Abstract
Patients with chronic kidney disease (CKD) have an elevated prevalence of atheromatous (ATH) and/or non-atheromatous (non-ATH) cardiovascular disease (CVD) due to an array of CKD-related risk factors, such as uremic toxins (UTs). Indeed, UTs have a major role in the emergence of a spectrum of CVDs, which constitute the leading cause of death in patients with end-stage renal disease. The European Uremic Toxin Work Group has identified over 100 UTs, more than 25 of which are dietary or gut-derived. Even though relationships between UTs and CVDs have been described in the literature, there are few reviews on the involvement of the most toxic compounds and the corresponding physiopathologic mechanisms. Here, we review the scientific literature on the dietary and gut-derived UTs with the greatest toxicity in vitro and in vivo. A better understanding of these toxins’ roles in the elevated prevalence of CVDs among CKD patients might facilitate the development of targeted treatments. Hence, we review (i) ATH and non-ATH CVDs and the respective levels of risk in patients with CKD and (ii) the mechanisms that underlie the influence of dietary and gut-derived UTs on CVDs.
Keywords: uremic toxins, atheromatous cardiovascular diseases, non-atheromatous cardiovascular diseases, chronic kidney disease
1. Introduction
With an estimated prevalence between 8% and 16%, chronic kidney disease (CKD) is a growing worldwide public health problem [1]. The Kidney Disease: Improving Global Outcomes (KDIGO) initiative has defined CKD as a decrease in kidney function for at least 3 months, referring a glomerular filtration rate (GFR) below 60 mL/min or an albumin-to-creatinine ratio above 30 mg/g (3.4 mg/mmol) [2]. Renal impairment has been directly linked to high rates of morbidity and mortality in general [3] and cardiovascular morbidity and mortality in particular [4,5]. In fact, CKD increases the risk of cardiovascular disease (CVD) by a factor of two to four [6], making them the leading cause of death in long-standing CKD patients [4]. In adults, CKD is associated with both atheromatous (ATH) CVDs (such as myocardial infarction (MI) and stroke) and non-atheromatous (non-ATH) CVDs (such as heart failure and atrial fibrillation) [7].
This increased risk of CVD is multifactorial given the combination of both, traditional cardiovascular risk factors that are often found as comorbidities in patients with CKD (e.g., hypertension, diabetes mellitus (DM), dyslipidemia, advanced age, male sex, smoking, high body mass index) and non-traditional cardiovascular risk factors, known as CKD-related risk factors (such as high albuminuria and uremic toxins’ (UTs) accumulation) [8,9].
UTs are defined by harmful solutes accumulated in the body when the kidneys’ filtration capabilities are gradually lost, whereas they normally should be excreted by healthy kidneys [4,10,11]. They originate from endogenous metabolism, microbial metabolism, or exogenous intake. In 2021, the European Uremic Toxin Work Group (EUTox) identified over 100 UTs, including more than 25 dietary and gut-derived compounds [12,13]. Based on their molecular weight and protein-binding ability, UTs are commonly classified into three categories: free water-soluble, low-molecular-weight solutes (<500 Da), water-soluble middle molecules (>500 Da), and protein-bound solutes [12,14]. In December 2021, Rosner et al. suggested a novel six-category classification of UTs [11]: small protein-bound molecules (<500 Da), small water-soluble molecules (<500 Da), small-middle molecules (500–15,000 Da), medium-middle molecules (>15,000–25,000 Da), large-middle molecules (>25,000–58,000 Da), and large molecules (>58,000 Da). This more holistic classification included the UTs’ physicochemical characteristics and their correlations with clinical symptoms and outcomes; however, Rosner et al.‘s classification has some limitations and has not yet been validated [11].
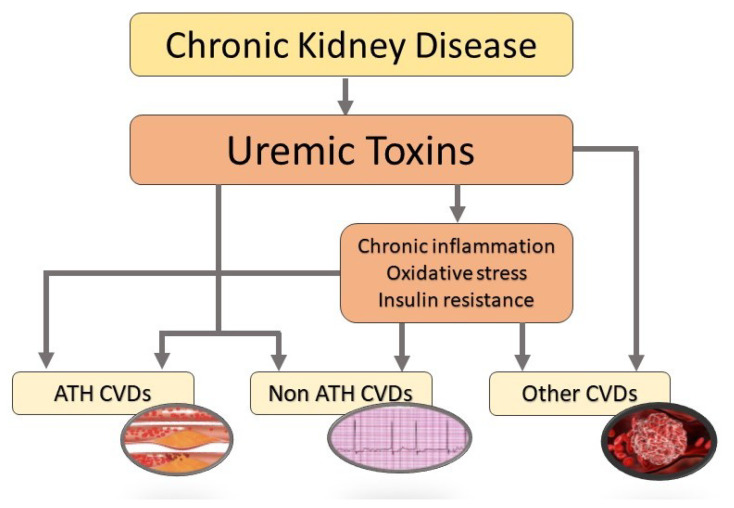
Dietary metabolites (such as 3-carboxy-4-ethyl-5-propyl-2-furanpropanoic acid (CMPF), phosphate, and urea) are known to be strongly associated with cardiovascular events [15,16]. Phosphate’s impact on CVDs does not occur independently: Phosphate has a direct effect on vascular smooth muscle cells (VSMCs) through the complex calcium/phosphate and an indirect effect via the fibroblast growth factor 23 (FGF23)/Klotho and PTH axes [17]. The gut microbiota metabolizes many dietary compounds, although most of the resulting metabolites are excreted by the kidneys in healthy individuals [18]. Subsequently, any disruption of the intestinal microbial composition, as seen in patients with CKD, will generate harmful metabolites [13]. Here, we only review data on the UTs with the greatest toxicity in vitro and in vivo [16]: indoles (indoxyl sulfate (IS), indole-3-acetic acid (IAA), kynurenine, and kynurenic acid (KA)), phenols (p-cresyl sulfate (PCS), p-cresyl glucuronide (PCG), and phenylacetylglutamine (PAG)), hippurates (hippuric acid (HA)), and others (CMPF, phosphate, urea, trimethylamine N-oxide (TMAO)). As ilustrated in Figure 1, the accumulation of these UTs has been implicated directly or indirectly in a spectrum of ATH and non-ATH CVDs [19].
Figure 1.
Schematic description of the effects of uremic toxins on cardiovascular diseases in a CKD setting. ATH, atheromatous; CVDs, cardiovascular diseases.
Previous reviews described the endogenously generated UTs (such as -2 microglobulin, interleukins, and other inflammatory markers), we aimed in the present narrative review to focus on the most toxic dietary and gut-derived compounds. Here, we review research published between 2002 and 2022 by (i) defining the various ATH and non-ATH CVDs and the associated risks in patients with CKD (ii) and listing the various mechanisms that underlie the influence of dietary and gut-derived UTs on the CVD risk.
2. Cardiovascular Diseases
The elevated incidence and prevalence of CVD is due to many conventional and non-conventional risk factors, of which CKD is the major non-modifiable one [20]. The prevalence of CVD is higher in patients with CKD than in healthy individuals, and the cardiovascular mortality rate is 10 to 30 times higher in dialysis patients than in the general population [21]. This elevated risk can be ascribed to the combination of conventional cardiovascular risk factors with those related directly to CKD such as oxidative stress, chronic inflammation, vascular calcification, and UTs [8,20,22]. According to Jankowski et al. [22], “CKD mimics accelerated aging of the cardiovascular system”.
In physiopathologic terms, CVDs can be classified as ATH or non-ATH [6] that are both highly prevalent in patients with CKD [8].
2.1. Atheromatous Cardiovascular Diseases
ATH CVD is characterized by the presence of occlusive lesions and plaques (called atheromas) inside the arterial wall; the aorta and the coronary arteries are primarily affected [23]. Atheromas in patients with CKD are mainly characterized by important thickening of the intima media [20,24]. According to the KDIGO [25] and the Cardiovascular Stroke Endpoint Definitions for Clinical Trials [26], ATH CVDs include stroke or MI (whether fatal or not) and hospitalization for silent ischemia, unstable angina, transient ischemic attacks, intrastent thrombosis, peripheral artery disease (PAD), percutaneous coronary interventions or coronary artery bypass grafts, vascular surgery, amputation, and revascularization for coronary artery disease (CAD) or PAD. Furthermore, PAD can be defined as a history of amputation, angioplasty, or lower limb bypass prompted by ATH distal ischemic lesions [8,27].
The prevalence of atherosclerotic CVD is higher in patients with moderate CKD than in healthy individuals, and atherosclerosis progression is strongly linked to the worsening of CKD [28]. The incidence of fatal MI was higher in dialysis patients than in the general population [20]. The results of observational studies have shown that the stroke risk increases with declining GFR [28,29], and that CKD is associated with a higher risk of PAD [30,31].
2.2. Non-Atheromatous Cardiovascular Diseases
Non-ATH CVD encompasses other types of CVD, including sudden cardiac death or death from heart failure (without a history of CAD), hospitalization for heart failure (again with no history of CAD), cardiac fibrosis, atrial fibrillation or other arrhythmia disorders, diastolic dysfunction, arterial stiffness, cardiomyocytes hypertrophy, vascular calcification, or valvular heart disease [8,27,32]. Prior cohorts concluded that the prevalence of heart failure increased markedly with CKD progression and affected 65 to 70% of patients with end-stage renal disease (ESRD) [33]. Patients with CKD had a significantly higher aortic pulse wave velocity [34]. The higher the CKD stage, the greater the risk of arterial stiffness [35]—the most prevalent arterial modification being in patients with CKD [36]. Arterial stiffness reflects vascular calcification [37], and both variables are independent predictors of CVD mortality [38]. Vascular calcification is mainly associated with low vessel elasticity [39], and medial artery calcification is the most common vascular calcification in patients with CKD [38]. Various studies have reported that CKD is a risk factor for cardiac arrhythmia [40,41]. Although atrial fibrillation represents the most prevalent type of arrhythmia, ventricular disorders are the most lethal [40,42]. For example, ventricular tachyarrhythmia accounted for 79% of cardiac arrests recorded in a study of hemodialysis (HD) patients [42].
In summary, CKD contributes significantly to severe non-ATH CVDs [33].
2.3. Other than Atheromatous and Non Atheromatous Cardiovascular Diseases
We also included cardiovascular complications that are neither ATH CVDs nor non-ATH CVDs (referred to hereafter as “other than ATH and non-ATH CVDs”). This group includes platelet aggregation, thrombus formation, and endothelial cell (EC) dysfunction. Many studies have characterized the vascular damage caused by CKD. It is well known that CKD progression is closely associated with levels of oxidative stress and inflammation, which enhance cardiovascular damage and mortality [43]. Numerous studies have also demonstrated that CKD is associated with significantly increased platelet activation [44,45], and with the risk of both venous and arterial thrombosis [46,47]. Patients with CKD frequently undergo endovascular procedures; the risk of post-angioplasty thrombosis or vascular access thrombosis is elevated in this high-risk population [48], and makes CKD the second-ranked risk factor for post-procedural complications [48,49].
3. Uremic Toxins and Risk for Cardiovascular Diseases
Some UTs markedly increase the relative risk of CVD in the CKD population. Many in vitro, in vivo, and observational studies have concluded for the impact of dietary and gut-derived UTs on adverse cardiac risk. We systematically searched the literature (the PubMed database, up until 28 February 2022) for publications on the relationship between UTs and CVDs in patients with CKD, by combining the following keywords: (“Chronic kidney disease” OR “renal impairment” OR “kidney disease” OR “chronic kidney failure” OR “chronic renal failure”) AND (“Cardiovascular disease” OR “vascular calcification” OR “oxidative stress” OR “coronary artery disease” OR “atherosclerosis” OR “peripheral artery disease” OR “cardiovascular damage” OR “Cardiovascular Diseases” [Mesh] OR “arrythmia” OR “stroke” OR “cardiovascular death” OR “myocardial infarction”) AND (“uremic toxin*” OR “uremic retention solute*” OR “cardiovascular toxin*” OR “ indoxyl sulfate” OR “p-Cresyl sulfate” OR “p-cresyl glucuronide ” OR “indole-3-acetic acid “OR” hippuric acid” OR “kynurenic acid” OR “kynurenine” OR “3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid” OR “Phenylacetylglutamine” OR “trimethylamine N-oxide”) AND (“in vivo” OR “in vitro” OR “animal*”) NOT “hemodialysis” NOT “peritoneal dialysis” NOT “dialysis”). A total of 262 articles were analyzed. We excluded 9 studies on pediatric patients, 25 reviews, and 15 studies investigating other UTs than the dietary and gut-derived ones included in the present review. A total of 213 original articles were selected.
3.1. Experimental Data: In Vitro Studies
Table 1 summarizes the in vitro studies of the UTs’ impact on CVDs in a CKD setting.
Table 1.
In vitro studies of the effects of UTs on cardiovascular complications.
| First Author, Year | Models | UT(s) Studied | Main Findings |
|---|---|---|---|
| Arinze [50], 2022 | Primary human dermal | IS | IS, kynurenine, and KA decreased Wnt/-catenin |
| microvascular ECs | Kynurenine | activity, which causes EC dysfunction and impairs | |
| KA | angiogenesis. | ||
| Lano [93], 2020 | HUVECs | IS | IS had a prothrombotic effect by increasing TF expression in ECs and peripheral blood mononuclear cells via AHR activation. |
| He [80], 2019 | HASMCs | IS | IS induced calcification of HASMCs via the NF-B signaling pathway. |
| Chen [81], 2016 | HASMCs | IS | IS decreased Klotho expression, promoting aortic calcification. |
| Tang [90], 2015 | Embryonic rat heart-derived cardiac H9c2 cells | IS | IS has a role in arrhythmogenesis: IS inhibited the inward rectifier potassium ion channels function, resulting in a prolonged QT interval. |
| Chitalia [94], 2013 | HVSMCs | IS | IS increased TF expression and decreased TF ubiquitination, leading to a thrombogenic milieu. |
| Liu [92], 2012 | Neonatal cardiac myocytes and fibroblasts from Sprague–Dawley rats | IS | IS was taken up by cardiomyocytes through OAT-1 and -3, leading to activation of the NF-B and MAPK pathways that are involved in cardiac hypertrophy and fibrosis. |
| Lekawanvijit [91], 2010 | Isolated NCMs, NCFs and THP-1 | IS | IS has a role in harmful cardiac remodeling: it has pro-fibrotic, pro-hypertrophic, and pro-inflammatory effects via the activation of MAPK and NF-B pathways. |
| Tumur [62], 2010 and Ito [63], 2010 | HUVECs | IS | IS increased the expression of the adhesion molecules ICAM-1, VCAM-1, MCP-1, and e-selectin, all of which are involved in the pathophysiology of atherosclerosis. |
| Muteliefu [51], 2009 | HASMCs | IS | IS induced ROS generation and the expression of Nox4, Cbfa1, ALP, and osteopontin in VSMCs. |
| Yamamoto [64], 2006 | VSMCs were isolated from the aortas of male Sprague–Dawley rats | IS | IS caused VSMC proliferation via activation of the p42/44 MAPK pathway, a mechanism involved in the progression of atherosclerotic lesions. |
| Dou [52], 2015 | Cultured human endothelial cells | IAA | IAA activated the inflammatory AHR/p38MAPK/NF-B pathway and increased the production of endothelial ROS. |
| Gao [96], 2015 | RBC from peripheral vein | IAA | IS and IAA caused RBC damage, which is involved |
| blood of eight healthy volunteers | IS | in thrombus formation. | |
| Gondouin [95], 2013 | HUVECs | IAA | IAA increased TF expression resulting in a prothrombotic effect. |
| Gross [65], 2015 | HUVECs and HVSMCs | PCS | PCS directly stimulated the Rho-associated protein kinase, which is involved in vascular dysfunction and vascular remodeling. |
| Watanabe [53], 2015 | HUVECs | PCS | PCS enhanced ROS production and NADPH oxidase expression. |
| Meijers [66], 2009 | HUVECs | PCS | PCS induced shedding of endothelial microparticles, causing endothelial dysfunction. |
| Schepers [58], 2007 | Blood from healthy donors incubated in the presence of PCS | PCS | The presence of PCS activated pro-inflammatory leukocyte free radical production. |
| Dou [67], 2004 | HUVECs | PCS | Both PCS and IS inhibited endothelial proliferation |
| IS | and wound repair. | ||
| Huang [60], 2018 | Human aortic endothelial cells | HA | HA contributed to mitochondrial fission by activating mitochondrial ROS production and Drp1 protein expression. |
| Shang [61], 2017 | HUVECs | HA | HA, IS, and IAA increased miR-92a levels, which im- |
| IS | pairs EC function. | ||
| IAA | |||
| Nagy [97], 2017 | Human islets of Langerhans from healthy donors | CMPF | CMPF inhibited insulin secretion. |
| Itoh [59], 2012 | HUVECs | CMPF | IS induced ROS production more intensely than |
| IS | CMPF did. | ||
| Bouabdallah [82], 2019 | HUVECs and HASMCs | Phosphate | Phosphate and IS induced the secretion of interleuk- |
| IS | in-8 from ECs, which is involved in VSMC calcification. | ||
| Jover [83], 2018 | VSMCs | Phosphate | High phosphate promoted extracellular matrix calcification and upregulated osteoblast markers. |
| Zhang [84], 2017 | HASMCs | Phosphate | High phosphate induced vascular calcification via the activation of TLR4/NF-B signaling. |
| Alesutan [85], 2017 | HASMCs | Phosphate | Hyperphosphatemia upregulated aldosterone synthase expression, inducing VSMCs osteogenic transdifferentiation and calcification. |
| Rahabi-Layachi [68], 2015 | HASMCs | Phosphate | Phosphate induced apoptosis and cell cycle arrest by blocking G1/S progression, thus reducing HASMCs proliferation. |
| M’Baya-Moutoula [86], 2015 | Peripheral blood mononuclear cells | Phosphate | Phosphate caused vascular calcification by modulating miR-223 and decreasing osteoclastogenesis. |
| Ciceri [87], 2015 | VSMCs | Phosphate | Phosphate caused VSMC osteoblastic differentiation and led to cell calcification. |
| Di Marco [69], 2013 | Human coronary artery ECs | Phosphate | Hyperphosphatemia decreased annexin II expression and stiffened ECs. |
| Six [70], 2012 | HUVECs | Phosphate | Phosphate exhibited a direct vasoconstrictor effect on aortic rings, increased phenylephrine-induced contraction, and lowered acetylcholine-induced relaxation—leading to endothelial dysfunction. |
| Guerrero [88], 2012 | Rat aortic rings and HVSMCs | Phosphate | Phosphate reduced expression of perlecan and induced BMP-2, which is involved in the osteogenic transdifferentiation pathways and would promote cells calcification. |
| Shroff [89], 2010 | VSMCs | Phosphate | Phosphate increased alkaline phosphatase activity and mediated calcification. |
| Di Marco [54], 2008 | HUVECs | Phosphate | Hyperphosphatemia caused EC apoptosis by increasing ROS generation and disrupting the mitochondrial membrane potential. |
| Shigematsu [71], 2003 | HVSMCs | Phosphate | Phosphate overload accelerated calcium deposition on arteriole walls. Moreover, phosphate led to vasoconstriction, decreased vasorelaxation, decreased NO production, stimulated ROS production, and induced ECs apoptosis. |
| Lee [72], 2021 | HUVECs | Urea | Urea led to excessive neutrophil extracellular trap formation and thus EC dysfunction. |
| Maciel [73], 2018 | An immortalized human EC line | Urea | Urea altered cell-to-cell junctions, leading to greater endothelial damage. |
| D’Apolito [55], 2018 | Human arterial ECs | Urea | Abnormal high urea levels had long-lasting effects on arterial cells: urea increased mitochondrial ROS production in arterial ECs even after dialysis, which typically promotes endothelial dysfunction. |
| D’Apolito [56], 2017 | Human endothelial progenitor cell | Urea | Urea caused ROS production and accelerated endothelial progenitor cell senescence. |
| Sun [75], 2016 | Human arterial EC | Urea | Urea levels were positively correlated with HDL carbamylation, which inhibited endothelial repair functions. |
| D’Apolito [57], 2015 | Human aortic ECs | Urea | Urea increased mitochondrial ROS production and inhibited GAPDH, which leads to the activation of the endothelial pro-inflammatory pathway. Furthermore, urea inactivated the anti-atherosclerosis enzyme PGI2 synthase. |
| Trécherel [74], 2012 | HASMCs | Urea | Urea induced BAD protein expression, sensitizing the HASMCs to apoptosis. |
| D’Apolito [98], 2010 | 3T3-L1 adipocytes treated with urea | Urea | Urea increased ROS levels and expression of the adipokines retinol binding protein 4 and resistin. |
| Zhang [76], 2020 | Aortic VSMCs from male “Sprague Dawley” rats and human VSMCs | TMAO | TMAO promoted vascular calcification through activation of the NLRP3 inflammasome and NF-B signals. |
| Ma [77], 2017 | HUVECs | TMAO | HUVECs showed impairment in cellular proliferation, and TMAO induced NF-B signaling pathway, increasing vascular inflammatory signals and EC dysfunction. |
| Boini [78], 2017 | Mouse carotid artery ECs | TMAO | TMAO activated NLRP3 inflammasomes, causing endothelial dysfunction. |
| Sun [79], 2016 | HUVECs | TMAO | TMAO activated NLRP3 inflammasomes, causing endothelial dysfunction. |
Abbreviations: AHR: aryl hydrocarbon receptor; ALP: alkaline phosphatase; Cbfa1: core binding factor 1; CMPF: 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid; CVD: cardiovascular disease; Drp: dynamin-related protein; ECs: endothelial cells; eNOS: endothelial nitric oxide synthase; ENPP1: ectonucleotide pyrophosphate/phosphodiesterase 1; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; HA: hippuric acid; HASMC: human aortic smooth muscle cell; HDL: high-density lipoprotein; HUVECs: human umbilical vein endothelial cells; HVSMC: human vascular smooth muscle cell; IAA: indole-3-acetic acid; ICAM-1: intercellular adhesion molecule-1; IS:indoxyl sulfate; KA: kynurenic acid; MAPK: mitogen-activated protein kinase; MCP-1: monocyte chemotactic protein-1; NADPH: nicotinamide adenine dinucleotide phosphate; NCM: neonatal rat cardiac myocyte; NCF: neonatal rat cardiac fibroblast; NF-kB: nuclear factor-kappa B; NLRP3: nucleotide-binding domain, leucine-rich containing family, pyrin domain-containing-3; NO: nitric oxide; PCS: para-cresyl sulfate; RBC: red blood cell; ROS: reactive oxygen species; TF: tissue factor; THP-1: human leukemia monocytic cell line; TLR4: tolllike receptor 4; TMAO: trimethylamine-N-oxide; UT: uremic toxin; VCAM-1: vascular cell adhesion molecule-1; VSMC: vascular smooth muscle cells.
Recent results have demonstrated that UTs contribute to CVDs in CKD patients through a variety of mechanisms, the most significant of which are endothelial dysfunction and vascular calcification caused mostly by oxidative stress and inflammation. For example, a recent study of primary human dermal microvascular ECs by Arinze et al. [50] showed that tryptophan-derived indoles (IS, kynurenine, and KA) were associated with the worsening of PAD. The UTs decreased Wnt/-catenin activity, causing EC dysfunction and impaired angiogenesis.
Studies in different in vitro models found that IS, IAA, PCS, phosphate, and urea induced oxidative stress by increasing reactive oxygen species (ROS) production [51,52,53,54,55,56,57] or by activating leukocyte free radical production [58], thus contributing to endothelial dysfunction and apoptosis. Itoh et al. [59] reported that IS induced ROS production in human umbilical vein endothelial cells (HUVECs) more intensely than CMPF did. HA and phosphate caused EC apoptosis by disrupting the mitochondrial membrane through increased production of ROS and Drp1 protein [54,60].
Shang et al. [61] concluded that IS, IAA, and HA increased levels of miR-92a, a microRNA induced by oxidative stress in ECs and which is involved in atherosclerosis. Furthermore, IS was involved in the pathophysiology of atherosclerosis by increasing the expression of the adhesion molecules intercellular adhesion molecule-1, vascular cell adhesion molecule-1, monocyte chemotactic protein-1, and e-selectin [62,63], and activating the p42/44 mitogen-activated protein kinase (MAPK) pathway and thus vascular smooth muscle cell (VSMC) proliferation [64].
Experiments on HUVECs demonstrated that PCS had a damaging effect on ECs by (i) directly stimulating the Rho-associated protein kinase [65], (ii) enhancing NADPH oxidase expression and ROS production [53], (iii) inducing the shedding of endothelial microparticles [66], and (iv) inhibiting (along with IS) endothelial proliferation and wound repair [67]. Phosphate (the UT most intensively studied in vitro) also damages ECs. Phosphate overloads blocked G1/S progression, reducing EC proliferation [68]. Moreover, phosphate overload decreased annexin II expression and stiffened ECs [69]. In experiments on aortic rings, excess of phosphate caused vasoconstriction, increased phenylephrine-induced contraction, and lowered acetylcholine-induced relaxation [70]. Furthermore, high phosphate levels accelerated calcium deposition on arteriole walls and decreased vasorelaxation and nitric oxide (NO) production in human vascular smooth muscle cells (HVSMCs), leading to vasoconstriction [71]. Similarly, urea disrupted ECs either via a direct effect on the cells or indirectly via protein carbamylation. Urea prompted the formation of excessive neutrophil extracellular trap in HUVECs [72], altered cell-to-cell junctions in an immortalized human EC line [73], induced Associated Agonist Of Cell Death (BAD) protein expression [74], and inhibited glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and Prostacyclin (PGI2) synthase (thus facilitating the activation of pro-atherosclerotic pathways) [57]. It is also noteworthy that high urea levels were associated with elevated mitochondrial ROS production in arterial ECs even after dialysis—suggesting that there is a “cellular memory” for urea-induced oxidative stress [55]. In terms of an indirect effect, urea levels were positively correlated with high-density lipoprotein (HDL) carbamylation, which then inhibited endothelial repair functions [75]. TMAO had the same endothelial effect in various models. It activated the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome and nuclear factor-kappa B (NF-B) signals, promoted leukocyte-EC adhesion, EC dysfunction, and vascular calcification and thus helped to enhance atherosclerosis processes [76,77,78,79].
Vascular calcification happens in various ways. Studies of human aortic smooth muscle cells (HASMCs) showed that IS induced aortic calcification by activating the NF-B signaling pathway [80], decreasing Klotho expression [81], and inducing ROS generation and the expression of NADPH oxidases (Nox1, Nox2, and Nox4), core binding factor 1, alkaline phosphatase, and osteopontin [51]. In Bouabdallah et al.’s study [82] of HUVECs and HASMCs, IS and phosphate induced the secretion of interleukin-8 from ECs and thus the promotion of vascular calcification. In addition, phosphate promoted extracellular matrix calcification and upregulated osteoblast marker expression by VSMCs [83]. High phosphate levels activated toll-like receptor 4 (TLR4)/NF-B signaling [84] and upregulated aldosterone synthase expression, which induced VSMCs osteogenic transdifferentiation and calcification [85]. In an in vitro study of peripheral blood mononuclear cells, phosphate modulated miR-223 expression and decreased osteoclastogenesis [86]. Similarly, phosphate caused osteoblastic differentiation in VSMCs [87], reduced perlecan expression in rat aortic rings (ex vivo) and HVSMCs, and induced BMP-2 (involved in osteogenic transdifferentiation pathways) [88]. Lastly, phosphate mediated vascular calcification by increasing alkaline phosphatase activity in VSMCs [89].
It has been reported that IS contributes to CVD through associations with arrhythmia, cardiac hypertrophy, and fibrosis. The toxin’s arrhythmogenic effect was evaluated in embryonic rat cardiomyocytes: IS inhibited the inward rectifier potassium ion channel—prolonging the action potential and the QT interval and inducing early after depolarization [90]. Lekawanvijit et al. [91] demonstrated the fibrotic effect of IS for the first time; this was later confirmed by Liu et al. [92]. IS exerted pro-fibrotic, pro-hypertrophic, and pro-inflammatory effects by activating MAPK and NF-B pathways [91]. Furthermore, IS increased collagen synthesis in neonatal rat cardiac fibroblasts, promoted myocyte hypertrophy, and stimulated tumor necrosis factor-alpha and interleukin-6 expression [91]. In rats, cardiomyocytes took up IS the organic anion transporters (OATs) 1 and 3, which led to the activation of the NF-B and MAPK pathways and favored cardiac hypertrophy and fibrosis [92].
Indoles are also known to have a prothrombotic effect. IS and IAA activated the aryl hydrocarbon receptor (AHR), which led to an increase in tissue factor (TF) expression [93,94,95]. Gao et al. [96] demonstrated that IS and IAA caused red blood cell damage, which might lead to thrombus formation.
UTs also have indirect effects on CVD. In particular, CMPF inhibited insulin secretion [97], and urea increased the expression of the adipokines retinol binding protein 4 and resistin [98].
Most of the following in vitro studies added human serum albumin (HSA) to the protein-bound UT to reflect their natural protein-bound state in uremia. This addition should be considered in such a context and, most importantly, while interpreting the results in order to reflect their actual clinical effects [99].
3.2. Experimental Data: Animal Studies
Animal studies investigating the UTs’ impact on ATH or non-ATH CVDs are summarized in Table 2.
Table 2.
Animal studies of the effects of UTs on cardiovascular complications.
| First Author, Year | Models | UT(s) Studied | Main Findings |
|---|---|---|---|
| Atheromatous CVDs | |||
| Arinze [50], 2022 | Adenine-induced | IS | IS, kynurenine, and KA suppressed Wnt/- |
| CKD mice and IS so- | Kynurenine | catenin signaling through increased AHR activity, | |
| lute-specific C57BL/6 | KA | leading to impaired angiogenesis and hindlimb | |
| mice | ischemia. | ||
| Hung [101], 2016 | Mice with subtotal nephrectomy | IS | IS decreased endothelial progenitor cells mobilization and impaired neovascularization, leading to PAD. |
| Han [103], 2016 | 5/6 nephrectomized ApoE –/– mice | PCS | PCS promoted the formation of atherosclerotic lesions, induced plaque instability and the migration and proliferation of VSMCs, and disturbed the balance between matrix metalloproteinases and tissue inhibitor of metalloproteinases within the plaques. |
| Huang [60], 2018 | 5/6 nephrectomized rat model | HA | HA caused pro-atherogenic effects by contributing to endothelial dysfunction via greater oxidative stress and impaired endothelium-dependent vasodilation. |
| Shang [61], 2017 | Male Wistar rats | HA | HA induced miR-92a, which is involved in angiogenic and atherosclerotic processes. |
| Massy [107], 2005 | ApoE −/− mice with partial kidney ablation | Urea | Urea contributed to arterial calcification and aggravated atherosclerosis. |
| Matsumoto [102], 2020 | Superior mesenteric arteries and femoral arteries of rat | TMAO | TMAO impaired endothelium-derived hyperpolarizing factor-type relaxation, which led to PAD. |
| Geng [104], 2018 | Apoe −/− mice fed a high-fat diet with or without TMAO | TMAO | TMAO enhanced the expression of CD36/MAPK/JNK pathway, promoting foam cells formation and, ultimately, atherosclerosis. |
| Seldin [105], 2016 | Female low-density lipoprotein receptor knockout mice injected with vehicle or TMAO | TMAO | TMAO induced vascular inflammation by activating MAPK and NF-B signaling, thus enhancing atherosclerosis. |
| Koeth [106], 2013 | Mice supplemented with dietary TMAO, carnitine, or choline | TMAO | TMAO accelerated atherosclerosis and was linked to major cardiac events. |
| Non-atheromatous CVDs | |||
| Kuo [108], 2020 | Nephrectomized male C57BL/6 mice | IS | IS promoted calcification in the aorta and peripheral arteries, with low NO production and high eNOS phosphorylation. |
| Opdebeeck [109], 2019 | 42 male Wistar rats ex- | IS | Both IS and PCS directly promoted severe calcifica- |
| posed to adenine sulfate for 10 days and then fed a phosphate-enriched diet | PCS | tion in the aorta and peripheral vessels via activation of inflammation and coagulation pathways. These changes were strongly associated with impaired glucose homeostasis. | |
| Chen [81], 2016 | 5/6 nephrectomized Sprague Dawley rats treated with IS | IS | IS decreased Klotho expression and promoted aortic calcification. |
| Chen [121], 2015 | Isolated rabbit left atrium, right atrium, pulmonary vein, and sinoatrial nodes before and after treatment with IS | IS | IS may contribute to atrial fibrillation: It increased pulmonary vein and atrial arrhythmogenesis through oxidative stress, inflammation, and fibrosis. |
| Yisireyili [119], 2013 and Lekawanvijit [120], 2012 | Dahl salt-sensitive hypertensive rats | IS | IS aggravated cardiac fibrosis and cardiomyocyte hypertrophy, with greater levels of oxidative stress and lower anti-oxidative defenses. |
| Muteliefu [110], 2012 | Aorta of subtotally nephrectomized Dahl salt-sensitive hypertensive rats | IS | IS accelerated VSMC senescence and vascular calcification, with upregulation of p21, p53, and prelamin A through oxidative stress. |
| Adijiang [111], 2010 | Dahl salt-sensitive hypertensive rats | IS | IS increased aortic calcification and wall thickness; induced expression of p16, p21, p53 and Rb in the calcification area; and thus promoted cell senescence. |
| Adijiang [112], 2008 | Dahl salt-sensitive hypertensive rats | IS | IS induced aortic calcification (with expression of osteoblast-specific proteins) and aortic wall thickening. |
| Han [126], 2015 | 5/6 nephrectomized mice | PCS | PCS promoted cardiac apoptosis and diastolic dysfunction by upregulating the expression of NADPH oxidase and the production of ROS. |
| Hu [123], 2015 | Two CKD rodent models: UNX-IRI26 and 5/6 nephrectomized | Phosphate | High phosphate was associated with lower Klotho levels, leading to cardiac hypertrophy and fibrosis. |
| Yamada [116], 2014 | Adenine-induced CKD male Sprague–Dawley rats | Phosphate | High phosphate directly increased the expression of TNF- and osteochondrogenic markers, inducing systemic inflammation and vascular calcification. |
| Lau [114], 2013 | DBA/2 mice with partial renal ablation | Phosphate | High phosphate was associated with arterial medial calcification. |
| Crouthamel [113], 2013 | Mice with targeted deletion of PiT-1 in VSMCs | Phosphate | High phosphate induced calcification of VSMCs. |
| El-Abbadi [115], 2009 | Female DBA/2 mice induced uremia with left total nephrectomy | Phosphate | High phosphate was associated with extensive arterial medial calcification. |
| Graciolli [117], 2009 | 5/6 nephrectomized Wistar rats with parathyroidectomy | Phosphate | Phosphate upregulated aortic expression of Runx2 and led to calcified VSMC. |
| Hosaka [118], 2009 | 5/6 nephrectomized male Sprague-Dawley rats | Phosphate | High phosphate induced elastin degradation via the upregulation of tissue-nonspecific alkaline phosphatase, accelerating the transformation of VSMCs into osteoblast-like cells and leading to medial layer calcification. |
| Zhu [122], 2021 | 25 nephrectomized SPF-grade male Sprague–Dawley rats | Urea | Urea caused myocardial hypertrophy. |
| Prommer [124], 2018 | 11 uremic mice and 8 controls | Urea | Urea led to systemic microvascular disease, with microvascular rarefaction, tissue hypoxia, and dysfunctional angiogenesis. |
| Carmona [125], 2011 | 2 groups of 30 Wistar male rats: 1 with renal ablation and the other with kidney manipulation only | Urea | Urea induced systemic inflammation and led to the thickening of subepicardiac arteries. |
| Other than ATH and non ATH CVDs | |||
| Yang [127], 2017 | C57BL/6J mice with left total nephrectomy | IS | IS activated ROS/p38 MAPK signaling and reduced Klotho expression, which induced platelet aggregation and thrombus formation. |
| Kolachalama [128], 2018 | A group of C57BL/6 mice administered Kyn, the excretion of which was inhibited by probenecid | Kynurenine | High kynurenine levels promoted clotting in response to vascular injury. |
| Koppe [133], 2017 | 5/6 nephrectomized | PCS | PCS (but not PCG) promoted insulin resistance. |
| mice | PCG | ||
| Koppe [134], 2013 | CD1 Swiss and C57BL/6J mice with 5/6 nephrectomy | PCS | PCS contributed to insulin resistance: It altered insulin signaling in skeletal muscle through the activation of extracellular signal-regulated kinases. |
| Nagy [97], 2017 | Male CD1 mice injected with CMPF | CMPF | CMPF inhibited insulin secretion. |
| Koppe [130], 2016 | C57BL/6N male mice with 5/6 nephrectomy | Urea | Urea increased oxidative stress and protein O-GlcNAcylation, impairing insulin secretion and glycolysis. |
| Carracedo [131], 2013 | 5/6 nephrectomized 40 male Wistar rats | Urea | Urea induced oxidative stress, leading to EC damage. |
| D’Apolito [98], 2010 | 5/6 nephrectomized C57BL/6J wild-type mice | Urea | Urea increased ROS production and induced insulin resistance and glucose intolerance. |
| Li [132], 2018 | 5/6 nephrectomized rats | TMAO | High TMAO levels decreased NO production, contributing to endothelial dysfunction. |
| Zhu [129], 2016 | Carotid artery thrombosis models of germ-free C57BL/6J female mice | TMAO | TMAO enhanced submaximal stimulus-dependent platelet activation, increasing the thrombosis risk. |
Abbreviations: CMPF: 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid; CVD: cardiovascular disease; eNOS:endothelial nitric oxide synthase; HA: hippuric acid; IS:indoxyl sulfate; KA: kynurenic acid; MAPK: mitogen-activated protein kinase; MI: myocardial infarction; NADPH: nicotinamide adenine dinucleotide phosphate; NO: nitric oxide; PAD: peripheral artery disease; PCS: para-cresyl sulfate; PCG: p-cresyl glucuronide; ROS: reactive oxygen species; Runx2: runt-related transcription factor 2; TMAO: trimethylamine-N-oxide; UT: uremic toxin; VSMC: vascular smooth muscle cell.
3.2.1. Atheromatous Cardiovascular Diseases
Arinze et al. [50,100] also recently studied the effect of tryptophan-derived indoles on PAD in adenine-induced CKD and IS solute–specific C57BL/6 mouse models [50]. IS, kynurenine, and KA increased AHR activity, resulting in the suppression of Wnt/-catenin signaling. This phenomenon led to impaired angiogenesis and caused hindlimb ischemia. This complication of PAD was also observed by Hung et al. [101], who concluded that IS caused PAD by decreasing the mobilization of endothelial progenitor cell and impairing neovascularization. TMAO was also found to engender PAD by impairing endothelium- derived, hyperpolarizing factor-type relaxation [102]. The results of in vivo studies suggest that PCS, HA, urea, and TMAO contribute to the acceleration of atherosclerosis. In a study of five of six nephrectomized apoE –/– mice, Han et al. [103] showed that PCS induced VSMC migration and proliferation and disturbed the balance between matrix metalloproteinases and tissue inhibitors of metalloproteinases within plaques. Huang et al. [60] and Shang et al. [61] added to their in vitro findings by studying HA’s role in atherosclerosis induction in animal models. HA induced oxidative stress, led to endothelial dysfunction, impaired endothelium-dependent vasodilation [60], and induced miR-92a (involved in the angiogenic process) [61]. Recent studies have found that TMAO is also involved in atherosclerosis induction. The compound activated the expression of components of the CD36/MAPK/JNK and NF-B signaling pathways [104,105], which promoted foam cell formation [104] and vascular inflammation [105]. Moreover, Koeth et al. [106] showed that TMAO was linked to major cardiac events—mainly MI and stroke. Massy et al. confirmed the role of urea [107] and concluded that the compound aggravated atherosclerosis by promoting arterial calcification.
3.2.2. Non-Atheromatous Cardiovascular Diseases
IS, PCS, phosphate, and urea also promote non-ATH CVDs in general and vascular calcification in particular. IS and PCS induced severe calcification of the aorta and peripheral arteries: IS decreased NO production and increased endothelial nitric oxide synthase (eNOS) phosphorylation [108], and both IS and PCS activated the inflammation and coagulation pathways [109]. According to Chen et al. [81], IS promoted aortic calcification by decreasing Klotho expression. Moreover, Muteliefu et al. [110] and Adijiang et al. [111,112] showed that IS accelerated wall thickening and vascular calcification through the upregulation of p16, p21, p53, prelamin A, and osteoblast-specific protein, which induced VSMC senescence. Phosphate also generated VSMCs calcification [113] and was associated with arterial medial calcification [114,115]. Phosphate overload increased the expression of Tumor Necrosis Factor alpha (TNF-), osteochondrogenic markers [116], and aortic runt-related transcription factor 2 [117], which induced systemic inflammation and VSMC calcification. In 5/6 nephrectomized male Sprague–Dawley rats, high phosphate caused medial calcification by increasing tissue-nonspecific alkaline phosphatase activity, which induced elastin degradation and accelerated the transformation of VSMCs into osteoblast-like cells [118].
UTs contributed to cardiac fibrosis, arrhythmia, and myocardial hypertrophy. It has been suggested that IS leads to atrial fibrillation. Exposure to IS accentuated cardiac fibrosis and cardiomyocyte hypertrophy by increasing oxidative stress and decreasing anti-oxidative defenses [119,120]. Chen et al. showed that these changes [121] led to greater pulmonary vein and atrial arrhythmogenesis. High phosphate and urea levels also lead to cardiac hypertrophy [122] and fibrosis [123]. For example, it was shown that high phosphate levels result in lower Klotho levels in rodent models of CKD [123]. Moreover, high urea levels led to systemic microvascular disease with microvascular rarefaction, tissue hypoxia, and dysfunctional angiogenesis [124]. High urea also induced systemic inflammation, which was responsible for subepicardiac artery thickening [125]. In 5/6 nephrectomized mice, PCS toxicity was linked to increases in NADPH oxidase expression and ROS production; these changes contributed to cardiomyocyte apoptosis, which in turn aggravated diastolic dysfunction (with a change in the ratio between early and late left ventricular transmitral peak flow velocities) [126].
3.2.3. Other than Atheromatous and Non-Atheromatous Cardiovascular Diseases
It has been suggested that IS, kynurenine, and TMAO increase the thrombosis risk. IS activated ROS/p38 MAPK signaling and reduced Klotho expression, which would aggravate the effect on platelet aggregation and thrombus formation [127]. Kynurenine promoted clotting as a consequence of vascular injury [128]. Zhu et al. [129] concluded that TMAO enhanced submaximal stimulus-dependent platelet activation and thus contributed to the thrombosis risk.
A number of mechanisms favor EC dysfunction. In 5/6 nephrectomized rats, urea increased ROS production and thus induced oxidative stress in the systemic circulation [98,130,131]. Furthermore, high TMAO levels are associated with a decrease in NO production [132].
The oxidative stress induced by urea contributed also to insulin resistance. Urea increases oxidative stress and protein O-GlcNAcylation, thus impairing insulin secretion and glycolysis [130]. D’Apolito et al. [98] showed that urea increased ROS production and promoted insulin resistance and glucose intolerance. IS and PCS also exert indirect effects given that they were strongly associated with impaired glucose homeostasis and thus hyperglycemia and insulin resistance [109]. Koppe et al. [133] concluded that only PCS (but not PCG) induced insulin resistance by activating extracellular signal-regulated kinases and thus altering insulin signaling in skeletal muscle [134]. Furthermore, Nagy et al.’s in vitro results [97] on CMPF’s role in promoting insulin resistance were confirmed in vivo [97].
3.3. Observational Studies
Observational studies of the UTs’ impact on ATH and/or non-ATH CVDs are summarized in Table 3.
Table 3.
Observational studies of the effects of UTs on cardiovascular complications.
| First Author, Year | Models | UT(s) Studied | Main Findings |
|---|---|---|---|
| Atheromatous CVDs | |||
| Arinze [50], 2022 | 20 HD patients and 15 | IS | Elevated plasma levels of IS, kynurenine, and KA in |
| controls | Kynurenine | HD patients showed a significant decrease in ECs | |
| KA | proliferation and migration, compared with the control group. | ||
| Arinze [50], 2022 | PAD patients: 35 without | IS | Elevated plasma levels of IS, kynurenine, KA, with |
| adverse limb event and | Kynurenine | suppressed Wnt activity in ECs were associated with | |
| 28 with | KA | an increased risk of future adverse limb events. | |
| Shafi et al. [153], 2015 | 394 incident HD patients | IS | Elevated serum levels of IS, PCS, PAG and HA were |
| PCS | associated with greater risk of fatal or nonfatal | ||
| PAG | atherosclerotic cardiovascular events in incident HD | ||
| HA | patients. | ||
| Hsu [154], 2013 | 191 mild-to-moderate CKD patients | IS | Elevated serum IS levels were associated with coronary atherosclerosis and correlated with the severity of the disease. |
| Melamed [167], 2013 | 521 incident HD patients | IS | IS and PCS were not associated with atherosclerotic |
| PCS | cardiovascular death. | ||
| Lin [155], 2012 | 70 pre-dialysis patients (CKD stage 3 to 5) | IS | Serum IS levels were positively correlated with atherosclerotic cardiovascular events. |
| Lin [152], 2012 | 100 stable HD patients | IS | Elevated serum levels of IS and PCS were associated |
| PCS | with PAD and arteriosclerosis markers. | ||
| Lin [157], 2010 | 100 HD patients | IS | Only elevated serum PCS levels were significantly |
| PCS | associated with fatal or nonfatal atherosclerotic cardiovascular events. | ||
| Taki [156], 2007 | 224 HD patients | IS | Plasma IS levels were significantly and negatively correlated with HDL cholesterol and were positively associated with atherosclerotic lesions. |
| Poesen [158], 2016 | 488 patients (all CKD | PCS | A lower serum PCS:PCG ratio and a higher |
| stages) | PCG | total PCS + PCG level were associated with fatal or nonfatal atherosclerotic CVDs. | |
| Wang [160], 2010 | 202 patients with stable angina and early-stage kidney failure | PCS | Elevated plasma PCS levels were associated with coronary artery disease and correlated with the severity of the disease. |
| Poesen [159], 2016 | 488 patients with CKD stages 1–5 | PAG | An elevated serum PAG level was a powerful, independent risk factor for major CVD (such as MI and stroke). |
| Merhi [162], 2017 | 3138 CKD patients | Phosphate | Hyperphosphatemia was associated with atherosclerotic CVD. |
| Eddington [163], 2010 | 1203 nondialyzed CKD patients | Phosphate | Hyperphosphatemia increased the risk of cardiovascular death from atheromatous CVD. |
| Kestenbaum [164], 2005 | 3490 CKD patients | Phosphate | Hyperphosphatemia was associated with MI. |
| Nakamura [161], 2002 | 525 HD patients | Phosphate | Hyperphosphatemia was associated with atherosclerotic diseases. |
| Stubbs [165], 2016 | 104 CKD patients | TMAO | Elevated TMAO concentrations were correlated with coronary atherosclerosis. |
| Kim [166], 2016 | 2529 patients (stages 3b and 4 CKD) | TMAO | Elevated serum TMAO levels were associated with ischemic cardiovascular events. |
| Non-atheromatous CVDs | |||
| Chinnappa [168], 2018 | 56 male patients with | IS | These serum UT levels showed significant negative |
| stage 2–5 CKD, nondia- | IAA | correlation with peak cardiac power and subclinical | |
| lyzed and free of heart | PCS | cardiac dysfunction, but no correlation with left ven- | |
| disease | PCG | tricular mass index was found. | |
| HA | |||
| Cao [169], 2015 | 258 HD patients | IS | Elevated plasma IS was associated with heart failure. |
| Sato [171], 2013 | 204 CKD patients with preserved left ventricular function | IS | Elevated plasma IS levels were associated with an increased risk of left ventricular diastolic dysfunction. |
| Shimazu [170], 2013 | 76 patients with mild-to-moderate CKD and dilated cardiomyopathy | IS | Elevated serum IS levels were associated with hospitalization for heart failure and cardiac death. |
| Barreto [172], 2009 | 139 patients with CKD from stage 2 to dialysis | IS | Being in the highest serum IS tertile was directly associated with pulse wave velocity, aortic calcification, and higher cardiovascular mortality. |
| Zapolski [173], 2020 | 100 CKD patients with persistent atrial fibrillation | KA | Serum KA levels were positively correlated with aortic stiffness and indices of diastolic dysfunction of left atrium and left ventricle. |
| Pawlak [174], 2010 | 106 CKD patients | KA | Elevated plasma kynurenine and KA levels were as- |
| Kynurenine | sociated with intima-media thickness. | ||
| Liabeuf [175], 2010 | 139 CKD patients | PCS | Elevated total and free serum PCS levels were significantly associated with vascular calcification, and free PCS was shown to be a predictor of cardiovascular death. |
| Yu [176], 2018 | 80 HD patients | HA | Elevated HA levels were significantly associated with left ventricular hypertrophy. |
| Petchey [177], 2012 | 120 CKD pre-dialysis patients | Phosphate | Serum phosphate was positively correlated with aortic pulse wave velocity, arterial stiffness, and the presence of vascular calcification. |
| Adeney [178], 2009 | 6814 patients with CKD aged 45–84 | Phosphate | Hyperphosphatemia was associated with vascular and valvular calcification. |
| Ix [180], 2009 | 440 patients with moderate CKD | Phosphate | Hyperphosphatemia was strongly associated with peripheral arterial stiffness. |
| Ketteler [179], 2003 | 312 HD patients | Phosphate | Hyperphosphatemia was associated with vascular calcification and cardiovascular mortality. |
| Drechsler [181], 2015 | 1255 HD patients | Urea | Higher blood urea levels were associated with higher tertile serum carbamylated albumin levels, which in turn were positively correlated with heart failure and arrhythmia. |
| Atheromatous and non-atheromatous CVDs | |||
| Chen [147], 2021 | 3407 participants with | IS | Lower 24-h kidney clearance of IS, KA, and PCS |
| CKD, excluding those | KA | was not found to be associated with heart failure and | |
| with a GFR < 20 mL/min/1.73 m2 | PCS | MI after adjustment for GFR. | |
| Fan [135], 2019 | 147 patients with CKD stage 1–5 | IS | Elevated plasma IS levels were associated with major adverse cardiovascular events, independently of GFR and nutritional status. |
| Shafi [148], 2017 | 1273 HD patients | IS | Overall, elevated serum IS, PCS, PAG and HA levels |
| PCS | were not associated with any cardiovascular event. | ||
| PAG | However, high IS levels were predictive of cardiac | ||
| HA | and sudden cardiac death in patients with low albumin levels. | ||
| Konje [136], 2021 | 92 CKD patients with a history of CVD, 46 with no history of CVD, and 46 with incident CVD | Kynurenine | Elevated serum kynurenine levels were associated with incident atheromatous and non-atheromatous CVDs. |
| Wu [137], 2012 | 112 HD patients aged from 65 to 90 | PCS | Elevated free PCS serum levels were associated with cardiovascular mortality. |
| Liabeuf [138], 2013 | 139 CKD patients | PCG | Elevated free and total serum PCG levels were correlated with cardiovascular mortality independently of survival predictors. |
| Luce [149], 2018 | 270 HD patients | CMPF | Elevated serum CMPF was not associated with any CVD. |
| McGovern [139], 2013 | 13,292 CKD patients at stages 3–5 | Phosphate | Hyperphosphatemia was correlated with increased CVDs. |
| Kimata [140], 2007 | 3973 HD patients | Phosphate | Hyperphosphatemia was significantly associated with cardiovascular mortality. |
| Menon [150], 2005 | 840 CKD patients | Phosphate | Hyperphosphatemia was significantly associated with increased cardiovascular mortality but only before adjustment for GFR. |
| Slinin [141], 2005 | 14829 HD patients | Phosphate | Hyperphosphatemia was associated with CVDs and mortality. |
| Young [142], 2005 | 17236 dialysis patients | Phosphate | Hyperphosphatemia was significantly associated with cardiovascular mortality. |
| Block [143], 2004 | 40538 HD patients | Phosphate | Hyperphosphatemia was significantly associated with cardiovascular hospitalization and mortality. |
| Laville [27], 2022 | 2507 CKD patients before RRT | Urea | Higher serum urea levels were associated with a greater risk of CVD. |
| Berg [146], 2013 | 187 HD patients | Urea | Urea was positively correlated with carbamylation of serum albumin, which is associated with CVDs and mortality. |
| Shafi [144], 2017 | 1846 prevalent HD patients | TMAO | An elevated serum TMAO concentration was associated with cardiovascular events and death. |
| Kaysen [151], 2015 | 235 HD patients | TMAO | There was no significant association between TMAO and cardiovascular hospitalizations or death. |
| Other than ATH and non ATH CVDs | |||
| Glorieux [182], 2021 | 523 nondialyzed patients | IS | Elevated serum levels of these UTs were correlated |
| (all stages of CKD) | IAA | with markers of endothelial damage (mainly angio- | |
| PCS | poietin-2). Elevated levels of free PCS and free PCG | ||
| PCG | had the strongest association with CVD, indepen- | ||
| HA | dently of the GFR. | ||
| Wang [184], 2019 | 110 patients with stage 3–5 CKD | IS | Elevated levels of serum IS were negatively correlated with vascular reactivity index values, leading to endothelial dysfunction. |
| Kolachalama [128], 2018 | 473 participants under- | IS | Elevated serum levels of IS and kynurenine were |
| going angioplasty for dialysis access dysfunction | Kynurenine | associated with postangioplasty thrombosis of dialysis grafts. | |
| Wu [190], 2016 | 306 patients undergoing angioplasty for dialysis access dysfunction | IS | Elevated serum levels of IS were associated with postangioplasty thrombosis of dialysis grafts. |
| Jourde-Chiche [185], 2009 | 38 HD patients and 21 | IS | Elevated serum levels of IS, IAA, and PCS were asso- |
| healthy controls | IAA | ciated with low numbers of endothelial progenitor | |
| PCS | cells. | ||
| Pawlak [187], 2010 | 64 patients on peritoneal dialysis | KA | Plasma KA levels were positively associated with TF inhibitor and negatively associated with prothrombin fragment 1 + 2 levels. |
| Pawlak [188], 2009 | 48 patients with ESRD | Kynurenine | Plasma kynurenine levels were positively associated with thrombomodulin and von Willebrand factor (markers of endothelial dysfunction). |
| Pawlak [186], 2009 | 146 CKD patients with 91 | Kynurenine | Elevated serum levels of kynurenine and KA were |
| ones on dialysis | KA | associated with increased oxidative stress, inflammation, and endothelial dysfunction. | |
| Pawlak [189], 2009 | 92 patients on dialysis | Kynurenine | Elevated serum levels of kynurenine and KA were |
| KA | independently and significantly associated with hypercoagulability. | ||
| Meijers [66], 2009 | 100 HD patients | PCS | Elevated serum PCS levels were associated with the levels of circulating endothelial microparticles. |
Abbreviations: CMPF: 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid; CKD: chronic kidney disease; CVD: cardiovascular disease; EC: endothelial cell; ESRD: end-stage renal disease; GFR: glomerular filtration rate; HA: hippuric acid; HD: hemodialysis; HDL: high-density lipoprotein; IAA: indole-3-acetic acid; IS:indoxyl sulfate; KA: kynurenic acid; MI: myocardial infarction; PAD: peripheral artery disease; PAG: phenylacetylglutamine; PCS: para-cresyl sulfate; PCG: p-cresyl glucuronide; RRT: renal replacement therapy; TF: tissue factor; TMAO: trimethylamine-N-oxide; UT: uremic toxin.
Some of the studies sought to elucidate the association between UTs with ATH and/or non-ATH CVDs in patients with CKD.
In 147 patients with stage 1 to 5 CKD, high plasma IS levels were associated with major adverse cardiovascular events independently of the GFR and nutritional status [135]. A recent study by Konje et al. [136] found a positive association between kynurenine and incident ATH and non-ATH CVDs, including MI, angina, coronary artery bypass grafting, angioplasty/stenting of a coronary artery, stroke, PAD, congestive heart failure, and arrhythmia. Wu et al. [137] showed that elevated free PCS serum levels were associated with cardiovascular mortality in HD patients. A study by Liabeuf et al. [138] demonstrated for the first time that serum PCG levels were positively correlated with cardiovascular mortality, independently of survival predictors. These results showed that even though PCG is the minor metabolite of p-cresol, it appears to have a substantial impact on mortality—as much as PCS and IS do. Several studies confirmed the link between phosphate and CVDs and mortality. In 13,292 stage 3 to 5 CKD patients, serum phosphate was positively correlated with increased risk of incident stroke, transient ischaemic attack, MI, advanced coronary artery disease, new cardiac failure, and death [139]. An elevated serum phosphate concentration was significantly associated with cardiovascular mortality in many study settings [140,141,142,143]. Similarly, high serum TMAO levels were associated with cardiovascular events and death in HD patients, including coronary events, arrhythmias, sudden cardiac death, and congestive heart failure [144].
Urea has direct and indirect effects on adverse cardiovascular outcomes in patients with CKD. A recent cohort conducted by Laville et al. in 2022 [27] was the first to show a direct association between elevated serum urea concentrations and a greater risk of ATH CVD, non-ATH CVD, and mortality in pre-ESRD patients; this was independent of other conventional risk factors, including renal function. In addition, elevated urea levels were positively correlated with CVD. Nevertheless, this correlation was indirect. There are various hypotheses for the toxicity of high urea levels, including protein carbamylation (a post-translational modification of proteins with various biological consequences—mainly related to atherogenesis) [145]. Berg et al. [146] affirmed that high urea was positively correlated with the carbamylation of serum albumin, and suggested that this carbamylation had an impact on CVD and mortality.
All the above-mentioned previous studies concluded that there was a significant, independent association between UTs and the risk of cardiovascular complications. However, a few studies did not find an association after adjusting for confounders, and others did not find any association between some UTs and cardiovascular morbidity/mortality. The Chronic Renal Insufficiency Cohort (CRIC) study by Chen et al. [147] included 3407 patients with CKD but not ESRD. The results showed that lower 24-h kidney clearance of IS, KA, and PCS was associated with incident heart failure and MI; however, this association was not statistically significant after adjustment for the GFR. In the HEMO study, Shafi et al. concluded [148] that IS, PCS, PAG, and HA were not associated with any cardiovascular event (coronary events, peripheral vascular disease, ischemic heart disease, congestive heart failure, or arrhythmias). However, high IS levels were predictive of cardiac and sudden cardiac death in patients with lower albumin. It has been shown that high serum CMPF levels were not associated with any type of CVD [149], and that higher phosphate levels were associated with increased cardiovascular mortality; however, the latter association was not statistically significant after adjustment for GFR [150]. In a study of 235 HD patients, Kaysen et al. [151] found that there was no significant association between TMAO and cardiovascular hospitalizations or death.
Even though some studies investigated the association of UTs with both ATH and non-ATH CVDs, many focused on one or the other.
3.3.1. Atheromatous Cardiovascular Diseases
The in vitro and in vivo effect of tryptophan-derived indoles on PAD (observed by Arinze et al. [50]) was confirmed in two cohorts. Firstly, a study of 20 HDs concluded that elevated plasma levels of IS, kynurenine, and KA were associated with a significant decrease in EC proliferation and migration, relative to 15 controls [50]. Secondly, 28 PAD patients with adverse limb events and 35 PAD patients without adverse limb events were followed up for 2 years. High plasma levels of IS, kynurenine, and KA, along with suppressed Wnt activity in ECs, were associated with an elevated risk of future adverse limb events [50]. In another study of 100 HD patients, elevated serum levels of IS and PCS were associated with PAD and arteriosclerosis markers [152].
Shafi et al. [153] concluded that higher serum levels of IS, PCS, PAG, and HA were associated with a greater risk of fatal or nonfatal atherosclerotic cardiovascular events. Previous studies had already shown that higher serum IS levels were associated with atherosclerotic cardiovascular events [154,155,156]. Hsu et al. [154] showed that higher serum IS levels were associated with coronary atherosclerosis and indicated that this elevation was correlated with the severity of the disease. In a cohort of 224 HD patients, plasma IS levels showed a significant negative correlation with HDL cholesterol, and they were associated with atherosclerotic lesions [156]. An older study by Lin et al. [157] in 2010 showed that high serum levels of PCS were significantly associated with atherosclerotic cardiovascular events. In 2016, Poesen et al. carried out two ancillary analyses of data from the Leuven Mild-to-Moderate CKD Study. One ancillary analysis showed that a lower serum PCS:PCG ratio and high total PCS and PCG levels were associated with fatal and nonfatal atherosclerotic CVDs [158]. The other demonstrated that an elevated serum PAG level was associated with CVD even after adjustment for age, sex, the presence of DM, prior CVD, and GFR; hence, elevated PAG was considered to be a powerful, independent risk factor for major CVDs (notably MI and stroke) [159].
The toxic effect of PCS on ATH CVDs dates back to a study conducted in 2010 on 202 stable angina patients with early stage of CKD, presenting that an elevated plasma level of PCS was associated with CAD and it was correlated with the severity of the disease [160]. The association between hyperphosphatemia and atherosclerotic diseases was reported from 2002 onwards [161] and again recently [162]. In 1203 non-dialyzed CKD patients, high serum phosphate levels were associated with an increased risk of fatal ATH CVD [163]. Hyperphosphatemia was associated with MI in a representative study of 3490 patients with CKD [164]. TMAO is the UT most frequently studied in animal models with regard to its link to ATH CVDs. Many cohorts from 2016 onwards have confirmed these findings. High TMAO concentrations were positively correlated with coronary atherosclerosis in one study [165] and with ischemic cardiovascular events in a study of 2529 stage 3b or 4 patients with CKD [166].
Only one study (by Melamed et al. [167]) failed to find an association between IS or PCS and fatal ATH CVD.
3.3.2. Non-Atheromatous Cardiovascular Diseases
While TMAO is most studied compound with regard to the effect of UTs on ATH CVDs in vivo, its counterpart for non-ATH CVDs is IS. Most observational clinical studies have confirmed the association between IS and vascular calcification. A recent study of IS, IAA, PCS, PCG, and HA showed that each UT was significantly and negatively correlated with peak cardiac power and significantly and positively correlated with subclinical cardiac dysfunction but not with the left ventricular mass index [168]. Cao et al. [169] and Shimazu et al. [170] reported that high plasma IS was associated with heart failure [169,170] and cardiac death [170]. In a study of 204 patients with CKD and preserved left ventricular function, greater plasma IS levels were associated with an elevated risk of left ventricular diastolic dysfunction [171]. In a cohort study by Barreto et al. [172], serum IS levels were directly associated with pulse wave velocity and aortic calcification, and being in the highest IS tertile was a strong predictor of cardiovascular mortality. In a study in 2020, KA also was associated with diastolic dysfunction [173]. Serum KA levels were positively correlated with aortic stiffness and with indices of left atrium and left ventricle diastolic dysfunction [173]. In addition, high plasma kynurenine and KA levels were associated with intima-media thickness in 106 CKD patients [174]. A 2010 study by Liabeuf et al. [175] proved that an elevated serum total and free PCS levels were significantly associated with vascular calcification, and free PCS was shown to be a predictor of cardiovascular mortality. HA was also investigated in 2018; high HA levels were significantly associated with left ventricular hypertrophy in HD patients [176]. Several studies showed that hyperphosphatemia was associated with vascular calcification [177,178,179]. Petchey et al. [177] further reported that serum phosphate was positively correlated with the aortic pulse wave velocity and thus with arterial stiffness. In a cohort of 6814 patients with CKD, high serum phosphate was associated with vascular and valvular calcification [178]. Moreover, Ix et al. [180] concluded that hyperphosphatemia was strongly associated with peripheral arterial stiffness. As mentioned above, urea exerts its toxicity indirectly through protein carbamylation. In 1255 HD patients, greater blood urea levels were associated with serum carbamylated albumin levels, and being in the upper urea tertile was positively correlated with heart failure and arrhythmia [181].
3.3.3. Other than Atheromatous and Non-Atheromatous Cardiovascular Diseases
Many observational studies have highlighted the detrimental effect of UTs on ECs. A novel study by Glorieux et al. [182] in 2021 demonstrated that high serum levels of IS, IAA, PCS, PCG, and HA were correlated with markers of endothelial damage—mainly angiopoietin-2, a protein with an essential role in angiogenesis and that is involved in carotid artery intima media thicknening, arterial stiffness, and left ventricular hypertrophy [182,183]. In a 2019 study by Wang et al. [184], elevated serum IS levels were negatively correlated with vascular reactivity index values. A study by Jourde-Chiche et al. [185] found that high serum levels of IS, IAA, and PCS were associated with low numbers of endothelial progenitor cells. Meijers et al. [66] investigated PCS in vitro and in an observational study; high serum PCS levels were associated with the presence of circulating endothelial microparticles. In 2009 and 2010, Pawlak et al. studied the toxicity of kynurenine and its metabolite KA; both were shown to be associated with elevated oxidative stress, inflammation, and EC dysfunction [186]. The researchers found that plasma KA levels were associated positively with TF inhibitor levels and negatively with prothrombin fragment 1 + 2 levels [187]. Pawlak et al. also concluded that plasma kynurenine levels were positively associated with thrombomodulin and von Willebrand factor (both markers of EC dysfunction) [188].
In addition, high serum kynurenine and KA levels were independently and significantly associated with hypercoagulability [189]. Elevated serum IS and kynurenine levels contribute to postangioplasty complications. High serum levels of IS [128,190] and kynurenine [128] were associated with postangioplasty thrombosis of dialysis grafts.
It is noteworthy that high levels of free PCS and free PCG had the strongest association with CVDs even after adjustment for the GFR [182].
4. Conclusions
We primarily reviewed gut-derived UTs because most of the latter are protein-bound solutes that are difficult to remove from the plasma; their accumulation in the circulation is associated with many harmful effects (including cardiovascular complications). We also reviewed phosphate and urea, whose toxicity has long been known but is still being explored. CVD is a major problem in patients with CKD and constitutes the leading cause of death in this population [19]. Strategies implemented to modify UT levels have been described in previous reviews [11,191]. A combined approach of targeting UTs with the other aspects of CVD could be necessary for its prevention. Noteworthy, the relation between CKD and CVDs is bidirectional: CVDs could also impact kidney’s health, causing kidney damage and disease progression, creating the cardio-renal syndrome [192].
Acknowledgments
We genuinely appreciate the assistance of M.David Fraser in English revision.
Author Contributions
Conceptualization, S.L. and Z.M.; methodology, C.E.C.; software, not applicable; validation, not applicable; investigation, not applicable; resources, C.E.C., Z.M. and S.L.; data curation, not applicable; writing—original draft preparation, C.E.C.; writing—review and editing, S.L. and Z.M.; visualization, not applicable; supervision, S.L. and Z.M.; project administration, not applicable; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
C.E.C and S.L. declare no conflict of interest. Z.M. reports having received grants for CKD REIN and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp and Dohme-Chibret, Sanofi-Genzyme, Lilly, Otsuka, Astra Zeneca, Vifor, and the French government, as well as fees and grants to charities from Astra Zeneca.
Key Contribution
This review provides an overview of the pathophysiological mechanisms of major dietary and gut-derived uremic toxins in the emergence and progression of cardiovascular diseases in a chronic kidney disease setting.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perico N., Remuzzi G. Chronic kidney disease: A research and public health priority. Nephrol. Dial. Transplant. 2012;27:iii19–iii26. doi: 10.1093/ndt/gfs284. [DOI] [PubMed] [Google Scholar]
- 2.Levey A.S., Eckardt K.U., Dorman N.M., Christiansen S.L., Hoorn E.J., Ingelfinger J.R., Inker L.A., Levin A., Mehrotra R., Palevsky P.M., et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97:1117–1129. doi: 10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Lv J.C., Zhang L.X. Prevalence and disease burden of chronic kidney disease. Ren. Fibros. Mech. Ther. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 4.Nlandu Y., Padden M., Seidowsky A., Hamaz S., Vilaine E., Cheddani L., Essig M., Massy Z.A. Toxines urémiques de moyen poids moléculaire: Un véritable regain d’intérêt. Néphrol. Thér. 2019;15:82–90. doi: 10.1016/j.nephro.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Wojtaszek E., Oldakowska-Jedynak U., Kwiatkowska M., Glogowski T., Malyszko J. Uremic toxins, oxidative stress, atherosclerosis in chronic kidney disease, and kidney transplantation. Oxidative Med. Cell. Longev. 2021;2021:6651367. doi: 10.1155/2021/6651367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R., Jafar T.H., Heerspink H.J.L., Mann J.F., Matsushita K., Wen C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 7.Drüeke T.B., Massy Z.A. Atherosclerosis in CKD: Differences from the general population. Nat. Rev. Nephrol. 2010;6:723–735. doi: 10.1038/nrneph.2010.143. [DOI] [PubMed] [Google Scholar]
- 8.Villain C., Metzger M., Combe C., Fouque D., Frimat L., Jacquelinet C., Laville M., Briançon S., Klein J., Schanstra J.P., et al. Prevalence of atheromatous and non-atheromatous cardiovascular disease by age in chronic kidney disease. Nephrol. Dial. Transplant. 2020;35:827–836. doi: 10.1093/ndt/gfy277. [DOI] [PubMed] [Google Scholar]
- 9.Liabeuf S., Drüeke T.B., Massy Z.A. Protein-bound uremic toxins: New insight from clinical studies. Toxins. 2011;3:911–919. doi: 10.3390/toxins3070911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanholder R., De Smet R., Glorieux G. Review on uremic toxins: Classification, concentration, and interindividual variability (volume 63, pg 1934, 2003) Kidney Int. 2020;98:1354. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosner M.H., Reis T., Husain-Syed F., Vanholder R., Hutchison C., Stenvinkel P., Blankestijn P.J., Cozzolino M., Juillard L., Kashani K., et al. Classification of uremic toxins and their role in kidney failure. Clin. J. Am. Soc. Nephrol. 2021;16:1918–1928. doi: 10.2215/CJN.02660221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duranton F., Cohen G., De Smet R., Rodriguez M., Jankowski J., Vanholder R., Argiles A., European Uremic Toxin Work Group Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graboski A.L., Redinbo M.R. Gut-derived protein-bound uremic toxins. Toxins. 2020;12:590. doi: 10.3390/toxins12090590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanholder R., Baurmeister U., Brunet P., Cohen G., Glorieux G., Jankowski J., European Uremic Toxin Work Group A bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008;19:863–870. doi: 10.1681/ASN.2007121377. [DOI] [PubMed] [Google Scholar]
- 15.Moradi H., Sica D.A., Kalantar-Zadeh K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am. J. Nephrol. 2013;38:136–148. doi: 10.1159/000351758. [DOI] [PubMed] [Google Scholar]
- 16.Pieniazek A., Bernasinska-Slomczewska J., Gwozdzinski L. Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD. Int. J. Mol. Sci. 2021;22:6196. doi: 10.3390/ijms22126196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross P., Six I., Kamel S., Massy Z.A. Vascular Toxicity of Phosphate in Chronic Kidney Disease–Beyond Vascular Calcification. Circ. J. 2014;78:2339–2346. doi: 10.1253/circj.CJ-14-0735. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z., Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9:416–431. doi: 10.1007/s13238-018-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim Y.J., Sidor N.A., Tonial N.C., Che A., Urquhart B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins. 2021;13:142. doi: 10.3390/toxins13020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarnak M.J., Levey A.S., Schoolwerth A.C., Coresh J., Culleton B., Hamm L.L., McCullough P.A., Kasiske B.L., Kelepouris E., Klag M.J., et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 21.Association A.H., Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., et al. Heart Disease and Stroke Statistics-2020 Update. 2020. [(accessed on 27 February 2022)]. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000757.
- 22.Jankowski J., Floege J., Fliser D., Böhm M., Marx N. Cardiovascular disease in chronic kidney disease: Pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.London G.M., Marchais S.J., Guerin A.P., Metivier F., Adda H. Arterial structure and function in end-stage renal disease. Nephrol. Dial. Transplant. 2002;17:1713–1724. doi: 10.1093/ndt/17.10.1713. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz U., Buzello M., Ritz E., Stein G., Raabe G., Wiest G., Mall G., Amann K. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol. Dial. Transplant. 2000;15:218–223. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 25.Herzog C.A., Asinger R.W., Berger A.K., Charytan D.M., Díez J., Hart R.G., Eckardt K.U., Kasiske B.L., McCullough P.A., Passman R.S., et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 26.Hicks K.A., Mahaffey K.W., Mehran R., Nissen S.E., Wiviott S.D., Dunn B., Solomon S.D., Marler J.R., Teerlink J.R., Farb A., et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502. [DOI] [PubMed] [Google Scholar]
- 27.Laville S.M., Couturier A., Lambert O., Metzger M., Mansencal N., Jacquelinet C., Laville M., Frimat L., Fouque D., Combe C., et al. Urea levels and cardiovascular disease in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2022:gfac045. doi: 10.1093/ndt/gfac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdivielso J.M., Rodríguez-Puyol D., Pascual J., Barrios C., Bermúdez-López M., Sánchez-Niño M.D., Pérez-Fernández M., Ortiz A. Atherosclerosis in chronic kidney disease: More, less, or just different? Arterioscler. Thromb. Vasc. Biol. 2019;39:1938–1966. doi: 10.1161/ATVBAHA.119.312705. [DOI] [PubMed] [Google Scholar]
- 29.Masson P., Webster A.C., Hong M., Turner R., Lindley R.I., Craig J.C. Chronic kidney disease and the risk of stroke: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2015;30:1162–1169. doi: 10.1093/ndt/gfv009. [DOI] [PubMed] [Google Scholar]
- 30.Chen J., Mohler E.R., Xie D., Shlipak M., Townsend R.R., Appel L.J., Ojo A., Schreiber M., Nessel L., Zhang X., et al. Traditional and non-traditional risk factors for incident peripheral arterial disease among patients with chronic kidney disease. Nephrol. Dial. Transplant. 2016;31:1145–1151. doi: 10.1093/ndt/gfv418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wattanakit K., Folsom A.R., Selvin E., Coresh J., Hirsch A.T., Weatherley B.D. Kidney function and risk of peripheral arterial disease: Results from the Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Soc. Nephrol. 2007;18:629–636. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 32.Odutayo A., Wong C.X., Hsiao A.J., Hopewell S., Altman D.G., Emdin C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 33.Silverberg D., Wexler D., Blum M., Schwartz D., Iaina A. The association between congestive heart failure and chronic renal disease. Curr. Opin. Nephrol. Hypertens. 2004;13:163–170. doi: 10.1097/00041552-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Sarafidis P.A., Loutradis C., Karpetas A., Tzanis G., Piperidou A., Koutroumpas G., Raptis V., Syrgkanis C., Liakopoulos V., Efstratiadis G., et al. Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all-cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension. 2017;70:148–157. doi: 10.1161/HYPERTENSIONAHA.117.09023. [DOI] [PubMed] [Google Scholar]
- 35.Briet M., Bozec E., Laurent S., Fassot C., London G., Jacquot C., Froissart M., Houillier P., Boutouyrie P. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350–357. doi: 10.1038/sj.ki.5000047. [DOI] [PubMed] [Google Scholar]
- 36.Pannier B., Guérin A.P., Marchais S.J., Safar M.E., London G.M. Stiffness of capacitive and conduit arteries: Prognostic significance for end-stage renal disease patients. Hypertension. 2005;45:592–596. doi: 10.1161/01.HYP.0000159190.71253.c3. [DOI] [PubMed] [Google Scholar]
- 37.London G.M. Arterial stiffness in chronic kidney disease and end-stage renal disease. Blood Purif. 2018;45:154–158. doi: 10.1159/000485146. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.J., Lee I.K., Jeon J.H. Vascular calcification—new insights into its mechanism. Int. J. Mol. Sci. 2020;21:2685. doi: 10.3390/ijms21082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vahed S.Z., Mostafavi S., Khatibi S.M.H., Shoja M.M., Ardalan M. Vascular calcification: An important understanding in nephrology. Vasc. Health Risk Manag. 2020;16:167. doi: 10.2147/VHRM.S242685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozcan C. Conduction Intervals and Atrial Fibrillation in Chronic Kidney Disease. Am. J. Nephrol. 2021;52:354–355. doi: 10.1159/000516153. [DOI] [PubMed] [Google Scholar]
- 41.Kaya B., Paydas S., Aikimbaev K., Altun E., Balal M., Deniz A., Kaypakli O., Demirtas M. Prevalence of cardiac arrhythmia and risk factors in chronic kidney disease patients. Saudi J. Kidney Dis. Transplant. 2018;29:567. doi: 10.4103/1319-2442.235178. [DOI] [PubMed] [Google Scholar]
- 42.Bonato F.O.B., Canziani M.E.F. Ventricular arrhythmia in chronic kidney disease patients. Braz. J. Nephrol. 2017;39:186–195. doi: 10.5935/0101-2800.20170033. [DOI] [PubMed] [Google Scholar]
- 43.Rapa S.F., Di Iorio B.R., Campiglia P., Heidland A., Marzocco S. Inflammation and oxidative stress in chronic kidney disease—potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2020;21:263. doi: 10.3390/ijms21010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gremmel T., Müller M., Steiner S., Seidinger D., Koppensteiner R., Kopp C.W., Panzer S. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol. Dial. Transplant. 2013;28:2116–2122. doi: 10.1093/ndt/gft103. [DOI] [PubMed] [Google Scholar]
- 45.Jain N., Wan F., Kothari M., Adelodun A., Ware J., Sarode R., Hedayati S.S. Association of platelet function with depression and its treatment with sertraline in patients with chronic kidney disease: Analysis of a randomized trial. BMC Nephrol. 2019;20:395. doi: 10.1186/s12882-019-1576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ocak G., Verduijn M., Vossen C.Y., Lijfering W.M., Dekker F.W., Rosendaal F.R., Gansevoort R.T., Mahmoodi B.K. Chronic kidney disease stages 1–3 increase the risk of venous thrombosis. J. Thromb. Haemost. 2010;8:2428–2435. doi: 10.1111/j.1538-7836.2010.04048.x. [DOI] [PubMed] [Google Scholar]
- 47.Folsom A.R., Lutsey P.L., Astor B.C., Wattanakit K., Heckbert S.R., Cushman M., Atherosclerosis Risk in Communities Study Chronic kidney disease and venous thromboembolism: A prospective study. Nephrol. Dial. Transplant. 2010;25:3296–3301. doi: 10.1093/ndt/gfq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura T., Morimoto T., Kozuma K., Honda Y., Kume T., Aizawa T., Mitsudo K., Miyazaki S., Yamaguchi T., Hiyoshi E., et al. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: Observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART) Circulation. 2010;122:52–61. doi: 10.1161/CIRCULATIONAHA.109.903955. [DOI] [PubMed] [Google Scholar]
- 49.Ting H.H., Tahirkheli N.K., Berger P.B., McCarthy J.T., Timimi F.K., Mathew V., Rihal C.S., Hasdai D., Holmes D.R., Jr. Evaluation of long-term survival after successful percutaneous coronary intervention among patients with chronic renal failure. Am. J. Cardiol. 2001;87:630–633. doi: 10.1016/S0002-9149(00)01442-9. [DOI] [PubMed] [Google Scholar]
- 50.Arinze N.V., Yin W., Lotfollahzadeh S., Napoleon M.A., Richards S., Walker J.A., Belghasem M., Ravid J.D., Kamel M.H., Whelan S.A., et al. Tryptophan metabolites suppress Wnt pathway and promote adverse limb events in CKD patients. J. Clin. Investig. 2022;132:e142260. doi: 10.1172/JCI142260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muteliefu G., Enomoto A., Jiang P., Takahashi M., Niwa T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol. Dial. Transplant. 2009;24:2051–2058. doi: 10.1093/ndt/gfn757. [DOI] [PubMed] [Google Scholar]
- 52.Dou L., Sallée M., Cerini C., Poitevin S., Gondouin B., Jourde-Chiche N., Fallague K., Brunet P., Calaf R., Dussol B., et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 2015;26:876–887. doi: 10.1681/ASN.2013121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe H., Miyamoto Y., Enoki Y., Ishima Y., Kadowaki D., Kotani S., Nakajima M., Tanaka M., Matsushita K., Mori Y., et al. p-Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol. Res. Perspect. 2015;3:e00092. doi: 10.1002/prp2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Marco G.S., Hausberg M., Hillebrand U., Rustemeyer P., Wittkowski W., Lang D., Pavenstadt H. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am. J. Physiol. Ren. Physiol. 2008;294:F1381–F1387. doi: 10.1152/ajprenal.00003.2008. [DOI] [PubMed] [Google Scholar]
- 55.D’Apolito M., Colia A.L., Manca E., Pettoello-Mantovani M., Sacco M., Maffione A.B., Brownlee M., Giardino I. Urea memory: Transient cell exposure to urea causes persistent mitochondrial ros production and endothelial dysfunction. Toxins. 2018;10:410. doi: 10.3390/toxins10100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Apolito M., Colia A.L., Lasalvia M., Capozzi V., Falcone M.P., Pettoello-Mantovani M., Brownlee M., Maffione A.B., Giardino I. Urea-induced ROS accelerate senescence in endothelial progenitor cells. Atherosclerosis. 2017;263:127–136. doi: 10.1016/j.atherosclerosis.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 57.D’Apolito M., Du X., Pisanelli D., Pettoello-Mantovani M., Campanozzi A., Giacco F., Maffione A.B., Colia A.L., Brownlee M., Giardino I. Urea-induced ROS cause endothelial dysfunction in chronic renal failure. Atherosclerosis. 2015;239:393–400. doi: 10.1016/j.atherosclerosis.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schepers E., Meert N., Glorieux G., Goeman J., Van der Eycken J., Vanholder R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol. Dial. Transplant. 2007;22:592–596. doi: 10.1093/ndt/gfl584. [DOI] [PubMed] [Google Scholar]
- 59.Itoh Y., Ezawa A., Kikuchi K., Tsuruta Y., Niwa T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem. 2012;403:1841–1850. doi: 10.1007/s00216-012-5929-3. [DOI] [PubMed] [Google Scholar]
- 60.Huang M., Wei R., Wang Y., Su T., Li P., Chen X. The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox Biol. 2018;16:303–313. doi: 10.1016/j.redox.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shang F., Wang S.C., Hsu C.Y., Miao Y., Martin M., Yin Y., Wu C.C., Wang Y.T., Wu G., Chien S., et al. MicroRNA-92a mediates endothelial dysfunction in CKD. J. Am. Soc. Nephrol. 2017;28:3251–3261. doi: 10.1681/ASN.2016111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tumur Z., Shimizu H., Enomoto A., Miyazaki H., Niwa T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kB activation. Am. J. Nephrol. 2010;31:435–441. doi: 10.1159/000299798. [DOI] [PubMed] [Google Scholar]
- 63.Ito S., Osaka M., Higuchi Y., Nishijima F., Ishii H., Yoshida M. Indoxyl sulfate induces leukocyte-endothelial interactions through up-regulation of E-selectin. J. Biol. Chem. 2010;285:38869–38875. doi: 10.1074/jbc.M110.166686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto H., Tsuruoka S., Ioka T., Ando H., Ito C., Akimoto T., Fujimura A., Asano Y., Kusano E. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006;69:1780–1785. doi: 10.1038/sj.ki.5000340. [DOI] [PubMed] [Google Scholar]
- 65.Gross P., Massy Z.A., Henaut L., Boudot C., Cagnard J., March C., Kamel S., Drueke T.B., Six I. Para-cresyl sulfate acutely impairs vascular reactivity and induces vascular remodeling. J. Cell. Physiol. 2015;230:2927–2935. doi: 10.1002/jcp.25018. [DOI] [PubMed] [Google Scholar]
- 66.Meijers B.K., Verbeke K., Dehaen W., Vanrenterghem Y., Hoylaerts M.F., Evenepoel P., Evenepoel P. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am. J. Kidney Dis. 2009;54:891–901. doi: 10.1053/j.ajkd.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 67.Dou L., Bertrand E., Cerini C., Faure V., Sampol J., Vanholder R., Berland Y., Brunet P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 68.Rahabi-Layachi H., Ourouda R., Boullier A., Massy Z.A., Amant C. Distinct effects of inorganic phosphate on cell cycle and apoptosis in human vascular smooth muscle cells. J. Cell. Physiol. 2015;230:347–355. doi: 10.1002/jcp.24715. [DOI] [PubMed] [Google Scholar]
- 69.Di Marco G.S., König M., Stock C., Wiesinger A., Hillebrand U., Reiermann S., Reuter S., Amler S., Köhler G., Buck F., et al. High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int. 2013;83:213–222. doi: 10.1038/ki.2012.300. [DOI] [PubMed] [Google Scholar]
- 70.Six I., Maizel J., Barreto F.C., Rangrez A.Y., Dupont S., Slama M., Tribouilloy C., Choukroun G., Maziere J.C., Bode-Boeger S., et al. Effects of phosphate on vascular function under normal conditions and influence of the uraemic state. Cardiovasc. Res. 2012;96:130–139. doi: 10.1093/cvr/cvs240. [DOI] [PubMed] [Google Scholar]
- 71.Shigematsu T., Kono T., Satoh K., Yokoyama K., Yoshida T., Hosoya T., Shirai K. Phosphate overload accelerates vascular calcium deposition in end-stage renal disease patients. Nephrol. Dial. Transplant. 2003;18:iii86–iii89. doi: 10.1093/ndt/gfg1022. [DOI] [PubMed] [Google Scholar]
- 72.Lee H.W., Nizet V., An J.N., Lee H.S., Song Y.R., Kim S.G., Kim J.K. Uremic serum damages endothelium by provoking excessive neutrophil extracellular trap formation. Sci. Rep. 2021;11:593. doi: 10.1038/s41598-021-00863-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maciel R.A., Cunha R.S., Busato V., Franco C.R., Gregório P.C., Dolenga C.J., Nakao L.S., Massy Z.A., Boullier A., Pecoits-Filho R., et al. Uremia impacts VE-cadherin and ZO-1 expression in human endothelial cell-to-cell junctions. Toxins. 2018;10:404. doi: 10.3390/toxins10100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trécherel E., Godin C., Louandre C., Benchitrit J., Poirot S., Mazière J.C., Massy Z.A., Galmiche A. Upregulation of BAD, a pro-apoptotic protein of the BCL2 family, in vascular smooth muscle cells exposed to uremic conditions. Biochem. Biophys. Res. Commun. 2012;417:479–483. doi: 10.1016/j.bbrc.2011.11.144. [DOI] [PubMed] [Google Scholar]
- 75.Sun J.T., Yang K., Lu L., Zhu Z.B., Zhu J.Z., Ni J.W., Han H., Chen N., Zhang R.Y. Increased carbamylation level of HDL in end-stage renal disease: Carbamylated-HDL attenuated endothelial cell function. Am. J.-Physiol. Ren. Physiol. 2016;310:F511–F517. doi: 10.1152/ajprenal.00508.2015. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X., Li Y., Yang P., Liu X., Lu L., Chen Y., Zhong X., Li Z., Liu H., Ou C., et al. Trimethylamine-N-Oxide promotes vascular calcification through activation of NLRP3 (nucleotide-binding domain, Leucine-Rich-Containing family, pyrin Domain-Containing-3) inflammasome and NF-κB (nuclear factor κB) signals. Arterioscler. Thromb. Vasc. Biol. 2020;40:751–765. doi: 10.1161/ATVBAHA.119.313414. [DOI] [PubMed] [Google Scholar]
- 77.Ma G., Pan B., Chen Y., Guo C., Zhao M., Zheng L., Chen B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. [(accessed on 18 March 2022)];Biosci. Rep. 2017 37 doi: 10.1042/BSR20160244. Available online: https://pdfs.semanticscholar.org/8ce6/2000e849c3249c7791eb0006a8cadc71d7dd.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boini K.M., Hussain T., Li P.L., Koka S.S. Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell. Physiol. Biochem. 2017;44:152–162. doi: 10.1159/000484623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun X., Jiao X., Ma Y., Liu Y., Zhang L., He Y., Chen Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016;481:63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 80.He X., Jiang H., Gao F., Liang S., Wei M., Chen L. Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF-κB signaling pathway. Microsc. Res. Tech. 2019;82:2000–2006. doi: 10.1002/jemt.23369. [DOI] [PubMed] [Google Scholar]
- 81.Chen J., Zhang X., Zhang H., Liu T., Zhang H., Teng J., Ji J., Ding X. Indoxyl sulfate enhance the hypermethylation of klotho and promote the process of vascular calcification in chronic kidney disease. Int. J. Biol. Sci. 2016;12:1236. doi: 10.7150/ijbs.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bouabdallah J., Zibara K., Issa H., Lenglet G., Kchour G., Caus T., Six I., Choukroun G., Kamel S., Bennis Y. Endothelial cells exposed to phosphate and indoxyl sulphate promote vascular calcification through interleukin-8 secretion. Nephrol. Dial. Transplant. 2019;34:1125–1134. doi: 10.1093/ndt/gfy325. [DOI] [PubMed] [Google Scholar]
- 83.Jover E., Silvente A., Marin F., Martinez-Gonzalez J., Orriols M., Martinez C.M., Puche C.M., Valdés M., Rodriguez C., Hernández-Romero D. Inhibition of enzymes involved in collagen cross-linking reduces vascular smooth muscle cell calcification. FASEB J. 2018;32:4459–4469. doi: 10.1096/fj.201700653R. [DOI] [PubMed] [Google Scholar]
- 84.Zhang D., Bi X., Liu Y., Huang Y., Xiong J., Xu X., Xiao T., Yu Y., Jiang W., Huang Y., et al. High phosphate-induced calcification of vascular smooth muscle cells is associated with the TLR4/NF-κb signaling pathway. Kidney Blood Press. Res. 2017;42:1205–1215. doi: 10.1159/000485874. [DOI] [PubMed] [Google Scholar]
- 85.Alesutan I., Voelkl J., Feger M., Kratschmar D.V., Castor T., Mia S., Sacherer M., Viereck R., Borst O., Leibrock C., et al. Involvement of vascular aldosterone synthase in phosphate-induced osteogenic transformation of vascular smooth muscle cells. Sci. Rep. 2017;7:2059. doi: 10.1038/s41598-017-01882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.M’Baya-Moutoula E., Louvet L., Metzinger-Le Meuth V., Massy Z.A., Metzinger L. High inorganic phosphate concentration inhibits osteoclastogenesis by modulating miR-223. Biochim. Biophys. Acta-(Bba)-Mol. Basis Dis. 2015;1852:2202–2212. doi: 10.1016/j.bbadis.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Ciceri P., Elli F., Cappelletti L., Tosi D., Braidotti P., Bulfamante G., Cozzolino M. A new in vitro model to delay high phosphate-induced vascular calcification progression. Mol. Cell. Biochem. 2015;410:197–206. doi: 10.1007/s11010-015-2552-6. [DOI] [PubMed] [Google Scholar]
- 88.Guerrero F., Montes de Oca A., Aguilera-Tejero E., Zafra R., Rodríguez M., López I. The effect of vitamin D derivatives on vascular calcification associated with inflammation. Nephrol. Dial. Transplant. 2012;27:2206–2212. doi: 10.1093/ndt/gfr555. [DOI] [PubMed] [Google Scholar]
- 89.Shroff R.C., McNair R., Skepper J.N., Figg N., Schurgers L.J., Deanfield J., Rees L., Shanahan C.M. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J. Am. Soc. Nephrol. 2010;21:103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang W.H., Wang C.P., Chung F.M., Huang L.L., Yu T.H., Hung W.C., Lu L.F., Chen P.Y., Luo C.H., Lee K.T., et al. Uremic retention solute indoxyl sulfate level is associated with prolonged QTc interval in early CKD patients. PLoS ONE. 2015;10:e0119545. doi: 10.1371/journal.pone.0119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lekawanvijit S., Adrahtas A., Kelly D.J., Kompa A.R., Wang B.H., Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Heart J. 2010;31:1771–1779. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 92.Liu S., Wang B.H., Kompa A.R., Lekawanvijit S., Krum H. Antagonists of organic anion transporters 1 and 3 ameliorate adverse cardiac remodelling induced by uremic toxin indoxyl sulfate. Int. J. Cardiol. 2012;158:457–458. doi: 10.1016/j.ijcard.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 93.Lano G., Laforêt M., Von Kotze C., Perrin J., Addi T., Brunet P., Poitevin S., Burtey S., Dou L. Aryl hydrocarbon receptor activation and tissue factor induction by fluid shear stress and indoxyl sulfate in endothelial cells. Int. J. Mol. Sci. 2020;21:2392. doi: 10.3390/ijms21072392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chitalia V.C., Shivanna S., Martorell J., Balcells M., Bosch I., Kolandaivelu K., Edelman E.R. Uremic serum and solutes increase post–vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation. 2013;127:365–376. doi: 10.1161/CIRCULATIONAHA.112.118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gondouin B., Cerini C., Dou L., Sallée M., Duval-Sabatier A., Pletinck A., Calaf R., Lacroix R., Jourde-Chiche N., Poitevin S., et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013;84:733–744. doi: 10.1038/ki.2013.133. [DOI] [PubMed] [Google Scholar]
- 96.Gao C., Ji S., Dong W., Qi Y., Song W., Cui D., Shi J. Indolic uremic solutes enhance procoagulant activity of red blood cells through phosphatidylserine exposure and microparticle release. Toxins. 2015;7:4390–4403. doi: 10.3390/toxins7114390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagy E., Liu Y., Prentice K.J., Sloop K.W., Sanders P.E., Batchuluun B., Hammond C.D., Wheeler M.B., Durham T.B. Synthesis and characterization of urofuranoic acids: In vivo metabolism of 2-(2-Carboxyethyl)-4-methyl-5-propylfuran-3-carboxylic acid (CMPF) and effects on in vitro insulin secretion. J. Med. Chem. 2017;60:1860–1875. doi: 10.1021/acs.jmedchem.6b01668. [DOI] [PubMed] [Google Scholar]
- 98.D’Apolito M., Du X., Zong H., Catucci A., Maiuri L., Trivisano T., Pettoello-Mantovani M., Campanozzi A., Raia V., Pessin J.E., et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Investig. 2010;120:203–213. doi: 10.1172/JCI37672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cohen G., Glorieux G., Thornalley P., Schepers E., Meert N., Jankowski J., Jankowski V., Argiles A., Anderstam B., Brunet P., et al. Review on uraemic toxins III: Recommendations for handling uraemic retention solutes in vitro—Towards a standardized approach for research on uraemia. Nephrol. Dial. Transplant. 2007;22:3381–3390. doi: 10.1093/ndt/gfm210. [DOI] [PubMed] [Google Scholar]
- 100.Massy Z.A., Drueke T.B. Role of uremic toxins in vascular disease-the end of nihilism? Kidney Int. 2022;22:S0085–S2538. doi: 10.1016/j.kint.2022.01.024. [DOI] [PubMed] [Google Scholar]
- 101.Hung S.C., Kuo K.L., Huang H.L., Lin C.C., Tsai T.H., Wang C.H., Chen J.W., Lin S.J., Huang P.H., Tarng D.C. Indoxyl sulfate suppresses endothelial progenitor cell–mediated neovascularization. Kidney Int. 2016;89:574–585. doi: 10.1016/j.kint.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 102.Matsumoto T., Kojima M., Takayanagi K., Taguchi K., Kobayashi T. Trimethylamine-N-oxide specifically impairs endothelium-derived hyperpolarizing factor-type relaxation in rat femoral artery. Biol. Pharm. Bull. 2020;43:569–573. doi: 10.1248/bpb.b19-00957. [DOI] [PubMed] [Google Scholar]
- 103.Han H., Chen Y., Zhu Z., Su X., Ni J., Du R., Zhang R., Jin W. p-Cresyl sulfate promotes the formation of atherosclerotic lesions and induces plaque instability by targeting vascular smooth muscle cells. Front. Med. 2016;10:320–329. doi: 10.1007/s11684-016-0463-x. [DOI] [PubMed] [Google Scholar]
- 104.Geng J., Yang C., Wang B., Zhang X., Hu T., Gu Y., Li J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed. Pharmacother. 2018;97:941–947. doi: 10.1016/j.biopha.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 105.Seldin M.M., Meng Y., Qi H., Zhu W., Wang Z., Hazen S.L., Lusis A.J., Shih D.M. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 2016;5:e002767. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Massy Z.A., Ivanovski O., Nguyen-Khoa T., Angulo J., Szumilak D., Mothu N., Phan O., Daudon M., Lacour B., Drüeke T.B., et al. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J. Am. Soc. Nephrol. 2005;16:109–116. doi: 10.1681/ASN.2004060495. [DOI] [PubMed] [Google Scholar]
- 108.Kuo K.L., Zhao J.F., Huang P.H., Guo B.C., Tarng D.C., Lee T.S. Indoxyl sulfate impairs valsartan-induced neovascularization. Redox Biol. 2020;30:101433. doi: 10.1016/j.redox.2020.101433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Opdebeeck B., Maudsley S., Azmi A., De Maré A., De Leger W., Meijers B., Verhulst A., Evenepoel P., D’Haese P.C., Neven E. Indoxyl sulfate and p-cresyl sulfate promote vascular calcification and associate with glucose intolerance. J. Am. Soc. Nephrol. 2019;30:751–766. doi: 10.1681/ASN.2018060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Muteliefu G., Shimizu H., Enomoto A., Nishijima F., Takahashi M., Niwa T. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation of p53, p21, and prelamin A through oxidative stress. Am. J. Physiol.-Cell Physiol. 2012;303:C126–C134. doi: 10.1152/ajpcell.00329.2011. [DOI] [PubMed] [Google Scholar]
- 111.Adijiang A., Higuchi Y., Nishijima F., Shimizu H., Niwa T. Indoxyl sulfate, a uremic toxin, promotes cell senescence in aorta of hypertensive rats. Biochem. Biophys. Res. Commun. 2010;399:637–641. doi: 10.1016/j.bbrc.2010.07.130. [DOI] [PubMed] [Google Scholar]
- 112.Adijiang A., Goto S., Uramoto S., Nishijima F., Niwa T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol. Dial. Transplant. 2008;23:1892–1901. doi: 10.1093/ndt/gfm861. [DOI] [PubMed] [Google Scholar]
- 113.Crouthamel M.H., Lau W.L., Leaf E.M., Chavkin N.W., Wallingford M.C., Peterson D.F., Li X., Liu Y., Chin M.T., Levi M., et al. Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: Redundant roles for PiT-1 and PiT-2. Arterioscler. Thromb. Vasc. Biol. 2013;33:2625–2632. doi: 10.1161/ATVBAHA.113.302249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lau W.L., Linnes M., Chu E.Y., Foster B.L., Bartley B.A., Somerman M.J., Giachelli C.M. High phosphate feeding promotes mineral and bone abnormalities in mice with chronic kidney disease. Nephrol. Dial. Transplant. 2013;28:62–69. doi: 10.1093/ndt/gfs333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Abbadi M.M., Pai A.S., Leaf E.M., Yang H.Y., Bartley B.A., Quan K.K., Ingalls C.M., Liao H.W., Giachelli C.M. Phosphate feeding induces arterial medial calcification in uremic mice: Role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009;75:1297–1307. doi: 10.1038/ki.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamada S., Tokumoto M., Tatsumoto N., Taniguchi M., Noguchi H., Nakano T., Masutani K., Ooboshi H., Tsuruya K., Kitazono T. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am. J.-Physiol.-Ren. Physiol. 2014;306:F1418–F1428. doi: 10.1152/ajprenal.00633.2013. [DOI] [PubMed] [Google Scholar]
- 117.Graciolli F.G., Neves K.R., dos Reis L.M., Graciolli R.G., Noronha I.L., Moysés R.M., Jorgetti V. Phosphorus overload and PTH induce aortic expression of Runx2 in experimental uraemia. Nephrol. Dial. Transplant. 2009;24:1416–1421. doi: 10.1093/ndt/gfn686. [DOI] [PubMed] [Google Scholar]
- 118.Hosaka N., Mizobuchi M., Ogata H., Kumata C., Kondo F., Koiwa F., Kinugasa E., Akizawa T. Elastin degradation accelerates phosphate-induced mineralization of vascular smooth muscle cells. Calcif. Tissue Int. 2009;85:523–529. doi: 10.1007/s00223-009-9297-8. [DOI] [PubMed] [Google Scholar]
- 119.Yisireyili M., Shimizu H., Saito S., Enomoto A., Nishijima F., Niwa T. Indoxyl sulfate promotes cardiac fibrosis with enhanced oxidative stress in hypertensive rats. Life Sci. 2013;92:1180–1185. doi: 10.1016/j.lfs.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 120.Lekawanvijit S., Kompa A.R., Manabe M., Wang B.H., Langham R.G., Nishijima F., Kelly D.J., Krum H. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS ONE. 2012;7:e41281. doi: 10.1371/journal.pone.0041281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen W.T., Chen Y.C., Hsieh M.H., Huang S.Y., Kao Y.H., Chen Y.A., Lin Y.K., Chen S.A., Chen Y.J. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J. Cardiovasc. Electrophysiol. 2015;26:203–210. doi: 10.1111/jce.12554. [DOI] [PubMed] [Google Scholar]
- 122.Zhu H., Pan L., Dai Y., Zheng D., Cai S. Role of TLR4/MyD88 Signaling Pathway in the Occurrence and Development of Uremia-Induced Myocardial Hypertrophy and Possible Mechanism. Evid.-Based Complement. Altern. Med. 2021;2021:7883643. doi: 10.1155/2021/7883643. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Hu M.C., Shi M., Cho H.J., Adams-Huet B., Paek J., Hill K., Shelton J., Amaral A.P., Faul C., Taniguchi M., et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J. Am. Soc. Nephrol. 2015;26:1290–1302. doi: 10.1681/ASN.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prommer H.U., Maurer J., von Websky K., Freise C., Sommer K., Nasser H., Samapati R., Reglin B., Guimarães P., Pries A.R., et al. Chronic kidney disease induces a systemic microangiopathy, tissue hypoxia and dysfunctional angiogenesis. Sci. Rep. 2018;8:3075. doi: 10.1038/s41598-018-23663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carmona A.A., Rigon B.G.d.S., Barroso M.P.B.S., Hauser A.B., Précoma D., Bucharles S., Noronha L.D., Pécoits-Filho R. Induction of systemic inflammation and thickening of subepicardiac arteries in an animal model of uremia. Braz. J. Nephrol. 2011;33:408–412. doi: 10.1590/S0101-28002011000400004. [DOI] [PubMed] [Google Scholar]
- 126.Han H., Zhu J., Zhu Z., Ni J., Du R., Dai Y., Chen Y., Wu Z., Lu L., Zhang R. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J. Am. Heart Assoc. 2015;4:e001852. doi: 10.1161/JAHA.115.001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang K., Du C., Wang X., Li F., Xu Y., Wang S., Chen S., Chen F., Shen M., Chen M., et al. Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease–associated thrombosis in mice. Blood J. Am. Soc. Hematol. 2017;129:2667–2679. doi: 10.1182/blood-2016-10-744060. [DOI] [PubMed] [Google Scholar]
- 128.Kolachalama V.B., Shashar M., Alousi F., Shivanna S., Rijal K., Belghasem M.E., Walker J., Matsuura S., Chang G.H., Gibson C.M., et al. Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. J. Am. Soc. Nephrol. 2018;29:1063–1072. doi: 10.1681/ASN.2017080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z., Li L., Fu X., Wu Y., Mehrabian M., et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koppe L., Nyam E., Vivot K., Fox J.E.M., Dai X.Q., Nguyen B.N., Trudel D., Attané C., Moullé V.S., MacDonald P.E., et al. Urea impairs β cell glycolysis and insulin secretion in chronic kidney disease. J. Clin. Investig. 2016;126:3598–3612. doi: 10.1172/JCI86181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carracedo J., Buendía P., Merino A., Soriano S., Esquivias E., Martín-Malo A., Aljama P., Ramírez R. Cellular senescence determines endothelial cell damage induced by uremia. Exp. Gerontol. 2013;48:766–773. doi: 10.1016/j.exger.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 132.Li T., Gua C., Wu B., Chen Y. Increased circulating trimethylamine N-oxide contributes to endothelial dysfunction in a rat model of chronic kidney disease. Biochem. Biophys. Res. Commun. 2018;495:2071–2077. doi: 10.1016/j.bbrc.2017.12.069. [DOI] [PubMed] [Google Scholar]
- 133.Koppe L., Alix P.M., Croze M.L., Chambert S., Vanholder R., Glorieux G., Fouque D., Soulage C.O. p-Cresyl glucuronide is a major metabolite of p-cresol in mouse: In contrast to p-cresyl sulphate, p-cresyl glucuronide fails to promote insulin resistance. Nephrol. Dial. Transplant. 2017;32:2000–2009. doi: 10.1093/ndt/gfx089. [DOI] [PubMed] [Google Scholar]
- 134.Koppe L., Pillon N.J., Vella R.E., Croze M.L., Pelletier C.C., Chambert S., Massy Z., Glorieux G., Vanholder R., Dugenet Y., et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J. Am. Soc. Nephrol. 2013;24:88–99. doi: 10.1681/ASN.2012050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fan P.C., Chang J.C.H., Lin C.N., Lee C.C., Chen Y.T., Chu P.H., Kou G., Lu Y.A., Yang C.W., Chen Y.C. Serum indoxyl sulfate predicts adverse cardiovascular events in patients with chronic kidney disease. J. Formos. Med. Assoc. 2019;118:1099–1106. doi: 10.1016/j.jfma.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 136.Konje V.C., Rajendiran T.M., Bellovich K., Gadegbeku C.A., Gipson D.S., Afshinnia F., Mathew A.V., The Michigan Kidney Translational Core CPROBE Investigator Group Tryptophan levels associate with incident cardiovascular disease in chronic kidney disease. Clin. Kidney J. 2021;14:1097–1105. doi: 10.1093/ckj/sfaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu I.W., Hsu K.H., Hsu H.J., Lee C.C., Sun C.Y., Tsai C.J., Wu M.S. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—a prospective cohort study. Nephrol. Dial. Transplant. 2012;27:1169–1175. doi: 10.1093/ndt/gfr453. [DOI] [PubMed] [Google Scholar]
- 138.Liabeuf S., Glorieux G., Lenglet A., Diouf M., Schepers E., Desjardins L., Choukroun G., Vanholder R., Massy Z.A., European Uremic Toxin (EUTox) Work Group Does p-cresylglucuronide have the same impact on mortality as other protein-bound uremic toxins? PLoS ONE. 2013;8:e67168. doi: 10.1371/journal.pone.0067168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McGovern A.P., de Lusignan S., van Vlymen J., Liyanage H., Tomson C.R., Gallagher H., Rafiq M., Jones S. Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: A large community based cohort study. PLos ONE. 2013;8:e74996. doi: 10.1371/journal.pone.0074996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kimata N., Albert J.M., Akiba T., Yamazaki S., Kawaguchi Y., Fukuhara S., Akizawa T., Saito A., Asano Y., Kurokawa K., et al. Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: The Japan dialysis outcomes and practice patterns study. Hemodial. Int. 2007;11:340–348. doi: 10.1111/j.1542-4758.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 141.Slinin Y., Foley R.N., Collins A.J. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J. Am. Soc. Nephrol. 2005;16:1788–1793. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 142.Young E.W., Albert J.M., Satayathum S., Goodkin D.A., Pisoni R.L., Akiba T., Akizawa T., Kurokawa K., Bommer J., Piera L., et al. Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 143.Block G.A., Klassen P.S., Lazarus J.M., Ofsthun N., Lowrie E.G., Chertow G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 144.Shafi T., Powe N.R., Meyer T.W., Hwang S., Hai X., Melamed M.L., Banerjee T., Coresh J., Hostetter T.H. Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J. Am. Soc. Nephrol. 2017;28:321–331. doi: 10.1681/ASN.2016030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Six I., Flissi N., Lenglet G., Louvet L., Kamel S., Gallet M., Massy Z.A., Liabeuf S. Uremic toxins and vascular dysfunction. Toxins. 2020;12:404. doi: 10.3390/toxins12060404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Berg A.H., Drechsler C., Wenger J., Buccafusca R., Hod T., Kalim S., Ramma W., Parikh S.M., Steen H., Friedman D.J., et al. Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Sci. Transl. Med. 2013;5:175ra29. doi: 10.1126/scitranslmed.3005218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen Y., Zelnick L.R., Huber M.P., Wang K., Bansal N., Hoofnagle A.N., Paranji R.K., Heckbert S.R., Weiss N.S., Go A.S., et al. Association Between Kidney Clearance of Secretory Solutes and Cardiovascular Events: The Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2021;78:226–235.e1. doi: 10.1053/j.ajkd.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shafi T., Sirich T.L., Meyer T.W., Hostetter T.H., Plummer N.S., Hwang S., Melamed M.L., Banerjee T., Coresh J., Powe N.R. Results of the HEMO Study suggest that p-cresol sulfate and indoxyl sulfate are not associated with cardiovascular outcomes. Kidney Int. 2017;92:1484–1492. doi: 10.1016/j.kint.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luce M., Bouchara A., Pastural M., Granjon S., Szelag J.C., Laville M., Arkouche W., Fouque D., Soulage C.O., Koppe L. Is 3-Carboxy-4-methyl-5-propyl-2-furanpropionate (CMPF) a Clinically Relevant Uremic Toxin in Haemodialysis Patients? Toxins. 2018;10:205. doi: 10.3390/toxins10050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Menon V., Greene T., Pereira A.A., Wang X., Beck G.J., Kusek J.W., Collins A.J., Levey A.S., Sarnak M.J. Relationship of phosphorus and calcium-phosphorus product with mortality in CKD. Am. J. Kidney Dis. 2005;46:455–463. doi: 10.1053/j.ajkd.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 151.Kaysen G.A., Johansen K.L., Chertow G.M., Dalrymple L.S., Kornak J., Grimes B., Dwyer T., Chassy A.W., Fiehn O. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J. Ren. Nutr. 2015;25:351–356. doi: 10.1053/j.jrn.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin C.J., Pan C.F., Liu H.L., Chuang C.K., Jayakumar T., Wang T.J., Chen H.H., Wu C.J. The role of protein-bound uremic toxins on peripheral artery disease and vascular access failure in patients on hemodialysis. Atherosclerosis. 2012;225:173–179. doi: 10.1016/j.atherosclerosis.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 153.Shafi T., Meyer T.W., Hostetter T.H., Melamed M.L., Parekh R.S., Hwang S., Banerjee T., Coresh J., Powe N.R. Free levels of selected organic solutes and cardiovascular morbidity and mortality in hemodialysis patients: Results from the retained organic solutes and clinical outcomes (ROSCO) investigators. PLoS ONE. 2015;10:e0126048. doi: 10.1371/journal.pone.0126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hsu C.C., Lu Y.C., Chiu C.A., Yu T.H., Hung W.C., Wang C.P., Lu L.F., Chung F.M., Lee Y.J., Tsai I.T. Levels of indoxyl sulfate are associated with severity of coronary atherosclerosis. Clin. Investig. Med. 2013;36:E42–E49. doi: 10.25011/cim.v36i1.19404. [DOI] [PubMed] [Google Scholar]
- 155.Lin C.J., Liu H.L., Pan C.F., Chuang C.K., Jayakumar T., Wang T.J., Chen H.H., Wu C.J. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch. Med. Res. 2012;43:451–456. doi: 10.1016/j.arcmed.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 156.Taki K., Tsuruta Y., Niwa T. Indoxyl sulfate and atherosclerotic risk factors in hemodialysis patients. Am. J. Nephrol. 2007;27:30–35. doi: 10.1159/000098542. [DOI] [PubMed] [Google Scholar]
- 157.Lin C.J., Wu C.J., Pan C.F., Chen Y.C., Sun F.J., Chen H.H. Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol. Dial. Transplant. 2010;25:3693–3700. doi: 10.1093/ndt/gfq251. [DOI] [PubMed] [Google Scholar]
- 158.Poesen R., Evenepoel P., de Loor H., Kuypers D., Augustijns P., Meijers B. Metabolism, protein binding, and renal clearance of microbiota–derived p-cresol in patients with CKD. Clin. J. Am. Soc. Nephrol. 2016;11:1136–1144. doi: 10.2215/CJN.00160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Poesen R., Claes K., Evenepoel P., de Loor H., Augustijns P., Kuypers D., Meijers B. Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J. Am. Soc. Nephrol. 2016;27:3479–3487. doi: 10.1681/ASN.2015121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang C.P., Lu L.F., Yu T.H., Hung W.C., Chiu C.A., Chung F.M., Yeh L.R., Chen H.J., Lee Y.J., Houng J.Y. Serum levels of total p-cresylsulphate are associated with angiographic coronary atherosclerosis severity in stable angina patients with early stage of renal failure. Atherosclerosis. 2010;211:579–583. doi: 10.1016/j.atherosclerosis.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 161.Nakamura S., Sasaki O., Nakahama H., Inenaga T., Kawano Y. Clinical characteristics and survival in end-stage renal disease patients with arteriosclerosis obliterans. Am. J. Nephrol. 2002;22:422–428. doi: 10.1159/000065267. [DOI] [PubMed] [Google Scholar]
- 162.Merhi B., Shireman T., Carpenter M.A., Kusek J.W., Jacques P., Pfeffer M., Rao M., Foster M.C., Kim S.J., Pesavento T.E., et al. Serum phosphorus and risk of cardiovascular disease, all-cause mortality, or graft failure in kidney transplant recipients: An ancillary study of the FAVORIT trial cohort. Am. J. Kidney Dis. 2017;70:377–385. doi: 10.1053/j.ajkd.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eddington H., Hoefield R., Sinha S., Chrysochou C., Lane B., Foley R.N., Hegarty J., New J., O’Donoghue D.J., Middleton R.J., et al. Serum phosphate and mortality in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010;5:2251–2257. doi: 10.2215/CJN.00810110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kestenbaum B., Sampson J.N., Rudser K.D., Patterson D.J., Seliger S.L., Young B., Sherrard D.J., Andress D.L. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 165.Stubbs J.R., House J.A., Ocque A.J., Zhang S., Johnson C., Kimber C., Schmidt K., Gupta A., Wetmore J.B., Nolin T.D., et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. 2016;27:305–313. doi: 10.1681/ASN.2014111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim R.B., Morse B.L., Djurdjev O., Tang M., Muirhead N., Barrett B., Holmes D.T., Madore F., Clase C.M., Rigatto C., et al. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89:1144–1152. doi: 10.1016/j.kint.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 167.Melamed M.L., Plantinga L., Shafi T., Parekh R., Meyer T.W., Hostetter T.H., Coresh J., Powe N.R. Retained organic solutes, patient characteristics and all-cause and cardiovascular mortality in hemodialysis: Results from the retained organic solutes and clinical outcomes (ROSCO) investigators. BMC Nephrol. 2013;14:2579. doi: 10.1186/1471-2369-14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chinnappa S., Tu Y.K., Yeh Y.C., Glorieux G., Vanholder R., Mooney A. Association between protein-bound uremic toxins and asymptomatic cardiac dysfunction in patients with chronic kidney disease. Toxins. 2018;10:520. doi: 10.3390/toxins10120520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao X.S., Chen J., Zou J.Z., Zhong Y.H., Teng J., Ji J., Chen Z.W., Liu Z.H., Shen B., Nie Y.X., et al. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2015;10:111–119. doi: 10.2215/CJN.04730514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shimazu S., Hirashiki A., Okumura T., Yamada T., Okamoto R., Shinoda N., Takeshita K., Kondo T., Niwa T., Murohara T. Association between indoxyl sulfate and cardiac dysfunction and prognosis in patients with dilated cardiomyopathy. Circ. J. 2013;77:390–396. doi: 10.1253/circj.CJ-12-0715. [DOI] [PubMed] [Google Scholar]
- 171.Sato B., Yoshikawa D., Ishii H., Suzuki S., Inoue Y., Takeshita K., Tanaka M., Kumagai S., Matsumoto M., Okumura S., et al. Relation of plasma indoxyl sulfate levels and estimated glomerular filtration rate to left ventricular diastolic dysfunction. Am. J. Cardiol. 2013;111:712–716. doi: 10.1016/j.amjcard.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 172.Barreto F.C., Barreto D.V., Liabeuf S., Meert N., Glorieux G., Temmar M., Choukroun G., Vanholder R., Massy Z.A., European Uremic Toxin Work Group (EUTox) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zapolski T., Kamińska A., Kocki T., Wysokiński A., Urbanska E.M. Aortic stiffness—Is kynurenic acid a novel marker? Cross-sectional study in patients with persistent atrial fibrillation. PLoS ONE. 2020;15:e0236413. doi: 10.1371/journal.pone.0236413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pawlak K., Myśliwiec M., Pawlak D. Kynurenine pathway–a new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv. Med. Sci. 2010;55:196–203. doi: 10.2478/v10039-010-0015-6. [DOI] [PubMed] [Google Scholar]
- 175.Liabeuf S., Barreto D.V., Barreto F.C., Meert N., Glorieux G., Schepers E., Temmar M., Choukroun G., Vanholder R., Massy Z.A., et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010;25:1183–1191. doi: 10.1093/ndt/gfp592. [DOI] [PubMed] [Google Scholar]
- 176.Yu T.H., Tang W.H., Lu Y.C., Wang C.P., Hung W.C., Wu C.C., Tsai I.T., Chung F.M., Houng J.Y., Lan W.C., et al. Association between hippuric acid and left ventricular hypertrophy in maintenance hemodialysis patients. Clin. Chim. Acta. 2018;484:47–51. doi: 10.1016/j.cca.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 177.Petchey W.G., Hawley C.M., Johnson D.W., Haluska B.A., Watkins T.W., Isbel N.M. Multimodality vascular imaging in CKD: Divergence of risk between measured parameters. Nephrol. Dial. Transplant. 2012;27:1004–1012. doi: 10.1093/ndt/gfr397. [DOI] [PubMed] [Google Scholar]
- 178.Adeney K.L., Siscovick D.S., Ix J.H., Seliger S.L., Shlipak M.G., Jenny N.S., Kestenbaum B.R. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J. Am. Soc. Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ketteler M., Bongartz P., Westenfeld R., Wildberger J.E., Mahnken A.H., Böhm R., Metzger T., Wanner C., Jahnen-Dechent W., Floege J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: A cross-sectional study. Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 180.Ix J.H., De Boer I.H., Peralta C.A., Adeney K.L., Duprez D.A., Jenny N.S., Siscovick D.S., Kestenbaum B.R. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin. J. Am. Soc. Nephrol. 2009;4:609–615. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Drechsler C., Kalim S., Wenger J.B., Suntharalingam P., Hod T., Thadhani R.I., Karumanchi S.A., Wanner C., Berg A.H. Protein carbamylation is associated with heart failure and mortality in diabetic patients with end-stage renal disease. Kidney Int. 2015;87:1201–1208. doi: 10.1038/ki.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Glorieux G., Vanholder R., Van Biesen W., Pletinck A., Schepers E., Neirynck N., Speeckaert M., De Bacquer D., Verbeke F. Free p-cresyl sulfate shows the highest association with cardiovascular outcome in chronic kidney disease. Nephrol. Dial. Transplant. 2021;36:998–1005. doi: 10.1093/ndt/gfab004. [DOI] [PubMed] [Google Scholar]
- 183.Scholz A., Plate K.H., Reiss Y. Angiopoietin-2: A multifaceted cytokine that functions in both angiogenesis and inflammation. Ann. N. Y. Acad. Sci. 2015;1347:45–51. doi: 10.1111/nyas.12726. [DOI] [PubMed] [Google Scholar]
- 184.Wang C.H., Lai Y.H., Kuo C.H., Lin Y.L., Tsai J.P., Hsu B.G. Association between serum indoxyl sulfate levels and endothelial function in non-dialysis chronic kidney disease. Toxins. 2019;11:589. doi: 10.3390/toxins11100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jourde-Chiche N., Dou L., Sabatier F., Calaf R., Cerini C., Robert S., Camoin-Jau L., Charpiot P., Argiles A., Dignat-George F., et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J. Thromb. Haemost. 2009;7:1576–1584. doi: 10.1111/j.1538-7836.2009.03540.x. [DOI] [PubMed] [Google Scholar]
- 186.Pawlak K., Domaniewski T., Mysliwiec M., Pawlak D. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis. 2009;204:309–314. doi: 10.1016/j.atherosclerosis.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 187.Pawlak K., Mysliwiec M., Pawlak D. Haemostatic system, biochemical profiles, kynurenines and the prevalence of cardiovascular disease in peritoneally dialyzed patients. Thromb. Res. 2010;125:e40–e45. doi: 10.1016/j.thromres.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 188.Pawlak K., Domaniewski T., Mysliwiec M., Pawlak D. Kynurenines and oxidative status are independently associated with thrombomodulin and von Willebrand factor levels in patients with end-stage renal disease. Thromb. Res. 2009;124:452–457. doi: 10.1016/j.thromres.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 189.Pawlak K., Mysliwiec M., Pawlak D. Hypercoagulability is independently associated with kynurenine pathway activation in dialysed uraemic patients. Thromb. Haemost. 2009;102:49–55. doi: 10.1160/TH08-10-0696. [DOI] [PubMed] [Google Scholar]
- 190.Wu C.C., Hsieh M.Y., Hung S.C., Kuo K.L., Tsai T.H., Lai C.L., Chen J.W., Lin S.J., Huang P.H., Tarng D.C. Serum indoxyl sulfate associates with postangioplasty thrombosis of dialysis grafts. J. Am. Soc. Nephrol. 2016;27:1254–1264. doi: 10.1681/ASN.2015010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Laville S.M., Massy Z.A., Kamel S., Chillon J.M., Choukroun G., Liabeuf S. Intestinal Chelators, Sorbants, and Gut-Derived Uremic Toxins. Toxins. 2021;13:91. doi: 10.3390/toxins13020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Loftus T.J., Filiberto A.C., Ozrazgat-Baslanti T., Gopal S., Bihorac A. Cardiovascular and renal disease in chronic critical illness. J. Clin. Med. 2021;10:1601. doi: 10.3390/jcm10081601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.