Abstract
Background: Bee venom acupuncture (BVA) is an effective treatment method for various diseases. Bee venom, however, can cause adverse effects, even rarely including life-threatening anaphylaxis, so safety-related evidence is required. In this study, we systematically estimated the incidence rate of anaphylaxis in response to BVA. Methods: We searched eight databases (MEDLINE (Pubmed), EMBASE, Cochrane Central Register of Controlled, KISS, KMBASE, Koreamed, OASIS, and NDSL) and systematically reviewed the articles that met the inclusion/exclusion criteria. Results: Among 225 potentially relevant articles, 49 were selected for this study. The overall incidence rate of anaphylaxis in response to BVA was 0.045% (95% CI 0.028–0.062). Women (0.083%, 95% CI 0.010–0.157) showed a higher incidence rate than men (0.019%, 95% CI −0.018 to 0.055), while the incidence for patients who had a skin test conducted (0.041%, 95% CI 0.011–0.072) was not significantly different compared to that obtained for patients for which there was no information about a skin test (0.047%, 95% CI 0.026–0.067). The publication year affected the incidence rate: it was highest before 1999 (1.099%, 95% CI −1.043 to 3.241), lower between 2000 and 2009 (0.049%, 95% CI 0.025–0.073), and lowest between 2010 and 2021 (0.037% 95% CI 0.014–0.060). Conclusions: In this study, we provide reference data about risk size and factors of BVA-related anaphylaxis, which is essentially required for BVA application in clinics.
Keywords: bee venom acupuncture, anaphylaxis, systematic review, incidence rate
1. Introduction
Bee venom is effective in treating pain and has recently been applied to various diseases such as arthritis, adhesive capsulitis, lupus, and cancer [1,2,3]. Bee venom acupuncture (BVA), one of the most frequently used techniques recently, involves intradermally or intramuscularly administering a small amount of refined bee venom into specific acupoints or painful areas [4].
Despite its clinical advantages, BVA often induces allergic reactions, ranging from local reactions such as rash, swelling, and itching to systemic reactions such as anaphylaxis [5]. Bee venom contains various enzymes, peptides, and biogenic amines, including melittin, phospholipase A2, and apamin [6]. Among these compounds, melittin is considered to be the main active ingredient associated with the therapeutic effect of bee venom [7]. Phospholipase A2 is known to be a major allergen that can induce anaphylaxis through inflammation and hypotension [8], even though it was also recently discovered to have immunomodulatory effects [9]. Anaphylaxis is a life-threatening systemic reaction, with clinical features such as lower blood pressure, blood-clotting tendency, and dyspnea; thus, it causes shock or seizures [10]. Therefore, it is very important to prevent anaphylaxis in clinics through the safe application of BVA.
Since the mid-2000s, in order to reduce the adverse reactions of bee venom, a method has been in use to remove its components that cause side effects, such as phospholipase A2, and extract and purify only the active ingredient, melittin, thereby creating what is called sweet bee venom (SBV) [11]. Some studies reviewing randomized controlled trials (RCTs) have reported that BVA has a higher risk of adverse events than normal acupuncture treatment, but the limitations of small-scale studies suggest a need for further research regarding the safety of BVA [12,13]. Our previous cohort study estimated a 0.047% (95% CI 0.001–0.092) incidence rate of anaphylactic reactions to BVA: there were 4 cases out of 8580 patients in one hospital over a span of 10 years [14].
Considering the impact of BVA anaphylaxis, clinic-derived data are still lacking and no comprehensive study has been conducted. We thus aimed to determine the overall incidence of anaphylaxis in response to BVA via a systematic review using all of the published literature.
2. Results
2.1. Characteristics of the Included Studies
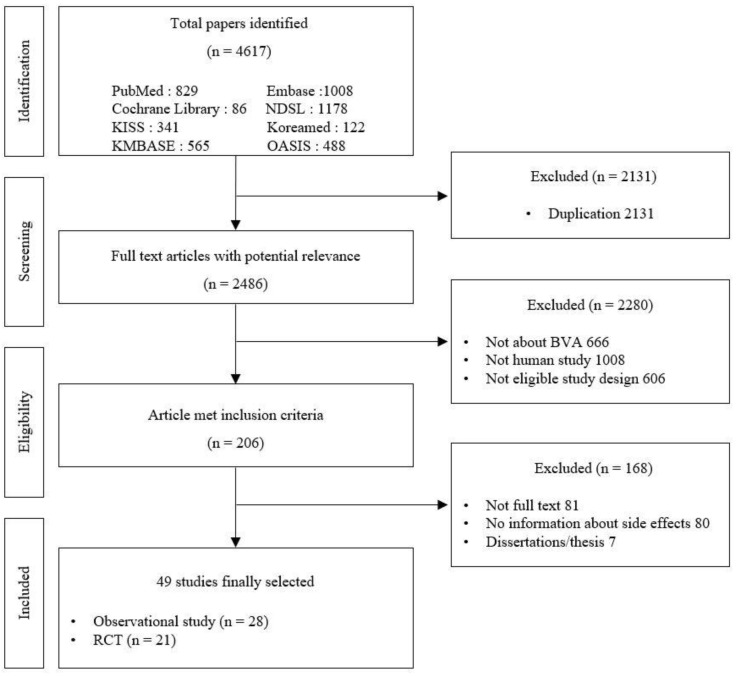
In total, 225 studies were identified in the initial screening, of which 49 articles (28 observational studies and 21 RCTs) met the inclusion criteria. Figure 1 shows a flow diagram of the procedure used to select the relevant studies. The characteristics of the included studies are summarized in Table 1.
Figure 1.
Flow diagram of the literature search process.
Table 1.
Characteristics of included studies.
| Items | Observational Studies | RCTs | Total |
|---|---|---|---|
| Number of studies (%) | 28 (57) | 21 (43) | 49 (100) |
| Number of participants (%) | 59,030 (99) | 703 (1) | 59,733 (100) |
| Men/Women | 5227 (48)/5739 (52) | 176 (33)/371 (67) | 5403/6110 |
| No information a | 48,064 | 156 | 48,220 |
| Mean age (years) b | 47.5 ± 8.6 | 55.5 ± 6.83 | 51.1 ± 8.8 |
| Main complaints (No. of participants, studies) | |||
| G00–G99 c | 93 (4) | 20 (1) | 113 (5) |
| S00–T98 d | 16 (1) | 61 (2) | 77 (3) |
| M00–M99 e | 57,897 (15) | 551 (16) | 58,448 (31) |
| Others (obesity) | 0 (0) | 51 (1) | 51 (1) |
| No information a | 1024 (8) | 20 (1) | 1044 (9) |
| Skin test (No. of participants, studies) | |||
| Yes | 16,585 (14) | 375 (13) | 16,960 (27) |
| No | 0 (0) | 0 (0) | 0 (0) |
| No information a | 42,445 (14) | 328 (8) | 42,773 (22) |
| Publication year (No. of participants, studies) | |||
| –1999 | 31 (1) | 60 (1) | 91 (2) |
| 2000–2009 | 32,350 (10) | 409 (9) | 32,759 (19) |
| 2010–2021 | 26,649 (17) | 234 (11) | 26,883 (28) |
| Country (No. of participants, studies) | |||
| South Korea | 58,953 (26) | 683 (20) | 59,636 (46) |
| France | 0 (0) | 20 (1) | 20 (1) |
| Romania | 26 (1) | 0 (0) | 26 (1) |
| USA | 51 (1) | 0 (0) | 51 (1) |
a The relevant information is not present in the articles; b mean age is the average of studies that presented the mean age of participants; c diseases of the nervous system; d injury, poisoning, and certain other consequences of external causes; e diseases of the musculoskeletal system and connective tissue.
The main complaints of patients who used BVA were musculoskeletal system or connective tissue disease in 31 patients, nervous system disease in 5 patients, and external causes such as injury in 3 patients. A skin test prior to BVA treatment was performed in 27 studies, while the rest did not specify whether a skin test was performed. Two studies were published between 1990 and 1999, nineteen studies between 2000 and 2009, and twenty-eight studies after 2010. These studies were from four countries: Korea (n = 46), France (n = 1), Romania (n = 1), and the USA (n = 1).
2.2. Incidence of Anaphylaxis in Response to BVA
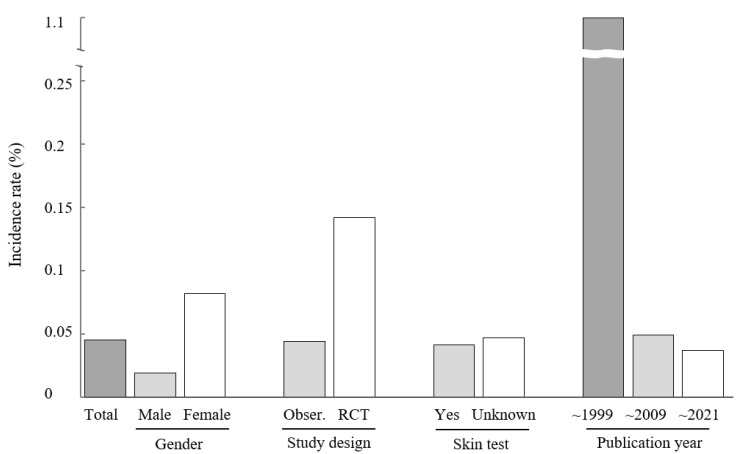
Of the total 59,733 participants treated with BVA, 27 cases of anaphylaxis were reported. The overall incidence of anaphylaxis in response to BVA was 0.045% (95% CI 0.028–0.055; Table 2 and Figure 2). The incidence was 0.044% (95% CI 0.027–0.061) in observational studies and 0.142% (95% CI −0.136 to 0.421) in RCTs.
Table 2.
Incidence of anaphylaxis due to BVA by subgroup.
| Group | Incidence (%, 95% CI) | ||
|---|---|---|---|
| Observational Studies (59,030 Participants) |
RCTs (703 Participants) |
Total (59,733 Participants) |
|
| No. of anaphylaxis | 26 | 1 | 27 |
| Overall incidence in all studies | 0.044 (0.027–0.061) | 0.142 (−0.136 to 0.421) | 0.045 (0.028–0.062) |
| Incidence by subgroup (No. studies that presented data) | |||
| Sex (37) | |||
| Men | 0.019 (−0.019 to 0.057) | 0 | 0.019 (−0.018 to 0.055) |
| Women | 0.089 (0.011–0.167) | 0 | 0.083 (0.010–0.157) |
| Main complaints (39) | |||
| G00-G99 a | 1.075 (−1.021 to 3.171) | 0 | 0.885 (−0.842 to 2.612) |
| S00-T98 b | 0 | 1.639 (−1.547 to 4.826) | 1.299 (−1.230 to 3.828) |
| M00-M99 c | 0.043 (0.026–0.060) | 0 | 0.043 (0.026–0.060) |
| Others (obesity) | 0 | 0 | 0 |
| No information d | 0 | 0 | 0 |
| Skin test (49) | |||
| Yes | 0.036 (0.007–0.065) | 0.267 (−0.255 to 0.789) | 0.041 (0.011–0.072) |
| No | |||
| No information d | 0.047 (0.026–0.068) | 0 | 0.047 (0.026–0.067) |
| Publication year (49) | |||
| –1999 | 3.226 (−2.994 to 9.446) | 0 | 1.099 (−1.043 to 3.241) |
| 2000–2009 | 0.049 (0.025–0.074) | 0 | 0.049 (0.025–0.073) |
| 2010–2021 | 0.034 (0.012–0.056) | 0.379 (−0.362 to 1.120) | 0.037 (0.014–0.060) |
a Diseases of the nervous system; b injury, poisoning, and certain other consequences of external causes; c diseases of the musculoskeletal system and connective tissue; d relevant information is not presented in the articles.
Figure 2.
The incidence rate of anaphylaxis due to BVA by subgroup. Obser.: observational study; Unknown: no description of whether or not the skin test was performed prior to BVA treatment.
2.3. Incidence of Anaphylaxis in Response to BVA by Subgroup
Thirty-seven studies describe the data by sex; women show a higher incidence rate than men (0.083% for women vs. 0.019% for men). Participants’ main complaints were available in 39 studies. Anaphylaxis was the most frequent after treatment for injury (1.299%), followed by neurological disorders (0.885%, representing one case of multiple sclerosis), and musculoskeletal disorders (0.043%).
Performing a skin test resulted in no significant difference in the incidence of anaphylaxis compared to cases in which the performing of a skin test was not described (0.041% in skin test performed vs. 0.047% in skin test not described). We identified that the more recently the paper was published, the lower the incidence of anaphylaxis: highest before 1999 (1.099%), lower between 2000 and 2009 (0.049%), and lowest between 2010 and 2021 (0.037%) (Table 2 and Figure 2).
2.4. Characteristics of Patients with Anaphylaxis
Out of a total of twenty-seven cases of anaphylaxis, information about twelve patients was available (Table 3). Nine patients showed anaphylaxis at the same concentration of bee venom, 10,000:1. Venom volume at time of anaphylaxis varied from 0.1 to 2 cc. The number of treatments until anaphylaxis reported varied from 1 to 24. Of the seven available studies, two administered bee venom intradermally and five performed an intramuscular injection. Four patients were grade IV (cardiovascular symptoms such as hypotension with/without cyanosis, collapse, arrhythmias, and angina pectoris), six patients were grade III (respiratory symptoms such as dyspnea, difficulty swallowing, hoarseness, and stridor), and the rest were unknown. Except for five cases for which treatment results were not described, twenty-two cases recovered from anaphylaxis.
Table 3.
Characteristics of anaphylaxis cases (27 cases).
| Patient Number | Sex/Age | Concentration during Anaphylaxis | Volume during Anaphylaxis | No. Treatments until Anaphylaxis | Administration | Anaphylaxis Grade c | Treatment Result |
|---|---|---|---|---|---|---|---|
| P1 | Unknown | Unknown | Unknown | 4 | Unknown | III | Recovered |
| P2–5 | 4 patients /(Unknown) |
Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| P6–16 a | 11 patients /(40.9 ± 12 a) |
Unknown | 1.6 cc ± 0.5 | 10.9 ± 6.8 | Unknown | Unknown | Recovered |
| P17 b | Unknown | Unknown | 0.4 cc | 24 | Intradermal | Unknown | Unknown |
| P18 | Unknown | 10,000:1 | Unknown | Unknown | Unknown | IV | Recovered |
| P19 | F/36 | Unknown | 2 cc | 6 | Intradermal | Unknown | Recovered |
| P20 | F/61 | 10,000:1 | 0.5 cc | 1 | Unknown | III | Recovered |
| P21 | F/65 | 10,000:1 | 0.1 cc | Test | Unknown | III | Recovered |
| P22 | F/77 | 10,000:1 | 1 cc | Unknown | Unknown | IV | Recovered |
| P23 | M/70 | 10,000:1 | 2 cc | 6 | Intramuscular | III | Recovered |
| P24 | F/59 | 10,000:1 | 2 cc | 2 | Intramuscular | IV | Recovered |
| P25 | F/62 | 10,000:1 | 2 cc | 13 | Intramuscular | III | Recovered |
| P26 | F/60 | 10,000:1 | 2 cc | 6 | Intramuscular | III | Recovered |
| P27 | F/42 | 10,000:1 | 2 cc | 22 | Intramuscular | IV | Recovered |
a All items are the mean values of 11 patients, b volume and treatment session are estimated from data presented, c Mueller grade.
3. Discussion
In clinical fields, bee venom is used to treat pain and inflammatory symptoms because of its various properties, such as being anti-inflammatory, antibiotic, or applicable in COVID-19 treatment or prevention [15]. In general, the incidence of mild adverse reactions to BVA, such as localized edema, pruritus, and skin rash, was reported to be 28.87% [16]. However, there were case reports of two deaths due to anaphylaxis after BVA treatment [17]. Clinical use of bee venom is limited owing to these severe cases of adverse effects; however, there is no clear evidence leading to the conclusion that bee venom therapy (BVT) is not safe [18]. In fact, two large cohort studies included in our review suggested that the incidence rate of anaphylaxis in response to BVA is 0.034% and 0.019% in 32,000 and 15,783 patients, respectively [19,20]. To overcome the limitations surrounding being outdated and individual studies using a small number of subjects to calculate incidence, we systematically reviewed the incidence rate of anaphylaxis in response to BVA.
Cases of anaphylaxis after BVA treatment are reported in 8 of 49 studies, with an incidence of 0.045% (95% CI 0.028–0.062), which is consistent with our previous single-hospital retrospective studies (0.047%, 95% CI 0.001–0.092) [14].
The main causes of anaphylaxis are foods, drugs, intravenous contrast agents, and venom [21]. Nonsteroidal anti-inflammatory drugs (NSAIDs) and antibiotics such as penicillins and sulfonamides are typical medications that can induce anaphylaxis, with incidence rates of 0.130%, 0.459%, and 0.151%, respectively [22]. Compared to these conventional drugs, the incidence of anaphylaxis due to BVA does not deviate significantly from normal levels.
Regarding the study design, RCTs include only specific populations according to selection and exclusion criteria; observational studies can be more representative of incidence rates, as they include broad and random population groups [23]. In our results, the more than three fold higher incidence of anaphylaxis in RCTs (0.142%) than in observational studies (0.044%) may be due to this limitation, along with the relatively small number of participants included.
Factors influencing allergic reactions to drugs are age, sex, and family history; older people and women are more likely to develop an allergic reaction [24,25]. Although the mechanism of susceptibility to anaphylaxis is unclear, these studies support our findings that women show a higher incidence of anaphylaxis in response to BVA than men [26]. The data on main complaints and incidence of anaphylaxis show for which disease BVA was most frequently used, not which disease increases the incidence of BVA-related anaphylaxis. Out of a total of 58,448 participants with musculoskeletal and connective tissue diseases for which BVA was most frequently applied in clinics, 25 of them had a BVA-related anaphylactic reaction. Looking at the nature of the subjects’ diseases, 98% of their chief complaints were musculoskeletal disorders such as herniated nucleus pulposus, osteoarthritis, rheumatoid arthritis, etc. In addition, BVA was applied to a total of 113 patients with neurological diseases (31 patients with Parkinson’s disease, 20 with facial paralysis, 11 with peripheral neuropathy, and 51 with multiple sclerosis), of which 1 patient with multiple sclerosis had an anaphylactic reaction after BVA treatment.
A skin test is a process performed prior to injection (for example, before antibiotic injection) to check whether an allergic reaction occurs with a small amount administered subcutaneously [27]. There is also evidence that skin tests are helpful in identifying possible anaphylaxis [28]. Surprisingly, in our study, the performance of a prior skin test did not affect the incidence rate of anaphylaxis due to BVA (0.041 vs. 0.047, Table 2 and Figure 2). Due to a lack of detailed information, the current data cannot provide evidence of a causal relationship between skin testing and incidence. We argue that the predictive ability of skin tests for BVA-induced anaphylaxis may be poorer than we expected. In our previous study, we found that 80% of patients with BVA-induced anaphylaxis had undergone a skin test or showed no reaction to several BVA treatments before anaphylaxis occurred [14]. These findings might indicate that clinicians should not rely on a skin test, but instead pay attention to other risk factors.
Along with the increase in research on the effectiveness of BVA, adverse-reaction-related studies have also been increasing recently. The reported number of anaphylaxis cases due to BVA is, fortunately, not increasing. The reason for this may be improved quality control regarding BVA and the use of BVA by well-educated physicians [29,30].
Our systematic review has some limitations. First, we must note the heterogeneity of the studies included. The studies’ participants, the control groups in RCTs, and the studies’ designs are diverse. Second, most of the studies were conducted in Korea, and so it is difficult to represent the global incidence rate. Moreover, the sample sizes used in the studies included in the analysis are too small. Lastly, there is no information on each individual patient who suffered anaphylaxis and was treated with BVA; thus, it is difficult to analyze the incidence rate according to the characteristics of each group. No details were provided regarding the dose of BVA, points, or depth of injection. Nevertheless, the major strength of this study is that it is an updated review involving the largest number of patients, to the best of our knowledge.
In conclusion, we provided physicians with comprehensive information about the rate of BVA-related anaphylaxis and its risk factors, especially its female predominance, but found no predictive ability of the skin test. However, because most of the studies included in this review were conducted in Korea, it is difficult to generalize to a global scale. Despite this limitation, our present results provide valuable reference data for clinicians and researchers looking into BVA-derived applications or developments in the future.
4. Materials and Methods
4.1. Search Strategy
A systemic literature survey was conducted using eight electronic databases: MEDLINE (Pubmed), EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL), KISS, KMBASE, Koreamed, OASIS, and NDSL. Both controlled terminology (MeSH and Emtree) and free text word searching were applied. A combination of search terms and keywords included bee venom (bee venom or bee or sweet bee or honey bee or wasp bee or self-administered bee or live bee or apitoxin or bong-chim) and acupuncture (acupuncture or needle or microneedle or pharmacopuncture or inject*) and their combination. Studies published up to December 2021 were searched, with no restrictions on the publication starting point.
4.2. Inclusion and Exclusion Criteria
4.2.1. Types of Study Design
We included observational studies and randomized controlled trials (RCTs) reporting the number of participants treated by BVA, regardless of language, and excluded those studies without full text. Types of studies such as case reports, case series, and experimental research (non-human subjects) were excluded because the incidence of BVA-related adverse events could not be estimated.
4.2.2. Types of Participants
Regarding the participants, only humans who received BVA were included. There were no restrictions on age, sex, or race.
4.2.3. Types of Interventions and Comparisons
Studies using bee venom through injection or acupuncture (i.e., bee venom acupuncture and bee venom injection) for the treatment of disease were included in this review. However, bee venom immunotherapy used to desensitize allergic reactions to bee venom was excluded. Live bee stings, propolis, and bee venom creams were excluded. Studies with other interventions (e.g., acupuncture, herbal medicine, massage, exercise, etc.) combined with BVA were included to confirm and estimate the incidence of BVA-related anaphylaxis. There were no restrictions on the comparison.
4.2.4. Type of Outcome Measures
Studies that did not report the presence or absence of BVA-related side effects were excluded, regardless of other outcome measures.
4.3. Data Extraction and Review Process
We extracted data on the number of participants, mean age, country, publication year, number of anaphylaxes, main complaints according to the international classification of diseases (ICD-10) [31], and whether a skin test was performed. The overall incidence and the incidence by subgroup were investigated. For each case of anaphylaxis, we specified the concentration and volume at the time of anaphylaxis, the cumulative number of treatments until anaphylaxis, administration method, anaphylaxis grade according to Mueller’s classification method [32], and treatment results.
Two reviewers (SHK and HMO) independently searched the titles and abstracts of the search results. Discrepancies were discussed by the two researchers, and when necessary, with a third reviewer (EJL). Potentially relevant articles not meeting all of the necessary inclusion criteria were excluded from this study. The incidence rate was determined by calculating the number of anaphylaxis occurrences among the total number of subjects.
4.4. Statistical Analysis
The pooled incidence rate is given as a percentage within the 95% confidence interval. Categorical variables (number of participants and studies) were analyzed as frequency and percentages by frequency analysis, and averages for the ages of total and subgroup participants are expressed as the means ± standard deviation (SD). All statistical analyses were performed with the SPSS statistical software package version 28.0 (SPSS Inc., Chicago, IL, USA).
Author Contributions
Conceptualization: E.-J.L. (Eun-Jung Lee); data curation: S.-H.K. and H.-M.O.; formal analysis: S.-H.K., H.-M.O., and E.-J.L. (Eun-Jin Lim); funding acquisition: E.-J.L. (Eun-Jung Lee); investigation: E.-J.L. (Eun-Jung Lee) and C.-G.S.; methodology: S.-H.K., H.-M.O., and E.-J.L. (Eun-Jin Lim); resources: J.-E.Y., D.-Y.K., B.-J.K., H.-J.H., and M.-S.O.; software: E.-J.L. (Eun-Jin Lim); supervision: E.-J.L. (Eun-Jung Lee) and C.-G.S.; validation: B.-J.K. and H.-J.H.; visualization: S.-H.K. and H.-M.O.; writing—original draft: S.-H.K. and H.-M.O.; writing—review and editing: E.-J.L. (Eun-Jung Lee) and C.-G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Daejeon University Research Grants (2018) and by the National Research Foundation of Korea grant funded by the Ministry of Science, ICT & Future Planning (NRF—2021R1A2C2013483).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used for this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Key Contribution
We estimated overall incidence rate of anaphylaxis in response to BVA, which was 0.045% (95% CI 0.028–0.062) by systematic review of the articles.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Castro H.J., Mendez-Inocencio J.I., Omidvar B., Omidvar J., Santilli J., Nielsen H.S., Jr., Pavot A.P., Richert J.R., Bellanti J.A. A phase I study of the safety of honeybee venom extract as a possible treatment for patients with progressive forms of multiple sclerosis. Allergy Asthma Proc. 2005;26:470–476. [PubMed] [Google Scholar]
- 2.Ali M.A.A.S.M. Studies on bee venom and its medical uses. Int. J. Adv. Res. Technol. 2012;1:69–83. [Google Scholar]
- 3.Rady I., Siddiqui I.A., Rady M., Mukhtar H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017;402:16–31. doi: 10.1016/j.canlet.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H.J. Principle of Herbal Acupuncture and Clinical Application. Iljoongsa; Seoul, Korea: 1999. [Google Scholar]
- 5.Park J.S., Lee M.J., Chung K.H., Ko D.K., Chung H. Live Bee Acupuncture (Bong-Chim) Dermatitis: Dermatitis due to Live Bee Acupuncture Therapy in Korea. Int. J. Dermatol. 2013;52:1519–1524. doi: 10.1111/ijd.12161. [DOI] [PubMed] [Google Scholar]
- 6.Abdela N., Jilo K. Bee Venom and its Therapeutic Values: A Review. Adv. Life Sci. Technol. 2016;44:18–22. [Google Scholar]
- 7.Komi D.E.A., Shafaghat F., Zwiener R.D. Immunology of bee venom. Clin. Rev. Allergy Immunol. 2018;54:386–396. doi: 10.1007/s12016-017-8597-4. [DOI] [PubMed] [Google Scholar]
- 8.Pucca M.B., Cerni F.A., Oliveira I.S., Jenkins T.P., Argemí L., Sørensen C.V., Ahmadi S., Barbosa J.E., Laustsen A.H. Bee updated: Current knowledge on bee venom and bee envenoming therapy. Front. Immunol. 2019;10:2090. doi: 10.3389/fimmu.2019.02090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magrioti V., Kokotos G. Phospholipase A2 inhibitors as potential therapeutic agents for the treatment of inflammatory diseases. Expert Opin. Ther. Pat. 2010;20:1–18. doi: 10.1517/13543770903463905. [DOI] [PubMed] [Google Scholar]
- 10.Cherniack E.P., Govorushko S. To bee or not to bee: The potential efficacy and safety of bee venom acupuncture in humans. Toxicon. 2018;154:74–78. doi: 10.1016/j.toxicon.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Choi Y.C., Kwon K.R., Choi S.H. Purification of Peptide Components including Melittin from Bee Venom using gel filtration chromatography and propionic acid urea polyacrylamide gel electrophoresis. J. Korean Pharmacopunct. Inst. 2006;9:105–111. [Google Scholar]
- 12.Yi W.S., Kim S.S. Reporting of adverse events in randomized controlled trials of bee venom acupuncture: A systematic review. J. Korean Med. Rehabil. 2014;24:97–109. [Google Scholar]
- 13.Jang S.B., Kim K.H. Clinical effectiveness and adverse events of bee venom therapy: A systematic review of randomized controlled trials. Toxins. 2020;12:558. doi: 10.3390/toxins12090558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee E.J., Ahn Y.C., Kim Y.I., Oh M.S., Park Y.C., Son C.G. Incidence Rate of Hypersensitivity Reactions to Bee-Venom Acupuncture. Front. Pharmacol. 2020;11:1–7. doi: 10.3389/fphar.2020.545555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weis W.A., Ripari N., Conte F.L., da Silva Honorio M., Sartori A.A., Matucci R.H., Sforcin J.M. An overview about apitherapy and its clinical applications. Phytomedicine. 2022;2:100239. doi: 10.1016/j.phyplu.2022.100239. [DOI] [Google Scholar]
- 16.Park J.H., Yim B.K., Lee J.-H., Lee S., Kim T.-H. Risk associated with bee venom therapy: A systematic review and meta-analysis. PLoS ONE. 2015;10:e0126971. doi: 10.1371/journal.pone.0126971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo N., Roh J. Systemic immediate hypersensitive reactions after treatment with sweet bee venom: A case report. J. Pharmacopunct. 2015;18:59. doi: 10.3831/KPI.2015.18.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K., Jeong H., Lee G., Jang S., Yook T. Characteristics of Adverse Events in Bee Venom Therapy Reported in South Korea: A Survey Study. Toxins. 2022;14:18. doi: 10.3390/toxins14010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang Y.J., Lee B.C. Clinical study of anaphylaxis on bee-venom acupuncture. J. Acupunct. Res. 2000;17:149–159. [Google Scholar]
- 20.Kim M.R., Shin J.S., Lee J., Lee Y.J., Ahn Y.J., Park K.B., Lee H.D., Lee Y., Kim S.G., Ha I.H. Safety of Acupuncture and Pharmacopuncture in 80,523 Musculoskeletal Disorder Patients: A Retrospective Review of Internal Safety Inspection and Electronic Medical Records. Medicine. 2016;95:e3635. doi: 10.1097/MD.0000000000003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tupper J., Visser S. Anaphylaxis: A review and update. Can. Fam. Physician. 2010;56:1009–1011. [PMC free article] [PubMed] [Google Scholar]
- 22.Dhopeshwarkar N., Sheikh A., Doan R., Topaz M., Bates D.W., Blumenthal K.G., Zhou L. Drug-induced anaphylaxis documented in electronic health records. J. Allergy Clin. Immunol. Pract. 2019;7:103–111. doi: 10.1016/j.jaip.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barton S. Which clinical studies provide the best evidence?: The best RCT still trumps the best observational study. BMJ. 2000;321:255–256. doi: 10.1136/bmj.321.7256.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudders S.A., Banerji A., Clark S., Camargo C.A., Jr. Age-related differences in the clinical presentation of food-induced anaphylaxis. J. Pediatr. 2011;158:326–328. doi: 10.1016/j.jpeds.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richter A.G., Nightingale P., Huissoon A.P., Krishna M.T. Risk factors for systemic reactions to bee venom in British beekeepers. Ann. Allergy Asthma Immunol. 2011;106:159–163. doi: 10.1016/j.anai.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Kim H.J., Kim S.H., Park H.D., Kim W.Y., Hong E.S. Predictors of Anaphylactic Shock in Patients with Anaphylaxis after Exposure to Bee Venom. J. Korean Soc. Clin. Toxicol. 2010;8:30–36. [Google Scholar]
- 27.Salvati L., Vitiello G., Parronchi P. Gender differences in anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2019;19:417–424. doi: 10.1097/ACI.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 28.Baker A., Empson M., The R., Fitzharris P. Skin testing for immediate hypersensitivity to corticosteroids: A case series and literature review. Clin. Exp. Allergy. 2015;45:669–676. doi: 10.1111/cea.12441. [DOI] [PubMed] [Google Scholar]
- 29.Chu C.S., Park H.S., Kim M.K., Cha B.C., Lee E., Kwon K.R. Expermental Studies of quantitative evaluation using HPLC and safety of Sweet Bee Venom. J. Pharmacopunct. 2007;10:81–86. doi: 10.3831/KPI.2007.10.2.081. [DOI] [Google Scholar]
- 30.Kang K.S., Kwon K.R. Experimental studies of validation and stability of sweet bee venom using HPLC. J. Pharmacopunct. 2009;12:33–50. doi: 10.3831/KPI.2009.12.4.033. [DOI] [Google Scholar]
- 31.The ICD-10 Version. [(accessed on 23 February 2022)]. Available online: https://icd.who.int/browse10/2019/en#/
- 32.Mueller H.L. Diagnosis and treatment of insect sensitivity. J. Asthma Res. 1966;3:331–333. doi: 10.3109/02770906609106941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study are available from the corresponding author upon request.