Abstract
Due to climate change in recent years, there has been an increasing water deficit during the winter wheat sowing period. This study evaluated six Croatian winter wheat varieties’ physiological, biochemical, and molecular responses under two drought stress levels at the germination/seedling growth stage. Lipid peroxidation was mainly induced under both drought stress treatments, while the antioxidative response was variety-specific. The most significant role in the antioxidative response had glutathione along with the ascorbate-glutathione pathway. Under drought stress, wheat seedlings responded in proline accumulation that was correlated with the P5CS gene expression. Expression of genes encoding dehydrins (DHN5, WZY2) was highly induced under the drought stress in all varieties, while genes encoding transcription factors were differentially regulated. Expression of DREB1 was upregulated under severe drought stress in most varieties, while the expression of WRKY2 was downregulated or revealed control levels. Different mechanisms were shown to contribute to the drought tolerance in different varieties, which was mainly associated with osmotic adjustment and dehydrins expression. Identifying different mechanisms in drought stress response would advance our understanding of the complex strategies contributing to wheat tolerance to drought in the early growth stage and could contribute to variety selection useful for developing new drought-tolerant varieties.
Keywords: antioxidative response, dehydrins, drought, osmotic adjustment, transcription factors, wheat
1. Introduction
Due to climate change, years with more extended periods of water deficit and heat stress are becoming more frequent and, thus, threatening global crop production [1,2]. Drought conditions particularly affect the yield and quality of wheat (Triticum aestivum L.), one of the most important and widespread grain crops, indispensable in human nutrition and animal feed production [3]. Although the region of Croatia is characterized by large seasonal variability, the increasing annual frequency of dry days was encountered all over the country in the past years [4]. In previous research, the evaluation of forty Croatian winter wheat genotypes showed a decrease in wheat yield by 14–50% under water deficit conditions [5].
The germination and seedling growth stage is one of the most sensitive stages in plant development, implying the importance of the plant’s tolerance to drought in the early growth stage [6,7]. In winter wheat, water deficit during the sowing period affects seed germination and causes a change in physiological and biochemical processes during seedling establishment, resulting in reduced growth, development, and overall productivity [8,9]. Osmotic stress caused by salinity and drought has a significant impact on plant productivity as a result of water limitations [10]. In response to osmotic stress, plants have adopted various drought tolerance mechanisms, including the formation of deeper roots, increased biomass, increased antioxidative metabolism, accumulation of osmoprotectants to facilitate osmotic adjustment, expression of different stress-responsive genes, and others [11,12,13,14,15].
Drought conditions in plant seedlings induce reactive oxygen species (ROS) production, which, in low concentrations, have a signaling role in abiotic stress response pathways. In contrast, higher ROS concentrations cause oxidative damage to the cellular biomolecules, such as proteins, lipids, and nucleic acids [16,17]. Lipid peroxidation induced by ROS disrupts membrane structure, leading to loss of its selectivity and integrity as well as disruption of water balance and nutrient uptake. Such imbalance can have detrimental effects on photosynthesis essential for biomass accumulation and therefore shoot and root elongation [18]. To maintain a balance between ROS production and scavenging, plants have developed an antioxidant defense system that includes nonenzymatic antioxidants and antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and the enzymes of the ascorbate-glutathione (AsA-GSH) pathway [19]. The AsA-GSH pathway is essential in detoxifying ROS and interacts with other defense systems to mitigate oxidative damage induced by abiotic stress [20]. This pathway entails the two potent antioxidants, glutathione and ascorbic acid, and four enzymes, namely ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR), that contribute to the maintenance of cellular glutathione and ascorbic acid pools in the plant cell, thus protecting plants from abiotic stress [20,21]. Glutathione is one of the most common nonenzymatic antioxidants, essential in maintaining the stability of the redox state in all plant cell compartments [22]. Glutathione has a role in plant growth and development as well as in defense mechanisms against various environmental stresses. It can directly neutralize ROS or indirectly act as a substrate of different enzymes for ROS and toxic substances removal [23,24]. Therefore. plants’ stress tolerance is extensively associated with the glutathione redox state maintained by the AsA-GSH pathway [20,21].
Under water-deficit conditions, plants have developed osmotic adjustment, one of the fundamental biochemical mechanisms of drought adaptation [14]. Osmotic adjustment changes cell osmotic potential due to the accumulation of osmolytes, thus preserving the physiological functions of the cell in stressful conditions. In addition to their role in osmoregulation, osmolytes have a role in maintaining membrane and other subcellular structures, in reactive oxygen species scavenging, and in gene expression regulation [25,26]. Depending on the plant metabolism, developmental stage, and environmental conditions, different plants species can accumulate different osmolites such as amino acids, sugar alcohols, sugars, quaternary amines, and other low-molecular-weight organic solutes [27,28]. Previous studies showed that wheat’s proline accumulation contributes to increased osmotic stress tolerance [29,30]. In plants, there are two pathways for proline biosynthesis, and the preferred one includes the conversion of glutamate to proline by two successive reactions catalyzed by the enzymes Δ1-pyrroline-5-carboxylate synthetase (P5CS) and Δ1-pyrroline-5-carboxylate reductase [31,32]. The expression of the P5CS gene encoding the P5CS enzyme in wheat was shown to be upregulated under osmotic stress and correlated with proline accumulation [29]. Moreover, overexpression of the P5CS gene in transgenic wheat resulted in increased stress tolerance to water-deficit conditions due to increased proline content [33].
One of the strategies to combat water-deficit conditions is an expression of protective proteins like dehydrins, an important group of late embryogenesis abundant proteins. Accumulation of dehydrins is induced by different developmental stages and by different abiotic stress factors. Several studies reported a positive correlation between the accumulation of dehydrin transcripts or proteins and drought tolerance [34,35]. Overexpression of wheat dehydrin gene (DHN5) enhances tolerance to salt and osmotic stress in Arabidopsis plants due to regulation of proline metabolism and antioxidative response [36,37,38].
In plants’ stress tolerance, stress recognition and signal transduction are crucial in inducing adequate cell response and in the regulation of stress-related genes [25,39]. In abiotic stress signal transduction, transcription factors are terminal transducers that directly regulate the expression of downstream stress-responsive genes by interacting with their promotor region [39]. The most characterized transcription factor families involved in plant abiotic stress are WRKY and AP2/EREBP family DREBs (dehydration-responsive element-binding proteins). Both transcription factor families regulate developmental, physiological, and metabolic processes [40,41]. WRKY transcription factors are defined by the WRKY domain, composed of a highly conserved WRKYGQK sequence important for protein–protein interaction, followed by a zinc-finger motif with DNA binding affinity. Based on the number of the WRKY domains and the type of zinc-finger motif, these transcription factors can be classified into three main groups (I, II, and III) [42,43]. The DREB subfamily is defined by the presence of a single highly conserved AP2/ethylene-responsive element-binding factor (ERF) DNA binding domain that specifically binds to dehydration-responsive element (DRE)/C-repeat element (CRT) cis-elements at the promoter of the dehydration/cold-regulated (RD/COR) genes, responsive to water deficit and low temperature [44,45]. Previous studies showed that many genes encoding different DREB and WRKY transcription factors in wheat are upregulated under exposure to water-deficit conditions, thereby improving the tendency of wheat to tolerate drought stress [46,47,48,49]. Some of the genes encoding DREB and WRKY transcription factors have been used in transgenic technology to improve stress tolerance in model and crop plants [40]. Overexpression of these genes in transgenic plants showed enhanced tolerance to multiple abiotic stresses [50,51,52,53]. Transgenic wheat seedlings overexpressing the TaWRKY2 gene exhibited enhanced tolerance to drought stress [52]. Overexpression of TaWRKY2 in transgenic Arabidopsis plants exhibited salt and drought tolerance, while overexpression of TaWRKY19 conferred tolerance to salt, drought, and freezing stresses in transgenic plants [54]. Transgenic Arabidopsis plants with overexpression of wheat TaDREB3A1 gene showed enhanced tolerance against heat, drought, and salt stresses [51].
The impact of drought stress has been well documented in many crop species, but this study includes an integrated approach on morpho-physiological, biochemical, and molecular levels in six winter wheat varieties under drought stress conditions at the seedling growth stage. The study includes Croatian genotypes selected due to their importance in production and based on previous field trials, which showed different susceptibility to drought conditions. Due to different susceptibilities to drought, we assumed different winter wheat varieties’ antioxidant responses, osmotic adjustment, expression of stress-responsive genes, and genes encoding transcription factors. The obtained results will enable us to select drought-tolerant varieties in the seedling stage of growth and understand the complex mechanisms contributing to their tolerance.
2. Materials and Methods
2.1. Plant Material and Treatments
For drought-tolerance evaluation, six commonly used Croatian winter wheat varieties originated from Agricultural Institute Osijek were selected (Silvija, Rujana, Bubnjar, Fifi, Anđelka, and Pepeljuga) and subjected to different levels of drought stress in the germination and seedling stage of growth. Before germination, wheat seeds were sterilized with 70% ethanol for 1 min and washed two times in dH2O. For each wheat variety, 40 seeds per treatment were germinated on a filter paper in glass jars (H 9 × W 10 cm, V = 0.5 L) supplied with 10 mL of polyethylene glycol 6000 (PEG) in final concentrations of 10 and 20% for drought stress induction, while seedlings growing in water were used as a control. Wheat seedlings were grown in a growth chamber under a 16/8 h light/dark photoperiod, 25/20 °C day/night temperature, and 60% relative humidity. During the growing period, 10 mL of the solution related to each treatment was added daily to the glass jars. After seven days of growth, wheat seedlings were sampled for morpho-physiological characterization and biochemical and molecular analysis. For biochemical and molecular analysis, the tissue of seedlings was frozen in liquid nitrogen and disrupted in 10 mL stainless steel jars containing a grinding ball (Ø 20 mm) for 1 min at 30 Hz using a TissueLyser II bead mill (Qiagen). Metabolites, proteins, and RNA were extracted from the tissue powder aliquots homogenized with an appropriate extraction solution.
2.2. Morpho-Physiological Traits Measurements
On the fourth day of germination, sprouted seeds were counted, and the germination energy was calculated, taking into account the total number of germinated seeds and the initial number of seeds according to the formula: (number of seeds germinated on the fourth day/total number of seeds) × 100. Wheat seedlings were sampled seven days after germination, whereas the shoots and roots were separated to assess the morphological traits. Shoots and roots length was measured and expressed in mm per plant. For the biomass estimation, a fresh mass of the shoots and roots was measured, while dry mass was determined after drying wheat tissue in an oven at 105 °C for 24 h. Shoot and root biomass was expressed in terms of dry weight (DW) per plant. For relative water content (RWC) estimation, fresh weight (FW) of young leaves was determined immediately after sampling, after which leaves were soaked in the dH2O for 24 h for hydration. After 24 h, turgid weight (TW) was determined, and the leaves were dried in the oven at 105 °C for 24 h for dry weight (DW) estimation. RWC (%) was calculated as (FW-DW)/(TW-DW) × 100 [55]. All morpho-physiological traits were determined from 15 seedlings per experimental group.
2.3. Determination of the Proline Content
Proline content was determined according to Carillo and Gibon [56]. Proline was extracted from the 0.1 g frozen tissue powder in 40% ethanol overnight at 4 °C. After cold extraction, the homogenate was centrifuged for 5 min at 14,000× g. An aliquot of extract (0.05 mL) was incubated with 0.1 mL of a ninhydrin reagent (1% (w/v) ninhydrin in 60% (v/v) acetic acid and 20% ethanol (v/v)) at 95 °C for 20 min on a TS-100 Thermo-Shaker (Biosan, Riga, Latvia). After cooling and brief centrifugation, an 0.1 mL aliquot of the reaction mixture was transferred to a 96-well microplate, and the absorbance was measured at 520 nm and 25 °C using Spark multimode microplate reader with SparkControl software (Tecan, Männedorf, Switzerland). Proline content was determined using a standard curve with proline, and the results were expressed in nmol/mg FW.
2.4. Determination of the Lipid Peroxidation Level
Lipid peroxidation levels in wheat seedlings were estimated by measuring the thiobarbituric acid reactive substances (TBARS), according to the method described by Verma and Dubey [57]. This method is based on the formation of red pigment, generated by the reaction of lipid peroxidation breakdown products like malondialdehyde (MDA) with thiobarbituric acid at an optimum pH of 3.5. Briefly, the frozen wheat powder was homogenized with 0.1% trichloroacetic acid (TCA) solution (1/5, w/v) and centrifuged for 10 min at 10,000× g and 4 °C. The reaction mixture that consisted of 0.5 mL of tissue extract and 1 mL of reagent (0.5% thiobarbituric acid in 20% TCA) was incubated for 30 min at 95 °C on a TS-100 Thermo-Shaker (Biosan, Riga, Latvia). After cooling the reaction mixture, the produced red pigment was measured at 532 and 600 nm on a LAMBDA 25 UV-Vis spectrophotometer equipped with UV WinLab v6.0.4 software package (PerkinElmer, Waltham, MA, USA). The results were expressed as nmol/g of FW.
2.5. Determination of the Glutathione Content
Total (tGSH) and oxidized glutathione (GSSG) content were determined using a kinetic method based on a continuous reduction of 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) to 5-thio-2-nitrobenzoic acid (TNB) by reduced glutathione (GSH), where NADPH reduces the GSSG in the presence of GR [58]. The method is modified for the microplate assay, and the measurements were performed using Greiner UV Star 96-well plates on a Spark multimode microplate reader with SparkControl software (Tecan, Männedorf, Switzerland). For glutathione content determination, the frozen wheat powder was homogenized with 5% 5-sulfosalicylic acid solution (1/10, w/v) and centrifuged for 10 min at 16,000× g and 4 °C. Reaction mixture consisted of 0.031 mg/mL DTNB, 0.115 U/mL of GR, 1 mM EDTA, and 10 μL of deproteinized extract in 100 mM phosphate buffer (pH 7.0), in a final volume of 0.21 mL. After 5 min of equilibration, the reaction was initiated by adding NADPH at a final concentration of 48 μM. The formation of TNB was continuously recorded at 412 nm for 5 min every 15 s, at 25 °C. The amount of tGSH was determined using a standard curve of GSH, and the results were expressed as nmol/g of FW. For GSSG determination, aliquots of deproteinized extracts were incubated with vinylpyridine and triethanolamine for one hour at room temperature for GSH removal. The measurements were performed in the same way as for the tGSH. The content of GSSG was determined using a standard GSSG curve, and the results were expressed in nmol/g of FW. The amount of GSH was obtained from the difference between tGSH and GSSG and expressed in nmol/g of FW.
2.6. Antioxidant Enzymes Activity Determination
Protein extracts were prepared by homogenizing an aliquot of frozen wheat powder with a 100 mM phosphate buffer (pH 7.0) containing 1 mM EDTA (1/5, w/v). Proteins were extracted after 15 min of incubation on ice and centrifugation at 20,000× g for 15 min at 4 °C. Protein extracts were stored at −80 °C until further analysis. The enzymes’ activities were measured at 25 °C using a LAMBDA 25 UV-Vis spectrophotometer equipped with UV WinLab v6.0.4 software package (PerkinElmer, Waltham, MA, USA) and Spark multimode microplate reader with SparkControl software (Tecan, Männedorf, Switzerland).
CAT (EC 1.11.1.6) activity was estimated according to the method described by Aebi [59] using H2O2 as a substrate. The reaction mixture consisted of 0.036% H2O2 in 50 mM phosphate buffer pH (7.0), and the reaction started with the addition of 50 μL of diluted protein extract. The decrease in absorbance due to the oxidation of H2O2 was measured at 240 nm for 3 min every 10 s. CAT activity was calculated using molar extinction coefficient (ε = 0.04 mM/cm) and expressed as U/mg of protein.
GST (EC 2.5.1.13) activity was determined by the method of Habig et al. [60], which is based on the formation of glutathione-2,4-dinitrobenzene (G-SDNB) due to the conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) with GSH. The reaction mixture consisted of 2 mM GSH, 1 mM CDNB, 1 mM EDTA, and 50 μL of protein extract in 100 mM phosphate buffer (pH 6.5), in a final volume of 1.5 mL. The increase in absorbance was recorded at 340 nm for 2 min every 15 s. GST activity was calculated using a molar extinction coefficient of glutathione-1-chloro-2,4-dinitrobenzene conjugate (ε = 9.6 mM/cm) and expressed as U/g of protein.
APX (EC 1.11.1.11) activity was determined according to the method described by Nakano and Asada [61]. The reaction mixture consisted of 0.5 mM ascorbic acid, 0.12 mM H2O2, 0.1 mM EDTA and 50 μL of diluted protein extract in 50 mM potassium phosphate buffer (pH 7.0), in a final concentration of 1 mL. The decrease in absorbance was measured at 290 nm for 2 min every 10 s. The APX activity was calculated using a molar extinction coefficient (ε = 2.8 mM/cm) and expressed in U/mg of protein.
GR (EC 1.6.4.2) activity was determined according to the method described by Racker [62], which is based on measuring NADPH during the reduction of GSSG. The reaction mixture consisted of 1 mM GSSG, 1 mM EDTA, and 25 μL of protein extract in 100 mM phosphate buffer (pH 7.5), in a final volume of 1 mL. After 10 min of equilibration at 25 °C, the reaction was started by adding NADPH in a final concentration of 0.1 mM. The decrease in absorbance was monitored at 340 nm for 2 min every 15 s. GR activity was calculated using the molar extinction coefficient for NADPH (ε = 6.22 mM/cm) and expressed in U/g protein.
DHAR (EC 1.8.5.1) activity was determined according to the method described by Ma and Cheng [63] and modified for microplate assay by Murshed et al. [64]. The method is based on glutathione-dependent reduction of dehydroascorbate (DHA). The reaction mixture consisted of 0.1 mM EDTA, 2.5 mM GSH, 0.2 mM DHA, and 10 μL of protein extract in 50 mm HEPES buffer (pH 7.0), in a final volume of 0.2 mL. The increase in absorbance was monitored at 265 nm for 3 min every 15 s. DHAR activity was calculated using molar extinction coefficient (ε = 8.33 mM/cm) and expressed in U/g protein.
The activity of MDHAR (EC 1.6.5.4) was determined by the method described by Hossain et al. [65] with modifications for the microplate assay. The method is based on reducing mono-dehydroascorbate, generated by the ascorbate oxidase, to ascorbate using NADH as the reducing agent. The reaction mixture comprised 2.5 mM ascorbate, 0.1 mM NADH, 0.14 U of ascorbate oxidase, and 10 μL of protein extract in 50 mM Tris-HCl buffer (pH 7.6), with a final volume of 0.2 mL. The decrease in absorbance was recorded at 340 nm for 3 min every 15 s. MDHAR activity was calculated using molar extinction coefficient (ε = 3.7 mM/cm) and expressed in U/g protein.
Total protein concentration in tissue extracts was estimated by the method of Bradford [66], using bovine serum albumin, ranging from 0.1 to 1.4 mg/mL, as a standard. For protein determination, a microwell plate assay was used where 5 μL of diluted protein extracts was added to 250 µL of Bradford reagent (Sigma-Aldrich, St. Louis, MI, USA), and, after a five-minute incubation, the sample was read at 595 nm on a Spark multimode microplate reader with SparkControl software (Tecan, Männedorf, Switzerland).
2.7. RNA Isolation, cDNA Synthesis, and Quantitative PCR
Total RNA was isolated from 50 mg of frozen wheat tissue powder using the NucleoZOL reagent (Macherey-Nagel), following the manufacturer’s instructions. Subsequently, residual DNA in the obtained RNA solution was removed by rDNase (Macherey-Nagel). For DNA digestion, 1/10 volume of the rDNase-buffer premix (1/10, v/v) was added to the RNA solution and incubated for 10 min at 37 °C. RNA was subsequently repurified by ethanol precipitation: 0.1 volume of 3 M sodium acetate (pH 5.2) and 2.5 volumes of 100% ethanol were added to one sample volume. After two hours of incubation at −20 °C, samples were centrifuged for 10 min at maximum speed. The RNA pellet was washed with 70% ethanol, dried, and resuspended in RNase-free water. RNA concentrations and purity were assessed using NanoPhotometer NP-80 (Implen, München, Germany). The average RNA yield was around 1000 ng/μL, while A260/A280 ratio was approximately 2.0. RNA integrity was verified on agarose gel electrophoresis and visualized by SYBR safe staining (Invitrogen).
First-strand cDNA was synthesized from 3 μg of total RNA using the GoTaq® 2-Step RT-qPCR System (Promega) according to the manufacturer’s recommendation. After denaturation of the RNA template and Oligo(dT)15 primer premix at 70 °C for 5 min, the cDNA was synthesized in a final volume of 20 μL by combining the denatured premix with the reaction mixture consisting of 1× GoScript buffer, 2.5 mM MgCl2, 0.5 mM nucleotide mix, 20 U of ribonuclease inhibitor, and 1U of reverse transcriptase. The cDNA synthesis was performed under the following conditions: primer annealing at 25 °C for 5 min, extension at 42 °C for 1 h, and enzyme inactivation at 70 °C for 5 min. All incubation steps were performed on the MiniAmp Plus Thermal PCR Cycler (Applied Biosystems, Waltham, MA, USA).
Following cDNA synthesis, quantitative PCR (QPCR) using dye-based detection was performed to analyze transcript levels of seven genes (P5CS, DHN5, WZY2, DREB1, WRKY2, actin, and ADP ribosylation factor). The specific oligonucleotide primers were designed based on sequences in the GeneBank database using Primer3 software (Table 1). Some primers were designed to span the exon–exon junction containing an intron to differentiate between RNA versus genomic DNA amplification, thus confirming the absence of DNA contamination. qPCR analysis was performed on StepOnePlus™ Real-Time PCR System with StepOnePlus™ Software v2.3 (Applied Biosystems, Waltham, MA, USA) and by using GoTaq® 2-Step RT-qPCR System (Promega), according to the manufacturer’s recommendation. The qPCR amplification of all target sequences was performed in a 25 μL reaction mixture containing 5 μL of five-fold diluted cDNA template, 12.5 μL of GoTaq qPCR Master Mix (2×), 200 nmol of each primer, and 0.25 μL CXR reference dye. The qPCR amplification was performed under the following conditions: GoTaq Hot Start Polymerase activation at 95 °C for 2 min, followed by 40 cycles consisting of denaturation at 95 °C for 15 s, primer annealing, and extension at 60 °C for 1 min. The specificity of the QPCR reaction was confirmed by melting curve analysis. Three biological replicates were used in quantification analysis, and three technical replicates were analyzed for each biological replicate. Relative gene expression was quantified using a relative standard curve based on five points, corresponding to a three-fold dilution series from pooled cDNA, and normalized using the geometric average of two reference genes, actin and ADP-ribosylation factor.
Table 1.
Oligonucleotide primers sequences.
| Target Gene | GenBank Accession No. |
Product Length (bp) | Forward Primer | Revers Primer |
|---|---|---|---|---|
| DHN5 | AY619566 | 99 | agaagaagggcatcatggac | ggcacctccactctcagaag |
| WZY2 | KF112871 | 142 | tcgttcgtcgtggtagtctg | atgaccttgctgtccgtagg |
| P5CS | KT868850 | 85 | ccggtgaatggcagagtaat | ccccacggagaactttaaca |
| WRKY2 | EU665425 | 131 | ctttggcttctcctttcacg | tgctgctcttgttgctcact |
| DREB1 | DQ195070 | 80 | gttggtacccaacccaagtg | aacagaacgaagcagggcta |
| actin [67] | AK457930 | 215 | tgaccgtatgagcaaggag | ccagacaactcgcaacttag |
| ADP-ribosylati factor [67] | XM_044502292 | 165 | gctctccaacaacattgccaac | gcttctgcctgtcacatacgc |
2.8. Data Analysis
Data were analyzed using the statistical program GraphPad Prism 5.03. The data were presented as the mean of six (or three for gene expression analysis) replicas ± standard deviations (SD). Differences among treatments in each variety separately were assessed by a one-way analysis of variance (ANOVA), followed by the Dunnett post hoc test. The Dunnett test was performed at a significance level of 5, 1, and 0.1% (* p < 0.05, ** p < 0.01, *** p < 0.001).
3. Results
3.1. Morpho-Physiological Traits
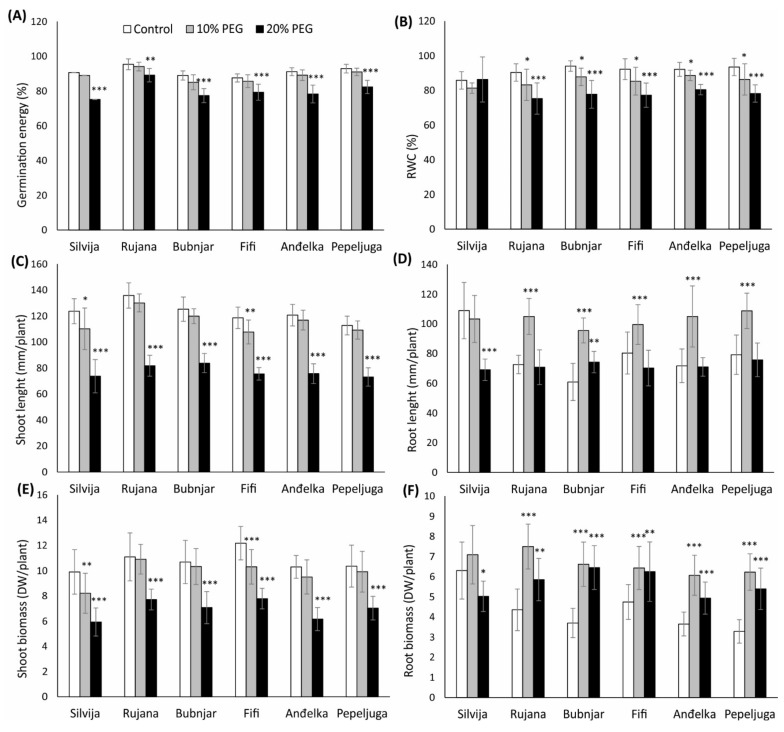
In all varieties, drought stress induced with 10% PEG did not significantly impact the grain germination, while treatment with 20% PEG significantly reduced the germination energy of all wheat varieties tested (Figure 1A). The reduction of germination energy ranged from 6.6 (p < 0.01) for the variety Rujana to 17% (p < 0.001) for the variety Silvija.
Figure 1.
Morpho-physiological traits: (A) germination energy, (B) relative water content (RWC), (C) shoot length; (D) root length; (E) shoot biomass, and (F) root biomass of seedlings of six Croatian wheat varieties (Silvija, Rujana, Bubnjar, Fifi, Anđelka, and Pepeljuga) under 10 and 20% PEG treatment. Expression data are presented as means of three independent biological replicates, and the error bars indicate standard deviations. Differences among treatments in each variety separately were assessed by a one-way analysis of variance (ANOVA), followed by the Dunnett post hoc test. The Dunnett test was performed at a significance level of 5, 1 and 0.1% (* p < 0.05, ** p < 0.01, *** p < 0.001).
Both levels of PEG treatments significantly reduced the RWC in almost all varieties in a concentration-dependent manner, with the exception of the variety Silvija, where no significant difference occurred in both treatments compared to the control (Figure 1B). RWC reduction under 10% PEG treatment ranged from 4 to 8% (p < 0.05) and under the 20% PEG treatment from 13 to 17% (p < 0.001).
Treatment under 20% PEG significantly reduced shoot length of all varieties (p < 0.001), while treatment with 10% PEG reduced the shoot length only in varieties Silvija and Fifi (Figure 1C). The highest shoot length reduction (11 and 40% under 10 and 20% PEG, respectively) was shown for variety Silvija, followed by variety Fifi (9 and 36% under 10 and 20% PEG, respectively). The lowest reduction of 33% under the 20% PEG had variety Bubnjar, followed by Pepeljuga and Anđelka, relative to the control.
Treatment under 10% PEG significantly induced root growth in almost all varieties (p < 0.001), with the exception of variety Silvija (Figure 1D). The increase in root length ranged from the highest 57% recorded for the variety Bubnjar, followed by the variety Anđelka (46%), to the lowest significant increase detected in the variety Fifi (24%), relative to the control. Additionally, the root length of the variety Bubnjar was also increased (22%, p < 0.01) under the 20% PEG treatment compared to the control. Only variety Silvija showed a significant reduction in root length (36%, p < 0.001) under the 20% PEG treatment.
Biomass of the shoots was significantly reduced in all varieties under treatment with 20% PEG (p < 0.001), while the treatment under 10% PEG significantly reduced biomass only of the variety Silvija and Fifi, compared to the control (Figure 1E). Thus, the highest reduction in shoot biomass was shown for the varieties Silvija, 17 (p < 0.01) and 40% (p < 0.001) under the 10 and 20% PEG, respectively, and variety Fifi, 15 (p < 0.001) and 36% (p < 0.001) under the 10 and 20% PEG-treatment, respectively, relative to the control.
Roots biomass increased in almost all varieties under both PEG treatments, with the exception of the variety Silvija, where 20% PEG significantly reduced the root biomass by 20% (p < 0.05) relative to the control seedlings (Figure 1F). Treatment with 10% PEG induced an increase in root biomass, ranging from 36 to 90%, while treatment under 20% PEG increased root biomass ranging from 32 to 74%. The highest significant increase was detected in varieties Bubnjar (80 and 74% under 10 and 20% PEG, respectively) and Pepeljuga (90 and 64% under 10 and 20% PEG, respectively), while the lowest significant increase was detected in variety Fifi (36 and 32% under the 10 and 20% PEG, respectively), relative to the control.
3.2. Proline Content in Wheat Seedlings
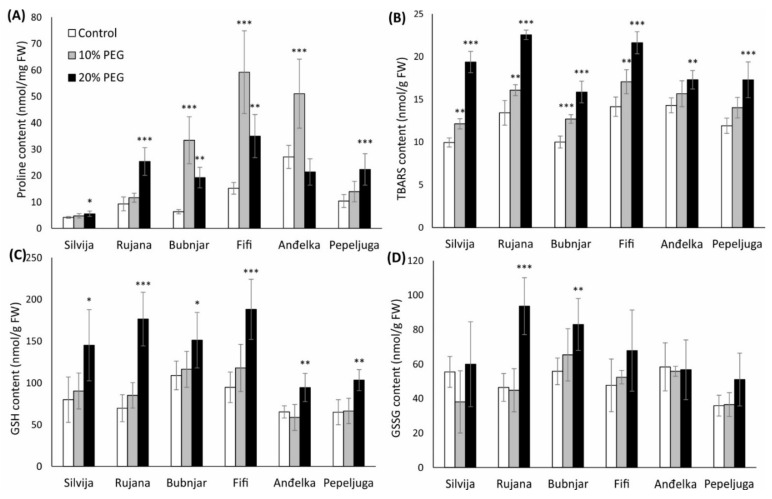
Treatment with 20% PEG significantly increased proline content in wheat seedlings of almost all varieties, except for variety Anđelka which showed no changes relative to the control (Figure 2A). Varieties Bubnjar, Fifi, and Anđelka showed a higher proline content after the treatment with 10% PEG (p < 0.001) than the 20% treatment. The highest increase of proline content was recorded in variety Bubnjar, with a 4-fold increase under 10% and a 2-fold increase under 20% PEG, relative to the control. Variety Silvija showed the lowest increase in proline content under severe stress, only 33% relative to the control (p < 0.05).
Figure 2.
Content of (A) thiobarbituric reactive substances (TBARS); (B) proline; (C) reduced glutathione (GSH); and (D) oxidized glutathione (GSSG) in wheat seedlings of six Croatian wheat varieties (Silvija, Rujana, Bubnjar, Fifi, Anđelka, and Pepeljuga) under 10 and 20% PEG treatment. Expression data are presented as means of three independent biological replicates, and the error bars indicate standard deviations. Differences among treatments in each variety separately were assessed by a one-way analysis of variance (ANOVA), followed by the Dunnett post hoc test. The Dunnett test was performed at a significance level of 5, 1 and 0.1% (* p < 0.05, ** p < 0.01, *** p < 0.001).
3.3. Lipid Peroxidation Levels in Wheat Seedlings
Treatments with PEG increased lipid peroxidation in seedlings of all wheat varieties in a concentration-dependent manner (Figure 2B). Treatment with 10% PEG caused a significant increase in TBARS content in the varieties Bubnjar (27%), Silvija (22%), Fifi (21%), and Rujana (20%), while no significant increase was recorded for the varieties Anđelka and Pepeljuga, compared to the control. Treatment with 20% PEG caused a significant increase of TBARS content in the seedlings of all varieties, with the most significant increase of 94% in variety Silvija (p < 0.001), and the lowest significant increase in varieties Pepeljuga and Anđelka, 21% (p < 0.001) and 45% (p < 0.01), respectively, compared to the control plants.
3.4. GSH and GSSG Content in Wheat Seedlings
Drought stress induced with 20% PEG increased the content of GSH in wheat seedlings of all varieties tested, while seedlings under 10% PEG treatment did not show a significant difference compared to the control (Figure 2C). The highest significant increase of 154% was recorded for the variety Rujana (p < 0.001), and the lowest increase of 39% for the variety Bubnjar (p < 0.05) under 20% PEG treatment relative to the control seedlings.
GSSG concentration was significantly higher in wheat seedlings of the varieties Rujana and Bubnjar under the 20% PEG treatment, while no significant changes in other varieties were observed (Figure 2D). The content of the GSSG in the variety Rujana was 102% (p < 0.001) and in the Bubnjar 49% (p < 0.01) higher compared to the control.
3.5. Antioxidant Enzymes Activities
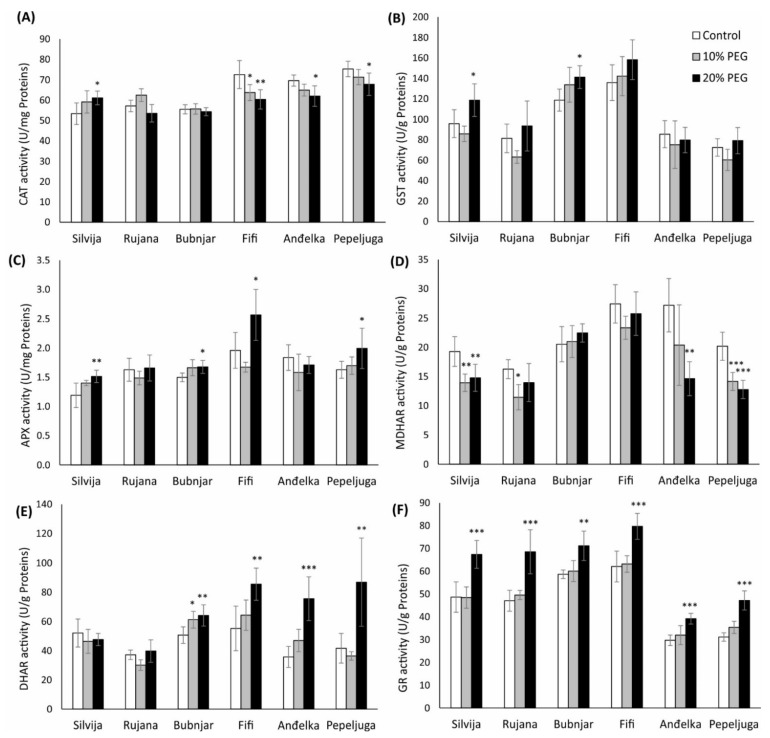
CAT activity was significantly increased only in seedlings of the variety Silvija after the treatment with 20% PEG (p < 0.05), while the same treatment significantly decreased the CAT activity in seedlings of varieties Fifi, Pepeljuga, and Anđelka (Figure 3A). Furthermore, CAT activity in variety Fifi was significantly decreased even after the treatment with 10% PEG (p < 0.05).
Figure 3.
The activity of (A) catalase (CAT); (B) glutathione S-transferase (GST); (C) ascorbate peroxidase (APX); (D) monodehydroascorbate reductase (MDHAR); (E) dehydroascorbate reductase (DHAR); and (F) glutathione reductase (GR) in wheat seedlings of six Croatian wheat varieties (Silvija, Rujana, Bubnjar, Fifi, Anđelka, and Pepeljuga) under 10 and 20% PEG treatment. Expression data are presented as means of three independent biological replicates, and the error bars indicate standard deviations. Differences among treatments in each variety separately were assessed by a one-way analysis of variance (ANOVA), followed by the Dunnett post hoc test. The Dunnett test was performed at a significance level of 5, 1, and 0.1% (* p < 0.05, ** p < 0.01, *** p < 0.001).
GST activity was significantly increased in wheat seedlings of the variety Silvija and Bubnjar treated with 20% PEG (p < 0.05) compared to control (Figure 3B).
APX activity was significantly increased in wheat seedlings of Silvija, Bubnjar, Fifi, and Pepeljuga under 20% PEG (Figure 3C). Thus, the largest increase in APX activity of 31% was recorded in the variety Fifi, followed by an increase of 27, 22, and 12%, respectively, in the varieties Silvija, Pepeljuga, and Bubnjar, compared to the control group.
Drought stress induced with 20% PEG significantly reduced MDHAR activity in varieties Anđelka, Pepeljuga, and Silvija (Figure 3D). The most significant reductions, of 46% (p < 0.01) and 37% (p < 0.001), were observed in varieties Anđelka and Pepeljuga, respectively. Treatment with 10% PEG significantly reduced enzyme activity in seedlings of Silvija (28%), Rujana (30%), and Pepeljuga (30%) relative to the control. Under both treatments, no significant changes in MDHAR activities were observed for Bubnjar and Fifi varieties.
DHAR activity was significantly increased in wheat seedlings of most varieties under the 20% PEG treatment (Figure 3E). Variety Anđelka had the most significant increase in enzyme activity of 111% (p < 0.001), followed by varieties Pepeljuga (109%), Fifi (55%), and Bubnjar (27%), relative to the control. Treatment with 10% PEG significantly induced DHAR activity only in variety Bubnjar, 21% relative to the control seedlings. No significant changes in DHAR activity for both treatments with PEG were detected for the varieties Silvija and Rujana.
Treatment with 20% PEG induced a significant GR activity in wheat seedlings of all investigated varieties compared to the control (Figure 3F). The most significant increase in GR activity of 52% (p < 0.001) was recorded for the variety Pepeljuga, followed by variety Rujana (46%), Silvija (38%), Anđelka (32%), Fifi (28%), and Bubnjar (21%), relative to control.
3.6. Genes Relative Expression Levels
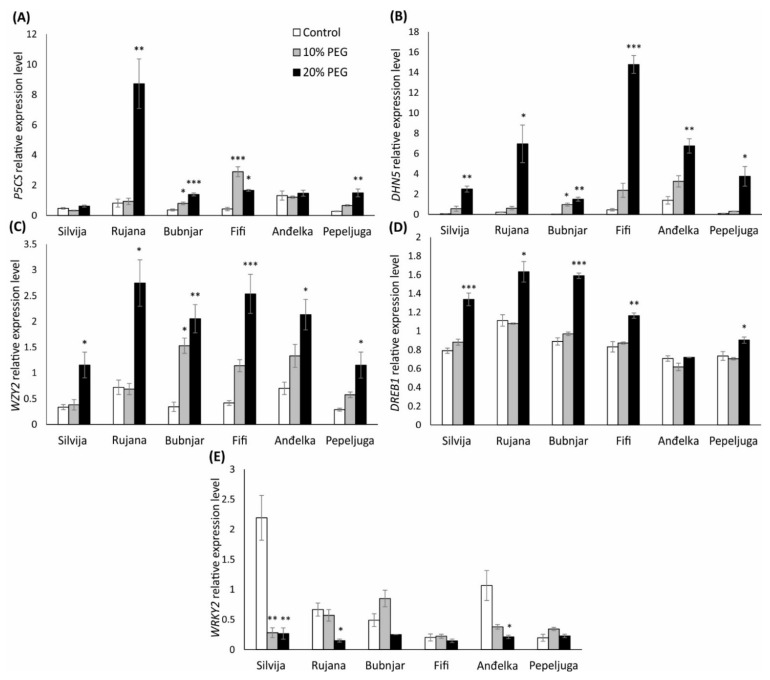
The expression of the P5CS gene encoding pyrroline-5-carboxylate synthetase was upregulated due to the treatment with 20% PEG in wheat seedlings of the variety Rujana, Bubnjar, Fifi, and Pepeljuga (Figure 4A). Treatment with 10% PEG induced expression of the genes in seedlings of the varieties Bubnjar and FiFi, which was in Fifi more pronounced than expression in seedlings treated with 20% PEG. Compared to the control, no significant changes in gene expression were observed for the varieties Silvija and Anđelka.
Figure 4.
Relative expression levels of P5CS (A), DHN5 (B), WZY2 (C), DREB1 (D), and WRKY2 (E) in wheat seedlings of six Croatian wheat varieties (Silvija, Rujana, Bubnjar, Fifi, Anđelka, and Pepeljuga) under 10 and 20% PEG treatment. Expression data are presented as means of three independent biological replicates, and the error bars indicate standard deviations. Differences among treatments in each variety separately were assessed by a one-way analysis of variance (ANOVA), followed by the Dunnett post hoc test. The Dunnett test was performed at a significance level of 5, 1 and 0.1% (* p < 0.05, ** p < 0.01, *** p < 0.001).
PEG-induced drought stress upregulated the expression of the genes encoding dehydrin proteins (DHN5 and WZY2) in all varieties’ wheat seedlings in a concentration-dependent manner (Figure 4B,C). Although the genes expressions under both treatments were higher in all varieties compared to the control, a significant increase was recorded only upon the treatment with 20% PEG, with the exception of the variety Bubnjar where both treatments caused a significant increase in the expression of both genes (DHN5 and WZY2) encoding dehydrins. The increase in the relative expression of the DHN5 gene was very high, ranging from 4-fold in wheat seedlings of variety Anđelka to the 51-fold in variety Bubnjar, relative to the control. The highest expression of the WZY2 gene was recorded in seedlings of varieties Bubnjar and Fifi, 5-fold higher relative to the control. In contrast, the lowest expression was observed in seedlings of Silvija and Anđelka, with approximately 2-fold higher expression than the control.
The expression of the DREB1 gene was induced by 20% PEG treatment in wheat seedlings of most varieties tested, while variety Anđelka did not show a difference in gene expression relative to the control (Figure 4D). The highest expression of the DREB1 gene was recorded in variety Bubnjar (78%, p < 0.001), followed by varieties Silvija (69%, p < 0.001), Rujana (47%, p < 0.01), Fifi (40%, p < 0.05), and Pepeljuga (23%, p < 0.05).
Treatments with both PEG concentrations significantly reduced the expression of the WRKY2 gene in wheat seedlings of the variety Silvija (Figure 4E). The reduction was 87 (p < 0.05) and 88% (p < 0.01) for 10 and 20% PEG, respectively, relative to the control. Treatment with 20% PEG also significantly reduced the expression of this gene in wheat seedlings of the varieties Rujana and Anđelka, 78 and 80%, relative to the control. No significant differences have occurred in the Bubnjar, Fifi, and Pepeljuga in both treatments relative to the control.
4. Discussion
Wheat seedlings’ response to drought stress depended on the variety and the severity of drought stress, and it was more pronounced at the drought stress induced by 20% PEG. PEG-induced drought stress affected seed germination and morpho-physiological traits of seedlings (RWC, root and shoot length and biomass) in a concentration-dependent manner. In order to tolerate water-deficit conditions, plants adapt their morpho-physiological traits that have been considered important criteria for characterizing drought tolerant and susceptible varieties [68,69,70,71]. Germination energy was decreased in all varieties under the severe drought stress induced by the 20% PEG, while the highest reduction was observed in the variety Silvija. Seed germination is the first most sensitive stage in plant development that could be significantly affected during water deficit conditions and, therefore, compromise seedling establishment [72,73,74]. Our results are in accordance with other studies that showed a reduction of seed germination under severe drought stress [13,75]. The RWC, an indicator of water status in plants, is mainly reduced under water-deficit conditions due to impaired root water absorption [76]. The RWC has been reported as a good indicator of drought stress tolerance in leaves and could be used for the selection of drought-tolerant wheat genotypes [69,71,77]. In our study, investigated varieties expressed very similar RWC responses under applied drought stress. Both PEG treatments reduced the leaf RWC concentration-dependent, while a significant decrease was observed under severe osmotic stress in most varieties. The exception was variety Silvija where no significant difference was observed under both treatments. Despite its application in the evaluation of drought tolerance [69,71,77], RWC could not be served in this study for the selection of resistant varieties.
As indicated by shoot length and biomass reduction, severe drought stress adversely affected shoot growth in all varieties. The reduction in shoot growth was the most pronounced in varieties Silvija and Fifi, while the lowest reduction was observed for variety Bubnjar, followed by Pepeljuga and Anđelka. A significant reduction of shoot growth and biomass accumulation under water-deficit conditions was also observed in other studies conducted on wheat [13,75,78]. Reduced shoot growth is correlated with reduced leaf transpiration and evaporation caused by water deficit. In plants, water deficit induces abscisic acid (ABA) root-to-leaf signaling, promoted by soil drying through the respiration system, and the main target of this signaling is the closure of stomata [15,79]. This stomatal closure will then limit leaf conductance and gas diffusion, thus limiting the photosynthetic rate and thereby shoot growth [80].
Unlike the shoot growth, applied drought stress significantly improved root growth, as seen from increased root biomass and length. Root length was increased in most varieties under the moderate stress treatment (10% PEG), while in Bubnjar it was increased even under severe stress, suggesting its tolerance to drought stress. Roots biomass increased in almost all varieties under both PEG-treatments, except the variety Silvija where root biomass and root length were reduced under severe stress (20% PEG). The highest increase in root length and biomass was observed for the variety Bubnjar, followed by Pepeljuga and Anđelka. Induction of ABA accumulation by water-deficit conditions induces the expression of transcription factors that downregulates genes involved in cytokinins biosynthesis and signaling and also upregulates cytokinin-degrading genes (CKX) [81]. Cytokinins are negative regulators of root growth whose reduction results in decreased shoot:root ratio (reduced shoot and enhanced root growth), important for plants’ adaptation to drought conditions [82,83]. Improved root growth enable plants to absorb water from deeper soil layer in water-deficit conditions [84,85]. Increased root elongation could be a consequence of larger root meristems formation as cytokinins control the exit of dividing cells from the root meristem [86]. On the other hand, cytokinins are required for the shoot growth and their reduction results in reduced shoot growth in order to save limited resources and the reallocation of the resources for root growth [82,87]. In a study conducted by Bayoumi et al. [77] on wheat, PEG-induced reduction in the shoot and root biomass and coleoptiles length was more pronounced in drought susceptible than tolerant genotypes. Based on these morpho-physiological traits in current research, the variety Bubnjar could be distinguished as the most drought-tolerant variety. Variety Bubnjar showed the lowest reduction in shoot growth and the highest increase in root growth under both stress treatments. Furthermore, considering morpho-physiological traits, the impact of drought stress was the most pronounced in the variety Silvija followed by the variety Fifi. Variety Silvija was found to be the most susceptible among the other experimental varieties showing the highest reduction in germination energy, shoot length and biomass, and root length and biomass.
As an indicator of oxidative stress, lipid peroxidation levels were measured. Lipid peroxidation is a consequence of increased ROS production in plant cells under water-deficit conditions [88]. Both drought stress levels induced lipid peroxidation in seedlings of most varieties in a concentration-dependent manner. These results are consistent with the results obtained by Chakraborty and Pradhan [89], where lipid peroxidation was induced by drought stress in wheat varieties tested, with a higher increase in susceptible varieties. Lipid peroxidation is also an important biomarker of the plants’ susceptibility to stress conditions [90,91]. Abid et al. [92] showed higher lipid peroxidation levels associated with higher ROS content in the sensitive variety. In our study, the most pronounced increase of lipid peroxidation was observed in the variety Silvija, indicating more cellular damage than in other varieties and confirming its sensitivity to drought stress. On the other hand, the lowest lipid peroxidation was shown for the varieties Anđelka and Pepeljuga, indicating a stronger antioxidative response in these varieties. In addition, to better performance in morpho-physiological traits compared to other varieties, lower lipid peroxidation levels in wheat seedlings of Pepeljuga and Anđelka distinguish these varieties as drought-tolerant to some extent.
The antioxidant defense system maintains a balance between ROS production and scavenging in plant cells [93,94,95]. Antioxidative status in wheat seedlings was determined by measuring glutathione contents (GSH and GSSG) and activities of antioxidant enzymes and enzymes of the AsA-GSH pathway (CAT, GST, APX, MDHAR, DHAR, and GR). Changes in enzyme activities were dependent on the variety and the intensity of the drought stress. The most significant role in the antioxidative response of wheat seedlings to drought stress had the AsA-GSH pathway. Numerous studies revealed that enhanced activity of the AsA-GSH pathway conferred better stress tolerance [20], while increased pool size of tGSH in wheat flag leaves implicates its role in drought stress [96]. To overcome the effect of drought stress, seedlings of all varieties displayed significantly higher GSH content under severe drought stress. By participating in direct or indirect ROS neutralization in plant cells, GSH is oxidized to GSSG form reduced back to GSH by the GR enzyme [23]. In seedlings of varieties Rujana and Bubnjar, GSSG concentrations were significantly higher under severe drought stress suggesting higher depletion and lower recycling of GSH under the applied stress. Despite the increased GR activity in these two varieties, GSH was not recycled sufficiently. Higher GST, APX, and DHAR activities in seedlings of variety Bubnjar implied enhanced GSH depletion. Unlike variety Bubnjar, higher concentrations of GSSG in seedlings of variety Rujana, despite the increased GR activity, suggested the involvement of GSH in other detoxication pathways that were not analyzed in this study, such as GPX activity, glyoxalase system, and phytochelatin synthesis [97]. Variety-specific effect on the GSH pool under water deficit conditions in wheat seedlings was found by Gietler et al. [98]. Their experiment showed that the GSH and tGSH pools were higher in drought-tolerant wheat seedlings.
The primary enzymatic component of the AsA-GSH pathway that catalyzes the detoxication of H2O2 using AsA as an electron donor is APX [61]. APX activity was increased in wheat seedlings of Silvija, Bubnjar, Fifi, and Pepeljuga under 20% PEG. In our study, severe drought stress reduced MDHAR activity in varieties Anđelka, Pepeljuga, and Silvija, while treatment with 10% PEG reduced enzyme activity in seedlings of Silvija, Rujana, and Pepeljuga. As a component of the AsA-GSH pathway, MDHAR is responsible for the reduction of monodehydroascorbate to AsA using NAD(P)H as an electron donor [21]. In case of decreased MDHAR activities, monodehydroascorbate could not be converted to AsA, and dehydroascorbate will be produced. Therefore, AsA is recycled by the DHAR that catalyzes the reduction of the dehydroascorbate to AsA using GSH as a reductant. In addition to MDHAR, DHAR has an important role in maintaining the AsA pool in the plant cells [21]. In our study, DHAR activity was significantly increased in wheat seedlings of most varieties under the 20% PEG treatment, while the most significant increase was observed in varieties Anđelka and Pepeljuga. Decreases in MDHAR and DHAR activity were not correlated in our study. In the AsA-GSH pathway, GR is responsible for GSH regeneration using NADPH as a reducing agent [99]. In a study conducted by Chakraborty and Pradhan [89], GR activity showed to be the most important in conferring wheat drought tolerance. Severe drought stress induced GR activity in wheat seedlings of all investigated varieties. The most significant increase in GR activity was recorded for the variety Pepeljuga. Lascano et al. [100] showed that drought-tolerant wheat varieties had increased APX and GR activities and higher tGSH content only under in vitro osmotic stress conditions, while the same response was omitted in drought field conditions. This paper confers different responses of AsA-GSH pathway antioxidative enzymes of the same wheat varieties in different drought stress conditions mainly related to the different magnitude of stress in the studies of water deficit. On the other hand, alternative water deficit protective systems, like drought-related increase in energy dissipation related to zeaxanthin [96,101], could decrease the oxidative load on the AsA-GSH pathway, thus changing its response.
Antioxidant enzymes, GST and CAT, showed less sensitivity to drought stress, and their different regulation in varieties suggested genotype-specific responses. In addition to APX, CAT is one of the major enzymatic scavengers for detoxifying H2O2. CAT activity was increased only in seedlings of the variety Silvija under severe stress. Reduced CAT activity in seedlings of varieties Fifi, Pepeljuga, and Anđelka under severe stress was negatively correlated to lipid peroxidation levels, suggesting that the absence of CAT induction resulted in increased lipid peroxidation in these varieties. Other varieties did not show a similar pattern. GST are enzymes that catalyze the conjugation of GSH to a variety of hydrophobic, electrophilic, and usually cytotoxic substrates, as well as the conversion of H2O2 at the expense of GSH, thereby producing GSSG [102]. Some GST isoforms have glutathione peroxidase activities, which catalyze the reduction of the toxic lipid peroxidation products, thus playing an important role in the maintenance of the membrane [103]. GST activity was increased only in wheat seedlings of varieties Silvija and Bubnjar treated with 20% PEG. Previous investigations about the role of GST in drought stress are relatively inconsistent. Galle et al. [104] found that GST activity was induced by osmotic stress in moderately drought tolerant and resistant wheat varieties. Moreover, Xu et al. [105] showed that transgenic Arabidopsis plants expressing tomato GST enhanced drought stress resistance. Conversely, Chen et al. [106] proposed a negative role of one GST isoform (AtGSTU17) as a component of stress-mediated signal transduction pathways in adaptive responses to drought. Namely, Arabidopsis atgstu17 mutated plants accumulated higher levels of GSH and ABA, better development of primary and lateral root systems.
Antioxidative response in plant cells under abiotic stress conditions is generally increased and correlates with cell protection and plant tolerance [107]. In a study conducted by Abid et al. [92], the tolerant wheat varieties exhibited higher antioxidant enzyme activities and lover lipid peroxidation under drought conditions compared to susceptible varieties. Enhanced antioxidant enzyme activities contribute to drought tolerance by decreasing oxidative damage [92]. Higher antioxidant enzyme activities have been reported to improve stress tolerance [108]. However, previous studies showed different and contradictory results [17]. The discrepancies in the results between different studies could be related to plant age, metabolism, tolerance to stress, duration, and the intensity of applied stress [17]. Additionally, the absence of a linear correlation between some results in our study confirmed the complexity of tolerance mechanisms in plants due to the involvement of different enzymes and genes.
In dehydration conditions, plant cells accumulate osmotically active compounds that, through osmotic adjustment, keep the main physiological functions of the cell [14]. This study analyzed the accumulation of proline as one of the main components of osmotic adjustment. In most varieties, both drought stress levels induced proline accumulation in wheat seedlings. These results are in agreement with the study of Abid et al. [92], who found an accumulation of proline during drought stress at tillering and jointing stages in wheat. Furthermore, in the study of Chakraborty and Pradhan [89], an increase in proline content was observed in the leaves of four wheat varieties exposed to drought, with higher accumulation recorded in drought-tolerant varieties. Other studies also showed that wheat’s proline accumulation contributes to increased osmotic stress tolerance [29,30,109]. In addition to osmoregulation, proline has a role in stabilizing protein and cell membrane structures and mitigating oxidative damage due to scavenging ROS [110]. This protective antioxidant role of proline is also connected with decreasing the TBARS levels, although there was no correlation between proline and TBARS content in our study. Proline accumulation was also recognized as a valuable drought tolerance indicator and could be used as a selection criterion in a wheat breeding program [30,77]. Previous studies reported higher proline accumulation under stress conditions in drought-tolerant varieties than drought-sensitive varieties [29,111]. The highest accumulation of proline was observed in seedlings of Bubnjar, with a 4-fold and 2-fold increase under 10 and 20% PEG treatment, respectively, confirming its tolerance to stress. On the contrary, the variety Silvija showed the lowest proline accumulation, consistent with its sensitivity to drought stress.
In this study, expression patterns of drought-responsive gene encoding P5CS (P5CS), the key enzyme in proline biosynthesis, and genes encoding dehydrins (DHN5 and WZY2) were analyzed. The increase in the relative expression of the analyzed stress-responsive genes, P5CS, WZY2, and especially DHN5, was very high and mainly upregulated under water-deficit conditions, while their expression under the control conditions was shallow. The expression of the P5CS gene was upregulated under severe stress (20% PEG) in wheat seedlings of varieties Rujana, Bubnjar, Fifi, and Pepeljuga, while 10% PEG induced expression of the gene only in seedlings of varieties Bubnjar and FiFi. Upregulation of P5CS under PEG-induced osmotic stress was also demonstrated by Ma et al. [112]. They showed that overexpression of TaP5CS in transgenic Arabidopsis plants increased proline content and decreased lipid peroxidation under osmotic stress. In our study, the expression of P5CS was mostly correlated with the accumulation of proline in wheat seedlings. Many studies have previously reported a strong correlation between increased P5CR enzyme activity or transcript levels and proline accumulation [29,113], leading to increased stress tolerance [33]. The absence of correlation between proline content and the expression of P5CS was observed in variety Anđelka under 10% PEG treatment. This discrepancy could be due to increased protein degradation and reduced proline catabolism in the plant cell under stress [109,114]. In addition to induced proline synthesis, proline accumulation in plant cells could also be a consequence of the inactivation of proline degradation by proline dehydrogenase and pyrroline-5-carboxylate dehydrogenase [114,115]. In the study of Abid et al. [92], a correlation between decreased protein content and increased proline and other amino acid concentrations was obtained.
DHN5 gene has been previously identified by Brini et al. [116]. In their study, Brini et al. [116] showed that expression of the DHN5 gene is induced during embryogenesis, salt stress, and by ABA in vegetative tissues. In a study conducted by Wang et al. [117], the expression of the DHN17 (the same as DHN5—accession no. AY619566) was also induced in leaves and roots of wheat seedlings treated with ABA, suggesting regulation by the ABA signal pathway. In transgenic studies, overexpression of TtDHN5 enhanced tolerance to osmotic and salt stress in transgenic Arabidopsis plants [36,37]. Under osmotic stress, transgenic plants exhibited better growth and higher proline accumulation than wild-type plants. Saibi et al. [36] showed that overexpression of TtDHN5 leads to salinity tolerance through proline and antioxidant metabolism regulation. In their study, TtDHN5 enhances P5CS activity in the transgenic Arabidopsis plants accompanied by proline accumulation. Furthermore, the activities of antioxidant enzymes (SOD, CAT, and POD) are increased in transgenic plants under stress conditions compared to wild type [36]. In transcriptome profiling of DHN5-overexpressing transgenic Arabidopsis plants, Brini et al. [38] identified different upregulated genes, including the MDHAR gene important for AsA recycling in AsA-GSH pathways. They found enhanced tolerance to oxidative stress caused by H2O2. In our study, the importance of the AsA-GSH pathway in stress response was revealed, as it was upregulated, but no correlation between expression of DHN5 and activity of MDHAR enzyme was observed. On the contrary, the activity of this enzyme was mostly reduced under the applied drought stress.
Dehydrin WZY2 gene was isolated from a drought-induced cDNA library of wheat and identified as a drought-stress responsive gene [118,119,120]. In their study, Liu et al. [121] elucidate the regulation of WZY2 expression using different approaches. The expression of the WZY2 is positively regulated by the interaction of the bHLH transcription factor (TabHLH49) with the WZY2 promotor, thus improving the drought stress resistance of wheat. Our study observed no linear correlation between investigated transcription factors and WZY2 gene expression. Liu et al. [122] showed that WZY2 could have an important role in the ABA signaling pathway through interaction with protein phosphatase 2C, a key protein in the ABA signaling pathway to regulate stress-responsive gene expression in wheat. Transgenic studies revealed that transgenic RNAi (WZY2) wheat exhibited lower RWC, antioxidative enzyme activity, and increased lipid peroxidation than wild-type wheat under osmotic stress. On the other hand, overexpression of the TaWZY2 in Arabidopsis plants showed a significant increase in tolerance to drought stress [123].
In order to understand molecular mechanisms of tolerance to drought stress in seedlings of different wheat varieties, genes encoding transcription factors (WRKY2 and DREB1) that regulate the expression of stress-responsive genes were analyzed. The expression of the DREB1 gene was induced under severe drought stress in wheat seedlings of most varieties tested. The DREB1 gene was previously isolated from a drought-induced cDNA library of wheat and identified by Shen et al. [46]. The role of DREB1 transcription factors in wheat under water-deficit conditions is evident from its upregulation in response to drought stress, salinity, and ABA [46,47]. According to Kurahashi et al. [47], more drought-tolerant wheat varieties accumulate more DREB1 gene transcripts under water-deficit conditions than susceptible varieties. Yousfi et al. [124] revealed induction of DREB1 in durum wheat under salinity and drought stress, with tolerant genotypes exhibiting lower expression than susceptible ones. In our study, the highest expression of the DREB1 gene was observed in seedlings of the variety Bubnjar, distinguished as drought-tolerant. Overexpression of AtDREB1A gen in transgenic wheat enhanced drought tolerance, indicating its role in adaptation to water-deficit stress [125]. In a study by Noor et al. [126], overexpression of the AtDREB1 gene in wheat under drought and salinity stress increased RWC, proline, and other metabolites content in transgenic wheat compared to wild-type.
In this study, we analyzed gene expression of the WRKY2 transcription factor, a member of group II consisting of one WRKY domain with a C2HC zink-finger motif [42]. Previously studies showed that overexpression of the TaWRKY2 gene in transgenic wheat seedlings and transgenic Arabidopsis plants enhanced tolerance to drought stress, as evidenced by improved morpho-physiological traits compared to wild-type plants [52,54]. Furthermore, the higher contents of proline and other metabolites provide protection from oxidative stress and osmotic damage. Due to its role in drought tolerance, Niu et al. [54] suggested the WRKY2 gene as a good candidate for improved drought tolerance of wheat varieties using transgenic technology. Multiple regulatory cis-elements were identified in the promoter region of the WRKY2 gene implying its regulation by multiple stress conditions (drought, salt, heat, and ABA) [52]. Contrary to the results obtained in mentioned studies, in our study, the expression of the TaWRKY2 gene was downregulated by the PEG-induced drought stress. Three varieties (Silvija, Rujana, and Anđelka) showed lower levels of WRKY2 gene transcript. The most pronounced impact of drought stress was observed for the variety Silvija where both PEG treatments reduced gene expression. No linear correlation between the expression of WRKY2 compared to other measured parameters was observed. Variety Silvija, with a higher degree of WRKY2 reduction, was previously distinguished as a drought-susceptible variety based on other parameters. It could be that WRKY2 was regulated by some transcriptional repressors that modulate plant stress responses. Such a large extent in suppression of WRKY2 gene expression in seedlings of the variety Silvija could be related to reduced morphological traits (root and shoot length and biomass) under the drought stress treatment. In their study, Hu et al. [127] identified the WRKY51 gene as the key factor in promoting lateral root formation through negative regulation of ethylene biosynthesis in wheat. Our results suggest that reduced WRKY2 gene expression could impact root formation, thus affecting seedling growth of the Silvija variety under drought stress conditions. This effect was not observed in other varieties, probably due to a more extensive reduction of WRKY2 gene expression in the variety Silvija compared to other varieties. Interestingly, in transcriptome profiling of transgenic Arabidopsis plants with DHN5 overexpression, Brini et al. [38] showed downregulation of some WRKY transcription factors (WRKY33 and WRKY70). Although WRKY2 was mostly downregulated and DHN5 upregulated under the drought stress conditions in our study, no linear correlation between the expression of the DHN5 and WRKY2 was observed.
Variety Bubnjar was distinguished as the most drought-tolerant variety. Enhanced drought resistance in Bubnjar was associated with osmotic adjustment, including the expression of genes encoding dehydrin proteins. Despite the active AsA-GSH pathway, high lipid peroxidation levels and high GSSG content in Bubnjar seedlings suggest lower antioxidative response in that variety. Varieties Anđelka and Pepeljuga showed drought tolerance to some extent, although mechanisms underlying their tolerance differed compared to the variety Bubnjar. In a variety Pepeljuga, high proline content and the expression of stress-responsive genes encoding dehydrins and P5CS contributed to osmotic adjustment as a mechanism of tolerance together with the activity of the AsA-GSH pathway. Tolerance strategies of variety Anđelka involved only the induction of antioxidative defense through the AsA-GSH pathway. Considering obtained data, variety Silvija was found to be drought-susceptible. In the variety Silvija, regardless of the antioxidative system induction (increased APX, GST, and CAT activity) and dehydrin genes expression, drought stress caused oxidative damage to lipids and impaired root and shoot growth. Variety Fifi revealed a high expression of the dehydrin genes, increased P5CS expression, and consequently proline accumulation. Reduced growth parameters could be due to the direction of the metabolism towards osmolyte and dehydrin synthesis.
5. Conclusions
Drought tolerance involves a complex network of different mechanisms comprising a number of proteins and genes, interactions between transcription factors and corresponding genes, and different signal transduction pathways and their mutual interactions. In the present study, different mechanisms have been shown to contribute to drought tolerance in different varieties, mainly related to osmotic adjustment and dehydrins expression. The most important role of antioxidant response was played by glutathione along with the AsA-GSH pathway. Genes encoding transcription factors were shown to be differentially regulated. The expression of DREB1 was upregulated under severe drought stress in most varieties, while the expression of WRKY2, unlike the other studies, was downregulated or revealed control levels, thus requiring further and broader investigation. Identifying different mechanisms in drought stress response would advance our understanding of the complex strategies contributing to wheat tolerance in the early growth stage and could contribute to variety selection useful for further development of new drought-tolerant varieties. Furthermore, elucidation of the proteins and genes involved in biochemical and molecular mechanisms under water-deficit conditions may lead to genetic improvement of wheat using transgenic technology. Considering obtained data, variety Silvija was drought-susceptible, while variety Bubnjar showed greater drought stress tolerance, suggesting better germination potential under water deficit conditions relative to other varieties.
Author Contributions
Conceptualization, V.Š., R.V. and I.Š.Č.; methodology, R.V., A.V. and I.Š.Č.; formal analysis, R.V., I.Š.Č., A.V., L.B. and K.Š.; investigation, R.V., V.Š., I.Š.Č., A.V., K.Š., L.B., S.M., N.S. and R.S.; resources, V.Š., R.V. and I.Š.Č.; writing—original draft preparation, R.V.; writing—review and editing, I.Š.Č. and V.Š.; visualization, R.V.; project administration, V.Š. and R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by European Union, who provided the EUROPEAN REGIONAL DEVELOPMENT FUND, grant number KK.01.1.1.04.0067.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arora N.K. Impact of climate change on agriculture production and its sustainable solutions. J. Environ. Sustain. 2019;2:95–96. doi: 10.1007/s42398-019-00078-w. [DOI] [Google Scholar]
- 2.Spinoni J., Naumann G., Vogt J.V., Barbosa P. The biggest drought events in Europe from 1950 to 2012. J. Hydrol. Reg. Stud. 2015;3:509–524. doi: 10.1016/j.ejrh.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javadinejad S., Dara R., Jafary F. Analysis and prioritization the effective factors on increasing farmers resilience under climate change and drought. Agric. Res. 2021;10:497–513. doi: 10.1007/s40003-020-00516-w. [DOI] [Google Scholar]
- 4.Marinović I., Cindrić Kalin K., Güttler I., Pasarić Z. Dry spells in Croatia: Observed climate change and climate projections. Atmosphere. 2021;12:652. doi: 10.3390/atmos12050652. [DOI] [Google Scholar]
- 5.Barić M., Kerfša S., Habus Jerčić I., Havrda S., Gelencir D. Evaluation and characterization of Croatian winter wheat genotypes (T. aestivum L.) for drought tolerance. Cereal Res. Commun. 2008;36:1031–1034. [Google Scholar]
- 6.Wolny E., Betekhtin A., Rojek M., Braszewska-Zalewska A., Lusinska J., Hasterok R. Germination and the early stages of seedling development in Brachypodium distachyon. Int. J. Mol. Sci. 2018;19:2916. doi: 10.3390/ijms19102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy Y.A.N., Reddy Y.N.P., Ramya V., Suma L.S., Reddy A.B.N., Krishna S.S. Chapter 8—Drought adaptation: Approaches for crop improvement. In: Singh M., Sood S., editors. Millets and Pseudo Cereals. Woodhead Publishing; Sawston, UK: 2021. pp. 143–158. [Google Scholar]
- 8.Kizilgeçi F., Tazebay N., Namli M., Albayrak Ö., Yıldırım M. The drought effect on seed germination and seedling growth in bread wheat (Triticum aestivum L.) Int. J. Agric. Environ. Food Sci. 2017;1:33–37. doi: 10.31015/jaefs.17005. [DOI] [Google Scholar]
- 9.Mahpara S., Zainab A., Ullah R., Kausar S., Bilal M., Latif M.I., Arif M., Akhtar I., Al-Hashimi A., Elshikh M.S., et al. The impact of PEG-induced drought stress on seed germination and seedling growth of different bread wheat (Triticum aestivum L.) genotypes. PLoS ONE. 2022;17:e0262937. doi: 10.1371/journal.pone.0262937. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Chun S.C., Paramasivan M., Chandrasekaran M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018;9:2525. doi: 10.3389/fmicb.2018.02525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulkarni M., Soolanayakanahally R., Ogawa S., Uga Y., Selvaraj M.G., Kagale S. Drought response in wheat: Key genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Front. Chem. 2017;5:106. doi: 10.3389/fchem.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuşvuran Ş., Daşgan Y., Abak K. Responses of different melon genotypes to drought stress. Yüzüncü Yıl Univ. J. Agric. Sci. 2011;21:209–219. [Google Scholar]
- 13.Qayyum A., Al Ayoubi S., Sher A., Bibi Y., Ahmad S., Shen Z., Jenks M.A. Improvement in drought tolerance in bread wheat is related to an improvement in osmolyte production, antioxidant enzyme activities, and gaseous exchange. Saudi J. Biol. Sci. 2021;28:5238–5249. doi: 10.1016/j.sjbs.2021.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders G.J., Arndt S.K. Osmotic adjustment under drought conditions. In: Aroca R., editor. Plant Responses to Drought Stress: From Morphological to Molecular Features. Springer; Berlin/Heidelberg, Germany: 2012. pp. 199–229. [Google Scholar]
- 15.Reddy A.R., Chaitanya K.V., Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. ROS signaling: The new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Cruz de Carvalho M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008;3:156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mickky B.M., Aldesuquy H.S. Impact of osmotic stress on seedling growth observations, membrane characteristics and antioxidant defense system of different wheat genotypes. Egypt. J. Basic Appl. Sci. 2019;4:47–54. doi: 10.1016/j.ejbas.2016.10.001. [DOI] [Google Scholar]
- 19.Ahmad P., Jaleel C.A., Salem M.A., Nabi G., Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010;30:161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- 20.Hasanuzzaman M., Bhuyan M., Anee T.I., Parvin K., Nahar K., Mahmud J.A., Fujita M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8:384. doi: 10.3390/antiox8090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey P., Singh J., Achary V.M.M., Reddy M.K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015;3:25. doi: 10.3389/fenvs.2015.00025. [DOI] [Google Scholar]
- 22.Noctor G., Foyer C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 23.Hasanuzzaman M., Nahar K., Anee T.I., Fujita M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants. 2017;23:249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossain M.A., Piyatida P., da Silva J.A.T., Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012;2012:872875. doi: 10.1155/2012/872875. [DOI] [Google Scholar]
- 25.Chaves M.M., Maroco J.P., Pereira J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- 26.De Carvalho K., de Campos M.K.F., Domingues D.S., Pereira L.F.P., Vieira L.G.E. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 2013;40:3269–3279. doi: 10.1007/s11033-012-2402-5. [DOI] [PubMed] [Google Scholar]
- 27.Slama I., Abdelly C., Bouchereau A., Flowers T., Savouré A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015;115:433–447. doi: 10.1093/aob/mcu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabados L., Kovács H., Zilberstein A., Bouchereau A. Plants in extreme environments. Adv. Bot. Res. 2011;57:105–150. doi: 10.1016/b978-0-12-387692-8.00004-7. [DOI] [Google Scholar]
- 29.Maghsoudi K., Emam Y., Niazi A., Pessarakli M., Arvin M.J. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J. Plant Interact. 2018;13:461–471. doi: 10.1080/17429145.2018.1506516. [DOI] [Google Scholar]
- 30.Mwadzingeni L., Shimelis H., Tesfay S., Tsilo T.J. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016;7:1276. doi: 10.3389/fpls.2016.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu C.A., Delauney A.J., Verma D.P. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl. Acad. Sci. USA. 1992;89:9354–9358. doi: 10.1073/pnas.89.19.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meena M., Divyanshu K., Kumar S., Swapnil P., Zehra A., Shukla V., Yadav M., Upadhyay R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon. 2019;5:e02952. doi: 10.1016/j.heliyon.2019.e02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vendruscolo E.C.G., Schuster I., Pileggi M., Scapim C.A., Molinari H.B.C., Marur C.J., Vieira L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007;164:1367–1376. doi: 10.1016/j.jplph.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Abedini R., GhaneGolmohammadi F., PishkamRad R., Pourabed E., Jafarnezhad A., Shobbar Z.S., Shahbazi M. Plant dehydrins: Shedding light on structure and expression patterns of dehydrin gene family in barley. J. Plant Res. 2017;130:747–763. doi: 10.1007/s10265-017-0941-5. [DOI] [PubMed] [Google Scholar]
- 35.Hu L., Wang Z., Du H., Huang B. Differential accumulation of dehydrins in response to water stress for hybrid and common bermudagrass genotypes differing in drought tolerance. J. Plant Physiol. 2010;167:103–109. doi: 10.1016/j.jplph.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Saibi W., Feki K., Ben Mahmoud R., Brini F. Durum wheat dehydrin (DHN-5) confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta. 2015;242:1187–1194. doi: 10.1007/s00425-015-2351-z. [DOI] [PubMed] [Google Scholar]
- 37.Brini F., Hanin M., Lumbreras V., Amara I., Khoudi H., Hassairi A., Pages M., Masmoudi K. Overexpression of wheat dehydrin DHN-5 enhances tolerance to salt and osmotic stress in Arab. thaliana. Plant Cell Rep. 2007;26:2017–2026. doi: 10.1007/s00299-007-0412-x. [DOI] [PubMed] [Google Scholar]
- 38.Brini F., Yamamoto A., Jlaiel L., Takeda S., Hobo T., Dinh H.Q., Hattori T., Masmoudi K., Hanin M. Pleiotropic effects of the wheat dehydrin DHN-5 on stress responses in Arabidopsis. Plant Cell Physiol. 2011;52:676–688. doi: 10.1093/pcp/pcr030. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Wang H., Shao H., Tang X. Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Front. Plant Sci. 2016;7:67. doi: 10.3389/fpls.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Javed T., Shabbir R., Ali A., Afzal I., Zaheer U., Gao S.J. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants. 2020;9:491. doi: 10.3390/plants9040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X., Li C., Wang H., Guo Z. WRKY transcription factors: Evolution, binding, and action. Phytopathol. Res. 2019;1:13. doi: 10.1186/s42483-019-0022-x. [DOI] [Google Scholar]
- 43.Chen F., Hu Y., Vannozzi A., Wu K., Cai H., Qin Y., Mullis A., Lin Z., Zhang L. The WRKY Transcription factor family in model plants and crops. Crit. Rev. Plant Sci. 2018;36:311–335. doi: 10.1080/07352689.2018.1441103. [DOI] [Google Scholar]
- 44.Sharoni A.M., Nuruzzaman M., Satoh K., Shimizu T., Kondoh H., Sasaya T., Choi I.-R., Omura T., Kikuchi S. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol. 2011;52:344–360. doi: 10.1093/pcp/pcq196. [DOI] [PubMed] [Google Scholar]
- 45.Riechmann J.L., Meyerowitz E.M. The AP2/EREBP family of plant transcription factors. Biol. Chem. 1998;379:633–646. doi: 10.1515/bchm.1998.379.6.633. [DOI] [PubMed] [Google Scholar]
- 46.Shen Y.G., Zhang W.K., He S.J., Zhang J.S., Liu Q., Chen S.Y. An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor. Appl. Genet. 2003;106:923–930. doi: 10.1007/s00122-002-1131-x. [DOI] [PubMed] [Google Scholar]
- 47.Kurahashi Y., Terashima A., Takumi S. Variation in dehydration tolerance, ABA sensitivity and related gene expression patterns in D-genome progenitor and synthetic hexaploid wheat lines. Int. J. Mol. Sci. 2009;10:2733–2751. doi: 10.3390/ijms10062733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zotova L., Kurishbayev A., Jatayev S., Khassanova G., Zhubatkanov A., Serikbay D., Sereda S., Sereda T., Shvidchenko V., Lopato S., et al. Genes encoding transcription factors TaDREB5 and TaNFYC-A7 are differentially expressed in leaves of bread wheat in response to drought, dehydration and ABA. Front. Plant Sci. 2018;9:1441. doi: 10.3389/fpls.2018.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu M., Wang Z., Xiao H.M., Yang Y. Characterization of TaDREB1 in wheat genotypes with different seed germination under osmotic stress. Hereditas. 2018;155:26. doi: 10.1186/s41065-018-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin Y., Tian Y., Liu X. A wheat salinity-induced WRKY transcription factor TaWRKY93 confers multiple abiotic stress tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2015;464:428–433. doi: 10.1016/j.bbrc.2015.06.128. [DOI] [PubMed] [Google Scholar]
- 51.Niu X., Luo T., Zhao H., Su Y., Ji W., Li H. Identification of wheat DREB genes and functional characterization of TaDREB3 in response to abiotic stresses. Gene. 2020;740:144514. doi: 10.1016/j.gene.2020.144514. [DOI] [PubMed] [Google Scholar]
- 52.Gao H., Wang Y., Xu P., Zhang Z. Overexpression of a WRKY transcription factor TaWRKY2 enhances drought stress tolerance in transgenic wheat. Front. Plant Sci. 2018;9:997. doi: 10.3389/fpls.2018.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C., Deng P., Chen L., Wang X., Ma H., Hu W., Yao N., Feng Y., Chai R., Yang G., et al. A Wheat WRKY transcription factor TaWRKY10 confers tolerance to multiple abiotic stresses in transgenic tobacco. PLoS ONE. 2013;8:e65120. doi: 10.1371/journal.pone.0065120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niu C.F., Wei W., Zhou Q.Y., Tian A.G., Hao Y.J., Zhang W.K., Ma B., Lin Q., Zhang Z.B., Zhang J.S., et al. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 2012;35:1156–1170. doi: 10.1111/j.1365-3040.2012.02480.x. [DOI] [PubMed] [Google Scholar]
- 55.Barrs H. Determination of water deficits in plant tissue. In: Kozlowski T., editor. Water Deficits and Plant Growth. Volume 1. Academic Press; Cambridge, MA, USA: 1968. pp. 235–368. [Google Scholar]
- 56.Carillo P., Gibon Y. PROTOCOL Extraction and Determination of Proline. 2011. [(accessed on 3 March 2022)]. Available online: https://www.researchgate.net/publication/211353600_PROTOCOL_Extraction_and_determination_of_proline.
- 57.Verma S., Dubey R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003;164:645–655. doi: 10.1016/S0168-9452(03)00022-0. [DOI] [Google Scholar]
- 58.Griffith O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 59.Aebi H. Methods in Enzymology. Volume 105. Academic Press; Cambridge, MA, USA: 1984. Catalase in vitro; pp. 121–126. [DOI] [PubMed] [Google Scholar]
- 60.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- 61.Nakano Y., Asada K. Hydrogen Peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- 62.Racker E. Glutathione reductase from bakers’ yeast and beef liver. J. Biol. Chem. 1955;217:855–865. doi: 10.1016/S0021-9258(18)65950-2. [DOI] [PubMed] [Google Scholar]
- 63.Ma F., Cheng L. Exposure of the shaded side of apple fruit to full sun leads to up-regulation of both the xanthophyll cycle and the ascorbate–glutathione cycle. Plant Sci. 2004;166:1479–1486. doi: 10.1016/j.plantsci.2004.01.024. [DOI] [Google Scholar]
- 64.Murshed R., Lopez-Lauri F., Sallanon H. Microplate quantification of enzymes of the plant ascorbate–glutathione cycle. Anal. Biochem. 2008;383:320–322. doi: 10.1016/j.ab.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 65.Hossain M.A., Nakano Y., Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395. doi: 10.1093/oxfordjournals.pcp.a076726. [DOI] [Google Scholar]
- 66.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 67.Paolacci A.R., Tanzarella O.A., Porceddu E., Ciaffi M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009;10:11. doi: 10.1186/1471-2199-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmad A., Aslam Z., Javed T., Hussain S., Raza A., Shabbir R., Mora-Poblete F., Saeed T., Zulfiqar F., Ali M.M., et al. Screening of Wheat (Triticum aestivum L.) Genotypes for drought tolerance through agronomic and physiological response. Agronomy. 2022;12:287. doi: 10.3390/agronomy12020287. [DOI] [Google Scholar]
- 69.Baloch M.J., Dunwell J., Khan N.U., Jatoi W.A., Khakhwani A.A., Vessar N.F., Gul S. Morpho-physiological characterization of spring wheat genotypes under drought stress. Int. J. Agric. Biol. 2013;15:945–950. [Google Scholar]
- 70.Ali M.A., Abbas A., Niaz S., Zulkiffal M., Ali S. Morpho-physiological criteria for drought tolerance in sorghum (Sorghum bicolor) at seedling and post-anthesis stages. Int. J. Agric. Biol. 2009;11:674–680. [Google Scholar]
- 71.Chowdhury M.K., Hasan M.A., Bahadur M.M., Islam M.R., Hakim M.A., Iqbal M.A., Javed T., Raza A., Shabbir R., Sorour S., et al. Evaluation of drought tolerance of some wheat (Triticum aestivum L.) genotypes through phenology, growth, and physiological indices. Agronomy. 2021;11:1792. doi: 10.3390/agronomy11091792. [DOI] [Google Scholar]
- 72.Albuquerque M.C., Carvalho N.M. Effect of the type of environmental stress on the emergence of sunflower (Helianthus annus L.), soybean (Glycine max (L.) Merril) and maize (Zea mays L.) seeds with different levels of vigor. Seed Sci. Technol. 2003;31:465–479. doi: 10.15258/sst.2003.31.2.23. [DOI] [Google Scholar]
- 73.Yadav P.V., Kumari M., Ahmed Z. Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in Capsicum. Res. J. Seed Sci. 2011;4:125–136. doi: 10.3923/rjss.2011.125.136. [DOI] [Google Scholar]
- 74.Bateman A., Lewandrowski W., Stevens J., Muñoz-Rojas M. The limitations of seedling growth and drought tolerance to novel soil substrates in arid systems: Implications for restoration success; Proceedings of the EGU General Assembly; Vienna, Austria. 17–22 April 2016; p. 1722. [Google Scholar]
- 75.Datta J.K., Mondal T., Banerjee A., Mondal N.K. Assessment of drought tolerance of selected wheat cultivars under laboratory condition. J. Agric. Sci. Technol. 2011;7:383–393. [Google Scholar]
- 76.Schonfeld M.A., Johnson R.C., Carver B.F., Mornhinweg D.W. Water Relations in Winter Wheat as Drought Resistance Indicators. Crop. Sci. 1988;28:526–531. doi: 10.2135/cropsci1988.0011183X002800030021x. [DOI] [Google Scholar]
- 77.Bayoumi T.Y., Eid M.H., Metwali E.M. Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. Afr. J. Biotechnol. 2008;7:2341–2352. [Google Scholar]
- 78.Fang Y., Du Y., Wang J., Wu A., Qiao S., Xu B., Zhang S., Siddique K.H.M., Chen Y. Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 2017;8:672. doi: 10.3389/fpls.2017.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Socias X., Correia M.J., Chaves M., Medrano H. The role of abscisic acid and water relations in drought responses of subterranean clover. J. Exp. Bot. 1997;48:1281–1288. doi: 10.1093/jxb/48.6.1281. [DOI] [Google Scholar]
- 80.Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- 81.Li W., Herrera-Estrella L., Tran L.-S.P. The Yin–Yang of cytokinin homeostasis and drought acclimation/adaptation. Trends Plant Sci. 2016;21:548–550. doi: 10.1016/j.tplants.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Werner T., Nehnevajova E., Köllmer I., Novák O., Stmad M., Krämer U., Schmülling T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell. 2010;22:3905–3920. doi: 10.1105/tpc.109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poorter H., Niklas K.J., Reich P.B., Oleksyn J., Poot P., Mommer L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012;193:30–50. doi: 10.1111/j.1469-8137.2011.03952.x. [DOI] [PubMed] [Google Scholar]
- 84.Liu D., Yang W., Zhao X. Studies on watermelon drought tolerance identification indices and method in gravel-mulched land of northwest China. China Veg. 2008;7:17–21. [Google Scholar]
- 85.Arai-Sanoh Y., Takai T., Yoshinaga S., Nakano H., Kojima M., Sakakibara H., Kondo M., Uga Y. Deep rooting conferred by DEEPER ROOTING 1 enhances rice yield in paddy fields. Sci. Rep. 2014;4:5563. doi: 10.1038/srep05563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dello Ioio R., Linhares F.S., Scacchi E., Casamitjana-Martinez E., Heidstra R., Costantino P., Sabatini S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007;17:678–682. doi: 10.1016/j.cub.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 87.Werner T., Holst K., Pörs Y., Guivarc’h A., Mustroph A., Chriqui D., Grimm B., Schmülling T. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J. Exp. Bot. 2008;59:2659–2672. doi: 10.1093/jxb/ern134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandey H.C., Bhatt R., Chandra A., Bhatt R. Drought stress induced changes in lipid peroxidation and antioxidant system in genus Avena. J. Environ. Biol. 2010;31:435–440. [PubMed] [Google Scholar]
- 89.Chakraborty U., Pradhan B. Drought stress-induced oxidative stress and antioxidative responses in four wheat (Triticum aestivum L.) varieties. Arch. Agron. Soil Sci. 2012;58:617–630. doi: 10.1080/03650340.2010.533660. [DOI] [Google Scholar]
- 90.Sachdev S., Ansari S.A., Ansari M.I., Fujita M., Hasanuzzaman M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants. 2021;10:277. doi: 10.3390/antiox10020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Sammarraie O.N., Alsharafa K.Y., Al-limoun M.O., Khleifat K.M., Al-Sarayreh S.A., Al-Shuneigat J.M., Kalaji H.M. Effect of various abiotic stressors on some biochemical indices of Lepidium sativum plants. Sci. Rep. 2020;10:21131. doi: 10.1038/s41598-020-78330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abid M., Ali S., Qi L.K., Zahoor R., Tian Z., Jiang D., Snider J.L., Dai T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.) Sci. Rep. 2018;8:4615. doi: 10.1038/s41598-018-21441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arora A., Sairam R.K., Srivastava G. Oxidative stress and antioxidative system in plants. Curr. Sci. 2001;82:1227–1238. [Google Scholar]
- 94.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 95.Dumanović J., Nepovimova E., Natić M., Kuča K., Jaćević V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021;11:2969. doi: 10.3389/fpls.2020.552969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herbinger K., Tausz M., Wonisch A., Soja G., Sorger A., Grill D. Complex interactive effects of drought and ozone stress on the antioxidant defence systems of two wheat cultivars. Plant Physiol. Biochem. 2002;40:691–696. doi: 10.1016/S0981-9428(02)01410-9. [DOI] [Google Scholar]
- 97.Štolfa I., Špoljarić Maronić D., Žuna Pfeiffer T., Lončarić Z. Glutathione and related enzymes in response to abiotic stress. In: Gupta D.K., Palma J.M., Corpas F.J., editors. Redox State as a Central Regulator of Plant-Cell Stress Responses. Springer International Publishing; Berlin/Heidelberg, Germany: 2016. pp. 183–211. [Google Scholar]
- 98.Gietler M., Nykiel M., Zagdańska B.M. Changes in the reduction state of ascorbate and glutathione, protein oxidation and hydrolysis leading to the development of dehydration intolerance in Triticum aestivum L. seedlings. Plant Growth Regul. 2016;79:287–297. doi: 10.1007/s10725-015-0133-z. [DOI] [Google Scholar]
- 99.Gill S., Anjum N., Hasanuzzaman M., Gill R., Trivedi D., Ahmad I., Pereira E., Tuteja N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013;70:204–212. doi: 10.1016/j.plaphy.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 100.Lascano H., Antonicelli G., Luna C., Melchiorre M., Gómez L., Racca R., Trippi V., Casano L. Antioxidant system response of different wheat cultivars under drought: Field and in vitro studies. Funct. Plant Biol. 2001;28:1095–1102. doi: 10.1071/PP01061. [DOI] [Google Scholar]
- 101.Tambussi E.A., Casadesús J., Munné-Bosch S., Araus J. Photoprotection in water-stressed plants of durum wheat (Triticum turgidum var. durum): Changes in chlorophyll fluorescence, spectral signature and photosynthetic pigments. Funct. Plant Biol. 2002;29:35–44. doi: 10.1071/PP01104. [DOI] [PubMed] [Google Scholar]
- 102.Marrs K.A. The functions and regulation of glutathione s-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- 103.Dixon D.P., Davis B.G., Edwards R. Functional divergence in the glutathione transferase superfamily in plants. Identification of two classes with putative functions in redox homeostasis in Arabidopsis thaliana. J. Biol. Chem. 2002;277:30859–30869. doi: 10.1074/jbc.M202919200. [DOI] [PubMed] [Google Scholar]
- 104.Galle A., Csiszár J., Szécsényi M., Erdei L., Benyó D., Györgyey J., Tari I. Induction and regulation of glutathione transferases in wheat species exposed to PEG induced osmotic stress. Acta Biol. Szeged. 2011;55:79–80. [Google Scholar]
- 105.Xu J., Xing X.-J., Tian Y.-S., Peng R.-H., Xue Y., Zhao W., Yao Q.-H. Transgenic Arabidopsis plants expressing tomato glutathione S-transferase showed enhanced resistance to salt and drought stress. PLoS ONE. 2015;10:e0136960. doi: 10.1371/journal.pone.0136960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen J.-H., Jiang H.-W., Hsieh E.-J., Chen H.-Y., Chien C.-T., Hsieh H.-L., Lin T.-P. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 2012;158:340–351. doi: 10.1104/pp.111.181875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 108.Mehrabad Pour-Benab S., Fabriki-Ourang S., Mehrabi A.-A. Expression of dehydrin and antioxidant genes and enzymatic antioxidant defense under drought stress in wild relatives of wheat. Biotechnol. Biotechnol. Equip. 2019;33:1063–1073. doi: 10.1080/13102818.2019.1638300. [DOI] [Google Scholar]
- 109.Bhaskara G.B., Yang T.H., Verslues P.E. Dynamic proline metabolism: Importance and regulation in water limited environments. Front. Plant Sci. 2015;6:484. doi: 10.3389/fpls.2015.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ashraf M., Foolad M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59:206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- 111.Saeedipour S. Relationship of grain yield, ABA and proline accumulation in tolerant and sensitive wheat cultivars as affected by water stress. Proc. Natl. Acad. Sci. USA. 2013;83:311–315. doi: 10.1007/s40011-012-0147-5. [DOI] [Google Scholar]
- 112.Ma L., Zhou E., Gao L., Mao X., Zhou R., Jia J. Isolation, expression analysis and chromosomal location of P5CR gene in common wheat (Triticum aestivum L.) S. Afr. J. Bot. 2008;74:705–712. doi: 10.1016/j.sajb.2008.05.003. [DOI] [Google Scholar]
- 113.Hien D.T., Jacobs M., Angenon G., Hermans C., Thu T.T., Son L.V., Roosens N.H. Proline accumulation and Δ1-pyrroline-5-carboxylate synthetase gene properties in three rice cultivars differing in salinity and drought tolerance. Plant Sci. 2003;165:1059–1068. doi: 10.1016/S0168-9452(03)00301-7. [DOI] [Google Scholar]
- 114.Sharma S., Villamor J.G., Verslues P.E. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011;157:292–304. doi: 10.1104/pp.111.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tateishi Y., Nakagawa T., Esaka M. Osmotolerance and growth stimulation of transgenic tobacco cells accumulating free proline by silencing proline dehydrogenase expression with double-stranded RNA interference technique. Physiol. Plant. 2005;125:224–234. doi: 10.1111/j.1399-3054.2005.00553.x. [DOI] [Google Scholar]
- 116.Brini F., Hanin M., Lumbreras V., Irar S., Pagès M., Masmoudi K. Functional characterization of DHN-5, a dehydrin showing a differential phosphorylation pattern in two Tunisian durum wheat (Triticum durum Desf.) varieties with marked differences in salt and drought tolerance. Plant Sci. 2007;172:20–28. doi: 10.1016/j.plantsci.2006.07.011. [DOI] [Google Scholar]
- 117.Wang Y., Xu H., Zhu H., Tao Y., Zhang G., Zhang L., Zhang C., Zhang Z., Ma Z. Classification and expression diversification of wheat dehydrin genes. Plant Sci. 2014;214:113–120. doi: 10.1016/j.plantsci.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 118.Zhu W., Zhang L., Lv H., Zhang H., Zhang D., Wang X., Chen J. The dehydrin wzy2 promoter from wheat defines its contribution to stress tolerance. Funct. Integr. Genom. 2014;14:111–125. doi: 10.1007/s10142-013-0354-z. [DOI] [PubMed] [Google Scholar]
- 119.Zhu W., Zhang L., Zhang N., Xing Y., Jiang B. The clone of wheat dehydrin-like gene wzy2 and its functional analysis in Pichia pastoris. Afr. J. Biotechnol. 2012;11:9549–9558. doi: 10.5897/AJB11.3470. [DOI] [Google Scholar]
- 120.Huang F., Zhang L., Wang L., Jiang B., Wen J. Cloning and sequence analysis of a new dehydrin gene (WZY2) from wheat. J. Northwest A F Univ. Nat. Sci. Ed. 2009;37:93–99. [Google Scholar]
- 121.Liu H., Yang Y., Liu D., Wang X., Zhang L. Transcription factor TabHLH49 positively regulates dehydrin WZY2 gene expression and enhances drought stress tolerance in wheat. BMC Plant Biol. 2020;20:259. doi: 10.1186/s12870-020-02474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu H., Yang Y., Zhang L. Identification of upstream transcription factors and an interacting PP2C protein of dehydrin WZY2 gene in wheat. Plant Signal. Behav. 2019;14:1678370. doi: 10.1080/15592324.2019.1678370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu Z., Wang X., Mu X., Zhang L. RNAi mediated silencing of dehydrin gene WZY2 confers osmotic stress intolerance in transgenic wheat. Funct. Plant Biol. 2019;46:877–884. doi: 10.1071/FP19068. [DOI] [PubMed] [Google Scholar]
- 124.Yousfi S., Márquez A.J., Betti M., Araus J.L., Serret M.D. Gene expression and physiological responses to salinity and water stress of contrasting durum wheat genotypes. J. Integr. Plant Biol. 2016;58:48–66. doi: 10.1111/jipb.12359. [DOI] [PubMed] [Google Scholar]
- 125.Pellegrineschi A., Reynolds M., Pacheco M., Brito R.M., Almeraya R., Yamaguchi-Shinozaki K., Hoisington D. Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome. 2004;47:493–500. doi: 10.1139/g03-140. [DOI] [PubMed] [Google Scholar]
- 126.Noor S., Ali S., Rahman H.-u., Farhatullah F., Ali G.M. Comparative study of transgenic (DREB1A) and non-transgenic wheat lines on relative water content, sugar, proline and chlorophyll under drought and salt stresses. Sarhad J. Agric. 2018;34:986–993. doi: 10.17582/journal.sja/2018/34.4.986.993. [DOI] [Google Scholar]
- 127.Hu Z., Wang R., Zheng M., Liu X., Meng F., Wu H., Yao Y., Xin M., Peng H., Ni Z., et al. TaWRKY51 promotes lateral root formation through negative regulation of ethylene biosynthesis in wheat (Triticum aestivum L.) Plant J. 2018;96:372–388. doi: 10.1111/tpj.14038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data is contained within the article.