Abstract
The genus Rhodotorula includes basidiomycetous oleaginous yeast species. Rhodotorula babjevae can produce compounds of biotechnological interest such as lipids, carotenoids, and biosurfactants from low value substrates such as lignocellulose hydrolysate. High-quality genome assemblies are needed to develop genetic tools and to understand fungal evolution and genetics. Here, we combined short- and long-read sequencing to resolve the genomes of two R. babjevae strains, CBS 7808 (type strain) and DBVPG 8058, at chromosomal level. Both genomes are 21 Mbp in size and have a GC content of 68.2%. Allele frequency analysis indicates that both strains are tetraploid. The genomes consist of a maximum of 21 chromosomes with a size of 0.4 to 2.4 Mbp. In both assemblies, the mitochondrial genome was recovered in a single contig, that shared 97% pairwise identity. Pairwise identity between most chromosomes ranges from 82 to 87%. We also found indications for strain-specific extrachromosomal endogenous DNA. A total of 7591 and 7481 protein-coding genes were annotated in CBS 7808 and DBVPG 8058, respectively. CBS 7808 accumulated a higher number of tandem duplications than DBVPG 8058. We identified large translocation events between putative chromosomes. Genome divergence values between the two strains indicate that they may belong to different species.
Keywords: Rhodotorula babjevae, de novo hybrid assembly, nanopore sequencing, genome divergence, ploidy
1. Introduction
Oleaginous yeasts have received considerable attention in recent years due to many potential biotechnological applications of microbial lipids. Rhodotorula species are basidiomycetous oleaginous yeasts whose lipid production accounts for more than 70% of dry cell weight. They show high tolerance to inhibitors, enabling them to convert lignocellulosic hydrolysates into lipids [1,2,3,4]. Microbial lipids from R. babjevae and other oleaginous yeasts have a fatty acid composition similar to vegetable oils and represent an environmentally and ethically suitable alternative raw material for the production of biofuels, oleochemicals, feed, and food additives [2,5,6]. Under nitrogen-limited conditions, R. babjevae can simultaneously accumulate biotechnologically important enzymes, glycolipids, and carotenoids [5]. Glycolipids from R. babjevae have promising environmental applications in biodegrading hydrocarbon pollutants and replacing synthetic compounds and chemical surfactants [7,8,9]. They are also attractive for other applications in various industrial sectors due to their antifungal, antibacterial, antiviral, and anti-carcinogenic activities [7,8,9,10]. However, it is desirable to obtain more robust R. babjevae strains to overcome the high production costs of microbial lipids and biosurfactants.
There are currently no methods described for the molecular manipulation of R. babjevae strains. To date, several genomes from Rhodotorula sp. have been sequenced including different strains of R. toruloides, R. graminis WP1, and R. glutinis ZHK. Of these, some have only been determined using short-read sequencing technologies or lack gene annotation [3,11,12,13,14,15,16,17,18]. To the best of our knowledge, no genome sequences are available for R. babjevae. The aim of this study was to obtain high-quality genome assemblies for R. babjevae as a prerequisite for the development of genetic tools, and to deepen our understanding of the biology and evolution of Rhodotorula species. To achieve this, we used a combination of short and long reads. This has previously been used successfully to generate high quality genome hybrid assemblies in terms of completeness, contiguity, and chromosome reconstruction [3,12,19,20]. We present here the de novo genome assemblies and annotations of two R. babjevae species strains, CBS 7808 (type strain) and DBVPG 8058, based on short- and long-read sequencing technologies. We also performed a genome divergence and ploidy analysis of both R. babjevae strains.
2. Materials and Methods
2.1. Yeast Strains
The type strain of R. babjevae (CBS 7808) was obtained from the CBS-KNAW collection (Utrecht, The Netherlands). Strain DBVPG 8058 was isolated and identified at the Swedish University of Agricultural Sciences, Uppsala (strain number in the strain collection of the Department of Molecular Sciences is J195) [2] and deposited in the Industrial Yeasts Collection (Perugia, Italy).
2.2. DNA Purification
The yeasts were cultivated in 50 mL Yeast–Peptone–Dextrose medium (YPD) until reaching exponential growth phase [21]. Cell wall degradation was performed according to [22] with some modifications. Briefly, the cells were suspended in 1 M sorbitol, 0.1 M sodium citrate, 0.01 M EDTA, and 0.03 M β-mercaptoethanol (SCEM), pH 5.8 after harvesting. Lyticase solution was added to the cell suspensions (100 U/mL) of CBS 7808 and DBVPG 8058, which were then incubated for 9 h or overnight, respectively. After Lyticase digestion, cells were harvested at 1200× g, suspended in SCEM buffer, and incubated overnight with Zymolyase (200 U/mL). Genomic DNA extraction from protoplasts was performed using the NucleoBond® CB 20 Kit (Macherey-Nagel, Düren, Germany). DNA concentration, purity, and quality were confirmed through Qubit™ 4 Fluorometer (Thermo Fisher Scientific, Singapore), NanoDrop® ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and agarose gel electrophoresis, respectively.
2.3. Library Preparation and Sequencing
The extracted DNA samples were sequenced using MinION (Oxford Nanopore Technologies, Oxford, UK) and the Illumina sequencing platform. Nanopore DNA libraries were prepared according to [23]. Briefly, 31.5 µL of AMPure magnetic beads were added to 5 µg of DNA for a “pre-cleaning” step. Library preparation was then performed according to a modified protocol [23] using a Ligation Sequencing Kit (SQK-LSK109, Oxford Nanopore Technologies, Oxford, UK). Each DNA library was loaded onto a FLO-MIN106 flow cell mounted on a MINION device (Oxford Nanopore Technologies). MinKNOW software (version 19.06.8) was used for sequencing as described by [23]. The base calling was run using Guppy version 3.2.4-1—195590e and model HAC-mod (modified base sensitive high accuracy model).
From the 6,665,174 long reads recovered from the CBS 7808 DNA library, the mean read length was 2789.7 bases and the read length N50 5553 bases yielding a total of 18,593 Mbp. For DBVPG 8058, 2,953,255 long reads were retrieved containing a total of 15,702 Mbp. The mean read length was 5317 bases and the read length N50 7411 bases. Aliquots of the extracted DNA from both R. babjevae strains were also subjected to short-read paired-end sequencing using the Illumina Novaseq platform (S prime, 2× 150 bp) and the TruSeq PCR free DNA library preparation kit (Illumina Inc., San Diego, CA, USA). 179,163,622 short reads were recovered from CBS 7808 DNA library, corresponding to a total of 27,053 Mbp. For DBVPG 8058, 203,873,550 short reads were retrieved containing a total of 30,784 Mbp.
2.4. Genome Assembly and Annotation
Genome assembly and annotation was performed using a custom pipeline described elsewhere [3], applying the program versions listed in Table S1. To further improve the annotation of transcripts and exon–intron boundaries, we additionally mapped RNA-Seq data from the closely related R. toruloides CBS 14 (PRJEB40807) to the R. babjevae genomes as previously described [3]. We used nQuire (v0.0) based on minimap2 short-read mappings (v2.17; no secondary alignments option) and the KmerCountExact script from the BBMap package (https://sourceforge.net/projects/bbmap/; accessed on 25 November 2021) (v38.86) to estimate the ploidy level of the R. babjevae strains [24,25]. To compare these methods and our ploidy results of the two R. babjevae strains with already published results, we also performed nQuire and KmerCountExact on Illumina sequencing data from Rhodotorula mucilaginosa JGTA-S1, accession number SRR5821556 [12].
The reconstruction of lipid metabolic pathway maps was performed using KEGG Mapper version 4.3. The KEGG Orthology (KO) identifiers were affiliated to the annotated transcripts of R. babjevae CBS 7808 and R. babjevae DBVPG 8058 using KofamKOALA [26] with an e-value cut-off of 0.01.
2.5. Genome Divergence Analysis
Synteny relationship analysis between R. babjevae CBS 7808 and R. babjevae DBVPG 8058 was performed using NUCmer (MUMmer, version 3.23). The maximum gap between adjacent matches in a cluster were set to 500 and the minimum cluster length to 100. Visualization of NUCmer alignments and other genomic features was performed with Circa (http://omgenomics.com/circa; accessed on 23 June 2021).
The level of sequence divergence between both R. babjevae strains as well as with other closely related Rhodotorula species, including R. glutinis ZHK (JAAGPT010000000.1), R. graminis WP1 (JTAO00000000.1) and R. toruloides strains CBS 14 (PRJEB40807), CGMCC 2.1609 (LKER00000000.1), VN1 (SJTE00000000.1) and NBRC 0880 (LCTV00000000.2), was evaluated using the alignment-free distance measure Kr [27]. We calculated Average Nucleotide Identity (ANI) values using the web-based calculator available at Kostas Lab [28]. DNA–DNA homology (DDH) was estimated with the Genome-to-Genome Distance Calculator (GGDC) 2.1 (http://ggdc.dsmz.de/distcalc2.php; accessed on 26 June 2021) using the program GBDP2_MUMMER [29].
Whole genome alignments of R. babjevae strains were performed using LASTZ (version 7.0.2) implemented in Geneious prime, version 2021.0.1 (Biomatters Ltd., Auckland, New Zealand) [30]. Nucleotide alignment and phylogenetic tree construction using MAFFT v7.450 [31] and PhyML 3.3.20180621 [32] with 100 bootstraps, respectively, were performed on the Geneious prime platform.
Whole genome comparison and identification of orthologous gene clusters and paralogous genes were performed on the web-based OrthoVenn2 platform (https://orthovenn2.bioinfotoolkits.net; accessed on 20 October 2021) using a threshold e-value of 1 × 10−15 and an inflation of 1.5 [33]. To identify duplicated genes (paralogs) with high sequence identity, an all-against-all sequence identity search was performed on the NCBI Genome Workbench version 3.7.0 [34] using BLASTp (BLOSUM62 matrix) with a cut-off e-value of 1 × 10−15. The output file was screened for protein sequences with at least 70% coverage and 70% sequence identity.
3. Results and Discussion
3.1. Genome Assembly, Ploidy Estimation, and Gene Annotation of R. babjevae Strains
The genome of both R. babjevae strains was assembled by a combined approach of long- and short-read sequencing with a coverage depth of about 2000 X. A summary of the genomic data is presented in Table 1. The CBS 7808 draft genome has an overall size of 21,862,387 bp and a GC content of 68.23%. Repetitive sequences make up 5.93% of the total length of the genome, of which 4.98% are single repeats and 0.96% are regions of low complexity. The draft genome of DBVPG 8058 has a total size of 21,522,072 bp and a GC content of 68.24%. The approach identified 6.73% as repetitive sequences, including 5.65% as single repeats and 1.09% as regions of low complexity. The similarity of genome features, such as genome size, GC content, and percentage of repetitive regions, confirms that they are closely related species. The genome size is comparable to that of other Rhodotorula species, but the GC content is slightly higher [3,11,12,13,15,18] (Table 1).
Table 1.
Genomic data from Rhodotorula species.
| Reference | This Study | This Study | [11] | [13] | [3] | [15] |
|---|---|---|---|---|---|---|
| Strain number | R. babjevae CBS 7808 | R. babjevae DBVPG 8058 | R. graminis WP1 | R. glutinis ZHK | R. toruloides CBS 14 | R. toruloides NP11 |
| Genome size (Mbp) | 21.9 | 21.5 | 21.0 | 21.8 | 20.5 | 20.2 |
| Coverage | 2058 | 2122 | 8.6 | 470 | 1514 | 96 |
| GC content (%) | 68.23 | 68.24 | 67.76 | 67.8 | 61.83 | 62.05 |
| Bases masked (%) | 5.93 | 6.73 | 6.5 | NA | 2.01 | 2.53 |
| No. Scaffolds | 3 | 1 | 26 | 30 | 3 | 34 |
| No. Contigs | 24 | 33 | 325 | NA | 23 | NA |
| Protein-coding genes | 7591 | 7481 | 7283 a | 6774 a | 9464 | 8171 |
| Avg. no. exons per gene | 4.0 | 3.9 | 6.2 | NA | 5.9 | NA |
| Sequencing platform | Nanopore and Illumina | Nanopore and Illumina | Sanger | PacBio and Illumina | Nanopore and Illumina | Illumina and Sanger |
NA—not available; a—refers to predicted genes.
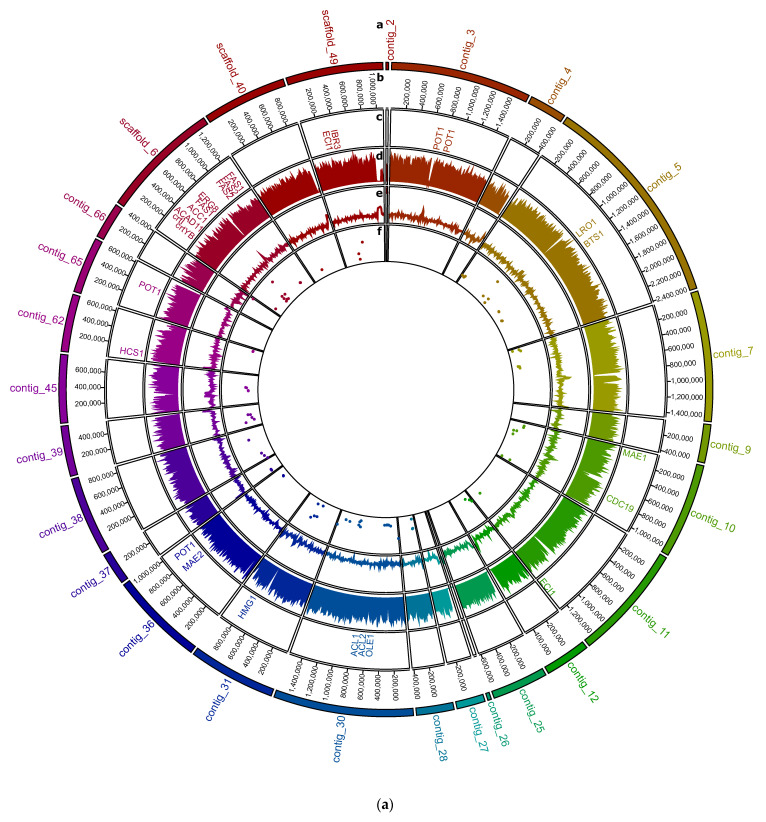
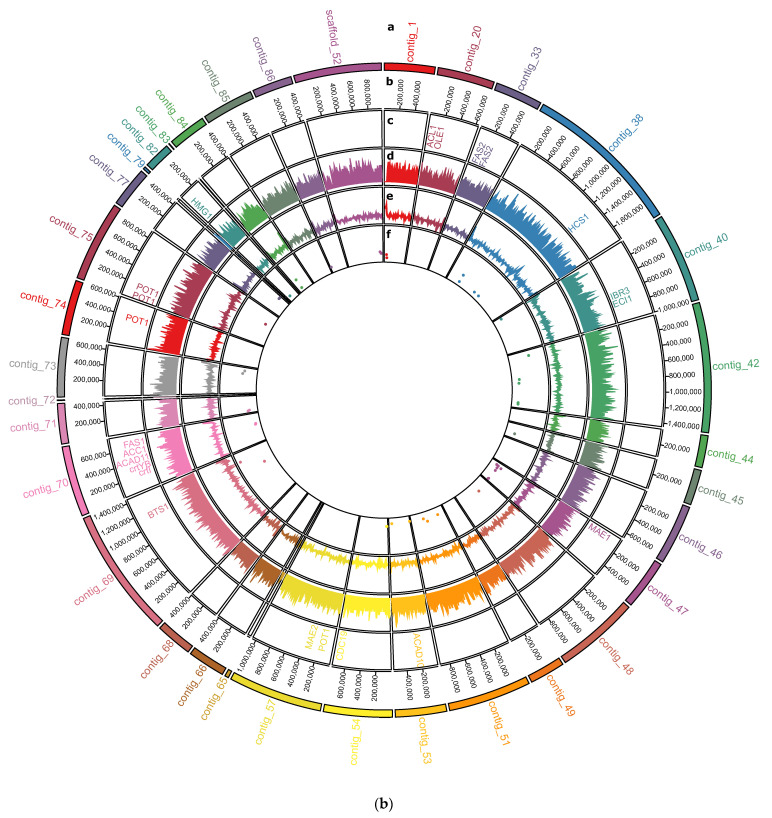
Sequence assembly resulted for R. babjevae CBS 7808 in 24 contigs and three scaffolds with a length N50 of 1,067,634 bp (Figure 1a, Table S2). A telomeric region was predicted at one of the termini for 13 contigs and scaffolds larger than 250,000 bp. The draft genome of strain DBVPG 8058 consists of 33 contigs and one scaffold with a length N50 of 789,767 bp (Figure 1b, Table S3). From the contigs and scaffolds with sizes larger than 250,000 bp in DBVPG 8058 genome assembly, two have telomere sequences at both termini and 15 at one terminus each. The low numbers of contigs and scaffolds in the genome assemblies from both R. babjevae strains indicate high accuracy, contiguity, and completeness. Two putative circular sequences were identified in each strain. Among them, contig_2 in CBS 7808 and contig_79 in DBVPG 8058 contained the mitochondrial genes. Both mitochondrial genomes are similar in size with 30.876 bp and 28.432 bp, respectively, and have a GC content of 38.9% (Tables S2 and S3).
Figure 1.
Overview of the genome assemblies of Rhodotorula babjevae strains: (a) CBS 7808, (b) DBVPG 8058. The concentric circles show from outside to inside: the contig name (a) and sizes (b), distribution of lipid and carotenoid metabolism related genes (c), and in non-overlapping 10 kb windows, the gene density (d), the deviation from the average GC content (e), and the density of duplicated genes with 70% sequence coverage (f).
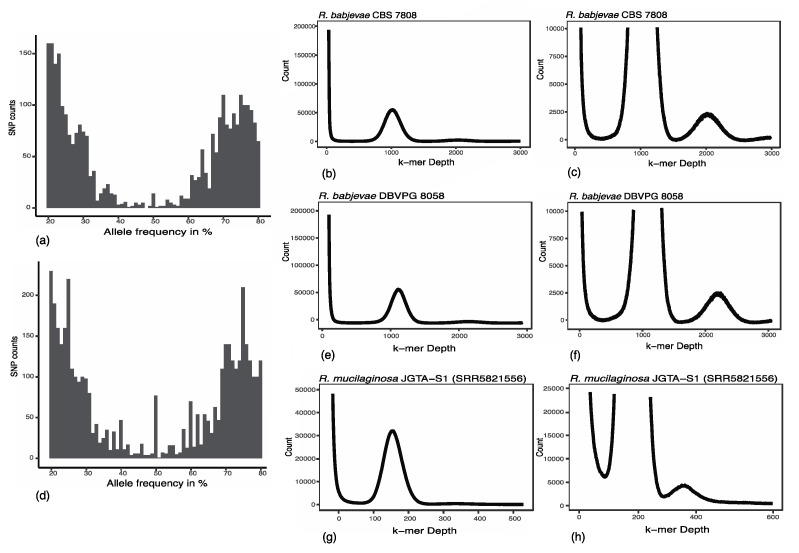
To estimate the ploidy in R. babjevae strains, we used nQuire. nQuire quantifies the distribution of the base frequencies at variable sites, and thus differentiates between different degrees of ploidy [24]. In both strains, the alleles occurred at frequencies of about 25% and 75%, indicating that both R. babjevae strains are tetraploid (Figure 2). Furthermore, we also used a k-mer counting approach to estimate ploidy. Using a k-mer length of 31, as recently shown by Sen et al. [12] for R. mucilaginosa JGTA-S1, only one peak appears in the plots. However, when the k-mer length is reduced to 17, as recently shown by Zou et al. [35], two distinct peaks appear for both R. mucilaginosa JGTA-S1 and the R. babjevae strains (Figure 2). The first and larger peak indicates tetraploidy while the second smaller peak indicates diploidy.
Figure 2.
Ploidy estimation of Rhodotorula babjevae and Rhodotorula mucilaginosa. R. babjevae CBS 7808: (a) Allele frequency values of single nucleotide polymorphisms (SNP) obtained through nQuire calculations using minimap2, (b) Distribution of 17-kmer frequencies using KmerCountExact from the BBmap package, (c) Zoomed in peaks of the 17-kmer frequency histogram. R. babjevae DBVPG 8058: (d) Allele frequency values of SNP (e) Distribution of 17-kmer frequencies, (f) Zoomed in peaks of the 17-kmer frequency histogram. R. mucilaginosa JGTA-S1: (g) Distribution of 17-kmer frequencies, (h) Zoomed in peaks of the 17-kmer frequency histogram. The reproduction of R. mucilaginosa JGTA-S1 ploidy estimation was performed using the Illumina data SRR5821556.
The ploidy level of R. babjevae strains has not been studied so far. The genomes of the closely related strains R. toruloides NP11 and R. mucilaginosa JGTA-S1 are considered to be haploid [12,15]. However, our analyses indicate that both R. mucilaginosa JGTA-S1 and R. babjevae CBS 7808 and DBVPG 8058 may be tetraploid. Tetraploidy has previously been widely recognized in yeast [36,37,38,39]. Knowing the ploidy level is of great importance for genetic engineering and for the development of efficient gene manipulation protocols.
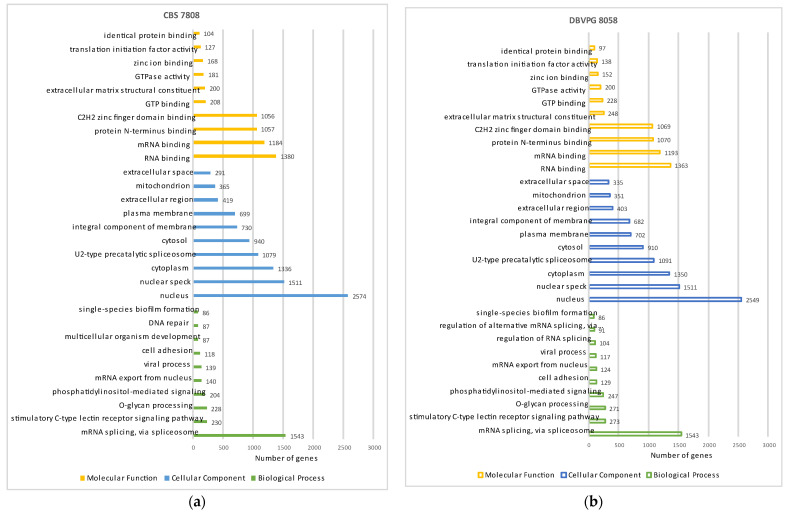
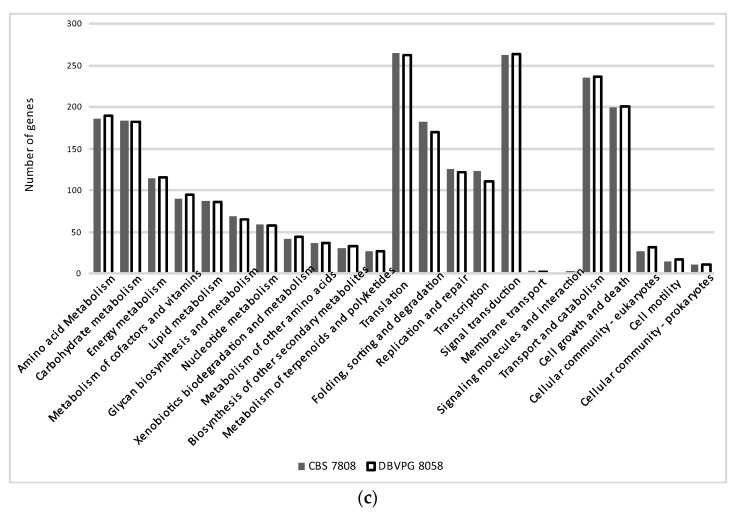
A total of 7591 protein-coding genes and 7607 associated transcripts were annotated in the CBS 7808 genome using MetaEuk (Table 1). The average number of estimated exons per gene is 3.97 (Table 1). The genome of DBVPG 8058 has 7481 protein-coding genes, 7516 associated transcripts and 3.93 estimated exons per gene (Table 1). The proportion of split genes in both genomes is correspondingly high, amounting to 6390 and 6305 for CBS 7808 and DBVPG 8058, respectively. This is consistent with previous findings for Rhodotorula spp. [3,12,15]. The distribution of exon counts in the genomes of R. babjevae strains CBS 7808 and DBVPG 8058 is shown in Table S4. 315 and 309 open reading frames (ORF) complementary to annotated genes were predicted in CBS 7808 and DBVPG 8058, respectively. The presence of antisense transcripts has previously been reported for the related species R. toruloides [3]. In yeast, the level of antisense transcription has been anti-correlated to sense mRNA, indicating antisense-dependent gene regulation through transcription interference under certain growth conditions [40,41]. Figures S1–S3 show the assignment of genes to the Gene Ontology (GO) categories’ biological processes, cellular components, and molecular functions, of which the top 10 are summarized in Figure 3a,b. A total of 2691 and 2660 CDS from CBS 7808 and DBVPG 8058, respectively, could be assigned KO numbers (Figure 3c). The biosynthesis of saturated and unsaturated fatty acids, glycerolipid metabolism, terpenoid backbone biosynthesis, carbon metabolism, and fatty acid metabolism are depicted in detail in Figures S4 and S5. Some examples of annotated genes that encode crucial enzymes for lipid and carotenoid metabolism are CDC19, MAE1, MAE2, ACL1, ACL2, ACC1, FAS1, FAS2, OLE1, ACAD10, ACAD11, IBR3, D6C81_05617, POT1, LRO1, HMG1, HCS1, ERG8, crtYB, crtI, and BTS1 (Tables S5 and S6). A difference in this respect is the absence of ACL2, and the presence of ACAD10 in DBVPG 8058.
Figure 3.
Assigned numbers of genes to the top 10 of the GO categories: biological processes, cellular components, and molecular functions in CBS 7808 (a) and DBVPG 8058 (b). (c) Assigned number of genes to the five KEGG top categories: metabolism, genetic information processing, environmental information processing, cellular processes, and organismal systems. KEGG (Kyoto Encyclopedia of Genes and Genomes), GO (Gene Ontology).
Benchmarking of universal single-copy orthologs (BUSCOs, using fungi_odb9) identified that 95.5% and 96.9% of the assessed genes in CBS 7808 and DBVPG 8058, respectively, were complete and single-copy (Figure S6). This supports the high quality of the draft genome assemblies reported here. Furthermore, 0.7% and 0.3% of the assessed genes in CBS 7808 and DBVPG 8058, respectively, were fragmented and the rest were missing (Figure S6). A small percentage of BUSCO genes might still be undetectable due to sequence regions with low coverage, repetitive elements, or assembly problems that cannot be solved even with the hybrid approach and would require additional sequencing and manual analysis. In addition, when a BUSCO gene was missing, there were either no significant matches or the BUSCO matches were below the range of values for the selected BUSCO profile. Finally, some marker genes that are part of the BUSCO “fungi” profile that we used as reference may not be part of the two R. babjevae strains.
3.2. Chromosome Organization
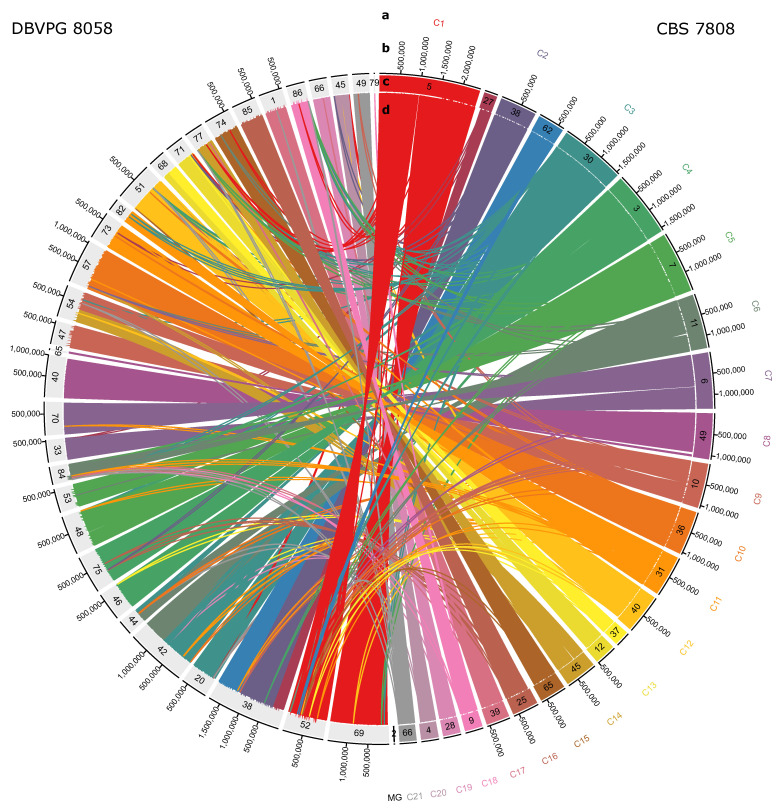
The R. babjevae genome assemblies were aligned for comparison using NUCmer. Out of a total of 27 contigs and scaffolds in CBS 7808, 24 matched 30 of the 34 assembled sequences in DBVPG 8058 (Figure 4). In general, the number of undisturbed segments is high. However, there are also major chromosomal rearrangements (Figure 4). LASTZ alignments of each contig from one R. babjevae strain with the whole genome of the other strain confirmed the results of the synteny analysis (Table S7, Figures S7 and S8). Based on these alignments we deduce that R. babjevae has a maximum of 21 chromosomes with sizes ranging from 0.4 to 2.4 Mbp (Table 2, Figure 4). The molecular karyotype of several Saccharomyces yeast strains has been identified as 16 [42]. Karyotyping studies in Rhodotorula species have identified at least 10 chromosomes in isolates of R. mucilaginosa and 11 in R. toruloides while Martín-Hernández et al. proposed that R. toruloides CBS 14 has at least 18 chromosomes [3,43,44]. The pairwise identity between chromosomes ranges from 82% to 87%. The mitochondrial genomes have 97% pairwise identity (Table S7, Figure S7). Four of the putative chromosomes are affected by large translocation events. This affects chromosomes 3 and 6, and chromosomes 9 and 14 (Table 2). Minor inversions were noticed in other chromosomes (Table S7, Figure S8). Each R. babjevae strain contains two contigs that are strain-specific (Tables S7 and S8). These are small linear contigs with higher read depths than the chromosomes, except for circular contig_26 in CBS 7808, which has a lower read depth than the chromosomes. These variations in read depth may indicate relaxed replication regulation. The linear DNA sequence from CBS 7808 contig_46 has two annotated genes, one of which encodes the Retrovirus-related Pol polyprotein from transposon 17.6. DNA plasmids have previously been found in filamentous fungi, including the close relative R. toruloides, with sizes ranging from 2.5 to 11 kb and typically encoding enzymes involved in plasmid replication [3,45,46]. This might indicate the presence of strain-specific extrachromosomal endogenous DNA.
Figure 4.
Genome alignment of Rhodotorula babjevae strains CBS 7808 and DBVPG 8058. Maximal unique matches between CBS 7808 and DBVPG 8058 were obtained using NUCmer 3.0 and visualized with Circa. The concentric circles show from outside to inside: putative chromosome names or mitochondrial genome, MG, in the reference strain CBS 7808 (a); contig and scaffolds’ sizes (b); and names (c). Ribbons are showing the unique and repetitive alignments using CBS 7808 contigs and scaffolds as the reference (d). Contigs from DBVPG 8058 are colored gray.
Table 2.
Putative chromosomes in Rhodotorula babjevae deduced from whole genome LASTZ alignments (Table S7, Figure S8).
| R. bajevae CBS 7808 | R. bajevae DBVPG 8058 | Genetic Structure | GC Content | Comments | Size (Mbp) |
|---|---|---|---|---|---|
| Contig_5 (2,415,752 bp) | Contig_69 (1,447,990 bp) | Putative chromosome 1 | 67–69% | Figure S8A | 2.4 |
| Scaffold_52 (977,625 bp) | |||||
| Contig_27 (320,063 bp) | Contig_38 (1,780,658 bp) | Putative chromosome 2 | 67–69% | Figure S8B | 1.8 |
| Contig_38 (881,966 bp) | |||||
| Contig_62 (644,441 bp) | |||||
| Contig_30 (1,569,459 bp) | Contig_20 (637,402 bp) | Putative chromosome 3 | 67–69% | Figure S8C | 1.6 |
| Contig_42 (1,446,680 bp) | Large translocation event between Chr. 3 and Chr.6 | ||||
| Contig_44 (357,974 bp) | |||||
| Contig_3 (1,574,520 bp) | Contig_46 (670,828 bp) | Putative chromosome 4 | 67–69% | Figure S8D | 1.6 |
| Contig_75 (900,917 bp) | |||||
| Contig_7 (1,460,653 bp) | Contig_48 (931,129 bp) | Putative chromosome 5 | 67–69% | Figure S8E | 1.5 |
| Contig_53 (571,073 bp) | |||||
| Contig_11 (1,300,441 bp) | Contig_42 (1,446,680 bp) | Putative chromosome 6 | 67–69% | Figure S8F | 1.3 |
| Contig_44 (357,974 bp) | Large translocation event between Chr. 3 and Chr.6 | ||||
| Contig_84 (425,340 bp) | |||||
| Scaffold_6 (1,337,997 bp) | Contig_33 (529,001 bp) | Putative chromosome 7 | 67–69% | Figure S8G | 1.3 |
| Contig_70 (789,767 bp) | |||||
| Scaffold_49 (1,089,446 bp) | Contig_40 (1,004,683 bp) | Putative chromosome 8 | 67–69% | Figure S8H | 1.1 |
| Contig_65 (41,334 bp) | |||||
| Contig_10 (1,067,634 bp) | Contig_47 (557,103 bp) | Putative chromosome 9 | 67–69% | Large translocation event between Chr. 9 and Chr.14 | 1.1 |
| Contig_54 (766,724 bp) | Figure S8I | ||||
| Contig_36 (1,056,323 bp) | Contig_57 (1,049,892 bp) | Putative chromosome 10 | 67–69% | Figure S8J | 1.1 |
| Contig_31 (979,228 bp) | Contig_73 (659,761 bp) | Putative chromosome 11 | 67–69% | Figure S8K | 1.0 |
| Contig_82 (299,180 bp) | |||||
| Scaffold_40 (948,604 bp) | Contig_51 (924,743 bp) | Putative chromosome 12 | 67–69% | Figure S8L | 0.9 |
| Contig 37 (362,520 bp) | Contig_68 (408,627 bp) | Putative chromosome 13 | 67–69% | Figure S8M | 0.9 |
| Contig_12 (511,897 bp) | Contig_71 (449,691 bp) | ||||
| Contig_45 (762,860 bp) | Contig_54 (766,724 bp) | Putative chromosome 14 | 67–69% |
Figure S8N Large translocation event between Chr. 9 and Chr.14 |
0.8 |
| Contig_77 (446,828 bp) | |||||
| Contig_65 (630,535 bp) | Contig_74 (614,034 bp) | Putative chromosome 15 | 67–69% | Figure S8O | 0.6 |
| Contig_25 (627,118 bp) | Contig_85 (573,802 bp) | Putative chromosome 16 | 67–69% | Figure S8P | 0.6 |
| Contig_39 (564,129 bp) | Contig_1 (565,532 bp) | Putative chromosome 17 | 67–69% | Figure S8Q | 0.6 |
| Contig_9 (429,397 bp) | Contig_86 (443,617 bp) | Putative chromosome 18 | 67–69% | Figure S8R | 0.4 |
| Contig_28 (422,133 bp) | Contig_66 (419,035 bp) | Putative chromosome 19 | 67–69% | Figure S8S | 0.4 |
| Contig_4 (418,972 bp) | Contig_45 (394,205 bp) | Putative chromosome 20 | 67–69% | Figure S8T | 0.4 |
| Contig_66 (406,102 bp) | Contig_49 (396,114 bp) | Putative chromosome 21 | 67–69% | Figure S8U | 0.4 |
Chr., chromosome.
3.3. Genome Divergence Analysis
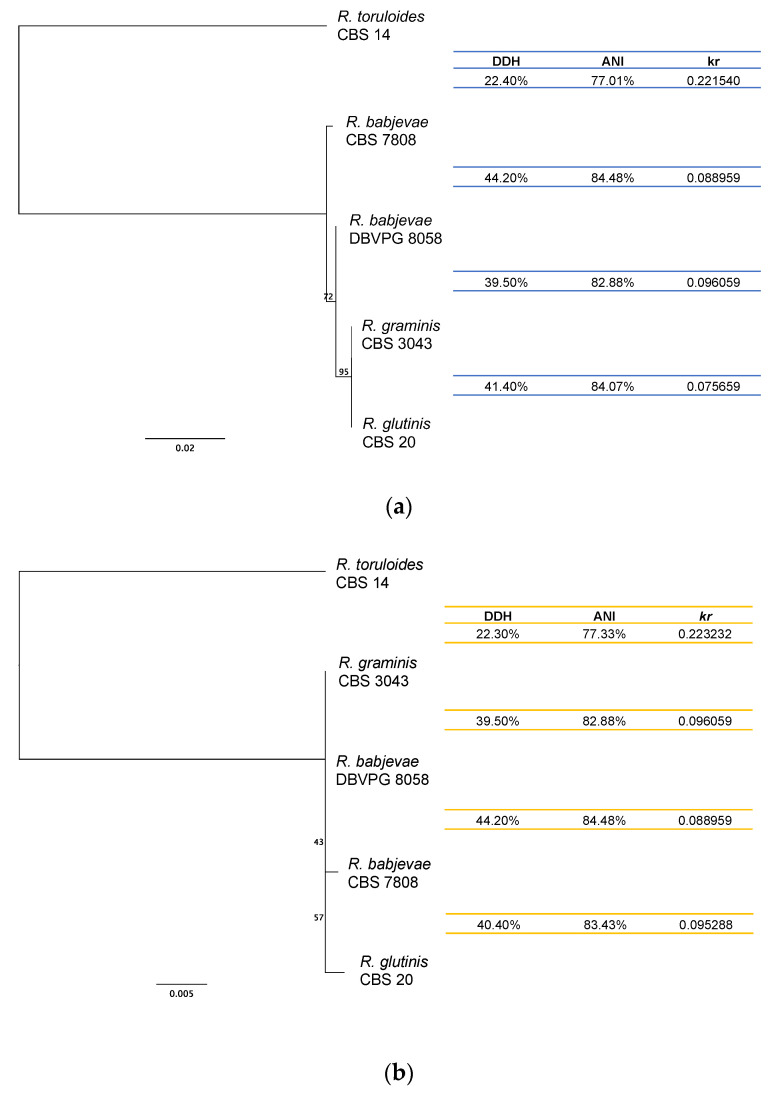
The genomes of the R. babjevae strains were compared to each other and to genomes of closely related Rhodotorula species in terms of DDH, ANI and Kr for tracing genome divergence (Figure 5, Table S9). The R. babjevae strains share 44.20% DDH estimates, 84.48% ANI and Kr values of 0.09. In general, the genetic divergence between R. babjevae strains was comparable to the divergence with R. graminis and R. glutinis, but higher than expected for strains of the same yeast species [47]. For instance, the divergence between strains of R. toruloides was much lower than that of the two R. babjevae strains (Table S9).
Figure 5.
Phylogenetic relationship of Rhodotorula babjevae strains and their placement within the Rhodotorula genus. The phylogenetic tree was built based on: (a) ITS; and (b) D1/D2 LSU of rRNA gene sequences. It was inferred using PhyML with 100 bootstraps on Geneious prime version 2021.0.1. Rhodotorula toruloides was selected as outgroup. Similarities between whole genome sequences of the corresponding strains are presented in terms of the alignment-free distance measure kr, Average Nucleotide Identity (ANI), and DNA–DNA homology (DDH). Rhodotorula graminis WP1 and R. glutinis ZHK genome sequences were used for the calculations instead of R. graminis CBS 3043 and R. glutinis CBS 20, respectively.
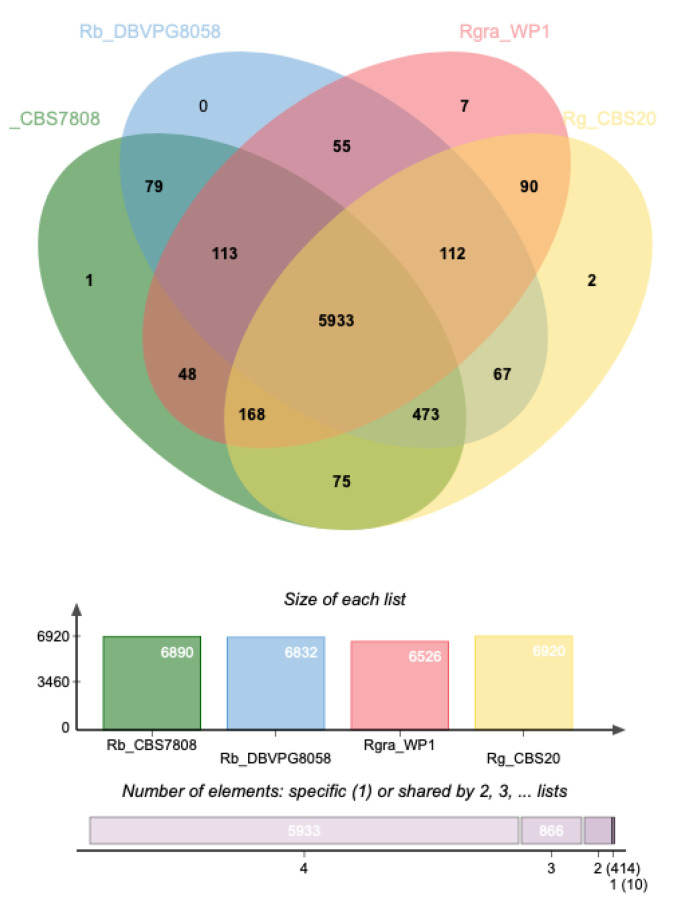
Moreover, the protein-coding sequences of the R. babjevae strains and their closest relatives R. graminis and R. glutinis were analyzed using OrthoVenn2 web platform to identify and compare orthologous gene clusters. The R. babjevae species share 6598 out of a total of 7223 orthologous clusters produced by OrthoVenn2, including both single-copy gene clusters and overlapping gene clusters such as paralogs (Figure 6). Of the shared clusters, 5933 are common within the three Rhodotorula species assessed, representing putative shared orthologous proteins that evolved from common ancestral genes. In addition, CBS 7808 has 389 single genes and one cluster that had no orthologs in the other genomes, while strain DBVPG 8058 has 355 single genes. These unique genes could account for the specific functional capabilities of the R. babjevae strains as a result of gene loss or gain events. Of the 79 orthologous clusters shared only between R. babjevae strains, some of the assigned GO terms are: Positive regulation of the unsaturated fatty acid biosynthetic process by positive regulation of transcription from RNA polymerase II promoter (GO:0036083), protein O-linked glycosylation (GO:0006493), glucan catabolic process (GO:0009251), cellular calcium ion homeostasis (GO:0006874), sulfate assimilation (GO:0000103), and carbohydrate transport (GO:0008643). The two R. babjevae strains show a high genome pairwise similarity and a high number of shared orthologous clusters, though not as high as for R. graminis and R. glutinis (Figure 6). In general, R. babjevae, R. glutinis, and R. graminis are very closely related species with a short evolutionary distance between them as compared to other species in the genus (i.e., R. toruloides). The strains CBS 7808 and DBVPG 8058 have high interstrain variability and a greater evolutionary distance to R. graminis than to R. glutinis.
Figure 6.
Distribution of shared orthologous clusters between Rhodotorula babjevae strains CBS 7808 and DBVPG 8058, R. graminis WP1 and R. glutinis CBS 20. The Venn diagram was generated using OrthoVenn2.
A total of 59 and 30 paralogous gene clusters were identified in CBS 7808 and DBVPG 8058, respectively, using OrthoVenn2 (Tables S10 and S11). Applying a cut-off value of 70% sequence coverage to them, we identified 29 and 19 duplicated genes, respectively, that potentially have not diverged in function. On the other hand, an all-against-all protein sequence similarity search was performed in each of the two strains using BLASTp with an e-value of 1 × 10−15, 70% coverage, and 70% sequence identity. This resulted in a total of 34 and 21 duplicated sequences in CBS 7808 and DBVPG 8058, respectively, and a total of 41 and 29 duplicated sequences with 70% sequence coverage, respectively, that were identified by any of the tools (Figure 1, Tables S12–S14). The higher accumulation of duplicated genes in CBS 7808 could be related to a higher number of gene duplication events due to faster evolution of the strain. The majority of these duplications lies adjacent to each other or in close proximity. Tandem duplications have been suggested as a mechanism of adaptative evolution to changing environments [48]. They may have arisen through homologous recombination between sequences on sister chromatids or homologous chromosomes [48]. It has been reported that considerable redundancy of duplicate gene pairs persists even after 100 million years of evolution in Saccharomyces cerevisiae [49]. Some of the predicted functions of the genes, which are duplicated only in CBS 7808, are Uncharacterized protein C17G8.02 (NAD biosynthesis), Mannose-6-phosphate isomerase and Phosphoenolpyruvate carboxykinase (ATP) (carbon metabolism), Acetyl-CoA carboxylase (fatty acid metabolism), Alpha-ketoglutarate-dependent sulfonate dioxygenase, Sulfite reductase [NADPH] hemoprotein beta-component and Sulfite reductase [NADPH] subunit beta (Sulfur metabolism), and Probable quinate permease (import of quinic acid as a carbon source). Some of the duplicated genes involved in metabolic processes identified only in DBVPG 8058 are mitochondrial Aspartate aminotransferase (intracellular NAD(H) redox balance) and Leucine-rich repeat extensin-like protein 3 (AtLRX3, cell morphogenesis). In both strains, the most frequently duplicated gene is SRRM2, which codes for the Ser/Arg repetitive matrix protein 2 and is involved in mRNA splicing. Cwc21p is encoded by CWC21, an ortholog of human SRRM2 in S. cerevisiae. It has been proposed that it resides in the catalytic center of the spliceosome and possibly fulfills its role in response to changing cellular environmental conditions [50]. The predicted function Ser/Arg repetitive matrix protein 2 was annotated in 1055 genes in CBS 7808 and 1068 in DBVPG 8058. Alternative splicing is an essential driver of proteomic diversity and may potentially provide a high level of evolutionary plasticity.
The type strain CBS7808 of R. babjevae investigated here, was first isolated from herbaceous plants in Moscow, Russia [51]. R. babjevae DBVPG 8058 was isolated from wild apples in Uppland locality, Sweden. The phylogenetic placement of DBVPG 8058 to the R. babjevae species was performed by the Industrial Yeasts Collection DBVPG by aligning 5.8S-ITS rDNA and D1/D2 26S rDNA regions in a similar manner as illustrated in Figure 5. However, the genome divergence values (DDH, ANI and Kr) proved to be more sensitive for delineating Rhodotorula species. Phylogenetic placement based on the standard rDNA regions may not be sufficient to understand yeast diversity and species delineation, as shown before [47,52,53]. These R. babjevae strains showed different behavior during enzymatic cell wall degradation for DNA purification both in this study and in another study where xylose medium was used [54]. Highly dynamic genome structures have already been found in closely related yeast species [20,55,56,57,58,59]. A dynamic genome structure of R. babjevae could enhance the physiological capabilities and thus the species’ environmental adaptability. [60,61,62,63]. However, their genetic divergence suggests that they may belong to different species. A genome comparison study using whole genome sequences from different strains of closely related Rhodotorula species would allow gaining a deeper knowledge about their genome structure and evolution, as well as identifying new species.
Taxonomic classification using Sourmash [64] and the GenBank reference (https://osf.io/4f8n3; accessed on 17 March 2022) assign to both genome assemblies: Eukaryota superkingdom, Basidiomycota phylum, Microbotryomycetes class, Sporidiobolales order, Sporidiobolaceae family, Rhodotorula genus, and Rhodotorula graminis species. The taxonomic classification might indicate that R. graminis was the closest relative of R. babjevae with available genomic data. Previous studies have shown a close evolutionary relationship between R. babjevae and R. graminis, which was also demonstrated here [13,65].
4. Conclusions
The hybrid sequencing approach resulted in high-resolution genomes of R. babjevae DBVPG 8058 and CBS 7808T. Both strains are tetraploid and have a maximum of 21 chromosomes. Some of the chromosomes show large-scale translocation events. Moreover, we demonstrated a high genome divergence between the R. babjevae strains, as high as the divergence to other closely related Rhodotorula species. This indicates that the two strains do not belong to the same species.
Acknowledgments
Illumina sequencing was performed by the SNP&SEQ Technology Platform in Uppsala, which is part of the National Genomics Infrastructure (NGI) Sweden and Science for Life Laboratory. The SNP&SEQ Platform is also supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation. A.V., C.B. and M.H. are shareholders of nanozoo GmbH.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof8040323/s1, Figure S1: Gene Ontology (GO) term summary related to the GO topic: molecular functions. Figure S2: Gene Ontology (GO) term summaries belonging to the GO topic: biological processes. Figure S3: Gene Ontology (GO) term summaries belonging to the GO topic: cellular components. Figure S4: Examples of lipid metabolism pathways in Rhodotorula babjevae CBS 7808 reconstructed by KEGG Mapper. Figure S5: Examples of lipid metabolism pathways in Rhodotorula babjevae DBVPG 8058 reconstructed by KEGG Mapper. Figure S6: Quantitative assessment of the hybrid genome assemblies and annotation completeness using Benchmarking Universal Single-Copy Orthologs (BUSCO). Figure S7: LASTZ alignment of the mitochondrial genome sequences of Rhodotorula babjevae CBS 7808 and DBVPG 8058. Figure S8: LASTZ alignment of contigs with assigned homology of Rhodotorula babjevae CBS 7808 and DBVPG 8058 representing putative chromosomes. Table S1: Program versions used for the genome assembly and annotation pipeline. Table S2: Characteristics from the contigs and scaffolds of Rhodotorula babjevae CBS 7808 genome assembly. Table S3: Characteristics from the contigs and scaffolds of Rhodotorula babjevae DBVPG 8058 genome assembly. Table S4: Distribution of exon counts in the two strains of Rhodotorula babjevae. Table S5: Examples of lipid and carotenoid metabolism related genes in Rhodotorula babjevae CBS 7808. Table S6: Examples of lipid and carotenoid metabolism related genes in Rhodotorula babjevae DBVPG 8058. Table S7: Scaffold and contigs with assigned homology and pairwise nucleotide identity between Rhodotorula babjevae strains. Table S8: Summary of features of strain-unique contigs in Rhodotorula babjevae. Table S9: Genetic divergence between Rhodotorula babjevae strains and closely related Rhodotorula species. Table S10: Alignment statistics of the duplicated genes identified in the Rhodotorula babjevae CBS 7808 genome by OrthoVenn2. Table S11: Alignment statistics of the duplicated genes identified in the Rhodotorula babjevae DBVPG 8058 genome by OrthoVenn2. Table S12: Alignment statistics of the duplicated genes identified in the Rhodotorula babjevae CBS 7808 genome by BLASTp. Table S13: Alignment statistics of the duplicated genes identified in the Rhodotorula babjevae DBVPG 8058 genome by BLASTp. Table S14: Duplicated genes in Rhodotorula babjevae identified by BLASTp and OrthoVenn2 with a minimum coverage of 70%.
Author Contributions
Conceptualization, V.P.; methodology, B.M.; validation, C.B., M.H. and A.V.; formal analysis, G.C.M.-H. and B.M.; investigation, G.C.M.-H. and B.M.; resources, V.P., C.B., M.H. and A.V.; data curation, C.B., M.H. and A.V.; writing—original draft preparation, G.C.M.-H.; writing—review and editing, B.M., V.P., C.B., M.H. and A.V.; visualization, G.C.M.-H.; supervision, B.M.; project administration, V.P.; funding acquisition, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas), grant number 2018-01877.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This project has been deposited at ENA under the accession PRJEB48745.
Conflicts of Interest
A.V., C.B., and M.H. are co-founders of nanozoo GmbH and hold shares in the company. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poontawee R., Yongmanitchai W., Limtong S. Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process Biochem. 2017;53:44–60. doi: 10.1016/j.procbio.2016.11.013. [DOI] [Google Scholar]
- 2.Brandenburg J., Poppele I., Blomqvist J., Puke M., Pickova J., Sandgren M., Rapoport A., Vedernikovs N., Passoth V. Bioethanol and lipid production from the enzymatic hydrolysate of wheat straw after furfural extraction. Appl. Microbiol. Biotechnol. 2018;102:6269–6277. doi: 10.1007/s00253-018-9081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martín-Hernández G.C., Müller B., Chmielarz M., Brandt C., Hölzer M., Viehweger A., Passoth V. Chromosome-level genome assembly and transcriptome—Based annotation of the oleaginous yeast Rhodotorula toruloides CBS 14. Genomics. 2021;113:4022–4027. doi: 10.1016/j.ygeno.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Chmielarz M., Blomqvist J., Sampels S., Sandgren M., Passoth V. Microbial lipid production from crude glycerol and hemicellulosic hydrolysate with oleaginous yeasts. Biotechnol. Biofuels. 2021;14:65. doi: 10.1186/s13068-021-01916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayadi I., Belghith H., Gargouri A., Guerfali M. Screening of new oleaginous yeasts for single cell oil production, hydrolytic potential exploitation and agro-industrial by-products valorization. Process Saf. Environ. Prot. 2018;119:104–114. doi: 10.1016/j.psep.2018.07.012. [DOI] [Google Scholar]
- 6.Blomqvist J., Pickova J., Tilami S.K., Sampels S., Mikkelsen N., Brandenburg J., Sandgren M., Passoth V. Oleaginous yeast as a component in fish feed. Sci. Rep. 2018;8:15945. doi: 10.1038/s41598-018-34232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerfali M., Ayadi I., Mohamed N., Ayadi W., Belghith H., Bronze M.R., Ribeiro M.H.L., Gargouri A. Triacylglycerols accumulation and glycolipids secretion by the oleaginous yeast Rhodotorula babjevae Y-SL7: Structural identification and biotechnological applications. Bioresour. Technol. 2019;273:326–334. doi: 10.1016/j.biortech.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 8.Sen S., Borah S.N., Bora A., Deka S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb. Cell Fact. 2017;16:95. doi: 10.1186/s12934-017-0711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seveiri R. Characterization and prospective applications of the exopolysaccharides produced by Rhodosporidium babjevae. Adv. Pharm. Bull. 2020;10:254–263. doi: 10.34172/apb.2020.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen S., Borah S.N., Kandimalla R., Bora A., Deka S. Sophorolipid Biosurfactant Can Control Cutaneous Dermatophytosis Caused by Trichophyton mentagrophytes. Front. Microbiol. 2020;11:329. doi: 10.3389/fmicb.2020.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Firrincieli A., Otillar R., Salamov A., Schmutz J., Khan Z., Redman R.S., Fleck N.D., Lindquist E., Grigoriev I.V., Doty S.L. Genome sequence of the plant growth promoting endophytic yeast Rhodotorula graminis WP1. Front. Microbiol. 2015;6:978. doi: 10.3389/fmicb.2015.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen D., Paul K., Saha C., Mukherjee G., Nag M., Ghosh S., Das A., Seal A., Tripathy S. A unique life-strategy of an endophytic yeast Rhodotorula mucilaginosa JGTA-S1—A comparative genomics viewpoint. DNA Res. 2019;26:131–146. doi: 10.1093/dnares/dsy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C.J., Zhao D., Cheng P., Zheng L., Yu G.H. Genomics and lipidomics analysis of the biotechnologically important oleaginous red yeast Rhodotorula glutinis ZHK provides new insights into its lipid and carotenoid metabolism. BMC Genomics. 2020;21:834. doi: 10.1186/s12864-020-07244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gan H.M., Thomas B.N., Cavanaugh N.T., Morales G.H., Mayers A.N., Savka M.A., Hudson A.O. Whole genome sequencing of Rhodotorula mucilaginosa isolated from the chewing stick (Distemonanthus benthamianus): Insights into Rhodotorula phylogeny, mitogenome dynamics and carotenoid biosynthesis. PeerJ. 2017;5:e4030. doi: 10.7717/peerj.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Z., Zhang S., Liu H., Shen H., Lin X., Yang F., Zhou Y.J., Jin G., Ye M., Zou H., et al. A multi-omic map of the lipid-producing yeast Rhodosporidium toruloides. Nat. Commun. 2012;3:1111–1112. doi: 10.1038/ncomms2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakankun I., Fristensky B., Levin D.B. Genome sequence analysis of the oleaginous yeast, Rhodotorula diobovata, and comparison of the carotenogenic and oleaginous pathway genes and gene products with other oleaginous yeasts. J. Fungi. 2021;7:320. doi: 10.3390/jof7040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tkavc R., Matrosova V.Y., Grichenko O.E., Gostincar C., Volpe R.P., Klimenkova P., Gaidamakova E.K., Zhou C.E., Stewart B.J., Lyman M.G., et al. Prospects for fungal bioremediation of acidic radioactive waste sites: Characterization and genome sequence of Rhodotorula taiwanensis MD1149. Front. Microbiol. 2018;8:2528. doi: 10.3389/fmicb.2017.02528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goordial J., Raymond-Bouchard I., Riley R., Ronholm J., Shapiro N., Woyke T., LaButti K.M., Tice H., Amirebrahimi M., Grigoriev I.V., et al. Improved high-quality draft genome sequence of the eurypsychrophile Rhodotorula sp. JG1b, isolated from permafrost in the hyperarid upper-elevation McMurdo Dry Valleys, Antarctica. Genome Announc. 2016;4:15–17. doi: 10.1128/genomeA.00069-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen R.A., Bunikis I., Tiukova I., Holmberg K., Lötstedt B., Pettersson O.V., Passoth V., Käller M., Vezzi F. De novo assembly of Dekkera bruxellensis: A multi technology approach using short and long-read sequencing and optical mapping. Gigascience. 2015;4:56. doi: 10.1186/s13742-015-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiukova I.A., Pettersson M.E., Hoeppner M.P., Olsen R.A., Käller M., Nielsen J., Dainat J., Lantz H., Söderberg J., Passoth V. Chromosomal genome assembly of the ethanol production strain CBS 11270 indicates a highly dynamic genome structure in the yeast species Brettanomyces bruxellensis. PLoS ONE. 2019;14:e0215077. doi: 10.1371/journal.pone.0215077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chmielarz M., Sampels S., Blomqvist J., Brandenburg J., Wende F., Sandgren M., Passoth V. FT-NIR: A tool for rapid intracellular lipid quantification in oleaginous yeasts. Biotechnol. Biofuels. 2019;12:169. doi: 10.1186/s13068-019-1513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pi H.W., Anandharaj M., Kao Y.Y., Lin Y.J., Chang J.J., Li W.H. Engineering the oleaginous red yeast Rhodotorula glutinis for simultaneous β-carotene and cellulase production. Sci. Rep. 2018;8:2–11. doi: 10.1038/s41598-018-29194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandt C., Bongcam-Rudloff E., Müller B. Abundance tracking by long-read nanopore sequencing of complex microbial communities in samples from 20 different biogas/wastewater plants. Appl. Sci. 2020;10:7518. doi: 10.3390/app10217518. [DOI] [Google Scholar]
- 24.Weiß C.L., Pais M., Cano L.M., Kamoun S., Burbano H.A. nQuire: A statistical framework for ploidy estimation using next generation sequencing. BMC Bioinform. 2018;19:122. doi: 10.1186/s12859-018-2128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aramaki T., Blanc-Mathieu R., Endo H., Ohkubo K., Kanehisa M., Goto S., Ogata H. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics. 2020;36:2251–2252. doi: 10.1093/bioinformatics/btz859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gremme G., Steinbiss S., Kurtz S. Genome tools: A comprehensive software library for efficient processing of structured genome annotations. IEEE/ACM Trans. Comput. Biol. Bioinform. 2013;10:645–656. doi: 10.1109/TCBB.2013.68. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-R L.M., Konstantinidis K.T. The enveomics collection: A toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ Prepr. 2016;4:e1900v1. doi: 10.7287/peerj.preprints.1900. [DOI] [Google Scholar]
- 29.Meier-Kolthoff J.P., Auch A.F., Klenk H.P., Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris R.S. Ph.D. Thesis. The Pennsylvania State University; State College, PA, USA: 2007. Improved Pairwise Alignment of Genomic DNA. [Google Scholar]
- 31.Katoh K., Standley D.M. MAFFT Multiple Sequence Alignment Software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guindon S., Dufayard J.-F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 33.Xu L., Dong Z., Fang L., Luo Y., Wei Z., Guo H., Zhang G., Gu Y.Q., Coleman-Derr D., Xia Q., et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019;47:W52–W58. doi: 10.1093/nar/gkz333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuznetsov A., Bollin C.J. NCBI Genome Workbench: Desktop software for comparative genomics, visualization, and GenBank data submission. Methods Mol. Biol. 2021;2231:261–295. doi: 10.1007/978-1-0716-1036-7_16. [DOI] [PubMed] [Google Scholar]
- 35.Zou C., Chen A., Xiao L., Muller H.M., Ache P., Haberer G., Zhang M., Jia W., Deng P., Huang R., et al. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017;27:1327–1340. doi: 10.1038/cr.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krahulec J., Lišková V., Boňková H., Lichvariková A., Šafranek M., Turňa J. The ploidy determination of the biotechnologically important yeast Candida utilis. J. Appl. Genet. 2020;61:275–286. doi: 10.1007/s13353-020-00544-w. [DOI] [PubMed] [Google Scholar]
- 37.Fijarczyk A., Hénault M., Marsit S., Charron G., Fischborn T., Nicole-Labrie L., Landry C.R. The genome sequence of the Jean-Talon strain, an archeological beer yeast from Québec, reveals traces of adaptation to specific brewing conditions. G3 Genes Genomes Genet. 2020;10:3087–3097. doi: 10.1534/g3.120.401149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallone B., Steensels J., Prahl T., Soriaga L., Saels V., Herrera-Malaver B., Merlevede A., Roncoroni M., Voordeckers K., Miraglia L., et al. Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell. 2016;166:1397–1410.e16. doi: 10.1016/j.cell.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peter J., Chiara M.D., Friedrich A., Yue J., Pflieger D., Bergström A., Sigwalt A., Barre B., Freel K., Llored A., et al. Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature. 2018;556:339–344. doi: 10.1038/s41586-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nevers A., Doyen A., Malabat C., Néron B., Kergrohen T., Jacquier A., Badis G. Antisense transcriptional interference mediates condition-specific gene repression in budding yeast. Nucleic Acids Res. 2018;46:6009–6025. doi: 10.1093/nar/gky342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wery M., Gautier C., Descrimes M., Yoda M., Vennin-Rendos H., Migeot V., Gautheret D., Hermand D., Morillon A. Native elongating transcript sequencing reveals global anti-correlation between sense and antisense nascent transcription in fission yeast. RNA. 2018;24:196–208. doi: 10.1261/rna.063446.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borovkova A.N., Michailova Y.V., Naumova E.S. Molecular Genetic Features of Biological Species of the Genus Saccharomyces. Microbiology. 2020;89:387–395. doi: 10.1134/S0026261720040037. [DOI] [Google Scholar]
- 43.Białkowska A.M., Szulczewska K.M., Krysiak J., Florczak T., Gromek E., Kassassir H., Kur J., Turkiewicz M. Genetic and biochemical characterization of yeasts isolated from Antarctic soil samples. Polar Biol. 2017;40:1787–1803. doi: 10.1007/s00300-017-2102-7. [DOI] [Google Scholar]
- 44.De Jonge P., De Jongh F.C.M., Meijers R., Steensma H.Y., Scheffers W.A. Orthogonal-field-alternation gel electrophoresis banding patterns of DNA from yeasts. Yeast. 1986;2:193–204. doi: 10.1002/yea.320020307. [DOI] [PubMed] [Google Scholar]
- 45.Cahan P., Kennell J.C. Identification and distribution of sequences having similarity to mitochondrial plasmids in mitochondrial genomes of filamentous fungi. Mol. Genet. Genom. 2005;273:462–473. doi: 10.1007/s00438-005-1133-x. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Zeng F., Hon C.C., Zhang Y., Leung F.C.C. The mitochondrial genome of the Basidiomycete fungus Pleurotus ostreatus (oyster mushroom) FEMS Microbiol. Lett. 2008;280:34–41. doi: 10.1111/j.1574-6968.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 47.Libkind D., Čadež N., Opulente D.A., Langdon Q.K., Rosa C.A., Sampaio J.P., Gonçalves P., Hittinger C.T., Lachance M.A. Towards yeast taxogenomics: Lessons from novel species descriptions based on complete genome sequences. FEMS Yeast Res. 2020;20:foaa042. doi: 10.1093/femsyr/foaa042. [DOI] [PubMed] [Google Scholar]
- 48.Lallemand T., Leduc M., Landès C., Rizzon C., Lerat E. An overview of duplicated gene detection methods: Why the duplication mechanism has to be accounted for in their choice. Genes. 2020;11:1046. doi: 10.3390/genes11091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dean E.J., Davis J.C., Davis R.W., Petrov D.A. Pervasive and persistent redundancy among duplicated genes in yeast. PLOS Genet. 2008;4:e1000113. doi: 10.1371/journal.pgen.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grainger R.J., Barrass J.D., Jacquier A., Rain J.-C., Beggs J.D. Physical and genetic interactions of yeast Cwc21p, an ortholog of human SRm300/SRRM2, suggest a role at the catalytic center of the spliceosome. RNA. 2009;15:2161–2173. doi: 10.1261/rna.1908309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golubev W. Rhodosporidium babjevae, a new heterothallic yeast species (Ustilaginales) Syst. Appl. Microbiol. 1993;16:445–449. doi: 10.1016/S0723-2020(11)80278-X. [DOI] [Google Scholar]
- 52.Chand Dakal T., Giudici P., Solieri L. Contrasting patterns of rDNA homogenization within the Zygosaccharomyces rouxii species complex. PLoS ONE. 2016;11:e0160744. doi: 10.1371/journal.pone.0160744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conti A., Corte L., Pierantoni D.C., Robert V., Cardinali G. What is the best lens? Comparing the resolution power of genome-derived markers and standard barcodes. Microorganisms. 2021;9:299. doi: 10.3390/microorganisms9020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brandenburg J., Blomqvist J., Shapaval V., Kohler A., Sampels S. Oleaginous yeasts respond differently to carbon sources present in lignocellulose hydrolysate. Biotechnol. Biofuels. 2021;14:124. doi: 10.1186/s13068-021-01974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q., Sun M., Zhang Y., Song Z., Zhang S., Zhang Q., Xu J.R., Liu H. Extensive chromosomal rearrangements and rapid evolution of novel effector superfamilies contribute to host adaptation and speciation in the basal ascomycetous fungi. Mol. Plant Pathol. 2020;21:330–348. doi: 10.1111/mpp.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Passoth V., Hansen M., Klinner U., Emeis C.C. The electrophoretic banding pattern of the chromosomes of Pichia stipitis and Candida shehatae. Curr. Genet. 1992;22:429–431. doi: 10.1007/BF00352445. [DOI] [PubMed] [Google Scholar]
- 57.Legrand M., Jaitly P., Feri A., D’Enfert C., Sanyal K. Candida albicans: An emerging yeast model to study eukaryotic genome plasticity. Trends Genet. 2019;35:292–307. doi: 10.1016/j.tig.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Narayanan A., Vadnala R.N., Ganguly P., Selvakumar P., Rudramurthy S.M., Prasad R., Chakrabarti A., Siddharthan R., Sanyal K. Functional and comparative analysis of centromeres reveals clade-specific genome rearrangements in Candida auris and a chromosome number change in related species. MBio. 2021;12:e00905-21. doi: 10.1128/mBio.00905-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagen F., Khayhan K., Theelen B., Kolecka A., Polacheck I., Sionov E., Falk R., Parnmen S., Lumbsch H.T., Boekhout T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015;78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Gordon J.L., Byrne K.P., Wolfe K.H. Mechanisms of chromosome number evolution in yeast. PLoS Genet. 2011;7:e1002190. doi: 10.1371/journal.pgen.1002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hellborg L., Piškur J. Complex nature of the genome in a wine spoilage yeast, Dekkera bruxellensis. Eukaryot. Cell. 2009;8:1739–1749. doi: 10.1128/EC.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang S.L., Lai H.Y., Tung S.Y., Leu J.Y. Dynamic large-scale chromosomal rearrangements fuel rapid adaptation in yeast populations. PLoS Genet. 2013;9:e1003232. doi: 10.1371/journal.pgen.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vassiliadis D., Wong K.H., Blinco J., Dumsday G., Andrianopoulos A., Monahan B. Adaptation to industrial stressors through genomic and transcriptional plasticity in a bioethanol producing fission yeast isolate. G3 Genes Genomes Genet. 2020;10:1375–1391. doi: 10.1534/g3.119.400986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pierce N.T., Irber L., Reiter T., Brooks P., Brown C.T. Large-scale sequence comparisons with sourmash. F1000 Res. 2019;8:1006. doi: 10.12688/f1000research.19675.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Civiero E., Pintus M., Ruggeri C., Tamburini E., Sollai F., Sanjust E., Zucca P. Physiological and phylogenetic characterization of Rhodotorula diobovata DSBCA06, a nitrophilous yeast. Biology. 2018;7:39. doi: 10.3390/biology7030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This project has been deposited at ENA under the accession PRJEB48745.