Abstract
The thrombotic microangiopathies (TMAs) are a heterogenous group of disorders with distinct pathophysiologies that cause occlusive microvascular or macrovascular thrombosis, and are characterized by microangiopathic hemolytic anemia, thrombocytopenia, and/or end‐organ ischemia. TMAs are associated with significant morbidity and mortality, and data on the management of certain TMAs are often lacking. The nomenclature, classification, and management of various TMAs is constantly evolving as we learn more about these rare syndromes. Thorough clinical and laboratory evaluation is essential to distinguish various TMAs and arrive at an accurate diagnosis, which is key for appropriate management. In this illustrated review, we focus on thrombotic thrombocytopenic purpura (TTP), Shiga toxin–associated hemolytic uremic syndrome, complement‐mediated hemolytic uremic syndrome, hematopoietic cell transplant‐associated TMA, and drug‐induced TMA, and describe their incidence, pathophysiology, diagnosis, and management. We also highlight emerging complement‐directed therapies under investigation for the management of complement‐mediated TMAs.
Keywords: atypical hemolytic uremic syndrome, hemolytic uremic syndrome, management, pathophysiology, thrombotic microangiopathies, thrombotic thrombocytopenic purpura
Essentials.
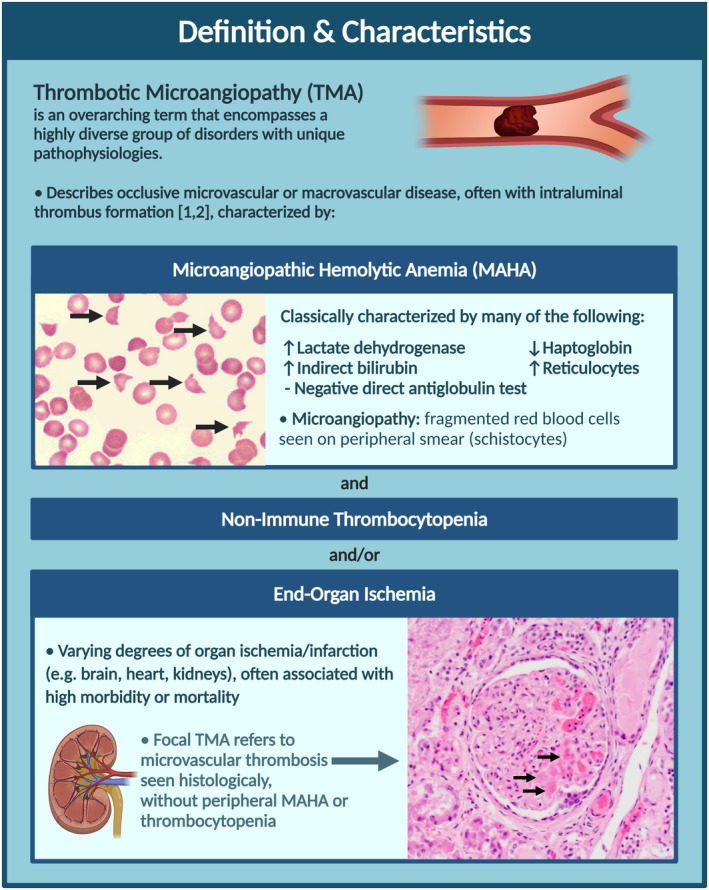
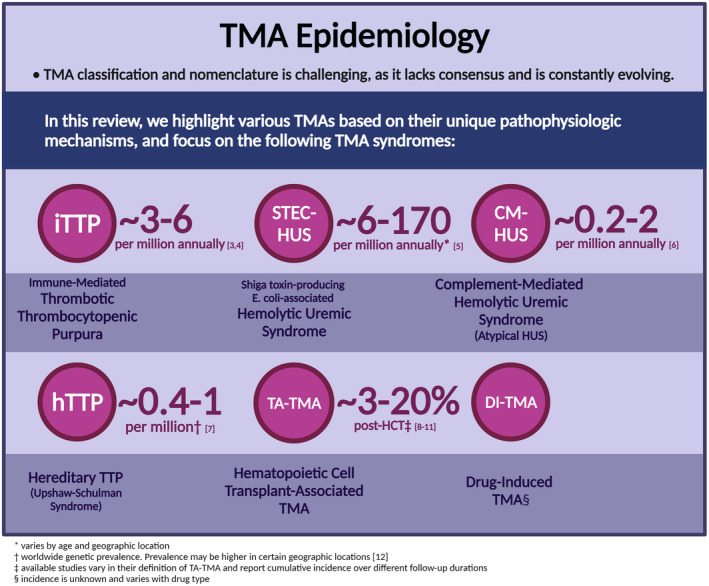
Thrombotic microangiopathies (TMAs) are a diverse group of rare, life‐threatening disorders.
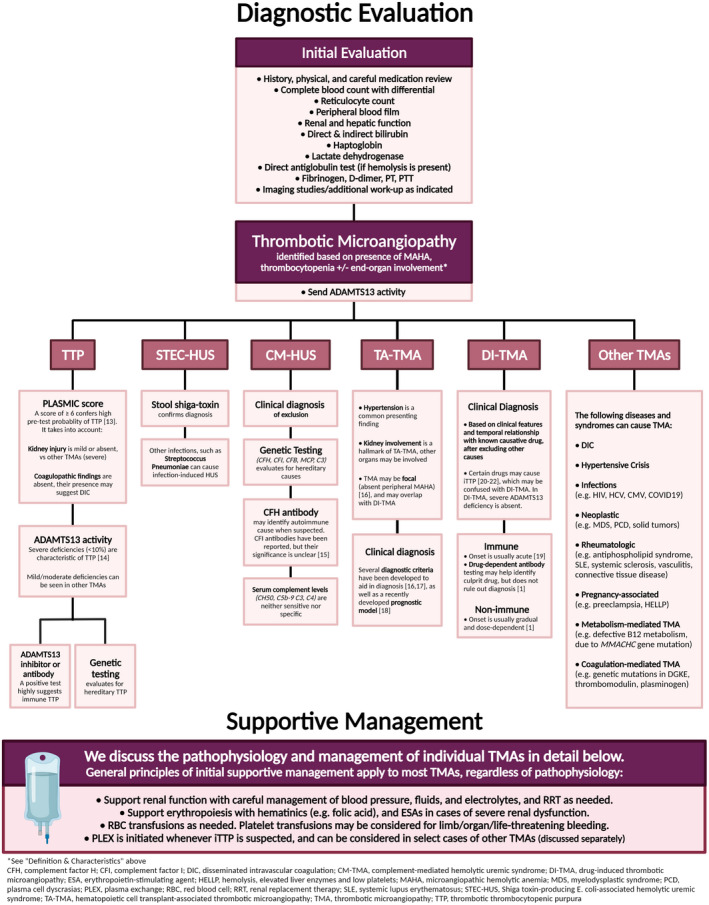
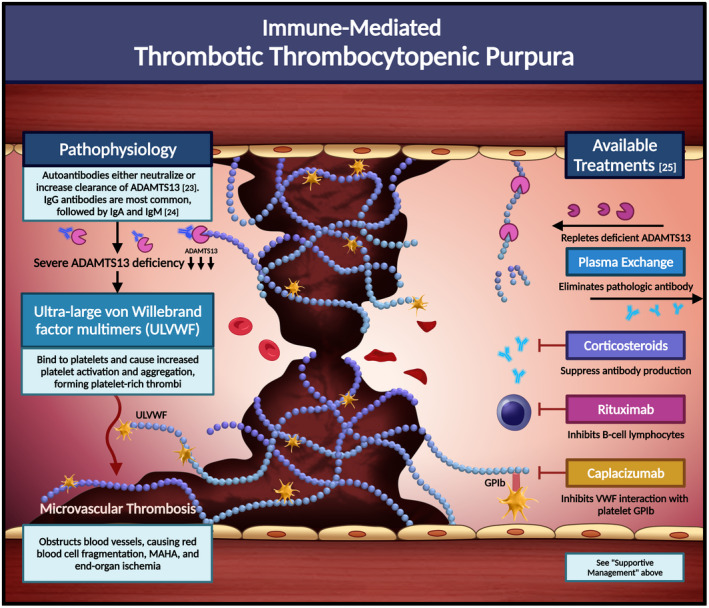
We review the incidence, pathophysiology, evaluation, and management of various TMAs.
Thorough evaluation is essential to distinguish TMAs, as management relies on accurate diagnosis.
Several complement‐directed therapies are emerging in the treatment of complement‐mediated TMAs.
RELATIONSHIP DISCLOSURE
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
MYA created all illustrations. MYA, SK, DCS, LN, and SA contributed to the scientific content and revised the manuscript.
ACKNOWLEDGMENTS
Created with BioRender.com. Micrographs of peripheral smear and kidney biopsy were taken and provided by Kristi Smock, MD, Division of Pathology at the University of Utah, ARUP Institute for Clinical and Experimental Pathology, Salt Lake City, Utah.
Abou‐Ismail MY, Kapoor S, Citla Sridhar D, Nayak L, Ahuja S. Thrombotic microangiopathies: An illustrated review. Res Pract Thromb Haemost. 2022;6:e12708. doi: 10.1002/rth2.12708
Handling Editor: Dr Michelle Sholzberg
Funding information
This work did not receive any funding.
Contributor Information
Mouhamed Yazan Abou‐Ismail, Email: yazan.abou-ismail@hsc.utah.edu, @DoctorYazanA.
Divyaswathi Citla Sridhar, @DivyaCitlaMD.
Lalitha Nayak, @nayaklalitha1.
Sanjay Ahuja, @ahujadoc.
REFERENCES
- 1. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654‐666. [DOI] [PubMed] [Google Scholar]
- 2. Scully M, Cataland S, Coppo P, et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(2):312‐322. [DOI] [PubMed] [Google Scholar]
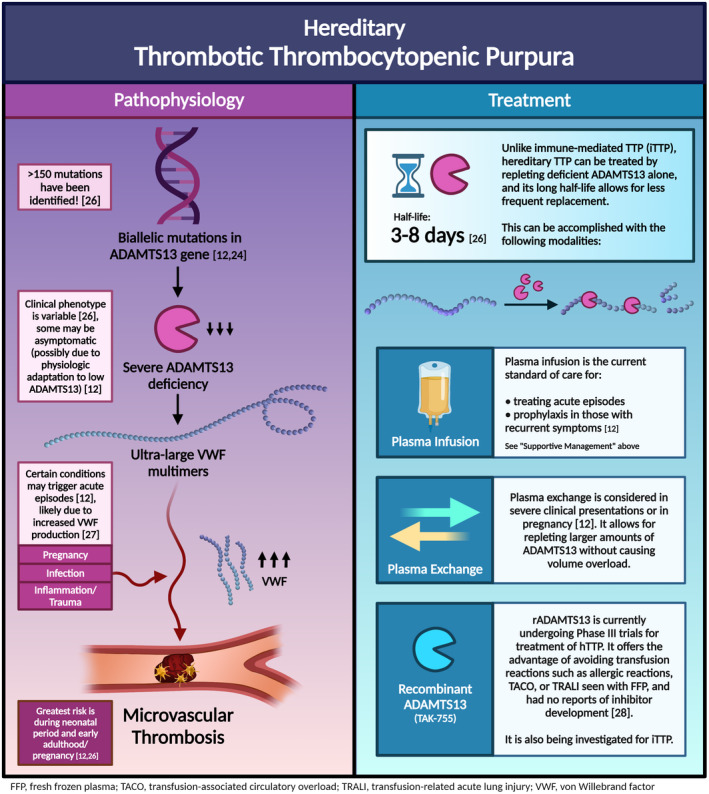
- 3. Reese JA, Muthurajah DS, Hovinga JAK, Vesely SK, Terrell DR, George JN. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired ADAMTS13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 2013;60(10):1676‐1682. [DOI] [PubMed] [Google Scholar]
- 4. Scully M, Yarranton H, Liesner R, et al. Regional UK TTP Registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol. 2008;142(5):819‐826. [DOI] [PubMed] [Google Scholar]
- 5. Zhao T, Fan S, Sun L. The global carrier frequency and genetic prevalence of Upshaw‐Schulman syndrome. BMC Genomic Data. 2021;22(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
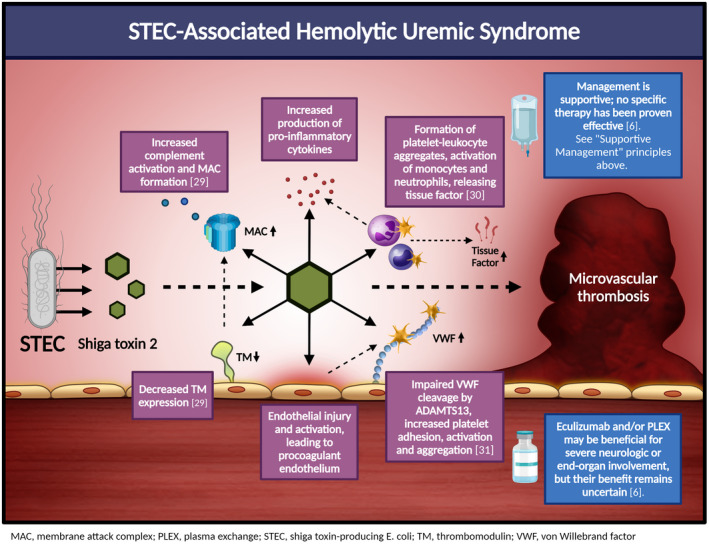
- 6. Fakhouri F, Zuber J, Frémeaux‐Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390(10095):681‐696. [DOI] [PubMed] [Google Scholar]
- 7. Yan K, Desai K, Gullapalli L, Druyts E, Balijepalli C. Epidemiology of atypical hemolytic uremic syndrome: a systematic literature review. Clin Epidemiol. 2020;12:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
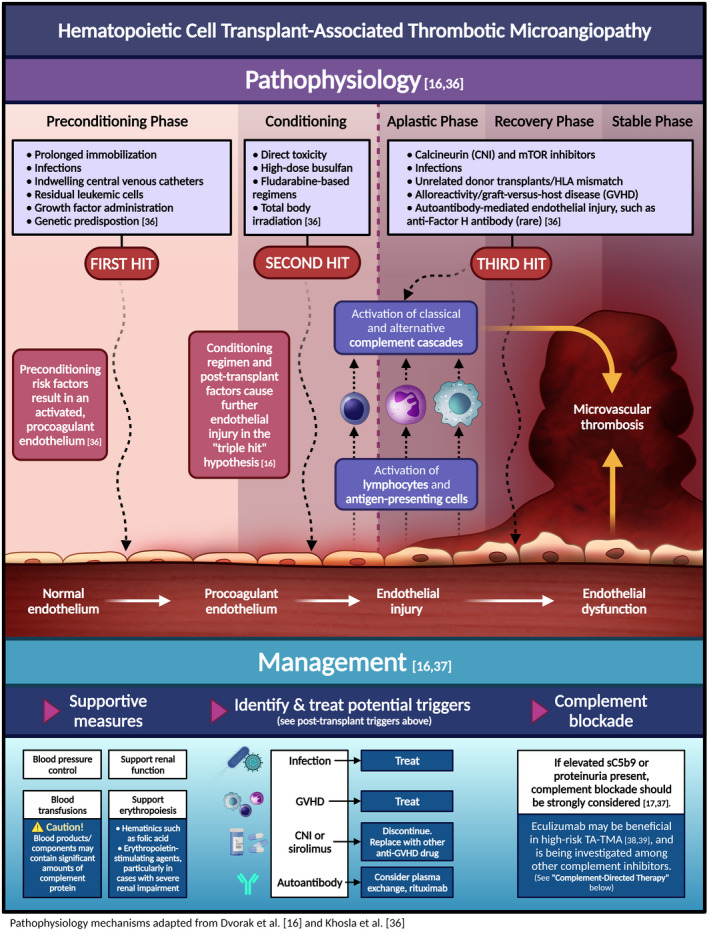
- 8. Epperla N, Li A, Logan B, et al. Incidence, risk factors for and outcomes of transplant‐associated thrombotic microangiopathy. Br J Haematol. 2020;189(6):1171‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Willems E, Baron F, Seidel L, Frère P, Fillet G, Beguin Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high‐dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45(4):689‐693. [DOI] [PubMed] [Google Scholar]
- 10. Changsirikulchai S, Myerson D, Guthrie KA, McDonald GB, Alpers CE, Hingorani SR. Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol. 2009;4(2):345‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakamae H, Yamane T, Hasegawa T, et al. Risk factor analysis for thrombotic microangiopathy after reduced‐intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2006;81(7):525‐531. [DOI] [PubMed] [Google Scholar]
- 12. Kremer Hovinga JA, George JN. Hereditary thrombotic thrombocytopenic purpura. N Engl J Med. 2019;381(17):1653‐1662. [DOI] [PubMed] [Google Scholar]
- 13. Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4(4):e157‐e164. [DOI] [PubMed] [Google Scholar]
- 14. Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2486‐2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kavanagh D, Pappworth IY, Anderson H, et al. Factor I autoantibodies in patients with atypical hemolytic uremic syndrome: disease‐associated or an epiphenomenon? Clin J Am Soc Nephrol. 2012;7(3):417‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dvorak CC. TA‐TMA: state of the art for diagnosis and treatment. Blood Adv. 2020;4(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young JA, Pallas CR, Knovich MA. Transplant‐associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 2021;56(8):1805‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao P, Wu Y‐J, He Y, et al. A prognostic model (BATAP) with external validation for patients with transplant‐associated thrombotic microangiopathy. Blood Adv. 2021;5(24):5479‐5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al‐Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug‐induced thrombotic microangiopathy: a systematic review of published reports. Blood. 2015;125(4):616‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacob S, Dunn BL, Qureshi ZP, et al. Ticlopidine‐, clopidogrel‐, and prasugrel‐associated thrombotic thrombocytopenic purpura: a 20‐year review from the Southern Network on Adverse Reactions (SONAR). Semin Thromb Hemost. 2012;38(8):845‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dickey MS, Raina AJ, Gilbar PJ, et al. Pembrolizumab‐induced thrombotic thrombocytopenic purpura. J Oncol Pharm Pract. 2020;26(5):1237‐1240. [DOI] [PubMed] [Google Scholar]
- 22. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836‐2846. [DOI] [PubMed] [Google Scholar]
- 23. Kangro K, Roose E, Joly BS, et al. Anti‐ADAMTS13 autoantibody profiling in patients with immune‐mediated thrombotic thrombocytopenic purpura. Blood Adv. 2021;5(17):3427‐3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sukumar S, Lämmle B, Cataland SR. Thrombotic thrombocytopenic purpura: pathophysiology, diagnosis, and management. J Clin Med. 2021;10(3):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2496‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scully M. Congenital TTP: next stop, acuity and therapy. Blood. 2021;137(25):3469‐3471. [DOI] [PubMed] [Google Scholar]
- 27. Yagi H, Yamaguchi N, Shida Y, et al. Highly elevated plasma level of von Willebrand factor accelerates the formation of platelet thrombus under high shear stress in plasma with deficient ADAMTS13 activity. Thromb Res. 2017;159:91‐95. [DOI] [PubMed] [Google Scholar]
- 28. Scully M, Knöbl P, Kentouche K, et al. Recombinant ADAMTS‐13: first‐in‐human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood. 2017;130(19):2055‐2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mayer CL, Leibowitz CS, Kurosawa S, Stearns‐Kurosawa DJ. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins (Basel). 2012;4(11):1261‐1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villysson A, Tontanahal A, Karpman D. Microvesicle involvement in Shiga toxin‐associated infection. Toxins (Basel). 2017;9(11):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nolasco LH, Turner NA, Bernardo A, et al. Hemolytic uremic syndrome‐associated Shiga toxins promote endothelial‐cell secretion and impair ADAMTS13 cleavage of unusually large von Willebrand factor multimers. Blood. 2005;106(13):4199‐4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
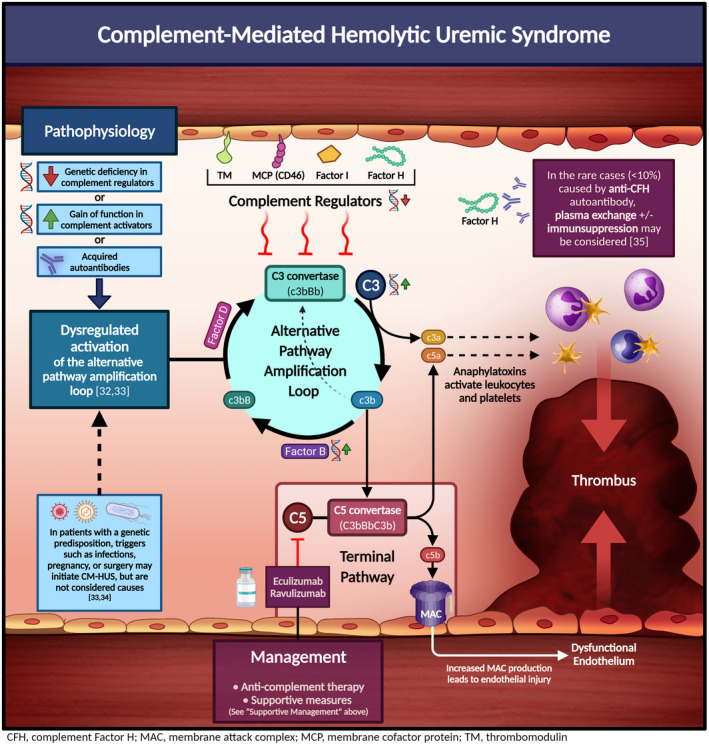
- 32. Brocklebank V, Wood KM, Kavanagh D. Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol. 2018;13(2):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noris M, Remuzzi G. Atypical hemolytic‐uremic syndrome. N Engl J Med. 2009;361(17):1676‐1687. [DOI] [PubMed] [Google Scholar]
- 34. Jokiranta TS. HUS and atypical HUS. Blood. 2017;129(21):2847‐2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cataland SR, Wu HM. How I treat: the clinical differentiation and initial treatment of adult patients with atypical hemolytic uremic syndrome. Blood. 2014;123(16):2478‐2484. [DOI] [PubMed] [Google Scholar]
- 36. Khosla J, Yeh AC, Spitzer TR, Dey BR. Hematopoietic stem cell transplant‐associated thrombotic microangiopathy: current paradigm and novel therapies. Bone Marrow Transplant. 2018;53(2):129‐137. [DOI] [PubMed] [Google Scholar]
- 37. Dvorak CC, Higham C, Shimano KA. Transplant‐associated thrombotic microangiopathy in pediatric hematopoietic cell transplant recipients: a practical approach to diagnosis and management. Front Pediatr. 2019;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jodele S, Dandoy CE, Lane A, et al. Complement blockade for TA‐TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135(13):1049‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang R, Zhou M, Qi J, et al. Efficacy and safety of eculizumab in the treatment of transplant‐associated thrombotic microangiopathy: a systematic review and meta‐analysis. Front Immunol. 2020;11:564647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. George JN, Morton JM, Liles NW, Nester CM. After the party's over. N Engl J Med. 2017;376(1):74‐80. [DOI] [PubMed] [Google Scholar]
- 41. Saleem R, Reese JA, George JN. Drug‐induced thrombotic microangiopathy: an updated systematic review, 2014–2018. Am J Hematol. 2018;93(9):E241‐E243. [DOI] [PubMed] [Google Scholar]


