Abstract
T-3811, the free base of T-3811ME (BMS-284756), a new des-F(6)-quinolone, showed a potent in vitro activity (MIC at which 90% of the isolates tested are inhibited [MIC90], 0.0313 μg/ml) against Mycoplasma pneumoniae. The MIC90 of T-3811 was 4-fold higher than that of clarithromycin but was 4- to 8-fold lower than those of trovafloxacin, gatifloxacin, gemifloxacin, and moxifloxacin and was 16- to 32-fold lower than those of levofloxacin, ciprofloxacin, and minocycline. In an experimental M. pneumoniae pneumonia model in hamsters, after the administration of T-3811ME (20 mg/kg of body weight as T-3811, once daily, orally) for 5 days, the reduction of viable cells of M. pneumoniae in bronchoalveolar lavage fluid was greater than those of trovafloxacin, levofloxacin, and clarithromycin (20 and 40 mg/kg, orally) (P < 0.05).
Mycoplasma pneumoniae is a major causative organism of pneumonia and accounts for as many as 20 to 30% of pneumonia cases (20). For the clinical treatment of M. pneumoniae infections, macrolides and tetracyclines are commonly used (9, 18, 25). However, mutants resistant to macrolides have been reported over the last 10 years (17, 21, 23). Thus, a viable alternative to macrolides in the treatment of pneumonia caused by M. pneumoniae is needed. New quinolones developed recently, such as moxifloxacin and gatifloxacin (5, 10), possess a wide antimicrobial spectrum and potent activity against various pathogens, including M. pneumoniae, and are expected to be useful in the treatment of this organism (4, 5, 6). T-3811ME (BMS-284756), a new des-F (6)-quinolone, which exhibits excellent activity against respiratory pathogens such as Streptococcus pneumoniae, Chlamydia pneumoniae, and M. pneumoniae, is under development for both oral and parenteral administration (24). In the present study, we studied in vitro and in vivo antimycoplasma activity of T-3811ME. The in vivo efficacy of T-3811ME was evaluated in hamsters with experimental pneumonia caused by M. pneumoniae and compared with those of trovafloxacin, levofloxacin, and clarithromycin.
Determination of MIC.
The following agents were employed: T-3811ME (T-3811 methanesulfonate monohydrate, also known as BMS-284756), T-3811 (the free base of T-3811ME), ciprofloxacin, levofloxacin, trovafloxacin, gatifloxacin, gemifloxacin, moxifloxacin, clarithromycin, and minocycline; T-3811, T-3811ME, trovafloxacin, gatifloxacin, gemifloxacin, and moxifloxacin were synthesized at Research Laboratories, Toyama Chemical Co., Ltd., Tokyo, Japan. Ciprofloxacin, levofloxacin, and clarithromycin were extracted from commercially available tablets. The purity of each of these three agents was above 99.8% as measured by high-performance liquid chromatography (HPLC). Minocycline was purchased from Lederle-Japan Ltd., Tokyo, Japan. T-3811, the free base of T-3811ME, was used for all in vitro studies.
M. pneumoniae FH, a standard strain, was used for experimental pneumonia studies with hamsters. Fifty clinical isolates of M. pneumoniae were used for MIC determination of nine antimicrobial agents. MICs were determined using a microdilution method (phenol red method) in 96-well microplates (12). A broth microdilution procedure was used according to the method of Whithear et al. (26). The medium used for MIC determination and dilution of agents was PPLO (Difco Inc., Detroit, Mich.) broth supplemented with 30% Mycoplasma Supplement S (Difco), 0.5% glucose (Wako Pure Chemicals Industries, Ltd., Osaka, Japan), and 0.002% phenol red (Tokyo Kasei Co., Ltd., Tokyo, Japan). Preculture was carried out with the same medium for 6 to 9 days of incubation at 37°C. Then, 10 μl of suspension (106 CFU/ml) was inoculated into 96-well microplates containing 90 μl of the antibiotic solution. The MICs were determined after 5 days of incubation at 37°C by color change from red to yellow. Phenol red indicated a yellow color (acidification) after cell growth resulting in consumption of glucose.
Therapeutic efficacies of T-3811ME, trovafloxacin, levofloxacin, and clarithromycin. (i) Experimental pneumonia model in hamsters.
For the experimental pneumonia model, hamsters were used as in previous reports (2, 3, 11). Four-week-old male Syrian hamsters were purchased from Japan SLC Inc., Shizuoka, Japan, and were assigned to the study after an acclimation period of 3 days. On the day of infection, the hamsters were randomly allocated to each group (control, n = 11; treated groups, n = 5 to 6). M. pneumoniae FH was cultured in PPLO broth at 37°C for 7 days and stored at −40°C (approximately 108 CFU/ml). The frozen cells were thawed at 37°C before use. Pulmonary infections were induced in hamsters anesthetized with sodium pentobarbital (Dainippon Pharmaceutical Co., Ltd., Osaka, Japan) by intratracheally injecting 0.1 ml of the suspension of M. pneumoniae.
(ii) Administration of antimicrobial agents.
T-3811ME, levofloxacin, trovafloxacin, and clarithromycin were suspended in 0.5% methylcellulose SM-400 (Shin-Etsu Chemical Co., Ltd., Tokyo, Japan). Oral administration of antimicrobial agents at 20 mg/kg of body weight (T-3811ME-treated groups) and 20 and 40 mg/kg (all other groups) was initiated on the 7th day after infection by M. pneumoniae and continued once daily for 2 and 5 days.
(iii) Evaluation of therapeutic effects of antimicrobial agents.
Animals were sacrificed at 24 h after final administration following anesthetization with ether. Bronchoalveolar lavage fluid (BALF) was obtained by injection into and subsequent suction of 2 ml of Hanks solution (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) from the trachea. This BALF and its 10-fold serial dilutions were used for viable cell count. The viable cell counts were measured on the plate of PPLO agar containing supplements. The limit of assay of CFUs in BALF by this method was 2.30 log CFU/ml.
(iv) Statistical analysis.
The significance of each group in terms of the viable cell count in BALF was analyzed by Tukey's multiple comparisons procedures in an imbalanced two-way ANOVA (SAS version 6.12 software).
The concentrations in serum, BALF, and lung tissue after administration at a dose of 20 mg/kg orally were studied for T-3811ME, trovafloxacin, levofloxacin, and clarithromycin (each group, n = 3 to 4). At 0.25, 0.5, 1, 2, 4, and 6 h after drug administration, blood, BALF, and lung tissue samples were collected. Animals were sacrificed by exsanguination from the cephalic vein under ether anesthesia at each sampling time. The blood serum (collected from the cephalic vein) was measured for drug concentrations. After blood was kept in Sepaclen-A-5 (Eiken Kizai Co., Ltd., Tokyo, Japan) at room temperature for more than 30 min, serum was obtained by centrifugation. Lung tissue was removed and homogenized in fourfold volumes of of phosphate-buffered solution (PBS) (pH 7.0, 1/15 mol/liter). BALF was obtained by injection into and subsequent suction of PBS (1 ml) from the trachea. We used this method because the volume of BALF was very small. However the actual concentration of BALF would be much higher if corrected for dilution. The homogenized tissue and BALF were centrifuged. The supernatants and serum were frozen at −40°C until required for HPLC assay. An equal volume of methanol was added to each of the samples, which were then mixed and centrifuged. Supernatants were used for measurement of T-3811, trovafloxacin, and levofloxacin concentrations by HPLC (LC series device [Shimadzu Co., Ltd., Tokyo, Japan] or L series device [Hitachi Co., Ltd., Tokyo, Japan]). The concentration of clarithromycin was measured by a bioassay method (paper disk method) using Micrococcus luteus ATCC 9341 as a test strain and heart infusion agar (Eiken Kagaku Co., Ltd., Tokyo, Japan) as a medium. Area under the plasma concentration-time curve from 0 h to ∞ (AUC0–∞) and the terminal half-life were calculated from the mean concentrations in plasma by noncompartmental analysis with WinNonlin software (Scientific Consulting, Inc.). The maximum plasma concentration (Cmax) and the time to reach Cmax were obtained directly from the actual mean concentrations in plasma.
The MIC at which 90% of the isolates tested are inhibited (MIC90) of T-3811 against 50 clinical isolates was 0.0313 μg/ml and was 4-fold higher than that of clarithromycin but was 4- to 8-fold lower than those of trovafloxacin, gatifloxacin, gemifloxacin, and moxifloxacin and was 16- to 32-fold lower than those of levofloxacin, ciprofloxacin, and minocycline. The MIC of T-3811 for M. pneumoniae FH was 0.0313 μg/ml and was 2- to 32-fold lower than those of other quinolones tested (Table 1).
TABLE 1.
Activities of antibacterial agents against 50 clinical isolates of M. pneumoniae and M. pneumoniae FH
| Antibacterial agent | MIC (μg/ml) for M. pneumoniaea
|
MIC (μg/ml) for M. pneumoniae FHb | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| T-3811 | 0.0156–0.0625 | 0.0313 | 0.0313 | 0.0313 |
| Ciprofloxacin | 0.5–2 | 1 | 1 | 1 |
| Levofloxacin | 0.25–1 | 0.5 | 0.5 | 0.5 |
| Trovafloxacin | 0.0625–0.25 | 0.125 | 0.25 | 0.125 |
| Gatifloxacin | 0.0625–0.25 | 0.125 | 0.125 | 0.125 |
| Gemifloxacin | 0.0625–0.25 | 0.25 | 0.25 | 0.125 |
| Moxifloxacin | 0.0625–0.125 | 0.125 | 0.125 | 0.0625 |
| Clarithromycin | 0.002–0.0156 | 0.0078 | 0.0078 | 0.002 |
| Minocycline | 0.0625–1 | 0.5 | 1 | 0.0625 |
50%, MIC50; 90%, MIC90.
The strain used for experimental pneumonia.
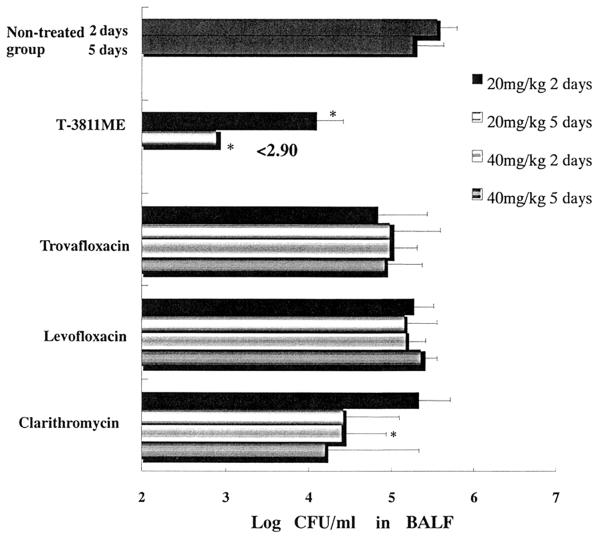
Figure 1 shows the viable cell counts in BALF from hamsters with experimental pneumonia caused by M. pneumoniae FH. The viable cells of M. pneumoniae in the nontreated group were 105 to 106 CFU/ml from 7 days (start of administration) through 12 days (1 day after administration over 5 days) after infection. After the administration of trovafloxacin (20 and 40 mg/kg), levofloxacin (20 and 40 mg/kg), and clarithromycin (20 mg/kg) once daily for 2 days, the viable cells of M. pneumoniae in BALF were comparable to those of the nontreated group. On the other hand, T-3811ME (20 mg/kg as T-3811) and clarithromycin (40 mg/kg) reduced the viable cell count in BALF, and the viable cells of M. pneumoniae in these groups were significantly fewer than in the nontreated group (P < 0.05). The viable cells of M. pneumoniae in BALF after administration of T-3811ME (20 mg/kg as T-3811) once daily for 5 days were significantly fewer than those in the clarithromycin-, trovafloxacin-, and levofloxacin-treated groups (20 and 40 mg/kg) and the nontreated groups (P < 0.05).
FIG. 1.
In vivo efficacy of T-3811ME and other antibacterial agents against experimental pneumonia caused by M. pneumoniae FH in hamsters. For results at 2 days an asterisk indicates a P of <0.05 for T-3811ME versus control, trovafloxacin, levofloxacin, and clarithromycin (except clarithromycin at 40 mg/kg), or for clarithromycin (40 mg/kg) versus control. For results at 5 days, an asterisk indicates a P of <0.05 for T-3811ME versus control, trovafloxacin, levofloxacin, and clarithromycin. Results are presented as Means + standard deviations (error bars) (untreated group, n = 11; treated groups, n = 5 to 6), for 2 or 5 days of therapy.
Table 2 shows the pharmacokinetic parameters. The Cmax of T-3811 in serum was 0.85 μg/ml and was lower than that of levofloxacin and comparable to those of trovafloxacin and clarithromycin. The Cmax of T-3811 in lung tissue was 2.1 μg/g and was lower than that of clarithromycin and comparable to those of levofloxacin and trovafloxacin. The Cmax of T-3811 in BALF was 0.09 μg/ml and was lower than that of levofloxacin and comparable to that of trovafloxacin. The Cmax of clarithromycin in BALF was lower than the limit of assay. The AUC0–∞ of T-3811 in lung tissue was 7.7 μg·h/g and was greater than that of trovafloxacin and lower than those of levofloxacin and clarithromycin. The Cmax/MIC and AUC0–∞/MIC of T-3811 in lung tissue were 67.1 and 246, respectively and were greater than those of trovafloxacin and levofloxacin. Clarithromycin possessed a potent in vitro activity against M. pneumoniae and showed a significant therapeutic effect (40 mg/kg, for 2 days) in the present study. However, the therapeutic effect of clarithromycin was inferior to that of T-3811ME in other cases. Because M. pneumoniae is an extracellular parasite (19), it was considered that the low distribution in BALF and weak bactericidal activity of clarithromycin compared with T-3811 (data not shown) resulted in low efficacy.
TABLE 2.
Pharmacokinetic parameters after oral administration of antibacterial agents (20 mg/kg) to hamsters with experimental pneumonia caused by M. pneumoniae FH
| Fluid | Antibacterial agent | Cmax (μg/ml or g) | Tmaxa (h) | t1/2b (h) | AUC0–∞ (μg · h/ml or g) | Cmax/MICc | AUC0–∞/MICc |
|---|---|---|---|---|---|---|---|
| Serum | T-3811MEd | 0.85 | 0.5 | 3.0 | 4.3 | 27.2 | 137 |
| Trovafloxacin | 1.1 | 0.5 | 1.7 | 3.1 | 8.80 | 24.8 | |
| Levofloxacin | 2.2 | 0.5 | 2.0 | 8.4 | 4.40 | 16.8 | |
| Clarithromycin | 0.62 | 1 | 1.3 | 1.0 | 310 | 500 | |
| Lung | T-3811ME | 2.1 | 1 | 2.4 | 7.7 | 67.1 | 246 |
| Trovafloxacin | 1.6 | 0.5 | 1.1 | 3.5 | 12.8 | 28.0 | |
| Levofloxacin | 2.5 | 0.25 | 2.2 | 10.3 | 5.00 | 20.6 | |
| Clarithromycin | 9.6 | 1 | 2.3 | 27.5 | 4,800 | 13,750 | |
| BALF | T-3811ME | 0.09 | 0.5 | 3.9 | 0.27 | ||
| Trovafloxacin | 0.08 | 1 | 1.1 | 0.22 | |||
| Levofloxacin | 0.17 | 0.5 | 2.1 | 0.46 | |||
| Clarithromycin | <0.20 |
Tmax, time to Cmax.
t1/2, terminal half-life.
MIC for M. pneumoniae FH.
Equivalent to T-3811.
Macrolides and tetracyclines are the most-active antibiotics against mycoplasmas in vitro (1, 16, 22, 25). However, improvements in therapy are needed to accommodate specific characteristics of the microorganism or unusual manifestations of the illness. Until now, it was reported that quinolones are less effective in mycoplasmal and chlamydial infection (16). The newer fluoroquinolones, such as moxifloxacin and gatifloxacin, which have recently undergone substantial development, exhibit in vitro activity against a broadened spectrum, including M. pneumoniae (5, 6, 7, 8, 10, 15). T-3811, the free base of T-3811ME (BMS-284756), also showed excellent in vitro activity against M. pneumoniae. The in vitro activity of T-3811 was four- to eight-fold greater than those of other new quinolones tested. Also, the in vivo efficacy of T-3811ME against experimental pneumonia caused by M. pneumoniae was superior to those of trovafloxacin, levofloxacin, and clarithromycin. Although the existing data on in vivo efficacy are only preliminary (11, 13, 14), the newer fluoroquinolones are promising agents for the treatment of M. pneumoniae infection and, more generally, for that of community-acquired pneumonia. Newer quinolones have attracted interest as potential therapy for community-acquired pneumonia because they are active against a wide range of pathogens, including M. pneumoniae.
In conclusion, T-3811ME possessed potent in vitro antimycoplasma activity and had a significantly superior therapeutic effect compared to clarithromycin, trovafloxacin, and levofloxacin on experimental pneumonia caused by M. pneumoniae FH. These data indicate that T-3811ME (BMS-284756) could be a viable alternative to macrolides, such as clarithromycin, in the treatment of pneumonia caused by M. pneumoniae.
Acknowledgments
We express our thanks to Mitsuo Kaku, Department of Molecular Diagnostics, Department of Laboratory Medicine, Tohoku University Graduate School of Medicine, for providing us with the M. pneumoniae strains and to Helen Lavin for reviewing the manuscript.
REFERENCES
- 1.Arai S, Gohara Y, Kuwano K, Kawashima T. Antimycoplasmal activities of new quinolones, tetracyclines, and macrolides against Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1992;36:1322–1324. doi: 10.1128/aac.36.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai S, Gohara Y, Akashi A, Kuwano K, Nishimoto M, Yano T, Oizumi K, Takeda K, Yamaguchi T. Effects of new quinolones on Mycoplasma pneumoniae-infected hamsters. Antimicrob Agents Chemother. 1993;37:287–292. doi: 10.1128/aac.37.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barile M F, Chandler D M, Yoshida H, Grabowski M W, Harasawa R, Razin S. Parameters of Mycoplasma pneumoniae infection in Syrian hamsters. Infect Immun. 1988;56:2443–2449. doi: 10.1128/iai.56.9.2443-2449.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bebear C, Dupon M, Renaudin H, de Barbeyrac B. Potential improvements in therapeutic options for mycoplasmal respiratory infections. Clin Infect Dis. 1993;17:S202–207. doi: 10.1093/clinids/17.supplement_1.s202. [DOI] [PubMed] [Google Scholar]
- 5.Bebear C M, Renaudin H, Boudjadja A, Bebear C. In vitro activity of BAY 12-8039, a new fluoroquinolone against mycoplasmas. Antimicrob Agents Chemother. 1998;42:703–704. doi: 10.1128/aac.42.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Child J, Andrews J, Boswell F, Brenwarld N, Wise R. The in-vitro activity of CP-99219, a new naphthyridone antimicrobial agent: a comparison with fluoroquinolone agents. J Antimicrob Chemother. 1995;35:869–876. doi: 10.1093/jac/35.6.869. [DOI] [PubMed] [Google Scholar]
- 7.Felmingham D, Robbins M J, Ingley K, Mathias I, Bhogal H, Leakey A. In-vitro activity of trovafloxacin, a new fluoroquinolone, against recent clinical isolates. J Antimicrob Chemother. 1997;39:43–50. doi: 10.1093/jac/39.suppl_2.43. [DOI] [PubMed] [Google Scholar]
- 8.Gohara Y, Arai S, Akashi A, Kuwano K, Tseng C C, Matsubara S, Matumoto M, Furudera T. In vitro and in vivo activities of Q-35, a new fluoroquinolone, against Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1993;37:1826–1830. doi: 10.1128/aac.37.9.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guay D R. Macrolide antibiotics in pediatric infectious diseases. Drugs. 1996;51:515–536. doi: 10.2165/00003495-199651040-00002. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka M, Yasue T, Fukuda H, Tomizawa H, Aoyama H, Hirai K. In vitro and in vivo antibacterial activities of AM-1155, a new 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1992;36:2108–2117. doi: 10.1128/aac.36.10.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida K, Kaku M, Irifune K, Mizukane R, Takemura H, Yoshida R, Tanaka H, Usui T, Tomono K, Suyama N. In-vitro and in-vivo activity of a new quinolone AM-1155 against Mycoplasma pneumoniae. J Antimicrob Chemother. 1994;34:875–883. doi: 10.1093/jac/34.6.875. [DOI] [PubMed] [Google Scholar]
- 12.Japan Society of Chemotherapy. Method for the determination of minimum inhibitory concentration (MIC) by micro-broth dilution method. Chemotherapy (Tokyo) 1992;40:184–189. [Google Scholar]
- 13.Kaku M, Ishida K, Irifune K, Mizukane R, Takemura H, Yoshida R, Tanaka H, Usui T, Tomono K, Suyama N. In vitro and in vivo activities of sparfloxacin against Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1994;38:738–741. doi: 10.1128/aac.38.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaku M, Ishida K, Kohno S, Koga H, Hara K, Usui T. Experimental and clinical studies of sparfloxacin in Mycoplasma pneumoniae infection. Drugs. 1995;49:412–413. doi: 10.2165/00003495-199500492-00117. [DOI] [PubMed] [Google Scholar]
- 15.Kenny G E, Cartwright F D. Susceptibilities of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum to a new quinolone, trovafloxacin (CP-99, 219) Antimicrob Agents Chemother. 1996;40:1048–1049. doi: 10.1128/aac.40.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny G E, Cartwright F D. Susceptibility of Mycoplasma pneumoniae to several new quinolones, tetracycline, and erythromycin. Antimicrob Agents Chemother. 1991;35:587–589. doi: 10.1128/aac.35.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosmidis J, Koratzanis G. Emergence of resistant bacterial strains during treatment of infections in the respiratory tract. Scand J Infect Dis. 1986;49:135–139. [PubMed] [Google Scholar]
- 18.Langtry H D, Balfour J A. Azithromycin. A review of its use in pediatric infectious diseases. Drugs. 1998;56:273–297. doi: 10.2165/00003495-199856020-00014. [DOI] [PubMed] [Google Scholar]
- 19.Leigh M W, Clyde W A., Jr Chlamydial and mycoplasmal pneumonias. Semin Respir Infect. 1987;2:152–158. [PubMed] [Google Scholar]
- 20.Lieberman D, Schlaeffer F, Lieberman D, Horowitz S, Horovitz O, Porath A. Mycoplasma pneumoniae community-acquired pneumonia: a review of 101 hospitalized adult patients. Respiration. 1996;63:261–266. doi: 10.1159/000196557. [DOI] [PubMed] [Google Scholar]
- 21.Lucier T S, Heitzman K, Liu S K, Hu P C. Transition mutations in the 23S rRNA of erythromycin-resistant isolates of Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1995;39:2770–2773. doi: 10.1128/aac.39.12.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlick W J. The problems of treating atypical pneumonia. Antimicrob Chemother. 1993;31:111–120. doi: 10.1093/jac/31.suppl_c.111. [DOI] [PubMed] [Google Scholar]
- 23.Stopler T, Branski D J. Resistance of Mycoplasma pneumoniae to macrolides, lincomycin, and streptogramin B. Antimicrob Agents Chemother. 1986;18:359–364. doi: 10.1093/jac/18.3.359. [DOI] [PubMed] [Google Scholar]
- 24.Takahata M, Mitsuyama J, Yamashiro Y, Yonezawa M, Araki H, Todo Y, Minami S, Watanabe Y, Narita H. In vitro and in vivo antimicrobial activities of T-3811ME, a novel des-F(6)-quinolone. Antimicrob Agents Chemother. 1999;43:1077–1084. doi: 10.1128/aac.43.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor-Robinson D, Bebear C J. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. Antimicrob Chemother. 1997;40:622–630. doi: 10.1093/jac/40.5.622. [DOI] [PubMed] [Google Scholar]
- 26.Whithear K G, Bowtell D D, Hughes K L. Evaluation and use of a micro-broth dilution procedure for testing sensitivity of fermentative avian mycoplasmas to antibiotics. Avian Dis. 1983;27:937–949. [PubMed] [Google Scholar]