Abstract
Background:
In-hospital acute declines in kidney function occur in approximately 20–30% of patients admitted with acute decompensated heart failure (ADHF) and may be associated with adverse outcomes.
Objective:
To examine whether incorporation of a comprehensive set of measures of decongestion modifies the association of acute declines with outcomes.
Methods:
Using data from the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial, we used multivariable Cox regression models to evaluate the association between in-hospital changes in estimated glomerular filtration rate (eGFR) with death and a composite outcome of cardiovascular death and heart failure hospitalization. We evaluated eGFR declines within the context of changes in markers of volume overload including b-type natriuretic peptide (BNP), N-terminal prohormone of b-type natriuretic peptide (NT-proBNP), and weight, as well as changes in measures of hemoconcentration including hematocrit, albumin and total protein.
Results:
Among 3,715 patients over a median follow-up of 9.9 months, every 30% decline in eGFR was associated with higher risk of both death (HR=1.19 [95% CI 1.07, 1.31] and the composite outcome (HR=1.09 [95% CI 1.01, 1.18]) in adjusted models. The acute decline in eGFR was no longer associated with higher risk of either outcome as long as there was evidence of decongestion, either by declines in BNP, NT-proBNP or weight or by increases in hematocrit, albumin or total protein. Interaction testing between decline in eGFR and changes in hematocrit, albumin and total protein was statistically significant (p-interaction of <0.01 for death and p-interaction of ≤0.01 for composite for all three biomarkers). Interaction between change in eGFR and changes in BNP (p-interaction=0.07 for death; 0.08 for composite), NT-proBNP (p-interaction=0.15 for death; 0.18 for composite) and weight (p-interaction=0.13 for death; 0.19 for composite) did not meet statistical significance.
Conclusion:
Overall, acute declines in eGFR are associated with adverse outcomes, with evidence of modification by changes in markers of decongestion, suggesting that they are no longer associated with adverse outcomes if these markers are concomitantly improving.
Keywords: decongestion, hemoconcentration, cardiorenal, worsening renal function
Condensed Abstract
Among 3,715 patients from the EVEREST trial, we observed that the relation between acute declines in estimated glomerular filtration rate (eGFR) and outcomes was modified by changes in markers of volume overload (b-type natriuretic peptide, N-terminal prohormone of b-type natriuretic peptide, weight) and markers of hemoconcentration (hematocrit, albumin, total protein). Among those with the least improvement in markers of volume overload or hemoconcentration, acute eGFR declines were associated with higher risk of death and cardiovascular events. However, among those displaying greater improvement in volume overload and hemoconcentration, there was no association between eGFR decline and risk of adverse outcomes.
Introduction
Approximately 20–30% of patients admitted for acute decompensated heart failure (ADHF) experience a decline in kidney function during their hospitalization (1, 2). The pathophysiology of this decline is not well understood and its association with post-discharge outcomes is controversial. Many mechanisms have been proposed to explain declines in kidney function among patients with ADHF, including renal congestion from volume overload (3, 4). Improvements in kidney function have been observed following fluid removal (5), however, fluid removal can also lead to acute declines in estimated glomerular filtration rate (eGFR) (6, 7).
Acute declines in eGFR pose a dilemma for clinicians, who may feel tempted to prematurely discontinue decongestion out of concern for the kidney function, given the association of baseline reduced kidney function with adverse outcomes. Prior post-hoc analyses have suggested that, as long as occurring within the setting of decongestion, acute declines in kidney function may be tolerated (8, 9). Many of these studies have relied on rise in a single biomarker of hemoconcentration such as hematocrit, but increases in hematocrit have not always been associated with degree of fluid removal. Natriuretic peptides, which are released in response to cardiac stretch in states of volume overload, can also serve as surrogates of congestion. A prior analysis has also shown that declines in kidney function may be associated with improved clinical outcomes if occurring in the context of decreases in N-terminal prohormone of b-type natriuretic peptide (NT-proBNP) (10).
Using data from the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial, we sought to investigate the relation between acute declines in kidney function with mortality and cardiovascular outcomes, as well as between changes in markers of decongestion with these clinical outcomes. Our primary goal was to evaluate and compare a comprehensive set of markers of volume overload, including changes in b-type natriuretic peptide (BNP), its prohormone NT-proBNP, and weight, as well as markers of hemoconcentration including changes in hematocrit, albumin, and total protein, in order to evaluate whether they can better inform clinicians in interpreting acute declines in kidney function.
Methods
Study Population and Design
The EVEREST trial was a multi-center randomized controlled trial that investigated the use of the vasopressin V2 receptor blocker tolvaptan in patients with ADHF (11). It enrolled patients with reduced left ventricular ejection fraction (≤ 40%) who had evidence of congestion based upon ≥2 clinical signs or symptoms, and were <48 hours into the hospitalization. Patients were randomized to either 30 mg of tolvaptan or placebo, in addition to their standard medical therapy. Key exclusion criteria included a serum creatinine >3.5 mg/dl and any comorbid condition with an expected survival of <6 months. A total of 4133 were enrolled into EVEREST, of whom patients without at least 2 in-hospital kidney function measurements (n=418) were excluded from this analysis. Participants in EVEREST provided informed consent at the time of enrollment; the present study was deemed exempt from review by the Tufts Health Sciences Institutional Review Board.
Exposure
Kidney Function:
The kidney function exposure of interest was change in eGFR. Estimates of GFR were determined by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula with serum creatinine (12). While the CKD-EPI equation has not yet been validated in the HF population, prior literature has shown its accuracy as compared to other estimating equations in both CKD and the general population and HF (12–14). Change in eGFR was determined as the difference between the log-transformed Day 7 eGFR and the baseline eGFR from the time of randomization. Log transformation by base 10/7 enabled assessment of hazard ratios per a 3/10 decline, synonymous to a 30% decline, which has been demonstrated as a surrogate endpoint in CKD literature (15).
Measures of volume overload:
We examined changes in BNP, NT-proBNP and weight. These markers were measured at baseline and at Day 7 (or day of discharge if discharged prior to Day 7). For administrative reasons, some study centers measured BNP and others measured NT-proBNP. Values for both BNP and NT-proBNP were transformed using log transformation given the skewed distribution of the data. Change was determined as the difference between the log-transformed Day 7 (or day of discharge) BNP or NT-proBNP and baseline BNP or NT-proBNP measurement. Weight was maintained on the raw scale and changes marked as the difference between Day 7 and baseline on the raw scale. Quartiles of change in BNP, NT-proBNP and weight were organized by order of change with Quartile 1 comprised of least decline in markers, and Quartile 4 of greatest decline.
Measures of hemoconcentration:
We examined changes in hematocrit, albumin, and total protein as reflection of intravascular volume. Increases in hematocrit as well as increases in albumin and total protein have been shown to be associated with decongestion (8, 9). These markers were measured at baseline and at Day 7 (or day of discharge if discharged prior to Day 7). Values were maintained on the raw scale and change determined as the difference between the Day 7 measurement and baseline measurement on the raw scale. Quartiles were organized by order of change with Quartile 1 comprised of least increase in markers, and Quartile 4 of greatest increase.
Outcomes
The primary outcome of interest was all-cause death. The secondary outcome of interest was a composite endpoint of cardiovascular death (CVD) or first rehospitalization for HF over the course of follow-up, consistent with the original pre-specified EVEREST trial primary and secondary outcomes (11). All events were adjudicated by a blinded clinical events committee. Vital status was reported as unknown for 25 (<1%) patients at the close of the trial (11). Day of hospital discharge or in-hospital Day 7 was used as time zero for the follow-up time at risk for each outcome.
Covariates
Several covariates were selected for analysis as potential confounding variables based on review of the literature and clinical relevance, including demographic characteristics (age, sex, race, body mass index [BMI]), baseline medication (angiotensin-converting enzyme inhibitors [ACEI] or angiotensin II receptor blockers [ARB], mineralicorticoid receptor antagonists [MRA]), as well as randomization arm (tolvaptan or placebo). Baseline eGFR was included as an adjustment variable in the final model, given its potential association with the exposure variable as well as the outcome variable. For analyses evaluating change in decongestion, the baseline decongestion biomarker (i.e., baseline BNP for change in BNP analysis) was also included as an adjustment variable.
Statistical Analysis
Values are presented as mean ± SD, or median [IQR] for non-normal distributions. Differences in the baseline characteristics of patients by quartiles of change in eGFR were examined using analysis of variance and Kruskal-Wallis tests, as well as by χ2 and Fisher’s exact tests for categorical and continouous variables, as appropriate.
Multivariable Cox proportional hazards regression models were used to evaluate the association between change in eGFR with the outcome of death and composite outcome of CVD or HF rehospitalization, as well as association between change in each biomarker of volume overload and hemoconcentration with outcomes. The association between decline in eGFR and outcomes was also further examined within each decongestion and hemoconcentration biomarker according to quartile of change from baseline to Day 7. Interaction testing was performed with decline in eGFR and change in each biomarker on the continuous scale. Patients were censored at the end-of-trial date (February 3, 2006), or at the date of last contact. We also tested for an interaction between treatment (tolvaptan vs placebo) and decline in eGFR with outcomes. Proportional hazards assumptions for models were checked by visualizing Shoenfeld residuals.
Several sensitivity analyses were also performed. First, multivariable Cox proportional hazards regression models were repeated using increase in serum creatinine, rather than decline in eGFR, given the possibility that eGFR may not be completely accurate in patients outside of steady-state. Second, to further evaluate whether the associations between decline in eGFR and outcomes are modified specifically by changes in decongestion or hemoconcentration, analyses were repeated using changes in sodium and chloride. Changes in serum sodium and chloride are less specific measures of changes in intravascular volume as they can be influenced by sodium intake, fluid intake, antidiuretic hormone levels, acid-base status or diuretic use, but increases in sodium and chloride have also been associated with improved mortality in prior studies (16, 17). Multivariable Cox proportional hazards regression models were repeated and interaction testing performed with decline in eGFR and change in sodium and change in chloride with each outcome. Because tolvaptan is known to affect sodium and chloride levels, these analyses were also performed within the placebo arm only.
Results
There were 3,715 patients with both baseline and follow-up kidney function data available. Median [25th, 75th] follow-up was 9.9 [5.3, 16.1] months.
Baseline Patient Characteristics
Baseline characteristics as separated by quartiles of change in eGFR are presented in Table 1. Overall, 2632 (71%) had hypertension, 1426 (39%) had diabetes and median [25th, 75th] baseline eGFR was 57 [42, 74] ml/min/1.73 m2. Demographic and basic cardiovascular characteristics including race, sex, EF and ischemic etiology of cardiac dysfunction were similar between the four groups, with the exception of age and BMI; those with greater declines in eGFR tended to be older and with higher BMI. Within the quartile with the least degree of kidney function decline (Quartile 1), baseline eGFR was slightly lower at 50 [38, 67] ml/min/1.73 m2 with median absolute change of 11 [8, 17] ml/min/1.73 m2 as opposed to baseline eGFR of 63 [47, 77] ml/min/1.73 m2 and absolute change of −16 [−21, −11] ml/min/1.73 m2 among those in Quartile 4. Most patients were taking either an ACEI or an ARB; there were no differences in baseline use of these medications amongst the four quartiles (Table 1). One notable difference, however, was that among those with the greatest decline in eGFR, 524 (56%) were randomized to tolvaptan whereas among the stable or improved eGFR only 391 (42%) were randomized to tolvaptan.
Table 1.
Baseline characteristics by quartiles of eGFR change on the log scale during hospitalization.
| Quartile 1 (n=930) 10.9 to 291.9% Change |
Quartile 2 (n=1011) 0 to 10.6% Change |
Quartile 3 (n=844) −15.9 to 0% Change |
Quartile 4 (n=930) −86.4 to −15.9% Change |
|
|---|---|---|---|---|
| Age | 64.8 ± 12.4 | 65.7 ± 11.8 | 65.4 ± 11.5 | 66.4 ± 11.3 |
| Female | 226 (24) | 241 (24) | 201 (24) | 261 (28) |
| Non-Black | 865 (93) | 933 (92) | 790 (94) | 873 (94) |
| BMI, kg/m2 | 28.2 ± 5.5 | 28.4 ± 5.6 | 29.0 ± 5.5 | 29.1 ± 5.6 |
| Ejection Fraction, % | 27 ± 8 | 28 ± 8 | 28 ± 8 | 28 ± 8 |
| Ischemic etiology | 602 (66) | 671 (67) | 546 (66) | 604 (66) |
| NYHA Functional Class 4 | 412 (44) | 378 (37) | 295 (35) | 398 (43) |
| Hypertension | 652 (70) | 704 (70) | 584 (69) | 692 (74) |
| Diabetes | 328 (35) | 392 (39) | 335 (40) | 371 (40) |
| Medications | ||||
| ACEI or ARB | 789 (85) | 854 (85) | 710 (84) | 801 (86) |
| Aldosterone antagonist | 516 (56) | 557 (55) | 487 (58) | 534 (57) |
| Laboratory Results | ||||
| Initial eGFR, ml/min/1.73 m2 | 50 [38, 67] | 55 [42, 73] | 60 [43, 78] | 63 [47, 77] |
| Change in eGFR, % | 22.4 [13.6, 33.1] | 0.0 [0.0, 6.4] | −10.0 [−12.0, −8.0] | −25.1 [−32.0, −19.8] |
| Initial BNP, pg/ml* | 847 [382, 1752] | 758 [301, 1523] | 586 [238, 1264] | 542 [234, 1183] |
| Change in BNP, % | −18.7 [−46.3, 20.8] | −25.6 [−51.8, 6.0] | −27.5 [−52.1, 6.3] | −36.2 [−61.7, −8.6] |
| Initial NT-proBNP, pg/ml* | 4556 [2149, 10687] | 4663 [2064, 8975] | 4646 [1976, 8043] | 4390 [2179, 8832] |
| Change in NT-proBNP, % | −31.0 [−54.4, −1.2] | −26.4 [−51.2, 0.3] | −27.0 [−56.2, −2.8] | −33.5 [−55.2, −4.2] |
| Initial Weight, kg | 82.3 ± 18.5 | 83.2 ± 19.2 | 84.4 ± 18.4 | 83.5 ± 18.8 |
| Change in weight, kg | −3.0 ± 3.3 | −3.0 ± 3.3 | −3.3 ± 3.3 | −3.5 ± 3.7 |
| Initial Hematocrit, % | 42.6 ± 6.2 | 42.0 ± 5.8 | 41.7 ± 5.6 | 41.6 ± 5.5 |
| Change in Hematocrit, % | 0.1 ± 3.7 | 0.8 ± 3.3 | 1.6 ± 3.4 | 1.8 ± 3.7 |
| Initial albumin, g/dL | 3.7 ± 0.5 | 3.8 ± 0.5 | 3.8 ± 0.5 | 3.8 ± 0.5 |
| Change in albumin, g/dL | −0.1 ± 0.4 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.2 ± 0.4 |
| Initial total protein, g/dL | 7.0 ± 0.8 | 7.1 ± 0.7 | 7.1 ± 0.7 | 7.1 ± 0.7 |
| Change in total protein, g/dL | 0.1 ± 0.6 | 0.2 ± 0.6 | 0.3 ± 0.5 | 0.4 ± 0.6 |
| Randomization Group | ||||
| Tolvaptan | 391 (42) | 478 (47) | 454 (54) | 524 (56) |
Values presented as either n (%), mean ± standard deviation or median [25th, 75th interquartile range].
For administrative reasons, some patients had baseline BNP measured (n=2,463) and some patients had baseline NT-proBNP measured (n=1,274).
BMI: body mass index; NYHA: New York Heart Association; eGFR: estimated glomerular filtration rate; BNP: b-type natriuretic peptide; NT-proBNP: N-terminal prohormone of b-type natriuretic peptide; ACEI:angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker
Those excluded due to missing either baseline or discharge or in-hospital Day 7 kidney function measurements 418 (10%) were largely similar in baseline characteristics (Supplemental Table S1).
Outcomes
Over a median follow-up of 9.9 months, there were 931 (25%) deaths and 1517 (41%) experienced the composite outcome of CVD or rehospitalization for HF.
In-hospital Decline in eGFR and Outcomes
An in-hospital decline in eGFR was not significantly associated with death in unadjusted models (HR=0.98 per every 30% eGFR decline; 95% CI 0.89, 1.09). When adjusting for baseline covariates it remained largely unchanged, but was associated with significantly increased risk when also adjusting for baseline eGFR (HR=1.19 per every 30% eGFR decline; 95% CI 1.07, 1.31) (Table 2, Central Illustration). For the composite of CVD or HF rehospitalization, decline in eGFR was not associated with outcomes in unadjusted models (HR=0.93 per every 30% eGFR decline; 95% CI 0.86, 1.01) or when adjusted for baseline covariates, but significantly associated with a 15% increased risk of the composite outcome in the fully adjusted model including baseline eGFR (Table 2). Plots of Schoenfeld residuals showed no violation of proportional hazards assumptions (Online Figure 3). There was no significant interaction between eGFR decline and randomization to tolvaptan or placebo with outcomes (p=0.58 for death; p=0.16 for composite).
Table 2.
Hazard ratio for death and composite outcome based on in-hospital decline in eGFR
| Hazard Ratio Per 30% decline in eGFR | |
|---|---|
| Death | |
| N | 3715 |
| N of events | 931 |
| Unadjusted HR | 0.98 (0.89, 1.09) |
| Adjusted HR, Model 1* | 1.01 (0.91, 1.12) |
| Adjusted HR, Model 2† | 1.19 (1.07, 1.31) |
| Composite of Cardiovascular Death or Heart Failure hospitalization | |
| N | 3715 |
| N of events | 1517 |
| Unadjusted HR | 0.93 (0.86, 1.01) |
| Adjusted HR, Model 1* | 0.93 (0.86, 1.01) |
| Adjusted HR, Model 2† | 1.09 (1.01, 1.18) |
Cox proportional hazards models used to evaluate association between decline in eGFR with death as well as a composite outcome of cardiovascular death and re-hospitalization for heart failure. Log transformation of eGFR by base 10/7 enabled hazard ratios to be assessed per a 3/10 decline, synonymous to a 30% decline.
adjusted for age, race, sex, BMI, randomization arm, use of ACEI/ARB, use of aldosterone antagonist
adjusted for all of the above as well as baseline eGFR
Abbreviations: BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP,b-type natriuretic peptide; eGFR, estimated glomerular filtration rate;
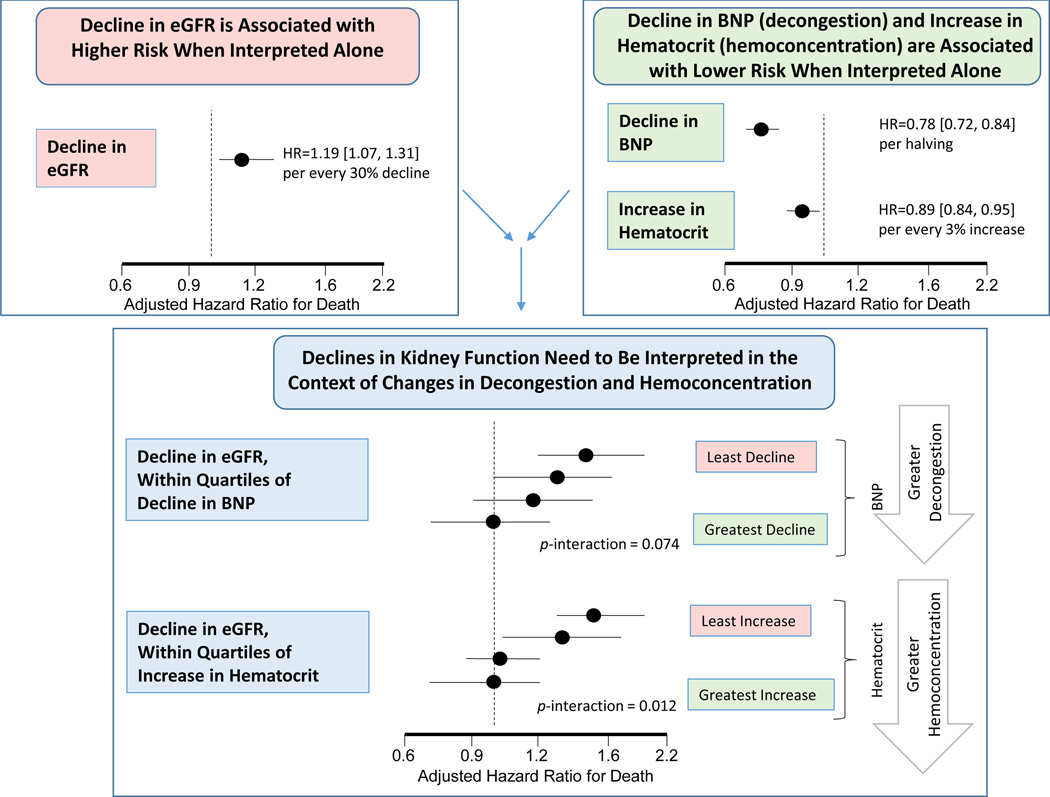
Central Illustration.
The acute decline in estimate glomerular filtration rate (eGFR) is associated with higher risk of death over a median 10-month follow-up. On the other hand, decongestion as measured by decline in b-type natriuretic peptide (BNP) and hemoconcentration as measured by increase in hematocrit, are associated with decreased risk of death. The association between decline in kidney function and death is modified by the change in these biomarkers of decongestion and hemoconcentration. That is, acute declines in kidney function are only associated with increased risk for death and cardiovascular outcomes when markers of decongestion worsened, but not when they improved.
In-hospital Changes in Measures of Volume Overload and Outcomes
Decreases in BNP and NT-proBNP were associated with lower risk of death and the composite outcome in both unadjusted and adjusted models (Table 3, Central Illustration). In contrast, change in weight was not significantly associated with either outcome (Table 3).
Table 3.
Hazard ratio for death and composite outcome based on in-hospital changes in biomarkers of volume overload and hemoconcentration
| Measures of Volume Overload | Measures of Hemoconcentration | |||||
|---|---|---|---|---|---|---|
|
BNP Per 50% decline |
NT-proBNP Per 50% decline |
Weight Per every 3 kg decrease |
Hematocrit Per every 3% increase |
Albumin Per every 1 g/dl increase |
Total protein Per every 1 g/dl increase |
|
| Death | ||||||
| N | 2463 | 1274 | 3862 | 3293 | 3618 | 3718 |
| N of events | 490 | 449 | 951 | 808 | 906 | 933 |
| Unadjusted HR | 0.87 (0.81, 0.94) |
0.68 (0.61, 0.76) |
1.00 (0.95, 1.06) |
0.90 (0.85, 0.95) |
0.77 (0.64, 0.93) |
0.88 (0.79, 0.98) |
| Adjusted HR* | 0.78 (0.72, 0.84) |
0.64 (0.57, 0.71) |
1.06 (1.00, 1.13) |
0.89 (0.84, 0.95) |
0.63 (0.52, 0.77) |
0.86 (0.76, 0.96) |
| Composite of CV Death or HF hospitalization | ||||||
| N | 2463 | 1274 | 3862 | 3293 | 3618 | 3718 |
| N of events | 871 | 685 | 1564 | 1318 | 1472 | 1518 |
| Unadjusted HR | 0.89 (0.84, 0.94) |
0.80 (0.74, 0.87 |
0.98 (0.93, 1.02) |
0.93 (0.89, 0.97) |
0.78 (0.68, 0.90) |
0.86 (0.80, 0.94) |
| Adjusted HR* | 0.82 (0.77, 0.87) |
0.76 (0.70, 0.83) |
0.99 (0.94, 1.04) |
0.90 (0.86, 0.94) |
0.68 (0.59, 0.80) |
0.81 (0.74, 0.89) |
Cox proportional hazards models used to evaluate association between decline in biomarkers with death as well as a composite outcome of cardiovascular death and re-hospitalization for heart failure. BNP and NT-proBNP were transformed by log-base 2.
adjusted for age, race, sex, BMI, randomization arm, use of ACEI/ARB, use of aldosterone antagonist and baseline eGFR. Baseline biomarker level was also included as an adjustment variable (ie for change in BNP with outcomes, model included baseline BNP).
Abbreviations: BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP,b-type natriuretic peptide; eGFR, estimated glomerular filtration rate; NT-proBNP: N-terminal prohormone of b-type natriuretic peptide
In-hospital Changes in Measures of Hemoconcentration and Outcomes
Increases in hematocrit, albumin and total protein were all associated with significantly lower risk of both all-cause mortality and the composite outcome in both unadjusted and adjusted models (Table 3, Central Illustration).
Interaction between Decline in eGFR and Change in Biomarkers of Volume Overload with Outcomes
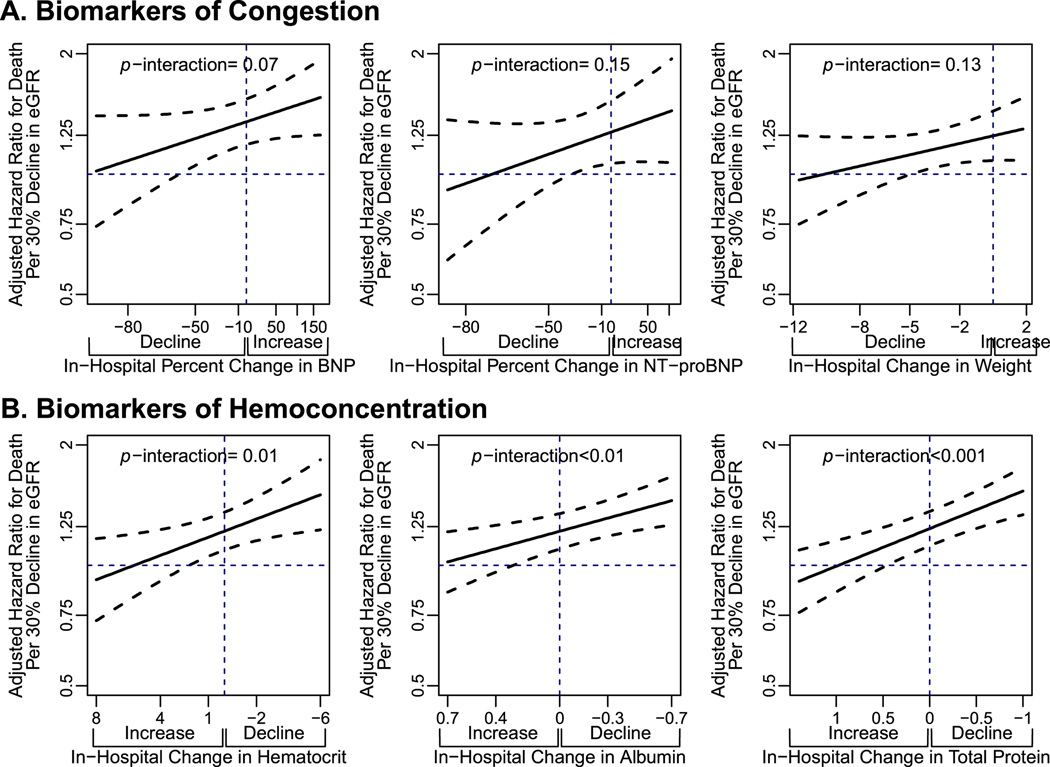
When decline of eGFR was examined within each group of biomarkers of volume overload, its relation with the outcomes of interest varied depending on direction of change in biomarkers. Within all three biomarkers of volume overload, the trend was consistent that decline in eGFR was associated with higher risk of mortality when these markers were rising, but less so as these markers were declining (Figure 1). Interaction testing was significant in unadjusted models for the interaction between decline in eGFR and change in BNP (p=0.020), change in NT-proBNP (p=0.05), and change in weight (p=0.04). The trend remained consistent, where risk was increased only when these biomarkers of change were increasing and not if they were decreasing, but the interaction no longer reached statistical significance in fully adjusted models (Figure 1). Results were similar for the composite outcome of CV death and HF hospitalization (Online Figure 1a).
Figure 1.
Adjusted hazards ratios for death per every 30% decline in eGFR according to varying levels of change in biomarkers of volume overload (a) and biomarkers of hemoconcentration (b).
Cox proportional hazards regression models were used to evaluate the interaction between decline in eGFR and change in surrogate measures of volume overload (BNP, NT-proBNP and weight) and by change in surrogate measures of hemoconcentration (hematocrit, albumin and total protein).
Abbreviations: BNP, b-type natriuretic peptide; eGFR, estimated glomerular filtration rate; NT-proBNP: N-terminal prohormone of b-type natriuretic peptide
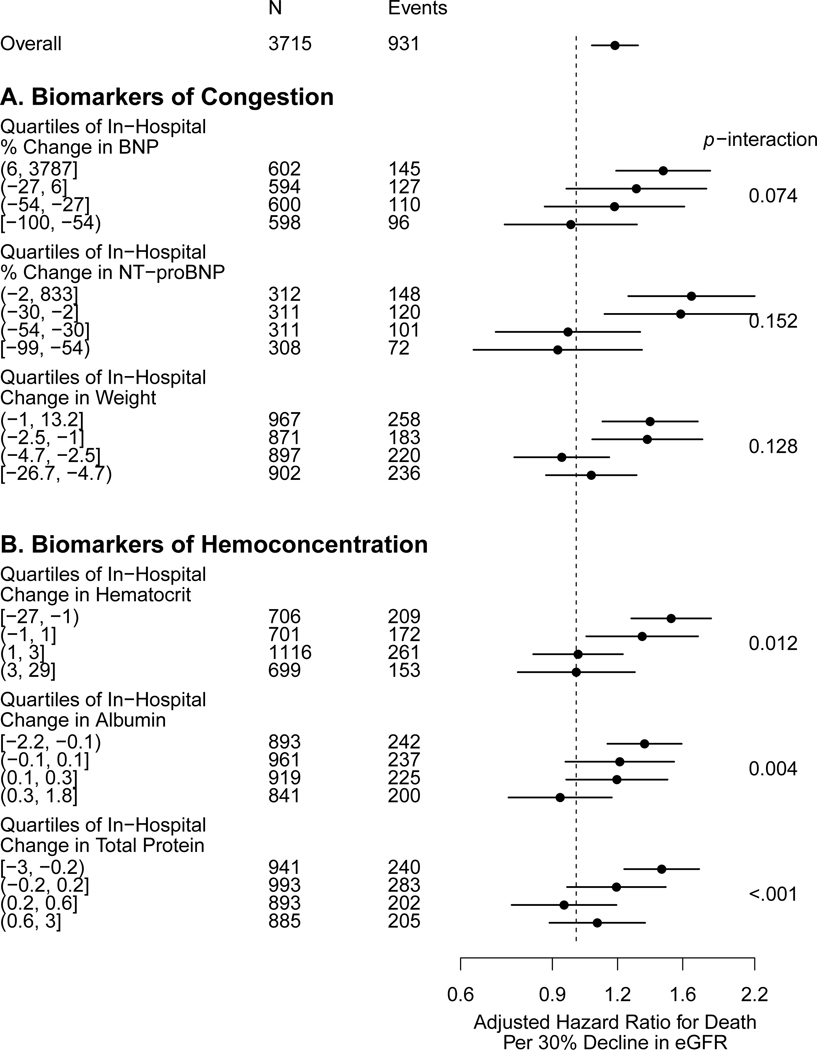
In models examining decline in eGFR within quartiles of change in markers of volume overload, there appeared to be a graded relation between decline in eGFR and risk of adverse outcomes (Central Illustration). Among those patients with the least degree of decline in markers of volume overload, a decline in eGFR was associated with significantly higher risk of death (Figure 2) and CV death or HF hospitalization (Online Figure 2). Among those with greater degrees of decline, there was no longer an association between decline in eGFR and either outcome (Figure 2, Online Figure 2, Online Table 2).
Figure 2.
Adjusted hazard ratios for death per every 30% eGFR decline within quartiles of change in biomarkers of volume overload (a) and biomarkers of hemoconcentration (b).
Cox proportional hazards regression models examining decline in eGFR within quartiles of change in measures of volume overload (BNP, NT-proBNP and weight) with quartile 1 representing the least decrease and quartile 4 representing the greatest decrease; and within quartiles of change in measures of hemoconcentration (hematocrit, albumin and total protein) with quartile 1 representing the least increase and quartile 4 representing the greatest increase. Interaction testing was performed with decline in eGFR and change in biomarkers both on the continuous scale (represented as p-interaction).
Abbreviations: BNP,b-type natriuretic peptide; eGFR, estimated glomerular filtration rate; NT-proBNP: N-terminal prohormone of b-type natriuretic peptide
Interaction between Decline in eGFR and Change in Hemoconcentration Biomarkers with Outcomes
When decline of eGFR was examined within each group of biomarkers of hemoconcentration, its relation with the outcomes of interest varied depending on direction of change in biomarkers. Within all three biomarkers of hemoconcentration, the trend was consistent that decline in eGFR was associated with higher risk of mortality when these markers were decreasing, but less so as these markers were increasing (Figure 1). Interaction testing between decline in eGFR and each marker of hemoconcentration was significant in both unadjusted (p<0.001 to 0.01) and adjusted models (p=0.001 to 0.01). Results were similar for the composite outcome of CV death and HF hospitalization (Online Figure 1b).
In models examining decline in eGFR within quartiles of change in biomarkers of hemoconcentration, there appeared to be a graded relation between decline in eGFR and risk of death (Central Illustration). Among patients with the least hemoconcentration, a decline in eGFR was associated with worse outcomes. However, among those displaying greater hemoconcentration (by any of the markers), there was progressive dissociation between eGFR decline and the risk of death (Figure 2). This pattern was also consistent for the composite outcome of CV death or HF hospitalization (Online Figure 2, Online Table 2).
Sensitivity Analyses
Results using change in serum creatinine instead of eGFR yielded very similar results (Online Table 3). Interactions with biomarkers of volume overload and hemoconcentration remained consistent (Online Figure 4, Online Figure 5).
When the relation between decline in eGFR and outcomes was examined within the context of changes in sodium and chloride, there was no significant interaction in fully adjusted models limited to the placebo arm (Online Table 4). For the outcome of death, there was no significant interaction (p-interaction of 0.35 and 0.76 for sodium and chloride respectively). Similarly, there was no significant interaction for the composite outcome.
Discussion
By providing a comprehensive panel of markers of decongestion, this study provides the most robust evidence that we are aware of to date suggesting that the relation between acute declines in eGFR during hospitalization for ADHF and clinical outcomes is modified by decongestion. As long as there is concomitant evidence of decongestion (either by decreases in BNP, NT-proBNP or weight, or by increases in hematocrit, albumin, or total protein), a decline in eGFR is not associated with harm. However, if acute declines in eGFR are observed without evidence of concomitant decongestion, the declines in kidney function are associated with worse survival and increased risk of CV death and HF hospitalization.
There has been a growing body of literature examining the relation between acute declines in eGFR during hospitalizations for ADHF and clinical outcomes. While some prior studies have shown that declines in eGFR are associated with worse outcomes,6,8,9 others have suggested that the risk varies depending on the clinical scenario. For example, analysis of the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) trial found that among patients who had an increase in creatinine of ≥0.3 mg/dl, there was an associated risk of either death or HF hospitalization at 30-days only if there were concomitant signs and symptoms of volume-overload, but not otherwise (18). Similarly, in an analysis of the Ultrafiltration in Decompensated Heart Failure with Cardiorenal Syndrome Study (CARRESS) and Diuretic Optimization Strategies Evaluation (DOSE) trials, an eGFR decline was associated with a 22% decreased risk of death or HF hospitalization if NT-proBNP was decreasing, but not if NT-proBNP was increasing (10). In the current analysis, the test of interaction between eGFR decline and change in BNP, NT-proBNP or weight did not meet statistical significance in adjusted models. It is possible that the relation between eGFR and natriuretic peptides is partly confounded as these peptides are filtered by the kidney and thus excretion may decrease as kidney function declines. Overall, however, the trend remained consistent, suggesting that decline in eGFR is reflective of much poorer prognosis if decongestion is not being achieved, but not associated with outcomes if there are declines in BNP, NT-proBNP and weight.
The tests of interaction between eGFR decline and changes in hematocrit, albumin and total protein in this current analysis were highly significant, even after adjusting for baseline eGFR (range of p<0.001 to 0.01). Increases in hematocrit and concentrations of intravascular albumin and total protein have served as surrogates for decongestion; that is, decreased intravascular fluid. Prior studies evaluating hemoconcentration during treatment of ADHF have shown that an eGFR decline was not associated with adverse outcomes in patients showing the greatest increase in hematocrit, albumin or total protein (8). Prior analysis of the placebo arm of the EVEREST trial showed a 19% reduction in risk of death for every 5% increase in hematocrit. There were also higher rates of eGFR decline among the partients in the quartile with greatest hematocrit rise (18%) compared to 8–11% among the other three quartiles (9). This current analysis has also taken the additional step of examining hemoconcentration side-by-side with markers of volume overload, as a comparison of change in kidney function in the context of a comprehensive panel of surrogate measures of decongestion. When hemoconcentration is evaluated in this manner, the changes in hemoconcentration seem to have a stronger modifying effect on the relation between changes in kidney function and outcomes than biomarkers of volume overload. Overall, however, the pattern remains consistent that a decline in kidney function during hospitalization for ADHF carries different prognostic value depending on the state of decongestion. Furthermore, we also evaluated whether the relation between decline in eGFR and outcomes is modified by changes in sodium and chloride. Although levels of sodium and chloride are affected by changes in volume, they are also influenced by many other factors including sodium intake, water intake, antidiuretic hormone levels, acid-base status and diuretic use. We observed that changes in sodium and chloride did not modify the association between decline in eGFR and outcomes, perhaps suggesting that the interaction is stronger with more direct measures of decongestion.
There are a number of limitations. Patients missing repeat kidney function measurements were excluded. However, there were no substantial differences in baseline characteristics between patients with complete data and those with missing measurements. Patients with a creatinine of >3.5 mg/dl were not included in EVEREST, but as this is the highest cut-off for any trial of patients with ADHF, our analyses may in fact be more generalizable to patients with CKD and HF. Tolvaptan can have hemodynamic effects on eGFR but formal testing for an interaction between declines in eGFR and randomization to tolvaptan with outcomes was not significant (19). It is possible that eGFR may not be completely accurate given that many of these patients were not in steady state. However, repeating the analysis with changes in serum creatinine produced similar results. Additionally, use of percent change in eGFR allows for easier translation of these results to clinical application. Determination of change in biomarkers during the hospitalization was limited to the difference between two measurements, which allows for the potential of misclassification if some patients experienced changes that were not captured by those two measurements.
Conclusions
The relation between acute declines in eGFR and clinical outcomes among patients admitted for ADHF is modified by change in a comprehensive panel of markers of decongestion. That is, acute declines in kidney function were only associated with an increased risk of mortality and cardiovascular outcomes when markers of decongestion worsened, but not when markers of congestion improved. The interactions were stronger between decline in kidney function and markers of hemoconcentration than with markers of volume overload, however the trends were consistent.
Supplementary Material
Clinical Perspectives.
Competency in patient care:
Inclusion of a comprehensive set of biomarkers of decongestion, including measures of volume overload and hemoconcentration, should be incorporated into the interpretation of declines in kidney function that occur during hospitalizations for acute decompensated heart failure.
Translational Outlook:
Declines in kidney function are associated with different prognosis depending on changes in biomarkers of decongestion. This suggests that the mechanism responsible for the decline in kidney function should be taken into account when interpreting declines in kidney function. Further research into the mechanisms of kidney function decline among patients undergoing treatment for acute decompensated heart failure may help further guide clinical management.
Acknowledgments
Funding: NIH training grant T32 DK007777
Disclosures: JMT receives grant support from Otsuka as well as grants and consulting fees from BMS, consulting fees from AstraZeneca, consulting fees from Novartis, grants and consulting fees from 3ive labs, consulting fees from Cardionomic, consulting fees from Bayer, grants and consulting fees from Boeringer Ingelheim, consulting fees from MagentaMed, consulting fees from Reprieve Medical, grants and consulting fees from Sanofi, grants and consulting fees from FIRE1, grants from Abbott, consulting fees from W.L. Gore. JEU received research support from Otsuka. MAK received research support from Otsuka, SC Pharma, and serves as a DSMB chair for BMS.
Social Media: @McCallumMD. Tweet Summary: “Acute declines in kidney function during ADHF need to be interpreted in context of changes in markers of decongestion”
Abbreviations
- ADHF
acute decompensated heart failure
- CVD
cardiovascular death
- HF
heart failure
- eGFR
estimated glomerular filtration rate
- BNP
b-type natriuretic peptide
- NT-proBNP
N-terminal prohormone of b-type natriuretic peptide
- EVEREST
Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan
- ACEI
angiotensin-converting enzyme inhibitor
- ARB
angiotensin II receptor blocker
- MRA
mineralocorticoid receptor antagonist
References
- 1.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J. Card. Fail 2007;13:599–608. [DOI] [PubMed] [Google Scholar]
- 2.Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J. Am. Coll. Cardiol 2004;43:61–67. [DOI] [PubMed] [Google Scholar]
- 3.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol 2009;53:582–588. [DOI] [PubMed] [Google Scholar]
- 5.Testani JM, Khera AV, St John Sutton MG, et al. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am. J. Cardiol 2010;105:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronson D, Abassi Z, Allon E, Burger AJ. Fluid loss, venous congestion, and worsening renal function in acute decompensated heart failure. Eur. J. Heart Fail 2013;15:637–643. [DOI] [PubMed] [Google Scholar]
- 7.Uthoff H, Breidthardt T, Klima T, et al. Central venous pressure and impaired renal function in patients with acute heart failure. Eur. J. Heart Fail 2011;13:432–439. [DOI] [PubMed] [Google Scholar]
- 8.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010;122:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene SJ, Gheorghiade M, Vaduganathan M, et al. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Eur. J. Heart Fail 2013;15:1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCallum W, Tighiouart H, Kiernan MS, Huggins GS, Sarnak MJ. Relation of Kidney Function Decline and NT-proBNP With Risk of Mortality and Readmission in Acute Decompensated Heart Failure. Am. J. Med 2019. [DOI] [PMC free article] [PubMed]
- 11.Konstam MA, Gheorghiade M, Burnett JC, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 12.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzano-Fernández S, Flores-Blanco PJ, Pérez-Calvo JI, et al. Comparison of risk prediction with the CKD-EPI and MDRD equations in acute decompensated heart failure. J. Card. Fail 2013;19:583–591. [DOI] [PubMed] [Google Scholar]
- 14.Oh J, Kang S-M, Hong N, et al. The CKD-EPI is more accurate in clinical outcome prediction than MDRD equation in acute heart failure: data from the Korean Heart Failure (KorHF) Registry. Int. J. Cardiol 2013;167:1084–1087. [DOI] [PubMed] [Google Scholar]
- 15.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014;311:2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madan VD, Novak E, Rich MW. Impact of change in serum sodium concentration on mortality in patients hospitalized with heart failure and hyponatremia. Circ Heart Fail 2011;4:637–643. [DOI] [PubMed] [Google Scholar]
- 17.Kondo T, Yamada T, Tamaki S, et al. Serial Change in Serum Chloride During Hospitalization Could Predict Heart Failure Death in Acute Decompensated Heart Failure Patients. Circ. J 2018;82:1041–1050. [DOI] [PubMed] [Google Scholar]
- 18.Metra M, Cotter G, Senger S, et al. Prognostic Significance of Creatinine Increases During an Acute Heart Failure Admission in Patients With and Without Residual Congestion: A Post Hoc Analysis of the PROTECT Data. Circ Heart Fail 2018;11:e004644. [DOI] [PubMed] [Google Scholar]
- 19.Boertien WE, Meijer E, de Jong PE, et al. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 2013;84:1278–1286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.