Abstract
Simple Summary
Recently, a crisis derived from foodborne infections, especially those are associated with food from animal origins caused by Clostridium perfringens (C. perfringens), has worsened. Unfortunately, the solutions to this crisis were restricted by an evolved resistance to antimicrobial agents. Therefore, we try to warn the world population about the hazards associated with this pathogen. The high diversity and polyclonality of C. perfringens strains depicted in our study show the urgent need to advance programs to control C. perfringens associated with foodborne infections. Additionally, the findings presented in this study are also of clinical importance, assisting in understanding the prevalence, origin, reservoir, and evolution of antimicrobial resistance of C. perfringens for establishing the control of this pathogen.
Abstract
Several food-poisoning outbreaks have been attributed to Clostridium perfringens (C. perfringens) worldwide. Despite that, this crisis was discussed in a few studies, and additional studies are urgently needed in this field. Therefore, we sought to highlight the prevalence, antimicrobial resistance, toxin profiles, and toxinotypes of C. perfringens isolates. In this study, 50 C. perfringens isolates obtained from 450 different animal origin samples (beef, chicken meat, and raw milk) were identified by phenotypic and genotypic methods. The antimicrobial susceptibility results were surprising, as most of the isolates (74%) showed multidrug-resistant (MDR) patterns. The phenotypic resistance to tetracycline, lincomycin, enrofloxacin, cefoxitin/ampicillin, and erythromycin was confirmed by the PCR detections of tet, lnu, qnr, bla, and erm(B) genes, respectively. In contrast to the toxinotypes C and E, toxinotype A prevailed (54%) among our isolates. Additionally, we found that the genes for C. perfringens enterotoxin (cpe) and C. perfringens beta2 toxin (cpb2) were distributed among the tested isolates with high prevalence rates (70 and 64%, respectively). Our findings confirmed that the C. perfringens foodborne crisis has been worsened by the evolution of MDR strains, which became the prominent phenotypes. Furthermore, we were not able to obtain a fixed association between the toxinotypes and antimicrobial resistance patterns.
Keywords: C. perfringens, MDR, antimicrobial resistance genes, toxin gene profiles, toxinotypes
1. Introduction
Several outbreaks have been attributed to foodborne infections, which cause several life-threatening diseases and public health problems. This crisis has grown and become more complex due to the emergence of multidrug-resistant (MDR) fungi [1] and bacteria [2] (i.e., Salmonella species, Staphylococcus aureus, Campylobacter jejuni, and Listeria monocytogenes) [3,4,5,6]. Clostridium perfringens (C. perfringens) is among the most important foodborne pathogens worldwide [7]. Recently, C. perfringens foodborne outbreaks were diagnosed during the Panhellenic Handball Championship for children [8]. The C. perfringens is a Gram-positive anaerobic bacterium that is able to form spores under unsuitable conditions, and it has spread widely in the environment [9]. Additionally, it occurs within the normal animal gut flora and becomes pathogenic upon a disturbance in the balance of the gut microbiota [10]. Moreover, stress conditions, starvation, and the continuous administration of antibiotics or anthelmintic drugs may increase the pathogenic power of this pathogen [11].
Approximately 13% of the gastrointestinal foodborne outbreaks have been associated with C. perfringens infections [12]. C. perfringens foodborne infections are always associated with meat and poultry products. The meat products can be contaminated with this pathogen during slaughtering via the contaminated surface or the contact of carcasses with feces [13,14]. The heat resistance of C. perfringens is associated with the formation of spores that can germinate at temperatures ranging from 15 to 55 °C [15]. The standard food service practices should be followed up in order to prevent the spread of this pathogen [16]. Therefore, it is recommended to cook food until the internal temperature reaches 70 °C. Notably, the gastrointestinal infection with C. perfringens in animals and humans occurs due to the production of potent exotoxins [10]. Accordingly, C. perfringens can be serotyped into five groups (A to E) based on the production of specific exotoxins [alpha (α), beta (β), epsilon (ε), and ι (iota)] [17]. The α-toxin, encoded by a plasmid mediated cpa gene, is associated with serotype A, as well as all other serotypes of C. perfringens [18]. Meanwhile, the serotype B harbors cpb and etx plasmid-mediated genes that encode β- and ε-toxins, respectively. The β- and ε-toxins are associated with serotypes C and D, respectively [19], but serotype E has the plasmid-mediated iap gene, which produces ι-toxin [20]. All serotypes can contain cpe and cpb2 genes, which produce C. perfringens enterotoxin (CPE) and C. perfringens beta2 toxin (CPB2) [21].
There are two general antimicrobial resistance mechanisms of C. perfringens, including the mutation of inherent genes or acquisition of resistance gene(s) [22]. The potential increase in the antimicrobial resistance of C. perfringens has been raised recently, with several reports announcing that most C. perfringens were MDR strains [23,24]. The resistance to tetracycline through TetA(P) protein, which regulates tetracycline active efflux, was common [25]. In addition, over 50% of C. perfringens isolates were resistant to lincomycin. However, 25% of this resistance was attributed to the expression of the lnu gene [26]. It is noteworthy that higher minimum inhibitory concentration (MIC) values of amoxicillin and ciprofloxacin were recorded due to the presence of the β-lactamase (bla) and quinolone (qnr) resistance genes, respectively [27]. In the same context, the macrolide-resistant C. perfringens may act as reservoirs for the erm gene, which assists in its conjugal transfer [28]. Owing to the increase in the emerging threat of foodborne-associated C. perfringens infections, we explored the prevalence of C. perfringens in food chains and spotlighted the evolution hazards of this pathogen in addition to the wide spread of MDR/toxigenic phenotypes in order to alarm the health organizations, especially in Egypt, to perform their duty and fulfill their responsibility.
2. Materials and Methods
2.1. Sample Collection
A total of 450 raw milk, beef, and chicken meat samples (150 each) were collected from various supermarkets in two different Governorates in Egypt. Thirty raw milk, beef, and chicken meat samples (10 each) were obtained from 8 and 7 different supermarkets in Sharkia and Port Said Governorates, respectively (Supplementary Table S1).
2.2. Isolation, Enumeration and Phenotypic Identification of C. perfringens
The collected samples were prepared according to the procedure recommended by the International Commission on Microbiological Specifications for Foods [29] to achieve ten-fold serial dilutions. Twenty-five grams or millimeters of each meat or milk sample were suspended into 225 mL of sterile peptone water (Oxoid, Basingstoke, UK)). The sample homogenates underwent heat shocking at 80 °C, in a water bath, for 15 min, to kill the non-spore forming aerobic bacteria, and then 1 mL of each sample was inoculated into 9 mL of fluid thioglycolate broth medium (Oxoid, UK), from which further ten-fold dilutions were prepared. A total of 1 mL from each of the previously prepared dilutions was streaked onto tryptose sulfite cycloserine (TSC) agar (Oxoid, Basingstoke, UK) plates, and all plates were incubated at 37 °C for 24 h in an anaerobic jar HP-11 (Oxoid, Basingstoke, UK), with gas generating kits (Oxoid, Basingstoke, UK). The plates were observed for the growth of C. perfringens, which was evident by the presence of characteristic black colonies [30]. The plates showing black colonies were selected, then the colonies were counted, and the results were interpreted as colony-forming units (CFU) per gram or mL of the sample. The presumed colonies on the agar plates were further subjected to purification by sub-culturing on TSC agar plates under the previously described conditions. The purified isolates were then identified by considering their cultural and morphological features, motility testing, double hemolysis on blood agar, and reverse Christie–Atkins–Munch–Petersen (CAMP) test. Moreover, the isolates were confirmed on the basis of some biochemical tests such as catalase, oxidase, nitrate reduction, indole, lactose fermentation, gelatin liquefaction, iron milk, and lecithinase production following the instructions specified in Bergey’s manual [31]. Additionally, further confirmation of the preliminary identified C. perfringens isolates was carried out using the commercially available API 20 A test system (bioMérieux, Marcy-l’Etoile, France) for identification of anaerobic bacteria according to the manufacturer’s instructions.
2.3. Genotypic Characterization of C. perfringens Isolates
The DNA of C. perfringens isolates was extracted using a QIAamp DNA Mini Kit Qiagen GmbH, Hilden, Germany) following the manufacturer’s instructions. The molecular characterization was conducted depending on the amplification of a unique region of C. perfringens 16S rRNA gene using species-specific PCR primers, ClPER-F (5′-AGATGGCATCATCATTCAAC-3′) and ClPER-R (5′-GCAAGGGATGTCAAGTGT-3′) [32]. The amplification of the target sequence was performed according to the following protocol: one cycle for 2 min at 94 °C, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s and extension at 72 °C for 45 s, and finally one cycle at 72 °C for 2 min. The C. perfringens ATCC 3626 and E. coli ATCC 25922 strains were used as positive and negative controls, respectively, during all PCR runs.
2.4. Antimicrobial Susceptibility Testing
The in vitro antimicrobial susceptibility patterns of all confirmed C. perfringens isolates were tested against ampicillin, amoxicillin/clavulanic acid, cefoxitin, enrofloxacin, imipenem, chloramphenicol, lincomycin, metronidazole, erythromycin, and tetracycline, using a broth microdilution method to determine the MIC values (Supplementary Table S2). Double-fold serial dilutions (0.125–512 μg/mL) of the tested antimicrobials were prepared in a sterile microtiter plate, and a fresh C. perfringens culture adjusted to 5 × 105 CFU/mL was added to each dilution. The plates were then incubated at 37 °C for 48 h under anaerobic conditions. Sterile broth and C. perfringens ATCC 3626 cultures were included as negative and positive controls in each run, respectively. The MIC values were determined according to the Clinical and Laboratory Standards Institute (CLSI) [33,34]. The MDR was defined as the resistance to at least one agent in three or more classes of the investigated antimicrobials.
2.5. Typing of C. perfringens Toxins
Clostridium perfringens toxins were typed using dermonecrotic tests in albino guinea pigs [35]. On the right side of guinea pig, a 0.2 mL of trypsinized 48 h supernatant of each C. perfringens culture was intradermally injected and the neutralized culture was injected on the left side in the same manner. The results were interpreted according to the degree of dermonecrotic reaction and its neutralization [36]. The neutralization tests were then performed in each albino guinea pig [37], using diagnostic C. perfringens antitoxin types A, B, C, D, and E (Burroguns, Welcome, Beckenham, London, UK).
2.6. Molecular Detection of C. perfringens Toxin and Antimicrobial Resistance Genes
The extracted DNAs of MDR and toxigenic C. perfringens isolates were subsequently subjected to PCR approaches to detect their relevant antibiotic resistance and toxin genes. Uniplex PCR assays were carried out to detect tet(K), tet(L), tet(M), lnu(A), lnu(B), erm(B), bla, qnrA, and qnrB genes associated with tetracycline, lincomycin, erythromycin, β-lactams, and enrofloxacin resistances, respectively as previously detailed [28,38,39,40,41,42]. Moreover, toxinogenic genotyping of C. perfringens was performed using a multiplex PCR procedure to detect C. perfringens alpha (cpa), beta (cpb), epsilon (etx), iota (iA), and enterotoxin (cpe) genes [43]. A uniplex PCR protocol was used for the amplification of the beta2 toxin (cpb2) gene [44]. The PCR reactions occurred in a total volume of 25 μL consisting of 12.5 μL ofDreamTaq TM Green Master Mix (2X) (Fermentas, Inc. Hanover, MD, USA), 1 μL of each primer (20 pmoL (Sigma-Aldrich, Co., St. Louis, MO, USA)), 5 uL of DNA template, and 5.5 μL of PCR-grade water. The primer sequences, product sizes and annealing temperatures used to amplify target resistance and toxin genes of C. perfringens are shown in Table 1. Ten microliters of amplified PCR products were subjected to gel electrophoresis in 1.5% agarose gel and visualized after staining with ethidium bromide (Sigma-Aldrich, Co., St. Louis, MO, USA), under UV illumination. Our PCR results were validated using both positive and negative controls. The DNAs from C. perfringens isolates, which harbor the tested genes, were used as the positive controls. Sterile saline was used as a negative control. The positive control DNAs were provided by the National Laboratory for Veterinary Quality Control on Poultry Production (NLQP).
Table 1.
Targeted resistance and toxin genes of C. perfringens and their primer sequences, expected amplicon sizes, and annealing temperatures.
| Target Gene | Primer Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|
| tet(K) | F: TTATGGTGGTTGTAGCTAGAAA R: AAAGGGTTAGAAACTCTTGAAA |
382 | 50 | [40] |
| tet(L) | F: ATAAATTGTTTCGGGTCGGTAAT R: AACCAGCCAACTAATGACAATGAT |
1077 | 50 | [38] |
| tet(M) | F: ACAGAAAGCTTATTATATAAC R: TGGCGTGTCTATGATGTTCAC |
171 | 55 | [39] |
| lnu(A) | F: GGTGGCTGGGGGGTAGATGTATTAACTGG R: GCTTCTTTTGAAATACATGGTATTTTTCGATC |
323 | 54 | [39] |
| lnu(B) | F: CCTACCTATTGTTTGTGGAA R: ATAACGTTACTCTCCTATTC |
906 | 45 | [39] |
| erm(B) | F: GAAAAGGTACTCAACCAAATA R: AGTAACGGTACTTAAATTGTTTAC |
638 | 57 | [28] |
| bla | F: ATGAAAGAAGTTCAAAAATATTTAGAG R: TTAGTGCCAATTGTTCATGATGG |
780 | 50 | [42] |
| qnrA | F: AGAGGATTTCTCACGCCAGG R: TGCCAGGCACAGATCTTGAC |
580 | 54 | [41] |
| qnrB | F: GGMATHGAAATTCGCCACTG R: TTTGCYGYYCGCCAGTCGAA |
264 | 54 | [41] |
| cpa | F: GCTAATGTTACTGCCGTTGA R: CCTCTGATACATCGTGTAAG |
324 | 54 | [41] |
| cpb | F: GCGAATATGCTGAATCATCTA R: GCAGGAACATTAGTATATCTTC |
196 | 54 | [43] |
| etx | F: GCGGTGATATCCATCTATTC R: CCACTTACTTGTCCTACTAAC |
655 | 54 | [43] |
| iA | F: ACTACTCTCAGACAAGACAG R: CTTTCCTTCTATTACTATACG |
446 | 54 | [43] |
| cpe | F: GGAGATGGTTGGATATTAGG R: GGACCAGCAGTTGTAGATA |
233 | 54 | [43] |
| cpb2 | F: AGATTTTAAATATGATCCTAACC R: CAATACCCTTCACCAAATACTC |
567 | 54 | [44] |
2.7. Statistical Analyses
The results of antimicrobial susceptibility test, toxins genes’ profiles, and toxinotypes distribution were plotted in a heatmap through the GraphPad software (version 8.0.1, GraphPad software Inc., LA Jolla, CA, USA). The other data were analyzed using the following R packages: heatmaply, corrplot, ggpubr, and hmisc.
3. Results
3.1. Prevalence, Loads, and Characterization of C. perfringens Isolates
Out of the 450 samples tested in this study, 50 were contaminated with C. perfringens with an overall prevalence rate of 11.1%. The samples from Sharkia governorate were more infected with C. perfringens (14.2%, 34 out of 240) than those from Port Said governorate (7.6%, 16 out of 210). The highest C. perfringens prevalence rate was found in chicken meat (12.6%, 19 out of 150), followed by raw milk (10.6%, 16 out of 150) and beef (10%, 15 out of 150). Interestingly, the number of C. perfringens in positive samples exceeded 102 CFU/g or /mL with mean values of 1.2 × 104, 2.4 × 103 and 9.7 × 102 CFU/g or /mL in the examined chicken meat, raw milk, and beef samples, respectively. The isolates were presumptively identified as C. perfringens on the basis of their cultural characters (Supplementary Figure S1) and morphological and biochemical features. Moreover, the API 20 A test and genetic detection of the specific 16S rRNA gene confirmed the identity of all recovered C. perfringens isolates.
3.2. Antimicrobial Susceptibility Results
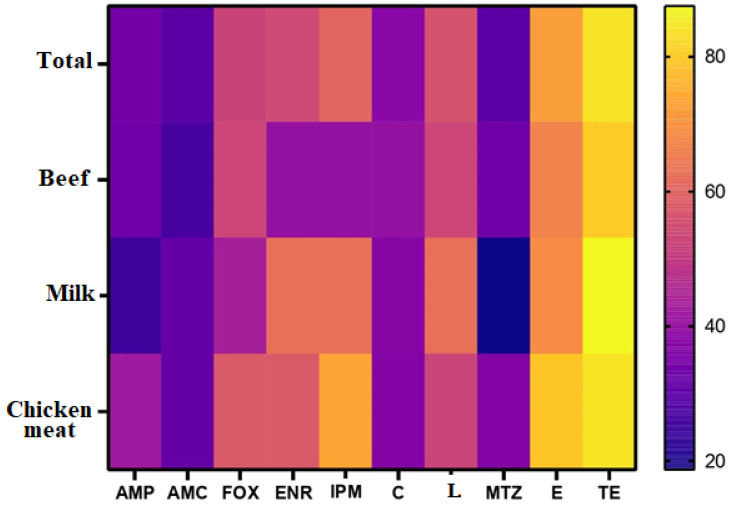
The highest resistance of C. perfringens isolates was recorded for tetracycline (84%) and erythromycin (72%). In turn, most of our isolates (70%) were sensitive to metronidazole and amoxicillin/clavulinic acid (Figure 1 and Figure 2). Tetracycline was the least effective drug for C. perfringens isolates, regardless of the source (beef, chicken meat, or milk) with resistance percentages of 80, 84.2, and 87.5%, respectively. Meanwhile, amoxicillin/clavulinic acid was the most effective drug for C. perfringens isolates in beef and chicken meat with susceptibility percentages of 73.3 and 68.4%, respectively. The highest susceptibility patterns for C. perfringens milk isolates were observed for metronidazole (81.2%), followed by ampicillin (75%) (Figure 1 and Figure 2). Generally, the overall resistance profiles of C. perfringens isolates were shocking as 74% (37 out of 50) were MDR. Additionally, three isolates, including two milk and one chicken meat, could not be treated with any of the tested antimicrobial agents.
Figure 1.
Frequency of resistance of C. perfringens isolates from beef, milk, and chicken meat samples to antimicrobials. AMP, ampicillin; AMC, amoxicillin/clavulanic acid; FOX, cefoxitin; ENR, enrofloxacin; IPM, imipenem; C, chloramphenicol; L, lincomycin; MTZ, metronidazole; E, erythromycin; TE, tetracycline. The percentages of resistance to antimicrobials are color-coded on the right of the figure.
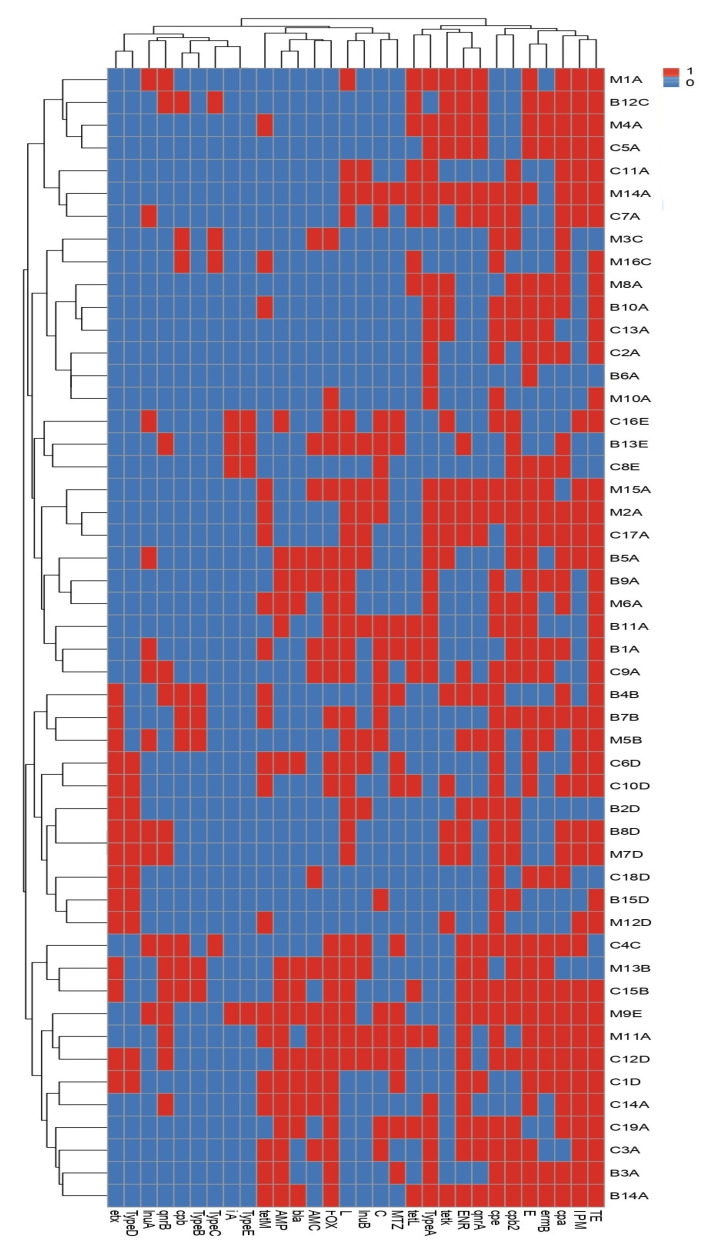
Figure 2.
Heat map and hierarchical clustering of the examined C. perfringens isolates based on the occurrence of antimicrobial resistance, antibiotic resistance, and toxin genes and toxinotypes. In the heat map, red and blue colors refer to the resistance/sensitivity to an antimicrobial agent and to the presence/absence of an antibiotic resistance, the toxin gene and toxinotype, respectively. The code numbers on the right of the heat map refer to the isolate numbers for beef (B), chicken meat (C), and milk (M) samples. AMP, ampicillin; AMC, amoxicillin/clavulanic acid; FOX, cefoxitin; ENR, enrofloxacin; IPM, imipenem; C, chloramphenicol; L, lincomycin; MTZ, metronidazole; E, erythromycin; TE, tetracycline. The tet(K), tet(L), and tet(M); lnu(A) and lnu(B); erm(B); bla; and qnrA and qnrB are genes associated with tetracycline, lincomycin, erythromycin, β-lactams, and enrofloxacin resistances, respectively. The cpa, cpb, etx, ia, and cpe are C. perfringens alpha, beta, epsilon, iota, and enterotoxin genes, respectively, and cpb2 is the C. perfringens beta2 toxin gene.
3.3. Molecular Basis of C. perfringens Resistance to Antimicrobials
A total of 85.7% (36 of 42) of the tetracycline resistant phenotypes harbored tet gene(s). Most tetracycline-resistant C. perfringens isolates harbored tet(K), tet(M), and tet(L) genes with prevalence rates of 45.2, 47.6, and 38%, respectively. Moreover, lnu gene(s) were found in 82.1% (23 of 28) of the lincomycin-resistant isolates. The lnu(A) and lnu(B) genes occurred in 39.2% (11/28) and 53.5% (15/28) of the lincomycin-resistant C. perfringens isolates, respectively. All enrofloxacin-resistant isolates (n = 27) harbored qnr gene(s). The qnrA and qnrB genes occurred in 74% (20/27) and 51.8% (14/27) of the enrofloxacin resistance isolates, respectively. Additionally, the bla gene occurred in 46.1% (12 of 26) of cefoxitin and 46.6% (7 of 15) of ampicillin resistant isolates. A total of 26 (72.2%) of the 36 erythromycin-resistant C. perfringens isolates carried the erm(B) gene (Figure 2). As expected, none of the tetracycline, lincomycin, enrofloxacin, and ampicillin or cefoxitin susceptible isolates carried tet, lnu, qnr, and bla genes, respectively. Moreover, the occurrence of relevant antibiotic resistance genes among resistant isolates was correlated with phenotypic antibiotic resistance and their molecular markers only. Meanwhile, our results confirmed that the absence of the resistance genes investigated did not predict the antimicrobial susceptibility, as some resistant strains lacked the common related resistance genes.
3.4. Toxinotyping and Toxin Gene Profiling of C. perfringens Isolates
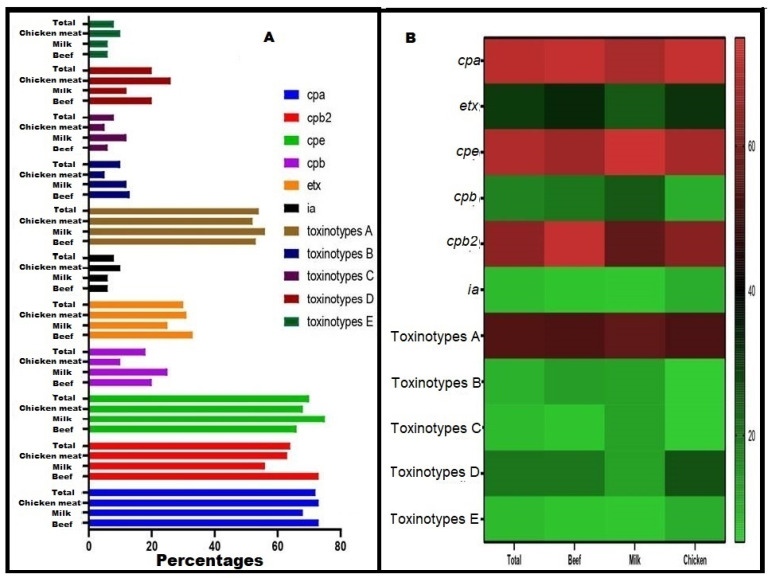
All recovered C. perfringens isolates were confirmed as toxigenic based on dermonecrotic reactions in albino guinea pigs. Interestingly, all toxinotypes were distributed among the tested C. perfringens isolates in our study. The toxin gene profiling clarified that C. perfringens type A had an occurrence that was higher than the other types, with a percentage of 54% (27 of 50). The least prevalent types were toxinotypes C and E (8% each, 4 out of 50). Most of the toxinotypes A and C were common among the milk isolates; however, the highest prevalence of toxinotypes B was detected among the beef isolates. The toxinotypes D and E prevailed among chicken meat isolates (Figure 3). The toxin genes cpa (72%), cpe (70%), and cpb2 (64%) were common, contrasting with the ia gene (8%), among the tested C. perfringens isolates (Figure 2).
Figure 3.
Distribution of toxinotypes and toxin genes among C. perfringens from chicken meat, milk, and beef samples. (A) Columns style using Graphpad prism, which showes the percentages of C. perfringens toxins and toxinotypes from each sample type. (B) Heat map style, in which the percentages of toxinotypes and toxin genes are color-coded on the right of the figure. The cpa, cpb, etx, ia, and cpe are C. perfringens alpha, beta, epsilon, iota, and enterotoxin genes, respectively; cpb2 is the C. perfringens beta2 toxin gene.
3.5. Phenotypic and Genotypic Diversity
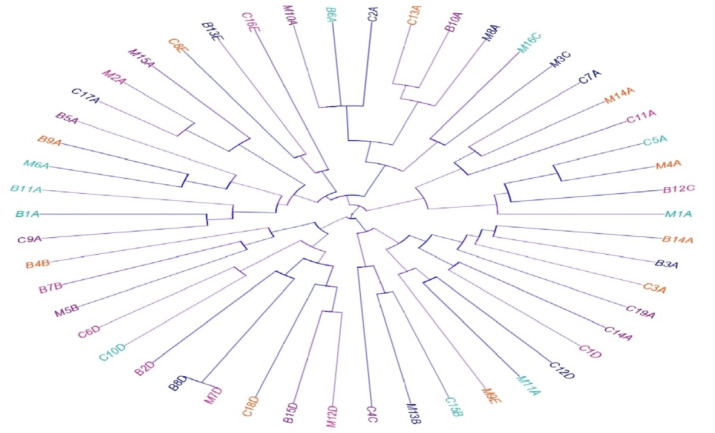
Our isolates showed high diversity and polyclonality based on the antimicrobial resistance and toxin gene profiles. Interestingly, all the tested isolates belonged to different lineages, with the exception of two isolates: one from beef and the other from milk samples (code numbers B8D and M7D, respectively; Figure 4).
Figure 4.
Dendrogram showing the relatedness of C. perfringens isolated from beef (B), chicken meat (C), and milk (M) samples as determined by the antimicrobial resistance and toxin gene profiles. The C. perfringens toxinotypes are indicated with different colors in the dendrogram to denote the specificity of various toxinotypes.
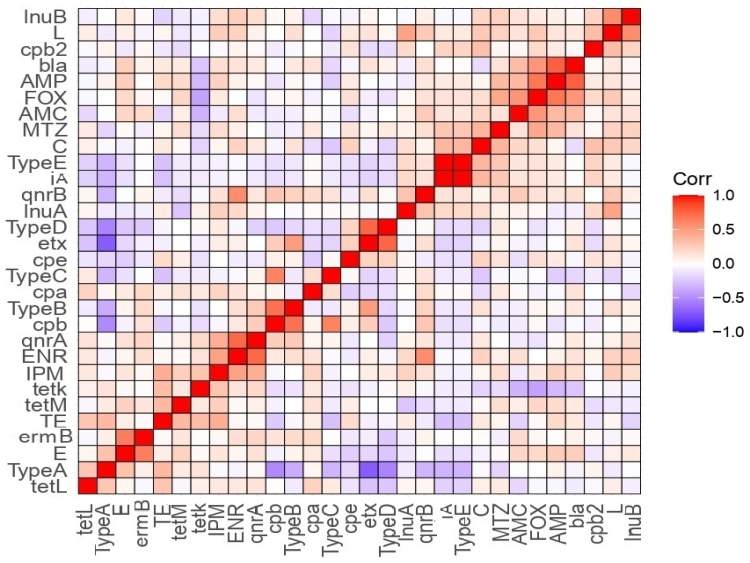
3.6. Correlation Analysis between Antimicrobial Susceptibility, Toxinotypes, and Toxin Gene Profiles
Toxinotype A was positively correlated with resistances to erythromycin, tetracycline, and the tet(L) gene, but it was negatively correlated with the resistance to metronidazole and the qnrB gene (Figure 5). We also recorded a positive correlation between toxinotype B and resistances to lincomycin, chloramephenicol, and enorfloxacin, and the erm(B), qnrA, qnrB, and bla genes, and a negative correlation between toxinotype B and the tet(K) gene. Moreover, toxinotype C was positively correlated with the qnrB and tet(L) genes and negatively correlated with the resistances to tetracycline, chloramphenicol, lincomycin, and ampicillin in addition to the bla gene. Toxinotype D was also correlated with the resistances to imipenem and metronidazole, and to the cpe gene. A negative correlation was recorded between toxinotype D and the resistances to erythromycin, chloramphenicol, and cefoxitin, and the tet(L), erm(B), qnrA, and cpb2 genes. Toxinotype E exhibited a positive correlation with the resistances to chloramphenicol and metronidazole, and the inu(A), qnrB, and cpb2 genes, and a negative correlation with the resistances to erythromycin and tetracycline, and the tet(L) gene. The cbp2 gene was positively correlated with toxinotype E, the resistances to chloramphenicol, lincomycin, and cefoxitin, and the inu(B), cpe, inu(A), and ia genes. The cbp2 gene was negatively correlated with toxinotype D, the resistances to tetracycline and erythromycin, and the etx and tet(M) genes. In addition, there was a positive correlation between the cpe gene and toxinotype D, the resistances to tetracycline, metronidazole, cefoxitin, ampicillin, lincomycin, and chloramphenicol, and the etx gene, and a negative correlation between the cpe gene and toxinotypes E and A, the resistances to erythromycin and ampicillin, and the cpa, ia, qnrB, tet(L), and tet(K) genes.
Figure 5.
Correlation (r) between antimicrobial resistance, antibiotic resistance, and toxin genes and toxinotypes of C. perfringens isolates from different sample types. Red and blue colors indicate positive and negative correlations, respectively. The color key refers to correlation coefficient (r). The darker red and blue colors imply stronger positive (R = 0.5:1) and negative (R = −0.5:−1) correlations, respectively. AMP, ampicillin; AMC, amoxicillin/clavulanic acid; FOX, cefoxitin; ENR, enrofloxacin; IPM, imipenem; C, chloramphenicol; L, lincomycin; MTZ, metronidazole; E, erythromycin; TE, tetracycline. The tet(K), tet(L), and tet(M); lnu(A) and lnu(B); erm(B); bla; and qnrA and qnrB are genes associated with tetracycline, lincomycin, erythromycin, β-lactams, and enrofloxacin resistances, respectively. The cpa, cpb, etx, ia, and cpe are C. perfringens alpha, beta, epsilon, iota, and enterotoxin genes, respectively, and cpb2 is the C. perfringens beta2 toxin gene.
4. Discussion
Risk-based food safety assessments are increasing worldwide. Foodborne pathogens are causing important outbreaks and diseases that have significant effects on the human health and economy. Food-poisoning outbreaks primarily comprise meat and meat products, but other food items, such as milk, may also be contaminated [45,46,47]. This crisis was compounded by the evolution of MDR/toxigenic strains [48,49,50]. Despite notable advances in the innovative next-generation therapies [51,52], the treatment failures for these pathogens were increased. Clostridium perfringens is an important foodborne pathogen, which causes several human and animal histotoxic and gastrointestinal diseases [53]. The high temperature used to cook meat products may inactivate the vegetative cells of C. perfringens; however, their spore can survive and then germinate, multiply, and produce toxins that lead to pronounced consumer health hazards [54]. On average, seven people in the United States and 50 to 100 people in the United Kingdom are killed by this foodborne illness per year [12,55]. Still, a limited number of studies have investigated this pathogen [56]. Therefore, this study attempted to break through this issue and examined the correlation between antimicrobial resistance, toxin profiles, and toxinotypes of C. perfringens.
The overall prevalence C. perfringens in our study (11.1%) was similar to that of studies in India (11%) [57] and Egypt (12.8%) [58]. In addition, our prevalence was lower than that recorded for chicken meat in China (23.1%) [59] and for American retail foods (30% and 80%) in the USA [60,61]. On the other hand, a lower prevalence was recorded in Pakistan (4%) [62] and Japan (8%) [63], and some reports did not detect C. perfringens in any of the examined chicken meat samples [64,65]. C. perfringens could be isolated from all of the samples tested (chicken meat, beef, and raw milk). The samples most infected with C. perfringens were chicken meat with a prevalence rate of 12.6%. A similar result was reported for Pakistan [62] and Japan [66]. Meanwhile, this contradicted a report in India [67], which reported that the highest contamination level of C. perfringens was recorded for goat meat. The varying prevalence of C. perfringens may be attributed to several factors such as differences in the hygienic conditions of the populations studied in the different studies, and the various techniques used to detect and isolate the organism in the collected samples [62]. Moreover, the variations in the hygienic practices during slaughtering, processing, and handling from production to consumption, the addition of additives, preservatives, and spices, and the stress conditions before and after the birds are slaughtered may affect the bacterial loads [68].
Several studies have referred to the increasing prevalence of MDR C. perfringens, which poses a serious threat to the efficient treatment for foodborne illness by limiting the therapeutic options [24,27]. The C. perfringens tested in this study showed frustrating susceptibility patterns, as 74% of the isolates were MDR. The antibiotics used in animal feed as growth promoters were the main causes for the evolution of C. perfringens resistance patterns as the bacteria become adapted as a consequence of the repeated use of antibiotics [69]. Therefore, there is an urgent need for solid guidelines defining the use of antibiotics and safe production of food products of animal origin.
All tested resistance genes were distributed with different prevalence among the resistant strains, and none of the susceptible strains had the related resistance genes. The tet genes were the most common resistance genes among our tested isolates. This result parallels with the phenotypic detection of resistance as most of our isolates were resistant to tetracycline. Several previous studies have established that tetracycline resistant strains were the most common phenotypes [70,71]. The continuous use of tetracycline as a growth promoter and the presence of numerous genes associated with the resistance to tetracycline shared among different C. perfringens isolates may explain the high prevalence of tetracycline resistant phenotypes [72].
As previously documented and supporting our results, the meat and meat products are commonly infected with C. perfringens type A [53,73]. Although the toxinotype A prevailed (54%), our C. perfringens isolates belonged to different linages as shown in Figure 2 and Figure 3. The tested isolates in this manuscript, as well as in other studies, showed high heterogeneity and belonged to several toxinotypes (A, B, C, D, and E) [74,75]. The heterogeneous nature of C. perfringens is possibly due to their recombination, in vivo and in vitro horizontal gene transfer, evolutionary dynamism, and, to a lesser extent, host specificity. Additionally, the non-outbreak isolates and randomly selected isolates showed high diversity in contrast to the isolates from the outbreaks [60,76].
In this report, the cpe and cpb2 genes were highly distributed among the tested isolates with prevalence rates of 70 and 64%, respectively. This was expected as all toxinotypes may potentially harbor these toxins in contrast to other toxins, which are linked to certain toxinotypes [21]. Another explanation for the high prevalence of cpe and cpb2 genes was that they are carried on both plasmid and chromosome [77,78] and the plasmid encoded genes can be transferred among the isolates, especially those recovered from the food chain [79]. Notably, C. perfringens type A can be modified to another distinct toxinotype upon acquisition of plasmid encoding toxins [80,81]. Therefore, the possibility of acquisition of a single toxin, such as CPE, is limited, supporting our results, once we recorded a negative correlation between the cpe gene and toxinotypes A and E. Several authors have correlated antimicrobial resistance patterns to only the administered antibiotics in animal feeds [82,83]. Moreover, the correlation between virulence and resistance was assessed in several other studies [84,85,86], and the genetic diversity of C. perfringens complicates these correlations [87]. In our report, we found weak positive and negative correlations. However, these correlations were variable, and we could not reach solid conclusions or fixed links. The treatment with antibiotics may increase the virulence expression such as of the cpb2 gene, due to antibiotic-induced ribosomal frameshifting [83]. Therefore, the antimicrobial resistance patterns may be unreliable markers to be correlated with the toxin profiles or toxinotypes of C. perfringens.
5. Conclusions
Beef, chicken meat, and raw milk samples from Egyptian supermarkets could be potentially contaminated with C. perfringens, especially with the toxinotype A, and the examined samples exceeded the permissible limits for C. perfringens, reflecting poor storage or poor processing conditions. Moreover, our results offer further evidence on the emergence of MDR C. perfringens strains. The antimicrobial resistance and toxin gene profiles expand knowledge on the high diversity and polyclonality of C. perfringens isolates. It is therefore recommended that the food safety standards and frequent inspections of the sanitary measures in supermarkets should be adequately enforced for efficient prevention of C. perfringens foodborne-associated infections among humans and animals. Moreover, control measures for proactive antimicrobial agents should be defined to limit the spread of MDR strains. The small number of the isolated strains (50) and the lack of whole-genome sequencing in this study prevent us from putting up solid correlations between antimicrobial resistance and toxinotypes of C. perfringens. Therefore, further studies in this point must be continued to provide a strong plane for the infection control protocols for C. perfringens.
Acknowledgments
The authors deeply acknowledge the Researchers Supporting program (TUMA-Project-2021-6), AlMaarefa University, Riyadh, Saudi Arabia for supporting the steps of this work.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology11040551/s1, Figure S1: Cultural characters of C. perfringens isolates on blood agar (A) showing double zone of hemolysis and on tryptose sulfite cycloserine agar (B) demonstrating typical characteristic black colonies. Table S1: Sources, numbers, locality, and distribution of the collected samples in the current study. Table S2: Interpretation of minimum inhibitory concentration values of different antimicrobial agents against Clostridium perfringens.
Author Contributions
M.M.B. and M.I.A.E.-H. designed the work; A.A.H., R.M.E.-T., R.M.A., and N.A.E. carried out the antimicrobial sensitivity test in this study. The molecular techniques and data analysis were conducted by M.M.B., M.I.A.E.-H., W.H.M., M.H.N., and M.M.G. Both the bioinformatics and statistical analyses of the data were performed by M.M.A.-S., M.I.A.E.-H., and M.M.B. Additionally, R.M.A., R.M.E.-T., A.A.H. and M.M.G. wrote the initial draft of this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All strains were isolated from food products at the Zagazig and Port Said Universities, and there were no human or animal clinical samples used in this study. Therefore, the informed consent and the ethical approval for this report were not necessary.
Informed Consent Statement
Not applicable, as only food samples, including chicken and bovine meats (processed meats), in addition to milk samples, were purchased from different supermarkets.
Data Availability Statement
All data generated or analyzed during this study are included in the published article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghaly M., Shaheen A., Bouhy A., Bendary M. Alternative therapy to manage otitis media caused by multidrug-resistant fungi. Arch. Microbiol. 2020;1:1231–1240. doi: 10.1007/s00203-020-01832-z. [DOI] [PubMed] [Google Scholar]
- 2.Okeke I.N., Laxminarayan R., Bhutta Z.A., Duse A.G., Jenkins P., O’Brien T.F., Pablos-Mendez A., Klugman K.P. Antimicrobial resistance in developing countries. Part I: Recent trends and current status. Lancet Infect. Dis. 2005;5:481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 3.Abd El-Aziz N.K., Abd El-Hamid M.I., Bendary M.M., El-Azazy A.A., Ammar A.M. Existence of vancomycin resistance among methicillin resistant S. aureues recovered from animal and human sources in Egypt. Slov. Vet. Res. 2018;55:221–230. [Google Scholar]
- 4.Abd El-Hamid M.I., Abd El-Aziz N.K., Samir M., El-Naenaeey E.Y., Abo Remela E.M., Mosbah R.A., Bendary M.M. Genetic Diversity of Campylobacter jejuni iolated from avian and human sources in Egypt. Front. Microbiol. 2019;10:2353. doi: 10.3389/fmicb.2019.02353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaly M.F., Nasr Z.M., Abousaty A.I., Seadawy H.G., Shaheen M.A.A., Albogami S., Al-Sanea M.M., Bendary M.M. Alternative and complementary therapies against foodborne Salmonella infections. Antibiotics. 2021;10:1453. doi: 10.3390/antibiotics10121453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim D., Abdelfattah-Hassan A., Badawi M., Ismail T.A., Bendary M.M., Abdelaziz A.M., Mosbah R.A., Mohamed D.I., Arisha A.H., Abd El-Hamid M.I. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella typhimurium. Sci. Rep. 2021;11:7742. doi: 10.1038/s41598-021-86990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross T.P., Kamara L.B., Hatheway C.L., Powers P., Libonati J.P., Harmon S.M., Israel E. Clostridium perfringens food poisoning: Use of serotyping in an outbreak setting. J. Clin. Microbiol. 1989;27:660–663. doi: 10.1128/jcm.27.4.660-663.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellou K., Kyritsi M., Chrysostomou A., Sideroglou T., Georgakopoulou T., Hadjichristodoulou C. Clostridium perfringens Foodborne Outbreak during an Athletic Event in Northern Greece, June 2019. Int. J. Environ. Res. Public Health. 2019;16:3967. doi: 10.3390/ijerph16203967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller R.W., Skinner J., Sulakvelidze A., Mathis G.F., Hofacre C.L. Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 2010;54:33–40. doi: 10.1637/8953-060509-Reg.1. [DOI] [PubMed] [Google Scholar]
- 10.Freedman J.C., Theoret J.R., Wisniewski J.A., Uzal F.A., Rood J.I., McClane B.A. Clostridium perfringens type A-E toxin plasmids. Res. Microbiol. 2015;166:264–279. doi: 10.1016/j.resmic.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prescott J.F. Brief Description of Animal Pathogenic Clostridia. In: Uzal F.A., Songer J.G., Prescott J.F., editors. Clostridial Diseases of Animals. 1st ed. John Wiley and Sons; Ames, IA, USA: 2016. pp. 13–19. [Google Scholar]
- 12.Adak G.K., Long S.M., O’Brien S.J. Trends in indigenous foodborne disease and deaths, England and Wales: 1992 to 2000. Gut. 2002;51:832–841. doi: 10.1136/gut.51.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung V.H., Phan Q., Costa C.E., Nishimura C., Pung K., Horn L., Sosa L. Notes from the Field: Clostridium perfringens outbreak at a catered lunch—Connecticut, September 2016. MMWR Morb. Morb. Mortal. Wkly. Rep. 2017;66:940–941. doi: 10.15585/mmwr.mm6635a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Food Safety Authority (EFSA) European Centre for Disease Prevention and Control (ECDC) The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2017. [(accessed on 5 September 2019)]. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5500.
- 15.Rhodehamel J., Harmon S.M. Clostridium Perfringens. U.S Food and Drug Adminstration, AOAC International; Gaithersburg, MD, USA: 1988. [(accessed on 31 October 2017)]. Bacteriological Analytical Manual. Chapter 16. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-Clostridium-perfringens. [Google Scholar]
- 16.Gizachew H. A Review on Clostridium Perfringens Food Poisoning. Glob. Res. J. Public Health Epidemiol. 2017;4:104–109. [Google Scholar]
- 17.Yoo H.S., Lee S.U., Park K.Y., Park Y.H. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. J. Clin. Microbiol. 1997;35:228–232. doi: 10.1128/jcm.35.1.228-232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper K.K., Songer J.G. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet. Microbiol. 2010;142:323–328. doi: 10.1016/j.vetmic.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 19.Chen J., Rood J.I., McClane B.A. Epsilon-toxin production by Clostridium perfringens type D strain CN3718 is dependent upon the agr operon but not the VirS/VirR two-component regulatory system. MBio. 2011;2:e00275-11. doi: 10.1128/mBio.00275-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Park J.Y., Kim S., Oh J.Y., Kim H.R., Jang I., Lee H.S., Kwon Y.K. Characterization of Clostridium perfringens isolates obtained from 2010 to 2012 from chickens with necrotic enteritis in Korea. Poult. Sci. 2015;94:1158–1164. doi: 10.3382/ps/pev037. [DOI] [PubMed] [Google Scholar]
- 21.Uzal F.A., Vidal J.E., McClane B.A., Gurjar A.A. Clostridium perfringens Toxins Involved in Mammalian Veterinary Diseases. Open Toxinol. J. 2010;2:24–42. doi: 10.2174/1875414701003020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall B.G. Predicting the evolution of antibiotic resistance genes. Nat. Rev. Microbiol. 2004;2:430–435. doi: 10.1038/nrmicro888. [DOI] [PubMed] [Google Scholar]
- 23.Ngamwongsatit B., Tanomsridachchai W., Suthienkul O., Urairong S., Navasakuljinda W., Janvilisri T. Multidrug resistance in Clostridium perfringens isolated from diarrheal neonatal piglets in Thailand. Anaerobe. 2016;38:88–93. doi: 10.1016/j.anaerobe.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y.H., Ye G.S. Determination of multidrug resistance mechanisms in Clostridium perfringens type A isolates using RNA sequencing and 2D-electrophoresis. Braz. J. Med. Biol. Res. 2018;51:8. doi: 10.1590/1414-431x20187044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bannam T.L., Johanesen P.A., Salvado C.L., Pidot S.J., Farrow K.A., Rood J.I. The Clostridium perfringens TetA(P) efflux protein contains a functional variant of the Motif A region found in major facilitator superfamily transport proteins. Microbiology. 2004;150:127–134. doi: 10.1099/mic.0.26614-0. [DOI] [PubMed] [Google Scholar]
- 26.Martel A., Devriese L.A., Cauwerts K., De Gussem K., Decostere A., Haesebrouck F. Susceptibility of Clostridium perfringens strains from broiler chickens to antibiotics and anticoccidials. Avian Pathol. 2004;33:3–7. doi: 10.1080/0307945031000163291. [DOI] [PubMed] [Google Scholar]
- 27.Ali M.Z., Islam M.M. Characterization of β-lactamase and quinolone resistant Clostridium perfringens recovered from broiler chickens with necrotic enteritis in Bangladesh. Iran. J. Vet. Res. 2021;22:48–54. doi: 10.22099/ijvr.2020.36848.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soge O.O., Tivoli L.D., Meschke J.S., Roberts M.C. A conjugativemacrolide resistance gene, mef(A), in environmental Clostridium perfringens carrying multiple macrolide and/or tetracyclineresistance genes. J. Appl. Microbiol. 2009;106:34–40. doi: 10.1111/j.1365-2672.2008.03960.x. [DOI] [PubMed] [Google Scholar]
- 29.ICMSF . Microorganisms in Foods. 1. Sampling Plans for Soft Drinks, Fruit Juices, Concentrates and Fruit Preserves. 2nd ed. University of Toronto Press; Toronto, ON, Canada: 1978. [Google Scholar]
- 30.Abudabos A.M., Alyemni A.H., Al-Marshad M.B. Bacillus subtilis PB6 based-probiotic (CloSTAT TM) improves intestinal morphological and microbiological status of broiler chickens under Clostridium perfringens challenge. Int. J. Agric. Biol. 2013;15:978–982. [Google Scholar]
- 31.Bergey D.H. Bergey’s Manual of Systematic Bacteriology, the Firmicutes, 2nd ed. Vol. 3. Williams & Wilkins; Athens, GA, USA: 2009. Clostridium; p. 738. [Google Scholar]
- 32.Kikuchi E., Miyamoto Y., Narushima S., Itoh K. Design of species-specific primers to identify 13 species of Clostridium harbored in human intestinal tracts. Microbiol. Immunol. 2002;46:353–358. doi: 10.1111/j.1348-0421.2002.tb02706.x. [DOI] [PubMed] [Google Scholar]
- 33.Clinical and Laboratory Standards Institute (CLSI) Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria. 9th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. CLSI standard M11. [Google Scholar]
- 34.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. CLSI supplement M100. [Google Scholar]
- 35.Bullen J.J. Clostridium welchii type D in the alimentary tract of normal sheep. J. Path Bact. 1952;64:201–210. doi: 10.1002/path.1700640120. [DOI] [PubMed] [Google Scholar]
- 36.Sterne M., Batty I. Pathogenic Clostridia. Butter Worth; London, UK: Boston, MA, USA: 1975. [Google Scholar]
- 37.Smith L.D.S., Holdeman L. The Pathogenic Anaerobic Bacteria. 1st ed. Charles Thomas Publisher; Athens, GA, USA: 1968. pp. 201–255. [Google Scholar]
- 38.Trzcinski K., Cooper B.S., Hryniewicz W., Dowson C.G. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2000;45:763–770. doi: 10.1093/jac/45.6.763. [DOI] [PubMed] [Google Scholar]
- 39.Aminov R.I., Garrigues-Jeanjean N., Mackie R.I. Molecular ecology of tetracycline resistance: Development and validation of primers for detection of tetracycline resistance genes encoding ribosomal protection proteins. Appl. Environ. Microbiol. 2001;67:22–32. doi: 10.1128/AEM.67.1.22-32.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masco L., Van-Hoorde K., De-Brandt E., Swings J., Huys G. Antimicrobial susceptibility of Bifidobacterium strains from humans, animals and probiotic products. J. Antimicrob. Chemother. 2006;58:85–94. doi: 10.1093/jac/dkl197. [DOI] [PubMed] [Google Scholar]
- 41.Cattoir V., Poirel L., Rotimi V., Soussy C.J., Nordmann P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBLproducing enterobacterial isolates. J. Antimicrob. Chemother. 2007;60:394–397. doi: 10.1093/jac/dkm204. [DOI] [PubMed] [Google Scholar]
- 42.Catalán A., Espoz M., Cortés W., Sagua H., González J., Araya J. Tetracycline and penicillin resistant Clostridium perfringens isolated from the fangs and venom glands of Loxosceles laeta: Its implications in loxoscelism treatment. Toxicon. 2010;56:890–896. doi: 10.1016/j.toxicon.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Meer R.R., Songer J.G. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am. J. Vet. Res. 1997;58:702–705. [PubMed] [Google Scholar]
- 44.Bueschel D.M., Helen-Jost B., Billington S.J., Trinh H.T., Glenn-Songer J. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: Correlation of genotype with phenotype. Vet. Microbiol. 2003;94:121–129. doi: 10.1016/S0378-1135(03)00081-6. [DOI] [PubMed] [Google Scholar]
- 45.Ammar A.M., Attia A.M., Abd El-Aziz N.K., Abd El Hamid M.I., El-Demerdash A.S. Class 1 integron and associated gene cassettes mediating multiple-drug resistance in some foodborne pathogens. Int. Food Res. J. 2016;23:332–339. [Google Scholar]
- 46.Ammar A.M., Attia A.M., Abd El-Hamid M.I., El-Shorbagy I.M., Abd El-Kader S.A. Genetic basis of resistance waves among methicillin resistant Staphylococcus aureus isolates recovered from milk and meat products in Egypt. Cell. Mol. Biol. 2016;62:7–15. [PubMed] [Google Scholar]
- 47.Ahmed H.A., Tahoun A.B., Abou Elez R.M., Abd El-Hamid M.I., Abd Ellatif S.S. Prevalence of Yersinia enterocolitica in milk and dairy products and the effects of storage temperatures on survival and virulence gene expression. Int. Dairy J. 2019;94:16–21. doi: 10.1016/j.idairyj.2019.02.010. [DOI] [Google Scholar]
- 48.Abd El-Hamid M.I., Bendary M.M. Comparative phenotypic and genotypic discrimination of methicillin resistant and susceptible Staphylococcus aureus in Egypt. Cell. Mol. Biol. 2015;61:106–117. [PubMed] [Google Scholar]
- 49.Abd El-Hamid M.I., Bendary M.M., Merwad A.M., Elsohaby I., Ghaith D.M., Alshareef W.A. What is behind phylogenetic analysis of hospital-, communityand livestock-associated methicillin-resistant Staphylococcus aureus? Transbound Emerg. Dis. 2019;66:1506–1517. doi: 10.1111/tbed.13170. [DOI] [PubMed] [Google Scholar]
- 50.Ammar A.M., Abd El-Hamid M.I., El-Malt R.S., Azab D.S., Albogami S., Al-Sanea M.M., Soliman W.E., Ghoneim M.M., Bendary M.M. Molecular detection of fluoroquinolone resistance among multidrug-, extensively drug-, and pan-drug-resistant Campylobacter species in Egypt. Antibiotics. 2021;10:1342. doi: 10.3390/antibiotics10111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosallam F.M., Helmy E.A., Bendary M.M., El-Batal I.A. Potency of a novel synthesized Ag- eugenol nanoemulsion for treating some bacterial and fungal pathogens. J. Mater. Res. 2021;36:1524–1537. doi: 10.1557/s43578-021-00226-1. [DOI] [Google Scholar]
- 52.Elfaky M.A., Abdel-Hamid M.I., Khalifa E., Alshareef W.A., Mosbah R.A., Elazab S.T., Ghoneim M.M., Al-Sanea M.M., Bendary M.M. Innovative next-generation therapies in combating multi-drug-resistant and multi-virulent Escherichia coli isolates: Insights from in vitro, in vivo, and molecular docking studies. Appl. Microbiol. Biotechnol. 2022;106:1691–1703. doi: 10.1007/s00253-022-11781-w. [DOI] [PubMed] [Google Scholar]
- 53.McClane B.A. Clostridium perfringens. In: Beuchat L.R., Doyle M.P., Montville T.J., editors. Food Microbiology: Fundamentals and Frontiers, 2nd ed. ASM Press; Washington, DC, USA: 2001. pp. 351–372. [Google Scholar]
- 54.Kamber U., Gokce H.I., Elmali M. Clostridium perfringens and its toxins in minced meat from Kars, Turkey. Food Addit. Contam. 2007;24:673–678. doi: 10.1080/02652030601186129. [DOI] [PubMed] [Google Scholar]
- 55.Mead P.S., Slutsker L., Dietz V., McCaig L.F., Bresee J.S., Shapiro C., Griffen P.M., Tauxe R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chalmers G., Bruce H.L., Hunter D.B., Parreira V.R., Kulkarni R.R., Jiang Y.F., Prescott J.F., Boerlin P. Multilocus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chicken populations. J. Clin. Microbiol. 2008;46:3957–3964. doi: 10.1128/JCM.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gurmu E.B., Hazarika R.A., Borah P., Barua A.G. Prevalence of enterotoxigenic Clostridium perfringens in foods of animal origin, Guwahati, India. J. Environ. Occup. Sci. 2013;2:45–50. doi: 10.5455/jeos.20130309121145. [DOI] [Google Scholar]
- 58.Emara M.S. Master’s Thesis. Cairo University; Cairo, Egypt: 2014. Anaerobic and Aerobic Microorganisms in Human Food. [Google Scholar]
- 59.Zhang T., Zhang W., Ai D., Zhang R., Lu Q., Luo Q., Shao H. Prevalence and characterization of Clostridium perfringens in broiler chickens and retail chicken meat in central China. Anaerobe. 2018;54:100–103. doi: 10.1016/j.anaerobe.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Lin Y.T., Labbe R. Enterotoxigenicity and genetic relatedness of Clostridium perfringens isolates from retail foods in the United States. Appl Environ. Microbiol. 2003;69:1642–1646. doi: 10.1128/AEM.69.3.1642-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen Q., McClane B.A. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl. Environ. Microbiol. 2004;70:2685–2691. doi: 10.1128/AEM.70.5.2685-2691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan M., Nazir J., Anjum A.A., Ahmad M.U., Nawaz M., Shabbir M.Z. Toxinotyping and antimicrobial susceptibility of enterotoxigenic Clostridium perfringens isolates from mutton, beef and chicken meat. J. Food Sci. Technol. 2015;52:5323–5328. doi: 10.1007/s13197-014-1584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miki Y., Miyamoto K., Kaneko-Hirano I., Fujiuchi K., Akimoto S. Prevalence and characterization of enterotoxin gene-carrying Clostridium perfringens isolates from retail meat products in Japan. Appl. Environ. Microbiol. 2008;74:5366–5372. doi: 10.1128/AEM.00783-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hashem H.M. Master’s Thesis. Benha University; Cairo, Egypt: 2015. Bacteriological criteria of dressed poultry with special reference to some microbial decontaminators. [Google Scholar]
- 65.Hemmat M.I., Amin R.A., El-Shater M.A., Hafez M.S. Bacteriological evaluation of freshly slaughtered chicken carcasses. BVMJ. 2015;28:74–82. [Google Scholar]
- 66.Miwa N., Nishina T., Kubo S., Atsumi M., Honda H. Amount of enterotoxigenic Clostridium perfringens in meat detected by nested PCR. Int. J. Food Microbiol. 1998;42:195–200. doi: 10.1016/S0168-1605(98)00082-8. [DOI] [PubMed] [Google Scholar]
- 67.Singh R.V., Bhilegaonkar K.N., Agarwal R.K. Studies on occurrence and characterization of Clostridium perfringens from select meats. J. Food Saf. 2005;25:146–156. doi: 10.1111/j.1745-4565.2005.00560.x. [DOI] [Google Scholar]
- 68.Kamal A. Master’s Thesis. Benha University; Cairo, Egypt: 2017. Clostridium perfringens in Meat and Chicken Received in University Hostel. [Google Scholar]
- 69.Arnold S., Gassner B., Giger T., Zwahlen R. Banning antimicrobial growth promoters in feedstuffs does not result in increased therapeutic use of antibiotics in medicated feed in pig farming. Pharmacoepidemiol. Drug Saf. 2004;13:323–331. doi: 10.1002/pds.874. [DOI] [PubMed] [Google Scholar]
- 70.Marks S.L., Kather E.J. Antimicrobial susceptibilities of canine Clostridium difficile and Clostridium perfringens isolates to commonly utilized antimicrobial drugs. Vet. Microbiol. 2003;94:39–45. doi: 10.1016/S0378-1135(03)00061-0. [DOI] [PubMed] [Google Scholar]
- 71.Chon J.W., Seo K.H., Bae D., Park J.H., Khan S., Sung K. Prevalence, toxin gene profile, antibiotic resistance, and molecular characterization of Clostridium perfringens from diarrheic and non-diarrheic dogs in Korea. J. Vet. Sci. 2018;19:368–374. doi: 10.4142/jvs.2018.19.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slavić D., Boerlin P., Fabri M., Klotins K.C., Zoethout J.K., Weir P.E., Bateman D. Antimicrobial susceptibility of Clostridium perfringens isolates of bovine, chicken, porcine, and turkey origin from Ontario. Can. J. Vet. Res. Rev. Can. Rech. Vet. 2011;75:89–97. [PMC free article] [PubMed] [Google Scholar]
- 73.Ghoneim N.H., Hamza D.A. Epidemiological studies on Clostridium perfringens food poisoning in retail foods. Rev. Sci. Tech. Int. Off. Epizoot. 2017;36:1025–1032. doi: 10.20506/rst.36.3.2734. [DOI] [PubMed] [Google Scholar]
- 74.Nauerby B., Pedersen K., Madsen M. Analysis by pulsed-field gel electrophoresis of the genetic diversity among Clostridium perfringens isolates from chickens. Vet. Microbiol. 2003;94:257–266. doi: 10.1016/S0378-1135(03)00118-4. [DOI] [PubMed] [Google Scholar]
- 75.Gholamiandekhordi A.R., Ducatelle R., Heyndrickx M., Haesebrouck F., Van-Immerseel F. Molecular and phenotypical characterization of Clostridium perfringens isolates from poultry flocks with different disease status. Vet. Microbiol. 2006;113:143–152. doi: 10.1016/j.vetmic.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura H., Ogasawara J., Monma C., Hase A., Suzuki H., Kai A., Haruki K., Nishikawa Y. Usefulness of a combination of pulsed-field gel electrophoresis and enrichment culture in labora-tory investigation of a foodborne outbreak due to Clostridium perfringens. Diagn. Microbiol. Infect. Dis. 2003;47:471–475. doi: 10.1016/S0732-8893(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 77.Fisher D.J., Miyamoto K., Harrison B., Akimoto S., Sarker M.R., McClane B.A. association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol. Microbiol. 2005;56:747–762. doi: 10.1111/j.1365-2958.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- 78.Xiao Y., Wagendorp A., Moezelaar R., Abee T., Wells-Bennik M.H. A wide variety of Clostridium perfringens type A food-borne isolates that carry a chromosomal cpe gene belong to one multilocus sequence typing cluster. Appl. Environ. Microbiol. 2012;78:7060–7068. doi: 10.1128/AEM.01486-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lacey J.A., Keyburn A.L., Ford M.E., Portela R.W., Johanesen P.A., Lyras D., Moore R.J. Conjugation-mediated horizontal gene transfer of Clostridium perfringens plasmids in the chicken gastrointestinal tract results in the formation of new virulent strains. Appl. Environ. Microbiol. 2017;83:e01814-17. doi: 10.1128/AEM.01814-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petit L., Gibert M., Popoff M.R. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999;7:104–110. doi: 10.1016/S0966-842X(98)01430-9. [DOI] [PubMed] [Google Scholar]
- 81.Rood J.I., Adams V., Lacey J., Lyras D., McClane B.A., Melville S.B., Moore R.J., Popoff M.R., Sarker M.R., Songer J.G. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobes. 2018;53:5–10. doi: 10.1016/j.anaerobe.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doyle M.E. Multidrug-resistant pathogens in the food supply. Foodborne Pathog. Dis. 2015;12:261–279. doi: 10.1089/fpd.2014.1865. [DOI] [PubMed] [Google Scholar]
- 83.Fayez M., El-Ghareeb W.R., Elmoslemany A., Alsunaini S.J., Alkafafy M., Alzahrani O.M., Mahmoud S.F., Elsohaby I. genotyping and antimicrobial susceptibility of Clostridium perfringens and clostridioides difficile in camel minced meat. Pathogens. 2021;10:1640. doi: 10.3390/pathogens10121640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beceiro A., Tomás M., Bou G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013;26:185–230. doi: 10.1128/CMR.00059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vilei E.M., Schlatter Y., Perreten V., Straub R., Popoff M.R., Gibert M., Grone A., Frey J. Antibiotic-induced expression of a cryptic cpb2 gene in equine β2-toxigenic Clostridium perfringens. Mol. Microbiol. 2005;57:1570–1581. doi: 10.1111/j.1365-2958.2005.04789.x. [DOI] [PubMed] [Google Scholar]
- 86.Ahn D., Prince A. Host-pathogen interface: Progress in understanding the pathogenesis of infection due to multidrug-resistant bacteria in the intensive care unit. J. Infect. Dis. 2017;215:S1–S8. doi: 10.1093/infdis/jiw405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei B., Cha S.Y., Zhang J.F., Shang K., Park H.C., Kang J., Lee K.J., Kang M., Jang H.K. Antimicrobial susceptibility and association with toxin determinants in Clostridium perfringens isolates from chickens. Microorganisms. 2020;8:1825. doi: 10.3390/microorganisms8111825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the published article and the Supplementary Materials.