Abstract
Background:
Glucocorticoid steroids are standard of care in Duchenne Muscular Dystrophy (DMD) to slow disease course. Use of glucocorticoids in other muscular dystrophies, including Becker (BMD) and Limb Girdle (LGMD), has been less explored. Recently, preclinical studies conducted in DMD and LGMD mouse models showed once-weekly prednisone was associated with improved muscle performance without activation of muscle atrophy genes.
Objective:
To determine safety and tolerability of once-weekly prednisone in patients with LGMD and BMD.
Methods:
We conducted an open label, exploratory single center study of of once-weekly prednisone at 0.75–1 mg/Kg in LGMD (n = 19) and BMD (n = 1) (mean age 35, range 18–60). The LGMD participants represented multiple different LGMD subtypes, and the study included ambulatory and non-ambulatory participants. Participants were assessed at baseline and 24 weeks for vital signs, blood biomarkers, and for patient-reported side effects. As secondary endpoints, functional muscle testing and body composition were measured.
Results:
Over the 24-week study, there were no significant changes in blood pressure, HgbA1C, or lipid profiles. We observed a reduction in serum creatine kinase over the study interval. Whole body DEXA scanning suggested a possible increase in lean mass and a reduction in adiposity. Functional measures suggested trends in improved muscle performance.
Conclusions:
In this single center, open label pilot study, once-weekly prednisone was safe and well tolerated. Additional investigation of once-weekly prednisone in a larger cohort and for a longer period of time is warranted.
Keywords: Muscular dystrophy, prednisone, Limb-Girdle Muscular Dystrophy, Becker Muscular Dystrophy, creatine kinase, neuromuscular diseases
INTRODUCTION
Corticosteroids are standard of care in Duchenne Muscular Dystrophy (DMD) [1]. Multiple steroids, including prednisone, deflazacort, and dosing regimens are under investigation for optimal steroid treatment in DMD patients to minimize side effects and maximize clinical benefit [2, 3]. Among steroid dosing strategies, the most studied is prednisone 0.75 mg/kg/day with multiple randomized clinical trials showing improved muscle strength and functional outcomes [4]. Attempting to minimize adverse effects including behavioral changes, investigators previously evaluated high dose weekend pulse steroids for efficacy in DMD models and in DMD patients [5–7]. In these studies, high dose weekend dosing was also found to be effective in stabilizing motor function in early DMD. Less is known about the efficacy of steroid use in Becker Muscular Dystrophy, in part, because of the rare nature of BMD. Small series and case reports of steroid use in BMD suggest potential benefit but randomized trials are lacking [8].
The limb-girdle muscular dystrophies (LGMD) are heterogeneous with some, but not all, forms sharing common pathological processes to DMD/BMD. Steroid use in LGMD, like BMD, is challenged by the rare nature of these disorders and clinical variability within specific genetic mutations making randomized clinical trials difficult to complete. One study in dysferlin-related LGMD suggested patients fared worse with steroid use [9]. Small case reports have shown potential benefit of steroids in other subtypes of LGMD. Two patients with FKRP-related LGMD were treated with 0.35 mg/kg/day of prednisolone and showed clinical improvement [10]. Two siblings with SGCB-related LGMD were treated with deflazacort 0.9 mg/kg/day for 22 months and showed signs of improved muscle strength and function [11]. Siblings with SGCA-related LGMD received deflazacort for 6 months and showed improved muscle strength and functional assessments [12]. A small retrospective study of 6 patients with LGMD on steroids for at least one year reported conflicting results [13].
Recent studies of steroid treatment in mouse models of DMD suggest that even lower doses of intermittent prednisone or deflazacort provide benefit with fewer adverse consequences [14]. Prednisone or deflazacort given once weekly at 1 mg/Kg improved functional performance with less steroid induced atrophy. A subsequent study documented that once weekly prednisone produced epigenetic remodeling of treated muscle and correlated with improved long-term benefit [15]. Furthermore, once weekly treatment with prednisone at 1 mg/Kg was extended to two LGMD models, dysferlinopathy and sarcoglycanopathy (Dysf and Sgcg, respectively), again showing long term benefit with improvement in muscle function and less obesity over time. Overall, mice treated with once weekly steroids had enhanced muscle repair without any atrophy or adipogenesis and improved muscle performance.
Based on preclinical studies, we now conducted the Weekly Steroids in Muscular Dystrophy (WSiMD) trial as a single center, open label 24-week clinical trial assesses the safety and potential efficacy of once weekly prednisone in LGMD and BMD. A cohort of 20 patients, mostly LGMD, tolerated weekly steroids well over the 24-week study with few adverse events. Secondary measures of muscle function suggest reduced disease progression. Larger and definitive trials of intermittent steroids in LGMD and BMD are needed.
METHODS
Protocol approval and trial registration
The study was approved by the Institutional Review Board at Northwestern University Feinberg School of Medicine (STU00208443). Written informed consent in accordance with universal good clinical practice guidelines in accordance with the ethical standards of the Helsinki declaration of 1975 were obtained from all participants after discussing the objectives, study design, risks and benefits of participation before enrolment into our study. The trial was registered at clinicaltrials.gov (NCT04054375).
Recruitment
Participants were recruited from the Muscular Dystrophy Association (MDA) clinic at Northwestern University from June 2019 to January 2020. The intent was to enroll 15 LGMD and 15 BMD patients (total 30). The Coalition to Cure Calpain, an advocacy organization for calpain-LGMD, advertised the study to its network, resulting more LGMD 2A patients. Due to COVID-19, we restricted enrollment at 20 subjects, and this included few BMD patients. Any participant who was not regularly seen in the Northwestern MDA clinic provided their most recent neurologist clinical note, genetic documentation of their mutation, and completed inclusion / exclusion criteria prior to their screening visit. COVID-19 impacted clinical assessments at 6 months (see below).
Patient eligibility
The inclusion criteria were: age between 18 –65 years old inclusive, echocardiogram with left ventricular ejection fraction greater than 25% within the prior 6 months, electrocardiogram within past 2 months without evidence of atrial fibrillation or recent myocardial infarction, no medication changes in past 3 months, and genetic confirmation of BMD or LGMD (CAPN3, DYSF, SGCG, SGCB, SGCD, FKRP, TTN, and ANO5). Patients were excluded from WSiMD if they had been on oral steroids in the past 3 years for greater than a 1-month period, history of diabetes mellitus, BMI greater than 35 kg/m2, uncontrolled hypertension, congestive heart failure, chronic kidney disease, full time ventilator dependency, orthopedic surgery within past 6 months, current pregnancy, recent history of myocardial infarction, and any history of tuberculosis.
Study design
WSiMD was a single center 24-week open label clinical trial. Participants had two in person visits (week 0 and week 24) and five monthly phone call check-ins (weeks 4, 8, 12, 16, 20, and 24). At week 0, baseline labs and exam were performed after informed consent. The following assessments were performed at both week 0 and week 24: blood pressure, heart rate, weight, whole body DEXA scan, safety clinical labs, and forced vital capacity testing (FVC). All patients, regardless of ambulatory status underwent the following physical therapy assessments at both visits: Vignos scale for lower extremity, Brooke scale for upper extremity [16], grip strength, and NorthStar Ambulatory assessment for Dysferlinopathy (NSAD). Ambulatory participants underwent 6-minute walk test and 10-meter run test. All assessments were performed at our site by certified study personnel including study coordinators and occupational therapist. Study coordinators performed monthly phone call with participants. Drug adherence and dosage was confirmed in addition to adverse events patients experienced in the prior 4-week period at each phone call.
Study drug
Once enrolled in study, the study coordinator received oral prednisone from the onsite clinical trial pharmacy and provided pills to the participant at baseline visit. Participants over 70 kg received 0.75 mg/kg/week whereas patients under 70 kg received 1.0 mg/kg/week. There was no maximum or minimum dose limit. The study team educated participants to take the defined weight-based dose of prednisone on Monday nights between 7–9 pm after dinner. Drug reconciliation was performed during monthly phone calls and confirmed at week 24 study visit as patients returned any unused drug or empty medication bottle.
Laboratory Testing
Blood and urine were collected by nurses or study coordinators. All patients were fasting for at least four hours prior to blood collection. Since patients were fasting, blood draws were routinely prioritized to be performed after informed consent was signed prior to any physical therapy assessments.
Whole body DEXA scan
DEXA (dual energy x-ray absorptiometry) scans were performed on a Horizon A scanner (Hologic). Bone density and reported Z-scores were compared to appropriate age and sex controls in the following areas: total body, lumbar (L1–L4), and left forearm. Whole body DEXA scan also provided total body lean mass, limb specific lean mass, total body fat mass, limb specific fat mass, total body fat %, and total body mass. The first patient enrolled underwent an earlier version of the DEXA protocol, and therefore did not have certain metrics collected. All subsequent DEXA scans were uniformly protocoled.
Physical therapy assessments
Physical therapy assessments were performed on site at our neurological clinical trial center. The same assessor completed both baseline and end of study visit. Brooke scale for upper extremities is scored from 1 to 6, with lower numbers indicating better upper extremity function. Vignos scale for lower extremities is scored from 1 to 10, with lower numbers indicating less severe disease progression. 6-minute walk test was performed with a 25-meter track delineated with two cones as endpoints with the study team member walking behind the patient. Participants maintained the fastest pace while maintaining safety for the entirety of 6-minutes with instructions to pause if they felt dizzy, lightheaded, or weak. Participants were informed of the time remaining in 1-min intervals. 10-meter run test was performed in the same site with instructions to complete the 10-meters as fast and as safely possible. Each participant repeated the test three times to get an average time to run 10-meters. Grip strength was collected with a dynamometer (JAMAR Hydraulic Hand Dynamometer). Each hand underwent three trials with 10 seconds of rest in between each trial and reporting the average of the 3.
Forced Vital Capacity (FVC)
FVC was collected on a KoKo Legend II spirometer (Nspire). Repeat FVC testing was limited due to COVID-19.
Statistical analyses
Prism (Graphpad) v8.0 was used for statistical analysis. Week 24 values were compared to week 0 values with nonparametric paired t-test (Wilcoxon match-pairs signed rank test). P-values less than 0.05 were considered significant and indicated with a asterisk. Data were presented as single values (dot plots, histograms) for all data points. Tables, dot plots, histograms, and marked line plots depict mean±SEM.
RESULTS
Study cohort
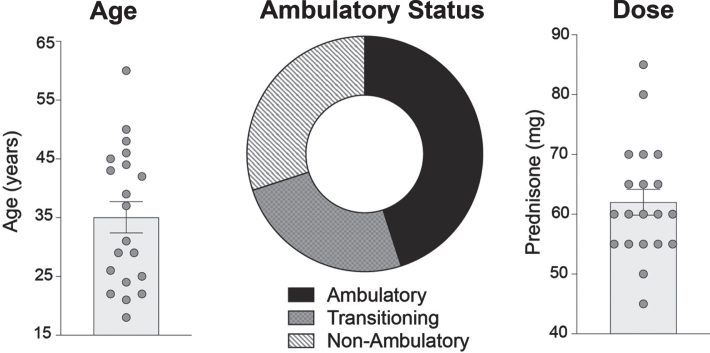
Twenty patients enrolled in WSiMD with sixteen completing all aspects of the study; 4/20 missed the on-site end of study assessments due to COVID-19. Out of the 20 participants, seven were female, nineteen had LGMD and one had BMD (Fig. 1). The average age was 35 years old, (range 18 to 60). Ambulatory and non-ambulatory patients were included. Of those with LGMD, the LGMD subtype is shown in Table 1. Eight participants had mutations in CAPN3, three had DYSF, one had SGCG, one had TTN, three had FKRP, and three had ANO5. The single BMD subject had a deletion in exons 45–47.
Fig. 1.
WSiMD study demographics. The average age of participants enrolled in the trial was 35 years (range 18 to 60 years). A total of twenty patients were enrolled (7 females and 13 males). Ambulatory status was determined using Vignos lower extremity score. 9 patients were ambulatory, 6 were non-ambulatory, and 5 were considered transitioning to non-ambulatory. Dosing determined by weight: < 70 kg treated with 1.0 mg/kg/week and > 70 kg treated with 0.75 mg/kg/week. Dosing occurred weekly on Monday nights, after dinner, between 7–9 pm. The average weekly dose was 62 mg, with a range of 45 to 85 mg.
Table 1.
Demographics of WSiMD participants
| Subject | Age Decade | Sex | Race | Dx | Gene | Dose | Ambulatory |
| 01 | 4th | M | WNH | LGMD | ANO5 | 85 mg | Yes |
| 02 | 4th | M | WNH | LGMD | ANO5 | 65 mg | Yes |
| 03 | 5th | M | WNH | BMD | DMD | 60 mg | No |
| 04 | 3rd | F | WH | LGMD | CAPN3 | 55 mg | Yes |
| 05 | 6th | F | WNH | LGMD | FKRP | 55 mg | No |
| 06 | 3rd | F | WNH | LGMD | SGCG | 50 mg | No |
| 07 | 3rd | M | WNH | LGMD | FKRP | 55 mg | No |
| 08 | 5th | M | SAI | LGMD | DYSF | 55 mg | No |
| 09 | 4th | F | WH | LGMD | DYSF | 65 mg | Yes |
| 10 | 5th | M | AA | LGMD | DYSF | 60 mg | No |
| 11 | 3rd | F | WH | LGMD | CAPN3 | 70 mg | Yes |
| 12 | 5th | M | WNH | LGMD | CAPN3 | 60 mg | No |
| 13 | 3rd | M | WNH | LGMD | CAPN3 | 45 mg | Yes |
| 14 | 7th | M | WH | LGMD | ANO5 | 80 mg | Yes |
| 15 | 5th | M | WNH | LGMD | CAPN3 | 65 mg | Yes |
| 16 | 3rd | M | WNH | LGMD | TTN | 70 mg | No |
| 17 | 5th | M | WNH | LGMD | CAPN3 | 70 mg | Yes |
| 18 | 2nd | M | WNH | LGMD | CAPN3 | 45 mg | Yes |
| 19 | 3rd | F | WNH | LGMD | CAPN3 | 60 mg | Yes |
| 20 | 3rd | F | WNH | LGMD | FKRP | 60 mg | Yes |
WNH: White Non-Hispanic. WH: White Hispanic. SAI: South Asian Indian. AA: African American.
Once weekly prednisone dosing
Oral prednisone was dosed based on weight. The median weekly dose was 60 mg (range 45 to 85) (Fig. 1). On average, patients completing study visits completed 24 doses, with a range of 23–26 doses. Based on monthly calls, three participants missed a single dose each. Drug reconciliation at week 24 visit confirmed that only three doses, one per patient, was missed.
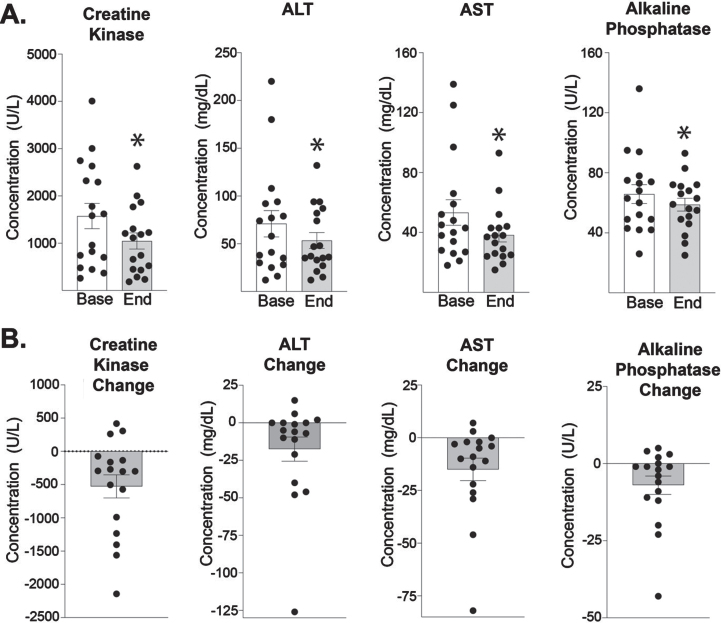
Serum CK in study participants
The majority of participants experienced a decrease in serum muscle-disease related biomarkers (Fig. 2). Creatine kinase (CK) levels decreased significantly from 1574 at baseline to 1047 at 24 weeks. Correspondingly, ALT and AST were also both significantly reduced. ALT decreased from 71 to 54 U/L at end of study. AST decreased from 53 to 38 U/L at end of study. Alkaline phosphatase was also reduced over the study interval, from 66 to 59 U/L at end of study.
Fig. 2.
Trends in muscle enzymes in study participants. Creatine kinase (CK) decreased from 1574 U/L (±269) at baseline to 1047 U/L (±171) at the end of 24 weeks of weekly prednisone (n = 17, p = 0.009). ALT decreased from 71 (±14) to 54 (±8) U/L (n = 17, p = 0.018). AST decreased from 53 (±9) to 38 (±5) U/L (n = 17, p = 0.001). Alkaline Phosphatase decreased from 66 (±6.6) to 59 (±4) U/L (n = 17, p = 0.032). Histograms depict single values and mean +/- SEM; *P < 0.05 vs baseline; paired t-test, nonparametric, Wilcoxon matched-pairs signed rank test.
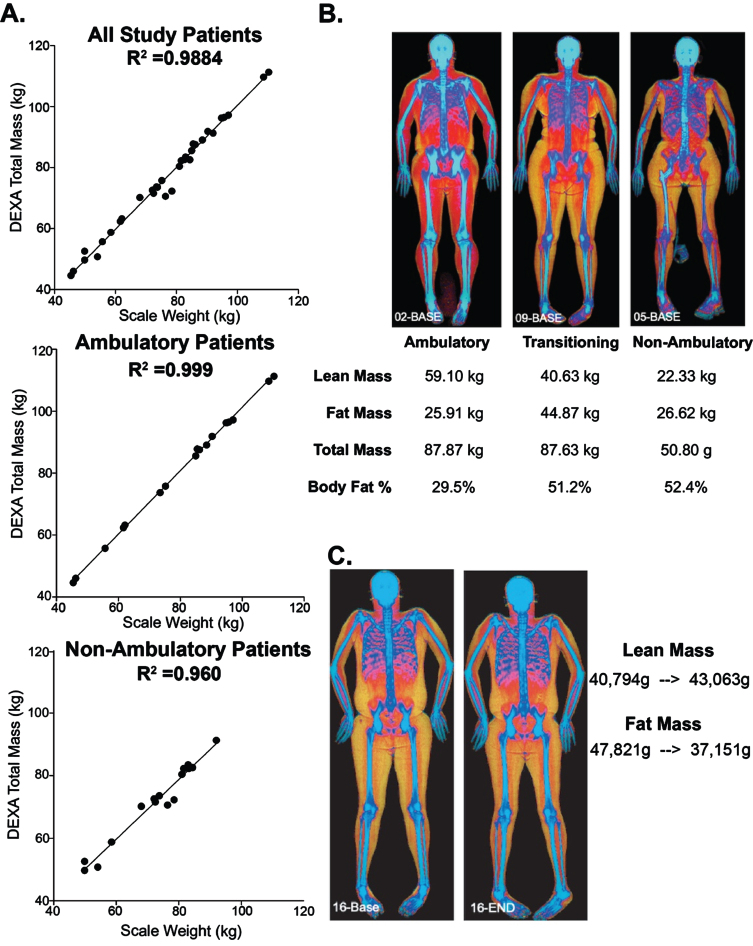
Whole body DEXA Scan to evaluate body composition
Whole body DEXA (dual energy x-ray absorptiometric) scans were used to determine body mass and these data were compared to weights collected at the same visit. In all study patients, R2 was 0.988, indicating a strong linear correlation between DEXA-measured total body mass and the patients’ measured body weights (Fig. 3A). The correlation between DEXA measured mass and measured weight, was slightly less for non-ambulatory compared to ambulatory (0.960 for ambulatory and 0.999 for non-ambulatory, n = 18 and n = 16, respectively.) We also compared the lean and fat mass, as determined by DEXA. Ambulatory patients had the highest amount of lean muscle mass and lowest body fat percentage whereas non-ambulatory patients had the least lean mass and greatest body fat percentage (Fig. 3B). DEXA scans detected changes in lean and fat muscle mass over time. Figure 3C depicts one subject who experienced an increase of 2.2 kg of lean muscle mass in additional to a 10 kg loss of fat mass over the study duration. This was the most extreme example, as other patients showed no significant changes on whole body DEXA scan at the end of 24 weeks.
Fig. 3.
Evaluation of body composition using whole body DEXA in WSiMD. (A) Patients scale weight taken in clinic were compared to same day DEXA calculated total mass. The correlation between scale weight and DEXA-determined weight was slightly better for ambulatory patients. (B) Examples of participants at different disease stages upon enrollment into the study. (C) Example of participant who improved in the study with increasing muscle mass and lower fat mass.
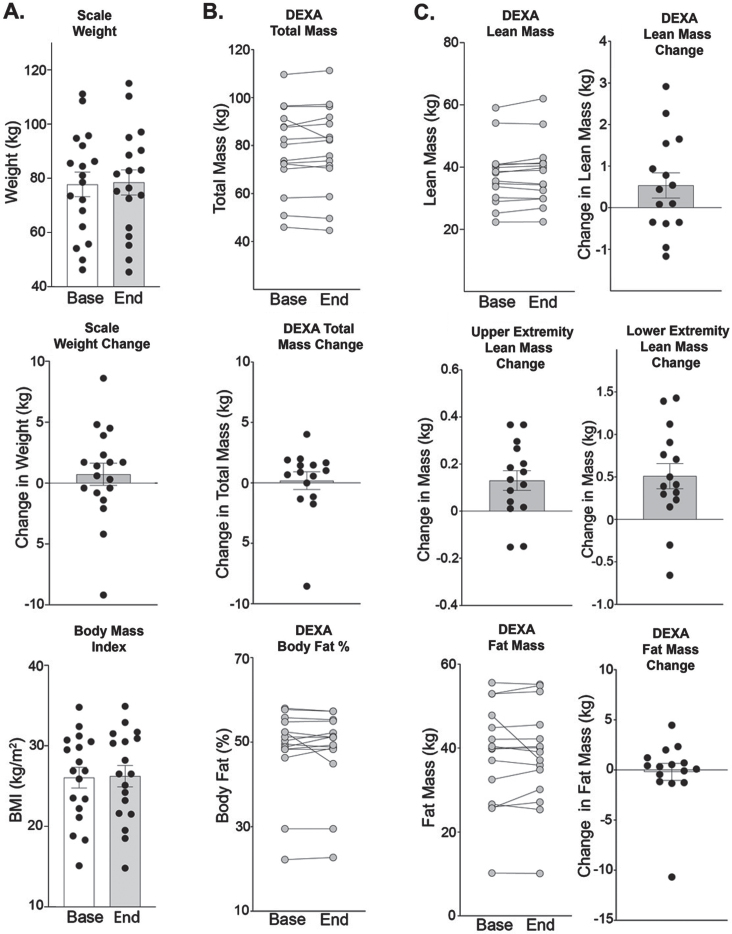
The average weight (kg) of patients at study onset was 77.7 (±4.5) kg with a range of 46.2 to 110.5 kg (Fig. 4A). The average weight slightly increased over study duration from 77.7 to 78.5 kg at study end. Similarly, body mass index (BMI) remained stable with a slight increase from 26.1 to 26.2 kg/m2. Weights extrapolated from whole body DEXA scans total mass, reported a baseline average weight of 78.4 (±4.5) kg with a slight average increase to 78.6 (±4.7) kg (n = 15, p = 0.276) (Fig. 4B). Participants had an overall slight increase in total body muscle mass of 538 g and a slight decrease in total body fat mass (–0.188 kg) and body fat % (-0.2%) (Fig. 4C). Although not significant, total body lean muscle mass increased from 37.54 (±2.5) to 38.08 (±2.62) kg (n = 15, p = 0.135). Ten out of the fifteen participants had an increase in muscle mass, and the majority had changes within the range of –1 kg to +1 kg (n = 11). Total body fat mass decreased from 38.29 to 38.10 kg. Upper extremity lean mass was calculated by adding right and left upper limb values computed by DEXA whole body scan. The majority of participants saw an improvement in lean muscle mass, with an average from 4.04 to 4.17 kg for an increase of 0.13 kg (n = 15, p = 0.008) (Fig. 4C). Lower extremity lean mass was calculated by adding right and left lower limb scores. The majority of participants saw an improvement in lower extremity lean muscle mass, with an average from 9.58 to 10.09 kg for an increase of 0.51 kg (n = 15, p = 0.005), (Fig. 4C).
Fig. 4.
Weekly steroids do not significantly alter fat mass. (A) Weights remained stable through the study duration (77.7 (±4.5) to 78.5 (±4.6) kg (n = 18, p = 0.269). Body Mass Index also remained stable (26.1 to 26.2, p = 0.363). (B) Using DEXA to estimate total mass, similar results were obtained 78.4 (±4.5) to 78.6 (±4.7) kg (n = 15, p = 0.277). Body fat % was 48.4 (±2.5) at study onset and 48.2 (±2.5) % at study end (n = 15, p = 0.903). (C) Lean mass was 37.5 (±2.5) at study onset compared to 38.1 (±2.6) kg at study end (n = 15, p = 0.135). Upper extremity lean mass increased by 0.13 kg, from 4.04 (±0.38) to 4169 (±0.40) kg at end of study (n = 15, p = 0.008). Lower extremity lean mass increased by 0.51 kg, from 9.58 (±0.80) to 10.09 (±0.76) kg at end of study (n = 15, p = 0.005). Fat mass remained stable from 38.2 (±3.2) to 38.1 (±3.1) kg at end of study (n = 15, p = 0.639). Histograms depict single values and mean ±SEM; curves depict individual patient changes; Paired t-test, nonparametric, Wilcoxon matched-pairs signed rank test were used.
Functional outcomes after 24 weeks of weekly steroids
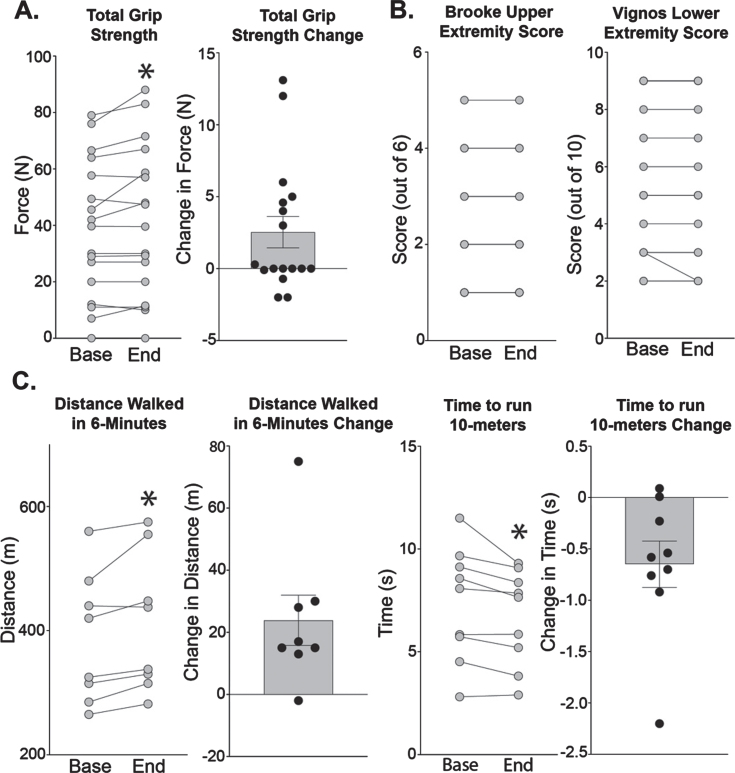
Ambulatory and non-ambulatory participants were considered separately since only ambulatory participants completed the 6-MWT and 10-meter run test. Consistent with the increase in upper extremity lean mass, grip strength improved from 38.6 (±5.9) to 41.1 (±6.5) N (n = 17, p = 0.040). Brooke upper extremity scale score did not change for any of the participants (Fig. 5B). Vignos lower extremity score remained stable in all but one participant who improved by one point. Ambulatory participants on average walked 24 meters more in 6-minutes (n = 9, p = 0.005) at the end of study and were able to run 10-meters 0.65 seconds faster (n = 8, p = 0.019).
Fig. 5.
Effects of weekly steroids on functional measures. (A) Grip strength increased from 38.6 (±5.9) to 41.1 (±6.5) N (n = 17, p = 0.040). (B) Brooke’s scale scores evaluate upper extremity function and range from 1 (least severe) to 6 (most severe). There were no changes in Brooke scores. Vignos scores evaluate lower extremity function and range from 1 (least severe) to 10 (most severe). One patient had a change in Vignos score with improvement from 3 to 2. (C) Participants able to ambulate improved in distance walked in six minutes from 386 (±37) to 410 (±40) meters (n = 8, p = 0.016). Participants ran ten meters faster, with a decrease in the time from 7.32 (±0.92) to 6.67 (±0.77) seconds (n = 9, p = 0.019). Histograms depict single values and mean+/- SEM; curves depict individual participant changes; *P < 0.05 vs baseline; paired t-test, nonparametric, Wilcoxon matched-pairs signed rank test.
Safety of once-weekly prednisone
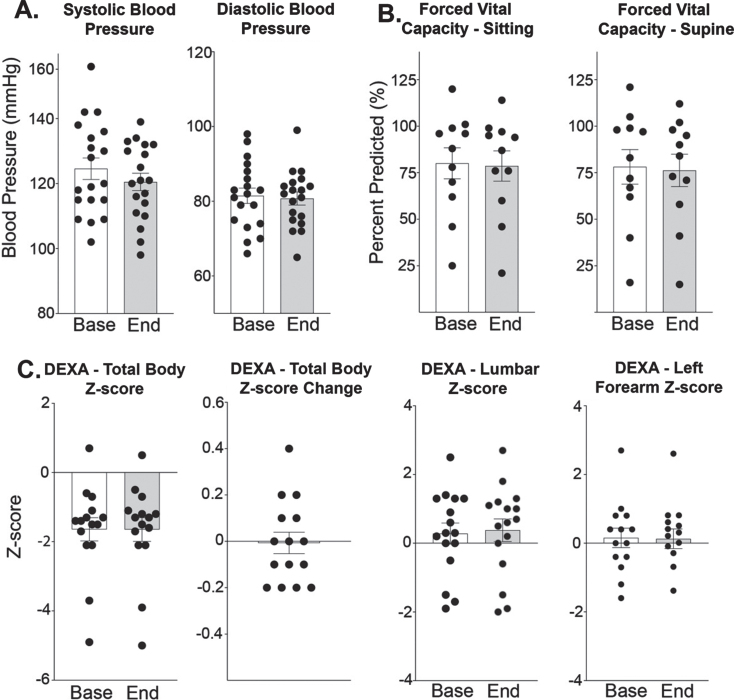
Blood pressure (BP), forced vital capacity (FVC), and bone density remained stable after treatment period (Fig. 6). Systolic and diastolic BP was comparable between study onset and end (systolic BP 125 vs 121 mmHg and diastolic BP 82 vs 81 mmHg). (Fig. 6A). FVC in both supine and seated positions did not significantly change, although FVC measurements were not completed by 9 participants due to COVID-19. FVC in the seated position was 80% at baseline and 79% at end of study. FVC in the supine position was 78% at baseline and 76% at end of study (Fig. 6B). Total body Z-scores for bone density were –1.64 (±0.33) indicating that many of the participants at baseline had osteopenia (Z-score > –1.5), of which two had met clinical levels defining for osteoporosis (Z-score > –2.5), (Fig. 6C). Overall, osteopenia as total bone density z-scores remained stable from –1.64 at baseline to –1.65. All but one patient was between a –0.2 to +0.2 change from baseline. Lumbar Z-scores and left forearm Z-scores had similar trends with minimal changes after treatment. Lumbar Z-scores were stable 0.28 to 0.38. Left forearm Z-score also remained stable from 0.16 to 0.13.
Fig. 6.
Weekly steroids over 6-month period do not significantly alter blood pressure, respiratory function, or bone density. (A) Systolic and diastolic blood pressure did not significantly increase. (B) Respiratory capacity remained stable in both the supine and sitting positions. (C) Total body bone density Z-score remained stable from –1.64 (±0.33) to –1.65 (±0.34) (n = 15, p = 0.828). Lumbar Z-scores were determined from L1–L4 and at study onset was 0.28 (±0.31) compared to 0.38 (±0.33) at study end (n = 16, p = 0.471). Left forearm Z-score remained stable from 0.16 (±0.28) to 0.13 (±0.29) (n = 15, p = 0.957). Histograms depict single values and mean +/- SEM; *P < 0.05 vs baseline; paired t-test, nonparametric, Wilcoxon matched-pairs signed rank test.
Metabolic changes
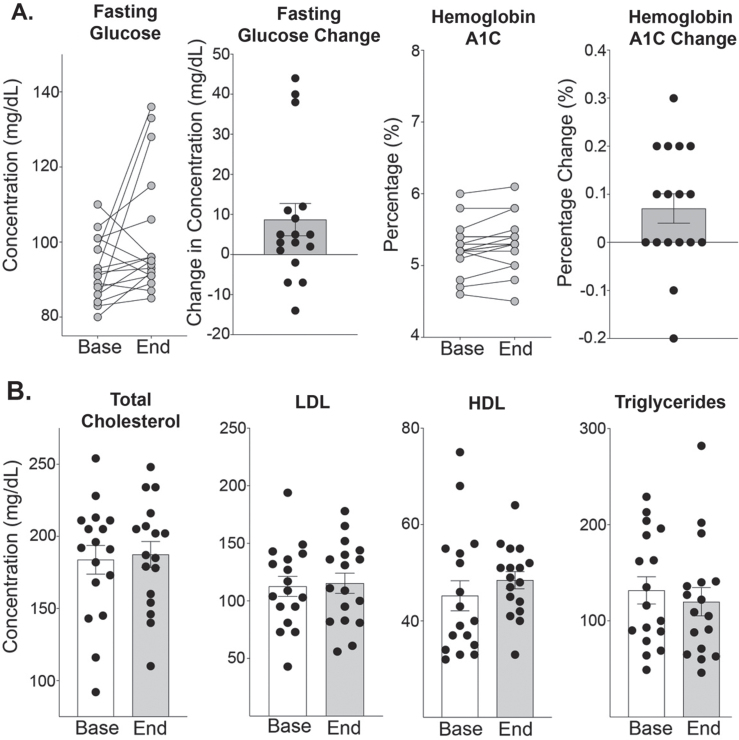
Three patients had an increase in fasting glucose levels (38, 40, and 44 mg/dL) (Fig. 7A). These three patients all were seen 10–14 hours after their weekly prednisone. No other patients had follow-up visits within this timeframe of taking the weekly prednisone. Hemoglobin A1c levels remained stable with a range of –0.2% to +0.3% (Fig. 7A). Fasting lipid panel did not significantly change with treatment. HDL levels were unchanged 45 to 49 mg/dL and triglycerides were stable 132 to 120 mg/dL.
Fig. 7.
Effect of Weekly steroids on fasting glucose and lipid profiles. (A) Glucose levels were collected after at least 4-hours of fasting. There was a trend towards increased fasting glucose, from 93 (±2) to 102 (±4) mg/dL (n = 17, p = 0.052). Three participants had glucose increases greater than 35, all drawn between 10–14 hours after their single weekly prednisone dose. Hemoglobin A1C levels was 5.25 (±0.08) at study onset compared to 5.32 (±0.09) % at study end (n = 17, p = 0.057). (B) Total cholesterol was 182 (±10) at study onset compared to 185 (±9) mg/dL at end of study (n = 17, p = 0.423). LDL remained stable from 113 (±8) mg/dL at baseline to 115 (±8) mg/dL at end of study (n = 17, p = 0.751). HDL was 45 (±3) mg/dL at study onset compared to 49 (±2) mg/dL (n = 17, p = 0.220). Triglycerides at study onset were 132 (±14) mg/dL compared to 120 (±15) mg/dL at study end (n = 17, p = 0.487). Histograms depict single values and mean +/- SEM; curves depict individual participant changes; *P < 0.05 vs baseline; paired t-test, nonparametric, Wilcoxon matched-pairs signed rank test.
Adverse effects
No participant experienced any serious adverse event, and everyone completed 24 weeks of dosing (Table 2). The three most common side effects were falls (n = 7, 35%), viral symptoms (n = 6, 30%), and increased anxiety on Monday night, the evening when the prednisone was taken (n = 4, 20%). Only two participants (10%) felt a subjective weight gain and one participant experienced worsening of cataracts. Three participants (15%) reported an increase in acne.
Table 2.
Adverse Effects
| Adverse Effects | Number of Patients (%) | Relation to Study Drug | Serious Adverse Event | Stopped Medication |
| Viral Symptoms | 6 / 20 (30%) | Unlikely | 0 / 20 | 0 / 20 |
| Bloating | 1 / 20 (5%) | Possible | 0 / 20 | 0 / 20 |
| Increased Anxiety | 4 / 20 (20%) | Likely | 0 / 20 | 0 / 20 |
| Hip / Shoulder Pain | 1 / 20 (5%) | Unlikely | 0 / 20 | 0 / 20 |
| Cataract Worsening | 1 / 20 (5%) | Possible | 0 / 20 | 0 / 20 |
| Epigastric Abdominal Pain | 1 / 20 (5%) | Unlikely | 0 / 20 | 0 / 20 |
| Urinary Tract Infection | 1 / 20 (5%) | Unlikely | 0 / 20 | 0 / 20 |
| Pins and Needle Sensations | 1 / 20 (5%) | Possible | 0 / 20 | 0 / 20 |
| Increase Acne | 3 / 20 (15%) | Likely | 0 / 20 | 0 / 20 |
| Decreased Libido | 1 / 20 (5%) | Possible | 0 / 20 | 0 / 20 |
| Constipation | 1 / 20 (5%) | Possible | 0 / 20 | 0 / 20 |
| Nighttime Sweating | 1 / 20 (5%) | Likely | 0 / 20 | 0 / 20 |
| Falls | 7 / 20 (35%) | Unlikely | 0 / 20 | 0 / 20 |
| Fractures | 1 / 20 (5%) | Unlikely | 0 / 20 | 0 / 20 |
| Weight Gain | 2 / 20 (10%) | Possible | 0 / 20 | 0 / 20 |
| Lightheadedness | 1 / 20 (5%) | Possible | 0 / 20 | 0 / 20 |
DISCUSSION
Safety and tolerability of once-weekly steroids in muscular dystrophy
Over this 24 week study, once-weekly prednisone appeared well tolerated in LGMD and BMD patients. None of the twenty participants stopped taking prednisone due to a serious adverse event, and the majority of side effects were temporary. Significant hypertension, hyperglycemia, and weight gain that would be expected with daily steroids was not observed [17]. We observed high compliance with once weekly prednisone with only 3 of 480 total doses being missed (0.6%). Nineteen out of twenty patients chose to continue weekly prednisone clinically after study completion.
In addition to being well tolerated, biomarkers and imaging data may hint at potential benefit. CK, which reflects muscle breakdown, was decreased at 24 weeks when compared to study onset values. Many muscle biomarkers decrease in parallel with declining muscle mass and also with reduced activity in muscular dystrophy, and therefore this could account for declining CK in our study [18]. However, whole body DEXA scans in this study suggest stabilization or even mild increase in muscle mass, although interpretation is limited due to lack of longitudinal measures from natural history studies for comparison. Thus, the reduction in CK we observed is not likely due to reduction in muscle mass over the 24-week study. Whole body DEXA imaging also suggested reduced adiposity over the 24-week study, rather than an increase, as might be expected from chronic daily steroid use. Consistent with this, we did not observe an increase in dyslipidemia or worsening hemoglobin A1C, which would reflect metabolic syndrome-type changes that are commonly seen from the use of chronic daily steroids. Interestingly, although more than half of participants had osteopenia at baseline, 24 weeks of once weekly steroids did not appear to worsen bone health during this relatively short timeframe.
Whole body DEXA scanning is useful in muscular dystrophy
DEXA scans have potential for greater utility in clinical management and clinical trials in adults with muscular dystrophy. Bone density change is a potential secondary endpoint as muscle, bone, and fat communicate on a molecular level and may act as a surrogate marker for improved muscle health. Based on the evidence for baseline osteopenia in this study cohort, at a minimum, patients should be screened for osteopenia as they become non-ambulatory since this might lead to treatments to prevent fracture. As more patients with muscular dystrophy take steroids, it will be important to track bone health long term, as consequences of chronic weekly steroids are currently unknown. Additionally, in this study Whole Body DEXA provided a relatively easy means to assess lean and fat mass.
Potential functional improvement with weekly steroids
We observed trends in functional improvement in both ambulatory and non-ambulatory patients for upper and lower extremity assessments. Not all patients improved, but as LGMD and BMD are progressive disorders, one would expect a decline of function over time in a control group. Patients on weekly steroids had an increase in lean muscle mass, stabilization of fat mass, and were able to walk faster and longer. Weekly steroids are thought to enhance muscle repair with decreased fibrofatty replacement of muscle tissue and unlike daily steroids, do not induce adipogenesis or atrophy leading to fewer side effects. Although steroids could improve function, this study had no control group and it is possible that the improvement could be due to uncontrolled factors including the placebo effect, greater activity levels, lack of long-term data, and closer monitoring as part of a clinical trial.
Study limitations and future directions
This study is limited by its single center nature, short duration, open label design without a control group, and small number of participants. Nonetheless, the relative apparent safety of once-weekly dosing supports the need for additional investigation of this regimen. In DMD, there is emerging data that boys taking weekend high dose steroids have similar skeletal muscle benefits without the same degree of side effects from taking chronic daily steroids [6, 7]. In older non-ambulatory DMD patients, those on steroids had higher baseline muscle function but experienced the same rate of decline as steroid naïve DMD participants [19]. The rate of decline in LGMD varies with LGMD subtype and differs from DMD, and studies longer than 6 months are needed to evaluate impact on disease progression. This study included adults with multiple different LGMD subtypes and one BMD patient, and studies aimed at earlier disease timepoints should be considered. Future trials are needed to determine optimal steroid dosing, including the amount and frequency. Natural history studies are lacking and difficult to achieve with a substantial cohort size due to the rare nature of LGMD and BMD. Furthermore, these disorders are heterogenous with variable disease progression even within the same genetic subtype. However, “basket-trial” approaches may be useful in this setting. Longer follow-up is needed to understand the long-term benefits and potential consequences from weekly steroids.
ACKNOWLEDGMENTS
This study was supported by the Kurt+Peter Foundation and National Institutes of Health.
The funders played no role in study design or interpretation.
CONFLICT OF INTERESTS
EMM is listed as a co-inventor on a patent application related to intermittent glucocorticoid use filed by Northwestern University.
REFERENCES
- [1]. Waldrop MA, Flanigan KM. Update in Duchenne and Becker muscular dystrophy. Curr Opin Neurol. 2019;32(5):722–7. [DOI] [PubMed] [Google Scholar]
- [2]. Rivera SR, Jhamb SK, Abdel-Hamid HZ, Acsadi G, Brandsema J, Ciafaloni E, et al. Medical management of muscle weakness in Duchenne muscular dystrophy. PLoS One. 2020;15(10):e0240687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Quattrocelli M, Zelikovich AS, Salamone IM, Fischer JA, McNally EM. Mechanisms and Clinical Applications of Glucocorticoid Steroids in Muscular Dystrophy. J Neuromuscul Dis. 2021;8(1):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2016;(5):CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Keeling RM, Golumbek PT, Streif EM, Connolly AM. Weekly oral prednisolone improves survival and strength in male mdx mice. Muscle Nerve. 2007;35(1):43–8. [DOI] [PubMed] [Google Scholar]
- [6]. Escolar DM, Hache LP, Clemens PR, Cnaan A, McDonald CM, Viswanathan V, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77(5):444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Connolly AM, Zaidman CM, Golumbek PT, Cradock MM, Flanigan KM, Kuntz NL, et al. Twice-weekly glucocorticosteroids in infants and young boys with Duchenne muscular dystrophy. Muscle Nerve. 2019;59(6):650–7. [DOI] [PubMed] [Google Scholar]
- [8]. Angelini C, Marozzo R, Pegoraro V. Current and emerging therapies in Becker muscular dystrophy (BMD). Acta Myol. 2019;38(3):172–9. [PMC free article] [PubMed] [Google Scholar]
- [9]. Walter MC, Reilich P, Thiele S, Schessl J, Schreiber H, Reiners K, et al. Treatment of dysferlinopathy with deflazacort: A double-blind, placebo-controlled clinical trial. Orphanet J Rare Dis. 2013;8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Darin N, Kroksmark AK, Ahlander AC, Moslemi AR, Oldfors A, Tulinius M. Inflammation and response to steroid treatment in limb-girdle muscular dystrophy 2I. Eur J Paediatr Neurol. 2007;11(6):353–7. [DOI] [PubMed] [Google Scholar]
- [11]. Wong-Kisiel LC, Kuntz NL. Two siblings with limb-girdle muscular dystrophy type 2E responsive to deflazacort. Neuromuscul Disord. 2010;20(2):122–4. [DOI] [PubMed] [Google Scholar]
- [12]. Angelini C, Fanin M, Menegazzo E, Freda MP, Duggan DJ, Hoffman EP. Homozygous alpha-sarcoglycan mutation in two siblings: One asymptomatic and one steroid-responsive mild limb-girdle muscular dystrophy patient. Muscle Nerve. 1998;21(6):769–75. [DOI] [PubMed] [Google Scholar]
- [13]. Albuquerque MA, Abath-Neto O, Maximino JR, Chadi G, Zanoteli E, Reed UC. Clinical aspects of patients with sarcoglycanopathies under steroids therapy. Arq Neuropsiquiatr. 2014;72(10):768–72. [DOI] [PubMed] [Google Scholar]
- [14]. Quattrocelli M, Salamone IM, Page PG, Warner JL, Demonbreun AR, McNally EM. Intermittent Glucocorticoid Dosing Improves Muscle Repair and Function in Mice with Limb-Girdle Muscular Dystrophy. Am J Pathol. . 2017;187(11):2520–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Quattrocelli M, Zelikovich AS, Jiang Z, Peek CB, Demonbreun AR, Kuntz NL, et al. Pulsed glucocorticoids enhance dystrophic muscle performance through epigenetic-metabolic reprogramming. JCI Insight. 2019;4(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Lord JP, Portwood MM, Fowler WM, Lieberman JS, Carson R. Upper vs lower extremity functional loss in neuromuscular disease. Arch Phys Med Rehabil. 1987;68(1):8–9. [PubMed] [Google Scholar]
- [17]. Gloss D, Moxley RT, 3rd, Ashwal S, Oskoui M. Practice guideline update summary: Corticosteroid treatment of Duchenne muscular dystrophy: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86(5):465–72. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [18]. Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, Furlong P, et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2015;112(23):7153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Connolly AM, Florence JM, Zaidman CM, Golumbek PT, Mendell JR, Flanigan KM, et al. Clinical trial readiness in non-ambulatory boys and men with duchenne muscular dystrophy: MDA-DMD network follow-up. Muscle Nerve. 2016;54(4):681–9. [DOI] [PubMed] [Google Scholar]