Abstract
Background
Previous famine studies reported the association between early life famine exposure and adulthood anthropometric profile. However, the findings were variable. Thus, a systematic review and meta-analysis was conducted to clarify the association of famine exposure in early life with the anthropometric profiles in adults.
Methods
Potentially relevant studies were searched through Scopus, Medline, Google Scholar and Google for gray literature and reference lists of previous studies. The random effects model (REM) and I2 test was used to adapt the pooling method and assess heterogeneity, respectively.
Results
Prenatal famine exposure was associated with increased risk of body mass index [SMD = 0.10 (95% CI: 0.02, 0.18)], waist circumference [SMD = 0.21 (95% CI: 0.11, 0.31)] in adults. Likewise, famine exposure during prenatal life was associated with decreased adult height [SMD) = − 0.26 (95% CI: − 0.44, − 0.09)]. Moreover, famine exposure during early childhood was associated with increased risk of waist circumference [SMD = 0.09 (95% CI: 0.01, 0.16)] and decreased adult height [SMD = − 0.16 (95% CI: − 0.27, − 0.04)].
Conclusion
Our finding indicates that exposure to famine during early life was associated with the anthropometric profile of adults. In terms of public health significance, the results of the study further underscore the importance of improving the nutritional status of mothers and children to prevent adulthood diseases in the long run.
Systematic review registration number
PROSPERO CRD42020168424
Supplementary Information
The online version contains supplementary material available at 10.1186/s40795-022-00523-w.
Keywords: Anthropometric profile, Famine exposure, Meta-analysis, Systematic review
Background
According to the Developmental Origins of Health and Disease (DOHaD) hypothesis nutritional deprivation during the critical periods of growth and development leads to structural and functional changes and increases the risk of developing adulthood disease later in life [1–6]. Early life, particularly intrauterine, first 2 years of postnatal and adolescence stage of life are the critical “window” period for for all rounded development of human capital, where optimal nutrition during this period is fundamental [7, 8]. These periods are exceptional periods where the body employs reductive adaptive mechanisms to sustain life at the expense of shaping the future adulthood for the worst [9, 10].
Naturally, growth and development is determined by our genome, but realization of this growth potential is only possible if nutrient supplies are maintained to the fullest, especially during the critical periods of life [11]. However, when these supplies are restricted, physiological adaptive process takes place to ensure survival, may leave behind a permanent damage of the exposure [2, 4, 12]. For example, the pancreas is fully formed by the time of birth and while the number of islets is set in utero [13]. Subtle developmental exposures that resulted in fewer islets formation may have no immediate impact upon pancreatic function but may mark pancreatic insufficiency in response to aging [14]. The kidney as well, may reflect ill function during adulthood due to impact of fetal adversaries on nephrogenesis [15].
In order to generate the best available evidence on the long-term impact of early life famine exposure on adulthood health, a natural study setting is required where the exposure was a natural phenomenon, such as famine. Famine studies can serve as a natural experimental setting which can provide unique insights into the effect of earl life undernutrition on the development adulthood diseases [16–18]. In light of an impending food insecurity today the famine studies are a compelling event from which to learn, look back, and look forward to preventing famine-related adverse outcomes [19, 20]. Moreover, the long-term anthropometric consequences of early-life undernutrition is very interesting and important to stimulate new thinking in the concepts of health and diseases [21, 22].
Previous famine studies have provided a number of evidences supporting the association between early famine exposure and adulthood anthropometric profile [23–35]. Yet, the findings were not always consistent. For example, positive associations of early life famine exposure with obesity [23, 27, 34] and adult height [26, 31, 35] were found in China and Dutch famine studies, but not in Leningrad studies [30, 36] and another Chinese study [35]. Two previous systematic review and meta-analysis reported the association between early life famine and the risks of obesity and overweight later in life [37, 38]. However, the potential impact of early life exposure to famine on height, BMI, and waist circumference has not been quantified. Given these considerations, we will conduct a systematic review and meta-analysis of observational studies in order to gain a better understanding of the links between early life famine experience and adult anthropometric measurements.
Methods
Search strategy and study selection
This review looked at both published and unpublished research to assess if there was a link between early life famine experience and anthropometric measurements in adults. Manual and electronic searches were used to locate the studies. The following databases were used to conduct an electronic search. An electronic search was conducted on Scopus, Medline, and Google Scholar databases. Gray literatures were retrieved using Google. Moreover, a manual search was performed to locate papers from previous studies. The Preferred Reporting Items of Systematic Review and Meta-Analysis (PRISMA) 2020 guideline was used [39], and the following keywords were used to search the articles: “Famine” OR “malnutrition” OR “undernutrition” OR “malnourishment” OR “starvation” OR “hunger” AND “early life” OR “pregnancy” OR “fetus” OR “infant” OR “child” or “adolescence” AND “height” OR “short stature” OR “body mass index” OR waist circumference” OR “waist to height ratio” AND “Adults”.
The research question was defined by the Participants, Interventions, Comparisons, Outcomes, and Study design (PICOS) criteria. In order to avoid double counting, only the article with the most relevant was included if several articles reported data from the same study population.
Inclusion and exclusion criteria’s
Both published and unpublished observational studies conducted among early life famine exposed adults (aged ≥19 years) in any setting across the world were included. The study included all articles published until October 30, 2020. The studies that did not fully accessed after accessing abstracts were excluded after at least two email contacts with the primary author. The exclusion of these articles reflects our inability to assess the quality of articles in the absence of full text. Furthermore, articles written in languages other than English were excluded.
Data extraction process
A standardized data extraction format was used to abstract data from the included articles (Supplementary file 1), which was adapted from the Johanna Briggs Institute’s data extraction format [40]. All relevant data for this review were extracted by two reviewers (GA and KHA). The disparities between to reviewers at the time of data abstraction were resolved through discussion. The corresponding author of the original research was contacted via email for further information or to clarify procedure details. The data extraction format included first author, study design, age sample size, publication year, country of origin, outcome, main findings between famine exposure in early life and adulthood.
Measurement of outcome variables
The primary interest of the study was to investigate the association of early life famine exposure and adulthood anthropometric measurements such as height, body mass index and waist circumference .
Measurement of exposure
This review was considered studies that report on the association between early life famine exposure (prenatal, early childhood, mid-childhood, adolescence) and adulthood anthropometric measurements. Prenatal exposure was defined as exposure to famine in during pregnancy, early childhood exposure was defined as exposure to famine during the first 2 years of life after birth, mid-childhood exposure was defined as exposure to famine during 4–9 years of age, adolescence exposure was defined as exposure to famine during 10–19 years of age [41].
Quality assessment
Two authors (GA and KHA) independently assessed the quality of each original study using the Newcastle Ottawa Scale, a three-part approach, for observational studies quality assessment [42] There are three key parts to the tool. The first component, which was assessed on a scale of one to five stars, was primarily concerned with the methodological quality of each piece. The second component assesses the study’s comparability, with a possible two-star rating. The third component, which was graded from three stars, focused on the outcomes and statistical analysis of each original research. Disagreements between the two reviewers were solved through discussion. Articles with a scale of ≥6 from 10 scales were categorized as high quality (Supplementary file 2).
Statistical analysis
Statistical analysis was performed using the Rev. Man 5.3 software (Rev Man 5.3) [43]. Odds Ratio (OR) pooled with 95% CI was determined to assess the strength of the association between exposure to famine and the risk of overweight, general obesity, and abdominal obesity. Standardized mean difference (SMD) was used to compare BMI, waist circumference, and height difference between exposed and nonexposed groups. The I square value (I2) was used to assess the heterogeneity between studies and the Random Effects Model (REM) was used as a pooling method. The I2 values of 0, 25, 50 and 75%, respectively, represent no, low, moderate, and high heterogeneity, while the P-values of chi-square statistics < 0:05 represent significant heterogeneity. Sensitivity analysis was performed by sequential failure of individual studies to further evaluate the source of heterogeneity [44]. Subgroup analyzes were conducted on the basis of gender. Publication bias was assessed through funnel plots.
Results
Characteristics of the study
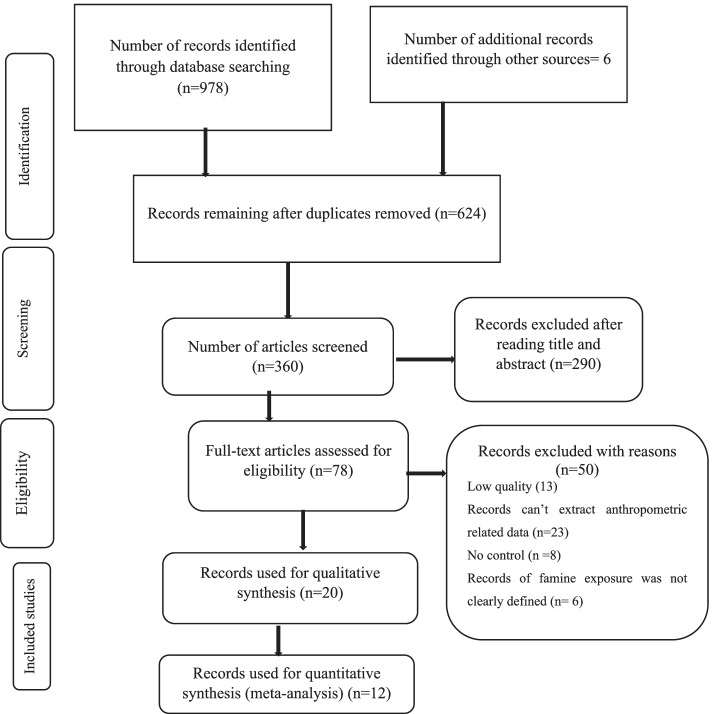
A total of 984 articles were retrieved on the basis of the search strategy. Approximately 624 potential articles were duplicated and removed, with the remaining 360 retrieved for further investigation. After the review of the titles and abstracts, 290 articles were excluded. The full text of the remaining 70 studies was retrieved for detailed evaluation, of which 50 were excluded. Of these 13 studies were excluded due to suboptimal quality (Supplementary file 3). The remaining 20 studies [23–26, 28–34, 45–56] have been included in the current systematic review. Of these, 12 studies were included in the meta-analysis to estimate the relationship between early-life exposure to famine and BMI [23–25, 29, 31, 33, 53], waist circumference [24, 29, 45, 49, 52, 53] and height [24, 26, 27, 31, 33, 53] in adulthood. The detailed characteristics of the studies have been shown in Table 1. The Flow chart diagrams to describe the selection of studies for a systematic review and meta-analysis is shown in Fig. 1.
Table 1.
Characteristics of studies reporting the association of early life exposure to famine and anthropometric profile in adults, 2021
| First Author, Year/country | Famine year /duration | Study Design | Sample size | Age at measure (years) | Outcome studied | Main findings |
|---|---|---|---|---|---|---|
| de Rooij et al., 2007 / Dutch | 1944–45 / 6 months | Historical cohort | 783 | Exposed ~ 58.5 Unexposed ~ 57.4 | WC (cm) | Waist circumference (cm): unexposed = 94.1 + 12.4, late gestation exposed = 92.6 + 13.9, mid-gestation = 92.0 + 12.9, early gestation = 89.6 + 11.4 |
| Han and Hon, 2019/ South Korea | 1950–53/ 4 year | Historical cohort | 25,708 |
Exposed ~ 59–73 Unexposed ~ 50–55 |
WC (cm) BMI (kg/m2) |
Waist circumference (cm): Men: Fetal exposed = 85.87 (0.42), early childhood exposed = 85.46 (0.43), late childhood exposed = 84.26 (0.64), adolescence exposed = 83.11 (0.92), unexposed = 85.86 (0.47), Women: Fetal exposed = 82.58 (0.43), early childhood exposed = 82.60 (0.44), late childhood exposed = 81.94 (0.64), adolescent exposed = 81.49 (0.92), unexposed = 81.26 (0.47) BMI: Fetal exposed = 24.19 (0.16), early childhood exposed = 24.09 (0.16), late childhood exposed = 23.70 (0.23), adolescent exposed = 23.36 (0.32), Unexposed = 24.24 (0.17), Women: Fetal exposed = 24.29 (0.15), early childhood exposed = 24.28 (0.15), late childhood exposed = 24.11 (0.22), adolescent exposed = 23.96 (0.31), unexposed = 23.92 (0.17) |
| Ning et al., 2019/ China | 1959–61 /3 year | Historical cohort | 9, 588 |
Exposed ~ 47–65 Unexposed ~ 40 |
WC (cm) BMI (kg/m2) |
Waist circumference (cm), men: Unexposed = 85.0 (0.56), fetal-exposed = 87.2 (0.71), childhood-exposed = 86.5 (0.33), adolescence-exposed = 88.3 (0.69), women: Unexposed = 82.1 (0.39), fetal-exposed = 82.8 (0.52), childhood-exposed = 83.2 (0.25), adolescence-exposed = 83.6 (0.55) BMI, all subjects: Unexposed = 24.6 (0.12), fetal-exposed = 25.3 (0.15), childhood-exposed = 25.7 (0.07), adolescence-exposed = 26.3 (0.15) |
| Wang et al., 2017/ China | 1959–61/3 year | Historical cohort | 6445 |
Exposed ~ 52–59 Unexposed 40–51 |
WC (cm) | Waist circumference, men: Unexposed = 82.9 ± 8.8, fetal-exposed = 83.9 ± 9.0, childhood-exposed = 83.6 ± 9.5, adolescence = 83.5 ± 9.7, women: Unexposed = 74.1 ± 8.2, fetal-exposed = 77.4 ± 8.7, childhood-exposed = 79.6 ± 9.3, adolescence = 81.6 ± 10.2 |
| Wang et al., 2019/ China | 1959–61/3 year | Historical cohort | 2148 |
Exposed = 51–55 Unexposed = 48 |
Height WC (cm) BMI (kg/m2) |
Waist circumference (cm): Unexposed = 85.43 (9.80), fetal exposed = 85.70 (10.62), infant = 84.96 (9.75), preschool = 85.50 (10.04) |
| Stanner et al., 1997/ Leningrad | 1941–44/ 6 months | Cross-sectional | 549 |
Exposed = 52–53 Unexposed = 52.8 |
BMI (kg/m2) Height (m) WHR |
BMI (kg/m2): Unexposed = 25.2 (24.1 to 26.3), intrauterine exposed = 24.6 (23.6 to 25.6), infant group = 25.4 (24.2 to 26.6), Height (m): Unexposed = 1.73 (1.71 to 1.75), intrauterine exposed = 1.72 (1.70 to 1.74), infant group = 1.74 (1.72 to 1.76) WHR: Unexposed = 0.87 (0.85 to 0.89), intrauterine exposed = 0.86 (0.84 to 0.88), infant group = 0.88 (0.84 to 0.92) |
| Shi, Nicholls et al. 2018/ China | 1959–61/3 year | Historical cohort | 5772 |
Exposed = 50–57 Unexposed = 47 |
BMI | BMI: Unexposed cohort = 24.2 (3.6), fetal exposed cohort = 24.3 (4.4), early child exposed = 23.9 (3.9), mid childhood exposed = 23.3 (3.6), late childhood exposed = 23.7 (3.8) |
| Chen et al., 2019/ China | 1959–61/3 year | Historical cohort | 5295 |
Exposed = 52–93 Unexposed = 40–51 |
WC (cm) BMI |
Waist circumference, men: Unexposed = 82.9 ± 8.8, fetal exposed = 83.9 ± 9.0, childhood exposed = 83.6 ± 9.5, adolescent exposed = 83.4 ± 9.7, women: Unexposed = 74.1 ± 8.2, fetal exposed: 77.4 ± 8.7, childhood exposed = 79.5 ± 9.3, adolescent exposed = 81.6 ± 10.1 BMI, men Unexposed = 24.8 ± 3.2, fetal exposed = 24.9 ± 3.1, childhood exposed = 24.7 ± 3.3, adolescent exposed = 23.9 ± 3.4, women: Unexposed = 23.6 ± 3.3, fetal exposed = 24.5 ± 3.4, childhood exposed = 24.7 ± 3.6, adolescent exposed = 24.4 ± 3.8, |
| Hult et al., 2010/ Nigeria | 1968–70/2 year | Historical cohort | 1338 |
Exposed = 40–43 Unexposed = 37 |
WC (cm) BMI (kg/m2) Height |
Height, cm (mean (SD): unexposed = 170 [8], fetal-infant = 169 [8], early childhood = 169 [8] Waist circumference, cm (mean (SD): Unexposed = 91 [11], fetal-infant = 94 [13], early childhood = 93 [11] BMI, kg/m2: unexposed = 26.5 (4.4), fetal-infant = 27.5 (4.6), early childhood = 26.7 (4.7) |
| Painter et al., 2006b/ Dutch | 1944–45 /6 months | Historical cohort | 721 | BMI | BMI, kg/m2: Born before famine = 28.4, late gestation exposure = 28.1, mid gestation exposure = 27.9, early gestation exposure = 27.9, conceived after famine = 28.8 | |
| Liu et al., 2019/China | 1959–61/3 year | Historical cohort | 18,984 | Exposed ~ 41.6–44.6 | Height | Height, cm, mean (SD): Unexposed = 161.2 (8.2), fetal-Exposed = 161.0 (8.1), infant-Exposed = 160.4 (8.2) |
| Unexposed ~ 38.6 | ||||||
| Meng et al., 2016/China | 1959–61/3 year | Historical cohort | 94,052 | NM | BMI | Exposed: BMI (β-coefficients (95% CI): 0.12, 0.03–0.22) |
| Portrait et al., 2017/Dutch | 1944–45/ 6 months | Historical cohort | 1008 | Age between 44 and 60 years | Adult Height |
Height = Mean (SD), Exposed during gestation to age 1 = 170.8 (8.1), early childhood = 171.8 (9.0), late childhood = 171.0 (8.3), puberty = 170.6 (9.0), Male: gestation to age 1 = 177.1 (4.9), early childhood = 177.9 (6.8), late childhood = 176.6 (6.0), puberty = 176.6 (6.8) Female: gestation to age 1 = 163.4 (3.4), early childhood = 165.5 (6.2), late childhood = 165.2 (6.1), puberty = 163.9 (5.8) Height = Mean (SD), Unexposed during gestation to age 1 = 173.4 (8.4), early childhood = 171.7 (9.3), late childhood = 170.9 (9.0), puberty = 169.3 (8.1), Male, gestation to age 1 = 179.7 (6.5), early childhood = 178.6 (6.8), late childhood = 178.5 (7.0), puberty = 175.8 (5.8), Female, gestation to age 1 = 168.2 (5.8), early childhood = 165.7 (6.6), late childhood = 165.3 (5.4), puberty = 164.5 (5.9) |
| Ravelli et al., 1999/Dutch | 1944–45/ 6 months | Historical cohort | 6445 |
Exposed ~ 52–59 Unexposed ~ 40–51 |
BMI (kg/m2) WC (cm) Height (cm) |
BMI (kg/m2): Born before famine = 26.7, late gestation = 26.7, mid gestation = 26.6, early gestation = 28.1 conceived after the famine = 27.2 Waist circumference (cm): Born before famine = 91.8, late gestation = 92.4, mid gestation = 91.0, early gestation = 95.6, conceived after the famine = 92.5 Height (cm): Born before famine = 171.0, late gestation = 170.9, mid gestation = 168.6, early gestation = 171.0, conceived after the famine = 170.9 |
| Song et al., 2020/China | 1959–61/3 year | Historical cohort | 8054 |
Exposed = 50.9(50.2–51) Unexposed = 48.4(47.8–49) |
WC (cm) BMI (kg/m2) |
BMI (kg/m2): Unexposed = 24.1(22.0,26.5), fetal exposed = 24.2(22.0,26.5), WC (cm): unexposed = 82.0(75.4,89.0), fetal exposed = 82.5(76.0,89.3) |
| Stein et al., 2007 /China | 1944–45/ 6 months | Historical cohort | 11,784 |
Exposed age = 58.9 ± 0.49 Control age = 58.8 ± 1.57 |
Height (cm) WC (cm) BMI (kg/m2) |
Height (cm), Men: Unexposed = 178.3 ± 6.3, exposed = 177.4 ± 6.2, Women: Unexposed = 165.4 ± 6.3, exposed = 165.4 ± 6.6, BMI (kg/m2), Men: Unexposed = 27.9 ± 4.0, exposed = 27.8 ± 3.6, Women: Unexposed = 26.9 ± 4.5, exposed = 28.8 ± 5.7, WC (cm), Men: Unexposed = 101.4 ± 10.5, exposed = 100.5 ± 10.1, Women: Unexposed = 93.9 ± 11.1, exposed = 99.0 ± 11.9 |
| van Abeelen et al., 2012c/Dutch | 1944–45/ 6 months | Historical cohort | 11,784 | Ages between 49 and 70 years |
BMI (kg/m2) WC (cm) |
BMI (kg/m2) Mean (SD), 0–9 years = Unexposed = 25.6 (3.9), Moderately exposed = 25.9 (4.0), Severely exposed = 26.2 (4.3), 10–17 years, Unexposed = 26.5 (4.0), Moderately = 26.7(4.0), Severely = 26.4 (4.0), ≥18 years = Unexposed = 26.9(4.3), Moderately = 27.1(4.5), Severely = 27.1 (3.8), WC (cm), Mean (SD), 0–9 years = Unexposed = 82.3 (9.6), Moderately = 83.0 (9.8), Severely = 83.8 (10.5), 10–17 years = Unexposed = 85.7(9.9), Moderately = 86.4(9.9), Severely = 85.8 (10.2) ≥18 years = Unexposed = 87.0 (9.6), Moderately = 87.2(9.9), Severely = 87.5 (9.9) |
| Wang, Wang et al. 2010/China | 1959–61/3 year | Historical cohort | 17,023 | Born during 1956–1964 | Height (cm) | Height, Female: Unexposed = 158.12 cm, gestational = 157.67 cm, toddler = 156.98 cm, Male: Unexposed = 168.45 cm, gestational = 168.52 cm, toddler = 167.86 cm |
| Woo et al., 2010/China | 1959–61/3 year | Cross sectional cohort | 3732 | Men and women aged ≥65 years |
BMI (kg/m2) Height (cm) |
BMI: Unexposed = 23.46 (3.25), exposed = 23.83 (3.31) Whole body % fat, Unexposed = 29.51 (7.16), exposed = 29.23 (7.19) Height (cm), Unexposed = 157.37 (8.19), exposed = 156.96 (8.24) |
AOR Adjusted odds ratio, BMI Body mass index, WC Waist circumference
Fig. 1.
Flow diagram of studies included in the systematic review and meta-analysis of famine exposure in early life and anthropometric profile in adults
Meta-analyses
Prenatal exposure to famine and anthropometric profile in adulthood
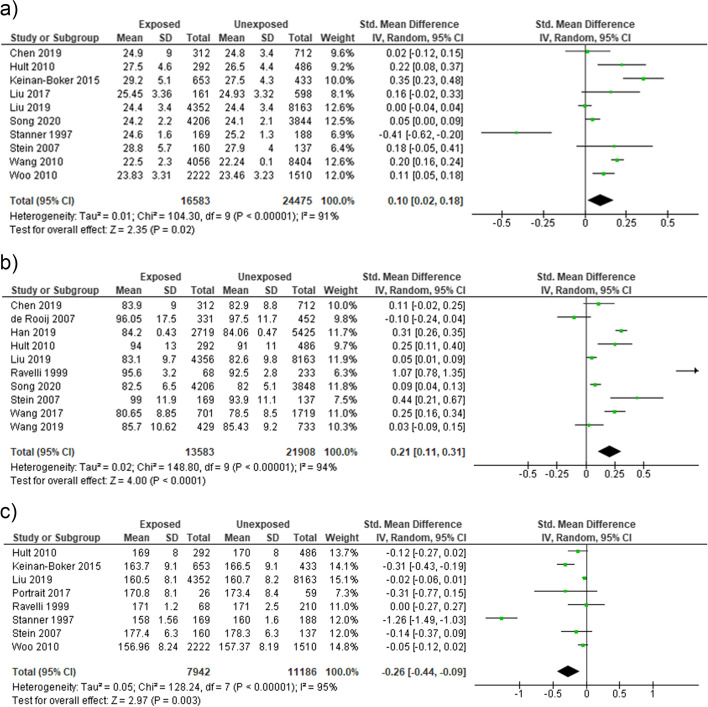
Prenatal famine exposure was associated with body mass index [OR = 0.17 (95% CI: 0.07, 0.27], waist circumference [OR = 0.46 (95% CI: 0.11, 0.82)], and adult height [OR = − 0.30 (95% CI: − 0.53, − 0.08)]. Nonetheless, higher heterogeneity was observed in the analysis of body mass index (I2 = 95%) and waist circumference (I2 = 100%), adult height (I2 = 98%). To further seek heterogeneity sources, sensitivity analysis was performed by omitting one study at a time. The result showed that famine exposure during prenatal life was associated with increased risk of BMI [Standardized Mean Difference (SMD) = 0.10 (95% CI: 0.02, 0.18)], (I2 = 91%) after omitting the study of Ravelli et al. (1999) [27] (Fig. 2a), and waist circumference [SMD = 0.21 (95% CI: 0.11, 0.31)], (I2 = 94%) after removing the study of Ning et al. (2019) [47] (Fig. 2b). Moreover, prenatal famine exposure to famine was associated with decreased adult height [SMD) = − 0.26 (95% CI: − 0.44, − 0.09)], (I2 = 95%) after omitting the study of Woo (2010) [33] (Fig. 2c).
Fig. 2.
Sensitivity analysis forest plot of prenatal exposure to famine and (a) BMI, (b) waist circumference (c) adult height, 2021
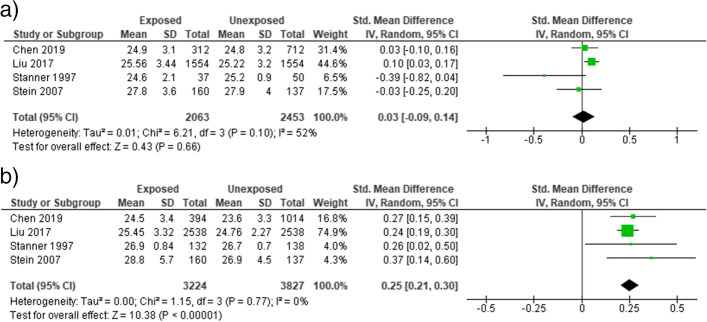
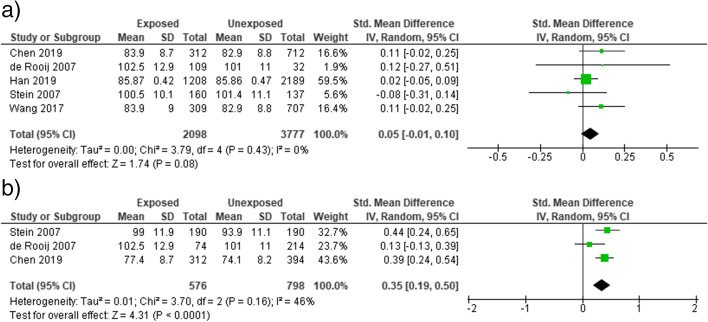
We also performed subgroup analysis based on the gender of participants. No significant association was observed between prenatal famine exposure and BMI [SMD = 0.03 (95% CI: − 0.09, 0.14), I2 = 52%] (Fig. 3a). In females, famine exposure during prenatal life was significantly associated with the higher BMI [SMD = 0.25 (95% CI: 0.21, 0.30)], (I2 = 0%) (Fig. 3b). similarly, prenatal famine exposure was not significantly associated with waist circumference in males [SMD = 0.05 (95% CI: − 0.0.01, 0.10)], (I2 = 0%) (Fig. 4a). However, the association was significant in male participants [SMD = 0.35 (95% CI: 0.19, 0.50)], (I2 = 46%) (Fig. 4b).
Fig. 3.
Forest plot of sex-specific effect of prenatal expose exposure to famine on BMI in adults (a) male (b) female, 2021
Fig. 4.
Forest plot of sex-specific effect of prenatal expose to famine on waist circumference in adults (a) male (b) female, 2021
Childhood exposure to famine and anthropometric profile in adulthood
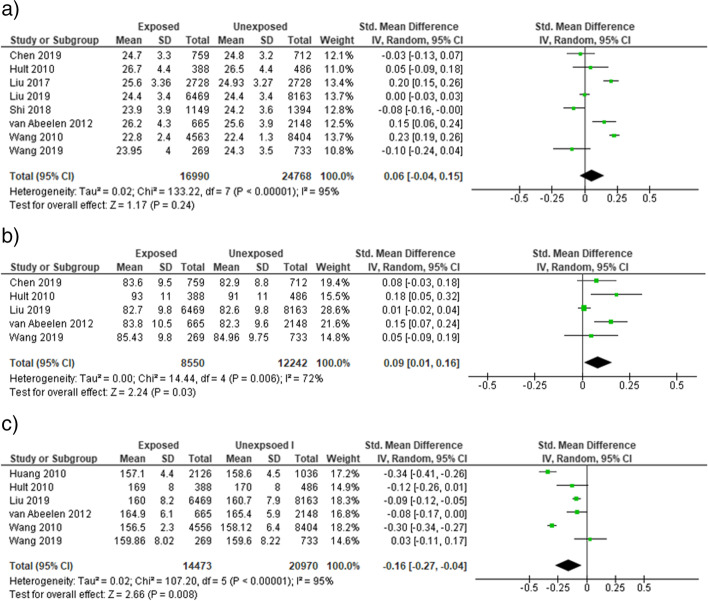
Famine exposure during early childhood period of life was not associated with body mass index [OR = 0.06 (95% CI: − 0.04, 0.15)], I2 = 95% (Fig. 5a) in adults. A significant association was observed between early childhood famine exposure and waist circumference [SMD = 0.09 (95% CI: 0.01, 0.16)], I2 = 72% (Fig. 5b) in adults. Similarly, famine exposure during early childhood life was associated with decreased adult height [SMD = − 0.16 (95% CI: − 0.27, − 0.04)], I2 = 95% (Fig. 5c). We performed sensitivity analysis to identify the sources of heterogeneity. However, no change was observed on the heterogeneity test (I2).
Fig. 5.
Sensitivity analysis forest plot of childhood exposure to famine and (a) BMI, (b) waist circumference (c) adult height, 2021
Publication bias
Publication bias was evaluated by funnel plots. As the study sourced out all quality gray literatures, less publication bias was reported for the analysis of metabolic syndrome, diabetes mellitus, and hypertension (Supplementary file 4).
Discussion
We found that famine exposure during prenatal life was also associated with increased BMI and waist circumference and decreased height in adulthood. Early life famine exposure was associated with increased BMI and waist circumference among female participants than males. However, no association was observed between childhood famine exposure and BMI.
Epigenetic change could be one of the mechanisms behind the link between famine experience in childhood and adulthood anthropometric measures. Evidence suggests that famine-induced epigenetic changes such as DNA methylation or programming of the hypothalamic pituitary adrenal (HPA) axis result in catch-up development and long-term impacts on the risk of increased body mass index. According to DOHaD hypothesis, famine exposure during the early stages could change the structure and function of important tissues and organs [1, 2]. Studies also revealed that childhood stunting as a result of nutrient deprivation in early life is associated with decreased height in adults [26, 35, 57].
The review may also reflect a possible sex-difference in the impact of effect of early life famine exposure on adulthood anthropometric measures. In certain parts of the world, particularly in Asian and African countries, parents tend to care sons more than daughters [58, 59]. These preferences may lead to poor health that may increase their susceptibility to increased body mass index, waist circumference and short stature in later life. Moreover, the sex-difference effect might be partly explained by mortality selection where men had higher mortality rates than women during famine [59, 60] Furthermore, females in low-income countries may have experience of physical inactivity, which modifies the effect of early life famine exposure on the increased risk of body mass index and waist circumference [61].
The contemporary relevance of our finding indicate the long term effects of earlier famine and undernutrition are far from over [62, 63]. It contributes to our understanding of the link between childhood malnutrition and a later risk of increased body mass index, waist circumference and short stature. We may be able to design targeted intervention and, eventually, preventative strategies once we have a better knowledge of these processes. As a result, our findings may be valuable in improving health-system awareness of those born during high-risk years, as well as emphasizing the importance of proper nutrition in infancy.
Strength and limitations
There are various advantages to this systematic review and meta-analysis. As there are potential differences in famine exposure during early life, the study looked into the effects of prenatal and early childhood famine exposure on BMI, waist circumference and adult height. Moreover, the sensitivity and subgroup analysis were performed in order to identify the sources of heterogeneity sources. However, certain potential limitations should be considered in our research. To begin with, the length of the famine varied between research, spanning from 1 to 4 years, which may have influenced the consistency of our findings. Second, the original article did not specify the extent of famine exposure. As a result, we were unable to investigate the relationship between famine severity and BMI, waist circumference and adult height. The other potential limitations of this study include subgroup analyses was not perform according to whether the studies adjusted for age and current lifestyle factors. It would be interesting to see if the results of the studies that performed adjustment for age and current lifestyle factors are different from those that did not perform such an adjustment.
Conclusion
Results from this study confirmed the relationships between early life, particularly prenatal life, exposure to famine and its association with BMI, waist circumference and adult height. The finding underpinning the nutritional status in early life, has a long-term effect on later life. Further studies on the mechanisms behind the association between early life famine exposure and adulthood anthropometric measures need to be clarified.
Supplementary Information
Acknowledgments
Not applicable.
Abbreviations
- BMI
Body mass index
- SMD
Standardized mean difference
- REM
Random effects model
- DOHaD
Developmental Origins of Health and Disease
- PRISMA
Preferred Reporting Items of Systematic Review and Meta-Analysis
- WC
Waist circumference
Authors’ contributions
GA, KHA and TB conceived and planned the study and did the analysis and interpretation. GA drafted the manuscript. GA, KHA and TB critically revised the manuscript. All authors have reviewed the manuscript and approved the final version for submission.
Funding
The study has not been funded.
Availability of data and materials
Data will be available upon reasonable request of the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Getachew Arage, Email: getachewarage2004@gmail.com.
Tefera Belachew, Email: Teferabelachew2@gmail.com.
Kalkidan Hassen Abate, Email: newewi333@gmail.com.
References
- 1.Charles M-A, Delpierre C, Bréant B. Developmental origin of health and adult diseases (DOHaD): evolution of a concept over three decades. Med Sci M/S. 2016;32(1):15–20. doi: 10.1051/medsci/20163201004. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71(5):1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA, Low FM. The role of developmental plasticity and epigenetics in human health. Birth Defects Res Part C: Embryo Today: Reviews. 2011;93(1):12–18. doi: 10.1002/bdrc.20198. [DOI] [PubMed] [Google Scholar]
- 5.Fall CH. Fetal programming and the risk of noncommunicable disease. Indian J Pediatr. 2013;80(1):13–20. doi: 10.1007/s12098-012-0834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman DJ, Reynolds RM, Hardy DB. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr Rev. 2017;75(12):951–970. doi: 10.1093/nutrit/nux053. [DOI] [PubMed] [Google Scholar]
- 7.Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30(1):15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Bogin B. Patterns of human growth: Cambridge University Press; 1999.
- 9.Sgarbieri VC, Pacheco MTB. Human development: from conception to maturity. Brazilian J Food Tech. 2017;20.
- 10.Langley-Evans SC. Nutrition in early life and the programming of adult disease: a review. J Human Nutr Diet: the official journal of the British Dietetic Association. 2015;28(Suppl 1):1–14. doi: 10.1111/jhn.12212. [DOI] [PubMed] [Google Scholar]
- 11.Jackson AA. Nutrients, growth, and the development of programmed metabolic function. Short and long term effects of breast feeding on child health: Springer; 2002. pp. 41–55. [DOI] [PubMed] [Google Scholar]
- 12.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 13.Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Neonatology. 1990;57(2):107–118. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- 14.Silveira PP, Portella AK, Goldani MZ, Barbieri MA. Developmental origins of health and disease (DOHaD) J Pediatr. 2007;83(6):494–504. doi: 10.2223/JPED.1728. [DOI] [PubMed] [Google Scholar]
- 15.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64(11):965–974. doi: 10.1016/S0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 16.Currie J, Vogl T. Early-life health and adult circumstance in developing countries. Annu Rev Econ. 2013;5(1):1–36. doi: 10.1146/annurev-economics-081412-103704. [DOI] [Google Scholar]
- 17.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Meng X, Qian N. The long term consequences of famine on survivors: evidence from a unique natural experiment using China's great famine. National Bureau Econ Res. 2009;Report No:0898–2937. [Google Scholar]
- 19.Rubin LP. Historical perspective of developmental origins of health and disease in humans. The Epigenome and Developmental Origins of Health and Disease: Elsevier; 2016. pp. 17–32. [Google Scholar]
- 20.Smith LC, Ramakrishnan U, Ndiaye A, Haddad L, Martorell R. The importance of Women's status for child nutrition in developing countries: international food policy research institute (Ifpri) research report abstract 131. Food Nutr Bull. 2003;24(3):287–288. doi: 10.1177/156482650302400309. [DOI] [Google Scholar]
- 21.Moraru A, De Almeida MM, Degryse J-M. PALTEM: what parameters should be collected in disaster settings to assess the long-term outcomes of famine? Int J Environ Res Public Health. 2018;15(5):857. doi: 10.3390/ijerph15050857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galler JR, Barrett LR. Children and famine: long-term impact on development. Ambulatory Child Health. 2001;7(2):85–95. doi: 10.1046/j.1467-0658.2001.00109.x. [DOI] [Google Scholar]
- 23.Chang X, Song P, Wang M, An L. The risks of overweight, obesity and abdominal obesity in middle age after exposure to famine in early life: evidence from the China’s 1959–1961 famine. J Nutr Health Aging. 2018;22(10):1198–1204. doi: 10.1007/s12603-018-1144-z. [DOI] [PubMed] [Google Scholar]
- 24.Liu D, Yu DM, Zhao LY, Fang HY, Zhang J, Wang JZ, et al. Exposure to Famine During Early Life and Abdominal Obesity in Adulthood: Findings from the Great Chinese Famine During 1959(−)1961. Nutrients. 2019;11(4). [DOI] [PMC free article] [PubMed]
- 25.Liu L, Pang ZC, Sun JP, Xue B, Wang SJ, Ning F, et al. Exposure to famine in early life and the risk of obesity in adulthood in Qingdao: evidence from the 1959-1961 Chinese famine. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2017;27(2):154–160. doi: 10.1016/j.numecd.2016.11.125. [DOI] [PubMed] [Google Scholar]
- 26.Portrait FRM, van Wingerden TF, Deeg DJH. Early life undernutrition and adult height: the Dutch famine of 1944-45. Econ Hum Biol. 2017;27(Pt B):339–348. doi: 10.1016/j.ehb.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Ravelli AC, van der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70(5):811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 28.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 29.Song C, Wang M, Chen Z, Yao Y, Feng G, Ma Y, et al. Fetal exposure to Chinese famine increases obesity risk in adulthood. Int J Environ Res Public Health. 2020;17(10):3649. doi: 10.3390/ijerph17103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanner SA, Bulmer K, Andres C, Lantseva OE, Borodina V, Poteen V, et al. Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study. Bmj. 1997;315(7119):1342–1348. doi: 10.1136/bmj.315.7119.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal–de Bruin K, Lumey L. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr. 2007;85(3):869–876. doi: 10.1093/ajcn/85.3.869. [DOI] [PubMed] [Google Scholar]
- 32.van Abeelen AF, Elias SG, Roseboom TJ, Bossuyt PM, van der Schouw YT, Grobbee DE, et al. Postnatal acute famine and risk of overweight: the dutch hungerwinter study. Int J Pediatr. 2012;2012. [DOI] [PMC free article] [PubMed]
- 33.Woo J, Leung J, Wong S. Impact of childhood experience of famine on late life health. J Nutr Health Aging. 2010;14(2):91–95. doi: 10.1007/s12603-009-0193-8. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Wang X, Kong Y, Zhang JH, Zeng Q. The great Chinese famine leads to shorter and overweight females in Chongqing Chinese population after 50 years. Obesity (Silver Spring) 2010;18(3):588–592. doi: 10.1038/oby.2009.296. [DOI] [PubMed] [Google Scholar]
- 35.Wang P-X, Wang J-J, Lei Y-X, Xiao L, Luo Z-C. Impact of fetal and infant exposure to the Chinese great famine on the risk of hypertension in adulthood. PLoS One. 2012;7(11):e49720. doi: 10.1371/journal.pone.0049720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koupil I, Shestov DB, Sparén P, Plavinskaja S, Parfenova N, Vågerö D. Blood pressure, hypertension and mortality from circulatory disease in men and women who survived the siege of Leningrad. Eur J Epidemiol. 2007;22(4):223–234. doi: 10.1007/s10654-007-9113-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Zhang L, Xuan P, Fan Y, Yang L, Hu C, et al. The relationship between famine exposure during early life and body mass index in adulthood: a systematic review and meta-analysis. PLoS One. 2018;13(2):e0192212. doi: 10.1371/journal.pone.0192212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hidayat K, Du X, Shi BM, Qin LQ. Foetal and childhood exposure to famine and the risks of cardiometabolic conditions in adulthood: a systematic review and meta-analysis of observational studies. Obes Rev. 2020;21(5):e12981. doi: 10.1111/obr.12981. [DOI] [PubMed] [Google Scholar]
- 39.Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 40.Munn Z, Tufanaru C, Aromataris E. JBI's systematic reviews: data extraction and synthesis. AJN Am J Nurs. 2014;114(7):49–54. doi: 10.1097/01.NAJ.0000451683.66447.89. [DOI] [PubMed] [Google Scholar]
- 41.Bogin B. Human growth and development. Basics in human evolution. Elsevier. 2015:285–93.
- 42.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 43.Collaboration C. Review Manager (RevMan) [Computer Program] Version 5.2. 3, The Nordic Cochrane Centre, Copenhagen, 2012. Health Psychol Rev 2014;17.
- 44.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Rooij SR, Painter RC, Holleman F, Bossuyt PM, Roseboom TJ. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am J Clin Nutr. 2007;86(4):1219–1224. doi: 10.1093/ajcn/86.4.1219. [DOI] [PubMed] [Google Scholar]
- 46.Han C, Hong Y-C. Fetal and childhood malnutrition during the Korean war and metabolic syndrome in adulthood. Nutrition. 2019;62:186–193. doi: 10.1016/j.nut.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Ning F, Ren J, Song X, Zhang D, Liu L, Zhang L, et al. Famine exposure in early life and risk of metabolic syndrome in adulthood: comparisons of different metabolic syndrome definitions. J Diabetes Res. 2019;2019:7954856. doi: 10.1155/2019/7954856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang N, Wang X, Li Q, Han B, Chen Y, Zhu C, et al. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr. 2017;36(1):253–259. doi: 10.1016/j.clnu.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Zou Z, Wang S, Yang Z, Ma J. Chinese famine exposure in infancy and metabolic syndrome in adulthood: results from the China health and retirement longitudinal study. Eur J Clin Nutr. 2019;73(5):724–732. doi: 10.1038/s41430-018-0211-1. [DOI] [PubMed] [Google Scholar]
- 50.Keinan-Boker L, Shasha-Lavsky H, Eilat-Zanani S, Edri-Shur A, Shasha SM. Chronic health conditions in Jewish holocaust survivors born during world war II. Israel Med Assoc J: IMAJ. 2015;17(4):206–212. [PubMed] [Google Scholar]
- 51.Shi Z, Nicholls SJ, Taylor AW, Magliano DJ, Appleton S, Zimmet P. Early life exposure to Chinese famine modifies the association between hypertension and cardiovascular disease. J Hypertens. 2018;36(1):54–60. doi: 10.1097/HJH.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 52.Chen C, Zhao L, Ning Z, Li Q, Han B, Cheng J, et al. Famine exposure in early life is associated with visceral adipose dysfunction in adult females. Eur J Nutr. 2019;58(4):1625–1633. doi: 10.1007/s00394-018-1707-0. [DOI] [PubMed] [Google Scholar]
- 53.Hult M, Tornhammar P, Ueda P, Chima C, Bonamy A-KE, Ozumba B, et al. Hypertension, diabetes and overweight: looming legacies of the Biafran famine. PLoS One. 2010;5(10):e13582. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng RR, Si JH, Lyu J, Guo Y, Bian Z, Yu CQ, et al. Association between famine exposure during early life and BMI in adulthood. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37(11):1450–1453. doi: 10.3760/cma.j.issn.0254-6450.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Zhao W, Zhang X, Mu R, Zhai Y, Kong L, et al. Impact of famine during pregnancy and infancy on health in adulthood. Obes Rev. 2008;9:95–99. doi: 10.1111/j.1467-789X.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 56.Painter RC, De Rooij SR, Bossuyt PM, Osmond C, Barker DJ, Bleker OP, et al. A possible link between prenatal exposure to famine and breast cancer: a preliminary study. Am J Hum Biol. 2006;18(6):853–856. doi: 10.1002/ajhb.20564. [DOI] [PubMed] [Google Scholar]
- 57.Huang C, Li Z, Wang M, Martorell R. Early life exposure to the 1959–1961 Chinese famine has long-term health consequences. J Nutr. 2010;140(10):1874–1878. doi: 10.3945/jn.110.121293. [DOI] [PubMed] [Google Scholar]
- 58.Lundberg S. Sons, daughters, and parental behaviour. Oxf Rev Econ Policy. 2005;21(3):340–356. doi: 10.1093/oxrep/gri020. [DOI] [Google Scholar]
- 59.Chen H, Nembhard WN, Stockwell HG. Sex-specific effects of fetal exposure to the 1959–1961 Chinese famine on risk of adult hypertension. Matern Child Health J. 2014;18(3):527–533. doi: 10.1007/s10995-013-1268-z. [DOI] [PubMed] [Google Scholar]
- 60.Mu R, Zhang X. Why does the great Chinese famine affect the male and female survivors differently? Mortality selection versus son preference. Econ Human Biol. 2011;9(1):92–105. doi: 10.1016/j.ehb.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Atkinson K, Lowe S, Moore S. Human development, occupational structure and physical inactivity among 47 low and middle income countries. Prev Med Rep. 2016;3:40–45. doi: 10.1016/j.pmedr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2020;395(10217):65–74. doi: 10.1016/S0140-6736(19)32497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Black RE, Alderman H, Bhutta ZA, Gillespie S, Haddad L, Horton S, et al. Maternal and child nutrition: building momentum for impact. Lancet 2013;382(9890):372–5. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available upon reasonable request of the corresponding author.