Abstract
Simple Summary
Knockdown resistance (kdr) mutations in the voltage-gated sodium channel (VGSC) of mosquitoes confer resistance to pyrethroid insecticides. Analysis of kdr mutations in Aedes aegypti mosquitoes collected from five different townships in the Mandalay area, Myanmar, revealed high levels of validated kdr mutations in domains II and III of vgsc. Moreover, high frequencies of concurrent kdr mutations were also detected. The results of this study suggest that kdr mutations associated with pyrethroid resistance are widespread in the Ae. aegypti population of the study area. Our results provide a valuable molecular basis to understand the pyrethroid resistance status of the Ae. aegypti population in the area and underscore the need for an effective vector control program in Myanmar.
Abstract
Aedes aegypti is an important mosquito vector transmitting diverse arboviral diseases in Myanmar. Pyrethroid insecticides have been widely used in Myanmar as the key mosquito control measure, but the efforts are constrained by increasing resistance. Knockdown resistance (kdr) mutations in the voltage-gated sodium channel (VGSC) are related to pyrethroid resistance in Ae. aegypti. We analyzed the patterns and distributions of the kdr mutations in Ae. aegypti in the Mandalay area of Myanmar. The segment 6 regions of domains II and III of vgsc were separately amplified from individual Ae. aegypti genomic DNA via polymerase chain reaction. The amplified gene fragments were sequenced. High proportions of three major kdr mutations, including S989P (54.8%), V1016G (73.6%), and F1534C (69.5%), were detected in the vgsc of Ae. aegypti from all studied areas. Other kdr mutations, T1520I and F1534L, were also found. These kdr mutations represent 11 distinct haplotypes of the vgsc population. The S989P/V1016G/F1534C was the most prevalent, followed by S989P/V1016V and V1016G/F1534C. A quadruple mutation, S989P/V1016G/T1520I/F1534C, was also identified. High frequencies of concurrent kdr mutations were observed in vgsc of Myanmar Ae. aegypti, suggesting a high level of pyrethroid resistance in the population. These findings underscore the need for an effective vector control program in Myanmar.
Keywords: Aedes aegypti, voltage-gated sodium channel, knockdown resistance, Myanmar
1. Introduction
Aedes aegypti is the primary mosquito vector transmitting diverse arboviral pathogens such as dengue, chikungunya, Zika, and yellow fever viruses to humans in wide geographical areas [1]. This anthropophilic mosquito species originated in Africa and rapidly spread worldwide by successfully adapting to artificial environments [2]. Their ability to thrive in urban environments and to travel long distances via transportation strengthens their vectorial competence. Rapid urbanization and climate change also accelerated the widespread distribution of Ae. aegypti in tropical and subtropical areas [3].
Ae. aegypti has been mainly controlled by environmental management and the use of insecticides such as pyrethroids, which are widely employed globally due to their photostability, low mammalian toxicity, high efficacy, and rapid paralysis or “knockdown” [4]. Pyrethroid insecticides target the voltage-gated sodium channel (VGSC) in insect neurons and alter the action potential, thereby resulting in paralysis and death of mosquitoes [4]. However, the extensive use of pyrethroids has led to the emergence of insecticide resistance in Ae. aegypti populations and global outbreaks of arboviral diseases [5,6]. VGSC is a transmembrane protein found in the mosquito’s nerve cell membranes. This protein consists of four homologous domains (domains I–IV), each of which comprises six hydrophobic subunits, segments 1–6 [7]. The helical segments 5 and 6 of domain II along with the helical segment 6 of domain III form a hydrophobic pocket for binding with insecticidal compounds [8]. However, the indiscriminate use of pyrethroids induces resistance mediated by knockdown resistance (kdr) mutations in the vgsc. The kdr mutations induce structural changes in the VGSC, which prevent the binding of pyrethroids to the target site, resulting in insecticidal resistance [9,10,11]. Until now, diverse kdr mutations contributing to pyrethroid resistance have been detected in the global Ae. aegypti population. These mutations, including V410L, G923V, L982W, S989P, I1011M/V, L1014F/S, V1016G/I, T1520I, F1534C, and D1763Y, generate diverse and continent-specific kdr genotypes in the global Ae. aegypti populations [9,12,13,14,15,16].
Ae. aegypti is the primary vector transmitting dengue fever in Myanmar. The warm and humid climate throughout the year and unsanitary environments provide ideal breeding conditions for the mosquito species, resulting in large numbers of annual dengue fever cases in the country. More than 20,000 annual cases have been reported in Myanmar during the last decade [17,18]. Pyrethroid insecticides have been widely used as the principal control measure for Ae. aegypti in Myanmar; however, the extensive and indiscreet use of the insecticides has increased concerns regarding insecticide resistance in the Ae. aegypti population, which threatens effective control of the mosquito species. In this respect, understanding the insecticide resistance status is imperative to implement appropriate guidelines or alternative strategies for Ae. aegypti control in Myanmar.
Molecular approaches to analyze kdr mutations in vgsc of Ae. aegypti are valid tools to monitor insecticide resistance [9,12,15]. Molecular studies revealed a substantial number of kdr mutations associated with insecticide resistance in the Myanmar Ae. aegypti population [19,20,21]. In this study, we analyzed the patterns and prevalence of kdr mutations in vgsc of Ae. aegypti collected in the Mandalay area, Myanmar, in order to extend our knowledge of the insecticide resistance status of the mosquito population in the country.
2. Materials and Methods
2.1. Mosquito Collection
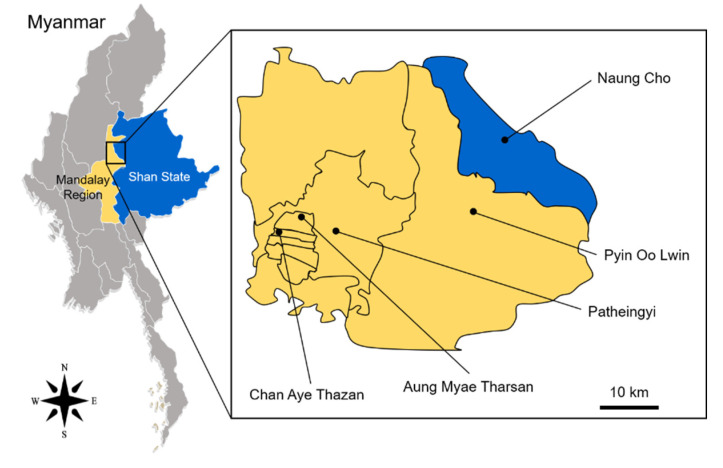
Aedes aegypti larvae and pupae were collected from 5 different townships: Patheingyi (21°57′44″ N, 96°24′20.9″ E), Pyin Oo Lwin (21°59′25.9″ N, 96°04′13.0″ E), Chan Aye Thazan (21°57′44.7″ N, 96°24′20.9″ E), and Aung Myae Tharsan (21°58′39.6″ N, 96°05′54.3″ E) in the Mandalay Region, and Naung Cho (22°19′48.7″ N, 96°47′24.0″ E) in Shan State (Figure 1). The mosquito samples were collected from artificial water containers, such as discarded tires, metal drums, water storage clay pots, enamel jars, concrete water storage tanks, or flower vases around households at different locations of each township during July to October 2019 at the peak of mosquito breeding and dengue fever transmission. The collected mosquito samples were transferred to clean water containers, transported to the Department of Medical Research Pyin Oo Lwin Branch, and reared to adults in the laboratory insectary at a temperature of 25 ± 2 °C and humidity of 80 ± 10%. Adult Ae. aegypti mosquitoes were further confirmed using standard mosquito classification methods [22].
Figure 1.
Map of the mosquito collection sites. Aedes aegypti larvae and pupae were collected at 4 townships in Mandalay Region [Patheingyi (21°57′44.7″ N, 96°24′20.9″ E), Pyin Oo Lwin (21°59′25.9″ N, 96°04′13.0″ E), Chan Aye Thazan (21°57′44.7″ N, 96°24′20.9″ E), and Aung Myae Tharsan (21°58′39.6″ N, 96°05′54.3″ E)] and 1 township in Shan State [Naung Cho (22°19′48.7″ N, 96°47′24.0″ E)]. Yellow and blue colors represent Mandalay Region and Shan State, respectively. Closed black circles indicated the collection sites of the mosquito samples.
2.2. DNA Extraction and Gene Amplification
The mosquito DNA was extracted from 411 Ae. aegypti mosquitoes individually using a Tissue Genomic DNA Extraction Kit (Bioneer, Daejeon, Korea) following the manufacturer’s instructions. Fragments of the vgsc flanking segment 6 of domain II (DII-S6) and segment 6 of domain III (DIII-S6) were amplified via polymerase chain reaction (PCR) with specific primer sets: AaNavF20 (5′-ACAATGTGGATCGCTTCCC-3′) and AaNavR21 (5′-TGGACAAAAGCAAGGCTAAG-3′) for DII-S6, and AaNavEx31P (5′-TCGCGGGAG-GTAAGTTATTG-3′) and AaNavEx31Q (5′-GTTGATGTGCGATGGAAATG-3′) for DIII-S6 [21,23]. The thermal cycling conditions were initial denaturing at 95 °C for 10 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s and extension at 72 °C for 30 s, and the final extension at 72 °C for 5 min. Each PCR product was analyzed with 1.2% agarose gel, purified from the gel, and ligated into the T&A cloning vector (Real Biotech Corporation, Banqiao City, Taiwan). Each ligation mixture was transformed into Escherichia coli DH5α competent cells (Real Biotech Corporation) and positive clones with correct inserts were selected with colony PCR. The nucleotide sequences of the cloned gene fragments were analyzed via Sanger DNA sequencing (Macrogen, Daejeon, Korea). To verify the sequence accuracy of the cloned gene fragments, plasmids from at least two to three individual clones from each mosquito sample were sequenced bidirectionally.
2.3. Sequence Analysis
The nucleotide and deduced amino acid sequences of vgsc DII-S6 and DIII-S6 were analyzed using Editseq and Seqman programs in the DNASTAR package (DNASTAR, Madison, WI, USA). The amino acid polymorphisms in the sequences were detected by comparing them with the reference sequence of Ae. aegypti vgsc (GenBank accession number: KY747529.1). The nucleotide sequences analyzed in this study were deposited at GenBank under the accession numbers OL338546–OL338939 for DII-S6 and OL338940–OL339333 for DIII-S6.
3. Results
3.1. kdr Mutations in vgsc of Myanmar Ae. aegypti
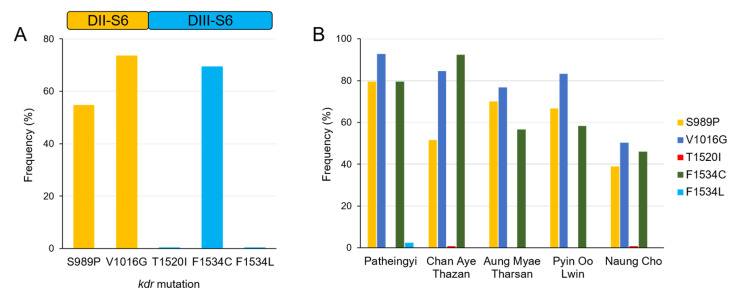
PCR of DII-S6 and DIII-S6 regions of vgsc from 411 Myanmar Ae. aegypti mosquitoes resulted in successful amplification of both fragments in 394 mosquitoes. However, both gene fragments were not successfully amplified in five samples, or only one of the two gene fragments was amplified in 12 samples, and thus excluded in further analysis. Due to differences in the number of mosquito samples collected in each township, the number of sequences obtained from each township varied. Most of the sequences originated from Ae. aegypti collected in Naung Cho (139, 35.3%) and Chan Aye Thazan (130, 33.0%), followed by Patheingyi (83, 21.1%), Aung Myae Tharsan (30, 7.6%), and Pyin Oo Lwin (12, 3.0%). Four major validated kdr mutations, S989P and V1016G in DII-S6, and T1520I and F1534C in DIII-S6, were identified in Myanmar Ae. aegypti (Figure 2A). Interestingly, F1534L was also found. Among these, V1016G was predominant (290/394, 73.6%) followed by F1534C (274/394, 69.5%) and S989P (216/394, 54.8%). Meanwhile, T1520I and F1534L were detected with lower frequencies of 0.5%. These mutations showed similar, but not identical, distribution patterns between and among Ae. aegypti populations collected from different townships (Figure 2B). Overall frequencies of V1016G and F1534C were relatively high, ranging from 50.4% to 92.8% in Ae. aegypti populations from all five townships. S989P was also detected with a high frequency in Ae. aegypti populations from Patheingyi (79.5%), Aung Myae Tharsan (70.0%), Pyin Oo Lwin (66.7%), Chan Aye Thazan (51.5%), and Naung Cho (38.9%). A low frequency (0.8%) of T1520I was identified only in Ae. aegypti from Chan Aye Thazan and Naung Cho. F1534L was also detected in two mosquito samples (2.4%) from Patheingyi. In addition to these major mutations, diverse minor mutations were also detected in two to five mosquito samples (Table 1). However, F1020S in DII-S6 was detected in 50 Ae. aegypti (12.7%) mosquitoes, mainly from Chan Aye Thazan and Naung Cho. E1553G in DIII-S6 was also identified in 10 mosquito samples (2.5%) from Chan Aye Thazan and Aung Myae Tharsan.
Figure 2.
Frequencies of major kdr mutations associated with pyrethroid resistance in Ae. aegypti populations collected in Mandalay area, Myanmar. (A) Frequencies of major kdr mutations detected in vgsc DII-S6 and DIII-S6 of Myanmar Ae. aegypti populations. (B) Frequencies of major kdr mutations detected in Myanmar Ae. aegypti populations collected at 5 townships, Mandalay area, Myanmar.
Table 1.
Minor mutations found in vgsc DII-S6 and DIII-S6 of Myanmar Ae. aegypti.
| Domain | Mutation | Patheingyi (n = 83) | Chan Aye Thazan (n = 130) |
Aung Myae Tharsan (n = 30) | Pyin Oo Lwin (n = 12) |
Naung Cho (n = 139) |
Total (n = 394) |
|---|---|---|---|---|---|---|---|
| DII-S6 | D960G | 1 | 1 | 2 | |||
| W966R | 2 | 2 | |||||
| N967D | 1 | 1 | 2 | ||||
| M972V | 2 | 2 | |||||
| M972I | 2 | 2 | |||||
| I977T | 1 | 2 | 3 | ||||
| E985G | 2 | 2 | |||||
| I987N | 2 | 2 | |||||
| W991R | 1 | 1 | 2 | ||||
| D992N | 2 | 2 | |||||
| M994V | 2 | 2 | |||||
| D998G | 2 | 2 | |||||
| P1003S | 2 | 2 | |||||
| F1004I | 2 | 2 | |||||
| F1020S | 4 | 20 | 2 | 2 | 22 | 50 | |
| DIII-S6 | V1512A | 2 | 2 | ||||
| K1514E | 5 | 5 | |||||
| M1524V | 1 | 2 | 3 | ||||
| L1526P | 1 | 1 | 2 | ||||
| Y1527F | 1 | 2 | 3 | ||||
| Y1527C | 4 | 4 | |||||
| F1528L | 2 | 2 | |||||
| I1533V | 2 | 2 | |||||
| I1533T | 2 | 2 | |||||
| F1543L | 1 | 1 | 2 | ||||
| I1544V | 2 | 1 | 3 | ||||
| I1548V | 1 | 1 | 2 | ||||
| E1553G | 9 | 1 | 10 | ||||
| G1581D | 1 | 1 | 2 | ||||
| K1584E | 1 | 1 | 2 |
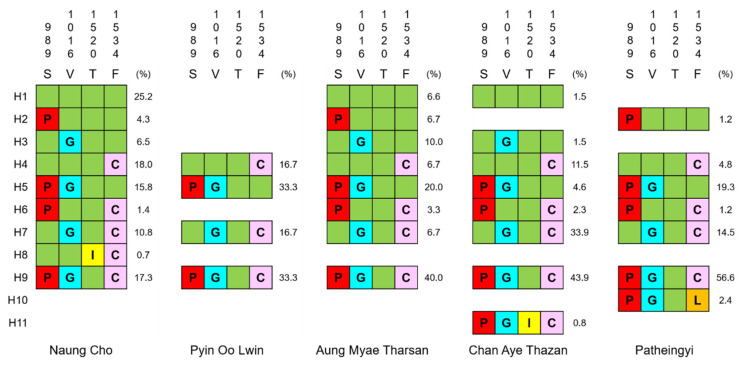
3.2. vgsc Haplotypes in Myanmar Ae. aegypti
Based on the overall patterns and distributions of major kdr mutations identified in DII-S6 and DIII-S6 of each vgsc sequence, the Ae. aegypti vgsc sequences from Myanmar were clustered into 11 different haplotypes, H1–H11 (Figure 3). These haplotypes were not equally distributed in the Ae. aegypti population from each township. The haplotypes identified in the mosquitoes collected from different townships were also distinct. Ae. aegypti from Naung Cho, Chan Aye Thazan, Aung Myae Tharsan, and Patheingyi showed greater haplotype diversity than that of Pyin Oo Lwin. H1, the wild-type haplotype, was found only in the mosquito samples obtained from Naung Cho, Aung Myae Tharsan, and Chan Aye Thazan. Among the 10 haplotypes carrying kdr mutations, seven haplotypes, i.e., excluding H2, H3, and H4, harbored at least two different kdr mutations. Four haplotypes, H4, H5, H7, and H9, were generally detected in the Ae. aegypti population from all five townships. In particular, H9 (S989P/V1016G/F1534C) was the most predominant haplotype in Ae. aegypti from Pyin Oo Lin, Aung Myae Tharsan, Chan Aye Thazan, and Patheingyi. H5 (S989P/V1016G) and H7 (V1016G/F1534C) were also prevalent in the population. Furthermore, H8 (T1520I/F1534C), H10 (S989P/V1016G/F1534L), and H11 (S989P/V1016G/ T1520I/F1534C) were unique to Ae. aegypti from Naung Cho, Patheingyi, and Chan Aye Thazan, respectively.
Figure 3.
Patterns and frequencies of vgsc haplotypes based on major kdr mutations in Ae. aegypti vgsc from 5 study sites. The wild type amino acids are the column rows and colored boxes indicate mutations. Single or concurrent kdr mutations in the vgsc DII-S6 and DIII-S6 generated 11 distinct haplotypes of the gene. Patterns and frequencies of each haplotype differed by study site.
4. Discussion
Diverse kdr mutations associated with pyrethroid resistance have been identified globally in the Ae. aegypti population [9,12,13,14,15,16]. Among these mutations, S989P, V1016G, and F1534C are the most common mutations found in Asian Ae. aegypti populations [15,19,21,24,25,26,27,28]. In our previous study, we analyzed the overall profiles of kdr mutations in vgsc of Ae. aegypti collected from the Mandalay area of Myanmar and found substantial levels of potent insecticide resistance in the population [21]. However, our previous study was a pooled analysis of Ae. aegypti samples, which prevented accurate determination of the frequency and pattern of kdr mutations in individual mosquitos to map insecticide resistance. In the present study, we analyzed the patterns and prevalence of kdr mutations in the vgsc of individual Ae. aegypti in the Mandalay area to investigate the insecticide resistance status of the Ae. aegypti population in the area. The three major Asian-type kdr mutations strongly linked to pyrethroid resistance, S989P, V1016G, and F1534C, were found in Ae. aegypti analyzed in this study with high frequencies: S989P (54.8%), V1016G (73.6%), and F1534C (69.5%). High frequencies of these mutations were also reported in our previous study using pooled Ae. aegypti samples collected from the Mandalay area in 2017, but with slightly different frequencies of S989P (68.6%), V1016G (73.5%), and F1534C (40.1%) [21]. High levels of S989P (78.8%), V1016G (84.4%), and F1534C (23.2%) were also reported in the Ae. aegypti population from Yangon, Myanmar [19]. These mutations were also routinely found in neighboring countries including India, Laos, and Thailand, but with different frequencies [15,24,25,26,27,28]. T1520I detected in Ae. aegypti in this study was also a recently identified vgsc mutation in Ae. aegypti from India, Pakistan, and China [29,30,31], and most recently in Myanmar Ae. aegypti [21]. T1520I alone does not confer pyrethroid or DDT resistance, but when combined with F1534C induces high levels of pyrethroid resistance [29,30]. Therefore, T1520I has been selected in populations with background kdr mutation F1534C established in the field [30]. Consistent with previous studies [29,30,31], a strong association of T1520I with F1534C was also observed in the Myanmar Ae. aegypti population analyzed in this study. F1534L is another recently identified mutation in Indian Ae. aegypti and is likely to be genetically associated with permethrin resistance [32]. This mutation associated with deltamethrin resistance has also been reported in Ae. albopictus [33,34]. The F1534L identified in Myanmar Ae. aegypti was also strongly linked to both S989P and V1016G in Myanmar Ae. aegypti. Interestingly, a substantial frequency (12.7%) of F1020S in DII-S6 was detected in Ae. aegypti population from all five study sites, especially Naung Cho and Chan Aye Thazan. This mutation was also identified in our previous study, but its frequency was not high in the mosquitoes collected from the Mandalay area in 2017 [21]. The association of F1020S with pyrethroid resistance is still unclear; however, the increasing frequency of this mutation in the Myanmar Ae. aegypti population suggests the need for additional studies to investigate its potent association with pyrethroid resistance.
Concurrent kdr mutations involving DII-S6 and DIII-S6 of vgsc confer high pyrethroid resistance in Ae. aegypti [20,29,30,31]. Diverse haplotypes of concurrent kdr mutations were reported in Ae. aegypti from neighboring countries including India [29], Thailand [26,35,36], China [37], Laos [28], Pakistan [31], Indonesia [38], and Malaysia [27]. High frequencies of concurrent kdr mutations were also detected in the Ae. aegypti population analyzed in this study. Seven haplotypes of vgsc found in the Myanmar Ae. aegypti population harbored co-occurrent kdr mutations. H9 (S989P/V1016G/F1534C) was the most predominant followed by H5 (S989P/V1016V) and H7 (V1016G/F1534C). Haplotypes carrying S989P/F1534C (H6), T1520I/F1534C (H8), S989P/V1016G/F1534L (H10), and S989P/V1016G/T1520I/F1534C (H11) were also identified, but their frequencies were not high. Similar patterns of complex and concurrent kdr mutations were also detected in our previous study [21]. In addition to these validated kdr mutations in DII-S6 and DIII-S6, minor mutations, including F1020S and E1553G, were also detected in Ae. aegypti from the Mandalay area, and many of them were concurrent with the major kdr mutations. Additional studies are also needed to investigate the mutations contributing to pyrethroid resistance in the mosquito population, despite their generally low frequencies.
5. Conclusions
This study clearly suggests that kdr mutations associated with pyrethroid resistance are widespread in the Ae. aegypti population of the Mandalay area, Myanmar. Continued strong pressure for pyrethroid resistance is driving the mutational changes. Our results also provide a valuable molecular basis to understand the pyrethroid resistance status of the Ae. aegypti population in the area. High levels of concurrent kdr mutations and the appearance of potent novel mutations suggest the need to investigate the pyrethroid resistance status and underlying resistance mechanisms in the Ae. aegypti population in the study area. Accurate assessment of the pyrethroid resistance status in the area requires larger numbers of Ae. aegypti samples from diverse sites. These efforts are highly relevant to the Ae. aegypti control program in Myanmar for prompt detection of pyrethroid resistance, in addition to updated Ae. aegypti control strategies in the country.
Acknowledgments
We thank the staff in the Department of Medical Research Pyin Oo Lwin Branch for their contribution and technical support for mosquito collections.
Author Contributions
H.N. and T.C.V. conducted the experiments. Y.Y.M. and M.K.M. collected and classified the mosquitoes. H.N., T.C.V., H.G.L., J.-M.K., T.-S.K., H.-J.S. and B.-K.N. analyzed and interpreted the data. T.-S.K., H.-J.S. and B.-K.N. funding acquisition. B.-K.N. designed and supervised the experiments. H.N., T.C.V. and B.-K.N. wrote the draft of the manuscript. H.G.L., J.-M.K., Y.Y.M., M.K.M., T.-S.K. and H.-J.S. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (2018M3A9H5055614).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data supporting the conclusions of this article are provided within the article. The original datasets analyzed in this study are available from the corresponding author upon request. The nucleotide sequences reported in this study have been deposited in the GenBank database under the accession numbers OL338546–OL338939 for DII-S6 and OL338940–OL339333 for DIII-S6.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Powell J.R. Perspective piece mosquito-borne human viral diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018;98:1563–1565. doi: 10.4269/ajtmh.17-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell J.R., Gloria-Soria A., Kotsakiozi P. Recent history of Aedes aegypti: Vector genomics and epidemiology records. Bioscience. 2018;68:854–860. doi: 10.1093/biosci/biy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Servadio J.L., Rosenthal S.R., Carlson L., Bauer C. Climate patterns and mosquito-borne disease outbreaks in South and Southeast Asia. J. Infect. Public Health. 2018;11:566–571. doi: 10.1016/j.jiph.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo N. Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019;95:378–400. doi: 10.2183/pjab.95.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores A.E., Ponce G., Silva B.G., Gutierrez S.M., Bobadilla C., Lopez B., Mercado R., Black W.C., IV. Wide spread cross resistance to pyrethroids in Aedes aegypti (Diptera: Culicidae) from Veracruz State Mexico. J. Econ. Entomol. 2013;106:959–969. doi: 10.1603/EC12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amelia-Yap Z.H., Chen C.D., Sofian-Azirun M., Low V.L. Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: Present situation and prospects for management. Parasites Vectors. 2018;11:332. doi: 10.1186/s13071-018-2899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lera Ruiz M., Kraus R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015;58:959–969. doi: 10.1021/jm501981g. [DOI] [PubMed] [Google Scholar]
- 8.Field L.M., Emyr Davies T.G., O’Reilly A.O., Williamson M.S., Wallace B.A. Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur. Biophys. J. 2017;46:675–679. doi: 10.1007/s00249-016-1195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemingway J., Hawkes N.J., McCarroll L., Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect. Biochem. Mol. Biol. 2004;34:653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Soderlund D.M. Pyrethroids, knockdown resistance and sodium channels. Pest Manag. Sci. 2008;64:610–616. doi: 10.1002/ps.1574. [DOI] [PubMed] [Google Scholar]
- 11.Dong K., Du Y., Rinkevich F., Nomura Y., Xu P., Wang L., Silver K., Zhorov B.S. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014;50:1–17. doi: 10.1016/j.ibmb.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver K.S., Du Y., Nomura Y., Oliveira E.E., Salgado V.L., Zhorov B.S., Dong K. Voltage-gated sodium channels as insecticide targets. Adv. Insect Phys. 2014;46:389–433. doi: 10.1016/B978-0-12-417010-0.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosme L.V., Gloria-Soriaid A., Caccone A., Powell J.R., Martins A.J. Evolution of kdr haplotypes in worldwide populations of Aedes aegypti: Independent origins of the F1534C kdr mutation. PLoS Negl. Trop. Dis. 2020;14:e0008219. doi: 10.1371/journal.pntd.0008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyes C.L., Vontas J., Martins A.J., Ng L.C., Koou S.Y., Dusfour I., Raghavendra K., Pinto J., Corbel V., David J.P., et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017;11:e0005625. doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Y., Nomura Y., Zhorov B.S., Dong K. Sodium channel mutations and pyrethroid resistance in Aedes aegypti. Insects. 2016;7:60. doi: 10.3390/insects7040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinkevich F.D., Du Y., Dong K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic. Biochem. Physiol. 2013;106:93–100. doi: 10.1016/j.pestbp.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyaw A.K., Ngwe Tun M.M., Moi M.L., Nabeshima T., Soe K.T., Thwe S.M., Myint A.A., Maung K.T.T., Aung W., Hayasaka D., et al. Clinical, virological and epidemiological characterization of dengue outbreak in Myanmar, 2015. Epidemiol. Infect. 2017;145:1886–1897. doi: 10.1017/S0950268817000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linn N.N., Kyaw K.W.Y., Shewade H.D., Kyaw A.M.M., Tun M.M., Khine S.K., Linn N.Y.Y., Thi A., Lin Z. Notified dengue deaths in Myanmar (2017-18): Profile and diagnosis delays. F1000Research. 2020;9:579. doi: 10.12688/f1000research.23699.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawada H., Oo S.Z.M., Thaung S., Kawashima E., Maung Y.N.M., Thu H.M., Thant K.Z., Minakawa N. Co-occurrence of Point Mutations in the Voltage-Gated Sodium Channel of Pyrethroid-Resistant Aedes aegypti Populations in Myanmar. PLoS Negl. Trop. Dis. 2014;8:e3032. doi: 10.1371/journal.pntd.0003032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Son-Un P., Choovattanapakorn N., Saingamsook J., Yanola J., Lumjuan N., Walton C., Somboon P. Effect of relaxation of deltamethrin pressure on metabolic resistance in a pyrethroid-resistant Aedes aegypti (Diptera: Culicidae) strain harboring fixed P989P and G1016G kdr alleles. J. Med. Entomol. 2018;55:975–981. doi: 10.1093/jme/tjy037. [DOI] [PubMed] [Google Scholar]
- 21.Naw H., Su M.N.C., Võ T.C., Lê H.G., Kang J.M., Jun H., Mya Y.Y., Myint M.K., Lee J., Sohn W.M., et al. Overall prevalence and distribution of knockdown resistance (Kdr) mutations in Aedes aegypti from Mandalay region, Myanmar. Korean J. Parasitol. 2020;58:709–714. doi: 10.3347/kjp.2020.58.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y.-M. The subgenus Stegomyia of Aedes in the Afrotropical region I. The Africanus group of species (Diptera: Culicidae). Gainesville, USA. Am. Entomol. Inst. 1990;26:10–42. [Google Scholar]
- 23.Sayono S., Hidayati A.P.N., Fahri S., Sumanto D., Dharmana E., Hadisaputro S., Asih P.B.S., Syafruddin D. Distribution of voltage-gated sodium channel (NAV) alleles among the Aedes aegypti populations in central Java province and its aociation with resistance to pyrethroid insecticides. PLoS ONE. 2016;11:e0150577. doi: 10.1371/journal.pone.0150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha P., Chatterjee M., Ballav S., Chowdhury A., Basu N., Maji A.K. Prevalence of kdr mutations and insecticide susceptibility among natural population of Aedes aegypti in West Bengal. PLoS ONE. 2019;14:e0215541. doi: 10.1371/journal.pone.0215541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawada H., Higa Y., Komagata O., Kasai S., Tomita T., Nguyen T.Y., Luu L.L., Sánchez R.A.P., Takagi M. Widespread distribution of a newly found point mutation in voltage-gated sodium channel in pyrethroid-resistant Aedes aegypti populations in Vietnam. PLoS Negl. Trop. Dis. 2009;3:e527. doi: 10.1371/journal.pntd.0000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanola J., Somboon P., Walton C., Nachaiwieng W., Somwang P., Prapanthadara L. High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop. Med. Int. Health. 2011;16:501–509. doi: 10.1111/j.1365-3156.2011.02725.x. [DOI] [PubMed] [Google Scholar]
- 27.Ishak I.H., Jaal Z., Ranson H., Wondji C.S. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit Vectors. 2015;8:181. doi: 10.1186/s13071-015-0797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcombe S., Fustec B., Cattel J., Chonephetsarath S., Thammavong P., Phommavanh N., David J.P., Corbel V., Sutherland I.W., Hertz J.C., et al. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl. Trop. Dis. 2019;13:e0007852. doi: 10.1371/journal.pntd.0007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kushwah R.B.S., Dykes C.L., Kapoor N., Adak T., Singh O.P. Pyrethroid-Resistance and Presence of Two Knockdown Resistance (kdr) Mutations, F1534C and a Novel Mutation T1520I, in Indian Aedes aegypti. PLoS Negl. Trop. Dis. 2015;9:e3332. doi: 10.1371/journal.pntd.0003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M., Du Y., Wu S., Nomura Y., Zhu G., Zhorov B.S., Dong K. Molecular evidence of sequential evolution of DDT-and pyrethroid-resistant sodium channel in Aedes aegypti. PLoS Negl. Trop. Dis. 2019;13:e0007432. doi: 10.1371/journal.pntd.0007432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman R.U., Souza B., Uddin I., Carrara L., Brito L.P., Costa M.M., Mahmood M.A., Khan S., Lima J.B.P., Martins A.J. Insecticide resistance and underlying targets-site and metabolic mechanisms in Aedes aegypti and Aedes albopictus from Lahore, Pakistan. Sci. Rep. 2021;11:4555. doi: 10.1038/s41598-021-83465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushwah R.B.S., Kaur T., Dykes C.L., Ravi Kumar H., Kapoor N., Singh O.P. A new knockdown resistance (kdr) mutation, F1534L, in the voltage-gated sodium channel of Aedes aegypti, co-occurring with F1534C, S989P and V1016G. Parasites Vectors. 2020;13:327. doi: 10.1186/s13071-020-04201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcombe S., Farajollahi A., Healy S.P., Clark G.G., Fonseca D.M. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE. 2014;9:e101992. doi: 10.1371/journal.pone.0101992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Xu J., Zhong D., Zhang H., Yang W., Zhou G., Su X., Wu Y., Wu K., Cai S., et al. Evidence for multiple-insecticide resistance in urban Aedes albopictus populations in southern China. Parasites Vectors. 2018;11:4. doi: 10.1186/s13071-017-2581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srisawat R., Komalamisra N., Apiwathnasorn C., Paeporn P., Roytrakul S., Rongsriyam Y., Eshita Y. Field-collected permethrin-resistant Aedes aegypti from central Thailand contain point mutations in the domain IIS6 of the sodium channel gene (kdr) Southeast Asian J. Trop. Med. Public Health. 2012;43:1380–1386. [PubMed] [Google Scholar]
- 36.Stenhouse S.A., Plernsub S., Yanola J., Lumjuan N., Dantrakool A., Choochote W., Somboon P. Detection of the V1016G mutation in the voltage-gated sodium channel gene of Aedes aegypti (Diptera: Culicidae) by allele-specific PCR assay, and its distribution and effect on deltamethrin resistance in Thailand. Parasites Vectors. 2013;6:253. doi: 10.1186/1756-3305-6-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C.X., Kaufman P.E., De Xue R., Zhao M.H., Wang G., Yan T., Guo X.X., Zhang Y.M., Dong Y.D., Xing D., et al. Relationship between insecticide resistance and kdr mutations in the dengue vector Aedes aegypti in Southern China. Parasites Vectors. 2015;8:325. doi: 10.1186/s13071-015-0933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wuliandari J.R., Lee S.F., White V.L., Tantowijoyo W., Hoffmann A.A., Endersby-Harshman N.M. Association between three mutations, F1565C, V1023G and S996P, in the voltage-sensitive sodium channel gene and knockdown resistance in Aedes aegypti from Yogyakarta, Indonesia. Insects. 2015;6:658–685. doi: 10.3390/insects6030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are provided within the article. The original datasets analyzed in this study are available from the corresponding author upon request. The nucleotide sequences reported in this study have been deposited in the GenBank database under the accession numbers OL338546–OL338939 for DII-S6 and OL338940–OL339333 for DIII-S6.