Abstract
The mechanism of glycopeptide resistance in Staphylococcus aureus is not known with certainty. Because the target of vancomycin is the d-Ala–d-Ala terminus of the stem peptide of the peptidoglycan precursor, by subjecting muropeptides to reversed-phase high-performance liquid chromatography, we investigated peptidoglycan obtained from glycopeptide-intermediate S. aureus (GISA) isolates for changes in composition and evaluated whether any peptidoglycan structural change was a consistent feature of clinical GISA isolates. GISA isolates Mu50 and Mu3 from Japan had the large glutamate-containing monomeric peak demonstrated previously, although strain H1, a vancomycin-susceptible MRSA isolate from Japan that was clonally related to Mu3 and Mu50, and a femC mutant that we studied, did also. For the U.S. GISA isolates, strain NJ had a large monomeric peak with a retention time identical to that described for the glutamate-containing monomer in strains H1, Mu3, and Mu50. However, a much smaller corresponding peak was seen in GISA MI, and this peak was absent from both GISA PC and a recent GISA isolate obtained from an adult patient in Illinois (strain IL). These data suggest that a uniform alteration in peptidoglycan composition cannot be discerned among the GISA isolates and indicate that a single genetic or biochemical change is unlikely to account for the glycopeptide resistance phenotype in the clinical GISA isolates observed to date. Furthermore, a large monomeric glutamate-containing peak is not sufficient to confer the resistance phenotype.
Clinical Staphylococcus aureus isolates with intermediate resistance to glycopeptides, so-called glycopeptide-intermediate S. aureus (GISA) isolates, have recently been recognized in Japan, (12, 13), the United States (3, 18, 19, 21), and elsewhere. On the basis of phenotypic differences with respect to vancomycin and teicoplanin susceptibility and bacterial population analyses, three distinct classes of resistant isolates (class A, vancomycin intermediate, teicoplanin intermediate; class B, vancomycin intermediate, teicoplanin susceptible; class C, vancomycin susceptible, teicoplanin intermediate) have been recognized (2). All clinical GISA isolates described to date are heteroresistant in that they contain a subpopulation of bacteria able to grow on medium containing vancomycin at relatively high concentrations. The mechanism of resistance in the clinical GISA isolates is not yet known, but the low level of resistance and the lack of hybridization with enterococcal vancomycin resistance genes suggest that the mechanism(s) is distinct from those that mediate vancomycin resistance in enterococci (15).
Because the target of vancomycin is the d-Ala–d-Ala terminus of the stem peptide of the peptidoglycan precursors (1), it has been appropriate to examine peptidoglycans obtained from GISA isolates to assess changes in composition (8, 10, 14, 19). In one study of two Japanese GISA isolates, strains Mu3 and Mu50 (11), the mutanolysin-digested peptide of the cell wall of Mu50 had an increased content of glutamate, a decreased level of cross-linking of peptidoglycan, and a decreased dimeric muropeptide/monomeric muropeptide ratio. The authors suggested that the increase in the amount of glutamate-containing muropeptides which bind to vancomycin more avidly than amidated, d-isoglutamine-containing muropeptides and the decreased amount of cross-linking which leads to an increase in the number of d-Ala–d-Ala sites in the preexisting cell wall might contribute to vancomycin resistance by increasing the level of vancomycin binding to the preexisting wall of Mu50. They also suggested that the observed thickening of the Mu50 cell wall may decrease the amount of vancomycin available to reach d-Ala–d-Ala pentapeptide peptidoglycan precursor termini.
To investigate whether the changes documented in strains Mu3 and Mu50 are consistently present in clinical GISA strains, we analyzed the peptidoglycan compositions of GISA isolates obtained from patients in the United States (isolates MI, NJ, PC, and IL) as well as those of Mu3 and Mu50. We also compared the peptidoglycan composition of H1, a methicillin-resistant S. aureus (MRSA) isolate from Japan, with those of the related GISA isolates Mu3 and Mu50.
Isolate IL provided an opportunity to study changes in peptidoglycan composition associated with acquisition of the GISA phenotype. GISA isolate IL was obtained from the blood of a 63-year-old female dialysis recipient in Illinois (3). This isolate was one of a series of MRSA isolates from the blood of this patient who was receiving vancomycin therapy. An earlier clonally related isolate from the blood of this patient was susceptible to glycopeptides and was available for study.
We also compared the peptidoglycan compositions of clinical GISA isolates with those of glycopeptide-susceptible revertant isolates that were isogenic with the glycopeptide-resistant clinical parent isolate. As reported previously (2), these were prepared by serial passage on a nonselective medium and were associated with decreases in the MICs of vancomycin and teicoplanin to the susceptible range (16) and a decrease or loss of the glycopeptide-resistant subpopulation.
MATERIALS AND METHODS
S. aureus isolates.
S. aureus isolates were routinely cultured at 37°C and were stored as frozen stocks in skim milk (Difco Laboratories, Detroit, Mich.) at −70°C as described previously (6) (Table 1). Glycopeptide-susceptible, MRSA strain H1 was isolated by Hiramatsu et al. (12) from the sputum of an elderly Japanese man with pneumonia who was successfully treated with vancomycin. GISA isolates representing all three phenotype classes were isolated from patients who did not respond to vancomycin treatment. Glycopeptide-susceptible revertant isolates were prepared by passage of clinical GISA isolates on nonselective medium as described previously (2).
TABLE 1.
Bacterial strainsa
| Isolate | Description | MIC (μg/ml)
|
Source | Reference | |
|---|---|---|---|---|---|
| Vmb | Tcob | ||||
| MI | Class A GISA clinical isolatec | 6 | 8 | CDC isolate HIP5827 (Fred Tenover) | 4, 5, 21 |
| MI (P60) | MI passaged on BHI for 60 days | 2 | 3 | 2 | |
| NJ | Class B GISA clinical isolate | 5 | 5 | CDC isolate HIP5836 (Fred Tenover) | 5, 21 |
| NJ (P15) | NJ passaged on BHI for 15 days | 3 | 4 | 2 | |
| PC | Class B GISA clinical isolate | 5 | 5 | CDC isolate HIP 6297 (Fred Tenover) | 18, 19 |
| PC (P15) | PC passaged on BHI for 15 days | 2 | 3 | 2 | |
| Mu50 | Class B GISA isolate | 5 | 5 | K. Hiramatsu (Tokyo, Japan) | 10, 12, 13 |
| Mu50 (P15) | Mu50 passaged on BHI for 15 days | 2 | 2 | 2 | |
| Mu3 | Class C GISA clinical isolate | 2 | 9 | K. Hiramatsu | 10, 12, 13 |
| Mu3 (P15) | Mu3 passaged on BHI for 15 days | 2 | 6 | 2 | |
| BB589 | femC mutant | 1d | 2d | B. Berger-Bachi (Zurich, Switzerland) | 22 |
| H1 | MRSA isolate from a Japanese patient with pneumonia believed to have responded favorably to vancomycin | 2e, 3/4-5d | 8e, 6-12/12d | K. Hiramatsu | 12 |
| IL (isolate X31325) | MRSA isolate | 1 | 4 | R. Carey (Maywood, Ill.) | 3 |
| IL (isolate T78628) | GISA isolate | 8 | ND | R. Carey | 3 |
Abbreviations: CDC, Centers for Disease Control and Prevention (Atlanta, Ga.); classes A, B, and C, glycopeptide resistance (class A, vancomycin intermediate, teicoplanin intermediate; class B, vancomycin intermediate, teicoplanin susceptible; class C, vancomycin susceptible, teicoplanin intermediate); BHI, brain heart infusion broth; Vm, vancomycin; Tco, teicoplainin; ND, not determined.
Values were published previously (2), except for those for isolates BB589 (femC mutant), H1, IL (isolate X31325), and IL (isolate T78628).
The MIC of teicoplanin for this isolate was determined to be 16 μg/ml by investigations performed at the Centers for Disease Control and Prevention. This value justified the class A designation. At the University of Chicago, a value of 8 μg/ml has been consistently obtained. This value would render the isolate class B.
Done at the University of Chicago in brain heart infusion broth at 24 h/48 h.
Done at the University of Chicago in Mueller-Hinton broth at 24 h.
The species of all isolates was confirmed by use of the Staphaurex latex agglutination test (Abbott Laboratories, Chicago, Ill.). GISA parent strains and revertants were coagulase positive and Staphaurex positive. When possible, the SmaI restriction pattern of whole-cell DNA obtained from each isolate was analyzed by pulsed-field gel electrophoresis and was confirmed to be similar to that published previously (12, 18, 19, 21). Moreover, as reported previously (2), the SmaI restriction pattern of each susceptible revertant isolate was identical to that of its GISA parent. The patterns of the MRSA, vancomycin-susceptible isolate from the patient in Illinois (isolate IL) and the subsequent GISA isolate from the same patient were also identical (S. Boyle-Vavra et al., unpublished data). Antimicrobial susceptibility testing was performed by the broth dilution method as described previously (2, 16), except that brain heart infusion broth was sometimes substituted for cation-supplemented Mueller-Hinton broth. To discern small changes in resistance phenotype, our procedure differed from the NCCLS protocol in our testing of glycopeptides in arithmetic dilutions instead of twofold dilutions. MIC testing was performed on at least six occasions. The variation between tests has been minimal. The values reported here represent the mode of multiple determinations. Population analysis for MRSA isolate H1 was performed as described previously (2).
Preparation of cell walls for HPLC.
Mutanolysin-digested muropeptides were prepared from S. aureus isolates as described previously (22), except that a bead beater (model no. 1107900; Bio Spec Products, Inc., Bartlesville, Okla.) was used to lyse the cells. In experiments in which vancomycin was added, this was done when the optical density at A578 reached 0.2; cells were then grown to an optical density at A578 of 0.7 before harvesting. The muropeptide mixture was separated by reversed-phase high-performance liquid chromatography (HPLC) as described previously (22). Briefly, the column (3-μm Hypersil ODS; 4.0 by 250 mm; CompAx-peek; Knauer, Berlin, Germany) was eluted with a linear gradient from 5% methanol in 50 mM sodium phosphate (pH 2.5) containing 0.0005% sodium azide to 30% methanol in 50 mM sodium phosphate (pH 2.8) within 210 min. Muropeptide peaks were detected by measurement of the absorption at 206 nm.
Data analysis.
The magnitude of each peak was calculated as the area under the reversed-phase HPLC tracing. The amount of peptidoglycan cross-linking was calculated as Σ (n − 1)/n (percent n-mers), where n is equal to 1, 2, 3, and 4. Since peaks with retention times longer than those of the tetramer region cannot be reliably separated, we assumed that all of them were oligomers and that the average number of cross-linked peptides was 15. The percentage of d-Ala–d-Ala-containing muropeptides was calculated as (1.0 × percent monomers) + (0.5 × percent dimers) + (0.33 × percent trimers) + (0.25 × percent tetramer) + (0.1 × percent oligomers). Typically, about 20% of the peptidoglycan subunits in the digested cell wall material carry the d-Ala–d-Ala terminus.
RESULTS
Analysis of peptidoglycans.
Peptidoglycans from the various S. aureus isolates were digested with mutanolysin, an enzyme which cleaves native peptidoglycan between the saccharide backbone residues, leaving the peptide cross-bridges intact. This digestion creates muropeptide species of various disaccharide pentapeptide units depending on the number of peptide cross-bridges in the S. aureus isolate.
We found that the HPLC chromatograms for all strains, irrespective of resistance phenotype, had the typical muropeptide species identified in previous investigations (7, 14, 17). The nomenclature used to identify relevant peaks was described previously (17, 22). The dimeric, trimeric, etc., muropeptides represent peptide-cross-linked combinations of muropeptide monomeric structures.
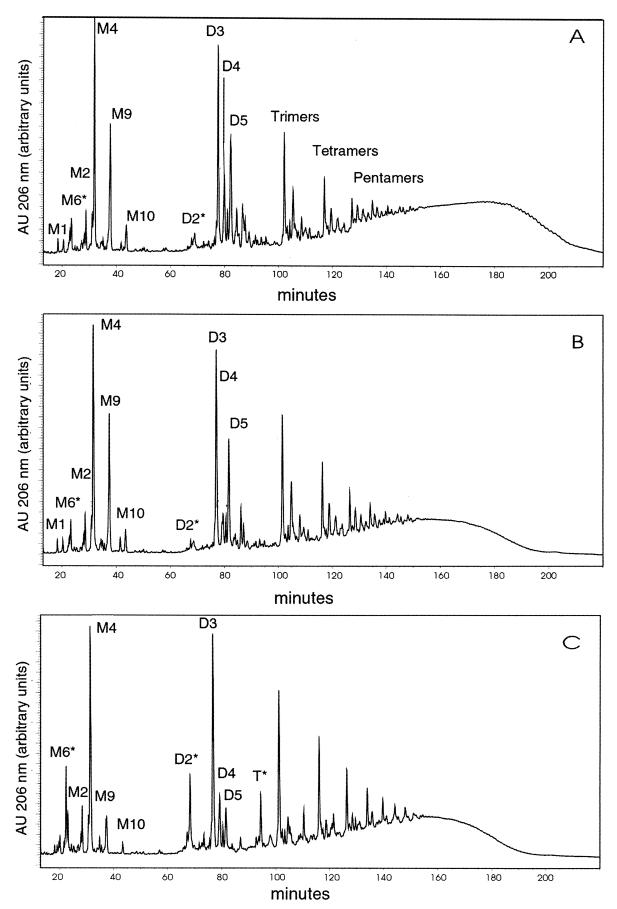
The HPLC chromatograms of peptidoglycan digested with mutanolysin from MRSA isolate H1 and class A GISA isolate Mu50 contained an unusually large M9 peak, a disaccharide-pentapeptide monomer with the pentaglycine bridge that is typical of the species but that contains d-glutamate instead of d-isoglutamine in the stem peptide (Fig. 1A and B). A somewhat smaller M9 peak was also seen in the chromatogram of digested peptidoglycan from class C GISA isolate Mu3 (data not shown). This M9 peak was greatly diminished in magnitude in Mu50 (P15) which had been passaged for 15 days (Fig. 1C). This finding indicates that the loss of resistance in Mu50 was associated with the loss of the M9 peak. For Mu3, the MIC of vancomycin did not decrease with serial passage, although the MIC of teicoplanin did decrease. The magnitude of the M9 peak in Mu3 (P15), the isolate obtained after serial passage on nonselective medium, did not appreciably change in magnitude. All three isolates from Japan also contained the M4 peak typical of all S. aureus isolates.
FIG. 1.
Separation of muropeptides obtained by digestion with mutanolysin by reversed phase HPLC from vancomycin-susceptible MRSA and GISA isolates from Japan. (A) H1; (B) Mu50; (C) Mu50 (P15). M, monomeric; D, dimeric; T, trimeric.
Among the U.S. GISA isolates (Fig. 2, left-hand panels), those from New Jersey (isolate NJ), Michigan (isolate MI), and Port Chester, N.Y. (isolate PC), a large M9 peak was found only for the peptidoglycan isolated from strain NJ . Very small M9 peaks comparable to those demonstrable in most wild-type S. aureus isolates were found in the chromatograms of peptidoglycans from strains MI and PC. As was the case for isolate Mu50, the GISA isolates whose results are depicted in Fig. 2 had a decrease in magnitude of the M9 peak uniformly noted for each susceptible, revertant isolate. The magnitude of the decrease associated with reversion was largest in isolate NJ. All the U.S. GISA isolates also had the normal M4 peak (Fig. 2, right-hand panels).
FIG. 2.
Separation of muropeptides obtained by digestion with mutanolysin by reversed phase HPLC from three GISA isolates obtained from patients in the United States. The GISA isolates are shown in the left hand panels, and the susceptible revertants of the clinical isolates are shown in the right hand panels. (A) NJ and NJ (P15); (B) MI and MI (P60); (C) PC and PC (P15).
A recently described patient from Illinois had sustained MRSA bacteremia, even though the patient received vancomycin therapy (3). Two isolates from the blood of this patient, a vancomycin-susceptible, MRSA isolate and a clonally related GISA isolate, were obtained from cultures of blood obtained 16 days apart. Mutanolysin-digested peptidoglycan from the vancomycin-susceptible, MRSA isolate yielded a small M9 peak whose size resembled those of the peaks observed in the chromatograms for GISA isolates MI and PC (Fig. 3A). The M9 peak of the clonally related GISA isolate subsequently obtained from the blood of the same patient later in the hospitalization was of a similar magnitude (Fig. 3B). Thus, in this instance, acquisition of intermediate resistance to vancomycin and teicoplanin was not associated with an increase in the size of the M9 peak.
FIG. 3.
Separation of muropeptides obtained by digestion with mutanolysin by reversed-phase HPLC from two isolates obtained from the blood of a patient in Illinois. (A) MRSA, vancomycin-susceptible isolate; (B) genetically related GISA isolate.
We calculated the relative M4 and M9 peak magnitudes by measuring the areas under these peaks and calculating the M4 peak area/M9 peak area ratio (M4/M9 ratio) (Table 2). Typically, S. aureus isolates have an M4/M9 ratio of 3 to 4, reflecting the relatively small area under the M9 peak that is typical for the species (H. Labischinski, unpublished data). For the U.S. GISA isolates, isolate NJ had a low M4/M9 ratio that did not change appreciably when the cells were grown in vancomycin at 0.5 × the MIC (data not shown). However, isolates MI and PC had higher M4/M9 ratios, reflecting the relatively smaller area under the M9 peak.
TABLE 2.
Peptidoglycan compositions of S. aureus isolates
| Isolate | Phenotypea | M4/M9 ratio | % Cross-linking | % d-Ala–d-Ala | Dimer/monomer ratio |
|---|---|---|---|---|---|
| 523 | MSSA | 4 | 75.9 | 22.0 | 2.1 |
| H1 | MRSA | 1.5 | 77 | 22.2 | 1.8 |
| Mu3 | GISA | 2.8 | 76.7 | 22.8 | 1.8 |
| Mu3 (P15) | MRSA | 2.4 | 76.8 | 22.3 | 1.6 |
| Mu50 | GISA | 2.1 | 72.0 | 25.7 | 1.6 |
| Mu50 (P15) | MRSA | 4.1 | 73.3 | 25.3 | 1.6 |
| NJ | GISA | 1.3 | 74.9 | 24.3 | 1.5 |
| NJ (P15) | MRSA | 2.4 | 77.6 | 21.6 | 1.6 |
| MI | GISA | 2.8 | 73.9 | 25.1 | 1.4 |
| MI (P60) | MRSA | 3.9 | 75.1 | 22.4 | 1.2 |
| PC | GISA | 3.5 | 81.1 | 18.3 | 1.9 |
| PC (P15) | MRSA | 3.6 | 82.3 | 19.5 | 2.2 |
| IL | MRSA | 3.9 | 79.3 | 17.8 | 1.6 |
| IL | GISA | 4.3 | 81.1 | 16.7 | 1.6 |
MSSA, methicillin-susceptible S. aureus; GISA, methicillin-resistant S. aureus that is also intermediately resistant to glycopeptides.
Our analysis of the peptidoglycan that we prepared from isolate Mu50 confirmed that this GISA isolate had a relatively low M4/M9 ratio, reflecting the relatively large area under the M9 peak reported earlier (11). The M4/M9 ratio of the class C vancomycin-resistant isolate Mu3 was somewhat higher. The area under its M9 peak was somewhat smaller than that of Mu50 but was still relatively large compared with that typically found for S. aureus isolates. It is noteworthy that vancomycin-susceptible (see below) isolate H1 from Japan had a very large area under its M9 peak, similar to that of Mu50, and, thus, a very low M4/M9 ratio. Indeed, this vancomycin-susceptible MRSA isolate had the lowest M4/M9 ratio and the highest M9 peak of isolates examined in our laboratory.
Reversion to susceptibility by serial passage of the clinical GISA isolates on nonselective medium had various impacts on the M4/M9 ratio. For the revertant isolates of parent clinical GISA isolates NJ, MI, and Mu50, the M4/M9 ratio uniformly increased, reflecting a decrease in the M9 peak area. The M4/M9 ratio of the NJ revertant, grown in vancomycin at 0.5 × the MIC, was identical to that of the NJ revertant grown in the absence of vancomycin (data not shown). In contrast, the M4/M9 ratio for the revertant of class B GISA isolate PC was essentially unchanged, as was that of the class C isolate Mu3 obtained after serial passage. The M4/M9 ratios for the vancomycin-susceptible MRSA-GISA IL isolate pair were similar to each other.
Peptidoglycan cross-linking.
Of the three isolates from Japan, isolate H1 was the most cross-linked (Table 2); Mu50 was the least cross-linked, and Mu3 was intermediately cross-linked. For isolates Mu3 and Mu50, these relative values are similar to those described previously (10) and, taken together, suggest an inverse relationship between vancomycin resistance and cross-linking. In contrast, cross-linking of the U.S. GISA isolates studied produced various results. Isolates NJ and MI were cross-linked at an intermediate level between those for isolates Mu3 and Mu50; isolate PC was more highly cross-linked. Comparison of the glycopeptide-susceptible and -resistant IL isolates indicates that the GISA isolate was more highly cross-linked than the vancomycin-susceptible MRSA antecedent isolate. Thus, in this background, cross-linking increased in association with the acquisition of vancomycin intermediate resistance.
Reversion of isolates Mu50, NJ, MI, and PC to vancomycin susceptibility was uniformly associated with increased cross-linking (Table 2), although some differences within the pairs were minimal.
A possible relationship between the magnitude of the M9 peak and the degree of cross-linking was studied by plotting the M4/M9 ratio (which reflected mostly changes in the magnitude of M9) versus the degree of cross-linking for all the isolates that we studied; a strong correlation was found (r = 0.71; P = 0.05).
Other analyses.
Among the isolates from Japan, isolates Mu3 and H1 had identical dimeric/monomeric muropeptide ratios, while that for Mu50 was somewhat lower (Table 2). Relative to Mu3, isolate Mu50 had increases in both the percent monomeric and the percent dimeric species in the digested peptidoglycan (data not shown). Another feature of the vancomycin-susceptible revertants derived from isolates Mu50 and PC was the increase in muropeptides, as marked by an asterisk in the peak nomenclature; e.g., M6*, D2*, and T* suggest increased endopeptidase activities in these revertants.
Characterization of susceptibility of MRSA isolate H1.
The MICs of glycopeptides for MRSA isolate H1 as determined by the broth dilution method (Table 1) revealed values in the susceptibile range by NCCLS standards. Population analysis on vancomycin-containing medium revealed the absence of a resistant subpopulation (data not shown). Interestingly, when MIC testing was done in brain heart infusion broth and the results were read after 48 h of incubation, methods alternative to those recommended by NCCLS, the MIC of teicoplanin was 12 μg/ml, a value in the intermediate range. This isolate therefore has properties different from those reported for GISA isolate Mu3 or Mu50 (2, 12); both were reported to have resistant subpopulations that grow on medium containing ≥4 μg of vancomycin/ml, although only the vancomycin MIC for Mu50 was in the intermediate range.
DISCUSSION
A uniform alteration in the peptidoglycan compositions of the GISA isolates could not be discerned. This finding indicates that it is not likely that a single genetic or biochemical change in the cell wall composition accounts for the glycopeptide resistance phenotype in all clinical isolates obtained to date. A striking change found in several of the clinical GISA isolates was the decreased M4/M9 ratio due to a large area under the M9 peak described previously in isolate Mu50 and to a lesser extent in Mu3 (11). A similar change was found in U.S. GISA isolate NJ, a GISA isolate from Germany (9), and, to a lesser extent, in strain MI but was absent from GISA isolates PC and IL. Thus, the presence of a large area under the M9 peak is a common but not a specific finding for the mutanolysin-digested peptidoglycan fragments from clinical GISA isolates. Furthermore, the different SmaI digestion patterns of DNA from strain NJ compared with those of DNAs from strains Mu50 and Mu3 demonstrate that GISA isolates of different genetic backgrounds may have large areas under their M9 peaks.
Surprisingly, one of the lowest M4/M9 ratios that we documented occurred in vancomycin-susceptible, MRSA isolate H1 isolated from Japan (12). This observation suggests that the presence of a large area under the M9 peak is not confined to vancomycin-resistant, MRSA isolates. However, the intermediate MIC of teicoplanin for this isolate that we found in brain heart infusion broth when the MIC was read at 48 h suggests that the large area under the M9 peak found for isolate H1 may be associated with teicoplanin resistance. Additional investigation will be required to understand the importance of the large area under the M9 peak found for H1 or the importance of the results obtained in brain heart infusion broth after extended incubation.
An increase in the area under the M9 peak has been shown to result from either or both of two alterations in peptidoglycan structure that would produce monomeric species with identical retention times: an alanine substitution for the glycine in the cross-bridge or a d-glutamate substitution for d-isoglutamine in the stem peptide. The increase in the area under the M9 peak is more likely to have resulted from the glutamate-substituted stem peptide. Hanaki et al. (11), who also analyzed the peptidoglycan structure in strain Mu3, demonstrated that the alanine-substituted cross-link contributed little to the magnitude of the area under the M9 peak. Moreover, in Mu50 the d-Ala-substituted cross-linker species was absent altogether, and, furthermore, in all other S. aureus strains studied to date, the d-Ala-containing cross-linker species has been extremely small (Labischinski, unpublished data).
Our data for strains Mu3 and H1 and two additional lines of evidence suggest that the large area under the M9 peak, documenting the presence of glutamate-containing pentapeptide precursors, may not be sufficient to produce the vancomycin resistance phenotype for which the vancomycin MIC is in the intermediate range. First, a femC mutant had decreased amidation of the d-glutamate residue of the peptidoglycan stem peptide (23) and, thus, had a large peak area with a retention time identical to that of M9 that reflected the d-glutamate residue in position 2 of the stem peptide. This mutant had decreased levels of expression of glnA, the gene encoding glutamine synthetase, as a result of a polar transposon insertion in glnR, the glutamine synthetase repressor gene. However, this change alone was insufficient to produce the glycopeptide-intermediate phenotype in the femC mutant when it was tested in our laboratory (unpublished data). Second, as mentioned above, strain H1 from Japan, the isolate with one of the lowest M4/M9 ratios that we observed, had a very large M9 peak area and was susceptible to vancomycin.
What might be the implications of a glutamate-substituted stem peptide for susceptibility to vancomycin or teicoplanin? Glutamate-containing cell wall peptides have been shown to bind to vancomycin more avidly than isoglutamine-containing peptides (11). Additionally, such a substitution would likely lead to decreased cross-linking, perhaps by producing a substrate with lower affinity for one or more transpeptidase enzymes. In support of this we found a strong correlation between the M4/M9 ratio in the clinical GISA isolates that we studied and the degree of cross-linking. Moreover, decreased peptidoglycan cross-linking was also found in the vancomycin-susceptible femC mutant with an identical “substitution” of glutamate for glutamine in the stem peptide (22). Thus, a glutamate substitution at position 2 of the stem peptide, irrespective of the molecular determinants responsible for its occurrence, is correlated with a decrease in the level of cross-linking but not necessarily with an increase in the level of vancomycin resistance.
A decrease in the level of cross-linking would lead to an increase in the d-Ala–d-Ala content since the terminal d-Ala is cleaved during the transpeptidation step in peptidoglycan synthesis. Thus, some have proposed that nonproductive vancomycin binding to the preexisting bacterial cell wall may be one mechanism that decreases the peribacterial vancomycin pool available for antimicrobial effect (11, 19, 20). This model would not explain the resistance mechanisms for isolates like PC and IL, which were highly cross-linked.
The decrease in the magnitude of the area under the M9 peak and the inferred decrease in the glutamate content of the stem peptide suggest an association between these changes and a return to vancomycin susceptibility in GISA clinical isolates (2). One might also have anticipated a change in the MIC of oxacillin for isolates with this cell wall reorganization, but such a change did not occur. The change in the magnitude of the area under the M9 peak had no apparent effect on susceptibility to β-lactams; little change in the MIC of oxacillin occurred, despite a decrease in the MIC of vancomycin (2).
Thus, a simple “vancomycin-trapping” model seems unlikely to account for resistance in all clinical isolates obtained to date. As yet, no correlation has been demonstrated between the amount of vancomycin binding and the magnitude of resistance. Furthermore, our data indicate that a large area under the M9 peak is neither a sensitive nor a specific finding for the vancomycin resistance phenotype in the clinical GISA isolates studied to date. Other cell surface changes such as the increased cell wall thickness observed in both laboratory-derived and clinical isolates may impede vancomycin's access to its peptidoglycan precursor target.
Taken together, these data suggest that cell wall reorganization occurs commonly in GISA clinical isolates; the different patterns that we observed suggest that a variety of molecular mechanisms may be mediating or accompanying these effects. These observations are consistent with those observed for laboratory-derived vancomycin-resistant isolates, in which cell wall reorganization was common but for which no consistent pattern could be discerned (14).
ACKNOWLEDGMENTS
We are grateful to Keichii Hiramatsu for supplying GISA strains Mu50 and Mu3 and vancomycin-susceptible, MRSA isolate H1, to Fred Tenover for supplying isolates MI, NJ, and PC, to Roberta Carey for the IL isolates, and to Brigitte Berger-Bachi for providing the femC mutant strain. We are grateful to Vasanthi Pallinti and Monica Koplas for technical assistance with preliminary preparation of partially purified peptidoglycan cell wall digests, MIC determination, and population analyses and to Karsten Servan for technical assistance with the HPLC analysis.
This work was supported by grants RO1 AI 40481-01 BM and RO3 1 AI 44999-0 from the National Institute of Allergy and Infectious Diseases and a grant from the Grant Healthcare Foundation to R.S.D. and S.B.-V.
REFERENCES
- 1.Barna J C J, Williams D H. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol. 1984;38:339–357. doi: 10.1146/annurev.mi.38.100184.002011. [DOI] [PubMed] [Google Scholar]
- 2.Boyle-Vavra S, Berke S, Lee J C, Daum R S. Reversion of glycopeptide resistance phenotype in Staphylococcus aureus clinical isolates. Antimicrob Agents Chemother. 2000;44:272–277. doi: 10.1128/aac.44.2.272-277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—Illinois, 1999. Morb Mortal Wkly Rep. 2000;48:1165–1167. [PubMed] [Google Scholar]
- 4.CDC. Centers for Disease Control and Prevention. 1997. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb. Mortal. Wkly. Rep. 46:765–766. [PubMed]
- 5.Centers for Disease Control and Prevention. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, Sept. 1997. Morb Mortal Wkly Rep. 1997;46:813–815. [PubMed] [Google Scholar]
- 6.Daum R S, Gupta S, Sabbagh R, Milewski W M. Characterization of Staphylococcus aureus isolates with decreased susceptibility to vancomycin and teicoplanin: isolation and purification of a constitutively produced protein associated with decreased susceptibility. J Infect Dis. 1992;166:1066–1072. doi: 10.1093/infdis/166.5.1066. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge B, Chang Y, Gage D, Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain: the role of penicillin binding protein 2A. J Biol Chem. 1992;41:11248–11254. [PubMed] [Google Scholar]
- 8.de Jonge B L, Gage M, D, Handwerger S. Peptidoglycan composition of vancomycin resistant enterococci. Microb Drug Resist. 1996;2:225–229. doi: 10.1089/mdr.1996.2.225. [DOI] [PubMed] [Google Scholar]
- 9.Geisel R, Schmitz F, Thomas L, Berns G, Zetsche O, Ulrich B, Fluit A, Labischinsky H, Witte W. Emergence of heterogeneous intermediate vancomycin resistance in Staphylococcus aureus isolates in the Dusseldorf area. J Antimicrob Chemother. 1999;43:846–848. doi: 10.1093/jac/43.6.846. [DOI] [PubMed] [Google Scholar]
- 10.Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum R S, Labischinski H, Hiramatsu K. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother. 1998;42:199–209. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- 11.Hanaki H, Labischinski H, Inaba Y, Kondo N, Murakami H, Hiramatsu K. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J Antimicrob Chemother. 1998;42:315–320. doi: 10.1093/jac/42.3.315. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Moreira B, Boyle-Vavra S, De Jonge B L M, Daum R S. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1788–1793. doi: 10.1128/aac.41.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray B. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342:710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- 16.NCCLS. Performance standards for antimicrobial susceptibility testing: ninth informational supplement, M100–S9. Vol. 19. Wayne, Pa: NCCLS; 1999. [Google Scholar]
- 17.Roos M, Pittenauer E, Schmid E, Beyer M, Reinike B, Allmaier G, Labischinski H. Improved high-performance liquid chromatographic separation of peptidoglycan isolated from various Staphylococcus aureus strains for mass spectrometric characterization. J Chromatogr B Biomed Sci Appl. 1998;705:183–192. doi: 10.1016/s0378-4347(97)00506-9. [DOI] [PubMed] [Google Scholar]
- 18.Rotun S S, McMath V, Schoonmaker D J, Maupin P S, Tenover F C, Hill B C, Ackman D M. Staphylococcus aureus with reduced susceptibility to vancomycin isolated from a patient with fatal bacteremia. Emerg Infect Dis. 1999;5:147–149. doi: 10.3201/eid0501.990118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieradzki K, Roberts R B, Haber S W, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med. 1999;340:517–523. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]
- 20.Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith T L, Pearson M L, Wilcox K R, Cruz C, Lancaster M V, Robinson-Dunn B, Tenover F, Zervos M J, Band J D, White E, Jarvis W R. Emergence of vancomycin resistance in Staphylococcus aureus. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 22.Stranden A, Ehlert K, Labischinski H, Berger-Bachi B. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997;179:9–16. doi: 10.1128/jb.179.1.9-16.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stranden A, Roos M, Berger-Bachi B. Glutamine synthetase and heteroresistance in methicillin-resistant Staphylococcus aureus. Microb Drug Resist. 1996;2:201–207. doi: 10.1089/mdr.1996.2.201. [DOI] [PubMed] [Google Scholar]