Abstract
Objective
To describe the unique case history of a patient with mGluR1 antibodies, with mainly limbic and without cerebellar symptoms.
Methods
A 50-year-old woman initially presented with focal seizures with epigastric rising and déjà-vu sensations, next to cognitive complaints, and musical auditory hallucinations. MRI, EEG, and neuronal autoantibody tests were performed.
Results
EEG findings showed slow and sharp activity (sharp waves and sharp-wave–slow-wave complex) in the left temporal lobe. A test for autoantibodies was negative initially. Because of persistent symptoms, serum and CSF were tested 4 years later and found positive for mGluR1 antibodies. Treatment started with monthly IV immunoglobulins and azathioprine that was replaced by mycophenolate mofetil later. Especially cognitive symptoms and hallucinations did not respond well to the treatment. During treatment, mGluR1 antibodies remained present in CSF.
Discussion
Whereas cerebellar symptoms are present in 97% of mGluR1-positive cases, our patient presented without ataxia. Therefore, we suggest that the clinical presentation of patients with mGluR1 antibodies is probably more diverse than previously described. Testing for mGluR1 antibodies should be considered in patients with limbic encephalitis and epilepsy, especially when negative for more common antibodies.
A 50-year-old woman, with a history of vasovagal syncope, cardiac ablation, and migraine, presented with an episode of acute distress and confusion, screaming and crying, followed by stuttering speech and vomiting. The patient was unable to recall this incident.
For several weeks, she had experienced a mild headache and episodes of musical hallucinations, lasting for hours. Moreover, the patient experienced multiple episodes of epigastric rising and déjà-vu sensations. There were no abnormalities in the physical and neurologic examinations and in routine blood tests and the ECG. An MRI examination of the brain showed 2 nonrecent vascular lesions, one in the vermis (Figure 1) and the other in the right frontal cortex. An EEG showed slow and sharp activity (sharp waves and sharp-wave–slow-wave complex) in the left temporal lobe (Figure 2). Symptoms and findings were consistent with focal epilepsy, with focal seizures with a sensory onset and intact awareness and one focal seizure with impaired awareness. After these findings, the patient started using lamotrigine and later, clobazam. In the etiologic workup, the CSF recurrently showed slight pleocytosis (6–10 leucocytes/µL), 100% mononuclear, and unique oligoclonal IgG bands. However, anti-GAD65 was absent in serum by ELISA (RSR Limited, Cardiff, UK).
Figure 1. MRI of the Brain.
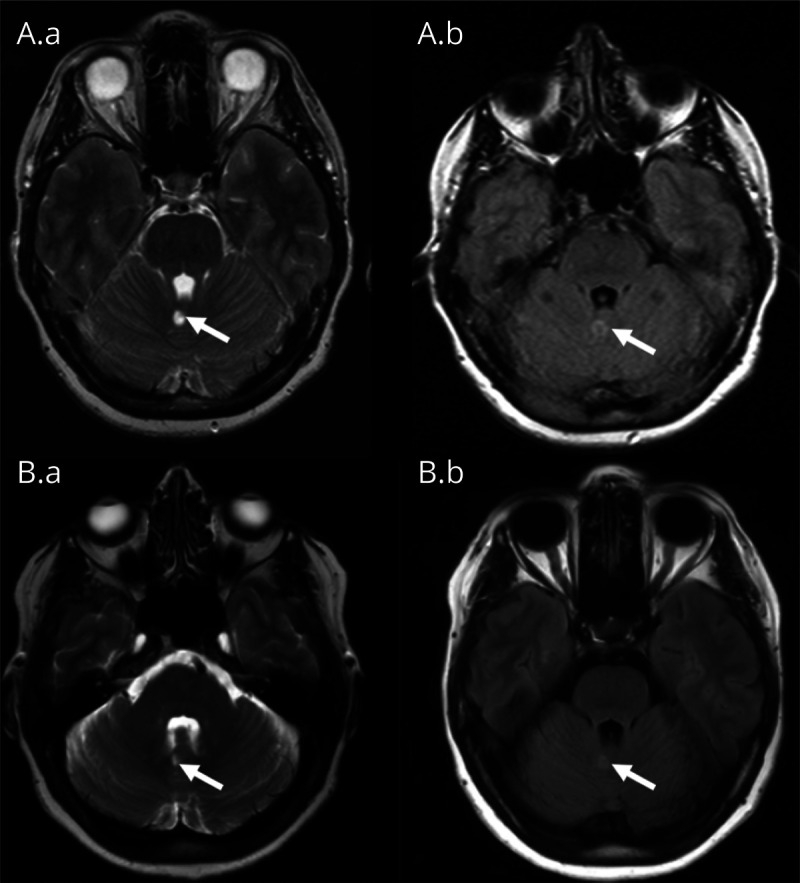
(A.a-b) MRI of the brain (A.a: axial T2-weighted image; A.b: axial fluid-attenuated inversion recovery [FLAIR] image) shortly after the first presentation. The arrow shows the spot of increased signal intensity in the cerebellar vermis caused by vascular damage. (B.a-b) An MRI examination of the brain performed recently (4 years after symptom onset) shows no progression of the cerebellar lesion or any other abnormal findings.
Figure 2. Electro-Encephalogram.
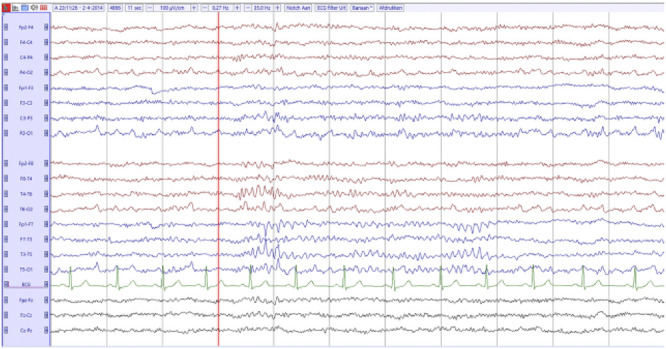
The electro-encephalogram (EEG) 6 months after initial presentation. It shows a normal background pattern, with a focal abnormality (sometimes with epileptiform features) in the left (more than in the right) temporal lobe. These abnormalities did not have a clinical correlate.
Tapering off antiepileptic drugs resulted in recurring focal seizures with sensory onset, 18 months after the first presentation. Then, she was treated with valproic acid with good response. However, no treatment reduced her auditory hallucinations. Furthermore, she experienced cognitive problems since the onset of symptoms. Formal neuropsychological testing showed reduced processing speed and a slight degree of word-finding difficulties, without prominent aphasia.
Because of the unclear etiology, we retested the serum taken initially, 48 months after the first test for autoantibodies, using immunohistochemistry (IHC),1 which resulted in staining with mGluR1 pattern (Figure 3A).2 We confirmed this with a cell-based assay (CBA) (Figure 3B).2 Newly collected CSF was tested and proved strongly positive for anti-mGluR1. The serum showed negative results, which suggested intrathecal production of anti-mGluR1 antibodies. Serum and CSF samples, tested for other neuronal autoantibodies, were all negative (including in-house CBA for GAD65/67, mGluR5, and a commercial CBA testing kit: autoimmune encephalitis Mosaic 1, Euroimmun, Lübeck, Germany).
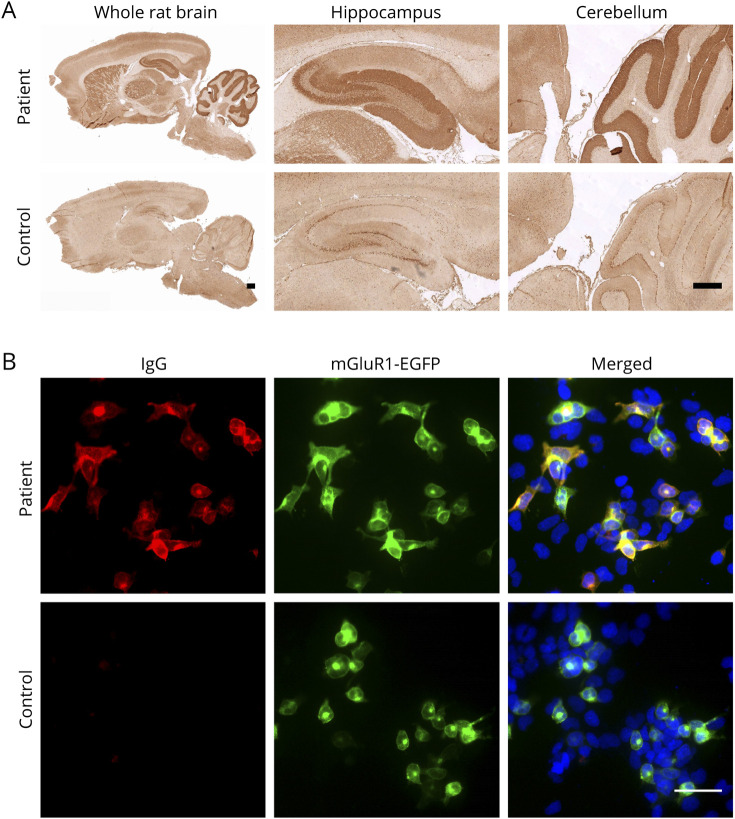
Figure 3. Immunohistochemistry on Rat Brain and Live Neuron Stainings.
(A) Tissue-based assay: serum from the patient showed strong reactivity on rat brain compared with that from a negative control. The reactivity was mainly in the CA3 area and dentate gyrus area of hippocampus and the molecular layer of cerebellum. Scale bar = 500 μm. (B) Cell-based assay: Serum from this patient showed clear staining on transfected cells compared with serum from a healthy individual, which did not show any IgG staining to the transfected cells. MGluR1-enhanced green fluorescent protein (EGFP) transfected human embryonic kidney (HEK) cells were used for the staining (green). IgG autoantibodies were stained with goat anti-human IgG 594 (red, #109-546-170, Jackson), and nuclei were stained with 4',6-diamidine-2'-phenylindole dihydrochloride (DAPI) (blue). Scale bar = 50 μm.
After the exclusion of a primary tumor causing the antibody production by total body CT/PET scan, the patient was treated with IV immunoglobulin (IVIG) (a monthly recurring course of 0.4 mg/kg/d IVIG for 5 consecutive days, for 5 months, in a tapering schedule). Maintenance therapy was started with azathioprine, which was later replaced by mycophenolate mofetil because of an increase in hallucinations. During therapy, the patient experienced improvement of her well-being, with more energy and less memory problems. Besides, the musical hallucinations were less frequent. However, over the last years, the patient had experienced relapsing episodes with an increase in musical hallucinations, headache, and fatigue. A repeated MRI examination of the brain showed no changes. Moreover, an EEG finding showed no electroencephalographic correlate for the musical hallucinations. Based on these findings, mycophenolate mofetil and valproic acid were continued. Throughout the disease history, the CSF remained positive for anti-mGluR1, even after treatment (CSF collected 67 months after initial clinical presentation).
Discussion
We found mGluR1 antibodies in a patient with the classic triad for limbic encephalitis (epilepsy and psychiatric and cognitive complaints), whereas previous studies reported ataxia as the most predominant symptom, occurring in 97% in a series of patients with mGluR1 antibodies.3 mGluR1 is not only highly expressed in Purkinje cell dendrites of the cerebellar cortex but also present in neurons located in the thalamus, hippocampus, globus pallidus, substantia nigra, deep nuclei of the cerebellum, and superior colliculus.4 The reason why our patient showed no signs of cerebellar involvement remains to be determined.
Our patient experienced only a partial and temporary improvement after the immunomodulatory therapy, which might be due to the long delay between start of the symptoms and start of immunomodulatory treatment.5 Another possible explanation could be the inability of most commonly used immune treatments to result in a rapid and sustained control of the immune response within the CNS.6 The delay of treatment could have been prevented because our patient had an antibodies contributing to focal epilepsy signs and symptoms (ACES) score of 2, which relates to a higher chance of an autoimmune cause for her epilepsy.7 Furthermore, IHC shows its advantage as a supplementary method for screening, whereas antibody screening in clinical practice by several specific CBA has its limitations.
In conclusion, presentation of anti-mGluR1–related disease can be more diverse than previously believed. Testing for mGluR1 antibodies should be considered in patients with limbic encephalitis and epilepsy, especially when negative for more common antibodies.
Acknowledgment
The authors are thankful for the financial support received from the following funding organizations: P. Martinez-Martinez was supported by an Aspasia/NWO grant (015.011.033), and S. Zong, C. Correia-Hoffmann, and M. Mané-Damas were supported by Kootstra Talent Fellowships. The authors also thank Vivianne van Kranen-Mastenbroek (Academic Center for Epileptology Kempenhaeghe/MUMC + Heeze and Maastricht) for providing the EEG image and Josep Dalmau from Instituto de Investigación Biomédica August Pi i Sunyer (IDIBAPS), Barcelona, for providing the mGluR1-EGFP plasmids and for his help with the detection of the autoantibodies.
Appendix. Authors
Footnotes
These authors contributed equally to this work.
The Article Processing Charge was funded by the authors.
Go to Neurology.org/NN for full disclosures. Funding information is provided at the end of the article.
Submitted and externally peer reviewed. The handling editor was Josep O. Dalmau, MD, PhD, FAAN.
Contributor Information
Anita M. Vinke, Email: anita.vinke@mumc.nl.
Shenghua Zong, Email: s.zong@maastrichtuniversity.nl.
Josien H. Janssen, Email: josien.janssen@mumc.nl.
Carolin Correia-Hoffmann, Email: cchoffmann@ualg.pt.
Marina Mané-Damas, Email: m.damas@maastrichtuniversity.nl.
Jan G.M.C. Damoiseaux, Email: jan.damoiseaux@mumc.nl.
J.M. de Vries, Email: j.m.devries@erasmusmc.nl.
Dirk Pröpper, Email: d.propper@zuyderland.nl.
Peter Molenaar, Email: p.molenaar@maastrichtuniversity.nl.
Mario Losen, Email: m.losen@maastrichtuniversity.nl.
Pilar Martinez Martinez, Email: p.martinez@maastrichtuniversity.nl.
Study Funding
The authors report no targeted funding.
Disclosure
All authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Zong S, Correia-Hoffmann C, Mané-Damas M, et al. Novel neuronal surface autoantibodies in plasma of patients with depression and anxiety. Transl Psychiatry. 2020;10(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedroso JL, Dutra LA, Espay AJ, Höftberger R, Barsottini OGP. Video NeuroImages: head titubation in anti-mGluR1 autoantibody-associated cerebellitis. Neurology. 2018;90(16):746-747. [DOI] [PubMed] [Google Scholar]
- 3.Spatola M, Petit Pedrol M, Maudes E, et al. Clinical features, prognostic factors, and antibody effects in anti-mGluR1 encephalitis. Neurology. 2020;95(22):e3012–e3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fotuhi M, Sharp AH, Glatt CE, et al. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993;13(5):2001-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bruijn M, Bastiaansen AEM, Mojzisova H, et al. Antibodies contributing to focal epilepsy signs and symptoms score. Ann Neurol. 2021;89:698-710. [DOI] [PMC free article] [PubMed] [Google Scholar]