Abstract
Gatifloxacin (GTX), a new fluoroquinolone with extended antibacterial activity, is an interesting candidate for the treatment of chronic bacterial prostatitis (CBP). Besides the antibacterial spectrum, the concentrations in the target tissues and fluids are crucial for the treatment of CBP. Thus, it was of interest to investigate its penetration into prostatic and seminal fluid. GTX concentrations in plasma, urine, ejaculate, prostatic and seminal fluid, and sperm cells were determined by a high-performance liquid chromatography method after oral intake of a single 400-mg dose in 10 male Caucasian volunteers in the fasting state. Simultaneous application of the renal contrast agent iohexol was used to estimate the maximal possible contamination of ejaculate and prostatic and seminal fluid by urine. GTX was well tolerated. The means (standard deviations) for the following parameters were as indicated: time to maximum concentration of drug in serum, 1.66 (0.91) h; maximum concentration of drug in serum, 2.90 (0.39) μg/ml; area under the concentration-time curve from 0 to 24 h, 25.65 μg · h/ml; and half life, 7.2 (0.90) h. Within 12 h about 50% of the drug was excreted unchanged into the urine. The mean renal clearance was 169 ml/min. The gatifloxacin concentrations in ejaculate, seminal fluid, and prostatic fluid were in the range of the corresponding plasma concentrations which were 1.92 (0.27) μg/ml at approximately the same time point (4 h after drug intake). The concentrations in sperm cells (0.195, 0.076, and 0.011 μg/ml) could be determined in three subjects. The good penetration into prostatic and seminal fluid, the good tolerance, and the previously reported broad antibacterial spectrum suggest that GTX may be a good alternative for the treatment of chronic bacterial prostatitis. Clinical studies should be performed to confirm this assumption.
Fluoroquinolones have already been used successfully in the treatment of chronic bacterial prostatitis (CBP) and are recommended as first-line treatment for this indication (1, 6). This recommendation is based on their antibacterial activity; on their ability to penetrate into prostatic tissue, prostatic fluid, seminal fluid, and ejaculate; and on clinical studies (6). Although in about 60% of patients with symptoms of chronic prostatitis significant prostatic inflammation can be demonstrated (4), an etiologically recognized pathogen, such as Escherichia coli, Klebsiella spp., Proteus spp., Enterococcus faecalis, or Pseudomonas aeruginosa, is only isolated in up to 10% of these patients (14). In the vast majority of patients, bacterial evaluation either fails to identify a pathogen (nonbacterial prostatitis), or identifies so-called atypical bacteria, like Mycoplasma spp., Ureaplasma spp., and Chlamydia spp. These atypical pathogens are, however, not well covered by the antibacterial activity of the classical fluoroquinolones, e.g., ciprofloxacin or ofloxacin. Thus, the treatment of CBP remains a challenging issue, and new fluoroquinolones with improved antibacterial activity also against gram-positive pathogens and Mycoplasma and Chlamydia species, as well as against anaerobes, may be considered for the treatment of CBP.
Gatifloxacin, a new fluoroquinolone antibiotic, has a broad spectrum of activity encompassing both gram-positive and gram-negative organisms, as well as anaerobes (2). It also has activity against Mycoplasma and Chlamydia spp. (5). Since the antibacterial spectrum and the concentrations in the target tissues are crucial for the treatment of CBP, it was of interest to investigate its penetration into prostatic and seminal fluid. The results of this study could serve as a basis for a clinical study protocol (dosage selection and estimate of clinical and bacteriological efficacy) to test gatifloxacin in the treatment of CBP and vesiculitis.
(This work was presented in part at the 21st International Congress of Chemotherapy, Birmingham, United Kingdom, 4 to 9 July 1999.)
MATERIALS AND METHODS
Study design.
This was a single-dose, one-way, open-labeled, noncontrolled, single-center, phase I study. The study was approved by the institutional and local ethics committees, and written informed consent was obtained from each volunteer.
Study subjects.
Ten male Caucasian volunteers, 18 to 33 years old (mean age, 23 years) with a body weight ranging from 63 to 97 kg (mean, 77 kg) and a body height ranging from 172 to 190 cm (mean, 180 cm) were included. The subjects were considered healthy according to history, physical examination, electrocardiogram, and standard laboratory tests, including hepatitis virus and human immunodeficiency virus screen. Prior to administration of the study drug, and 24 h after dosing, routine hematology, urine, biochemistry, and electrocardiogram analyses were repeated. Vital signs (blood pressure, pulse rate, and oral temperature) were assessed, and each subject underwent a full physical examination.
The subjects had no known or suspected intolerance or hypersensitivity to quinolones or related drugs and no evidence or history of psychiatric illness, suicide risk, epilepsy, or alcohol or drug abuse. Before drug intake drug screening of urine for benzodiazepine, opiate, amphetamine, and cannabis was performed by a rapid enzyme immune assay (Boehringer, Mannheim, Germany) in the Laboratory Schubach, Passau, Germany. Blood and breath alcohol tests were negative for all subjects before drug intake.
The subjects did not use any medication in the 2 weeks prior to the study, any enzyme-inducing or -inhibiting drug during 2 months prior to the study, or any experimental drugs during 3 months prior to the study start and were not likely to require any medication during the study period, except one subject who used a local antiviral cream (aciclovir [Zovirax]) 1 day prior to study for the treatment of herpes labialis. Six of the subjects reported current tobacco use, while four reported that they had never used tobacco. Five subjects reported regular use of alcohol, while five reported that they did not regularly consume alcohol (i.e., consumed fewer than 3 to 7 pints of beer per week).
Study procedure.
No recreational drugs or alcohol consumption were allowed during the course of the study, 24 h before drug administration to 48 h postdose. Subjects were not allowed to consume antacids or other drugs containing zinc, magnesium, or calcium within 6 h of gatifloxacin ingestion. All subjects complied with this.
After an overnight fast, baseline urine and blood samples were taken. Subsequently the subjects received one tablet of 400 mg of gatifloxacin (product no. CG5501; batch no. 0112097; expiration date July 2000) provided by Grünenthal GmbH, Stolberg, Germany. The tablet was taken by the oral route under medical supervision with 200 ml of mineral water, including mouth check to assure swallowing. At the time of oral study drug administration, subjects also received 5 ml of a single intravenous dose of the renal contrast medium iohexol (Omnipaque-300; Schering, Berlin, Germany), corresponding to 3,235 g of iohexol (1.5 g of iodine). Standard breakfast and lunch were served 3 and 5 h after drug administration, respectively. Supper was ad libitum.
Blood samples were taken immediately prior to and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, and 24 h after drug administration into heparinized tubes. Prostatic fluid was obtained by prostatic massage within 5 days prior to (baseline) and 4 h after study drug administration. Subsequently, at each occasion of prostatic fluid collection ejaculate was obtained by masturbation. Ejaculate samples were divided: one aliquot was taken without further treatment for the measurement of gatifloxacin concentration in ejaculate and the other aliquot was used to measure the concentration of gatifloxacin in seminal fluid and sperm cells following the separation of seminal fluid from the sperm cells as follows. The ejaculate (50 μl) was pipetted on a silicone layer (100 μl) and centrifuged using a Microfuge (Beckman, Munich, Germany) at 10,000 rpm for approximately 1 min. The lower aqueous layer was 20% perchloric acid. Subsequent high-performance liquid chromatography (HPLC) analysis was performed on the perchloric acid layer as described for ejaculate.
After prostatic fluid and ejaculate were obtained, the subjects were allowed to empty their bladders for the first time after drug administration, and the urine (0- to 4-h period) was collected. In addition to the 4-h sample, urine samples of the 4- to 8-h and 8- to 12-h periods were also collected from all subjects.
Safety.
All subjects who received a dose of gatifloxacin were included in the evaluation of safety, which included review of treatment-emergent, clinical adverse events (AEs) and laboratory AEs.
Analytical methods.
Plasma and urine samples for gatifloxacin analysis were measured at CEPHAC, Saint Benoit Cedex, France. Prostatic fluid, ejaculate, cell-free seminal fluid, and sperm cell samples were analyzed for gatifloxacin at IBMP, Nürnberg-Heroldsberg, Germany. Iohexol concentrations in urine, ejaculate, and seminal and prostatic fluid were also determined at IBMP.
Assay of gatifloxacin in plasma and urine.
Gatifloxacin concentrations in plasma were assayed at CEPHAC by a validated HPLC method, with fluorimetric detection. Gatifloxacin was extracted by using a liquid-liquid extraction method at pH 7.0 followed by a back extraction with 1 N hydrochloric acid. The method used has previously been implemented and validated for plasma and urine samples at CEPHAC and was reported elsewhere (Grünenthal internal report FE-PK 479). Gatifloxacin in urine was analyzed using the same procedure as that for the determination of gatifloxacin in plasma. The limit of quantification was 0.01 μg/ml in plasma and 0.10 μg/ml in urine. The resulting coefficients from linear regressions in plasma and urine were at least 0.998 and 0.999, respectively. The quality control (QC) samples were measured and treated in the same manner as subjects' samples. The interday precision of QC samples of gatifloxacin in plasma and that in urine were ≤3.4%, with an interday accuracy of ±8.1% over the whole concentration range.
Assay of gatifloxacin in prostatic fluid, ejaculate, cell-free seminal fluid, and sperm cells.
Gatifloxacin in prostatic fluid, ejaculate, cell-free seminal fluid and sperm cells was analyzed by a validated HPLC method with fluorescence detection at IBMP. Prostatic fluid, ejaculate, and seminal fluid (50 μl) were deproteinized by addition of precipitating solution, mixed, and centrifuged for 30 min at 11,000 rpm. The sperm cell samples (50 μl) were diluted with distilled water. Fifteen microliters of samples were in this way analyzed by HPLC with fluorescence detection. The Turbochrom 3 software (1991 release, version 3.2; PE Nelson, Cupertino, Calif.) was used for evaluation of chromatograms.
For validation and calibration, ejaculate, seminal fluid, and sperm cell calibration curves using nine standards (including a blank sample) and sets of spiked QC samples were prepared. The coefficients from linear regressions of the standard curve were at least 0.999. For prostatic fluid and for sperm cells no calibration curve could be prepared due to the low volume of drug-free prostatic fluid or sperm cells available. Therefore, prostatic fluid and sperm cell samples were analyzed against the seminal fluid calibration curve.
The QC samples were measured and treated in the same manner as subjects' samples. The interday precision of QC samples of gatifloxacin in ejaculate and that in cell-free seminal fluid were ≤9.3%, with an interday accuracy of ±7.9% over the whole concentration range. The intraday precision of the assay for gatifloxacin in ejaculate, cell-free seminal fluid, prostatic fluid, and cells was ≤7.2%, with an intraday accuracy of ±7.3%.
Assay of iohexol.
Iohexol in urine, prostatic fluid, ejaculate, and cell-free seminal fluid was analyzed at IBMP by an HPLC technique employing UV detection. The method used has been implemented at that laboratory. Urine, prostatic fluid, ejaculate, and cell-free seminal fluid samples (50 μl) were deproteinized by addition of 25 μl of acetonitrile-perchloric acid, mixed, and centrifuged for 30 min at 11,000 rpm. Ten microliters of such prepared samples were analyzed by HPLC with UV detection (254 nm). For human urine three iohexol standards were prepared (12.5, 25, and 50 mg/ml) in human urine. Using the theoretical concentrations of these standards and measured peak heights, concentrations were determined and clinical samples were analyzed against these standards. Concentrations of iohexol in clinical urine samples ranged from 0.48 to 2.0 mg/ml.
For validation and calibration, ejaculate and cell-free seminal fluid calibration curves using seven standards (including a blank sample) and sets of spiked QC samples were prepared. Calibration was performed by weighted (reciprocal of standard concentration) linear regression. The resulting coefficients from linear progressions were at least 0.999. The limit of quantification of iohexol was set to 1.95 μg/ml for human ejaculate and 1.89 μg/ml for both human cell-free seminal fluid and prostatic fluid. For validation of the iohexol concentration in ejaculate and cell-free seminal fluid, three QC samples for each fluid were prepared.
The gatifloxacin assay was cross-checked for iohexol and the iohexol assay was cross-checked for gatifloxacin, and no interference was found. This result was expected, since iohexol is a polar compound unlikely to be extracted by the solvent used to extract gatifloxacin (methylene dichloride at pH 2.8). In addition, the mobile phase used for the assay of gatifloxacin is more polar than that used for iohexol, suggesting that iohexol would elute much earlier than gatifloxacin.
Analytical data management.
For plasma and urine measurements of gatifloxacin the outputs of the analytical detector were digitized by means of an analog-to-digital converter connected to a personal computer. The signals were identified and processed for peak height calculation with the Turbochrom 4 software. For calibrations, the nominal concentrations associated with these measurements were computed through weighted linear regression (weighting factor: reciprocal of standard concentration squares). Concentrations in the subject's samples and the QC samples were calculated using the slope and the intercept obtained from the calibration lines. Concentrations were expressed as micrograms of anhydrous gatifloxacin per milliliter of fluid.
The evaluation of the calibration standards of gatifloxacin and iohexol in prostatic fluid, ejaculate, cell-free seminal fluid, and sperm cells was performed by a weighted linear regression (reciprocal of standard concentration) with theoretical concentrations of calibration standards and measured peak height ratios (for gatifloxacin concentration) or peak heights (for iohexol concentrations) by employing Turbochrom 3 software (version 3.2). All statistical calculations were performed on a PC using the program Microsoft Excel (version 7.0) without rounding.
Pharmacokinetic analysis methods.
Pharmacokinetic parameters, derived from concentrations in plasma and urine measured in samples collected immediately before and within 24 h after dosing, included the following: maximum concentration in plasma (Cmax), time at which Cmax occurs (Tmax), area under the curve from 0 to 24 h (AUC0–24), plasma elimination half-life (t1/2), and renal clearance (CLR), calculated as UR (amount excreted unchanged in urine, in milligrams)/AUC (in milligrams · hour/milliliter).
In addition, the body fluid/plasma ratios were determined using the concentration in plasma at the corresponding sampling time. Single-dose, noncompartmental pharmacokinetic parameters of gatifloxacin were calculated for each subject dosed with gatifloxacin according to standard methods by using PHAR-NCA software (Innaphase, Paris, France; version 1.3).
Urinary contamination of prostatic fluid, ejaculate, and seminal fluid.
The theoretical (maximum) urinary contamination of prostatic fluid, ejaculate, and seminal fluid was estimated assuming that the total iohexol concentration of these fluids could have been derived from the corresponding iohexol urinary concentration according to the following formula: urinary contamination of fluid (%) = fluid iohexol (micrograms per milliliter) × 100/urinary iohexol (micrograms per milliliter).
RESULTS
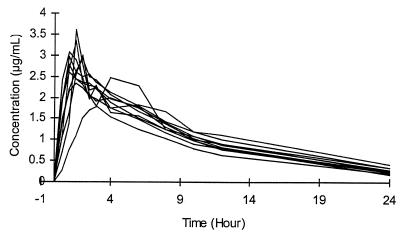
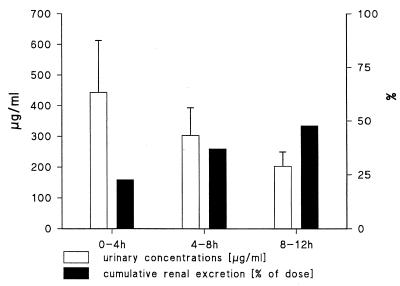
The individual concentrations of gatifloxacin in plasma are shown in Fig. 1. The pharmacokinetic parameters (Table 1) showed a mean (standard deviation [SD]) Tmax of 1.66 (0.91) h, with a Cmax of 2.90 (0.39) μg/ml and a t1/2 of 7.20 (0.90) h. The AUC0–24 was 25.65 (2.53) μg · h/ml. A mean AUC0–12 has been calculated (18.83 μg · h/ml) in order to estimate CLR. The mean (SD) urinary concentration and cumulative excretion of gatifloxacin are shown in Fig. 2. Within 12 h on average 47.9% (5.1%) or 191.38 (20.28) mg of the administered dose was excreted unchanged. Given the excreted amount of gatifloxacin (191.4 mg) and the AUC0–12 (18.8 μg · h/ml) a mean renal clearance of 169 ml/min was calculated. The individual gatifloxacin concentrations in urine (0 to 4 h), plasma, ejaculate, seminal fluid, sperm cells, and prostatic fluid and the respective fluid/plasma ratios at 4 h after drug administration are shown in Table 2. Median (range) plasma concentration amounted to 1.92 (1.53 to 2.46) μg/ml. Median ejaculate-, seminal fluid-, and prostatic fluid-to-plasma ratios were 1.03, 1.02, and 1.29, respectively.
FIG. 1.
Composite individual profiles of gatifloxacin concentrations in plasma versus time following a single 400-mg oral dose of gatifloxacin to healthy, male volunteers (n = 10).
TABLE 1.
Pharmacokinetic parameters of gatifloxacin following a single oral dose of 400 mg administered to healthy, male volunteers (n = 10)
| Parameter | Mean | SD | Median | Range |
|---|---|---|---|---|
| Tmax (h) | 1.66 | 0.91 | 1.50 | 1.00–4.00 |
| Cmax (μg/ml) | 2.90 | 0.39 | 2.93 | 2.33–3.59 |
| t1/2 (h) | 7.20 | 0.90 | 7.10 | 5.86–8.83 |
| AUC0–24 (μg · h/ml) | 25.65 | 2.53 | 25.36 | 21.32–29.90 |
FIG. 2.
Mean (SD [error bars]) urinary concentrations and cumulative renal excretion of gatifloxacin following a single 400-mg oral dose of gatifloxacin administered to healthy, male volunteers (n = 10).
TABLE 2.
Gatifloxacin concentrations and ratios of fluid-to-plasma gatifloxacin concentration, measured 4 h after oral administration of 400 mg of gatifloxacin
| Patient no. or value | Gatifloxacin concn (μg/ml) in:
|
Ratio of gatifloxacin concn in fluid to that in plasma
|
||||||
|---|---|---|---|---|---|---|---|---|
| Plasma (4 h) | Urine (0–4 h) | Ejaculate (4 h) | Seminal fluid (4 h) | Prostatic fluid (4 h) | Ejaculate | Seminal fluid | Prostatic fluid | |
| 1 | 1.96 | 649 | 1.91 | 1.58 | 2.23 | 0.98 | 0.81 | 1.14 |
| 2 | 1.63 | 468 | 1.71 | 1.72 | 1.72 | 1.05 | 1.05 | 1.05 |
| 3 | 2.46 | 111 | 2.68 | 2.67 | NSa | 1.09 | 1.09 | |
| 4 | 1.88 | 309 | 1.72 | 1.59 | NS | 0.91 | 0.84 | |
| 5 | 1.98 | 603 | 1.70 | 1.75 | 2.55 | 0.86 | 0.88 | 1.29 |
| 6 | 1.75 | 399 | 1.58 | 1.84 | 1.91 | 0.90 | 1.05 | 1.09 |
| 7 | 2.08 | 460 | 2.58 | 2.52 | NS | 1.24 | 1.21 | |
| 8 | 1.76 | 601 | 1.81 | 1.75 | 2.03 | 1.03 | 1.00 | 1.31 |
| 9 | 1.53 | 543 | NS | NS | 2.62 | 1.72 | ||
| 10 | 2.13 | 288 | 2.80 | NS | 3.10 | 1.32 | 1.46 | |
| n | 10 | 10 | 9 | 8 | 7 | 9 | 8 | 7 |
| Mean | 1.92 | 443.1 | 2.05 | 1.93 | 2.35 | 1.07 | 1.01 | 1.23 |
| SD | 0.27 | 169.3 | 0.49 | 0.42 | 0.16 | 0.16 | 0.14 | 0.23 |
| CV (%)b | 14 | 44 | 24 | 22 | 15 | 15 | 14 | 18 |
| Median | 1.92 | 464.0 | 1.81 | 1.75 | 1.03 | 1.03 | 1.02 | 1.29 |
| Range | 1.53–2.46 | 111–649 | 1.58–2.80 | 1.58–2.67 | 0.86–1.32 | 0.86–1.32 | 0.81–1.21 | 1.05–1.72 |
NS, no sample.
CV, coefficient of variation.
Theoretical maximum percent urinary contaminations of ejaculate, seminal fluid, and prostatic fluid were calculated from the iohexol concentrations in urine and the corresponding fluids. The theoretical maximum percent urinary concentration of the ejaculate ranged between 0.01 and 0.05%, that of the seminal fluid ranged between 0.01 and 0.04%, and that of prostatic fluid ranged between 0.01 and 0.16%. Thus, the highest possible urinary contamination of 0.16% in prostatic fluid could have contributed up to 0.64 μg/ml to the prostatic fluid concentration of gatifloxacin (patient 6), considering the urinary gatifloxacin concentration of 399 μg/ml. The measured gatifloxacin concentration of that prostatic fluid was 1.91 μg/ml. If urinary contamination is assumed as mentioned before, the real concentration in prostatic fluid would have been 1.27 μg/ml, with a corresponding concentration in plasma of 1.75 μg/ml, resulting in a true prostatic fluid-to-plasma ratio of 0.73. If all fluid-to-plasma ratios are corrected by this means, the median (range) true fluid-to-plasma ratios of gatifloxacin can be calculated as 1.10 (0.73 to 1.61) for prostatic fluid, 0.99 (0.77 to 1.17) for seminal fluid, and 0.99 (0.83 to 1.25) for ejaculate. Concentrations in sperm cells could only be determined in three subjects and were 0.195, 0.076, and 0.011 μg/ml.
Gatifloxacin was well tolerated. A total of two treatment-emergent AEs (headaches) were reported by 2 of the 10 (20%) subjects. Both were classified to be of moderate intensity and were judged to have no relationship to study medication but rather to study conditions, e.g., no caffeine intake.
DISCUSSION
This study of 10 healthy volunteers shows that a single oral dose of 400 mg of gatifloxacin is well tolerated and is rapidly and well absorbed with a mean (SD) Tmax of 1.66 (0.91) h, an estimated Cmax of 2.90 (0.39) μg/ml, and a mean t1/2 of 7.20 (0.90) h. The AUC0–24 was 25.65 (2.53) μg · h/ml. Thus, the Cmax and the t1/2 of gatifloxacin are higher than those observed for comparable doses of enoxacin, norfloxacin, and ciprofloxacin in a similar setting (15). In this study we investigated urinary excretion only up to 12 h after oral administration, and within this time about 50% of the substance was excreted unchanged into urine. Earlier investigations showed that approximately 65 to 90% of gatifloxacin is excreted unchanged into the urine over 24 to 72 h (12, 17; H. Stahlberg, K. Goehler, M. Guillaume, and A. Mignot, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. 8–8, 1998). The concentrations in plasma and urinary excretion of gatifloxacin found in this study are somewhat lower than those found by other investigators (3, 12, 17; Stahlberg et al., 38th ICAAC). Since the CLR was practically identical with that found by other authors (3, 12), we assume that lower absorption in our volunteers was the reason for the lower concentrations in plasma and urinary excretion.
The determination of the concentrations of antibiotics in prostatic and seminal fluid may be biased by urinary contamination (16). To estimate potential contaminations of prostatic and seminal fluid by urine, we used a model which has been published earlier (7–11). It has been proposed that by using this model it is possible to estimate the maximum possible amount of urinary contamination. In brief, the renal contrast agent iohexol, which is excreted almost exclusively via glomerular filtration by the kidneys, was administered intravenously to the subjects at the same time the study drug was given. Since the iohexol concentrations in urine are several hundred-fold greater than those in plasma or prostatic fluid, possible urinary contamination can be detected with a sensitivity of even less than 1%. By simply dividing the fluid concentration of iohexol by the corresponding urinary concentration, this model allows one to correct the measured study drug concentrations by the calculated amount of maximal possible urinary contamination.
Since gatifloxacin is, like other quinolones, a zwitterion (pKa1 6.0 and pKa2 9.2) with its isoelectric point at pH 7.7, the degree of ionization is low in plasma (pH 7.4) and should be higher in the more-acidic prostatic fluid or the more alkaline seminal fluid. According to the principle of nonionic diffusion (13) the concentration of such a substance can be expected to be higher in prostatic fluid as well as in seminal fluid than that achieved with weak acids such as, e.g., β-lactam antibiotics (9, 10).
We used gatifloxacin concentrations in prostatic and seminal fluid, corrected by maximal urinary contamination, to estimate the true fluid/plasma ratios. These median (range) ratios were 1.10 (0.73 to 1.61) for prostatic fluid, 0.99 (0.77 to 1.17) for seminal fluid, and 0.99 (0.83 to 1.25) for ejaculate, respectively. Thus, gatifloxacin concentrations in ejaculate, seminal fluid, and prostatic fluid after a 400-mg oral dose are in the range of the corresponding concentrations in plasma. The median seminal fluid/plasma ratio is comparable to that of lomefloxacin (1.0 to 1.3) but is somewhat lower than that of other fluoroquinolones such as fleroxacin (1.3 to 1.7), enoxacin (2.2), ciprofloxacin (5.8 to 7.1) (6), or ofloxacin (2.6 to 4.0) (7, 8, 11). In contrast, the median prostatic fluid/plasma ratio was at least twofold higher than those reported from similar studies with norfloxacin, ciprofloxacin, lomefloxacin, enoxacin, or ofloxacin (6–8, 11), which were found to be in the range of 0.12 to 0.48.
Conclusion.
The relatively high concentrations of gatifloxacin in prostatic and seminal fluid as compared to those of other fluoroquinolones, along with the extended antibacterial spectrum, indicate that gatifloxacin may be an appropriate therapeutic agent for CBP, which should be investigated in clinical trials. According to its pharmacokinetic properties and its good penetration into prostatic and seminal fluid, an oral dosage of 400 mg once daily appears to be suitable for investigating the efficacy of gatifloxacin in CBP.
ACKNOWLEDGMENT
This work was supported by Grünenthal GmbH, Aachen, Germany.
REFERENCES
- 1.Bjerklund Johansen T E, Grüneberg R N, Guibert J, Hofstetter A, Lobel B, Naber K G, Palou Redorta J, van Cangh P J. The role of antibiotics in the treatment of chronic prostatitis: a consensus statement. Eur Urol. 1998;34:457–466. doi: 10.1159/000019784. [DOI] [PubMed] [Google Scholar]
- 2.Hosaka M, Yaasue T, Fukuda H, Tomizawa H, Aoyama H, Hirai K. In vitro and in vivo antibacterial activities of AM-1155, a new 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1992;36:2108–2117. doi: 10.1128/aac.36.10.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lober S, Ziege S, Rau M, Schreiber G, Mignot A, Koeppe P, Lode H. Pharmacokinetics of gatifloxacin and interaction with an antacid containing aluminum and magnesium. Antimicrob Agents Chemother. 1999;43:1067–1071. doi: 10.1128/aac.43.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luizzi G. The prostatitis syndromes. Int J Sex Transm Dis Acquir Immune Defic Syndr. 1996;7:471–478. [Google Scholar]
- 5.Miyashita N, Niki Y, Kishimoto T, Nakajima M, Matsushima T. In vitro and in vivo activities of AM-1155, a new fluoroquinolone, against Chlamydia spp. Antimicrob Agents Chemother. 1997;41:1331–1334. doi: 10.1128/aac.41.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naber K G. Role of quinolones in treatment of chronic bacterial prostatitis. In: Hooper D C, Wolfson J S O, editors. Quinolone antimicrobial agents. 2nd ed. Washington, D.C.: American Society for Microbiology; 1993. pp. 285–297. [Google Scholar]
- 7.Naber K G, Sörgel F, Kees F, Schumacher H, Metz R, Grobecker H. In-vitro activity of fleroxacin against isolates causing complicated urinary tract infections and concentrations in seminal and prostatic fluid and in prostatic adenoma tissue. J Antimicrob Chemother. 1988;22(Suppl. D):199–207. doi: 10.1093/jac/22.supplement_d.199. [DOI] [PubMed] [Google Scholar]
- 8.Naber K G, Sörgel F, Kinzig M, Weigel D M. Penetration of ciprofloxacin into prostatic fluid, ejaculate and seminal fluid in volunteers after an oral dose of 750 mg. J Urol. 1993;150:1718–1721. doi: 10.1016/s0022-5347(17)35877-9. [DOI] [PubMed] [Google Scholar]
- 9.Naber K G, Sörgel F, Kinzig M, Weigel D E. Pharmakokinetik und Penetration von Tazobactam/Piperacillin (Tazobac®) in das Prostatasekret und das Prostataadenomgewebe bei älteren Patienten. Fortschr Antimikrob Antineoplas Chemother. 1992;11:457–463. [Google Scholar]
- 10.Naber K G, Kinzig M, Adam D, Sörgel F, Bajorski A H, Kiehn R. Concentrations of cefpodoxime in plasma, ejaculate and in prostatic fluid and adenoma tissue. Infection. 1991;19:34–39. doi: 10.1007/BF01643755. [DOI] [PubMed] [Google Scholar]
- 11.Naber K G, Kinzig M, Sörgel F, Weigel D. Penetration of ofloxacin into prostatic fluid, ejaculate and seminal fluid. Infection. 1993;21:98–100. doi: 10.1007/BF01710740. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima M, Uematsu T, Kosuge K, Kusajima H, Ooie T, Masuda Y, Ishida R, Uchida H. Single- and multiple-dose pharmacokinetics of AM-1155, a new 6-fluoro-8-methoxy quinolone, in humans. Antimicrob Agents Chemother. 1995;9:265–270. doi: 10.1128/aac.39.12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamey T A, Meares E M, Jr, Winningham D G. Chronic bacterial prostatitis and the diffusion of drugs into prostatic fluid. J Urol. 1970;103:187. doi: 10.1016/s0022-5347(17)61919-0. [DOI] [PubMed] [Google Scholar]
- 14.Weidner W, Schiefer H G, Frauss H, Jantos Ch, Friedrich H J, Altmannsberger M. Chronic prostatitis: a thorough search for etiologically involved microorganisms in 1,461 patients. Infection. 1991;19(Suppl. 3):119–125. doi: 10.1007/BF01643680. [DOI] [PubMed] [Google Scholar]
- 15.Well M, Naber K G, Kinzig-Schippers M, Sörgel F. Urinary bactericidal activity and pharmacokinetics of enoxacin versus norfloxacin and ciprofloxacin in healthy volunteers after a single oral dose. Int J Antimicrob Agents. 1998;10:31–38. doi: 10.1016/s0924-8579(98)00014-4. [DOI] [PubMed] [Google Scholar]
- 16.Winningham D G, Nemoy N J, Stamey T A. Diffusion of antibiotics from plasma into prostatic fluid. Nature. 1968;291:139. doi: 10.1038/219139a0. [DOI] [PubMed] [Google Scholar]
- 17.Wise R, Andrews J. M. J M, Ashby J P, Marshall J. A study to determine the pharmacokinetics and inflammatory fluid penetration of gatifloxacin following a single oral dose. J Antimicrob Chemother. 1999;44:701–704. doi: 10.1093/jac/44.5.701. [DOI] [PubMed] [Google Scholar]