Abstract
Background
Bariatric (weight loss) surgery for obesity is considered when other treatments have failed. The effects of the available bariatric procedures compared with medical management and with each other are uncertain. This is an update of a Cochrane review first published in 2003 and most recently updated in 2009.
Objectives
To assess the effects of bariatric surgery for overweight and obesity, including the control of comorbidities.
Search methods
Studies were obtained from searches of numerous databases, supplemented with searches of reference lists and consultation with experts in obesity research. Date of last search was November 2013.
Selection criteria
Randomised controlled trials (RCTs) comparing surgical interventions with non‐surgical management of obesity or overweight or comparing different surgical procedures.
Data collection and analysis
Data were extracted by one review author and checked by a second review author. Two review authors independently assessed risk of bias and evaluated overall study quality utilising the GRADE instrument.
Main results
Twenty‐two trials with 1798 participants were included; sample sizes ranged from 15 to 250. Most studies followed participants for 12, 24 or 36 months; the longest follow‐up was 10 years. The risk of bias across all domains of most trials was uncertain; just one was judged to have adequate allocation concealment.
All seven RCTs comparing surgery with non‐surgical interventions found benefits of surgery on measures of weight change at one to two years follow‐up. Improvements for some aspects of health‐related quality of life (QoL) (two RCTs) and diabetes (five RCTs) were also found. The overall quality of the evidence was moderate. Five studies reported data on mortality, no deaths occurred. Serious adverse events (SAEs) were reported in four studies and ranged from 0% to 37% in the surgery groups and 0% to 25% in the no surgery groups. Between 2% and 13% of participants required reoperations in the five studies that reported these data.
Three RCTs found that laparoscopic Roux‐en‐Y gastric bypass (L)(RYGB) achieved significantly greater weight loss and body mass index (BMI) reduction up to five years after surgery compared with laparoscopic adjustable gastric banding (LAGB). Mean end‐of‐study BMI was lower following LRYGB compared with LAGB: mean difference (MD) ‐5.2 kg/m² (95% confidence interval (CI) ‐6.4 to ‐4.0; P < 0.00001; 265 participants; 3 trials; moderate quality evidence). Evidence for QoL and comorbidities was very low quality. The LRGYB procedure resulted in greater duration of hospitalisation in two RCTs (4/3.1 versus 2/1.5 days) and a greater number of late major complications (26.1% versus 11.6%) in one RCT. In one RCT the LAGB required high rates of reoperation for band removal (9 patients, 40.9%).
Open RYGB, LRYGB and laparoscopic sleeve gastrectomy (LSG) led to losses of weight and/or BMI but there was no consistent picture as to which procedure was better or worse in the seven included trials. MD was ‐0.2 kg/m² (95% CI ‐1.8 to 1.3); 353 participants; 6 trials; low quality evidence) in favour of LRYGB. No statistically significant differences in QoL were found (one RCT). Six RCTs reported mortality; one death occurred following LRYGB. SAEs were reported by one RCT and were higher in the LRYGB group (4.5%) than the LSG group (0.9%). Reoperations ranged from 6.7% to 24% in the LRYGB group and 3.3% to 34% in the LSG group. Effects on comorbidities, complications and additional surgical procedures were neutral, except gastro‐oesophageal reflux disease improved following LRYGB (one RCT). One RCT of people with a BMI 25 to 35 and type 2 diabetes found laparoscopic mini‐gastric bypass resulted in greater weight loss and improvement of diabetes compared with LSG, and had similar levels of complications.
Two RCTs found that biliopancreatic diversion with duodenal switch (BDDS) resulted in greater weight loss than RYGB in morbidly obese patients. End‐of‐study mean BMI loss was greater following BDDS: MD ‐7.3 kg/m² (95% CI ‐9.3 to ‐5.4); P < 0.00001; 107 participants; 2 trials; moderate quality evidence). QoL was similar on most domains. In one study between 82% to 100% of participants with diabetes had a HbA1c of less than 5% three years after surgery. Reoperations were higher in the BDDS group (16.1% to 27.6%) than the LRYGB group (4.3% to 8.3%). One death occurred in the BDDS group.
One RCT comparing laparoscopic duodenojejunal bypass with sleeve gastrectomy versus LRYGB found BMI, excess weight loss, and rates of remission of diabetes and hypertension were similar at 12 months follow‐up (very low quality evidence). QoL, SAEs and reoperation rates were not reported. No deaths occurred in either group.
One RCT comparing laparoscopic isolated sleeve gastrectomy (LISG) versus LAGB found greater improvement in weight‐loss outcomes following LISG at three years follow‐up (very low quality evidence). QoL, mortality and SAEs were not reported. Reoperations occurred in 20% of the LAGB group and in 10% of the LISG group.
One RCT (unpublished) comparing laparoscopic gastric imbrication with LSG found no statistically significant difference in weight loss between groups (very low quality evidence). QoL and comorbidities were not reported. No deaths occurred. Two participants in the gastric imbrication group required reoperation.
Authors' conclusions
Surgery results in greater improvement in weight loss outcomes and weight associated comorbidities compared with non‐surgical interventions, regardless of the type of procedures used. When compared with each other, certain procedures resulted in greater weight loss and improvements in comorbidities than others. Outcomes were similar between RYGB and sleeve gastrectomy, and both of these procedures had better outcomes than adjustable gastric banding. For people with very high BMI, biliopancreatic diversion with duodenal switch resulted in greater weight loss than RYGB. Duodenojejunal bypass with sleeve gastrectomy and laparoscopic RYGB had similar outcomes, however this is based on one small trial. Isolated sleeve gastrectomy led to better weight‐loss outcomes than adjustable gastric banding after three years follow‐up. This was based on one trial only. Weight‐related outcomes were similar between laparoscopic gastric imbrication and laparoscopic sleeve gastrectomy in one trial. Across all studies adverse event rates and reoperation rates were generally poorly reported. Most trials followed participants for only one or two years, therefore the long‐term effects of surgery remain unclear.
Plain language summary
Surgery for obesity
Review question
What are the effects of weight loss (bariatric) surgery for overweight or obese adults?
Background
Obesity is associated with many health problems and a higher risk of death. Bariatric surgery for obesity is usually only considered when other treatments have failed. We aimed to compare surgical interventions with non‐surgical interventions for obesity (such as drugs, diet and exercise) and to compare different surgical procedures. Bariatric surgery can be considered for people with a body mass index (BMI = kg/m²) greater than 40, or for those with a BMI less than 40 and obesity‐related diseases such as diabetes.
Study characteristics
We included 22 studies comparing surgery with non‐surgical interventions, or comparing different types of surgery. Altogether 1496 participants were allocated to surgery and 302 participants to non‐surgical interventions. Most studies followed participants for 12 to 36 months, the longest follow‐up was 10 years. The majority of participants were women and, on average, in their early 30s to early 50s.
Key results
Seven studies compared surgery with non‐surgical interventions. Due to differences in the way that the studies were designed we decided not to generate an average of their results. The direction of the effect indicated that people who had surgery achieved greater weight loss one to two years afterwards compared with people who did not have surgery. Improvements in quality of life and diabetes were also found. No deaths occurred, reoperations in the surgical intervention groups ranged between 2% and 13%, as reported in five studies.
Three studies found that gastric bypass (GB) achieved greater weight loss up to five years after surgery compared with adjustable gastric band (AGB): the BMI at the end of the studies was on average five units less. The GB procedure resulted in greater duration of hospitalisation and a greater number of late major complications. AGB required high rates of reoperation for removal of the gastric band.
Seven studies compared GB with sleeve gastrectomy (SG). Overall there were no important differences for weight loss, quality of life, comorbidities and complications, although gastro‐oesophageal reflux disease improved in more patients following GB in one study. One death occurred in the GB group. Serious adverse events occurred in 5% of the GB group and 1% of SG group, as reported in one study. Two studies reported 7% to 24% of people with GB and 3% to 34% of those with SG requiring reoperations.
Two studies found that biliopancreatic diversion with duodenal switch resulted in greater weight loss than GB after two or four years in people with a relatively high BMI. BMI at the end of the studies was on average seven units lower. One death occurred in the biliopancreatic diversion group. Reoperations were higher in the biliopancreatic diversion group (16% to 28%) than the GB group (4% to 8%).
One study comparing duodenojejunal bypass with SG versus GB found weight loss outcomes and rates of remission of diabetes and hypertension were similar at 12 months follow‐up. No deaths occurred in either group, reoperation rates were not reported.
One study found that BMI was reduced by 10 units more following SG at three years follow‐up compared with AGB. Reoperations occurred in 20% of the AGB group and in 10% of the SG group.
One study found no relevant difference in weight‐loss outcomes following gastric imbrication compared with SG. No deaths occurred; 17% of participants in the gastric imbrication group required reoperation.
Quality of the evidence
From the information that was available to us about the studies, we were unable to assess how well designed they were. Adverse events and reoperation rates were not consistently reported in the publications of the studies. Most studies followed participants for only one or two years, therefore the long‐term effects of surgery remain unclear.
Few studies assessed the effects of bariatric surgery in treating comorbidities in participants with a lower BMI. There is therefore a lack of evidence for the use of bariatric surgery in treating comorbidities in people who are overweight or who do not meet standard criteria for bariatric surgery.
Currentness of data
This evidence is up to date as of November 2013.
Summary of findings
Summary of findings for the main comparison. Surgery compared with no surgery for obesity.
| Surgery compared with no surgery for obesity | ||||||
| Patient or population: participants with obesity Settings: any Intervention: surgery Comparison: no surgery | ||||||
| Outcomes | No surgery | Surgery | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| BMI at study end [kg/m²] Follow‐up: 12 to 24 months | See comment | See comment | Not estimablea | 582 (5) | ⊕⊕⊕⊝ moderateb | The direction of the effect was consistently in favour of surgery |
| Health‐related quality of life Short Form Health Survey (SF‐36) Follow‐up: mean 2 years | See comment | See comment | Not estimablea | 140 (2) | ⊕⊕⊕⊝ moderatec | Improvements were seen in both studies for some aspects of health‐related quality of life but not others |
| Comobidities: diabetes Different definitions used Follow‐up: 12 to 24 months | See comment | See comment | Not estimablea | 442 (5) | ⊕⊕⊕⊝ moderateb | More people experienced remission of disease following surgery |
|
Mortality Follow‐up: 12 to 24 months |
See comment | See comment | Not estimablea | 478 (5) |
⊕⊕⊕⊝ moderated | 5 of 7 studies reported data: no deaths occurred |
|
Serious adverse events (SAEs) [%] Follow‐up: 12 to 24 months |
See comment | See comment | Not estimablea | 438 (4) |
⊕⊝⊝⊝ very lowe | 4 of 7 studies reported data: SAEs ranged from 0% to 37% in the surgery group and from 0% to 25% in the no surgery group |
|
Reoperations [%] Follow‐up: 12 to 24 months |
See comment | See comment | Not estimablea | 470 (5) |
⊕⊝⊝⊝ very lowe | 5 studies reported data: 2% to 13% of participants in the surgery group underwent reoperations |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aStudies could not be pooled due to differences in participants, interventions (types of surgery), and comparators bDowngraded by one level because allocation concealment was unclear in most studies. Blinding was not possible in trials of surgery versus no surgery, however this was judged to have little impact on measures of weight/BMI cDowngraded by one level because allocation concealment was unclear in one trial. No or unclear blinding of outcome assessors could affect subjective outcomes dDowngraded by one level because only 5 of 7 studies provided data eDowngraded by three levels because of inconsistent reporting, risk of bias and imprecision
Summary of findings 2. Laparoscopic gastric bypass compared with laparoscopic adjustable gastric banding for obesity.
| Laparoscopic gastric bypass compared with laparoscopic adjustable gastric banding for obesity | ||||||
| Patient or population: participants with obesity Settings: any Intervention: laparoscopic gastric bypass Comparison: laparoscopic adjustable gastric banding | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Laparoscopic adjustable gastric banding | Laparoscopic gastric bypass | |||||
| BMI at study end [kg/m²] Follow‐up: 1 to 10 years | The mean BMI at study end ranged across control groups from 36 to 37 | The mean BMI at study end in the intervention groups was 5.2 lower (6.4 to 4.0 lower) | ‐ | 265 (3) | ⊕⊕⊕⊝ moderatea | ‐ |
| Health‐related quality of life Short Form Health Survey (SF‐36) Follow‐up: mean 12 months | See comment | See comment | Not estimable | 250 (1) | ⊕⊝⊝⊝ very lowb | Data not reported. Trial states that scores were comparable to US norms in both groups |
|
Comorbidities:diabetes Follow‐up: 10 years |
See comment | See comment | Not estimable | 51 (1) | ⊕⊝⊝⊝ very lowc | Only one participant had diabetes at baseline, this was not observed after 5 years of follow‐up. |
|
Mortality Follow‐up: 4 to 10 years |
See comment | See comment | Not estimable | 301 (2) |
⊕⊕⊕⊝ moderated | 2 studies reported data: 1 death was observed in the laparoscopic gastric bypass group |
| Serious adverse events (SAEs) | See comment | See comment | Not estimable | See comment | See comment | Not reported |
|
Reoperations [%] Follow‐up: 4 to 10 years |
See comment | See comment | Not estimable | 240 (2) |
⊕⊝⊝⊝ very lowe | 2 studies reported data: 12.6% to 28.6% vs 12.8% to 40.9% in the laparoscopic gastric bypass group vs laparoscopic adjustable gastric banding group, respectively |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by one level because of high or unclear risk of attrition bias bDowngraded by three levels because of one trial only with few participants and high risk of attrition bias cDowngraded by three levels because of one trial only with few participants and high risk of selective reporting and 'other' bias dDowngraded one level because only 2 of 3 studies provided data eDowngraded by three levels because of inconsistent reporting, risk of bias and imprecision; data partly reported as revision rates/reoperations, however not specified as SAEs
Summary of findings 3. Laparoscopic Roux‐en‐Y gastric bypass compared with laparoscopic sleeve gastrectomy for obesity.
| Laparoscopic gastric bypass compared with laparoscopic sleeve gastrectomy for obesity | ||||||
| Patient or population: participants with obesity Settings: any Intervention: laparoscopic gastric bypass Comparison: laparoscopic sleeve gastrectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Laparoscopic sleeve gastrectomy | Laparoscopic Roux‐en‐Y gastric bypass | |||||
| BMI at study end [kg/m²] Follow‐up: 12 to 36 months | The mean BMI at study end ranged across control groups from 27 to 33 | The mean BMI at study end in the intervention groups was 0.2 lower (1.8 lower to 1.3 higher) | ‐ | 353 (6) | ⊕⊕⊝⊝ lowa | ‐ |
| Health‐related quality of life Follow‐up: mean 12 months | See comment | See comment | ‐ | 217 (1) | ⊕⊝⊝⊝ very lowb | Interim analysis showed no statistically significant differences between groups |
| Comorbidities: diabetes [different definitions used] Follow‐up: 12 to 36 months | See comment | See comment | Not estimable | 353 (6) | ⊕⊕⊝⊝ lowc | Diabetes was reported in different ways by the studies but no relevant difference between groups was found |
|
Mortality Follow‐up: 12 to 36 months |
See comment | See comment | Not estimable | 600 (6) |
⊕⊕⊕⊝ moderated | 6 studies reported data: 1 death was observed in the laparoscopic Roux‐en‐Y gastric bypass group |
|
Serious adverse events (SAEs) [%] Follow‐up: 12 months |
See comment | See comment | Not estimable | 217 (1) |
⊕⊝⊝⊝ very lowe | 1 study reported data: 4.5% in the laparoscopic gastric bypass group and 0.9% in the laparoscopic sleeve gastrectomy group |
|
Reoperations [%] Follow‐up: 12 months |
See comment | See comment | Not estimable | 277 (2) |
⊕⊝⊝⊝ very lowe | 2 of 6 studies reported data: 6.7% to 23.6% in the laparoscopic gastric bypass group and 3.3% to 33.6% in the laparoscopic sleeve gastrectomy group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by two levels because of inconsistency, imprecision and some trials showing attrition bias bDowngraded by three levels because one trial only with few participants and high risk of performance, detection and 'other' risk of bias cDowngraded by two levels because of few patients and few events, and some studies showing high risk of attrition, performance, detection and selective reporting bias dDowngraded by one level because only 6 of 8 studies provided data eDowngraded by three levels because of inconsistent reporting, risk of bias and imprecision
Summary of findings 4. Gastric bypass versus biliopancreatic diversion with duodenal switch (laparoscopic or open) for obesity.
| Gastric bypass compared with biliopancreatic diversion with duodenal switch for obesity | ||||||
| Patient or population: participants with obesity Settings: any Intervention: gastric bypass (open or laparoscopic) Comparison: biliopancreatic diversion with duodenal switch (open or laparoscopic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Biliopancreatic diversion with duodenal switch | Gastric bypass | |||||
| BMI reduction at study end [kg/m²] Follow‐up: 24 to 48 months | The mean BMI reduction at study end ranged across control groups from 23 to 25 | The mean BMI reduction at study end in the intervention groups was 7.3 lower (9.3 lower to 5.4 lower) | ‐ | 107 (2) |
⊕⊕⊕⊝ moderatea | ‐ |
|
Health‐related quality of life Follow‐up: 24 months |
See comment | See comment | Not estimable | 60 (1) | ⊕⊝⊝⊝ very lowb | Only 1 of 8 SF‐36 domains showed a statistically significant difference in favour of gastric bypass |
| Comorbidities: diabetes Follow‐up: 24 to 48 months | See comment | See comment | Not estimable | 60 (1) | ⊕⊝⊝⊝ very lowb | Three years after surgery 82% to 100% of participants had an HbA1c < 5% |
|
Mortality Follow‐up: 24 to 48 months |
See comment | See comment | Not estimable | 107 (2) |
⊕⊕⊕⊝ moderatea | One death was observed in the open biliopancreatic diversion with duodenal switch group |
| Serious adverse events (SAEs) | See comment | See comment | Not estimable | See comment | See comment | Not reported |
|
Reoperations [%] Follow‐up: 24 to 48 months |
See comment | See comment | Not estimable | 107 (2) |
⊕⊝⊝⊝ very lowc | Both studies reported data: 4.3% to 16.1% vs 8.3% to 27.6% in the gastric bypass group vs biliopancreatic diversion with duodenal switch group, respectively |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; RR: risk ratio; SF: short‐form survey | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by one level because of few trials and participants, and risk of 'other' bias bDowngraded by three levels because of one trial only with few participants, indirectness, selective reporting and 'other' risk of bias cDowngraded by three levels because of few trials and participants, risk of bias and inconsistency
Summary of findings 5. Laparoscopic gastric bypass compared with laparoscopic duodenojejunal bypass with sleeve gastrectomy for obesity.
| Laparoscopic gastric bypass compared with laparoscopic duodenojejunal bypass with sleeve gastrectomy for obesity | ||||||
| Patient or population: participants with obesity Settings: any Intervention: laparoscopic gastric bypass Comparison: laparoscopic duodenojejunal bypass with sleeve gastrectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Laparoscopic duodenojejunal bypass with sleeve gastrectomy | Laparoscopic gastric bypass | |||||
| BMI at study end [kg/m²] Follow‐up: mean 12 months | The mean BMI at study end in the control group was 28.2 | The mean BMI at study end in the intervention group was 0.7higher (0.3 lower to 1.6 higher) | ‐ | 57 (1) | ⊕⊝⊝⊝ very lowa | ‐ |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | |
| Comorbiditites: diabetes [Proportions with complete remission and partial remission] Follow‐up: mean 12 months | See comment | See comment | Not estimable | 57 (1) | ⊕⊝⊝⊝ very lowa | Reports no difference in complete or partial remission of diabetes in those with diabetes at baseline |
| Mortality Follow‐up: mean 12 months | See comment | See comment | Not estimable | 57 (1) | ⊕⊝⊝⊝ very lowa | No deaths in either group were reported |
| Serious adverse events (SAEs) | See comment | See comment | Not estimable | See comment | See comment | Not reported |
| Reoperations [%] | See comment | See comment | Not estimable | See comment | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by three levels due to one trial only with few participants and unclear risk of bias across all domains
Summary of findings 6. Laparoscopic adjustable gastric banding compared with laparoscopic isolated sleeve gastrectomy for obesity.
| Laparoscopic adjustable gastric banding compared with laparoscopic isolated sleeve gastrectomy for obesity | ||||||
| Patient or population: participants with obesity Settings: any Intervention: laparoscopic adjustable gastric banding Comparison: laparoscopic isolated sleeve gastrectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Laparoscopic isolated sleeve gastrectomy | Laparoscopic adjustable gastric banding | |||||
| Reduction in BMI [kg/m²] Follow‐up: mean 36 months | The mean reduction in BMI in the control group was 27.5 | The mean reduction in BMI in the intervention group was 9.5 lowera | ‐ | 80 (1) | ⊕⊝⊝⊝ very lowb | ‐ |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not reported |
| Comorbidities: diabetes | See comment | See comment | Not estimable | See comment | See comment | Not reported |
| Mortality | See comment | See comment | Not estimable | See comment | See comment | Not reported |
| Serious adverse events (SAEs) | See comment | See comment | Not estimable | See comment | See comment | Not reported |
|
Reoperations [%] Follow‐up: mean 36 months |
See comment | See comment | Not estimable | 80 (1) |
⊕⊝⊝⊝ very lowb | 20% in the laparoscopic gastric banding group and 10% in the laparoscopic isolated sleeve gastrectomy group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aTrial reports median (range), P = 0.0004 bDowngraded by three levels due to one trial only with few participants and unclear risk of bias across all domains
Summary of findings 7. Laparaoscopic gastric imbrication compared with laparoscopic sleeve gastrectomy for obesity.
| Laparaoscopic gastric imbrication compared with laparoscopic sleeve gastrectomy for obesity | ||||||
| Patient or population: participants with obesity Settings: any Intervention: laparoscopic gastric imbrication Comparison: laparoscopic sleeve gastrectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Laparoscopic sleeve gastrectomy | Laparaoscopic gastric imbrication | |||||
| BMI at study end [kg/m²] Follow‐up: mean 36 months | The mean BMI at study end in the control group was 32.1 | The mean BMI at study end in the intervention group was 4.8 higher (0.1 lower to 9.7 higher) | ‐ | 30 (1) | ⊕⊝⊝⊝ very lowa | ‐ |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not reported |
| Comorbidities | See comment | See comment | Not estimable | See comment | See comment | Not reported |
|
Mortality Follow‐up: mean 36 months |
See comment | See comment | Not estimable | 30 (1) | ⊕⊝⊝⊝ very lowa | No deaths occurred |
| Serious adverse events (SAEs) | See comment | See comment | Not estimable | See comment | See comment | Not reported |
|
Reoperations [%] Follow‐up: mean 36 months |
See comment | See comment | Not estimable | 30 (1) | ⊕⊝⊝⊝ very lowa | 2 (16.7%) participants in the laparoscopic gastric imbrication group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by three levels due to one trial only with few participants, and high risk of 'other' bias and unclear risk of bias across the other domains
Background
Description of the condition
Obesity is defined as abnormal or excessive fat accumulation that may impair health, and studies suggest that, without intervention, reversal of obesity is uncommon.
The most commonly used measure for classifying obesity is the body mass index (BMI), calculated as body weight in kilograms divided by height in metres squared (kg/m2). In adults a desirable BMI is between 18.5 to 25 and overweight is between 25 to 30. Obesity is defined as BMI over 30, while severe or morbid obesity is defined as BMI over 40. A BMI of 30 is equivalent to a weight of 97.5 kg in a person 1.8 m tall or a weight of 77 kg in a person 1.6 m tall. However, different populations have different associations between BMI, percentage of body fat, and health risks, and a desirable BMI is lower in some Asian populations (WHO 2004).
Projections by the World Health Organization (WHO) indicated that globally in 2005 at least 400 million adults were obese (WHO 2006). In some countries, including the USA, UK, and Australia, the rates of obesity have more than doubled in the last 25 years (Lobstein 2007). In England, the prevalence of obesity in people aged 16 and over is 24.8% (NHS IC 2012) and the prevalence of morbid obesity is 2.5% (3.2% of women and 1.7% of men) (NHS IC 2012). In the US 6.6% of adults are morbidly obese (Sturm 2013).
Health consequences in adults
The predominant serious health consequences associated with obesity in adults include type 2 diabetes, cardiovascular disease, musculoskeletal disorders such as osteoarthritis, and certain cancers. Some of these health consequences may constitute the principal cause of death such as heart disease, stroke, some cancers; whilst others such as type 2 diabetes lead to a reduced life expectancy. Other important health consequences that have a negative impact on quality of life are obstructive sleep apnoea, infertility, obstetric complications and psychiatric comorbidity.
The WHO 2000 found that the relative risks of particular diseases in obese people, compared to lean people, are fairly similar throughout the world and have classified these into three broad categories: greatly increased risk (relative risk much greater than 3), including type 2 diabetes, dyslipidaemia, insulin resistance, breathlessness, sleep apnoea and gall bladder diseases; moderately increased risk (relative risk 2 to 3), including cardiovascular disease, hypertension osteoarthritis of the knees and hyperuricaemia and gout; and slightly increased risk (relative risk 1 to 2), including colon cancer, breast cancer in postmenopausal women, endometrial cancer, reproductive hormone abnormalities, polycystic ovary syndrome, impaired fertility, foetal defects, low back pain and risk of anaesthesia complications. A more detailed description of the health consequences of overweight and obesity can be found in Picot 2009.
Description of the intervention
Bariatric surgery for obesity is a major surgical intervention with a risk of significant early and late morbidity and of perioperative mortality. Contraindications for bariatric surgery include poor myocardial reserve, significant chronic obstructive airways disease or respiratory dysfunction, non‐compliance of medical treatment and psychological disorders of a significant degree. Many types of bariatric surgery require long‐term supplementation with vitamins and iron, and patients often have a very restricted liquid diet in the immediate weeks after surgery. Hospital stay is generally between two to seven days for most procedures, typically one to two days for sleeve gastrectomy, and zero to one day for gastric banding.
Surgery aims to reduce weight and maintain any loss through restriction of intake or malabsorption of food, or a combination of these. Several different surgical procedures have been used; this review will focus on the principal types of surgical procedure in current use. Of these, gastric bypass, sleeve gastrectomy and adjustable gastric banding are much more commonly performed than the others. These procedures are usually performed laparoscopically. Laparoscopic surgery has been a major advance in bariatric surgery and has decreased the time spent in hospital and the recovery time for the patient. Open procedures are commonly not routinely used unless there is a need for conversion during laparoscopic surgery. The following section briefly discusses these procedures and their complications, but does not provide a comprehensive discussion of the many variants of these procedures that have developed.
Gastric bypass
The Roux‐en‐Y and resectional gastric bypass procedures combine restriction and malabsorption techniques, creating both a small gastric pouch and a bypass that prevents the patient from absorbing all they have eaten. The Roux‐en‐Y procedure entails partition of the upper part of the stomach using surgical staples to create a small pouch (50 mL or less) with a small outlet (gastroenterostomy stoma) to the intestine that is attached to the pouch. The Roux‐en‐Y technique is used to avoid a loop gastroenterostomy and the bile reflux that may ensue. Adaptations of the procedure have been used to increase malabsorption and increase weight loss. Often a prosthetic band is used to stabilise the gastroenterostomy, preventing late stretching of the opening and improving long‐term weight control. It is technically possible to reverse a gastric bypass.
Complications associated with gastric bypass include failure of the staple partition, leaks at the junction of the stomach and small intestine, acute gastric dilatation, and delayed gastric emptying either spontaneously or secondary to a blockage. Other complications may occur following surgery including: vomiting caused by narrowing of the stoma due to scar tissue development, wound hernias and intestinal obstruction. Dumping syndrome can also occur (an adverse event caused by eating refined sugar, symptoms of which include rapid heart rate, nausea, tremor, faint feeling and diarrhoea). It is thought that the dumping syndrome aids weight loss by conditioning the patient against eating sweet foods. Nutritional deficiencies, such as calcium, vitamin D, vitamin B12, and some iron deficiency anaemias may occur, necessitating routine monitoring and supplementation where required.
Adjustable gastric banding
Adjustable gastric banding is the least invasive of the purely restrictive bariatric surgery procedures. It limits food intake by placing a constricting ring completely around the top end (fundus) of the stomach. While early bands were non‐adjustable, those used currently incorporate an inflatable balloon within their lining to allow adjustment of the size of the stoma to regulate food intake. Adjustment is undertaken without the need for surgery by adding or removing saline through a subcutaneous access port. As a restrictive procedure, gastric banding avoids the problems associated with malabsorptive techniques. Gastric banding is technically a reversible procedure.
Complications include those associated with the operative procedure: splenic injury, oesophageal injury, wound infection, band slippage, band erosion (or migration), reservoir deflation/leak, persistent vomiting, failure to lose weight and acid reflux. Some complications may result in a need for revisional or band‐removal surgery (Lee 2007).
Biliopancreatic diversion with duodenal switch
Biliopancreatic diversion with duodenal switch is a modification of the biliopancreatic diversion procedure, which is no longer commonly used. Biliopancreatic diversion is primarily a malabsorptive procedure. The standard procedure involves the removal of part of the stomach (a limited horizontal gastrectomy) to limit oral intake and induce weight loss. The gastric pouch that is created is larger than that of gastric bypass or the restrictive procedures, therefore allowing larger meals, and patients remain on a less restricted diet than would be the case following gastric bypass. Part of the small intestine is also bypassed (the malabsorptive component) by the construction of a long limb Roux‐en‐Y anastomosis with a short common ‘alimentary’ channel of 50 cm length. Biliopancreatic diversion is only a partially reversible procedure. The combination of biliopancreatic diversion with duodenal switch is an additional adaptation of the standard procedure. It has a sleeve gastrectomy rather than a horizontal gastrectomy.
Biliopancreatic diversion with duodenal switch tends to be used only with patients with 'superobesity' (usually meaning BMI > 50kg), due to the high rates of complications associated with it. Historically, biliopancreatic diversion alone resulted in the complication of postgastrectomy syndrome (including, for example, dumping syndrome, bile reflux, diarrhoea) in a high proportion of patients who underwent the operation. The duodenal switch adaptation was incorporated to address this, and the combined procedure has resulted in a decrease in the proportion of patients who experience this post‐operative complication. However, other complications are similar to biliopancreatic diversion and include nutritional deficiencies (particularly protein, calcium, zinc, iron and fat soluble vitamins), foul smelling stools and flatus. Nutritional monitoring and supplementation when required is needed. The most common complication is bowel obstruction. Biliopancreatic diversion with duodenal switch is associated with an approximately 1% operative mortality rate, which rises to 2.5% when the procedure is performed laparoscopically (Moshiri 2013).
Sleeve gastrectomy
For some patients who are at high risk from bariatric surgery a sleeve gastrectomy is considered. This was originally used as the first part of a two‐part surgical procedure, being followed at a later date by a conversion to either a gastric bypass or a duodenal switch. However, for some, enough weight is lost with the sleeve gastrectomy alone, and it is now increasingly used as a stand‐alone procedure. The sleeve gastrectomy divides the stomach vertically to reduce its size to about 25%. It leaves the pyloric valve at the bottom of the stomach intact which means that the stomach function and digestion are unaltered. After six to 12 months the stomach may have expanded and not restrict intake as much; this is when the gastric bypass can then be added if necessary. The sleeve gastrectomy is not reversible.
Complications are reduced as digestion is unaffected, however patients are at risk from leaking from the newly formed stomach or vomiting due to over‐eating. This operation is relatively quick to perform, which reduces the risk of complications.
Sleeve gastrectomy with duodenojejunal bypass
Duodenojejunal bypass has been used as an additional procedure with sleeve gastrectomy. The addition of it to sleeve gastrectomy was developed in an Asian population with the aim of investigating whether it could be used instead of Roux‐en‐Y gastric bypass. In Asian countries, there is a high rate of gastric cancer and therefore it is important that surgeons can examine the excluded stomach following Roux‐en‐Y gastric bypass to check for this, but doing so can result in complications. Sleeve gastrectomy does not involve exclusion of the stomach and represents an alternative procedure. However, due to concerns that sleeve gastrectomy may not result in long‐term weight loss (Kasama 2009) or be as effective as Roux‐en‐Y gastric bypass in treating co‐morbidities (Praveen Raj 2012c), investigators have added duodenojejunal bypass to the procedure. Duodenojejunal bypass involves bypassing the proximal small intestine, resulting in food moving directly to the more distal small intestine. It has been hypothesised that bypassing the proximal small intestine may also improve diabetes and glucose tolerance (Kasama 2009). Duodenojejual bypass (without sleeve gastrectomy) has been used for treating diabetes in non‐obese patients (Ferzli 2009); this is now the primary use for duodenojejunal bypass, with or without a sleeve.
Preliminary complications data from one study of 38 patients who underwent laparoscopic duodenojejunal bypass with sleeve gastrectomy showed that one patient had to have a reoperation due to internal herniation. Otherwise, there were no major or minor complications and no operative mortalities (Praveen Raj 2012c).
Gastric imbrication
Gastric imbrication (or gastric plication) is a relatively new laparoscopic procedure that reduces the stomach volume without removing any stomach tissue. The stomach is folded into itself and stitched to create a narrow tube shape, similar to that of laparoscopic sleeve gastrectomy procedure. However, unlike sleeve gastrectomy, imbrication does not involve any cutting or stapling and the stomach tissue is not removed.
How the intervention might work
As described earlier, surgical procedures for obesity aim to reduce weight and maintain any loss through restricting food intake or causing malabsorption of food or a combination of these. It is hoped that as a consequence eating behaviour is modified, with patients consuming smaller quantities of food more slowly. In addition to modifying eating habits, patients are encouraged to commit to daily exercise as part of a wider change in lifestyle.
Whilst the success of weight‐loss interventions are often expressed in terms of the amount of weight lost, improvements in health‐related quality of life and comorbidities are generally a more meaningful indication of success for individuals (Avenell 2006; Kral 2006; Lean 2006). A systematic review of the long‐term effects of obesity treatments on body weight, risk factors for disease, and disease (Avenell 2004), found that weight loss from surgical and non‐surgical interventions for people suffering from obesity was associated with a decreased risk of the development of diabetes, and a reduction in low‐density lipoprotein cholesterol, total cholesterol and blood pressure, in the long term. The effects of bariatric (weight loss) surgery on weight and type 2 diabetes have also been reviewed (Levy 2007). The authors reported that bariatric surgery not only led to weight reduction, but also that preoperative diabetes resolved post‐surgery in more than 75% of cases. A further systematic review of the long‐term weight loss effects on all‐cause mortality in overweight and obese populations (Poobalan 2007) concludes that there is some evidence that intentional weight loss has long‐term benefits on all‐cause mortality for women and more so for people with diabetes. However, the long‐term effects for men are not clear. Weight loss in obese patients with knee osteoarthritis has also been systematically reviewed and the results of meta‐analysis indicated that disability could be significantly improved when weight was reduced over 5.1%, or at the rate of greater than 0.24% reduction per week (Christensen 2007). Weight loss has not been found to have a beneficial effect on risk of stroke (Curioni 2006).
Why it is important to do this review
The current edition of the review is an update. The original version was published in 2003, Issue 2 (Colquitt 2003), and was updated in 2005, Issue 4 (Colquitt 2005), and again in 2009, Issue 2 (Colquitt 2009).
The prevalence of obesity (BMI greater than 30) and morbid obesity (BMI greater than 40) among adults is increasing. The previous versions of this review found that although surgery appeared effective in terms of weight change, there was limited evidence addressing the long‐term consequences and its influence on the health‐related quality of life of patients. The reviews identified a need for good quality randomised controlled trials (RCTs) comparing either surgery with non‐surgical interventions, or comparing one type of surgical procedure with another surgical procedure. Further key implications for research were the need for an assessment of outcomes over longer time periods (at least five years), inclusion of health‐related quality of life outcomes and a more standardised approach to measuring and reporting important adverse events. The previous version of this review also identified a need for trials that compare procedures which combine restrictive and malabsorption components such as gastric bypass with purely restrictive procedures, such as adjustable gastric banding. Since the previous review was conducted, some of the surgical procedures included are no longer used in clinical practice. In addition, surgery is now proposed to be used to control for comorbidities of excess weight, such as type 2 diabetes, as well as for weight‐loss outcomes alone.
An update of the review is therefore required that will include data from more recent trials, including any that may have assessed new bariatric surgical techniques. Certain interventions that were included in the previous version but are not in current use will be excluded from this update. Further details can be found in Differences between protocol and review.
Objectives
To assess the effects of bariatric (weight loss) surgery for overweight and obesity, including the control of comorbidities.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Short‐term weight loss is common, therefore studies were only included if they reported measurements after a minimum of one year.
Types of participants
Adults who are overweight or obese as defined by the study.
Types of interventions
Surgical procedures in current use, performed either as open procedures or laparoscopically.
Types of comparators
Non‐surgical treatment (usual care, no treatment or medical management, for example very low calorie diet).
Different surgical procedures in current use, performed either as open procedures or laparoscopically.
Exclusions
Comparisons of variations of surgical techniques rather than different procedures.
Comparisons of open versus laparoscopic procedures (of the same bariatric surgery procedure).
-
Procedures no longer in current use:
Jejunoileal bypass
Horizontal gastroplasty
Vertical banded gastroplasty or vertical gastroplasty (not banded)
Banded gastroplasty that is not adjustable
Banded gastric bypass
Biliopancreatic diversion (without duodenal switch)
Types of outcome measures
Primary outcomes
Studies were included if they reported one or more of the following outcomes after at least 12 months follow‐up.
Measures of weight change, fat content (for example, BMI) or fat distribution (for example, waist‐hip ratio).
Health‐related quality of life, measured using a validated instrument.
Obesity‐related comorbidities (for example, diabetes, hypertension).
Secondary outcomes
Mortality (perioperative and total).
Adverse events (for example, perioperative morbidity such as staple line breakdown and wound infection, gastrointestinal disturbances, reoperations).
Revision rates (reversal or conversions to normal or other procedures).
Search methods for identification of studies
Electronic searches
We searched the following sources from inception to the specified date.
The Cochrane Library (2013, Issue 4).
MEDLINE (until 12/11/2013).
EMBASE (until 12/11/2013).
PsycINFO (until 12/11/2013).
CINAHL (until 12/11/2013).
Web of Knowledge SCI‐EXPANDED, and CPCI‐S (until 12/11/2013).
Zetoc British Library (until 12/11/2013).
Databases of grey literature
BIOSIS (until 12/11/2013).
Ongoing trials
UK Clinical Research Network (until 6/11/13).
ClinicalTrials.gov (until 6/11/13).
Controlled‐trials.com (until 6/11/13).
WHO International Clinical Trials Registry Platform (WHO ICTRP) (until 6/11/13).
For detailed search strategies please see Appendix 1.
It was anticipated that additional key words of relevance might be identified during any of the electronic or other searches, and if this had been the case, the electronic search strategies would have been modified to incorporate these terms. There were, however, no additional key words added to the search strategy.
Studies published in any language were eligible.
Searching other resources
We contacted relevant experts to obtain additional references, unpublished trials, and any ongoing trials.
Reference lists
We examined the reference lists of relevant trials and systematic reviews identified.
Data collection and analysis
Selection of studies
For this update, two review authors (two of KP, GF, EL, JC) independently scanned the titles, abstract sections and keywords of every record retrieved. Full articles were retrieved for further assessment if the information given suggested that the study:
included adults with obesity;
compared surgery with another surgical procedure, medical management or no treatment;
assessed one or more relevant clinical outcome measures;
had a minimum duration of 12 months.
If there was any doubt regarding these criteria from the information given in the title and abstract, the full article was retrieved for clarification. Eligibility criteria were applied to the full article using a pre‐designed form by two review authors independently. Where differences in opinion existed, they were resolved by discussion with a third review author.The PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses) flow‐chart of study selection is attached (Liberati 2009).
Data extraction and management
For studies that fulfilled the inclusion criteria, relevant population and intervention characteristics were extracted by one review author and checked by a second review author (any of KP, GF, EL, JC) using standard data extraction templates (for details see Characteristics of included studies; Table 8; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10) with any disagreements resolved by discussion, or if required by a third party. In the event of unclear information in an included trial, we contacted the primary author(s) of the article. See Appendix 11 for details.
1. Overview of study populations.
| Intervention(s) and comparator(s) | Screened/eligible [N] | Randomised [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] | Follow‐up | |
| (1) Angrisani 2007 | Laparoscopic Roux‐en‐Y gastric bypass | 24 | N/A | 21 | 87.5 | 10 years | |
| Laparoscopic adjustable gastric banding | 27 | N/A | 22a | 81.5 | |||
| total: | ‐ | 51 | N/A | 43 | 84.3 | ||
| (2) Aasheim 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 31 | N/A | 31 | 100 | 2 years | |
| Laparoscopic biliopancreatic diversion with duodenal switch | 29 | N/A | 27 | 93.1 | |||
| total: | 64 | 60 | N/A | 58 | 96.7 | ||
| (3) Demerdash 2013 | Laparoscopic Roux‐en‐Y gastric bypass | ‐ | N/A | 16 | ‐ | 1 year | |
| Laparoscopic adjustable gastric band | ‐ | N/A | 18 | ‐ | |||
| total: | ‐ | 40 | 34 | 85 | |||
| (4) Dixon 2008 | Laparoscopic gastric banding in addition to the conventional therapy | 30 | 30 | 29 | 96.7 | 2 years | |
| Conventional therapy | 30 | 30 | 26 | 86.7 | |||
| total: | 158 | 60 | 60 | 55 | 91.7 | ||
| (5) Dixon 2012 | Laparoscopic adjustable gastric banding and lifestyle programme | 30 | 30 | 28 | 93.3 | 2 years | |
| 2‐year conventional weight loss programme and lifestyle programme | 30 | 30 | 26 | 86.7 | |||
| total: | 130 | 60 | 60 | 54 | 90 | ||
| (6) Hedberg 2012 | Open biliopancreatic diversion with duodenal switch | 24 | N/A | 21 | 87.5 | 4 years | |
| Open Roux‐en‐Y gastric bypass | 23 | N/A | 20 | 87 | |||
| total: | 99 | 47 | N/A | 41 | 87.2 | ||
| (7) Himpens 2006 | Laparoscopic gastric banding | 40 | ‐ | ‐ | ‐ | 3 years | |
| Laparascopic isolated sleeve gastrectomy | 40 | ‐ | ‐ | ‐ | |||
| total: | ‐ | 80 | N/A | ‐ | ‐ | ||
| (8) Ikramuddin 2013 | Laparoscopic Roux‐en‐Y gastric bypass + lifestyle programme with medical management | 60 | 60 | 57b | 95 | 1 year | |
| Lifestyle programme with medical management | 60 | 60 | 57b | 95 | |||
| total: | 2648 | 120 | 120 | 114 | 95 | ||
| (9) Karamanakos 2008 | Laparoscopic Roux‐en‐Y gastric bypass | 30 | N/A | 29 | 96.7 | 3 years | |
| Laparoscopic sleeve gastrectomy | 30 | N/A | 28 | 93.3 | |||
| total: | 60 | 60 | N/A | 57 | 95 | ||
| (10) Keidar 2013 | Laporoscopic Roux‐en‐Y gastric bypass | 22 | N/A | 19 | 86.4 | 1 year | |
| Laparoscopic sleeve gastrectomy | 19 | N/A | 18 | 94.7 | |||
| total: | ‐ | 41 | N/A | 37 | 90.2 | ||
| (11) Lee 2011 | Simplified laparoscopic mini‐gastric bypass with duodenum exclusion | 30 | 30 | 30 | 100 | 1 year | |
| Laparoscopic sleeve gastrectomy without duodenum exclusion | 30 | 30 | 30 | 100 | |||
| total: | 209 | 60 | 60 | 60 | 100 | ||
| (12) Liang 2013 | Usual care | 36 | N/A | 36 | 100 | 1 year | |
| Usual care + exenatide | 36 | N/A | 34 | 94.4 | |||
| Laparoscopic Roux‐en‐Y gastric bypass | 36 | N/A | 31 | 86.1 | |||
| total: | ‐ | 108 | N/A | 101 | 93.5 | ||
| (13) Mingrone 2012 | Gastric bypass | 20 | N/A | 19 | 95 | 2 years | |
| Medical therapy | 20 | N/A | 18 | 90 | |||
| total: | 72 | 40 | N/A | 37 | 92.5 | ||
| (14) Nguyen 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 125 | N/A | 71 | 56.8 | 4 years | |
| Laparoscopic adjustable gastric banding | 125 | N/A | 30 | 24 | |||
| total: | ‐ | 250 | N/A | 101 | 40.4 | ||
| (15) Nogués 2010 | Laparascopic Roux‐en‐Y gastric bypass | 7 | 7 | 7 | 100 | 1 year | |
| Laparoscopic sleeve gastrectomy | 8 | 8 | 8 | 100 | |||
| total: | 30 | 15 | 15 | 15 | 100 | ||
| (16) O'Brien 2006 | Laparoscopic adjustable gastric band | 40 | N/A | 39 | 97.5 | 2 years | |
| Intensive non‐surgical programme | 40 | N/A | 40 | 100 | |||
| total: | 158 | 80 | N/A | 79 | 98.8 | ||
| (17) Paluszkiewicz 2012 | Open Roux‐en‐Y gastric bypass | 36 | ‐ | 35 | 97.2 | 1 year | |
| Laparoscopic sleeve gastrectomy | 36 | ‐ | 34 | 94.4 | |||
| total: | 86 | 72 | ‐ | 69 | 95.8 | ||
| (18) Peterli 2012 | Laparoscopic Roux‐en‐Y gastric bypass | 110 | N/A | N/A | N/A | 3 years | |
| Laparoscopic sleeve gastrectomy | 107 | N/A | N/A | N/A | |||
| total: | ‐ | 217c | N/A | N/Ad | N/A | ||
| (19) Praveen Raj 2012 | Laparoscopic duodenojejunal bypass with sleeve gastrectomy | 28 | ‐ | ‐ | ‐ | 1 year | |
| Laparoscopic Roux‐en‐Y gastric bypass | 29 | ‐ | ‐ | ‐ | |||
| total: | ‐ | 57 | ‐ | ‐ | ‐ | ||
| (20) Schauer 2012 | Intensive medical therapy alone | 50 | N/A | 41 | 82 | 1 year | |
| Intensive medical therapy plus laparoscopic Roux‐en‐Y gastric bypass | 50 | N/A | 50 | 100 | |||
| Intensive medical therapy plus laparoscopic sleeve gastrectomy | 50 | N/A | 49 | 98 | |||
| total: | 218 | 150 | N/A | 140 | 93.3 | ||
| (21) Sharma 2013 | Laparoscopic gastric imbrication | ‐ | 15 | N/A | 12 | 80 | 3 years |
| Laparoscopic sleeve gastrectomy | ‐ | 15 | N/A | 14 | 93.3 | ||
| total: | ‐ | 30 | N/A | 26 | 86.7 | ||
| (22) Vix 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 45 | N/A | 44 | 97.8 | 1 year | |
| Laparoscopic sleeve gastrectomy | 55 | N/A | 48 | 87.3 | |||
| total: | 410 | 100 | N/A | 92e | 92 | ||
| Grand total | All surgical interventions | 1496 | |||||
| All non‐surgical comparators | 302 | ||||||
| All surgical interventions and non‐surgical comparators | 1798 | ||||||
"‐" denotes not reported
aNine patients with band removal excluded from analysis at 10 years (therefore 13 patients included at 10 years) bData for missing patients were included in the ITT analysis using multiple imputation (statistical method specified) cAuthors state that 225 patients were randomised, but 8 patients were excluded after randomisation dTrial is ongoing, presented results were based on an interim analysis eVix 2013 reported 8 were lost to follow‐up (1 laparoscopic Roux‐en‐Y gastric bypass, 7 laparoscopic sleeve gastrectomy) but also reported one per group was lost to follow‐up. Data extracted here are from first statement.
ITT: intention‐to‐treat; N/A: not applicable
Dealing with duplicate publications and companion papers
In the case of duplicate publications and companion papers of a primary study, we tried to maximise yield of information by simultaneous evaluation of all available data. In cases of differences, the original publication was given priority.
Assessment of risk of bias in included studies
For this update, two review authors (two of KP, GF, EL, JC) assessed the risk of bias of each included study independently. Disagreements were resolved by consensus, or by consultation with a third party.
Risk of bias was assessed using The Cochrane Collaboration's tool (Higgins 2011a; Higgins 2011b). The following criteria were used.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of outcome assessors (detection bias),
Blinding of participants on subjective outcomes (performance bias)
Incomplete outcome data for weight loss, quality of life (QoL), comorbidity (attrition bias).
Selective reporting (reporting bias).
Other bias.
The assessment of blinding of participants (performance bias) was made on studies reporting self‐reported outcomes (e.g. health‐related quality of life measures). Detection bias (blinding of outcome assessors) was assessed on any type of outcome. Attrition bias (incomplete outcome data) was evaluated for weight loss, health‐related QoL and comorbidity outcomes separately.
'Risk of bias' criteria were judged as 'low risk', 'high risk' or 'unclear risk' and individual bias items were evaluated as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). A 'Risk of bias' summary and a 'Risk of bias' graph are presented.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MD) with 95% CI.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials (although none was identified) and multiple observations for the same outcome.
Dealing with missing data
We obtained relevant missing data from authors, if feasible, and evaluated important numerical data such as screened, eligible, randomised patients as well as intention‐to‐treat (ITT), as‐treated and per‐protocol (PP) populations. We investigated attrition rates, for example drop‐outs, losses to follow‐up and withdrawals, and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, we did not report study results as meta‐analytically pooled effect estimates.
We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We examined heterogeneity using the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011a).
When we found heterogeneity, we attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
We expected the following characteristics to introduce clinical heterogeneity.
Baseline BMI.
Presence of comorbidities at baseline.
Assessment of reporting biases
In cases of 10 studies or more for a given outcome, we intended to use funnel plots to assess small‐study effects. Due to several explanations for funnel plot asymmetry, we planned to interpret results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across studies, we planned primarily to summarise low‐risk of bias data by means of a random‐effects model (Wood 2008). In addition, we planned to perform statistical analyses according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analysis where data allowed.
Obese (BMI 30 to 40), morbidly obese (BMI 40 to 50) or superobese (BMI greater than 50).
Sex.
Length of follow‐up: 12 to 24 months, 25 to 36 months, 37 to 48 months, 49 months or greater.
Type of surgical procedure.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect sizes.
Restricting the analysis to published studies.
Restricting the analysis taking into account risk of bias, as specified at Assessment of risk of bias in included studies.
Restricting the analysis to very long or large studies to establish how much they dominate the results.
Restricting the analysis to studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
We also planned to test the robustness of the results by repeating the analysis using different measures of effect size (RR, odds ratio (OR) etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
Results of the search
Searches have been conducted for four previous versions of this review of bariatric surgery (Clegg 2002; Colquitt 2003; Colquitt 2005; Colquitt 2009); each version differs in the studies included as the review has evolved. In the 2009 version of the review there were 26 included studies. Three of these studies were non‐RCTs and have now been excluded from the review. Furthermore, 18 RCTs included in the 2009 version of the review that examined biliopancreatic diversion without duodenal switch, vertical banded gastroplasty, banded gastric bypass, or compared open versus laparoscopic procedures have been excluded from the review on advice from our expert advisory group, as these procedures and open surgery are no longer commonly used (see Differences between protocol and review). Five of the 26 studies included in the previous version are therefore included in the current review.
Update searches in November 2013 identified 2581 bibliographic records after removal of duplicates, of which 2474 were excluded and 107 full‐text articles and conference abstracts were retrieved for detailed examination. Of the 107 publications examined in detail, 67 were excluded and a further three abstracts are awaiting classification. The remaining 37 publications reported 17 RCTs which met the inclusion criteria (see Figure 1). Together with the five RCTs (reported in six publications) from the previous versions of the review, a total of 22 RCTs reported in 43 publications were therefore included.
1.
Study flow diagram
Ongoing studies
Twelve RCTs which appear to meet the review's inclusion criteria were identified as being in progress at November 2013. The anticipated completion dates range from August 2013 (NCT01073020) to September 2021 (NCT01501201). Although the NCT01073020 study was due to be completed during 2013, it is considered as ongoing since results have not yet been reported.
Seven of the ongoing studies are recruiting patients with varying degrees of obesity who also have type 2 diabetes (NCT01486680; NCT01821508; NCT01047735; NCT01073020; NCT01778738; NCT01501201; NCT00432809) and one study is specifically excluding patients with diabetes (NCT01581801). Three are recruiting participants with varying degrees of obesity who may have other comorbidities (which may or may not include type 2 diabetes) (NCT01352403; ISRCTN 00786323; NCT01929850). The remaining ongoing study is recruiting obese participants with stage 3‐4 chronic kidney disease (NCT01053130). The NCT01929850 study is notable in that it is restricted specifically to under served minority women.
Of the 12 ongoing trials, four are comparing two different surgical procedures (ISRCTN 00786323; NCT01486680; NCT01778738; NCT01581801); five are comparing a surgical procedure against a non‐surgical procedure (medical therapy or lifestyle intervention) (NCT01352403; NCT01929850; NCT01821508; NCT01053130; NCT01501201) and three (three‐arm) trials are comparing two different surgical procedures and a non‐surgical procedure (NCT01047735; NCT01073020; NCT00432809).
The surgical procedures that are being compared in these RCTs are: laparoscopic sleeve gastrectomy (NCT01486680; NCT01929850; NCT01053130; NCT01581801; NCT00432809); laparoscopic Roux‐en‐Y gastric bypass (NCT01486680; NCT01047735; NCT01073020; NCT01581801; NCT01501201; NCT00432809); laparoscopic adjustable gastric banding (ISRCTN 00786323; NCT01047735; NCT01073020); laparoscopic gastric bypass (ISRCTN 00786323); Roux‐en‐Y gastric bypass (NCT01821508); gastric bypass (NCT01778738) and sleeve gastrectomy (NCT01778738).
Included studies
Participants
Of the studies that reported the participant inclusion criteria (Himpens 2006 did not report criteria),10 limited inclusion to participants with morbid obesity (Aasheim 2009; Demerdash 2013; Hedberg 2012; Mingrone 2012; Nguyen 2009; Nogués 2010; Paluszkiewicz 2012; Peterli 2012; Sharma 2013; Vix 2013). Where morbid obesity was described further, a definition of BMI greater than 40 was commonly used, often with the additional criterion of BMI greater than 35 or 37 with comorbid disease. Two of these studies focused on the upper end of the obesity continuum. Hedberg 2012 required participants to have a BMI greater than 48 and Aasheim 2009 included those with super‐obesity (BMI 50 to 60). Five further studies included participants with both obesity and morbid obesity (Angrisani 2007; Dixon 2012; Keidar 2013; Liang 2013; Praveen Raj 2012). Angrisani 2007 included participants with BMI greater than 35 and an upper limit of BMI of 50; Praveen Raj 2012 included participants with a BMI of greater than 37 or 32 with comorbid disease; Dixon 2012 included participants with a BMI of 35 to 55; Keidar 2013 included people with BMI greater than 35 and type 2 diabetes; and Liang 2013 included people with BMI greater than 28 and type 2 diabetes. Three other studies focused on the lower end of the obesity continuum. O'Brien 2006 included participants with a BMI of 30 to 35 and identifiable comorbidities. Dixon 2008 and Ikramuddin 2013 limited inclusion to people diagnosed with type 2 diabetes and a BMI of 30 to 40. A further two studies had lower BMI inclusion limits of 27 to 43 (Schauer 2012) and greater than 25 but less than 35 (Lee 2011). In both these studies, inclusion was also limited to participants with type 2 diabetes.
The individual study sample size ranged from 15 (Nogués 2010) to 250 (Nguyen 2009). The majority of participants in the studies were female in all but four studies (Dixon 2012 42%; Hedberg 2012 47%; Liang 2013 31%; Keidar 2013 46% female) and mean age ranged from 34 years in Karamanakos 2008 to 51 years in Liang 2013. Excluding the seven studies with inclusion criteria that focused on the upper and lower ends of the obesity continuum (Aasheim 2009; Dixon 2008; Hedberg 2012; Ikramuddin 2013; Lee 2011; O'Brien 2006; Schauer 2012), mean baseline BMI ranged from 37 in Himpens 2006 to 49 in Praveen Raj 2012. Mean baseline BMI in the study focusing on mild to moderate obesity was 34 in each group (O'Brien 2006), and was 37 in each group in one study focusing on type 2 diabetes (Dixon 2008) and 35 in the other study (Ikramuddin 2013). In the two studies with the lowest BMI inclusion criteria, the mean baseline BMI was 30 in Lee 2011 and 36 to 37 in each group in Schauer 2012. Hedberg 2012 focused on those with BMI greater than 48 and the mean BMI was 55 in each group of the study. In the study focusing on those with super obesity (BMI 50 to 60) (Aasheim 2009), the mean BMI in the included participants was 55 in both groups.
Baseline characteristics were similar between groups in most of the studies. There were some differences between groups at baseline in six studies (Aasheim 2009; Karamanakos 2008; Mingrone 2012; Nguyen 2009; Nogués 2010; Praveen Raj 2012; see Characteristics of included studies, Appendix 3 and Appendix 4).
Interventions
The included studies compared a variety of interventions, which are summarised in Characteristics of included studies and Appendix 2. Although these studies have been grouped according to the type of surgery for the purposes of this systematic review, there may be variations in surgical technique or procedure within the groupings. Seven RCTs compared surgery with non‐surgical interventions. The remaining RCTs compared different surgical procedures, including various types of gastric bypass, adjustable gastric banding, sleeve gastrectomy biliopancreatic diversion with duodenal switch, duodenojejunal bypass with sleeve gastrectomy, and gastric imbrication, performed with open or laparoscopic surgery. Gastric bypass (usually Roux‐en‐Y gastric bypass) and laparoscopic sleeve gastrectomy were the most commonly investigated procedures and formed the majority of the evidence base.
Outcomes
Several different measures of weight change were reported by the studies including BMI, weight loss, and excess weight loss. Some of the studies did not report measures of variability such as confidence intervals or standard deviations.
Health‐related quality of life was reported by five studies (Aasheim 2009; Dixon 2012; Nguyen 2009; O'Brien 2006; Peterli 2012) and comorbidities were reported by all but four studies (Demerdash 2013; Nguyen 2009; Sharma 2013; Vix 2013). A summary of outcomes reported by the included studies can be seen in Appendix 5.
Follow‐up
The minimum duration of follow‐up for inclusion in this review was 12 months, and most studies followed participants for 12, 24 or 36 months. The studies with the longest follow‐up periods were Hedberg 2012 (median of four years), Nguyen 2009 (mean of 4.2 years and 3.6 years in each group for the complications outcomes) and Angrisani 2007 (10 years). Some studies did not follow all participants for the reported length of time.
Country
Three studies were conducted in Australia (Dixon 2008; Dixon 2012; O'Brien 2006) and two studies were conducted in each of Sweden (Aasheim 2009 [also in Norway]; Hedberg 2012) USA (Nguyen 2009; Schauer 2012) and Italy (Angrisani 2007; Mingrone 2012). One study was conducted in each of Greece (Karamanakos 2008), Spain (Nogués 2010), Taiwan (Lee 2011), Belgium (Himpens 2006), India (Praveen Raj 2012), Switzerland (Peterli 2012), Poland (Paluszkiewicz 2012), China (Liang 2013), Egypt (Demerdash 2013), France (Vix 2013), India (Sharma 2013), and Israel (Keidar 2013). One study was conducted both in Taiwan and the USA (Ikramuddin 2013).
Excluded studies
After examination of 107 full‐text articles and conference abstracts, 67 were excluded. The publications were often excluded for more than one reason, but the most common reason for exclusion (in 47 of the 67 excluded publications) was that the study design did not meet the specified inclusion criteria (see Figure 1).
Studies awaiting classification
An eligibility decision could not be reached for one reference (see Characteristics of studies awaiting classification). This was a conference abstract comparing laparoscopic adjustable gastric banding against Roux‐en‐Y gastric bypass (Dadan 2011). It appeared to be potentially eligible for inclusion in the review, but was judged to be ‘unclear’ during the full text inclusion screening as it provided insufficient information for a judgement to be made. Authors have been contacted to obtain further information, and the status of this abstract will be reconsidered if sufficient information becomes available. Two additional relevant studies published only as abstracts were identified prior to submission of this updated review (Cesana 2013; Darabi 2013). Full details will be obtained and included in the next update of this review.
Risk of bias in included studies
A summary of review authors' judgements about risk of bias for the included RCTs can be seen in Figure 2 and Figure 3.
2.
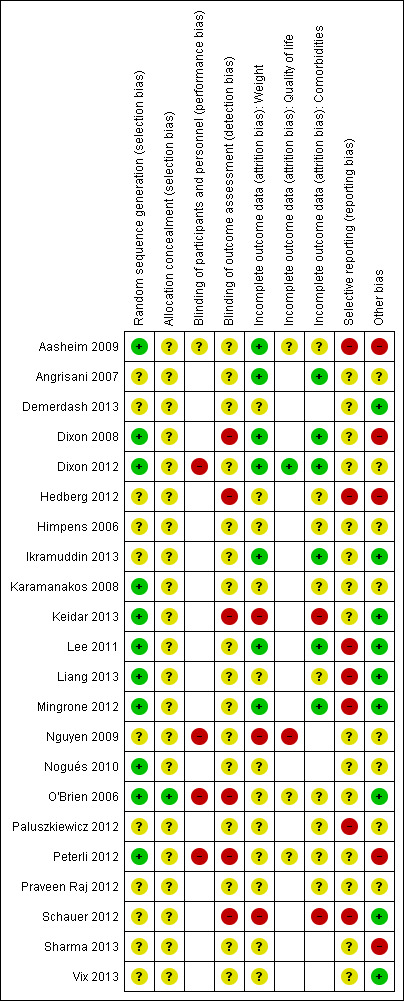
'Risk of bias' summary (blank cells indicate that the study did not report that particular outcome)
3.
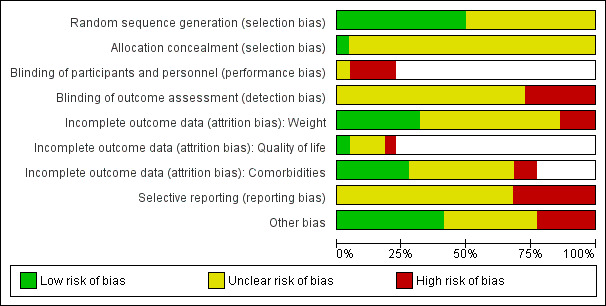
'Risk of bias' graph (blank cells indicate that the particular outcome was not investigated in some studies)
Allocation
Eleven RCTs described adequate allocation sequence generation (Aasheim 2009; Dixon 2008; Dixon 2012; Karamanakos 2008; Keidar 2013; Lee 2011; Liang 2013; Mingrone 2012; Nogués 2010; O'Brien 2006; Peterli 2012), and one had adequate concealment of allocation (O'Brien 2006). The method of allocation sequence generation and concealment was not reported by the remaining studies, therefore they were judged to be of uncertain risk of bias.
Blinding
Five RCTs assessed outcomes self‐reported by participants. In four of these studies participants were not blinded to the intervention received (Dixon 2012; Nguyen 2009; O'Brien 2006; Peterli 2012), and in one study blinding of participants was not reported or unclear (Aasheim 2009).
Only one RCT reported that outcome assessors were blinded to the intervention assignment, but as no details were given about the blinding method or whether it may have been broken, this was judged to be of unclear risk of bias (Karamanakos 2008). Outcome assessors were not blinded to the intervention assignments in six RCTs (Dixon 2008; Hedberg 2012; Keidar 2013; O'Brien 2006; Peterli 2012; Schauer 2012), therefore they were judged to be at high risk of bias. This information was not reported by the remaining RCTs.
Incomplete outcome data
Incomplete outcome data for weight loss were adequately addressed by seven RCTs (Aasheim 2009; Angrisani 2007; Dixon 2008; Dixon 2012; Ikramuddin 2013; Lee 2011; Mingrone 2012). Of the remaining 15 RCTs, 12 were judged to be at uncertain risk of bias and three at high risk of bias (Keidar 2013; Nguyen 2009; Schauer 2012).
Five RCTs assessed quality of life (Aasheim 2009; Dixon 2012; Nguyen 2009; O'Brien 2006; Peterli 2012). One RCT adequately addressed incomplete outcome data (Dixon 2012), three others were judged to be at uncertain risk of bias and one at high risk of bias (Nguyen 2009).
Comorbidities were assessed by 17 RCTs. Incomplete outcome data for co‐morbidities were adequately addressed by six studies (Angrisani 2007; Dixon 2008; Dixon 2012; Ikramuddin 2013; Lee 2011; Mingrone 2012). Two RCTs were judged to be at high risk of bias (Keidar 2013; Schauer 2012) but the remaining nine studies were judged to be of uncertain risk of bias (Aasheim 2009; Hedberg 2012; Himpens 2006; Karamanakos 2008; Liang 2013; O'Brien 2006; Paluszkiewicz 2012; Peterli 2012; Praveen Raj 2012).
Selective reporting
Seven studies (Aasheim 2009; Hedberg 2012; Lee 2011; Liang 2013; Mingrone 2012; Paluszkiewicz 2012; Schauer 2012) were judged not to be free of selective outcome reporting. The remaining studies were judged to be of uncertain risk of reporting bias.
Other potential sources of bias
Five RCTs were judged to be at high risk of bias from other potential sources (Aasheim 2009; Dixon 2008; Hedberg 2012; Peterli 2012; Sharma 2013). One used block randomisation in an unblinded trial (with either fixed block sizes or no reported details), which can mean it is possible to predict future assignments (Dixon 2008). Aasheim 2009 was judged to be at high risk of bias as the surgeons and multidisciplinary treatment teams were more experienced in one procedure (laparoscopic Roux‐en‐Y gastric bypass) than the other procedure (laparoscopic biliopancreatic diversion with duodenal switch), which may have impacted their results. Also, responses to a questionnaire item in a related publication were re‐categorised post‐hoc during analysis. Hedberg 2012 was judged to be at high risk of bias because the required sample size was not achieved due to patients declining randomisation because of their own preferences. Instead, an interim analysis of 47 patients showed significant differences between the two groups and the inclusion was stopped. It was also stated that for both groups after initial evaluations, abnormalities were treated before surgery. Peterli 2012 was judged to be at high risk of bias because the results presented were from an interim analysis that was not based on all the patients randomised. Sharma 2013 was judged to be at high risk of bias as the surgeons were reported as being less skilled in one of the interventions. No evidence bias from other sources was detected in nine RCTs (Demerdash 2013; Ikramuddin 2013; Keidar 2013; Lee 2011; Liang 2013; Mingrone 2012; O'Brien 2006; Schauer 2012; Vix 2013). The remaining RCTs were judged to be of uncertain risk of other potential sources of bias, because there was insufficient rationale or evidence that an identified problem will introduce bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
1. Surgery versus non‐surgical interventions
Seven RCTs compared surgery with non‐surgical interventions; however, the participants, types of surgery and the comparators differed between the studies. Two RCTs (Dixon 2008; Dixon 2012) compared laparoscopic adjustable gastric banding with a conventional therapy group. One RCT (O'Brien 2006) compared laparoscopic adjustable gastric banding with an intensive medical programme. One RCT (Mingrone 2012) compared gastric bypass with medical therapy (a third arm in this RCT comprised biliopancreatic diversion without duodenal switch but this is not considered in the present review as it did not meet the inclusion criteria). Three RCTs compared laparoscopic Roux‐en‐Y gastric bypass to different non‐surgical interventions. One (Schauer 2012) included three arms and compared laparoscopic Roux‐en‐Y gastric bypass plus medical therapy, laparoscopic sleeve gastrectomy plus medical therapy, and medical therapy alone. One (Ikramuddin 2013) compared laparoscopic Roux‐en‐Y gastric bypass and a lifestyle programme with medical management versus the lifestyle programme with medical management alone. The final RCT (Liang 2013), included three arms and compared laparoscopic Roux‐en‐Y gastric bypass with usual care (diet, exercise and biochemical goals), and usual care with a pharmacological treatment (exenatide). For a summary of finding of major outcomes see Table 1.
Primary outcomes
Measures of weight change, fat content or fat distribution
Meta‐analysis of weight loss outcomes for surgery versus non‐surgical interventions was considered inappropriate since the RCTs differed in the characteristics of their participants, interventions and comparators. Instead, outcomes are synthesised narratively below. Where data permit, mean differences (MD) in outcomes between surgery and non‐surgical study groups are displayed in forest plots.
Compared with non‐surgical interventions, surgery had a consistent effect on each of the outcome measures related to weight, regardless of the type of procedure. This can be seen in the data tables and forest plots as summarised in the bullet points here. A more detailed narrative description of each of the trials is also presented below.
The absolute mean BMI at follow‐up was reported by all seven RCTs, after one year (Ikramuddin 2013; Liang 2013; O'Brien 2006; Schauer 2012), 18 months (O'Brien 2006) and two years (Dixon 2008; Dixon 2012; Mingrone 2012; O'Brien 2006) (data are displayed in Analysis 1.1). In all seven RCTs the mean BMI was lower following surgery than following the non‐surgery therapy, however, statistical significance was not reported by Dixon 2008 or Dixon 2012. Five of these RCTs (Ikramuddin 2013; Liang 2013; Mingrone 2012; O'Brien 2006; Schauer 2012) provided sufficient data to display in a forest plot (Analysis 1.2). For Liang 2013, the comparison between the surgery versus usual care arm is displayed. The evidence was of moderate quality (GRADE).
Four of the RCTs reported mean BMI reduction after one year (Schauer 2012) or after two years (Dixon 2008; Dixon 2012; Mingrone 2012) (data are displayed in Analysis 1.3). In all these RCTs, BMI was reduced to a greater degree following the surgical intervention than the non‐surgical therapy. However, statistical analysis of the differences between groups was only reported by Schauer 2012 (P < 0.001 for each surgical procedure compared to medical therapy alone).
Absolute weight in kilograms at follow‐up was reported by four RCTs after one year (Ikramuddin 2013, O'Brien 2006; Schauer 2012), 18 months (O'Brien 2006) and two years (Dixon 2012; O'Brien 2006) (data are displayed in Analysis 1.4 and in a forest plot Analysis 1.5). In all four RCTs, weight was statistically significantly lower following surgery than the non‐surgical therapy (P < 0.001 for all comparisons or demonstrated by 95% confidence intervals (CI)).
Three RCTs reported weight loss in kilograms, after one year (Schauer 2012) or two years (Dixon 2008; Dixon 2012) (data are displayed in Analysis 1.6 and in a forest plot Analysis 1.7). In all three RCTs weight loss was statistically significantly greater following surgery than non‐surgical therapy (P < 0.001 for all comparisons).
Five RCTs reported weight change as the percentage of initial weight loss, after 12 months (Ikramuddin 2013) or after two years of follow‐up (Dixon 2008; Dixon 2012; Mingrone 2012; O'Brien 2006) (data are displayed in Analysis 1.8 and in a forest plot Analysis 1.9). Percentage of initial weight loss was consistently higher in the surgical intervention group than in the non‐surgical therapy group, with the differences being statistically significant (P < 0.001 for all comparisons), where reported.
Four RCTs reported weight change as the percentage of excess weight loss, after one year (O'Brien 2006; Schauer 2012) and two years (Dixon 2008; Mingrone 2012; O'Brien 2006) (data are displayed in Analysis 1.10). Percentage of excess weight loss was consistently higher in the surgical intervention groups than in the non‐surgical therapy groups, with the differences being statistically significant (P < 0.001 for all comparisons where reported). Two of these RCTs (Mingrone 2012; O'Brien 2006) provided sufficient data to display in a forest plot (Analysis 1.11).
Six of the RCTs reported information on other changes related to weight (data are displayed in Analysis 1.12). The outcomes included waist circumference (Dixon 2008; Dixon 2012; Ikramuddin 2013; Mingrone 2012; Schauer 2012), waist‐hip ratio (Dixon 2008; Schauer 2012), neck circumference (Dixon 2012), and the proportion of patients achieving excess weight loss or achieving satisfactory weight loss (O'Brien 2006). Most outcome measures favoured surgery, with statistically significant differences where reported. The exception to this was waist to hip ratio at 12 months (laparoscopic Roux‐en‐Y gastric bypass versus no surgery, P = 0.12, Schauer 2012) and change in neck circumference at two years (Dixon 2012).
1.1. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 1 Mean BMI [kg/m2].
| Mean BMI [kg/m2] | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P value |
| Dixon 2008 | BMI at 2 years, mean | 29.5 | 36.6 | |
| Dixon 2008 | ||||
| Dixon 2008 | ||||
| Dixon 2012 | BMI at 2 years, mean | 36.6 | 42.3 | |
| Dixon 2012 | ||||
| Dixon 2012 | ||||
| Ikramuddin 2013 | BMI at 12 months, mean (SD) | 25.8 (3.5) | 31.6 (3.7) | Difference ‐5.5 (95% CI ‐6.8 to ‐4.2) |
| Ikramuddin 2013 | ||||
| Ikramuddin 2013 | ||||
| Liang 2013 | BMI at 12 months. mean (SD): LRYGB v no surgery | 24.51 (0.91) | 30.38 (1.66) | P < 0.01 |
| Liang 2013 | BMI at 12 months, mean (SD): LRYGB v no surgery + exenatide | 24.51 (0.91) | 26.84 (1.21) | P < 0.05 |
| Liang 2013 | ||||
| Mingrone 2012 | BMI at 2 years, mean (SD) | 29.31 (2.64) | 43.07 (6.44) | P < 0.001 |
| Mingrone 2012 | ||||
| Mingrone 2012 | ||||
| O'Brien 2006 | BMI at 12 months (mean (95% CI) | 27.0 (26.2 to 27.8) | 29.9 (29.1 to 30.8) | P < 0.001 |
| O'Brien 2006 | BMI at 18 months (mean (95% CI) | 26.7 (25.9 to 27.5) | 30.9 (30.0 to 31.8) | P < 0.001 |
| O'Brien 2006 | BMI at 24 months (mean (95% CI) | 26.4 (25.6 to 27.2) | 31.5 (30.6 to 32.4) | P < 0.001 |
| Schauer 2012 | BMI at 12 months, mean (SD): LYRGB | 26.8 (3.2) | 34.4 (3.7) | P < 0.001 |
| Schauer 2012 | BMI at 12 months, mean (SD): LSG | 27.2 (3.5) | 34.4 (3.7) | P < 0.001 |
| Schauer 2012 | ||||
1.2. Analysis.
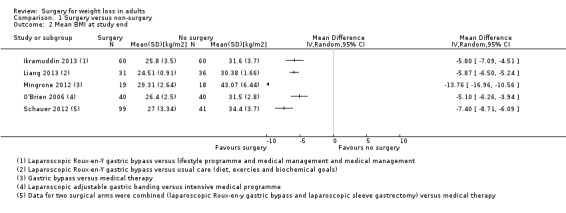
Comparison 1 Surgery versus non‐surgery, Outcome 2 Mean BMI at study end.
1.3. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 3 BMI reduction.
| BMI reduction | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P‐value |
| Dixon 2008 | Reduction in BMI at 2 years, mean | 7.4 | 0.5 | |
| Dixon 2008 | ||||
| Dixon 2012 | BMI loss at 2 years, mean | 9.7 | 1.5 | |
| Dixon 2012 | ||||
| Mingrone 2012 | BMI change from baseline at 2 years, mean (SD) | ‐33.31 (7.88) | ‐4.73 (6.37) | |
| Mingrone 2012 | ||||
| Schauer 2012 | BMI reduction at 12 months, mean (SD): LRYGB | ‐10.2 (3.1) | ‐1.9 (2.9) | P < 0.001 |
| Schauer 2012 | BMI reduction at 12 months, mean (SD): LSG | ‐9.0 (2.7) | ‐1.9 (2.9) | |
1.4. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 4 Weight [kg].
| Weight [kg] | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P‐value |
| Dixon 2012 | Weight at 2 years, kg, mean (95% CI) | 107 (99 to 116) | 121.8 (113 to 129) | ‐ |
| Dixon 2012 | ||||
| Dixon 2012 | ||||
| Ikramuddin 2013 | Weight at 12 mo, mean (SD) | 73.0 (13.6) | 90.1 (17.0) | Difference ‐16.0 (95% CI ‐21.1 to ‐10.8) |
| Ikramuddin 2013 | ||||
| Ikramuddin 2013 | ||||
| O'Brien 2006 | Weight at 12 months, kg, (mean (95% CI)) | 76.3 (74.1 to 78.5) | 85.3 (83.0 to 87.5) | P < 0.001 |
| O'Brien 2006 | Weight at 18 months, kg, (mean (95% CI)) | 75.2 (73.1 to 77.4) | 87.7 (79.9 to 83.0) | P < 0.001 |
| O'Brien 2006 | Weight at 24 months, kg, (mean (95% CI)) | 74.5 (72.4 to 76.7) | 89.5 (80.5 to 83.6) | P < 0.001 |
| Schauer 2012 | Weight at 12 months, kg, mean (SD): LRYGB | 77.3 (13.0) | 99.0 (16.4) | P < 0.001 |
| Schauer 2012 | Weight at 12 months, kg, mean (SD): LSG | 75.5 (12.9) | 99.0 (16.4) | P < 0.001 |
| Schauer 2012 | ||||
1.5. Analysis.
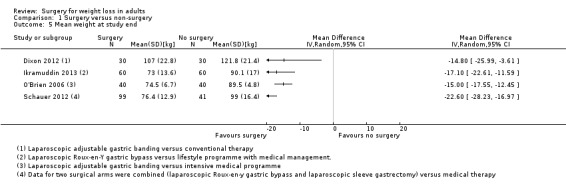
Comparison 1 Surgery versus non‐surgery, Outcome 5 Mean weight at study end.
1.6. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 6 Weight loss [kg].
| Weight loss [kg] | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P value |
| Dixon 2008 | Weight loss at 2 years, mean (SD) | ‐21.1 (10.5) | ‐1.5 (5.4) | Difference ‐19.6 (‐23.8, ‐15.2); P < 0.001 |
| Dixon 2008 | ||||
| Dixon 2012 | Weight loss at 2 years, mean (95% CI) | ‐27.8 (‐34.7 to ‐20.9) | ‐5.1 (‐9.3 to ‐0.8) | ‐22.7 (‐31.1 to ‐14.3); P < 0.001 |
| Dixon 2012 | ||||
| Schauer 2012 | Weight loss at 12 months, mean (SD): LRYGB | ‐29.4 (8.9) | ‐5.4 (8.0) | P < 0.001 |
| Schauer 2012 | Weight loss at 12 months, mean (SD): LSG | ‐25.1 (8.5) | ‐5.4 (8.0) | P < 0.001 |
1.7. Analysis.
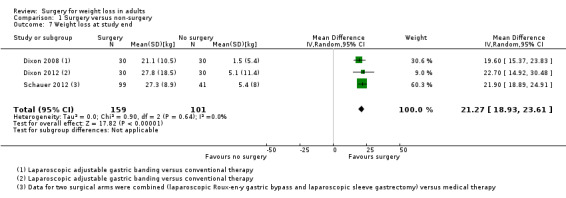
Comparison 1 Surgery versus non‐surgery, Outcome 7 Weight loss at study end.
1.8. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 8 Initial weight loss [%].
| Initial weight loss [%] | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P‐value |
| Dixon 2008 | % Initial weight loss at 2 years, mean (SD) | 20.0 (9.4) | 1.4 (4.9) | P < 0.001 |
| Dixon 2012 | Weight loss at 2 years, %, mean (95% CI) | 20.6 (15.4 to 25.7) | 2.9 (0.6 to 7.3) | P < 0.001 |
| Ikramuddin 2013 | % weight change at 12 mo, mean (SD) | ‐26.1 (8.7) | ‐7.9 (7.8) | Difference ‐17.5 (95% CI ‐20.7 to ‐14.2) |
| Mingrone 2012 | Weight loss at 2 years, % (SD) | ‐33.31 (7.88) | ‐4.74 (6.37) | P < 0.001 |
| O'Brien 2006 | % of initial weight lost at 2 years (mean (95% CI)) | 21.6 (19.3 to 23.9) | 5.5 (3.2 to 7.9) | |
1.9. Analysis.
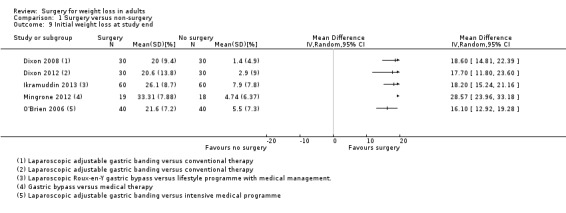
Comparison 1 Surgery versus non‐surgery, Outcome 9 Initial weight loss at study end.
1.10. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 10 Excess weight loss [%].
| Excess weight loss [%] | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P‐value |
| Dixon 2008 | % Excess weight loss at 2 years | 62.5 | 4.3 | |
| Dixon 2008 | ||||
| Mingrone 2012 | % Excess weight lost at 2 years, (SD) | 68.08 (12.70) | 9.29 (12.94) | P < 0.001 |
| Mingrone 2012 | ||||
| O'Brien 2006 | % Excess weight lost at 12 months (mean (95% CI)) | 78.6 (69.2 to 88.1) | 41.1 (31.2 to 50.9) | P < 0.001 |
| O'Brien 2006 | % Excess weight lost at 2 years (mean (95% CI)) | 87.2 (77.7 to 96.6) | 21.8 (11.9 to 31.6) | P < 0.001 |
| Schauer 2012 | % Excess weight lost at 12 months, median (interquartile range): LRYGB | 88 (72, 101) | 13 (0.8, 23) | P < 0.001 |
| Schauer 2012 | % Excess weight lost at 12 months, median (interquartile range): LSG | 81 (65, 97) | 13 (0.8, 23) | P < 0.001 |
1.11. Analysis.
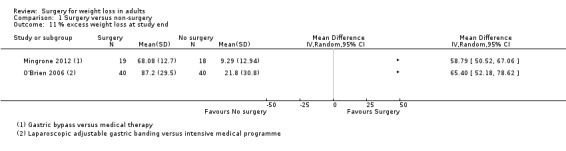
Comparison 1 Surgery versus non‐surgery, Outcome 11 % excess weight loss at study end.
1.12. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 12 Other weight change data.
| Other weight change data | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P value |
| Dixon 2008 | Waist to hip ratio at 2 years, cm, mean (SD) | 0.90 (0.06) | 0.95 (0.08) | Difference in change ‐0.05 (95% CI ‐0.07 to ‐0.007); P = 0.02 |
| Dixon 2008 | Waist circumference at 2 years, cm, mean (SD) | 95.8 (10.3) | 112.7 (10.3) | Difference (in change) ‐13.9 (95% CI ‐19.0 to ‐8.7); P < 0.001 |
| Dixon 2008 | ||||
| Dixon 2008 | ||||
| Dixon 2008 | ||||
| Dixon 2008 | ||||
| Dixon 2008 | ||||
| Dixon 2008 | ||||
| Dixon 2012 | Waist circumference at 2 years, cm, mean (95% CI) | 119.8 (112 to 126) | 124 (119 to 128) | Not reported |
| Dixon 2012 | Change in waist circumference, baseline to 2 years, cm, mean (95% CI) | ‐18.1 (‐12.7 to ‐23.6) | ‐2.9 (‐5.6 to 0.0) | ‐15.2 (‐21.1 to ‐9.33); P = 0.01 |
| Dixon 2012 | Neck circumference at 2 years, cm, mean (95% CI) | 42 (39.1 to 45) | 44.6 (6.0) | Not reported |
| Dixon 2012 | Change in neck circumference, baseline to 2 years, cm, mean (95% CI) | ‐5.2 (‐8.3 to ‐2.07) | ‐1.8 (‐3.3 to ‐0.23) | ‐3.4 (‐7.5 to 0.65); P = 0.10 |
| Dixon 2012 | ||||
| Dixon 2012 | ||||
| Dixon 2012 | ||||
| Dixon 2012 | ||||
| Ikramuddin 2013 | Waist circumference, cm, at 12 mo, mean (SD) | 90 (11) | 105 (11) | Difference ‐15 (95% CI ‐18 to ‐11) |
| Ikramuddin 2013 | ||||
| Ikramuddin 2013 | ||||
| Ikramuddin 2013 | ||||
| Ikramuddin 2013 | ||||
| Ikramuddin 2013 | ||||
| Ikramuddin 2013 | ||||
| Ikramuddin 2013 | ||||
| Mingrone 2012 | Waist, cm at 2 years, mean (SD) | 98.58 (13.06) | 116.33 (12.14) | P < 0.001 |
| Mingrone 2012 | Waist, cm change from baseline at 2 years, mean (SD) | ‐19.91 (8.44) | ‐7.69 (7.80) | |
| Mingrone 2012 | ||||
| Mingrone 2012 | ||||
| Mingrone 2012 | ||||
| Mingrone 2012 | ||||
| Mingrone 2012 | ||||
| Mingrone 2012 | ||||
| O'Brien 2006 | Proportion achieving excess weight loss (> 50%) at 2 years (%) | 33/39 (85%) | 8/31 (26%) | P < 0.001 |
| O'Brien 2006 | Proportion achieving satisfactory weight loss (> 25%) at 2 years (%) |
39/40 (98%) | 14/40 (35%) | P < 0.001 |
| O'Brien 2006 | ||||
| O'Brien 2006 | ||||
| O'Brien 2006 | ||||
| O'Brien 2006 | ||||
| O'Brien 2006 | ||||
| O'Brien 2006 | ||||
| Schauer 2012 | Waist at 12 months, cm, mean (SD): LRYGB | 93.4 (9.0) | 108.8 (10.8) | P < 0.001 |
| Schauer 2012 | Waist at 12 months, cm, mean (SD): LSG | 93.5 (8.8) | 108.8 (10.8) | P < 0.001 |
| Schauer 2012 | Change in waist at 12 months, cm, mean (SD): LRYGB | ‐23.0 (8.3) | ‐4.1 (8.5) | P < 0.001 |
| Schauer 2012 | Change in waist at 12 months, cm, mean (SD): LSG | 20.1 (9.0) | ‐4.1 (8.5) | P < 0.001 |
| Schauer 2012 | Waist:hip ratio at 12 months, mean (SD): LRYGB | 0.91 (0.06) | 0.93 (0.08) | P = 0.12 |
| Schauer 2012 | Waist:hip ratio at 12 months, mean (SD): LSG | 0.92 (0.07) | 0.93 (0.08) | P = 0.07 |
| Schauer 2012 | Change in waist:hip ratio at 12 months, mean (SD): LRYGB | ‐0.05 (0.06) | ‐0.01 (0.04) | P < 0.001 |
| Schauer 2012 | Change in waist:hip ratio at 12 months, mean (SD): LSG | ‐0.05 (0.07) | ‐0.01 (0.04) | P = 0.02 |
The following paragraphs provide a more detailed description of the trials summarised above.
In a comparison of laparoscopic adjustable gastric banding with non‐surgical interventions in people with a BMI ranging from 30 to 35 and identifiable co‐morbidities, O'Brien 2006 reported a statistically significant (P < 0.001) difference in the weight of participants at 12, 18 and 24 months. While people in the laparoscopic adjustable gastric banding group consistently lost weight during the two‐year follow‐up, those in the non‐surgical group increased in weight, despite an initial loss of weight at six months. The differences in weight change were reflected in their respective BMIs, with statistically significant (P < 0.001) differences beyond the six‐month follow‐up. Participants in the laparoscopic adjustable gastric banding group experienced a decrease in their BMI from 33.7 at baseline to 26.4 at two years compared with a decrease from a BMI of 33.5 at baseline to 31.5 at two years for those in the non‐surgical group. By two years people receiving laparoscopic adjustable gastric banding had lost 87.2% of excess weight, statistically significantly (P < 0.001) more than the 21.8% lost by people in the non‐surgical group. Of those people with laparoscopic adjustable gastric banding, 98% had achieved a satisfactory weight loss (greater than 25% of excess weight loss) at two years, compared to 35% of people in the non‐surgical group.
Dixon 2008, who assessed the effectiveness of laparoscopic adjustable gastric banding and conventional therapy on obese people (BMI 30 to 40) diagnosed with type 2 diabetes at two years follow‐up, found a statistically significantly (P < 0.001) greater mean percentage weight loss following laparoscopic adjustable gastric banding (20.0%) compared with conventional therapy (1.4%). This equated to a statistically significant (P < 0.001) difference in mean weight loss with those receiving laparoscopic adjustable gastric banding losing an additional 19.6 kg. The change in weight resulted in a reduction in the mean BMI for people in the laparoscopic adjustable gastric banding group from 36.9 to 29.5, while those in the conventional therapy group declined from a BMI of 37.1 to 36.6. Dixon 2008 reported that the loss of weight represented a loss of 62.5% of excess weight (using BMI 25 as ideal weight) for people with the laparoscopic adjustable gastric banding and 4.3% for people receiving conventional therapy. Similar benefits were noted on measures of waist circumference and waist‐hip ratio for those in the laparoscopic adjustable gastric banding group compared to the conventional therapy group.
In a comparison of laparoscopic adjustable gastric banding with a conventional weight‐loss programme in obese people (BMI 35 to 55) who had a confirmed diagnosis of obstructive sleep apnoea, Dixon 2012 reported a statistically significant difference in weight loss (kg) at two years, in favour of laparoscopic adjustable gastric banding (P < 0.001). The proportion of weight lost at two years was also seen to be statistically significantly different between the two groups, in favour of surgery (P < 0.001). BMI at two years was reported for the two study groups but no statistical analyses were presented for this outcome. Similarly, waist circumference and neck circumference values at two years were reported. The change in waist circumference between the two groups was seen to favour laparoscopic adjustable gastric banding (P = 0.01) at two years, however. The change in neck circumference between the two groups was not statistically significantly different (P = 0.10).
Ikramuddin 2013 compared laparoscopic Roux‐en‐Y gastric bypass and a lifestyle programme with medical management versus the lifestyle programme with medical management alone in obese people (BMI 30 to 39.9) with type 2 diabetes and inadequate glycaemic control. The study found a BMI difference at 12 months follow‐up of ‐5.5 kg/m2 (95% CI ‐6.8 to ‐4.2) favouring the surgical intervention. There was also lower weight, a greater proportion of weight change and a lower waist circumference at 12 months in those undergoing the surgical intervention compared with the lifestyle programme.
In a three‐arm RCT, Liang 2013 compared laparoscopic Roux‐en‐Y gastric bypass with a usual care group and a usual care plus exenatide in those with a BMI greater than 28 together with type 2 diabetes and hypertension. At 12 months, gastric bypass led to a statistically significantly lower BMI than usual care (P < 0.01); gastric bypass also led to a statistically significantly lower BMI at 12 months compared with usual care and exenatide (P < 0.05), although the difference was smaller. No other weight‐related outcomes were reported in this RCT.
In a three‐arm RCT, Mingrone 2012 compared both gastric bypass and biliopancreatic diversion with a medical therapy group in those with a BMI of 35 or more and with type 2 diabetes (the biliopancreatic diversion arm was excluded from this review). In this trial, gastric bypass was found to result in a statistically significantly (P < 0.001) greater percentage of weight loss and excess weight loss, and waist circumference was lower at two years than those treated with medical therapy only. This was similarly reflected in the participants’ BMI, which was statistically significantly lower in the gastric surgery group (mean 29.3) compared to the medical therapy group (mean 43.1) (P < 0.001). Changes from baseline values were also presented for BMI and waist circumference but these were not analysed statistically.
Schauer 2012 compared both intensive medical therapy plus laparoscopic Roux‐en‐Y gastric bypass, and intensive medical therapy plus laparoscopic sleeve gastrectomy, against intensive medical therapy alone in participants with type 2 diabetes and a BMI of 27 to 43. In this RCT, both surgical procedures resulted in statistically significant greater weight loss at 12 months than medical therapy alone on all the measures used (change in weight in kilograms, BMI, waist circumference, waist‐hip ratio and percentage of excess weight lost).
Health‐related quality of life
Two of the seven RCTs that compared surgical and non‐surgical interventions reported validated measures of health‐related quality of life (Dixon 2012; O'Brien 2006). The quality of the evidence was moderate.
O'Brien 2006 compared short form health survey (SF‐36) domain scores at two years follow‐up for people undergoing laparoscopic adjustable gastric banding and non‐surgical therapy (Analysis 1.13). Statistically significantly higher scores were reported for five of the eight domains for laparoscopic adjustable gastric banding compared to the non‐surgical group.
1.13. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 13 Health‐related quality of life.
| Health‐related quality of life | ||||
|---|---|---|---|---|
| Study | SF‐36 scores | Surgery | No surgery | Mean (95% CI) of between‐group differences; P value |
| Dixon 2012 | Physical function, mean change at 2 years (95% CI) | 29.6 (16.1 to 43.2) | 12.8 (1.4 to 24.2) | 16.8 (‐3.4 to 37); P = 0.1 |
| Dixon 2012 | Physical Role, mean change at 2 years (95% CI) | 39.2 (17.3 to 61.2) | 5.7 (‐12.9 to 24.3) | 33.5 (2.2 to 64.8); P = 0.04 |
| Dixon 2012 | Bodily Pain, mean change at 2 years (95% CI) | 14.6 (4.7 to 24.5) | 7.2 (0.8 to 13.7) | 7.4 (‐6.5 to 21.2); P = 0.29 |
| Dixon 2012 | General Health, mean change at 2 years (95% CI) | 30 (20.8 to 39.1) | 11.6 (2.3 to 20.8) | 18.4 (3.6 to 33.2); P = 0.02 |
| Dixon 2012 | Vitality, mean change at 2 years (95% CI) | 22.5 (13.4 to 31.7) | 5.2 (‐5.7 to 16.0) | 17.3 (0.4 to 34.3); P = 0.05 |
| Dixon 2012 | Social Functioing, mean change at 2 years (95% CI) | 16.3 (2.4 to 30.3) | 5.7 (‐5.0 to 16.4) | 10.6 (‐9.1 to 30.3); P = 0.29 |
| Dixon 2012 | Emotional Role, mean change at 2 years (95% CI) | 20.5 (‐3.3 to 44.3) | 4.9 (‐12.0 to 21.9) | 15.6 (‐19.7 to 50.9); P = 0.38 |
| Dixon 2012 | Mental Health, mean change at 2 years (95% CI) | 9.1 (‐0.3 to 18.4) | 4.8 (‐4.6 to 14.1) | 4.3 (‐10.5 to 19.0); P = 0.57 |
| Dixon 2012 | Physical Component summary score, mean change at 2 years (95% CI) | 12.6 (7.3 to 17.9) | 3.4 (‐1.6 to 8.4) | 9.3 (0.5 to 18.0); P = 0.04 |
| Dixon 2012 | Mental Component summary score, mean change at 2 years (95% CI) | 0.5 (‐3.0 to 4.0) | 0.8 (‐2.2 to 3.8) | ‐0.3 (‐5.3 to 4.8); P = 0.92 |
| O'Brien 2006 | Physical function at 2 years, mean | 90 | 87 | P < 0.05 |
| O'Brien 2006 | Physical Role at 2 years, mean | 92 | 70 | P < 0.05 |
| O'Brien 2006 | Pain at 2 years, mean | 83 | 78 | P = ns |
| O'Brien 2006 | General Health at 2 years, mean | 73 | 68 | P < 0.05 |
| O'Brien 2006 | Vitality at 2 years, mean | 66 | 57 | P < 0.05 |
| O'Brien 2006 | Social Functioning at 2 years, mean | 85 | 81 | P = ns |
| O'Brien 2006 | Emotional Role at 2 years, mean | 92 | 72 | P < 0.05 |
| O'Brien 2006 | Mental Health at 2 years, mean | 76 | 72 | P = ns |
| O'Brien 2006 | ||||
| O'Brien 2006 | ||||
Dixon 2012 also reported outcomes at two years on the SF‐36, reporting both the individual domains and the component summary scores (Analysis 1.13). Statistically significant greater improvements from baseline SF‐36 scores were reported for two (role‐physical, general health) of the eight domains for laparoscopic adjustable gastric banding compared to the conventional weight‐loss programme. On the physical component score a statistically significant difference in improvement between groups was seen in favour of laparoscopic adjustable gastric banding (P = 0.04); however, on the mental component summary score there was no statistically significant difference between the two treatment groups (P = 0.92).
Obesity‐related comorbidities
All seven of the RCTs that compared surgical and non‐surgical interventions reported effects of the interventions on comorbidities, although the types of comorbidities reported differed between the RCTs. Meta‐analysis of comorbidity outcomes was not feasible due to differences between the RCTs in the way comorbidity outcomes were reported. Instead, comorbidity outcomes are summarised narratively below.
Five of the RCTs reported diabetes‐related outcomes (patients with diabetes remission, diabetes medication or specified levels of glycosylated haemoglobin) (Dixon 2008; Ikramuddin 2013; Liang 2013; Mingrone 2012; Schauer 2012) (data are displayed in Analysis 1.14). The quality of the evidence was moderate. Each of these trials specifically included participants who had type 2 diabetes at baseline. Dixon 2008 reported that remission of type 2 diabetes after two years was statistically significantly (P < 0.001) higher following laparoscopic adjustable gastric banding (73%) than conventional therapy (13%) (RR 5.5; 95% CI 2.2 to 14.00). At two years follow‐up a greater proportion of those receiving laparoscopic adjustable gastric banding no longer required diabetes medication compared to conventional therapy (change from baseline 83% versus 15%, respectively, not tested for statistical significance). There were similar improvements from baseline to two years follow‐up for those in the laparoscopic adjustable gastric banding group compared to the conventional therapy group in their use of metformin (86.3% versus 30.8%), other hypoglycaemics (27.6% versus 3.2%), and insulin (3.4% versus 11.5%), although these differences between the groups were also not tested for statistical significance. Ikramuddin 2013 reported that at 12 months, 44% of those in the laparoscopic Roux‐en‐Y gastric bypass group had a glycosylated haemoglobin level of < 6% compared with 9% in the lifestyle programme with medical management group (see Analysis 1.14 for details). The proportion with a glycosylated haemoglobin level < 7% at 12 months was also greater in the surgically treated group than those treated with the lifestyle programme (75% versus 32% respectively, see Analysis 1.14). Liang 2013 reported a greater proportion of people with diabetes remission in the laparoscopic Roux‐en‐Y gastric bypass group (90%) than the usual care group (0%) or usual care and exenatide therapy group (0%). Mingrone 2012 reported that after two years, 75% of those in the gastric bypass group but none of those in the medical therapy group were classed as having a diabetes remission (P < 0.001). All participants in the gastric bypass group discontinued pharmacological treatment for diabetes within 15 days, although it is unclear if this analysis is based on the intention‐to‐treat (ITT) population. Schauer 2012 reported that proportionally more participants in the laparoscopic Roux‐en‐Y gastric bypass plus intensive medical therapy and laparoscopic sleeve gastrectomy plus intensive medical therapy groups achieved a glycosylated haemoglobin level of ≤ 6% at 12 months than patients in the intensive medical therapy alone group (42%, 37% and 12%, respectively; P = 0.002 for gastric bypass versus medical therapy alone; P = 0.008 for sleeve gastrectomy versus medical therapy alone). Proportionally more patients in the surgery groups than in the medical therapy alone group achieved a glycosylated haemoglobin level of ≤ 6% and also were not using any diabetes medications (42%, 27% and none, respectively; P < 0.001 for gastric bypass versus medical therapy alone and for sleeve gastrectomy versus medical therapy alone). A higher proportion of patients in the gastric bypass and sleeve gastrectomy groups were taking no diabetes medications than in the medical therapy alone group (78%, 51% and none, respectively; P < 0.05 for gastric bypass versus medical therapy alone and for sleeve gastrectomy versus medical therapy alone).
1.14. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 14 Comorbitidies: diabetes.
| Comorbitidies: diabetes | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P value |
| Dixon 2008 | Remission of type 2 diabetes at 2‐years | 22/30 (73%) | 4/30 (13%) | RR 5.5 (95% CI 2.2 to 14.0); P < 0.001 |
| Dixon 2008 | No diabetes medication at baseline | 2/29 (6.9%) | 4/26 (15.4%) | |
| Dixon 2008 | No diabetes medication at baseline at 2 years | 26/29 (89.7%) | 8/26 (30.8%) | |
| Dixon 2008 | ||||
| Ikramuddin 2013 | % with fasting glucose <100 mg/dl at 12 months, n (%) | 25 (44) | 7 (14) | OR 5.8 (95% CI 2.1 to 15.9) |
| Ikramuddin 2013 | % with HbA1c < 6.0% at 12 months, n (%) | 25 (44) | 5 (9) | OR 7.9 (95% CI 2.7 to 23.4) |
| Ikramuddin 2013 | % with HbA1c < 7.0% at 12 months, n (%) | 43 (75) | 18 (32) | OR 6.0 (95% CI 2.6 to 13.9) |
| Ikramuddin 2013 | ||||
| Liang 2013 | Diabetes remission at 12 months: LRYGB v no surgery | 28/31 (90%) | 0/36 (0%) | |
| Liang 2013 | Diabetes remission at 12 months: LRYGB v no surgery + exenatide | 28/31 (90%) | 0/34 (0%) | |
| Liang 2013 | ||||
| Liang 2013 | ||||
| Mingrone 2012 | Diabetes remission at 2 years, n/N (%) | 15/20 (75%) | 0/18 (0%) | P < 0.001 |
| Mingrone 2012 | ||||
| Mingrone 2012 | ||||
| Mingrone 2012 | ||||
| Schauer 2012 | Glycosylated haemoglobin ≤6% at 12 months, n (%): LRYGB | 21 (42) | 5 (12) | P = 0.002 |
| Schauer 2012 | Glycosylated haemoglobin ≤6% at 12 months, n (%): LSG | 18 (37) | 5 (12) | P = 0.008 |
| Schauer 2012 | n (%) of patients taking no diabetes medications: LRYGB | 38 (78) | 0 | P < 0.05 |
| Schauer 2012 | n (%) of patients taking no diabetes medications: LSG | 25 (51) | 0 | p < 0.05 |
Two RCTs reported use of hypertension medication (Dixon 2008; Mingrone 2012) (data are displayed in Analysis 1.15). Dixon 2008 reported improvements from baseline to two years follow‐up for those in the laparoscopic adjustable gastric banding group compared to the conventional therapy group in their use of anti‐hypertensives (49.3% versus 0%) although these differences between the groups were not tested for statistical significance. Mingrone 2012 reported that the proportions of participants with a reduction/discontinuation of antihypertensive therapies were 80% in the laparoscopic adjustable gastric banding group and 70% in the conventional therapy group, but no analyses were undertaken on these data. Ikramuddin 2013 found no difference in the proportion of people with systolic blood pressure < 130 mmgHg (odds ratio (OR) 1.7, 95% CI 0.6 to 4.6).
1.15. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 15 Comorbitidies: hypertension.
| Comorbitidies: hypertension | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P value |
| Dixon 2008 | Antihypertensive agents at baseline, n/N (%) | 20/29 (70%) | 15/26 (57.7%) | |
| Dixon 2008 | Antihypertensive agents at 2 years, n/N (%) | 6/29 (20.7%) | 15/26 (57.7%) | |
| Ikramuddin 2013 | % with systolic BP < 130 mm Hg at 12 months, n (%) | 48 (84) | 44 (79) | OR 1.7 (95% CI 0.6 to 4.6) |
| Ikramuddin 2013 | ||||
| Mingrone 2012 | Reduction/discontinuation of antihypertensive therapy, % | 80 | 70 | |
| Mingrone 2012 | ||||
Four RCTs reported on the metabolic syndrome, although definitions of this differed (Dixon 2008; Dixon 2012; O'Brien 2006; Schauer 2012) (data are displayed in Analysis 1.16). Dixon 2008 reported that a greater proportion of people undergoing laparoscopic adjustable gastric banding than conventional therapy did not have metabolic syndrome after two years (70% versus 13%, P < 0.001). Dixon 2012 reported that after two years the proportion of participants who had metabolic syndrome relative to those with metabolic syndrome at baseline was lower (53%) in the laparoscopic adjustable gastric banding group than the conventional therapy group (92%), with the changes from baseline (‐47% and ‐8% respectively) differing significantly between the groups (P = 0.005). O'Brien 2006 reported that both study groups had a similar proportion of patients with metabolic syndrome at baseline (37.5%), but after two years the proportion with metabolic syndrome differed significantly between the groups, being 2.7% in the laparoscopic adjustable gastric banding group and 24% in the intensive medical programme group (P = 0.006). Schauer 2012 found that after one year, a higher proportion of patients in the gastric bypass and sleeve gastrectomy groups than in the medical therapy alone group experienced a resolution of metabolic syndrome (65.2%, 58.7% and 35.1%, respectively; P = 0.01 for gastric bypass versus medical therapy alone and P = 0.03 for sleeve gastrectomy versus medical therapy alone).
1.16. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 16 Comorbitidies: metabolic syndrome.
| Comorbitidies: metabolic syndrome | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P value |
| Dixon 2008 | Metabolic syndrome (NOT meeting criteria) at baseline 2 yrs, n (%) |
1 (3%) | 1 (3%) | |
| Dixon 2008 | Metabolic syndrome (NOT meeting criteria) at 2 years, n (%) | 21 (70%) | 4 (13%) | P < 0.001 |
| Dixon 2008 | ||||
| Dixon 2012 | Metabolic syndrome at baseline, n/N | 19/30 | 24/30 | |
| Dixon 2012 | Metabolic syndrome at 2 years, n/N (% of baseline) | 10/19 (53) | 22/24 (92) | |
| Dixon 2012 | Change in metabolic syndrome, baseline to 2 years, n (%) | ‐9 (47) | ‐2 (8) | P = 0.005 |
| O'Brien 2006 | Metabolic syndrome at baseline, n/N (%) | 15/40 (37.5%) | 15/40 (37.5%) | |
| O'Brien 2006 | Metabolic syndrome at 2 years, n/N (%) | 1/39 (2.7%) | 8/33 (24%) | P = 0.006 |
| O'Brien 2006 | ||||
| Schauer 2012 | Resolution of metabolic syndrome at 12 months, n (%): LRYGB | 30 (65.2) | 13 (35.1) | P = 0.01 |
| Schauer 2012 | Resolution of metabolic syndrome at 12 months, n (%): LSG | 27 (58.7) | 13 (35.1) | P = 0.03 |
| Schauer 2012 | ||||
Two RCTs reported lipid normalisation or use of lipid medication (Dixon 2008; Mingrone 2012) (data are displayed in Analysis 1.17). Dixon 2008 reported improvements from baseline to two years follow‐up for those in the laparoscopic adjustable gastric banding group compared to the conventional therapy group in their use of lipid‐lowering agents (27.6% versus 3.9%) although the difference between the groups was not tested for statistical significance. Mingrone 2012 reported that the proportion of participants with normalisation of lipids after two years was significantly higher in the gastric bypass group than the medical therapy group, for total cholesterol (100% versus 27.3%; P < 0.001), high density lipoprotein (HDL) cholesterol (100% versus 11.1%; P < 0.005) and triglycerides (85.7% versus 0%; P < 0.001). Ikramuddin 2013 reported no difference in the proportion with low density lipoprotein (LDL) cholesterol < 100 mg/dL at 12 months (OR 1.6, 95% CI 0.7 to 3.8).
1.17. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 17 Comorbitidies: Lipids.
| Comorbitidies: Lipids | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P value |
| Dixon 2008 | Lipid lowering agents at baseline, n/N (%) | 12/29 (41.4%) | 8/26 (30.8%) | |
| Dixon 2008 | Lipid lowering agents at 2‐years, n/N (%) | 4/29 (13.8%) | 7/26 (26.9%) | |
| Dixon 2008 | ||||
| Ikramuddin 2013 | % with LDL cholesterol < 100 mg/dl at 12 months, n (%) | 45 (79) | 38 (70) | OR 1.6 (95% CI 0.7 to 3.8) |
| Ikramuddin 2013 | ||||
| Ikramuddin 2013 | ||||
| Mingrone 2012 | Total cholesterol normalisation at 2 years, % | 100 | 27.3 | P < 0.001 |
| Mingrone 2012 | HDL cholesterol normalisation at 2 years, % | 100 | 11.1 | P < 0.005 |
| Mingrone 2012 | Triglyceride normalisation at 2 years, % | 85.7 | 0 | P < 0.001 |
One RCT reported the effects of the interventions on sleep (Dixon 2012) (data are displayed in Analysis 1.18). Dixon 2012 compared laparoscopic adjustable gastric banding with conventional weight‐loss therapy in obese people with sleep apnoea. The proportion of participants that achieved a diagnosis of ‘mild’ obstructive sleep apnoea after two years was statistically significantly higher in those treated with laparoscopic adjustable gastric banding (27%) compared with conventional therapy (7%) (P = 0.04). One participant in the conventional therapy group and none in the laparoscopic adjustable gastric banding group achieved remission of sleep apnoea. The proportion who were adherent to continuous positive airway pressure after two years was also reported but did not differ significantly between the study groups.
1.18. Analysis.
Comparison 1 Surgery versus non‐surgery, Outcome 18 Comorbitidies: Sleep.
| Comorbitidies: Sleep | ||||
|---|---|---|---|---|
| Study | Outcome | Surgery | No surgery | P value |
| Dixon 2012 | CPAP initiated, n/N (%) | 28/30 (93) | 25/30 (83) | Stated not significant |
| Dixon 2012 | CPAP adherent at 2 years, n/N (%) | 14/28 (50) | 18/25 (72) | Stated not significant |
| Dixon 2012 | Achieved mild OSA at 2 years, n/N (%) | 8/30 (27) | 2/30 (7) | P = 0.04 |
| Dixon 2012 | Achieved OSA remission at 2 years, n/N (%) | 0/0 (0) | 1/30 (3) | Not reported |
Secondary outcomes
Adverse events, mortality and revision rates
All seven of the RCTs that compared surgical and non‐surgical interventions reported complications and additional operative procedures, although these were defined differently in each RCT, precluding meta‐analysis. A narrative summary of each study is provided below.
Dixon 2008 reported several adverse events among people in the laparoscopic adjustable gastric banding group (n = 30), including a superficial wound infection (one patient), gastric pouch enlargement requiring revisional surgery (two patients), eating difficulties and persistent regurgitation requiring band removal (one patient), post‐operative febrile episode (one patient), minor hypoglycaemic episode (one patient), and gastrointestinal tract intolerance to metformin (one patient). People in the conventional therapy group (n = 30) suffered minor adverse events associated with their medication which resolved following discontinuation of treatment, including gastrointestinal problems (two patients), persistent diarrhoea with metformin (one patient), and vasculitic rash (one patient). Other adverse events included multiple hypoglycaemic episodes (one patient), angina and a transient cerebral ischaemic episode requiring admission to hospital (one patient) and intolerance to very low‐calorie meal replacement (two patients). Dixon 2008 noted that the mean procedure time for placement of the laparoscopic adjustable gastric banding was 54 minutes and that 80% of patients were kept in hospital for only one day.
Dixon 2012 reported the number of participants with adverse events, serious adverse events and minor adverse events, and the total number of adverse events, serious adverse events and minor adverse events for those in the laparoscopic adjustable gastric banding group and the conventional weight‐loss programme group, although rates were not compared statistically. There were 14 people with adverse events in total in the laparoscopic adjustable gastric banding group and 13 in the conventional weight‐loss programme group. Frequency of serious adverse events was the same (17%) in both treatment groups, with five events being recorded in each of the two groups. Serious events in the surgically treated group were cholecystitis with pancreatitis, pouch dilation requiring repositioning, pneumonia, severe headaches and strangulated umbilical hernia. Serious adverse events in the conventional therapy group were acute abdomen, asthma, cardiac and renal failure, angina and peri‐anal abscess and fistula. Minor adverse events were experienced by 40% of the participants in the laparoscopic adjustable gastric banding group compared with 30% of participants in the conventional therapy group. There were no deaths in either group. Five participants in each group were hospitalised during follow‐up.
Ikramuddin 2013 reported there were four early serious adverse events in the laparoscopic Roux‐en‐Y gastric bypass group but no events in the lifestyle programme group. The events were two anastomotic leaks, one wound infection and one wound hernia. There were six late complications of surgery, including stricture (n = 2) and small bowel obstruction (n = 2). In total, there were 22 serious adverse events in the surgical group compared with 15 in the non‐surgical group. Revisional surgery was undertaken on one patient in the surgical intervention group but there were no conversions to other surgical interventions for weight loss. Selected minor adverse events related to diabetes or the procedure were reported to be higher in the surgical group than the non‐surgical group although this was not tested for statistical significance (45 versus 18 for the two groups respectively). Iron deficiency was observed in 13 (22%) of those treated with gastric bypass and vitamin D deficiency in 4 (7%). In people in the lifestyle programme there were no cases of iron deficiency and 5 (8%) cases of vitamin D deficiency. No deaths occurred.
Few data are reported on complications and adverse events in the study by Liang 2013 where it is reported that there were no serious adverse events or deaths in any of the three treatment groups.
Mingrone 2012 reported no operative deaths from gastric bypass, and reported low numbers of late complications (three in the gastric bypass group). Two participants in the medical therapy group had persistent diarrhoea associated with metformin use.
O'Brien 2006 found a higher proportion of adverse events among those people in the non‐surgical therapy group (58%, n = 31) than in the laparoscopic adjustable gastric banding group (18%, n = 39). For those receiving non‐surgical therapy the most common adverse events were intolerance to orlistat (26%), acute cholecystitis (13%), the need for operative interventions (13%) and intolerance to very low calorie diet (3%). Adverse events reported by people in the laparoscopic adjustable gastric banding group included operative interventions (13%), laparoscopic revision (prolapse or posterior) (10%), 5 mm port site infection (2.6%), and acute cholecystitis (2.6%). Loss to follow‐up was higher in the non‐surgical group (16%) compared to laparoscopic adjustable gastric banding group (2.6%) (but reasons not given).
In the RCT that compared laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy (each in addition to intensive medical therapy) with intensive medical therapy alone in patients with type 2 diabetes and a BMI of 27 to 43 (Schauer 2012), there were no deaths in any group. Proportionally more patients who underwent gastric bypass (22%, n = 11) were hospitalised due to a serious adverse event than patients who underwent sleeve gastrectomy (8%, n = 4) or medical therapy alone (9%, n = 4). More patients in the gastric bypass group (n = 3) than in the sleeve gastrectomy (n = 1) and medical therapy alone (n = 0) groups also underwent reoperation. However, proportionally more patients who underwent sleeve gastrectomy (80%, n = 39) and medical therapy alone (81%, n = 35) had a hypoglycaemic episode during the 12 months following surgery than patients who underwent gastric bypass (56%, n = 28) (P values not reported).
2. Comparisons of different surgical procedures: laparoscopic gastric bypass versus laparoscopic adjustable gastric banding
Three RCTs (Angrisani 2007; Demerdash 2013; Nguyen 2009) compared laparoscopic Roux‐en‐Y gastric bypass with laparoscopic adjustable gastric banding. The Demerdash 2013 study had follow‐up of 12 months with a sample size of 34 participants. Two of the studies were relatively long‐term studies; Angrisani 2007 reported five year outcomes for 51 participants and 10‐year outcomes for 34 of these, and the Nguyen 2009 study randomised 250 participants and had four years follow‐up. It should be noted that in the Nguyen 2009 RCT, the proportion of drop‐outs immediately after randomisation was relatively large (11% to 31%) and unbalanced across the study groups, leading us to classify this study as being at high risk of attrition bias, whilst the Angrisani 2007 and Demerdash 2013 RCTs were classified as being mostly at unclear risk of bias. The percentage excess weight lost was specified as the primary, powered outcome in the RCT by Nguyen 2009. The Angrisani 2007 and Demerdash 2013 RCTs did not report whether any outcomes were powered statistically nor whether any outcomes were designated as primary. For a summary of finding of major outcomes see Table 2.
Primary outcomes
Measures of weight change, fat content or fat distribution
BMI showed a consistent pattern in all three RCTs, being lower in the laparoscopic Roux‐en‐Y gastric bypass group at all the follow‐up assessments, despite the pre‐surgery BMI having initially been higher in the laparoscopic Roux‐en‐Y gastric bypass group than the LAGB group in the Nguyen 2009 RCT (data are displayed in Analysis 2.1). When these trials were pooled in a meta‐analysis, the mean end‐of‐study BMI was lower following laparoscopic Roux‐en‐Y gastric bypass compared with laparoscopic adjustable gastric banding (MD ‐5.2 kg/m² (95% CI ‐6.4 to ‐4.0); P < 0.00001; 265 participants; 3 trials; moderate quality evidence; Analysis 2.2). No statistical heterogeneity was evident (Chi² = 0.18, P = 0.91, I2 = 0%).
2.1. Analysis.
Comparison 2 Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding, Outcome 1 Mean BMI [kg/m2].
| Mean BMI [kg/m2] | ||||
|---|---|---|---|---|
| Study | Outcome | LRYGB | LAGB | P value |
| Angrisani 2007 | Mean BMI at 12‐months | 35.4 | 38.7 | |
| Angrisani 2007 | Mean BMI at 36‐months | 29.1 | 35.6 | |
| Angrisani 2007 | Mean BMI at 5‐years (range 60‐66 months) | 29.8 | 34.9 | P < 0.001 |
| Angrisani 2007 | Mean BMI at 10‐years (range 120‐130 months) | 30.4 (5) | 36.5 (7) | P = 0.003 |
| Demerdash 2013 | Mean (SD) BMI at 12 months | 32.0 (2.8) | 37.1 (1.6) | P = 0.0013 |
| Demerdash 2013 | ||||
| Demerdash 2013 | ||||
| Demerdash 2013 | ||||
| Nguyen 2009 | Mean BMI, 1 year | 31.6 | 37.3 | P < 0.05 |
| Nguyen 2009 | Mean BMI, 2 years | 30.6 | 35.8 | P < 0.05 |
| Nguyen 2009 | Mean BMI, 3 years | 30.8 | 35.8 | P < 0.05 |
| Nguyen 2009 | Mean (SD) BMI, 4 years | 30.5 (5.5) | 35.7 (8.1) | P < 0.05 |
2.2. Analysis.
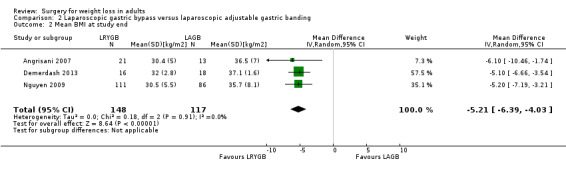
Comparison 2 Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding, Outcome 2 Mean BMI at study end.
Only the Angrisani 2007 RCT reported patients’ mean weight at follow‐up and this was lower in the laparoscopic Roux‐en‐Y gastric bypass group than the laparoscopic adjustable gastric banding group at all follow‐up assessments (P < 0.001 at five years, and P = 0.002 at 10 years, data are displayed in Analysis 2.3).
2.3. Analysis.
Comparison 2 Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding, Outcome 3 Mean weight [kg].
| Mean weight [kg] | ||||
|---|---|---|---|---|
| Study | Outcome, mean (SD) | LRYGB | LAGB | P value |
| Angrisani 2007 | Mean weight, kg at 12‐months | 92.8 | 102.4 | |
| Angrisani 2007 | Mean weight, kg at 36‐months | 83.5 | 98.7 | |
| Angrisani 2007 | Mean weight, kg at 5‐years (range 60‐66 months) | 84 | 97.9 | P < 0.001 |
| Angrisani 2007 | Mean weight, kg (SD) at 10‐years (range 120‐130 months) | 83.2 (18) | 101.3 (22) | P = 0.002 |
In two RCTs (Angrisani 2007; Nguyen 2009) the percentage excess weight loss was consistently larger in the laparoscopic Roux‐en‐Y gastric bypass group than the laparoscopic adjustable gastric banding group at all follow‐up assessments (data are displayed in Analysis 2.4). When these trials were combined in a meta‐analysis, mean end‐of‐study percentage excess weight lost was significantly higher following laparoscopic Roux‐en‐Y gastric bypass compared with laparoscopic adjustable gastric banding (MD 23.0% (95% CI 13.6 to 32.5) ; P < 0.00001; 135 participants; 2 trials; Analysis 2.5). No statistical heterogeneity was evident (Chi² = 0.00, P = 0.99, I2 = 0%).
2.4. Analysis.
Comparison 2 Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding, Outcome 4 Excess weight loss [%].
| Excess weight loss [%] | ||||
|---|---|---|---|---|
| Study | Outcome | LRYGB | LAGB | P value |
| Angrisani 2007 | % EWL at 12‐months | 51.3 | 34.7 | |
| Angrisani 2007 | % EWL at 36‐months | 67.3 | 47.3 | |
| Angrisani 2007 | % EWL at 5‐years (range 60‐66 months) | 66.6 | 47.5 | P < 0.001 |
| Angrisani 2007 | % EWL at 10‐years (range 120‐130 months) | 69.0 (29) | 45.9 (27) | P = 0.03 |
| Nguyen 2009 | Mean (SD) % EWL, 1 year | 64.3 ( ‐ ) (n=111) | 36.5 ( ‐ ) (n=86) | P < 0.05 |
| Nguyen 2009 | Mean (SD) % EWL, 2 years | 68.9 (16.1) (n=94) | 41.8 (20) (n=79) | P < 0.05 |
| Nguyen 2009 | Mean (SD) % EWL, 3 years | 67.5 (16.9) (n=81) | 41.5 (21.4) (n=62) | P < 0.05 |
| Nguyen 2009 | Mean (SD) % EWL, 4 years | 68.4 (19.5) (n=71) | 45.4 (27.6) (n=30) | P < 0.05 |
2.5. Analysis.
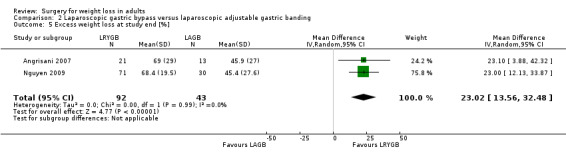
Comparison 2 Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding, Outcome 5 Excess weight loss at study end [%].
Two RCTs (Angrisani 2007; Nguyen 2009) were consistent in reporting that the proportion of patients who experienced failure of weight‐loss treatment was lower in the laparoscopic Roux‐en‐Y gastric bypass group than the laparoscopic adjustable gastric banding group (statistical significance was reported only by Angrisani 2007 (P < 0.001)), although the RCTs each used different definitions of treatment failure (the need for conversion to another bariatric procedure due to failure of weight loss, or having less than 20% excess weight loss (Nguyen 2009); or having a BMI > 35 at five‐year follow‐up Angrisani 2007) (data are displayed in Analysis 2.6). Demerdash 2013 reported that the proportion of body weight decreased at 12 months was greater in the laparoscopic Roux‐en‐Y gastric bypass group than the laparoscopic adjustable gastric banding group (P = 0.025).
2.6. Analysis.
Comparison 2 Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding, Outcome 6 Other weight change data.
| Other weight change data | ||||
|---|---|---|---|---|
| Study | Outcome | LRYGB | LAGB | P value |
| Angrisani 2007 | Weight loss failure (BMI > 35) at 5‐years | 1/24 (4.2%) | 9/26 (34.6%) | P < 0.001 |
| Angrisani 2007 | BMI <30 at 5‐years | 15/24 (62.5%) | 3/26 (11.5%) | P < 0.001 |
| Angrisani 2007 | Proportion with EWL <=25% at 10 years | 1/21 (4.7%) | 4/13 (30.8%) | |
| Angrisani 2007 | Proportion with EWL 25% to 50% at 10 years | 4/21 (19.1%) | 3/13 (23%) | |
| Angrisani 2007 | Proportion with EWL >=50% at 10 years | 16/21 (76.2) | 6/13 (46.2%) | |
| Angrisani 2007 | ||||
| Demerdash 2013 | % body weight decrease at 12 months, mean (SD) | 31.5 (19.58) | 26.25 (22.13) | P = 0.025 |
| Demerdash 2013 | ||||
| Demerdash 2013 | ||||
| Demerdash 2013 | ||||
| Demerdash 2013 | ||||
| Demerdash 2013 | ||||
| Nguyen 2009 | Weight loss <20% (poor/failure) [%] (time of assessment unknown) | 0.0 | 16.7 | |
| Nguyen 2009 | Weight loss 20‐39.9% (adequate) [%] (time of assessment unknown) | 5.1 | 33.3 | |
| Nguyen 2009 | Weight loss 40‐59.9% (good) [%] (time of assessment unknown) | 30.8 | 34.6 | |
| Nguyen 2009 | Weight loss 60‐79.9% (excellent) [%] (time of assessment unknown) | 51.3 | 11.5 | |
| Nguyen 2009 | Weight loss >80% (exceptional) [%] (time of assessment unknown) | 12.8 | 3.8 | |
| Nguyen 2009 | Treatment failure, including patients lost to follow up classified as failures [%] (time of assessment unknown) | 15.3 | 23.3 | |
Health‐related quality of life
Health‐related quality of life was assessed only in the Nguyen 2009 RCT. The SF‐36 instrument was employed but only limited results were presented and this outcome was considered at high risk of bias due to incomplete reporting. The only relevant information reported was that all the eight domains of the SF‐36 that were assessed at 12 months post‐surgery had scores comparable to US norms in both study groups. The quality of the evidence was very low.
Obesity‐related comorbidities
The Nguyen 2009 and Demerdash 2013 RCTs did not specifically assess the impact of the two procedures on weight‐related comorbidities. In the Angrisani 2007 RCT, baseline rates of comorbidities were low with two participants in the laparoscopic Roux‐en‐Y gastric bypass group having hyperlipidaemia, one hypertension, and one type 2 diabetes. In the laparoscopic adjustable gastric banding group, three participants had hypertension and one sleep apnoea at baseline. The authors reported that after five years there was resolution of the diabetes, and hyperlipidaemia (in the laparoscopic Roux‐en‐Y gastric bypass group) and sleep apnoea (in the laparoscopic adjustable gastric banding group), and those that were followed up after 10 years (5 of 8) were still in remission. The quality of the evidence for diabetes was very low.
Secondary outcomes
Adverse events, mortality and revision rates
Two of the RCTs (Angrisani 2007; Nguyen 2009) that compared laparoscopic gastric bypass against laparoscopic adjustable gastric banding reported complications and additional operative procedures, although these were defined differently in each RCT, precluding meta‐analysis. A narrative summary of each study is provided below.
One death was reported in the Nguyen 2009 RCT eight months after surgery in the laparoscopic Roux‐en‐Y gastric bypass group, but was not considered related to the bariatric treatment. No deaths occurred during the Angrisani 2007 RCT.
Both RCTs reported that mean length of hospital stay was significantly longer in the laparoscopic Roux‐en‐Y gastric bypass group than the laparoscopic adjustable gastric banding group (4 versus 2 days, P < 0.05, Angrisani 2007; and 3.1 versus 1.5 days, P < 0.01, Nguyen 2009). The proportion of patients requiring intensive care unit stay was reported only by Nguyen 2009 (2.7% in the laparoscopic Roux‐en‐Y gastric bypass group compared to 1.2% in the laparoscopic adjustable gastric banding group; difference not statistically significant), whilst Angrisani 2007 mentioned that a patient in the laparoscopic Roux‐en‐Y gastric bypass group required an intensive care unit stay of 40 days. In the Nguyen 2009 RCT, the proportion of patients requiring reoperations within 30 days was larger in the laparoscopic Roux‐en‐Y gastric bypass group (5.4% compared to 1.2%) whilst the proportion requiring late reoperations was smaller in the laparoscopic Roux‐en‐Y gastric bypass group (7.2% compared to 11.6%) (differences not statistically significant; P ≥ 0.05). In the Angrisani 2007 RCT the proportions of patients requiring reoperations were 28.6% (6 patients) in the laparoscopic Roux‐en‐Y gastric bypass group (cholecystectomy (4), internal hernia (1), incisional hernia (1)), and 40.9% (9 patients) in the laparoscopic adjustable gastric banding group (all band removal: 4 due to unsatisfactory weight loss and had other bariatric procedures (2 Roux‐en‐Y gastric bypass, 2 biliopancreatic diversion); 5 had no further procedures, 1 for band erosion 3 for pouch dilation, 1 for untreatable reflux symptoms)) (Appendix 9). Nguyen 2009 reported that in the laparoscopic Roux‐en‐Y gastric bypass group 6 readmissions were required within 30 days after surgery compared to none in the laparoscopic adjustable gastric banding group (P = 0.04).
Complications were classified in the Nguyen 2009 RCT in four groups according to time (early/late) and severity (major/minor) (Appendix 8). Overall, there were significantly more complications in the laparoscopic Roux‐en‐Y gastric bypass group than the laparoscopic adjustable gastric banding group (45% versus 17.4%; P < 0.01), with the differences being statistically significant for early minor complications (15.3% versus 4.7%; P = 0.02), late minor complications (13.5% versus 0%; P < 0.01), and late major complications (26.1% versus 11.6%; P = 0.01) (group differences for early major complications were not significant; P ≥ 0.05). The most frequent early major complication was gastrointestinal obstruction (laparoscopic Roux‐en‐Y gastric bypass 3.6% versus laparoscopic adjustable gastric banding 1.2%) whilst the most frequent late major complication was anastomotic stricture, which affected only laparoscopic Roux‐en‐Y gastric bypass patients (15.3%). The most frequent of the minor complications were early wound infection and late marginal ulcer, which occurred only in the laparoscopic Roux‐en‐Y gastric bypass group and affected 6.3% and 8.1% of the patients respectively.
Two (8.4%) early complications requiring surgery were reported by Angrisani 2007 in the laparoscopic Roux‐en‐Y gastric bypass group (one posterior pouch leak intraoperatively causing conversion to open surgery, one sepsis caused by jejunal perforation (sutured and intestine resected). No early complications requiring surgery were noted in the laparoscopic adjustable gastric banding group.
3. Comparisons of different surgical procedures: gastric bypass versus sleeve gastrectomy
Eight trials are discussed in this section. Six RCTs (Karamanakos 2008; Keidar 2013; Nogués 2010; Peterli 2012; Schauer 2012;Vix 2013) compared laparoscopic Roux‐en‐Y gastric bypass with laparoscopic sleeve gastrectomy, one RCT (Paluszkiewicz 2012) compared open Roux‐en‐Y gastric bypass with laparoscopic sleeve gastrectomy and one RCT (Lee 2011) compared simplified laparoscopic mini‐gastric bypass with duodenum exclusion against laparoscopic sleeve gastrectomy without duodenum exclusion. Two studies included participants with lower BMIs than the other studies. Schauer 2012 limited inclusion to patients with BMI 27 to 43 and type 2 diabetes, however the mean BMIs at baseline (Appendix 3) in this study suggest the majority of participants were obese.The study by Lee 2011 included patients with a BMI of between 25 to 35 and poorly controlled type 2 diabetes. Due to differences in the surgical procedures and participants, Lee 2011 is considered separately below and not combined in the meta‐analyses. When interpreting the findings of these studies it should be kept in mind that the sample sizes were relatively small in the laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic sleeve gastrectomy comparisons by Nogués 2010 (7 to 8 participants per group), and that the Peterli 2012 study was considered to be at high risk of bias since the outcomes reported were from an interim analysis that was not based on all patients randomised in an ongoing trial. The trial by Keidar 2013 was assessed as being of high risk of detection bias (outcome assessors not blinded to treatment), and attrition bias (higher rates of drop‐out in one arm) for weight and comorbidity outcomes. Only one of these studies specified that they were powered statistically for weight or BMI outcomes (Peterli 2012). For a summary of finding of major outcomes see Table 3.
Primary outcomes
Measures of weight change, fat content or fat distribution
Six of the seven RCTs that compared laparoscopic Roux‐en‐Y gastric bypass against laparoscopic sleeve gastrectomy (Karamanakos 2008; Keidar 2013; Nogués 2010; Peterli 2012; Schauer 2012) or open Roux‐en‐Y gastric bypass against laparoscopic sleeve gastrectomy (Paluszkiewicz 2012) reported BMI at one or three years after surgery (data are displayed in Analysis 3.1). Results from Karamanakos 2008; Keidar 2013 and Paluszkiewicz 2012 favoured sleeve gastrectomy, whilst the other trials favoured gastric bypass. However, differences were statistically significant in only one of the studies, with BMI 4.3 units lower in the laparoscopic Roux‐en‐Y gastric bypass group one year after surgery (P = 0.01, Nogués 2010). Overall, mean BMI at study end was non‐significantly lower following gastric bypass compared with sleeve gastrectomy: MD ‐0.2 kg/m² (95% CI ‐1.8 to 1.3); P = 0.78; 353 participants; 6 trials; low quality evidence; Analysis 3.2. Substantial statistical heterogeneity was present (Chi2 = 14.60, P = 0.001, I2 = 66%).
3.1. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 1 Mean BMI [kg/m2].
| Mean BMI [kg/m2] | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Karamanakos 2008 | BMI at 3 years, mean (SD) | 31.3 (3.9) | 29.6 (4.1) | P = 0.11 |
| Karamanakos 2008 | ||||
| Karamanakos 2008 | ||||
| Keidar 2013 | BMI at 12 months | 31.4 (4.2) | 30.4 (3.8) | ns |
| Keidar 2013 | ||||
| Keidar 2013 | ||||
| Lee 2011 | BMI at 12 months, mean (SD) | 22.8 (2.2) | 24.4 (2.4) | P = 0.009 |
| Lee 2011 | ||||
| Lee 2011 | ||||
| Nogués 2010 | BMI at 12 months, mean (SD) | 26.2 (2.6) | 30.5 (2.6) | P = 0.01 |
| Nogués 2010 | ||||
| Nogués 2010 | ||||
| Paluszkiewicz 2012 | BMI at 12 months, mean (SD) | 33.8 (5.4) | 32.8 (5.6) | ns |
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Peterli 2012 | BMI at 12 months, mean (SD) | 29.9 (4.8) (n=109) | 30.7 (5.0) (n=107) | P = 0.25 |
| Peterli 2012 | BMI at 2 years, mean (SD) | 30.1 (5.7) (n=52) | 31.1 (4.7) (n=60) | P = 0.28 |
| Peterli 2012 | BMI at 3 years, mean (SD) | 31.7 (6.7 (n=32) | 32.5 (5.6) (n=38) | P = 0.56 |
| Schauer 2012 | BMI at 12 months, mean (SD) | 26.8 (3.2) | 27.2 (3.5) | P = 0.61 |
| Schauer 2012 | ||||
| Schauer 2012 | ||||
3.2. Analysis.
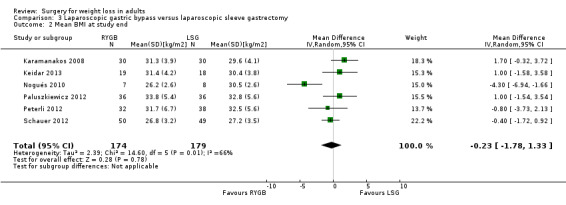
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 2 Mean BMI at study end.
Two trials (Nogués 2010; Schauer 2012) reported a greater reduction in BMI following gastric bypass, but this was statistically significant in only one of these trials (P = 0.03; Analysis 3.3). The pooled mean BMI reduction at 12 months was non‐significantly greater following laparoscopic Roux‐en‐Y gastric bypass compared with laparoscopic sleeve gastrectomy (MD 1.8 kg/m² (95% CI ‐0.34 to 3.93); P = 0.10; 114 participants; 2 trials; Analysis 3.4). Although some statistical heterogeneity was present (Chi2 = 1.55, P = 0.21, I2 = 35%), the direction of the effect was consistent in these two trials.
3.3. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 3 BMI reduction.
| BMI reduction | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P‐value |
| Nogués 2010 | BMI change at 12 months, mean (SD) | ‐16.8 (4.1) | ‐13.0 (3.6) | NS |
| Schauer 2012 | BMI change at 12 months, mean (SD) | ‐10.2 (3.1) | ‐9.0 (2.7) | P = 0.03 |
3.4. Analysis.
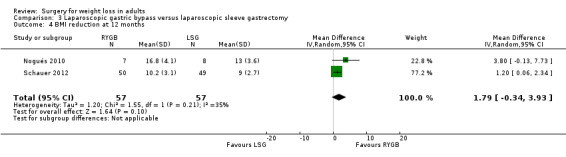
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 4 BMI reduction at 12 months.
Five studies (Keidar 2013;Nogués 2010; Peterli 2012; Schauer 2012;Paluszkiewicz 2012) reported the final weight one year after surgery, and one study also reported it at two and three years after surgery (Peterli 2012) (data are displayed in Analysis 3.5). None of these studies found that the final weight differed significantly between Roux‐en‐Y gastric bypass and sleeve gastrectomy at any time point. There was no statistically significant difference in pooled end of study mean weight: MD 1.2 kg/m² (95% CI ‐2.0 to 4.5); P = 0.46; 293 participants; five trials Analysis 3.6. No statistical heterogeneity was present (Chi2 = ,3.72 P = 0.45, I2 = 0%).
3.5. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 5 Mean weight [kg].
| Mean weight [kg] | ||||
|---|---|---|---|---|
| Study | Outcomes | RYGB | LSG | P value |
| Keidar 2013 | Weight kg at 12 months | 87.8 (14.1) | 84.1 (11.8) | ns |
| Keidar 2013 | ||||
| Keidar 2013 | ||||
| Lee 2011 | Weight kg at 12 months, mean (SD) | 60.7 (10.1) | 65.7 (7.9) | P = 0.03 |
| Lee 2011 | ||||
| Lee 2011 | ||||
| Nogués 2010 | weight at 12 months, kg, mean (SD) | 71.4 (8.2) | 76.5 (8.2) | NS |
| Nogués 2010 | ||||
| Nogués 2010 | ||||
| Paluszkiewicz 2012 | Weight at 12 months, kg, mean (SD) | 96.8 (17.4) | 91.7 (17.4) | ns |
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Peterli 2012 | Weight at 12 months, kg, mean (SD) | 84.7 (16.8) (n=110) | 86.9 (16.9) (n=107) | P = 0.34 |
| Peterli 2012 | Weight at 2 years, kg, mean (SD) | 85.8 (17.9) (n=52) | 87.3 (14.8) (n=60) | P = 0.61 |
| Peterli 2012 | Weight at 3 years, kg, mean (SD) | 90.3 (21.0) (n=32) | 91.3 (18.1) (n=38) | P = 0.83 |
| Schauer 2012 | Weight at 12 months, kg, mean (SD) | 77.3 (13.0) | 75.5 (12.9) | P = 0.50 |
| Schauer 2012 | ||||
| Schauer 2012 | ||||
3.6. Analysis.
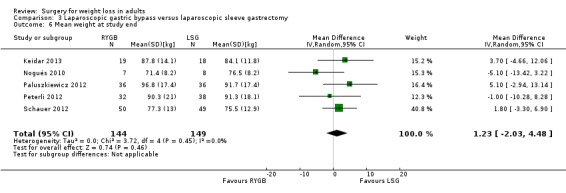
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 6 Mean weight at study end.
Three of the studies reported absolute weight loss one year after surgery (Karamanakos 2008; Nogués 2010; Schauer 2012). Weight loss ranged from 29.4 to 45.3 kg in the laparoscopic Roux‐en‐Y gastric bypass group and 25.1 to 43.6 kg in the sleeve gastrectomy group (data are displayed in Analysis 3.7). In Nogués 2010 mean weight loss after one year was significantly greater in the Roux‐en‐Y gastric bypass group by 13 kg (P = 0.015), however mean pre‐operative weight was already significantly higher in the Roux‐en‐Y gastric bypass group by 7.8 kg (P = 0.025). The pooled mean weight loss after one year was non‐significantly greater following Roux‐en‐Y gastric bypass compared with sleeve gastrectomy: MD 4.1 kg/m² (95% CI ‐3.31 to 11.49); 146 participants; 3 trials; Analysis 3.8. Considerable statistical heterogeneity was present (Chi2 = 8.23, P = 0.02, I2 = 76%).
3.7. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 7 Weight loss [kg].
| Weight loss [kg] | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Karamanakos 2008 | Weight loss at 12 months, mean (SD) | 40.0 (8.3) | 43.6 (11.7) | P = 0.322 |
| Nogués 2010 | weight loss at 12 months, mean (SD) | 45.3 (9.1) | 32.4 (8.7) | P = 0.015 |
| Schauer 2012 | weight loss at 12 months, mean (SD) | 29.4 (8.9) | 25.1 (8.5) | P = 0.02 |
3.8. Analysis.
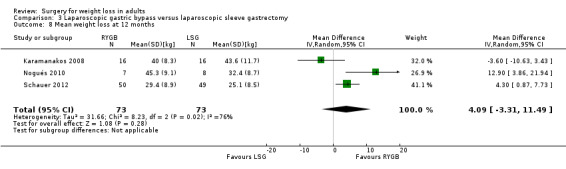
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 8 Mean weight loss at 12 months.
The percentage excess weight lost was reported by five RCTs (Analysis 3.9), two of which reported non‐statistically significant results favouring gastric bypass (Paluszkiewicz 2012; Schauer 2012). Two trials reported results favouring sleeve gastrectomy (Karamanakos 2008; Vix 2013), one of which reported non‐statistically significant results (Vix 2013) and the remaining trial found greater percentage excess weight loss following sleeve gastrectomy that approached statistical significance at 1 and 2 years post‐surgery (P = 0.05). By three years the difference was not statistically significant (P = 0.13, Karamanakos 2008).
3.9. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 9 Excess weight loss [%].
| Excess weight loss [%] | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Karamanakos 2008 | % EWL at 12 months | 65.6 | 72.9 | P = 0.05 |
| Karamanakos 2008 | % EWL at 2 years | 65.3 | 73.2 | P = 0.05 |
| Karamanakos 2008 | % EWL at 3 years | 62.1 | 68.5 | P = 0.13 |
| Lee 2011 | % EWL at 12 months, mean (SD) | 94.4 (33.1) | 76.3 (38.9) | P = 0.06 |
| Lee 2011 | ||||
| Lee 2011 | ||||
| Paluszkiewicz 2012 | % EWL at 12 months | 64.2 | 37.6 | ns |
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Schauer 2012 | % EWL, median (interquartile range) | 88 (72, 101) | 81 (65, 97) | P = 0.32 |
| Schauer 2012 | ||||
| Schauer 2012 | ||||
| Vix 2013 | % EWL at 12 months, mean | 80.38 | 82.97 | P ≥ 0.05 |
| Vix 2013 | ||||
| Vix 2013 | ||||
The excess percentage of BMI lost was reported in three studies and did not differ significantly between the study groups, either at one year (Peterli 2012; Vix 2013), two years (Peterli 2012), or three years post‐surgery (Karamanakos 2008; Peterli 2012) (data are displayed in Analysis 3.10). Paluszkiewicz 2012 and Karamanakos 2008 reported no statistically significant difference in the proportion of patients with greater than 50% excess weight loss at 12 months (Analysis 3.10). The same outcome was reported for two years and three years post‐surgery in the Karamanakos 2008 study where results were also not statistically significant. Other outcomes reported in these studies include percentage body fat, percentage fat mass, percentage fat‐free mass and waist circumference at 12 months (Keidar 2013) and waist circumference and waist‐ hip ratio at 12 months (Schauer 2012). Results can be seen in Analysis 3.10.
3.10. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 10 Other weight change data.
| Other weight change data | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Karamanakos 2008 | achieved >50% of EWL 1 year post‐surgery [%] | 83 | 93 | P = 0.42 |
| Karamanakos 2008 | achieved >50% of EWL 2 years post‐surgery [%] | 83 | 87 | P = 0.9 |
| Karamanakos 2008 | achieved >50% of EWL 3 years post‐surgery [%] | 77 | 83 | P = 0.74 |
| Karamanakos 2008 | % excess BMI lost at 3 years | 61.4 | 68.2 | P = 0.12 |
| Karamanakos 2008 | ||||
| Karamanakos 2008 | ||||
| Karamanakos 2008 | ||||
| Keidar 2013 | % weight loss from baseline, mean (SD) | 25.9 (5.4) | 28.4 (5.9) | ns |
| Keidar 2013 | % body fat at 12 months, mean (SD) | 30.0 (6.4) | 31.5 (8.3) | ns |
| Keidar 2013 | Fat mass (kg) at 12 months, mean (SD) | 25.7 (5.1) | 26.5 (8.7) | ns |
| Keidar 2013 | % Fat mass change from baseline, mean (SD) | 23.7 (8.7) | 24.2 (9.6) | ns |
| Keidar 2013 | Fat‐free mass (kg) at 12 months, mean (SD) | 61.5 (13.5) | 56.8 (9.7) | ns |
| Keidar 2013 | % Fat‐free mass change from baseline, mean (SD) | 6.9 (6.1) | 9.1 (6.3) | ns |
| Keidar 2013 | Waist (cm) at 12 months, mean (SD) | 100.9 (10.4) | 98.6 (9.3) | ns |
| Lee 2011 | % Weight loss at 12 months | 23.3 | 19.9 | P = 0.02 |
| Lee 2011 | Waist circumference cm at 12 months, mean (SD) | 79.7 (7.4) | 85.3 (5.7) | P = 0.002 |
| Lee 2011 | ||||
| Lee 2011 | ||||
| Lee 2011 | ||||
| Lee 2011 | ||||
| Lee 2011 | ||||
| Paluszkiewicz 2012 | % EWL > 50%, n (%) at 12 months | 28 (77.8) | 27 (75) | ns |
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Peterli 2012 | % excess BMI loss at 12 months, mean (SD) | 76.6 (21.0) (n=109) | 72.3 (22.0) (n=107) | P = 0.14 |
| Peterli 2012 | % excess BMI loss at 2 years, mean (SD) | 77.0 (21.7) (n=52) | 69.1 (22.0) (n=60) | P = 0.06 |
| Peterli 2012 | % excess BMI loss at 3 years, mean (SD) | 72.8 (21.2) (n=32) | 63.3 (23.3) (n=38) | P= 0.08 |
| Peterli 2012 | ||||
| Peterli 2012 | ||||
| Peterli 2012 | ||||
| Peterli 2012 | ||||
| Schauer 2012 | Waist circumference cm at 12 months, mean (SD) | 93.4 (9.0) | 93.5 (8.8) | P = 0.96 |
| Schauer 2012 | Waist circumference change from baseline, mean (SD) | ‐23.0 (8.3) | ‐20.1 (9.0) | P = 0.11 |
| Schauer 2012 | Waist:hip ratio at 12 months, mean (SD) | 0.91 (0.06) | 0.92 (0.07) | P = 0.71 |
| Schauer 2012 | Waist:hip ratio change from baseline, mean (SD) | ‐0.05 (0.06) | ‐0.05 (0.07) | P = 0.68 |
| Schauer 2012 | ||||
| Schauer 2012 | ||||
| Schauer 2012 | ||||
| Vix 2013 | % excess BMI loss at 12 months, mean | 71.79 | 70.62 | P ≥ 0.05 |
| Vix 2013 | ||||
| Vix 2013 | ||||
| Vix 2013 | ||||
| Vix 2013 | ||||
| Vix 2013 | ||||
| Vix 2013 | ||||
Lee 2011 examined the weight‐loss effects of simplified laparoscopic mini‐gastric bypass with duodenum exclusion compared to laparoscopic sleeve gastrectomy without duodenum exclusion at 12 months after surgery among patients with a BMI of > 25 to < 35 and who had poorly controlled type 2 diabetes. At 12 months, the mini‐gastric bypass group had a statistically significant lower mean BMI (22.8 (standard deviation (SD) 2.2) versus 24.4 (SD 2.4); P = 0.009; Analysis 3.1), lower mean weight (60.7 kg (SD 10.1 kg) versus 65.7 kg (SD 7.9 kg); P = 0.03; Analysis 3.5), greater mean percentage of weight loss (23.3% versus 19.9%, P = 0.02; Analysis 3.10), and smaller waist circumference (79.7 cm (SD 7.4 cm) versus 85.3 cm (SD 5.7 cm); P = 0.002) (Analysis 3.10) than the sleeve gastrectomy group. The mean percentage of excess weight loss was higher in the mini‐gastric bypass group than the laparoscopic sleeve gastrectomy group, but this difference was not statistically significant (94.4% (SD 33.1) versus 76.3% (SD 38.9), P = 0.06) (Analysis 3.9).
Health‐related quality of life
Only one of the RCTs that compared gastric bypass with sleeve gastrectomy reported health‐related quality of life outcomes (Peterli 2012). In their interim analysis, Peterli 2012 found that health‐related quality of life, assessed using the Gastrointestinal Quality of Life Index (GIQLI), did not statistically significantly differ between groups one year after surgery (data are displayed in Analysis 3.11). The quality of the evidence was very low.
3.11. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 11 Health related quality of life.
| Health related quality of life | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Peterli 2012 | GIQLI score, baseline, mean (SD) | 98.8 (17.4) | 99.0 (20.5) | P ≥ 0.05 |
| Peterli 2012 | GIQLI score at 12 months, mean | 128 | 127 | P ≥ 0.05 |
Obesity‐related comorbidities
Comparisons of comorbidities across these RCTs are limited because the studies tended to report different outcomes. Diabetes‐related outcomes are displayed in Analysis 3.12. Karamanakos 2008 reported the number of cases of diabetes that "resolved" (term used by publication) following surgery. In this study, five patients in each study group had diabetes, and four cases in each group resolved. Keidar 2013 reported the proportion of patients with normal fasting glucose and glycosylated haemoglobin at 12 months, which were reported as 31% in the gastric bypass group and 47% in the sleeve gastrectomy group. The study also reported the proportion with impaired fasting glucose and normal glycosylated haemoglobin, the use of oral hypoglycaemic medication and insulin (see Analysis 3.12). No analysis of statistical differences between groups were reported for any of these outcomes. Nogués 2010 reported normalisation of insulin resistance in patients who fulfilled criteria for insulin resistance at baseline and also withdrawal of diabetic medication among a subgroup of patients who had diabetes at baseline, but neither of these outcomes differed notably between the study groups (no statistical analysis was reported). Peterli 2012 found no statistically significant differences between interventions (P ≥ 0.05) in the proportion of patients who discontinued medication for type 2 diabetes (67.9% versus 57.7%, respectively) or experienced diabetes improvement (28.6% versus 42.3%, respectively). Paluszkiewicz 2012 also reported no statistically significant difference in the proportion of people who had type 2 diabetes at baseline and experienced resolution at 12 months (gastric bypass: 9 of 14 (64.3%) versus sleeve gastrectomy: 4 of 10 (40%)). Schauer 2012, who limited inclusion to people with type 2 diabetes, reported the proportion of people with HbA1c 6% or below and found that this did not differ between groups. The proportion of participants taking no diabetes medications at 12 months appeared to be higher in the laparoscopic Roux‐en‐Y gastric bypass group compared with the laparoscopic sleeve gastrectomy group but no P value was reported. The quality of the evidence for diabetes was low.
3.12. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 12 Comorbidities: diabetes.
| Comorbidities: diabetes | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Karamanakos 2008 | Resolution or improvement of type 2 diabetes, n (%), at 3 years | 4/5 (80) | 4/5 (80) | P > 0.05 |
| Karamanakos 2008 | Resolution or improvement of impaired glucose tolerance, n (%) at 3 years | 5/5 (100) | 5/5 (100) | P > 0.05 |
| Karamanakos 2008 | ||||
| Karamanakos 2008 | ||||
| Keidar 2013 | Normal fasting glucose and HbA1c at 12 months, n (%) | 5/16 (31) | 7/15 (47) | |
| Keidar 2013 | Impaired fasting glucose with normal HbA1c at 12 months, n (%) | 4/16 (25) | 7/15 (47) | |
| Keidar 2013 | Oral hypoglycaemic use at 12 months, n (%) | 8/19 (42) | 3/18 (17) | |
| Keidar 2013 | Insulin use at 12 months, n (%) | 2/19 (11) | 1/18 (6) | |
| Lee 2011 | Remission of diabetes mellitus (HbA1c < 6.5%) at 12 months, n (%) | 28 (93) | 14 (47) | P = 0.02 |
| Lee 2011 | Successful treatment of diabetes mellitus (HbA1c < 7%, LDL‐C < 100 mg/dL, and triglycerides < 150 mg/dL at 12 months, n (%) | 17 (57) | 0 (0) | P < 0.001 |
| Lee 2011 | ||||
| Lee 2011 | ||||
| Nogués 2010 | Withdrawal of use of diabetic medication among a subgroup of patients with diabetes at baseline (n/N), at 12 months | 2/2 | 2/2 | |
| Nogués 2010 | Normalisation of insulin resistance (HOMA‐IR) in patients who fulfilled criteria for insulin resistance at baseline (n/N), at 12 months | 6/6 | 3/4 | |
| Nogués 2010 | ||||
| Nogués 2010 | ||||
| Paluszkiewicz 2012 | Resolution of type 2 diabetes at 12 months, n (%) | 9/14 (64.3) | 4/10 (40) | ns |
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Peterli 2012 | Discontinued medication for type 2 diabetes, % at 1 year | 67.9 | 57.7 | P ≥ 0.05 |
| Peterli 2012 | Type 2 diabetes cured, % at 1 year | 67.9 | 57.7 | ns |
| Peterli 2012 | Type 2 diabetes improved, % at 1 year | 28.6 | 42.3 | ns |
| Peterli 2012 | ||||
| Schauer 2012 | Glycosylated haemoglobin at 12 months ≤ 6%, n (%) | 21 (42) | 18 (37) | P = 0.59 |
| Schauer 2012 | n (%) of patients taking no diabetes medications at 12 months | 38 (78) | 25 (51) | |
| Schauer 2012 | ||||
| Schauer 2012 | ||||
Three RCTs reported resolution or improvement of hypertension at 12 months (Paluszkiewicz 2012; Peterli 2012) or at three years (Karamanakos 2008). However, none of these outcomes differed significantly between the Roux‐en‐Y gastric bypass and sleeve gastrectomy groups (data are displayed in Analysis 3.13).
3.13. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 13 Comorbidities: hypertension.
| Comorbidities: hypertension | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Karamanakos 2008 | Resolution or improvement of hypertension at 3 years, n (%) | 3/5 (60) | 3/4 (75) | P > 0.05 |
| Karamanakos 2008 | ||||
| Paluszkiewicz 2012 | Resolution of hypertension at 12 months, n (%) | 11/30 (36.7) | 8/25 (32) | ns |
| Paluszkiewicz 2012 | ||||
| Peterli 2012 | Hypertension cured, % at 1 year | 33.0 | 33.0 | ns |
| Peterli 2012 | Hypertension improved, % at 1 year | 62.0 | 57.0 | ns |
Four RCTs reported outcomes related to dyslipidaemia (data are displayed in Analysis 3.14). The outcomes included resolution or improvement of high‐density lipoprotein and triglycerides three years after surgery relative to pre‐specified thresholds (Karamanakos 2008), resolution of dyslipidaemia at 12 months (Paluszkiewicz 2012), improvement or cure of dyslipidaemia after one year (Peterli 2012) and abnormal triglycerides at 12 months (Vix 2013). The frequency of resolution of dyslipidaemia after 12 months was statistically significantly higher following laparoscopic Roux‐en‐Y gastric bypass (41.9%) than following laparoscopic sleeve gastrectomy (16.1%) (P < 0.05, Paluszkiewicz 2012), but none of the other lipidaemia‐related outcomes differed significantly between the study groups.
3.14. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 14 Comorbidities: dyslipidaemia.
| Comorbidities: dyslipidaemia | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Karamanakos 2008 | Resolution or improvement of HDL < threshold at 3 years | 4/4 (100) | 2/3 (67) | P > 0.05 |
| Karamanakos 2008 | Resolution or improvement of LDL > threshold at 3 years | 9/10 (90) | 6/8 (75) | P > 0.05 |
| Karamanakos 2008 | Resolution or improvement of triglycerides > threshold at 3 years | 5/5 (100) | 2/3 (67) | P > 0.05 |
| Paluszkiewicz 2012 | Resolution of dysplipidaemia at 12 months, n (%) | 13/31 (41.9) | 5/31 (16.1) | P < 0.05 |
| Paluszkiewicz 2012 | ||||
| Paluszkiewicz 2012 | ||||
| Peterli 2012 | Dyslipidaemia cured, % at 1 year | 47.0 | 26.0 | ns |
| Peterli 2012 | Dyslipidaemia improved, % at 1 year | 50.0 | 59.0 | ns |
| Peterli 2012 | ||||
| Vix 2013 | Abnormal triglycerides at baseline, n (%) | 8 (17.8) | 15 (27.3) | |
| Vix 2013 | Abnormal triglycerides at 12 months, n (%) | 0 (0) | 0 (0) | |
| Vix 2013 | ||||
One RCT reported metabolic syndrome (data are displayed in Analysis 3.15). The proportion with resolution of metabolic syndrome after one year did not differ statistically significantly between the study groups (Schauer 2012).
3.15. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 15 Comorbidities: metabolic syndrome.
| Comorbidities: metabolic syndrome | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Lee 2011 | Metabolic syndrome at 12 months, n (%) | 2 (6.6) | 18 (60.0) | P < 0.001 |
| Schauer 2012 | Resolution of metabolic syndrome at 12 months, n (%) | 30 (65.2) | 27 (58.7) | P = 0.52 |
Two RCTs reported obstructive sleep apnoea (data are displayed in Analysis 3.16). The proportions of patients experiencing resolution or improvement after one year did not differ significantly between the laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy study groups in either of the two RCTs (Karamanakos 2008; Peterli 2012).
3.16. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 16 Comorbidities: sleep.
| Comorbidities: sleep | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Karamanakos 2008 | Resolution or improvement of obstructive sleep apnoea at 3 years, n/N (%) | 2/3 (67) | 4/6 (67) | P > 0.05 |
| Karamanakos 2008 | ||||
| Peterli 2012 | Obstructive sleep apnoea syndrome cured, [%] at 1 year | 33.0 | 52.0 | ns |
| Peterli 2012 | Obstructive sleep apnoea syndrome improved, [%] at 1 year | 67.0 | 45.0 | ns |
Other co‐morbidities that were reported in the RCTs were the frequency of resolution, improvement or new onset of gastro‐oesophageal reflux disease (Karamanakos 2008; Peterli 2012), improvement or cure of back/joint pain, hyperuricaemia, and depression (Peterli 2012), and resolution or improvement of degenerative arthritis and menstrual irregularities (Karamanakos 2008) (data are displayed in Analysis 3.17). Among these outcomes, only one differed significantly between the study groups: Peterli 2012 found that proportionally more patients who underwent laparoscopic Roux‐en‐Y gastric bypass than laparoscopic sleeve gastrectomy experienced remission or improvement in existing pre‐operative gastro‐oesophageal reflux disease (76.5% versus 50%; P = 0.008). Karamanakos 2008, however, found no difference in this outcome, with resolution or improvement occurring in all patients in both groups.
3.17. Analysis.
Comparison 3 Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy, Outcome 17 Comorbidities: other co‐morbidities.
| Comorbidities: other co‐morbidities | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | LSG | P value |
| Karamanakos 2008 | Resolution or improvement of GERD at 3 years, n/N (%) | 5/5 (100) | 2/2 (100) | P > 0.05 |
| Karamanakos 2008 | Resolution or improvement of degenerative arthritis at 3 years, n/N (%) | 5/6 (83) | 4/5 (80) | P > 0.05 |
| Karamanakos 2008 | Resolution or improvement of menstrual irregularities at 3 years, n/N (%) | 7/7 (100) | 7/7 (100) | P > 0.05 |
| Karamanakos 2008 | ||||
| Karamanakos 2008 | ||||
| Karamanakos 2008 | ||||
| Karamanakos 2008 | ||||
| Karamanakos 2008 | ||||
| Peterli 2012 | New‐onset GERD, %, at 1 year | 4 | 12.5 | P = 0.12 |
| Peterli 2012 | GERD cured or improved, % at 1 year | 76.5 | 50 | P = 0.008 |
| Peterli 2012 | Back/joint pain cured, % at 1 year | 17.0 | 22.0 | ns |
| Peterli 2012 | Back/joint pain improved, % at 1 year | 71.0 | 67.0 | ns |
| Peterli 2012 | Hyperuricaemia cured, % at 1 year | 62.5 | 55.0 | ns |
| Peterli 2012 | Hyperuricaemia improved, % at 1 year | 37.5 | 45.0 | ns |
| Peterli 2012 | Depression cured, % at 1 year | 6.0 | 17.0 | ns |
| Peterli 2012 | Depression improved at 1 year | 83.0 | 78.0 | ns |
All of the patients in Lee 2011 had poorly controlled type 2 diabetes and the aim of the trial was to examine the effects of simplified laparoscopic mini‐gastric bypass with duodenum exclusion compared with laparoscopic sleeve gastrectomy without duodenum exclusion in treating type 2 diabetes. The primary outcome was the proportion of patients who achieved remission of type 2 diabetes. Nearly all the patients who underwent gastric bypass achieved remission (93%) compared to 47% of patients who underwent laparoscopic sleeve gastrectomy (P = 0.02). Successful treatment of diabetes (for definition see Analysis 3.12) was achieved in significantly more participants in the gastric bypass group (57%) than the sleeve gastrectomy group (0%) (P < 0.001). Furthermore, proportionally fewer patients in the gastric bypass group than in the sleeve gastrectomy group had metabolic syndrome at 12 months (6.6% (n = 2) versus 60.0% (n = 18), P < 0.001) (Analysis 3.15). However, the authors did not report the proportion of patients in each group with metabolic syndrome at baseline, so it is unclear whether or not this difference was due to the surgical procedures or baseline imbalances between groups.
Secondary outcomes
Adverse events, mortality and revision rates
Four of the six RCTs that compared laparoscopic Roux‐en‐Y gastric bypass against laparoscopic sleeve gastrectomy explicitly reported mortality. Karamanakos 2008, Keidar 2013 and Schauer 2012 stated that no deaths occurred in either group during the study and Peterli 2012 stated that there was one death in the laparoscopic Roux‐en‐Y gastric bypass group and none in the laparoscopic sleeve gastrectomy group.
Four RCTs comparing laparoscopic procedures provided some information about complications and additional operative procedures. Nogués 2010 reported that there were no complications during or after surgery in either study group, with no further details or definitions given.
Karamanakos 2008 reported that there were no conversions to open surgery and no intraoperative and post‐operative complications. Karamanakos 2008 reported that both the laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy groups had the same numbers of early major post‐operative complications (2/30; 7%) and late major post‐operative complications (1/30; 3%) and that neither group experienced any dysphagia or obstruction at any time or required any conversions from laparoscopic to open surgery. The early major complications that occurred were intestinal obstruction and enterocutaneous fistula in the laparoscopic Roux‐en‐Y gastric bypass group (both revised by open surgery); and, in the laparoscopic sleeve gastrectomy group, gastric obstruction (revised by reoperation and supplemental gastric resection) and leakage at the cardio‐oesophageal junction (managed with intravenous (IV) antibiotics and drainage). The late major complications were ileus obstruction in the laparoscopic Roux‐en‐Y gastric bypass group (managed conservatively) and abdominal abscess in the laparoscopic sleeve gastrectomy group (managed by drainage and antibiotics).
Peterli 2012 reported one surgical conversion in each group and that similar numbers of patients in the laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy groups underwent additional operations (26 (23.6%) versus 36 (33.6%), P = 0.09). Similar proportions of patients in the laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy groups experienced a complication within 30 days of surgery (17.2% versus 8.4%, P = 0.067). Eleven laparoscopic Roux‐en‐Y gastric bypass patients had a major complication compared with two laparoscopic sleeve gastrectomy patients (P value not provided). Five patients in the laparoscopic Roux‐en‐Y gastric bypass group (4.5%) and one patient in the laparoscopic sleeve gastrectomy group had a severe complication requiring reoperation (P = 0.21). Other reported complications one year after surgery in the Peterli 2012 study were severe gastro‐oesophageal reflux disease symptoms (two patients in the laparoscopic sleeve gastrectomy group), anastomotic ulcer at the gastro‐enterostomy (one patient in the laparoscopic Roux‐en‐Y gastric bypass group) and stricture requiring endoscopic dilatation (one patient in the laparoscopic Roux‐en‐Y gastric bypass group). One year after surgery, none of the patients underwent further surgery for insufficient weight loss or internal hernia.
Schauer 2012 reported the proportion of patients with serious adverse events who required hospitalisation (22% following laparoscopic Roux‐en‐Y gastric bypass and 8% following laparoscopic sleeve gastrectomy); the most commonly reported serious adverse events were requirement for intravenous infusion for dehydration, reoperation, blood transfusions, gastro‐intestinal leak and arrhythmias. Other adverse events were also reported, the most common event was a hypoglycaemic episode, which occurred in 56% of participants treated with laparoscopic Roux‐en‐Y gastric bypass and 80% of participants treated with laparoscopic sleeve gastrectomy. No statistical analyses were reported for differences between groups.
Three of the RCTs reported micronutrient deficiencies. In the Peterli 2012 RCT, within one year after surgery, similar proportions of patients in the laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy groups experienced micronutrient deficiency (24.5% versus 26.2%, P value not reported). The authors stated that the most frequent deficiency was vitamin D. Vitamin B12 deficiency occurred in 15 laparoscopic Roux‐en‐Y gastric bypass and 7 laparoscopic sleeve gastrectomy patients (P < 0.12). Karamanakos 2008 also reported the proportions of patients in each group who had a range of nutritional deficiencies three years after surgery. Of these, vitamin B12 deficiency was more frequent in the laparoscopic Roux‐en‐Y gastric bypass group (24%) than the laparoscopic sleeve gastrectomy group (4%), but this difference was not statistically significant (P = 0.05). Vix 2013 reported that the proportion of participants with vitamin D deficiency was lower in those treated with sleeve gastrectomy than those undergoing gastric bypass (48% versus 82% respectively, no P value reported). Baseline rates of vitamin D deficiency were 84.6% and 85.7% for the two groups, respectively.
The single RCT that compared open Roux‐en‐Y gastric bypass against laparoscopic sleeve gastrectomy reported that no deaths occurred in either group during the study (Paluszkiewicz 2012). Both groups had similar lengths of hospital stay (median six days) and frequencies of ‘early morbidity’ (< 30 days after surgery: leak, bleeding, venous thrombosis, wound infection, wound fluid collection) (Roux‐en‐Y gastric bypass 16.6%, laparoscopic sleeve gastrectomy 19.4%). Both groups also had similar overall frequencies of ‘late morbidity’ (≥30 days after surgery: incisional hernia, cholelithiasis, serum iron deficiency, serum vitamin B12 deficiency) (both groups 61.1%). The most notable differences between groups, none of which were statistically significant, were: reoperations were required in two laparoscopic sleeve gastrectomy patients (reasons reported) but not in any Roux‐en‐Y gastric bypass patients; there were three major complications in the laparoscopic sleeve gastrectomy group but none in the Roux‐en‐Y gastric bypass group; and vitamin B12 deficiency affected more patients in the Roux‐en‐Y gastric bypass group (30.6%) than the laparoscopic sleeve gastrectomy group (13.8%).
Lee 2011 reported that there were no deaths in either the simplified laparoscopic mini‐gastric bypass with duodenum exclusion or laparoscopic sleeve gastrectomy without duodenum exclusion groups. One patient in each group experienced a complication that required hospitalisation for “conservative treatment” and three patients in each group (10%) experienced minor complications. There were no major complications among patients who underwent either surgical procedure.
4. Comparisons of different surgical procedures: gastric bypass versus biliopancreatic diversion with duodenal switch (laparoscopic or open)
One RCT (Hedberg 2012) compared open Roux‐en‐Y gastric bypass against open biliopancreatic diversion with duodenal switch in patients with a BMI greater than 48, and another RCT (Aasheim 2009) compared laparoscopic Roux‐en‐Y gastric bypass against laparoscopic biliopancreatic diversion with duodenal switch in patients with a BMI of 50 to 60. Both trials had a high risk of selective reporting and 'other' bias.
Primary outcomes
Measures of weight change, fat content or fat distribution
Mean BMI was lower two years following biliopancreatic diversion with duodenal switch than following Roux‐en‐Y gastric bypass (BMI 30.1 (95% CI 28.5 to 31.7) versus BMI 37.5 (95% CI 36.0 to 39.1)) in Aasheim 2009. Both studies found that biliopancreatic diversion with duodenal switch resulted in a greater BMI reduction than gastric bypass (data are displayed in Analysis 4.2): mean 23.2 (SD 6.9) BMI units versus mean 16.2 (SD 4.9) BMI units at four years, P < 0.001 (Hedberg 2012); and mean 24.8 (95% CI 23.0 to 26.5) versus mean 17.3 (95% CI 15.7 to 19.0) at two years, P < 0.001 (Aasheim 2009), respectively. The pooled end‐of‐study mean BMI loss was statistically significantly lower in the gastric bypass group than the biliopancreatic diversion with duodenal switch group (MD ‐7.3 kg/m² (95% CI ‐9.3 to ‐5.4; P < 0.00001; 107 participants; 2 trials; moderate quality evidence; Analysis 4.3).
4.2. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 2 Mean BMI reduction.
| Mean BMI reduction | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | BPD+switch | P value |
| Aasheim 2009 | BMI reduction at 1 year, mean (SD) | 16.3 (4.3) | 22.8 (4.7) | P < 0.001 |
| Aasheim 2009 | BMI reduction at 2 years, mean (95% CI) | 17.3 (15.7 to 19.0) | 24.8 (23.0 to 26.5) | Mean between‐group difference, 7.44 (95% CI 5.24 to 9.64); P < 0.001 |
| Hedberg 2012 | BMI reduction at 4 years, mean (SD) | 16.2 (4.9) | 23.2 (6.9) | |
| Hedberg 2012 | ||||
4.3. Analysis.
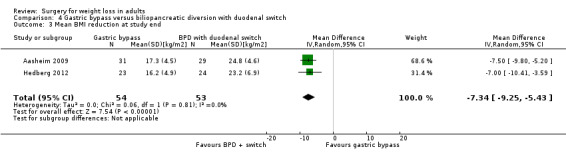
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 3 Mean BMI reduction at study end.
Percentage of excess BMI loss was also consistently lower in the gastric bypass group (data are displayed in Analysis 4.4). In the Hedberg 2012 RCT, the mean percentage excess BMI loss after four years was 80% (SD 15%) and 51% (SD 23%) in the biliopancreatic diversion and gastric bypass groups, respectively (P < 0.001). In the Aasheim 2009 RCT, the mean percentage excess BMI loss after one year was 74.8% (SD 11.2%) and 54.4% (SD 12.8%) in the biliopancreatic diversion and gastric bypass groups, respectively (P < 0.001). The end‐of study pooled mean percentage excess BMI loss was statistically significantly lower following gastric bypass than following biliopancreatic diversion with duodenal switch (MD ‐23% (95% CI ‐31 to ‐15); P < 0.00001; 107 participants; 2 trials; Analysis 4.5). Additionally, Hedberg 2012 reported that proportionally fewer patients in the biliopancreatic diversion with duodenal switch group failed to achieve a greater than 50% loss of excess BMI (4.8% versus 40.0%, P < 0.001) (Analysis 4.9).
4.4. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 4 Excess BMI loss [%].
| Excess BMI loss [%] | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | BPD+switch | P value |
| Aasheim 2009 | % excess BMI lost at 1 year, mean (SD) | 54.4 (12.8) | 74.8 (11.2) | P < 0.001 |
| Hedberg 2012 | % excess BMI lost at 4 years, mean (SD) | 51 (23) | 80 (15) | P < 0.001 |
4.5. Analysis.
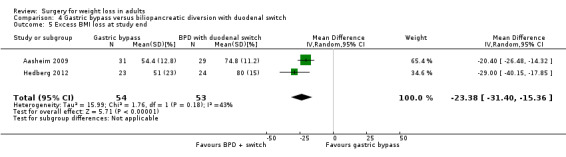
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 5 Excess BMI loss at study end.
4.9. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 9 Other weight change data.
| Other weight change data | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | BPD+switch | P value |
| Aasheim 2009 | Waist circumference at 1 year, cm, mean (95% CI) | 120 (116 to 123) | 105 (102 to 109) | |
| Aasheim 2009 | Waist circumference at 2 years, cm, mean (95% CI) | 115 (111 to 119) | 100 (96.0 to 104) | |
| Aasheim 2009 | Change in waist circumference at 2 years, cm, mean (95% CI) | ‐36.7 (‐41.0 to ‐32.4) | ‐51.5 (‐56.0 to ‐47.0) | Mean between‐group change (95% CI): 14.8 (9.29 to 20.3); P < 0.001 |
| Aasheim 2009 | Hip circumference at 1 year, cm, mean (95% CI) | 127 (124 to 130) | 116 (113 to 119) | |
| Aasheim 2009 | Hip circumference at 2 years, cm, mean (95% CI) | 124 (120 to 127) | 110 (106 to 113) | |
| Aasheim 2009 | Change in hip circumference at 2 years, cm, mean (95% CI) | ‐31.7 (‐35.7 to ‐27.8) | ‐45.6 (‐49.7 to ‐41.6) | Mean between‐group change (95% CI): 13.9 (9.07 to 18.8); P< 0.001 |
| Aasheim 2009 | Saggital diameter at 1 year, cm, mean (95% CI) | 25.9 (24.7 to 27.1) | 23.1 (21.8 to 24.3) | |
| Aasheim 2009 | Saggital diameter at 2 years, cm, mean (95% CI) | 24.5 (23.3 to 25.6) | 21.7 (20.6 to 22.8) | |
| Aasheim 2009 | Change in sagital diameter at 2 years, cm, mean (95% CI) | ‐11.8 (‐13.0 to ‐10.6) | ‐14.6 (‐15.8 to ‐13.4) | Mean between‐group change (95% CI): 2.78 (1.24 to 4.32); P < 0.001 |
| Hedberg 2012 | Failure to achieve > 50% of excess BMI loss, %, mean | 40.0 | 4.8 | P < 0.001 |
| Hedberg 2012 | ||||
| Hedberg 2012 | ||||
| Hedberg 2012 | ||||
| Hedberg 2012 | ||||
| Hedberg 2012 | ||||
| Hedberg 2012 | ||||
| Hedberg 2012 | ||||
| Hedberg 2012 | ||||
Of the two RCTs, only Aasheim 2009 reported weight outcomes in kilograms. The absolute weight at one and two years was higher in the gastric bypass group than the biliopancreatic diversion group (statistical analysis of differences between groups not reported, Analysis 4.6). After two years the biliopancreatic diversion group had lost more weight than the gastric bypass group (‐73.5 kg (95% CI ‐79.0 to ‐68.1)) compared to ‐50.6 kg (95% CI ‐55.8 to ‐45.4), P < 0.001) (Analysis 4.7). After two years, the percentage of body weight loss was lower in the gastric bypass group (statistical analysis of differences between groups not reported, Analysis 4.8).
4.6. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 6 Mean weight [kg].
| Mean weight [kg] | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | BPD+switch | P value |
| Aasheim 2009 | Weight at 1 year, kg, mean (95% CI) | 110 (104 to 115) | 89.4 (84.1 to 94.8) | |
| Aasheim 2009 | Weight at 2 years, kg, mean (95% CI) | 111 (106 to 117) | 88.3 (82.6 to 93.9) | |
4.7. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 7 Weight loss in kg.
| Weight loss in kg | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | BPD+switch | P value |
| Aasheim 2009 | Weight loss at 2 years, kg, mean (95% CI) | ‐50.6 (‐55.8 to ‐45.4) | ‐73.5 (‐79.0 to ‐68.1) | Mean between‐group change (95% CI): 23.0 (16.2 to 29.7); P < 0.001 |
4.8. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 8 Body weight loss [%].
| Body weight loss [%] | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | BPD+switch | P value |
| Aasheim 2009 | % of body weight loss at 2 years, mean (95% CI) | 31.2 (29.2 to 33.2) | 44.8 (42.8 to 46.8) | |
Other outcomes related to weight loss were reported only by Aasheim 2009 (data are displayed in Analysis 4.9). Between baseline and two years, the biliopancreatic diversion with duodenal switch group showed greater mean improvements than the gastric bypass group in waist circumference, hip circumference and sagittal diameter (P < 0.001 for all comparisons). There was no statistically significant difference between the procedures in the percentage of weight lost as fat‐free mass at two years (mean between‐group difference 1.0 percentage points (95% CI ‐2.4 to 4.4); P = 0.54). At two years, none of the patients who underwent biliopancreatic diversion with duodenal switch had a BMI of 40 or more compared to 26% of patients who underwent gastric bypass (P = 0.006).
Health‐related quality of life
Health‐related quality of life was measured in the Aasheim 2009 RCT only, using the Norwegian and Swedish versions of the SF‐36 (data are displayed in Analysis 4.10). The only statistically significant difference between groups in improvement in health‐related quality of life between baseline and two years was that patients who underwent biliopancreatic diversion with duodenal switch reported less improvement in bodily pain than patients who underwent gastric bypass (mean improvement: 8.6 (95% CI ‐2 to 19.2) points versus 28.8 (95% CI 18.9 to 38.8) points, P = 0.003). No statistically significant difference between groups in mean change from baseline on the obesity‐related problems scale was reported by Aasheim 2009 (Analysis 4.11). The quality of the evidence was very low .
4.10. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 10 Health‐related quality of life: SF‐36.
| Health‐related quality of life: SF‐36 | ||||
|---|---|---|---|---|
| Study | SF‐36 domain | LRYGB | BPD+switch | Mean between group difference (CI); P value |
| Aasheim 2009 | Physical functioning change from baseline at 24 months, mean (95% CI) | 36.0 (27.9 to 44.0) | 32.9 (24.6 to 41.3) | 3.04 (‐5.45 to 11.5); P = 0.48 |
| Aasheim 2009 | Role limitations due to physical health problems change from baseline at 24 months, mean (95% CI) | 32.7 (20.3 to 45.0) | 22.3 (9.38 to 35.2) | 10.4 (‐3.51 to 24.3); P = 0.143 |
| Aasheim 2009 | Bodily pain change from baseline at 24 months, mean (95% CI) | 28.8 (18.9 to 38.8) | 8.63 (‐1.98 to 19.2) | 20.2 (6.71 to 33.7); P = 0.003 |
| Aasheim 2009 | General health perceptions change from baseline at 24 months, mean (95% CI) | 29.3 (21.2 to 37.4) | 27.0 (18.4 to 35.6) | 2.33 (‐8.24 to 12.9); P = 0.67 |
| Aasheim 2009 | Vitality change from baseline at 24 months, mean (95% CI) | 20.4 (11.3 to 29.4) | 19.9 (10.3 to 29.4) | 0.49 (‐11.4 to 12.4); P = 0.94 |
| Aasheim 2009 | Social functioning change from baseline at 24 months, mean (95% CI) | 14.6 (2.77 to 26.4) | 18.5 (6.12 to 30.9) | ‐3.92 (‐17.8 to 9.93); P = 0.58 |
| Aasheim 2009 | Role limitations due to emotional problems change from baseline at 24 months, mean (95% CI) | 12.6 (1.85 to 23.3) | 10.9 (‐0.47 to 22.3) | 1.67 (‐12.5 to 15.8); P = 0.82 |
| Aasheim 2009 | General mental health change from baseline at 24 months, mean (95% CI) | 4.09 (‐3.40 to 11.6) | 7.89 (‐0.06 to 15.8) | ‐3.80 (‐13.8 to 6.21); P = 0.46 |
4.11. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 11 Health‐related quality of life: Obesity‐related problems scale.
| Health‐related quality of life: Obesity‐related problems scale | ||||
|---|---|---|---|---|
| Study | Obesity‐related problems scale score | LRYGB | BPD+switch | Mean between group difference (CI); P value |
| Aasheim 2009 | Baseline, mean (95% CI) | 59.2 (50.3 to 68.1) | 61.4 (52.2 to 70.7) | Not reported |
| Aasheim 2009 | Mean change from baseline at 2 years, mean (95% CI) | ‐27.7 (‐37.1 to ‐18.3) | ‐32.5 (‐42.2 to ‐22.8) | 4.81 (‐8.69 to 18.3); P = 0.23 |
Obesity‐related comorbidities
Both RCTs provided limited information on the effects of the weight‐loss interventions on comorbidities related to either diabetes (Hedberg 2012) or sleep (Aasheim 2009). Hedberg 2012 reported that at three years after surgery all patients (100%) in the biliopancreatic diversion with duodenal switch group had an HbA1c level of less than 5% compared to 82% of patients who had undergone gastric bypass (P value not reported) (Analysis 4.12). Medication use was measured in a patient self‐report questionnaire at ≥ 2 years after surgery, but Hedberg 2012 has not reported these data. Aasheim 2009 reported there were no statistically significant differences between the procedures in the number of patients reporting snoring and sleep apnoea symptoms (P > 0.05 for all reported symptoms) at two years (detailed questionnaire data reported but not tabulated here). The authors reported that the numbers of patients in the whole sample using antihypertensive drugs, insulin and lipid‐lowering therapy with statins reduced after surgery, but they did not provide a breakdown of medication use by treatment group. The quality of the evidence for diabetes was very low.
4.12. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 12 Co‐morbidities: diabetes.
| Co‐morbidities: diabetes | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | BPD+switch | P value |
| Hedberg 2012 | HbA1c < 5% at 3 years post‐surgery, % | 82 | 100 | ‐ |
Secondary outcomes
Adverse events, mortality and revision rates
In the Hedberg 2012 RCT using open surgery, there was one death in the biliopancreatic diversion with duodenal switch group, due to pulmonary embolism, and none in the gastric bypass group (P = 0.511) (Appendix 7). Aasheim 2009 reported no deaths in patients undergoing either procedure laparoscopically (Appendix 7). Hedberg 2012 reported that two patients in the biliopancreatic diversion with duodenal switch group and one in the gastric bypass group underwent reoperation (P = 0.516) for suspected perioperative leaks (with negative findings for the patient who underwent gastric bypass). None of the patients in either group received revisional surgery. Aasheim 2009 found that similar numbers of patients in the gastric bypass and biliopancreatic diversion with duodenal switch groups underwent reoperation in the perioperative period of up to 30 days after surgery (two versus one, P = 1.000) and between the end of the perioperative period and one year post‐surgery (none versus three, P = 0.107). Three patients in the gastric bypass compared to seven patients in the biliopancreatic diversion with duodenal switch groups underwent a new surgical procedure between the end of the perioperative period and two years follow‐up, but this difference was not statistically significant (P = 0.155).
Hedberg 2012 reported that in the biliopancreatic diversion with duodenal switch group, one patient (4%) was readmitted to hospital for cholecystitis and three (13%) for incisional hernia repair. In the gastric bypass group, one patient (4%) was readmitted to hospital for abdominal pain and two (9%) for incisional hernia. Aasheim 2009 reported that similar numbers of patients in each group were readmitted to hospital during the perioperative period (4 patients in each group, P = 1.000), and between the end of the perioperative period and two years after surgery (7 patients in the gastric bypass group versus 16 patients in the biliopancreatic diversion with duodenal switch group, P = 0.28).
Surgery complications reported in Hedberg 2012 are shown in Appendix 9. Aasheim 2009 found that the number of patients with complications during the perioperative period or late complications were similar between groups. Aasheim 2009 found that the proportion of patients who experienced adverse events between surgery and two years follow‐up was higher in the biliopancreatic diversion with duodenal switch group than the gastric bypass group (62% (n = 18) versus 32% (n = 10), P = 0.021), with a variety of adverse events reported in each group (see Appendix 10).
5. Comparisons of different surgical procedures: laparoscopic gastric bypass versus laparoscopic duodenojejunal bypass with sleeve gastrectomy
One RCT with an uncertain risk of bias across all domains compared laparoscopic duodenojejunal bypass with sleeve gastrectomy against laparoscopic Roux‐en‐Y gastric bypass (Praveen Raj 2012).
Primary outcomes
Measures of weight change, fat content or fat distribution
At 12 months follow‐up there were no statistically significant differences in BMI (Analysis 5.1), excess weight loss Analysis 5.2), or percentage excess weight loss (Analysis 5.3) between laparoscopic duodenojejunal bypass with sleeve gastrectomy and laparoscopic Roux‐en‐Y gastric bypass. The quality of the evidence was very low.
5.1. Analysis.
Comparison 5 Laparoscopic gastric bypass versus laparoscopic duodenojejunal bypass with sleeve gastrectomy, Outcome 1 Mean BMI [kg/m2].
| Mean BMI [kg/m2] | ||||
|---|---|---|---|---|
| Study | Outcome | LRYGB | LDJB+SG | P value |
| Praveen Raj 2012 | Mean BMI at 12 months (SD) | 28.84 (1.57) | 28.19 (2.14) | P = 0.194 |
5.2. Analysis.
Comparison 5 Laparoscopic gastric bypass versus laparoscopic duodenojejunal bypass with sleeve gastrectomy, Outcome 2 Excess weight loss [kg].
| Excess weight loss [kg] | ||||
|---|---|---|---|---|
| Study | Outcome | LRYGB | LDJB+SG | P value |
| Praveen Raj 2012 | Excess weight loss at 12 months, kg, mean (SD) | 53.21 (6.04) | 51.40 (8.37) | P = 0.303 |
5.3. Analysis.
Comparison 5 Laparoscopic gastric bypass versus laparoscopic duodenojejunal bypass with sleeve gastrectomy, Outcome 3 Excess weight loss [%].
| Excess weight loss [%] | ||||
|---|---|---|---|---|
| Study | Outcome | LRYGB | LDJB+SG | P value |
| Praveen Raj 2012 | % EWL at 12 months, mean (SD) | 79.98 (4.77) | 81.94 (9.51) | P = 0.326 |
Health‐related quality of life
Health‐related quality of life was not assessed by Praveen Raj 2012.
Obesity‐related comorbidities
At baseline, 20 (71%) of participants in the laparoscopic duodenojejunal bypass with sleeve gastrectomy group and 16 (55%) in the laparoscopic Roux‐en‐Y gastric bypass group had diabetes. There were no statistically significant differences between groups in the proportion with a ‘complete remission’ or an ‘improvement’ in diabetes (Analysis 5.4). The study appeared to use different criteria for an improvement in diabetes in each arm, which may have a bearing on the results seen. Hypertension was seen in 36% and 41% of participants in the two groups respectively at baseline. There were no statistically significant differences between the two surgical procedures in the proportions of participants in the categories ‘remission’, ‘improvement’ or ‘no improvement’ of hypertension (Analysis 5.4). However, the timing of the assessment of these comorbidities was not stated in the trial publication. (Analysis 5.4). The quality of the evidence for diabetes was very low.
5.4. Analysis.
Comparison 5 Laparoscopic gastric bypass versus laparoscopic duodenojejunal bypass with sleeve gastrectomy, Outcome 4 Comorbidites.
| Comorbidites | ||||
|---|---|---|---|---|
| Study | Outcome | LRYGB | LDJB+SG | P value |
| Praveen Raj 2012 | Complete remission of type 2 diabetes, n (%) at 12 months | 13/16 (81) | 16/20 (80) | ns |
| Praveen Raj 2012 | Improvement in type 2 diabetes, n (%) at 12 months | 3/16 (19) | 4/20 (20) | ns |
| Praveen Raj 2012 | Remission of hypertension, n (%) at 12 months | 9/12 (75) | 8/10 (80) | ns |
| Praveen Raj 2012 | Improvement in hypertension, n (%) at 12 months | 2/12 (17) | 0/10 | ns |
| Praveen Raj 2012 | No improvement in hypertension, n (%) at 12 months | 1/12 (8) | 2/10 (20) | ns |
Secondary outcomes
Adverse events, mortality and revision rates
No deaths in either group were reported in the RCT by Praveen Raj 2012. One adverse event was reported in the laparoscopic duodenojejunal bypass with sleeve gastrectomy group only. This was an internal herniation through the retrocolic window one month after surgery. It is unclear if any other adverse events were measured or monitored during the study.
6. Comparisons of different surgical procedures: laparoscopic adjustable gastric banding versus laparoscopic isolated sleeve gastrectomy
One RCT (Himpens 2006) with an uncertain risk of bias across all domains compared laparoscopic adjustable gastric banding with laparoscopic isolated sleeve gastrectomy.
Primary outcomes
Measures of weight change, fat content or fat distribution
Himpens 2006 reported that the reduction in BMI was statistically significantly greater in participants in the laparoscopic isolated sleeve gastrectomy group than the laparoscopic adjustable gastric banding group three years after surgery (27.5 versus 18, P < 0.0004, Analysis 6.1). Weight loss (three years: 29.5 kg versus 17 kg, P < 0.0001, Analysis 6.2) and the proportion of excess weight loss at one year (57.7% versus 41.4%, P = 0.0004) and three years after surgery (66% versus 48%, P = 0.0025) (Analysis 6.3) were also statistically significantly improved in laparoscopic isolated sleeve gastrectomy participants in comparison to the laparoscopic adjustable gastric banding participants. All of these data were presented by the trial authors as medians and ranges, so care should be taken when interpreting the results. The quality of the evidence was very low.
6.1. Analysis.
Comparison 6 Laparoscopic adjustable gastric banding versus laparoscopic isolated sleeve gastrectomy, Outcome 1 BMI decrease.
| BMI decrease | ||||
|---|---|---|---|---|
| Study | Outcome | LAGB | LISG | P value |
| Himpens 2006 | BMI decrease at 1 year, median (range) | 15.5 (5 to 39) | 25 (0 to 45) | P < 0.0001 |
| Himpens 2006 | BMI decrease at 3 years, median (range) | 18 (0 to 39) | 27.5 (0 to 48) | P = 0.0004 |
6.2. Analysis.
Comparison 6 Laparoscopic adjustable gastric banding versus laparoscopic isolated sleeve gastrectomy, Outcome 2 Weight loss [kg].
| Weight loss [kg] | ||||
|---|---|---|---|---|
| Study | Outcome | LAGB | LISG | P value |
| Himpens 2006 | Weight loss at 1 year, kg, median (range) | 14 (‐5 to 38) | 26 (0 to 46) | P < 0.0001 |
| Himpens 2006 | Weight loss at 3 years, kg, median (range) | 17 (0 to 40) | 29.5 (1 to 48) | P < 0.0001 |
6.3. Analysis.
Comparison 6 Laparoscopic adjustable gastric banding versus laparoscopic isolated sleeve gastrectomy, Outcome 3 Excess weight loss [%].
| Excess weight loss [%] | ||||
|---|---|---|---|---|
| Study | Outcome | LAGB | LISG | P value |
| Himpens 2006 | % EWL at 1 year, median (range) | 41.4 (‐11.8 to 130.5) | 57.7 (0 to 125.5) | P = 0.0004 |
| Himpens 2006 | % EWL at 3 years, median (range) | 48 (0 to 124.8) | 66 (‐3.1 to 152.4) | P = 0.0025 |
Health‐related quality of life
Quality of life was not assessed by Himpens 2006.
Obesity‐related comorbidities
At baseline, gastro‐oesophageal reflux disease requiring drug therapy with proton pump inhibitors was a problem for 15% (6/40) of the laparoscopic adjustable gastric banding participants and 20% (8/40) of the participants in the laparoscopic isolated sleeve gastrectomy group. After one year, gastro‐oesophageal reflux disease had resolved in 83% and 75% of these participants in the two groups respectively, and this remained the same at three years (statistical significance was not reported) (Analysis 6.4). In those without gastro‐oesophageal reflux disease at baseline, no statistically significant differences in rates of appearance of gastro‐oesophageal reflux disease between the intervention groups were observed at one year (laparoscopic adjustable gastric banding 3/34 (8.8%), versus laparoscopic isolated sleeve gastrectomy 7/32 (21.8%), P = not significant (ns)) or three years [(laparoscopic adjustable gastric banding 7/34 (20.5%) versus laparoscopic isolated sleeve gastrectomy 1/32 (3.1%), P = ns).
6.4. Analysis.
Comparison 6 Laparoscopic adjustable gastric banding versus laparoscopic isolated sleeve gastrectomy, Outcome 4 Comorbidities: other.
| Comorbidities: other | ||||
|---|---|---|---|---|
| Study | Outcome | LAGB | LISG | P value |
| Himpens 2006 | Resolution of GERD, % | 83 | 75 | |
Secondary outcomes
Adverse events, mortality and revision rates
No early postoperative complications were seen in the laparoscopic adjustable gastric banding group of the Himpens 2006 RCT. Two participants in the laparoscopic isolated sleeve gastrectomy group (5%) had an early post operative complication; both required revisional surgery and in one this was a total gastrectomy due to gastric ischaemia (Appendix 9). Late complications requiring surgery were observed in the laparoscopic adjustable gastric banding participants, with three pouch dilations (treated with band removal in two and conversion to Roux‐en‐Y gastric bypass in one); one gastric erosion (treated with Roux‐en‐Y gastric bypass) and three disconnections of the port (treated with reconnection). There were no late complications requiring surgery in the laparoscopic isolated sleeve gastrectomy group. Complications not requiring surgery that were observed at one and three years can be seen in Appendix 10. There appeared to be higher frequencies of complications in the laparoscopic adjustable gastric banding group than in the laparoscopic isolated sleeve gastrectomy group but this is based on observation of the data only, as no statistical analysis was undertaken.
In addition, two participants in each group had ‘insufficient weight loss’ noted as a complication in the Himpens 2006 study. The two participants in the laparoscopic adjustable gastric banding group were converted to Roux‐en‐Y gastric bypass and the two participants in the laparoscopic isolated sleeve gastrectomy group were converted to laparoscopic duodenal switch.
7. Comparisons of different surgical procedures: laparoscopic gastric imbrication versus laparoscopic sleeve gastrectomy
One unpublished RCT (Sharma 2013) with a high risk of 'other' bias compared laparoscopic gastric imbrication with laparoscopic sleeve gastrectomy.
Primary outcomes
Measures of weight change, fat content or fat distribution
Sharma 2013 reported that there were no statistically significant differences in mean BMI (Analysis 7.1) or excess weight loss (Analysis 7.2) between those treated with laparoscopic gastric imbrication and those treated with laparoscopic sleeve gastrectomy at 12 months or at 3 years. The quality of the evidence was very low (GRADE).
7.1. Analysis.
Comparison 7 Laparaoscopic gastric imbrication versus laparoscopic sleeve gastrectomy, Outcome 1 Mean BMI [kg/m2].
| Mean BMI [kg/m2] | ||||
|---|---|---|---|---|
| Study | Outcome | Gastric imbrication | Sleeve gastrectomy | P value |
| Sharma 2013 | BMI, mean (SD) at 12 months | 35.3 (6.1) | 32.5 (5.8) | |
| Sharma 2013 | BMI, mean (SD) at 3 years | 36.9 (7.7) | 32.1 (5.9) | |
7.2. Analysis.
Comparison 7 Laparaoscopic gastric imbrication versus laparoscopic sleeve gastrectomy, Outcome 2 Excess weight loss.
| Excess weight loss | ||||
|---|---|---|---|---|
| Study | Outcome | Gastric imbrication | Sleeve gastrectomy | P value |
| Sharma 2013 | Excess weight loss (unit unclear) at 12 months, mean (SD) | 42.1 (13.0) | 53.8 (19.5) | |
| Sharma 2013 | Excess weight loss (unit unclear) at 3 years, mean (SD) | 39.5 (14.4) | 50.0 (20.3) | |
Health‐related quality of life
Health‐related quality of life was not assessed by Sharma 2013.
Obesity‐related comorbidities
Comorbidities were not reported by Sharma 2013.
Secondary outcomes
Adverse events, mortality and revision rates
No major complications were seen in the laparoscopic sleeve gastrectomy group of the Sharma 2013 trial. In the laparoscopic gastric imbrication group, two (16.7%) of participants had major complications requiring reoperation (Appendix 9), one of which was a conversion to a sleeve gastrectomy (Appendix 9). However, the authors noted that the surgeons were less experienced in this procedure.
Discussion
Summary of main results
Surgery versus non‐surgical interventions
Seven randomised controlled trials (RCTs) (one with a low risk of selection bias and six of uncertain risk of selection bias) were included. Regardless of the surgical intervention used or the type of participants included, all studies found statistically significant benefits on measures of weight change compared with no surgery at one to two years follow‐up. One RCT found more improvement in five of eight domains of the SF‐36 following laparoscopic adjustable gastric banding compared with no surgery, and one other found more improvement in two of the eight domains, but in only one of the two component scores (physical health). The RCTs of people with type 2 diabetes found significantly higher remission of the disease following surgery than conventional therapy or diet only. The effects of surgery on hypertension and lipids were less clear. All four of the RCTs reporting metabolic syndrome found significantly fewer people with the syndrome after surgery. One RCT of people with obstructive sleep apnoea found the proportion who achieved ‘mild’ sleep apnoea at follow‐up was higher in the laparoscopic adjustable gastric banding group than the conventional therapy group. All seven RCTs reported adverse events from surgery (e.g. operative interventions, revisional surgery, port site infection) and from conventional therapy (e.g. intolerance to medication, acute cholecystitis, need for operative intervention, gastrointestinal problems). Adverse events also occurred in the non‐surgery groups.
Comparisons of different surgical procedures
Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding
Three RCTs with uncertain risk of bias compared laparoscopic Roux‐en‐Y gastric bypass against laparoscopic adjustable gastric banding and showed that laparoscopic Roux‐en‐Y gastric bypass achieved significantly greater weight loss and BMI reduction up to five years after surgery compared to laparoscopic adjustable gastric banding. The laparoscopic Roux‐en‐Y gastric bypass procedure resulted in greater duration of hospitalisation and, in one RCT, a greater number of late major complications when compared with laparoscopic adjustable gastric banding. In another RCT, a high proportion of the laparoscopic adjustable gastric banding group required reoperation for band removal. The reliability of outcomes from one of the RCTs may be questionable because relatively large and unbalanced proportions of patients dropped out from each study group after randomisation.
Gastric bypass versus sleeve gastrectomy
Laparoscopic or open Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy all led to losses of weight and/or BMI but the seven included studies did not provide a clear and consistent picture as to which procedure was better or worse for achieving loss of weight or BMI. Overall, no statistically significant difference was found between the procedures. All studies had a high or uncertain risk of bias, generally with small sample sizes, limited duration of follow‐up and other methodological limitations.
Only one of the RCTs that compared Roux‐en‐Y gastric bypass with sleeve gastrectomy reported health‐related quality of life outcomes (Peterli 2012). This study found similar health‐related quality of life scores one year after surgery in both surgical groups. One death occurred in one of the four studies that reported mortality, and this was in the laparoscopic Roux‐en‐Y gastric bypass group (Peterli 2012). Comorbidities, complications and additional surgical procedures were reported in different ways in the different RCTs but they did not differ significantly between the surgery groups, except for improvement in pre‐existing gastro‐oesophageal reflux disease, which improved in proportionally more patients in the laparoscopic Roux‐en‐Y gastric bypass than laparoscopic sleeve gastrectomy group in the Peterli 2012 RCT.
The one RCT (Lee 2011) that compared simplified laparoscopic mini‐gastric bypass with duodenum exclusion against laparoscopic sleeve gastrectomy, found that gastric bypass may be superior to laparoscopic sleeve gastrectomy for weight loss and treating diabetes in patients with type 2 diabetes and a BMI of > 25 to < 35, whilst resulting in similar levels of complications. The risk of attrition bias in this study was judged to be low, but the risk of selection bias was uncertain and the risk of reporting bias was high.
Gastric bypass versus biliopancreatic diversion with duodenal switch
Two RCTs found that biliopancreatic diversion with duodenal switch resulted in greater weight loss than Roux‐en‐Y gastric bypass in people with a very high BMI. Limited comorbidity data were reported. In one RCT, biliopancreatic diversion with duodenal switch was associated with more improvement in HbA1c levels; in the other RCT, patient self‐reported sleep apnoea symptoms were similar between groups at two years. Adverse event rates, however, were higher with biliopancreatic diversion with duodenal switch and patients who underwent this procedure also experienced less improvement in the bodily pain domain of health‐related quality of life two years after surgery than patients who underwent gastric bypass. Hedberg 2012 had a high risk of performance bias, detection bias, reporting bias and other bias. Aasheim 2009 had a high risk of reporting bias and other bias.
Laparoscopic gastric bypass versus laparoscopic duodenojejunal bypass with sleeve gastrectomy
In one small RCT with an uncertain risk of bias, BMI and excess weight loss at 12 months follow‐up were similar between laparoscopic duodenojejunal bypass with sleeve gastrectomy and laparoscopic Roux‐en‐Y gastric bypass. Rates of remission of diabetes and hypertension were also similar between groups.
Laparoscopic adjustable gastric banding versus laparoscopic isolated sleeve gastrectomy
On measures of weight, participants undergoing laparoscopic isolated sleeve gastrectomy showed more improvement than participants undergoing laparoscopic adjustable gastric banding in a single RCT with an uncertain risk of bias. Early complications requiring surgery only occurred in the laparoscopic isolated sleeve gastrectomy group whilst late complications requiring surgery only occurred in the laparoscopic adjustable gastric banding group.
Laparoscopic gastric imbrication versus laparoscopic sleeve gastrectomy
One small unpublished RCT with a high risk of bias found no significant differences in weight‐loss outcomes between laparoscopic gastric imbrication versus laparoscopic sleeve gastrectomy. Health‐related quality of life and comorbidities were not reported.
Overall completeness and applicability of evidence
All 22 RCTs included in this review examined one or more of the currently most commonly performed bariatric surgery procedures in practice: gastric bypass, sleeve gastrectomy and adjustable gastric banding. The majority compared surgical with non‐surgical procedures (seven RCTs) or gastric bypass with sleeve gastrectomy (eight RCTs). The evidence‐base for comparisons of other surgical procedures was more limited, with only one to three RCTs available, making it difficult to draw conclusions about the relative effectiveness of some procedures.
The majority of participants included in the trials were women, and, on average, participants were in their early 30s to early 50s and were morbidly obese. However, greater benefit may occur among younger adults who have a longer period to accrue benefit, if weight loss and effects on comorbidity are maintained. Few studies included participants aged over 60 years so the findings may not be generalisable to older adults. Furthermore, expert opinion indicates the patient populations included in the studies may not fully represent those seen in clinical practice, because many focused on low risk patients, and, until recently in the UK, much surgery was performed on more unwell and generally more obese patients with more advanced complications.
Part of the objective of the review was to examine the effects of bariatric surgery on the control of obesity‐related comorbidities. Eighteen RCTs measured changes in comorbidities post‐surgery, but they differed in the conditions examined. Diabetes‐related outcomes and hypertension were most commonly assessed. However, there was variation in how studies measured and reported outcomes, making it difficult to compare findings. For example, measures of diabetes‐related outcomes included remission or improvement in diabetes or insulin resistance, use of diabetes medications, and the proportion of patients achieving specified HbA1c or fasting plasma glucose levels. Some studies used the term 'resolved' regarding type 2 diabetes (e.g. Angrisani 2007 and Liang 2013), however it should be noted that type 2 diabetes does not 'resolve'; it may go into remission but recurrence is fairly common over time. These studies also did not report the criteria used for defining ‘resolution’, making it uncertain how relevant the results are to clinical practice. Fewer RCTs examined sleep apnoea, metabolic syndrome, dyslipidaemia or normalisation of lipid profiles, gastroesophageal reflux disease (GERD), degenerative arthritis, menstrual irregularities, back or joint pain, hyperuricaemia, or depression, so there is currently only limited evidence for whether or not surgery is effective in treating these conditions. None of the studies examined longer‐term complications of diabetes, which are important treatment outcomes.
Few RCTs assessed the effectiveness of bariatric surgery in treating comorbidities in patients with a lower BMI. There is therefore a lack of evidence for the use of bariatric surgery in treating comorbidities in patients who are overweight or who do not meet standard criteria for bariatric surgery.
Only five of the RCTs included in this review reported any assessment of health‐related quality of life issues. It is therefore difficult to make any judgment about the impact of weight‐loss interventions on the health‐related quality of an obese person’s daily life.
An important question concerning interventions used in managing weight loss is whether the procedure offers a long‐lasting effect. Expert opinion suggests that follow‐up should consider outcomes beyond five years. The follow‐up period in all but one of the studies in this review ranged between one and four years, and was particularly short in the studies comparing surgery versus medical management. Only one study examined outcomes at 10 years post‐surgery (Angrisani 2007). Therefore, the longer‐term impact of surgery on weight loss or comorbidities is unclear. The short duration of the RCTs also meant that the impact of late complications (such as gastric ulcers, stomal stenosis and erosions, and band slippage) and the need for revisional surgery are likely to have been underestimated.
Expert opinion indicates that there are little data on outcomes with optimal treatment of control groups in the studies comparing surgery with non‐surgical interventions. They may therefore overestimate the benefits of surgery.
It was beyond the remit of this research to assess the impact of pre‐and post‐intervention education, counselling and support on the outcomes of the interventions. However, the majority of the studies included in this review did not provide such details which may be important for understanding patient compliance to the lifestyle and diet modifications that are necessary for successful weight‐loss maintenance.
Quality of the evidence
The review identified 22 relevant RCTs that included a total of 1798 participants, with seven RCTs comparing surgery to non‐surgical interventions (618 participants) and 15 RCTs comparing different surgical procedures (1180 participants). Many of the RCTs had an uncertain risk of bias as the reporting was unclear. Just one RCT reported adequate allocation concealment and was therefore at low risk of selection bias. The majority of studies did not mention whether outcomes assessors were blinded to intervention assignments. The reporting of incomplete outcome data for weight loss, health‐related quality of life or co‐morbidity was either unclear or judged to be of high risk of bias for most of the studies.
The overall quality of evidence was assessed using GRADE.
The quality of evidence for the comparison of surgery versus surgery was moderate. Quality was downgraded due to serious limitations in design or execution of the included RCTs (risk of bias).
The quality of evidence for the comparison of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding was moderate (BMI outcome) or very low (health‐related quality of life and diabetes outcomes). Quality was downgraded due to serious or very serious limitations in design or execution of the included RCTs (risk of bias), serious imprecision for diabetes outcomes, and suspected reporting bias for both health‐related quality of life and diabetes outcomes.
The quality of evidence for the comparison of laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy was low (BMI and diabetes outcomes) or very low (health‐related quality of life outcome). Quality was downgraded due to limitations in design or execution of the included RCTs (risk of bias), serious inconsistency in the BMI outcome, serious imprecision in health‐related quality of life and diabetes outcomes and suspected reporting bias in health‐related quality of life outcomes.
The quality of evidence for the comparison of gastric bypass versus biliopancreatic diversion with duodenal switch was moderate (BMI outcome) or very low (health‐related quality of life and diabetes outcomes). Quality was downgraded due to serious limitations in design or execution of the included RCTs (risk of bias), and serious imprecision and suspected reporting bias in health‐related quality of life and diabetes outcomes.
The quality of evidence for the comparisons of: laparoscopic gastric bypass versus laparoscopic duodenojejunal bypass with sleeve gastrectomy; laparoscopic adjustable gastric banding versus laparoscopic isolated sleeve gastrectomy; and laparoscopic gastric imbrication versus laparoscopic sleeve gastrectomy, was very low. Each of these comparisons was assessed by only one RCT, and quality was downgraded due to serious limitations in study design or execution of the included RCTs (risk of bias), serious imprecision and suspected reporting bias.
Potential biases in the review process
A strength of this review was that we carried out a comprehensive search of the literature, including one database of grey literature, minimising the risk of bias in study selection. A further strength was that we were able to perform meta‐analysis for some comparisons and outcomes. However, only one study was available for some comparisons, for example laparoscopic adjustable gastric banding versus laparoscopic isolated sleeve gastrectomy, precluding meta‐analysis. Even when the same procedures were compared by more than one RCT, limitations in the literature often prevented us from proceeding with meta‐analysis: there were often differences in the outcomes reported or the patient groups and interventions (in the case of the studies comparing surgery with non‐surgical interventions).
Overall, 11 of the 15 studies comparing different surgical procedures were included in one or more meta‐analytic quantitative comparisons, with two to six studies included in each comparison. Due to the small number of studies included in the meta‐analyses, only limited conclusions can be drawn from them. The small number of studies in each meta‐analysis also made it unfeasible for us to explore subgroup effects (e.g. BMI category or gender effects) or to conduct sensitivity analyses (e.g. to explore the impact of the quality or funding source on outcomes). We were also unable to assess publication biases due to the low number of studies available for each of the comparisons.
Deaths, adverse events and some complications are generally rare events and therefore it is not likely that evidence presented here provides reliable estimates of the incidence of these events since most of the RCTs were of a limited size and duration. Adverse events were also reported in a variety of ways across studies, making it difficult to compare between studies. Often no standard definitions or classification systems were used and it was unclear how comprehensive recording and reporting was. Deaths and reoperations were not reported in seven and eight, respectively, of the included studies. This may have led to an underestimate of some of the more frequently encountered complications such as failure of gastric bands, e.g. due to band slip or erosion, complications that usually necessitate band removal.
Within the review, types of surgery were broadly classified into types of procedures. Limited attention is given to the numerous modifications developed by different clinicians within these categories. We have also not investigated the impact of surgical team experience on outcomes, which may have affected the results of some studies, particularly those investigating newer procedures. We did, however, consider this to be an ‘other source’ of potential bias in the 'Risk of bias' assessments, and have therefore reported where this was the case when studies have made this information available.
Agreements and disagreements with other studies or reviews
In accordance with the previous version of this systematic review (Colquitt 2009), we found that surgery results in greater weight loss, reductions in some comorbidities and improvements in some aspects of health‐related quality of life than conventional treatment – this conclusion still holds when considering only the bariatric procedures currently in use in clinical practice in this updated review. The findings of other recent systematic reviews of RCTs and observational studies concur that surgery results in greater short‐term weight loss (Chan 2013; Gloy 2013; Moldovan 2011) and improvement in comorbidities (Chan 2013; Gloy 2013) than conventional treatment. High quality RCTs of the long‐term effects of surgery compared to conventional treatment, however, are still lacking. The wider literature suggests that conventional treatment may not be successful in promoting longer‐term beneficial outcomes. Data from the Swedish Obese Subjects (SOS) study (Sjöström 2013) – a large, prospective, controlled trial – indicates that at 10 to 20 years, surgery results in greater weight loss, lower overall mortality and reduced incidence of comorbidities than usual care, with the highest level of weight loss achieved at two years post‐surgery and some regains thereafter before overall weight loss stabilises at eight to 10 years. Another systematic review suggests weight‐management programmes result in small weight reductions in overweight and obese adults, but weight regain often occurs in the long term (Loveman 2011).
The number of RCTs comparing different surgical procedures has increased since our last review (Colquitt 2009), particularly those comparing sleeve gastrectomy and gastric bypass, but the evidence‐base remains limited meaning that again, few conclusions can be drawn about the relative effectiveness of different procedures from direct evidence. A network meta‐analysis by Padwal 2011a indicates that for weight loss, diversionary procedures are the most effective, followed by diversionary/restrictive procedures, with restrictive procedures resulting in the least weight loss. Other systematic reviews, including those incorporating non‐RCT evidence (O'Brien 2013b), support our finding that adjustable gastric banding results in less weight loss than gastric bypass, while resulting in fewer adverse effects (Padwal 2011b), but higher revision rates (O'Brien 2013), and that biliopancreatic diversion with duodenal switch results in more weight loss than gastric bypass (O'Brien 2013b). The RCT evidence in our review currently provides no clear indication about whether different procedures may have different benefits in improving comorbidities, although there is some indication from a systematic review of RCTs and observational studies (Meijer 2011) that proportionally more patients treated with Roux‐en‐Y gastric bypass experience reversal of diabetes than those treated with adjustable gastric banding.
In our review, the number of deaths reported by the included studies within the surgical trial arms ranged from 0% (none) to 4.2%, with the majority of studies reporting that no deaths occurred. Gloy 2013 similarly found in a recent systematic review of 11 studies comparing surgery with no surgery that no deaths occurred after surgery. However, due to the number of RCTs not reporting whether or not deaths occurred in our review, it remains uncertain if the RCT evidence is accurately capturing mortality rates. A systematic review and meta‐analysis of mortality in bariatric surgery, which included RCT and non‐RCT evidence, reported that total mortality at 30 days or less was 0.28% (95% CI 0.22 to 0.34) with restrictive operations having the lowest mortality (Buchwald 2007).
In line with our previous review (Colquitt 2009), we found there is still a need for RCTs to examine outcomes over longer‐time periods (at least five years), to include quality of life outcomes and use a more standardised approach to measuring and reporting important adverse events. We have identified and described relevant trials that were in progress as of November 2013. Of 12 ongoing studies identified, seven include people with varying degrees of obesity who also have type 2 diabetes and will contribute to the evidence of the effects of surgery in this group. Unfortunately only one of the 12 studies plans to follow patients for five years, therefore, evidence on the long‐term effects of surgery remains an unmet need.
Authors' conclusions
Implications for practice.
Surgery for obesity results in greater weight loss than conventional treatment in the short term (e.g. up to two years post‐surgery). Furthermore, the weight loss is associated with reductions in comorbidities, such as diabetes, metabolic syndrome and sleep apnoea, although the benefits for hypertension and improvement in lipid profiles are less clear. Compared to conventional treatment, surgery is also associated with greater short‐term improvements in some aspects of health‐related quality of life, but not others. Currently, there are no RCTs that examine the longer‐term effects of surgery in comparison with conventional treatment on weight loss, comorbidities (including the prevention of diabetes complications) and health‐related quality of life, so it is unclear if the benefits are maintained over time.
Surgery and conventional treatment were both associated with adverse effects. In the case of surgery, possible gains in health‐related quality of life need to be considered against the risks of reoperations and the possibility of postoperative mortality.
There are a number of different bariatric procedures available. Nine of these have been compared with other bariatric procedures in RCTs, but some of the comparisons were assessed by just one trial. The largest evidence base was for gastric bypass versus sleeve gastrectomy, which suggests that gastric bypass results in similar weight loss to sleeve gastrectomy. More limited evidence suggests that weight loss following gastric bypass is also similar to duodenojejunal bypass with sleeve gastrectomy, but greater than adjustable gastric banding. Other limited evidence suggests that biliopancreatic diversion with duodenal switch seems to result in more weight loss than gastric bypass in morbidly obese patients, that isolated sleeve gastrectomy appears to result in greater weight loss than adjustable gastric banding and that simplified laparoscopic mini‐gastric bypass with duodenum exclusion results in greater weight loss than sleeve gastrectomy in people with a lower BMI. One small trial at a high risk of bias indicates that gastric imbrication and sleeve gastrectomy may be similarly effective in reducing weight. Regarding the treatment of comorbidities, simplified laparoscopic mini‐gastric bypass with duodenum exclusion appears to be more effective in treating diabetes than sleeve gastrectomy in people with a low BMI. Apart from this, there was no clear indication from the evidence whether any procedure was more effective than another in controlling comorbidities.
Data on the comparative safety of the bariatric procedures were limited. All procedures were associated with adverse events, but many of the comparisons of different procedures showed no clear pattern that any of the interventions are associated consistently with particular adverse events. Limited evidence suggests biliopancreatic diversion with duodenal switch is associated with a higher rate of adverse events than gastric bypass. Limited evidence also indicates that Roux‐en‐Y gastric bypass results in more complications than adjustable gastric banding, but adjustable gastric banding has a higher need for reoperation.
Due to the limited evidence and poor quality of the trials, caution is required when interpreting the comparative safety and effectiveness of these procedures.
Implications for research.
There continues to be a need for good‐quality, long‐term RCTs comparing different operative techniques and surgery with conventional treatment for obesity that include an assessment of patient health‐related quality of life. Expert opinion suggests that follow‐up should consider outcomes beyond five years.
There is also a need for RCTs that examine the long‐term effectiveness of surgery in controlling comorbidities, particularly to ascertain whether the short‐term favourable benefits for surgery compared to conventional treatment found in this review persist over time. Control groups in these studies need to be optimally treated, with surgery compared with the current standard of care. We did not identify any studies that examined the impact of surgery on longer‐term complications of type 2 diabetes and we recommend that researchers consider measuring these outcomes in future studies.
The evidence base for the clinical effectiveness of bariatric surgery for treating patients who do not meet standard eligibility criteria for bariatric surgery, including adults with a lower BMI and comorbidities such as type 2 diabetes, is very limited. Further good‐quality RCTs are required to provide clinical effectiveness and health‐related quality of life evidence for this population, which might help inform clinicians' decisions about when might be the right time to perform surgery for optimal outcomes (e.g. when patients are relatively fit without complications versus when they have more advanced complications). Studies recruiting younger and older adults are also needed, as evidence is lacking for these groups.
Assessing the risks of different bariatric procedures is still hampered by a lack of consistency in the reporting of adverse outcomes. A core set of important adverse outcomes should be identified so that a standardised approach to describing adverse outcomes can be developed. All studies should report whether or not deaths occurred and the number of patients who underwent reoperations.
Overall, there is a need for researchers to improve their reporting of methodological features of primary studies, such as allocation concealment, blinding of outcome assessors, how incomplete outcome data were dealt with and whether or not intention‐to‐treat analyses were used.
Feedback
Eligibility of studies, 4 June 2009
Summary
First, I would like to congratulate you for this well‐written review. I have a simple question. Although your literature searches lasted until July 2008, two key articles published in 2007 are neither included nor mentioned as being excluded: 1. Sjoestroem et al., N Engl J Med 2007; 357(8): 741‐52. 2. Adams et al., N Engl J Med 2007; 357(8): 753‐61. Perhaps you can provide a reason, why these influential articles are not referenced. Yours sincerely, Stefan Sauerland.
Reply
Thank you for your comment.
1. The reference by Sjostrom and colleagues 2007 belongs to the included SOS 1997‐2007 study, and data from this publication are summarised in Table 9. However, this reference and three others from the SOS 1997‐2007 study appear to have been omitted in error. They have now been added to the included studies list, so thank you for bringing this to our attention.
2. The study by Adams and colleagues 2007 was a retrospective cohort study, and as such was excluded at the initial screening of titles and abstracts. With over 5000 references identified by our searches it is not possible to list all the potentially relevant excluded studies.
Contributors
Comments made by Dr. Stefan Sauerland (stefan.sauerland@ifom‐uni‐wh.de).
Jill Colquitt replied to the comments on behalf of the review authors for the review.
What's new
| Date | Event | Description |
|---|---|---|
| 13 October 2014 | Amended | Minor corrections of plain language summary |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 1 February 2014 | New search has been performed | Third update of first version published in 2003 |
| 1 February 2014 | New citation required and conclusions have changed | Study selection criteria changed. Findings from new studies included. Conclusions changed. New review authors. Title changed from 'Surgery for obesity' to 'Surgery for weight loss in adults'. |
| 20 July 2009 | Feedback has been incorporated | Added four references to the 'SOS 1997‐2007' study that had previously been omitted from the reference list. |
| 27 October 2008 | New citation required and conclusions have changed | Substantive amendment |
| 27 October 2008 | New search has been performed | Title changed. Study selection criteria changed. Findings from new studies included. Authors changed. |
| 15 August 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
An earlier version of this project was funded by the NIHR Health Technology Assessment Programme (project number 08/06/01) and was published in full in Health Technology Assessment. See the HTA Programme website (www.hta.ac.uk) for further project information.
We would like to acknowledge the contribution of four authors who contributed to earlier versions of this review: Pamela Royle (searching for trials, quality assessment, data extraction); Manpreet Sidhu (selection of studies, data extraction); Joanna Picot (protocol draft, selection of studies, data extraction and review drafting) and Andy Clegg (protocol draft, selection of studies, data extraction and review drafting).
We would like to thank members of our advisory group panel who provided expert advice and comments on this version of the systematic review: Professor John Wilding, Professor of Medicine & Honorary Consultant Physician, University Hospital Aintree, Liverpool; Ms Jane Munro, Project Manager/Research Lead, Surgical Obesity Treatment Study (SCOTS), University of Glasgow; Mr Ken Clare, Service User (KC is chair of trustees of weight‐loss surgery information and support, a registered charity. He is a self‐employed bariatric nurse and a council member of BOMSS); Mr David Galloway, Surgeon, Gartnavel General Hospital, Glasgow; Mr Afshin Alijani, Surgical lead for bariatric surgery in Tayside.
We are grateful to Karen Welch, information specialist (SHTAC, University of Southampton), for conducting the update searches and Angela Vincent (Research Programme Administrator, SHTAC) for retrieving references.
Appendices
Appendix 1. Search strategies
| Search terms and databases |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (MEDLINE medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) substitutes one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent (i.e. number of words within range of search term). |
| The Cochrane Library |
| #1 MeSH descriptor: [Obesity] explode all trees #2 MeSH descriptor: [Overweight] this term only #3 MeSH descriptor: [Weight Loss] explode all trees #4 (obes* or overweight or "over weight")(obes* or overweight or "over weight") #5 #1 or #2 or #3 or #4#1 or #2 or #3 or #4 #6 MeSH descriptor: [Bariatric Surgery] explode all trees #7 (bariatric near/5 surg*)(bariatric near/5 surg*) #8 (obes* near/5 surg*)(obes* near/5 surg*) #9 ((antiobesity or anti‐obesity or "anti obesity") near/5 (surg*))((antiobesity or anti‐obesity or "anti obesity") near/5 (surg*)) #10(gastroplasty or gastrogastrostomy or gastro?gastrostomy or gastroenterostomy or "gastric bypass" or "gastric surgery" or "restrictive surgery") #11 MeSH descriptor: [Gastric Bypass] explode all trees #12 MeSH descriptor: [Jejunoileal Bypass] explode all trees #13 ((jejunoileal or "jejuno‐ilial" or "jejuno ilial") next (bypass))((jejunoileal or "jejuno‐ilial" or "jejuno ilial") next (bypass)) #14 gastrointestinal next surg*gastrointestinal next surg* #15 gastrointestinal next diversion*gastrointestinal next diversion* #16 biliopancreatic diversionbiliopancreatic diversion #17 MeSH descriptor: [Biliopancreatic Diversion] #18 "gastric band*""gastric band*" #19 "silicon band*""silicon band*" #20 MeSH descriptor: [Gastroenterostomy] explode all trees #21 gastrectomygastrectomy #22 MeSH descriptor: [Gastroplasty] explode all trees #23 LAGB:ti,abLAGB:ti,ab #24 stomach near/5 stapl*stomach near/5 stapl* #25 gastric near/5 stapl*gastric near/5 stapl* #26 lap next band*lap next band* #27 mason* next proceduremason* next procedure #28 "roux‐en‐Y""roux‐en‐Y" #29 MeSH descriptor: [Anastomosis, Roux‐en‐Y] explode all trees #30 #malabsorpti* next procedure* #31 malabsorpti* next surg* #32r duodenal next switch* #33 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 #34 #5 and #33 |
| MEDLINE |
|
| EMBASE |
| 1 exp OBESITY/ or exp MORBID OBESITY/ 2 over?weight.ti,ab. 3 over weight.ti,ab. 4 overeating.ti,ab. 5 over?eating.ti,ab. 6 exp Weight Reduction/ 7 (weight adj1 los*).ti,ab. 8 (weight adj1 loos*).ti,ab. 9 weightloss.ti,ab. 10 weight?loss.ti,ab. 11 (weight adj3 reduc*).ti,ab. 12 weight?reduc*.ti,ab. 13 or/1‐12 14 "bariatric surg*".ti,ab. 15 exp Bariatric Surgery/ 16 (surg* adj5 bariatric).ti,ab. 17 (anti?obesity adj3 surg*).ti,ab. 18 (antiobesity adj3 surg*).ti,ab. 19 anti obesity surg*.ti,ab. 20 (obesity adj5 surgery).ti,ab. 21 (obesity adj5 surgical).ti,ab. 22 (gastroplasty or gastrogastrostomy or gastro?gastrostomy or gastroenterostomy or "gastric bypass" or "gastric surgery" or "restrictive surgery").ti,ab. 23 exp Stomach Bypass/ 24 exp Jejunoileal Bypass/ 25 jejuno?ileal bypass.ti,ab. 26 jejunoileal bypass.ti,ab. 27 gastrointestinal surg*.ti,ab. 28 gastrointestinal diversion*.ti,ab. 29 (gastro‐intestinal adj5 diversion).ti,ab. 30 exp Biliopancreatic Bypass/ 31 Biliopancreatic Bypass.ti,ab. 32 Biliopancreatic diversion.ti,ab. 33 bilio?pancreatic diversion.ti,ab. 34 bilio?pancreatic bypass.ti,ab. 35 gastric band*.ti,ab. 36 exp Gastric Banding/ 37 silicon band*.ti,ab. 38 exp GASTROENTEROSTOMY/ 39 gastroenterostomy.ti,ab. 40 exp GASTRECTOMY/ 41 gastrectomy.ti,ab. 42 exp GASTROPLASTY/ 43 LAGB.ti,ab. 44 stomach stapl*.ti,ab. 45 gastric stapl*.ti,ab. 46 lap band*.ti,ab. 47 lap‐band*.ti,ab. 48 malabsorptive surg*.ti,ab. 49 mason* procedure.ti,ab. 50 "roux‐en‐Y".ti,ab. 51 exp Roux y Anastomosis/ 52 malabsorpti* procedure*.ti,ab. 53 malabsorpti* surg*.ti,ab. 54 duodenal switch*.ti,ab. 55 or/14‐54 56 13 and 55 57 OBESITY/su [Surgery] 58 Morbid Obesity/su [Surgery] 59 57 or 58 60 13 and 59 61 56 or 60 62 Randomized Controlled Trial/ 63 Randomization/ 64 Single Blind Procedure/ 65 Double Blind Procedure/ 66 ((single or doubl* or trebl* or tripl*) adj (mask* or blind*)).tw. 67 (placebo* and control* and trial*).tw. 68 randomi?ed control* trial*.tw. 69 (random* adj2 allocat*).tw. 70 (placebo* and random* and (trial* or study or studies)).tw. 71 (randomized or randomised).tw. 72 Controlled Clinical Trial/ 73 Meta Analysis/ 74 (meta‐analys* or meta analys* or metaanalys*).tw. 75 (systematic* adj3 review*).tw. 76 health technology assessment*.ti,ab,in. 77 biomedical technology assessment/ 78 or/62‐77 79 61 and 78 80 limit 79 to yr="2010 ‐Current" 81 limit 80 to human |
| CINAHL |
| S34 .S28 OR S30 OR S32 S33 .S28 OR S30 OR S32 S32 .S26 AND S31 S31 .S7 OR S8 S30 .S18 AND S26 AND S29 S S29 .(MH "Body Mass Index") S28 .S19 AND S26 S27 .S19 AND S26 S26 .S20 OR S21 OR S22 OR S23 OR S24 OR S25 S25 .(MH "Placebos") S24 .TX placebo* AND TX control* S23 .TX random* AND TX control* S22 .TX randomized OR TX randomised S21 .(MH "Random Assignment") S20 .(MH "Randomized Controlled Trials") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") S19 .S6 AND S18 S18 .S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 S17 .(MH "Anastomosis, Roux‐en‐Y") S16 .TX "roux‐en‐Y" S15 .TX LAGB S14 .TX "silicon band*" OR TX "lap band*" OR TX "gastric band*" S13 .TX "biliopancreatic diversion" OR TX "biliopancreatic bypass" S12 .TX "jejunoileal bypass" S11 .(MH "Jejunoileal Bypass") S10 .(MH "Gastric Bypass") S9 .TX .(gastroplasty or gastrogastrostomy or "gastro‐gastrostomy" or gastroenterostomy or "gastric bypass" or "gastric surgery" or "restrictive surgery" or gastrectomy) S8 .bariatric N/3 surg* S7 .(MH "Bariatric Surgery+") S6 .S1 OR S2 OR S3 OR S4 OR S5 S5 .TX "weight loss" S4 .(MH "Weight Loss") S3 .TX overweight OR TX "over weight" OR TX "over‐weight" S2 .TX obes* S1 .(MH "Obesity+") Search modes ‐ Boolean/Phrase |
| PsychINFO |
| S1 .DE "Obesity" S2 .TX obes* S3 .DE "Overweight" S4 .TX overweight OR TX "over weight" S5 .DE "Weight Loss" S6 .TX "weight loss" S7 .S1 OR S2 OR S3 OR S4 OR S6 S8 .S1 OR S2 OR S3 OR S4 OR S6 S9 .DE "Bariatric Surgery" S10 .TX bariatric surg* S11 .TX (gastroplasty or gastrogastrostomy or gastro?gastrostomy or gastroenterostomy or "gastric bypass" or "gastric surgery" or "restrictive surgery S12 .TX "gastric bypass" Limiters ‐ Publication Year from: 2010‐2013 S13 .TX "jejunoilial bypass" OR TX "biliopancreatic bypass" S14 .TX Gastrectomy S15 .TX "roux en‐Y" S16 .TX gastric band* Limiters ‐ Publication Year from: 2010‐2013 S17 .TX silicon band Limiters ‐ Publication Year from: 2010‐2013 S18 .TX LAGB S19 .S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 S20 .S8 AND S19 S21 .TX (random* and (trial* or study or studies or allocat S22 .TX randomized OR TX randomised S23 .DE "Placebo" S24 .TX (placebo* and control* and trial*) S25 .TX (placebo* and control* and stud*) S26 .TX ((single or doubl* or trebl* or tripl*) N5 (mask* or blind*)) S27 .DE "Clinical Trials" S28 .S21 OR S22 OR S24 OR S25 OR S26 OR S27 S29 .S20 AND |
| Web of Knowledge SCI‐EXPANDED, and CPCI‐S |
| # 1 52,600 TS=(obes*) # 2 3,262 TS=(gastroplasty or gastrogastrostomy or gastroenterostomy or "gastric bypass" or "gastric surgery" or "restrictive surgery") # 3 360 TS=("gastrointestinal diversion*" or "biliopancreatic diversion") # 4 975 TS=("gastric band*" or "silicon band*") # 5 1 TS=("stomach stapl*") # 6 1,612 TS=( "Roux‐en‐Y") # 7 132 TS=(malabsorpti* procedure*) # 8 9 TS=("malabsorpti* surg*") # 9 256 TS=("duodenal switch") # 10 265 TS=(LAGB) # 11 4 TS=("mason* procedure") # 12 3,748 TS=(bariatric near surg*) # 13 6,107 #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 # 14 3,448 #13 AND #1 # 15 113,963 TS=(random* NEAR (trial* or study or studies or allocat*)) # 16 115,239 TS=(randomized or randomised) # 17 33,131 TS=((single or doubl* or trebl* or tripl*) NEAR (mask* or blind*)) # 18 19,607 TS=(placebo* and control* and trial*) # 19 16,602 TS=(placebo* and control* and stud*) # 20 147,128 #19 OR #18 OR #17 OR #16 OR #15 # 21 394 #20 AND #14 |
| Zetoc British Library |
| Bariatric surg* in title and random* in any field Gastric band* in title and random* in any field |
Appendix 2. Description of interventions
| Intervention(s) | Comparator(s) | |
| Aasheim 2009 | Laparoscopic Roux‐en‐Y gastric bypass | Laparoscopic biliopancreatic diversion with duodenal switch |
| Angrisani 2007 | Laparoscopic Roux‐en‐Y gastric bypass | Laparoscopic adjustable gastric banding |
| Demerdash 2013 | Laparoscopic Roux‐en‐Y gastric bypass | Laparoscopic adjustable gastric banding |
| Dixon 2008 | Laparoscopic adjustable gastric banding | Conventional therapya |
| Dixon 2012 | Laparoscopic adjustable gastric banding | Conventional therapya |
| Hedberg 2012 | Biliopancreatic diversion with duodenal switch | Roux‐en‐Y gastric bypass |
| Himpens 2006 | Laparoscopic gastric banding | Laparascopic isolated sleeve gastrectomy |
| Ikramuddin 2013 | Roux‐en‐Y gastric bypass | Lifestyle programme with medical managementb |
| Karamanakos 2008 | Laparoscopic Roux‐en‐Y gastric bypass | Laparoscopic sleeve gastrectomy |
| Keidar 2013 | Laparoscopic Roux‐en‐Y gastric bypass | Laparoscopic sleeve gastrectomy |
| Lee 2011 | Laparoscopic gastric bypass with duodenum exclusion | Laparoscopic sleeve gastrectomy without duodenum exclusion |
| Liang 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 1) Usual carec 2) Exenatide (drug therapy) + usual care |
| Mingrone 2012 | 1) Gastric bypass 2) Biliopancreatic diversion |
Medical therapyd |
| Nguyen 2009 | Laparoscopic Roux‐en‐Y gastric bypass | Laparoscopic adjustable gastric banding |
| Nogues 2010 | Laparoscopic Roux‐en‐Y gastric bypass | Laparoscopic sleeve gastrectomy |
| O'Brien 2006 | Laparoscopic adjustable gastric band (Lap‐Band system) | Intensive non‐surgical programmee |
| Paluszkiewicz 2012 | Roux‐en‐Y gastric bypass | Laparoscopic sleeve gastrectomy |
| Peterli 2012 | Laparoscopic Roux‐en‐Y gastric bypass | Laparoscopic sleeve gastrectomy |
| Praveen Raj 2012 | Laparoscopic duodenojejunal bypass with sleeve gastrectomy | Laparoscopic Roux‐en‐Y gastric bypass |
| Schauer 2012 | 1) Laparoscopic Roux‐en‐Y gastric bypass 2) Laparoscopic sleeve gastrectomy |
Intensive medical therapyf |
| Sharma 2013 | Laparoscopic sleeve gastrectomy | Laparoscopic gastric Imbrication |
| Vix 2013 | Laparoscopic Roux‐en‐Y gastric bypass | Laparoscopic sleeve gastrectomy |
|
aBest medical practice for treatment, education and follow‐up of type 2 diabetes. Visits at least every 6 weeks throughout the 2 years. Lifestyle modification programs individually structured to reduce energy intake, fat (< 30%) and saturated fats, to encourage low glycaemic index and high fibre foods. Physical activity advice to encourage 10,000 steps per day and 200 minutes per week of structured activity. Low calorie diets and medications discussed with all participants and used in some cases.
bLifestyle modification designed to produce maximum achievable weight loss and medications to control glycaemia and cardiovascular disease risk factors while facilitating weight loss. Used only US Food and Drug Administration‐approved medications. Included regular counselling meetings with a trained interventionist to discuss strategies for facilitating weight management and increasing physical activity, including self‐monitoring, stimulus control, problem solving, social support, cognitive behavior modification, recipe modification, eating away from home, and relapse prevention.
cPatients were assessed and treated by a multidisciplinary team that included an endocrinologist, a dietitian, a cardiologist, and a nurse. Medical therapy was adjusted according to the seven‐point glycaemic profile during the first 3 months and according to HbA1c levels thereafter. The dose of oral hypoglycaemic medications, antihypertensive drugs and insulin was optimised on an individual basis with the aim of reaching HbA1c < 7% and blood pressure (BP) 140/90 mm Hg. The nutrition goal was based on an individual energy intake and reducing fat intake to < 30%, saturated fat to < 10% and increasing high fibre intake and for physical exercise 30 min of brisk walking every day associated with moderate‐intensity aerobic activity twice a week.
dTreated by a multidisciplinary team including a diabetologist, dietitian and nurse, visits at baseline, 1, 3, 6,9,12 and 24 months. Oral hypoglycaemic agents and insulin doses optimised on an individual basis to reach a glycosylated haemoglobin A1c levels < 7%. Programs for diet and lifestyle modification, including reduced overall energy and fat intake (details provided) and increased physical exercise. eThe non surgical programme centred on the use of behavioural modification, very‐low‐calorie diet, and pharmacotherapy with education and professional support on appropriate eating and exercise behaviour. The programme began with a 6 month VLCD (500 ‐ 550 kcal/d) which used Optifast for 12 weeks, then over 4 weeks some VLC meals with 120 mg orlistat before the non‐VLC meals, and then 120 mg orlistat before all meals. The 6 month intensive phase was followed by further courses of VLCD or orlistat as tolerated, as well as continual behavioural, dietary, and exercise advice. Physician saw each patient every 2 weeks during the VLCD programme, and every 4 ‐ 6 weeks during the rest of the study. Common programme: all patients were instructed and encouraged to follow appropriate lifestyle behaviour of good eating practices and increased exercise and activity. All participants were encouraged to exercise for at least 200 minutes a week. fLifestyle counselling, weight management, home glucose monitoring, new drug therapies, sessions with a diabetes speciality educator, encouraged to participate in weight watchers. | ||
Appendix 3. Baseline characteristics (I)
| Intervention(s) and comparator(s) | Mean duration of follow‐up | Description of participants | Year(s) of study [year to year]a | Country | Setting | Ethnic groups [%] | Duration of condition [mean/range years (SD), or as reported] | |
| Aasheim 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 2 years | BMI 50‐60 | 2006‐2009 | Norway, Sweden | Public health care centres | "Europoid" 95 | ‐ |
| Laparoscopic biliopancreatic diversion with duodenal switch | ||||||||
| Angrisani 2007 | Laparoscopic Roux‐en‐Y gastric bypass | 10 years | BMI > 35 to < 50 | 2000 (randomisation) | Italy | ‐ | ‐ | ‐ |
| Laparoscopic adjustable gastric banding | ||||||||
| Demerdash 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 1 year | BMI > 40, or > 35 with comorbidities | 2008‐2010 | Egypt | Hospital surgical department | ‐ | ‐ |
| Laparoscopic adjustable gastric band | ||||||||
| Dixon 2008 | Laparoscopic gastric banding in addition to the conventional therapy | 2 years | BMI 30‐40, type 2 diabetes ≤ 2 years | 2002‐2006 | Australia | University research centre | ‐ | ‐ |
| Conventional therapy | ||||||||
| Dixon 2012 | Laparoscopic adjustable gastric banding | 2 years | BMI >35 and <55 with recently diagnosed obstructive sleep apnoea and apnoea‐hypopnoea index of ≥20 events/hour | 2006‐2009 | Australia | 1‐2 day outpatient or inpatient | ‐ | ‐ |
| Conventional therapy | ‐ | |||||||
| Hedberg 2012 | Biliopancreatic diversion with duodenal switch | 4 years | BMI > 48 | 2004‐2007 (recruitment) | Sweden | Secondary care (after 2 years in primary care) | ‐ | ‐ |
| Roux‐en‐Y gastric bypass | ||||||||
| Himpens 2006 | Laparoscopic gastric banding | 3 years | ‐ | 2002 (surgery) | Belgium | ‐ | ‐ | ‐ |
| Laparascopic isolated sleeve gastrectomy | ||||||||
| Ikramuddin 2013 | Laparoscopic Roux‐en‐Y gastric bypass + lifestyle programme with medical management | 1 year | BMI 30.0 to 39.9, type 2 diabetes, inadequate glycaemic control | 2008‐2011 | Taiwan and USA | Teaching hospitals | Non‐Hispanic white 55, East Asian 27, non‐Hispanic black 8, Hispanic 7, native American 3, other 0 | Years since diabetes diagnosis = 8.9 (6.1) |
| Lifestyle programme with medical management | Non‐Hispanic white 50, East Asian 28, non‐Hispanic black 10, Hispanic 7, native American 2, other 3 | Years since diabetes diagnosis = 9.1 (5.6) | ||||||
| Karamanakos 2008 (including Kehagias 2011) | Laparoscopic Roux‐en‐Y gastric bypass | 3 years | BMI ≤ 50 | 2005‐2007 (recruitment) | Greece | ‐ | Greek 100 | ‐ |
| Laparoscopic sleeve gastrectomy | ||||||||
| Keidar 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 12 months | BMI > 35, type 2 diabetes | 2008‐2010 | Israel | Obesity clinic | ‐ | 5.4 (5.0) |
| Laparoscopic sleeve gastrectomy | 6.7 (5.3) | |||||||
| Lee 2011 | Simplified laparoscopic mini‐gastric bypass with duodenum exclusion | 1 year | BMI > 25 to < 35, poorly controlled type 2 diabetes | 2007‐2009 | Taiwan | Secondary care | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy without duodenum exclusion | ||||||||
| Liang 2013 | Usual care | 1 year | BMI > 28, type 2 diabetes, hypertension | 2008‐2011 (recruitment) | China | Secondary care | ‐ | Type 2 diabetes: 7.24 (1.61) Hypertension: 8.15 (0.96) |
| Usual care + exenatide | Type 2 diabetes: 7.17 (1.80) Hypertension: 7.78 (1.47) | |||||||
| Laparoscopic Roux‐en‐Y gastric bypass | Type 2 diabetes: 7.39 (1.69) Hypertension: 7.94 (1.58) | |||||||
| Mingrone 2012 | Gastric bypass | 2 years | BMI ≥35, type 2 diabetes | 2009‐2011 (recruitment) | Italy | Diabetes day clinic | ‐ | 6.03 (1.18) |
| Medical therapy | 6.08 (1.24) | |||||||
| Nguyen 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 4 years | BMI 40‐60 or 35 with comorbidities | 2002‐2007 (recruitment) | USA | Bariatric surgery clinic | ‐ | ‐ |
| Laparoscopic adjustable gastric banding | ||||||||
| Nogues 2010 | Laparascopic Roux‐en‐Y gastric bypass | 1 year | BMI > 40 or > 35 with comorbidity | 2007‐2008 (recruitment) | Spain | Secondary care | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | ||||||||
| O'Brien 2006 | Laparoscopic adjustable gastric band | 2 years | BMI 30‐35 with obesity‐related co‐morbidity/problem | 2000‐2003 | Australia | Private community hospital | ‐ | ‐ |
| Intensive non‐surgical programme | ‐ | |||||||
| Paluszkiewicz 2012 | Roux‐en‐Y gastric bypass | 1 year | BMI ≥ 40 or ≥ 35 with comorbidity | 2008‐2011 (recruitment) | Poland | Secondary care | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | ||||||||
| Peterli 2012 | Laparoscopic Roux‐en‐Y gastric bypass | 3 years | BMI > 40, or BMI > 35 with comorbidity | 2007‐2011 | Switzerland | Secondary care | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | ||||||||
| Praveen Raj 2012 | Laparoscopic duodenojejunal bypass with sleeve gastrectomy | 1 year | BMI > 37 or > 32 with comorbidities | 2009‐2010 | India | ‐ | ‐ | ‐ |
| Laparoscopic Roux‐en‐Y gastric bypass | ||||||||
| Schauer 2012 | Laparoscopic Roux‐en‐Y gastric bypass + intensive medical therapy | 1 year | Type 2 diabetes, BMI 27 to 43 | 2007‐2011 (recruitment) | USA | ‐ | White 74 | 8.2 (5.5) |
| Laparoscopic sleeve gastrectomy + intensive medical therapy | White 72 | 8.5 (4.8) | ||||||
| Intensive medical therapy alone | White 74 | 8.9 (5.8) | ||||||
| Sharma 2013 | Laparoscopic gastric imbrication | 3 years | BMI > 40, or > 35 with ≥ 1 comorbidity | Started 2009 | India | 'ASIAN Surgical Centre' | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | ||||||||
| Vix 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 1 year | BMI 40‐60 | Started 2009 | France | Secondary care (hospital surgical department) | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | ||||||||
| "‐" denotes not reported aIn some cases the study period reported by the authors excludes follow‐up (e.g. refers to recruitment or surgery period only) | ||||||||
Appendix 4. Baseline characteristics (II)
| Intervention(s) and comparator(s) | Sex [female %] | Age [mean years SD)/range] | BMI [mean kg/m² (SD)] | Weight [mean kg (SD)] | Co‐medications / Co‐interventions [%] | Co‐morbidities [N, % or as stated] | |
| Aasheim 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 74 | 35 (7) | 54.8 (3.2) | 162 (24) | ‐ | N (rounded %): type 2 diabetes 6 (19), joint pain 16 (52), depression 5 (16), hypertension 8 (26), asthma 8 (26), urinary incontinence 5 (16), sleep apnoea 5 (16), GERD 5 (16), diabetes mellitus 5 (16), hypothyroidism 3 (10), gallstones 2 (7), hyperlipidaemia 0 (0), gout 1 (3) |
| Laparoscopic biliopancreatic diversion with duodenal switch | 66 | 36 (5) | 55.2 (3.5) | 162 (20) | ‐ | N (rounded %): type 2 diabetes 6 (21), joint pain 13 (45), depression 12 (41), hypertension 8 (28), asthma 5 (17), urinary incontinence 7 (24), sleep apnoea 6 (21), GERD 4 (14), diabetes mellitus 3 (10), hypothyroidism 3 (10), gallstones 1 (3), hyperlipidaemia 3 (10), gout 1 (3) | |
| all: | 70 | ‐ | ‐ | ‐ | ‐ | ||
| Angrisani 2007 | Laparoscopic Roux‐en‐Y gastric bypass | 83 | 34.1 (8.9) | 43.8 (4.1) | 118.2 (13.2) | ‐ | N: 2 hyperlipaemia, 1 hypertension, 1 type 2 diabetes |
| Laparoscopic adjustable gastric banding | 81 | 33.8 (9.1) | 43.4 (4.2) | 117.1 (12.8) | ‐ | N: 3 hypertension, 1 sleep apnoea | |
| Demerdash 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 89a | 39 (4.5) | 46.2 (2.56) | 142.7 (22.6) | ‐ | ‐ |
| Laparoscopic adjustable gastric band | 83 | 37 (6) | 45.8 (2.7) | 138.7 (22.98) | ‐ | ‐ | |
| Dixon 2008 | Laparoscopic gastric banding in addition to the conventional therapy | 50 | 46.6 (7.4) | 37.0 (2.7) | 105.6 (13.8) | n/N (%)b: Metformin: 28/29 (97); Other hypoglycaemic agents: 9/29 (31); Insulin: 1/29 (3); Antihypertensive agents: 20/29 (69); Lipid‐lowering agents: 12/29 (41) | N (%): type 2 diabetes 30 (100), hypertension 28 (93), metabolic syndrome 29 (97), coronary artery disease 0 (0) |
| Conventional therapy | 57 | 47.1 (8.7) | 37.2 (2.5) | 105.9 (14.2) | n/N (%)b: Metformin: 26/26 (100); Other hypoglycaemic agents: 8/26 (31); Insulin: 0/26 (0); Antihypertensive agents: 15/26 (58); Lipid‐lowering agents: 8/26 (31) | N (%): type 2 diabetes 30 (100), hypertension 27 (90), metabolic syndrome 29 (97), coronary artery disease 1 (3) | |
| Dixon 2012 | Laparoscopic adjustable gastric banding | 43 | 47.4 (8.8) | 46.3 (6.0) | 134.9 (22.1) | ‐ | N (%): obstructive sleep apnoea 30 (100), hypertension 15 (50), diabetes 10 (33), depression 12 (40), metabolic syndrome 19 (63) |
| Conventional therapy | 40 | 50.0 (8.2) | 43.8 (4.9) | 126.0 (19.3) | ‐ | N (%): obstructive sleep apnoea 30 (100), hypertension 17 (57), diabetes 10 (33), depression 11 (37), metabolic syndrome 24 (80) | |
| Hedberg 2012 | Biliopancreatic diversion with duodenal switch | 50 | 40.2 (9.5) | 54.5 (6.7) | ‐ | N (%): oral diabetes medication 6 (25), insulin 1 (4), any diabetes medication 7 (29) | N (%): hypertension 6 (25), hyperlipidaemia 0 (0), sleep apnoea 4 (17) |
| Roux‐en‐Y gastric bypass | 43 | 37.9 (10.4) | 54.5 (5.6) | ‐ | N (%): oral diabetes medication 1 (4), insulin 0 (0), any diabetes medication 1 (4) | N (%): hypertension 7 (30), hyperlipidaemia 0 (0), sleep apnoea 3 (13) | |
| all: | 47 | 39.1 (9.9) | 54.5 (6.1) | ‐ | |||
| Himpens 2006 | Laparoscopic gastric banding | 83 | median 36 (20‐61) | median 37 (30‐47) | ‐ | ‐ | N (%): GERD requiring proton pump inhibitor 6 (15) |
| Laparascopic isolated sleeve gastrectomy | 78 | median 40 (22‐65) | median 39 (30‐53) | ‐ | ‐ | N (%): GERD requiring proton pump inhibitor 8 (20) | |
| Ikramuddin 2013 | Laparoscopic Roux‐en‐Y gastric bypass + lifestyle programme with medical management | 63 | 49 (9) | 34.9 (3.0) | 98.8 (14.0) | insulin 62, other glycaemic medicines 87, dyslipidaemia medicines 65, blood pressure medicines 68 | % inferred from inclusion criteria: type 2 diabetes 100, elevated HbA1c (> 8%) 100 |
| Lifestyle programme with medical management | 57 | 49 (8) | 34.3 (3.1) | 97.9 (17.0) | insulin 43, other glycaemic medicines 95, dyslipidaemia medicines 68, blood pressure medicines 73 | % inferred from inclusion criteria: type 2 diabetes 100, elevated HbA1c (> 8%) 100 | |
| all: | 34.6 (3.1) | ||||||
| Karamanakos 2008 (including Kehagias 2011) | Laparoscopic Roux‐en‐Y gastric bypass | 73 | 36 (8.4) | 45.8 (3.7) | 123.1 (13.9) | ‐ | N (%): hypertension 5 (17), type 2 diabetes 5 (17), impaired glucose tolerance 5 (17), HDL < threshold 4 (13), LDL > threshold 10 (33), triglycerides > threshold 5 (17), obstructive sleep apnoea 3 (10), GERD 5 (17), degenerative arthritis 6 (20), menstrual irregularities 7 (23), ≥ 1 obesity‐related co‐morbidity 23 (77) |
| Laparoscopic sleeve gastrectomy | 73 | 33.7 (9.9) | 44.9 (3.4) | 126.9 (18.0) | ‐ | N (%): hypertension 4 (13), type 2 diabetes 5 (17), impaired glucose tolerance 5 (17), HDL < threshold 3 (10), LDL > threshold 8 (27), triglycerides > threshold 3 (10), obstructive sleep apnoea 6 (20), GERD 2 (7), degenerative arthritis 5 (17), menstrual irregularities 7 (23), ≥ 1 obesity‐related co‐morbidity 20 (67) | |
| all: | 73 | ‐ | ‐ | ‐ | |||
| Keidar 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 42c | 51.45 (8.3) | 42 (4.8) | 118.04 (16.5) | oral hypoglycaemics: 63%; insulin: 21% | Type 2 diabetes: 100% |
| Laparoscopic sleeve gastrectomy | 50c | 47.7 (11.7) | 42.5 (5.2) | 117.9 (17.8) | oral hypoglycaemics: 50%; insulin: 22% | Type 2 diabetes: 100% | |
| all: | 46 | ||||||
| Lee 2011 | Simplified laparoscopic mini‐gastric bypass with duodenum exclusion | ‐ | ‐ | ‐ | ‐ | ‐ | %: poorly‐controlled type 2 diabetes 100 |
| Laparoscopic sleeve gastrectomy without duodenum exclusion | ‐ | ‐ | ‐ | ‐ | ‐ | %: poorly‐controlled type 2 diabetes 100 | |
| Liang 2013 | Usual care | 33.3 | 51.75 (6.70) | 30.34 (1.96)d | 81.31 (4.97) | %: insulin therapy in combination with (unspecified) oral agents 100 | %: type 2 diabetes 100, hypertension 100 |
| Usual care + exenatide | 29.4 | 50.94 (5.89) | 30.28 (1.44) | 81.76 (3.67) | %: insulin therapy in combination with (unspecified) oral agents 100 | %: type 2 diabetes 100, hypertension 100 | |
| Laparoscopic Roux‐en‐Y gastric bypass | 29 | 50.81 (5.44) | 30.48 (0.94) | 81.97 (3.53) | %: insulin therapy in combination with (unspecified) oral agents 100 | %: type 2 diabetes 100, hypertension 100 | |
| all: | ‐ | ‐ | 30.3 [25.0‐34.0] | ‐ | ‐ | ||
| Mingrone 2012 | Gastric bypass | 60 | 43.90 (7.57) | 44.85 (5.16) | 129.84 (22.58) | ‐ | %: type 2 diabetes 100, elevated HbA1c (≥ 7%) 100 |
| Medical therapy | 50 | 43.45 (7.27) | 45.62 (6.24) | 136.40 (21.94) | ‐ | %: type 2 diabetes 100, elevated HbA1c (≥ 7%) 100 | |
| all: | 53 | ‐ | ‐ | ‐ | |||
| Nguyen 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 77.4 | 41.4 (11.0) | 47.5 (5.5) | 133 (21) | ‐ | %: diabetes 20.7, hypertension 38.7, previous abdominal surgery 45.9 |
| Laparoscopic adjustable gastric banding | 75.6 | 45.8 (9.8) | 45.5 (5.4) | 129 (21) | ‐ | %: diabetes 26.7, hypertension 51.1, previous abdominal surgery 47.7 | |
| Nogues 2010 | Laparascopic Roux‐en‐Y gastric bypass | 100 | 45.86 (8.6)e | 43.1 (3.9)e | 116.7 (5.5) | N (%): metformin 2 (28.6) | N (rounded %): hypertension 5 (71), diabetes mellitus 2 (29), dyslipidaemia 5 (71), arthropathy 4 (57), GERD 0 (0), urinary incontinence 3 (43), depression 4 (57), obstructive sleep apnoea 2 (29), insulin resistance 6 (86) |
| Laparoscopic sleeve gastrectomy | 100 | 49.63 (9.6) | 43.5 (3.2) | 108.9 (6.3) | N (%): metformin 2 (25.0) | N (rounded %): hypertension 7 (88), diabetes mellitus 2 (25), dyslipidaemia 5 (63), arthropathy 6 (75), GERD 1 (13), urinary incontinence 6 (75), depression 7 (88), obstructive sleep apnoea 2 (25), insulin resistance 4 (50) | |
| all: | 100 | 47.8 (9.0) | 43.3 (3.4) | ‐ | |||
| O'Brien 2006 | Laparoscopic adjustable gastric band | 75 | 41.8 (6.4) | 33.7 (1.8) | 96.1 (11.2) | ‐ | %: hypertension 22.5, metabolic syndrome 37.5, coronary artery disease 0 |
| Intensive non‐surgical programme | 77.5 | 40.7 (7.0) | 33.5 (1.4) | 93.6 (11.9) | ‐ | %: hypertension 17.5, metabolic syndrome 37.5, coronary artery disease 0 | |
| Paluszkiewicz 2012 | Roux‐en‐Y gastric bypass | 64 | 43.9 (10.8) | 48.6 (5.4) | 137.7 (17.7) | ‐ | N (rounded %): hypertension 30 (83), type 2 diabetes 14 (39), dyslipidaemia 31 (86) |
| Laparoscopic sleeve gastrectomy | 72 | 44.9 (10.6) | 46.1 (5.9) | 130.7 (15.5) | ‐ | N (rounded %): hypertension 25 (69), type 2 diabetes 10 (28), dyslipidaemia 31 (86) | |
| all: | 68 | ‐ | ‐ | ‐ | |||
| Peterli 2012 | Laparoscopic Roux‐en‐Y gastric bypass | 72 | 42.1 (11.2) | 44.2 (5.3) | 124.8 (19.8) | ‐ | %: hypertension 59, type 2 diabetes 26, dyslipidaemia 51, obstructive sleep apnoea 42, GERD 46, back/joint arthralgia 68, depression 11 |
| Laparoscopic sleeve gastrectomy | 72 | 43.0 (11.1) | 43.6 (5.3) | 123.5 (19.4) | ‐ | %: hypertension 63, type 2 diabetes 24, dyslipidaemia 67, obstructive sleep apnoea 48, GERD 44, back/joint arthralgia 61, depression 20 | |
| all: | 72 | 43.0 (5.3) | 44 (11.1) | ‐ | |||
| Praveen Raj 2012 | Laparoscopic duodenojejunal bypass with sleeve gastrectomy | 64 | 39.5 | 48.28 (3.80) | ‐ | ‐ | N (%): type 2 diabetes 20 (71), hypertension 10 (36) |
| Laparoscopic Roux‐en‐Y gastric bypass | 55 | 43.5 | 49.29 (3.63) | ‐ | ‐ | N (%): type 2 diabetes 16 (55), hypertension 12 (41) | |
| all: | 60 | ‐ | ‐ | ‐ | |||
| Schauer 2012 | Laparoscopic Roux‐en‐Y gastric bypass | 58 | 48.3 (8.4) | 37.0 (3.3) | 106.7 (14.8) | N (%): insulin 22 (44) | N (%): type 2 diabetes 50 (100), elevated HbA1c (> 7%) 50 (100), metabolic syndrome 45 (90), dyslipidaemia history 44 (88), hypertension history 35 (70) |
| Laparoscopic sleeve gastrectomy | 78 | 47.9 (8.0) | 36.2 (3.9) | 100.8 (16.4) | N (%): insulin 22 (44) | N (%): type 2 diabetes 50 (100), elevated HbA1c (> 7%) 50 (100), metabolic syndrome 47 (94), dyslipidaemia history 40 (80), hypertension history 30 (60) | |
| Intensive medical therapy | 62 | 49.7 (7.4) | 36.8 (3.0) | 106.5 (14.7) | N (%): insulin 22 (44) | N (%): type 2 diabetes 50 (100), elevated HbA1c (> 7%) 50 (100), metabolic syndrome 46 (92), dyslipidaemia history 36 (84), hypertension history 26 (60) | |
| Sharma 2013 | Laparoscopic gastric imbrication | ‐ | 40.5 | 44.7 (6.1) | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | ‐ | 39.9 | 44.0 (7.8) | ‐ | ‐ | ‐ | |
| Vix 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 87 | 35.23 (9.37) | 47.09 (5.64) | 129.58 (21.17) | ‐ | %: type 2 diabetes 8.9, hypertension 37.8, sleep apnoea syndrome 20.0, vitamin D deficiency 85.7, hypercholesterolaemia 26.7, abnormal LDL 11.1, abnormal HDL 6.7, abnormal triglycerides 17.8, hyperparathyroidism 24.0 |
| Laparoscopic sleeve gastrectomy | 78 | 35.13 (9.7) | 45.57 (4.79) | 128.68 (18.27) | ‐ | %: type 2 diabetes 7.3, hypertension 21.8, sleep apnoea syndrome 9.1, vitamin D deficiency 84.6, hypercholesterolaemia 27.3, abnormal LDL 9.1, abnormal HDL 5.5, abnormal triglycerides 27.3, hyperparathyroidism 28.13 | |
| "‐" denotes not reported aUnclear whether data are based on 16 or 18 participants in this group ‐ the data extracted here assume 18 (16 females and 2 males) bN = number completing study cBaseline characteristics of per protocol population only presented dThis was reported as 30.34 in table 1 and 30.94 in table 2 eData here are from the Nogues paper ‐ those from the associated Ramon paper are slightly different GERD: gastroesophageal reflux disease; HbA1c: glycosylated haemoglobin A1c: HDL: high density lipoprotein; LDL: low density lipoprotein | |||||||
Appendix 5. Matrix of study endpoints (publications)
| Endpoint reported in publication | Endpoint not measured or reported in publication | Time of measurementa | ||
| Aasheim 2009 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 0, 6 wk, 6, 12, 24 mo | ||
| Health‐related quality of life | x | Reported results for only two of the four measures of quality of life specified in the protocol. | 0, 12, 24 mo | |
| Obesity related co‐morbidities | x | 0, 12, 24 mo and after surgery | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | ≤ 30 d | ||
| Adverse effects | x | ≤ 30 d , 24 mo | ||
| Revision rates | x | ≤ 30 d , 12, 24 mo | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Total body potassium, folate, parathyroid hormone, riboflavin, thiamine, 25‐hydroxyvitamin D, vitamin A, vitamin B‐6, vitamin B‐12 , vitamin C, vitamin E, haemoglobin, ionised calcium, number of patients taking dietary supplements, systolic blood pressure, diastolic blood pressure, total cholesterol level, high‐density lipoprotein cholesterol level, low‐density lipoprotein cholesterol, triglyceride level, plasma glucose level, insulin level, C‐reactive protein level | |||
| Subgroups reported in publication | % oxygen saturation (lying supine; sitting), forced vital capacity (FVC) (lying supine; sitting), peak expiratory flow (PEF) (lying supine; sitting), % predicted FVC, and % predicted PEF assessed in a sub‐study of patients at one study centre only. Self‐reported sleep apnoea and snoring symptoms were also only assessed in this sub‐study of patients | |||
| Angrisani 2007 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 0, 12, 36, 60, 120 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | 60 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | ‐ | ||
| Adverse effects | x | < 30 d and late | ||
| Revision rates | x | ‐ | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | N/A | |||
| Subgroups reported in publication: | N/A | |||
| Demerdash 2013 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 0, 12 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | xc | N/A | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | N/A | ||
| Adverse effects | x | N/A | ||
| Revision rates | x | N/A | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Blood pressure (systolic, diastolic), apolipoprotein A‐IV, fasting blood glucose, serum insulin, homeostasis model assessment (HOMA) index, cholesterol (total, HDL, LDL), triglycerides | |||
| Subgroups reported in publication | N/A | |||
| Dixon 2008 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 24 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | 24 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | N/A | ||
| Adverse effects | x | ‐ | ||
| Revision rates | x | ‐ | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | % change in glycosylated haemoglobin A1c (HbA1c), blood pressure, fasting lipids (including total cholesterol, triglycerides, and high‐density lipoprotein cholesterol). Change in self‐reported rates of physical activity and relationship with weight loss; association between weight loss and lower HbA1c / remission | |||
| Subgroups reported in publication | N/A | |||
| Dixon 2012 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 0, 24 mo | ||
| Health‐related quality of life | x | 0, 24 mo | ||
| Obesity related co‐morbidities | x | 0, 24 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | 24 mo | ||
| Adverse effects | x | |||
| Revision rates | x | N/A | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Apnea‐hypopnea index (AHI), AHI change, systolic blood pressure (BP), change in systolic BP, diastolic BP, change in diastolic BP, resting heart rate, change in resting heart rate, % HbA1c, change in HbA1c, plasma glucose, change in plasma glucose, plasma insulin at 2 years, change in plasma insulin, total cholesterol, change in total cholesterol, triglycerides, change in triglycerides, HDL cholesterol, change in HDL cholesterol, Epworth Sleepiness Scale score, Beck Depression Inventory score, sleep patterns (polysomnography outcomes), continuous positive airway pressure (CPAP) adherence, 6‐minute walk test distance | |||
| Subgroups reported in publication | N/A | |||
| Hedberg 2012 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 48 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | Medication use at ≥ 24 mo not reported | 0, ≥ 24 mo, 36 mo | |
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | In‐hospital and total (perioperative) | ||
| Adverse effects | x | Not reported: abdominal pain, abdominal symptoms 'extended enquiry', dumping, heartburn, soiling, vomiting | ≥ 24 mo (only some of those measured reported) | |
| Revision rates | x | ‐ | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | C‐reactive protein, fasting glucose, % HbA1c. Measured at baseline, 12 mo and 24 mo: anaemia (baseline not reported), folate (baseline not reported), haemoglobin (baseline not reported), albumin, glucose, HbA1c, vitamin B12, fasting glucose, high‐density lipoprotein (not reported), low‐density lipoprotein (not reported), triglycerides (not reported) (hyperlipidaemia at baseline only reported) | |||
| Subgroups reported in publication | N/A | |||
| Himpens 2006 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 12, 36 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | 12, 36 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | N/A | ||
| Adverse effects | x | 12, 36 mo | ||
| Revision rates | x | Reported postoperatively in sleeve gastrectomy group and as late in gastric bypass group | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Feelings of hunger, craving for eating sweets | |||
| Subgroups reported in publication | N/A | |||
| Ikramuddin 2013 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 0, 12 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | 0, 12 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | 12 mo | ||
| Adverse effects | x | 12 mo | ||
| Revision rates | x | 12 mo | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Fasting glucose (mg/mL), HbA1c (%), HDL cholesterol, triglycerides, systolic and diastolic blood pressure, pulse rate | |||
| Subgroups reported in publication | N/A | |||
| Karamanakos 2008 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 1, 3, 6, 12, 24, 36 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | 1, 3, 6, 12, 24, 36 mo (overall morbidity also reported for ≤ 30 d and late) | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | ‐ | ||
| Adverse effects | x | ≤ 30 d and late | ||
| Revision rates | x | ‐ | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Ghrelin levels, peptide‐YY levels, appetite | |||
| Subgroups reported in publication | N/A | |||
| Keider 2013 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 3, 12 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | 3, 12 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | 12 mo | ||
| Adverse effects | X | N/A | ||
| Revision rates | X | N/A | ||
| Economic costs | X | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | HbA1c % and mmol/mol, glucose tolerance (fasting glucose or 2‐hour glucose test, normalisation), insulin, C‐peptide levels | |||
| Subgroups reported in publication | N/A | |||
| Lee 2011 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 1, 3, 6, 12 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | Medication use not reported | 12 mo | |
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | ‐ | ||
| Adverse effects | x | ‐ | ||
| Revision rates | x | N/A | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Changes in indirect measures of insulin resistance was assessed using the homeostasis model assessments, % reduced HbA1c, % HbA1c, C‐peptide, glucose, insulin, blood pressure, lipids | |||
| Subgroups reported in publication: | N/A | |||
| Liang 2013 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 12 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | 12 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | 12 mo | ||
| Adverse effects | x | Hypoglycaemic events measured but not reported | 12 mo | |
| Revision rates | x | N/A | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Left ventricle mass index, other left ventricle parameters (relative wall thickness, ejection fraction), fasting plasma glucose, fasting insulin, glycated haemoglobin, systolic blood pressure, cholesterol (total, LDL, HDL), triglycerides, serum Hs‐CRP, HMW adiponectin, TNF‐α, HOMA index (all continuous outcomes) | |||
| Subgroups reported in publication | N/A | |||
| Mingrone 2012 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 24 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | Diabetes remission not reported in the way pre‐specified | 24 mo | |
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | Operative deaths | ||
| Adverse effects | x | Late complications | ||
| Revision rates | x | N/A | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Time to normalisation of fasting glucose and glycated haemoglobin, glucose, glucose change, glycated haemoglobin, change in glycated haemoglobin, total cholesterol, total cholesterol change, HDL cholesterol, HDL cholesterol change, LDL cholesterol, LDL cholesterol change, triglycerides, triglycerides change, systolic blood pressure, systolic blood pressure change, diastolic blood pressure, diastolic blood pressure change | |||
| Subgroups reported in publication | N/A | |||
| Nguyen 2009 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 12, 24, 36, 48 mo | ||
| Health‐related quality of life | x | 0, 1, 3, 9, 12 mo | ||
| Obesity related co‐morbidities | x | N/A | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | At any time (reported for ≤ 30 d and in hospital, 90 d, 12 mo) | ||
| Adverse effects | x | ≤ 30 d and > 30 d | ||
| Revision rates | x | ≤ 30 d or > 30 d | ||
| Economic costs | x | ‐ | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | N/A | |||
| Subgroups reported in publication | Weight loss by starting body mass index (BMI) subgroup (BMI < 50 subgroup vs ≥ 50) | |||
| Nogues 2010 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 3, 12 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | 0, 3, 12 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | N/A | ||
| Adverse effects | x | During and after surgery | ||
| Revision rates | x | N/A | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Biochemistry (calcium, phosphorus, 25 hydroxy‐vitamin D, intact parathyroid hormone) and bone turnover markers. Gastrointestinal hormone outcomes, including: ghrelin, leptin, glucagon‐like peptide 1 (GLP‐1), PYY, and PP. Fasting plasma glucose, fasting insulin levels, HOMA‐IR | |||
| Subgroups reported in publication | Withdrawal of use of diabetic medication at 3 months among a subgroup of patients with diabetes at baseline, normalisation of insulin resistance (HOMA‐IR) in patients who fulfilled criteria for insulin resistance at baseline | |||
| O'Brien 2006 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 6, 12, 18, 24 mo | ||
| Health‐related quality of life | x | 0, 12, 24 mo | ||
| Obesity related co‐morbidities | x | 12, 24 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | N/A | ||
| Adverse effects | x | ‐ | ||
| Revision rates | x | ‐ | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Health status, diastolic blood pressure, systolic blood pressure, fasting plasma glucose, insulin sensitivity index, HDL cholesterol, LDL cholesterol, triglyceride level, total cholesterol, total cholesterol‐HDL cholesterol ratio | |||
| Subgroups reported in publication | Related paper reports on body composition measurements for those participants who completed all of the body composition studies (voluntary aspect of the study) | |||
| Paluszkiewicz 2012 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 6, 12 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | 6, 12 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | ≤ 30 d or > 30 d | ||
| Adverse effects | x | ≤ 30 d or > 30 d | ||
| Revision rates | x | ‐ | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Impaired glucose tolerance (not reported) | |||
| Subgroups reported in publication | N/A | |||
| Peterli 2012 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 0, 1 wk, 3, 6, 12, 24, 36 mo | ||
| Health‐related quality of life | x | One of two quality of life measures reported. One not reported, due to insufficient data collected | 0, 12 mo | |
| Obesity related co‐morbidities | x | 0, 1 wk, 3, 6, 12 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | ≤ 30 d | ||
| Adverse effects | x | ≤ 30 d, 12 mo | ||
| Revision rates | x | ‐ | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | HOMA index, glucose, fasting plasma glucose, insulin, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, total/HDL cholesterol ratio. Hormones: foregut: cholecystokinin (CCK), ghrelin; hindgut: glucagon‐like peptide 1 (GLP‐1), peptide YY (PYY) | |||
| Subgroups reported in publication | N/A | |||
| Praveen Raj 2012 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 0, 3, 6, 12 months | ||
| Health‐related quality of life | x | |||
| Obesity related co‐morbidities | x | 0, 6 mo for lipid profile; otherwise not reported | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | ‐ | ||
| Adverse effects | x | ‐ (only one adverse event reported, which occurred at 1 mo) | ||
| Revision rates | x | N/A | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | N/A | |||
| Subgroups reported in publication | Improvement in type 2 diabetes mellitus (DM), among a subsample with DM at baseline, improvement in hypertension, among a subsample with hypertension at baseline | |||
| Schauer 2012 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 12 mo | ||
| Health‐related quality of life | x (protocol states measured) | N/A | ||
| Obesity related co‐morbidities | x | 12 mo | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | ‐ | ||
| Adverse effects | x | 12 mo | ||
| Revision rates | x | 12 mo | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Fasting plasma glucose, change in fasting plasma glucose, fasting insulin, lipids, and high‐sensitivity C‐reactive protein, the homeostasis model assessment of insulin resistance (HOMA‐IR) index, blood pressure, high‐density lipoprotein cholesterol, insulin, low‐density lipoprotein cholesterol, triglycerides, total cholesterol | |||
| Subgroups reported in publication | N/A | |||
| Sharma 2013 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 0, 6, 12, 36 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | x | N/A | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | ‐ | ||
| Adverse effects | xd | ‐ | ||
| Revision rates | x | ‐ | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | x | N/A | ||
| Subgroups reported in publication | N/A | |||
| Vix 2013 | Review's primary outcomes | |||
| Measures of weight change, fat content or fat distribution | x | 0, 1, 3, 6, 12 mo | ||
| Health‐related quality of life | x | N/A | ||
| Obesity related co‐morbidities | xb | N/A | ||
| Review's secondary outcomes | ||||
| Mortality (perioperative and total) | x | N/A | ||
| Adverse effects | x | N/A | ||
| Revision rates | x | N/A | ||
| Economic costs | x | N/A | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | Fasting plasma glucose, fasting serum insulin, HOMA indices, glycated haemoglobin, triglycerides and cholesterol (total, HDL, LDL), vitamin D concentrations, vitamin D deficiency, calcium, serum parathyroid hormone, secondary hyperparathyroidism rate | |||
| Subgroups reported in publication | Subgroups compared patients whose baseline characteristics indicated normoglycaemia/hyperglycaemia, and normal/abnormal status for triglycerides, total cholesterol, LDL cholesterol and HDL cholesterol; not stated whether subgroups were planned a priori | |||
| "‐" denotes not reported aUnderlined data denote times of measurement for primary and secondary review outcomes, if measured and reported in the results section of the publication (other times represent planned but not reported points in time) b(P) Primary or (S) secondary endpoint(s) refer to verbatim statements in the publication, (O) other endpoints relate to outcomes which were not specified as 'primary' or 'secondary' outcomes in the publication cComorbidities reported but not as dichotomous outcomes dComplications d: day(s); mo: month(s); N/A: not applicable | ||||
Appendix 6. Definition of endpoint measurement
| Major/minor reoperation | Health‐related quality of life | Measures of weight change, fat content or fat distribution | Mortality (perioperative) | Immediate/early/late operative complications | Review's obesity related comorbidities | Revisional surgery | Serious/severe adverse events | |
| Aasheim 2009 | N/A | Norwegian and Swedish versions of the Short‐Form‐36 Health Survey (SF‐36) (4‐week recall, version 2.0); obesity‐related Problems scale, score 0 to 100, high score more dysfunction | BMI, BMI reduction, % of excess BMI lost, weight (kg), % of body weight loss, change in weight, waist circumference, hip circumference, sagittal diameter, fat mass (bioelectrical impedance analysis), fat‐free mass (bioelectrical impedance analysis), fat‐free mass (total body potassium measurement), percentage of weight lost as fat‐free mass, percentage of weight lost as fat‐free mass (total body potassium measurement) | Within 30 days of surgery | Perioperative complications = those occurring within 30 days of surgery Late complications = those occurring between 30 days and two years after surgery |
Number of participants using antihypertensive drugs, oral hypoglycaemic drugs, insulin and lipid‐lowering therapy with statins. Snoring and sleep apnoea (measured in a sub‐study of participants at one study centre only) were assessed using a questionnaire developed for the study which measured self‐reported snoring and experience of sleep apnoea symptoms | ‐ | N/A |
| Angrisani 2007 | N/A | N/A | Percentage of excess weight loss, BMI, decrease in BMI, weight (kg), weight loss failure (BMI > 35 kg/m² at 5‐years), BMI < 30 kg/m² at 5 years and 10 years | Any deaths | Early complications = those occurring within 30 days of surgery Late complications reported but not defined. |
Resolution of diabetes, sleep apnoea and hyperlipaemia (criteria for resolution not reported) | ‐ | N/A |
| Dixon 2008 | N/A | N/A | Weight loss (kg), % weight loss, waist circumference, waist to hip ratio | N/A | Complications reported, but not reported as early and late | Proportion of participants achieving remission of type 2 diabetes (fasting plasma glucose <126 mg/dL and HbA1c < 6.2% without the use of oral hypoglycaemic agents or insulin), changes in medication use and in proportion of patients with metabolic syndrome (as defined by the National Cholesterol Education Program Adult Treatment Panel III criteria) Changes in indirect measures of insulin resistance (using homeostatic model assessment method). Remission of type 2 diabetes, metabolic syndrome (number and proportion NOT meeting criteria), HbA1c, proportion with HbA1c <6.2%, systolic blood pressure, diastolic blood pressure, plasma glucose, plasma insulin, total cholesterol, triglycerides, HDL‐C, total cholesterol to HDL‐C ratio, use of diabetes medication, use of non diabetes medication (antihypertensive agents, lipid‐lowering agents) | ‐ | ‐ |
| Demerdash 2013 | N/A | N/A | BMI, % body weight decrease | N/A | N/A | ‐ | N/A | N/A |
| Dixon 2012 | N/A | SF‐36 (domain scores and component summary scores) | Weight (kg), weight loss (kg), % weight loss, BMI, BMI loss, waist circumference, change in waist circumference, neck circumference, change in neck circumference | N/A | Complications reported, but not reported as early and late | Achieved mild obstructive sleep apnoea (OSA) (apnoea‐hypopnoea index [AHI] < 15 events/hour), achieved OSA remission (AHI < 5 events/hour), metabolic syndrome status | N/A | Those requiring urgent hospitalisation |
| Hedberg 2012 | N/A | N/A | BMI, % excess BMI lost, failure to achieve > 50% of excess BMI loss. Weight at ≥ 24 mo measured in a self‐report patient questionnaire | Perioperative period not defined | Perioperative and late complications reported, but measurement period not defined. Some adverse events were measured by a self‐report participant symptom questionnaire and were not reported in the publication (reported: diarrhoea, malodorous flatus, reoperations and revisional surgery; not reported: abdominal pain, abdominal symptoms 'extended enquiry', dumping, heartburn, soiling, vomiting) | Medication use (not reported), proportion of patients with HbA1c < 5% | ‐ | N/A |
| Himpens 2006 | N/A | N/A | Weight loss (kg), BMI, % excess weight loss | N/A | Complications not defined as immediate/early/late Complications reported as 'not requiring surgery' and 'requiring surgery' | Modification of gastroesophageal reflux disease (GERD) (number of patients on proton pump inhibitor (PPI) medication) | ‐ | N/A |
| Ikramuddin 2013 | N/A | N/A | Waist circumference (cm), weight (kg), BMI, percent weight change | N/A | Complications were reported as postoperative and late surgical but the time periods were not defined explicitly; the postoperative complications were also referred to as 'perioperative' and appear to have occurred early in the post‐operative period | Composite comorbidity endpoint defined as HbA1c < 7.0%, LDL‐C < 100 mg/dL, and systolic blood pressure < 130 mm Hg, at the 12‐month visit; number of medications used to control glycaemia, dyslipidaemia and blood pressure; n and % of participants with HbA1c < 6.0% and n and % of participants with fasting glucose < 100 mg/dL | Reported but not defined explicitly (unclear whether all cases of revision reported) | Reported but not defined explicitly |
| Karamanakos 2008 | N/A | N/A | % excess weight loss, BMI, achieved >50% of excess weight lost | N/A | Perioperative/early morbidity (≤ 30 days), late morbidity | Resolution or improvement of preoperative comorbidities (n and %): hypertension (systolic blood pressure ≥140 and/or diastolic blood pressure ≥90 mm Hg or antihypertensive drug therapy), type 2 diabetes mellitus (fasting plasma glucose ≥126 mg/dL or 2‐h plasma glucose ≥200 mg/dL during OGTT or antidiabetic drug with or without insulin therapy), impaired glucose tolerance (2‐h plasma glucose ≥140 mg/dL and ≤200 mg/dL during oral glucose tolerance test (OGTT)), HDL < threshold (<40 mg/dL for men, <50 mg/dL for women), LDL > threshold (>100 mg/dL), triglycerides > threshold (> 150), obstructive sleep apnoea (repeated episodes of upper airway occlusion during sleep, with or without sleepiness, and high apnoea‐hypopnoea index and need for nasal continuous positive airway pressure during sleep), GERD (need for PPI agents and/or oesophagitis revealed on endoscopy), degenerative arthritis (clinical and radiological documentation), menstrual irregularities (clinical and/or hormonal documentation) | ‐ | Major complications reported, but not defined |
| Keider 2013 | N/A | N/A | Weight, BMI, body fat (%), fat mass (kg), fat‐free mass (kg), Waist (cm) | Period not defined | N/A | HBA1c (% and mmol/mol); diabetes treatments (oral, insulin, diet), 'off glucose lowering medications', 'normal fasting glucose and HbA1c', 'impaired fasting glucose with normal HbA1c' (no further details provided for these three outcomes) | ‐ | ‐ |
| Lee 2011 | N/A | N/A | % weight loss, BMI, weight (kg), % excess weight loss, waist circumference | N/A | An early complication was defined as a complication that occurred ≤ 30 days post‐surgery A late complication was defined as a complication that occurred > 30 days post‐surgery or required re‐admission |
Glycaemic control (defined as the proportion of patients achieving remission of type 2 diabetes, defined as a fasting plasma glucose level of < 126 mg/dL, plus a HbA1c level of < 6.5% without the use of oral hypoglycaemics or insulin), successful treatment of diabetes mellitus (defined as HbA1c < 7%, LDL‐C < 100 mg/dL, and triglycerides < 150 mg/dL), metabolic syndrome (defined by the National Cholesterol Education Program Adult Treatment Panel III criteria), changes in medication use | N/A | A major complication was defined as a complication requiring intervention and hospitalisation for more than 14 days. |
| Liang 2013 | N/A | N/A | Weight and BMI measured using the International Collaborative Study on Hypertension in Blacks (ICSHIB) standardised protocol | N/A | Adverse events were measured but not defined as early or late | Type 2 diabetes resolution (not defined); discontinuation of diabetes and hypertension drugs; presence of hypertension; hypertension defined as systolic blood pressure 140 mmHg and/or diastolic (DBP) 90 mmHg as per 1999 WHO/ISH criteria | N/A | A serious adverse event was defined as an adverse event that resulted in death, hospitalisation, disability, life‐threatening experience, or that required medical or surgical intervention to prevent one of the other outcomes |
| Mingrone 2012 | N/A | N/A | % weight loss, % excess weight loss, BMI, BMI change, waist circumference | N/A | Late complications reported, but not defined | Rate of remission of type 2 diabetes (a fasting plasma glucose level of < 100 mg/dL (5.6 mmol/L and a HbA1c level of < 6.5% for at least 1 year without active pharmacologic therapy (based on recommendations by the American Diabetes Association)), discontinuation of pharmacological treatment for diabetes, total cholesterol normalisation (not defined), HDL cholesterol normalisation (not defined), LDL cholesterol normalisation (not defined), triglyceride normalisation (not defined), reduction/discontinuation of antihypertensive therapy | N/A | ‐ |
| Nguyen 2009 | N/A | SF‐36 (operationalised as the number of domains with improved scores, and the number of domains with scores comparable to US norms; criteria for defining improvement in scores was not reported) | Excess weight lost (EWL) (pre‐operative weight minus post‐operative weight, divided by pre‐operative weight minus ideal body weight and multiplied by 100), weight loss was also categorized into 5 groups according to the % EWL: poor/failure (<20%), acceptable (20%–39.9%), good (40%–59.9%), excellent (60%–79.9%), and exceptional (≥80%), BMI, treatment failure (defined as (1) the need for conversion to another bariatric procedure due to failure of weight loss or (2) having <20% EWL) | In hospital or within 30 days of surgery | Early complications (≤ 30 days after surgery) Late complications (> 30 days after surgery) |
N/A | ‐ | Complications were graded as follows: surgical complications grade I (alterations from the ideal postoperative course, non‐life‐threatening, and with no lasting disability), grade II (potentially life‐threatening but without residual disability, subdivided in to 2 groups: IIa, requiring blood transfusions, total parenteral nutrition, drug therapy, or a hospital stay twice the median stay; and IIb, requiring therapeutic procedures such as endoscopy or reoperation), grade III (with residual disability or requiring organ resection), grade IV (death). Major complications were defined as grade IIb, III, IV complications. |
| Nogues 2010 | N/A | N/A | Weight (kg), weight change, BMI, BMI change | N/A | Complications during and after surgery reported, but measurement periods not defined | Normalisation of insulin resistance (HOMA‐IR) in participants who fulfilled criteria for insulin resistance at baseline; withdrawal of use of diabetic medication at 3 months among a subgroup of patients with diabetes at baseline | N/A | N/A |
| O'Brien 2006 | N/A | SF‐36 (domain scores) | Change in absolute weight (kg), body mass index, percentage of initial weight lost and excess weight lost, proportion of patients losing more than 50% of excess weight, proportion of patients achieving satisfactory weight loss (greater than 25% of excess weight lost) | N/A | Total events reported, not reported as early or late | Number and proportion of patients with metabolic syndrome (defined by the Adult Treatment Panel III criteria) | ‐ | Major complications were defined as those that required hospitalisation or major outpatient treatment (major events were defined but not reported; only total and specific events reported) |
| Paluszkiewicz 2012 | N/A | N/A | BMI, weight (kg), % EWL, %EWL > 50%. Excess weight was defined as initial body weight in excess of the upper limit of the normal weight ranges estimated at the BMI of 25 kg/m² for a given participant height | ≤ 30 d | Early complications (< 30 day) Late complications (> 30 day) |
Number and proportion of patients with remission or improvement in comorbidities. Hypertension, change from baseline in hypertension, type 2 diabetes, change from baseline in type 2 diabetes, dyslipidaemia, change from baseline in dyslipidaemia. Remission of co‐morbidities assessed according to the clinical, biochemical, hormonal and radiological documentation. Improvement defined as a reduction of medication taken and improvement of the symptoms or blood investigation specific to the comorbidity. Remission of hypertension: normal systolic and diastolic arterial pressure without active antihypertensive treatment. Remission of type 2 diabetes: normal fasting glucose levels (<100 mg/dL) and HbA1c < 6% in the absence of active antidiabetic treatment. Remission of dyslipidaemia: normal levels of total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol in the absence of active lipid‐lowering treatment | ‐ | A major complication was defined as a complication resulting in death or reoperation, a hospital stay of more than 7 days after the procedure, or a need for blood transfusion of four or more units |
| Peterli 2012 | N/A | Gastrointestinal Quality of Life Index (GIQLI) questionnaire score, BAROS‐QoL score (only reported for a subgroup of participants as not all study centres returned these data) | Weight (kg), BMI, % excess BMI loss | Within 30 days of surgery | Perioperative complications (within 30 d surgery) | Discontinuation of medication for type 2 diabetes, new‐onset GERD. Proportion of patients with remission or improvement of comorbidities, including hypertension, diabetes mellitus type 2, dyslipidaemia, obstructive sleep apnoea syndrome, back/joint pain, hyperuricaemia, GERD and depression. "Remission and improvement of comorbidities were defined by the endocrinologist/physician responsible for follow‐up" | ‐ | Perioperative complications were graded using the Clavien/Dindo grading system: Grade I complications are defined as minor deteriorations from the normal postoperative course, grade II complications require treatment by drugs, blood transfusion, physiotherapy or nutritional support, grade III complications need interventional or operative treatment, grade IV complications are life‐threatening and managed by ICU, and grade V is death. |
| Praveen Raj 2012 | N/A | N/A | BMI, excess weight loss (kilograms), percent excess weight loss | N/A | Complications reported, unclear at which time points complications were measured (only one complication was reported and this occurred at 1 mo) | Improvement in type 2 diabetes mellitus (DM), among a subsample with DM at baseline, improvement in hypertension among a subsample with hypertension at baseline, lipid profile. Remission of DM defined as achieving a HbA1C of < 7 without the need for oral hypoglycaemic agents (OHA) or insulin. ‘Improvement’ did not appear to be pre‐defined, and was characterised slightly differently for each arm in the results. Remission of hypertension (no requirement of medication by one year), improvement of hypertension (reduced requirement of medication), lipid profile (normalisation of all parameters) | ‐ | N/A |
| Schauer 2012 | N/A | N/A | Weight (kg), change in weight, BMI, change in BMI, % excess weight loss, waist circumference, waist‐ hip ratio | N/A | Adverse events reported up to 12 months, but not defined as early or late | Proportion of patients with an HbA1c level of 6% or less (with or without diabetes medications), coexisting illnesses, changes in medication, HbA1c categorisation, resolution of metabolic syndrome | ‐ | Serious adverse events reported but not defined |
| Sharma 2013 | N/A | N/A | BMI and EWL | N/A | Two major complications reported but not explicitly defined (however, they both required reoperations) | N/A | Not defined in study, but trial reported that two reoperations were required in gastric imbrication group. A gastric outlet obstruction was revised by a removal of the sutures that were blocking the outflow of the pouch. A leak was reoperated on and converted to a sleeve gastrectomy | N/A |
| Vix 2013 | N/A | N/A | %EWL | N/A | N/A | N/A | N/A | N/A |
| "‐" denotes not reported BMI: body mass index; d: day(s); EWL: excess weight lost; GERD: gastroesophageal reflux disease; HbA1c: glycosylated haemoglobin A1c; HDL(‐C): high density lipoprotein (cholesterol); ICU: intensive care unit; ISH: International Society of Hypertension; LDL(‐C): low density lipoprotein (cholesterol); mo: month(s); N/A: not applicable; OGTT: oral glucose tolerance test; PPI: proton pump inhibitor; WHO: World Health Organization | ||||||||
Appendix 7. Adverse events (I)
| Intervention(s) and comparator(s) | Participants included in analysis [N]a | Deaths [N (%)] | All adverse events [N (%)]b | Severe/serious adverse events [N (%)] | |
| Angrisani 2007 | Laparoscopic Roux‐en‐Y gastric bypass | 21c | 0 (0) | ‐ | ‐ |
| Laparoscopic adjustable gastric banding | 22c | 0 (0) | ‐ | ‐ | |
| Aasheim 2009 | Laparoscopic Roux‐en‐Y gastric bypass | ‐ | 0 (0) | 10 (32.0) | ‐ |
| Laparoscopic biliopancreatic diversion with duodenal switch (LDS) | ‐ | 0 (0) | 18 (62.0) | ‐ | |
| Demerdash 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 16d | ‐ | ‐ | ‐ |
| Laparoscopic adjustable gastric band | 18d | ‐ | ‐ | ‐ | |
| Dixon 2008 | Laparoscopic gastric banding in addition to the conventional therapy | 30e | ‐ | ‐ | ‐ |
| Conventional therapy | 30e | ‐ | ‐ | ‐ | |
| Dixon 2012 | Laparoscopic adjustable gastric banding and lifestyle programme | 30e | 0 (0) | 14 (46.7) | 5 (16.7) |
| 2‐year conventional weight‐loss programme and lifestyle programme | 30e | 0 (0) | 13 (43.3) | 5 (16.7) | |
| Hedberg 2012 | Open biliopancreatic diversion with duodenal switch | 24f | 1 (4.2) | ‐ | ‐ |
| Open Roux‐en‐Y gastric bypass | 23f | 0 (0) | ‐ | ‐ | |
| Himpens 2006 | Laparoscopic gastric banding | 40f | ‐ | ‐ | ‐ |
| Laparascopic isolated sleeve gastrectomy | 40f | ‐ | ‐ | ‐ | |
| Ikramuddin 2013 | Laparoscopic Roux‐en‐Y gastric bypass + lifestyle programme with medical management | 60g | 0 (0) | ‐ | 22 (36.7) |
| Lifestyle programme with medical management | 60g | 0 (0) | ‐ | 15 (25) | |
| Karamanakos 2008 | Laparoscopic Roux‐en‐Y gastric bypass | 30h | 0 (0) | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 30h | 0 (0) | ‐ | ‐ | |
| Keidar 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 22h | 0 (0) | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 19h | 0 (0) | ‐ | ‐ | |
| Lee 2011 | Simplified laparoscopic mini‐gastric bypass with duodenum exclusion | 30e | 0 (0) | ‐ | 0 (0) |
| Laparoscopic sleeve gastrectomy without duodenum exclusion | 30e | 0 (0) | ‐ | 0 (0) | |
| Liang 2013 | Usual care | 36i | 0 (0) | ‐ | 0 (0) |
| Usual care + exenatide | 34j | 0 (0) | ‐ | 0 (0) | |
| Laparoscopic Roux‐en‐Y gastric bypass | 31j | 0 (0) | ‐ | 0 (0) | |
| Mingrone 2012 | Gastric bypass | 20h | 0 (0) | ‐ | ‐ |
| Medical therapy | 20h | 0 (0) | ‐ | ‐ | |
| Nguyen 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 111k | 1 (0.9) | 50 (45.0) | ‐ |
| Laparoscopic adjustable gastric banding | 86k | 0 (0) | 15 (17.4) | ‐ | |
| Nogués 2010 | Laparascopic Roux‐en‐Y gastric bypass | 7e | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 8e | ‐ | ‐ | ‐ | |
| O'Brien 2006 | Laparoscopic adjustable gastric band | 39k | ‐ | 7 (17.9) | ‐ |
| Intensive non‐surgical programme | 31k | ‐ | 18 (58.1) | ‐ | |
| Paluszkiewicz 2012 | Open Roux‐en‐Y gastric bypass | ‐ | 0 (0) | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | ‐ | 0 (0) | ‐ | ‐ | |
| Peterli 2012 | Laparoscopic Roux‐en‐Y gastric bypass | 110l | 1 (0.9) | ‐ | 5 (4.5) |
| Laparoscopic sleeve gastrectomy | 107l | 0 (0) | ‐ | 1 (0.9) | |
| Praveen Raj 2012 | Laparoscopic duodenojejunal bypass with sleeve gastrectomy | 28e | ‐ | 1 (3.6) | ‐ |
| Laparoscopic Roux‐en‐Y gastric bypass | 29e | ‐ | 0 (0) | ‐ | |
| Schauer 2012 | Intensive medical therapy alone | 41k | 0 (0) | ‐ | 4 (9) |
| Intensive medical therapy plus laparoscopic Roux‐en‐Y gastric bypass | 50k | 0 (0) | ‐ | 11 (22) | |
| Intensive medical therapy plus laparoscopic sleeve gastrectomy | 49k | 0 (0) | ‐ | 4 (8) | |
| Sharma 2013 | Laparoscopic gastric imbrication | 12d | 0 (0) | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 14d | 0 (0) | ‐ | ‐ | |
| Vix 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 44d | ‐m | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 48d | ‐m | ‐ | ‐ | |
| "‐" denotes not reported aNot all of the studies used the ITT population in the adverse events analyses; footnotes show the population on which each of the analyses were based b'All adverse events' refers to the total number of complications and/or adverse events cStudy completers from 10 year follow‐up dNumber of completers eITT population fThis is the randomised n; unclear if analyses were ITT or not gITT population (missing data included by multiple imputation) hThis is the randomised n iAnalysis population after dropouts (not ITT) ‐ however there were no dropouts in this group jAnalysis population after dropouts (not ITT) kNumber included in the analyses; analyses were not ITT lITT population; analyses based on one year follow‐up data and all participants completed one year follow‐up mNo mortality displayed in CONSORT chart but unclear if mortality occurred among those lost to follow‐up CONSORT: consolidated standards of reporting trials; ITT: intention to treat | |||||
Appendix 8. Adverse events (II)
| Intervention(s) and comparator(s) | Participants included in analysis [N]a | Left study due to adverse events [N (%)] | Total complications [N (%)] | Late complications [N (%)] | |
| Angrisani 2007 | Laparoscopic Roux‐en‐Y gastric bypass | 24c | ‐ | ‐ | ‐ |
| Laparoscopic adjustable gastric banding | 26c | ‐ | ‐ | ‐ | |
| Aasheim 2009 | Laparoscopic Roux‐en‐Y gastric bypass | ‐ | ‐ | ‐ | 9 (29.0) |
| Laparoscopic biliopancreatic diversion with duodenal switch (LDS) | ‐ | ‐ | ‐ | 12 (41.0) | |
| Demerdash 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 16d | ‐ | ‐ | ‐ |
| Laparoscopic adjustable gastric band | 18d | ‐ | ‐ | ‐ | |
| Dixon 2008 | Laparoscopic gastric banding in addition to the conventional therapy | 30e | ‐ | ‐ | ‐ |
| Conventional therapy | 30e | ‐ | ‐ | ‐ | |
| Dixon 2012 | Laparoscopic adjustable gastric banding and lifestyle programme | 30e | ‐ | ‐ | ‐ |
| 2‐year conventional weight‐loss programme and lifestyle programme | 30e | ‐ | ‐ | ‐ | |
| Hedberg 2012 | Open biliopancreatic diversion with duodenal switch | 24f | ‐ | ‐ | ‐ |
| Open Roux‐en‐Y gastric bypass | 23f | ‐ | ‐ | ‐ | |
| Himpens 2006 | Laparoscopic gastric banding | 40f | ‐ | ‐ | ‐ |
| Laparascopic isolated sleeve gastrectomy | 40f | ‐ | ‐ | ‐ | |
| Ikramuddin 2013 | Laparoscopic Roux‐en‐Y gastric bypass + lifestyle programme with medical management | 60g | ‐g2 | ‐ | ‐ |
| Lifestyle programme with medical management | 60g | ‐ | ‐ | ‐ | |
| Karamanakos 2008 | Laparoscopic Roux‐en‐Y gastric bypass | 30h | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 30h | ‐ | ‐ | ‐ | |
| Keidar 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 22h | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 19h | ‐ | ‐ | ‐ | |
| Lee 2011 | Simplified laparoscopic mini‐gastric bypass with duodenum exclusion | 30e | 0 (0) | ‐ | 1 (3.3) |
| Laparoscopic sleeve gastrectomy without duodenum exclusion | 30e | 0 (0) | ‐ | 1 (3.3) | |
| Liang 2013 | Usual care | 36i | ‐ | ‐ | ‐ |
| Usual care + exenatide | 34j | ‐ | ‐ | ‐ | |
| Laparoscopic Roux‐en‐Y gastric bypass | 31j | ‐ | ‐ | ‐ | |
| Mingrone 2012 | Gastric bypass | 20h | 1 (5.0) | ‐ | 3 (15) |
| Medical therapy | 20h | ‐ | ‐ | ‐ | |
| Nguyen 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 111k | ‐ | ‐ | 43 (38.7) |
| Laparoscopic adjustable gastric banding | 86k | ‐ | ‐ | 10 (11.6) | |
| Nogués 2010 | Laparascopic Roux‐en‐Y gastric bypass | 7e | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 8e | ‐ | ‐ | ‐ | |
| O'Brien 2006 | Laparoscopic adjustable gastric band | 39k | ‐ | ‐ | ‐ |
| Intensive non‐surgical programme | 31k | ‐ | ‐ | ‐ | |
| Paluszkiewicz 2012 | Open Roux‐en‐Y gastric bypass | ‐ | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | ‐ | ‐ | ‐ | ‐ | |
| Peterli 2012 | Laparoscopic Roux‐en‐Y gastric bypass | 110l | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 107l | ‐ | ‐ | ‐ | |
| Praveen Raj 2012 | Laparoscopic duodenojejunal bypass with sleeve gastrectomy | 28e | ‐ | ‐ | ‐ |
| Laparoscopic Roux‐en‐Y gastric bypass | 29e | ‐ | ‐ | ‐ | |
| Schauer 2012 | Intensive medical therapy alone | 41k | 0 (0) | ‐ | ‐ |
| Intensive medical therapy plus laparoscopic Roux‐en‐Y gastric bypass | 50k | 0 (0) | ‐ | ‐ | |
| Intensive medical therapy plus laparoscopic sleeve gastrectomy | 49k | 1 (2.0) | ‐ | ‐ | |
| Sharma 2013 | Laparoscopic gastric imbrication | 12d | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 14d | ‐ | ‐ | ‐ | |
| Vix 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 44d | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 48d | ‐ | ‐ | ‐ | |
| "‐" denotes not reported aNot all of the studies used the ITT population in the adverse events analyses; footnotes show the population on which each of the analyses were based cStudy completers dNumber of completers eITT population fThis is the randomised n; unclear if analyses were ITT or not gITT population (missing data included by multiple imputation) g2All participants were included in the analysis; 3 were lost to follow‐up in each group but reasons not stated hThis is the randomised n iAnalysis population after dropouts (not ITT) ‐ however there were no dropouts in this group jAnalysis population after dropouts (not ITT) kNumber included in the analyses; analyses were not ITT lITT population; analyses based on one year follow‐up data and all participants completed one year follow‐up CONSORT: consolidated standards of reporting trials; ITT: intention to treat | |||||
Appendix 9. Adverse events (III)
| Intervention(s) and comparator(s) | Participants included in analysis [N]a | Immediate/early operative complications [N (%)] | Revisional surgery/reoperations [N (%)]b | Early reoperation [N (%)] | |
| Angrisani 2007 | Laparoscopic Roux‐en‐Y gastric bypass | 21c | 2 (9.5) | 6 (28.6) | ‐ |
| Laparoscopic adjustable gastric banding | 22c | 0 (0) | 9 (40.9) | ‐ | |
| Aasheim 2009 | Laparoscopic Roux‐en‐Y gastric bypass | ‐ | 4 (13.0) | ‐ | 2 (6.5) |
| Laparoscopic biliopancreatic diversion with duodenal switch (LDS) | ‐ | 7 (24.0) | ‐ | 1 (3.4) | |
| Demerdash 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 16d | ‐ | ‐ | ‐ |
| Laparoscopic adjustable gastric band | 18d | ‐ | ‐ | ‐ | |
| Dixon 2008 | Laparoscopic gastric banding in addition to the conventional therapy | 30e | ‐ | 3 (10) | ‐ |
| Conventional therapy | 30e | N/A | N/A | N/A | |
| Dixon 2012 | Laparoscopic adjustable gastric banding and lifestyle programme | 30e | ‐ | 1 (3.3) | ‐ |
| 2‐year conventional weight‐loss programme and lifestyle programme | 30e | N/A | N/A | N/A | |
| Hedberg 2012 | Open biliopancreatic diversion with duodenal switch | 24f | ‐ | 2 (8.3) | ‐ |
| Open Roux‐en‐Y gastric bypass | 23f | ‐ | 1 (4.3) | ‐ | |
| Himpens 2006 | Laparoscopic gastric banding | 40f | ‐ | ‐ | 0 |
| Laparascopic isolated sleeve gastrectomy | 40f | ‐ | ‐ | 2 (5) | |
| Ikramuddin 2013 | Laparoscopic Roux‐en‐Y gastric bypass + lifestyle programme with medical management | 60g | ‐ | 1 (1.7)g2 | ‐ |
| Lifestyle programme with medical management | 60g | N/A | N/A | N/A | |
| Karamanakos 2008 | Laparoscopic Roux‐en‐Y gastric bypass | 30h | ‐ | 2 (6.7) | ‐ |
| Laparoscopic sleeve gastrectomy | 30h | ‐ | 1 (3.4) | ‐ | |
| Keidar 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 22h | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 19h | ‐ | ‐ | ‐ | |
| Lee 2011 | Simplified laparoscopic mini‐gastric bypass with duodenum exclusion | 30e | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy without duodenum exclusion | 30e | ‐ | ‐ | ‐ | |
| Liang 2013 | Usual care | 36i | N/A | N/A | N/A |
| Usual care + exenatide | 34j | N/A | N/A | N/A | |
| Laparoscopic Roux‐en‐Y gastric bypass | 31j | ‐ | ‐ | ‐ | |
| Mingrone 2012 | Gastric bypass | 20h | ‐ | ‐ | ‐ |
| Medical therapy | 20h | N/A | N/A | N/A | |
| Nguyen 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 111k | 24 (21.6) | ‐ | 6 (5.4) |
| Laparoscopic adjustable gastric banding | 86k | 6 (7.0) | ‐ | 1 (1.2) | |
| Nogués 2010 | Laparascopic Roux‐en‐Y gastric bypass | 7e | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 8e | ‐ | ‐ | ‐ | |
| O'Brien 2006 | Laparoscopic adjustable gastric band | 39k | ‐ | 5 (13) | ‐ |
| Intensive non‐surgical programme | 31k | N/A | N/A | N/A | |
| Paluszkiewicz 2012 | Open Roux‐en‐Y gastric bypass | ‐ | 6 (16.6) | 0 | ‐ |
| Laparoscopic sleeve gastrectomy | ‐ | 7 (19.4) | 2 (5.6) | ‐ | |
| Peterli 2012 | Laparoscopic Roux‐en‐Y gastric bypass | 110l | 19 (17.3) | 1 (0.9) | ‐ |
| Laparoscopic sleeve gastrectomy | 107l | 9 (8.4) | 1 (0.9) | ‐ | |
| Praveen Raj 2012 | Laparoscopic duodenojejunal bypass with sleeve gastrectomy | 28e | ‐ | ‐ | ‐ |
| Laparoscopic Roux‐en‐Y gastric bypass | 29e | ‐ | ‐ | ‐ | |
| Schauer 2012 | Intensive medical therapy alone | 41k | N/A | N/A | N/A |
| Intensive medical therapy plus laparoscopic Roux‐en‐Y gastric bypass | 50k | ‐ | 3 (6) | ‐ | |
| Intensive medical therapy plus laparoscopic sleeve gastrectomy | 49k | ‐ | 1 (2) | ‐ | |
| Sharma 2013 | Laparoscopic gastric imbrication | 12d | ‐ | 1 (8.3) | ‐ |
| Laparoscopic sleeve gastrectomy | 14d | ‐ | 0 (0) | ‐ | |
| Vix 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 44d | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 48d | ‐ | ‐ | ‐ | |
| "‐" denotes not reported aNot all of the studies used the ITT population in the adverse events analyses; footnotes show the population on which each of the analyses were based bThis includes conversions from laparoscopic to open procedures cStudy completers from 10 year follow‐up dNumber of completers eITT population fThis is the randomised n; unclear if analyses were ITT or not gITT population (missing data included by multiple imputation) g2Mentioned by study authors for one participant but unclear whether other reoperations occurred hThis is the randomised n iAnalysis population after dropouts (not ITT) ‐ however there were no dropouts in this group jAnalysis population after dropouts (not ITT) kNumber included in the analyses; analyses were not ITT lITT population; analyses based on one year follow‐up data and all participants completed one year follow‐up CONSORT: consolidated standards of reporting trials; ITT: intention to treat; N/A: not applicable | |||||
Appendix 10. Adverse events (IV)
| Intervention(s) and comparator(s) | Participants included in analysis [N]a | Late reoperation [N (%)] | Infection [N (%)] | Conversion of surgery [N (%)]b | |
| Angrisani 2007 | Laparoscopic Roux‐en‐Y gastric bypass | 24c | ‐ | ‐ | 0 (0) |
| Laparoscopic adjustable gastric banding | 26c | ‐ | ‐ | 1 (3.8) | |
| Aasheim 2009 | Laparoscopic Roux‐en‐Y gastric bypass | ‐ | 3 (10.0) | ‐ | ‐ |
| Laparoscopic biliopancreatic diversion with duodenal switch (LDS) | ‐ | 7 (24.0) | ‐ | ‐ | |
| Demerdash 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 16d | ‐ | ‐ | ‐ |
| Laparoscopic adjustable gastric band | 18d | ‐ | ‐ | ‐ | |
| Dixon 2008 | Laparoscopic gastric banding in addition to the conventional therapy | 30e | ‐ | ‐ | ‐ |
| Conventional therapy | 30e | N/A | ‐ | N/A | |
| Dixon 2012 | Laparoscopic adjustable gastric banding and lifestyle programme | 30e | ‐ | ‐ | ‐ |
| 2‐year conventional weight‐loss programme and lifestyle programme | 30e | N/A | ‐ | N/A | |
| Hedberg 2012 | Open biliopancreatic diversion with duodenal switch | 24f | ‐ | ‐ | 0 (0) |
| Open Roux‐en‐Y gastric bypass | 23f | ‐ | ‐ | 0 (0) | |
| Himpens 2006 | Laparoscopic gastric banding | 40f | 7 (17.5) | ‐ | 2 (5) |
| Laparascopic isolated sleeve gastrectomy | 40f | 0 | ‐ | 2 (5) | |
| Ikramuddin 2013 | Laparoscopic Roux‐en‐Y gastric bypass + lifestyle programme with medical management | 60g | ‐ | ‐ | 0 (0) |
| Lifestyle programme with medical management | 60g | N/A | ‐ | N/A | |
| Karamanakos 2008 | Laparoscopic Roux‐en‐Y gastric bypass | 30h | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 30h | ‐ | ‐ | ‐ | |
| Keidar 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 22h | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 19h | ‐ | ‐ | ‐ | |
| Lee 2011 | Simplified laparoscopic mini‐gastric bypass with duodenum exclusion | 30e | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy without duodenum exclusion | 30e | ‐ | ‐ | ‐ | |
| Liang 2013 | Usual care | 36i | N/A | ‐ | N/A |
| Usual care + exenatide | 34j | N/A | ‐ | N/A | |
| Laparoscopic Roux‐en‐Y gastric bypass | 31j | ‐ | ‐ | ‐ | |
| Mingrone 2012 | Gastric bypass | 20h | ‐ | ‐ | ‐ |
| Medical therapy | 20h | N/A | ‐ | N/A | |
| Nguyen 2009 | Laparoscopic Roux‐en‐Y gastric bypass | 111k | 8 (7.2) | ‐ | ‐ |
| Laparoscopic adjustable gastric banding | 86k | 10 (11.6) | ‐ | ‐ | |
| Nogués 2010 | Laparascopic Roux‐en‐Y gastric bypass | 7e | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 8e | ‐ | ‐ | ‐ | |
| O'Brien 2006 | Laparoscopic adjustable gastric band | 39k | ‐ | 1 (2.6) | ‐ |
| Intensive non‐surgical programme | 31k | N/A | 0 (0) | N/A | |
| Paluszkiewicz 2012 | Open Roux‐en‐Y gastric bypass | ‐ | ‐ | 2 (5.5) | ‐ |
| Laparoscopic sleeve gastrectomy | ‐ | ‐ | 1 (2.7) | ‐ | |
| Peterli 2012 | Laparoscopic Roux‐en‐Y gastric bypass | 110l | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 107l | ‐ | ‐ | ‐ | |
| Praveen Raj 2012 | Laparoscopic duodenojejunal bypass with sleeve gastrectomy | 28e | ‐ | ‐ | ‐ |
| Laparoscopic Roux‐en‐Y gastric bypass | 29e | ‐ | ‐ | ‐ | |
| Schauer 2012 | Intensive medical therapy alone | 41k | N/A | ‐ | N/A |
| Intensive medical therapy plus laparoscopic Roux‐en‐Y gastric bypass | 50k | ‐ | ‐ | ‐ | |
| Intensive medical therapy plus laparoscopic sleeve gastrectomy | 49k | ‐ | ‐ | ‐ | |
| Sharma 2013 | Laparoscopic gastric imbrication | 12d | ‐ | ‐ | 1 (8.3) |
| Laparoscopic sleeve gastrectomy | 14d | ‐ | ‐ | 0 | |
| Vix 2013 | Laparoscopic Roux‐en‐Y gastric bypass | 44d | ‐ | ‐ | ‐ |
| Laparoscopic sleeve gastrectomy | 48d | ‐ | ‐ | ‐ | |
| "‐" denotes not reported aNot all of the studies used the ITT population in the adverse events analyses; footnotes show the population on which each of the analyses were based bWe defined conversion as when a patient is converted to a different bariatric surgery procedure cStudy completers dNumber of completers eITT population fThis is the randomised n; unclear if analyses were ITT or not gITT population (missing data included by multiple imputation) hThis is the randomised n iAnalysis population after dropouts (not ITT) ‐ however there were no dropouts in this group jAnalysis population after dropouts (not ITT) kNumber included in the analyses; analyses were not ITT lITT population; analyses based on one year follow‐up data and all participants completed one year follow‐up CONSORT: consolidated standards of reporting trials; ITT: intention to treat; N/A: not applicable | |||||
Appendix 11. Survey of authors providing information on trials
| Study author contacted | Study author replied | Study author asked for additional information | Study author provided data | |
| Aasheim 2009 | Y | Y | Y | Y |
| Angrisani 2007 | N | N/A | N/A | N/A |
| Cesana 2013 | N | N/A | N/A | N/A |
| Dadan 2011 | Y | N | N/A | N/A |
| Darabi 2013 | N | N/A | N/A | N/A |
| Demerdash 2013 | N | N/A | N/A | N/A |
| Dixon 2008 | N | N/A | N/A | N/A |
| Dixon 2012 | N | N/A | N/A | N/A |
| Hedberg 2012 | N | N/A | N/A | N/A |
| Himpens 2006 | N | N/A | N/A | N/A |
| Ikramuddin 2013 | N | N/A | N/A | N/A |
| Karamanakos 2008 | Y | Y | Y | Y |
| Keidar 2013 | N | N/A | N/A | N/A |
| Lee 2011 | N | N/A | N/A | N/A |
| Liang 2013 | N | N/A | N/A | N/A |
| Mingrone 2012 | N | N/A | N/A | N/A |
| Nguyen 2009 | N | N/A | N/A | N/A |
| Nogués 2010 | Y | N | Y | N/A |
| O'Brien 2006 | N | N/A | N/A | N/A |
| Paluszkiewicz 2012 | Y | N | Y | N/A |
| Peterli 2012 | Y | Y | Y | Y |
| Praveen Raj 2012 | N | N/A | N/A | N/A |
| Schauer 2012 | N | N/A | N/A | N/A |
| Sharma 2013 | N | N/A | N/A | N/A |
| Vix 2013 | N | N/A | N/A | N/A |
| N: no; Y: yes; N/A: not applicable | ||||
Data and analyses
Comparison 1. Surgery versus non‐surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BMI [kg/m2] | Other data | No numeric data | ||
| 2 Mean BMI at study end | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 BMI reduction | Other data | No numeric data | ||
| 4 Weight [kg] | Other data | No numeric data | ||
| 5 Mean weight at study end | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Weight loss [kg] | Other data | No numeric data | ||
| 7 Weight loss at study end | 3 | 260 | Mean Difference (IV, Random, 95% CI) | 21.27 [18.93, 23.61] |
| 8 Initial weight loss [%] | Other data | No numeric data | ||
| 9 Initial weight loss at study end | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Excess weight loss [%] | Other data | No numeric data | ||
| 11 % excess weight loss at study end | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Other weight change data | Other data | No numeric data | ||
| 13 Health‐related quality of life | Other data | No numeric data | ||
| 14 Comorbitidies: diabetes | Other data | No numeric data | ||
| 15 Comorbitidies: hypertension | Other data | No numeric data | ||
| 16 Comorbitidies: metabolic syndrome | Other data | No numeric data | ||
| 17 Comorbitidies: Lipids | Other data | No numeric data | ||
| 18 Comorbitidies: Sleep | Other data | No numeric data |
Comparison 2. Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BMI [kg/m2] | Other data | No numeric data | ||
| 2 Mean BMI at study end | 3 | 265 | Mean Difference (IV, Random, 95% CI) | ‐5.21 [‐6.39, ‐4.03] |
| 3 Mean weight [kg] | Other data | No numeric data | ||
| 4 Excess weight loss [%] | Other data | No numeric data | ||
| 5 Excess weight loss at study end [%] | 2 | 135 | Mean Difference (IV, Random, 95% CI) | 23.02 [13.56, 32.48] |
| 6 Other weight change data | Other data | No numeric data |
Comparison 3. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BMI [kg/m2] | Other data | No numeric data | ||
| 2 Mean BMI at study end | 6 | 353 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.78, 1.33] |
| 3 BMI reduction | Other data | No numeric data | ||
| 4 BMI reduction at 12 months | 2 | 114 | Mean Difference (IV, Random, 95% CI) | 1.79 [‐0.34, 3.93] |
| 5 Mean weight [kg] | Other data | No numeric data | ||
| 6 Mean weight at study end | 5 | 293 | Mean Difference (IV, Random, 95% CI) | 1.23 [‐2.03, 4.48] |
| 7 Weight loss [kg] | Other data | No numeric data | ||
| 8 Mean weight loss at 12 months | 3 | 146 | Mean Difference (IV, Random, 95% CI) | 4.09 [‐3.31, 11.49] |
| 9 Excess weight loss [%] | Other data | No numeric data | ||
| 10 Other weight change data | Other data | No numeric data | ||
| 11 Health related quality of life | Other data | No numeric data | ||
| 12 Comorbidities: diabetes | Other data | No numeric data | ||
| 13 Comorbidities: hypertension | Other data | No numeric data | ||
| 14 Comorbidities: dyslipidaemia | Other data | No numeric data | ||
| 15 Comorbidities: metabolic syndrome | Other data | No numeric data | ||
| 16 Comorbidities: sleep | Other data | No numeric data | ||
| 17 Comorbidities: other co‐morbidities | Other data | No numeric data |
Comparison 4. Gastric bypass versus biliopancreatic diversion with duodenal switch.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BMI [kg/m2] | Other data | No numeric data | ||
| 2 Mean BMI reduction | Other data | No numeric data | ||
| 3 Mean BMI reduction at study end | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐7.34 [‐9.25, ‐5.43] |
| 4 Excess BMI loss [%] | Other data | No numeric data | ||
| 5 Excess BMI loss at study end | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐23.38 [‐31.40, ‐15.36] |
| 6 Mean weight [kg] | Other data | No numeric data | ||
| 7 Weight loss in kg | Other data | No numeric data | ||
| 8 Body weight loss [%] | Other data | No numeric data | ||
| 9 Other weight change data | Other data | No numeric data | ||
| 10 Health‐related quality of life: SF‐36 | Other data | No numeric data | ||
| 11 Health‐related quality of life: Obesity‐related problems scale | Other data | No numeric data | ||
| 12 Co‐morbidities: diabetes | Other data | No numeric data |
4.1. Analysis.
Comparison 4 Gastric bypass versus biliopancreatic diversion with duodenal switch, Outcome 1 Mean BMI [kg/m2].
| Mean BMI [kg/m2] | ||||
|---|---|---|---|---|
| Study | Outcome | RYGB | BPD+switch | P value |
| Aasheim 2009 | BMI at 1 year, mean (SD) | 38.5 (4.0) | 32.5 (3.2) | P < 0.001 |
| Aasheim 2009 | BMI at 2 years, mean (95 % CI) | 37.5 (36.0 to 39.1) | 30.1 (28.5 to 31.7) | |
Comparison 5. Laparoscopic gastric bypass versus laparoscopic duodenojejunal bypass with sleeve gastrectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BMI [kg/m2] | Other data | No numeric data | ||
| 2 Excess weight loss [kg] | Other data | No numeric data | ||
| 3 Excess weight loss [%] | Other data | No numeric data | ||
| 4 Comorbidites | Other data | No numeric data |
Comparison 6. Laparoscopic adjustable gastric banding versus laparoscopic isolated sleeve gastrectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BMI decrease | Other data | No numeric data | ||
| 2 Weight loss [kg] | Other data | No numeric data | ||
| 3 Excess weight loss [%] | Other data | No numeric data | ||
| 4 Comorbidities: other | Other data | No numeric data |
Comparison 7. Laparaoscopic gastric imbrication versus laparoscopic sleeve gastrectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BMI [kg/m2] | Other data | No numeric data | ||
| 2 Excess weight loss | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aasheim 2009.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not stated Superiority design |
|
| Participants |
Inclusion criteria: superobese (BMI: 50‐60); aged 20‐50 years; non‐achievement of sustained weight loss through non‐surgical methods; signed informed consent. Exclusion criteria: previous bariatric or major abdominal surgery; severe cardio‐pulmonary disease; malignancy; oral steroid treatment; drug abuse; severe psychiatric illness. Diagnostic criteria: BMI: 50‐60 |
|
| Interventions |
Number of study centres: 2 Treatment before study: low calorie diet (1000 kcal/day) (to reduce liver size) for 3 weeks before surgery (note: unclear if treatment was given pre‐ or post‐randomisation) Titration period: n/a Interventions: 1. Laparoscopic Roux‐en‐Y gastric bypass (LRYGB), with nutritional intervention post‐surgery (multivitamin + vitamin D + calcium + iron supplementation, including vitamin B12) 2. Laparoscopic biliopancreatic diversion with duodenal switch (LDS), with nutritional intervention post‐surgery (multivitamin + vitamin D + calcium + iron supplementation, not including vitamin B12) Patients received a low molecular weight heparin daily from the day after the operation. Patients were also prescribed ursodeoxycholic acid for 6 months (except patients who had undergone cholecystectomy; n = 1 per group) (Aasheim 2009) Sub‐study of respiratory function, pulmonary complications and sleep apnoea, in one study centre (Sweden): patients received surgery as above, but also received pre‐operative information from a physical therapist. “The patients were instructed to perform 3 sessions of 10 deep breaths of positive expiratory pressure (PEP) using a mouthpiece … every second hour during daytime.” (Olsen 2012, p. 29) |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, quality of life, complications and additional procedures | |
| Study details |
Run‐in period: “patients followed a very‐low‐calorie diet (1000 kcal) for 3 wk immediately before surgery to reduce their liver size” (Aasheim 2009, p. 16). (Note: unclear whether or not this was delivered pre‐ or post‐randomisation.) Sub‐study of respiratory function, pulmonary complications and sleep apnoea, in one study centre (Sweden): breathing exercises with PEP and early ambulation (Olsen 2012). Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "to describe changes in vitamin status in superobese patients who underwent gastric by‐pass or duodenal switch in an unblinded, prospective, randomized controlled trial" (Aasheim 2009, p. 15‐16). Quote: “this report presents the perioperative results and 1‐year morbidity and weight loss data” (Søvik 2010, p.160‐161). Quote: “to determine whether duodenal switch leads to greater weight loss and more favourable improvements in cardiovascular risk factors and quality of life than gastric bypass” (Søvik 2011, p. 281). Quote: “to investigate respiratory function, pulmonary complications and experience of sleep apnoea after bariatric surgery in superobese patients following laparoscopic gastric bypass or duodenal switch” (Olsen 2012, p. 29). Quote: "in the present report, the gastrointestinal side effects, calorific intake, and changes in obesity‐specific quality of life were evaluated at 2 years after gastric bypass and duodenal switch" (Sovik 2013 p642) |
|
| Notes | BMI: body mass index; LDS: laparoscopic biliopancreatic diversion with duodenal switch; LRYGB: laparoscopic Roux‐en‐Y gastric bypass; n/a: not applicable Ongoing study, due to finish in April 2014. Related publications: Related study identified from Søvik 2011: Aasheim ET, Elshorbagy AK, My Diep L, Søvik TT, Mala T, Valdivia‐Garcia M et al. Effect of bariatric surgery on sulphur amino acids and glutamate. Br J Nutr, 2011; 106; 432‐40. (No outcome data of relevance. Identified in 2013 update searches; excluded.) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: a computer‐driven randomisation procedure was used, using the minimisation method. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information |
| Incomplete outcome data (attrition bias) Weight | Low risk | Comment: one of the 61 patients randomised withdrew from the study as he wished to undergo non‐surgical management of his weight. He was not aware of which treatment arm he had been randomised to. One patient did not complete the 1‐yr follow‐up (reason not provided). Missing data were not imputed for the statistical analyses, but the low rate of missing data is unlikely to have impacted on the effect sizes for this outcome. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: one participant in each arm did not complete the QOL measure after surgery and these participants were excluded from the analyses. |
| Incomplete outcome data (attrition bias) Comorbidities | Unclear risk | Comment: sample sizes are not consistently reported for biochemical outcomes so it is difficult to make an informed judgement about risk of bias |
| Selective reporting (reporting bias) | High risk | Comment: all the outcomes mentioned in the methods section are reported as results. However, results for haemoglobin, total cholesterol and triacylglycerols were reported, yet these were not mentioned in the methods section. Furthermore, results of other measures of nutritional status were reported in online supplementary data to the paper for patients in one study centre, yet these measures were not described in the methods. It is also not clear why data were only available for patients from one study centre. Also, results for only two of four quality of life measures specified in the protocol were reported. |
| Other bias | High risk | Comment: there was differential use of nutritional supplements by patients in each arm, which could have biased the nutritional status outcomes, although it is not clear if this differential use was due to different levels of compliance or due to different levels of prescribing based on nutrition needs – which was part of the protocol for nutritional supplementation after surgery. Also, gastric bypass patients received a vitamin B‐12 supplement while duodenal switch patients did not. Furthermore, it is stated that the surgeons and multidisciplinary treatment teams were more experienced in LRYGB procedures than LDS, which may have impacted the results. Also, responses to questionnaire item about snoring in the sub‐study (Olsen 2012) were re‐categorised into different response options (a dichotomous yes/no) post‐hoc during analysis |
Angrisani 2007.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: BMI > 35 to < 50 kg/m2, age > 16 years but < 50 years, willingness to accept randomisation Exclusion criteria: history of hiatal hernia, previous major abdominal surgery Diagnostic criteria: BMI > 35 to < 50 kg/m2 |
|
| Interventions |
Number of study centres: not reported but appears to be single centre Treatment before study: none reported Titration period: n/a Interventions: 1. Laparoscopic Roux‐en‐Y gastric bypass (LRYGBP) 2. Laparoscopic adjustable gastric banding (LAGB) |
|
| Outcomes | Outcomes reported in abstract of publication: complications and additional procedures, co‐morbidities, weight loss | |
| Study details |
Run‐in period: none reported Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: not reported Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: “to perform a prospective randomized comparison of the outcomes of LAGB and LRYGB in patients followed up for a minimum of 5 years” (Angrisani 2007, p. 128) Quote "to compare outcomes of patients randomly assigned to undergo LAGB or LRYGB at 10 years" (Angrisani 2013, p 405) |
|
| Notes | BMI: body mass index; LAGB: laparoscopic adjustable gastric banding; LRYGBL laparoscopic Roux‐en‐Y gastric bypass; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: described as randomised but no detail of the method used to generate the randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: randomisation by sealed envelopes but no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding of outcome assessors not reported. Patients were informed of the operation to which they had been randomised pre‐operatively (but no self‐reported outcomes) |
| Incomplete outcome data (attrition bias) Weight | Low risk | Comment: 8 patients were excluded after randomisation because they refused to undergo the procedure to which they had been assigned (5 LRYGB, 3 LAGB) 1 LAGB reported to be lost to follow‐up at 5 years. At 10‐year follow‐up, 5 of 27 LAGB and 3 of 24 RYGB patients were lost to follow‐up. |
| Incomplete outcome data (attrition bias) Comorbidities | Low risk | Comment: as for incomplete outcome data ‐ weight |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcomes listed in methods section all reported in results but no way to check if all results reported in protocol are reported in paper |
| Other bias | Unclear risk | Comment: authors state that for LRYGB they were in the early phase of the learning curve, whereas for LAGB approximately 150 people had been operated by the senior author. |
Demerdash 2013.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: satisfaction of the minimal criteria for bariatric surgical treatment, as determined by the Consensus Development Panel of the National Institutes of Health. In brief, surgery may be considered in those persons with body mass index (BMI) greater than 40 kg/m2, or greater than 35 kg/m2, when there are comorbidities which are life‐threatening or detrimental to activities of daily living. Exclusion criteria: not reported Diagnostic criteria: BMI > 40, or > 35 with comorbidities life‐threatening or detrimental to activities of daily living |
|
| Interventions |
Number of study centres: 1 Treatment before study: none reported Titration period: not applicable Interventions: 1. Laparoscopic Roux‐en‐Y gastric bypass 2. Laparoscopic adjustable gastric band |
|
| Outcomes | Outcomes reported in abstract of publication: weight | |
| Study details |
Run‐in period: none reported Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: not reported Publication status: peer reviewed journal |
|
| Stated aim for study | Quote:"to study the effect of 2 commonly performed bariatric surgical procedures; laparoscopic Roux‐en‐Y gastric bypass (RYGBP) and laparoscopic gastric band (BAND), on the cardiovascular risk profile in morbidly obese patients and its correlation with the plasma apolipoprotein apo A‐IV level" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: stated ‘patients were randomly assigned by using sealed envelope technique’ but no details reported (e.g. whether envelopes were opaque or sequentially numbered) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: number randomised per group not explicitly clear; slight imbalance in dropouts (small starting number); reasons for attrition not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: results were reported for all outcomes mentioned in the methods; a study protocol with a priori definitions would help to clarify risk of reporting bias |
| Other bias | Low risk | Comment: no evidence of other bias |
Dixon 2008.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: aged 20‐60 years, BMI of 30‐40, diagnosed with clearly documented type 2 diabetes within the previous 2 years, had no evidence of renal impairment or diabetic retinopathy, and were able to understand and comply with the study process. Exclusion criteria: history of type 1 diabetes, diabetes secondary to a specific disease, previous bariatric surgery, history of medical problems such as mental impairment, drug or alcohol addiction, recent major vascular event, internal malignancy, or portal hypertension; or a contraindication for either study group. Also excluded if did not attend 2 initial information visits. Diagnostic criteria: BMI 30‐40 and diagnosed with type 2 diabetes within the previous 2 years |
|
| Interventions |
Number of study centres: not reported (3 hospitals named) Treatment before study: prior to randomisation, participants were “assessed by a dietician, a general physician, and a consultant endocrinologist specialising in diabetes … to suggest any changes required to maximize current management” (Dixon 2008, p. 317). Over a period of three months, patients received suggestions for alterations to their eating, exercise, glucose self‐monitoring and medications. Titration period: n/a Interventions 1. LAGB in addition to the conventional‐therapy programme 2. Conventional therapy. Best medical practice for treatment, education and follow‐up of type 2 diabetes. Visits at least every 6 weeks throughout the 2 years. Lifestyle modification programmes individually structured to reduce energy intake, fat (< 30%) and saturated fats, to encourage low glycaemic index and high fibre foods. Physical activity advice to encourage 10,000 steps per day and 200 minutes per week of structured activity. Low calorie diets and medications discussed with all participants and used in some cases. |
|
| Outcomes | Outcomes reported in abstract of publication: comorbidities, complications, weight loss | |
| Study details |
Run‐in period: not reported. Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: commercial and non‐commercial Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: “to compare surgically induced weight loss with conventional therapy for the management of recently diagnosed type 2 diabetes (< 2 years).” (Dixon 2008, p. 317) | |
| Notes | BMI: body mass index; LAGB: laparoscopic adjustable gastric banding; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was computer‐derived, with blocking into 3 groups to allow for orderly recruitment into both study groups and to reduce the risk of uneven recruitment late in the series |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: states study not blinded |
| Incomplete outcome data (attrition bias) Weight | Low risk | Comment: of the 30 randomised to LAGB one withdrew preoperatively, of the 30 randomised to conventional therapy, 4 withdrew after randomisation, reasons not given |
| Incomplete outcome data (attrition bias) Comorbidities | Low risk | Comment: as for incomplete outcome data ‐ weight |
| Selective reporting (reporting bias) | Unclear risk | Comment: all the outcomes mentioned in the methods section seem to be reported as results, although physical activity is not mentioned in the methods but results are reported. Protocol not available |
| Other bias | High risk | Comment: participants took part in at least 3‐months of run‐in where alterations to eating, exercise, glucose self‐monitoring and medications were suggested. Compliance was measured during this time. The endocrinologist then independently determined when a participant was ready for randomisation. Of 158 potentially eligible participants, only 60 were randomised. Reasons for exclusions before randomisation were noted No statistically significant differences in baseline characteristics Block randomisation used in an unblinded trial, which may be possible to predict assignments |
Dixon 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: age 18‐60 years; BMI 35‐55; AHI ≥ 20 events/hour diagnosed within the previous 6 months with recommendations to commence CPAP therapy; at least 3 prior significant weight loss attempts. Exclusion criteria: previous bariatric surgery; obesity hypoventilation syndrome requiring bi‐level positive airway pressure; contraindications to bariatric surgery including cognitive impairment, drug or alcohol addiction, and significant cardiopulmonary, neurological, vascular, gastrointestinal, or neoplastic disease. Diagnostic criteria: BMI 35‐55 |
|
| Interventions |
Number of study centres: not reported (recruitment was from 7 centres) Treatment before study: not reported Titration period: n/a 1. Laparoscopic adjustable gastric banding (LAGB) (including initial very low energy diet (VLED) to reduce liver size) and lifestyle programme 2. 2‐year conventional weight‐loss programme (CON) (including offer of VLED, individualised dietary, physical activity and behavioural programmes, and regular consultations with a dietician and physician) and lifestyle programme. Stated (top of p. 1143) that management of OSA, and intensity and nature of the lifestyle programme were common to both groups. |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, co‐morbidities | |
| Study details |
Run‐in period: LAGB patients underwent 2 weeks of VLED within 1 month of randomisation to reduce liver size prior to surgery. In the CON group, all participants were offered an initial VLED program, with the meal replacements provided. Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial; in addition, LAGBs and laparoscopic ports were provided without charge by the manufacturers Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "To determine whether surgically induced weight loss is more effective than conventional weight‐loss therapy in the management of OSA" (Dixon 2012, abstract) | |
| Notes | AHI: apnoea‐hypopnoea index; CON: conventional weight‐loss programme; CPAP: continuous positive airway pressure; LAGB: laparoscopic adjustable gastric banding; n/a: not applicable; OSA: obstructive sleep apnoea; VLED: very low energy diet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: stated computer‐derived randomisation was used |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information provided, but not feasible to blind participants to surgery or no surgery |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: staff assessing the primary outcome (AHI) and polysomnographic outcomes were blinded to randomisation group However, method and effectiveness of blinding not reported, and not reported whether assessors of other outcomes were blinded. No information provided on how or by whom the patient‐reported outcomes were collected and prepared for analysis. |
| Incomplete outcome data (attrition bias) Weight | Low risk | Comment: missing data imputed in analysis (limited description); missing data and reasons reported; no major imbalance between groups. |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | Comment: missing data imputed in analysis (limited description); missing data and reasons reported; no major imbalance between groups. |
| Incomplete outcome data (attrition bias) Comorbidities | Low risk | Comment: missing data imputed in analysis (limited description); missing data and reasons reported; no major imbalance between groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: most of the outcomes were pre‐specified in the methods but new cases of type 2 diabetes (which only occurred in the CON group), were not counted as adverse events; no protocol available to check completeness of reporting. |
| Other bias | Unclear risk | Comment: inadequate information provided. |
Hedberg 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: BMI > 48kg/m2 Exclusion criteria: stated only that of the eligible patients, 9 were excluded on medical grounds or because of language difficulties and a further 43 refused to be randomised. Diagnostic criteria: BMI > 48 kg/m2 |
|
| Interventions |
Number of study centres: not reported but appears to be a single centre Treatment before study: for both groups stated that after initial evaluation of the internist, dietician and psychologist, education provided on post‐operative diet and [unspecified] abnormalities were treated [using unspecified methods] before surgery. Titration period: n/a Interventions: 1: Open (laparotomic) biliopancreatic diversion with duodenal switch (BPD/DS) 2: Open (laparotomic) Roux‐en‐Y gastric bypass (RYGB) All patients received post‐operative multivitamin supplements |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, co‐morbidities, complications | |
| Study details |
Run‐in period: not reported Study terminated before regular end: yes (recruitment was terminated before planned sample size was reached) |
|
| Publication details |
Language of publication: English Funding: not reported Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "to compare the weight loss (primary outcome), perioperative results, and complications in the long and short term, as well as the gastrointestinal symptoms and biochemical profiles (secondary outcomes) in a prospective, randomised controlled trial of [BPD/DS] versus [RYGB] in patients with a BMI > 48 kg/m2" (Hedberg 2012, p. 339) | |
| Notes | BMI: body mass index; BPD/DS: open (laparotomic) biliopancreatic diversion with duodenal switch; n/a: not applicable; RYGB: open (laparotomic) Roux‐en‐Y gastric bypass | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: randomisation was achieved with sealed envelopes, which were opened after the patient had been anaesthetised – however, it was not stated whether envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: study was not blinded, except that “the type of procedure was unknown to the patient and staff until 2 days postoperatively” |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: attrition is reported and overall was balanced across the groups (3 patients in each group declined follow‐up or did not reply); however, the timing of these dropouts is not stated and no sample sizes are provided for outcomes (the timings of which were also unclear in many cases). It was not stated whether any of the patients who did not drop out failed to provide weight‐loss data. |
| Incomplete outcome data (attrition bias) Comorbidities | Unclear risk | Comment: as for incomplete outcome data ‐ weight |
| Selective reporting (reporting bias) | High risk | Comment: there is considerable scope for selective reporting as some outcomes were reported only at baseline whilst others were reported only post‐surgery; also the timing of assessments is unclear in many cases. Overall, many of the measurements stated in the methods appear to be missing from the results. For the patient reported questionnaire, the investigators appear to have chosen specific sets of outcomes to report or exclude. The baseline number and % of patients with diabetes was higher in the BPD/DS than RYGB group (29% versus 4%) but this difference was not reported, except indirectly for two diabetes medication subgroups. |
| Other bias | High risk | Comment: the required sample size was not achieved due to patients declining randomisation because of their own preferences. Instead, an interim analysis of 47 patients showed significant differences between the 2 groups and the inclusion was stopped. It was stated that for both groups after initial evaluation by the internist, dietician and psychologist, [unspecified] abnormalities were treated [using unspecified methods] before surgery. |
Himpens 2006.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: only inclusion criteria mentioned are candidates for laparoscopic restrictive operation Exclusion criteria: not reported Diagnostic criteria: not reported (baseline BMI range 30‐53) |
|
| Interventions |
Number of study centres: not reported but appears to be a single centre Treatment before study: prior to surgery, 6 GB and 8 SG patients experienced GERD and needed daily treatment with proton pump inhibitor (unclear whether treatment was given before or after randomisation). Titration period: n/a Interventions: 1. Laparoscopic adjustable gastric band (LAGB) 2. Laparascopic isolated Sleeve Gastrectomy (LISG). |
|
| Outcomes | Outcomes reported in abstract of publication: co‐morbidities, complications and additional operative procedures, weight loss | |
| Study details |
Run‐in period: none reported Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: not reported Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: “to compare the laparoscopic adjustable GB and laparoscopic isolated SG in terms of weight loss, feeling of hunger, craving for eating sweets, gastroesophageal reflux disease (GERD), complications and re‐operations, reporting the results after 1 year and 3 years” (Himpens 2006, p. 1451) | |
| Notes | BMI: body mass index; GB: laparoscopic adjustable gastric banding; GERD: gastroesophageal reflux disease; LAGBL: laparoscopic adjustable gastric band; LISG: laparoscopic isolated sleeve gastrectomy; n/a: not applicable; SG: solated sleeve gastrectomy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: states patients operated consecutively and randomly assigned. No details of randomisation sequence reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details reported |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: states that 80 randomised, 40 in each group. No discussion of any attrition or exclusions, appears to be no losses at 3 years but unable to check as numbers not presented in any details of weight loss results |
| Incomplete outcome data (attrition bias) Comorbidities | Unclear risk | Comment: GERD outcomes ‐ all numbers were reported, but data were statistically analysed by subgroup for this outcome ‐ those without GERD at baseline to see if it appeared, those with it at baseline to see if it disappeared. |
| Selective reporting (reporting bias) | Unclear risk | Comment: reports data on outcomes listed in methods, but study protocol not available, Only reports mean change and range, not standard deviations |
| Other bias | Unclear risk | Comment: the characteristics of the patients were reported to be similar for the two groups, although states medians and ranges were performed unclear what the reason is for this. Insufficient information to assess whether an important risk of bias exists |
Ikramuddin 2013.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: aged 30 to 67 years; under physician's care for type 2 diabetes for ≥ 6 mo before recruitment; HbA1c ≥ 8.0% at time of entry; serum C‐peptide level > 1.0 ng/mL 90 min after a liquid mixed meal; BMI 30.0 to 39.9; willing to accept randomisation to either treatment group and follow full treatment protocol. Exclusion criteria: conditions that would contraindicate surgery, such as serious cardiovascular disease, previous gastrointestinal surgery, psychological concerns, or history of malignancy. Diagnostic criteria: BMI 30.0 to 39.9, type 2 diabetes, inadequate glycaemic control |
|
| Interventions |
Number of study centres: 4 Treatment before study: not reported, but inclusion criteria specify patients had to have received physician treatment for type 2 diabetes for ≥ 6 mo Titration period: not applicable Interventions: 1. Laparoscopic Roux‐en‐Y gastric bypass + lifestyle programme with medical management 2. Lifestyle programme with medical management |
|
| Outcomes | Outcomes reported in abstract of publication: weight, comorbidities, complications | |
| Study details |
Run‐in period: patients in the surgery group were placed on a low calorie diet with meal replacements 2 wk before the operation Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: study was supported by Covidien, Mansfield, Massachusetts; publication was supported in part by a grant both from the National Center for Advancing Translational Sciences, and National Institutes of Health, formerly the National Center for Research Resources. Authors declared that the sponsoring agency had no role in the collection, management, analysis, and interpretation of the study data; and had no part in the preparation of the manuscript. The sponsor was allowed to review the manuscript prior to submission but had no role in the decision to submit the manuscript for publication. Publication status: peer reviewed journal |
|
| Stated aim for study | Quote:"To compare Roux‐en‐Y gastric bypass with lifestyle and intensive medical management to achieve control of comorbid risk factors" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication "The randomization schedule used permuted blocks of random length within each site so that each site would have nearly equal proportions in each group" Comment: randomisation method not explicitly stated but, based on the approach for permutation of the blocks would appear to be computer‐based |
| Allocation concealment (selection bias) | Unclear risk | Quote from publication "allocation between treatment groups was concealed to the study staff until after randomization" Comment: method of concealment not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote from publication: "Randomization assignment was unblinded" Quote from publication: “Investigators, data collectors, and outcome adjudicators were blinded to aggregate outcomes until the final patient completed the 12‐month follow‐up" Comment: not clear what “blinded to aggregate outcomes” means; unclear if blinded to allocation group |
| Incomplete outcome data (attrition bias) Weight | Low risk | Comment: number lost to follow‐up small (5%) and evenly balanced across the study groups, although reasons for attrition not reported; analysis includes all patients with multiple imputation for the 5% who dropped out |
| Incomplete outcome data (attrition bias) Comorbidities | Low risk | Comment: as above for weight‐loss outcomes |
| Selective reporting (reporting bias) | Unclear risk | Comment: results were reported for all outcomes mentioned in the methods. A number of comorbidities were not defined or had threshold values which might be arbitrary. |
| Other bias | Low risk | Comment: no evidence of other bias |
Karamanakos 2008.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: stated only that patients had BMI ≤ 50 and were on the waiting list pool for bariatric surgery. In response to a request for further information, the author clarified that the BMI inclusion criteria were 40 to 50 and 35 to 50 for patients with type 2 diabetes. Exclusion criteria: chronic medical or psychiatric illness, substance abuse, previous gastrointestinal surgery. Diagnostic criteria: BMI 40 to 50, and 35 to 50 for patients with type 2 diabetes. |
|
| Interventions |
Number of study centres: not reported but appears to be a single centre Treatment before study: none reported Titration period: n/a Interventions: 1. Laparoscopic Roux‐en‐Y gastric bypass (LRYGBP) + daily multivitamin and mineral supplementation including intramuscular vitamin B12 (+ daily iron supplement for all premenopausal women – time period of supplementation not stated). 2. Laparoscopic sleeve gastrectomy (LSG) + multivitamin and mineral supplementation for 6 months then according to requirement. |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, co‐morbidities, complications and additional procedures | |
| Study details |
Run‐in period: none reported Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: not reported Publication status: peer review journal |
|
| Stated aim for study | Quote: “to evaluate and compare the effects of LRYGBP to the effects of LSG … on body weight, appetite and also on ghrelin and PYY levels.” (Karamanakos 2008, p. 402) Quote: "...to compare the mid‐term outcomes in non‐superobese patients undergoing LRYGB and LSG" (Kehagias 2011, p. 1650) |
|
| Notes | BMI: body mass index; LRYGB: laparoscopic Roux‐en‐Y gastric bypass; LSG: laparoscopic sleeve gastrectomy; n/a = not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: “Computer‐generated random numbers were used to assign the type of surgery” (Kehagias 2011, p. 1651) |
| Allocation concealment (selection bias) | Unclear risk | Quote from publication: "... random numbers were used to assign the type of surgery which was written on a card sealed in a completely opaque envelope" (Kehagias 2011, p. 1651) Comment: unclear whether envelopes sequentially numbered, and when and to whom the information in the envelopes was disclosed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote from publication: "Blinding as to the type of procedure involved the patient and the medical staff and the independent data collector" (Kehagias 2011, p. 1651). Comment: no details were given about the blinding method or whether it may have been broken |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: for 3‐year follow‐up it is unclear whether dropouts were included in the analysis; reasons for dropout were not reported |
| Incomplete outcome data (attrition bias) Comorbidities | Unclear risk | Comment: unclear whether all comorbidities were reported; timing of some comorbidities inconsistently defined; statistical significance of co‐morbidities reported inconsistently; unclear whether 3 missing patients at year 3 were analysed |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcomes were assessed at 1, 3, 6, 12, 24 and 36 months but only reported yearly Duration of anaesthesia and length of stay were recorded but not reported Minor complications were reported narratively only, not separately by intervention group: Quote from publication: “…minor complications such as acid regurgitation, heartburn and vomiting were present in approximately 20% of LSG group patients during the first six post‐operative months and, in most cases, were not severe” |
| Other bias | Unclear risk | Comment: extent of vitamin supplementation unclear: stated in discussion that LSG group did not require supplementation but implied in methods section that they did receive supplements for at least 6 months. Overall, supplementation was more extensive in LRYGB than LSG group |
Keidar 2013.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not stated (assume 1:1) Superiority design |
|
| Participants |
Inclusion criteria: patients with type 2 diabetes (based on baseline oral glucose tolerance test with medication discontinued), BMI greater than 35, aged 18 to 65 years. Exclusion criteria: previous gastrointestinal surgery Diagnostic criteria: BMI > 35 |
|
| Interventions |
Number of study centres: one Treatment before study: none Titration period: none Interventions: 1. Laparoscopic Roux‐en‐Y gastric bypass (LRYGB) 2. Laparoscopic sleeve gastrectomy (LSG) |
|
| Outcomes | Outcomes reported in abstract of publication: weight, co‐morbidities | |
| Study details |
Run‐in period: none Study terminated before regular end: no |
|
| Publication details |
Complete and delete as appropriate Language of publication: English Funding: commercial and non‐commercial funding Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "to compare RYGB and sleeve gastrectomy (SB) in obese patients with type 2 diabetes using a randomised trial to evaluate glucose tolerance and changes in body composition over 12 months post‐surgery" | |
| Notes | BMI: body mass index; LRYGB: laparoscopic Roux‐en‐Y gastric bypass; LSG: laparoscopic sleeve gastrectomy; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Randomisation using online randomisation software" Comment: URL provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: details not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from publication: "allocation to treatments was not concealed and patients knew which procedure they were to undergo" |
| Incomplete outcome data (attrition bias) Weight | High risk | Comment: drop‐outs reported by group, numbers were small however the overall sample size was small and there were more drop‐outs from one surgical group than the other |
| Incomplete outcome data (attrition bias) Comorbidities | High risk | Comment: drop‐outs reported by group, numbers were small however the overall sample size was small and there were more drop‐outs from one surgical group than the other |
| Selective reporting (reporting bias) | Unclear risk | Comment: results were reported for all outcomes mentioned in the methods, protocol not available. |
| Other bias | Low risk | Comment: no evidence of other bias |
Lee 2011.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: aged between > 30 and < 60 years old; BMI > 25 to < 35; poorly controlled type II diabetes; had been receiving treatment from an endocrinologist for 6 months or longer; no evidence of renal impairment or diabetic retinopathy; ability to understand and comply with study process. Exclusion criteria: presence of a “specific disease” (Lee 2011, p. 144); previous bariatric surgery; history of major medical problems, including mental impairment, drug or alcohol addiction, recent major vascular event, internal malignant neoplasm, and portal hypertension; contraindication for either surgery; C‐peptide level below 1.0; non‐attendance at initial two information visits. Diagnostic criteria: BMI > 25 to < 35 and type II diabetes. |
|
| Interventions |
Number of study centres: 1 Treatment before study: not applicable Titration period: not applicable Interventions: 1. Laparoscopic gastric bypass with duodenum exclusion (LGBD) 2. Laparoscopic sleeve gastrectomy without duodenum exclusion (LSG) |
|
| Outcomes | Outcomes reported in abstract of publication: weight and co‐morbidities | |
| Study details |
Run‐in period: ≤ 2 weeks, during which patients received suggestions for changing their eating, glucose monitoring and vitamin supplementation. Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "to evaluate the efficacy of 2 different gastrointestinal metabolic operations for the treatment of T2DM and to test the foregut hypothesis" (Lee 2011, p. 143). | |
| Notes | BMI: body mass index; LGBD: laparoscopic gastric bypass with duodenum exclusion; LSG: laparoscopic sleeve gastrectomy without duodenum exclusion; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "A computer‐generated variable block schedule was used for randomisation" (Lee 2011, p. 144) |
| Allocation concealment (selection bias) | Unclear risk | Comment: variable block randomisation was performed onsite in the operation theatre, but it is unclear by whom and how the allocation sequence was concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote from publication: "The study was double‐blinded" (Lee 2011, p. 144) Comment: states double blinded, but no other details provided , so unclear if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) Weight | Low risk | Comment: All randomised patients were followed up |
| Incomplete outcome data (attrition bias) Comorbidities | Low risk | Comment: all randomised patients were followed up |
| Selective reporting (reporting bias) | High risk | Comment: medication use was pre‐specified as an outcome in the methods section, but results were not reported. Study protocol is unavailable. |
| Other bias | Low risk | Comment: no evidence of other bias |
Liang 2013.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: T2DM diagnosed according to WHO criteria. Other inclusion criteria were: (1) obesity (body mass index [BMI] > 28 kg/m2) in accordance with the WHO Asia‐Pacific classification for obesity; (2) T2DM with hypertension of 5–10 years with hypertension defined as systolic blood pressure (SBP) 140 mmHg and/or diastolic blood pressure (DBP) 90 mmHg as per 1999 WHO/ISH criteria; (3) insulin therapy in combination with oral administration of drugs for 12 months; (4) glycated haemoglobin (HbA1c) > 7%; (5) age: 30–60 years; (6) seronegative for antibodies against insulin, islet cells and glutamic acid decarboxylase (GAD); (7) C‐peptide level 0.3 mg/L. Exclusion criteria: (1) people without diabetes; (2) type 1 diabetes mellitus, presence of autoimmune diabetes indicated by antibodies to insulin, islet cells, and GAD, and gestational diabetes; (3) patients with heart, liver, or renal function impairment; (4) presence of severe infections or cerebrovascular disease; (5) fasting serum insulin was less than one‐third of the normal value; (6) diabetes of more than 10 years duration; (7) age > 60 years or <30 years. Diagnostic criteria: type 2 diabetes requiring insulin and oral drugs for 12 months, hypertension, BMI > 28 kg/m2 |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin and oral diabetes drugs taken for 12 months (an inclusion criterion) Titration period: not applicable Interventions: 1. Usual care (multidisciplinary team; diet, exercise and biochemical goals) 2. Usual care plus exenatide 3. Laparoscopic Roux‐en‐Y gastric bypass |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, comorbidities | |
| Study details |
Run‐in period: none reported Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: research grants from the National Natural Science Foundation of China Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: (stated in abstract) "to evaluate the effect of laparoscopic Roux‐en‐Y gastric bypass (RYGB) surgery compared with usual care with and without Exenatide therapy in obese people with type 2 diabetes mellitus (T2DM) and hypertension" Also stated (p. 52): "The primary aim of this trial was the change in cardiac function in patients undergoing RYGB surgery, usual care or GLP‐1 therapy. The secondary aims were to assess changes in metabolic parameters (BMI, HbA1c, HOMA‐IR and lipids) and inflammation (hs‐CRP, TNF‐a, HMW‐adiponectin) after a 12‐month treatment period" |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication "use of a computerized system for generating random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: analysis population unclear. Seven patients dropped out after randomisation (usual care = 0, usual care + exenatide = 2, LRYGB = 5) – however stated all patients were followed up; it was not reported why they withdrew, nor at what time they withdrew |
| Incomplete outcome data (attrition bias) Comorbidities | Unclear risk | Comment: as stated above for weight loss |
| Selective reporting (reporting bias) | High risk | Comment: hypoglycaemic episodes stated as measured but no data reported. Considered a key outcome given that a glycaemia‐modifying drug was part of the intervention |
| Other bias | Low risk | Comment: no evidence of other bias |
Mingrone 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: age 30‐60 years, BMI ≥35, type 2 diabetes for at least 5 years, glycated haemoglobin ≥7.0%, ability to understand and comply with study protocol. Exclusion criteria: type 1 diabetes, diabetes secondary to specific disease or glucocorticoid therapy, previous bariatric surgery, pregnancy, other medical conditions requiring short‐term hospitalisation, severe diabetes complications, other severe medical conditions, geographical inaccessibility. Diagnostic criteria: BMI ≥ 35 with type 2 diabetes. |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Titration period: n/a Interventions: 1. Gastric bypass (plus daily nutritional supplementation) 2. Medical therapy (treated by a multidisciplinary team including a diabetologist, dietitian and nurse, visits at baseline, 1, 3, 6,9,12 and 24 months. Oral hypoglycaemic agents and insulin doses optimised on an individual basis to reach a glycated haemoglobin level <7%. Programs for diet and lifestyle modification, including reduced overall energy and fat intake (details provided) and increased physical exercise). |
|
| Outcomes | Outcomes reported in abstract of publication: comorbidities | |
| Study details |
Run‐in period: not reported Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial: supported by the Catholic University of Rome Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "comparing the efficacy of two types of bariatric surgery (gastric bypass and biliopancreatic diversion) with conventional medical therapy in severely obese patients with Type 2 diabetes" (Mingrone 2012, p. 1578) | |
| Notes | BMI: body mass index; GB: gastric bypass; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "use of a computerised system for generating random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details reported |
| Incomplete outcome data (attrition bias) Weight | Low risk | Comment: missing numbers were small and appeared to be balanced however unclear whether dropouts are related to outcome |
| Incomplete outcome data (attrition bias) Comorbidities | Low risk | Comment: missing numbers were small and appeared to be balanced however unclear whether dropouts are related to outcome |
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is available. The primary outcome of diabetes remission has not been reported in the way it was pre‐specified. The protocol states that diabetes remission would be assessed in terms of both full and partial remission. In the paper, only “diabetes remission” is reported (unclear if this is full, partial or a composite of both) |
| Other bias | Low risk | Comment: no evidence of other bias |
Nguyen 2009.
| Methods |
Parallel controlled clinical trial Randomisation ratio: not stated Superiority design |
|
| Participants |
Inclusion criteria: BMI 40‐60 kg/m2 or 35 kg/m2 with comorbidities, acceptable operative risk, aged 18‐60 years. Exclusion criteria: large ventral hernia, hiatal hernia, or previous gastric or bariatric surgery Diagnostic criteria: BMI 40‐60 |
|
| Interventions |
Number of study centres: 1 Treatment before study: not reported Titration period: not applicable Interventions: 1: Laparoscopic Roux‐en‐Y gastric bypass (LRYGB) 2: Laparoscopic adjustable gastric banding (LAGB) |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, QoL, complications and additional procedures | |
| Study details |
Run‐in period: not reported Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "to compare the outcomes, convalescence, quality of life, and costs of laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic adjustable gastric banding" (Nguyen 2009, p. 631) | |
| Notes | BMI: body mass index; LAGB: laparoscopic adjustable gastric banding; LRYGB: laparoscopic Roux‐en‐Y gastric bypass; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "they were randomly assigned to laparoscopic gastric bypass or laparoscopic gastric banding by use of sealed envelopes with a block of 3 groups to allow for even recruitment" Comment: method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: see above, sealed envelopes were used but not stated whether sequentially numbered and opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from publication: “The randomized assignment was discussed with the patient at the second office visit, when the patient had the right to withdraw from the study protocol" Comment: QoL was the only self‐reported outcome; participants were not blinded for any outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided relating to whether outcome assessors were blinded Comment: participants were not blinded; no information provided on whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Weight | High risk | Comment: the proportion excluded immediately after randomisation differed notably between LRYGB and LAGB groups (11.2% and 31.2% respectively). Dropouts at years 2‐4 were reported (40 LRYGB and 56 LAGB at year 4) but reasons not given |
| Incomplete outcome data (attrition bias) Quality of life | High risk | Comment: as above |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available; all outcomes specified in the methods section were reported; however, the methods did not give full details of the way all outcomes were to be measured and assessed; some surgical outcomes were not explicitly mentioned a priori; subgroups of weight loss by starting BMI were reported but not mentioned a priori |
| Other bias | Unclear risk | Quote from publication: "Our study has several limitations. First, the baseline BMI was significantly higher (47 vs. 45 kg/m2, respectively) and age was significantly lower (41 vs. 45 years, respectively) in the gastric bypass group than in the gastric banding group. These differences occurred by chance though the randomization process" (Nguyen 2009). In addition, for complications, mean duration of follow‐up differed (LYRGB 4.2 years, LAGB 3.6 years). No protocol to reduce the risk of differential behaviours by patients and healthcare providers in the absence of blinding was reported. |
Nogués 2010.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not stated Superiority design |
|
| Participants |
Inclusion criteria: morbidly obese women, aged 18‐55 years (NB Ramon 2012 states age range for inclusion was 18‐60 years), BMI > 40 or > 35 with comorbidity (type 2 diabetes, sleep apnoea, obesity hypoventilation disorder, severe arthropathy in weight bearing joints, cardiovascular disease, dyslipidaemia). Exclusion criteria: obesity secondary to endocrine diseases and psychiatric disorders, disease that contraindicates surgery, BMI > 50 kg/m2; not suitable for bariatric laparoscopic surgery; currently receiving revisional surgery Diagnostic criteria: BMI > 40 or > 35 with comorbidity |
|
| Interventions |
Number of study centres: 1 Treatment before study: not reported Titration period: not applicable Interventions: 1: Laparoscopic Roux‐en‐Y gastric bypass (LRYGB) 2: Laparoscopic sleeve gastrectomy (LSG) |
|
| Outcomes | Outcomes reported in abstract of publication: none of the review's primary outcomes reported in the abstract | |
| Study details |
Run‐in period: not applicable Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "to compare the impact of these two surgical techniques [LRYGB and LSG] on mineral metabolism and bone mass in patients undergoing bariatric surgery." (Nogués 2010, p104) Quote: "to compare the effects of LRYGB and LSG on glucose metabolism and levels of gastrointestinal hormones … in morbid obese patients" (Ramon 2012, p. 1117). |
|
| Notes | BMI: body mass index; LSG: laparoscopic sleeve gastrectomy; LRYGB: laparoscopic Roux‐en‐Y gastric bypass; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "sequence of treatment allocation was generated in the randomisation module of the True Epistat statistical software". |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: no information provided on whether there were any missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: pilot study with minimal reporting of outcomes, unclear whether other outcomes were captured |
| Other bias | Unclear risk | Comment: insufficient information to make a judgement |
O'Brien 2006.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: age between 20 and 50 years, BMI 30 to 35 kg/m2 with identifiable problems, including an obesity‐related co‐morbid condition (such as hypertension, dyslipidaemia, diabetes, obstructive sleep apnoea, or gastroesophageal reflux disease), severe physical limitations, or clinically significant psychosocial problems associated with their obesity; had attempted to reduce weight over at least the previous 5 years; could understand the options offered and the randomisation process; and were willing to comply with the requirements of each programme Exclusion criteria: candidates with a history of bariatric surgery or medical problems that contraindicated treatment in either study group, such as impaired mental status, drug or alcohol addiction, or portal hypertension. Participants were also excluded if they had undergone an intensive, physician‐supervised programme that used very‐low‐calorie diets or pharmacotherapy or if they did not attend the two initial patient information visits. Diagnostic criteria: BMI 30 to 35 kg/m2 with identifiable obesity‐related problems |
|
| Interventions |
Number of study centres: not reported but appears to be a single centre Treatment before study: none reported Titration period: n/a Interventions: 1. Laparoscopic adjustable gastric band (Lap‐Band system) (LAGB) 2. Intensive non‐surgical programme (Nonsurgical) The non surgical programme centred on the use of behavioural modification, very‐low‐calorie diet, and pharmacotherapy with education and professional support on appropriate eating and exercise behaviour. The programme began with a 6‐month VLCD (500‐550 kcal/d) which used Optifast for 12‐weeks, then over 4‐weeks some VLC meals with 120mg orlistat before the non‐ VLC meals, and then 120mg orlistat before all meals. The 6 month intensive phase was followed by further courses of VLCD or orlistat as tolerated, as well as continual behavioural, dietary, and exercise advice. Physician saw each patient every 2 weeks during the VLCD programme, and every 4‐6 weeks during the rest of the study. Common programme: all patients were instructed and encouraged to follow appropriate lifestyle behaviour of good eating practices and increased exercise and activity. All participants were encouraged to exercise for at least 200 minutes a week. |
|
| Outcomes | Outcomes reported in abstract of publication: co‐morbidities, QoL, weight loss | |
| Study details |
Run‐in period: none reported Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: commercial and non‐commercial Publication status: peer review journal |
|
| Stated aim for study | Quote: “We hypothesized that surgical therapy would induce more weight loss, health benefit, and improvement in quality of life than non surgical therapy and have conducted a randomized, controlled trial comparing the effectiveness of current non surgical therapy with laparoscopic adjustable gastric banding in a group of mildly to moderately obese adults (body mass index, 30 kg/m2 to 35 kg/m2)” (O'Brien 2006, p. 626) | |
| Notes | BMI: body mass index; LAGB: laparoscopic adjustable gastric band; n/a: not applicable; VLC: very low calorie; VLCD: very low calorie diet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐derived random allocation sequence prepared at the trial office. No blocking or stratification |
| Allocation concealment (selection bias) | Low risk | Comment: trial co‐ordinator contacted the trial office by telephone to obtain the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants could not have been blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: states that the study was not blinded (outcome assessors not specified but assume not blinded) |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: withdrawals noted in both groups: one LAGB participant withdrew preoperatively, 5 non‐surgical participants withdrew (weeks 4, 6, 8, 10, and 52), and 2 non‐surgical participants moved overseas. Uneven withdrawals between groups but as reasons not provided for all withdrawals it is unclear whether withdrawals were related to the outcome. States intention‐to‐treat analysis conducted, but in the surgical group the one patient who withdrew preoperatively was not included in the analysis |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: as above, however for quality of life data were analysed only for those who completed the study (case analysis, LAGB n = 39/40, non surgical n = 33/40) |
| Incomplete outcome data (attrition bias) Comorbidities | Unclear risk | Comment: as above quality of life with a case analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. Outcomes listed in the methods reported on |
| Other bias | Low risk | Comment: no evidence of other bias |
Paluszkiewicz 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: BMI ≥40 kg/m2 or ≥ 35kg/m2 with at least one comorbidity (type 2 diabetes, hypertension, dyslipidaemia, obstructive sleep apnoea), 18‐60 years Exclusion criteria: BMI > 60, poorly controlled significant medical or psychiatric disorder, active alcohol or substance abuse, active duodenal/gastric ulcer disease, difficult to treat gastro‐oesophageal reflux disease with a large hiatal hernia, previous major gastrointestinal surgery, diagnosed or suspected malignancy. Diagnostic criteria: BMI ≥ 40 or ≥ 35kg/m2 with comorbidity |
|
| Interventions |
Number of study centres: 1 Treatment before study: not reported Titration period: not applicable Interventions: 1: Open roux‐en‐Y gastric bypass (RYGB) 2: Laparoscopic sleeve gastrectomy (LSG) GF note ‐ update SG to LSG in DX form All participants received Multivitamin and minerals (1 tablet per day), iron (0.1g per day), vitamin B12 (1000 µg per month). Cholesystectomy performed at surgery if gallstones were symptomatic, no numbers provided. |
|
| Outcomes | Outcomes reported in abstract of publication: complications, weight loss, additional procedures | |
| Study details |
Run‐in period: treated for peptic ulcer disease and/or Helicobacter pylori infection preoperatively if diagnosed. Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: peer reviewed journal and conference abstract |
|
| Stated aim for study | Quote: "to compare 6‐month and 1‐year outcomes in patients undergoing LSG and open RYGB in a single teaching hospital in Poland." (Paluszkiewicz 2012, p226) | |
| Notes | BMI: body mass index; LSG: laparoscopic sleeve gastrectomy; n/a: not applicable; RYGB: open roux‐en‐Y gastric bypass Paluszkiewicz 2011 (abstract) appears to report baseline and endpoint data from a smaller subgroup of participants, data therefore extracted from the main publication only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "simple randomisation was used to assign patients to treatment groups" Comment: no further details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details reported |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: uncertainty over the number of participants included in the analyses with little information in the full publication, and conflicting information in the abstract |
| Incomplete outcome data (attrition bias) Comorbidities | Unclear risk | Comment: as for incomplete outcome data ‐ weight |
| Selective reporting (reporting bias) | High risk | Comment: states impaired glucose tolerance was an outcome but no data reported |
| Other bias | Unclear risk | Comment: insufficient details reported to make a judgment |
Peterli 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: BMI > 40 kg/m2 or BMI > 35 kg/m2 with presence of at least one comorbidity, age 18‐65 years, failure of conservative treatment over 2 years. Exclusion criteria: contraindications to major abdominal surgery, severe symptomatic gastro‐oesophageal reflux disease despite medication, large hiatal hernia, expected dense adhesions at the level of the small bowel, the need for endoscopic follow‐up of the duodenum, patients with inflammatory bowel disease. Diagnostic criteria: BMI > 40, or BMI > 35 with comorbidity. |
|
| Interventions |
Number of study centres: 4 Treatment before study: not reported Titration period: not reported Interventions: 1: Laparoscopic Roux‐en‐Y gastric bypass (LRYGB) 2: Laparoscopic sleeve gastrectomy (LSG) Stated that vitamin supplementation and postoperative thrombosis prophylaxis were performed according to the policy of each participating centre (no further details reported). |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, co‐morbidities, complications and additional procedures | |
| Study details |
Run‐in period: not reported Study terminated before regular end: no; however, note that follow‐up was intended to be 5 years but to date results have only been published up to 1 year for most outcomes and 3 years for weight and BMI; at the time of analysis, median follow‐up was 2 years. |
|
| Publication details |
Language of publication: English Funding: commercial and non‐commercial Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: “… to perform a large multicentre RCT assessing the efficacy and safety of LSG and LRYGB…” (Peterli in press, p.4) | |
| Notes | BMI: body mass index; LSG: laparoscopic sleeve gastrectomy; LRYGB: laparoscopic Roux‐en‐Y gastric bypass; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were assigned to either LSG or LRYGB using a computer based randomization with sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Comment: sealed envelopes were used, but it is unclear if they were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information about blinding provided. ClinicalTrials.gov record describes the trial as ‘open label’ |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information about blinding provided. ClinicalTrials.gov record describes the trial as ‘open label’ |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: data presented are for an interim analysis during an ongoing study. Follow‐up data not presented for all the patients randomised – this is probably because it is not available yet as data is for an interim analysis at one year, but this is not fully explained in the publication (i.e. no information about the number of participants who had completed one‐year follow‐up) |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: authors have not provided the number of patients included in the quality of life analysis. This is an interim analysis |
| Incomplete outcome data (attrition bias) Comorbidities | Unclear risk | Comment: authors have not provided the number of patients included in the co‐morbidities analyses. This is an interim analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: results for outcomes pre‐specified in the protocol have been reported. The only measure pre‐specified that results were not provided for was an additional measure of quality of life, BAROS QoL, and the authors explained in their answers to our request for clarifications that data were not provided for this as not all study centres delivered the results. BAROS QoL data are provided in a conference abstract for a small number of patients only |
| Other bias | High risk | Comment: interim analysis, that does not present data on all patients randomised. |
Praveen Raj 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: BMI > 37 kg m2 or BMI > 32 kg m2 with diabetes mellitus or another two significant comorbidities related to obesity; unable to lose or maintain weight through dietary or other forms of medical management; aged 18‐65 years old. Exclusion criteria: patients with sliding hernia (contraindication for sleeve gastrectomy). Diagnostic criteria: BMI > 37 kg m2 or BMI > 32 kg m2 with comorbidities |
|
| Interventions |
Number of study centres: not reported Treatment before study: none Titration period: not applicable Intervention: 1: Laparoscopic duodenojejunal bypass with sleeve gastrectomy (DJB) 2: Laparoscopic Roux‐en‐Y gastric bypass (LRYGB) |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, co‐morbidities, and complications and additional procedures | |
| Study details |
Run‐in period: not applicable Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: none Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "we required a procedure that is as effective as Roux en Y gastric bypass but still addressing the drawbacks mentioned. So, with our initial experience of laparoscopic duodenojejunal bypass with sleeve gastrectomy, we began a randomized trial comparing it with laparoscopic roux en Y gastric bypass" (Praveen Raj 2012, p. 423) | |
| Notes | BMI: body mass index; DJB: laparoscopic duodenojejunal bypass with sleeve; LRYGB: laparoscopic Roux‐en‐Y gastric bypass; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "patients … were randomized by closed envelope technique" Comment: unclear if there was a random component to the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: closed envelopes used, but no other details provided (i.e. whether or not they were sequentially numbered) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details about blinding or assessment provided |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: attrition and missing data rate not clearly reported |
| Incomplete outcome data (attrition bias) Comorbidities | Unclear risk | Comment: attrition and missing data rate not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial protocol not available and paper does not detail outcomes measured in the methods section |
| Other bias | Unclear risk | Comment: insufficient reporting to determine whether any other source of bias was present |
Schauer 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: aged 20–60 years; diagnosis of type 2 diabetes (glycated haemoglobin level, >7.0%); and BMI of 27 to 43. Exclusion criteria: previous bariatric surgery or complex abdominal surgery; and poorly controlled medical or psychiatric disorders. Diagnostic criteria: type 2 diabetes: glycated haemoglobin level, >7.0%, BMI 27 to 43. |
|
| Interventions |
Number of study centres: 1 Treatment before study: not applicable Titration period: not applicable Interventions: 1. Intensive medical therapy alone (MT) 2. Intensive medical therapy plus laparoscopic Roux‐en‐Y gastric bypass (LRYGB) 3. Intensive medical therapy plus laparoscopic sleeve gastrectomy (LSG) (All patients were treated with lipid‐lowering and antihypertensive medications. Patients in the surgical procedures group received nutrient supplementation following surgery, which differed slightly according to the procedures underwent.) Intensive medical therapy consisted of lifestyle counselling, weight management, home glucose monitoring, new drug therapies, sessions with a diabetes speciality educator, and encouragement to participate in weight watchers. |
|
| Outcomes | Outcomes reported in abstract of publication: comorbidities, weight loss, complications and additional procedures | |
| Study details |
Run‐in period: not applicable Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: commercial and non‐commercial Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "to compare intensive medical therapy with surgical treatment (gastric bypass or sleeve gastrectomy) as a means of improving glycaemic control in obese patients with Type 2 diabetes" (Schauer 2012, p. 1568). "to evaluate the effects of three treatments on glucose regulation, pancreatic B‐cell function (insulin secretion and body composition in a subset of 60 subjects" (Kashyap 2013 p.2176, no data extracted) |
|
| Notes | BMI: body mass index; LRYGB: intensive medical therapy plus laparoscopic Roux‐en‐Y gastric bypass; LSG: intensive medical therapy plus laparoscopic sleeve gastrectomy; MT: intensive medical therapy alone; n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: non‐blinded trial |
| Incomplete outcome data (attrition bias) Weight | High risk | Comment: ITT analysis not conducted. There is an imbalance in missing data across the groups, with more patients withdrawing from the trial in the medical therapy arm than in the gastric bypass or sleeve gastrectomy arms |
| Incomplete outcome data (attrition bias) Comorbidities | High risk | Comment: ITT analysis not conducted. There is an imbalance in missing data across the groups, with more patients withdrawing from the trial in the medical therapy arm than in the gastric bypass or sleeve gastrectomy arms |
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is available. This states that patient self‐report measures of health‐related quality of life were used in the study, but this outcome has not been reported in this publication. |
| Other bias | Low risk | Comment: no evidence of other bias |
Sharma 2013.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: recruited patients all needed to meet the NIH criteria for Bariatric surgery (BMI over 40 or greater than 35 with at least one comorbidity) Exclusion criteria: none reported Diagnostic criteria: BMI > 40, or > 35 with ≥ 1 comorbidity |
|
| Interventions |
Number of study centres: 1 Treatment before study: none reported Titration period: not applicable Interventions: 1. Laparoscopic gastric imbrication 2. Laparoscopic sleeve gastrectomy |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss; complications and additional operative procedures | |
| Study details |
Run‐in period: not reported Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: not reported (authors declared they had no conflicts of interests) Publication status: unpublished manuscript (pending journal decision) |
|
| Stated aim for study | Quote: 'To compare the surgical outcome after sleeve gastrectomy and gastric imbrication in patients with morbid obesity' (Narwaria 2011) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: outcome assessors were blinded to allocation group for the first year, but unblinded for years 2 and 3. However also stated (in Discussion) that ‘the third party administrator who followed the patients for weight‐loss outcomes also randomized the patients. We tried to account for this by having two different people perform each function and not having them communicate with one another about the study’ |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: per protocol analysis. The authors imply that a patient flow chart is available but it was not provided with the manuscript. Without this, numbers of dropouts, and any reasons for dropouts, are not known. Stated 100% follow up but also dropouts and crossovers occurred – no reasons given Comment: baseline and year 2 data not reported for EWL |
| Selective reporting (reporting bias) | Unclear risk | Comment: the manuscript focuses on weight outcomes; results were reported for all outcomes mentioned in the methods. A study protocol with a priori definitions would help to clarify risk of reporting bias |
| Other bias | High risk | Quote: "The one leak in our LGI group can be directly attributed to our lack of experience with the LGI. We are certain if our study had been conducted by one of the prominent LGI groups around the world the leak we experienced would have been avoided" Comment: a source of bias if the surgeons were consistently less skilled at gastric imbrication than sleeve gastrectomy but this only appears to have resulted in a serious outcome in one operation |
Vix 2013.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not reported Inferiority design |
|
| Participants |
Inclusion criteria: not reported other than patients meeting the criteria for bariatric surgery Exclusion criteria: BMI < 40 and > 60; patient preference for a specific procedure; inability to provide informed consent; age < 18 or > 60 years; previous upper or lower gastrointestinal surgery; and hiatal hernia > 2 cm. Diagnostic criteria: BMI 40‐60 |
|
| Interventions |
Number of study centres: 1 Treatment before study: patients presenting with vitamin D deficiency were supplied with cholecalciferol in the preoperative (and also postoperative period) and continued until normalization was achieved. Titration period: n/a Interventions: 1. Laparoscopic Roux‐en‐Y gastric bypass 2. Laparoscopic sleeve gastrectomy |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss | |
| Study details |
Run‐in period: not applicable Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: not reported Publication status: peer reviewed journal |
|
| Stated aim for study | Quote: "To assess postoperative outcomes of sleeve gastrectomy (SG) versus Roux‐en‐Y gastric bypass (RYGB)" | |
| Notes | n/a: not applicable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: stated (Surg Endosc paper) that patients were assigned to group by sealed‐envelope randomisation. Stated (Obes Res paper) “Randomization was achieved using closed envelopes. For the first 100 patients, 120 envelopes were prepared, with an estimated minimum of 10 % failure rate after randomization to the allocated procedure for specific French health insurance issues. Patients in which the randomization procedure failed after medical adviser decision‐making were excluded from the study.” Comment: Neither statement gives sufficient details to judge low risk of bias (e.g. unclear whether envelopes were opaque). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) Weight | Unclear risk | Comment: there are discrepancies in the reported attrition rate between the two linked papers. Obes Surg paper states 8 were lost to follow up of which 7 were in the LSG group (13% of those randomised); Surg Endosc paper states only 1 per group was lost to follow up. Reason given for loss to follow up was lost contact. |
| Selective reporting (reporting bias) | Unclear risk | Comment: results were reported for all outcomes mentioned in the methods; a study protocol with a priori definitions would help to clarify risk of reporting bias |
| Other bias | Low risk | Comment: no evidence of other bias |
Note: where the judgement is 'Unclear' and the description is blank, the study did not report that particular outcome. ITT: intention‐to‐treat QoL: quality of life RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2009 | Study design |
| Albanopoulos 2013 | Outcomes |
| Angrisani 2009 | Intervention |
| Anon 2011 | Study design |
| Arceo‐Olaiz 2008 | Intervention |
| Bockelbrink 2008 | Study design (health technology assessment review) |
| Bond 2009 | Study design, intervention, population, outcomes |
| Bose 2010 | Study design |
| Brimas 2013 | Not an RCT, interventions |
| Buchwald 2009 | Study design |
| Bueter 2011a | Outcomes |
| Bueter 2011b | Outcomes |
| Burguera 2011 | Study design |
| Chronaiou 2012 | Intervention |
| Colquitt 2009 | Study design (systematic review) |
| De Groot 2009 | Study design |
| Fredheim 2011 | Study design |
| Fredheim 2013 | Not an RCT |
| Friedrich 2013 | Not an RCT, outcomes |
| Frige' 2009 | Study design |
| Gagner 2011 | Study design, intervention |
| Garb 2009 | Study design |
| Gehrer 2010 | Study design |
| Guelinckx 2009 | Study design |
| Heindorff 1997 | Outcomes |
| Hofso 2010 | Study design |
| Hofso 2011 | Study design |
| Holty 2011 | Intervention, outcomes |
| Hussain 2009 | Study design |
| Inci 2011 | Intervention |
| Inge 2009 | Study design, population |
| Jonnalagadda 2012 | Intervention |
| Keating 2009 | Study design |
| Kolotkin 2009 | Study design |
| Lancaster 2008 | Study design, outcomes |
| Lanzi 2011 | Study design |
| Lee 2005 | Intervention (comparison of surgical technique ‐ gastric bypass vs mini gastric bypass) |
| Lee 2011b | Population |
| Lewis 2012 | Study design |
| Lin 2011 | Study design |
| Mummadi 2008 | Study design |
| Mundet 2008 | Study design (commentary article) |
| Nordstrand 2011 | Study design |
| Nordstrand 2012 | Study design, intervention |
| O'Brien 2010 | Population |
| O'Brien 2013 | Not an RCT |
| Oude 2009a | Study design |
| Oude 2009b | Study design |
| Papalazarou 2010 | Intervention |
| Picot 2009 | Study design (systematic review) |
| Pietri 2012 | Study design |
| Pokala 2012 | Study design |
| Pollock 2013 | Not an RCT |
| Pontiroli 2009 | Study design |
| Praveen Raj 2012b | Study design, intervention |
| Rebecchi 2011 | Redundant intervention |
| Rico Hernandez 2009 | Intervention |
| Schouten 2010 | Study design, outcomes |
| Schouten 2010b | Redundant intervention |
| Scozzari 2009 | Redundant intervention |
| Sjostrom 2008 | Outcomes |
| Sjostrom 2009 | Study design |
| Skroubis 2013 | Not an RCT |
| Tice 2008 | Study design |
| Treadwell 2008 | Study design |
| Varela 2011 | Study design |
| Werling 2013 | Redundant intervention |
BMI: body mass index RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Cesana 2013.
| Methods | RCT |
| Participants | Patients with morbid obesity (duration unspecified); sample size not stated |
| Interventions | Laparoscopic gastric plication Laparoscopic sleeve gastrectomy |
| Outcomes | Assessed at 12 months: Weight loss: BMI reduction, percentage excess weight lost. Postoperative complications. Operative time. |
| Notes |
Dadan 2011.
| Methods | RCT |
| Participants | Patients with 'morbid obesity at least six months after surgical intervention'. Sample size unclear ‐ stated 45 patients 'qualified to the study' |
| Interventions | Laparoscopic adjustable gastric banding Roux‐en‐Y gastric bypass (not stated whether laparoscopic) |
| Outcomes | Timing of assessment not reported: Quality of life: based on Bariatric Analysis and Reporting Outcome System (BAROS) questionnaire. Result of surgical treatment of obesity (appears to be based on nominal classes ranging from 'excellent' to 'unsuccessful'). |
| Notes |
Darabi 2013.
| Methods | RCT |
| Participants | Patients with morbid obesity (duration unspecified), 20 randomised per group |
| Interventions | Laparoscopic gastric plication Laparoscopic mini‐gastric bypass |
| Outcomes | Assessed at 12 months: Weight loss: percentage excess weight lost. Comorbidities including hyperlipidaemia and iron deficiency. Re‐hospitalisation and reoperation. Operative time. Length of hospital stay. |
| Notes |
BMI: body mass index RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ISRCTN 00786323.
| Trial name or title | BY‐BAND |
| Methods | RCT, parallel group |
| Participants | BMI 40 or more, or BMI 35 to 40 and other co‐morbidities (e.g. type 2 diabetes), that could improve with weight loss |
| Interventions | Laparoscopic adjustable gastric banding Laparoscopic gastric bypass |
| Outcomes | Proposed assessment at 3 years: Weight loss. Surgical complications. Nutritional outcomes. Symptoms. Quality of life. National Health Service value for money. |
| Starting date | Not reported; estimated completion date March 2016 |
| Contact information | Professor Jane Blazeby, University of Bristol, UK |
| Study identifier | ISRCTN 00786323 |
| Official title | Gastric BYpass or adjustable gastric BANDing surgery to treat morbid obesity: a multi‐centre randomised controlled trial ‐ The BY‐BAND Trial |
| Stated purpose of study | To compare the effectiveness, cost effectiveness and acceptability of laparoscopic adjustable gastric banding and laparoscopic gastric bypass |
| Notes | Estimated enrolment 724 patients in eight hospitals |
NCT00432809.
| Trial name or title | STAMPEDE trial |
| Methods | RCT, parallel group |
| Participants | BMI > 27 and < 43; type 2 diabetes mellitus with HbA1c > 7.0% |
| Interventions | Laparoscopic Roux‐en‐Y gastric bypass Laparoscopic sleeve gastrectomy Intensive medical therapy for diabetes |
| Outcomes | Proposed assessment at 12 months: Body weight, BMI and their changes from baseline. Resolution of type 2 diabetes (defined on glycated haemoglobin) (primary outcome). Obesity‐related comorbidities (hypertension, dyslipidaemia). Quality of life (instrument(s) not specified). Adverse events, complications, hospitalisations. Insulin resistance and secretion, glycated haemoglobin. Cost‐effectiveness of each intervention. Blood pressure. Use of diabetes and cardiovascular medications. |
| Starting date | February 2007; estimated completion date January 2016 |
| Contact information | Philip R Schauer, MD, Director, Bariatric and Metabolic Institute, Cleveland Clinic Foundation, Cleveland, Ohio, United States |
| Study identifier | NCT00432809 |
| Official title | STAMPEDE: Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently |
| Stated purpose of study | To compare the relative clinical outcomes between advanced medical therapy alone or advanced medical therapy combined with bariatric surgery [either Roux‐en‐Y gastric bypass (RYGBP) or laparoscopic sleeve gastrectomy] in patients with type 2 diabetes and a body mass index (BMI) between 27 and 43 kg/m2 |
| Notes | Estimated enrolment 150 |
NCT01047735.
| Trial name or title | The TRIABETES study: A trial to compare surgical and medical treatments for type 2 diabetes |
| Methods | RCT, parallel group |
| Participants | BMI 30‐35 with difficult to control type 2 diabetes mellitus requiring antidiabetic medication; BMI 35‐40 with type 2 diabetes mellitus |
| Interventions | Laparoscopic Roux‐en‐Y gastric bypass Laparoscopic adjustable gastric banding Lifestyle weight‐loss intervention |
| Outcomes | Proposed assessment at 12 months: Weight loss. Feasibility of performing an RCT comparing these three interventions (stated primary outcome). |
| Starting date | September 2009; estimated completion date April 2015 |
| Contact information | Anita P Courcoulas, MD, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States |
| Study identifier | NCT01047735 |
| Official title | A Randomized Trial to Compare Surgical and Medical Treatments for Type 2 Diabetes |
| Stated purpose of study | To obtain preliminary information regarding the effectiveness of two major types of bariatric surgery, Laparoscopic Roux‐en‐Y Gastric Bypass and Laparoscopic Adjustable Gastric Banding versus an intensive lifestyle intervention to induce weight loss with diet and increased physical activity |
| Notes | Estimated enrolment 60 |
NCT01053130.
| Trial name or title | Not reported |
| Methods | RCT, parallel group |
| Participants | BMI 35‐45 with stage 3‐4 chronic kidney disease |
| Interventions | Laparoscopic sleeve gastrectomy Lifestyle modification with diet, exercise and pharmacotherapy |
| Outcomes | Proposed assessment at 12, 24, 36 months unless stated: Weight, BMI. Body composition (waist and hip circumference, body fat). Quality of life and depression. Composite cardiovascular and mortality outcome. Insulin resistance (12 months). Kidney function (primary outcome) (12 months). Kidney function, immunological and obesity markers (12 months). |
| Starting date | January 2010; estimated completion date January 2014 |
| Contact information | Ms Helen L MacLaughlin, King's College Hospital, London, UK |
| Study identifier | NCT01053130 |
| Official title | The Effect of Weight Loss Surgery on Preservation of Kidney Function and Cardiovascular Disease Risk Factors in Obese Patients With Stages 3‐4 Chronic Kidney Disease: a Randomised Controlled Trial |
| Stated purpose of study | To evaluate weight loss surgery vs lifestyle modification in patients with chronic kidney disease with estimated kidney function of 20‐60% and morbid obesity (BMI 35‐45) in terms of kidney function, cardiovascular disease risk factors and all‐cause mortality. |
| Notes | Estimated enrolment 60 |
NCT01073020.
| Trial name or title | Surgery or Lifestyle with Intensive Medical Management in the Treatment of Type 2 Diabetes (SLIMM‐T2D) |
| Methods | RCT, parallel group |
| Participants | BMI 30‐45 (comparison 1) or BMI 30‐42 (comparison 2); type 2 diabetes of duration ≥ 1 year |
| Interventions | Laparoscopic adjustable gastric band Laparoscopic Roux‐en‐Y gastric bypass Intensive medical diabetes and weight management programme ('Why WAIT?') Comparison 1 = Laparoscopic adjustable gastric band compared to Intensive medical diabetes and weight management programme; Comparison 2 = Laparoscopic Roux‐en‐Y gastric bypass compared to Intensive medical diabetes and weight management programme |
| Outcomes | Proposed assessment at 12 months: Glycaemic control (defined in terms of fasting plasma glucose levels) (primary outcome). Quality of life (instrument(s) not specified). Cost utility. Metabolic factors and cardiovascular risk markers. |
| Starting date | January 2010; estimated completion date August 2013 (results remain unpublished as at 15 January 2014) |
| Contact information | Allison B. Goldfine, MD, Joslin Diabetes Center, Boston, Massachusetts, United States |
| Study identifier | NCT01073020 |
| Official title | Surgery or Lifestyle With Intensive Medical Management in the Treatment of Type 2 Diabetes (SLIMM‐T2D) |
| Stated purpose of study | 'This trial investigates the utility of currently practiced and available bariatric surgical procedures as compared with multidisciplinary intensive medical and weight management for the treatment of T2DM with class 1 and 2 obesity.' |
| Notes | Estimated enrolment 100 |
NCT01352403.
| Trial name or title | Wurzburg Adipositas Study (WAS) |
| Methods | RCT, parallel group |
| Participants | BMI > 40 without concomitant diseases; BMI > 35 with concomitant diseases |
| Interventions | Gastric bypass Intensive lifestyle intervention |
| Outcomes | Proposed assessment at 12 months: Quality of life (SF‐36). Cardiac fitness (VO2) (primary outcome). |
| Starting date | May 2011; estimated completion date June 2016 |
| Contact information | Prof. Dr. Bruno Allolio, University hospital of Wuerzburg, Wuerzburg, Germany |
| Study identifier | NCT01352403 |
| Official title | Severe Obesity: Bariatric Surgery vs. Life‐Style‐Intervention Wurzburg Adipositas Study – WAS |
| Stated purpose of study | To investigate the effects of gastric bypass in comparison to a intensive life style intervention on cardiac function and quality of life in patients with morbid obesity. |
| Notes | Estimated enrolment 60 |
NCT01486680.
| Trial name or title | Not reported |
| Methods | RCT, parallel group |
| Participants | BMI 35‐65; type 2 diabetes mellitus for at least 6 months |
| Interventions | Laparoscopic Roux‐en‐Y gastric bypass Laparoscopic sleeve gastrectomy |
| Outcomes | Proposed assessment at 5 years unless stated: Weight loss. Remission of type 2 diabetes (complete and partial) (primary outcome). Comorbidity resolution. Perioperative and postoperative morbidity and mortality (also at 1 and 5 years). Body composition, bone density and resting energy expenditure (1 and 5 years). Quality of life (SF‐36, Anxiety depression scale) (1 and 5 years). |
| Starting date | September 2011; estimated completion date October 2018 |
| Contact information | Contact: Dr Michael Booth, North Shore Hospital, Auckland, New Zealand |
| Study identifier | NCT01486680 |
| Official title | Prospective Randomised Controlled Trial Comparing the Efficacy of Laparoscopic Silastic Ring Roux‐en‐Y Gastric Bypass Versus Laparoscopic Sleeve Gastrectomy for the Management of Type 2 Diabetes Mellitus in Obese Patients |
| Stated purpose of study | To compare which of these two surgical procedures is most effective at treating T2DM in obese patients, as well as comparing whether there are any differences in the amount of weight lost, side effects and quality of life. |
| Notes | Estimated enrolment 106 |
NCT01501201.
| Trial name or title | Comparison of gastric by‐pass and optimized medical treatment in obese diabetic patients (DIABSURG) |
| Methods | RCT, parallel group, phase IV |
| Participants | BMI > 35 and < 50; type 2 diabetes mellitus with HbA1c > 7.5 %; treated with GLP1 (glucagon‐like peptide) analogue or insulin |
| Interventions | Laparoscopic Roux‐en‐Y gastric bypass Optimised medical management (for obesity and poorly controlled type 2 diabetes) |
| Outcomes | Proposed assessment: Weight loss (at 2 years). Glycemic control (at 2 years). Quality of life (instrument(s) not specified) (at 2 years). Mortality (up to 7 and 10 years) (primary outcome). Cost, cost‐effectiveness and cost‐utility (2 years). |
| Starting date | February 2011; estimated completion date September 2021 |
| Contact information | Francois Pattou, Professor, University Hospital, Lille, France |
| Study identifier | NCT01501201 |
| Official title | Comparison of Gastric By‐Pass and Optimized Medical Treatment in Obese Diabetic Patients in Terms of Mortality, Glycemic Control, and Cost Effectiveness ‐ Prospective, Multicenter, Randomized Study |
| Stated purpose of study | To compare the results of the Gastric By‐Pass (GBP) to that of optimised medical therapy in patients with obesity and poorly controlled type 2 diabetes in terms of mortality, weight loss, glycaemic control, quality of life, cost, cost‐effectiveness and cost utility of these two strategies. |
| Notes | Estimated enrolment 490, multi‐centre (number of centres not reported) |
NCT01581801.
| Trial name or title | Not reported |
| Methods | RCT, parallel group |
| Participants | BMI 35 (in presence of complications e.g. sleep apnoea, severe coxarthritis or gonarthritis, severe hypertension) up to BMI 50; excluding patients with history of type 1 or 2 diabetes |
| Interventions | Laparoscopic Roux‐en‐Y gastric bypass Laparoscopic sleeve gastrectomy |
| Outcomes | Proposed assessment at 12 months unless stated: Weight, BMI. Body composition including abdominal circumference. Lipid profile. Reactive hypoglycaemia incidence (primary outcome). Hypoglycaemia symptoms (within 5 years of surgery). Insulin sensitivity and secretion. Cardiovascular system abnormalities. |
| Starting date | October 2012; estimated completion date December 2014 |
| Contact information | Geltrude Mingrone, MD, Catholic University School of Medicine, Rome, Italy |
| Study identifier | NCT01581801 |
| Official title | Randomized Clinical Study Comparing the Effect of Roux‐en‐Y Gastric Bypass and Sleeve Gastrectomy on Reactive Hypoglycemia |
| Stated purpose of study | To conduct a 1‐year randomised trial to compare the incidence of hypoglycaemia after laparoscopic Roux‐en‐Y gastric bypass or laparoscopic sleeve gastrectomy |
| Notes | Estimated enrolment 50 |
NCT01778738.
| Trial name or title | Type 2 Diabetes after sleeve gastrectomy and Roux‐en‐Y gastric bypass: A randomised single centre study (OSEBERG) |
| Methods | RCT, parallel group |
| Participants | BMI ≥ 35; type 2 diabetes with current HbA1c ≥ 6.5 % or use of oral anti‐diabetic medications; excluding patients with previous bariatric surgery |
| Interventions | Gastric bypass Sleeve gastrectomy |
| Outcomes | Proposed assessment at 12 months: Weight loss, BMI. Remission of type 2 diabetes (primary outcome). Nocturnal blood pressure reduction. Carotid‐to‐femoral pulse wave velocity. |
| Starting date | January 2013; estimated completion date January 2020 |
| Contact information | Jøran Hjelmesæth, MD. PhD, The Morbid Obesity Center, Vestfold Hospital Trust, Vestfold, Norway |
| Study identifier | NCT01778738 |
| Official title | Glycaemia, Insulin Secretion and Action in Morbidly Obese Subjects With Type 2 Diabetes After Sleeve Gastrectomy and Roux‐en‐Y Gastric Bypass: A Randomised Single Centre Study |
| Stated purpose of study | Not explicitly stated |
| Notes | Estimated enrolment 120 |
NCT01821508.
| Trial name or title | Not reported |
| Methods | RCT, parallel group |
| Participants | BMI 30‐35; patients with microalbuminuria and receiving pharmacological treatment for type 2 diabetes mellitus; diabetes diagnosis not more than 15 years before recruitment |
| Interventions | Roux‐en‐Y gastric bypass Clinical treatment of type 2 diabetes mellitus (best and most modern available) |
| Outcomes | Proposed assessment at 24 months unless stated: Quality of life (SF‐36) (12 and 24 months). Glycaemic control (fasting glucose and glycated haemoglobin). Discontinuation of type 2 diabetes mellitus medication. Normalisation of lipids. Normalisation of blood pressure. Normalisation of albumin/creatinine ratio (primary outcome). Retinopathy reversal. Development or worsening of peripheral neuropathy. |
| Starting date | March 2013; estimated completion date April 2015 |
| Contact information | Ricardo V Cohen, MD, PhD, Hospital Alemão Oswaldo Cruz, São Paulo, Brazil |
| Study identifier | NCT01821508 |
| Official title | Prospective, Open, Randomized, Unicenter Study Comparing Roux‐en‐Y Gastric Bypass With the Best Clinical Treatment Regarding Improvement of Microvascular Complications of Type 2 Diabetes Mellitus in Obese Patients |
| Stated purpose of study | To evaluate the effects of Roux‐en‐Y gastric bypass in the control of diabetic nephropathy in diabetic patients with BMI between 30 and 35 |
| Notes | Estimated enrolment 72 |
NCT01929850.
| Trial name or title | Not reported |
| Methods | RCT, cross‐over design |
| Participants | Female; of a medically underserved, rural, poor or under‐represented minority (based on specified criteria); history of obesity at least 2.5 years; BMI > 40 and < 55, or BMI > 35 and <55 with one or more significant comorbidities (defined as diabetes, pulmonary disease, cardiac disease, or hypertension). |
| Interventions | Laparoscopic sleeve gastrectomy Intensive medically supervised nutritional and exercise therapy ('Weight Watchers 360') |
| Outcomes | Proposed assessment at 12 months: BMI (primary outcome). Quality of life (instrument(s) not reported). |
| Starting date | October 2013; estimated completion date October 2018 |
| Contact information | John P Cello, MD, University of California, San Francisco / San Francisco General Hospital, California, United States |
| Study identifier | NCT01929850 |
| Official title | Bariatric Surgery Plus Weight Watchers vs. Weight Watchers in Underserved Minorities: Randomized Controlled Cross‐over Trial |
| Stated purpose of study | Not explicitly stated |
| Notes | Estimated enrolment 100 |
BMI: body mass index RCT: randomised controlled trial SF‐36: Short form 36
Differences between protocol and review
To ensure that this review is kept relevant to current practice, a number of changes were made to the protocol of the current update.
Participants
Two changes to the inclusion criteria of participants have been made:
The current update is limited to adults. The 2009 version of this review had been expanded to include people of all ages undergoing surgery for obesity to reflect current guidelines (Buchwald 2005; NICE 2006) and indications from the literature that weight‐loss surgery is undertaken in people under the age of 18. However, it has since been decided that bariatric surgery in children and adolescents would be better considered within the Cochrane review 'Interventions for treating obesity in children'.
The definition of obesity was altered to include overweight or obesity as defined by eligible studies. In the 2009 version of this review, obesity was defined as BMI greater than 30 with serious comorbid disease. However, bariatric surgery may now be undertaken in people with a BMI less than 30.
Interventions
The following interventions were excluded from the current update as they are no longer in current practice.
Vertical banded gastroplasty.
Banded gastric bypass.
Biliopancreatic diversion (without duodenal switch).
In addition, comparisons of the same procedure undertaken with open surgery versus laparoscopic surgery were excluded.
Study design
Only randomised controlled trials (RCTs) were eligible for the current update. The 2009 version of this review included controlled clinical trials (CCTs) and prospective cohort studies comparing surgical interventions with non‐surgical treatment, as few RCTs were anticipated. However, the evidence base has since increased.
Searches
The following searches for ongoing studies were conducted for the 2009 version of this review.
National Research Register (until 30/7/2008).
UK Clinical Rearch Network (until 30/7/2008).
Clinical Trials.gov (until 30/7/2008).
Controlled Clinical Trials (until 30/7/2008).
Australia NZ Clinical Trial Register (until 30/7/2008).
However, National Research Register no longer exists, and Australia and New Zealand Clinical Trials are adequately covered by WHO International Clinical Trails Registry Platform (WHO ICTRP). For the current update the following databases were searched for ongoing studies.
UK Clinical Rearch Network.
ClinicalTrials.gov.
Controlled‐trials.com.
WHO International Clinical Trails Registry Platform (WHO ICTRP).
Authors
Two authors (Andrew Clegg and Joanna Picot) of the previous version of the review were not involved in the current update. Two additional authors (Karen Pickett and Geoff Frampton) contributed to this update.
Assessment of reporting bias
We intended to use funnel plots to assess small‐study effects where there were 10 studies or more for a given outcome, however, there were no instances where this was possible.
Subgroup analysis
We planned to carry out subgroup analyses on different degrees of obesity (as measured by the BMI (BMI 30 to 40), (BMI 40 to 50) (BMI > 50)); sex, length of follow‐up; and type of surgical procedure, however there were not sufficient data available for these analyses to be undertaken.
Sensitivity analysis
We planned to perform a range of sensitivity analyses to explore their influence on effect sizes (restricting the analysis to published studies, to account for risk of bias, to very long or large studies, and to studies using filters such as diagnostic criteria, language of publication, source of funding or county), however there were not sufficient data available for these analyses to be undertaken.
Contributions of authors
Jill L Colquitt (JC): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and update draft.
Karen Pickett (KP): acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and update draft
Emma Loveman (EL): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and update draft.
Geoff Frampton (GF): acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and update draft
Sources of support
Internal sources
NIHR HealthTechnology Assessment Programme (project number 08/06/01), UK.
External sources
No sources of support supplied
Declarations of interest
JC: None known.
KP: None known.
EL: None known.
GF: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Aasheim 2009 {published data only}
- Aasheim ET, Bjorkman S, Sovik TT, Engstrom M, Hanvold SE, Mala T, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. American Journal of Clinical Nutrition 2009;90:15‐22. [DOI] [PubMed] [Google Scholar]
- Olsén MF, Wiklund M, Lönroth H, Olbers T. Respiratory function in superobese patients before and after bariatric surgery ‐ a randomised controlled trial. Open Obesity Journal 2012;4:28‐34. [Google Scholar]
- Sovik TT, Aasheim ET, Taha O, Engström M, Fagerland MW, Björkman S, et al. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: A randomized trial. Annals of Internal Medicine 2011;155(5):281‐91. [DOI] [PubMed] [Google Scholar]
- Sovik TT, Karlsson J, Aasheim ET, Fagerland MW, Björkman S, Engström M, et al. Gastrointestinal function and eating behaviour after gastric bypass and duodenal switch. Surgery for Obesity and Related Diseases 2013;9(4):641‐7. [DOI] [PubMed] [Google Scholar]
- Sovik TT, Taha O, Aasheim ET, Engström M, Kristinsson J, Björkman S, et al. Randomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesity. British Journal of Surgery 2010;97:160‐6. [DOI] [PubMed] [Google Scholar]
Angrisani 2007 {published data only}
- Angrisani A, Lorenzo M, Borrrelli V. Laparoscopic adjustable gastric banding versus Roux‐en‐Y gastric bypass: 5‐year results of a prospective randomized trial. Surgery for Obesity and Related Diseases 2007;3:127‐33. [DOI] [PubMed] [Google Scholar]
- Angrisani L, Cutulo PP, Formisano G, Nosso G, Vitolo G. Laparoscopic adjustable gastric banding versus Roux‐en‐Y gastric bypass: 10‐year results of a prospective randomised trial. Surgery for Obesity and Related Diseases 2013;9(3):405‐13. [DOI] [PubMed] [Google Scholar]
Demerdash 2013 {published data only}
- Demerdash HM, Sharara G, Katri K. Differential effects of gastric bypass and banding on the cardiovascular risk profile in morbidly obese subjects: The correlation with plasma apolipoprotein A‐IV concentration. Alexandria Journal of Medicine 2013;49:17‐23. [Google Scholar]
Dixon 2008 {published data only}
- Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299(3):316‐23. [DOI] [PubMed] [Google Scholar]
Dixon 2012 {published data only}
- Dixon J, Schachter L, O'Brien P, Jones K, Grima M, Lambert G, et al. Surgical versus conventional therapy for weight loss treatment of obstructive sleep apnea: A randomized controlled trial. Sleep and Biological Rhythms 2012;10 Suppl 1:40‐1. [DOI] [PubMed] [Google Scholar]
- Dixon JB, Schachter LM, O'Brien PE, Jones K, Grima M, Lambert G, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012;308:1142‐9. [DOI] [PubMed] [Google Scholar]
- Dixon JB, Schachter LM, O'Brien PE, Jones KM, Grima MT, Brown WA, et al. Surgical versus conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2012;185:A3854. [DOI] [PubMed] [Google Scholar]
Hedberg 2012 {published data only}
- Hedberg J, .Sundbom M. Superior weight loss and lower HbA1c 3 years after duodenal switch compared with Roux‐en‐Y gastric bypass‐‐a randomized controlled trial. Surgery for Obesity & Related Diseases 2012;8:338‐43. [DOI] [PubMed] [Google Scholar]
Himpens 2006 {published data only}
- Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obesity Surgery 2006;16(11):1450‐6. [DOI] [PubMed] [Google Scholar]
Ikramuddin 2013 {published data only}
- Ikramuddin S, Korner J, Lee W‐J, Connett JE, Inabnet WB, Billington CJ, et al. Roux‐en‐Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia. JAMA 2013;309(21):2240‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karamanakos 2008 {published data only}
- Karamanakos SN, Kehagias I, Argentou M, Alexandridis T, Kalfarentzos F. Is sleeve gastrectomy more effective than Roux‐en‐Y gastric bypass? Results from a randomized clinical trial. Obesity Surgery 2011;21:956‐1156. [DOI] [PubMed] [Google Scholar]
- Karamanakos SN, Kehagias I, Argentou M, AlexandridisT, Kalfarentzos F. Is sleeve gastrectomy more effective than Roux‐en‐Y gastric bypass? Results from a randomized clinical trial. Obesity Surgery 2011;21(8):959‐960. [DOI] [PubMed] [Google Scholar]
- Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide‐YY levels after Roux‐en‐Y gastric bypass and sleeve gastrectomy. A prospective double blind study. Annals of Surgery 2008;247(3):401‐7. [DOI] [PubMed] [Google Scholar]
- Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI <50 kg/m2. Obesity Surgery 2011;21:1650‐6. [DOI] [PubMed] [Google Scholar]
Keidar 2013 {published data only}
- Hershkop KJ, Keidar A, schweiger C, Hecht L, Weiss R. Roux‐en‐Y gastric bypass vs. sleeve gastrectomy for obese patients with Type 2 diabetes: A randomized trial. Diabetes 2012;Conference:A505. [DOI] [PubMed] [Google Scholar]
- Keidar A, Hershkop KJ, Marko L, Schweiger C, Hecht L, Bartov N, et al. Roux‐en‐Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia 2013;56:1914‐8. [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
Liang 2013 {published data only}
- Liang Z, Wu Q, Chen B, Yu, P, Zhao H, Ouyang X. Effect of laparoscopic Roux‐en‐Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: A randomized controlled trial. Diabetes Research and Clinical Practice 2013;101:50‐6. [DOI] [PubMed] [Google Scholar]
Mingrone 2012 {published data only}
- Mingrone G, Panunzi S, De GA, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes.. New England Journal of Medicine 2012;366:1577‐85. [DOI] [PubMed] [Google Scholar]
Nguyen 2009 {published data only}
- Nguyen NT, Slone JA, Nguyen X‐M, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: Outcomes, quality of life, and costs.. Annals of Surgery 2009;250:631‐9. [DOI] [PubMed] [Google Scholar]
Nogués 2010 {published data only}
- Nogués X, Goday A, Pena MJ, Benaiges D, de RM, Crous X, et al. Bone mass loss after sleeve gastrectomy: A prospective comparative study with gastric bypassPerdida de masa osea tras gastrectomia tubular: Estudio prospectivo comparativo con el bypass gastrico.. Cirugia Espanola 2010;88:103‐9. [DOI] [PubMed] [Google Scholar]
- Ramon JM, Salvans S, Crous X, Puig S, Goday A, Benaiges D, et al. Effect of Roux‐en‐Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial.. Journal of Gastrointestinal Surgery 2012;16:1116‐22. [DOI] [PubMed] [Google Scholar]
O'Brien 2006 {published data only}
- Dixon JB, Strauss BJ, Laurie C, O'Brien PE. Changes in body composition with weight loss: obese subjects randomized to surgical and medical programs. Obesity 2007;15(5):1187‐98. [DOI] [PubMed] [Google Scholar]
- O'Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Annals of Internal Medicine 2006;144(9):625‐33. [DOI] [PubMed] [Google Scholar]
Paluszkiewicz 2012 {published data only}
- Paluszkiewicz R, Kalinowski P, Wroblewski T, Bartoszewicz Z, Biaolbrzeska‐Paluszkiewicz J, Ziarkiewicz‐Wroblewska B, et al. Prospective randomized clinical trial of laparoscopic sleeve gastrectomy versus open Roux‐en‐Y gastric bypass for the management of patients with morbid obesity. Videosurgery and other miniinvasive techniques 2012;7:225‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz R, Kalinowski P, Wróblewski T, Bialobrzeska‐Paluszkiewicz J, Ziarkiewicz‐Wróblewska B, Remiszewski P, et al. Improvement of metabolic parameters after laparoscopic sleeve gastrectomy (LSG) versus Roux‐En‐Y gastric bypass (RYGB) ‐ Early results of a prospective randomized trial. Obesity Surgery 2011;21(8):983‐4. [Google Scholar]
Peterli 2012 {published and unpublished data}
- Gass M, Kern B, Peters T, Christoff‐Courtin C, Hendrickson P, Flue M, et al. Laparoscopic sleeve‐gastrectomy (LSG) and laparoscopic Roux‐en‐Y gastric bypass (LRYGB): mid‐term results of a prospective randomized trial. Obesity Surgery 2011;21:1109. [Google Scholar]
- Peterli R, Borbély Y, Kern B, Gass M, Peters T, Thurnheer M, et al. Early Results of the Swiss Multicentre Bypass Or Sleeve Study (SM‐BOSS): A prospective randomized trial comparing laparoscopic Sleeve Gastrectomy and Roux‐Y‐Gastric Bypass. Annals of Surgery 2013;258(5):690‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterli R, Kern B, Peters T, Christoffel‐Courtin C, Hendrickson P, Fle M. Equal effectiveness of laparoscopic sleeve‐gastrectomy (LSG)and laparoscopic Roux‐Y‐gastric bypass (LRYGB): mid‐term results of a prospective randomized trial. Obesity Surgery 2009;19(8):1046. [Google Scholar]
- Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel‐Court, Gass M, et al. Metabolic and hormonal changes after laparoscopic Roux‐en‐Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial.. Obesity Surgery 2012;22:740‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Praveen Raj 2012 {published data only}
- Praveen Raj P, Kumaravel R, Chandramaliteeswaran C, Rajpandian S, Palanivelu C. Is laparoscopic duodenojejunal bypass with sleeve an effective alternative to Roux en Y gastric bypass in morbidly obese patients: preliminary results of a randomized trial. Obesity Surgery 2012;22:422‐6. [DOI] [PubMed] [Google Scholar]
Schauer 2012 {published data only}
- Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul‐Ghani M, Abood B, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and Type 2 diabetes. Analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care 2013;36:2175‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SK, Samat A, Wolski K, Abood B, Pothier CE, Bhatt DL, et al. Improved acylated grehlin supression at 2 years in obese patients with type 2 duabetes: effects of bariatric surgery versus standard medical therapy. International Journal of Obesity in press. [DOI: 10.1038/ijo2013.196] [DOI] [PMC free article] [PubMed]
- Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. New England Journal of Medicine 2012;366(17):1567‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2013 {published and unpublished data}
- Narwaria M, Cottam D, Sharma S. Laparoscopic gastric imbrication versus sleeve gastrectomy ‐ a prospective single blind randomized trial. Obesity Surgery. 2011; Vol. 21:1017. [DOI] [PubMed]
- Sharma S, Narwaria M, Cottam DR. Randomized double blinded trial of laparoscopic gastric imbrication v laparoscopic sleeve gastrectomy at a single Indian institution. Unpublished manuscript provided by author. [DOI] [PubMed]
Vix 2013 {published data only}
References to studies excluded from this review
Adams 2009 {published data only}
- Adams TD, Pendleton RC, Strong MB, Kolotkin RL, Walker JM, Litwin E, et al. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity 2009;18(1):121‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albanopoulos 2013 {published data only}
- Albanopoulos K, Tsamis D, Natoudi M, Memos N, Flessas I, Louizos A, et al. Laparoscopic gastric mini bypass vs. Laparoscopic sleeve gastrectomy for treatment of morbid obesity. Preliminary results of a prospective randomized study. Obesity Surgery 2013;23(8):1138. [Google Scholar]
Angrisani 2009 {published data only}
- Angrisani L, Cutolo PP, Ciciriello MB, Vitolo G, Persico F, Lorenzo M, et al. Laparoscopic adjustable gastric banding with truncal vagotomy versus laparoscopic adjustable gastric banding alone: interim results of a prospective randomized trial. Surgery for Obesity & Related Diseases 2009;5(4):435‐8. [DOI] [PubMed] [Google Scholar]
Anon 2011 {published and unpublished data}
- Anonymous. Laparoscopic sleeve gastrectomy for obesity. OR Manager 2011;27(5):15‐9. [PubMed] [Google Scholar]
Arceo‐Olaiz 2008 {published data only}
- Arceo‐Olaiz R, Espana‐Gomez MN, Montalvo‐Hernandez J, Velazquez‐Fernandez D, Pantoja JP, Herrera MF. Maximal weight loss after banded and unbanded laparoscopic Roux‐en‐Y gastric bypass: a randomized controlled trial. Surgery for Obesity & Related Diseases 2008;4(4):507‐11. [DOI] [PubMed] [Google Scholar]
Bockelbrink 2008 {published and unpublished data}
- Bockelbrink A, Stoeber Y, Roll S, Vauth C, Willich SN, Greiner W. Evaluation of medical and health economic effectiveness of bariatric surgery (obesity surgery) versus conservative strategies in adult patients with morbid obesity. GMS Health Technology Assessment 2008;4:1‐10. [PMC free article] [PubMed] [Google Scholar]
Bond 2009 {published data only}
- Bond DS, Phelan S, Leahey TM, Hill JO, Wing RR. Weight‐loss maintenance in successful weight losers: surgical vs non‐surgical methods. International Journal of Obesity 2009;33(1):173‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bose 2010 {published data only}
- Bose M, Machineni S, Olivan B, Teixeira J, McGinty JJ, Bawa B, et al. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity 2010;18(6):1085‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brimas 2013 {published data only}
- Brimas E, Brimas G. Gastroesophageal reflux disease after laparoscopic adjustable gastric banding in morbidly obese patients. Obesity Surgery 2013;23(8):1062‐3. [Google Scholar]
Buchwald 2009 {published data only}
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta‐analysis. American Journal of Medicine 2009;122(3):248‐56. [DOI] [PubMed] [Google Scholar]
Bueter 2011a {published data only}
- Bueter M, Theis N, Werling M, Ashrafian H, Lowenstein C, Bloom SR, et al. Roux‐en‐y gastric bypass affects the preference for high fat food in rats and humans. Obesity Surgery 2011;Conference(var.pagings):1016. [Google Scholar]
Bueter 2011b {published data only}
- Bueter M, Olbers T, Lutz TA, Roux CW. Changes in hormones and energy expenditure after bariatric surgery. Obesity Surgery 2011;Conference(var.pagings):8‐967. [Google Scholar]
Burguera 2011 {published data only}
- Burguera B, Tur J, Luque L, Alos M, Pagan A, Salinas R, et al. TRAMOMTANA (multidisciplinary treatment of morbid obesity: Behavioral therapy, nutritional support and physical activity). Obesity 2011;Conference(var.pagings):S117. [DOI] [PubMed] [Google Scholar]
Chronaiou 2012 {published data only}
- Chronaiou A, Tsoli M, Kehagias I, Leotsinidis M, Kalfarentzos F, Alexandrides TK. Lower ghrelin levels and exaggerated postprandial peptide‐YY, glucagon‐like peptide‐1, and insulin responses, after gastric fundus resection, in patients undergoing Roux‐en‐Y gastric bypass: a randomized clinical trial. Obesity Surgery 2012;22(11):1761‐70. [DOI] [PubMed] [Google Scholar]
Colquitt 2009 {published data only}
- Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. [Review] [157 refs][Update of Cochrane Database Syst Rev 2005;(4):CD003641;PMID:16235331]. [DOI] [PubMed] [Google Scholar]
De Groot 2009 {published data only}
- Groot NL, Burgerhart JS, Meeberg PC, Vries DR, Smout AJPM, Siersema PD. Systematic review: the effects of conservative and surgical treatment for obesity on gastro‐oesophageal reflux disease. Alimentary Pharmacology & Therapeutics 2009;30(11‐12):1091‐102. [DOI] [PubMed] [Google Scholar]
Fredheim 2011 {published data only}
- Fredheim JM, Rollheim J, Hjelmesaeth J. Effect of bariatric surgery and intensive lifestyle intervention on obstructive sleep apnea: A controlled clinical trial. Obesity Reviews 2011;Conference(var.pagings):207. [Google Scholar]
Fredheim 2013 {published data only}
- Fredheim JM, Rollheim J, Sandbu R, Hofso D, Omland T, Roislien J, et al. Obstructive spleep apnea after weightloss: a clinical trial comparing gastric bypass and intensive lifestyle intervention. Journal of Clinical Sleep Medicine 2013;9(5):427‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedrich 2013 {published data only}
- Friedrich AE, Meile T, Damms‐Machado A, Kueper M, Kramer KM, Konigsrainer A, et al. Effect of laparoscopic sleeve gastectomy on lipid profiles, inflammation markers and liver enzymes in obese patients. Clinical Nutrition. 2013; Vol. 31, issue 1:S106.
Frige' 2009 {published data only}
- Frige' F, Laneri M, Veronelli A, Folli F, Paganelli M, Vedani P, et al. Bariatric surgery in obesity: changes of glucose and lipid metabolism correlate with changes of fat mass. Nutrition Metabolism & Cardiovascular Diseases 2009;19(3):198‐204. [DOI] [PubMed] [Google Scholar]
Gagner 2011 {published data only}
- Gagner M. Laparoscopic BPD‐DS for weight regain and failure of treatment after proximal gastric bypass. Obesity Surgery 2011;Conference(var.pagings):8. [Google Scholar]
Garb 2009 {published data only}
- Garb J, Welch G, Zagarins S, Kuhn J, Romanelli J. Bariatric surgery for the treatment of morbid obesity: a meta‐analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obesity Surgery 2009;19(10):1447‐55. [DOI] [PubMed] [Google Scholar]
Gehrer 2010 {published data only}
- Gehrer S, Kern B, Peters T, Christofiel‐Court, Peterli R. Fewer nutrient Deficiencies after laparoscopic sleeve gastrectomy (LSG) than after Laparoscopic Roux‐Y‐gastric bypass (LRYGB)‐a prospective study. Obesity Surgery 2010;20(4):447‐53. [DOI] [PubMed] [Google Scholar]
Guelinckx 2009 {published data only}
- Guelinckx I, Devlieger R, Vansant G. Reproductive outcome after bariatric surgery: a critical review. Human Reproduction Update 2009;15(2):189‐201. [DOI] [PubMed] [Google Scholar]
Heindorff 1997 {published data only}
- Heindorff H, Hougaard K, Larsen PN. Laparscopic adjustable gastric band increases weight loss compared to dietary treatment: a randomized study (abstract). Obesity Surgery 1997;7:300‐1. [Google Scholar]
Hofso 2010 {published data only}
- Hofso D, Nordstrand N, Johnson LK, Karlsen TI, Hager H, Jenssen T, et al. Obesity‐related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. European Journal of Endocrinology 2010;163(5):735‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofso 2011 {published data only}
- Hofso D, Jenssen T, Bollerslev J, Ueland T, Godang K, Stumvoll M, et al. Beta cell function after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. European Journal of Endocrinology 2011;164(2):231‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holty 2011 {published data only}
- Holty J‐E, Parimi N, Ballesteros M, Blackwell T, Cirangle PT, Jossart GH, et al. Does surgically induced weight loss improve daytime sleepiness?. Obesity Surgery 2011;21(10):1535‐45. [DOI] [PubMed] [Google Scholar]
Hussain 2009 {published data only}
- Hussain A, Mahmood H, El‐Hasani S. Can Roux‐en‐Y gastric bypass provide a lifelong solution for diabetes mellitus?. Canadian Journal of Surgery 2009;52(6):E269‐E275. [PMC free article] [PubMed] [Google Scholar]
Inci 2011 {published data only}
- Inci K, Arkhammar SK, Lonroth H, Fandriks L, Kral JG. T2DM prevention by combining truncal vagotomy with gastric restriction compared to restriction alone: 22‐Yr follow‐up. Obesity 2011;Conference(var.pagings):S189. [Google Scholar]
Inge 2009 {published data only}
- Inge TH, Miyano G, Bean J, Helmrath M, Courcoulas A, Harmon CM, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics 2009;123(1):214‐22. [DOI] [PubMed] [Google Scholar]
Jonnalagadda 2012 {published data only}
- Jonnalagadda SS, Gupta N, Eagon JC, Bessler M, Mull LN, DiGiorgi M, et al. Preliminary results of a randomized, blinded, sham‐controlled trial of transoral gastroplasty for the treatment of morbid obesity. Gastroenterology 2012;142(5):S78. [Google Scholar]
Keating 2009 {published data only}
- Keating CL, Dixon JB, Moodie ML, Peeters A, Playfair J, O'Brien PE. Cost‐efficacy of surgically induced weight loss for the management of type 2 diabetes: a randomized controlled trial. Diabetes Care 2009;32(4):580‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolotkin 2009 {published data only}
- Kolotkin RL, Crosby RD, Gress RE, Hunt SC, Adams TD. Two‐year changes in health‐related quality of life in gastric bypass patients compared with severely obese controls. Surgery for Obesity & Related Diseases 2009;5(2):250‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lancaster 2008 {published data only}
- Lancaster RT, Hutter MM. Bands and bypasses: 30‐day morbidity and mortality of bariatric surgical procedures as assessed by prospective, multi‐center, risk‐adjusted ACS‐NSQIP data. Surgical Endoscopy 2008;22(12):2554‐63. [DOI] [PubMed] [Google Scholar]
Lanzi 2011 {published data only}
- Lanzi P, Noè D, Ventura P, Vitali T, Giuffrè M, Spiti R, et al. Outcomes of bariatric surgery: clinical benefits versus short‐term and long‐term complications. Nutritional Therapy & Metabolism 2011;29(3):124‐33. [Google Scholar]
Lee 2005 {published data only}
- Lee WJ. Laparoscopic Roux‐en‐Y versus mini‐gastric bypass for the treatment of morbid obesity: A prospective randomized controlled clinical trial. Annals of Surgery 2005;242(1):20‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2011b {published data only}
- Lee WJ, Chen CY, Chong K, Lee YC, Chen SC, Lee SD. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surgery for Obesity & Related Diseases 2011;7(6):683‐90. [DOI] [PubMed] [Google Scholar]
Lewis 2012 {published data only}
- Lewis K. Bariatric surgery significantly improves rates of diabetes resolution compared with medical therapy alone. Journal of Clinical Outcomes Management 2012;19(5):197‐200. [Google Scholar]
Lin 2011 {published data only}
- Lin CH, Kurup S, Herrero P, Schechtman KB, Eagon JC, Klein S, et al. Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity 2011;19(9):1804‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mummadi 2008 {published data only}
- Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta‐analysis. Clinical Gastroenterology & Hepatology 2008;6(12):1396‐402. [DOI] [PubMed] [Google Scholar]
Mundet 2008 {published data only}
- Mundet TX. Treatment of obesity by means of bariatric surgery not only reverts diabetes mellitus type 2, but also improves metabolic control. FMC Formacion Medica Continuada en Atencion Primaria 2008;15(9):623. [Google Scholar]
Nordstrand 2011 {published data only}
- Nordstrand N, Hofso D, Saltvedt E, Hjelmesaeth J. Nocturnal hypertension and systolic blood pressure dip in morbidly obese subjects: The effect of bariatric surgery and lifestyle intervention therapy. Obesity Reviews 2011;12(Suppl 1):38‐62. [Google Scholar]
Nordstrand 2012 {published data only}
- Nordstrand N, Hertel JK, Hofso D, Sandbu R, Saltvedt E, Roislien J, et al. A controlled clinical trial of the effect of gastric bypass surgery and intensive lifestyle intervention on nocturnal hypertension and the circadian blood pressure rhythm in patients with morbid obesity. Surgery 2012;151(5):674‐80. [DOI] [PubMed] [Google Scholar]
O'Brien 2010 {published data only}
- O'Brien PE, Sawyer SM, Laurie C, Brown WA, Skinner S, Veit F, et al. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. JAMA 2010;303(6):519‐526. [DOI] [PubMed] [Google Scholar]
O'Brien 2013 {published data only}
- O'Brien PE, Brennan L, Laurie C, Brown W. Intensive medical weight loss or laparoscopic adjustable gastric banding in the treatment of mild to moderate obesity: long‐term follow‐up of a prospective randomised trial. Obesity Surgery 2013;23(9):1345‐53. [DOI] [PubMed] [Google Scholar]
Oude 2009a {published data only}
- Oude LH, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. [Review] [273 refs][Update of Cochrane Database Syst Rev 2003;(3):CD001872;PMID:12917914]. [Google Scholar]
Oude 2009b {published data only}
- Oude LH, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001872.pub2] [DOI] [PubMed] [Google Scholar]
Papalazarou 2010 {published data only}
- Papalazarou A, Yannakoulia M, Kavouras SA, Komesidou V, Dimitriadis G, Papakonstantinou A, et al. Lifestyle intervention favourably affects weight loss and maintenance following obesity surgery. Obesity 2010;18(7):1348‐53. [DOI] [PubMed] [Google Scholar]
Picot 2009 {published data only}
- Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost‐effectiveness of bariatric (weight loss) surgery for obesity: A systematic review and economic evaluation. Health Technology Assessment 2009;13(41):ix‐214. [DOI] [PubMed] [Google Scholar]
Pietri 2012 {published and unpublished data}
- Pietri P, Alessandro A, Mattia P, Lorenzis G. BMI‐EWL Trends, short and long‐term slippages in patients operated with the new‐HAGA LAGB (Heliogast system) for morbid obesity, according to four different techniques. Definitive results in a pilot prospective randomized study. Obesity Surgery 2012;22(9):1316‐7. [Google Scholar]
Pokala 2012 {published data only}
- Pokala S. Gastric bypass or biliopancreatic diversion increases remission from type 2 diabetes in obese adults. Annals of Internal Medicine 2012;157(2):JC1‐12. [DOI] [PubMed] [Google Scholar]
Pollock 2013 {published data only}
- Pollock RF, Muduma G, Valentine WJ. Evaluating the cost‐effectiveness of laparoscopic adjustable gastric banding versus standard medical management in obese patients with type 2 diabetes in the UK. Diabetes, Obesity & Metabolism 2013;15(2):121‐9. [DOI] [PubMed] [Google Scholar]
Pontiroli 2009 {published data only}
- Pontiroli AE, Laneri M, Veronelli A, Frige F, Micheletto G, Folli F, et al. Biliary pancreatic diversion and laparoscopic adjustable gastric banding in morbid obesity: their long‐term effects on metabolic syndrome and on cardiovascular parameters. Cardiovascular Diabetology 2009;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Praveen Raj 2012b {published data only}
- Praveen Raj P, Kumaravel R, Chandramaliteeswaran C, Vaithiswaran V, Palanivelu C. Laparoscopic duodenojejunal bypass with sleeve gastrectomy: preliminary results of a prospective series from India. Surgical Endoscopy 2012;26(3):688‐92. [DOI] [PubMed] [Google Scholar]
Rebecchi 2011 {published data only}
- Rebecchi F, Rocchietto S, Giaccone C, Talha A, Morino M. Gastroesophageal reflux disease and esophageal motility in morbidly obese patients submitted to laparoscopic adjustable silicone gastric banding or laparoscopic vertical banded gastroplasty. Surgical Endoscopy 2011;25(3):795‐803. [DOI] [PubMed] [Google Scholar]
Rico Hernandez 2009 {published data only}
- Rico Hernandez MA, Martinez SE, Armero FM, Diaz GJ, Calvo VI. Five‐year comparison of two techniques of bariatric surgery in patients with morbid obesity followed up in a nurse consultation. Nutricion Hospitalaria 2009;24(6):667‐75. [PubMed] [Google Scholar]
Schouten 2010 {published data only}
- Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, Janssen IM, et al. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Annals of Surgery 2010;251(2):236‐43. [DOI] [PubMed] [Google Scholar]
Schouten 2010b {published data only}
- Schouten R, Wiryasaputra DC, Dielen FMH, Gemert WG, Greve JWM. Long‐term results of bariatric restrictive procedures: A prospective study. Obesity Surgery 2010;20(12):1617‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scozzari 2009 {published data only}
- Scozzari G, Farinella E, Bonnet G, Toppino M, Morino M. Laparoscopic adjustable silicone gastric banding vs laparoscopic vertical banded gastroplasty in morbidly obese patients: long‐term results of a prospective randomized controlled clinical trial. Obesity Surgery 2009;19(8):1108‐15. [DOI] [PubMed] [Google Scholar]
Sjostrom 2008 {published data only}
- Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. International Journal of Obesity 2008;32 Suppl 7:S93‐S97. [DOI] [PubMed] [Google Scholar]
Sjostrom 2009 {published data only}
- Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncology 2009;10(7):653‐62. [DOI] [PubMed] [Google Scholar]
Skroubis 2013 {published data only}
- Skroubis G, Kouri N, Mead N, Kalfarentzos F. Long‐term results of a prospective comparison of Roux‐en‐Y gastric bypass versus a variant of biliopancreatic diversion in a non‐superobese population (BMI 35‐50 kg/m(2)). Obesity Surgery 2014;24:197‐204. [DOI: 10.1007/s11695-013-1081-1] [DOI] [PubMed] [Google Scholar]
Tice 2008 {published data only}
- Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. American Journal of Medicine 2008;121(10):885‐93. [DOI] [PubMed] [Google Scholar]
Treadwell 2008 {published data only}
- Treadwell JR, Sun F, Schoelles K. Systematic review and meta‐analysis of bariatric surgery for pediatric obesity. Annals of Surgery 2008;248(5):763‐76. [DOI] [PubMed] [Google Scholar]
Varela 2011 {published data only}
- Varela JE. Laparoscopic sleeve gastrectomy versus laparoscopic adjustable gastric banding for the treatment severe obesity in high risk patients. Jsls‐Journal of the Society of Laparoendoscopic Surgeons 2011;15(4):486‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Werling 2013 {published data only}
- Werling M, Fandriks L, Bjorklund P, Maleckas A, Brandberg J, Lonroth H, et al. Long‐term results of a randomized clinical trial comparing Roux‐en‐Y gastric bypass with vertical banded gastroplasty. British Journal of Surgery 2013;100(2):222‐30. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cesana 2013 {published data only}
- Cesana G, Uccelli M, Ciccaarese F, Castello G, Olmi S. Laparoscopic gastric plication versus sleeve gastrectomy, a randomised study. Obesity Surgery 2013;23(8):1024‐5. [Google Scholar]
Dadan 2011 {published data only}
- Dadan J, Iwacewicz P, Hady HR, Myśliwiec P, Golaszewski P. Quality of life after chosen bariatric procedures basing on Baros. Obesity Surgery 2011;21:1090. [Google Scholar]
Darabi 2013 {published data only}
- Darabi S, Talebpour M. Laparoscopic gastric plication versus mini‐gastric bypass surgery in the treatment of morbid obesity: a randomized clinica trial. Obeisty Surgery 2013;23(8):1025‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN 00786323 {unpublished data only}
- ISRCTN 00786323. BY‐BAND: Gastric BYpass or adjustable gastric BANDing surgery to treat morbid obesity: a multi‐centre randomised controlled trial ‐ The BY‐BAND Trial. http://public.ukcrn.org.uk/Search/StudyDetail.aspx?StudyID=11911 (accessed 5 November 2013).
NCT00432809 {unpublished data only}
- NCT00432809. Advanced medical therapy versus advanced medical therapy plus bariatric surgery for the resolution of Type 2 diabetes [Official Title: STAMPEDE: Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently]. http://clinicaltrials.gov/show/NCT00432809 (accessed 5 November 2013).
NCT01047735 {unpublished data only}
- NCT01047735. The TRIABETES Study: A trial to compare surgical and medical treatments for Type 2 diabetes. http://clinicaltrials.gov/show/NCT01047735 (accessed 5 November 2013).
NCT01053130 {unpublished data only}
- NCT01053130. Weight loss interventions in obese patients with stages 3‐4 chronic kidney disease: a randomised controlled trial. http://clinicaltrials.gov/show/NCT01053130 (accessed 5 November 2013).
NCT01073020 {unpublished data only}
- NCT01073020. Surgery or Lifestyle with Intensive Medical Management in the Treatment of Type 2 Diabetes (SLIMM‐T2D). http://clinicaltrials.gov/show/NCT01073020 (accessed 5 November 2013).
NCT01352403 {unpublished data only}
- NCT01352403. Severe obesity: Bariatric surgery vs. life‐style‐intervention (WAS). http://clinicaltrials.gov/show/NCT01352403 (accessed 5 November 2013).
NCT01486680 {unpublished data only}
- NCT01486680. Silastic ring gastric bypass versus sleeve gastrectomy for Type 2 diabetes mellitus in obese patients. http://clinicaltrials.gov/show/NCT01486680 (accessed 5 November 2013).
NCT01501201 {unpublished data only}
- NCT01501201. Comparison of gastric by‐pass and optimized medical treatment in obese diabetic patients (DIABSURG). http://clinicaltrials.gov/show/NCT01501201 (accessed 5 November 2013).
NCT01581801 {unpublished data only}
- NCT01581801. Bariatric surgery and reactive hypoglycaemia. http://clinicaltrials.gov/show/NCT01581801 (accessed 5 November 2013).
NCT01778738 {unpublished data only}
- NCT01778738. Type 2 diabetes after sleeve gastrectomy and Roux‐en‐Y Gastric Bypass: a randomised single centre study (OSEBERG). http://clinicaltrials.gov/show/NCT01778738 (accessed 5 November 2013).
NCT01821508 {unpublished data only}
- NCT01821508. Clinical study on metabolic surgery compared to the best clinical treatment in patients with Type 2 diabetes mellitus. http://clinicaltrials.gov/show/NCT01821508 (accessed 5 November 2013).
NCT01929850 {unpublished data only}
- NCT01929850. Laparoscopic sleeve gastrectomy plus weight watchers vs. weight watchers alone in underserved minority women. http://clinicaltrials.gov/show/NCT01929850 (accessed 5 November 2013).
Additional references
Avenell 2004
Avenell 2006
- Avenell A, Sattar N, Lean M. Management: Part I‐‐Behaviour change, diet, and activity. BMJ 2006;333(7571):740‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchwald 2005
- Buchwald H. Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third‐party payers. Surgery for Obesity and Related Diseases 2005;1(3):371‐81. [DOI] [PubMed] [Google Scholar]
Buchwald 2007
- Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta‐analysis. Surgery 2007;142(4):621‐32. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan C‐P, Wang B‐Y, Cheng C‐Y, Lin C‐H, Hsieh M‐C, Tsou J‐J, et al. Randomized controlled trials in bariatric surgery. Obesity Surgery 2013;23:118‐30. [DOI] [PubMed] [Google Scholar]
Christensen 2007
- Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta‐analysis. Annals of the Rheumatic Diseases 2007;66(4):433‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curioni 2006
- Curioni C, Andre C, Veras R. Weight reduction for primary prevention of stroke in adults with overweight or obesity. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006062.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferzli 2009
- Ferzli GS, Dominique E, Ciaglia M, Bluth MH, Gonzalez A, Fingerhit A. Clinical improvement after duodejejunal bypass for nonobese type 2 diabetes despite minimal improvement in glycemic homeostasis. World Journal of Surgery 2009;33(5):972‐9. [DOI] [PubMed] [Google Scholar]
Gloy 2013
- Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non‐surgical treatment for obesity: a systematic review and meta‐analysis of randomised controlled trials. BMJ 2013;347:f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasama 2009
- Kasama K, Tagaya N, Kanehira E, Oshiro T, Seki Y, Kinouchi M, et al. Laparoscopic sleeve gastrectomy with duodenojejunal bypass: technique and preliminary results. Journal of Metabolic Surgery and Allied Care 2009;19(10):1341‐5. [DOI] [PubMed] [Google Scholar]
Kehagias 2011
- Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI<50 kg/m2. Obesity Surgery 2011;21:1650‐6. [DOI] [PubMed] [Google Scholar]
Kral 2006
- Kral John G. Management: Part III ‐ Surgery. BMJ 2006;333(7574):900‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lean 2006
- Lean M, Finer N. Management: Part II ‐ Drugs. BMJ 2006;333(7572):794‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2007
- Lee CW, Kelly JJ, Wassef WY. Complications of bariatric surgery. Current Opinion in Gastroenterology 2007;23(6):636‐43. [DOI] [PubMed] [Google Scholar]
Levy 2007
- Levy P, Fried M, Santini F, Finer N. The comparative effects of bariatric surgery on weight and type 2 diabetes. Obesity Surgery 2007;17(9):1248‐56. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobstein 2007
- Lobstein, T, Jackson Leach, R. International comparisons of obesity trends, determinants and responses. Evidence review. Foresight Tackling Obesities: Future Choices Vol. 2007, issue http://www.foresight.gov.uk.
Loveman 2011
- Loveman E, Frampton GK, Shepherd J, Picot J, Cooper K, Bryant J, et al. The clinical effectiveness and cost‐effectiveness of long‐term weight management schemes for adults: a systematic review. Health Technology Assessment 2011;15(2):1‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meijer 2011
- Meijer RI, Wagensveld BA, Siegert CE, Eringa EC, Serne EH, Smulders YM. Bariatric surgery as a novel treatment for type 2 diabetes mellitus. A systematic review. Archives of Surgery 2011;146(6):744‐50. [DOI] [PubMed] [Google Scholar]
Moldovan 2011
- Moldovan AR, David D. Effect of obesity treatments on eating behavior: Psychosocial interventions versus surgical interventions. A systematic review. Eating Behaviors 2011;12:161‐7. [DOI] [PubMed] [Google Scholar]
Moshiri 2013
- Moshiri M, Osman S, Robinson TJ, Khandelwal S, Bhargava P, Rohrmann CA. Evolution of bariatric surgery: A historical perspective. American Journal of Roentgenology 2013;201(1):W40‐W48. [DOI] [PubMed] [Google Scholar]
NHS IC 2012
- The NHS Information Centre for Health and Social Care. Health Survey for England – 2011: Adult trend tables.. http://www.hscic.gov.uk/searchcatalogue?productid=10152&topics=2%2fPublic+health%2fLifestyle%2fPhysical+activity&sort=Relevance&size=10&page=1#top 2012.
NICE 2006
- National Institute for Health and Clinical Excellence. Obesity ‐ Guidance on the prevention, identification, assessment and management of overweight and obesity in adults and children. Clinical Guideline. London: The Stationary Office, 2006; Vol. 43. [PubMed]
O'Brien 2013b
- O’Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long‐term outcomes after bariatric surgery. Fifteen‐year follow‐up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Annals of Surgery 2013;257(1):87‐94. [DOI] [PubMed] [Google Scholar]
Olsen 2012
- Olsen MF, Wiklund M, LOnroth H, Olbers T. Respiratory function in superobese patients before and after bariatric surgery‐ a randomised controlled trial. Open Obesity Journal 2012;4:28‐34. [Google Scholar]
Padwal 2011a
- Padwal R, Klarenbach S, Wiebe N, Birch D, Karmali S, Manns B, et al. Bariatric surgery: a systematic review and network meta‐analysis of randomized trials. Obesity Reviews 2011;12:602‐21. [DOI] [PubMed] [Google Scholar]
Padwal 2011b
- Padwal R, Klarenbach S, Wiebe N, Hazel M, Birch D, Karmali S, Sharma, AM, Manns B, Tonelli M. Bariatric surgery: A systematic review of the clinical and economic evidence. Journal of General Internal Medicine 2011;26(10):1183‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paluszkiewicz 2011
- Paluszkiewicz R, Kalinowski P, Wroblewski T, Bialobrzeska‐Paluszkiewicz J, Ziarkiewicz‐Wroblewska B, Remiszewski P, et al. Improvement of metabolic parameters after laparoscopic sleeve gastrectomy (LSG) versus Roux‐En‐Y gastric bypass (RYGB) ‐ Early results of a prospective randomized trial. Obesity Surgery 2011;21(8):983‐4. [Google Scholar]
Poobalan 2007
- Poobalan AS. Long‐term weight loss effects on all cause mortality in overweight/obese populations. Obesity Reviews 2007;8(6):503‐13. [DOI] [PubMed] [Google Scholar]
Praveen Raj 2012c
- Praveen Raj P, Kumaravel R, Chandramaliteeswaran C, Vaithiswaran V, Palanivelu C. Laparoscopic duodenojejunal bypass with sleeve gastrectomy: preliminary results of a prospective series from India. Surgical Endoscopy 2012;26(3):688‐92. [DOI] [PubMed] [Google Scholar]
Ramon 2012
- Ramon JM, Salvans S, Crous X, Puig S, Goday A, Benaiges D, et al. Effect of Roux‐en‐Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. Journal of Gastrointestinal Surgery 2012;16:1116‐22. [DOI] [PubMed] [Google Scholar]
Sjöström 2013
- Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial ‐ a prospective controlled intervention study of bariatric surgery. Journal of Internal Medicine 2013;273(3):219‐34. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Sturm 2013
- Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the US. International Journal of Obesity 2013;37(6):889‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Søvik 2010
- Søvik TT, Taha O, Aasheim ET, Engström M, Kristinsson J, Björkman S, et al. Randomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesity. British Journal of Surgery 2010;97:160‐6. [DOI] [PubMed] [Google Scholar]
Søvik 2011
- Søvik TT, Aasheim ET, Taha O, Engström M, Fagerland MW, Björkman S, Kristinsson J, Birkeland KI, Mala T, Olbers T. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: a randomized trial. Annals of Internal Medicine 2011;155:281‐291. [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO Technical Report Series 2000; Vol. 894. [PubMed]
WHO 2004
- World Health Organization. Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. The Lancet 2004;363:157‐63. [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. Overweight and Obesity. Factsheet no.311. http://www.who.int/mediacentre/factsheets/fs311/en/index.html September 2006.
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Clegg 2002
Clegg 2003
- Clegg A, Colquitt J, Sidhu M, Royle P, Walker A. Clinical and cost effectiveness of surgery for morbid obesity: a systematic review and economic evaluation. International Journal of Obesity 2003;27:1167‐77. [DOI] [PubMed] [Google Scholar]
Colquitt 2003
- Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003641] [DOI] [PubMed] [Google Scholar]
Colquitt 2005
- Colquitt J, Clegg A, Loveman E, Royle P, Sidhu MK. Surgery for morbid obesity. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003641.pub2] [DOI] [PubMed] [Google Scholar]
Colquitt 2009
- Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003641.pub3] [DOI] [PubMed] [Google Scholar]


