovascular dysfunction in patients with either type 1 or type 2 diabetes is limited. Therefore, future studies need to address this important gap in our knowledge.
Figure 2:
Summary of the O-GlcNAc mediated effects of diabetes on the cardiovascular system. On the left, the effects of increased O-GlcNAc levels on the molecular events and functional consequence in the vascular system, including smooth muscle and endothelial cells. On the right, the effects of increased O-GlcNAc levels on the molecular events and functional consequence in cardiomyocytes. The citations for the effects of O-GlcNAc on these processes, which are also included in the main body of the text, are as follows: AKT activity [29]; CaMKII activation [42]; Circadian clock [72]**; Collagen synthesis [65]; ET-1 activation [30]; FOXO1 activity [66]; ICAM-1 levels [63]; eNOS activity [26]; miR-200a/200b levels [31]; Mitochondrial bioenergetics [47,51]; p53 activation [33]; PGC1α activity [68]; PLB phosphorylation [37]; PLC-β activity [44]; Runx2 activation [29]; SERCA protein levels [35,36]; SOCE/STIM1 function [46]; Sp1 activity [35,61]; VEFG levels [64]. These citations are examples only and should not be considered a complete list.
Table 1:
O-GlcNAcvlated proteins implicated in the adverse effects of diabetes on the cardiovascular system*
| Protein | Effects of O-GlcNAc on protein function |
|---|---|
| Transcriptional regulators | |
| Sp1 | Both increases and decreases transcriptional activity depending on which sites are modified |
| p53 | Increases stability and transcriptional activity |
| FOXO1 | Increases target gene transcription |
| PGC-1α | Enhances stability and upregulated downstream genes |
| PPARγ | Reduces its transcriptional activity |
| GATA4 | Influences hypertrophic signaling |
| MEF2C | Influences hypertrophic signaling |
| HDAC4 | Facilitates HDAC4 proteolysis and attenuates development of heart failure |
| Bmal1 | Increases stability, shifts phase of clock |
| Clock | Increases stability, shifts phase of clock |
| Per2 | O-GlcNAc increases its suppressor activity |
| Ca2+ signaling and contractility | |
| SERCA | Not known, but likely decreases activity |
| Phospholamban | Decreases phosphorylation and increases interaction with SerCa, impairs relaxation |
| Actin | Reduces myofilament Ca2+ sensitivity |
| Myosin heavy chain | Reduces myofilament Ca2+ sensitivity |
| αTropomyosin | Reduces myofilament Ca2+ sensitivity |
| TroponinT | Decreases phosphorylation and reduces contractility |
| Myosin light chain 2 | Decreases phosphorylation and reduces contractility |
| CaMKII | Increases Ca2+-independent activity, increased Ca2+ spark frequency. |
| STIM1 | Inhibits puncta formation and Ca2+ entry |
| Nav1.5 | Increases arrhythmogenesis |
| PLC-β | Decreases activity |
| Mitochondria and Metabolism | |
| OPA1 | Imbalance between mitochondrial fission and fusion |
| DRP1 | Imbalance between mitochondrial fission and fusion |
| OGG1 | Decreases activity leading to increase in mitochondrial DNA damage |
| PDH - α subunit | Regulates activity |
| CPT1B | Impaired mitochondrial function |
| VDAC | Impaired mitochondrial function |
| COXI | Decreased complex activity |
| NDUFA9 | Decreased complex activity |
| ATP5A/B | Decreased activity |
| AKT | Attenuates and increases activity depending on specific modification sites |
| GSK3β | Decreased activity |
| Others | |
| eNOS | Attenuates activity |
| SNAP29 | Attenuates autophagic flux |
| Acetaldehyde dehydrogenase 2 (ALDH2) | Decreased activity exacerbates I/R injury |
A small but growing number of studies suggest that increasing cardiac O-GlcNAc levels alone is sufficient to recapitulate the adverse effects of diabetes on the heart; whereas a reduction of O-GlcNAc levels in the setting of diabetes attenuates these effects and improves vascular and cardiomyocyte function. Consequently, targeting O-GlcNAc levels as a therapeutic approach for managing diabetic complications is potentially attractive; however, our fundamental understanding of how O-GlcNAc levels are regulated in the heart remains very limited. In diabetes the increase in O-GlcNAc is usually attributed to hyperglycemia leading excessive HBP flux and subsequent O-GlcNAcylation; however, this assumption has yet to be verified experimentally. It should be noted that cardiac O-GlcNAc levels are also chronically increased in hypertrophy, heart failure and aging none of which are associated primarily with systemic metabolic dysfunction [19]. Consequently, it remains unclear why in diabetes as well as other such disease states there is a resetting of steady state cardiac O-GlcNAc levels outside of the “optimal range”.
In addition, given the wide-ranging effects of O-GlcNAc on cardiomyocyte function, including transcription, metabolism, contractility and Ca2+ signaling, we need to understand how to modulate O-GlcNAcylation of specific pathways rather than globally. Therefore, future studies will require the development of techniques that enable us to visualize O-GlcNAc turnover on individual proteins, improve our understanding of spatial and temporal changes in cellular O-GlcNAcylation that occur in response to physiological (i.e. insulin) and pathological (i.e., phenylephrine) stimuli as well as a better knowledge of the factors that regulate HBP flux and the activities of OGT and OGA.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute (grant R01HL142216 to J.Z., J.C.C., and M.E.Y.). We would also like to thank our numerous colleagues at UAB and other institutions for insightful and wide-ranging discussions on O-GlcNAc biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Varki A, Kornfeld S: Historical Background and Overview. In Essentials of Glycobiology, edn 3rd. Edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, et al. ; 2015. [Google Scholar]
- 2. Torres CR, Hart GW: Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984, 259:3308–3317. *Pioneering study demontrating for first time single terminal O-GlcNAc modification of nuclear and cytoplasmic proteins
- 3.Zachara N, Akimoto Y, Hart GW: The O-GlcNAc Modification. In Essentials of Glycobiology. Edited by rd, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, et al. ; 2015:239–251. [Google Scholar]
- 4.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. : Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 5.Ritchie RH, Abel ED: Basic Mechanisms of Diabetic Heart Disease. Circ Res 2020, 126:1501–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin CX, Sleaby R, Davidoff AJ, Bell JR, De Blasio MJ, Delbridge LM, Chatham JC, Ritchie RH: Insights into the role of maladaptive hexosamine biosynthesis and O-GlcNAcylation in development of diabetic cardiac complications. Pharmacol Res 2016. [DOI] [PubMed] [Google Scholar]
- 7.McLarty JL, Marsh SA, Chatham JC: Post-translational protein modification by O-linked N-acetyl-glucosamine: its role in mediating the adverse effects of diabetes on the heart. Life Sci 2013, 92:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buse MG: Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol - Endo and Met 2006, 290:E1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milewski S: Glucosamine-6-phosphate synthase--the multi-facets enzyme. Biochim Biophys Acta 2002, 1597:173–192. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, Criollo A, Luo X, Tan W, Jiang N, et al. : Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 2014, 156:1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran DH, May HI, Li Q, Luo X, Huang J, Zhang G, Niewold E, Wang X, Gillette TG, Deng Y, et al. : Chronic activation of hexosamine biosynthesis in the heart triggers pathological cardiac remodeling. Nat Commun 2020, 11:1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marshall S, Bacote V, Traxinger RR: Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 1991, 266:4706–4712. *Identified increased flux through the hexosamine biosythesis pathway as a factor contributing to insulin resistance.
- 13.Gibb AA, Lorkiewicz PK, Zheng YT, Zhang X, Bhatnagar A, Jones SP, Hill BG: Integration of flux measurements to resolve changes in anabolic and catabolic metabolism in cardiac myocytes. Biochem J 2017, 474:2785–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olson AK, Bouchard B, Zhu WZ, Chatham JC, Des Rosiers C: First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart. J Biol Chem 2020, 295:2018–2033. **Established methodolgy for quantifying simultaneously the rate of glucose metabolism via glycolysis and the hexosamine biosynthesis pathway.
- 15.King DT, Males A, Davies GJ, Vocadlo DJ: Molecular mechanisms regulating O-linked N-acetylglucosamine (O-GlcNAc)-processing enzymes. Curr Opin Chem Biol 2019, 53:131–144. [DOI] [PubMed] [Google Scholar]
- 16.Levine ZG, Walker S: The Biochemistry of O-GlcNAc Transferase: Which Functions Make It Essential in Mammalian Cells? Annu Rev Biochem 2016, 85:631–657. [DOI] [PubMed] [Google Scholar]
- 17.Durand P, Golinelli-Pimpaneau B, Mouilleron S, Badet B, Badet-Denisot MA: Highlights of glucosamine-6P synthase catalysis. Arch Biochem Biophys 2008, 474:302–317. [DOI] [PubMed] [Google Scholar]
- 18.Gelinas R, Mailleux F, Dontaine J, Bultot L, Demeulder B, Ginion A, Daskalopoulos EP, Esfahani H, Dubois-Deruy E, Lauzier B, et al. : AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat Commun 2018, 9:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatham JC, Zhang J, Wende AR: Role of O-linked N-acetylglucosamine (O-GlcNAc) protein modification in cellular (patho)physiology. Physioloigcal Reviews In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruan H-B, Ma Y, Torres S, Zhang B, Feriod C, Heck RM, Qian K, Fu M, Li X, Nathanson MH, et al. : Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation. Genes & Development 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP: Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res 2009, 104:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright JN, Collins HE, Wende AR, Chatham JC: O-GlcNAcylation and cardiovascular disease. Biochem Soc Trans 2017, 45:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Blasio MJ, Huynh N, Deo M, Dubrana LE, Walsh J, Willis A, Prakoso D, Kiriazis H, Donner DG, Chatham JC, et al. : Defining the Progression of Diabetic Cardiomyopathy in a Mouse Model of Type 1 Diabetes. Front Physiol 2020, 11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han I, Kudlow JE: Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol 1997, 17:2550–2558. *Demonstrated that changes in hexosamine biosynthesis flux changed O-GlcNAc levels on Sp1, there by altering its transcriptional activity.
- 25.Akimoto Y, Kreppel LK, Hirano H, Hart GW: Hyperglycemia and the O-GlcNAc transferase in rat aortic smooth muscle cells: elevated expression and altered patterns of O-GlcNAcylation. Arch Biochem Biophys 2001, 389:166–175. [DOI] [PubMed] [Google Scholar]
- 26. Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M: Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 2001, 108:1341–1348. **Demontrated that hyperglycemia and increased HBP flux resulted in increased O-GlcNAc modification of serine 1177 of eNOS concomitant with a decrease in phosphorylation and a reduction in eNOS activity. Proposed this as a potential mechanism for hyperglycemia-mediated inhibition of eNOS and impaired endothelial function.
- 27.Issad T, Masson E, Pagesy P: O-GlcNAc modification, insulin signaling and diabetic complications. Diabetes Metab 2010, 36:423–435. [DOI] [PubMed] [Google Scholar]
- 28.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R: Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 2002, 106:466–472. [DOI] [PubMed] [Google Scholar]
- 29. Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell’Italia LJ, Chatham JC, Wu H, Chen Y: Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res 2014, 114:1094–1102. *Reported direct link between increased AKT O-GlcNAcylation and increased vascular calcification associated with hyperglycemia and diabetes.
- 30.Lima VV, Giachini FR, Hardy DM, Webb RC, Tostes RC: O-GlcNAcylation: a novel pathway contributing to the effects of endothelin in the vasculature. Am J Physiol Regul Integr Comp Physiol 2011, 300:R236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo WY, Yang WK, Peng CT, Pai WY, Wang HJ: MicroRNA-200a/200b Modulate High Glucose-Induced Endothelial Inflammation by Targeting O-linked N-Acetylglucosamine Transferase Expression. Front Physiol 2018, 9:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerjee PS, Lagerlöf O, Hart GW: Roles of O-GlcNAc in chronic diseases of aging. Molecular Aspects of Medicine 2016, 51:1–15. [DOI] [PubMed] [Google Scholar]
- 33.Si R, Zhang Q, Tsuji-Hosokawa A, Watanabe M, Willson C, Lai N, Wang J, Dai A, Scott BT, Dillmann WH, et al. : Overexpression of p53 due to excess protein O-GlcNAcylation is associated with coronary microvascular disease in type 2 diabetes. Cardiovasc Res 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ren J, Gintant GA, Miller RE, Davidoff AJ: High extracellular glucose impairs cardiac E-C coupling in a glycosylation-dependent manner. Am J Physiol 1997, 273:H2876–2883. *First report linking increased HBP flux as a mechanism for impaired cardiomyoycte contractility resulting from hyperglycemia and by impliation diabetes.
- 35. Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH: Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. Journal of Biological Chemistry 2003, 278:44230–44237. *Reported that impaired Ca2+ uptake in to the SR that occurs with hyperglycemia was due to increased HBP flux and O-GlcNAcylation of Sp1 along with a reduction in MEF-2 protein levels
- 36. Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH: Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 2005, 96:1006–1013. **Showed that AAV mediated increase in O-GlcNAcase improved contractility in hearts from diabetic mice and increased SR Ca2+ load. O-GlcNAcase expression increased SERCA protein levels
- 37.Yokoe S, Asahi M, Takeda T, Otsu K, Taniguchi N, Miyoshi E, Suzuki K: Inhibition of phospholamban phosphorylation by O-GlcNAcylation: implications for diabetic cardiomyopathy. Glycobiology 2010, 20:1217–1226. [DOI] [PubMed] [Google Scholar]
- 38.Fricovsky ES, Suarez J, Ihm SH, Scott BT, Suarez-Ramirez JA, Banerjee I, Torres-Gonzalez M, Wang H, Ellrott I, Maya-Ramos L, et al. : Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol 2012, 303:R689–R699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramirez-Correa G, Ma J, Slawson C, Zeidan Q, Lugo-Fagundo NS, Xu M, Shen X, Gao WD, Caceres V, Chakir K, et al. : Removal of abnormal myofilament O-GlcNAcylation restores Ca2+ sensitivity in diabetic cardiac muscle. Diabetes 2015, 64:3573–3587. **Identification of O-GlcNAc modification sites on multiple contractile proteins in the heart. Demonstrated that reducing myofilament O-GlcNAc levels improved contractility. Found that diabetes resulted in delocalization of OGT and OGA from Z- and A-lines respectively.
- 40.Hedou J, Cieniewski-Bernard C, Leroy Y, Michalski JC, Mounier Y, Bastide B: O-linked N-acetylglucosaminylation is involved in the Ca2+ activation properties of rat skeletal muscle. J Biol Chem 2007, 282:10360–10369. [DOI] [PubMed] [Google Scholar]
- 41.Chang AN, Kamm KE, Stull JT: Role of myosin light chain phosphatase in cardiac physiology and pathophysiology. J Mol Cell Cardiol 2016, 101:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, et al. : Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 2013, 502:372–376. **Demonstrated that O-GlcNAc modification of CaMKII at Ser 279 leads to autonomous activation of CaMKII. CaMKII O-GlcNAcylation is increased in the heart of diabetic humans and rats. Increase in CaMKII O-GlcNAcylation may contribute to arrhythmogenesis.
- 43.Kronlage M, Dewenter M, Grosso J, Fleming T, Oehl U, Lehmann LH, Falcao-Pires I, Leite-Moreira AF, Volk N, Grone HJ, et al. : O-GlcNAcylation of Histone Deacetylase 4 Protects the Diabetic Heart From Failure. Circulation 2019, 140:580–594. [DOI] [PubMed] [Google Scholar]
- 44.Kim YH, Song M, Oh YS, Heo K, Choi JW, Park JM, Kim SH, Lim S, Kwon HM, Ryu SH, et al. : Inhibition of phospholipase C-beta1-mediated signaling by O-GlcNAc modification. J Cell Physiol 2006, 207:689–696. [DOI] [PubMed] [Google Scholar]
- 45.Nagy T, Champattanachai V, Marchase RB, Chatham JC: Glucosamine inhibits angiotensin II-induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-linked N-acetylglucosamine. Am J Physiol Cell Physiol 2006, 290:C57–65. [DOI] [PubMed] [Google Scholar]
- 46.Zhu-Mauldin X, Marsh SA, Zou L, Marchase RB, Chatham JC: Modification of STIM1 by O-linked N-acetylglucosamine (O-GlcNAc) attenuates store-operated calcium entry in neonatal cardiomyocytes. J Biol Chem 2012, 287:39094–39106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Hu Y, Oyeleye MO, Dillmann WH: Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem 2009, 284:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, Dillmann WH: Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol 2011, 300:R1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, Han X, Yates J, Hoshijima M, Dillmann WH: Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dassanayaka S, Readnower RD, Salabei JK, Long BW, Aird AL, Zheng YT, Muthusamy S, Facundo HT, Hill BG, Jones SP: High glucose induces mitochondrial dysfunction independently of protein O-GlcNAcylation. Biochem J 2015, 467:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Banerjee PS, Ma J, Hart GW: Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria. Proc Natl Acad Sci U S A 2015, 112:6050–6055. **Demonstrated that in the diabetic rat heart mitochondrial OGT was increased and was localized to the matrix. Identified the pyrimidine nucleotide carrier as transporter of UDP-GlcNAc from cytosol into mitochondria.
- 52. Ma J, Banerjee P, Whelan SA, Liu T, Wei AC, Ramirez-Correa G, McComb ME, Costello CE, O’Rourke B, Murphy A, et al. : Comparative Proteomics Reveals Dysregulated Mitochondrial O-GlcNAcylation in Diabetic Hearts. J Proteome Res 2016, 15:2254–2264. *Comparative O-GlcNAc profiling of mitochondria from control and diabetic rat hearts. Identified 86 O-GlcNAcylated mitochondrial proteins with many exhibiting differential responses to diabetes.
- 53.Wright JN, Benavides GA, Johnson MS, Wani W, Ouyang X, Zou L, Collins HE, Zhang J, Darley-Usmar V, Chatham JC: Acute increases in O-GlcNAc indirectly impair mitochondrial bioenergetics through dysregulation of LonP1-mediated mitochondrial protein complex turnover. Am J Physiol Cell Physiol 2019, 316:C862–C875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma J, Liu T, Wei AC, Banerjee P, O’Rourke B, Hart GW: O-GlcNAcomic Profiling Identifies Widespread O-Linked beta-N-Acetylglucosamine Modification (O-GlcNAcylation) in Oxidative Phosphorylation System Regulating Cardiac Mitochondrial Function. J Biol Chem 2015, 290:29141–29153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cividini F, Scott BT, Dai A, Han W, Suarez J, Diaz-Juarez J, Diemer T, Casteel DE, Dillmann WH: O-GlcNAcylation of 8-Oxoguanine DNA Glycosylase (Ogg1) Impairs Oxidative Mitochondrial DNA Lesion Repair in Diabetic Hearts. J Biol Chem 2016, 291:26515–26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trapannone R, Mariappa D, Ferenbach AT, van Aalten DM: Nucleocytoplasmic human O-GlcNAc transferase is sufficient for O-GlcNAcylation of mitochondrial proteins. Biochem J 2016, 473:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sacoman JL, Dagda RY, Burnham-Marusich AR, Dagda RK, Berninsone PM: Mitochondrial O-GlcNAc Transferase (mOGT) Regulates Mitochondrial Structure, Function, and Survival in HeLa Cells. J Biol Chem 2017, 292:4499–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sciarretta S, Maejima Y, Zablocki D, Sadoshima J: The Role of Autophagy in the Heart. Annu Rev Physiol 2018, 80:1–26. [DOI] [PubMed] [Google Scholar]
- 59. Marsh SA, Powell PC, Dell’italia LJ, Chatham JC: Cardiac O-GlcNAcylation blunts autophagic signaling in the diabetic heart. Life Sci 2013, 92:648–656. *Demonstrated that increasing O-GlcNAc levels in adult cardiomyocytes attenuated autophagic response to nutrient depletion. Showed that Bcl2 and Beclin-1 were potential O-GlcNAc targets.
- 60.Huang L, Yuan P, Yu P, Kong Q, Xu Z, Yan X, Shen Y, Yang J, Wan R, Hong K, et al. : O-GlcNAc-modified SNAP29 inhibits autophagy-mediated degradation via the disturbed SNAP29-STX17-VAMP8 complex and exacerbates myocardial injury in type I diabetic rats. Int J Mol Med 2018, 42:3278–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jackson SP, Tjian R: O-glycosylation of eukaryotic transcription factors: Implications for mechanisms of transcriptional regulation. Cell 1988, 55:125–133. [DOI] [PubMed] [Google Scholar]
- 62.Hart GW: Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem 1997, 66:315–335. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Qu Y, Niu T, Wang H, Liu K: O-GlcNAc modification of Sp1 mediates hyperglycaemia-induced ICAM-1 up-regulation in endothelial cells. Biochem Biophys Res Commun 2017, 484:79–84. [DOI] [PubMed] [Google Scholar]
- 64.Donovan K, Alekseev O, Qi X, Cho W, Azizkhan-Clifford J: O-GlcNAc modification of transcription factor Sp1 mediates hyperglycemia-induced VEGF-A upregulation in retinal cells. Invest Ophthalmol Vis Sci 2014, 55:7862–7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aguilar H, Fricovsky E, Ihm S, Schimke M, Maya-Ramos L, Aroonsakool N, Ceballos G, Dillmann W, Villarreal F, Ramirez-Sanchez I: Role for high-glucose-induced protein O-GlcNAcylation in stimulating cardiac fibroblast collagen synthesis. Am J Physiol Cell Physiol 2014, 306:C794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW: O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem 2008, 283:16283–16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeon JH, Suh HN, Kim MO, Ryu JM, Han HJ: Glucosamine-induced OGT activation mediates glucose production through cleaved Notch1 and FoxO1, which coordinately contributed to the regulation of maintenance of self-renewal in mouse embryonic stem cells. Stem cells and development 2014, 23:2067–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, Hart GW: A PGC-1α-O-GlcNAc Transferase Complex Regulates FoxO Transcription Factor Activity in Response to Glucose. Journal of Biological Chemistry 2009, 284:5148–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, et al. : Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest 2012, 122:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schilling J, Kelly DP: The PGC-1 cascade as a therapeutic target for heart failure. J Mol Cell Cardiol 2011, 51:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Chatham J, Young ME: Circadian Regulation of Cardiac Physiology: Rhythms That Keep the Heart Beating. Annu Rev Physiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, et al. : O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem 2011, 286:44606–44619. **First report that core components of the mammalian clock are O-GlcNAc modified and increasing O-GlcNAc levels shifts phase of circadian clock Demonstrated that in the heart O-GlcNAcylation exhibits a diurnal rhythm which is absent when cardiomyocyte specific clock was ablated.
- 73.Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H: Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol 2002, 34:223–231. [DOI] [PubMed] [Google Scholar]
- 74.Su W, Xie Z, Guo Z, Duncan MJ, Lutshumba J, Gong MC: Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol 2012, 302:H621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Umapathi P, Mesubi O, Banerjee P, Zachara N, Wang Q, Granger J, Luczak E, Wu Y, Florea L, Talbot C, et al. : Elevated O-Glcnacylation : An Independent Driver of Cardiomyopathy. Circulation 2019, 140 A13696. [Google Scholar]
- 76.Prakoso D, Kiriazis H, Tate M, Qian H, Deo M, Parry LJ, Gregorevic P, Du X, Chatham J, De Blasio M, et al. : Manipulation of cardiac O-GlcNAc modification alters cardiac function and remodelling in the setting of diabetic cardiomyopathy. Eur Heart J. 2018, 39 (Suppl 1):ehy566.5213. [Google Scholar]
- 77.Marsh SA, Dell’Italia LJ, Chatham JC: Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids 2011, 40:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ortiz-Meoz RF, Jiang J, Lazarus MB, Orman M, Janetzko J, Fan C, Duveau DY, Tan ZW, Thomas CJ, Walker S: A small molecule that inhibits OGT activity in cells. ACS Chem Biol 2015, 10:1392–1397. *Characterization of small molecule inhibitor of OGT that is cell permeable and decreases O-GlcNAc levels in mammalian cell lines without affecting cell surface N- and O-linked glycans.
- 79.Dias WB, Cheung WD, Hart GW: O-GlcNAcylation of kinases. Biochem Biophys Res Commun 2012, 422:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ducheix S, Magré J, Cariou B, Prieur X: Chronic O-GlcNAcylation and Diabetic Cardiomyopathy: The Bitterness of Glucose. Frontiers in Endocrinology 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]