Abstract
Rat lungworm (Angiostrongylus cantonensis) is a neurotropic nematode, and the leading cause of eosinophilic meningitis worldwide. The parasite is usually contracted through ingestion of infected gastropods, often hidden in raw or partially cooked produce. Pharmaceutical grade pyrantel pamoate was evaluated as a post-exposure prophylactic against A. cantonensis. Pyrantel pamoate is readily available over-the-counter in most pharmacies in the USA and possesses anthelmintic activity exclusive to the gastrointestinal tract (GIT). Administering pyrantel pamoate immediately after exposure should theoretically paralyze the larvae in the GIT, causing the larvae to be expelled via peristalsis without entering the systemic circulation. In this study, pyrantel pamoate (11 mg/kg) was orally administered to experimentally infected rats at 0, 2-, 4-, 6-, or 8-h post-infection. The rats were euthanized six weeks post-infection, and worm burden was evaluated from the heart-lung complex. This is the first in vivo study to evaluate its efficacy against A. cantonensis. This study demonstrates that pyrantel pamoate can significantly reduce worm burden by 53–72% (P = 0.004), and thus likely reduce the severity of infection that is known to be associated with worm burden. This paralyzing effect of pyrantel pamoate on the parasite may also be beneficial for delaying the establishment of infection until a more suitable anthelmintic such as albendazole is made available to the patient.
Keywords: Rat lungworm; Angiostrongylus cantonensis, prophylactic treatment; pyrantel pamoate
Graphical abstract
Highlights
-
•
Pyrantel pamoate (PP) shows significant in vivo efficacy against A. cantonensis.
-
•
Efficacy of PP was evaluated for the first time in an experimental animal model.
-
•
Administering PP between 4 and 8 h post-exposure significantly reduces worm burden.
-
•
PP is a new post-exposure prophylactic for rat lungworm disease.
1. Introduction
Angiostrongylus cantonensis is an obligate, digenetic, parasitic nematode and the causative agent for the clinical condition known as neuroangiostrongyliasis (rat lungworm disease) that often results in eosinophilic meningitis. The first human neuroangiostrongyliasis case was reported from Taiwan in 1945 (Nomura and Lin, 1945), and according to various reports, the parasite has been spreading throughout Asia, the Caribbean, Africa, South America, and the Pacific, including Hawaii (USA) (Wang et al., 2008; Jarvi et al., 2017). Recently A. cantonensis has also been detected in other states in the USA, including Florida, Louisiana, Texas, Oklahoma, Tennessee, and South Carolina (Kim et al., 2002; Stockdale Walden et al., 2017; Teem et al., 2013; Underwood et al., 2019). Additionally, autochthonous human cases have been reported from several states including Texas, and Tennessee (Flerlage et al., 2017; Foster et al., 2016), and also in Europe (Nguyen et al., 2017). The State of Hawaii is considered a hotspot for neuroangiostrongyliasis in the USA, with over 82 reported cases between 2007 and 2017 (Johnston et al., 2019). Infections are mainly caused by accidental ingestion of third stage larvae (L3) from infected mollusks, typically hidden in produce. The severity of the disease can range from mild symptoms to serious life-long neurological anomalies, paralysis, and even death (Wang et al., 2008). This dramatic range of symptoms is thought to be correlated with the number of parasites involved in the infection, with higher worm burdens causing more severe symptoms (Ji et al., 2017; Wang et al., 2008). Currently, in the USA, specific treatments are only initiated after diagnostic confirmation by detecting A. cantonensis DNA in the cerebrospinal fluid (Ansdell et al., 2021; Qvarnstrom et al., 2016).
Pyrantel pamoate is an FDA-approved, over-the-counter (OTC) anthelmintic that showed significant in vitro efficacy against A. cantonensis L3s in our previous studies (Jacob et al., 2021b). Pyrantel pamoate is commonly used for pinworms (Enterobius vermicularis) as well as for lungworms, filariae, and arthropods (Martin and Geary, 2016; Pickering et al., 2006). The significant activity shown by pyrantel pamoate against A. cantonensis in vitro, and its availability as a safe OTC drug with anthelmintic activity exclusive to the gastrointestinal tract (GIT) made its candidacy as a potential post-exposure prophylactic (Jacob et al., 2021b). In theory, administering pyrantel pamoate immediately after a known ingestion of rat lungworm is expected to paralyze the larvae in the GIT, allowing the larvae to be expelled via peristalsis without entry into the systemic circulation.
Based on the results from our previous in vitro study (Jacob et al., 2021b), Hilo Medical Center hospital, in Hawaii, has added pyrantel pamoate to their treatment guidelines as a prophylactic after a known exposure (Hilo Medical Center, 2020). According to these guidelines, the first-line response after a known exposure is to induce emesis as soon as possible, then obtain pyrantel pamoate from the nearest pharmacy and administer it according to the manufacturer's instructions, followed by care from the primary care provider. Other in vitro studies have also suggested pyrantel to be a good candidate by evaluating the motility patterns of adult worms, post-exposure (Mentz and Graeff-teixeira, 2003; Terada et al., 1983, 1982; Terada and Sano, 1986). Additionally, Lämmler and Weidner (1975), investigated the efficacy of pyrantel against A. cantonensis, albeit in its tartrate form, in multimammate rats. In their study, pyrantel tartrate (200 mg/kg) was administered from the fifth day post-infection for five consecutive days and showed 60.6% reduction of worm burden. Pyrantel tartrate is more hydrophilic than its pamoate form, allowing the pyrantel to be systemically absorbed. Hence, pyrantel tartrate would continue to exert its effects even after A. cantonensis larvae have entered the circulatory system (Martin and Geary, 2016). However, this superior systemic absorption of pyrantel tartrate is also associated with significant toxicity in humans (PubChem Pyrantel, 2021) and thus its use is limited to veterinary applications (Nielsen, 2015). Hence, formulations of pyrantel intended for human use attach a pamoate moiety, making the molecule highly lipophilic, thereby minimizing systemic absorption from the GIT and associated toxicity. This study is the first in vivo investigation of the efficacy of pyrantel pamoate against A. cantonensis.
2. Materials and methods
2.1. Animal care
Wistar rats (Rattus norvegicus) IGC outbred strain (Code 003) were purchased from Charles River Labs (Raleigh, NC, USA). All rats were immediately weighed upon arrival, individual animals were identified with a cage card with animal number IDs, and each was housed in a polycarbonate shoebox cage (21 × 47 × 26 cm) at the USDA APHIS Wildlife Services National Wildlife Research Center (NWRC) Hawaii Field Station, Hilo, HI, USA. Rats were between 8 and 9 weeks of age at the beginning of the study. Rats were maintained on Laboratory Rodent Diet 5001 (LabDiet, St. Louis, MO, USA) and provided both food and water ad libitum. Rats were allowed to acclimate for a minimum 7–10 days before initiating the study.
2.2. Study design
A total of 48 rats were used in this study, equally divided into 8 study groups with 6 rats in each group. The study groups consisted of: (1) untreated control (2) infected control; (3) pyrantel pamoate (uninfected) control; with pyrantel administration at different time points post-infection (PI): (4) 0-h (immediately after infection); (5) 2-h PI; (6) 4-h PI; (7) 6-h PI; and (8) 8-h PI. Each group consisted of an equal number of male and female rats.
2.3. Larval (L3) isolation
Semi-slugs (Parmarion martensi) were collected from the east region of Hawaii Island, and L3s were isolated as previously described Jarvi et al. (2019). Briefly, approximately 50 semi-slugs were individually drowned in 50 ml Falcon tubes filled with tap water for 72 h, after which slugs were removed, and the bottom 10 ml of water (containing larvae) from each of the tubes was collected into separate culture plates (100 mm × 15 mm). Larvae were isolated under a dissecting microscope (Leica S9 D and Wild Heerbrugg M4A APO) and pooled into a single separate culture plate containing dH2O, using a 20 μl micropipette. These larvae (n = 50) were then handpicked and transferred into separate 1.5 ml Eppendorf tubes containing 500 μl of dH2O (n = 36 tubes), which was later used for gavage. All animal procedures were conducted in accordance with the approved protocols (see ethical standards).
2.4. Experimental infection
Rats in the infected control group and the hourly treatment groups (n = 36 rats) were gavaged with 50 larvae each, in 500 μl of dH2O, into the distal esophagus, using sterile flexible plastic gavage tubes (Instech Laboratories, Inc., Plymouth Meeting, PA, USA) and 1 ml sterile tuberculin syringes (Norm-ject, Tuttlingen, Germany). Similarly, rats in the untreated control and pyrantel control groups (n = 12) were gavaged with 500 μl of dH2O without larvae. All rats were sedated prior to the gavage with a mixture of isoflurane – oxygen gas using a Tec III 300P vaporizer (Vaporizer Sales & Services, Rockmart GA, USA) placed within a DWS36-A ductless fume hood (Air Science DWS Downflow Workstation, Fort Myers, FL, USA).
2.5. Pyrantel treatment
Pharmaceutical grade pyrantel pamoate (Reese's pinworm medicine, Cleveland, OH, USA) was used in this study. The typical dosage of pyrantel pamoate for humans is 11 mg/kg, with dosing determined by the amount of pyrantel base (Papich, 2016). Pharmaceutical grade pyrantel pamoate contains 50 mg/ml pyrantel base, a high dose to be directly used in rats due to their smaller body weight. Therefore, we reformulated the pharmaceutical product to contain the required dose of pyrantel (11 mg/kg) in a volume of 500 μl dH2O for gastric gavaging in rats. The average body weight of male and female rats was calculated, and 11 mg/kg unit doses were formulated accordingly for both sexes, using dH2O, making the final volume of 500 μl per 1.5 ml Eppendorf tube.
All the rats in the pyrantel control group and the hourly treatment groups (n = 36 rats) were gavaged with this pyrantel formulation based on the weight of the rats. For the hourly treatment groups, pyrantel formulation was gavaged according to the respective treatment group, that is, 0, 2-, 4-, 6- and 8-h PI. Similarly, rats from the untreated and infection control groups (n = 12) were gavaged with 500 μl of dH2O. The pyrantel formulation was also gavaged in the same manner as the larvae, as described above. All rats were sedated prior to gavaging.
2.6. Necropsy
Rats were held for 6 weeks post-infection and humanely euthanized in a CO2 chamber. The heart-lung complex was dissected and placed into individual culture plates and was examined using fine-tip forceps under a Leica 10–40X dissecting microscope for the presence of adult worms. The number of worms recovered from each rat was recorded. One worm from each rat was used for the confirmation of A. cantonensis using PCR (Sears et al., 2021).
2.7. Statistical analysis
After testing for heteroscedasticity, one-way ANOVA was used to evaluate the number of worms extracted post-infection across study groups. The worm burden after no drug treatment and pyrantel pamoate treatment (all hourly treatment groups combined) was analyzed using orthogonal contrasts. Tukey's test was used to determine the significance of worms recovered over time. A two-way ANOVA was used to examine the effect of sex of the rat on the number of worms extracted after hourly treatments. All statistical analyses were performed using Minitab (Minitab, LLC., State College, PA) software, version 18.0. Level of significance for all analyses was set at an alpha equal to 0.05.
3. Results
3.1. Worm recovery
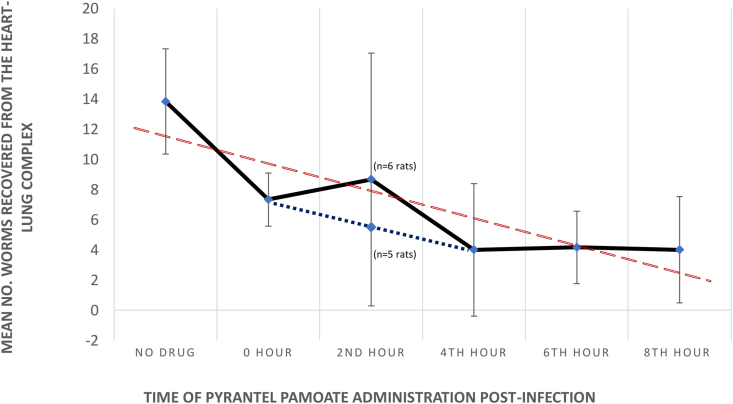
A total of 252 adult worms were recovered in this study and were genetically identified as A. cantonensis using PCR (Sears et al., 2021). The mean of the number of worms recovered from the no drug control group and each hourly treatment group, along with their standard deviations (SD) were used to plot a time-response curve (Fig. 1). The red dotted trend line represents the time-dependent, post-exposure prophylactic activity of pyrantel pamoate against A. cantonensis (P = 0.004). There was a significant difference in the overall mean number of worms recovered between the no drug control group (13.83 ± 3.49 SD) and all the pyrantel pamoate hourly treatment groups (5.63 ± 4.81 SD) (P = 0.0004), with a reduction of 53% in the number of worms recovered from 0-h treatment group as compared to the infected control group, where the drug was administered immediately after gavaging the infective larvae.
Fig. 1.
Time-response curve representing post-exposure prophylactic activity of pyrantel pamoate against A. cantonensis. The red trend line represents the time-dependent activity of pyrantel pamoate. In the 2-h treatment, the mean number of worms recovered is calculated inclusive of the outliers with 24 worms (n = 6), as well as excluding this outlier (n = 5) with the change in the time-response curve represented by the dotted blue line.
** Statistical significance as compared to the (no drug) controls (P < 0.005). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The mean number of worms recovered from the heart-lung complex from each of the treatment group post-euthanasia, standard deviations and their ranges within each group are shown in Table 1. In the 2-h treatment group, the range for most of the rats (n = 5) was 2–12 worms, however, there was a single outlier with 24 worms recovered, resulting in a large standard deviation (Table 1, Fig. 1). The dotted blue line in Fig. 1 represents the mean worm recovery excluding the outlier with 24 worms (n = 5 rats). The means and ranges for the 4-, 6-, and 8-h groups were relatively consistent with means of 4.0, 4.17, and 4.0 and ranges of 0–10, 2–8, and 1–10 worms recovered, respectively. Thus, pyrantel pamoate was most effective when administered at 4, 6 and 8-h post-infection (P=0.009, P=0.010, and P = 0.009, respectively). There was no significance correlation between the sex of the rat and worm recovery (P = 0.72).
Table 1.
Summary of worms recovered from heart-lung complex of experimentally infected rats. Groups include a no-drug control, and treatments with pyrantel pamoate (11 mg/kg) at 0 h (immediate), 2-h, 4-h, 6-h, and 8-h post-L3 exposure.
| Study group | Mean number of worms recovered | Standard deviations | Range of worms recovered |
|---|---|---|---|
| No drug (n=6) | 13.83 | 3.49 | 9–19 |
| 0-h treatment (n=6) | 7.33 | 1.75 | 5–10 |
| 2-h treatment (n=6) | 8.67 | 8.38 | 2–24 |
| 2-h treatment (n=5)a | 5.6 | 4.15 | 2–12 |
| 4-h treatment (n=6) | 4.0 | 4.38 | 0–10 |
| 6-h treatment (n=6) | 4.17 | 2.4 | 2–8 |
| 8-h treatment (n=6) | 4.0 | 3.52 | 1–10 |
Excluding the outlier with 24 worms.
4. Discussion
The World Health Organization's list of essential medicines consists of those medications considered to be most effective and safe to meet the most important needs in a health system (World Health Organization, 2021). Pyrantel pamoate has been included in this list as an anthelmintic since 1983. In the US, pyrantel pamoate suspension formulations are available (OTC) in most pharmacies. Pyrantel is a cholinergic agonist, which binds to the nicotinic receptors at the neuromuscular junction (NMJ). Such binding initiates the influx of cations, causing depolarization of muscles, ultimately resulting in the spastic paralysis of parasitic nematodes (Martin and Geary, 2016). Although pyrantel does not directly kill the intestinal parasites, due to the induced paralysis they are anticipated to then be expelled out of the body via peristalsis (Saari et al., 2019).
Pyrantel pamoate's potential as an early intervention complements the use of albendazole, currently considered as the most suitable anthelmintic for the management of neuroangiostrongyliasis. Albendazole has a broad spectrum of nematocidal activity, and it is the only approved nematicide capable of crossing the blood-brain barrier (Jacob et al., 2021a; Ramírez et al., 2001). Nonetheless, it has some significant disadvantages. Albendazole is one of the most expensive drugs on the US market and requires a clinical prescription (Shahriar and Alpern, 2020). As previously discussed, in the US treatments specific to neuroangiostrongyliasis are initiated only after diagnostic confirmation, even for episodes of known exposure (Ansdell et al., 2021; Qvarnstrom et al., 2016). During this time, the parasite will likely have already migrated to the brain and caused neurological damage. Thus, there is a great need for an affordable and more immediately available anthelmintic to prevent or minimize the severity of infection.
This study shows that pyrantel pamoate can significantly reduce worm burden if administered early after exposure (P = 0.0004). The severity of symptoms is thought to be correlated with the number of parasites involved in the infection (Ji et al., 2017; Wang et al., 2008). The ability of pyrantel pamoate to reduce the number of worms involved in the infection, particularly if administered hours after a known exposure, has the potential to reduce the severity of symptoms. However, unlike intestinal nematodes for which pyrantel pamoate is intended, A. cantonensis larvae will attempt to enter the systemic circulation hours after ingestion. According to Mackerras and Sandars (1955), A. cantonensis larvae were detected in the blood by the fourth hour post-infection in experimentally infected rats. Therefore, the timing of pyrantel pamoate administration is very critical in the case of A. cantonensis exposure. However, in humans, for larvae to enter the bloodstream may be delayed considering the greater volume and associated surface area of the human GIT, as compared with the rat GIT.
Angiostrongylus costaricensis is also a digenetic parasitic nematode with a lifecycle very similar to that of A. cantonensis. However, A. costaricensis is not a neurotropic parasite but an intestinal parasite and the causative agent of the clinical condition known as abdominal angiostrongyliasis. Although A. costaricensis is a different species, pyrantel pamoate may also be beneficial for the management of abdominal angiostrongyliasis. Previous in vitro studies that investigated pyrantel's efficacy against A. costaricensis based on motility and egg development have reported it to be effective (Ishih et al., 2001; Mentz and Graeff-teixeira, 2003; Terada et al., 1986).
Our study shows that post-exposure administration of pyrantel pamoate significantly reduces A. cantonensis worm burden, however, our results seem counter-intuitive with a longer delay in the administration of pyrantel pamoate resulting in a greater reduction in worm burden (i.e., 0- and 2-h post-exposure vs. 4-, 6- and 8-h post-exposure). To explain such results, it must be reiterated that pyrantel pamoate will only impart its anthelmintic activity while remaining in contact with A. cantonensis larvae (Jacob et al., 2021b). Thus, we believe the trendline in Fig. 1 illustrates the effects of various GI events on the duration of pyrantel pamoate retention within the GIT and the reduction in worm burden as a result. Several factors could affect the duration of contact between pyrantel pamoate and A. cantonensis larvae, such as food/liquid contents and the co-location of the larvae in the GIT at the time of pyrantel administration. Additionally, the viscosity of pyrantel preparation, gastric emptying time, and peristalsis are also factors that may influence the duration of contact between the larvae and pyrantel pamoate.
In this study, rats were neither deprived of food or water prior to the administration of pyrantel pamoate, as to mimic the real-life scenario of accidental exposure. The rats involved in this study were continuously provided with food and water ad libitum. The amount of food could delay or even prevent the uniform distribution of pyrantel within the stomach, allowing a few A. cantonensis larvae to migrate into the circulation. Similarly, large volumes of liquid in the stomach could dilute pyrantel, retarding its anthelmintic activity or making it ineffective. These factors may offer an explanation for the variation in the number of worms recovered from individual rats within the same treatment group, such as the outlier in the 2-h treatment, resulting in large standard deviations (Table 1, Fig. 1).
Perhaps most critically, the gastric emptying time for solid meals in rats is approximately 1 h (Purdon and Bass, 1973) while that for liquids is more immediate (Bennink et al., 2003). When administering pharmaceutical grade pyrantel pamoate, its high viscosity (due to solvents used such as glycerin), results in having an extended duration of contact with the larvae in the stomach. However, in this study, the necessary reformulation of pyrantel pamoate using dH2O considerably reduced its viscosity. Considering this, it is possible that during the more immediate post-exposure hours of treatment (0–2 h), the reformulated pyrantel may have been more rapidly flushed down into the smaller intestine, with a minimum duration of contact with the larvae situated in the stomach, where the majority would be expected to be at this point in time.
However, by the 4th-hour post-exposure, though few larvae might have already made entry into the circulation (Mackerras and Sandars, 1955) (the plateau with an average of 4 worms seen from the 4th-hour treatment in Fig. 1, Table 1), the majority would have moved to the small intestine along with the food contents. The less viscous pyrantel pamoate administered during this 4th hour period is likely to have rapidly passed into the small intestine, putting the majority of the larvae in contact with the drug. Furthermore, peristaltic motion should evenly distribute pyrantel pamoate within the intestine, providing more chance of contact with these larvae for an extended duration. However, this may not be the case when the original (undiluted) pharmaceutical grade pyrantel pamoate is used. Due to its high viscosity, it is expected that the retention time in the stomach and the duration of contact with larvae will both be greater during the early hours post-ingestion, providing minimal opportunities for these larvae to make entry into the circulation.
This study clearly demonstrates a significant reduction in the number of adult worms recovered after treatment with pyrantel pamoate in rats. However, additional studies are required to establish clinical relevance for this post-exposure prophylactic in humans.
5. Conclusion
Though the administration of pyrantel pamoate significantly reduced the number of A. cantonensis, our results made it clear that further investigation into the timing of administration of pyrantel pamoate is needed, given the limited period for the ingested larvae and pyrantel pamoate to co-locate within the same GI compartment. The post-exposure prophylactic efficacy of pyrantel pamoate when reformulated in a more viscous solvent such as glycerin or liquid paraffin may also impact results. Our research clearly shows that post-exposure administration of pyrantel pamoate can reduce the worm burden under this condition, with a potential reduction in severity of infection. Furthermore, the paralyzing effects of OTC pyrantel pamoate may also delay the establishment of infection until a more suitable anthelmintic such as albendazole is made available to the patient.
Ethical standards
All animal procedures were conducted according to the Guidelines of the American Society of Mammologists for the use of mammals in research (Sikes and Gannon, 2011) and following approved University of Hawaii Institutional Animal Care and Use Committee (21–3576) protocol and IACUC protocols USDA NWRC QA-3346. Any use of trade, firm, or product names is for descriptive purpose only and does not imply endorsement by the U.S. Government. The findings and conclusions in this publication have not been formally disseminated by the U.S. Department of Agriculture and should not be construed to represent any agency determination or policy.
Funding
This work was supported by the Hawaii State Legislature, The Daniel K. Inouye College of Pharmacy. This research was also supported in part by the U.S. Department of Agriculture, National Wildlife Research Center, and the Daniel K. Inouye U.S. Pacific Basin Agriculture Research Service.
Acknowledgments
The authors thank Michael McBride, DVM, Supervisory Veterinary Medical Officer at National Wildlife Research Center, APHIS Wildlife Services, and Alfred J. Mina, DVM, Maikai Veterinarian Clinic, Hilo, Hawaii, for veterinary consultation and support. The United States Department of Agriculture is an equal opportunity provider and employer.
References
- Ansdell V., Kramer K.J., McMillan J.K., Gosnell W.L., Murphy G.S., Meyer B.C., Blalock E.U., Yates J., Lteif L., Smith O.A., Melish M. Guidelines for the diagnosis and treatment of neuroangiostrongyliasis: updated recommendations. Parasitology. 2021;148:227–233. doi: 10.1017/S0031182020001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennink R.J., de Jonge W.J., Symonds E.L., van den Wijngaard R.M., Spijkerboer A.L., Benninga M.A., Boeckxstaens G.E. Validation of gastric-emptying scintigraphy of solids and liquids in mice using dedicated animal pinhole scintigraphy. J. Nucl. Med. 2003;44:1099. [PubMed] [Google Scholar]
- Flerlage T., Qvarnstrom Y., Noh J., Devincenzo J., Madni A., Bagga B., Hysmith N. Angiostrongylus cantonensis eosinophilic meningitis in an infant, Tennessee, USA. Emerg. Infect. Dis. 2017;23:1756–1757. doi: 10.1007/978-3-642-85397-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C.E., Nicholson E.G., Chun A.C., Gharfeh M., Anvari S., Seeborg F.O., Lopez M.A., Campbell J.R., Marquez L., Starke J.R., Palazzi D.L. Angiostrongylus cantonensis infection: a cause of fever of unknown origin in pediatric patients. Clin. Infect. Dis. 2016;63:1475–1478. doi: 10.1093/cid/ciw606. [DOI] [PubMed] [Google Scholar]
- Hilo Medical Center Treatment protocol for rat lungworm, angiostrongyliasis. 2020. https://www.hilomedicalcenter.org/wp-content/uploads/2020/02/Rat-Lungworm-for-ED-Doctors-2020.pdf 10.10.21.
- Ishih A., Yanoh M., Ikeya C., Ban A., Terada M. Effects of anthelmintics on the development of eggs of Angiostrongylus costaricensis in vitro. J. Helminthol. 2001;75:351–354. [PubMed] [Google Scholar]
- Jacob J., Steel A., Lin Z., Berger F., Zöeller K., Jarvi S. Clinical efficacy and safety of albendazole and other benzimidazole anthelmintics for rat lungworm disease (neuroangiostrongyliasis): a systematic analysis of clinical reports and animal studies. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Tan G., Lange I., Saeed H., Date A., Jarvi S. In vitro efficacy of anthelmintics on Angiostrongylus cantonensis L3 larvae. Parasitology. 2021;148:240–250. doi: 10.1017/S0031182020001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvi S.I., Jacob J., Sugihara R.T., Leinbach I.L., Klasner I.H., Kaluna L.M., Snook K.A., Howe M.K., Jacquier S.H., Lange I., Atkinson A.L., Deane A.R., Niebuhr C.N., Siers S.R. Validation of a death assay for Angiostrongylus cantonensis larvae (L3) using propidium iodide in a rat model (Rattus norvegicus) Parasitology. 2019;146:1421–1428. doi: 10.1017/S0031182019000908. [DOI] [PubMed] [Google Scholar]
- Jarvi S.I., Quarta S., Jacquier S., Howe K., Bicakci D., Dasalla C., Lovesy N., Snook K., McHugh R., Niebuhr C.N. High prevalence of Angiostrongylus cantonensis (rat lungworm) on eastern Hawai'i Island: a closer look at life cycle traits and patterns of infection in wild rats (Rattus spp.) PLoS One. 2017;12 doi: 10.1371/journal.pone.0189458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L., Yiyue X., Xujin H., Minghui Z., Mengying Z., Yue H., Yanqi W., Langui S., Xin Z., Datao L., Shuo W., Huanqin Z., Zhongdao W., Zhiyue L. Study on the tolerance and adaptation of rats to Angiostrongylus cantonensis infection. Parasitol. Res. 2017;116:1937–1945. doi: 10.1007/s00436-017-5472-4. [DOI] [PubMed] [Google Scholar]
- Johnston D.I., Dixon M.C., Elm J.L., Calimlim P.S., Sciulli R.H., Park S.Y. Review of cases of angiostrongyliasis in Hawaii, 2007-2017. Am. J. Trop. Med. Hyg. 2019;101:608–616. doi: 10.4269/ajtmh.19-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.Y., Stewart T.B., Bauer R.W., Mitchell M. Parastrongylus (Angiostrongylus) cantonensis now endemic in Louisiana wildlife. J. Parasitol. 2002;88:1024–1026. doi: 10.1645/0022-3395(2002)088[1024:PACNEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lämmler V.G., Weidner E. Zur larviziden wirkung von anthelminthika gegen Angiostrongylus cantonensis. Berl. Münchener Tierärztliche Wochenschr. 1975;88:152–156. [PubMed] [Google Scholar]
- Mackerras M.J., Sandars D.F. The life history of the rat lung-worm, Angiostrongylus cantonensis (Chen) (Nematoda: metastrongylidae) Aust. J. Zool. 1955;3:1–21. [Google Scholar]
- Martin R., Geary T. In: Pyrantel Parasiticide Therapy in Humans and Domestic Animals. Marchiondo A., editor. Academic Press; Massachusetts, USA: 2016. Pharmacology of pyrantel; pp. 21–45. [Google Scholar]
- Mentz B.M., Graeff-teixeira C. Review drug trials for treatment of human angiostrongyliasis. Rev. do Inst. Med. Trop. São Paulo. 2003;45:179–184. doi: 10.1590/s0036-46652003000400001. [DOI] [PubMed] [Google Scholar]
- Nguyen Y., Rossi B., Argy N., Baker C., Nickel B., Marti H., Zarrouk V., Houzé S., Fantin B., Lefort A. Autochthonous case of eosinophilic meningitis caused by Angiostrongylus cantonensis, France, 2016. Emerg. Infect. Dis. 2017;23:1045–1046. doi: 10.3201/eid2306.161999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.K. In: Robinson's Current Therapy in Equine Medicine. seventh ed. Sprayberry K.A., Robinson N.E., editors. W.B. Saunders; St. Louis: 2015. Internal parasite screening and control; pp. 336–340. [DOI] [Google Scholar]
- Nomura S., Lin H. First clinical case of Haemostrongylus ratti. Taiwan no ikai. 1945;3:589–592. [Google Scholar]
- Papich M.G. In: Saunders Handbook of Veterinary Drugs. fourth ed. Papich M.G., editor. W.B. Saunders; St. Louis: 2016. Pyrantel pamoate, pyrantel tartrate; pp. 693–694. [DOI] [Google Scholar]
- Pickering L., Baker C., Long S., McMillan J. In: Red Book: Report of the Committee on Infectious Diseases. Pickering L.K., Baker C.J., Long S.S., McMillan J.A., editors. American academy of pediatrics; Illinois, USA: 2006. American academy of pediatrics. pinworm infection; pp. 520–522. [Google Scholar]
- Pyrantel PubChem. National center for biotechnology information. Compound summary for CID 708857, pyrantel. 2021. https://pubchem.ncbi.nlm.nih.gov/compound/Pyrantel 10.10.21.
- Purdon R.A., Bass P. Gastric and intestinal transit in rats measured by a radioactive test meal. Gastroenterology. 1973;64:968–976. doi: 10.1016/S0016-5085(73)80009-5. [DOI] [PubMed] [Google Scholar]
- Qvarnstrom Y., Xayavong M., da Silva A.C.A., Park S.Y., Whelen A.C., Calimlim P.S., Sciulli R.H., Honda S.A.A., Higa K., Kitsutani P., Chea N., Heng S., Johnson S., Graeff-Teixeira C., Fox L.M., da Silva A.J. Real-time polymerase chain reaction detection of Angiostrongylus cantonensis DNA in cerebrospinal fluid from patients with eosinophilic meningitis. Am. J. Trop. Med. Hyg. 2016;94:176–181. doi: 10.4269/ajtmh.15-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez T., Benítez-Bribiesca L., Ostrosky-Wegman P., Herrera L.A. In vitro effects of albendazole and its metabolites on the cell proliferation kinetics and micronuclei frequency of stimulated human lymphocytes. Arch. Med. Res. 2001;32:119–122. doi: 10.1016/s0188-4409(01)00259-4. [DOI] [PubMed] [Google Scholar]
- Saari S., Näreaho A., Nikander S. In: Canine Parasites and Parasitic Disease. Saari S., Näreaho A., Nikander S., editors. Academic Press; Massachusetts, USA: 2019. Therapy and control; pp. 247–254. [Google Scholar]
- Sears W.J., Qvarnstrom Y., Dahlstrom E., Snook K., Kaluna L., Baláž V., Feckova B., Šlapeta J., Modry D., Jarvi S., Nutman T.B. AcanR3990 qPCR: a novel, highly sensitive, bioinformatically-informed assay to detect Angiostrongylus cantonensis infections. Clin. Infect. Dis. 2021;73:e1594–e1600. doi: 10.1093/cid/ciaa1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahriar A.A., Alpern J.D. Antiparasitic drugs in the United States—two roads to high prices. Frontier. Sociol. 2020;5:1–5. doi: 10.3389/fsoc.2020.540478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes R.S., Gannon W.L. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011;92:235–253. doi: 10.1644/10-MAMM-F-355.1. The animal care and use committee of the American society of Mammologists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale Walden H.D., Slapcinsky J.D., Roff S., Mendieta Calle J., Diaz Goodwin Z., Stern J., Corlett R., Conway J., McIntosh A. Geographic distribution of Angiostrongylus cantonensis in wild rats (Rattus rattus) and terrestrial snails in Florida, USA. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teem J.L., Qvarnstrom Y., Bishop H.S., da Silva A.J., Carter J., White-McLean J., Smith T. The occurrence of the rat lungworm, Angiostrongylus cantonensis, in nonindigenous snails in the Gulf of Mexico region of the United States. Hawai‘i J. Med. Public Health. 2013;72:11–14. [PMC free article] [PubMed] [Google Scholar]
- Terada M., Ishii A., Kino H., Sano M. Studies on chemotherapy of parasitic helminths (XVIII). Mechanism of spastically paralyzing action of pyrantel in Angiostrongylus cantonensis. Experientia. 1983;39:1383–1385. doi: 10.1007/BF01990115. [DOI] [PubMed] [Google Scholar]
- Terada M., Ishii A.I., Kino H., Sano M. Studies on chemotherapy of parasitic helminths (VII). Effects of various cholinergic agents on the motility of Angiostrongylus cantonensis. Jpn. J. Pharmacol. 1982;32:633–642. doi: 10.1254/jjp.32.633. [DOI] [PubMed] [Google Scholar]
- Terada M., Rodriguez B.O., Dharejo A.M., Ishii A.I., Sano M. Studies on chemotherapy of parasitic helminths (XXVI). Comparative in vitro effects of various anthelmintics on the motility of Angiostrongylus costaricensis and A. cantonensis. Jpn. J. Parasitol. 1986;35:365–367. [Google Scholar]
- Terada M., Sano M. Effects of diethylcarbamazine on the motility of Angiostrongylus cantonensis and Dirofilaria immitis. Z. für Parasitenkd. 1986;72:375–385. doi: 10.1007/BF00928748. [DOI] [PubMed] [Google Scholar]
- Underwood E.B., Walker M.J., Darden T.L., Kingsley-Smith P.R. Frequency of occurrence of the rat lungworm parasite in the invasive island apple snail in South Carolina, USA. J. Aquat. Anim. Health. 2019;31:168–172. doi: 10.1002/aah.10063. [DOI] [PubMed] [Google Scholar]
- Wang Q.P., Lai D.H., Zhu X.Q., Chen X.G., Lun Z.R. Human angiostrongyliasis. Lancet Infect. Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization List of essential medicines. 2021. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 twenty-second ed. 10.10.21.