Abstract
Modern biomedicine aims to develop integrated solutions that use medical, biotechnological, materials science, and engineering concepts to create functional alternatives for the specific, selective, and accurate management of medical conditions. In the particular case of tissue engineering, designing a model that simulates all tissue qualities and fulfills all tissue requirements is a continuous challenge in the field of bone regeneration. The therapeutic protocols used for bone healing applications are limited by the hierarchical nature and extensive vascularization of osseous tissue, especially in large bone lesions. In this regard, nanotechnology paves the way for a new era in bone treatment, repair and regeneration, by enabling the fabrication of complex nanostructures that are similar to those found in the natural bone and which exhibit multifunctional bioactivity. This review aims to lay out the tremendous outcomes of using inorganic nanoparticles in bone healing applications, including bone repair and regeneration, and modern therapeutic strategies for bone-related pathologies.
Keywords: bone regeneration, inorganic nanoparticles, bioceramic nanoparticles, oxide nanoparticles, metallic nanoparticles
1. Introduction
Bone is a dynamic tissue that is constantly renewed and repaired through its intrinsic remodeling process, which involves interactions between resident cells (osteoclasts and osteoblasts) and signaling factors, that remove old and damaged tissue and create new bone, respectively [1,2]. This fine-tuned synergy is responsible for the preservation of bone balance. The healing of bone fractures and the restoration of critical bone anomalies are difficult tasks for orthopedics, traumatologists, and maxillofacial surgeons [3]. Given the specific patient-related requirements and limitations in bone regeneration, the clinical use of synthetic bone substitutes represents one of the most important updates in bone regenerative therapy [4,5]. The current progress in nanotechnology-derived biomaterials enables the development of bone implants that are osteoconductive and osteoinductive, as well as biocompatible, biodegradable, and bioresorbable [6,7,8].
Nanobiomaterials include nanometer-sized and nanostructured bioactive materials, which peculiar behavior and new properties strongly impact the emerging trends of modern biomedicine and biotechnology [9,10]. Nanostructured biomaterials possess improved and superior bone regeneration ability thanks to their particular physicochemical properties and biological behavior, which are quite different from their bulk counterparts [11,12]. During the last decade, various nanoparticle-based protocols have been successfully evaluated for the diagnosis and targeted treatment of orthotopic and metastatic bone cancers [13,14]. The size of nanoparticles (NPs, 1–100 nm size range) permits their passage through biological barriers, while their size-related features (including a high surface area-to-volume ratio, surface energy and reactivity, mechanical, thermal, optical, electrical and magnetic properties governed by quantum effects, and intrinsic biological activity) enable them to attain significant therapeutic efficacy [15,16]. Moreover, nanoengineered platforms may increase drug solubility and improve drug bioavailability, but also enhance pharmacokinetics and pharmacodynamics, and provide specific and selective targeted and/or controlled therapeutic effects [17,18].
With the aim to overcome the drawbacks of classical restorative and replacement procedures of hard tissues (including herein the limited bioavailability and increased immunogenicity of autografts and allografts, but also the bioinertness and limited bioactivity of clinically approved biomaterials) [19,20], an impressive amount of progress has been reported in the development of bone regeneration materials during the last few decades. Biomaterials for hard tissue engineering applications include the following categories: (i) first-generation biomaterials—prosthetic devices made from biologically inert materials, such as metals and alloys, certain synthetic polymers, and bioceramics; (ii) second-generation biomaterials—osteoconductive and osteoinductive devices made from bioactive, biodegradable, and bioresorbable materials, such as calcium phosphates, bioactive glasses, and polyesters; and (iii) third-generation biomaterials—advanced and multifunctional biomaterials with osteogenic properties and the ability to regulate the body’s functions [21,22,23].
As the size-related behavior of NPs is also responsible for the occurrence of circumstantial toxic effects, a real challenge consists in maximizing their therapeutic effects by properly tuning the biocompatibility/multifunctionality balance. Nanosized particles can invade surrounding cells or tissues, and they frequently cluster or migrate inside blood vessels, causing additional damage to distant tissues or organs [24,25]. The toxicity of nanoparticles is determined by various parameters, including shape, size, composition, porosity, surface chemistry and coating, but other factors—such as the aggregation state and interactions with biomolecules—may influence their toxicity in humans [26,27].
Nanoparticle-based delivery systems have many advantages over conventional pharmaceutical formulations. These include reduced side effects, enhanced therapeutic effects, prolonged circulation half-life, improved permeability, and patient compliance [28,29]. Designing and developing performance-enhanced platforms for targeted or non-targeted drug delivery generally implies the precise selection of the nanocarrier, which can be (i) inorganic, including quantum dots (semiconductor-based nanoparticles), metallic (noble metals) and oxide nanoparticles, or (ii) organic, including carbon-based nanostructures, such as polymers, dendrimers, exosomes, micelles, liposomes, and solid lipid NPs [30,31].
Thanks to their high surface reactivity, unique surface physics and chemistry, increased chemical stability and photostability, facile surface modification, quantum yields, improved bioavailability, reduced or absent intrinsic toxicity, extended lifetime, great drug-loading capacity, and controlled drug release ability, inorganic NPs have indisputable advantages as active therapeutic carriers [32,33]. Moreover, by coating the inorganic NPs with additional surface ligands (i.e., proteins, peptides, carbohydrates, etc.), higher reactivity and enhanced functionality can be achieved [34,35]. In general, nanocarriers based on inorganic NPs consist of an inorganic core (metal-/oxide-based nanostructures) and an organic shell (carbon-based compounds, which serve as substrates for bio-macromolecular conjugation and/or as shields that protect the inner core from undesirable physicochemical interactions with the biological microenvironment) [36,37]. Biocompatible nanomaterials based on pristine and metal-doped calcium phosphates [38,39,40], bioceramics [41,42] and vitroceramics [43,44], oxides (such as alumina, ceria, silica, titania, and zirconia) [45,46,47,48,49], and metallic nanostructures [50,51,52] are extensively investigated for the unconventional management of bone tissue injuries.
This review aims to point out the significance of inorganic nanoparticles in bone healing by including relevant and recent data on the NP-based repair and regeneration of bone tissue.
2. Bioceramic Nanoparticles
2.1. Hydroxyapatite
The conventional therapeutic strategy in bone grafting mainly includes the use of allografts and autogenous grafts, and also different isolated or combined substitutes based on calcium phosphate (CaP) materials [53,54]. CaP-based nanoparticles have been extensively investigated in preclinical and clinical studies as bone graft alternatives [55,56]. The use of CaP nanoparticles can be expanded towards cell-/tissue-specific drug delivery platforms owing to their intrinsic features, such as unique biocompatibility and bioactivity, high adsorptive capacity, composition-/microstructure-related tunable properties, and application-related adjustable biodegradability [57,58].
Particularly successful and promising outcomes in designing biomaterials for hard tissue repair and replacement are related to synthetic hydroxyapatite (HA), Ca10(PO4)6(OH)2 [58,59]. Naturally, HA is present in metamorphic and igneous rocks as a natural mineral, but it is also present in teeth and bones as the major inorganic component [60,61]. Tremendous interest has been lately oriented towards the revaluation of naturally-derived HA, which can be extracted from sustainable biogenic sources or wastes [62,63,64,65]. Representative sources for extracting natural HA include: (i) mammalian sources—bovine [66,67,68], ovine [69,70], and swine [71,72] bones; (ii) marine or aquatic sources—fish bones [72,73,74], cuttlefish bones [75,76], and corals [77,78]; (iii) shells—cockle shell [79,80], clam shell [81,82], mussel shell [83,84], snail shell [85,86], and egg shell [87,88]; and (iv) mineral sources [89,90].
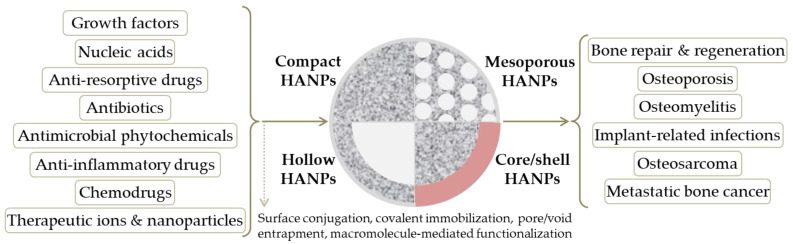
Nanosized HA particles have more unique properties than micro-sized HA particles. For example, it has been reported that nanosized HA exhibits greater protein adsorption, improved cell adhesion, and superior bioactivity when compared to micro-sized HA [60,91]. It also possesses a significant capability to decrease apoptotic death in healthy cells and, therefore, improve cell proliferation and cell activity related to bone growth [91,92]. Given their compositional similarity with the natural bone tissue and their ability to increase new bone formation [93,94,95], HA nanoparticles (HANPs) are regarded as safe candidates for bone-targeted therapy, as summarized in Figure 1.
Figure 1.
Schematic representation of hydroxyapatite nanoparticles (HANPs) in bone healing applications.
Possessing excellent biocompatibility and being highly bioactive and biodegradable, HA is widely used for orthopedic, dental, and maxillofacial applications, especially thanks to the unique features of HANPs, which include anti-tumor activity and drug/gene delivery potential [96,97,98]. Even though the intrinsic biocompatibility of nano-hydroxyapatite has been extensively confirmed, recent studies have argued that a thorough screening of HANPs’ toxicity should be conducted to assess their biological effects, as the potential biotoxicity of HANPs (affected by particle diameter, exposure dose, and contact method) was reported [91,99].
Although HA is considered to be a suitable material for bone tissue repair and regeneration, its osteoinductive qualities are insufficient to allow large bone defects to mend. To circumvent these drawbacks, several bioactive compounds including growth factors that play a key role during the bone remodeling process, have been employed in bone tissue engineering [100,101,102,103,104]. Osteoinductive growth factors have been utilized in restorative and regenerative procedures for dental [7,105] and orthopedic (craniofacial, spinal fusion and non-union deformities) [54,106,107] pathologies, either alone or combined with ceramic and polymeric or composite materials, with little indication that they are superior to autografts. Bone morphogenetic protein-2 (BMP-2) is the gold standard growth factor for enhancing bone healing, and it has been successfully used in various research studies. In terms of osteogenic activity and augmented bone healing, superior results were reported for BMP-2-modified nanostructured formulations based on HA/natural polymers [108,109] and HA/synthetic polymers [110,111]. However, due to its short half-life in vivo, the clinical applicability of BMP-2 is limited, as a suitable BMP-2-loaded bone substitute should accurately provide initial large doses and subsequent constant therapeutic concentrations [112]. Promising HANP-based formulations for orthopedic and orthodontic applications have also been developed via modification with other bone morphogenetic proteins (BMPs) [113,114], fibroblast growth factor (FGF) [101,115], and vascular endothelial growth factor (VEGF) [116,117] (Figure 2), which beneficially contribute to bone matrix mineralization, osteoblastogenesis and new bone formation, implant osteointegration, and vascularization.
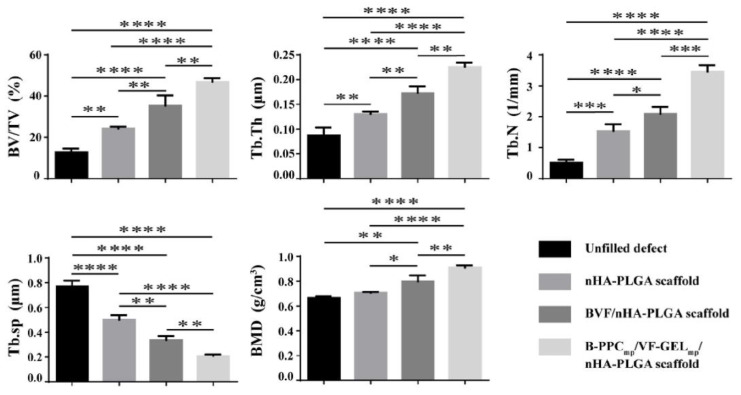
Figure 2.
Quantitative representation of bone regeneration induced in rat femur defects by bare nano-hydroxyapatite/poly(lactide-co-glycolide) scaffolds (nHA-PLGA), nHA/PLGA scaffolds modified with BMP-2, VEGF, and FGF-2 (BVF/nHA-PLGA), and nHA/PLGA scaffolds modified with BMP-2-loaded poly(lactic-co-glycolic acid)-poly(ethylene glycol)-carboxyl microparticles and VEGF/FGF-2-loaded gelatin microparticles (B-PPCmp/VF-GELmp/nHA-PLGA), evidenced at 12 weeks post-implantation by bone volume fractions (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.n), trabecular spacing (Tb.Sp), and bone mineral density (BMD). Each data point represents the mean ± standard deviation (n = 3), and statistically significant differences are indicated as ∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001, and ∗∗∗∗ p < 0.0001. See Ref. [117]. Reprinted from an open access source.
The synergistic efficacy of HANPs coupled with anti-osteoporotic compounds has been demonstrated. Nitrogen-containing bisphosphonates inhibit specific protein and enzyme mechanisms within osteoclasts, thus interfering with their activity by triggering the cellular apoptosis and disrupting the cellular ultrastructure [118,119]. Several studies evidenced the anti-osteoporotic efficiency of HANP-based materials modified with alendronate [97,120], risedronate [121,122], and zoledronate [123,124]. By inhibiting osteoclast-mediated bone resorption, bisphosphonate-modified HA-based constructs—such as coatings [125,126], scaffolds [109,127], and injectable formulations [128,129]—determine a net improvement in osteogenic processes. Recently, HANPs loaded with salmon calcitonin polypeptide were proposed for the sublingual management of osteoporosis [130]. Promising results were also evidenced for HA-based biomaterials loaded with an anti-resorptive agent (denosumab) [131] or an anabolic agent (teriparatide) [132].
Following the development of the promising strontium ranelate (SR) (Protelos®/Protos®, Servier Laboratories, Surene, France) anti-osteoporotic drug, a variety of studies have been conducted, ranging from strontium (Sr) mapping in bones and teeth to investigating Sr incorporation into bone mineral (in particular, in the crystal surface and lattice) and a decrease in calcium content, and to evaluating Sr effects in synthetic HA. Sr has a dual positive effect during osteogenesis and bone remodeling, by boosting the development of pre-osteoblastic cells, while suppressing the generation and functionality of osteoclastic cells [133,134]. By gathering the distinctive advantages of HA and Sr, their composites represent a suitable choice for the controlled and targeted therapy of bone tissue [135,136,137].
Other studies revealed the significance of zinc (Zn)-enriched HA nanomaterials for the repair and regeneration of traumatic and osteoporotic bone tissues, as it has been evidenced that Zn addition is beneficial for enhanced osteogenesis and the prevention of osteoclast-mediated resorption [138,139,140].
Selenium (Se) is a vital micronutrient for human health, as it plays an important role in disease prevention and cellular pathophysiological balance maintenance. In this respect, Se-modified HA nanomaterials proved to be promising alternatives for bone tissue therapy, since the presence of Se synergistically determines enhanced cellular processes in healthy cells (adhesion, migration, proliferation, and osteogenic differentiation) [141,142] and significant apoptotic damage in cancerous cells [143,144].
In order to increase the structural integrity and to modulate interactions between the biological microenvironment and inorganic nanostructures, the surface modification of HA-based nanomaterials was explored [145,146]. The hydroxyl-abundant surface of HA is responsible for beneficial interactions with organic compounds, resulting in surface silanization and covalent bonding [147,148,149], immobilization and grafting [150,151,152]. Coupling natural [153,154] or synthetic [155,156] polymers onto the surface of HANPs has been shown to improve the NPs’ colloidal stability and mechanical qualities, together with their biofunctional outcomes. When used as bone-filling materials, such composite or hybrid structures can additionally act as active depots for the long-term release of pharmaceuticals, including drugs [157,158,159] and biomolecules [160,161,162].
Particular attention was oriented towards the fabrication of HANP-modified polymeric scaffolds, given the fact that a higher amount of nanoparticles triggers and accelerates the nucleation of biomimetic apatite, finally resulting in increased bone formation [146,163]. Designing HA/polymer constructs for bone tissue engineering requires fulfilling some essential aspects: (i) structural requirements: tissue-mimicking composition and architecture, adequate mechanical behavior, and highly porous interconnected structure, which are responsible for the osteoconductive and osteoinductive outcomes, as well as for proper cellular migration and normal development, oxygenation and nutrition, and vascularization; and (ii) biological requirements: biocompatibility, nontoxicity, non-immunogenicity, and biodegradability, which are vital aspects for enhanced osteogenesis and host integration [164,165,166].
Owing to their excellent biodegradability and nontoxicity, and particular resemblance with the natural extracellular matrix, natural polymers—such as proteins (e.g., collagen [109,167], gelatin [168,169], silk fibroin [170,171]) and polysaccharides (e.g., chitosan [172,173], cellulose [174,175], alginate [176,177])—are indisputable candidates for bone healing applications. The modification of such scaffolds with HA-based formulations represents an attractive strategy to overcome their intrinsic restrictions (improper mechanical properties, uncontrollable degradability, immunogenicity, and microbial contamination susceptibility).
In comparison with natural polymers, synthetic polyesters (e.g., polylactide (PLA) [178,179], poly(lactide-co-glycolide) (PLGA) [180,181], polycaprolactone (PCL) [182,183], and polyhydroxyalkanoates [184,185]) provide superior mechanical performance, increased chemical and structural stability, and tunable biodegradability. However, due to their intrinsic limitations (including hydrophobicity, slower degradation rate, and problematical metabolization/excretion of their byproducts), additional alterations are required to fabricate superior HA-modified bioactive scaffolds for bone healing.
As particular representatives of HANPs, mesoporous nanostructures have gained great attention regarding the development of nanostructured platforms for the controlled therapy of bone tissue [186,187]. It has been demonstrated that mesoporous HANPs represent efficient nanocarriers for growth factors [188,189,190], antimicrobial ions [191,192] (Figure 3), antibiotics [193,194], and anti-tumor drugs [195,196,197], as a result of their uniform, accessible, and highly organized porous microstructure.
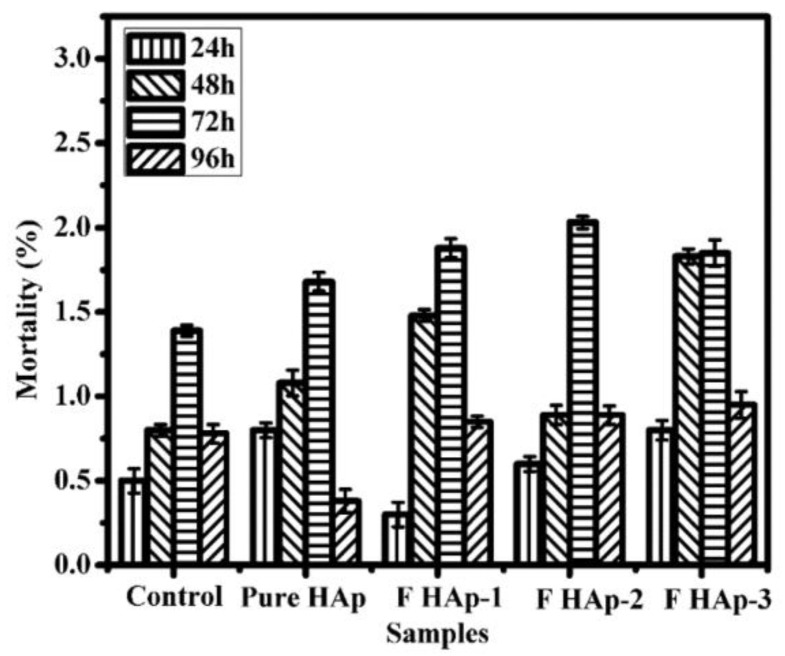
Figure 3.
Quantitative representation of mortality (death rate, %) in zebrafish embryos treated with mesoporous fluoride-doped nano-hydroxyapatite (0.6, 1.2, and 3.2 at.% for FHAp-1, FHAp-2, and FHAp-3, respectively) with respect to time and concentration. The as-developed FHAp nanorods also exhibited important concentration-dependent antibacterial effects against Pseudomonas aeruginosa and Bacillus subtilis. See Ref. [192]. Reprinted from an open access source.
HANP-based therapeutic strategies have a lot of promise for bone tissue engineering, which represents a complex and challenging research field of modern biomedicine [198]. The characteristics of HA-based nanomaterials can be accurately optimized during the synthesis, in order to fabricate low-cost and performance-enhanced advanced biomaterials for therapeutic usage [199,200]. Nanofabrication techniques can provide precise control over the physicochemical and microstructural features of HANPs, which are mandatory for achieving spatial control over cell behavior, while imparting the necessary structural properties [201,202].
2.2. Bioactive Glass
Bioactive glasses (BGs), with their indisputable and versatile silica-based representatives, are amorphous solids which compositional and structural characteristics have been proved beneficial for the development of bioactive substitutes and platforms for bone tissue repair and regeneration [112,203]. BGs, firstly introduced in the early 1970s, opened up a new direction towards bone tissue therapy, as their intrinsic features (rapid and stable bonding with living tissues and surface-mediated reactions that encourage biomimetic apatite formation under physiological conditions) became prototypical requirements for designing bioactive materials [203,204].
An increased SiO2 content in silica-based BGs (of maximum 60%) is responsible for their strong bonding with the bone tissue (i.e., direct BG/bone interface, without fibrous connective tissue), which further provides enhanced interactions between surface-generated bone-like apatite layer and collagen fibers [203,205]. Besides the intrinsic osteostimulative characteristics of silicon-containing bioceramics [206,207], it has been evidenced that subsidiary ions released by the dissolution of BGs (calcium, sodium, and phosphorous) contribute to bone repair and regeneration by accelerating mineralization, stimulating cellular processes (proliferation, migration, and differentiation), and regulating the molecular mechanisms (protein and gene expression) involved in osteogenesis and angiogenesis [208,209,210]. The bioactivity of silica-based BGs can be further boosted by incorporating other ions that provide additional immunomodulatory and/or antimicrobial functions, such as magnesium [211,212], zinc [213,214], copper [215,216], silver [217,218], and strontium [219,220]. In addition to conventional BGs, phosphate-based [221,222,223] and borate-based [224,225,226] bioactive glasses have been explored for bone healing applications, but they require extensive composition-related control over their stability, dissolution, and biological activity [227,228].
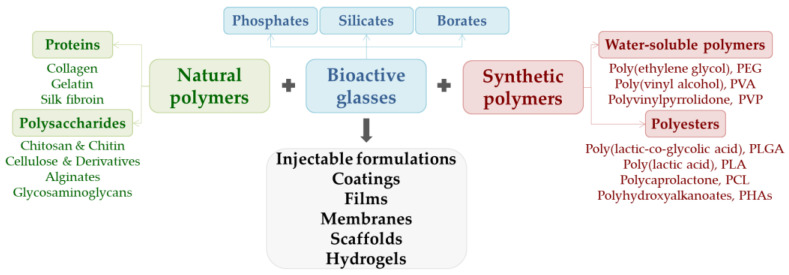
Besides encouraging stable bonding with host tissues, BGs also provide active sites for favorable interactions with polymers, both natural and synthetic, as briefly evidenced in Figure 4 [210,229,230]. BG/polymer composites possess advanced functionality in terms of mechanical performance, microstructure, reactivity, biodegradability, osteostimulation, and osteogenesis, thus representing suitable candidates for bone tissue engineering and regenerative medicine [210,229,231]. Since the key features of BGs, such as solubility and bioactivity, can be enhanced by changing the structure and particle size (at the nanoscale level), nanosized BGs are attractive and versatile fillers for biodegradable polymers when it comes to the fabrication of advanced composites for bone healing [232,233,234].
Figure 4.
Schematic representation of bioactive glass/polymer composites in bone healing applications.
Because of their large specific surface area and rapid ion release rate in biological fluids, nanoscale bioactive glass particles display higher bioactivity than microscale bioactive glass particles. However, the conventional synthesis of bioactive glass nanoparticles (BGNPs) is challenging and problematic due to the difficulty of doping high amounts of calcium ions within the silica network, resulting in uneven distribution and low calcium content. Furthermore, BGNPs are often synthesized by using dilute solutions in order to avoid nanoparticle aggregation, thus reducing the production efficiency and raising the costs. Reactive flash nanoprecipitation [235] and ultrasound-assisted sol–gel [236,237] were proposed as successful alternatives for the traditional sol–gel synthesis of BGNPs, resulting in particles with a more homogenous calcium-enriched composition, smaller size and narrower size dispersion, and superior bioactivity.
The ability to incorporate active ions within their composition is a significant advantage of BGNPs over other inorganic nanoparticles, as the release of such ions during dissolution opens up a world of possibilities for enhancing the biofunctional outcome of nanoengineered composites. Doping BGs with antimicrobial ions represents a promising strategy for the fabrication of bone fillers or bone grafts that can allow bone repair and regeneration without the risk of post-implant infections [238,239]. Therefore, the potential use of BGs doped with zinc (Zn)—Zn-BGs—was thoroughly investigated [240], as the presence of Zn determined antibacterial effects, and also contributed to enhanced mineralization and osteogenic activity [241,242]. Beneficial effects with respect to in vitro mineralization, cellular development, and antimicrobial efficiency, were also evidenced in the case of silver (Ag)-doped BGs (Ag-BGs) [218,243].
Despite the promising results reported in BGNP-based composites and devices, significant efforts must be made in order to fully explore and beneficially revalue the biological potential of such nanomaterials, as there is a lack of data regarding the long-term in vivo safety and performance of BGNPs [244,245].
In comparison with conventional BGNPs, mesoporous bioactive glass nanoparticles (MBGNPs) provide additional advantages regarding the microstructure-related ability to load and release therapeutic agents, representing multifunctional platforms for bone healing applications. MBGNPs are usually obtained by sol–gel-mediated protocols [246,247], and their versatile composition enable the incorporation of different therapeutic compounds, including copper [248,249], silver [218,250], and zinc [251,252] for antimicrobial effects, osteogenic activity, and immunomodulation; strontium for pro-osteogenic and pro-angiogenic effects [253,254]; cerium and gallium for antibacterial activity and bioactivity [255,256]; cobalt [257], iron [258], selenium [259], and tellurium [260] for anti-cancer effects.
In addition to their intrinsic capabilities (osteoconductive, osteoinductive, and angiogenic effects), MBGNPs represent attractive nanocarriers for the controlled and targeted delivery of antibiotics [261,262] (Figure 5), anti-osteoporotic drugs [263,264], chemotherapeutic agents [265,266], and biomolecules [267,268], thus providing an unrivaled and prospective edge towards designing innovative smart materials for bone tissue therapy [246,269,270].
Figure 5.
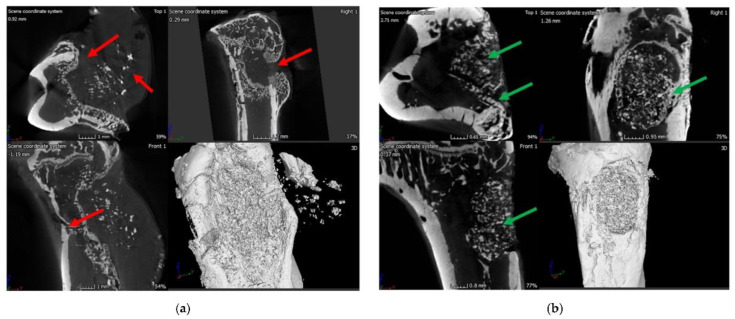
Micro-computed tomography (μ-CT) images of the infected rat tibia (control group), evidencing signs of infection at 8 weeks: narrowing of marrow space, presence of puss-filled fibrous capsule, sinus tract, and deformed bone with ectopic bone growth (red arrows) (a). μ-CT images of the infected rat tibia treated with vancomycin-loaded polymer/BG bone void-filling putty at 8 weeks post-implantation, evidencing signs of healing bone, as well as the formation of cortical and cancellous bone in the drilling space (green arrows) (b). The as-developed scaffolds also determined the in vivo eradication of Staphylococcus aureus. See Ref. [262]. Reprinted from an open access source.
3. Oxide Nanoparticles
3.1. Mesoporous Silica
Silicon (Si) is naturally found in the human body, and it has a regulatory role during the normal development of the skeleton and connective tissues, and also has beneficial effects during collagen synthesis and matrix mineralization [271,272]. Besides representing a major source of Si ions, silica (SiO2)-based nanomaterials—especially mesoporous silica nanoparticles (MSNs)—provide attractive and tunable characteristics for biomedical applications, including drug/biomolecule delivery systems [273,274,275], tissue engineering [276,277,278], regenerative medicine [279,280,281], and cancer therapy [282,283,284].
A large surface area and pore volume ratio, adjustable particle size, well-structured internal and external porosity, uniform and controllable pore size, impressive surface functionalization, and intrinsic biocompatibility, represent the key features of MSNs used for the fabrication of therapeutic biomaterials and devices [285,286,287]. The porosity characteristics of MSNs can be explored for loading various therapeutics, including biomolecules, soluble and insoluble drugs, targeting molecular drugs, and imaging agents, as well as their different combinations, which may be simultaneously released within the impaired tissues to achieve improved local concentration and synergistic drug therapy and diagnostics (theranostics) [288,289,290]. Moreover, the pore-opening gating mechanisms distinguished in MSNs provide indisputable advantages over the controlled release of the therapeutic cargo in response to internal (e.g., weakly acidic local microenvironment, cancer-overexpressed enzymes, or other biomolecules) and external (e.g., light, ultrasound, and magnetic field exposure) stimuli [291,292].
Although MSNs represent one of the most appealing nanomaterials for the fabrication of performance-enhanced constructs for bone healing applications, some critical parameters must be considered in order to achieve the desired therapeutic effects. By optimizing the synthesis parameters (such as the type of silica precursor, the pH and temperature during the reaction, and the type and concentration of surfactant), the size, morphology, and porosity of MSNs can be modified [293,294]. Conventional and modified sol–gel, evaporation-induced self-assembly, and core-templating synthesis (in the case of hollow MSNs) represent the most explored strategies for fabricating MSNs with controllable particle and pore sizes [295,296].
Vital events involved in bone repair and regeneration, including cellular proliferation and differentiation, bone matrix mineralization, osteoinduction, and osteogenesis, can all be triggered or boosted by means of Si-enriched nanosized and nanostructured materials [297,298]. Through their modulatory effects on the specific molecular complexes involved in bone homeostasis, MSNs stimulate pro-osteoblastic action and mineralization, induce osteogenic differentiation and angiogenesis, and inhibit osteoclasts, thus influencing the osteoblast/osteoclast ratio [47,299,300]. Moreover, the bone healing process can be promoted or accelerated by loading osteoinductive proteins [301,302] and related encoding peptides [303] or encoding plasmids [302,304] (Figure 6) within MSN-based formulations. Besides their intrinsic bioactivity, MSNs exhibit impressive opportunities for fabricating multifunctional platforms for bone healing therapy, as their distinctive open porous microstructure enables the incorporation and release of various therapeutic molecules [305,306].
Figure 6.
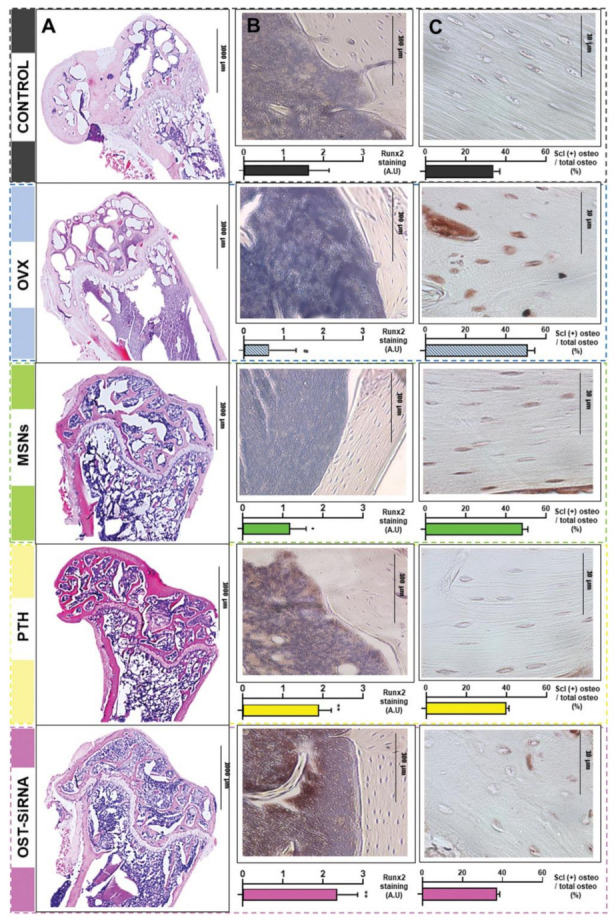
Histological analysis and immunostaining in the femur of osteoporotic mice at 3 weeks post-treatment with mesoporous silica nanoparticles (MSNs) grafted with alendronate-modified poly(ethylene glycol) and poly(ethylene imine) (MSNs-PA@PEI), parathyroid hormone (PTH), and MSNs-PA@PEI loaded with osteostatin and sclerostin-encoding plasmid (OST-SiRNA), evidencing: representative micrographs of different femur histological sections after hematoxylin/eosin and Masson–Goldner trichrome staining (A); representative Runx2 immunostaining in mice femurs, revealed by the abundant positivity (brown stains) for the transcription factor in cells after PTH or OST-siRNA treatments (B); total and sclerostin-positive osteocytes in the cortical femur (C). Data are represented as mean ± standard error of mean of five independent mice (n = 5), and the statistical significance is indicated as # p < 0.001 vs. control, * p < 0.05 vs. ovariectomized mice (OVX), and ** p < 0.001 vs. OVX. See Ref. [302]. Reprinted from an open access source.
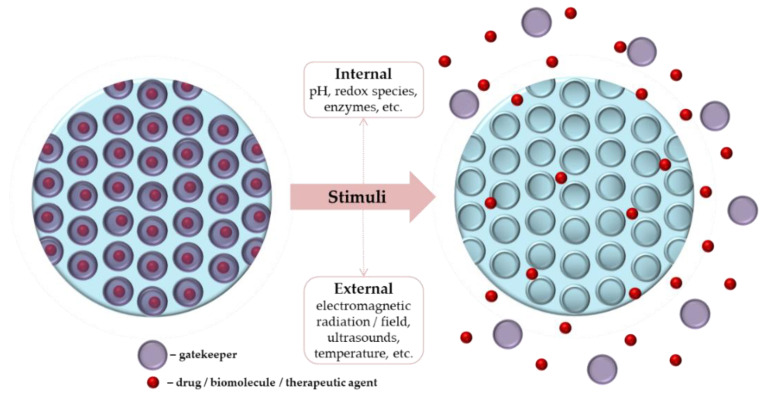
MSNs possess an impressive ability to transport therapeutic biomolecules and active targeting molecules into impaired bone cells, thus representing attractive multifunctional platforms for bone tissue therapy. In addition, the premature and non-specific release of the therapeutic cargo can be limited or even eliminated by using gatekeepers (e.g., nucleotides, natural or synthetic polymers, and metallic nanoparticles) that block pores and provide on-demand pore opening and closing in response to certain stimuli (Figure 7) [307,308,309]. The as-fabricated MSN-based platforms can act as active carriers for chemo drugs, anti-resorptive agents, antibiotics, and genes, providing targeted and controlled therapy for bone-related pathologies, in addition to their intrinsic bone healing effects [307,310].
Figure 7.
Schematic representation of stimuli-responsive mesoporous silica nanoparticles (MSNs).
The incorporation of MSNs within three-dimensional nanoengineered networks provides tremendous possibilities for the specific and selective management of bone infection and bone cancer [310,311,312]. Besides their compositional and structural resemblance with the natural tissue, artificial scaffolds exhibit increased loading efficiency and modulated release of pristine or nanosystem-conjugated drugs/biomolecules [313].
MSN-based nanosystems have been evaluated as efficient loading/releasing vehicles for several antibiotics [314,315,316]. Moreover, composite scaffolds incorporating cephalexin-loaded MSNs [276] and vancomycin-loaded MSNs [317] proved to represent promising candidates for the local treatment of bone infection, while promoting bone healing.
The specific and selective management of bone cancer can be achieved with MSNs-based carriers that target particular receptors that are overexpressed in cancer cells [318,319,320]. The cellular uptake of such nanostructures can also be improved by considering particular features of the tumor microenvironment [321,322] or by altering the intrinsic regulatory mechanisms of highly metabolically active cancerous cells [323,324,325]. Moreover, the versatile functionality of MSNs can also be explored for developing unconventional anti-cancer strategies by means of non-radioactive and controlled alternatives mediated by nanostructures conjugated with active targeting molecules and loaded with reduced drug concentrations or/and sono/photosensitizers [326,327,328].
3.2. Iron Oxide
Magnetic nanoparticles (MNPs) possess magnetic, semiconductor, nontoxic, and bioactive properties all at once, and play a critical role in the progress of modern biomedicine, with particular outcomes towards the specific and selective therapy of bone tissue [329,330]. The biomedical versatility of iron oxide nanoparticles, as particular representatives of the magneto-responsive nanostructures, relies on their multifunctional size-related features, such as intrinsic biocompatibility and biodegradability, surface chemistry and reactivity, and tunable magnetism (with particular superparamagnetic behavior for ultra-small MNPs) [331,332].
Besides their intrinsic size-governed anti-infective [333,334,335] and anti-tumor effects [336,337,338], the surface modification of MNPs with inorganic capping layers [339,340,341], therapeutic molecules [342,343,344], and biomolecule-conjugated macromolecule layers [345,346,347] paves the way towards the fabrication of accurate and efficient strategies for bone healing. The impressive functionalization potential of superparamagnetic iron oxide nanoparticles (SPIONs) enables the fabrication of active platforms for bone repair and regeneration, as well as for bone infection and cancer. Such magnetic nanostructures can act as active vehicles and therapeutic enhancers for their cargo, but their functionality can be extended by means of external triggers (electromagnetic radiation and fields), which represent the leading advantage of MNP-based biomedicine [348,349,350].
Following their exposure to an alternating magnetic field, MNPs undergo important magnetic relaxation, as their magnetic moment (given by unpaired spin electrons in the outermost electron shell) rapidly flips its orientation between two stable states, but they also can undergo physical rotation and circumstantial collisions, finally resulting in converting the external energy into heat [351,352]. This peculiar behavior of MNPs gives them an impressive potential for the local thermally-induced alteration of pathological cells by means of magnetic hyperthermia, which is being extensively investigated for cancer management [353,354]. Moreover, if therapeutic agents are conjugated to MNPs, their local release can be externally triggered and controlled. Even if the clinical application of magnetically targeted therapy by means of magnetized medications still requires regulatory protocols [355,356], the preclinical evaluation of SPION-mediated bone cancer therapy is of great interest. Besides acting as mechanical reinforcements for polymeric scaffolds, SPIONs contribute to the normal development of bone cells and promote the mineralization process and osteogenic activity [357,358,359], and also promote the in vivo bone repair and regeneration [359,360,361]. In addition to their ability to generate localized hyperthermia while avoiding the impairment of surrounding normal tissues when combined with SPIONs, it has been reported that magnetic fields are beneficial for promoting the osteogenic activity of progenitor cells. Magnetic fields regulate the cellular uptake of SPIONs via stem cells and preosteoblasts and promote their osteogenic differentiation and bone matrix mineralization, and also contribute to their proliferation, migration, and organization inside scaffolds [362,363], finally resulting in magnetically guided osteogenesis and angiogenesis [364,365,366]. SPION-loaded constructs (e.g., porous inorganic scaffolds, polymer sponges, and hydrogels) and external magnetic fields synergistically act to provide successful therapeutic alternatives for bone healing [329,367].
Magneto-responsive HA/SPIONs composites have been investigated particularly for bone healing applications owing to their synergistic effects. HA/SPIONs formulations exhibit intrinsic antimicrobial effects [368,369] while promoting osteogenesis and neovascularization and inhibiting osteoclastogenesis [370,371,372] (Figure 8). In addition, their drug carrier ability opens the way for efficient and accelerated infection-free bone repair [97,373].
Figure 8.
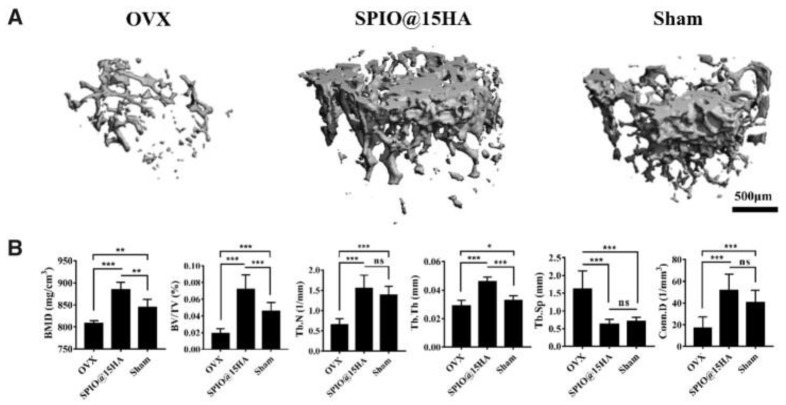
Three-dimensional μ-CT reconstruction images of trabecular bone in ovariectomized mice (OVX), OVX treated with hydroxyapatite-coated superparamagnetic iron oxide nanocomposites (SPIO@15HA) and sham group (A), and trabecular bone mass parameters (B), evidenced after 3 months post-injection. BMD—bone mineral density, BV/TV—bone volume fractions, Tb.N—trabecular number, Tb.Th—trabecular thickness, Tb.Sp—trabecular spacing, Conn.D—connectivity density. Data are expressed as mean ± standard deviation of seven independent mice (n = 7), ns means no significance, and the statistical significance is indicated as * p < 0.05, ** p < 0.01, and *** p < 0.001. See Ref. [370]. Reprinted from an open access source.
Given the extensive use of metallic implants in the clinical restoration and replacement of bone tissue, an attractive nanotechnology-derived approach consists of enhancing their bioactivity and osteogenic activity using surface coatings [374,375,376]. It has been reported that the incorporation of SPIONs within HA [377,378] or polymer [379,380] coatings leads to significant improvements in the wettability and corrosion resistance of titanium-based biomaterials, and also enhanced apatite-forming ability and cellular events. As the direct interactions between SPIONs and therapeutic agents determine the formation of highly stable nanosystems with potentiated therapeutic effects, such nanostructures have been extensively investigated with respect to the development of new pharmaceuticals [35,381,382]. The therapeutic outcome of metallic implants can be achieved by means of synthetic polyester coatings embedded with MNPs conjugated with natural antimicrobial extracts [383], electroactive polymer coatings embedded with antibiotic-functionalized MNPs [379,380], and chemo drug-loaded SPIONs/cyclodextrin coatings [384].
3.3. Other Oxides
The therapeutic implications of other oxide nanoparticles in bone healing applications have been also explored [385,386]. For instance, magnesium oxide (MgO) and zinc oxide (ZnO) nanoparticles have been investigated for the fabrication of functional bone substitutes [387,388]. MgO and ZnO NPs exert strong antimicrobial and anti-biofilm activity [389,390], and also antioxidant effects [391,392], making them suitable candidates for boosting the performance of HA-based substitutes [393,394,395,396].
Following their dissolution, MgO NPs provide mineral nutrients that are essential for most biological processes, including new bone formation, by promoting osteogenic proliferation and differentiation and bone-like mineral deposition [397,398,399]. By exerting positive immunomodulatory effects, MgO NPs indirectly suppress the activity of osteoclasts [400]. Besides acting as mechanical reinforcements for polymeric scaffolds, MgO NPs also modulate their hydrophilicity and degradation, whilst the polymeric matrix enables the gradual release of therapeutic ions, finally resulting in enhancing the bone healing ability of such composites [401,402].
Given the fact that an imbalance in the normal zinc deposits and cellular zinc homeostasis may occur after bone tissue injuries (as the human skeleton is a major source of zinc), producing zinc-enriched substitutes is of great importance for bone healing and normal skeletal development [403,404]. ZnO NPs synergistically act on the bone cells involved in bone formation and remodeling by inducing osteogenic effects [405,406] and modulating the osteoclastogenic events [407,408]. The oxidative events induced by ZnO NPs (mediated by free zinc ions and reactive oxygen species) can be further explored for bone tissue regeneration and bone cancer therapy through their pro-angiogenic [409,410] and anti-angiogenic [411,412] properties, respectively.
It has been evidenced that cerium oxide (ceria) NPs stimulate the osteogenic differentiation of stem cells and regulate bone mineralization, and also exhibit antioxidant effects (which are beneficial for limiting the oxidative events that may occur during slow bone regeneration and bone-related inflammatory pathologies) [413,414,415]. Nano-ceria also modulates the angiogenesis process of ceramic and polymeric biomaterials following their implantation, resulting in accelerated new bone formation [416,417] (Figure 9). Moreover, the stimuli-responsive ability of ceria NPs [418,419], together with their radio-protective effects [420,421] and intrinsic antibacterial effects (evidenced against extracellular and intracellular pathogens) [422,423], open up new ways for the efficient treatment of bone diseases.
Figure 9.
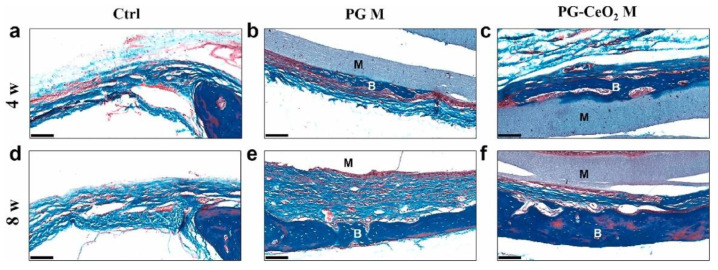
Histological analysis of rat cranial defects treated with bare and nano-ceria-loaded polycaprolactone/gelatin membranes (PG M and PG-CeO2 M, respectively) for 4 and 8 weeks (w), evidenced by Masson’s trichrome staining. Control group (a,d), PG M group (b,e), and PG-CeO2 M group (c,f). M—membrane, B—bone, scale bar—100 μm. See Ref. [417]. Reprinted from an open access source.
Recently, hollow manganese oxide NPs were proposed as efficient platforms for the immunotherapy of osteosarcoma, with their additional tumor-targeting ability and imaging-guided drug delivery [424]. These oxide nanoparticles exhibit important osteogenic activity and bone-forming ability [425,426], while their excellent antioxidant effects proved to be beneficial for the management of osteoarthritis [427,428].
A significant improvement in the mechanical behavior and thermal stability of polymeric biomaterials has been evidenced after the incorporation of titanium oxide (titania) NPs, with such nanostructured platforms being proposed for the long-term use in bone regeneration [429,430]. The efficiency of nano-titania on osteoblast/osteoclast homeostasis [431,432] and collagen deposition (by inducing the secretion of biomolecules that actively regulate bone repair) [433], without affecting the differentiation and mineralization of osteoblasts [433,434], has been reported.
4. Metallic Nanoparticles
This review also covers the implications of metal-based nanoparticles in bone tissue therapy. Owing to their peculiar nanosize-related characteristics, which include biomechanics and thermochemistry, stability and optical behavior, reduced toxicity and good biocompatibility, proliferative and intrinsic osteogenic potential, cellular development modulation, and intrinsic antimicrobial and anti-cancer effects, metallic NPs are versatile candidates for bone healing applications [385,435].
4.1. Gold
Gold nanoparticles (AuNPs) are biocompatible and inert nanosized structures with high monodispersity, electroconductivity, and excellent optical properties [436,437]. The impressive use of AuNPs in modern biomedicine relies on their highly remarkable surface functionalization potential, and includes targeted therapeutic formulations (drug, macromolecule, peptide, protein, and gene delivery), biomedical imaging and diagnosis (biodetection and biosensing), and complex therapy (photothermal, photodynamic, and radiation therapy) [438,439,440].
In relation to bone healing therapy, it has been evidenced that AuNPs exhibit intrinsic osteogenic effects (by promoting the differentiation of pluripotent cells and biomimetic apatite formation) [441,442], inhibit osteoclastogenesis [443,444], and accelerate de novo bone formation [445,446]. Several molecular mechanisms were proposed for AuNP-mediated osteogenic differentiation [439,447]. Stem cells may undergo osteogenic differentiation in response to extracellular AuNPs (physical and/or chemical modification of the microenvironment) and intracellular AuNPs (mechanical stress) by means of the integrin-mediated signaling pathway [448,449], transcellular pathway [441,450], and autophagy [442,451]. It has also been evidenced that the osteogenic ability of AuNPs is strongly related to their concentration [452], size [445], and shape [453].
What is more, AuNPs also exhibit important antimicrobial [454,455] and anti-cancer [456,457] activity. By considering the multifunctional therapeutic effects of AuNPs, and also their impressive functionalization versatility, substantial efforts have been oriented towards the fabrication of AuNP-embedded composites and complex formulations for bone repair and regeneration [439,458]. Moreover, given their peculiar electrical and optical behavior, AuNPs have been explored for the targeted and controlled management of bone infections and bone cancers [385,459].
4.2. Silver
Silver nanoparticles (AgNPs) are one of the most explored nanosized noble metals in modern industrial and biomedical applications, owing to their intrinsic catalytic effect, chemical stability, good electrical conductivity, optical behavior, and versatile biological activity [460,461]. In its ionic, metallic, and nanoparticulate forms, silver has been extensively used as an antibacterial agent [462,463]. The particular anti-pathogenic effects of nano-silver have been assigned to their ability to adhere to bacterial cell walls and produce oxidative stress, resulting in the bacterial cell wall and membrane impairment and subsequent cytoplasmic leakage, and the denaturation of bacterial macromolecules and alteration of vital cellular processes, respectively [464,465,466]. Silver ions released by AgNPs mediate bacterial death by impairing the peptidoglycan component of cell walls, hindering bacterial protein synthesis and obstructing replication signals and energy-dependent survival processes by binding to nucleic acids [467,468].
In the realm of orthopedics and dentistry, where the infection susceptibility of implanted devices is a continuous danger, the clinical potential of nano-silver is of special interest [469,470,471]. Since AgNPs stimulate osteogenesis and inhibit osteoclastogenesis [472,473], their use in bone healing applications gives rise to multifunctional platforms, and such nanostructures can be used to induce or potentiate the antimicrobial effects of nanoengineered constructs and clinically used devices, while stimulating the osteogenic activity [474,475,476] (Figure 10).
Figure 10.
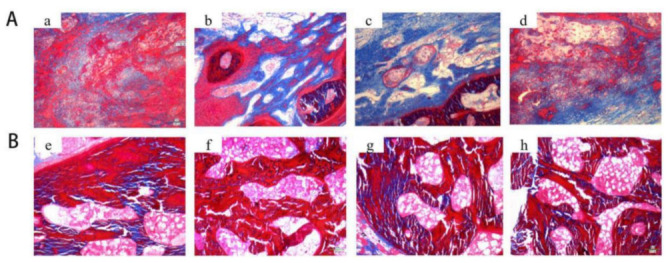
Histological analysis of rabbit skull defects treated with bare and nano-silver-loaded gelatin/alginate scaffolds (Gel/Alg and AgNP–Gel/Alg, respectively) for 4 weeks (A) and 8 weeks (B), evidenced by Masson staining (100×). Gel/Alg group (a,e); 200 μM AgNP–Gel/Alg group (b,f); 400 μM AgNP–Gel/Alg group (c,g); 600 μM AgNP–Gel/Alg group (d,h). See Ref. [475]. Reprinted from an open access source.
Modifying surface coatings with AgNPs represents an attractive strategy to enhance the bioactivity and osseointegration of metallic implants used in orthopedics and orthodontics. The use of AgNPs in oxide and non-oxide ceramic coatings can minimize the infection susceptibility of metallic implants by modulating the coating’s resistance to bacterial contamination and colonization, and exerting broad-spectrum antibacterial effects, while they maintain or improve their beneficial effects on osteogenic activity [477,478,479]. Furthermore, embedding AgNPs within polymer coatings represents an attractive strategy to generate antimicrobial surfaces for bone implants, with the additional osteogenic ability and bone-forming potential [480,481,482]. Such nanostructured layers act synergistically, as the inorganic nanosystems locally exert their antimicrobial effects, while the polymer matrix prevents their agglomeration, protects them from external damage, and provides an active carrier for their local release [483,484], and also prevents AgNP-mediated local tissue reactions [485].
In bone healing therapy, particular attention was oriented towards the incorporation of AgNPs within biomimetic constructs, such as HA-based coatings and polymer/HA scaffolds. Besides their anti-pathogenic effects, such nanomaterials proved to be beneficial substrates for mineralization and osteogenic differentiation, finally resulting in enhanced osseointegration of the metallic implants [486,487,488] and functional bone substitutes [489,490], respectively.
AgNPs exhibit nanosize-governed intrinsic anti-cancer activity (as evidenced against various cancer types) [491,492], and they also exert potentiating effects on chemotherapeutic agents [493,494] and alter tumor angiogenesis [495,496]. The local release of silver ions after cellular uptake determines cellular oxidative damage, impairment of cellular substructures, and subsequent apoptosis and necrosis [497,498,499,500]. The efficiency of AgNP-based formulations on bone cancers has been investigated against osteosarcoma [501,502,503], rhabdomyosarcoma [504,505], Ewing’s sarcoma [506], and chondrosarcoma [507].
4.3. Copper
Copper (Cu) is one essential trace element found in the human body that has a vital role in the cellular events that maintain the normal function of bones, blood vessels, and nerves, and it also contributes to wound healing speed, antioxidant defense, and immune function [508,509,510].
Copper deficiency has been linked with several disorders that mostly affect the connective and bone tissues. Cu plays a vital role in bone metabolism, and its lack may cause bone anomalies and deformities [386,511]. It has been evidenced that Cu deficiency leads to an inhibited activity of the oxidases (enzymes which normal function requires trace element cofactors) that are involved in collagen synthesis and vitamin D activation, thus resulting in the increased solubility of bone collagen, damaged peptide chain connections, impaired bone collagen stability, and reduced bone strength [512,513].
Given its beneficial role in bone metabolism, the use of Cu-based formulations—with particular emphasis on metallic ions and nanoparticles—is of great interest for bone healing applications. Following their incorporation or immobilization within different materials, copper nanoparticles (CuNPs) exhibit increased chemical stability and a self-tuned ability to gradually release the metallic ions without affecting the stability of matrix materials [514,515].
Furthermore, all forms of copper, including ions, nanoparticles, and alloys, possess excellent antibacterial properties, alongside osteogenic and angiogenic effects [508,516]. In a similar way to AgNPs, the antibacterial action of CuNPs relies on the conjunction between the nanosize-related impairment of cellular structures and metallic ion-mediated events (oxidative damage, obstructed protein synthesis, inhibited replication, and altered cellular survival processes) [517,518,519]. CuNPs also exhibit powerful antioxidant action (thus neutralizing free radicals and preventing cell damage) [520,521] and anti-cancer activity [522,523].
Even if substantial studies must be performed to properly and accurately revalue their therapeutic potential [524,525], Cu-based formulations represent multifaceted candidates for bone tissue therapy, as evidenced by their bone healing ability (enhanced mineralization, osteogenesis and angiogenesis, and modulated osteoclastogenesis) [526,527,528], extended antibacterial activity [515,529,530], and anti-tumor efficiency [531,532]. CuNPs have also been investigated with respect to dental applications, as efficient antimicrobials for denture base resins [533], endodontic treatment [534], and periodontitis management [535].
5. Conclusions and Perspectives
Designing successful devices and substitutes for bone therapy still represents a challenge for modern biomedicine, as it implies the accurate understanding of bone pathophysiology, the proper selection of biomaterials and fabrication protocols, and maximal therapeutic efficiency.
Nanoparticle-based biomaterials and biotechnologies have been lately validated as viable alternatives to traditional scaffolding protocols. In particular, bioceramic, oxide, and metallic nanoparticles demonstrated impressive therapeutic outcomes for bone repair and regeneration, and also for bone pathologies management.
Owing to their bioactivity, biomimetic composition, and good incorporation within the natural bone structure, bioceramic nanoparticles represent the best choice for reparative and regenerative bone therapy. Their acknowledged cytocompatibility and beneficial interactions with living tissues can be explored in conjunction with polymeric constructs and other inorganic (ions, nanoparticles, alloys, and composites) or organic substances (drugs and biomolecules) in order to fabricate bone-mimicking platforms for the specific and selective management of bone pathologies.
Even if substantial efforts should be made to completely understand and finely tune the implications of oxide and metallic nanoparticles in bone healing, their functional versatility (as nanocarriers, imaging agents, and sensitizers) and intrinsic therapeutic activity are impressive. Such peculiar characteristics pave the way towards the development of multifunctional bone substitutes, including platforms for targeted and localized drug delivery (antimicrobial, anti-inflammatory, anti-resorptive, and anti-cancer therapy), specific and selective detection and diagnosis, and effective combined therapy.
Besides being active components for bone processes (contributing with their osteoconductive, osteoinductive, and osteogenic effects), the previously discussed inorganic nanomaterials exhibit additional biological activities (antimicrobial, antioxidant, immunomodulatory, anti-resorptive, and anti-cancer). The nanosize-governed surface chemistry of these nanoparticles provides active sites for the conjugation of various therapeutic agents (e.g., ions, nanostructures, drugs, biomolecules, and nucleic acids), and also enables their immobilization or incorporation into more complex constructs, finally resulting in the development of versatile and performance-enhanced candidates for bone healing applications.
Author Contributions
A.-C.B., O.G., E.A., A.M.G. and A.F. designed and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
All authors would like to acknowledge and thank for the financial support provided by the University Politehnica of Bucharest. This paper acknowledges the support of the Ministry of Education and Research, CNCS UEFISCDI, project no. 524PED/2020 (PN-III-P2-2.1-PED-2019).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ralston S.H. Bone structure and metabolism. Medicine. 2021;49:567–571. doi: 10.1016/j.mpmed.2021.06.009. [DOI] [Google Scholar]
- 2.Abe K., Shimozaki S., Domoto T., Yamamoto N., Tsuchiya H., Minamoto T. Glycogen synthase kinase 3β biology in bone and soft tissue sarcomas. J. Cancer Metastasis Treat. 2020;6:51. doi: 10.20517/2394-4722.2020.117. [DOI] [Google Scholar]
- 3.Chen J., Ashames A., Buabeid M.A., Fahelelbom K.M., Ijaz M., Murtaza G. Nanocomposites drug delivery systems for the healing of bone fractures. Int. J. Pharm. 2020;585:119477. doi: 10.1016/j.ijpharm.2020.119477. [DOI] [PubMed] [Google Scholar]
- 4.Kupikowska-Stobba B., Kasprzak M. Fabrication of nanoparticles for bone regeneration: New insight into applications of nanoemulsion technology. J. Mater. Chem. B. 2021;9:5221–5244. doi: 10.1039/D1TB00559F. [DOI] [PubMed] [Google Scholar]
- 5.Sohn H.-S., Oh J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019;23:9. doi: 10.1186/s40824-019-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra G., Pandey A. Biodegradable bone implants in orthopedic applications: A review. Biocybern. Biomed. Eng. 2020;40:596–610. doi: 10.1016/j.bbe.2020.02.003. [DOI] [Google Scholar]
- 7.Tahmasebi E., Alam M., Yazdanian M., Tebyanian H., Yazdanian A., Seifalian A., Mosaddad S.A. Current biocompatible materials in oral regeneration: A comprehensive overview of composite materials. J. Mater. Res. Technol. 2020;9:11731–11755. doi: 10.1016/j.jmrt.2020.08.042. [DOI] [Google Scholar]
- 8.Collon K., Gallo M.C., Lieberman J.R. Musculoskeletal tissue engineering: Regional gene therapy for bone repair. Biomaterials. 2021;275:120901. doi: 10.1016/j.biomaterials.2021.120901. [DOI] [PubMed] [Google Scholar]
- 9.Kumar P., Saini M., Dehiya B.S., Sindhu A., Kumar V., Kumar R., Lamberti L., Pruncu C.I., Thakur R. Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials. 2020;10:2019. doi: 10.3390/nano10102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H., Feng W., Chen Y., Shi J. Inorganic nanoparticles in clinical trials and translations. Nano Today. 2020;35:100972. doi: 10.1016/j.nantod.2020.100972. [DOI] [Google Scholar]
- 11.Lyons J.G., Plantz M.A., Hsu W.K., Hsu E.L., Minardi S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020;8:922. doi: 10.3389/fbioe.2020.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W., Yeung K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017;2:224–247. doi: 10.1016/j.bioactmat.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X., Li L., Cai X., Huang Q., Xiao J., Cheng Y. Targeting nanoparticles for diagnosis and therapy of bone tumors: Opportunities and challenges. Biomaterials. 2021;265:120404. doi: 10.1016/j.biomaterials.2020.120404. [DOI] [PubMed] [Google Scholar]
- 14.Ojo O.A., Olayide I.I., Akalabu M.C., Ajiboye B.O., Ojo A.B., Oyinloye B.E., Ramalingam M. Nanoparticles and their biomedical applications. Biointerface Res. Appl. Chem. 2020;11:8431–8445. [Google Scholar]
- 15.Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 16.Zheng K., Xie J. Engineering Ultrasmall Metal Nanoclusters as Promising Theranostic Agents. Trends Chem. 2020;2:665–679. doi: 10.1016/j.trechm.2020.04.011. [DOI] [Google Scholar]
- 17.van der Meel R., Sulheim E., Shi Y., Kiessling F., Mulder W.J.M., Lammers T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019;14:1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldwin P., Li D.J., Auston D.A., Mir H.S., Yoon R.S., Koval K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma. 2019;33:203–213. doi: 10.1097/BOT.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 20.Hu C., Ashok D., Nisbet D.R., Gautam V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials. 2019;219:119366. doi: 10.1016/j.biomaterials.2019.119366. [DOI] [PubMed] [Google Scholar]
- 21.Hench L.L., Thompson I. Twenty-first century challenges for biomaterials. J. R. Soc. Interface. 2010;7((Suppl. S4)):S37–S391. doi: 10.1098/rsif.2010.0151.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fattahian H., Mansouri K., Mansouri N. Biomaterials, substitutes, and tissue engineering in bone repair: Current and future concepts. Comp. Clin. Pathol. 2019;28:879–891. doi: 10.1007/s00580-017-2507-2. [DOI] [Google Scholar]
- 23.Jin S., Xia X., Huang J., Yuan C., Zuo Y., Li Y., Li J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021;127:56–79. doi: 10.1016/j.actbio.2021.03.067. [DOI] [PubMed] [Google Scholar]
- 24.Wu F., Harper B.J., Harper S.L. Differential dissolution and toxicity of surface functionalized silver nanoparticles in small-scale microcosms: Impacts of community complexity. Environ. Sci. Nano. 2017;4:359–372. doi: 10.1039/C6EN00324A. [DOI] [Google Scholar]
- 25.Wang N., Dheen S.T., Fuh J.Y.H., Kumar A.S. A review of multi-functional ceramic nanoparticles in 3D printed bone tissue engineering. Bioprinting. 2021;23:e00146. doi: 10.1016/j.bprint.2021.e00146. [DOI] [Google Scholar]
- 26.Wang N., Maskomani S., Meenashisundaram G.K., Fuh J.Y.H., Dheen S.T., Anantharajan S.K. A study of Titanium and Magnesium particle-induced oxidative stress and toxicity to human osteoblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;117:111285. doi: 10.1016/j.msec.2020.111285. [DOI] [PubMed] [Google Scholar]
- 27.Tortella G.R., Rubilar O., Durán N., Diez M.C., Martínez M., Parada J., Seabra A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020;390:121974. doi: 10.1016/j.jhazmat.2019.121974. [DOI] [PubMed] [Google Scholar]
- 28.Khan M.A., Singh D., Ahmad A., Siddique H.R. Revisiting inorganic nanoparticles as promising therapeutic agents: A paradigm shift in oncological theranostics. Eur. J. Pharm. Sci. 2021;164:105892. doi: 10.1016/j.ejps.2021.105892. [DOI] [PubMed] [Google Scholar]
- 29.Gherasim O., Popescu R.C., Gherasim T.G., Grumezescu V., Andronescu E. Nanoparticles in Pharmacotherapy. William Andrew (Elsevier); Oxford, UK: 2019. Pharmacotherapy and nanotechnology; pp. 1–21. [Google Scholar]
- 30.Chenthamara D., Subramaniam S., Ramakrishnan S.G., Krishnaswamy S., Essa M.M., Lin F.-H., Qoronfleh M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019;23:20. doi: 10.1186/s40824-019-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Y., Zhou Y., Liu L., Xu Y., Chen Q., Wang Y., Wu S., Deng Y., Zhang J., Shao A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020;7:193. doi: 10.3389/fmolb.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heuer-Jungemann A., Feliu N., Bakaimi I., Hamaly M., Alkilany A., Chakraborty I., Masood A., Casula M.F., Kostopoulou A., Oh E., et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019;119:4819–4880. doi: 10.1021/acs.chemrev.8b00733. [DOI] [PubMed] [Google Scholar]
- 33.Chandrakala V., Aruna V., Angajala G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022 doi: 10.1007/s42247-021-00335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakraborty I., Parak W.J. Protein-Induced Shape Control of Noble Metal Nanoparticles. Adv. Mater. Interfaces. 2019;6:1801407. doi: 10.1002/admi.201801407. [DOI] [Google Scholar]
- 35.Mihai A.D., Chircov C., Grumezescu A.M., Holban A.M. Magnetite nanoparticles and essential oils systems for advanced antibacterial therapies. Int. J. Mol. Sci. 2020;21:7355. doi: 10.3390/ijms21197355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiozzi V., Rossi F. Inorganic–organic core/shell nanoparticles: Progress and applications. Nanoscale Adv. 2020;2:5090–5105. doi: 10.1039/D0NA00411A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarrintaj P., Paran S.M.R., Jafari S., Mozafari M. Compatibilization of Polymer Blends. Elsevier; Amsterdam, The Netherlands: 2020. Application of Compatibilized Polymer Blends in Biomedical Fields; pp. 511–537. [Google Scholar]
- 38.Marques C.F., Olhero S., Abrantes J.C.C., Marote A., Ferreira S., Vieira S.I., Ferreira J.M.F. Biocompatibility and antimicrobial activity of biphasic calcium phosphate powders doped with metal ions for regenerative medicine. Ceram. Int. 2017;43:15719–15728. doi: 10.1016/j.ceramint.2017.08.133. [DOI] [Google Scholar]
- 39.Samanta S.K., Devi K.B., Das P., Mukherjee P., Chanda A., Roy M., Nandi S.K. Metallic ion doped tri-calcium phosphate ceramics: Effect of dynamic loading on in vivo bone regeneration. J. Mech. Behav. Biomed. Mater. 2019;96:227–235. doi: 10.1016/j.jmbbm.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 40.Strutynska N., Livitska O., Prylutska S., Yumyna Y., Zelena P., Skivka L., Malyshenko A., Vovchenko L., Strelchuk V., Prylutskyy Y., et al. New nanostructured apatite-type (Na+,Zn2+,CO32−)-doped calcium phosphates: Preparation, mechanical properties and antibacterial activity. J. Mol. Struct. 2020;1222:128932. doi: 10.1016/j.molstruc.2020.128932. [DOI] [Google Scholar]
- 41.Kaur P., Singh K.J., Kaur S., Kaur S., Singh A.P. Sol-gel derived strontium-doped SiO2–CaO–MgO–P2O5 bioceramics for faster growth of bone like hydroxyapatite and their in vitro study for orthopedic applications. Mater. Chem. Phys. 2020;245:122763. doi: 10.1016/j.matchemphys.2020.122763. [DOI] [Google Scholar]
- 42.Sarin N., Singh K., Singh D., Arora S., Singh A.P., Mahajan H.J.M.C. Preliminary studies of strontium and selenium binary doped CaO–SiO2–P2O5–MgO bioceramics for faster growth of hydroxyapatite and bone regeneration applications. Mater. Chem. Phys. 2020;253:123329. doi: 10.1016/j.matchemphys.2020.123329. [DOI] [Google Scholar]
- 43.Thompson F.C., Matsumoto M.A., Biguetti C.C., Rennó A.C.M., de Andrade Holgado L., Santiago Junior J.F., Munerato M.S., Saraiva P.P. Distinct healing pattern of maxillary sinus augmentation using the vitroceramic Biosilicate®: Study in rabbits. Mater. Sci. Eng. C. 2019;99:726–734. doi: 10.1016/j.msec.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Munerato M.S., Biguetti C.C., Parra da Silva R.B., Rodrigues da Silva A.C., Zucon Bacelar A.C., Lima da Silva J., Rondina Couto M.C., Húngaro Duarte M.A., Santiago-Junior J.F., Bossini P.S., et al. Inflammatory response and macrophage polarization using different physicochemical biomaterials for oral and maxillofacial reconstruction. Mater. Sci. Eng. C. 2020;107:110229. doi: 10.1016/j.msec.2019.110229. [DOI] [PubMed] [Google Scholar]
- 45.Zafar B., Mottaghitalab F., Shahosseini Z., Negahdari B., Farokhi M. Silk fibroin/alumina nanoparticle scaffold using for osteogenic differentiation of rabbit adipose-derived stem cells. Materialia. 2020;9:100518. doi: 10.1016/j.mtla.2019.100518. [DOI] [Google Scholar]
- 46.Li X., Qi M., Sun X., Weir M.D., Tay F.R., Oates T.W., Dong B., Zhou Y., Wang L., Xu H.H.K. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019;94:627–643. doi: 10.1016/j.actbio.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Kanniyappan H., Venkatesan M., Panji J., Ramasamy M., Muthuvijayan V. Evaluating the inherent osteogenic and angiogenic potential of mesoporous silica nanoparticles to augment vascularized bone tissue formation. Microporous Mesoporous Mater. 2021;311:110687. doi: 10.1016/j.micromeso.2020.110687. [DOI] [Google Scholar]
- 48.Yang R., Yan Y., Wu Z., Wei Y., Song H., Zhu L., Zhao C., Xu N., Fu J., Huo K. Resveratrol-loaded titania nanotube coatings promote osteogenesis and inhibit inflammation through reducing the reactive oxygen species production via regulation of NF-κB signaling pathway. Mater. Sci. Eng. C. 2021;131:112513. doi: 10.1016/j.msec.2021.112513. [DOI] [PubMed] [Google Scholar]
- 49.Goldschmidt G.M., Krok-Borkowicz M., Zybała R., Pamuła E., Telle R., Conrads G., Schickle K. Biomimetic in situ precipitation of calcium phosphate containing silver nanoparticles on zirconia ceramic materials for surface functionalization in terms of antimicrobial and osteoconductive properties. Dent. Mater. 2021;37:10–18. doi: 10.1016/j.dental.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Cao H., Zhang W., Meng F., Guo J., Wang D., Qian S., Jiang X., Liu X., Chu P.K. Osteogenesis Catalyzed by Titanium-Supported Silver Nanoparticles. ACS Appl. Mater. Interfaces. 2017;9:5149–5157. doi: 10.1021/acsami.6b15448. [DOI] [PubMed] [Google Scholar]
- 51.Radwan-Pragłowska J., Janus L., Piatkowski M., Bogdał D., Matysek D. 3D hierarchical, nanostructured chitosan/PLA/HA scaffolds doped with TiO2/Au/Pt NPs with tunable properties for guided bone tissue engineering. Polymers. 2020;12:792. doi: 10.3390/polym12040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heidari F., Tabatabaei F.S., Razavi M., Bazargan-Lari R., Tavangar M., Romanos G.E., Vashaee D., Tayebi L. 3D construct of hydroxyapatite/zinc oxide/palladium nanocomposite scaffold for bone tissue engineering. J. Mater. Sci. Mater. Med. 2020;31:85. doi: 10.1007/s10856-020-06409-2. [DOI] [PubMed] [Google Scholar]
- 53.Tiomnova O.T., Coelho F., Pellizaro T.A.G., Enrique J., Chanfrau R., de Oliveira Capote T.S., Basmaji P., Pantoja Y.V., Guastaldi A.C. Preparation of Scaffolds of Amorphous Calcium Phosphate and Bacterial Cellulose for Use in Tissue Regeneration by Freeze-Drying Process. Biointerface Res. Appl. Chem. 2021;11:7357–7367. doi: 10.33263/BRIAC111.73577367. [DOI] [Google Scholar]
- 54.Gillman C.E., Jayasuriya A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C. 2021;130:112466. doi: 10.1016/j.msec.2021.112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao R., Yang R., Cooper P.R., Khurshid Z., Shavandi A., Ratnayake J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules. 2021;26:3007. doi: 10.3390/molecules26103007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridi F., Meazzini I., Castroflorio B., Bonini M., Berti D., Baglioni P. Functional calcium phosphate composites in nanomedicine. Adv. Colloid Interface Sci. 2017;244:281–295. doi: 10.1016/j.cis.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Parent M., Baradari H., Champion E., Damia C., Viana-Trecant M. Design of calcium phosphate ceramics for drug delivery applications in bone diseases: A review of the parameters affecting the loading and release of the therapeutic substance. J. Control. Release. 2017;252:1–17. doi: 10.1016/j.jconrel.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 58.Andronescu E., Grumezescu A.M., Guşă M.I., Holban A.M., Ilie F.C., Irimia A., Nicoară I.F., Ţone M. Nanobiomaterials in Hard Tissue Engineering: Applications of Nanobiomaterials. William Andrew (Elsevier); Oxford, UK: 2016. Nano-hydroxyapatite: Novel approaches in biomedical applications; pp. 189–213. [Google Scholar]
- 59.Florea D.A., Chircov C., Grumezescu A.M. Hydroxyapatite particles-directing the cellular activity in bone regeneration processes: An up-to-date review. Appl. Sci. 2020;10:3483. doi: 10.3390/app10103483. [DOI] [Google Scholar]
- 60.Liang W., Ding P., Li G., Lu E., Zhao Z. Hydroxyapatite Nanoparticles Facilitate Osteoblast Differentiation and Bone Formation Within Sagittal Suture During Expansion in Rats [Corrigendum] Drug Des. Dev. Ther. 2021;15:3617–3618. doi: 10.2147/DDDT.S334630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dasgupta S., Mondal S., Ray S., Singh Y., Maji K. Hydroxyapatite-Collagen Nanoparticles Reinforced Polyanhydride Based Injectable Paste for Bone Substitution: Effect of Dopant Addition in Vitro. J. Biomater. Sci. Polym. Ed. 2021;32:1312–1336. doi: 10.1080/09205063.2021.1916867. [DOI] [PubMed] [Google Scholar]
- 62.Mohd Pu’ad N.A.S., Koshy P., Abdullah H.Z., Idris M.I., Lee T.C. Syntheses of hydroxyapatite from natural sources. Heliyon. 2019;5:e01588. doi: 10.1016/j.heliyon.2019.e01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fiume E., Magnaterra G., Rahdar A., Verné E., Baino F. Hydroxyapatite for biomedical applications: A short overview. Ceramics. 2021;4:542–563. doi: 10.3390/ceramics4040039. [DOI] [Google Scholar]
- 64.Duta L., Dorcioman G., Grumezescu V. A review on biphasic calcium phosphate materials derived from fish discards. Nanomaterials. 2021;11:2856. doi: 10.3390/nano11112856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tite T., Popa A.C., Balescu L.M., Bogdan I.M., Pasuk I., Ferreira J.M.F., Stan G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials. 2018;11:2081. doi: 10.3390/ma11112081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rincón-López J.A., Hermann-Muñoz J.A., Giraldo-Betancur A.L., De Vizcaya-Ruiz A., Alvarado-Orozco J.M., Muñoz-Saldaña J. Synthesis, characterization and in vitro study of synthetic and bovine-derived hydroxyapatite ceramics: A comparison. Materials. 2018;9:333. doi: 10.3390/ma11030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duta L., Neamtu J., Melinte R.P., Zureigat O.A., Popescu-Pelin G., Chioibasu D., Oktar F.N., Popescu A.C. In vivo assessment of bone enhancement in the case of 3d-printed implants functionalized with lithium-doped biological-derived hydroxyapatite coatings: A preliminary study on rabbits. Coatings. 2020;10:992. doi: 10.3390/coatings10100992. [DOI] [Google Scholar]
- 68.Ramesh N., Ratnayake J.T.B., Moratti S.C., Dias G.J. Effect of chitosan infiltration on hydroxyapatite scaffolds derived from New Zealand bovine cancellous bones for bone regeneration. Int. J. Biol. Macromol. 2020;160:1009–1020. doi: 10.1016/j.ijbiomac.2020.05.269. [DOI] [PubMed] [Google Scholar]
- 69.Duta L., Mihailescu N., Popescu A.C., Luculescu C.R., Mihailescu I.N., Çetin G., Gunduz O., Oktar F.N., Popa A.C., Kuncser A., et al. Comparative physical, chemical and biological assessment of simple and titanium-doped ovine dentine-derived hydroxyapatite coatings fabricated by pulsed laser deposition. Appl. Surf. Sci. 2017;413:129–139. doi: 10.1016/j.apsusc.2017.04.025. [DOI] [Google Scholar]
- 70.Ekren N. Reinforcement of sheep-bone derived hydroxyapatite with bioactive glass. J. Ceram. Proces. Res. 2017;18:64–68. [Google Scholar]
- 71.Sobczak-Kupiec A., Pluta K., Drabczyk A., Włoś M., Tyliszczak B. Synthesis and characterization of ceramic—Polymer composites containing bioactive synthetic hydroxyapatite for biomedical applications. Ceram. Int. 2018;44:13630–13638. doi: 10.1016/j.ceramint.2018.04.199. [DOI] [Google Scholar]
- 72.Ramirez-Gutierrez C.F., Londoño-Restrepo S.M., del Real A., Mondragón M.A., Rodriguez-García M.E. Effect of the temperature and sintering time on the thermal, structural, morphological, and vibrational properties of hydroxyapatite derived from pig bone. Ceram. Int. 2017;43:7552–7559. doi: 10.1016/j.ceramint.2017.03.046. [DOI] [Google Scholar]
- 73.Mahmoud E.M., Sayed M., El-Kady A.M., Elsayed H., Naga S.M. In vitro and in vivo study of naturally derived alginate/hydroxyapatite bio composite scaffolds. Int. J. Biol. Macromol. 2020;165:1346–1360. doi: 10.1016/j.ijbiomac.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 74.Surya P., Nithin A., Sundaramanickam A., Sathish M. Synthesis and characterization of nano-hydroxyapatite from Sardinella longiceps fish bone and its effects on human osteoblast bone cells. J. Mech. Behav. Biomed. Mater. 2021;119:104501. doi: 10.1016/j.jmbbm.2021.104501. [DOI] [PubMed] [Google Scholar]
- 75.Balu S., Sundaradoss M.V., Andra S., Jeevanandam J. Facile biogenic fabrication of hydroxyapatite nanorods using cuttlefish bone and their bactericidal and biocompatibility study. Beilstein J. Nanotechnol. 2020;11:285–295. doi: 10.3762/bjnano.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arjama M., Mehnath S., Rajan M., Jeyaraj M. Injectable cuttlefish HAP and macromolecular fibroin protein hydrogel for natural bone mimicking matrix for enhancement of osteoinduction progression. React. Funct. Polym. 2021;160:104841. doi: 10.1016/j.reactfunctpolym.2021.104841. [DOI] [Google Scholar]
- 77.Karacan I., Ben-Nissan B., Wang H.A., Juritza A., Swain M.V., Müller W.H., Chou J., Stamboulis A., Macha I.J., Taraschi V. Mechanical testing of antimicrobial biocomposite coating on metallic medical implants as drug delivery system. Mater. Sci. Eng. C. 2019;104:109757. doi: 10.1016/j.msec.2019.109757. [DOI] [PubMed] [Google Scholar]
- 78.Lin X., Hunziker E.B., Liu T., Hu Q., Liu Y. Enhanced biocompatibility and improved osteogenesis of coralline hydroxyapatite modified by bone morphogenetic protein 2 incorporated into a biomimetic coating. Mater. Sci. Eng. C. 2019;96:329–336. doi: 10.1016/j.msec.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 79.Hussein A.I., Ab-Ghani Z., Mat A.N.C., Ghani N.A.A., Husein A., Rahman I.A. Synthesis and characterization of spherical calcium carbonate nanoparticles derived from cockle shells. Appl. Sci. 2020;10:7170. doi: 10.3390/app10207170. [DOI] [Google Scholar]
- 80.Citradewi P.W., Hidayat H., Purwiandono G., Fatimah I., Sagadevan S. Clitorea ternatea-mediated silver nanoparticle-doped hydroxyapatite derived from cockle shell as antibacterial material. Chem. Phys. Lett. 2021;769:138412. doi: 10.1016/j.cplett.2021.138412. [DOI] [Google Scholar]
- 81.Hembrick-Holloman V., Samuel T., Mohammed Z., Jeelani S., Rangari V.K. Ecofriendly production of bioactive tissue engineering scaffolds derived from egg- and sea-shells. J. Mater. Res. Technol. 2020;9:13729–13739. doi: 10.1016/j.jmrt.2020.09.093. [DOI] [Google Scholar]
- 82.Wan Jusoh W.N., Matori K.A., Zaid M.H.M., Zainuddin N., Khiri M.Z.A., Rahman N.A.A., Jalil R.A., Kul E. Incorporation of hydroxyapatite into glass ionomer cement (Gic) formulated based on alumino-silicate-fluoride glass ceramics from waste materials. Materials. 2021;14:954. doi: 10.3390/ma14040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scialla S., Carella F., Dapporto M., Sprio S., Piancastelli A., Palazzo B., Adamiano A., Esposti L.D., Iafisco M., Piccirillo C. Mussel shell-derived macroporous 3D scaffold: Characterization and optimization study of a bioceramic from the circular economy. Mar. Drugs. 2020;18:309. doi: 10.3390/md18060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karunakaran G., Cho E.B., Kumar G.S., Kolesnikov E., Janarthanan G., Pillai M.M., Rajendran S., Boobalan S., Sudha K.G., Rajeshkumar M.P. Mesoporous Mg-doped hydroxyapatite nanorods prepared from bio-waste blue mussel shells for implant applications. Ceram. Int. 2020;46:28514–28527. doi: 10.1016/j.ceramint.2020.08.009. [DOI] [Google Scholar]
- 85.Yinka K.M., Olayiwola A.J., Sulaiman A., Ali A., Iqbal F. Preparation and characterization of hydroxyapatite powder for biomedical applications from giant african land snail shell using a hydrothermal technique. Eng. Appl. Sci. Res. 2020;47:275–286. doi: 10.14456/easr.2020.30. [DOI] [Google Scholar]
- 86.Januariyasa I.K., Ana I.D., Yusuf Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C. 2020;107:110347. doi: 10.1016/j.msec.2019.110347. [DOI] [PubMed] [Google Scholar]
- 87.Chuysinuan P., Nooeaid P., Thanyacharoen T., Techasakul S., Pavasant P., Kanjanamekanant K. Injectable eggshell-derived hydroxyapatite-incorporated fibroin-alginate composite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2021;193:799–808. doi: 10.1016/j.ijbiomac.2021.10.132. [DOI] [PubMed] [Google Scholar]
- 88.Dumitrescu C.R., Neacsu I.A., Surdu V.A., Nicoara A.I., Iordache F., Trusca R., Ciocan L.T., Ficai A., Andronescu E. Nano-hydroxyapatite vs. Xenografts: Synthesis, characterization, and in vitro behavior. Nanomaterials. 2021;11:2289. doi: 10.3390/nano11092289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miculescu F., Mocanu A.C., Stan G.E., Miculescu M., Maidaniuc A., Cîmpean A., Mitran V., Voicu S.I., Machedon-Pisu T., Ciocan L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018;438:147–157. doi: 10.1016/j.apsusc.2017.07.144. [DOI] [Google Scholar]
- 90.Hartatiek, Yudyanto, Wuriantika M.I., Utomo J., Nurhuda M., Masruroh, Santjojo D.J.D.H. Nanostructure, porosity and tensile strength of PVA/Hydroxyapatite composite nanofiber for bone tissue engineering. Mater. Today Proc. 2021;44:3203–3206. doi: 10.1016/j.matpr.2020.11.438. [DOI] [Google Scholar]
- 91.Lara-Ochoa S., Ortega-Lara W., Guerrero-Beltrán C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics. 2021;13:1642. doi: 10.3390/pharmaceutics13101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khalifehzadeh R., Arami H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020;279:102157. doi: 10.1016/j.cis.2020.102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oryan A., Hassanajili S., Sahvieh S., Azarpira N. Effectiveness of mesenchymal stem cell-seeded onto the 3D polylactic acid/polycaprolactone/hydroxyapatite scaffold on the radius bone defect in rat. Life Sci. 2020;257:118038. doi: 10.1016/j.lfs.2020.118038. [DOI] [PubMed] [Google Scholar]
- 94.Wang K., Cheng W., Ding Z., Xu G., Zheng X., Li M., Lu G., Lu Q. Injectable silk/hydroxyapatite nanocomposite hydrogels with vascularization capacity for bone regeneration. J. Mater. Sci. Technol. 2021;63:172–181. doi: 10.1016/j.jmst.2020.02.030. [DOI] [Google Scholar]
- 95.Kazimierczak P., Przekora A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings. 2020;10:971. doi: 10.3390/coatings10100971. [DOI] [Google Scholar]
- 96.Zhao S., Cui W., Rajendran N.K., Su F., Rajan M. Investigations of Gold nanoparticles-mediated Carbon Nanotube Reinforced Hydroxyapatite Composite for Bone Regenerations. J. Saudi Chem. Soc. 2021;25:101261. doi: 10.1016/j.jscs.2021.101261. [DOI] [Google Scholar]
- 97.Zhao X., Zhu L., Fan C. Sequential alendronate delivery by hydroxyapatite-coated maghemite for enhanced bone fracture healing. J. Drug Deliv. Sci. Technol. 2021;66:102761. doi: 10.1016/j.jddst.2021.102761. [DOI] [Google Scholar]
- 98.Guo X., Xue M., Chen F., Guo Q., Zhou X., Lin H., Chen Y. Local delivery and controlled release of miR-34a loaded in hydroxyapatite/mesoporous organosilica nanoparticles composite-coated implant wire to accelerate bone fracture healing. Biomaterials. 2022;280:121300. doi: 10.1016/j.biomaterials.2021.121300. [DOI] [PubMed] [Google Scholar]
- 99.Hadji H., Bouchemal K. Effect of micro- and nanoparticle shape on biological processes. J. Control. Release. 2022;342:93–110. doi: 10.1016/j.jconrel.2021.12.032. [DOI] [PubMed] [Google Scholar]
- 100.Murahashi Y., Yano F., Nakamoto H., Maenohara Y., Iba K., Yamashita T., Tanaka S., Ishihara K., Okamura Y., Moro T., et al. Multi-layered PLLA-nanosheets loaded with FGF-2 induce robust bone regeneration with controlled release in critical-sized mouse femoral defects. Acta Biomater. 2019;85:172–179. doi: 10.1016/j.actbio.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 101.Yanagisawa Y., Ito A., Hara Y., Mutsuzaki H., Murai S., Fujii K., Sogo Y., Hirose M., Oyane A., Kobayashi F., et al. Initial clinical trial of pins coated with fibroblast growth factor-2–apatite composite layer in external fixation of distal radius fractures. J. Orthop. 2019;16:69–73. doi: 10.1016/j.jor.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raftery R.M., Mencía-Castaño I., Sperger S., Chen G., Cavanagh B., Feichtinger G.A., Redl H., Hacobian A., O’Brien F.J. Delivery of the improved BMP-2-Advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair. J. Control. Release. 2018;283:20–31. doi: 10.1016/j.jconrel.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 103.Chen S., Shi Y., Zhang X., Ma J. Evaluation of BMP-2 and VEGF loaded 3D printed hydroxyapatite composite scaffolds with enhanced osteogenic capacity in vitro and in vivo. Mater. Sci. Eng. C. 2020;112:110893. doi: 10.1016/j.msec.2020.110893. [DOI] [PubMed] [Google Scholar]
- 104.Casarrubios L., Gómez-Cerezo N., Sánchez-Salcedo S., Feito M.J., Serrano M.C., Saiz-Pardo M., Ortega L., de Pablo D., Díaz-Güemes I., Fernández-Tomé B., et al. Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta Biomater. 2020;101:544–553. doi: 10.1016/j.actbio.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 105.Yazdanian M., Arefi A.H., Alam M., Abbasi K., Tebyaniyan H., Tahmasebi E., Ranjbar R., Seifalian A., Rahbar M. Decellularized and biological scaffolds in dental and craniofacial tissue engineering: A comprehensive overview. J. Mater. Res. Technol. 2021;15:1217–1251. doi: 10.1016/j.jmrt.2021.08.083. [DOI] [Google Scholar]
- 106.Zhang Y., Jiang Y., Zou D., Yuan B., Ke H.Z., Li W. Therapeutics for enhancement of spinal fusion: A mini review. J. Orthop. Transl. 2021;31:73–79. doi: 10.1016/j.jot.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Safari B., Davaran S., Aghanejad A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 2021;175:544–557. doi: 10.1016/j.ijbiomac.2021.02.052. [DOI] [PubMed] [Google Scholar]
- 108.Fitzpatrick V., Martín-Moldes Z., Deck A., Torres-Sanchez R., Valat A., Cairns D., Li C., Kaplan D.L. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials. 2021;276:120995. doi: 10.1016/j.biomaterials.2021.120995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee D., Wufuer M., Kim I., Choi T.H., Kim B.J., Jung H.G., Jeon B., Lee G., Jeon O.H., Chang H., et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci. Rep. 2021;11:746. doi: 10.1038/s41598-020-80608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rittipakorn P., Thuaksuban N., Mai-Ngam K., Charoenla S., Noppakunmongkolchai W. Bioactivity of a novel polycaprolactone-hydroxyapatite scaffold used as a carrier of low dose bmp-2: An in vitro study. Polymers. 2021;13:466. doi: 10.3390/polym13030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bal Z., Korkusuz F., Ishiguro H., Okada R., Kushioka J., Chijimatsu R., Kodama J., Tateiwa D., Ukon Y., Nakagawa S., et al. A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J. 2021;21:865–873. doi: 10.1016/j.spinee.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 112.Sonatkar J., Kandasubramanian B. Bioactive glass with biocompatible polymers for bone applications. Eur. Polym. J. 2021;160:110801. doi: 10.1016/j.eurpolymj.2021.110801. [DOI] [Google Scholar]
- 113.Li X., Zhang R., Tan X., Li B., Liu Y., Wang X. Synthesis and Evaluation of BMMSC-seeded BMP-6/nHAG/GMS Scaffolds for Bone Regeneration. Int. J. Med. Sci. 2019;16:1007–1017. doi: 10.7150/ijms.31966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gherasim O., Grumezescu A.M., Grumezescu V., Andronescu E., Negut I., Bîrcă A.C., Gălățeanu B., Hudiță A. Bioactive coatings loaded with osteogenic protein for metallic implants. Polymers. 2021;13:4303. doi: 10.3390/polym13244303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu Y., Zheng L., Zhang J., Lin L., Shen Y., Zhang X., Wu B. Dual delivery of bone morphogenetic protein-2 and basic fibroblast growth factor from nanohydroxyapatite/collagen for bone tissue engineering. Appl. Biol. Chem. 2019;62:49. doi: 10.1186/s13765-019-0453-1. [DOI] [Google Scholar]
- 116.Godoy-Gallardo M., Portolés-Gil N., López-Periago A.M., Domingo C., Hosta-Rigau L. Immobilization of bmp-2 and vegf within multilayered polydopamine-coated scaffolds and the resulting osteogenic and angiogenic synergy of co-cultured human mesenchymal stem cells and human endothelial progenitor cells. Int. J. Mol. Sci. 2020;21:6418. doi: 10.3390/ijms21176418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kang F., Yi Q., Gu P., Dong Y., Zhang Z., Zhang L., Bai Y. Controlled growth factor delivery system with osteogenic-angiogenic coupling effect for bone regeneration. J. Orthop. Transl. 2021;31:110–125. doi: 10.1016/j.jot.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santora A.C., Sharma A. Osteoporosis: Pathophysiology and Clinical Management. Humana; Cham, Switzerland: 2020. Bisphosphonates: Mechanisms of Action and Role in Osteoporosis Therapy; pp. 277–307. [DOI] [Google Scholar]
- 119.Oryan A., Sahvieh S. Effects of bisphosphonates on osteoporosis: Focus on zoledronate. Life Sci. 2021;264:118681. doi: 10.1016/j.lfs.2020.118681. [DOI] [PubMed] [Google Scholar]
- 120.Chen S., Guo R., Xie C., Liang Q., Xiao X. Biomimetic mineralization of nanocrystalline hydroxyapatites on aminated modified polylactic acid microspheres to develop a novel drug delivery system for alendronate. Mater. Sci. Eng. C. 2020;110:110655. doi: 10.1016/j.msec.2020.110655. [DOI] [PubMed] [Google Scholar]
- 121.Sahana H., Khajuria D.K., Razdan R., Mahapatra D.R., Bhat M.R., Suresh S., Rao R.R., Mariappan L. Improvement in bone properties by using risedronate adsorbed hydroxyapatite novel nanoparticle based formulation in a rat model of osteoporosis. J. Biomed. Nanotechnol. 2013;9:193–201. doi: 10.1166/jbn.2013.1482. [DOI] [PubMed] [Google Scholar]
- 122.Gyanewali S., Kesharwani P., Sheikh A., Ahmad F.J., Trivedi R., Talegaonkar S. Formulation development and in vitro–in vivo assessment of protransfersomal gel of anti-resorptive drug in osteoporosis treatment. Int. J. Pharm. 2021;608:121060. doi: 10.1016/j.ijpharm.2021.121060. [DOI] [PubMed] [Google Scholar]
- 123.Xu Y., Zhang Z., Wang H., Zhong W., Sun C., Sun W., Wu H. Zoledronic Acid-Loaded Hybrid Hyaluronic Acid/Polyethylene Glycol/Nano-Hydroxyapatite Nanoparticle: Novel Fabrication and Safety Verification. Front. Bioeng. Biotechnol. 2021;9:28. doi: 10.3389/fbioe.2021.629928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khajuria D.K., Razdan R., Mahapatra D.R. Development, in vitro and in vivo characterization of zoledronic acid functionalized hydroxyapatite nanoparticle based formulation for treatment of osteoporosis in animal model. Eur. J. Pharm. Sci. 2015;66:173–183. doi: 10.1016/j.ejps.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 125.Pyo S.W., Kim Y.M., Kim C.S., Lee I.S., Park J.U. Bone formation on biomimetic calcium phosphate-coated and zoledronate-immobilized titanium implants in osteoporotic rat tibiae. Int. J. Oral Maxillofac. Implant. 2014;29:478–484. doi: 10.11607/jomi.3423. [DOI] [PubMed] [Google Scholar]
- 126.Shen X., Ma P., Hu Y., Xu G., Xu K., Chen W., Ran Q., Dai L., Yu Y., Mu C., et al. Alendronate-loaded hydroxyapatite-TiO2 nanotubes for improved bone formation in osteoporotic rabbits. J. Mater. Chem. B. 2016;4:1423–1436. doi: 10.1039/C5TB01956G. [DOI] [PubMed] [Google Scholar]
- 127.Cometa S., Bonifacio M.A., Tranquillo E., Gloria A., Domingos M., De Giglio E. A 3d printed composite scaffold loaded with clodronate to regenerate osteoporotic bone: In vitro characterization. Polymers. 2021;13:150. doi: 10.3390/polym13010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kettenberger U., Luginbuehl V., Procter P., Pioletti D.P. In vitro and in vivo investigation of bisphosphonate-loaded hydroxyapatite particles for peri-implant bone augmentation. J. Tissue Eng. Regen. Med. 2017;11:1974–1985. doi: 10.1002/term.2094. [DOI] [PubMed] [Google Scholar]
- 129.Demir-Oğuz Ö., Ege D. Effect of zoledronic acid and graphene oxide on the physical and in vitro properties of injectable bone substitutes. Mater. Sci. Eng. C. 2021;120:111758. doi: 10.1016/j.msec.2020.111758. [DOI] [PubMed] [Google Scholar]
- 130.Kotak D.J., Devarajan P.V. Bone targeted delivery of salmon calcitonin hydroxyapatite nanoparticles for sublingual osteoporosis therapy (SLOT) Nanomed. Nanotechnol. Biol. Med. 2020;24:102153. doi: 10.1016/j.nano.2020.102153. [DOI] [PubMed] [Google Scholar]
- 131.Watanabe H., Ikoma T., Sotome S., Okawa A. Local administration and enhanced release of bone metabolic antibodies from hydroxyapatite/chondroitin sulfate nanocomposite microparticles using zinc cations. J. Mater. Chem. B. 2021;9:757–766. doi: 10.1039/D0TB02050H. [DOI] [PubMed] [Google Scholar]
- 132.Dave J.R., Dewle A.M., Mhaske S.T., Phulpagar P.T., Mathe V.L., More S.E., Khan A.A., Murthy A.V.R., Datar S.S., Jog A.J., et al. Hydroxyapatite nanorods loaded with parathyroid hormone (PTH) synergistically enhance the net formative effect of PTH anabolic therapy. Nanomed. Nanotechnol. Biol. Med. 2019;15:218–230. doi: 10.1016/j.nano.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 133.Martín-del-Campo M., Sampedro J.G., Flores-Cedillo M.L., Rosales-Ibañez R., Rojo L. Bone Regeneration Induced by Strontium Folate Loaded Biohybrid Scaffolds. Molecules. 2019;24:1660. doi: 10.3390/molecules24091660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Surmenev R.A., Shkarina S., Syromotina D.S., Melnik E.V., Shkarin R., Selezneva I.I., Ermakov A.M., Ivlev S.I., Cecilia A., Weinhardt V., et al. Characterization of biomimetic silicate- and strontium-containing hydroxyapatite microparticles embedded in biodegradable electrospun polycaprolactone scaffolds for bone regeneration. Eur. Polym. J. 2019;113:67–77. doi: 10.1016/j.eurpolymj.2019.01.042. [DOI] [Google Scholar]
- 135.Luz E.P.C.G., das Chagas B.S., de Almeida N.T., de Fátima Borges M., Andrade F.K., Muniz C.R., Castro-Silva I.I., Teixeira E.H., Popat K., de Freitas Rosa M., et al. Resorbable bacterial cellulose membranes with strontium release for guided bone regeneration. Mater. Sci. Eng. C. 2020;116:111175. doi: 10.1016/j.msec.2020.111175. [DOI] [PubMed] [Google Scholar]
- 136.Stipniece L., Wilson S., Curran J.M., Chen R., Salma-Ancane K., Sharma P.K., Meenan B.J., Boyd A.R. Strontium substituted hydroxyapatite promotes direct primary human osteoblast maturation. Ceram. Int. 2021;47:3368–3379. doi: 10.1016/j.ceramint.2020.09.182. [DOI] [Google Scholar]
- 137.Cirillo M., Martelli G., Boanini E., Rubini K., Di Filippo M., Torricelli P., Pagani S., Fini M., Bigi A., Giacomini D. Strontium substituted hydroxyapatite with β-lactam integrin agonists to enhance mesenchymal cells adhesion and to promote bone regeneration. Colloids Surf. B Biointerfaces. 2021;200:111580. doi: 10.1016/j.colsurfb.2021.111580. [DOI] [PubMed] [Google Scholar]
- 138.Hidalgo-Robatto B.M., López-Álvarez M., Azevedo A.S., Dorado J., Serra J., Azevedo N.F., González P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018;333:168–177. doi: 10.1016/j.surfcoat.2017.11.006. [DOI] [Google Scholar]
- 139.Fernandes M.H., Alves M.M., Cebotarenco M., Ribeiro I.A.C., Grenho L., Gomes P.S., Carmezim M.J., Santos C.F. Citrate zinc hydroxyapatite nanorods with enhanced cytocompatibility and osteogenesis for bone regeneration. Mater. Sci. Eng. C. 2020;115:111147. doi: 10.1016/j.msec.2020.111147. [DOI] [PubMed] [Google Scholar]
- 140.Maleki-Ghaleh H., Hossein Siadati M., Fallah A., Zarrabi A., Afghah F., Koc B., Dalir Abdolahinia E., Omidi Y., Barar J., Akbari-Fakhrabadi A., et al. Effect of zinc-doped hydroxyapatite/graphene nanocomposite on the physicochemical properties and osteogenesis differentiation of 3D-printed polycaprolactone scaffolds for bone tissue engineering. Chem. Eng. J. 2021;426:131321. doi: 10.1016/j.cej.2021.131321. [DOI] [Google Scholar]
- 141.He L., Li H., Chen X., Xu T., Sun T., Huang H., Lu M., Yin Y., Ge J., Weng J., et al. Selenium-substituted hydroxyapatite particles with regulated microstructures for osteogenic differentiation and anti-tumor effects. Ceram. Int. 2019;45:13787–13798. doi: 10.1016/j.ceramint.2019.04.075. [DOI] [Google Scholar]
- 142.Muthusamy S., Mahendiran B., Sampath S., Jaisankar S.N., Anandasadagopan S.K., Krishnakumar G.S. Hydroxyapatite nanophases augmented with selenium and manganese ions for bone regeneration: Physiochemical, microstructural and biological characterization. Mater. Sci. Eng. C. 2021;126:112149. doi: 10.1016/j.msec.2021.112149. [DOI] [PubMed] [Google Scholar]
- 143.Barbanente A., Palazzo B., Degli Esposti L., Adamiano A., Iafisco M., Ditaranto N., Migoni D., Gervaso F., Nadar R., Ivanchenko P., et al. Selenium-doped hydroxyapatite nanoparticles for potential application in bone tumor therapy. J. Inorg. Biochem. 2021;215:111334. doi: 10.1016/j.jinorgbio.2020.111334. [DOI] [PubMed] [Google Scholar]
- 144.Li X., Wang Y., Chen Y., Zhou P., Wei K., Wang H., Wang J., Fang H., Zhang S. Hierarchically constructed selenium-doped bone-mimetic nanoparticles promote ROS-mediated autophagy and apoptosis for bone tumor inhibition. Biomaterials. 2020;257:120253. doi: 10.1016/j.biomaterials.2020.120253. [DOI] [PubMed] [Google Scholar]
- 145.Peñaflor T., Chai Y., Tagaya M. Hydroxyapatite Nanoparticle Coating on Polymer for Constructing Effective Biointeractive Interfaces. J. Nanomater. 2019;2019:6495239. doi: 10.1155/2019/6495239. [DOI] [Google Scholar]
- 146.Anita Lett J., Sagadevan S., Fatimah I., Hoque M.E., Lokanathan Y., Léonard E., Alshahateet S.F., Schirhagl R., Oh W.C. Recent advances in natural polymer-based hydroxyapatite scaffolds: Properties and applications. Eur. Polym. J. 2021;148:110360. doi: 10.1016/j.eurpolymj.2021.110360. [DOI] [Google Scholar]
- 147.Siniscalco D., Dutreilh-Colas M., Hjezi Z., Cornette J., El Felss N., Champion E., Damia C. Functionalization of hydroxyapatite ceramics: Raman mapping investigation of silanization. Ceramics. 2019;2:29. doi: 10.3390/ceramics2020029. [DOI] [Google Scholar]
- 148.Sandomierski M., Buchwald Z., Voelkel A. Calcium montmorillonite and montmorillonite with hydroxyapatite layer as fillers in dental composites with remineralizing potential. Appl. Clay Sci. 2020;198:105822. doi: 10.1016/j.clay.2020.105822. [DOI] [Google Scholar]
- 149.Salim S.A., Loutfy S.A., El-Fakharany E.M., Taha T.H., Hussien Y., Kamoun E.A. Influence of chitosan and hydroxyapatite incorporation on properties of electrospun PVA/HA nanofibrous mats for bone tissue regeneration: Nanofibers optimization and in-vitro assessment. J. Drug Deliv. Sci. Technol. 2021;62:102417. doi: 10.1016/j.jddst.2021.102417. [DOI] [Google Scholar]
- 150.Tian J., Zhou H., Jiang R., Chen J., Mao L., Liu M., Deng F., Liu L., Zhang X., Wei Y. Preparation and biological imaging of fluorescent hydroxyapatite nanoparticles with poly(2-ethyl-2-oxazoline) through surface-initiated cationic ring-opening polymerization. Mater. Sci. Eng. C. 2020;108:110424. doi: 10.1016/j.msec.2019.110424. [DOI] [PubMed] [Google Scholar]
- 151.Tham D.Q., Huynh M.D., Linh N.T.D., Van D.T.C., Van Cong D., Dung N.T.K., Trang N.T.T., Van Lam P., Hoang T., Lam T.D. Pmma bone cements modified with silane-treated and pmma-grafted hydroxyapatite nanocrystals: Preparation and characterization. Polymers. 2021;13:3860. doi: 10.3390/polym13223860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heragh B.K., Javanshir S., Mahdavinia G.R., Jamal M.R.N. Hydroxyapatite grafted chitosan/laponite RD hydrogel: Evaluation of the encapsulation capacity, pH-responsivity, and controlled release behavior. Int. J. Biol. Macromol. 2021;190:351–359. doi: 10.1016/j.ijbiomac.2021.08.220. [DOI] [PubMed] [Google Scholar]
- 153.Zhou Q., Wang T., Wang C., Wang Z., Yang Y., Li P., Cai R., Sun M., Yuan H., Nie L. Synthesis and characterization of silver nanoparticles-doped hydroxyapatite/alginate microparticles with promising cytocompatibility and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2020;585:124081. doi: 10.1016/j.colsurfa.2019.124081. [DOI] [Google Scholar]
- 154.Ma L., Su W., Ran Y., Ma X., Yi Z., Chen G., Chen X., Deng Z., Tong Q., Wang X., et al. Synthesis and characterization of injectable self-healing hydrogels based on oxidized alginate-hybrid-hydroxyapatite nanoparticles and carboxymethyl chitosan. Int. J. Biol. Macromol. 2020;165:1164–1174. doi: 10.1016/j.ijbiomac.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 155.Chen R., Shi J., Zhu B., Zhang L., Cao S. Mesoporous hollow hydroxyapatite capped with smart polymer for multi-stimuli remotely controlled drug delivery. Microporous Mesoporous Mater. 2020;306:110447. doi: 10.1016/j.micromeso.2020.110447. [DOI] [Google Scholar]
- 156.Li K., Chen J., Xue Y., Ding T., Zhu S., Mao M., Zhang L., Han Y. Polymer brush grafted antimicrobial peptide on hydroxyapatite nanorods for highly effective antibacterial performance. Chem. Eng. J. 2021;423:130133. doi: 10.1016/j.cej.2021.130133. [DOI] [Google Scholar]
- 157.Ram Prasad S., Jayakrishnan A., Sampath Kumar T.S. Hydroxyapatite-poly(vinyl alcohol) core-shell nanoparticles for dual delivery of methotrexate and gemcitabine for bone cancer treatment. J. Drug Deliv. Sci. Technol. 2019;51:629–638. doi: 10.1016/j.jddst.2019.03.041. [DOI] [Google Scholar]
- 158.Verma G., Shetake N.G., Pandrekar S., Pandey B.N., Hassan P.A., Priyadarsini K.I. Development of surface functionalized hydroxyapatite nanoparticles for enhanced specificity towards tumor cells. Eur. J. Pharm. Sci. 2020;144:105206. doi: 10.1016/j.ejps.2019.105206. [DOI] [PubMed] [Google Scholar]
- 159.Rial R., Hassan N., Liu Z., Ruso J.M. The design and green nanofabrication of noble hydrogel systems with encapsulation of doped bioactive hydroxyapatite toward sustained drug delivery. J. Mol. Liq. 2021;343:117598. doi: 10.1016/j.molliq.2021.117598. [DOI] [Google Scholar]
- 160.Vázquez-Hernández F., Mendoza-Acevedo S., Mendoza-Barrera C.O., Mendoza-Álvarez J., Luna-Arias J.P. Antibody-coupled hydroxyapatite nanoparticles as efficient tools for labeling intracellular proteins. Mater. Sci. Eng. C. 2017;71:909–918. doi: 10.1016/j.msec.2016.10.082. [DOI] [PubMed] [Google Scholar]
- 161.Cipreste M.F., Mussel W.D.N., Batista da Silva J., Freitas Marques M.B.D., Batista R.J.C., Gastelois P.L., Augusto de Almeida Macedo W.A.D.A., Sousa E.M.B.D. A new theranostic system for bone disorders: Functionalized folate-MDP hydroxyapatite nanoparticles with radiolabeled copper-64. Mater. Chem. Phys. 2020;254:123265. doi: 10.1016/j.matchemphys.2020.123265. [DOI] [Google Scholar]
- 162.Kim H.S., Lee J.H., Mandakhbayar N., Jin G.Z., Kim S.J., Yoon J.Y., Jo S.B., Park J.H., Singh R.K., Jang J.H., et al. Therapeutic tissue regenerative nanohybrids self-assembled from bioactive inorganic core/chitosan shell nanounits. Biomaterials. 2021;274:120857. doi: 10.1016/j.biomaterials.2021.120857. [DOI] [PubMed] [Google Scholar]
- 163.Xue Z., Yang M., Xu D. Nucleation of Biomimetic Hydroxyapatite Nanoparticles on the Surface of Type I Collagen: Molecular Dynamics Investigations. J. Phys. Chem. C. 2019;123:2533–2543. doi: 10.1021/acs.jpcc.8b10342. [DOI] [Google Scholar]
- 164.Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020;5:584–603. doi: 10.1038/s41578-020-0204-2. [DOI] [Google Scholar]
- 165.Jain S., Gujjala R., Abdul Azeem P., Ojha S., Samudrala R.K. A review on mechanical and In-vitro studies of polymer reinforced bioactive glass-scaffolds and their fabrication techniques. Ceram. Int. 2021;48:5908–5921. doi: 10.1016/j.ceramint.2021.11.206. [DOI] [Google Scholar]
- 166.Bahremandi-Toloue E., Mohammadalizadeh Z., Mukherjee S., Karbasi S. Incorporation of inorganic bioceramics into electrospun scaffolds for tissue engineering applications: A review. Ceram. Int. 2021;48:8803–8837. doi: 10.1016/j.ceramint.2021.12.125. [DOI] [Google Scholar]
- 167.Lackington W.A., Gehweiler D., Zderic I., Nehrbass D., Zeiter S., González-Vázquez A., O’Brien F.J., Stoddart M.J., Thompson K. Incorporation of hydroxyapatite into collagen scaffolds enhances the therapeutic efficacy of rhBMP-2 in a weight-bearing femoral defect model. Mater. Today Commun. 2021;29:102933. doi: 10.1016/j.mtcomm.2021.102933. [DOI] [Google Scholar]
- 168.El-Habashy S.E., El-Kamel A.H., Essawy M.M., Abdelfattah E.Z.A., Eltaher H.M. 3D printed bioinspired scaffolds integrating doxycycline nanoparticles: Customizable implants for in vivo osteoregeneration. Int. J. Pharm. 2021;607:121002. doi: 10.1016/j.ijpharm.2021.121002. [DOI] [PubMed] [Google Scholar]
- 169.Echave M.C., Erezuma I., Golafshan N., Castilho M., Kadumudi F.B., Pimenta-Lopes C., Ventura F., Pujol A., Jimenez J.J., Camara J.A., et al. Bioinspired gelatin/bioceramic composites loaded with bone morphogenetic protein-2 (BMP-2) promote osteoporotic bone repair. Mater. Sci. Eng. C. 2021. in press . [DOI] [PubMed]
- 170.Yu X., Shen G., Shang Q., Zhang Z., Zhao W., Zhang P., Liang D., Ren H., Jiang X. A Naringin-loaded gelatin-microsphere/nano-hydroxyapatite/silk fibroin composite scaffold promoted healing of critical-size vertebral defects in ovariectomised rat. Int. J. Biol. Macromol. 2021;193:510–518. doi: 10.1016/j.ijbiomac.2021.10.036. [DOI] [PubMed] [Google Scholar]
- 171.Deininger C., Wagner A., Heimel P., Salzer E., Vila X.M., Weißenbacher N., Grillari J., Redl H., Wichlas F., Freude T., et al. Enhanced bmp-2-mediated bone repair using an anisotropic silk fibroin scaffold coated with bone-like apatite. Int. J. Mol. Sci. 2022;23:283. doi: 10.3390/ijms23010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jahan K., Manickam G., Tabrizian M., Murshed M. In vitro and in vivo investigation of osteogenic properties of self-contained phosphate-releasing injectable purine-crosslinked chitosan-hydroxyapatite constructs. Sci. Rep. 2020;10:11603. doi: 10.1038/s41598-020-67886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li T.T., Zhang Y., Ren H.T., Peng H.K., Lou C.W., Lin J.H. Two-step strategy for constructing hierarchical pore structured chitosan–hydroxyapatite composite scaffolds for bone tissue engineering. Carbohydr. Polym. 2021;260:117765. doi: 10.1016/j.carbpol.2021.117765. [DOI] [PubMed] [Google Scholar]
- 174.Sofi H.S., Akram T., Shabir N., Vasita R., Jadhav A.H., Sheikh F.A. Regenerated cellulose nanofibers from cellulose acetate: Incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater. Sci. Eng. C. 2021;118:111547. doi: 10.1016/j.msec.2020.111547. [DOI] [PubMed] [Google Scholar]
- 175.Cao S., Li Q., Zhang S., Liu K., Yang Y., Chen J. Oxidized bacterial cellulose reinforced nanocomposite scaffolds for bone repair. Colloids Surf. B Biointerfaces. 2022;211:112316. doi: 10.1016/j.colsurfb.2021.112316. [DOI] [PubMed] [Google Scholar]
- 176.Iglesias-Mejuto A., García-González C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C. 2021;131:112525. doi: 10.1016/j.msec.2021.112525. [DOI] [PubMed] [Google Scholar]
- 177.Luo Y., Chen B., Zhang X., Huang S., Wa Q. 3D printed concentrated alginate/GelMA hollow-fibers-packed scaffolds with nano apatite coatings for bone tissue engineering. Int. J. Biol. Macromol. 2022;202:366–374. doi: 10.1016/j.ijbiomac.2022.01.096. [DOI] [PubMed] [Google Scholar]
- 178.Zimina A., Senatov F., Choudhary R., Kolesnikov E., Anisimova N., Kiselevskiy M., Orlova P., Strukova N., Generalova M., Manskikh V., et al. Biocompatibility and physico-chemical properties of highly porous PLA/HA scaffolds for bone reconstruction. Polymers. 2020;12:2938. doi: 10.3390/polym12122938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang W., Zhang B., Li M., Li J., Zhang C., Han Y., Wang L., Wang K., Zhou C., Liu L., et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos. Part B Eng. 2021;224:109192. doi: 10.1016/j.compositesb.2021.109192. [DOI] [Google Scholar]
- 180.Chang P.C., Luo H.T., Lin Z.J., Tai W.C., Chang C.H., Chang Y.C., Cochran D.L., Chen M.H. Preclinical evaluation of a 3D-printed hydroxyapatite/poly(lactic-co-glycolic acid) scaffold for ridge augmentation. J. Formos. Med. Assoc. 2021;120:1100–1107. doi: 10.1016/j.jfma.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 181.Wei J., Yan Y., Gao J., Li Y., Wang R., Wang J., Zou Q., Zuo Y., Zhu M., Li J. 3D-printed hydroxyapatite microspheres reinforced PLGA scaffolds for bone regeneration. Mater. Sci. Eng. C. 2022. in press . [DOI] [PubMed]
- 182.Ciocca L., Giorgio L.I., Sara R., Sabrina G., Andrea V., Annapaola P., Alessandro S., Barbara D., Paolo M., Adriano P., et al. Nanostructured surface bioactive composite scaffold for filling of bone defects. Biointerface Res. Appl. Chem. 2020;10:5038–5047. doi: 10.33263/BRIAC102.038047. [DOI] [Google Scholar]
- 183.Yedekçi B., Tezcaner A., Yılmaz B., Demir T., Evis Z. 3D porous PCL-PEG-PCL/strontium, magnesium and boron multi-doped hydroxyapatite composite scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2022;125:104941. doi: 10.1016/j.jmbbm.2021.104941. [DOI] [PubMed] [Google Scholar]
- 184.Volkov A.V., Muraev A.A., Zharkova I.I., Voinova V.V., Akoulina E.A., Zhuikov V.A., Khaydapova D.D., Chesnokova D.V., Menshikh K.A., Dudun A.A., et al. Poly(3-hydroxybutyrate)/hydroxyapatite/alginate scaffolds seeded with mesenchymal stem cells enhance the regeneration of critical-sized bone defect. Mater. Sci. Eng. C. 2020;114:110991. doi: 10.1016/j.msec.2020.110991. [DOI] [PubMed] [Google Scholar]
- 185.Senatov F., Zimina A., Chubrik A., Kolesnikov E., Permyakova E., Voronin A., Poponova M., Orlova P., Grunina T., Nikitin K. Effect of recombinant BMP-2 and erythropoietin on osteogenic properties of biomimetic PLA/PCL/HA and PHB/HA scaffolds in critical-size cranial defects model. Mater. Sci. Eng. C. 2022. in press . [DOI] [PubMed]
- 186.Molino G., Palmieri M.C., Montalbano G., Fiorilli S., Vitale-Brovarone C. Biomimetic and mesoporous nano-hydroxyapatite for bone tissue application: A short review. Biomed. Mater. 2020;15:022001. doi: 10.1088/1748-605X/ab5f1a. [DOI] [PubMed] [Google Scholar]
- 187.Pan P., Yue Q., Li J., Gao M., Yang X., Ren Y., Cheng X., Cui P., Deng Y. Smart Cargo Delivery System based on Mesoporous Nanoparticles for Bone Disease Diagnosis and Treatment. Adv. Sci. 2021;8:2004586. doi: 10.1002/advs.202004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qiu Y., Xu X., Guo W., Zhao Y., Su J., Chen J. Mesoporous Hydroxyapatite Nanoparticles Mediate the Release and Bioactivity of BMP-2 for Enhanced Bone Regeneration. ACS Biomater. Sci. Eng. 2020;6:2323–2335. doi: 10.1021/acsbiomaterials.9b01954. [DOI] [PubMed] [Google Scholar]
- 189.Liao Y., Li H., Shu R., Chen H., Zhao L., Song Z., Zhou W. Mesoporous Hydroxyapatite/Chitosan Loaded with Recombinant-Human Amelogenin Could Enhance Antibacterial Effect and Promote Periodontal Regeneration. Front. Cell. Infect. Microbiol. 2020;10:180. doi: 10.3389/fcimb.2020.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Piard C., Luthcke R., Kamalitdinov T., Fisher J. Sustained delivery of vascular endothelial growth factor from mesoporous calcium-deficient hydroxyapatite microparticles promotes in vitro angiogenesis and osteogenesis. J. Biomed. Mater. Res.-Part A. 2021;109:1080–1087. doi: 10.1002/jbm.a.37100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Aggarwal A., Singh R.P., Danewalia S.S., Saggu H.S. Novel mesoporous anionic substituted hydroxyapatite particles for multipurpose applications. Ceram. Int. 2021;48:6313–6321. doi: 10.1016/j.ceramint.2021.11.174. [DOI] [Google Scholar]
- 192.Karunakaran G., Cho E.B., Kumar G.S., Kolesnikov E., Sudha K.G., Mariyappan K., Han A., Choi S.S. Citric Acid-Mediated Microwave-Hydrothermal Synthesis of Mesoporous F-Doped HAp Nanorods from Bio-Waste for Biocidal Implant Applications. Nanomaterials. 2022;12:315. doi: 10.3390/nano12030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lett J.A., Sagadevan S., Prabhakar J.J., Hamizi N.A., Badruddin I.A., Johan M.R., Marlinda A.R., Wahab Y.A., Khan T.M.Y., Kamangar S. Drug leaching properties of Vancomycin loaded mesoporous hydroxyapatite as bone substitutes. Processes. 2019;7:826. doi: 10.3390/pr7110826. [DOI] [Google Scholar]
- 194.Singh G., Jolly S.S., Singh R.P. Cerium substituted hydroxyapatite mesoporous nanorods: Synthesis and characterization for drug delivery applications. Mater. Today Proc. 2020;28:1460–1466. doi: 10.1016/j.matpr.2020.04.821. [DOI] [Google Scholar]
- 195.Meshkini A., Oveisi H. Methotrexate-F127 conjugated mesoporous zinc hydroxyapatite as an efficient drug delivery system for overcoming chemotherapy resistance in osteosarcoma cells. Colloids Surf. B Biointerfaces. 2017;158:319–330. doi: 10.1016/j.colsurfb.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 196.Izadi A., Meshkini A., Entezari M.H. Mesoporous superparamagnetic hydroxyapatite nanocomposite: A multifunctional platform for synergistic targeted chemo-magnetotherapy. Mater. Sci. Eng. C. 2019;101:27–41. doi: 10.1016/j.msec.2019.03.066. [DOI] [PubMed] [Google Scholar]
- 197.Huang H., Du M., Chen J., Zhong S., Wang J. Preparation and characterization of abalone shells derived biological mesoporous hydroxyapatite microspheres for drug delivery. Mater. Sci. Eng. C. 2020;113:110969. doi: 10.1016/j.msec.2020.110969. [DOI] [PubMed] [Google Scholar]
- 198.Lowe B., Hardy J.G., Walsh L.J. Optimizing Nanohydroxyapatite Nanocomposites for Bone Tissue Engineering. ACS Omega. 2020;5:1–9. doi: 10.1021/acsomega.9b02917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fong M.K., Rahman M.N.A., Arifin A.M.T., Haq R.H.A., Hassan M.F., Taib I. Characterization, thermal and biological properties of PCL/PLA/PEG/N-HA composites. Biointerface Res. Appl. Chem. 2021;11:9017–9026. [Google Scholar]
- 200.Francisco E.M., Zoccolotti J.d.O., Tiomno Tiomnova O., Gustavo Tolaba A., Rodriguez Chanfrau J.E., Jorge J.H., Basmaji P., Guastaldi A.C. Sterilization of Scaffolds of Calcium Phosphates and Bacterial Cellulose for their Use in Tissue Regeneration. Biointerface Res. Appl. Chem. 2021;11:10089. [Google Scholar]
- 201.Redondo F.L., Giaroli M.C., Ciolino A.E., Ninago M.D. Preparation of Porous Poly (Lactic Acid)/Tricalcium Phosphate Composite Scaffolds for Tissue Engineering. Biointerface Res. Appl. Chem. 2022;12:5610–5624. doi: 10.33263/BRIAC124.56105624. [DOI] [Google Scholar]
- 202.Krishna E.S., Suresh G. Bioactive Titanium-Hydroxyapatite Composites by Powder Metallurgy Route. Biointerface Res. Appl. Chem. 2022;12:5375–5383. doi: 10.33263/BRIAC124.53755383. [DOI] [Google Scholar]
- 203.Hench L.L., Jones J.R. Bioactive glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015;3:194. doi: 10.3389/fbioe.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.van Vugt T.A., Geurts J.A.P., Arts J.J., Lindfors N.C. Management of Periprosthetic Joint Infections (PJIs) Woodhead Publishing (Elsevier); Duxford, UK: 2017. Biomaterials in treatment of orthopedic infections; pp. 41–68. [Google Scholar]
- 205.Kaur G., Kumar V., Baino F., Mauro J., Pickrell G., Evans I., Bretcanu O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C. 2019;104:109895. doi: 10.1016/j.msec.2019.109895. [DOI] [PubMed] [Google Scholar]
- 206.Brunello G., Elsayed H., Biasetto L. Bioactive glass and silicate-based ceramic coatings on metallic implants: Open challenge or outdated topic? Materials. 2019;12:2929. doi: 10.3390/ma12182929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Srinath P., Abdul Azeem P., Venugopal Reddy K. Review on calcium silicate-based bioceramics in bone tissue engineering. Int. J. Appl. Ceram. Technol. 2020;17:2450–2464. doi: 10.1111/ijac.13577. [DOI] [Google Scholar]
- 208.Hench L.L. Opening paper 2015- some comments on bioglass: Four eras of discovery and development. Biomed. Glasses. 2015;1:1–11. doi: 10.1515/bglass-2015-0001. [DOI] [Google Scholar]
- 209.Kargozar S., Baino F., Hamzehlou S., Hill R.G., Mozafari M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018;36:430–444. doi: 10.1016/j.tibtech.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 210.Sergi R., Bellucci D., Cannillo V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials. 2020;13:5560. doi: 10.3390/ma13235560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Karakuzu-Ikizler B., Terzioğlu P., Basaran-Elalmis Y., Tekerek B.S., Yücel S. Role of magnesium and aluminum substitution on the structural properties and bioactivity of bioglasses synthesized from biogenic silica. Bioact. Mater. 2020;5:66–73. doi: 10.1016/j.bioactmat.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.de Araujo Bastos Santana L., Oliveira Junior P.H., Damia C., dos Santos Tavares D., dos Santos E.A. Bioactivity in SBF versus trace element effects: The isolated role of Mg2+ and Zn2+ in osteoblast behavior. Mater. Sci. Eng. C. 2021;118:111320. doi: 10.1016/j.msec.2020.111320. [DOI] [PubMed] [Google Scholar]
- 213.Chen Y.H., Tseng S.P., Wu S.M., Shih C.J. Structure-dependence of anti-methicillin-resistant staphylococcus aureus (MRSA) activity on ZnO-containing bioglass. J. Alloys Compd. 2020;848:156487. doi: 10.1016/j.jallcom.2020.156487. [DOI] [Google Scholar]
- 214.Marin C.P., Crovace M.C., Zanotto E.D. Competent F18 bioglass-Biosilicate® bone graft scaffold substitutes. J. Eur. Ceram. Soc. 2021;41:7910–7920. doi: 10.1016/j.jeurceramsoc.2021.08.056. [DOI] [Google Scholar]
- 215.Chitra S., Bargavi P., Balasubramaniam M., Chandran R.R., Balakumar S. Impact of copper on in-vitro biomineralization, drug release efficacy and antimicrobial properties of bioactive glasses. Mater. Sci. Eng. C. 2020;109:110598. doi: 10.1016/j.msec.2019.110598. [DOI] [PubMed] [Google Scholar]
- 216.Akhtach S., Tabia Z., El Mabrouk K., Bricha M., Belkhou R. A comprehensive study on copper incorporated bio-glass matrix for its potential antimicrobial applications. Ceram. Int. 2021;47:424–433. doi: 10.1016/j.ceramint.2020.08.149. [DOI] [Google Scholar]
- 217.Vale A.C., Pereira P.R., Barbosa A.M., Torrado E., Alves N.M. Optimization of silver-containing bioglass nanoparticles envisaging biomedical applications. Mater. Sci. Eng. C. 2019;94:161–168. doi: 10.1016/j.msec.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 218.Mortazavi S., Rahsepar M., Hosseinzadeh S. Modification of mesoporous structure of silver-doped bioactive glass with antibacterial properties for bone tissue applications. Ceram. Int. 2021;48:8276–8285. doi: 10.1016/j.ceramint.2021.12.032. [DOI] [Google Scholar]
- 219.Araujo M., Silva A., Cabal B., Bartolomé J., Mello-Castanho S. In vitro bioactivity and antibacterial capacity of 45S5 Bioglass®-based compositions containing alumina and strontium. J. Mater. Res. Technol. 2021;13:154–161. doi: 10.1016/j.jmrt.2021.04.053. [DOI] [Google Scholar]
- 220.Katunar M.R., Pastore J.I., Cisilino A., Merlo J., Alonso L.S., Baca M., Haddad K., Cere S., Ballarre J. Early osseointegration of strontium-doped coatings on titanium implants in an osteoporotic rat model. Surf. Coat. Technol. 2022;433:128159. doi: 10.1016/j.surfcoat.2022.128159. [DOI] [Google Scholar]
- 221.Babu M.M., Prasad P.S., Venkateswara Rao P., Govindan N.P., Singh R.K., Kim H.W., Veeraiah N. Titanium incorporated Zinc-Phosphate bioactive glasses for bone tissue repair and regeneration: Impact of Ti4+ on physico-mechanical and in vitro bioactivity. Ceram. Int. 2019;45:23715–23727. doi: 10.1016/j.ceramint.2019.08.087. [DOI] [Google Scholar]
- 222.Matamoros-Veloza A., Hossain K.M.Z., Scammell B.E., Ahmed I., Hall R., Kapur N. Formulating injectable pastes of porous calcium phosphate glass microspheres for bone regeneration applications. J. Mech. Behav. Biomed. Mater. 2020;102:103489. doi: 10.1016/j.jmbbm.2019.103489. [DOI] [PubMed] [Google Scholar]
- 223.Babu M.M., Rao P.V., Singh R.K., Kim H.W., Veeraiah N., Özcan M., Prasad P.S. ZnO incorporated high phosphate bioactive glasses for guided bone regeneration implants: Enhancement of in vitro bioactivity and antibacterial activity. J. Mater. Res. Technol. 2021;15:633–646. doi: 10.1016/j.jmrt.2021.08.020. [DOI] [Google Scholar]
- 224.Cui X., Huang W., Zhou J., Wang H., Zhou N., Wang D., Cui X., Huang C., Yu Z., Wang L., et al. Evaluation of an injectable bioactive borate glass cement to heal bone defects in a rabbit femoral condyle model. Mater. Sci. Eng. C. 2017;73:585–595. doi: 10.1016/j.msec.2016.12.101. [DOI] [PubMed] [Google Scholar]
- 225.Yin H., Yang C., Gao Y., Wang C., Li M., Guo H., Tong Q. Fabrication and characterization of strontium-doped borate-based bioactive glass scaffolds for bone tissue engineering. J. Alloys Compd. 2018;743:564–569. doi: 10.1016/j.jallcom.2018.01.099. [DOI] [Google Scholar]
- 226.Ali A., Singh B.N., Yadav S., Ershad M., Singh S.K., Mallick S.P., Pyare R. CuO assisted borate 1393B3 glass scaffold with enhanced mechanical performance and cytocompatibility: An In vitro study. J. Mech. Behav. Biomed. Mater. 2021;114:104231. doi: 10.1016/j.jmbbm.2020.104231. [DOI] [PubMed] [Google Scholar]
- 227.Danewalia S.S., Singh K. Bioactive glasses and glass–ceramics for hyperthermia treatment of cancer: State-of-art, challenges, and future perspectives. Mater. Today Bio. 2021;10:100100. doi: 10.1016/j.mtbio.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zeimaran E., Pourshahrestani S., Fathi A., Razak N.A.B.A., Kadri N.A., Sheikhi A., Baino F. Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration. Acta Biomater. 2021;136:1–36. doi: 10.1016/j.actbio.2021.09.034. [DOI] [PubMed] [Google Scholar]
- 229.Erol-Taygun M., Unalan I., Idris M.I.B., Mano J.F., Boccaccini A.R. Bioactıve Glass-Polymer Nanocomposites for Bone Tıssue Regeneration Applicatıons: A Revıew. Adv. Eng. Mater. 2019;21:1900287. doi: 10.1002/adem.201900287. [DOI] [Google Scholar]
- 230.Skallevold H.E., Rokaya D., Khurshid Z., Zafar M.S. Bioactive glass applications in dentistry. Int. J. Mol. Sci. 2019;20:5960. doi: 10.3390/ijms20235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Thomas A., Johnson E., Agrawal A.K., Bera J. Preparation and characterization of glass–ceramic reinforced alginate scaffolds for bone tissue engineering. J. Mater. Res. 2019;34:3798–3809. doi: 10.1557/jmr.2019.343. [DOI] [Google Scholar]
- 232.Tian T., Xie W., Gao W., Wang G., Zeng L., Miao G., Lei B., Lin Z., Chen X. Micro-nano bioactive glass particles incorporated porous scaffold for promoting osteogenesis and angiogenesis in vitro. Front. Chem. 2019;7:186. doi: 10.3389/fchem.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tabia Z., Akhtach S., Bricha M., El Mabrouk K. Tailoring the biodegradability and bioactivity of green-electrospun polycaprolactone fibers by incorporation of bioactive glass nanoparticles for guided bone regeneration. Eur. Polym. J. 2021;161:110841. doi: 10.1016/j.eurpolymj.2021.110841. [DOI] [Google Scholar]
- 234.Daskalakis E., Huang B., Vyas C., Acar A.A., Fallah A., Cooper G., Weightman A., Koc B., Blunn G., Bartolo P. Novel 3D Bioglass Scaffolds for Bone Tissue Regeneration. Polymers. 2022;14:445. doi: 10.3390/polym14030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ji L., Xu T., Gu J., Liu Q., Zhou S., Shi G., Zhu Z. Preparation of bioactive glass nanoparticles with highly and evenly doped calcium ions by reactive flash nanoprecipitation. J. Mater. Sci. Mater. Med. 2021;32:48. doi: 10.1007/s10856-021-06521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lins C.E.C., Oliveira A.A.R., Gonzalez I., Macedo W.A.A., Pereira M.M. Structural analysis of fluorine-containing bioactive glass nanoparticles synthesized by sol–gel route assisted by ultrasound energy. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018;106:360–366. doi: 10.1002/jbm.b.33846. [DOI] [PubMed] [Google Scholar]
- 237.Pinto E., Souz I.E., De Carvalho S.M., Martins T., De Magalhães Pereira M. Fluorine-containing bioactive glass spherical particles synthesized by sol-gel route assisted by ultrasound energy or mechanical mixing. Mater. Res. 2020;23:e20200070. doi: 10.1590/1980-5373-MR-2020-0070. [DOI] [Google Scholar]
- 238.Jones J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015;23:S53–S82. doi: 10.1016/j.actbio.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 239.Pajares-Chamorro N., Chatzistavrou X. Bioactive Glass Nanoparticles for Tissue Regeneration. ACS Omega. 2020;5:12716–12726. doi: 10.1021/acsomega.0c00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kim D., Shim Y.S., An S.Y., Lee M.J. Role of Zinc-Doped Bioactive Glass Encapsulated with Microspherical Gelatin in Localized Supplementation for Tissue Regeneration: A Contemporary Review. Molecules. 2021;26:1823. doi: 10.3390/molecules26071823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Neščáková Z., Zheng K., Liverani L., Nawaz Q., Galusková D., Kaňková H., Michálek M., Galusek D., Boccaccini A.R. Multifunctional zinc ion doped sol—Gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019;4:312–321. doi: 10.1016/j.bioactmat.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Paramita P., Ramachandran M., Narashiman S., Nagarajan S., Sukumar D.K., Chung T.-W., Ambigapathi M. Sol–gel based synthesis and biological properties of zinc integrated nano bioglass ceramics for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2021;32:5. doi: 10.1007/s10856-020-06478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Akhtach S., Tabia Z., Bricha M., El Mabrouk K. Structural characterization, in vitro bioactivity, and antibacterial evaluation of low silver-doped bioactive glasses. Ceram. Int. 2021;47:29036–29046. doi: 10.1016/j.ceramint.2021.07.066. [DOI] [Google Scholar]
- 244.Schatkoski V.M., Larissa do Amaral Montanheiro T., Canuto de Menezes B.R., Pereira R.M., Rodrigues K.F., Ribas R.G., Morais da Silva D., Thim G.P. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceram. Int. 2021;47:2999–3012. doi: 10.1016/j.ceramint.2020.09.213. [DOI] [Google Scholar]
- 245.Liang W., Wu X., Dong Y., Shao R., Chen X., Zhou P., Xu F. In vivo behavior of bioactive glass-based composites in animal models for bone regeneration. Biomater. Sci. 2021;9:1924–1944. doi: 10.1039/D0BM01663B. [DOI] [PubMed] [Google Scholar]
- 246.Wang Y., Pan H., Chen X. The preparation of hollow mesoporous bioglass nanoparticles with excellent drug delivery capacity for bone tissue regeneration. Front. Chem. 2019;7:283. doi: 10.3389/fchem.2019.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mao Y., Liao J., Wu M., Wen J., Xu J., Li Y., Xie Y., Ying Q. Preparation of nano spherical bioglass by alkali-catalyzed mixed template. Mater. Res. Express. 2020;7:105202. doi: 10.1088/2053-1591/abc373. [DOI] [Google Scholar]
- 248.Zheng K., Kang J., Rutkowski B., Gawȩda M., Zhang J., Wang Y., Founier N., Sitarz M., Taccardi N., Boccaccini A.R. Toward highly dispersed mesoporous bioactive glass nanoparticles with high cu concentration using cu/ascorbic acid complex as precursor. Front. Chem. 2019;7:497. doi: 10.3389/fchem.2019.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Baino F. Copper-doped ordered mesoporous bioactive glass: A promising multifunctional platform for bone tissue engineering. Bioengineering. 2020;7:45. doi: 10.3390/bioengineering7020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kumar A., Mittal A., Das A., Sen D., Mariappan C.R. Mesoporous electroactive silver doped calcium borosilicates: Structural, antibacterial and myogenic potential relationship of improved bio-ceramics. Ceram. Int. 2021;47:3586–3596. doi: 10.1016/j.ceramint.2020.09.207. [DOI] [Google Scholar]
- 251.Westhauser F., Decker S., Nawaz Q., Rehder F., Wilkesmann S., Moghaddam A., Kunisch E., Boccaccini A.R. Impact of zinc-or copper-doped mesoporous bioactive glass nanoparticles on the osteogenic differentiation and matrix formation of mesenchymal stromal cells. Materials. 2021;14:1864. doi: 10.3390/ma14081864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sun H., Zheng K., Zhou T., Boccaccini A.R. Incorporation of zinc into binary SiO2-CaO mesoporous bioactive glass nanoparticles enhances anti-inflammatory and osteogenic activities. Pharmaceutics. 2021;13:2124. doi: 10.3390/pharmaceutics13122124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fiorilli S., Molino G., Pontremoli C., Iviglia G., Torre E., Cassinelli C., Morra M., Vitale-Brovarone C. The incorporation of strontium to improve bone-regeneration ability of mesoporous bioactive glasses. Materials. 2018;11:678. doi: 10.3390/ma11050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fiorilli S., Pagani M., Boggio E., Gigliotti C.L., Dianzani C., Gauthier R., Pontremoli C., Montalbano G., Dianzani U., Vitale-Brovarone C. Sr-containing mesoporous bioactive glasses bio-functionalized with recombinant ICOS-Fc: An in vitro study. Nanomaterials. 2021;11:321. doi: 10.3390/nano11020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Shruti S., Andreatta F., Furlani E., Marin E., Maschio S., Fedrizzi L. Cerium, gallium and zinc containing mesoporous bioactive glass coating deposited on titanium alloy. Appl. Surf. Sci. 2016;378:216–223. doi: 10.1016/j.apsusc.2016.03.209. [DOI] [Google Scholar]
- 256.Kurtuldu F., Mutlu N., Michálek M., Zheng K., Masar M., Liverani L., Chen S., Galusek D., Boccaccini A.R. Cerium and gallium containing mesoporous bioactive glass nanoparticles for bone regeneration: Bioactivity, biocompatibility and antibacterial activity. Mater. Sci. Eng. C. 2021;124:112050. doi: 10.1016/j.msec.2021.112050. [DOI] [PubMed] [Google Scholar]
- 257.El-Fiqi A., Kim H.W. Sol-gel synthesis and characterization of novel cobalt ions-containing mesoporous bioactive glass nanospheres as hypoxia and ferroptosis-inducing nanotherapeutics. J. Non-Cryst. Solids. 2021;569:120999. doi: 10.1016/j.jnoncrysol.2021.120999. [DOI] [Google Scholar]
- 258.El-Fiqi A., Kim H.W. Iron ions-releasing mesoporous bioactive glass ultrasmall nanoparticles designed as ferroptosis-based bone cancer nanotherapeutics: Ultrasonic-coupled sol–gel synthesis, properties and iron ions release. Mater. Lett. 2021;294:129759. doi: 10.1016/j.matlet.2021.129759. [DOI] [Google Scholar]
- 259.Zhang Y., Hu M., Zhang W., Zhang X. Homology of selenium (Se) and tellurium (Te) endow the functional similarity of Se-doped and Te-doped mesoporous bioactive glass nanoparticles in bone tissue engineering. Ceram. Int. 2022;48:3729–3739. doi: 10.1016/j.ceramint.2021.10.155. [DOI] [Google Scholar]
- 260.Zhang Y., Hu M., Zhang W., Zhang X. Construction of tellurium-doped mesoporous bioactive glass nanoparticles for bone cancer therapy by promoting ROS-mediated apoptosis and antibacterial activity. J. Colloid Interface Sci. 2022;610:719–730. doi: 10.1016/j.jcis.2021.11.122. [DOI] [PubMed] [Google Scholar]
- 261.Heras C., Jiménez-Holguín J., Doadrio A.L., Vallet-Regí M., Sánchez-Salcedo S., Salinas A.J. Multifunctional antibiotic- and zinc-containing mesoporous bioactive glass scaffolds to fight bone infection. Acta Biomater. 2020;114:395–406. doi: 10.1016/j.actbio.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hasan R., Schaner K., Mulinti P., Brooks A. A bioglass-based antibiotic (Vancomycin) releasing bone void filling putty to treat osteomyelitis and aid bone healing. Int. J. Mol. Sci. 2021;22:7736. doi: 10.3390/ijms22147736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang X., Zeng D., Weng W., Huang Q., Zhang X., Wen J., Wu J., Jiang X. Alendronate delivery on amino modified mesoporous bioactive glass scaffolds to enhance bone regeneration in osteoporosis rats. Artif. Cells Nanomed. Biotechnol. 2018;46:171–181. doi: 10.1080/21691401.2018.1453825. [DOI] [PubMed] [Google Scholar]
- 264.Ravanbakhsh M., Labbaf S., Karimzadeh F., Pinna A., Houreh A.B., Nasr-Esfahani M.H. Mesoporous bioactive glasses for the combined application of osteosarcoma treatment and bone regeneration. Mater. Sci. Eng. C. 2019;104:109994. doi: 10.1016/j.msec.2019.109994. [DOI] [PubMed] [Google Scholar]
- 265.Hu M., Fang J., Zhang Y., Wang X., Zhong W., Zhou Z. Design and evaluation a kind of functional biomaterial for bone tissue engineering: Selenium/mesoporous bioactive glass nanospheres. J. Colloid Interface Sci. 2020;579:654–666. doi: 10.1016/j.jcis.2020.06.122. [DOI] [PubMed] [Google Scholar]
- 266.ur Rahman M.S., Tahir M.A., Noreen S., Yasir M., Khan M.B., Mahmood T., Bahadur A., Shoaib M. Osteogenic silver oxide doped mesoporous bioactive glass for controlled release of doxorubicin against bone cancer cell line (MG-63): In vitro and in vivo cytotoxicity evaluation. Ceram. Int. 2020;46:10765–10770. doi: 10.1016/j.ceramint.2020.01.086. [DOI] [Google Scholar]
- 267.Berkmann J.C., Herrera Martin A.X., Pontremoli C., Zheng K., Bucher C.H., Ellinghaus A., Boccaccini A.R., Fiorilli S., Brovarone C.V., Duda G.N., et al. In vivo validation of spray-dried mesoporous bioactive glass microspheres acting as prolonged local release systems for bmp-2 to support bone regeneration. Pharmaceutics. 2020;12:823. doi: 10.3390/pharmaceutics12090823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Xin T., Mao J., Liu L., Tang J., Wu L., Yu X., Gu Y., Cui W., Chen L. Programmed Sustained Release of Recombinant Human Bone Morphogenetic Protein-2 and Inorganic Ion Composite Hydrogel as Artificial Periosteum. ACS Appl. Mater. Interfaces. 2020;12:6840–6851. doi: 10.1021/acsami.9b18496. [DOI] [PubMed] [Google Scholar]
- 269.Jiménez-Holguín J., Sánchez-Salcedo S., Vallet-Regí M., Salinas A.J. Development and evaluation of copper-containing mesoporous bioactive glasses for bone defects therapy. Microporous Mesoporous Mater. Off. J. Int. Zeolite Assoc. 2020;308:110454. doi: 10.1016/j.micromeso.2020.110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lalzawmliana V., Anand A., Roy M., Kundu B., Nandi S.K. Mesoporous bioactive glasses for bone healing and biomolecules delivery. Mater. Sci. Eng. C. 2020;106:110180. doi: 10.1016/j.msec.2019.110180. [DOI] [PubMed] [Google Scholar]
- 271.Uribe P., Johansson A., Jugdaohsingh R., Powell J.J., Magnusson C., Davila M., Westerlund A., Ransjö M. Soluble silica stimulates osteogenic differentiation and gap junction communication in human dental follicle cells. Sci. Rep. 2020;10:9923. doi: 10.1038/s41598-020-66939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Rondanelli M., Faliva M.A., Peroni G., Gasparri C., Perna S., Riva A., Petrangolini G., Tartara A. Silicon: A neglected micronutrient essential for bone health. Exp. Biol. Med. 2021;246:1500–1511. doi: 10.1177/1535370221997072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Almáši M., Matiašová A.A., Šuleková M., Beňová E., Ševc J., Váhovská L., Lisnichuk M., Girman V., Zeleňáková A., Hudák A., et al. In vivo study of light-driven naproxen release from gated mesoporous silica drug delivery system. Sci. Rep. 2021;11:20191. doi: 10.1038/s41598-021-99678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Galhano J., Marcelo G.A., Duarte M.P., Oliveira E. Ofloxacin@Doxorubicin-Epirubicin functionalized MCM-41 mesoporous silica–based nanocarriers as synergistic drug delivery tools for cancer related bacterial infections. Bioorg. Chem. 2022;118:105470. doi: 10.1016/j.bioorg.2021.105470. [DOI] [PubMed] [Google Scholar]
- 275.Wang Y., Shahi P.K., Wang X., Xie R., Zhao Y., Wu M., Roge S., Pattnaik B.R., Gong S. In vivo targeted delivery of nucleic acids and CRISPR genome editors enabled by GSH-responsive silica nanoparticles. J. Control. Release. 2021;336:296–309. doi: 10.1016/j.jconrel.2021.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Paris J.L., Lafuente-Gómez N., Cabañas M.V., Román J., Peña J., Vallet-Regí M. Fabrication of a nanoparticle-containing 3D porous bone scaffold with proangiogenic and antibacterial properties. Acta Biomater. 2019;86:441–449. doi: 10.1016/j.actbio.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Li Y., Xu T., Tu Z., Dai W., Xue Y., Tang C., Gao W., Mao C., Lei B., Lin C. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics. 2020;10:4929–4943. doi: 10.7150/thno.41839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yu Y., Yu X., Tian D., Yu A., Wan Y. Thermo-responsive chitosan/silk fibroin/amino-functionalized mesoporous silica hydrogels with strong and elastic characteristics for bone tissue engineering. Int. J. Biol. Macromol. 2021;182:1746–1758. doi: 10.1016/j.ijbiomac.2021.05.166. [DOI] [PubMed] [Google Scholar]
- 279.Hou Y.T., Wu K.C.W., Lee C.Y. Development of glycyrrhizin-conjugated, chitosan-coated, lysine-embedded mesoporous silica nanoparticles for hepatocyte-targeted liver tissue regeneration. Materialia. 2020;9:100568. doi: 10.1016/j.mtla.2019.100568. [DOI] [Google Scholar]
- 280.Zhang B., Ding Z., Dong J., Lin F., Xue Z., Xu J. Macrophage-mediated degradable gelatin-coated mesoporous silica nanoparticles carrying pirfenidone for the treatment of rat spinal cord injury. Nanomed. Nanotechnol. Biol. Med. 2021;37:102420. doi: 10.1016/j.nano.2021.102420. [DOI] [PubMed] [Google Scholar]
- 281.Serati-Nouri H., Rasoulpoor S., Pourpirali R., Sadeghi-Soureh S., Esmaeilizadeh N., Dadashpour M., Roshangar L., Zarghami N. In vitro expansion of human adipose-derived stem cells with delayed senescence through dual stage release of curcumin from mesoporous silica nanoparticles/electrospun nanofibers. Life Sci. 2021;285:119947. doi: 10.1016/j.lfs.2021.119947. [DOI] [PubMed] [Google Scholar]
- 282.Chen M., Hu J., Wang L., Li Y., Zhu C., Chen C., Shi M., Ju Z., Cao X., Zhang Z. Targeted and redox-responsive drug delivery systems based on carbonic anhydrase IX-decorated mesoporous silica nanoparticles for cancer therapy. Sci. Rep. 2020;10:14447. doi: 10.1038/s41598-020-71071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhang B.B., Chen X.J., Fan X.D., Zhu J.J., Wei Y.H., Zheng H.S., Zheng H.Y., Wang B.H., Piao J.G., Li F.Z. Lipid/PAA-coated mesoporous silica nanoparticles for dual-pH-responsive codelivery of arsenic trioxide/paclitaxel against breast cancer cells. Acta Pharmacol. Sin. 2021;42:832–842. doi: 10.1038/s41401-021-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kundu M., Sadhukhan P., Ghosh N., Ghosh S., Chatterjee S., Das J., Brahmachari G., Sil P.C. In vivo therapeutic evaluation of a novel bis-lawsone derivative against tumor following delivery using mesoporous silica nanoparticle based redox-responsive drug delivery system. Mater. Sci. Eng. C. 2021;126:112142. doi: 10.1016/j.msec.2021.112142. [DOI] [PubMed] [Google Scholar]
- 285.Chircov C., Spoială A., Păun C., Crăciun L., Ficai D., Ficai A., Andronescu E., Turculeƫ Ș.C. Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents. Molecules. 2020;25:3814. doi: 10.3390/molecules25173814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kankala R.K., Han Y.-H., Xia H.-Y., Wang S.-B., Chen A.-Z. Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotechnol. 2022;20:126. doi: 10.1186/s12951-022-01315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Živojević K., Mladenović M., Djisalov M., Mundzic M., Ruiz-Hernandez E., Gadjanski I., Knežević N. Advanced mesoporous silica nanocarriers in cancer theranostics and gene editing applications. J. Control. Release Off. J. Control. Release Soc. 2021;337:193–211. doi: 10.1016/j.jconrel.2021.07.029. [DOI] [PubMed] [Google Scholar]
- 288.Jafari S., Derakhshankhah H., Alaei L., Fattahi A., Varnamkhasti B.S., Saboury A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019;109:1100–1111. doi: 10.1016/j.biopha.2018.10.167. [DOI] [PubMed] [Google Scholar]
- 289.Rastegari E., Hsiao Y.-J., Lai W.-Y., Lai Y.-H., Yang T.-C., Chen S.-J., Huang P.-I., Chiou S.-H., Mou C.-Y., Chien Y. An Update on Mesoporous Silica Nanoparticle Applications in Nanomedicine. Pharmaceutics. 2021;13:1067. doi: 10.3390/pharmaceutics13071067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sábio R.M., Meneguin A.B., Martins dos Santos A., Monteiro A.S., Chorilli M. Exploiting mesoporous silica nanoparticles as versatile drug carriers for several routes of administration. Microporous Mesoporous Mater. 2021;312:110774. doi: 10.1016/j.micromeso.2020.110774. [DOI] [Google Scholar]
- 291.He Q., Chen J., Yan J., Cai S., Xiong H., Liu Y., Peng D., Mo M., Liu Z. Tumor microenvironment responsive drug delivery systems. Asian J. Pharm. Sci. 2020;15:416–448. doi: 10.1016/j.ajps.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wang K., Lu J., Li J., Gao Y., Mao Y., Zhao Q., Wang S. Current trends in smart mesoporous silica-based nanovehicles for photoactivated cancer therapy. J. Control. Release. 2021;339:445–472. doi: 10.1016/j.jconrel.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 293.Eivazzadeh-Keihan R., Chenab K.K., Taheri-Ledari R., Mosafer J., Hashemi S.M., Mokhtarzadeh A., Maleki A., Hamblin M.R. Recent advances in the application of mesoporous silica-based nanomaterials for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;107:110267. doi: 10.1016/j.msec.2019.110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ghosh S., Webster T.J. Mesoporous Silica Based Nanostructures for Bone Tissue Regeneration. Front. Mater. 2021;8:213. doi: 10.3389/fmats.2021.692309. [DOI] [Google Scholar]
- 295.Narayan R., Nayak U.Y., Raichur A.M., Garg S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics. 2018;10:118. doi: 10.3390/pharmaceutics10030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pal N., Lee J.-H., Cho E.-B. Recent Trends in Morphology-Controlled Synthesis and Application of Mesoporous Silica Nanoparticles. Nanomaterials. 2020;10:2122. doi: 10.3390/nano10112122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gandhimathi C., Quek Y.J., Ezhilarasu H., Ramakrishna S., Bay B.-H., Srinivasan D.K. Osteogenic Differentiation of Mesenchymal Stem Cells with Silica-Coated Gold Nanoparticles for Bone Tissue Engineering. Int. J. Mol. Sci. 2019;20:5135. doi: 10.3390/ijms20205135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hosseinpour S., Walsh L.J., Xu C. Modulating Osteoimmune Responses by Mesoporous Silica Nanoparticles. ACS Biomater. Sci. Eng. 2021 doi: 10.1021/acsbiomaterials.1c00899. [DOI] [PubMed] [Google Scholar]
- 299.Beck G.R., Ha S.W., Camalier C.E., Yamaguchi M., Li Y., Lee J.K., Weitzmann M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012;8:793–803. doi: 10.1016/j.nano.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Shi M., Zhou Y., Shao J., Chen Z., Song B., Chang J., Wu C., Xiao Y. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015;21:178–189. doi: 10.1016/j.actbio.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 301.Xu C., Xiao L., Cao Y., He Y., Lei C., Xiao Y., Sun W., Ahadian S., Zhou X., Khademhosseini A., et al. Mesoporous silica rods with cone shaped pores modulate inflammation and deliver BMP-2 for bone regeneration. Nano Res. 2020;13:2323–2331. doi: 10.1007/s12274-020-2783-z. [DOI] [Google Scholar]
- 302.Mora-Raimundo P., Lozano D., Benito M., Mulero F., Manzano M., Vallet-Regí M. Osteoporosis Remission and New Bone Formation with Mesoporous Silica Nanoparticles. Adv. Sci. 2021;8:2101107. doi: 10.1002/advs.202101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Cui W., Liu Q., Yang L., Wang K., Sun T., Ji Y., Liu L., Yu W., Qu Y., Wang J., et al. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2018;4:211–221. doi: 10.1021/acsbiomaterials.7b00506. [DOI] [PubMed] [Google Scholar]
- 304.Zhou X., Zhang Q., Chen L., Nie W., Wang W., Wang H., Mo X., He C. Versatile Nanocarrier Based on Functionalized Mesoporous Silica Nanoparticles to Codeliver Osteogenic Gene and Drug for Enhanced Osteodifferentiation. ACS Biomater. Sci. Eng. 2019;5:710–723. doi: 10.1021/acsbiomaterials.8b01110. [DOI] [PubMed] [Google Scholar]
- 305.Castillo R.R., Lozano D., Vallet-Regí M. Mesoporous Silica Nanoparticles as Carriers for Therapeutic Biomolecules. Pharmaceutics. 2020;12:432. doi: 10.3390/pharmaceutics12050432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Abbasi M., Hafez Ghoran S., Niakan M.H., Jamali K., Moeini Z., Jangjou A., Izadpanah P., Amani A. Mesoporous silica nanoparticle: Heralding a brighter future in cancer nanomedicine. Microporous Mesoporous Mater. 2021;319:110967. doi: 10.1016/j.micromeso.2021.110967. [DOI] [Google Scholar]
- 307.Gisbert-Garzarán M., Manzano M., Vallet-Regí M. Mesoporous Silica Nanoparticles for the Treatment of Complex Bone Diseases: Bone Cancer, Bone Infection and Osteoporosis. Pharmaceutics. 2020;12:83. doi: 10.3390/pharmaceutics12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhu H., Zheng K., Boccaccini A.R. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021;129:1–17. doi: 10.1016/j.actbio.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 309.Kuang Y., Zhai J., Xiao Q., Zhao S., Li C. Polysaccharide/mesoporous silica nanoparticle-based drug delivery systems: A review. Int. J. Biol. Macromol. 2021;193:457–473. doi: 10.1016/j.ijbiomac.2021.10.142. [DOI] [PubMed] [Google Scholar]
- 310.Zhou S., Zhong Q., Wang Y., Hu P., Zhong W., Huang C.B., Yu Z.Q., Ding C.D., Liu H., Fu J. Chemically engineered mesoporous silica nanoparticles-based intelligent delivery systems for theranostic applications in multiple cancerous/non-cancerous diseases. Coord. Chem. Rev. 2022;452:214309. doi: 10.1016/j.ccr.2021.214309. [DOI] [Google Scholar]
- 311.Bernardos A., Piacenza E., Sancenón F., Hamidi M., Maleki A., Turner R.J., Martínez-Máñez R. Mesoporous Silica-Based Materials with Bactericidal Properties. Small. 2019;15:1900669. doi: 10.1002/smll.201900669. [DOI] [PubMed] [Google Scholar]
- 312.Carvalho G.C., Sábio R.M., de Cássia Ribeiro T., Monteiro A.S., Pereira D.V., Ribeiro S.J.L., Chorilli M. Highlights in Mesoporous Silica Nanoparticles as a Multifunctional Controlled Drug Delivery Nanoplatform for Infectious Diseases Treatment. Pharm. Res. 2020;37:191. doi: 10.1007/s11095-020-02917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Seljak K.B., Kocbek P., Gašperlin M. Mesoporous silica nanoparticles as delivery carriers: An overview of drug loading techniques. J. Drug Deliv. Sci. Technol. 2020;59:101906. doi: 10.1016/j.jddst.2020.101906. [DOI] [Google Scholar]
- 314.Andrade G.F., Faria J.A.Q.A., Gomes D.A., de Barros A.L.B., Fernandes R.S., Coelho A.C.S., Takahashi J.A., da Silva Cunha A., de Sousa E.M.B. Mesoporous silica SBA-16/hydroxyapatite-based composite for ciprofloxacin delivery to bacterial bone infection. J. Sol-Gel Sci. Technol. 2018;85:369–381. doi: 10.1007/s10971-017-4557-y. [DOI] [Google Scholar]
- 315.Szewczyk A., Skwira A., Konopacka A., Sadej R., Prokopowicz M. Mesoporous silica-bioglass composite pellets as bone drug delivery system with mineralization potential. Int. J. Mol. Sci. 2021;22:4708. doi: 10.3390/ijms22094708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Yao C., Zhu M., Han X., Xu Q., Dai M., Nie T., Liu X. A Bone-Targeting Enoxacin Delivery System to Eradicate Staphylococcus Aureus-Related Implantation Infections and Bone Loss. Front. Bioeng. Biotechnol. 2021;9:749910. doi: 10.3389/fbioe.2021.749910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhou X., Weng W., Chen B., Feng W., Wang W., Nie W., Chen L., Mo X., Su J., He C. Mesoporous silica nanoparticles/gelatin porous composite scaffolds with localized and sustained release of vancomycin for treatment of infected bone defects. J. Mater. Chem. B. 2018;6:740–752. doi: 10.1039/C7TB01246B. [DOI] [PubMed] [Google Scholar]
- 318.Lu Y., Li L., Lin Z., Li M., Hu X., Zhang Y., Peng M., Xia H., Han G. Enhancing Osteosarcoma Killing and CT Imaging Using Ultrahigh Drug Loading and NIR-Responsive Bismuth Sulfide@Mesoporous Silica Nanoparticles. Adv. Healthc. Mater. 2018;7:1800602. doi: 10.1002/adhm.201800602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Martínez-Carmona M., Lozano D., Colilla M., Vallet-Regí M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018;65:393–404. doi: 10.1016/j.actbio.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 320.Hu H., Yang W., Liang Z., Zhou Z., Song Q., Liu W., Deng X., Zhu J., Xing X., Zhong B., et al. Amplification of oxidative stress with lycorine and gold-based nanocomposites for synergistic cascade cancer therapy. J. Nanobiotechnol. 2021;19:221. doi: 10.1186/s12951-021-00933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Moodley T., Singh M. Polymeric mesoporous silica nanoparticles for enhanced delivery of 5-fluorouracil in vitro. Pharmaceutics. 2019;11:288. doi: 10.3390/pharmaceutics11060288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Moodley T., Singh M. Polymeric mesoporous silica nanoparticles for combination drug delivery in vitro. Biointerface Res. Appl. Chem. 2020;11:11905–11919. [Google Scholar]
- 323.Shin T.H., Seo C., Lee D.Y., Ji M., Manavalan B., Basith S., Chakkarapani S.K., Kang S.H., Lee G., Paik M.J., et al. Silica-coated magnetic nanoparticles induce glucose metabolic dysfunction in vitro via the generation of reactive oxygen species. Arch. Toxicol. 2019;93:1201–1212. doi: 10.1007/s00204-019-02402-z. [DOI] [PubMed] [Google Scholar]
- 324.Tong F., Ye Y., Chen B., Gao J., Liu L., Ou J., Van Hest J.C.M., Liu S., Peng F., Tu Y. Bone-Targeting Prodrug Mesoporous Silica-Based Nanoreactor with Reactive Oxygen Species Burst for Enhanced Chemotherapy. ACS Appl. Mater. Interfaces. 2020;12:34630–34642. doi: 10.1021/acsami.0c08992. [DOI] [PubMed] [Google Scholar]
- 325.Shao L., Li Y., Huang F., Wang X., Lu J., Jia F., Pan Z., Cui X., Ge G., Deng X., et al. Complementary autophagy inhibition and glucose metabolism with rattle-structured polydopamine@mesoporous silica nanoparticles for augmented low-temperature photothermal therapy and in vivo photoacoustic imaging. Theranostics. 2020;10:7273–7286. doi: 10.7150/thno.44668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Moodley T., Singh M. Current Stimuli-Responsive Mesoporous Silica Nanoparticles for Cancer Therapy. Pharmaceutics. 2021;13:71. doi: 10.3390/pharmaceutics13010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Abdo G.G., Zagho M.M., Khalil A. Recent advances in stimuli-responsive drug release and targeting concepts using mesoporous silica nanoparticles. Emergent Mater. 2020;3:407–425. doi: 10.1007/s42247-020-00109-x. [DOI] [Google Scholar]
- 328.Castillo R.R., Vallet-Regí M. Functional Mesoporous Silica Nanocomposites: Biomedical applications and Biosafety. Int. J. Mol. Sci. 2019;20:929. doi: 10.3390/ijms20040929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Friedrich R.P., Cicha I., Alexiou C. Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials. 2021;11:2337. doi: 10.3390/nano11092337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Fan D., Wang Q., Zhu T., Wang H., Liu B., Wang Y., Liu Z., Liu X., Fan D., Wang X. Recent Advances of Magnetic Nanomaterials in Bone Tissue Repair. Front. Chem. 2020;8:745. doi: 10.3389/fchem.2020.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Popescu R.C., Andronescu E., Vasile B.S. Recent advances in magnetite nanoparticle functionalization for nanomedicine. Nanomaterials. 2019;9:1791. doi: 10.3390/nano9121791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ramazanov M., Karimova A., Shirinova H. Magnetism for drug delivery, mri and hyperthermia applications: A review. Biointerface Res. Appl. Chem. 2021;11:8654–8668. doi: 10.33263/BRIAC112.86548668. [DOI] [Google Scholar]
- 333.Sathishkumar G., Logeshwaran V., Sarathbabu S., Jha P.K., Jeyaraj M., Rajkuberan C., Senthilkumar N., Sivaramakrishnan S. Green synthesis of magnetic Fe3O4 nanoparticles using Couroupita guianensis Aubl. fruit extract for their antibacterial and cytotoxicity activities. Artif. Cells Nanomed. Biotechnol. 2018;46:589–598. doi: 10.1080/21691401.2017.1332635. [DOI] [PubMed] [Google Scholar]
- 334.Vasantharaj S., Sathiyavimal S., Senthilkumar P., LewisOscar F., Pugazhendhi A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J. Photochem. Photobiol. B Biol. 2019;192:74–82. doi: 10.1016/j.jphotobiol.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 335.Armijo L.M., Wawrzyniec S.J., Kopciuch M., Brandt Y.I., Rivera A.C., Withers N.J., Cook N.C., Huber D.L., Monson T.C., Smyth H.D.C., et al. Antibacterial activity of iron oxide, iron nitride, and tobramycin conjugated nanoparticles against Pseudomonas aeruginosa biofilms. J. Nanobiotechnol. 2020;18:35. doi: 10.1186/s12951-020-0588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Sakthi Sri S.P., Taj J., George M. Facile synthesis of magnetite nanocubes using deep eutectic solvent: An insight to anticancer and photo-Fenton efficacy. Surf. Interfaces. 2020;20:100609. doi: 10.1016/j.surfin.2020.100609. [DOI] [Google Scholar]
- 337.Yusefi M., Shameli K., Yee O.S., Teow S.Y., Hedayatnasab Z., Jahangirian H., Webster T.J., Kuča K. Green synthesis of Fe3O4 nanoparticles stabilized by a garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomed. 2021;16:2515–2532. doi: 10.2147/IJN.S284134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Badry M.D., Wahba M.A., Khaled R.K., Ali M.M. Hydrothermally assisted synthesis of magnetic iron oxide-chitosan nanocomposites: Electrical and biological evaluation. Biointerface Res. Appl. Chem. 2022;12:2229–2241. doi: 10.33263/BRIAC122.22292241. [DOI] [Google Scholar]
- 339.Divband B., Gharehaghaji N., Atashi Z. High Transverse Relaxivity and Anticancer Agent Loading/Release Characteristics of Porous Calcium Phosphate Coated Iron Oxide Nanoparticles. Biointerface Res. Appl. Chem. 2020;11:10402–10411. [Google Scholar]
- 340.Miola M., Bellare A., Gerbaldo R., Laviano F., Vernè E. Synthesis and characterization of magnetic and antibacterial nanoparticles as filler in acrylic cements for bone cancer and comorbidities therapy. Ceram. Int. 2021;47:17633–17643. doi: 10.1016/j.ceramint.2021.03.082. [DOI] [Google Scholar]
- 341.Wu V.M., Huynh E., Tang S., Uskoković V. Brain and bone cancer targeting by a ferrofluid composed of superparamagnetic iron-oxide/silica/carbon nanoparticles (earthicles) Acta Biomater. 2019;88:422–447. doi: 10.1016/j.actbio.2019.01.064. [DOI] [PubMed] [Google Scholar]
- 342.Popescu R.C., Andronescu E., Vasile B.Ș., Truşcă R., Boldeiu A., Mogoantă L., Mogoșanu G.D., Temelie M., Radu M., Grumezescu A.M., et al. Fabrication and cytotoxicity of gemcitabine-functionalized magnetite nanoparticles. Molecules. 2017;22:1080. doi: 10.3390/molecules22071080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Raghubir M., Rahman C.N., Fang J., Matsui H., Mahajan S.S. Osteosarcoma growth suppression by riluzole delivery via iron oxide nanocage in nude mice. Oncol. Rep. 2020;43:169–176. doi: 10.3892/or.2019.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Pang Y., Su L., Fu Y., Jia F., Zhang C., Cao X., He W., Kong X., Xu J., Zhao J., et al. Inhibition of furin by bone targeting superparamagnetic iron oxide nanoparticles alleviated breast cancer bone metastasis. Bioact. Mater. 2021;6:712–720. doi: 10.1016/j.bioactmat.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zarei S., Sadighian S., Rostamizadeh K., Khalkhali M. Theragnostic magnetic core-shell nanoparticle as versatile nanoplatform for magnetic resonance imaging and drug delivery. Biointerface Res. Appl. Chem. 2021;11:13276–13289. [Google Scholar]
- 346.Gong M., Liu H., Sun N., Xie Y., Yan F., Cai L. Polyethylenimine-dextran-coated magnetic nanoparticles loaded with miR-302b suppress osteosarcoma in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2020;15:711–723. doi: 10.2217/nnm-2019-0218. [DOI] [PubMed] [Google Scholar]
- 347.Khodaei A., Jahanmard F., Madaah Hosseini H.R., Bagheri R., Dabbagh A., Weinans H., Amin Yavari S. Controlled temperature-mediated curcumin release from magneto-thermal nanocarriers to kill bone tumors. Bioact. Mater. 2022;11:107–117. doi: 10.1016/j.bioactmat.2021.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Popescu R.C., Straticiuc M., Mustăciosu C., Temelie M., Trușcă R., Vasile B.Ș., Boldeiu A., Mirea D., Andrei R.F., Cenușă C., et al. Enhanced internalization of nanoparticles following ionizing radiation leads to mitotic catastrophe in MG-63 human osteosarcoma cells. Int. J. Mol. Sci. 2020;21:7220. doi: 10.3390/ijms21197220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Dong S., Chen Y., Yu L., Lin K., Wang X. Magnetic Hyperthermia–Synergistic H2O2 Self-Sufficient Catalytic Suppression of Osteosarcoma with Enhanced Bone-Regeneration Bioactivity by 3D-Printing Composite Scaffolds. Adv. Funct. Mater. 2020;30:1907071. doi: 10.1002/adfm.201907071. [DOI] [Google Scholar]
- 350.Lu J.W., Yang F., Ke Q.F., Xie X.T., Guo Y.P. Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomed. Nanotechnol. Biol. Med. 2018;14:811–822. doi: 10.1016/j.nano.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 351.Musielak M., Piotrowski I., Suchorska W.M. Superparamagnetic iron oxide nanoparticles (SPIONs) as a multifunctional tool in various cancer therapies. Rep. Pract. Oncol. Radiother. 2019;24:307–314. doi: 10.1016/j.rpor.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Samrot A.V., Sahithya C.S., Selvarani A.J., Purayil S.K., Ponnaiah P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021;4:100042. doi: 10.1016/j.crgsc.2020.100042. [DOI] [Google Scholar]
- 353.Gavilán H., Avugadda S.K., Fernández-Cabada T., Soni N., Cassani M., Mai B.T., Chantrell R., Pellegrino T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021;50:11614–11667. doi: 10.1039/D1CS00427A. [DOI] [PubMed] [Google Scholar]
- 354.Schneider M.G.M., Martín M.J., Otarola J., Vakarelska E., Simeonov V., Lassalle V., Nedyalkova M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics. 2022;14:204. doi: 10.3390/pharmaceutics14010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Ignatovich Z., Novik K., Abakshonok A., Koroleva E., Beklemisheva A., Panina L., Kaniukov E., Anisovich M., Shumskaya A. One-Step Synthesis of Magnetic Nanocomposite with Embedded Biologically Active Substance. Molecules. 2021;26:937. doi: 10.3390/molecules26040937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Senthilkumar N., Kumar Sharma P., Sood N., Bhalla N. Designing magnetic nanoparticles for in vivo applications and understanding their fate inside human body. Coord. Chem. Rev. 2021;445:214082. doi: 10.1016/j.ccr.2021.214082. [DOI] [Google Scholar]
- 357.Bin S., Wang A., Guo W., Yu L., Feng P. Micro Magnetic Field Produced by Fe3O4 Nanoparticles in Bone Scaffold for Enhancing Cellular Activity. Polymers. 2020;12:2045. doi: 10.3390/polym12092045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Saraiva A.S., Ribeiro I.A.C., Fernandes M.H., Cerdeira A.C., Vieira B.J.C., Waerenborgh J.C., Pereira L.C.J., Cláudio R., Carmezim M.J., Gomes P., et al. 3D-printed platform multi-loaded with bioactive, magnetic nanoparticles and an antibiotic for re-growing bone tissue. Int. J. Pharm. 2021;593:120097. doi: 10.1016/j.ijpharm.2020.120097. [DOI] [PubMed] [Google Scholar]
- 359.Shuai C., Yang W., He C., Peng S., Gao C., Yang Y., Qi F., Feng P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2020;185:108275. doi: 10.1016/j.matdes.2019.108275. [DOI] [Google Scholar]
- 360.Li M., Liu J., Cui X., Sun G., Hu J., Xu S., Yang F., Zhang L., Wang X., Tang P. Osteogenesis effects of magnetic nanoparticles modified-porous scaffolds for the reconstruction of bone defect after bone tumor resection. Regen. Biomater. 2019;6:373–381. doi: 10.1093/rb/rbz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Jia L., Yang Z., Sun L., Zhang Q., Guo Y., Chen Y., Dai Y., Xia Y. A three-dimensional-printed SPION/PLGA scaffold for enhanced palate-bone regeneration and concurrent alteration of the oral microbiota in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;126:112173. doi: 10.1016/j.msec.2021.112173. [DOI] [PubMed] [Google Scholar]
- 362.Paun I.A., Calin B.S., Mustaciosu C.C., Mihailescu M., Moldovan A., Crisan O., Leca A., Luculescu C.R. 3D superparamagnetic scaffolds for bone mineralization under static magnetic field stimulation. Materials. 2019;12:2834. doi: 10.3390/ma12172834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Tanasa E., Zaharia C., Hudita A., Radu I.C., Costache M., Galateanu B. Impact of the magnetic field on 3T3-E1 preosteoblasts inside SMART silk fibroin-based scaffolds decorated with magnetic nanoparticles. Mater. Sci. Eng. C. 2020;110:110714. doi: 10.1016/j.msec.2020.110714. [DOI] [PubMed] [Google Scholar]
- 364.Filippi M., Dasen B., Guerrero J., Garello F., Isu G., Born G., Ehrbar M., Martin I., Scherberich A. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials. 2019;223:119468. doi: 10.1016/j.biomaterials.2019.119468. [DOI] [PubMed] [Google Scholar]
- 365.Wu D., Chang X., Tian J., Kang L., Wu Y., Liu J., Wu X., Huang Y., Gao B., Wang H., et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: Release of exosomal miR-1260a improves osteogenesis and angiogenesis. J. Nanobiotechnol. 2021;19:209. doi: 10.1186/s12951-021-00958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Zhao Y.Z., Chen R., Xue P.P., Luo L.Z., Zhong B., Tong M.Q., Chen B., Yao Q., Yuan J.D., Xu H.L. Magnetic PLGA microspheres loaded with SPIONs promoted the reconstruction of bone defects through regulating the bone mesenchymal stem cells under an external magnetic field. Mater. Sci. Eng. C. 2021;122:111877. doi: 10.1016/j.msec.2021.111877. [DOI] [PubMed] [Google Scholar]
- 367.Piñeiro Y., González Gómez M., de Castro Alves L., Arnosa Prieto A., García Acevedo P., Seco Gudiña R., Puig J., Teijeiro C., Yáñez Vilar S., Rivas J. Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine. Magnetochemistry. 2020;6:4. doi: 10.3390/magnetochemistry6010004. [DOI] [Google Scholar]
- 368.Singh S., Singh G., Bala N. Synthesis and characterization of iron oxide-hydroxyapatite-chitosan composite coating and its biological assessment for biomedical applications. Prog. Org. Coat. 2021;150:106011. doi: 10.1016/j.porgcoat.2020.106011. [DOI] [Google Scholar]
- 369.Wei X., Zhang X., Yang Z., Li L., Sui H. Osteoinductive potential and antibacterial characteristics of collagen coated iron oxide nanosphere containing strontium and hydroxyapatite in long term bone fractures. Arab. J. Chem. 2021;14:102984. doi: 10.1016/j.arabjc.2020.102984. [DOI] [Google Scholar]
- 370.Li M., Fu S., Cai Z., Li D., Liu L., Deng D., Jin R., Ai H. Dual regulation of osteoclastogenesis and osteogenesis for osteoporosis therapy by iron oxide hydroxyapatite core/shell nanocomposites. Regen. Biomater. 2021;8:rbab027. doi: 10.1093/rb/rbab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ferreira-Ermita D.A., Valente F.L., Carlo-Reis E.C., Araújo F.R., Ribeiro I.M., Cintra C.C., Borges A.P. Characterization and in vivo biocompatibility analysis of synthetic hydroxyapatite compounds associated with magnetite nanoparticles for a drug delivery system in osteomyelitis treatment. Results Mater. 2020;5:100063. doi: 10.1016/j.rinma.2020.100063. [DOI] [Google Scholar]
- 372.Liu Q., Feng L., Chen Z., Lan Y., Liu Y., Li D., Yan C., Xu Y. Ultrasmall Superparamagnetic Iron Oxide Labeled Silk Fibroin/Hydroxyapatite Multifunctional Scaffold Loaded with Bone Marrow-Derived Mesenchymal Stem Cells for Bone Regeneration. Front. Bioeng. Biotechnol. 2020;8:697. doi: 10.3389/fbioe.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Wang G., Xu W., Zhang J., Tang T., Chen J., Fan C. Induction of bone remodeling by raloxifene-doped iron oxide functionalized with hydroxyapatite to accelerate fracture healing. J. Biomed. Nanotechnol. 2021;17:932–941. doi: 10.1166/jbn.2021.3068. [DOI] [PubMed] [Google Scholar]
- 374.Gherasim O., Grumezescu V., Socol G., Ficai A. Nanoarchitectonics in Biomedicine. William Andrew (Elsevier); Oxford, UK: 2019. Nanoarchitectonics prepared by laser processing and their biomedicinal applications; pp. 23–53. [Google Scholar]
- 375.Hussain M., Askari Rizvi S.H., Abbas N., Sajjad U., Shad M.R., Badshah M.A., Malik A.I. Recent developments in coatings for orthopedic metallic implants. Coatings. 2021;11:791. doi: 10.3390/coatings11070791. [DOI] [Google Scholar]
- 376.Montazerian M., Hosseinzadeh F., Migneco C., Fook M.V., Baino F. Bioceramic coatings on metallic implants: An overview. Ceram. Int. 2022;48:8987–9005. doi: 10.1016/j.ceramint.2022.02.055. [DOI] [Google Scholar]
- 377.Montiel A., Bustamante E., Escudero M. Synthesis and Electrochemical Characterisation of Magnetite Coatings on Ti6Al4V-ELI. Metals. 2020;10:1640. doi: 10.3390/met10121640. [DOI] [Google Scholar]
- 378.Predoi D., Iconaru S.L., Ciobanu S.C., Predoi S.-A., Buton N., Megier C., Beuran M. Development of Iron-Doped Hydroxyapatite Coatings. Coatings. 2021;11:186. doi: 10.3390/coatings11020186. [DOI] [Google Scholar]
- 379.Popescu-Pelin G., Fufă O., Popescu R.C., Savu D., Socol M., Zgură I., Holban A.M., Vasile B.Ş., Grumezescu V., Socol G. Lincomycin–embedded PANI–based coatings for biomedical applications. Appl. Surf. Sci. 2018;455:653–666. doi: 10.1016/j.apsusc.2018.06.016. [DOI] [Google Scholar]
- 380.Visan A.I., Popescu-Pelin G., Gherasim O., Grumezescu V., Socol M., Zgura I., Florica C., Popescu R.C., Savu D., Holban A.M., et al. Laser processed antimicrobial nanocomposite based on polyaniline grafted lignin loaded with Gentamicin-functionalized magnetite. Polymers. 2019;11:283. doi: 10.3390/polym11020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Rodrigues G.R., López-Abarrategui C., de la Serna Gómez I., Dias S.C., Otero-González A.J., Franco O.L. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 2019;555:356–367. doi: 10.1016/j.ijpharm.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 382.Iranpour S., Bahrami A.R., Saljooghi A.S., Matin M.M. Application of smart nanoparticles as a potential platform for effective colorectal cancer therapy. Coord. Chem. Rev. 2021;442:213949. doi: 10.1016/j.ccr.2021.213949. [DOI] [Google Scholar]
- 383.Negut I., Grumezescu V., Ficai A., Grumezescu A.M., Holban A.M., Popescu R.C., Savu D., Vasile B.S., Socol G. MAPLE deposition of Nigella sativa functionalized Fe3O4 nanoparticles for antimicrobial coatings. Appl. Surf. Sci. 2018;455:513–521. doi: 10.1016/j.apsusc.2018.05.202. [DOI] [Google Scholar]
- 384.Puiu R.A., Balaure P.C., Constantinescu E., Grumezescu A.M., Andronescu E., Oprea O.C., Vasile B.S., Grumezescu V., Negut I., Nica I.C., et al. Anti-cancer nanopowders and maple-fabricated thin coatings based on spions surface modified with paclitaxel loaded β-cyclodextrin. Pharmaceutics. 2021;13:1356. doi: 10.3390/pharmaceutics13091356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Wang N., Fuh J.Y.H., Dheen S.T., Senthil Kumar A. Functions and applications of metallic and metallic oxide nanoparticles in orthopedic implants and scaffolds. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2021;109:160–179. doi: 10.1002/jbm.b.34688. [DOI] [PubMed] [Google Scholar]
- 386.Ghosh S., Webster T.J. Metallic nanoscaffolds as osteogenic promoters: Advances, challenges and scope. Metals. 2021;11:1356. doi: 10.3390/met11091356. [DOI] [Google Scholar]
- 387.Bedair T.M., Heo Y., Ryu J., Bedair H.M., Park W., Han D.K. Biocompatible and functional inorganic magnesium ceramic particles for biomedical applications. Biomater. Sci. 2021;9:1903–1923. doi: 10.1039/D0BM01934H. [DOI] [PubMed] [Google Scholar]
- 388.Li Y., Yang Y., Qing Y., Li R., Tang X., Guo D., Qin Y. Enhancing zno-np antibacterial and osteogenesis properties in orthopedic applications: A review. Int. J. Nanomed. 2020;15:6247–6262. doi: 10.2147/IJN.S262876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Noori A.J., Kareem F.A. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon. 2019;5:e02568. doi: 10.1016/j.heliyon.2019.e02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Spirescu V.A., Șuhan R., Niculescu A.G., Grumezescu V., Negut I., Holban A.M., Oprea O.C., Bîrcă A.C., Vasile B.Ș., Grumezescu A.M., et al. Biofilm-resistant nanocoatings based on ZnO nanoparticles and linalool. Nanomaterials. 2021;11:2564. doi: 10.3390/nano11102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Younis I.Y., El-Hawary S.S., Eldahshan O.A., Abdel-Aziz M.M., Ali Z.Y. Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci. Rep. 2021;11:16868. doi: 10.1038/s41598-021-96377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Jan H., Shah M., Andleeb A., Faisal S., Khattak A., Rizwan M., Drouet S., Hano C., Abbasi B.H. Plant-Based Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Aqueous Leaf Extract of Aquilegia pubiflora: Their Antiproliferative Activity against HepG2 Cells Inducing Reactive Oxygen Species and Other in Vitro Properties. Oxidative Med. Cell. Longev. 2021;2021:4786227. doi: 10.1155/2021/4786227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Coelho C.C., Araújo R., Quadros P.A., Sousa S.R., Monteiro F.J. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections. Mater. Sci. Eng. C. 2019;97:529–538. doi: 10.1016/j.msec.2018.12.059. [DOI] [PubMed] [Google Scholar]
- 394.Kumar S., Gautam C., Chauhan B.S., Srikrishna S., Yadav R.S., Rai S.B. Enhanced mechanical properties and hydrophilic behavior of magnesium oxide added hydroxyapatite nanocomposite: A bone substitute material for load bearing applications. Ceram. Int. 2020;46:16235–16248. doi: 10.1016/j.ceramint.2020.03.180. [DOI] [Google Scholar]
- 395.Safari Gezaz M., Mohammadi Aref S., Khatamian M. Investigation of structural properties of hydroxyapatite/zinc oxide nanocomposites; an alternative candidate for replacement in recovery of bones in load-tolerating areas. Mater. Chem. Phys. 2019;226:169–176. doi: 10.1016/j.matchemphys.2019.01.005. [DOI] [Google Scholar]
- 396.Yan T., Jiang Z., Li P., Chen Q., Zhou J., Cui X., Wang Q. Novel hydroxyapatite whiskers modified by silver ion and nano zinc oxide used for bone defect repairment. Coatings. 2021;11:957. doi: 10.3390/coatings11080957. [DOI] [Google Scholar]
- 397.Shen J., Wang W., Zhai X., Chen B., Qiao W., Li W., Li P., Zhao Y., Meng Y., Qian S., et al. 3D-printed nanocomposite scaffolds with tunable magnesium ionic microenvironment induce in situ bone tissue regeneration. Appl. Mater. Today. 2019;16:493–507. doi: 10.1016/j.apmt.2019.07.012. [DOI] [Google Scholar]
- 398.Wu Z., Meng Z., Wu Q., Zeng D., Guo Z., Yao J., Bian Y., Gu Y., Cheng S., Peng L., et al. Biomimetic and osteogenic 3D silk fibroin composite scaffolds with nano MgO and mineralized hydroxyapatite for bone regeneration. J. Tissue Eng. 2020;11:2041731420967791. doi: 10.1177/2041731420967791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Niknam Z., Golchin A., Rezaei–Tavirani M., Ranjbarvan P., Zali H., Omidi M., Mansouri V. Osteogenic differentiation potential of adipose-derived mesenchymal stem cells cultured on magnesium oxide/polycaprolactone nanofibrous scaffolds for improving bone tissue reconstruction. Adv. Pharm. Bull. 2020;12:142–154. doi: 10.34172/apb.2022.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Yin Y., Huang Q., Yang M., Xiao J., Wu H., Liu Y., Li Q., Huang W., Lei G., Zhou K. MgO Nanoparticles Protect against Titanium Particle-Induced Osteolysis in a Mouse Model because of Their Positive Immunomodulatory Effect. ACS Biomater. Sci. Eng. 2020;6:3005–3014. doi: 10.1021/acsbiomaterials.9b01852. [DOI] [PubMed] [Google Scholar]
- 401.Xing X., Cheng G., Yin C., Cheng X., Cheng Y., Ni Y., Zhou X., Deng H., Li Z. Magnesium-containing silk fibroin/polycaprolactone electrospun nanofibrous scaffolds for accelerating bone regeneration. Arab. J. Chem. 2020;13:5526–5538. doi: 10.1016/j.arabjc.2020.03.031. [DOI] [Google Scholar]
- 402.Zhao Y., Liang H., Zhang S., Qu S., Jiang Y., Chen M. Effects of magnesium oxide (MgO) shapes on in vitro and in vivo degradation behaviors of PLA/MgO composites in long term. Polymers. 2020;12:1074. doi: 10.3390/polym12051074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Huang T., Yan G., Guan M. Zinc homeostasis in bone: Zinc transporters and bone diseases. Int. J. Mol. Sci. 2020;21:1236. doi: 10.3390/ijms21041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.O’Connor J.P., Kanjilal D., Teitelbaum M., Lin S.S., Cottrell J.A. Zinc as a therapeutic agent in bone regeneration. Materials. 2020;13:2211. doi: 10.3390/ma13102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Zhao Z., Wan Y., Yu M., Wang H., Cai Y., Liu C., Zhang D. Biocompability evaluation of micro textures coated with zinc oxide on Ti-6Al-4V treated by nanosecond laser. Surf. Coat. Technol. 2021;422:127453. doi: 10.1016/j.surfcoat.2021.127453. [DOI] [Google Scholar]
- 406.Tang Y., Rajendran P., Veeraraghavan V.P., Hussain S., Balakrishna J.P., Chinnathambi A., Alharbi S.A., Alahmadi T.A., Rengarajan T., Mohan S.K. Osteogenic differentiation and mineralization potential of zinc oxide nanoparticles from Scutellaria baicalensis on human osteoblast-like MG-63 cells. Mater. Sci. Eng. C. 2021;119:111656. doi: 10.1016/j.msec.2020.111656. [DOI] [PubMed] [Google Scholar]
- 407.Zhang R., Liu X., Xiong Z., Huang Q., Yang X., Yan H., Ma J., Feng Q., Shen Z. The immunomodulatory effects of Zn-incorporated micro/nanostructured coating in inducing osteogenesis. Artif. Cells Nanomed. Biotechnol. 2018;46:1123–1130. doi: 10.1080/21691401.2018.1446442. [DOI] [PubMed] [Google Scholar]
- 408.Negrescu A.M., Necula M.G., Gebaur A., Golgovici F., Nica C., Curti F., Iovu H., Costache M., Cimpean A. In vitro macrophage immunomodulation by poly(ε-caprolactone) based-coated AZ31 Mg Alloy. Int. J. Mol. Sci. 2021;22:909. doi: 10.3390/ijms22020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Ahtzaz S., Nasir M., Shahzadi L., Iqbal F., Chaudhry A.A., Yar M., Rehman I.U., Amir W., Anjum A., Arshad R. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017;132:409–418. doi: 10.1016/j.matdes.2017.07.023. [DOI] [Google Scholar]
- 410.Maimaiti B., Zhang N., Yan L., Luo J., Xie C., Wang Y., Ma C., Ye T. Stable ZnO-doped hydroxyapatite nanocoating for anti-infection and osteogenic on titanium. Colloids Surf. B Biointerfaces. 2020;186:110731. doi: 10.1016/j.colsurfb.2019.110731. [DOI] [PubMed] [Google Scholar]
- 411.Rahimi Kalateh Shah Mohammad G., Homayouni Tabrizi M., Ardalan T., Yadamani S., Safavi E. Green synthesis of zinc oxide nanoparticles and evaluation of anti-angiogenesis, anti-inflammatory and cytotoxicity properties. J. Biosci. 2019;44:40. doi: 10.1007/s12038-019-9845-y. [DOI] [PubMed] [Google Scholar]
- 412.Poier N., Hochstöger J., Hackenberg S., Scherzad A., Bregenzer M., Schopper D., Kleinsasser N. Effects of zinc oxide nanoparticles in huvec: Cyto-and genotoxicity and functional impairment after long-term and repetitive exposure in vitro. Int. J. Nanomed. 2020;15:4441–4452. doi: 10.2147/IJN.S246797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Purohit S.D., Singh H., Bhaskar R., Yadav I., Chou C.F., Gupta M.K., Mishra N.C. Gelatin—Alginate—Cerium oxide nanocomposite scaffold for bone regeneration. Mater. Sci. Eng. C. 2020;116:111111. doi: 10.1016/j.msec.2020.111111. [DOI] [PubMed] [Google Scholar]
- 414.Mani M.P., Jaganathan S.K., Khudzari A.Z.M. Evaluation of electrospun polyurethane scaffolds loaded with cerium oxide for bone tissue engineering. J. Ind. Text. 2021 doi: 10.1177/15280837211006668. [DOI] [Google Scholar]
- 415.Wei F., Neal C.J., Sakthivel T.S., Kean T., Seal S., Coathup M.J. Multi-functional cerium oxide nanoparticles regulate inflammation and enhance osteogenesis. Mater. Sci. Eng. C. 2021;124:112041. doi: 10.1016/j.msec.2021.112041. [DOI] [PubMed] [Google Scholar]
- 416.Shan J., Wang S., Xu H., Zhan H., Geng Z., Liang H., Dai M. Incorporation of cerium oxide into zirconia toughened alumina ceramic promotes osteogenic differentiation and osseointegration. J. Biomater. Appl. 2022;36:976–984. doi: 10.1177/08853282211036535. [DOI] [PubMed] [Google Scholar]
- 417.Ren S., Zhou Y., Zheng K., Xu X., Yang J., Wang X., Miao L., Wei H., Xu Y. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact. Mater. 2022;7:242–253. doi: 10.1016/j.bioactmat.2021.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Yu Y., Zhao S., Gu D., Zhu B., Liu H., Wu W., Wu J., Wei H., Miao L. Cerium oxide nanozyme attenuates periodontal bone destruction by inhibiting ROS-NFκB pathway. Nanoscale. 2022;14:2628–2637. doi: 10.1039/D1NR06043K. [DOI] [PubMed] [Google Scholar]
- 419.Dou C., Li J., He J., Luo F., Yu T., Dai Q., Chen Y., Xu J., Yang X., Dong S. Bone-targeted pH-responsive cerium nanoparticles for anabolic therapy in osteoporosis. Bioact. Mater. 2021;6:4697–4706. doi: 10.1016/j.bioactmat.2021.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Caputo F., Giovanetti A., Corsi F., Maresca V., Briganti S., Licoccia S., Traversa E., Ghibelli L. Cerium oxide nanoparticles reestablish cell integrity checkpoints and apoptosis competence in irradiated HaCaT cells via novel redox-independent activity. Front. Pharmacol. 2018;9:1183. doi: 10.3389/fphar.2018.01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Wei F., Neal C.J., Sakthivel T.S., Seal S., Kean T., Razavi M., Coathup M. Cerium oxide nanoparticles protect against irradiation-induced cellular damage while augmenting osteogenesis. Mater. Sci. Eng. C. 2021;126:112145. doi: 10.1016/j.msec.2021.112145. [DOI] [PubMed] [Google Scholar]
- 422.Iqbal N., Anastasiou A., Aslam Z., Raif E.M., Do T., Giannoudis P.V., Jha A. Interrelationships between the structural, spectroscopic, and antibacterial properties of nanoscale (<50 nm) cerium oxides. Sci. Rep. 2021;11:20875. doi: 10.1038/s41598-021-00222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Matter M.T., Doppegieter M., Gogos A., Keevend K., Ren Q., Herrmann I.K. Inorganic nanohybrids combat antibiotic-resistant bacteria hiding within human macrophages. Nanoscale. 2021;13:8224–8234. doi: 10.1039/D0NR08285F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Fu L., Zhang W., Zhou X., Fu J., He C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact. Mater. 2022;17:221–233. doi: 10.1016/j.bioactmat.2022.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Esmaeilnejad A., Mahmoudi P., Zamanian A., Mozafari M. Synthesis of titanium oxide nanotubes and their decoration by MnO nanoparticles for biomedical applications. Ceram. Int. 2019;45:19275–19282. doi: 10.1016/j.ceramint.2019.06.177. [DOI] [Google Scholar]
- 426.Westhauser F., Wilkesmann S., Nawaz Q., Schmitz S.I., Moghaddam A., Boccaccini A.R. Osteogenic properties of manganese-doped mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res.-Part A. 2020;108:1806–1815. doi: 10.1002/jbm.a.36945. [DOI] [PubMed] [Google Scholar]
- 427.Kumar S., Adjei I.M., Brown S.B., Liseth O., Sharma B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials. 2019;224:119467. doi: 10.1016/j.biomaterials.2019.119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Chen L., Tiwari S.R., Zhang Y., Zhang J., Sun Y. Facile Synthesis of Hollow MnO2 Nanoparticles for Reactive Oxygen Species Scavenging in Osteoarthritis. ACS Biomater. Sci. Eng. 2021;7:1686–1692. doi: 10.1021/acsbiomaterials.1c00005. [DOI] [PubMed] [Google Scholar]
- 429.Rezk A.I., Bhattarai D.P., Park J., Park C.H., Kim C.S. Polyaniline-coated titanium oxide nanoparticles and simvastatin-loaded poly(ε-caprolactone) composite nanofibers scaffold for bone tissue regeneration application. Colloids Surf. B Biointerfaces. 2020;192:111007. doi: 10.1016/j.colsurfb.2020.111007. [DOI] [PubMed] [Google Scholar]
- 430.Tovar C.D.G., Castro J.I., Valencia C.H., Zapata P.A., Solano M.A., López E.F., Chaur M.N., Zapata M.E.V., Hernandez J.H.M. Synthesis of chitosan beads incorporating graphene oxide/titanium dioxide nanoparticles for in vivo studies. Molecules. 2020;25:2308. doi: 10.3390/molecules25102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Cheng W., Xu X., Lang Y., Cheng Z., Rizwan M., Tang X., Xie L., Liu Y., Xu H., Liu Y. Anatase and rutile TiO2 nanoparticles lead effective bone damage in young rat model via the igf-1 signaling pathway. Int. J. Nanomed. 2021;16:7233–7247. doi: 10.2147/IJN.S333632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Valencia-Llano C.H., Solano M.A., Grande-Tovar C.D. Nanocomposites of chitosan/graphene oxide/titanium dioxide nanoparticles/blackberry waste extract as potential bone substitutes. Polymers. 2021;13:2877. doi: 10.3390/polym13223877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Souza W., Piperni S.G., Laviola P., Rossi A.L., Rossi M.I.D., Archanjo B.S., Leite P.E., Fernandes M.H., Rocha L.A., Granjeiro J.M., et al. The two faces of titanium dioxide nanoparticles bio-camouflage in 3D bone spheroids. Sci. Rep. 2019;9:9309. doi: 10.1038/s41598-019-45797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Ren Y., Feng X., Lang X., Wang J., Du Z., Niu X. Evaluation of Osteogenic Potentials of Titanium Dioxide Nanoparticles with Different Sizes and Shapes. J. Nanomater. 2020;2020:8887323. doi: 10.1155/2020/8887323. [DOI] [Google Scholar]
- 435.Eivazzadeh-Keihan R., Noruzi E., Jafari A., Radinekiyan F., Hashemi S., Chenab K., Ahmadpour F., Behboudi A., Mokhtarzadeh A., Maleki A., et al. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020;14:1687–1714. doi: 10.1002/term.3131. [DOI] [PubMed] [Google Scholar]
- 436.Dykman L.A., Khlebtsov N.G. Gold nanoparticles in chemo-, immuno-, and combined therapy: Review. Biomed. Opt. Express. 2019;10:3152–3182. doi: 10.1364/BOE.10.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Nejati K., Dadashpour M., Gharibi T., Mellatyar H., Akbarzadeh A. Biomedical Applications of Functionalized Gold Nanoparticles: A Review. J. Clust. Sci. 2022;33:1–16. doi: 10.1007/s10876-020-01955-9. [DOI] [Google Scholar]
- 438.Vodyashkin A.A., Rizk M.G., Kezimana P., Kirichuk A.A., Stanishevskiy Y.M. Application of Gold Nanoparticle-Based Materials in Cancer Therapy and Diagnostics. ChemEngineering. 2021;5:69. doi: 10.3390/chemengineering5040069. [DOI] [Google Scholar]
- 439.Li H., Pan S., Xia P., Chang Y., Fu C., Kong W., Yu Z., Wang K., Yang X., Qi Z. Advances in the application of gold nanoparticles in bone tissue engineering. J. Biol. Eng. 2020;14:14. doi: 10.1186/s13036-020-00236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Hu X., Zhang Y., Ding T., Liu J., Zhao H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020;8:990. doi: 10.3389/fbioe.2020.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Liang H., Xu X., Feng X., Ma L., Deng X., Wu S., Liu X., Yang C. Gold nanoparticles-loaded hydroxyapatite composites guide osteogenic differentiation of human mesenchymal stem cells through Wnt/β-catenin signaling pathway. Int. J. Nanomed. 2019;14:6151–6163. doi: 10.2147/IJN.S213889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Zhang Y., Wang P., Wang Y., Li J., Qiao D., Chen R., Yang W., Yan F. Gold nanoparticles promote the bone regeneration of periodontal ligament stem cell sheets through activation of autophagy. Int. J. Nanomed. 2021;16:61–73. doi: 10.2147/IJN.S282246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Bai X., Gao Y., Zhang M., Chang Y.N., Chen K., Li J., Zhang J., Liang Y., Kong J., Wang Y., et al. Carboxylated gold nanoparticles inhibit bone erosion by disturbing the acidification of an osteoclast absorption microenvironment. Nanoscale. 2020;12:3871–3878. doi: 10.1039/C9NR09698A. [DOI] [PubMed] [Google Scholar]
- 444.Nah H., Lee D., Lee J.S., Lee S.J., Heo D.N., Lee Y.H., Bang J.B., Hwang Y.S., Moon H.J., Kwon I.K. Strategy to inhibit effective differentiation of RANKL-induced osteoclasts using vitamin D-conjugated gold nanoparticles. Appl. Surf. Sci. 2020;527:146765. doi: 10.1016/j.apsusc.2020.146765. [DOI] [Google Scholar]
- 445.Zhang Y., Wang P., Mao H., Zhang Y., Zheng L., Yu P., Guo Z., Li L., Jiang Q. PEGylated gold nanoparticles promote osteogenic differentiation in in vitro and in vivo systems. Mater. Des. 2021;197:109231. doi: 10.1016/j.matdes.2020.109231. [DOI] [Google Scholar]
- 446.Samadian H., Khastar H., Ehterami A., Salehi M. Bioengineered 3D nanocomposite based on gold nanoparticles and gelatin nanofibers for bone regeneration: In vitro and in vivo study. Sci. Rep. 2021;11:13877. doi: 10.1038/s41598-021-93367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Shi Y., Han X., Pan S., Wu Y., Jiang Y., Lin J., Chen Y., Jin H. Gold Nanomaterials and Bone/Cartilage Tissue Engineering: Biomedical Applications and Molecular Mechanisms. Front. Chem. 2021;9:546. doi: 10.3389/fchem.2021.724188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Niu C., Yuan K., Ma R., Gao L., Jiang W., Hu X., Lin W., Zhang X., Huang Z. Gold nanoparticles promote osteogenic differentiation of human periodontal ligament stem cells via the p38 MAPK signaling pathway. Mol. Med. Rep. 2017;16:4879–4886. doi: 10.3892/mmr.2017.7170. [DOI] [PubMed] [Google Scholar]
- 449.Li L., Zhang Y., Wang M., Zhou J., Zhang Q., Yang W., Li Y., Yan F. Gold Nanoparticles Combined Human β-Defensin 3 Gene-Modified Human Periodontal Ligament Cells Alleviate Periodontal Destruction via the p38 MAPK Pathway. Front. Bioeng. Biotechnol. 2021;9:35. doi: 10.3389/fbioe.2021.631191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Zhou J., Zhang Y., Li L., Fu H., Yang W., Yan F. Human β-defensin 3-combined gold nanoparticles for enhancement of osteogenic differentiation of human periodontal ligament cells in inflammatory microenvironments. Int. J. Nanomed. 2018;13:555–567. doi: 10.2147/IJN.S150897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Zhang S., Zhou H., Kong N., Wang Z., Fu H., Zhang Y., Xiao Y., Yang W., Yan F. L-cysteine-modified chiral gold nanoparticles promote periodontal tissue regeneration. Bioact. Mater. 2021;6:3288–3299. doi: 10.1016/j.bioactmat.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Huang C., Dong J., Zhang Y., Chai S., Wang X., Kang S., Yu D., Wang P., Jiang Q. Gold Nanoparticles-Loaded Polyvinylpyrrolidone/Ethylcellulose Coaxial Electrospun Nanofibers with Enhanced Osteogenic Capability for Bone Tissue Regeneration. Mater. Des. 2021;212:110240. doi: 10.1016/j.matdes.2021.110240. [DOI] [Google Scholar]
- 453.Yuan L., Qi X., Qin G., Liu Q., Zhang F., Song Y., Deng J. Effects of gold nanostructures on differentiation of mesenchymal stem cells. Colloids Surf. B Biointerfaces. 2019;184:110494. doi: 10.1016/j.colsurfb.2019.110494. [DOI] [PubMed] [Google Scholar]
- 454.Emilin Renitta R., Smitha I., Sahithya C.S., Samrot A.V., Abirami S., Dhiva S., Anand D.A. Synthesis, characterization, and antibacterial activity of biosynthesized gold nanoparticles. Biointerface Res. Appl. Chem. 2021;11:9619–9628. doi: 10.33263/BRIAC112.96199628. [DOI] [Google Scholar]
- 455.Sathiyaraj S., Suriyakala G., Dhanesh Gandhi A., Babujanarthanam R., Almaary K.S., Chen T.W., Kaviyarasu K. Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J. Infect. Public Health. 2021;14:1842–1847. doi: 10.1016/j.jiph.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 456.Singh N., Das M.K., Ansari A., Mohanta D., Rajamani P. Biogenic nanosized gold particles: Physico-chemical characterization and its anticancer response against breast cancer. Biotechnol. Rep. 2021;30:e00612. doi: 10.1016/j.btre.2021.e00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Saqr A.A., Khafagy E.S., Alalaiwe A., Aldawsari M.F., Alshahrani S.M., Anwer M.K., Khan S., Abu Lila A.S., Arab H.H., Hegazy W.A.H. Synthesis of gold nanoparticles by using green machinery: Characterization and in vitro toxicity. Nanomaterials. 2021;11:808. doi: 10.3390/nano11030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Tan H.-L., Teow S.-Y., Pushpamalar J. Application of Metal Nanoparticle⁻Hydrogel Composites in Tissue Regeneration. Bioengineering. 2019;6:17. doi: 10.3390/bioengineering6010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Sun J., Xing F., Braun J., Traub F., Rommens P.M., Xiang Z., Ritz U. Progress of phototherapy applications in the treatment of bone cancer. Int. J. Mol. Sci. 2021;22:1354. doi: 10.3390/ijms222111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Burdușel A.-C., Gherasim O., Grumezescu A.M., Mogoantă L., Ficai A., Andronescu E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials. 2018;8:681. doi: 10.3390/nano8090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Gherasim O., Puiu R.A., Bîrcă A.C., Burdușel A.-C., Grumezescu A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials. 2020;10:2318. doi: 10.3390/nano10112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Li W.R., Sun T.L., Zhou S.L., Ma Y.K., Shi Q.S., Xie X.B., Huang X.M. A comparative analysis of antibacterial activity, dynamics, and effects of silver ions and silver nanoparticles against four bacterial strains. Int. Biodeterior. Biodegrad. 2017;123:304–310. doi: 10.1016/j.ibiod.2017.07.015. [DOI] [Google Scholar]
- 463.Lagashetty A., Ganiger S.K.P., Reddy S. Green Synthesis, Characterization and Antibacterial Study of Ag-Au Bimetallic Nanocomposite using Tea Powder Extract. Biointerface Res. Appl. Chem. 2020;11:8087–8095. doi: 10.33263/BRIAC111.80878095. [DOI] [Google Scholar]
- 464.Singh M., Renu V.K., Upadhyay S.K., Singh R. Biomimetic Synthesis of Silver Nanoparticles from Aqueous Extract of Saraca indica and its Profound Antibacterial Activity. Biointerface Res. Appl. Chem. 2021;11:8110–8120. [Google Scholar]
- 465.Thiruvengadam V., Bansod A.V. Green Synthesis of Silver Nanoparticles Using Melia Azedarach and its Characterization, Corrosion and Antibacterial Properties. Biointerface Res. Appl. Chem. 2021;11:8577–8586. [Google Scholar]
- 466.Quinteros M.A., Viviana C.A., Onnainty R., Mary V.S., Theumer M.G., Granero G.E., Paraje M.G., Páez P.L. Biosynthesized silver nanoparticles: Decoding their mechanism of action in Staphylococcus aureus and Escherichia coli. Int. J. Biochem. Cell Biol. 2018;104:87–93. doi: 10.1016/j.biocel.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 467.Qing Y., Cheng L., Li R., Liu G., Zhang Y., Tang X., Wang J., Liu H., Qin Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018;13:3311–3327. doi: 10.2147/IJN.S165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Poon T.K.C., Iyengar K.P., Jain V.K. Silver Nanoparticle (AgNP) Technology applications in trauma and orthopaedics. J. Clin. Orthop. Trauma. 2021;21:101536. doi: 10.1016/j.jcot.2021.101536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Chen Y., Guan M., Ren R., Gao C., Cheng H., Li Y., Gao B., Wei Y., Fu J., Sun J., et al. Improved Immunoregulation of Ultra-Low-Dose Silver Nanoparticle-Loaded TiO(2) Nanotubes via M2 Macrophage Polarization by Regulating GLUT1 and Autophagy. Int. J. Nanomed. 2020;15:2011–2026. doi: 10.2147/IJN.S242919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Marques L., Martinez G., Guidelli É., Tamashiro J., Segato R., Payão S.L.M., Baffa O., Kinoshita A. Performance on Bone Regeneration of a Silver Nanoparticle Delivery System Based on Natural Rubber Membrane NRL-AgNP. Coatings. 2020;10:323. doi: 10.3390/coatings10040323. [DOI] [Google Scholar]
- 471.Coman A.N., Mare A., Tanase C., Bud E., Rusu A. Silver-Deposited Nanoparticles on the Titanium Nanotubes Surface as a Promising Antibacterial Material into Implants. Metals. 2021;11:92. doi: 10.3390/met11010092. [DOI] [Google Scholar]
- 472.He W., Zheng Y., Feng Q., Elkhooly T.A., Liu X., Yang X., Wang Y., Xie Y. Silver nanoparticles stimulate osteogenesis of human mesenchymal stem cells through activation of autophagy. Nanomed. Nanotechnol. Biol. Med. 2020;15:337–353. doi: 10.2217/nnm-2019-0026. [DOI] [PubMed] [Google Scholar]
- 473.Lee D., Ko W.K., Kim S.J., Han I.B., Hong J.B., Sheen S.H., Sohn S. Inhibitory effects of gold and silver nanoparticles on the differentiation into osteoclasts in vitro. Pharmaceutics. 2021;13:462. doi: 10.3390/pharmaceutics13040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Nguyen A.K., Patel R., Noble J.M., Zheng J., Narayan R.J., Kumar G., Goering P.L. Effects of Subcytotoxic Exposure of Silver Nanoparticles on Osteogenic Differentiation of Human Bone Marrow Stem Cells. Appl. Vitr. Toxicol. 2019;5:123–133. doi: 10.1089/aivt.2019.0001. [DOI] [Google Scholar]
- 475.Zhao Y., Liu J., Zhang M., He J., Zheng B., Liu F., Zhao Z., Liu Y. Use of silver nanoparticle–gelatin/alginate scaffold to repair skull defects. Coatings. 2020;10:948. doi: 10.3390/coatings10100948. [DOI] [Google Scholar]
- 476.Ramyaa Shri K., Subitha P., Narasimhan S., Murugesan R., Narayan S. Fabrication of dexamethasone-silver nanoparticles entrapped dendrimer collagen matrix nanoparticles for dental applications. Biointerface Res. Appl. Chem. 2021;11:14935–14955. doi: 10.33263/BRIAC116.1493514955. [DOI] [Google Scholar]
- 477.Hu C.C., Chang C.H., Chang Y., Hsieh J.H., Ueng S.W. Beneficial Effect of TaON-Ag Nanocomposite Titanium on Antibacterial Capacity in Orthopedic Application. Int. J. Nanomed. 2020;15:7889–7900. doi: 10.2147/IJN.S264303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Zhang C., Lan J., Wang S., Han S., Yang H., Niu Q., Wang J., Wang Q., Xiang Y., Wu Y., et al. Silver nanowires on acid-alkali-treated titanium surface: Bacterial attachment and osteogenic activity. Ceram. Int. 2019;45:24528–24537. doi: 10.1016/j.ceramint.2019.08.180. [DOI] [Google Scholar]
- 479.Saubade F.J., Hughes S., Wickens D.J., Wilson-Nieuwenhuis J., Dempsey-Hibbert N., Crowther G.S., West G., Kelly P., Banks C.E., Whitehead K.A. Effectiveness of titanium nitride silver coatings against Staphylococcus spp. in the presence of BSA and whole blood conditioning agents. Int. Biodeterior. Biodegrad. 2019;141:44–51. doi: 10.1016/j.ibiod.2018.06.016. [DOI] [Google Scholar]
- 480.Bakhsheshi-Rad H.R., Ismail A.F., Aziz M., Akbari M., Hadisi Z., Khoshnava S.M., Pagan E., Chen X. Co-incorporation of graphene oxide/silver nanoparticle into poly-L-lactic acid fibrous: A route toward the development of cytocompatible and antibacterial coating layer on magnesium implants. Mater. Sci. Eng. C. 2020;111:110812. doi: 10.1016/j.msec.2020.110812. [DOI] [PubMed] [Google Scholar]
- 481.Oleshko O., Liubchak I., Husak Y., Korniienko V., Yusupova A., Oleshko T., Banasiuk R., Szkodo M., Matros-Taranets I., Kazek-Kęsik A., et al. In vitro biological characterization of silver-doped anodic oxide coating on titanium. Materials. 2020;13:4359. doi: 10.3390/ma13194359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Wenhao Z., Zhang T., Yan J., Li Q., Xiong P., Li Y., Cheng Y., Zheng Y. In vitro and in vivo evaluation of structurally-controlled silk fibroin coatings for orthopedic infection and in-situ osteogenesis. Acta Biomater. 2020;116:223–245. doi: 10.1016/j.actbio.2020.08.040. [DOI] [PubMed] [Google Scholar]
- 483.Gaviria J., Alcudia A., Begines B., Beltrán A.M., Villarraga J., Moriche R., Rodríguez-Ortiz J.A., Torres Y. Synthesis and deposition of silver nanoparticles on porous titanium substrates for biomedical applications. Surf. Coat. Technol. 2021;406:126667. doi: 10.1016/j.surfcoat.2020.126667. [DOI] [Google Scholar]
- 484.Mallakpour S., Abbasi M. Hydroxyapatite mineralization on chitosan-tragacanth gum/silica@silver nanocomposites and their antibacterial activity evaluation. Int. J. Biol. Macromol. 2020;151:909–923. doi: 10.1016/j.ijbiomac.2020.02.167. [DOI] [PubMed] [Google Scholar]
- 485.Ferdous Z., Nemmar A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020;21:2375. doi: 10.3390/ijms21072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Trincă L.C., Mareci D., Souto R.M., Lozano-Gorrín A.D., Izquierdo J., Burtan L., Motrescu I., Vulpe V., Pavel G., Strungaru S., et al. Osseointegration evaluation of ZrTi alloys with hydroxyapatite-zirconia-silver layer in pig’s tibiae. Appl. Surf. Sci. 2019;487:127–137. doi: 10.1016/j.apsusc.2019.05.003. [DOI] [Google Scholar]
- 487.Lapaj L., Wozniak W., Markuszewski J. Osseointegration of hydroxyapatite coatings doped with silver nanoparticles: Scanning electron microscopy studies on a rabbit model. Folia Morphol. 2019;78:107–113. doi: 10.5603/FM.a2018.0055. [DOI] [PubMed] [Google Scholar]
- 488.Sulej-Chojnacka J., Woźniak W., Andrzejewski D. The effect of hydroxyapatite coating with silver nanoparticles on osseointegration of titanium implants. Eng. Biomater. 2020;23:9–15. [Google Scholar]
- 489.Yang Y., Cheng Y., Deng F., Shen L., Zhao Z., Peng S., Shuai C. A bifunctional bone scaffold combines osteogenesis and antibacterial activity via in situ grown hydroxyapatite and silver nanoparticles. Bio-Des. Manuf. 2021;4:452–468. doi: 10.1007/s42242-021-00130-x. [DOI] [Google Scholar]
- 490.Abdelaziz D., Hefnawy A., Al-Wakeel E., El-Fallal A., El-Sherbiny I.M. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J. Adv. Res. 2021;28:51–62. doi: 10.1016/j.jare.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Miranda R.R., Sampaio I., Zucolotto V. Exploring silver nanoparticles for cancer therapy and diagnosis. Colloids Surf. B Biointerfaces. 2022;210:112254. doi: 10.1016/j.colsurfb.2021.112254. [DOI] [PubMed] [Google Scholar]
- 492.Kovács D., Igaz N., Gopisetty M.K., Kiricsi M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? Int. J. Mol. Sci. 2022;23:839. doi: 10.3390/ijms23020839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Gounden S., Daniels A., Singh M. Chitosan-modified silver nanoparticles enhance cisplatin activity in breast cancer cells. Biointerface Res. Appl. Chem. 2021;11:10572–10584. doi: 10.33263/BRIAC113.1057210584. [DOI] [Google Scholar]
- 494.Karuppaiah A., Siram K., Selvaraj D., Ramasamy M., Babu D., Sankar V. Synergistic and enhanced anticancer effect of a facile surface modified non-cytotoxic silver nanoparticle conjugated with gemcitabine in metastatic breast cancer cells. Mater. Today Commun. 2020;23:100884. doi: 10.1016/j.mtcomm.2019.100884. [DOI] [Google Scholar]
- 495.Ghandehari S., HomayouniTabrizi M., Ardalan P. Evaluation of Anti-angiogenic Activity of Silver Nanoparticle Synthesis by Rubina tinctorum L (Ru-AgNPs) Using Chicken Chorioallantoic Membrane (CAM) Assay. J. Arak Univ. Med. Sci. 2018;21:82–90. [Google Scholar]
- 496.Baghani M., Es-Haghi A. Characterization of silver nanoparticles biosynthesized using Amaranthus cruentus. Bioinspired Biomim. Nanobiomater. 2020;9:129–136. doi: 10.1680/jbibn.18.00051. [DOI] [Google Scholar]
- 497.Kumari R., Saini A.K., Chhillar A.K., Saini V., Saini R.V. Antitumor effect of bio-fabricated silver nanoparticles towards ehrlich ascites carcinoma. Biointerface Res. Appl. Chem. 2021;11:12958–12972. doi: 10.33263/BRIAC115.1295812972. [DOI] [Google Scholar]
- 498.Li J., Zhang B., Chang X., Gan J., Li W., Niu S., Kong L., Wu T., Zhang T., Tang M., et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ. Pollut. 2020;256:113430. doi: 10.1016/j.envpol.2019.113430. [DOI] [PubMed] [Google Scholar]
- 499.Ferreira L.A.B., Garcia-Fossa F., Radaic A., Durán N., Fávaro W.J., de Jesus M.B. Biogenic silver nanoparticles: In vitro and in vivo antitumor activity in bladder cancer. Eur. J. Pharm. Biopharm. 2020;151:162–170. doi: 10.1016/j.ejpb.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 500.Baker A., Iram S., Syed A., Elgorban A.M., Al-Falih A.M., Bahkali A.H., Khan M.S., Kim J. Potentially bioactive fungus mediated silver nanoparticles. Nanomaterials. 2021;11:3227. doi: 10.3390/nano11123227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Wen X., Wang Q., Dai T., Shao J., Wu X., Jiang Z., Jacob J.A., Jiang C. Identification of possible reductants in the aqueous leaf extract of mangrove plant Rhizophora apiculata for the fabrication and cytotoxicity of silver nanoparticles against human osteosarcoma MG-63 cells. Mater. Sci. Eng. C. 2020;116:111252. doi: 10.1016/j.msec.2020.111252. [DOI] [PubMed] [Google Scholar]
- 502.Danışman-Kalındemirtaş F., Kariïper İ.A., Hepokur C., Erdem-Kuruca S. Selective cytotoxicity of paclitaxel bonded silver nanoparticle on different cancer cells. J. Drug Deliv. Sci. Technol. 2021;61:102265. doi: 10.1016/j.jddst.2020.102265. [DOI] [Google Scholar]
- 503.Michalakis K., Bakopoulou A., Papachristou E., Vasilaki D., Tsouknidas A., Michailidis N., Johnstone E. Evaluation of the Response of HOS and Saos-2 Osteosarcoma Cell Lines When Exposed to Different Sizes and Concentrations of Silver Nanoparticles. BioMed Res. Int. 2021;2021:5013065. doi: 10.1155/2021/5013065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Khan T., Yasmin A., Townley H.E. An evaluation of the activity of biologically synthesized silver nanoparticles against bacteria, fungi and mammalian cell lines. Colloids Surf. B Biointerfaces. 2020;194:111156. doi: 10.1016/j.colsurfb.2020.111156. [DOI] [PubMed] [Google Scholar]
- 505.Tariq H., Rafi M., Amirzada M.I., Muhammad S.A., Yameen M.A., Mannan A., Ismail T., Shahzadi I., Murtaza G., Fatima N. Photodynamic cytotoxic and antibacterial evaluation of Tecoma stans and Narcissus tazetta mediated silver nanoparticles. Arab. J. Chem. 2022;15:103652. doi: 10.1016/j.arabjc.2021.103652. [DOI] [Google Scholar]
- 506.Da Silva Ferreira V., Eugenio M.F.C., Del Nery Dos Santos E., De Souza W., Santanna C. Cellular toxicology and mechanism of the response to silver-based nanoparticle exposure in Ewing’s sarcoma cells. Nanotechnology. 2020;32:115101. doi: 10.1088/1361-6528/abcef3. [DOI] [PubMed] [Google Scholar]
- 507.Rolim W.R., Lamilla C., Pieretti J.C., Nascimento M.H.M., Ferreira F.F., Tortella G.R., Diez M.C., Barrientos L., Rubilar O., Seabra A.B. Antibacterial Activity and Cytotoxicity of Silver Chloride/Silver Nanocomposite Synthesized by a Bacterium Isolated from Antarctic Soil. BioNanoScience. 2020;10:136–148. doi: 10.1007/s12668-019-00693-1. [DOI] [Google Scholar]
- 508.Wang Y., Zhang W., Yao Q. Copper-based biomaterials for bone and cartilage tissue engineering. J. Orthop. Transl. 2021;29:60–71. doi: 10.1016/j.jot.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Szabo R., Bodolea C., Mocan T. Iron, copper, and zinc homeostasis: Physiology, physiopathology, and nanomediated applications. Nanomaterials. 2021;11:2985. doi: 10.3390/nano11112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Zoroddu M.A., Aaseth J., Crisponi G., Medici S., Peana M., Nurchi V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019;195:120–129. doi: 10.1016/j.jinorgbio.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 511.Rondanelli M., Faliva M.A., Infantino V., Gasparri C., Iannello G., Perna S., Riva A., Petrangolini G., Tartara A., Peroni G. Copper as dietary supplement for bone metabolism: A review. Nutrients. 2021;13:2246. doi: 10.3390/nu13072246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Gaffney-Stomberg E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019;188:26–34. doi: 10.1007/s12011-018-1583-8. [DOI] [PubMed] [Google Scholar]
- 513.Lin W., Xu L., Li G. Molecular Insights into Lysyl Oxidases in Cartilage Regeneration and Rejuvenation. Front. Bioeng. Biotechnol. 2020;8:359. doi: 10.3389/fbioe.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Mitra D., Li M., Kang E.T., Neoh K.G. Transparent Copper-Based Antibacterial Coatings with Enhanced Efficacy against Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces. 2019;11:73–83. doi: 10.1021/acsami.8b09640. [DOI] [PubMed] [Google Scholar]
- 515.van Hengel I.A.J., Tierolf M.W.A.M., Valerio V.P.M., Minneboo M., Fluit A.C., Fratila-Apachitei L.E., Apachitei I., Zadpoor A.A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B. 2020;8:1589–1602. doi: 10.1039/C9TB02434D. [DOI] [PubMed] [Google Scholar]
- 516.Shen Q., Qi Y., Kong Y., Bao H., Wang Y., Dong A., Wu H., Xu Y. Advances in Copper-Based Biomaterials with Antibacterial and Osteogenic Properties for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2022;9:795425. doi: 10.3389/fbioe.2021.795425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Asghar M.A., Asghar M.A. Green synthesized and characterized copper nanoparticles using various new plants extracts aggravate microbial cell membrane damage after interaction with lipopolysaccharide. Int. J. Biol. Macromol. 2020;160:1168–1176. doi: 10.1016/j.ijbiomac.2020.05.198. [DOI] [PubMed] [Google Scholar]
- 518.Vijayakumar G., Kesavan H., Kannan A., Arulanandam D., Kim J.H., Kim K.J., Song H.J., Kim H.J., Rangarajulu S.K. Phytosynthesis of copper nanoparticles using extracts of spices and their antibacterial properties. Processes. 2021;9:1341. doi: 10.3390/pr9081341. [DOI] [Google Scholar]
- 519.Nieto-Maldonado A., Bustos-Guadarrama S., Espinoza-Gomez H.Z., Flores-López L., Ramirez-Acosta K., Alonso-Nuñez G., Cadena-Nava R.D. Green synthesis of copper nanoparticles using different plant extracts and their antibacterial activity. J. Environ. Chem. Eng. 2022;10:107130. doi: 10.1016/j.jece.2022.107130. [DOI] [Google Scholar]
- 520.Hemmati S., Ahmeda A., Salehabadi Y., Zangeneh A., Zangeneh M.M. Synthesis, characterization, and evaluation of cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing effects of copper nanoparticles using the aqueous extract of Strawberry fruit and L-Ascorbic acid. Polyhedron. 2020;180:114425. doi: 10.1016/j.poly.2020.114425. [DOI] [Google Scholar]
- 521.Ginting B., Maulana I., Karnila I. Biosynthesis Copper Nanoparticles using Blumea balsamifera Leaf Extracts: Characterization of its Antioxidant and Cytotoxicity Activities. Surf. Interfaces. 2020;21:100799. doi: 10.1016/j.surfin.2020.100799. [DOI] [Google Scholar]
- 522.Xu D., Li E., Karmakar B., Awwad N.S., Ibrahium H.A., Osman H.E.H., El-kott A.F., Abdel-Daim M.M. Green preparation of copper nanoparticle-loaded chitosan/alginate bio-composite: Investigation of its cytotoxicity, antioxidant and anti-human breast cancer properties. Arab. J. Chem. 2022;15:103638. doi: 10.1016/j.arabjc.2021.103638. [DOI] [Google Scholar]
- 523.Gholami-Shabani M., Sotoodehnejadnematalahi F., Shams-Ghahfarokhi M., Eslamifar A., Razzaghi-Abyaneh M. Physicochemical properties, anticancer and antimicrobial activities of metallic nanoparticles green synthesized by Aspergillus kambarensis. IET Nanobiotechnol. 2022;16:1–13. doi: 10.1049/nbt2.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Karekar N., Karan A., Khezerlou E., Prajapati N., Pernici C.D., Murray T.A., DeCoster M.A. Self-assembled metal-organic biohybrids (MOBs) using copper and silver for cell studies. Nanomaterials. 2019;9:1282. doi: 10.3390/nano9091282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Prajapati N., Karan A., Khezerlou E., DeCoster M.A. The Immunomodulatory Potential of Copper and Silver Based Self-Assembled Metal Organic Biohybrids Nanomaterials in Cancer Theranostics. Front. Chem. 2021;8:1296. doi: 10.3389/fchem.2020.629835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Wang X., Molino B.Z., Pitkänen S., Ojansivu M., Xu C., Hannula M., Hyttinen J., Miettinen S., Hupa L., Wallace G. 3D Scaffolds of Polycaprolactone/Copper-Doped Bioactive Glass: Architecture Engineering with Additive Manufacturing and Cellular Assessments in a Coculture of Bone Marrow Stem Cells and Endothelial Cells. ACS Biomater. Sci. Eng. 2019;5:4496–4510. doi: 10.1021/acsbiomaterials.9b00105. [DOI] [PubMed] [Google Scholar]
- 527.Bozorgi A., Mozafari M., Khazaei M., Soleimani M., Jamalpoor Z. Fabrication, characterization, and optimization of a novel copper-incorporated chitosan/gelatin-based scaffold for bone tissue engineering applications. BioImpacts. 2021;11 doi: 10.34172/bi.2021.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Wu H., Yang S., Xiao J., Ouyang Z., Yang M., Zhang M., Zhao D., Huang Q. Facile synthesis of multi-functional nano-composites by precise loading of Cu2+ onto MgO nano-particles for enhanced osteoblast differentiation, inhibited osteoclast formation and effective bacterial killing. Mater. Sci. Eng. C. 2021;130:112442. doi: 10.1016/j.msec.2021.112442. [DOI] [PubMed] [Google Scholar]
- 529.Ryan E.J., Ryan A.J., González-Vázquez A., Philippart A., Ciraldo F.E., Hobbs C., Nicolosi V., Boccaccini A.R., Kearney C.J., O’Brien F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials. 2019;197:405–416. doi: 10.1016/j.biomaterials.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 530.Zou F., Jiang J., Lv F., Xia X., Ma X. Preparation of antibacterial and osteoconductive 3D-printed PLGA/Cu(I)@ZIF-8 nanocomposite scaffolds for infected bone repair. J. Nanobiotechnol. 2020;18:39. doi: 10.1186/s12951-020-00594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Ma H., Ma Z., Chen Q., Li W., Liu X., Ma X., Mao Y., Yang H., Ma H., Wang J. Bifunctional, Copper-Doped, Mesoporous Silica Nanosphere-Modified, Bioceramic Scaffolds for Bone Tumor Therapy. Front. Chem. 2020;8:1099. doi: 10.3389/fchem.2020.610232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Pang L., Zhao R., Chen J., Ding J., Chen X., Chai W., Cui X., Li X., Wang D., Pan H. Osteogenic and anti-tumor Cu and Mn-doped borosilicate nanoparticles for syncretic bone repair and chemodynamic therapy in bone tumor treatment. Bioact. Mater. 2022;12:1–15. doi: 10.1016/j.bioactmat.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Patnaik A., Aiyer P., Gali S., Deveswaran R. Flexural strength and anti-fungal activity of copper nano-particles on poly-methyl methacrylate denture base resins. Mater. Today Proc. 2021;46:8761–8766. doi: 10.1016/j.matpr.2021.04.085. [DOI] [Google Scholar]
- 534.Rojas B., Soto N., Villalba M., Bello-Toledo H., Meléndrez-Castro M., Sánchez-Sanhueza G. Antibacterial activity of copper nanoparticles (Cunps) against a resistant calcium hydroxide multispecies endodontic biofilm. Nanomaterials. 2021;11:2254. doi: 10.3390/nano11092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Gad El-Rab S.M.F., Basha S., Ashour A.A., Enan E.T., Alyamani A.A., Felemban N.H. Green Synthesis of Copper Nano-Drug and Its Dental Application upon Periodontal Disease-Causing Microorganisms. J. Microbiol. Biotechnol. 2021;31:1656–1666. doi: 10.4014/jmb.2106.06008. [DOI] [PMC free article] [PubMed] [Google Scholar]