Abstract
Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) is a very rare type of leukemia in children, Although BPDCN is a chemo-sensitive tumor, the relapse rate is very high. Tagraxofusp, which is a CD123-directed cytotoxin has been used as a targeted therapy and has shown promising results in patients with either untreated or relapsed BPDCN. There is also a good response with Venetoclax, a selective BCL2 inhibitor, as a single agent or in combination with chemotherapy.
Here, we described a case of a pediatric patient with BPDCN who was treated initially with ALL-based regimen followed by Allogeneic hematopoietic stem-cell transplantation (HSCT) and salvaged with Hyper-CVAD combined with Venetoclax after testicular relapse 11 months post Allogeneic HSCT.
Keywords: Plasmacytoid, Dendritic, Neoplasm, Leukemia, Venetoclax
1. Introduction
Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) is an extremely rare aggresive hematologic malignancy that most commonly affects older adults, with a median age at diagnosis of 65 to 67 years [1]. The exact incidence of BPDCN is unknown and it may represent around 0.5% of all hematologic malignancies [2]. The incidence in children is even lower with fewer than 80 cases reported [3]. There is no consensus about the treatment options for patients with BPDCN and the management remains challenging. The most common regimens used include acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) type therapies. BPDCN is chemo-sensitive but unfortunately, the relapse rate is very high with a median survival time of less than 2 years [4]. However, children tend to have a more favorable outcome [3].
Targeted therapy has been developed for BPDCN. Tagraxofusp, which is a CD123-directed cytotoxin consisting of recombinant human interleukin-3 fused to a truncated diphtheria toxin [5], was approved by the US Food and Drug Administration for use in adults and children older than 2 years of age with BPDCN and has shown promising results in untreated or relapsed BPDCN [6]. Some reports have recently shown a good response with Venetoclax, a selective BCL2 inhibitor, as a single agent or in combination with chemotherapy [7, 8].
Here, we describe a pediatric patient with BPDCN who was treated initially with ALL-based regimen followed by allogeneic hematopoietic stem-cell transplantation (HSCT) and salvaged with Hyper-CVAD combined with Venetoclax after testicular relapse 11 months post HSCT.
2. Case presentation
The patient is an 11-year-old male who was previously healthy presented with anemia, thrombocytopenia, and 83% blasts in peripheral blood. Hiswhite blood cell count was 12,000/cu.mm with 83% atypical lymphocytes, and blast-like cells, hemoglobin 9.6 g/dL and platelets count 93,000/cu.mm. On physical examination, he had no detectable hepatosplenomegaly or skin lesions. The testicular exam was normal. Bone marrow aspiration and biopsy were both hypercellular (100%) with 91% blasts. Flow cytometry revealed a significant population of blasts in the dim CD45 positive blast gate that were positive for CD4, partial CD10, CD16/56, CD123 HLA-DR., possible very dim TDT, and negative for all other markers. By Immunohistochemistry, the tumor cells were positive for CD56, TCL-1, and CD123 and negative for CD3, PAX-5, MPO, CD68, and lysozyme. Cytogenetics showed a reciprocal translocation between chromosomes 1 and 6, and a derivative chromosome 7. Bone marrow morphology and phenotype confirmed the diagnosis of BPDCN. Cerebrospinal fluid was negative for malignant cells. PET scan showed heterogeneous uptake within the bone marrow.
Chemotherapy was initiated as per St. Jude Total XV Protocol with a four-drug induction (prednisone, vincristine, daunorubicin, and asparaginase) with weekly triple intrathecal therapy TIT (methotrexate, cytarabine, and hydrocortisone). The patient had an uncomplicated hospital course. A repeat bone marrow performed at 6 weeks after initiation of therapy, following myeloid recovery showed no evidence of acute leukemia or increased blasts.
He then recived consolidation chemotherapy consisting of four cycles of methotrexate 5 g/m2. Disease evaluation after the consolidation phase including bone marrow aspirates and CSF studies demonstrated a complete remission status.
Since BPDCN is usually characterized by an aggressive clinical course and due to the dismal outcomes following chemotherapy alone in many patients, he underwent allogeneic stem cell transplantation from a fully matched sibling donor. He received total body irradiation 12 Gy and cyclophosphamide (60 mg/kg x2) as a preparative regimen, with cyclosporine and methotrexate for GVHD prophylaxis. He engrafted by day 22. He did not develop GVHD. Chimerism analysis by multiplex PCR amplification of STRs demonstrated 99% donor on days 30, 90, and 180 posts HSCT. The patient received CNS prophylaxis post-transplant with 10 monthly intrathecal cytarabine doses.
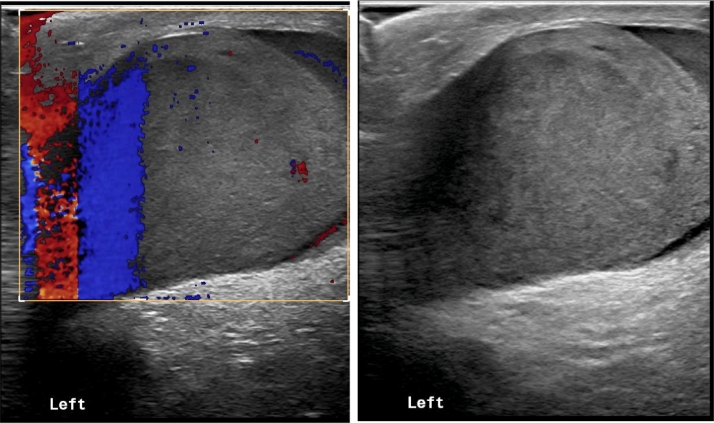
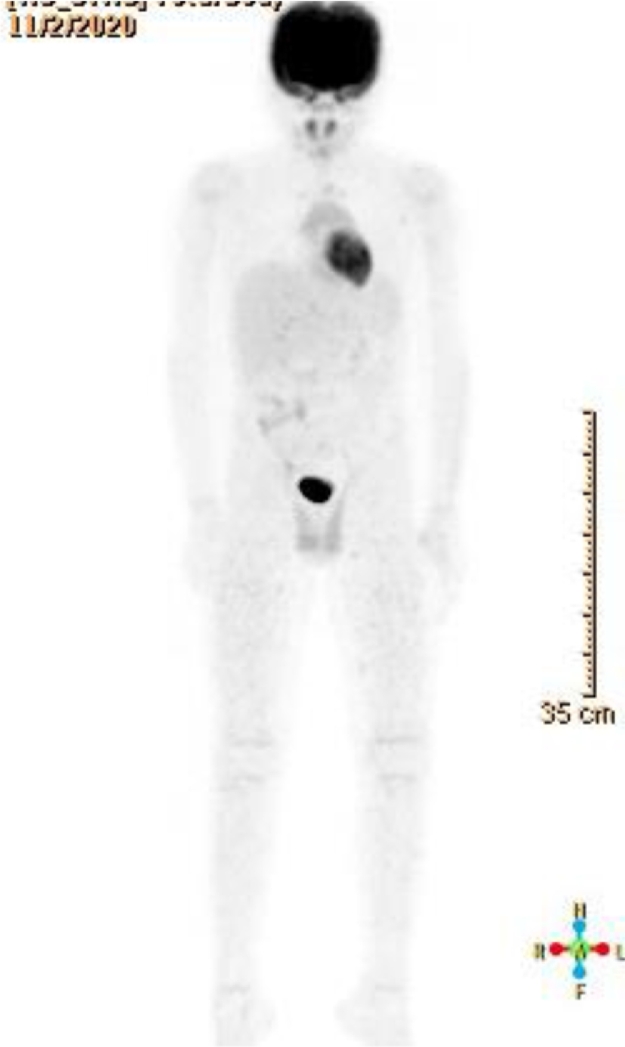
He was well and in complete remission until 11 months post HSCT, when he presented with abdominal pain and testicular enlargement, physical exam showed only bilateral hard enlarged testicles. Laboratory investigations were within normal limits. Doppler testicular ultrasound revealed bilateral disturbed echotexture with areas of increased echogenicity and flow, suggestive of disease infiltration (Fig. 1). Testicular biopsy confirmed relapse with diffuse infiltrate of atypical cells positive for TCL-1, CD4, CD10 (partial), CD56, CD123, and negative for muramidase, MPO, PAX-5, CD3, and CD34. A bone marrow aspiration was consistent with involvement by BPDCN (1% of total cells), and PET scan showed mild uptake in the testicles and spermatic cords with no abnormal uptake elsewhere (Fig. 2). CSF cytology was negative.
Fig. 1.
Left testicle ultrasound showing enlarged testicle with areas of increased echogenicity and flow, suggestive of disease infiltration.
Fig. 2.
PET scan showing enlargement of the testicles and spermatic cord which show mildly increased FDG uptake suggestive of disease involvement.
Due to unavailability of Tagraxofusp he was started on a second-line treatment with Hyper-CVAD in combination with Venetoclax (Cycle A: Cyclophosphamide, Vincristine, Doxorubicin, Dexamethasone alternating with cycle B: Methotrexate and Cytarabine) Venetoclax was given 240 mg/m2/day orally daily for 7 days with cycle A and for 14 days with cycle B. Reduced doses of methotrexate (250 mg/m2) and cytarabine (2 g/m2/dose) were used in cycle B to avoid neurotoxicity given the prior TBI. TIT was administered twice in each cycle. He received a total of 3 cycles. Treatment initiation led to a rapid response with nearly complete resolution of the testicular disease by ultrasound and a negative bone marrow exam after cycle 1. PET scan demonstrated complete remission after cycle 2. Treatment was complicated by COVID 19 pneumonia requiring hospitalization, febrile neutropenia, and E. coli ESBL bacteremia.
We opted to consolidate with a second allogeneic HSCT from a different matched sibling, using thiotepa, busulfan, fludarabine and antithymocyte globulin for conditioning. He did well, engrafted by day 18 and was discharged on day 20. He continues to do well 200 days post HSCT.
3. Discussion
BPDCN is a rare leukemia initially described as an acute agranular CD4-positive natural killer (NK) cell leukemia in 1995 [9], it was identified as a distinct entity in the 2008 and 2016 WHO classification of tumors of the hematopoietic and lymphoid tissues [10] [11]. Diagnosis is established by either immunohistochemistry or flow cytometry. Cells express CD4 and CD56 [12], in addition to plasmacytoid dendritic markers including [13]. In our case, we relied on CD4, CD56, TCL-1, and CD123 positivity to confirm the diagnosis. Most patients present with cutaneous lesions with or without multiorgan involvement including lymph nodes, bone marrow, liver, and spleen [14]. However, some may lack skin manifestations [15]. In a systematic review of 74 pediatric patients 24% presented without skin lesions, like our patient [3].
The management of BPDCN remains controversial. The current recommendation is to use tagraxofusp (CD123-directed cytotoxin) for remission induction in adults and children over 2 years [6].The alternative induction regimens for BPDCN vary. A retrospective multicenter analysis showed that patients who received ALL-like therapy had a more favorable outcome compared with patients who received AML-like therapy [16]. In a large retrospective case series, 43 patients received acute leukemia-type regimens, complete remission (CR) were achieved in 17 patients (7 after AML and 10 after an ALL regimens) [2]. Based on this data, we chose the St. Jude Total XV ALL regimen for front-line treatment of our patient.
The role of allogeneic HSCT in first remission is still unclear in pediatric BPDCN patients due to the paucity of cases. In contrast, retrospective studies in adults report favorable outcomes in patients undergoing allogeneic HCT in first remission [17] [18]. We preferred allogeneic HSCT in our patient due to the availability of a matched sibling donor.
Although BPDCN is a highly recurrent neoplasm, testicular relapse in pediatric patients has not been widely reported. Moreover, initial testicular presentation is extremely rare. Dhariwal et al. described a 13-year-old male who initially presented with a testicular mass followed by bone marrow involvement [19]. Park et al. reported the first case of relapsed BPDCN presenting as a unilateral testicular tumor in a 54-year-old patient [20].
Optimal treatment of relapsed BPDCN is not well defined and the treatment options depend on prior therapy. Venetoclax, a BCL2 inhibitor, has shown a significant response with relapsed BPDCN alone or in combination with chemotherapy [7] [8]. In our case, Hyper-CVAD in combination with Venetoclax led to rapid complete remission after 2 cycles and was well tolerated.
In summary, This is a rare case of a pediatric patient with testicular relapse BPDCN who was salvaged with Hyper-CVAD in combination with Venetoclax. Further follow-up is needed for a better assessment of the outcome.
Informed consent
Informed consent is not required as this case report does not include any identifying information or photos of the patient
Declaration of Competing Interest
No potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Julia F., Petrella T., Beylot-Barry M., et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br. J. Dermatol. 2013;169:579. doi: 10.1111/bjd.12412. [DOI] [PubMed] [Google Scholar]
- 2.Pagano L., Valentini C.G., Pulsoni A., Fisogni S., Carluccio P., Mannelli F., et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98:239–246. doi: 10.3324/haematol.2012.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M.J., Nasr A., Kabir B., et al. Pediatric blastic plasmacytoid dendritic cell neoplasm: a systematic literature review. J. Pediatr. Hematol. Oncol. 2017;39:528–537. doi: 10.1097/MPH.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 4.Venugopal S., Zhou S., El Jamal S.M., Lane A.A., Mascarenhas J. Blastic plasmacytoid dendritic cell neoplasm-current insights. Clin. Lymphoma Myeloma Leuk. 2019;19(9):545–554. doi: 10.1016/j.clml.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Frankel A., Liu J.S., Rizzieri D., Hogge D. Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk. Lymphoma. 2008;49:543–553. doi: 10.1080/10428190701799035. [DOI] [PubMed] [Google Scholar]
- 6.Pemmaraju N., Lane A.A., Sweet K.L., et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N. Engl. J. Med. 2019;380:1628. doi: 10.1056/NEJMoa1815105. [DOI] [PubMed] [Google Scholar]
- 7.Montero J., Stephansky J., Cai T., Griffin G.K., Cabal-Hierro L., Togami K., Hogdal L.J., Galinsky I., Morgan E.A., Aster J.C., Davids M.S., LeBoeuf N.R., Stone R.M., Konopleva M., Pemmaraju N., Letai A., Lane A.A. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer Discov. 2017;7(2):156–164. doi: 10.1158/2159-8290.CD-16-0999. FebEpub 2016 Dec 16. PMID: 27986708; PMCID: PMC5296248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pemmaraju N., Konopleva M., Lane A.A. More on blastic plasmacytoid dendritic-cell neoplasms. N. Engl. J. Med. 2019;380(7):695–696. doi: 10.1056/NEJMc1814963. [DOI] [PubMed] [Google Scholar]
- 9.Brody J.P., Allen S., Schulman P., et al. Acute agranular CD4-positive natural killer cell leukemia. Comprehensive clinicopathologic studies including virologic and in vitro culture with inducing agents. Cancer. 1995;75:2474. doi: 10.1002/1097-0142(19950515)75:10<2474::aid-cncr2820751013>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Facchetti F., Jones D.M., Petrella T., et al. In: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Swerdlow SH, Campo E, Harris NL, et al., editors. IARC; Lyon: 2008. Blastic plasmacytoid dendritic cell neoplasm; p. 145. [Google Scholar]
- 11.Arber D.A., Orazi A., Hasserjian R., et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edition. International Agency for Research on Cancer (IARC); Lyon: 2017. revised. revised. [Google Scholar]
- 13.Marafioti T., Paterson J.C., Ballabio E., et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008;111:3778. doi: 10.1182/blood-2007-10-117531. [DOI] [PubMed] [Google Scholar]
- 14.Feuillard J., Jacob M.C., Valensi F., et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002;99:1556. doi: 10.1182/blood.v99.5.1556. [DOI] [PubMed] [Google Scholar]
- 15.Rauh M.J., Rahman F., Good D., et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation, lacking cutaneous involvement: case series and literature review. Leuk. Res. 2012;36:81. doi: 10.1016/j.leukres.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Taylor J., Haddadin M., Upadhyay V.A., et al. Multicenter analysis of outcomes in blastic plasmacytoid dendritic cell neoplasm offers a pretargeted therapy benchmark. Blood. 2019;134:678. doi: 10.1182/blood.2019001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roos-Weil D., Dietrich S., Boumendil A., et al. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2013;121:440. doi: 10.1182/blood-2012-08-448613. [DOI] [PubMed] [Google Scholar]
- 18.Aoki T., Suzuki R., Kuwatsuka Y., et al. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood. 2015;125:3559. doi: 10.1182/blood-2015-01-621268. [DOI] [PubMed] [Google Scholar]
- 19.Dhariwal S., Gupta M. A case of blastic plasmacytoid dendritic cell neoplasm with unusual presentation. Turk. J. Hematol. 2019;36(1):55–56. doi: 10.4274/tjh.galenos.2018.2018.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pak Daniel M., Tretiakova Maria S. An Unusual Manifestation of Blastic Plasmacytoid Dendritic Cell Neoplasm as a Testicular Tumor. Case Rep. Pathol. 2019;2019:4. doi: 10.1155/2019/9196167. Article ID 9196167pages. [DOI] [PMC free article] [PubMed] [Google Scholar]