Abstract
Laser interstitial thermal therapy (LITT) has become an increasingly utilized alternative to surgical resection for the treatment of glioma in patients. However, treatment outcomes in isocitrate dehydrogenase 1 and 2 (IDH1/2) mutant glioma, specifically, have not been reported. The objective of this study was to characterize a single institution’s cohort of IDH1/2 mutant grade 2/3 glioma patients treated with LITT. We collected data on patient presentation, radiographic features, tumor molecular profile, complications, and outcomes. We calculated progression-free survival (PFS) and tested factors for significant association with longer PFS. Overall, 22.7% of our cohort experienced progression at a median follow up of 1.8 years. The three- and five-year estimates of PFS were 72.5% and 54.4%, respectively. This is the first study to characterize outcomes in patients with IDH1/2 mutant glioma after LITT. Our results suggest that LITT is an effective treatment option for IDH1/2 mutant glioma.
Keywords: glioma, IDH1 mutation, IDH2 mutation, laser interstitial thermal therapy, astrocytoma, oligodendroglioma
1. Introduction
Since its first description in 1983 [1] and application in the treatment of brain lesions in 1990 [2], indications for laser interstitial thermal therapy (LITT) within the central nervous system (CNS) have continued to expand. While initially used in the treatment of recurrent glioblastoma (GBM), LITT has become more common in the treatment of primary CNS neoplasms [3,4,5,6], brain metastases [7,8], radiation necrosis [7,9,10], and epilepsy [11,12]. Retrospective studies have shown that LITT is a reasonable and at times favorable alternative to standard of care surgical resection in gliomas, particularly in cases where lesions are deep-seated or if the patient is not a good candidate for open surgical resection [4,7,13,14].
Gliomas with mutations in isocitrate dehydrogenase (IDH) 1 and 2, first discovered in genomic analysis of GBM, are a molecularly distinct subtype of diffuse glioma associated with younger age of diagnosis and longer overall survival compared to IDH1/2 wild-type gliomas [15,16,17]. While many studies of glioma have historically combined IDH1/2 mutant and wild-type cohorts when analyzing treatment outcomes in glioma patients, recently, more concerted efforts have been made to analyze the outcomes of IDH1/2 mutant gliomas as a distinct entity [18,19,20]. Currently, the standard of care in the treatment of IDH1/2 mutant gliomas is maximal, safe surgical resection, with adjuvant radiotherapy and specific chemotherapy dependent on molecular subtype and grade [21]. Repeat surgery, particularly in the case of recurrent high-grade glioma, is also commonplace [22,23].
Over the last decade, LITT has become an increasingly utilized modality in the treatment of gliomas. While LITT has been shown to be an effective, well-tolerated alternative to open surgical resection in both low- [4,24,25] and high-grade [14,26,27] gliomas, LITT treatment outcomes specific to IDH1/2 mutant grade 2 and 3 gliomas have not been studied. The objective of the present study was to describe the clinical and histopathological presentation, treatment, and outcomes of patients with IDH1/2 mutant grade 2/3 gliomas treated with LITT at a single institution, to determine the progression-free survival of these patients, and to compare their clinical outcomes with previously published findings.
2. Materials and Methods
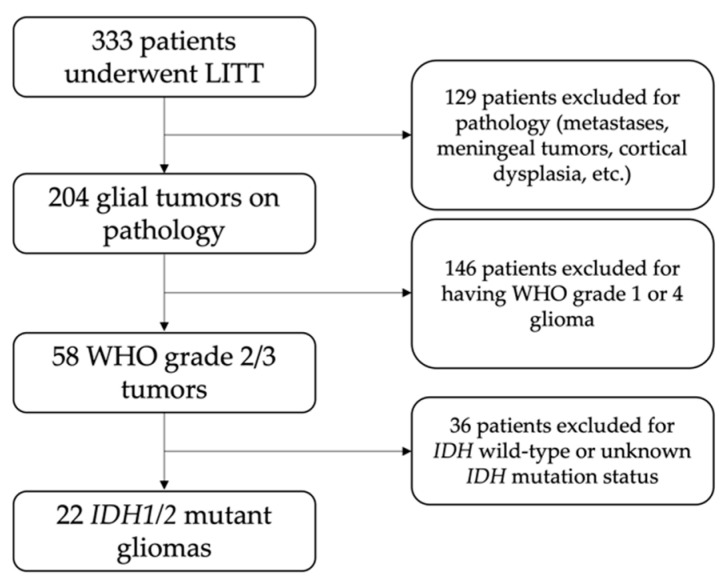
The study was conducted with the approval of the institutional review board (IRB ID# 201409046, approval date: 3 February 2022). This study was a single-institution, retrospective case series of patients treated at Washington University, Barnes Jewish Hospital, and St. Louis Children’s Hospital with LITT for IDH1/2 mutant grade 2 or 3 gliomas from 2014 to 2021. Patients who underwent LITT at our institution were identified via combination of case log databases and departmental billing records. Patients were excluded if their pathology was not consistent with a glial neoplasm, was determined to be WHO grades I or IV, was IDH1/2 wild-type, or was of an unknown IDH1/2 mutation status (Figure 1).
Figure 1.
Flowchart of patient selection.
Data extracted from the electronic medical record included patient demographics, clinical presentation, radiographic characteristics, treatment prior to LITT, LITT operative details, adjuvant treatment, and outcomes after treatment to better classify the presentation and outcomes of these patients. Tumor volume was calculated using the formula (tumor volume) = (antero-posterior diameter) × (transverse diameter) × (craniocaudal diameter)/2. In cases where a patient presented with more than one lesion, tumor volumes were combined for analysis.
We collected tumor genetic information to classify gliomas by molecular subtype according to 2021 WHO guidelines [28]. IDH1/2 mutation status was determined by immunohistochemistry (R132H antibody) or DNA sequencing. Chromosome 1p and 19q deletion status was determined using fluorescence in situ hybridization. Using this information, we reclassified all tumors into the following categories: (1) oligodendroglioma (ODG; IDH1/2 mutant with 1p/19q co-deletion) and (2) diffuse astrocytoma (DA; IDH1/2 mutant without 1p/19q co-deletion or with TP53 and ATRX mutations). Patients with non-glial neoplasms, no IDH1/2 mutation on immunohistochemistry or sequencing, or inconclusive IDH1/2 status were excluded from this study.
Our primary outcome of interest in this study was progression-free survival (PFS). Progression was defined as an increase in area of enhancement of a previously treated enhancing lesion and/or new or increasing mass lesions on MRI. PFS was measured from the date of initial LITT to the date of documented radiographic progression. Secondary outcomes of interest included complications, operative details, and need for repeat LITT. In our analysis, we considered pre-planned, staged LITT treatments as one treatment.
A literature search was performed to identify studies analyzing treatment outcomes and progression-free survival of IDH-mutant cohorts who underwent craniotomy or LITT. Studies analyzing IDH-mutant grade 2 or 3 glioma cohorts that reported survival outcomes were reviewed.
Statistical analyses were performed using SPSS version 28 (IBM Corporation, Armonk, NY, USA). Categorical variables were reported as percentage of the total cohort. Continuous variables were evaluated for normality; normally distributed continuous variables were reported with mean and standard deviation, and non-normally distributed continuous variables were reported with median and range.
Median time to progression was obtained using Kaplan–Meier (KM) product-limit estimates of the survival function and were graphically represented using KM curves. If the survival estimate was above 50% at the end of observation time, a “not reached” indicator was used. KM survival tables were used to estimate PFS at 3 and 5 years. KM curves were constructed for the overall cohort, first line versus salvage treatment, prior extent of resection, extent of ablation, and tumor pathology. Log-rank testing was conducted to identify factors independently associated with differences in PFS. Univariate logistic regression was used to identify factors independently associated with progression. Factors with p < 0.10 were retained for multivariable analysis (Cox proportional hazard testing and multivariable logistic regression).
Patient consent was not required due to the retrospective nature of the study.
3. Results
3.1. Baseline Characteristics and Presentation
Twenty-two patients who underwent LITT for IDH1/2 mutant grade 2/3 glioma at a mean age of 46.6 years were included in this study. Their demographics, presenting symptoms, and pre-LITT treatment details can be found in Table 1. Of these patients, 63.6% were male, 86.4% were white, and 95.5% were alive at the time of chart review. The series included one death due to an unknown cause but thought not to be tumor-related (Case #21). Sixteen patients (72.7%) were symptomatic at presentation, with seizure (40.9%) and headache (22.7%) as the most common symptoms. The median (range) pre-LITT Karnofsky Performance Score (KPS) was 85 (60–100). The median (range) initial tumor volume was 5.6 (0.18–48.38) cm3.
Table 1.
Cohort demographics and initial presentation.
| Case | Age | Sex | Presenting Symptoms | Pre-LITT KPS | Pre-LITT Treatment | Lesion Location | Tumor Volume (cm3) |
|---|---|---|---|---|---|---|---|
| 1 | 43.8 | F | Sensory changes, headache, syncopal episodes, fatigue, change in taste/smell | 90 | None | L frontotemporal, insula | 21.39 |
| 2 | 44.0 | M | Seizures | 80 | None | R frontoparietal, cingulate gyrus | 2.42 |
| 3 | 65.9 | M | Seizures | 90 | None | L parietooccipital | 0.84 |
| 4 | 35.0 | F | Headache, vision problems, neurocognitive deficits, behavioral changes | 70 | None | R parietooccipital | 10.35 |
| 5 | 67.3 | F | Seizures, neurocognitive deficits, tremor | 70 | Bx | L frontotemporal, basal ganglia, insula | 37.02 |
| 6 | 60.7 | M | Seizures | 80 | GTR ×2, RT, C | R frontal | 10.67 |
| 7 | 49.8 | M | Seizures, speech difficulties | 80 | STR, RT, C | L occipital | 5.95 |
| 8 | 46.9 | F | None | 100 | GTR, RT, | R frontal | 15.75 |
| 9 | 56.9 | M | Muscle weakness/paralysis, speech difficulties | 70 | GTR ×2, RT, Bx | L frontoparietal | 1.69 |
| 10 | 46.4 | F | None | 100 | GTR | L frontal | 1.08 |
| 11 | 53.3 | M | None | 100 | STR, RT, C | R temporal, insula | 22.28 |
| 12 | 60.0 | M | Muscle weakness/paralysis, headache | 80 | STR, C, | R frontoparietal | 10.01 |
| 13 | 21.8 | M | Seizures | 80 | Bx | L frontotemporal, insula | 48.38 |
| 14 | 47.4 | F | None | 100 | STR, RT, | R temporal | 10.29 |
| 15 | 36.0 | M | Seizures | 80 | STR ×2, RT, C, GK | L temporal | 3.02 |
| 16 | 45.4 | M | None | 100 | GTR, RT, C, Bx | L frontal | 0.38 |
| 17 | 31.3 | M | Headache | 90 | None | L insula | 15.91 |
| 18 | 48.5 | M | Muscle weakness/paralysis, headache, speech difficulties | 60 | STR ×2, RT, C | L frontoparietal | 0.18 |
| 19 | 44.2 | F | Seizures | 100 | GTR, RT, C | R frontal | 1.05 |
| 20 | 41.9 | M | Earaches | 90 | None | L insula | 3.15 |
| 21 | 34.3 | M | None | 100 | GTR, RT, C | L insula | 3.78, 1.46 * |
| 22 | 44.8 | F | Seizures | 80 | STR, RT, C | R temporal | 5.30 |
Abbreviations: KPS = Karnosfsky Performance Score, F = female, M = male, L = left, R = right, GTR = gross total resection, STR = subtotal resection, RT = radiation therapy, C = chemotherapy, GK = gamma knife radiosurgery, Bx = biopsy. * This patient presented with two lesions.
Treatment prior to LITT was common, with 72.7% of patients having undergone pre-LITT treatment. Surgical resection was the most common pre-LITT treatment (14 patients, 63.6%), with 57.1% of these patients undergoing gross total resection (GTR) and 42.9% undergoing subtotal resection (STR). Ten patients (45.5%) underwent chemotherapy, with most patients (90%) undergoing chemotherapy with temozolomide. Radiation therapy was also common, with 12 (54.4%) of patients undergoing treatment. Biopsy was less common (four patients, 18.2%), and radiosurgery was the least common pre-LITT treatment (one patient).
3.2. Tumor Pathology
IDH1/2 mutant gliomas were re-classified according to the 2021 WHO guidelines into oligodendroglioma (ODG, IDH1/2 mutant and 1p/19q co-deleted) and diffuse astrocytoma (DA, IDH1/2 mutant without 1p/19q co-deletion). Of the 22 patients in the study, 12 patients (54.5%) had ODG and 10 (45.5%) had DA. Most tumors (59.1%) were grade 2, while the remaining nine (40.9%) were grade 3. Only one patient (Case #2) had a mutation in IDH2 (R127K); the remaining patients had mutations in IDH1. The most common IDH1 mutation was R132H (90.1%), followed by R132C (4.5%). Other mutations in each patient’s tumor, along with Ki-67 proliferative index information, can be found in Table 2.
Table 2.
Histopathologic findings.
| Case | Tumor Type | WHO Grade | Mutations | Ki-67 Index | Tumor Mutational Burden |
|---|---|---|---|---|---|
| 1 | Diffuse astrocytoma | 2 | IDH1, TP53, ATRX | ≈4 | 1 |
| 2 | Oligodendroglioma | 2 | IDH2, TERTp, CIC, 1p/19q co-deletion | 17 | 0 |
| 3 | Oligodendroglioma | 2 | IDH1, TERTp, CIC, 1p/19q co-deletion | 6 | 3 |
| 4 | Diffuse astrocytoma | 2 | IDH1, TP53, ATRX | 2.5 | 6 |
| 5 | Oligodendroglioma | 2 | IDH1, 1p/19q co-deletion | NT | NT |
| 6 | Diffuse astrocytoma | 2 | IDH1, TP53, ATRX | <10 | NT |
| 7 | Diffuse astrocytoma | 2 | IDH1 | 5.7 | NT |
| 8 | Diffuse astrocytoma | 2 | IDH1, TP53, ATRX | 2.3 | 3 |
| 9 | Oligodendroglioma | 2 | IDH1, 1p/19q co-deletion | 8.2 | NT |
| 10 | Oligodendroglioma | 2 | IDH1, 1p/19q co-deletion | 2.1 | NT |
| 11 | Oligodendroglioma | 2 | IDH1, TERTp, CIC, 1p/19q co-deletion | 3.7 | 0 |
| 12 | Oligodendroglioma | 2 | IDH1, TERTp, CIC, 1p/19q co-deletion | NT | 42 |
| 13 | Diffuse astrocytoma | 2 | IDH1 | <5 | NT |
| 14 | Diffuse astrocytoma | 3 | IDH1, TP53, ATRX | 1.4 | 1 |
| 15 | Oligodendroglioma | 3 | IDH1, TERTp, CIC, 1p/19q co-deletion | 40 | 16 |
| 16 | Oligodendroglioma | 3 | IDH1, TERTp, 1p/19q co-deletion | 9.9 | 13 |
| 17 | Diffuse astrocytoma | 3 | IDH1, TP53, ATRX | NT | NT |
| 18 | Oligodendroglioma | 3 | IDH1, 1p/19q co-deletion | 24 | NT |
| 19 | Oligodendroglioma | 3 | IDH1, TERTp, CIC, 1p/19q co-deletion | 20 | NT |
| 20 | Diffuse astrocytoma | 3 | IDH1, TP53, ATRX | 5–10 | 4 |
| 21 | Diffuse astrocytoma | 3 | IDH1, TP53, ATRX | 31.5 | 1 |
| 22 | Oligodendroglioma | 3 | IDH1, TERTp, 1p/19q co-deletion | 50 | 3 |
Abbreviations: NT = not tested.
3.3. LITT Treatment
LITT operative details and outcomes are detailed for each case in Table 3. LITT was the first-line treatment in 36.4% of our cohort. The median (range) volume ablated in this cohort overall was 95% (65–100%). The mean ± standard deviation (SD) operative time was 211.2 ± 77.7 min, and the mean anesthesia time was 342.5 ± 80.3 min. Overall, three patients in our cohort (13.6%) experienced perioperative complications after LITT. Two patients (9%) in the cohort suffered from perioperative complications after their initial LITT procedure. Of these, one patient (Case #12) developed seizures in the post-operative period, while the other (Case #19) was treated for deep vein thrombosis. A third patient (Case #1) suffered from perioperative complications after a second, non-staged LITT procedure for residual tumor. Her course was complicated by severe cerebral edema requiring decompressive hemicraniectomy and characterized by new onset aphasia, right facial droop, right hemiparesis, and dysarthria. She was later discharged to inpatient rehabilitation.
Table 3.
Operative details and outcomes.
| Case | First Line vs. Salvage | Indication for LITT | EOA (%) | Perioperative Complications | Adjuvant Tx | Repeat LITT | Time to Progression (Months) | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | First line | Tumor location | 80 | Severe edema, new FND | RT, C | Yes | No progression | 9.85 |
| 2 | First line | Tumor location | 95 | None | RT, C | No | No progression | 7.36 |
| 3 | First line | Unknown | 85 | None | RT, C | No | No progression | 16.52 |
| 4 | First line | Tumor location | 95 | None | RT, C | No | No progression | 33.80 |
| 5 | First line | Shorter LOS | 65 | None | RT, C | No | No progression | 73.13 |
| 6 | Salvage | Refractory to Tx | 95 | None | None | No | No progression | 62.12 |
| 7 | Salvage | Recurrence | NR | None | STR, RT, C | No | 7.42 | 70.14 |
| 8 | Salvage | Recurrence | 95 | None | RT, C | No | No progression | 19.48 |
| 9 | Salvage | Recurrence | 98 | None | C | No | 45.34 | 88.50 |
| 10 | Salvage | Recurrence | 100 | None | None | No | No progression | 23.55 |
| 11 | Salvage | Recurrence | 80 | None | C | No | 18.82 | 18.82 |
| 12 | Salvage | Recurrence | 80 | Seizure | None | No | No progression | 16.29 |
| 13 | First line | Aborted craniotomy | 70 | None | None | Yes | 32.69 | 85.32 |
| 14 | Salvage | Recurrence | NR | None | RT, C | No | No progression | 39.13 |
| 15 | Salvage | Recurrence | 99 | None | RT, C | No | 8.25 | 15.01 |
| 16 | Salvage | Recurrence | 80 | None | C, GK | No | No progression | 11.66 |
| 17 | First line | Lower percieved risk | 95 | None | C | No | No progression | 31.57 |
| 18 | Salvage | Recurrence | 100 | None | RT | No | No progression | 89.82 |
| 19 | Salvage | Recurrence | 100 | DVT | None | No | No progression | 24.77 |
| 20 | First line | Favorable safety profile | 80 | None | None | No | No progression | 40.97 |
| 21 | Salvage | Recurrence | 99 | None | RT, C | No | No progression | 2.89 |
| 22 | Salvage | Recurrence | 95 | None | C x2 | No | No progression | 7.78 |
Abbreviations: EOA = extent of ablation, FND = focal neurologic deficit, LOS = length of stay, Tx = treatment, DVT = deep vein thrombosis, RT = radiation therapy, C = chemotherapy, GK = gamma knife, STR = subtotal resection, NR = not reported.
On follow-up, she continued to have fluctuations in memory and cognitive function, although her hemiparesis, dysarthria, aphasia, and facial droop had resolved.
The median (range) intensive care unit length of stay was 0 (0–2) days, while the median (range) hospital stay was 1 (0–6) days. The majority of patients were discharged home from the hospital (95.5%), while one patient was discharged to inpatient rehabilitation. Many patients (77.3%) in this cohort received adjuvant treatment after their LITT procedure, with 68.2% receiving chemotherapy and 50.0% receiving radiation therapy.
Three patients (13.6%) underwent staged LITT (Cases #5, 11, and 13), and all of them tolerated the staged procedures without complication. One of these patients (Case #13) underwent a third staged treatment. Two patients underwent repeat, non-staged LITT due to residual (Case #1) or recurrent (Case #13) tumor 2 months and 2 years after initial treatment, respectively. One patient (Case #7) underwent subtotal resection due to tumor progression after LITT. Repeat pathology in this patient demonstrated malignant transformation of the tumor from grade 2 diffuse astrocytoma to grade 3 anaplastic astrocytoma.
3.4. Progression-Free Survival
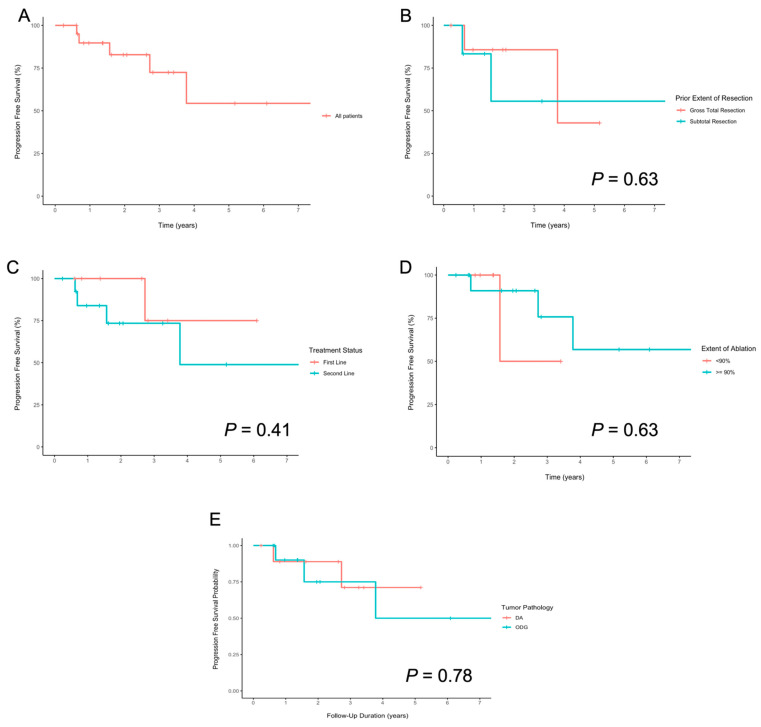
Kaplan–Meier curves were used to graphically display PFS (Figure 2), and log-rank tests were used to compare the differences between groups. The cohort’s median (range) follow-up duration was 2.0 (0.2–7.5) years. In the overall cohort, the median PFS was not reached at the time of analysis; the mean (standard error) PFS was 5.2 (0.8) years. For the overall cohort, the 3-year estimated PFS was 72.5% (95% CI 47.8–97.2), and the 5-year estimate was 54.4% (18.5–90.3%). Mean, median, and estimate PFS stratified by extent of ablation, extent of resection, pathology, and treatment status can be found in Table 4. Of note, several cut-offs for extent of ablation were examined (90%, 95%, 99%), with no difference in PFS.
Figure 2.
Kaplan–Meier plots showing progression-free survival (A) overall and stratified by (B) extent of prior resection, (C) first line versus salvage, (D) extent of ablation, and (E) tumor pathology.
Table 4.
PFS stratified by extent of resection, treatment status, extent of ablation, and pathology.
| Variable | Mean (SE) PFS, Years | Median (SE) PFS, Years | Three-Year PFS (SE) Estimate |
Five-year PFS (SE) Estimate |
|---|---|---|---|---|
| Prior extent of resection | ||||
| Gross total resection | 3.9 (0.7) | 3.8 (NC) | 85.7% (13.2%) | 42.9% (31.0%) |
| Subtotal resection | 4.7 (1.5) | Not reached | 55.6% (24.8%) | 55.6% (24.8%) |
| Treatment status | ||||
| First line | 5.3 (0.7) | Not reached | 75.0% (21.7%) | 75.0% (21.7%) |
| Salvage | 4.9 (1.0) | 3.8 (NC) | 73.4% (13.4%) | 49.0% (21.9%) |
| Extent of ablation | ||||
| <90% | 2.5 (0.7) | 1.6 (NC) | 50.0% (35.4%) | 50.0% (35.4%) |
| ≥90% | 5.4 (0.9) | Not reached | 75.8% (15.6%) | 56.8% (20.1%) |
| Pathology | ||||
| DA | 4.2 (0.6) | Not reached | 71.1% (18.0%) | 71.1% (18.0%) |
| ODG | 5.0 (1.1) | 3.8 (NC) | 75.0% (15.8%) | 50.0% (23.0%) |
Abbreviations: SE = standard error, NC = not calculated, DA = diffuse astrocytoma, ODG = oligodendroglioma.
Five patients (22.7%) had progression of their tumors at a median time (range) of 18.8 (7.4–45.3) months after LITT; importantly, this group was both small and heterogenous in nature. We then evaluated for factors associated with tumor progression. We considered age, extent of ablation, WHO grade, pathology, first line versus salvage, adjuvant therapy, and prior extent of resection in our analysis. We found that none of these factors were independent risk factors for PFS on univariate or multivariate analysis (Table S1), although this analysis is admittedly limited by low total number and events.
4. Discussion
Presently, maximally safe surgical resection is the standard of care for IDH1/2 mutant glioma. Although the use of LITT in the treatment of grades II and III glioma has increased over the last few decades for both primary [3,4] and recurrent gliomas [24,29], to our knowledge, this is the first multi-patient study analyzing the outcomes of IDH1/2 mutant grade 2/3 gliomas treated with LITT.
In our cohort of 22 patients, there was relatively equal representation of IDH1/2 mutant oligodendroglioma and diffuse astrocytoma. LITT appeared to be tolerated well, with only three patients experiencing perioperative complications, two of which were neurologic in nature. The vast majority of patients were discharged home after a short hospital stay. These findings are in line with other studies analyzing the rate of complications after LITT for glioma, as well as other pathologies, in addition to being in line with the rate of complications seen in some craniotomy cohorts (Table 5) [30,31,32,33].
Table 5.
Studies analyzing PFS in IDH1/2 mutant cohorts.
| Study (Year) | WHO Grade | Median (Range) Follow-Up, Months | Pathology | Median (95% CI) PFS, months | Three-Year PFS Estimate (95% CI) | Five-Year PFS Estimate (95% CI) | Ten-Year PFS Estimate (95% CI) | Complication Rates a | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Our study | II, III | 24.1 (2.9–89.8) | ODG + DA (n = 22) | Median not reached | 72.5% (57.8–97.2) | 54.4% (18.5–90.3) | NR | Perioperative: 14% | Mean (SE) PFS: ODG + DA: 62.4 (10.0) ODG: 59.9 (13.2) DA: 50.76 (6.96) |
| ODG (n = 12) | 45.6 | 75.0% (44.1–100) | 50.0% (27.0–73.0) | NR | |||||
| DA (n = 10) | Median not reached | 71.1% (35.8–100) | 71.1% (35.8–100) | NR | |||||
| Craniotomy Cohorts | |||||||||
| Navarria et al. (2020) | III | 40 (16–146) | ODG + DA (n = 96) | 69 (51–89) | 62.4% (61.3–63.5) | 53.0% (51.6–54.4) | NR | Perioperative: 16% Worsening of preoperative deficits: 14% | |
| ODG (n = 42) | 76 (32–89) | 63.4% (60.9–65.9) | 63.4% (60.9–65.9) | NR | |||||
| DA (n = 54) | 52 (34–57) | 59.9% (57.8–62.0) | 38.8% (35.8–41.8) | NR | |||||
| Patel et al. (2018) | II | 44.4 (0.6–187.2) | ODG + DA (n = 52) | 78 (NR) | 88.8% (79.6–98.1) | NR | Malignant PFS only | ||
| Kavouridis et al. (2019) | II | 64.8 (NR) | ODG (n = 140) | NR | NR | 38.5% (27.6–49.4) | 24.1% (12.8–37.4) | NR | |
| DA (n = 154) | NR | NR | 19.3% (12.2–27.7) | 3.2% (0.6–9.6) | |||||
| Tom et al. (2019) | II | NR | ODG (n = 18) | 113 (NR) | NR | NR | NR | All patients with GTR | |
| DA (n = 30) | 56 (NR) | NR | NR | ||||||
| Choi et al. (2020) | II | 66.9 (5.3–171.3) | ODG (n = 45) | NR | NR | NR | 73.6% (NR) | NR | |
| DA (n = 80) | NR | NR | NR | 32.5% (NR) | |||||
| Pal’a et al. (2019) | II | 72 (95% CI 57.6–75.6) | ODG + DA (n = 144) | 46.8 (NR) | NR | NR | NR | NR | |
| Miller et al. (2019) | II, III | 76.8 (NR) | ODG + DA (n = 275) | PFS1: 68.4 (56.4–76.8) PFS2: 37.2 (25.2–49.2) |
NR | NR | NR | NR | PFS1: resection to first recurrence PFS2: first recurrence to second recurrence |
| DA (n = 180) | 68.2 (NR) | NR | NR | NR | |||||
| ODG (n = 95) | 67.9 (NR) | NR | NR | NR | |||||
| Thon et al. (2012) | II | 173 (36–306) | DA (n = 89) | 47 (range 35–60) | NR | 37.8% (NR) | 10.5% (NR) | NR | Supratentorial only |
| LITT Cohorts | |||||||||
| Mohommadi et al. (2014) * | III | 7.2 (0.1–23.0) | DA (n = 6) ODG (n = 4) |
5.6 (NR) | NR | NR | NR | Any complication: 37% Worsening of preoperative deficits: 20% Seizure: 3% Infection: 6% |
|
| Leonardi and Lumeta (2002) * | II, III | NR | Low-grade DA (n = 7) | Mean: 16 (9–233) | NR | NR | NR | Neurologic deterioration: 17% Seizure: 4% Infection: 8% |
|
| Anaplastic ODG + DA (n = 11) | Mean: 10 (6–14) | NR | NR | NR | |||||
| Reimer et al. (1998) * | III | 12 (NR) | DA (n = 3) | 6 (range 6–12) | NR | NR | NR | Transient aphasia: 25% | |
| Murayi et al. (2020) * | III | NR | DA (n = 2) | Pt 1: 2.9 Pt 2: death POD3 |
NR | NR | NR | Permanent morbidity: 46% Perioperative mortality: 15% |
|
Abbreviations: PFS = progression-free survival, CI = confidence interval, ODG = oligodendroglioma, DA = diffuse astrocytoma, SE = standard error, NR = not reported, GTR = gross total resection. * Indicates cohorts with unclear IDH mutational status. a Complication rates were taken as a proportion of the total cohort, including those that were not grades 2 and 3.
In our cohort, the median PFS was not reached within our median (range) follow-up time of 2.0 (0.2–7.5) years. Our 3- and 5-year PFS estimates were 72.5% and 54.4%, respectively. In our cohort, 22.7% of our patients experienced tumor progression at a median time of 18.8 months after LITT. Reported PFS after LITT in grade 2 and 3 astrocytoma and oligodendroglioma have ranged from 3 to 16 months (Table 5) [34,35,36,37]. However, data are limited because many prior case series and reports of gliomas treated with LITT did not analyze PFS duration. They instead focused on safety profile and complication rates. These cases also did not routinely report the IDH1/2 status of the tumors, and cohorts were presumed to be a mixture of IDH1/2 mutant and wild type. The current case series represents the first of its kind to analyze treatment outcomes in IDH1/2 mutant glioma in particular.
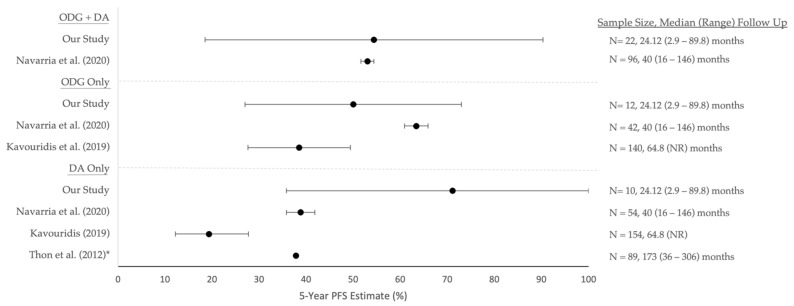
As a comparison, we also looked at studies analyzing outcomes in IDH1/2 mutant tumors treated with open resection (Table 5). In cohorts treated primarily by surgical resection and adjuvant therapy, median PFS for IDH1/2 mutant grade 2 and 3 gliomas ranged from 46.8 to 78.0 months [18,30,38,39]. Stratified by tumor pathology, ODG median PFS ranged from 76 to 113 months [38,40], and DA from 52 to 56 months [38,40]. In addition, mean PFS and PFS estimates at various time points have been reported in the previous literature (Table 5) [31,32,39,40,41]. Our cohort did not reach the median PFS; thus, we can only conclude that our cohort’s PFS was greater than our median follow-up time of 2.0 years. The overall mean PFS, however, was 5.2 years, and our 3- and 5- year estimates of PFS were 72.5% and 54.4%, respectively. Importantly, these estimates are at least comparable those found in the surgical resection literature (Figure 3) [32,38,39,41].
Figure 3.
Five-year PFS estimates (95% confidence intervals) of prior surgical resection studies. Corresponding median (range) follow-up durations and sample sizes can be found on the right side for each study. * Indicates that a confidence interval was not provided or able to be calculated for that study. Abbreviations: PFS = progression-free survival, ODG = oligodendroglioma, DA = diffuse astrocytoma, NR = not reported.
We must acknowledge selection bias when comparing PFS in our cohort with the surgical resection literature. In many cases, LITT at our institution is offered to patients who are otherwise not suitable surgical candidates or as salvage therapy. When comparing the PFS interval between the first and second episode of recurrence in the Miller et al. cohort (PFS2, 3.1 years) with the median PFS of our patients for whom LITT was salvage or second line (3.8 years), our PFS interval was found to be similar to that found in the surgical resection cohort [18].
Several questions remain about the treatment of IDH1/2 mutant glioma with LITT, and one is the question of malignant progression rates post-treatment. In our cohort, one patient exhibited malignant transformation approximately 7 months after LITT. Of note, this was the patient’s second overall recurrence, and the tumor molecular analysis demonstrated polysomy 7, which has been associated with malignant degeneration of low-grade glioma [33]. Overall, the proportion of patients whose tumors underwent malignant transformation was 4.5%, which is lower than reported rates in IDH1/2 mutant glioma after resection [32]. Further validation is required to test the intriguing hypothesis that LITT may also decrease rates of malignant transformation.
This study has several limitations to acknowledge. Our cohort size, although the largest to date examining outcomes in IDH1/2 mutant glioma after LITT, is still small, and as such is limited in its power to detect subtle-to-moderate differences between groups. For this reason, we were unable to perform subgroup analysis, particularly comparing patients treated with first-line versus salvage therapy. Additionally, we were unable to account for heterogeneity in pre-LITT and adjuvant treatments, including those who underwent surgical resection before LITT and those who did not. Our median duration of follow-up was shorter than prior literature analyzing outcomes in IDH1/2 mutant gliomas after surgical resection, although our median approached the recommended threshold for IDH1/2 mutant gliomas [16]. Notably, our cohort did not reach the median PFS within our duration of follow-up. While out of the scope of this preliminary, retrospective analysis, a larger, multi-institutional cohort analysis or prospective study is needed to further characterize outcomes in IDH1/2 mutant grade 2/3 gliomas treated with LITT and further elucidate its effects on PFS compared to both open surgical resection and IDH1/2 wild-type gliomas; this will be the focus of future work.
5. Conclusions
LITT is an effective alternative to open resection in patients with glioma. In this study, we analyzed the outcomes of patients with IDH1/2 mutant grade 2 and 3 gliomas treated with LITT at a single institution. We found that our cohort had a relatively low rate of complications that was on par with complication rates seen in craniotomy and LITT cohorts, and malignant progression after LITT was rare. The median time to progression and three- and five-year PFS estimates in our cohort were on par with those reported in the literature after surgical resection. While further, multi-institutional studies are needed to better characterize treatment outcomes after LITT in patients with IDH1/2 mutant glioma and to elucidate risk factors for progression, the findings in this study suggest that LITT may be an effective treatment option for this molecular subtype.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/curroncol29040209/s1, Table S1: Univariate time to event and regression analysis.
Author Contributions
Conceptualization, A.H.K. and R.H.H.; methodology, A.H.K., R.H.H. and G.W.J.; formal analysis, G.W.J.; data curation, G.W.J. and R.H.H.; writing—original draft preparation, G.W.J.; writing—review and editing, E.C.L., M.D.S., A.H.K. and R.H.H.; supervision, A.H.K., M.D.S. and E.C.L.; funding acquisition, A.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Christopher Davidson and Knight Family Fund (to A.H.K.) and the Duesenberg Research Fund (to A.H.K). R.H.H. was supported by a postdoctoral fellowship, PF-21-149-01-CDP, from the American Cancer Society.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Washington University in St. Louis School of Medicine (ID# 201409046, approval date: 3 February 2022).
Informed Consent Statement
Patient consent was waived by the institution’s Institutional Review Board due to the retrospective nature of the study.
Data Availability Statement
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
A.H.K. is a consultant for Monteris Medical and has received research grants from Monteris Medical for a mouse laser therapy study as well as from Stryker and Collagen Matrix for clinical outcomes studies about a dural substitute, which have no direct relation to this study. E.C.L.’s disclosures are as follows: Stock ownership: Neurolutions, General Sensing, Osteovantage, Pear Therapeutics, Face to Face Biometrics, Immunovalent, Caeli Vascular, Acera, Sora Neuroscience, Inner Cosmos, Kinetrix, NeuroDev. Consultant: Monteris Medical, E15, Acera, Alcyone, Intellectual Ventures, Neurolutions, Osteovantage, Pear Therapeutics, Inc., Sante Ventures, Microbot. SAB: Pear Therapeutics, Microbot. Licensing from Intellectual Property: Neurolutions, Osteovantage, Caeli Vascular. Licensing/product development agreements or royalties for inventions/IP: Cerovations, Intellectual Ventures. Washington University owns equity in Neurolutions. M.D.S. is a consultant for Monteris Medical. R.H.H. and G.W.J. have nothing to disclose.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bown S.G. Phototherapy of Tumors. World J. Surg. 1983;7:700–709. doi: 10.1007/BF01655209. [DOI] [PubMed] [Google Scholar]
- 2.Sugiyama K., Sakai T., Fujishima I., Ryu H., Uemura K., Yokoyama T. Stereotactic Interstitial Laser-Hyperthermia Using Nd-YAG Laser. Stereotact. Funct. Neurosurg. 1990;54:501–505. doi: 10.1159/000100263. [DOI] [PubMed] [Google Scholar]
- 3.Karampelas I., Sloan A.E. Laser-Induced Interstitial Thermotherapy of Gliomas. Prog. Neurol. Surg. 2018;32:14–26. doi: 10.1159/000469676. [DOI] [PubMed] [Google Scholar]
- 4.Chen C., Lee I., Tatsui C., Elder T., Sloan A.E. Laser Interstitial Thermotherapy (LITT) for the Treatment of Tumors of the Brain and Spine: A Brief Review. J. Neuro-Oncol. 2021;151:429–442. doi: 10.1007/s11060-020-03652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahmathulla G., Recinos P.F., Kamian K., Mohammadi A.M., Ahluwalia M.S., Barnett G.H. MRI-Guided Laser Interstitial Thermal Therapy in Neuro-Oncology: A Review of Its Current Clinical Applications. Oncology. 2014;87:67–82. doi: 10.1159/000362817. [DOI] [PubMed] [Google Scholar]
- 6.Ashraf O., Patel N.V., Hanft S., Danish S.F. Laser-Induced Thermal Therapy in Neuro-Oncology: A Review. World Neurosurg. 2018;112:166–177. doi: 10.1016/j.wneu.2018.01.123. [DOI] [PubMed] [Google Scholar]
- 7.Hong C.S., Deng D., Vera A., Chiang V.L. Laser-Interstitial Thermal Therapy Compared to Craniotomy for Treatment of Radiation Necrosis or Recurrent Tumor in Brain Metastases Failing Radiosurgery. J. Neuro-Oncol. 2019;142:309–317. doi: 10.1007/s11060-019-03097-z. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier A., McNichols R.J., Stafford R.J., Guichard J.-P., Reizine D., Delaloge S., Vicaut E., Payen D., Gowda A., George B. Laser Thermal Therapy: Real-Time MRI-Guided and Computer-Controlled Procedures for Metastatic Brain Tumors. Lasers Surg. Med. 2011;43:943–950. doi: 10.1002/lsm.21138. [DOI] [PubMed] [Google Scholar]
- 9.Rao M.S., Hargreaves E.L., Khan A.J., Haffty B.G., Danish S.F. Magnetic Resonance-Guided Laser Ablation Improves Local Control for Postradiosurgery Recurrence and/or Radiation Necrosis. Neurosurgery. 2014;74:658–667. doi: 10.1227/NEU.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 10.Rahmathulla G., Recinos P.F., Valerio J.E., Chao S., Barnett G.H. Laser Interstitial Thermal Therapy for Focal Cerebral Radiation Necrosis: A Case Report and Literature Review. Stereotact. Funct. Neurosurg. 2012;90:192–200. doi: 10.1159/000338251. [DOI] [PubMed] [Google Scholar]
- 11.Miller B.A., Salehi A., Limbrick D.D., Smyth M.D. Applications of a Robotic Stereotactic Arm for Pediatric Epilepsy and Neurooncology Surgery. J. Neurosurg. Pediatrics. 2017;20:364–370. doi: 10.3171/2017.5.PEDS1782. [DOI] [PubMed] [Google Scholar]
- 12.Tovar-Spinoza Z., Carter D., Ferrone D., Eksioglu Y., Huckins S. The Use of MRI-Guided Laser-Induced Thermal Ablation for Epilepsy. Child’s Nerv. Syst. 2013;29:2089–2094. doi: 10.1007/s00381-013-2169-6. [DOI] [PubMed] [Google Scholar]
- 13.Buckley R.T., Wang A.C., Miller J.W., Novotny E.J., Ojemann J.G. Stereotactic Laser Ablation for Hypothalamic and Deep Intraventricular Lesions. Neurosurg. Focus. 2016;41:E10. doi: 10.3171/2016.7.FOCUS16236. [DOI] [PubMed] [Google Scholar]
- 14.Barnett G.H., Voigt J.D., Alhuwalia M.S. A Systematic Review and Meta-Analysis of Studies Examining the Use of Brain Laser Interstitial Thermal Therapy versus Craniotomy for the Treatment of High-Grade Tumors in or near Areas of Eloquence: An Examination of the Extent of Resection and Major Complication Rates Associated with Each Type of Surgery. Stereotact. Funct. Neurosurg. 2016;94:164–173. doi: 10.1159/000446247. [DOI] [PubMed] [Google Scholar]
- 15.Parsons D.W., Jones S., Zhang X., Lin J.C.-H., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.-M., Gallia G.L., et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller J.J., Shih H.A., Andronesi O.C., Cahill D.P. Isocitrate Dehydrogenase-Mutant Glioma: Evolving Clinical and Therapeutic Implications. Cancer. 2017;123:4535–4546. doi: 10.1002/cncr.31039. [DOI] [PubMed] [Google Scholar]
- 17.Yan H., Parsons D.W., Jin G., McLendon R., Rasheed B.A., Yuan W., Kos I., Batinic-Haberle I., Jones S., Riggins G.J., et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J.J., Loebel F., Juratli T.A., Tummala S.S., Williams E.A., Batchelor T.T., Arrillaga-Romany I., Cahill D.P. Accelerated Progression of IDH Mutant Glioma after First Recurrence. Neuro-Oncology. 2019;21:669–677. doi: 10.1093/neuonc/noz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olar A., Wani K.M., Alfaro-Munoz K.D., Heathcock L.E., van Thuijl H.F., Gilbert M.R., Armstrong T.S., Sulman E.P., Cahill D.P., Vera-Bolanos E., et al. IDH Mutation Status and Role of WHO Grade and Mitotic Index in Overall Survival in Grade II–III Diffuse Gliomas. Acta Neuropathol. 2015;129:585–596. doi: 10.1007/s00401-015-1398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuss D.E., Mamatjan Y., Schrimpf D., Capper D., Hovestadt V., Kratz A., Sahm F., Koelsche C., Korshunov A., Olar A., et al. IDH Mutant Diffuse and Anaplastic Astrocytomas Have Similar Age at Presentation and Little Difference in Survival: A Grading Problem for WHO. Acta Neuropathol. 2015;129:867–873. doi: 10.1007/s00401-015-1438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weller M., van den Bent M., Preusser M., le Rhun E., Tonn J.C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021;18:170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natsume K., Sakakima H., Kawamura K., Yoshida A., Akihiro S., Yonezawa H., Yoshimoto K., Shimodozono M. Factors Influencing the Improvement of Activities of Daily Living during Inpatient Rehabilitation in Newly Diagnosed Patients with Glioblastoma Multiforme. J. Clin. Med. 2022;11:417. doi: 10.3390/jcm11020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montemurro N., Fanelli G.N., Scatena C., Ortenzi V., Pasqualetti F., Mazzanti C.M., Morganti R., Paiar F., Naccarato A.G., Perrini P. Surgical Outcome and Molecular Pattern Characterization of Recurrent Glioblastoma Multiforme: A Single-Center Retrospective Series. Clin. Neurol. Neurosurg. 2021;207:106735. doi: 10.1016/j.clineuro.2021.106735. [DOI] [PubMed] [Google Scholar]
- 24.Easwaran T., Lion A., Vortmeyer A., Kingery K., Bc M.D., Raskin J. Seizure Freedom from Recurrent Insular Low-Grade Glioma Following Laser Interstitial Thermal Therapy. Child’s Nerv. Syst. 2020;36:1055–1059. doi: 10.1007/s00381-019-04493-6. [DOI] [PubMed] [Google Scholar]
- 25.Hafez D.M., Liekweg C., Leuthardt E.C. Staged Laser Interstitial Thermal Therapy (LITT) Treatments to Left Insular Low-Grade Glioma. Clin. Neurosurg. 2020;86:E337–E342. doi: 10.1093/neuros/nyz120. [DOI] [PubMed] [Google Scholar]
- 26.Avecillas-Chasin J.M., Atik A., Mohammadi A.M., Barnett G.H. Laser Thermal Therapy in the Management of High-Grade Gliomas. Int. J. Hyperth. 2020;37:44–52. doi: 10.1080/02656736.2020.1767807. [DOI] [PubMed] [Google Scholar]
- 27.Hawasli A.H., Kim A.H., Dunn G.P., Tran D.D., Leuthardt E.C. Stereotactic Laser Ablation of High-Grade Gliomas. Neurosurg. Focus. 2014;37:E1. doi: 10.3171/2014.9.FOCUS14471. [DOI] [PubMed] [Google Scholar]
- 28.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee I., Kalkanis S., Hadjipanayis C.G. Stereotactic Laser Interstitial Thermal Therapy for Recurrent High-Grade Gliomas. Neurosurgery. 2016;79:S24–S34. doi: 10.1227/NEU.0000000000001443. [DOI] [PubMed] [Google Scholar]
- 30.Paľa A., Coburger J., Scherer M., Ahmeti H., Roder C., Gessler F., Jungk C., Scheuerle A., Senft C., Tatagiba M., et al. To Treat or Not to Treat? A Retrospective Multicenter Assessment of Survival in Patients with IDH-Mutant Low-Grade Glioma Based on Adjuvant Treatment. J. Neurosurg. 2020;133:273–280. doi: 10.3171/2019.4.JNS183395. [DOI] [PubMed] [Google Scholar]
- 31.Choi J., Kim S.H., Ahn S.S., Choi H.J., Yoon H.I., Cho J.H., Roh T.H., Kang S.G., Chang J.H., Suh C.O. Extent of Resection and Molecular Pathologic Subtype Are Potent Prognostic Factors of Adult WHO Grade II Glioma. Sci. Rep. 2020;10:2086. doi: 10.1038/s41598-020-59089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thon N., Eigenbrod S., Kreth S., Lutz J., Tonn J.C., Kretzschmar H., Peraud A., Kreth F.W. IDH1 Mutations in Grade II Astrocytomas Are Associated with Unfavorable Progression-Free Survival and Prolonged Postrecurrence Survival. Cancer. 2012;118:452–460. doi: 10.1002/cncr.26298. [DOI] [PubMed] [Google Scholar]
- 33.Idbaih A., Carvalho Silva R., Crinière E., Marie Y., Carpentier C., Boisselier B., Taillibert S., Rousseau A., Mokhtari K., Ducray F., et al. Genomic Changes in Progression of Low-Grade Gliomas. J. Neuro-Oncol. 2008;90:133–140. doi: 10.1007/s11060-008-9644-z. [DOI] [PubMed] [Google Scholar]
- 34.Murayi R., Borghei-Razavi H., Barnett G.H., Mohammadi A.M. Laser Interstitial Thermal Therapy in the Treatment of Thalamic Brain Tumors: A Case Series. Oper. Neurosurg. 2020;19:641–650. doi: 10.1093/ons/opaa206. [DOI] [PubMed] [Google Scholar]
- 35.Reimer P., Bremer C., Horch C., Morgenroth C., Allkemper T., Schuierer G. MR-Monitored LITT as a Palliative Concept in Patients with High Grade Gliomas: Preliminary Clinical Experience. J. Magn. Reson. Imaging. 1998;8:240–244. doi: 10.1002/jmri.1880080140. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadi A.M., Hawasli A.H., Rodriguez A., Schroeder J.L., Laxton A.W., Elson P., Tatter S.B., Barnett G.H., Leuthardt E.C. The Role of Laser Interstitial Thermal Therapy in Enhancing Progression-free Survival of Difficult-to-access High-grade Gliomas: A Multicenter Study. Cancer Med. 2014;3:971–979. doi: 10.1002/cam4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonardi M.A., Lumenta C.B. Stereotactic Guided Laser-Induced Interstitial Thermotherapy (SLITT) in Gliomas with Intraoperative Morphologic Monitoring in an Open MR: Clinical Expierence. MIN Minim. Invasive Neurosurg. 2002;45:201–207. doi: 10.1055/s-2002-36203. [DOI] [PubMed] [Google Scholar]
- 38.Navarria P., Pessina F., Clerici E., Rossini Z., Franceschini D., D’Agostino G., Franzese C., Comito T., Loi M., Simonelli M., et al. Is IDH Status the Only Factor Predicting Prognosis in Newly Diagnosed Anaplastic Glioma Patients? Outcome Evaluation and Prognostic Factor Analysis in a Single-Institution Large Series. J. Neurosurg. 2021;135:64–77. doi: 10.3171/2020.5.JNS201116. [DOI] [PubMed] [Google Scholar]
- 39.Patel T., Bander E.D., Venn R.A., Powell T., Cederquist G.Y.M., Schaefer P.M., Puchi L.A., Akhmerov A., Ogilvie S., Reiner A.S., et al. The Role of Extent of Resection in IDH1 Wild-Type or Mutant Low-Grade Gliomas. Neurosurgery. 2018;82:808–814. doi: 10.1093/neuros/nyx265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tom M.C., Varra V., Leyrer C.M., Park D.Y., Chao S.T., Yu J.S., Suh J.H., Reddy C.A., Balagamwala E.H., Broughman J.R., et al. Risk Factors for Progression Among Low-Grade Gliomas After Gross Total Resection and Initial Observation in the Molecular Era. Int. J. Radiat. Oncol. Biol. Phys. 2019;104:1099–1105. doi: 10.1016/j.ijrobp.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Kavouridis V.K., Boaro A., Dorr J., Cho E.Y., Iorgulescu J.B., Reardon D.A., Arnaout O., Smith T.R. Contemporary Assessment of Extent of Resection in Molecularly Defined Categories of Diffuse Low-Grade Glioma: A Volumetric Analysis. J. Neurosurg. 2020;133:1291–1301. doi: 10.3171/2019.6.JNS19972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.