Abstract
Background
Traditional Chinese herbal medicines have been used for a long time to treat diabetes, and many controlled trials have been done to investigate their efficacy.
Objectives
To assess the effects of Chinese herbal medicines in patients with type 2 diabetes mellitus.
Search methods
We searched the following electronic databases: The Cochrane Library (CENTRAL), the Chinese BioMedical Database, MEDLINE, EMBASE, and LILACS, combined with hand searches on Chinese journals and conference proceedings. No language restriction was used.
Selection criteria
Randomised trials of herbal medicines (with at least two months treatment duration) compared with placebo, pharmacological or non‐pharmacological interventions were included.
Data collection and analysis
Data were extracted independently by two reviewers. The methodological quality of trials was evaluated using the parameters of randomisation, allocation concealment, double blinding, and drop‐out rates. Meta‐analyses were performed where data were available.
Main results
Sixty‐six randomised trials, involving 8302 participants, met the inclusion criteria. Methodological quality was generally low. Sixty‐nine different herbal medicines were tested in the included trials, which compared herbal medicines with placebo, hypoglycaemic drugs, or herbal medicines plus hypoglycaemic drugs. Compared with placebo, Holy basil leaves, Xianzhen Pian, Qidan Tongmai, traditional Chinese formulae (TCT), Huoxue Jiangtang Pingzhi, and Inolter showed significantly hypoglycaemic response. Compared with hypoglycaemic drugs including glibenclamide, tolbutamide, or gliclazide, seven herbal medicines demonstrated a significant better metabolic control, including Bushen Jiangtang Tang, Composite Trichosanthis, Jiangtang Kang, Ketang Ling, Shenqi Jiangtang Yin, Xiaoke Tang, and Yishen Huoxue Tiaogan. In 29 trials that evaluated herbal medicines combined with hypoglycaemic drugs, 15 different herbal preparations showed additional better effects than hypoglycaemic drugs monotherapy. Two herbal therapies combined with diet and behaviour change showed better hypoglycaemic effects than diet and behaviour change alone. No serious adverse effects from the herbal medicines were reported.
Authors' conclusions
Some herbal medicines show hypoglycaemic effect sin type 2 diabetes. However, these findings should be carefully interpreted due to the low methodological quality, small sample size, and limited number of trials. In the light of some positive findings, some herbal medicines deserve further examination in high‐quality trials.
Plain language summary
Chinese herbal medicines for type 2 diabetes mellitus
We are still waiting for firm evidence on Chinese herbal medicines for treatment of non‐insulin‐dependent diabetes. Although the use of herbal medicines for treatment of diabetes has a long history especially in the East, current evidence cannot warrant to support the routine use in clinical practice. This systematic review evaluates the effects of various herbal preparations (including single herbs or mixtures of different herbs) for treating people with type 2 diabetes. The review shows that some herbal medicines lower blood sugar and relieving symptoms in patients with diabetes. However, the methodological quality of the clinical trials evaluating these herbs is generally poor. The analyses also indicate that trials with positive findings are more likely to be associated with exaggerated effects. However, the trials did not report significant adverse effects. In conclusion, herbal medicines should not be recommended for routine use in diabetic patients of type 2 diabetes until we get scientifically sound trials. Testing the herbs in larger, well‐designed trials is needed in order to establish the necessary evidence for their use.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (i.e. elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group on The Cochrane Library (see 'About the Cochrane Collaboration', 'Collaborative Review Groups‐CRGs'). For an explanation of methodological terms, see the main Glossary on The Cochrane Library.
There are two types of diabetes mellitus: type 1 insulin dependent (IDDM) and type 2 non‐insulin dependent (NIDDM). Type 2 diabetes mellitus, the most common form, is the fourth leading cause of death in developed countries with a two fold excess mortality and two to four fold increased risk of coronary heart disease and stroke (McKinlay 2000). Diabetes affects women and men of all ages and every ethnic category, and many cases of diabetes remain undiagnosed for an average of 4 to 7 years (McKinlay 2000). Diabetes profoundly affects quality of life and represents a life‐long burden on a patient's social support system. Diabetes places large financial demands on the health care system (WHO 1998).
Description of the intervention
For the management of type 2 diabetes mellitus, the initial recommendations include adaptation of diet, weight loss if appropriate, and exercise. If this regimen does not keep blood glucose levels within the normal range, anti‐hyperglycaemic agents are prescribed, which include metformin and sulphonylurea drugs, or insulin. Because of the chronicity of diabetes, the impact of the disease on quality of life, the possibility of severe complications, and the requirements for self‐care, it is not unlikely that diabetic patients will seek complementary / alternative therapies (McGrady 1999), for example using food supplements or herbal medicines. Some goals of these therapies are to lower blood glucose levels, to decrease dosage of oral anti‐hyperglycaemic drugs, to decrease insulin resistance, and to assist in managing the complications of diabetes (Bailey 1989; Ivorra 1989; McGrady 1999).
Herbal medicine forms the main part of Traditional Chinese Medicine, which is a 3000‐year‐old holistic system of medicine combining medicinal herbs, acupuncture, food therapy, massage, and therapeutic exercise for both treatment and prevention of diseases (Fulder 1996). Traditional Chinese Medicine has its unique theories for concepts of aetiology, systems of diagnosis, and treatment which are vital to its practice. The theories of Traditional Chinese Medicine include Yin‐Yang (representing the opposite principles and balance between positive and negative systems in the body),the five elements(fire, earth, metal, water, and wood), Qi (vital energy) and blood, Zhang‐Fu (five viscerae and six bowels), and channels and collaterals (Meridian doctrine) (Liu 1991; Cheng 2000). Diseases are considered to result from internal causes as well as external causes, which are defined as disturbances e.g. the imbalance between Yin and Yang. The drug treatment of Chinese medicine consists typically in complex prescriptions of a combination of several components. This combination is based on specialChinese diagnostic patterns i.e. inspection, listening, smelling, inquiry, and palpation.Herbs are used for correcting the imbalance of Yin‐Yang in the body and maintaining kinetic balance under the movement of five elements. Bianzheng Lunzhi (differentiation of symptoms and prescription of drugs) is the application of these theories.
Medicinal herbs have been widely used for more than 2000 years to treat type 2 diabetes mellitus ('Xiao Ke Bing' in ancient records of Traditional Chinese Medicine). In the late 1970's, clinical investigations were reported on the use of herbal medicines (both different single herbs and mixtures of herbs) as a means of treating diabetes and its complications (Zhou 1980). The mechanism of action of the herbal medicines involves regulating glycaemic metabolism, decreasing cholesterol levels, eliminating free radicals, increasing secretion of insulin, and improving microcirculation (Chen 1997; Luo 1998; Shen 1997; Zhu 1997; Zhu 1999). Until March 1999, 14 herbal medicines (13 mixture of herbs and one extract of single herb) [see Appendix 9] were officially approved for the treatment of diabetes by the State Drug Regulatory Authority of China (CMH 1999); and 13 herbal medicines [see Appendix 8] were listed in the National Essential Drugs by the State Drug Administration of China (SDA 2000). Almost all the herbal medicines are so called 'Chinese proprietary medicines', i.e. they are usually based on well‐established and long‐standing recipes and formulated as tablets or capsules for commerce, convenience, or palatability. However, active ingredients of these herbal medicines are largely unknown and they are combined to formulate herbal medicines. A number of clinical trials have been reported on the subject in Chinese medical journals during the past 20 years. The first randomised trial was reported in 1991 (Chen 1991).
Why it is important to do this review
The results of the trials suggest that treatment with Chinese herbal medicines may have great potentials for reducing hyperglycaemia and complications of type 2 diabetes mellitus. However, there are reports of liver toxicity and kidney damage or even cancer associated with using Chinese herbal medicines (Ishizaki 1996; Melchart 1999; Gottieb 2000; Tomlinson 2000). The potential role and safety for long‐term use of herbal medicines in patients with type 2 diabetes mellitus needs to be systematically reviewed to assess the current practice and direct the continued search for new treatment regimens.
Objectives
To assess the effects of Chinese herbal medicines inpatients with type 2 diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised clinical trials fulfilling the inclusion criteria were eligible for this review. Ideally, trial participants, people administering the treatment and outcome assessors would all have been blinded, but single‐blinded and unblinded trials were also considered and their effect on the overall results were assessed in a sensitivity analysis.
Types of participants
Adults (18 years or older) with type 2 diabetes mellitus were included. To be consistent with changes in diagnostic criteria of type 2 diabetes mellitus through the years (WHO 1980; WHO 1985; ADA 1997; WHO 1998; ADA 1999), the diagnosis should have been established using the diagnostic criteria valid at the time of the beginning of the trial. Ideally, diagnostic criteria should have been described. These changes may have produced significant variability in the inclusion criteria, in the clinical characteristics of the patients included as well as in the results obtained. These differences we reconsidered and explored using sensitivity analyses.
Types of interventions
The intervention of Chinese herbal medicines includes extract from herbs, single herbs, Chinese proprietary medicines (see under Appendices), or a compound of herbs that is prescribed (individualised treatment) by Chinese practitioner.
The control intervention includes placebo, a non‐pharmacological intervention (for example, exercise or diet), or any active intervention used with the intention of lowering blood glucose levels (for example, metformin, sulphonylureas, acarbose, insulin).
Herbal medicines plus other therapies such as a holistic treatment, for example, herbs plus acupuncture, were excluded. Trials were only included if the treatment was given for a minimum of two months.
Co‐interventions were allowed as long as both arms of the randomised trial received the same co‐intervention(s).
Types of outcome measures
Primary outcomes
mortality (diabetes‐related and all‐cause);
quality of life (ideally measured using a validated instrument);
diabetes complications (neuropathy, retinopathy, nephropathy, sexual dysfunction).
Trials in which the major goal of the intervention was the treatment of the diabetic complications were considered in separate reviews.
Secondary outcomes
glycaemic control (glycated haemoglobin levels (HbA1c) and fasting blood glucose levels);
weight or body mass index (BMI);
fasting insulin levels;
adverse events (for example liver toxicity, kidney damage);
costs.
Timing of outcome assessment
The main outcome measures require trials of five years or more to yield meaningful results. For other outcomes, we included trials of short duration (two to three months), medium duration (three months to six months) and long duration (more than six months).
Search methods for identification of studies
Electronic searches
The following electronic databases were searched regardless of language and publication status:
The Cochrane Library, including the Cochrane Controlled Trials Register (CENTRAL, Issue 1, 2004);
MEDLINE (search 1966 to 04/2004);
EMBASE (from 1974 to 4/2004);
Chinese BioMedical disk (CD‐ROM) (from 1979 to 2004);
LILACS (www.bireme.br/bvs/I/ibd.htm) (from 1986 to 2004);
Database of the grey literature (Sigle).
We will also search databases of ongoing trials: 'Current Controlled Trials' (www.controlled‐trials.com ‐ with links to other databases of ongoing trials).
For detailed search strategies please see under Appendix 1.
Additional key words of relevance identified during any of the electronic or other searches. If this would have been the case, electronic search strategies would have been modified to incorporate these terms.
Searching other resources
We handsearched the Chinese Journal of Diabetes and Chinese Journal of Endocrinology and Metabolism from the first publication date onwards. We tried to identify potentially eligible studies by searching the reference lists of relevant trials and reviews identified.
Authors of relevant identified studies and experts were contacted in order to obtain additional references, unpublished trials, ongoing trials or to obtain missing data not reported in the original trials. Similarly, manufacturers of the reviewed Chinese medicines were contacted in order to retrieve information on herb trials, published and unpublished.
Data collection and analysis
Selection of studies
Two reviewers (MZ, WW) assessed the titles, abstract sections and keywords of every record retrieved independently. Full articles were retrieved for further assessment if the information given suggested that the study: 1. included patients with diabetes mellitus, 2. compared Chinese herbal medicine with placebo or any other active intervention, 3. assessed one or more relevant outcome measure, 4. used random allocation to the comparison groups. If there were any unclear information in the title or abstract, the full article was retrieved for clarification. Interrater agreement for study selection was measured using the kappa statistic (Fleiss 1981). Where differences in opinion existed, they were resolved by a third party (JL). If resolving disagreement was not possible, the article was added to those 'awaiting assessment' and the authors were contacted for clarification.
Data extraction and management
Data concerning details of study population, intervention and outcomes were extracted independently by two reviewers (MZ, WW) using a standard data extraction form. The standard data extraction form included at least the following items:
General information: published/unpublished, title, authors, source, contact address, country, urban/rural etc., language of publication, year of publication, duplicate publications, sponsoring, setting.
Trial characteristics: design, duration, randomisation (and method), allocation concealment (and method), blinding (patients, people administering treatment, outcome assessors), check of blinding.
Intervention(s): placebo included, intervention(s) (single herb or compound of herbs, dose, route, timing, mode of treatment, expertise of the practitioner), comparison intervention(s) (dose, route, timing), co‐medication(s) (dose, route, timing).
Patients: sampling (random / convenience), exclusion criteria, total number and number in comparison groups, sex, age, baseline characteristics, diagnostic criteria, duration of diabetes, similarity of groups at baseline (including any co‐morbidity), assessment of compliance, withdrawals / losses to follow‐up (reasons / description), subgroups.
Outcomes: outcomes specified above, any other outcomes assessed, other events, length of follow‐up, quality of reporting of outcomes.
Results: for outcomes and times of assessment (including a measure of variation), if necessary converted to measures of effect specified below; intention‐to‐treat analysis.
Differences in data extraction was resolved by consensus, referring back to the original article. When necessary, information was sought from the authors of the primary studies.
Assessment of risk of bias in included studies
The quality of reporting each trial was assessed based largely on the quality criteria specified by Schulz and Jadad (Schulz 1995; Jadad 1996). In particular, the following factors we restudied:
Minimisation of selection bias ‐ a) was the randomisation procedure adequate? b) was the allocation concealment adequate?
Minimisation of performance bias ‐ were the patients and people administering the treatment blind to the intervention if blinding was possible?
Minimisation of attrition bias ‐ a) were withdrawals and dropouts completely described? b) was analysis by intention‐to‐treat?
Minimisation of detection bias ‐ were outcome assessors blind to the intervention?
Based on these criteria, studies were broadly subdivided into the following three categories (see Cochrane Handbook): A ‐ all quality criteria met: low risk of bias. B ‐ one or more of the quality criteria only partly met: moderate risk of bias. C ‐ one or more criteria not met: high risk of bias.
This classification would have been used as the basis of a sensitivity analysis. Additionally, we would have explored the influence of individual quality criteria in a sensitivity analysis.
Each trial was assessed independently by two reviewers (MZ, WW) and verified by JL. Interrater agreement was calculated using the kappa statistic. Any disagreement with the quality assessment was resolved through discussion and a judgement was made based on consensus.
Assessment of heterogeneity
Heterogeneity was tested for using the Z score and the Chi square statistic with significance being set at P < 0.10. Possible sources of heterogeneity would have been assessed by subgroup and sensitivity analyses as described below. Potential bias would have been tested for using the funnel plot or other corrective analytical methods depending on the number of clinical trials included in the systematic review (Egger 1997).
The analyses were carried out using RevMan Analyses 1.0.2 in Review Manager 4.2.5 (Cochrane software).
Data synthesis
Data were summarised statistically if they were available, of sufficient quality and sufficiently similar. We expect both event (dichotomous) data and continuous data.
Dichotomous data were expressed as relative risk (RR) with 95% confidence interval (CI). We calculated the risk difference (RD) and converted the RD into the number needed to treat (NNT) or the number needed to harm (NNH) if follow‐up was similar for the different trials. Continuous data wereexpressed as weighted mean differences (WMD) with 95% CI and an overall WMD was calculated. Overall results were calculated based on the random effects model.
Subgroup analysis and investigation of heterogeneity
We would have aimed to perform subgroup analyses in order to explore effect size differences in case there would have been a significant result for at least one of the major outcome measures:
glycosylated haemoglobin level at baseline (if this is unavailable, mean level of fasting plasma glucose at baseline will be used) (subdividing into three groups of low, medium and high level ‐ based on data);
age (18 to 40 years, 41 to 64 years, older than 65 years);
gender;
weight (normal (BMI: women less than 25, men less than 27), overweight (BMI: women 25‐30, men 27‐30), obese (BMI more than 30)) (Garrow 1988);
different herbs / herbal preparations;
duration of intervention (short, medium, long ‐ based on data).
Sensitivity analysis
We would have performed sensitivity analyses in order to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies (if there were any).
repeating the analysis taking account of study quality, as specified above.
repeating the analysis excluding any very long or large studies to establish how much they dominate the results.
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, publication status, source of funding (industry versus other), country.
The robustness of the results were also testedby repeating the analysis using different measures of effects size (RD, RR) and different statistic models (fixed and random effects models).
Results
Description of studies
Our initial searches identified 713 references, 674 from the electronic searches and 39 from handsearches. After reading titles and abstracts, 570 of these articles were excluded because they were duplicates, non‐clinical studies, or had study objectives different from this review. A total of 143 references published in three languages (Chinese, English, and Spanish) were retrieved for further assessment. From a recently published review article, we identified five (Hale 1989; Sharma 1990; Sitprija 1987; Sotaniemi 1995; Velussi 1997)additional references (Yeh 2003). Of these, 82 references were excluded because they did not meet our inclusion criteria. The reasons for exclusion were listed under 'Characteristics of excluded studies'.
In total 66 randomised clinical trials were included in this review. They reported random allocation of patients with type 2 diabetes mellitus to herbal medicines versus controls (placebo in 10 trials, glibenclamide, gliclazide, glipizide, metformin, tolbutamide, or glurenorm in 25 trials, and dietary plus life style modification in two trials) or herbal medicines plus hypoglycaemic drugs versus hypoglycaemic drugs (29 trials). Two trials reported four herbal interventions versus placebos (Russo 1990; Wu YN 2003), and other four trials reported three arms in the trials. The 66 randomised trials were listed under 'Characteristics of included studies', of which six trials were published in English and 60 in Chinese.
Participants
A total of 8302 patients with type 2 diabetes mellitus were randomised in 66 trials, among which one trial also included 18 patients with type 1 diabetes (Chang ZQ 1998). Five trials included in‐patients, 14 trials included outpatients, and 26 trials included both out‐ and in‐patients. The remaining 21 trials did not specify the origin of the patients. The ethnic groups of patients were Hindu in three trials (Agrawal 1996; Agrawal 2002; Namdul 2001), Brazilian in one trial (Russo 1990), Finnish in one trial (Sotaniemi 1995), and Chinese in the remaining trials.
All randomised clinical trials included adults with a mean age of 53 years in 53 trials providing data. Eight trials did not report data on sex and/or age (Deng QW 2003; Lan QF 2000; Li YM 2003; Mao L 2002; Ren PA 2003; Wu HM 1996; Xu YS 1997; Zhang FB 2003), and the rest reported a male to female ratio of 1:1 (3986:3818). The average size of the trials was 123 patients, ranging from 32 to 336 patients per trial.
Diagnosis
The diagnostic criteria for type 2 diabetes mellitus were mainly based on WHO criteria (14 trials by criteria in 1980; 13 by 1985; one by 1997; six by 1999; 13 without specification), and two trials used the diagnostic criteria of ADA 1997, one trial of NDDG 1979. Two trials based their diagnostic criteriaon textbook criteria, and 14 trials without specification.
Interventions
Sixty‐nine different herbal medicines were tested in 66 randomised trials. Herbal medicine Xianzhen Pian was tested in three trials. According to the category of medicinal herbs, seven trials tested single herbs (Agrawal 1996; Chen SH 1997; Huang CL 2003; Russo 1990; Sotaniemi 1995; Yao LD 2003; Zhang J 2003) and the remaining trials tested compounds of herbs. The compositions and treatment regimens of herbal medicines varied (Appendix 2: Preparations and compositions of the medicinal herbs). The median duration of treatment was 2.6 months (ranging from 2 to 6 months). The control interventions included placebo in 10 trials (Agrawal 1996; Agrawal 2002; Chen YB 1995; Pan MZ 1997; Shen T 1998; Sotaniemi 1995; Vray 1995; Wu YN 2003; Zhang M 2001a), and hypoglycaemic drugs or dietary control in 56 trials.
Outcomes
None of the 66 trials reported mortality, incidence of diabetic complications, quality of life, and health economics. The outcomes reported were mainly surrogate parametersincluding fasting blood glucose, HbA1c, fasting insulin, insulin sensitivity indices, body weight, as well as symptoms. Adverse effects were reported in 17 trials. All the reported outcomes were measured at the end of thetreatment.
Risk of bias in included studies
Two trials were cross‐over randomised trials (Agrawal 1996; Russo 1990), and others were parallel‐group design. One trial was amulticentre randomised trial (Vray 1995). Of the 66 included randomised trials, seven reported themethods for generation of allocation randomisation. Among them, four used random number table (Chen XL 2001; Huang CL 2003; Wu YN 2003; Zhang M 2001a), one used drawing (Ni HX 2000), one used tossing coins (Wang JS 2000), and one used alternative allocation (Feng YM 1998). Three trials was obviously assessed as inadequate based on the methods of randomisation (Feng YM 1998; Ni HX 2000; Wang JS 2000), and the remaining trials did not provide information about allocation concealment. Double blinding was reported in four trials published in English (Agrawal 2002; Russo 1990; Sotaniemi 1995; Vray 1995). Three trials were single blinded, onetrial reported blinding of the outcomeassessor (Agrawal 1996), and two trials did not report detailed information on blinding (Chen YB 1995; Pan MZ 1997).
One trial reported a pre‐trial estimation of sample size (Vray 1995), and one trial stated performing intention‐to‐treat analysis (Namdul 2001). Six trials reported withdrawals (Chen SH 1997; Guo GY 1998; Li YM 2003; Namdul 2001; Vray 1995; Wang JS 2000). Data from 16 Chinese trials showed significant skewed distribution of the participants among the allocatedgroups (Chen XH 1993; Hua SG 1997; Lan QF 2000; Miao WH 2003; Qing ZQ 2001; Ren PA 2003; Shao CP 2001; Tao XY 2002; Tong J 2003; Wu HM 1996; Yang TB 2002; Yao LD 2003; You BW 1999; Zhang FB 2003; Zhou C 2001; Zhou JH 2001a).
Effects of interventions
No trial was assessed as low risk of bias since they did not meet all quality criteria in terms of minimisation of selection, performance, attrition, and detection biases. Few trials could be assessed as moderate risk of bias since they partly met one or more quality criteria. Majority of the trials was assessed as trials with high risk of bias since they did not meet one or more quality criteria.
Blood glucose and serum insulin responses
Herbal medicines versus placebo
Ten trials (involving 804 patients) compared herbal medicines with placebo (Agrawal 1996; Agrawal 2002; Chen YB 1995; Pan MZ 1997; Russo 1990; Shen T 1998; Sotaniemi 1995; Vray 1995; Wu YN 2003; Zhang M 2001a). The tested herbal medicines included Myrcia uniflora, Bauhinian forficata, Ginseng, Holy basil, Inolter, Xianzhen Pian, traditional Chinese treatment (TCT), Qidan Tongmai, Huoxue Jiangtang Pingzhi formula, and Liuwei Dihuang Tang. Among them, only Xianzhen Pian was tested in three trials. The reported outcomes included fasting blood glucose, postprandial blood glucose, HbA1c, fasting serum insulin levels, symptoms, and adverse effects (Appendix 3).
Normalisation of fasting blood glucose
Compared with placebo, Xianzhen Pian showed significantly better effect on normalisation of fasting blood glucose (RR 2.50; 95% CI 1.38 to 4.54). There was no significant heterogeneity among the comparisons. Qidan Tongmai appeared better than placebo regarding normalisation of fasting blood glucose (Zhang M 2001a).
Fasting blood glucose levels
Xianzhen Pian showed a significant reduction in fasting blood glucose levels (WMD ‐0.85 mmol/L; 95% CI ‐1.64 to ‐ 0.05) in three trials. TCT showed asignificant effect on reducing fasting blood glucose levels (Vray 1995). Huoxue Jiangtang Pingzhi formula reduced fasting blood glucose levels significantly, while Liuwei Dihuang Tang was significantly inferior to placebo for reducingfasting blood glucose levels (Wu YN 2003). Among other comparisons, Ginseng, Bauhinia forficata, Myrcia uniflora, Inolter, Qidan Tongmai versus placebo showed no statistically significant effects in fasting blood glucose levels.
Glycaeted haemoglobin levels and fasting serum insulin levels
Compared with placebo, Inolter reduced significantly HbA1c levels, Qidan Tongmai was better than placebo, and TCT better than placebo (Agrawal 2002; Vray 1995; Zhang M 2001a). There was no significant difference between Ginseng and placebo in HbA1c and fasting serum insulin levels (Sotaniemi 1995). Both Huoxue Jiangtang Pingzhi formula and Liuwei Dihuang Tang reduced significantly fasting serum insulin levels, respectively (Wu YN 2003).
Herbal medicines versus hypoglycaemic drugs
Twenty‐five trials (involving 3563 patients) compared 28 different herbal medicines with hypoglycaemic drugs (Cao FK 1997; Chen SH 1997; Chen XH 1993; Chen XL 2001; Deng QW 2003; Feng YM 1998; Guo GY 1998; Hua SG 1997; Lan QF 2000; Li RG 2001; Li YM 2003; Pang DR 2002; Qing ZQ 2001; Ren PA 2003; Wang JS 2000; Wang KF 1993; Wang KP 1999; Xie CG 1996; Xu Q 2003; Yang TB 2002; Yao LD 2003; You BW 1999; Zhao XY 2001; Zhou C 2001; Zhou P 1997). Two trials included three arms comparing different herbs with controls (Chen XH 1993; Zhou C 2001). The tested herbs included berberine, Bushen Jiangtang Tang, Composite Trichosanthis, Jianpi Jiangtang Tang, Jiangtang Kang, Jiangtang No. 1‐3, Jinqi Jiangtang, Kelening, Ketang Ling, Maziren Wan, Potentilla chinensis, Shenqi Jiangtang Yin, Shenqi Yuxiao Tang, Shengqing Jiangtang recipe, Shugan Huoxue recipe, Shuizhi Sanhuang Tang, Tangfu Kang, Tangning Pian, Tangzhi Xiao, Tianyuan Jiangtang Wan, Xiaoke Ling, Xiaoke Tang, Xiaoke Yin, Xiaoyao San, Yiqi Yangyin Huayu Tang, Yiqi Yangyin Huoxue recipe, Yishen Huoxue Tiaogan, and Yuquan Wan. The compared hypoglycaemic drugs included glibenclamide, tolbutamide, gliclazide, glurenorm, phenformin, and insoral. We were not able to pool the data due to the variations of the tested herbal medicines and control interventions (Appendix 4).
All outcomes
Bushen Jiangtang Tang versus glibenclamide Bushen Jiangtang Tang appeared better than glibenclamide regarding normalisation of fasting blood glucose (Li RG 2001). Similar significant effect was showed on reducing fasting blood glucose levels (WMD ‐0.95 mmol/L; 95% CI ‐1.75 to ‐0.15). No data were available for other outcomes.
Composite Trichosanthis versus tolbutamide Composite Trichosanthis showed significant better effect than tolbutamide on the normalisation of fasting blood glucose (Chen XH 1993). No data were available for other outcomes.
Jianpi Jiangtang Tang versus gliclazide plus alginric sodium diester There was no significant difference between the comparison regarding fasting blood glucose levels in one small trial (Wang KP 1999). No data were available for other outcomes.
Jiangtang Kang versus glibenclamide Compared with glibenclamide, Jiangtang Kang showed significantly better effect on the normalisation of fasting blood glucose (Chen SH 1997). Jiangtang Kang also showed a significant better effect on reducing fasting blood glucose levels both at the end of two months treatment and at the end of six months treatment. Similar positive effect of Jiangtang Kang was found on reducing HbA1c levels at the end of two months, and at the end of six months). However, there was no significant difference regarding fasting serum insulin levels.
Jiangtang No. 1‐3 capsules versus glibenclamide Jiangtang No. 1‐3 were three herbal mixtures prescribed by the trial investigators, and were applied at different time of a day, i.e., No. 1 to be taken in the morning, No. 2 to be taken in the midday, and No. 3 to be taken in the evening (Zhou C 2001). There was no significant difference between Jiangtang No. 1‐3 and glibenclamide in both the normalisation of fasting blood glucose (84/200 versus 22/50) and the fasting blood glucose levels. No data were available for other outcomes.
Jinqi Jiangtang versus glibenclamide There was no significant difference between Jinqi Jiangtang capsule and glibenclamide regarding normalisation of fasting blood glucose (14/50 versus 22/50) (Zhou C 2001). However, Jinqi Jiangtang was significantly inferior to glibenclamide regarding reducing the fasting blood glucose levels. No data were available for other outcomes.
Kelening versus glibenclamide Kelening did not differ significantly from glibenclamide regarding normalisation of fasting blood glucose (8/33 versus 12/32) (Zhou P 1997). But, Kelening was significantly inferior to glibenclamide regarding reducing fasting blood glucose levels, and HbA1c levels. The fasting serum insulin levels was significantly lower in patients treated with Kelening than those treated with glibenclamide.
Ketang Ling versus gliclazide Ketang Ling showed significantly better effect than gliclazide on the normalisation of fasting blood glucose (Lan QF 2000). No data were available for other outcomes.
Maziren Wan versus glibenclamide plus phenformin There was no significant difference between Maziren Wan and glibenclamide plus phenformin regarding the normalisation of fasting blood glucose (72/118 versus 21/32) and fasting blood glucose levels (Ren PA 2003).
Potentilla chinensis plus berberine versus metformin plus glipizide There was no significant difference between the comparison regarding the fasting blood glucose levels (Yao LD 2003). No data were available for other outcomes.
Shenqi Jiangtang Yin versus tolbutamide Shenqi Jiangtang Yin appeared significantly better than tolbutamide regarding the normalisation of fasting blood glucose and the fasting blood glucose levels (Yang TB 2002). No data were available for other outcomes.
Shenqi Yuxiao Tang versus gliclazide There was no significant difference between Shenqi Yuxiao Tang and gliclazide regarding the normalisation of fasting blood glucose (24/60 versus 12/30) (Qing ZQ 2001). However, Shenqi Yuxiao Tang appeared better than gliclazide in reducing fasting blood glucose levels. No data were available for other outcomes.
Shengqing Jiangtang recipe versus glibenclamide There was no significant difference between the comparison regarding the normalisation of fasting blood glucose (15/35 versus 10/27) and fasting blood glucose levels (Cao FK 1997). No data were available for other outcomes.
Shugan Huoxue recipe versus gliclazide There was no significant difference between the comparison regarding the normalisation of fasting blood glucose (46/72 versus 17/36) and fasting blood glucose levels (You BW 1999). No data were available for other outcomes.
Shuizhi Sanhuang Tang versus glibenclamide There was no significant difference between the comparison regarding the normalisation of fasting blood glucose (11/20 versus 4/12) (Wang KF 1993). However, Shuizhi Sanhuang Tang significantly reduced the fasting blood glucose levels. No data were available for other outcomes.
Tangfu Kang versus gliclazide There was no significant difference between the comparison regarding the levels of fasting blood glucose, HbA1c, and fasting serum insulin (Xie CG 1996).
Tangning Pian versus glibenclamide There was no significant difference between the comparison regarding the normalisation of fasting blood glucose (29/50 versus 22/49) and fasting blood glucose levels (Zhao XY 2001). However, HbA1c levels were lower in patients treated with Tangning Pian than those treated with glibenclamide.
Tangzhi Xiao versus gliclazide Tangzhi Xiao significantly reduced the fasting blood glucose level (Li YM 2003). The fasting serum insulin level in Tangzhi Xiao group was significantly lower than that in gliclazide group.
Tianyuan Jiangtang Wan versus gliclazide There was no significant difference between the comparison regarding the fasting blood glucose levels (Guo GY 1998). No data were available for other outcomes.
Xiaoke Ling versus gliclazide There was no significant difference between the comparison regarding the normalisation of fasting blood glucose (75/150 versus 52/100) (Feng YM 1998). Xiaoke Ling was significantly inferior to gliclazide regarding lowering the fasting blood glucose levels. No data were available for other outcomes.
Xiaoke Tang versus glibenclamide Xiaoke Tang showed significantly better effect than glibenclamide on the normalisation of fasting blood glucose (48/82 versus 20/64) (Pang DR 2002). No data were available for other outcomes.
Xiaoke Yin versus glibenclamide plus Insoral There was no significant difference between the comparison regarding the normalisation of fasting blood glucose (59/100 versus 38/82) (Chen XL 2001). No data were available for other outcomes.
Xiaoyao San versus glibenclamide Xiaoyao San appeared significantly better effect than glibenclamide on the fasting blood glucose level (Deng QW 2003). However, there was no significant difference between the comparison in HbA1c level.
Yiqi Yangyin Huayu Tang versus glibenclamide There was no significant difference between the comparison regarding the fasting blood glucose levels (Hua SG 1997). No data were available for other outcomes.
Yiqi Yangyin Huoxue recipe versus glurenorm There was no significant difference between the comparison regarding the fasting blood glucose levels (Wang JS 2000). No data were available for other outcomes.
Yishen Huoxue Tiaogan versus gliclazide Yishen Huoxue Tiaogan showed significantly better effect than gliclazide on the normalisation of fasting blood glucose (34/55 versus 20/49) and on the fasting blood glucose levels (Xu Q 2003). No data were available for other outcomes.
Yuquan Wan versus tolbutamide There was no significant difference between the comparison regarding the normalisation of fasting blood glucose (6/25 versus 6/28) (Chen XH 1993). No data were available for other outcomes.
Herbal medicines plus hypoglycaemic drugs versus hypoglycaemic drugs
Twenty‐nine trials (involving 3577 patients) compared different herbal medicines plus hypoglycaemic drugs versus hypoglycaemic drugs (Chang ZQ 1998; Chen H 2003; Guo HF 1996; Hou LY 2003; Hu YP 2003; Huang CL 2003; Li HW 2002; Li Y 2000; Mao L 2002; Miao WH 2003; Ni HX 2000; Peng SJ 1995; Shao CP 2001; Tao XY 2002; Wang BS 1988; Wang YS 2003; Wei JL 2003; Wen S 2000; Wu HM 1996; Xu YS 1997; Yang PL 2003; Yao LD 2003; Zeng Y 2001; Zhang HZ 1999; Zheng X 2001; Zhou JH 2001a; Zhou P 1997; Zhou XT 2001; Zhu ZZ 1997). The tested herbs included Astragalus, Buqi Zhiyin Huoxue Huayu, Danzhi Xiaoyao San, Herbal mixtures, Huatan Huoxue recipe, Jiangtang Fang, Jiangtang Tiaozhi Tang, Jianpi Huatan Huoxue, Jianpi Zhuyun Yishen Huayu, Jinli Da, Kelening, Potentilla chinensis, Potentilla composite, Qimai Dahuang Tang, Qingre Huatan Huoxue Huayu, Sanhuang Jiangtang recipe, Shengqi Huafen Tang, Shenqi Jiangtang Tang, Shugan Jianpi Huoxue Tang, Sihuang capsule, Tangniaobing No. 2, Tianyuan Jiangtang Wan, Xiaoke Wan, Xiaotang Ling, Xiaoyao San, Xingyu Huashi recipe, Xuange Yin, Yiqi Yangyin Huoxue Huayu, Yiqi Yangyin Qingre recipe, Yisheng Jiangtang Fang, and Zhonghui Chuanhuang Ye. We did not perform a meta‐analysis due to the variability of the interventions and no herbal remedy was tested twice. The results were also summarised in Appendix 5.
All outcomes
Astragalus plus gliclazide and metformin versus gliclazide and metformin The combined therapy was significantly better than hypoglycaemic agents alone regarding fasting blood glucose levels, HbA1c levels, and fasting serum insulin (Huang CL 2003).
Buqi Zhiyin Huoxue Huayu plus glibenclamide and metformin versus glibenclamide and metformin There was significantly lower serum insulin levels in patients treated with the combination therapy than those treated with hypoglycaemic drugs alone (Chang ZQ 1998). Data for outcomes of fasting blood glucose and HbA1c could not be extracted because the trial included both type 1 and 2 diabetic patients and reported the outcomes aggregately.
Danzhi Xiaoyao San plus hypoglycaemic drug versus hypoglycaemic drug The combination therapy of Danzhi Xiaoyao San and hypoglycaemic drug showed a tendency toward better effect than hypoglycaemic drug monotherapy on the normalisation of fasting blood glucose (17/31 versus 5/20) (Ni HX 2000). Danzhi Xiaoyao San plus hypoglycaemic drug reduced significantly the fasting blood glucose levels and HbA1c. There was no significant difference between the combination therapy and hypoglycaemic drug alone in fasting serum insulin levels. The trial did not specify the hypoglycaemic drug.
Herbal mixtures plus tolbutamide versus tolbutamide One trial used either of two herbal mixtures (Yiqi Yangyin Qingre and Jianpi Zhuyun Yishen Huayu) according to the syndromes of patients (Wu HM 1996). It showed that herbal mixture plus tolbutamide was significantly better than tolbutamide alone in reducing fasting blood glucose levels. No data were available for other outcomes.
Huatan Huoxue recipe plus glibenclamide and metformin versus glibenclamide and metformin The combined treatment of herbal remedy with hypoglycaemic drugs did not show significantly additional benefit in the normalisation of fasting blood glucose (19/103 versus 9/61) (Tao XY 2002). No data were available for other outcomes.
Jiangtang Fang plus glibenclamide versus glibenclamide The combined treatment of Jiangtang Fang and glibenclamide showed significant better effects on the normalisation of fasting blood glucose (32/52 versus 20/51; RR 1.57, 95% CI 1.05 to 2.35) and on reducing the fasting blood glucose levels (Wen S 2000). The combined treatment also showed significant effect on HbA1c levels. There was no significant difference between the combined treatment and glibenclamide alone in serum insulin levels.
Jiangtang Tiaozhi Tang plus glibenclamide versus glibenclamide The combined treatment of Jiangtang Tiaozhi Tang and glibenclamide showed significant better effects on the normalisation of fasting blood glucose (36/68 versus 13/52) and on reducing the fasting blood glucose levels (Wei JL 2003). No data were available for other outcomes.
Jianpi Huatan Huoxue plus metformin versus metformin The combined treatment of Jianpi Huatan Huoxue and metformin showed significant better effects on the normalisation of fasting blood glucose (36/88 versus 9/46) and on reducing the fasting blood glucose levels (Yang PL 2003). No data were available for other outcomes.
Jinli Da plus glibenclamide versus glibenclamide A combination of herbal extracts and glibenclamide showed significantly better effect than glibenclamide alone on normalisation of fasting blood glucose (20/40 versus 2/28) and on reducing the fasting blood glucose levels (Zheng X 2001). No data were available for other outcomes.
Kelening plus glibenclamide versus glibenclamide There was no significant difference between the combined treatment and glibenclamide alone regarding the normalisation of fasting blood glucose (14/33 versus 12/32) and the fasting blood glucose levels (Zhou P 1997). However, the combined treatment appeared significant effects on HbA1c levels and serum insulin levels.
Potentilla chinensis plus metformin and glipizide versus metformin and glipizide There was no statistically significant difference between single herb Potentilla chinensis combined with metformin and glipizide against metformin and glipizide regarding fasting blood glucose levels (Yao LD 2003). No other outcomes were available.
Potentilla composite plus glipizide versus glipizide The herbal mixture of Potentilla combined with glipizide showed significantly effect on reducing the fasting blood glucose levels (Shao CP 2001). No data were available for other outcomes.
Qimai Dahuang Tang plus gliclazide versus gliclazide The combined treatment showed significantly better effects than gliclazide on normalisation of fasting blood glucose (87/98 versus 25/49) and on reducing the fasting blood glucose levels (Zhou JH 2001a). No data were available for other outcomes.
Qingre Huatan Huoxue Huayu plus glibenclamide versus glibenclamide There was no significantly additional benefit from the combination of Qingre Huatan Huoxue Huayu and glibenclamide regarding normalisation of fasting blood glucose compared with glibenclamide alone (Zeng Y 2001). However, a significant benefit was showed from the combination therapy in reducing the fasting blood glucose levels. No data were available for other outcomes.
Sanhuang Jiangtang recipe plus hypoglycaemic drug versus glipizide There was no significant difference between the combination therapy and glipizide regarding normalisation (23/53 versus 18/42) or fasting blood glucose levels (Zhu ZZ 1997). There was no significant difference regarding serum insulin levels between the comparison.
Shengqi Huafen Tang plus glibenclamide and Insoral versus glibenclamide and Insoral The combination of Shengqi Huafen Tang with two hypoglycaemic drugs did not show significantly benefit regarding normalisation of fasting blood glucose (20/57 versus 8/36) compared with the two drugs treatment (Li Y 2000). On the contrary, this combination appeared significantly inferior to glibenclamide and Insoral regarding reducing fasting blood glucose levels. No data were available for serum insulin and HbA1c levels.
Shenqi Jiangtang Tang plus glibenclamide versus glibenclamide There was no significant difference between the combination therapy and glibenclamide alone regarding normalisation of fasting blood glucose (35/88 versus 14/46) (Miao WH 2003). However, the combined therapy showed statistically significant effect on fasting blood glucose. No data were available for other outcomes.
Shugan Jianpi Huoxue Tang plus gliclazide and metformin versus gliclazide and metformin The combination therapy showed significantly better effect on normalisation of fasting blood glucose (20/38 versus 10/37) than hypoglycaemic drugs alone (Li HW 2002). However, this benefit was not consistent in reducing fasting blood glucose levels. There was no significant difference between the comparison regarding fasting serum insulin levels. No data on HbA1c were available.
Sihuang Jiaonang plus glibenclamide versus glibenclamide There was no significant difference between the combination therapy and glibenclamide alone regarding normalisation of fasting blood glucose (14/82 versus 8/75) (Hu YP 2003). No data were available for other outcomes.
Tangniaobing No. 2 plus glipizide versus glipizide There was no significant difference between the combination therapy and glipizide alone regarding normalisation (8/54 versus 4/44) or fasting blood glucose levels (Mao L 2002). No data were available for other outcomes.
Tianyuan Jiangtang Wan plus hypoglycaemic drug versus hypoglycaemic drug One trial reported a design of four arms with two different comparisons (Guo GY 1998). In two arms patients who were newly diagnosed and untreated were allocated to receive either Tianyuan Jiangtang Wan or gliclazide (see Comparison 02). While in another two arms, patients who had previous hypoglycaemic drug treatment were allocated to continue either their original hypoglycaemic drugs treatment alone or hypoglycaemic drugs plus Tianyuan Jiangtang Wan. The trial did not specify the hypoglycaemic drugs. Tianyuan Jiangtang Wan plus hypoglycaemic drugs appeared significant effects on reducing fasting blood glucose levels and on HbA1c levels compared with hypoglycaemic drugs alone. No other outcome was reported.
Xiaoke Fuzheng capsule plus glipizide or glibenclamide versus glipizide or glibenclamide There was no significant difference between the combination therapy and hypoglycaemic agent alone regarding normalisation of fasting blood glucose (28/58 versus 16/38) (Hou LY 2003). No data were available for other outcomes.
Xiaoke Wan plus glibenclamide versus glibenclamide Xiaoke Wan that contained seven herbs and glibenclamide was compared with glibenclamide in one trial (Zhang HZ 1999). There was no significant difference between Xiaoke Wan and glibenclamide regarding normalisation (39/86 versus 28/80) or fasting blood glucose levels. No data were available for other outcomes.
Xiaotang Ling plus glibenclamide versus glibenclamide The combined therapy showed statistically significant effects on fasting blood glucose and HbA1c (Wang YS 2003). There was no significant difference between the comparison in fasting serum insulin levels.
Xiaoyao San plus glibenclamide versus glibenclamide Xiaoyao San plus glibenclamide showed significantly better effects than glibenclamide alone on normalisation of fasting blood glucose (98/146 versus 48/132) and on reducing fasting blood glucose levels (Zhou XT 2001). The combination appeared to increase the fasting serum insulin levels. The trial did not provided data on HbA1c.
Xingyu Huashi recipe plus gliclazide versus gliclazide There was no significant difference between the combination therapy and gliclazide alone regarding fasting blood glucose levels (Guo HF 1996). No data were available for other outcomes.
Xuange Yin plus glipizide versus glipizide The combination of Xuange Yin and glipizide showed significantly better effects than glipizide alone on normalisation of fasting blood glucose (19/37 versus 10/37) and on reducing fasting blood glucose levels (Chen H 2003).
Yiqi Yangyin Bushen plus glibenclamide and metformin versus glibenclamide and metformin There was no statistically significant difference between the combination therapy and hypoglycaemic drugs alone regarding normalisation of fasting blood glucose (33/34 versus 13/16) (Zhang FB 2003). No data were available for other outcomes.
Yiqi Yangyin Huoxue Huayu recipe plus tolbutamide and persantine versus tolbutamide and persantine There was no significant difference between the combination therapy and hypoglycaemic drugs regarding normalisation of fasting blood glucose (29/56 versus 21/56) (Wang BS 1988). No data were available for other outcomes.
Yishen Jiangtang Fang plus glibenclamide versus glibenclamide The combination of Yisheng Jiangtang Fang and glibenclamide showed significantly better effects than glibenclamide alone on normalisation of fasting blood glucose (38/48 versus 22/40) and on reducing fasting blood glucose levels (Peng SJ 1995). The serum insulin levels in patients treated with the combination were significantly higher than in those treated only with glibenclamide.
Zhonghui Chuanhuang Ye plus hypoglycaemic drug versus hypoglycaemic drug One trial allocated patients to receive either Zhonghui Chuanhuang Ye added to their original hypoglycaemic treatment or continuing their original hypoglycaemic treatment (Xu YS 1997). The trial did not specify hypoglycaemic agent. The combination therapy showed a significant effect on reducing fasting blood glucose levels. No data were available for other outcomes.
Herbal medicine plus dietary and lifestyle modification versus dietary and lifestyle modification (Comparison 04) Two trials compared combined herbal medicines with non‐pharmacological therapy versus non‐pharmacological therapy alone (Namdul 2001; Zhang J 2003). Two herbal medicines (Qihuang capsule plus berberine) showed significant better effects than diet and exercise alone on normalisation of fasting blood glucose (18/30 versus 6/30), reducing fasting blood glucose levels, HbA1c, and fasting serum insulin (Zhang J 2003). Tibetan medicines plus dietary and lifestyle modification showed a significantly better effect than dietary and lifestyle modification alone on fasting blood glucose levels (Namdul 2001). The data on HbA1c and body weight could not be extracted due to inadequate reporting in this trial.
Symptoms improvement
Twenty‐eight trials stated the measurement of symptoms, but in most trials reporting was inadequate. Only data from six trials were available (Pan MZ 1997; Shen T 1998; Wang KP 1999; Xie CG 1996; Zhang HZ 1999; Zhou C 2001). The symptoms included thirstand dry mouth, fatigue, constipation, low back pain, limb numbness and pain, etc (Appendix 6). Overall, it appeared that improvement rates from the herbal intervention group were higher than in the control intervention group.
Adverse events
Seventeen trials out of 66 included trials reported adverse effects (Agrawal 1996; Agrawal 2002; Cao FK 1997; Chen XH 1993; Chen YB 1995; Guo GY 1998; Li YM 2003; Pan MZ 1997; Qing ZQ 2001; Russo 1990; Sotaniemi 1995; Vray 1995; Wei JL 2003; Wu YN 2003; Xie CG 1996; Zhang M 2001a; Zhou P 1997) (Appendix 7). Twelve of thesereported no adverse effects during herbal treatment. Five trials reported non‐serious adverse events. One patient developed urticaria after taking Tianyuan Jiangtang Wan this was relieved after stopping the treatment (Guo GY 1998). Two patients stopped a formula of traditional Chinese herbs (TCT) due to diarrhoea in one case and dry mouth in theother case (Vray 1995). Among 100 patients treated with Tangfu Kang, 12 patients developed mild diarrhoea, and five developed mild abdominal pain (Xie CG 1996). The symptoms were relieved after taking the herbal treatment for two to three days. In another trial, two patients had nausea and two had mild diarrhoea from 98 patients treated with Kelening (Zhou P 1997). Among 29 patients treated by herbal compound Tangzhi Xiao, four patients had diarrhoea, two had abdominal distension, and one had poor appetite (Li YM 2003). Similar adverse effects in gliclazide treated patients, including four with diarrhoea, one abdominal distension, one poor appetite, and one abdominal pain. There was no significant difference between herbal treatment and hypoglycaemic drugs regarding the incidence of adverse effects. No serious adverse events were observed.
Discussion
Sixty‐six randomised trials were included in this review. We excluded 35 randomised trials due to a treatment duration of less than two months, or outcomes reported not relevant to this review. We also excluded 18 randomised trials wherethe study objectives were to treat diabetic complications such as neuropathy, nephropathy, diabetic foot,or retinopathy. The included trials compared different herbal medicines or combinations with hypoglycaemic agents versus placebo, hypoglycaemic agents, or dietary and lifestyle modification in patients with type 2 diabetes mellitus. All trials measured surrogate outcomes, some evaluated symptoms or adverse events. Some herbal medicines seemed to improve blood glucose control in terms ofanormalisation or a reduction of fasting blood glucose, reduction of HbA1c or an increase of serum insulin levels. However, one should be cautious in interpreting the findings due to low methodological quality, general small sample size, and limited number of the trials identified for individual herbal medicine.
Compared with placebo, six herbal preparations, Holy basil leaves, Xianzhen Pian, Qidan Tongmai, Traditional Chinese Medicine (TCT), Huoxue Jiangtang Pingzhi, and Inolter, seemed to improve blood glucose control. While single herbal preparations Bauhinia forficata, Myrcia uniflora, and Ginseng appeared not to be effective on blood glucose control. An interesting positive finding from Xianzhen Pian in a meta‐analysis of three trials warrants further trial.
Comparisons of herbal medicines with hypoglycaemic drugs showed better hypoglycaemic responses for Bushen Jiangtang Tang, Composite Trichosanthis, Jiangtang Kang, Ketang Ling, Shenqi Jiangtang Yin, Xiaoke Tang, and Yishen Huoxue Tiaogan. The oral antidiabetic drugsinclude glibenclamide, tolbutamide, or gliclazide. Herbal medicines that showed no significant difference compared to hypoglycaemic agents included Jianpi Jiangtang Tang, Jiangtang No 1, 2, 3, Jinqi Jiangtang Tang, Shenqi Yuxiao Tang, Shengqing Jiangtang recipe, Shuizhi Sanhuang Tang, Tangfu Kang, Tangning Pian, Tianyuan Jiangtang Wan, Xiaoke Yin, Yiqi Yangyin Huayu Tang, Yiqi Yangyin Huoxue recipe, and Yuquan Wan. Two herbal medicines Kelening and Xiaoke Ling seemed to beless effective than glibenclamide.
The evidence appearsnot strong enough to arrive at a clinical recommendation due to the following limitations.
All herbal medicines that were tested against hypoglycaemic agents were mixtures composed of several different herbs. The majority of the herbal preparations were prescribed by the investigators, and the formulae were usually tailored to individual patients based on differentiation of the patients 'syndrome'. Therefore, the quality of the preparations cannot be assured.
No herbal medicine was tested more than once except Xianzhen Pian. Any of the eventual positive findings need to be confirmed.
The sample size was generally small.
Few of the trials used blinding methods. If a trial is not blinded, especially the outcome assessors, there may be a possibility of performance bias and detection bias (Schulz 1995; Moher 1998).
Twenty‐nine out of 66 included trials evaluate combination treatment of herbal medicines with hypoglycaemic agents compared with hypoglycaemic agents alone. Additional hypoglycaemic benefit from the combined treatment as found in the herbal preparations including Danzhi Xiaoyao San, Jiangtang Fang, Jiangtang Tiaozhi Tang, Jianpi Huatan Huoxue, Jinli Da, Qimai Dahuang Tang, Shugan Jianpi Huoxue Tang, Xiaoyao San, Yiqi Yangyin Qingre and Jianpi Zhuyun Yishen Huayu, Potentilla composite, Qingre Huatan Huoxue Huayu, Tianyuan Jiangtang Wan, Yishen Jiangtang Fang, and Zhonghui Chuanhuang Ye. All these herbal preparations are mixtures of herbs, and most of them were prescribed by the investigators without definite quality standard regarding the preparation process. Interpretation of these findings is difficult considering the above mentionedlimitations of trials. This systematic review tries topoint to some herbal medicines which deservefurther examination. Patient relevant outcomes such as incidence of mortality, complications, quality of life, and costs were not investigated. Symptoms improvement was inadequately reported.
This systematic review has several limitations. First, most of the included trials suffer from methodological issues such as poor quality in terms of randomisation and blinding. This may be associated with exaggerated effects of the herbal interventions due to subjected to systematic errors (bias). Potential bias may happen in selection of patients, administration of treatment, and assessment of outcomes. Methodologically less rigorous trials show significantly larger intervention effects than trials with more rigor (Schulz 1995; Moher 1998; Kjaergard 2001; Egger 2003). Empirical study has shown that Chinese trials are significantly affected by publication bias (Vickers 1998). Accordingly, when interpreting the present findings, publication bias should be taken into consideration. Second, most of the trials had small sample size. Although some data analyses did not demonstrate a statistically significant difference between herbal medicines and hypoglycaemic drugs, the results are likely to have been underpowered. Therefore, the analyses from the size of these trials may not establish with confidence that two interventions have equivalent effects (Pocock 1991; Piaggio 2001). Third, we do not have evidence that any of the herbs compared with hypoglycaemic agents has beneficial effects on type 2 diabetes mellitus from placebo controlled trials. All trials reported end‐of‐treatment responses, and we do not know long term responses due to lack of follow‐up. Fourth, almost all herbal medicines in the included trials were tested only once except Xianzhen Pian, which made it impossible to pool data. Fifth, ninety per cent of the trials was conducted on Chinese participants. It is therefore not possible to perform subgroup analysis on ethnic origin. We are not sure if the results are valid and applicable to other ethnic group, e.g., Caucasian patients.
Safety of herbal medicines in type 2 diabetes
The herbal medicines evaluated in this review generally appeared to be safe. However, we do not conclude on the safety of using herbal medicines in diabetic patients as adverse effects were not sufficiently reported.In clinical trials beneficial and harmful effects should receive equal attention, and the recording and reporting ofadverse effects should be improved.
Our review findings conform to a finding from recently published systematic review on herbs and dietary supplements for glycaemic control in diabetes (Yeh 2003). This review concluded that there is still insufficient evidence to draw substantial conclusions about the efficacy of individual herbs for diabetes. However, our review differs from this review in several aspects. First, we only included randomised clinical trials comparing herbal medicines with placebo or hypoglycaemic drugs in type 2 diabetes mellitus with anintervention duration exceeding two months. The review by Yeh and colleagues included both randomised and non‐randomised controlled trials with interventions including both herbs and dietary supplements in both types of diabetic patients and healthy individuals. Secondly, we did not limit our search strategy to studies published in English only. Actually, we thought non‐English literature to beimportant and identified alarge number of Chinese trials, many of which were not indexed and found in other databases. Thirdly, the criteria for quality assessment were different. We did not only use Jadad scale (Jadad 1996) for methodological quality assessment but also analysed individual components of methodological quality (Kjaergard 2001).
Western herbal medicines are often standardised extracts of single herbs used for particular conditions. In comparison, Chinese herbal medicines are quite often composed of mixtures of up to 20 different herbs. Besides, they are sometimes customised for each individual patient by the practitioners based on the differentiation of the patients' 'syndrome'. Therefore, trial design, could be adapted to the 'individualised treatment' by stratification of practitioners of the 'syndromes'. On the other hand, it is very important to investigate the herbal medicines according to a set of criteria which include preparation consistent with description in the pharmacopoeia, chemical standardisation, biological assays, animal models, and clinical testing (Yuan 2000). At least, in future trials it is necessary to improve the description of herbal medicines being tested, for example, plant species, geographical origin, harvest season, preparation procedures and quality of the products.
Authors' conclusions
Implications for practice.
Based on this systematic review, some herbal medicines have beneficial effects on blood glucose control in people with type 2 diabetes mellitus. However, at the momentwe cannot recommend any of the examined herbs for clinical routine use, since the majority of the trials had low methodological quality and the benefit has not been confirmed by large trials of high‐quality.
Implications for research.
Trialsin comparing Chinese herbal medicine with established hypoglycaemic drugs should be designed according to 'equivalence principle'. It is also interesting to verify additional benefits from herbal medicine combined with established hypoglycaemic drugs versus hypoglycaemic drug monotherapy. Outcome measures should include patient relevant outcome parameters. Standardised monitoring and reporting should be used for assessment of adverse events.
The methodological quality of randomised trials of herbal medicines for type 2 diabetes needs to be improved. The following aspects should be addressed: (i) detailed reporting of the methods used to generate allocation sequence and allocation concealment; (ii) sufficient application of double blinding with the use of adequate placebo; (iii) clear descriptions of withdrawal/drop‐out during the trial and use of intention‐to‐treat analysis; (iv) reporting of clinically important outcome measures from long‐term follow‐up; and (v) reporting of the trial according to the CONSORT statement (www.consort_statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 30 September 2008 | Amended | Converted to new review format. |
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. 1. exp Medicine, Chinese Traditional/ 2. exp Drugs, Chinese Herbal/ 3. exp Medicine, Oriental Traditional/ 4. exp Plants, Medicinal/ 5. (medicin$ adj5 (chines$ or oriental$ or tibetan$)).tw. 6. (herbal medicin$ or medicin$ herbal$).tw. 7. plant$ medicin$.tw. 8. medicin$ plant$.tw. 9. or/1‐8 10. exp diabetes mellitus, non‐insulin‐dependent/ 11. exp insulin resistance/ 12. impaired glucose toleranc$.tw. 13. glucose intoleranc$.tw. 14. insulin$ resistanc$.tw. 15. exp obesity in diabetes/ 16. (obes$ adj diabet$).tw. 17. (MODY or NIDDM).tw. 18. (non insulin$ depend$ or noninsulin$ depend$ or noninsulin?depend$ or non insulin?depend$).tw. 19. ((typ$ 2 or typ$ II) adj diabet$).tw. 20. ((keto?resist$ or non?keto$) adj diabet$).tw. 21. ((adult$ or matur$ or late or slow or stabl$) adj diabet$).tw. 22. (insulin$ defic$ adj relativ$).tw. 23. pluri?metabolic$ syndrom$.tw. 24. or/10‐23 25. exp diabetes insipidus/ 26. diabet$ insipidus.tw. 27. 25 or 26 28. 24 not 27 29. 9 and 28 30. randomized controlled trial.pt. 31. controlled clinical trial.pt. 32. randomized controlled trials.sh. 33. random allocation.sh. 34. double‐blind method.sh. 35. single‐blind method.sh. 36. 30 or 31 or 32 or 33 or 34 or 35 37. limit 36 to animal 38. limit 36 to human 39. 37 not 38 40. 36 not 39 41. clinical trial.pt. 42. exp clinical trials/ 43. (clinic$ adj25 trial$).tw. 44. ((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).tw. 45. placebos.sh. 46. placebo$.tw. 47. random$.tw. 48. research design.sh. 49. (latin adj square).tw. 50. or/41‐49 51. limit 50 to animal 52. limit 50 to human 53. 51 not 52 54. 50 not 53 55. comparative study.sh. 56. exp evaluation studies/ 57. follow‐up studies.sh. 58. prospective studies.sh. 59. (control$ or prospectiv$ or volunteer$).tw. 60. cross‐over studies.sh. 61. or/55‐60 62. limit 61 to animal 63. limit 61 to human 64. 62 not 63 65. 61 not 64 66. 40 or 54 or 65 67. 29 and 66 |
Appendix 2. The preparation and composition of the herbal medicines of the included trials
| Name of herbs | Preparation | Composition | Study ID |
| Astragalus | Injection | Extract of Astragalus membranaceus. | Huang CL 2003 |
| Bauhinian forficata | Herbal tea | Single herb of Bauhinia forficata. | Russo 1990 |
| Berberine | Tablet | Alkaloid extracted from herb Coptis chinensis. | Yao LD 2003 Zhang J 2003 |
| Buqi Zhiyin Huoxue Huayu | Decoction | Investigator‐prescribed herbal compound of gensing, Angelicae sinensis, Astragalus, Rehmanniae glutinosae, Lycii, Salviae miltiorrhizae, Ligustici chuanxiong, etc. | Chang ZQ 1998 |
| Bushen Huoxue No. 1 (also see 'Xianzhen Pian') | Tablet | Herbal compound composed of 9 herbs: Astragalus membranaceus, Epimedii, Ligustri lucidi, Cuscutae chinensis, Polygoni multiflori, Fructus Lycii, Salviae miltiorrhizae, Fructus Crataegi, Scutellariae Baicalensis. | Chen YB 1995 |
| Bushen Jiangtang Tang | Decoction | Investigator‐prescribed formula composed of 16 herbs: Rehmanniae glutinosae, Astragalus membranaceus, Polygonati odorati, Corni officinalis, Dioscoreae oppositae, Radix Puerariae, Cuscutae chinensis, Moutan Radicis, Alismatis orientalis, Poriae Cocos, Trichosanthis kirilowii, Ophiopogonis japonici, Scrophulariae ningpoensis, Secretio Bufonis, etc. | Li RG 2001 |
| Composita Trichosanthis | Tablet | Self‐developed herbal mixture (no details on composition). | Chen XH 1993 |
| Danzhi Xiaoyao San | Decoction | Herbal mixture composed of 13 herbs: Radix bupleuri, Paeoniae lactiflorae, Angelicae sinensis, Rhizoma atractylodis macrocephalae, Moutan radicis, Gardeniae jasminoidis, Citri Sarcodactylis, Rosae rugosae, Tuber curcumae, Rehmanniae glutinosae, Ophiopogonis japonici, Dendrobii, etc. | Ni HX 2000 |
| Fan Bai Cao (Potentilla composite) | Decoction | Investigator‐prescribed herbal mixture composed of 12 herbs: Potentilla discolor, Astragali membranacei, Secretio Bufonis, Rhizoma Atractylodis macrocephalae, Dioscoreae oppositae, Radix puerariae, Trichosanthis kirilowii, Lycii radicis, Polygoni multiflori, Semen Cassiae, Leonuri heterophylli, Schisandrae chinensis. | Shao CP 2001 |
| Fufang Tianhuafeng | Tablet | Investigator‐prescribed herbal mixture (no details on composition). | Chen XH 1993 |
| Ginseng | Tablet | Single herb (Dansk Droge, Copenhagen). | Sotaniemi 1995 |
| Herbal mixtures | Decoction | Two herbal mixtures prescribed based on two different syndroms. For type deficiency of qi‐yin, Yiqi Yangyin Qingre recipe was used, and for type of deficiency of spleen and kidney, Jianpi Zhuyun Yishen Huayu recipe was used. | Wu HM 1996 |
| Holy basil leaves | Powder | Dried leaf powder made from fresh leaves of holy basil. | Agrawal 1996 |
| Huatan Huoxue recipe | Decoction | Investigator‐prescribed herbal mixture composed of 13 herbs: Bombyx Batryticatus, Salviae miltiorrhizae, Trichosanthis kirilowii, Coicis lachryma‐jobi, Hirudo seu whitmania, Semen Persicae, Tuber Curcumae, Platycodi grandiflori, Rhizoma atractylodis macrocephalae, Alismatis orientalis, Ligustici chuanxiong, Sinapis albae, etc. | Tao XY 2002 |
| Huoxue Jiangtang Pingzhi formula | Decoction | Investigator‐prescribed herbal mixture composed of Angelica sinensis, Carthanmus tinctorius, Salviae miltiorrhizae, Crataegus pinnatifida, Ligustici Chuanxiong, Moutan radicis, Cinnamomi cassiae, Bupleuri chinensis, Rheum, Astragalus membranaceus, Polygonum multiflorum, Pueraria lobata. | Wu YN 2003 |
| Inolter | Capsule | Herbal formulation containing 5 herbs: Momordica charantis, Trigonella foenum graeceum, Asphalt, Gymnema sylvestre, Eugenia jambolena. | Agrawal 2002 |
| Jiangtang Fang | Decoction | Herbal mixture composed of 9 herbs: Astragalus membranaceus, Dioscorea opposita, Pueraria lobata, Ophiopogon japonicum, Rehmanniae glutinosae, Trichosanthis kirilowii, Lycii radicis, Rhizoma Coptidis, Salviae miltiorrhizae. | Wen S 2000 |
| Jiangtang Kang | Granule | Single herb of Chrysanthemi morifolii. | Chen SH 1997 |
| Jiangtang No. 1, 2, 3 | Pill | Investigator‐developed herbal preparations of three different herbal mixtures were applied for each patient to be taken No. 1 in the morning, No. 2 after lunch, No. 3 in the evening. | Zhou C 2001 Tong J 2003 |
| Jiangtang Tiaozhi Tang | Decoction | Herbal mixture composed of Astragalus membranaceus, Pseudostellaria heterophylla, Rehmanniae glutinosae, Ophiopogon japonicum, Pueraria lobata, Salviae miltiorrhizae, Anemarrhenae asphodeloidis, Paeonia veitchii, Schisandra chinensis, Poria cocos, Rheum, Coptis chinensis. | Wei JL 2003 |
| Jianpi Huatan Huoxue | Decoction | Herbal mixture composed of Astragalus membranaceus, Pseudostellaria heterophylla, Poria cocos, Pinelliae ternatae, Citrus reticulata, Atractylodis macrocephalae, Pueraria lobata, Polygomatum sibiricum, Eupatorii fortunei, Leonurus heterophyllus, Salviae miltiorrhizae, Crataegi pinnatifida. | Yang PL 2003 |
| Jianpi Jiangtang Tang | Decoction | Herbal mixture composed of 14 herbs: Astragalus membranaceus, Trichosanthis kirilowii, Dioscorea opposita, Pueraria lobata, Codonopsitis pilosulae, Atractylodis macrocephalae, Anemarrhena asphodeloides, Atractylodes lancea, Panax pseudo‐ginseng, Poria cocos, Alisma plantago‐aquatica, Salviae miltiorrhizae, Lycium barbarum, Crataegus pinnatifida. | Wang KP 1999 |
| Jinli Da | Pill | Condensed herbal pill composed mainly of Ginseng, Astragalus membranaceus, Dioscorea opposita, Atractylodis macrocephalae, Pueraria lobata, Corni officinalis, etc. | Zheng X 2001 |
| Jinqi Jiangtang capsule | Capsule | Herbal medicine manufactured by Jilin Aodong Pharmaceutical Group Company. | Zhou C 2001 |
| Kelening capsule | Capsule | Herbal preparation manufactured by Shandong Weihai Kunlunshan Pharmaceutical Factory, and composed of Astragalus membranaceus, Rhizoma Polygonati, Rehmanniae glutinosae, Trichosanthis kirilowii, Pseudostellariae heterophyllae. | Zhou P 1997 |
| Ketangling | Decoction | Investigator‐prescribed formula composed of 8 herbs: Mori albae radicis, Astragalus membranaceus, Morindae officinalis, Cucurbitae moschatae, Trichosanthis kirilowii, Salviae miltiorrhizae, Corni officinalis, Secretio Bufonis. | Lan QF 2000 |
| Liuwei Dihuang Tang | Decoction | Herbal mixture composed of Rehmanniae glutinosae, Corni officinalis, Dioscoreae oppositae, Alismatis orientalis, Poriae cocos, Moutan radicis. | Wu YN 2003 |
| Maziren Wan | Decoction | Herbal mixture composed of Cannabis sativae, Paeoniae lactiflorae, Pruni armeniacae, Citri aurantii, Magnoliae officinalis, Polygonati sibirici, Rehmanniae glutinosae, Dioscoreae oppositae, Trichosanthis kirilowii. | Ren PA 2003 |
| Myrcia uniflora | Herbal tea | Single herb of Myrcia uniflora. | Russo 1990 |
| Potentilla chinensis | Herbal tea | Single herb of Potentilla chinensis. | Yao LD 2003 |
| Potentilla discolor preparation (also see Fan Bai Cao) | Oral liquid | Herbal mixture composed of 12 herbs: Potentilla discolor, Astragalus membranaceus, Atractylodes lancea, Atractylodis macrocephalae, Dioscorea opposita, Pueraria lobata, Trichosanthis kirilowii, Lycii radicis, Polygoni multiflori, Cassiae, Leonuri heterophylli, Schisandra chinensis. | Shao CP 2001 |
| Qidan Tongmai | Tablet | Herbal mixture composed of 10 herbs: Astragalus membranaceus, Angelica sinensis, Cinnamomi cassiae, Carthamus tinctorius, Salviae miltiorrhizae, Hirudo seu whitmania, Polygoni multiflori, Ligustici chuanxiong, radix et rhizoma Rhei, Fructus Crataegi. | Zhang M 2001a |
| Qihuang Jiaonang | Capsule | Herbal mixture composed of raw Astragalus membranaceus, Rehmanniae glutinosae, Pueraria lobata, Polygonatum sibiricum, Salvia miltiorrhizae, Rheum, Coptis chinensis, Hirudo seu whitmania. | Zhang J 2003 |
| Qimai Dahuang Tang | Decoction | Investigator‐prescribed herbal formula composed of 13 herbs: Astragalus membranaceus, Ophiopogon japonicum, Rehmanniae glutinosae conquitae, Ginseng, Trichosanthis kirilowii, Polygonatum odoratum, Schisandra chinensis, Dioscorea opposita, Herba Dendrobii, Salviae miltiorrhizae, Alisma plantago‐aquatica, Rehmanniae glutinosae, Ligustici chuanxiong. | Zhou JH 2001a |
| Qingre Huatan Huoxue Huayu recipe | Decoction | Investigator‐prescribed herbal formula composed of Coptidis chinensis, Anemarrhena asphodeloides, Atractylodes lancea, Pueraria lobata, Euonymus alatus, Alisma plantago‐aquatica, Moutan radicis, radix et rhizoma Rhei, Mori albae radicis. | Zeng Y 2001 |
| Sanhuang Jiangtang recipe | Decoction and Tablet | Herbal prescription composed of 9 herbs: Radix et Rhizoma Rhei, Prunus persica, Cinnamomi cassiae, Mirabilitum purum, Glycyrrhizae uralensis, Scrophulariae ningpoensis, Rehmanniae glutinosae, Ophiopogon japonicum, Astragalus membranaceus. | Zhu ZZ 1997 |
| Shengqi Huafen Tang | Decoction | Investigator‐prescribed herbal formula composed of 12 herbs: Rehmanniae glutinosae, Astragalus membranaceus, Polygoni multiflori, Salviae miltiorrhizae, Fructus Lycii, Trichosanthis kirilowii, Radix puerariae, Leonuri heterophylli, Alismatis orientalis, Paeoniae rubrae, Polygonati, Glycyrrhizae uralensis. | Li Y 2000 |
| Shengqing Jiangtang recipe | Decoction | Investigator‐prescribed herbal mixture composed of Bupleuri, Cimicifugae, Astragalus, Bufonis, Platycodi Grandiflori, Scutellariae Baicalensis, Coptidis, Corneum Gigeriae Galli, etc. | Cao F 1997 |
| Shenqi Jiangtang Tang | Decoction | Investigator‐prescribed herbal mixture composed of raw Astragalus membranaceus, Dioscoreae oppositae, Polygonati sibirici, Lycii radicis, Codonopsitis pilosulae, Pueraria lobata, Poria cocos, Corni officinalis, Salviae miltiorrhizae, Paeoniae rubrae, Ligustici chuanxiong, Notoginseng. | Miao WH 2003 |
| Shenqi Jiangtang Yin | Decoction | Herbal mixture composed of Ginseng, Astragalus membranaceus, Rehmanniae glutinosae, Trichosanthis kirilowii, Pueraria lobata, Salviae miltiorrhizae, Dioscorea opposita, Schisandra chinensis, Ophiopogon japonicum, Lycium barbarum, Herba Epimedii, Glycyrrhizae uralensis. | Yang TB 2002 |
| Shenqi Yuxiao Tang | Decoction | Investigator‐prescribed herbal compound of 11 herbs including Ginseng, Corni officinalis, Pseudostellariae heterophyllae, Dioscoreae oppositae, Astragali membranacei, Adenophorae seu glehniae, Scrophulariae ningpoensis, Polygonati, Rehmanniae glutinosae, Schisandrae chinensis, Polygonati odorati. | Qing ZQ 2001 |
| Shugan Huoxue recipe | Decoction | Investigator‐prescribed herbal mixture composed of 12 herbs: Bupleuri chinensis, Paeoniae lactiflorae, Meliae toosendan, Tribulus terrestris, Dioscoreae oppositae, Atractylodes lancea, Scrophularia ningpoensis, Oueraria lobata, Hordei vulgaris germinantus, Salviae miltiorrhizae, Leonurus heterophyllus, Lumbricus. | You BW 1999 |
| Shugan Jianpi Huaxue Tang | Decoction | Investigator‐prescribed formula composed of 11 herbs: Radix Bupleuri, Atractylodis macrocephalae, Tuber Curcumae, Paeoniae Rubrae, Paeoniae Lactiflorae, Poriae Cocos, Citri Aurantii, Angelicae sinensis, Ligustici chuanxiong, Menthae haplocalycis, Glycyrrhizae uralensis. | Li HW 2002 |
| Shuizhi Sanhuang Tang | Decoction | Investigator‐prescribed herbal formula composed of 9 herbs: Hirudo seu whitmania, Astragalus membranaceus, Rehmanniae glutinosae, Radix et Rhizoma Rhei, Salviae miltiorrhizae, secretio Bufonis, Radix Puerariae, Herba Dendrobii, Scrophulariae ningpoensis. | Wang KF 1993 |
| Sihuang Jiaonang | Capsule | Herbal mixture composed of more than 17 herbs: Polygomatum sibiricum, Coptis chinensis, Scutellariae baicalensis, Astragalus membranaceus, Dendrobium nobile, Anemarrhena asphodeloides, Trigonellae foeni‐graeci, Scrophularia ningpoensis, Cornus officinalis, Trichosanthis kirilowii, Poria cocos, Dioscorea opposita, Salviae miltiorrhizae, Panax pseudo‐ginseng, Paeonia veitchii, Rehmanniae glutinosae, Coicis lachryma‐jobi, etc. | Hu YP 2003 |
| Tangfu Kang | Pill | Condensed pill composed of Astragalus membranaceus, Rehmanniae glutinosae, Dioscorea opposita, Cornus officinalis, Prunus persica, radix et rhizoma Rhei, Scrophularia ningpoensis, etc. | Xie CG 1996 |
| Tangniaobing No. 2 | Oral liquid | Investigator‐prescribed herbal formula composed of 17 herbs: Astragalus membranaceus, Panacis quinquefolii, Morindae officinalis, Psoraleae corylifoliae, Hirudo seu whitmania, Salviae miltiorrhizae, Scrophulariae ningpoensis, Dioscoreae oppositae, Corni officinalis, Secretio bufonis, Radix puerariae, Rehmanniae glutinosae conquitae, Alismatis orientalis, Litchi chinensis, Ophiopogonis japonici, Paeoniae lactiflorae, etc. | Mao L 2002 |
| Tangning Pian | Tablet | Self‐developed herbal preparation mainly composed of Astragalus membranaceus, Salviae miltiorrhizae, Ophiopogon japonicum, Lycii radicis, Dioscorea opposita, etc. | Zhao XY 2001 |
| Tangzhi Xiao | Capsule | Self‐prepared formulation composed of Salviae miltiorrhizae, Stephaniae tetrandrae, Coptis chinensis, Hirudo seu whitmania, Astragalus membranaceus, Dioscorea opposita, Moutan radicis. | Li YM 2003 |
| Tianyuan Jiangtang Wan | Water pills | Chinese patent medicine composed of 13 herbs: Rehmanniae glutinosae, Corni officinalis, Angelicae sinensis, Bombyx batryticatus, Schisandrae chinensis, etc. | Guo GY 1998 |
| Tibetan medicines | Powder or pills | At least two of four Tibetan medicines (Kyura‐6, Aru‐18, Yungwa‐4, and Sugmel‐19). | Namdul 2001 |
| Traditional Chinese herbs (TCT) | Capsule | A powder mixture of three herbs: Astragalus membranaceus, Coptes chinensis, Lonicera japonica. | Vray 1995 |
| Xianzhen Pian | Tablet | Chinese patent herbal medicine composed of more than 12 herbs including Astragalus membranaceus, Salviae miltiorrhizae, Rehmanniae glutinosae, Ligustri lucidi, Herba Epimedii, Cuscutae chinensis, Fructus Lycii, Cassiae, Anemarrhenae asphodeloidis, Coptidis, Scutellariae baicalensis, Hirudo seu whitmania, etc. | Pan MZ 1997; Shen T 1998 |
| Xiaoke Fuzheng Jiaonang | Capsule | Herbal mixture composed of more than 20 herbs including Panax quinquefolium, Lilii, Ligustici chuanxiong, Zizyphi spinosae, Cucurbitae moschatae, Acanthopanacis gracilistyli radicis, Pruni mume, Dioscorea opposita, Euryales ferocis, Trichosanthis kirilowii, Glycyrrhizae uralensis, et al. | Hou LY 2003 |
| Xiaoke Ling | Capsule | Self‐developed herbal formula composed of 12 herbs: Ginseng, Aconiti Carmichaeli praeparata, Astragalus, Corni officinalis, Ophiopogonis Japonici, Polygonati odorati, Rhizoma Polygonati, Cistanches deserticolae, Dioscoreae oppositae, Radix Puerariae, Salviae miltiorrhizae, Hirudo seu whitmania. | Feng YM 1998 |
| Xiaoke Tang | Decoction | Investigator‐prescribed herbal compound of 12 herbs including Rehmanniae glutinosae, Trichosanthis kirilowii, Ophiopogonis japonici, Polygonati odorati, Coptidis, Ginseng, Astragali membranacei, Dioscoreae oppositae, Corni officinalis, Salviae miltiorrhizae, Radix puerariae, Schisandrae chinensis. | Pang DR 2002 |
| Xiaoke Wan | Pill | Chinese patented medicine manufactured by Guangzhou First Chinese Medicine Factory. It is a combined medicine composed of Trichosanthis kirilowii, Rehmanniae glutinosae, Pueraria lobata, Astragalus membranaceus, Dioscorea opposita, Zeae mays, Schisandra chinensis, and western drug glibenclamide (2.5 mg/pill). | Zhang HZ 1999 |
| Xiaoke Yin | Decoction | Investigator‐prescribed herbal compound of 15 herbs: Trichosanthis kirilowii, Rehmanniae glutinosae, Dioscoreae oppositae, Paeoniae rubrae, Paeoniae lactiflorae, Salviae miltiorrhizae, Polygonati, Bufonis secretio, Anemarrhenae asphodeloidis, Lycii, Lycii radicis, Angelicae sinensis, Astragalus, Pseudostellariae heterophyllae, Schisandrae chinensis. | Chen XL 2001 |
| Xiaotang Ling Jiaonang | Capsule | Herbal mixture composed of Panax quinquefolium, Astragalus membranaceus, Coptis chinensis, Salviae miltiorrhizae, et al. | Wang YS 2003 |
| Xiaoyao San | Decoction | Herbal formula composed of Bupleuri chinensis, Angelica sinensis, Paeoniae lactiflorae, Ligustici chuanxiong, Atractylodis macrocephalae, Poria cocos, Pueraria lobata, Astragalus membranaceus, Nelumbinis nuciferae, Euonymus alatus, Portulacae oleraceae. | Zhou XT 2001 Deng QW 2003 |
| Xingyu Huashi recipe | Decoction | Investigator‐prescribed herbal compound of 8 herbs: Angelicae sinensis, Ligustici chuanxiong, Carthami tinctorii, Mori albae, Radix Puerariae, Alismatis orientalis, etc. | Guo HF 1996 |
| Xuange Yin | Granule | Investigator‐prescribed herbal compound of 8 herbs: Astragalus membranaceus, Scrophularia ningpoensis, Dioscorea opposita, Polygonum multiflorum, Nelumbinis nuciferae, Pueraria lobata, Poria cocos, Atractylodes lancea. | Chen H 2003 |
| Yiqi Yangyin Bushen recipe | Decoction | Investigator‐prescribed herbal formulation composed of raw Astragalus membranaceus, Atractylodes lancea, Dioscorea opposita, Rehmanniae glutinosae, Pueraria lobata, Scrophularia ningpoensis, Polygonatum odoratum, Dendrobium nobile, Trichosanthis kirilowii, Lycii barbarii. | Zhang FB 2003 |
| Yiqi Yangyin Huayu Tang | Decoction | Investigator‐prescribed herbal formula composed of 10 herbs: Pseudostellariae heterophyllae, Astragalus membranacei, Rehmanniae glutinosae, Trichosanthis kirilowii, Radix Puerariae, Ligustici chuanxiong, Salviae miltiorrhizae, Leonuri heterophylli, Hirudo seu whitmania, Fructus Crataegi. | Hua SG 1997 |
| Yiqi Yangyin Huoxue Huayu | Decoction | Investigator‐prescribed formula composed of 12 herbs: Rehmanniae glutinosae, Scrophulariae ningpoensis, Herba Dendrobii, Polygonati odorati, Rhizoma Polygonati, Trichosanthis kirilowii, Astragalus membranaceus, Pseudostellariae heterophyllae, Polygoni Cuspidati, Salviae miltiorrhizae, Angelicae sinensis, Paeoniae Rubrae. | Wang BS 1988 |
| Yiqi Yangyin Huoxue recipe | Decoction | Investigator‐prescribed herbal formula composed of 13 herbs: Astragalus membranaceus, Pseudostellariae heterophyllae, Scrophulariae ningpoensis, Ophiopogonis japonici, Polygonati odorati, Herba Dendrobii, Salviae miltiorrhizae, Fructus Lycii, Citri sarcodactylis, Fructus amomi, Paeoniae rubrae, Ligustici chuanxiong, Semen Persicae. | Wang JS 2000 |
| Yishen Huoxue Tiaogan | Decoction | Herbal mixture composed of Epimedii grandiflori, Ginseng, Lycii barbari, Eucommiae ulmoidis, Rehmanniae glutinosae conquitae, Salviae miltiorrhizae, Achyranthis bidentatae, Buthus martensi, Alismatis orientalis, Schisandrae chinensis, Astragalus membranaceus, Citri aurantii, Paeoniae lactiflorae, Scutellariae baicalensis. | Xu Q 2003 |
| Yishen Jiangtang Fang | Decoction | Investigator‐prescribed formula composed of 11 herbs including Rehmanniae glutinosae conquitae, Corni officinalis, Polygonati, Schisandrae chinensis, Astragali membranacei, Pseudostellariae heterophyllae, Trichosanthis kirilowii, Scrophulariae ningpoensis, Ophiopogonis japonici, Salviae miltiorrhizae, etc. | Peng SJ 1995 |
| Yuquan Wan | Pill | Chinese patent medicine manufactured by Chengdu Chinese Medicine Factory, Sichuan, China. | Chen XH 1993 |
| Zhonghui Chuanhuang Ye | Oral liquid | Formulated herbal preparation composed of more than 10 herbs and manufactured by Huiyuan Pharmaceutical Company. | Xu YS 1997 |
Appendix 3. Hypoglycaemic effects of herbal medicines compared with placebo
| Interventions | Normalisation of FBG | FBG levels (mmol/L) | HbA1c (%) | FSI levels (mU/L) | Study ID |
| Bauhinia forficata extracts vs placebo | ‐0.20 (‐2.76 to 2.36) | Russo 1990 | |||
| Ginseng vs placebo | ‐0.80 (‐1.64 to 0.04) | ‐0.25 (‐1.30 to 0.80) | 0.30 (‐6.65 to 7.25) | Sotaniemi 1995 | |
| Holy basil leaves vs placebo | ‐1.30 (‐1.92 to ‐0.68) | Agrawal 1996 | |||
| Huoxue Jiangtang Pingzhi formula vs placebo | 1.67 (0.69 to 4.00) | ‐1.10 (‐1.63 to ‐0.57) | ‐2.24 (‐3.13 to ‐1.35) | Wu YN 2003 | |
| Inolter vs placebo | ‐1.47 (‐3.47 to 0.53) | ‐0.80 (‐0.93 to ‐0.67) | Agrawal 2002 | ||
| Liuwei Dihuang Tang vs placebo | 1.17 (0.44 to 3.06) | 1.00 (0.41 to 1.59) | ‐2.19 (‐3.11 to ‐1.27) | Wu YN 2003 | |
| Myrcia uniflora extracts vs placebo | 0.50 (‐3.89 to 4.89) | Russo 1990 | |||
| Qidan Tongmai tablet vs placebo | 2.28 (1.02 to 5.12) | ‐0.35 (‐0.89 to 0.19) | ‐1.40 (‐1.87 to ‐0.93) | Zhang M 2001a | |
| TCT vs placebo | ‐0.22 (‐0.36 to ‐0.08) | ‐0.64 (‐0.76 to ‐0.52) | Vray 1995 | ||
| Xianzhen Pian vs placebo | 2.50 (1.38 to 4.54) | ‐0.85 (‐1.64 to ‐0.05) | Chen YB 1995 Pan MZ 1997 Shen T 1998 | ||
| FBG: fasting blood glucose | HbA1c: glycated haemoglobobin | FSI: fasting serum insulin |
Appendix 4. Hypoglycaemic effects of herbal medicines compared with drugs
| Interventions | Normalisation of FBG | FBG levels (mmol/L) | HbA1c (%) | FSI levels (mU/L) | Study ID |
| Bauhinia forficata extracts vs placebo | ‐0.20 (‐2.76 to 2.36) | Russo 1990 | |||
| Ginseng vs placebo | ‐0.80 (‐1.64 to 0.04) | ‐0.25 (‐1.30 to 0.80) | 0.30 (‐6.65 to 7.25) | Sotaniemi 1995 | |
| Holy basil leaves vs placebo | ‐1.30 (‐1.92 to ‐0.68) | Agrawal 1996 | |||
| Huoxue Jiangtang Pingzhi formula vs placebo | 1.67 (0.69 to 4.00) | ‐1.10 (‐1.63 to ‐0.57) | ‐2.24 (‐3.13 to ‐1.35) | Wu YN 2003 | |
| Inolter vs placebo | ‐1.47 (‐3.47 to 0.53) | ‐0.80 (‐0.93 to ‐0.67) | Agrawal 2002 | ||
| Liuwei Dihuang Tang vs placebo | 1.17 (0.44 to 3.06) | 1.00 (0.41 to 1.59) | ‐2.19 (‐3.11 to ‐1.27) | Wu YN 2003 | |
| Myrcia uniflora extracts vs placebo | 0.50 (‐3.89 to 4.89) | Russo 1990 | |||
| Qidan Tongmai tablet vs placebo | 2.28 (1.02 to 5.12) | ‐0.35 (‐0.89 to 0.19) | ‐1.40 (‐1.87 to ‐0.93) | Zhang M 2001a | |
| TCT vs placebo | ‐0.22 (‐0.36 to ‐0.08) | ‐0.64 (‐0.76 to ‐0.52) | Vray 1995 | ||
| Xianzhen Pian vs placebo | 2.50 (1.38 to 4.54) | ‐0.85 (‐1.64 to ‐0.05) | Chen YB 1995 Pan MZ 1997 Shen T 1998 | ||
| FBG: fasting blood glucose | HbA1c: glycated haemoglobobin | FSI: fasting serum insulin |
Appendix 5. Hypoglycaemic effects of herbal medicine combined therapy
| Interventions | Normalisation of FBG | FBG levels (mmol/L) | HbA1c (%) | FSI levels (mU/L) | Study ID |
| Astragalus plus gliclazide and metformin vs gliclazide and metformin | ‐1.47 (‐1.90 to ‐1.04) | ‐1.21 (‐1.47 to ‐0.95) | ‐2.84 (‐4.07 to ‐1.61) | Huang CL 2003 | |
| Buqi Zhiyin Huoxue Huayu plus glibenclamide and metformin vs glibenclamide and metformin | ‐1.60 (‐2.98 to ‐0.22) | Chang ZQ 1998 | |||
| Danzhi Xiaoyao San plus hypoglycemic drug vs hypoglycemic drug | 2.19 (0.96 to 5.00) | ‐1.30 (‐1.92 to ‐0.68) | ‐1.21 (‐1.40 to ‐1.02) | ‐1.30 (‐4.65 to 2.05) | Ni HX 2000 |
| Herbal mixture plus tolbutamide vs tolbutamide | ‐1.66 (‐2.17 to ‐1.15) | Wu HM 1996 | |||
| Huatan Huoxue recipe plus glibenclamide and metformin vs glibenclamide and metformin | 1.25 (0.60 to 2.59) | Tao XY 2002 | |||
| Jiangtang Fang plus glibenclamide vs glibenclamide | 1.57 (1.05 to 2.35) | ‐0.65 (‐1.16 to ‐0.14) | ‐1.13 (‐1.41 to ‐0.85) | 0.81 (‐0.88 to 2.50) | Wen S 2000 |
| Jiangtang Tiaozhi Tang plus glibenclamide vs glibenclamide | 2.12 (1.26 to 3.57) | ‐1.06 (‐1.57 to ‐0.55) | Wei JL 2003 | ||
| Jianpi Huatan Huoxue plus metformin vs metformin | 2.09 (1.11 to 3.96) | ‐0.96 (‐1.80 to ‐0.12) | Yang PL 2003 | ||
| Jinli Da plus glibenclamide vs glibenclamide | 7.00 (1.78 to 27.57) | ‐2.35 (‐3.68 to ‐1.02) | Zheng X 2001 | ||
| Kelening plus glibenclamide vs glibenclamide | 1.13 (0.62 to 2.06) | ‐0.35 (‐0.98 to 0.28) | ‐1.04 (‐1.35 to ‐0.73) | ‐2.41 (‐4.51 to ‐0.31) | Zhou P 1997 |
| Potentilla chinensis plus metformin and glipizide vs metformin and glipizide | ‐0.50 (‐1.01 to 0.01) | Yao LD 2003 | |||
| Potentilla composita plus glipizide vs glipizide | ‐1.81 (‐2.32 to ‐1.30) | Shao CP 2001 | |||
| Qimai Dahuang Tang plus gliclazide vs gliclazide | 1.74 (1.31 to 2.31) | ‐1.74 (‐2.68 to ‐0.80) | Zhou JH 2001a | ||
| Qingre Huatan Huoxue Huayu plus glibenclamide vs glibenclamide | 1.61 (0.76 to 3.38) | ‐2.27 (‐2.86 to ‐1.68) | Zeng Y 2001 | ||
| Sanhuang Jiangtang recipe plus hypoglycemic drug vs glipizide | 1.01 (0.64 to 1.61) | ‐0.58 (‐1.95 to 0.79) | 1.17 (‐34.05 to 36.39) | Zhu ZZ 1997 | |
| Shengqi Huafen Tang plus glibenclamide and Insoral vs glibenclamide and Insoral | 1.58 (0.78 to 3.20) | 1.38 (0.48 to 2.28) | Li Y 2000 | ||
| Shenqi Jiangtang Tang plus glibenclamide vs glibenclamide | 1.31 (0.79 to 2.17) | ‐1.41 (‐2.24 to ‐0.58) | Miao WH 2003 | ||
| Shugan Jianpi Huoxue Tang plus gliclazide and metformin vs gliclazide and metformin | 1.95 (1.06 to 3.58) | ‐0.70 (‐1.73 to 0.33) | ‐1.26 (‐3.17 to 0.65) | Li HW 2002 | |
| Sihuang Jiaonang plus glibenclamide vs glibenclamide | 1.60 (0.71 to 3.60) | Hu YP 2003 | |||
| Tangniaobing No. 2 plus glipizide vs glipizide | 1.63 (0.53 to 5.06) | 0.01 (‐0.59 to 0.61) | Mao L 2002 | ||
| Tianyuan Jiangtang Wan plus hypoglycemic drug vs hypoglycemic drug | ‐0.72 (‐1.40 to ‐0.04) | ‐1.45 (‐2.26 to ‐0.64) | Guo GY 1998 | ||
| Xiaoke Fuzheng capsule plus glipizide or glibenclamide vs glipizide or glibenclamide | 1.15 (0.73 to 1.81) | Hou LY 2003 | |||
| Xiaoke Wan (containing glibenclamide) vs glibenclamide | 1.30 (0.89 to 1.89) | ‐0.04 (‐0.29 to 0.21) | Zhang HZ 1999 | ||
| Xiaotang Ling plus glibenclamide vs glibenclamide | ‐0.56 (‐1.05 to ‐0.07) | ‐0.57 (‐1.13 to ‐0.01) | ‐2.19 (‐5.59 to 1.21) | Wang YS 2003 | |
| Xiaoyao San plus glibenclamide vs glibenclamide | 1.85 (1.43 to 2.38) | ‐1.27 (‐1.74 to ‐0.80) | 2.03 (1.78 to 2.28) | Zhou XT 2001 | |
| Xingyu Huashi recipe plus gliclazide vs gliclazide | ‐0.37 (‐1.41 to 0.67) | Guo HF 1996 | |||
| Xuange Yin plus glipizide vs glipizide | 1.90 (1.03 to 3.52) | ‐1.33 (‐2.44 to ‐0.22) | Chen H 2003 | ||
| Yiqi Yangyin Bushen plus glibenclazide and metformin vs glibenclazide and metformin | 1.19 (0.94 to 1.52) | Zhang FB 2003 | |||
| Yiqi Yangyin Huoxue Huayu recipe plus tolbutamide and persantine vs tolbutamide and persantine | 1.38 (0.91 to 2.11) | Wang BS 1988 | |||
| Yisheng Jiangtang Fang plus glibenclamide vs glibenclamide | 1.44 (1.05 to 1.97) | ‐2.77 (‐3.11 to ‐2.43) | 2.01 (1.69 to 2.33) | Peng SJ 1995 | |
| Zhonghui Chuanhuang Ye plus hypoglycemic drug vs hypoglycemic drug | ‐1.97 (‐3.01 to ‐0.93) | Xu YS 1997 | |||
| FBG: fasting blood glucose | HbA1c: glycated haemoglobulin | FSI: fasting serum insulin |
Appendix 6. Improvement rates of symptoms reported in six trials
| Study ID | Herbs | Dry mouth | Diabetes | Fatigue | Sweating | Constipation | Numb limbs | Low back pain |
| Li YM 2003 | Tangzhixiao gliclazide | 90%(19/21) 74%(17/23) | 86%(18/21) 70%(16/23) | 90%(19/21) 65%(15/23) | NA | NA | NA | NA |
| Pan MZ 1997 | Xiaoke Tang glibenclamide | 50% (7/14) 0 % (13/14) | NA | 34%(11/32) 14%(5/35) | 9% (2/22) 0% (0/26) | 70%(14/20) 33%(5/15) | 48%(15/31) 16%(5/32) | 33%(11/33) 15%(5/33) |
| Shen T 1998* | Xianzhen Pian placebo | 40% 9% | NA | NA | 67% 13% | 57% 20% | NA | 40% 17% |
| Wang KP 1999 | Jianpi Jiangtang Tang gliclazide | 81%(17/21) 65%(11/17) | 95%(18/19) 76%(13/17) | 87%(20/23) 75%(15/20) | NA | NA | NA | NA |
| Xie CG 1996 | Tangfu Kang gliclazide | 100%(62/62) 97%(64/66) | NA | 96%(75/78) 80%(48/60) | NA | 100%(48/48) 76%(39/51) | 100%(39/39) 82%(27/33) | 92%(69/75) 82%(54/66) |
| Zhang HZ 1999 | Xiaoke Wan glibenclamide | 71%(40/56) 46%(24/52) | NA | 75%(51/68) 33%(21/64) | 52%(22/42) 31%(12/39) | 55%(28/51) 12%(6/49) | NA | NA |
| Zhou C 2001 | Jiangtang No. 1,2, 3 Jinqi Jiangtang Jiaonang glibenclamide | 96%(141/146) 84%(31/37) 77%(27/35) | 96%(139/145) 67%(23/34) 70%(24/34) | 86%(152/177) 76%(29/38) 49%(19/39) | 89%(77/86) 69%(11/16) 47%(8/17) | NA | NA | 74%(60/81) 62%(10/16) 57%(8/14) |
| * no raw data | NA: not available |
Appendix 7. Herbal medicines and adverse effects in the included trials
| Herbs | Formulation | adverse events | study ID |
| Bushen Huoxue No. 1 | tablet | No adverse effects were observed. | Chen YB 1995 |
| Composite radix Trichosanthin | tablet | No obvious adverse effects were observed. | Chen XH 1993 |
| Ginseng | capsule | No adverse effects were observed. | Sotaniemi 1995 |
| Holy basil leaves | herbal tea | No adverse effects were observed. | Agrawal 1996 |
| Huoxue Jiangtang Pingzhi | decoction | No adverse effects were observed. | Wu YN 2003 |
| Inolter | capsule | No adverse effects were observed. | Agrawal 2002 |
| Jiangtang Tiaozhi Tang | decoction | No obvious adverse effects were observed. | Wei JL 2003 |
| Kelening | capsule | Two patients developed nausea and two developed mild diarrhoea. While in glibenclamide group, five patients developed nausea, four had dizziness, and four had hypoglycaemic effect. | Zhou P 1997 |
| Liuwei Dihuang Tang | decoction | No adverse effects were observed. | Wu YN 2003 |
| Myrcia uniflora | herbal tea | No adverse effects were observed. | Russo 1990 |
| Qidan Tongmai | tablet | No adverse effects were observed. | Zhang M 2001a |
| Shengqing Jiangtang recipe | decoction | No adverse effects were observed. | Cao FK 1997 |
| Shenqi Yuxiao Tang | decoction | No adverse effects were observed. | Qing ZQ 2001 |
| Tangfu Kang | pill | Twelve patients developed mild diarrhoea, and five had abdominal pain. While in gliclazide control group, 11 patients had nausea, eight had diarrhoea, and one appeared urticaria. | Xie CG 1996 |
| Tangzhi Xiao | capsule | Four patients had diarrhoea, two had abdominal distension, and one had poor appetite. Similar adverse effects in gliclazide treated patients, including four with diarrhoea, one abdominal distension, one poor appetite, and one abdominal pain. | Li YM 2003 |
| Tianyuan Jiangtang Wan | pill | One patient developed urticaria, and relieved by stopping the herbal treatment. | Guo GY 1998 |
| TCT (traditional Chinese treatment) | capsule | Two patients stopped treatment due to adverse effects including one patient with diarrhoea and one with dry mouth. One patient from placebo group developed vertigo. | Vray 1995 |
| Xiaoyao San | decoction | No hypoglycaemic effect was observed. | Deng QW 2003 |
| Xianzhen Pian | tablet | No adverse effects were observed. | Pan MZ 1997 |
| Yuquan Wan | pill | No obvious adverse effects were observed. | Chen XH 1993 |
Appendix 8. Herbal medicines for diabetes treatment in Chinese National Essential Drugs
| Drug Name | Formulations | Drug code |
| Yuquan Wan | pill | 588 |
| Yusanxiao Jiaonang | capsule | 589 |
| Jiangtang Shu Jiaonang | capsule | 590 |
| Tangmai Kang Keli | granule | 591 |
| Xiaoke Wan | pill | 592 |
| Jinqi Jiangtang Pian | tablet | 593 |
| Jiangtang Jia Pian | tablet | 594 |
| Kele Ning Jiaonang | capsule | 595 |
| Xiaotang Ling Jiaonang | capsule | 596 |
| Xiaoke Ping Pian | tablet | 597 |
| Yangyin Jiangtang Pian | tablet | 598 |
| Shenqi Jiangtang Keli | capsule and tablet | 599 |
| Xiaoke An Jiaonang | capsule | 600 |
Appendix 9. Approved herbal medicines for diabetes by the Chinese State Drug Administration
| Drug name | Year apporved | Types of drugs | Formulations |
| Yijin Jiangtang Koufuye | 1993 | 3 | oral liquid |
| Xiaole Ning Jiaonang | 1993 | 4 | capsule |
| Yusanxiao Jiaonang | 1994 | 3 | capsule |
| Qizhi Jiangtang Jiaonang | 1995 | 3 | capsule |
| Shenqi Jiangtang Keli | 1995 | 4 | capsule |
| Shenqi Jiangtang Jiaonang | 1997 | 4 | capsule |
| Shenqi Jiangtang Jiaonang | 1997 | 4 | tablet |
| Xiaoke Ning Keli | 1998 | 4 | granule |
| Jinqi Jiangtang Jiaonang | 1998 | 4 | tablet |
| Renshen Tangtai Zhusheye | 1998 | 2 | injection |
| Jinqi Jiangtang Jiaonang | 1999 | 4 | capsule |
| Jinqi Jiangtang Keli | 1999 | 4 | granule |
| Jinqi Jiangtang Keli | 1999 | 4 | tablet |
| Xiaoke An Jiaonang | 1999 | 3 | capsule |
Data and analyses
Comparison 1. Herbal medicine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Normalisation of fasting blood glucose (< 7.2 mmol/L) | 5 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.39, 2.98] |
| 1.1 Huoxue Jiangtang Pingzhi formula versus placebo | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.69, 4.00] |
| 1.2 Liuwei Dihuang Tang versus placebo | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.44, 3.06] |
| 1.3 Qidan Tongmai tablet versus placebo | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.02, 5.12] |
| 1.4 Xianzhen Pian versus placebo | 3 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [1.38, 4.54] |
| 2 Fasting blood glucose levels (mmol/L) | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Bauhinia forficata extracts versus placebo | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.76, 2.36] |
| 2.2 Ginseng versus placebo | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.64, 0.04] |
| 2.3 Holy basil versus placebo | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.92, ‐0.68] |
| 2.4 Huoxue Jiangtang Pingzhi formula versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.63, ‐0.57] |
| 2.5 Inolter versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.47 [‐3.47, 0.53] |
| 2.6 Liuwei Dihuang Tang versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.41, 1.59] |
| 2.7 Myrcia uniflora extracts versus placebo | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐3.89, 4.89] |
| 2.8 Qidan Tongmai tablet versus placebo | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.89, 0.19] |
| 2.9 TCT versus placebo | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.36, ‐0.08] |
| 2.10 Xianzhen Pian versus placebo | 3 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.64, ‐0.05] |
| 3 Glycated haemoglobin levels (%) | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ginseng versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Inolter versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Qidan Tongmai tablet versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 TCT versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fasting serum insulin levels (mU/L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Ginseng versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Huoxue Jiangtang Pingzhi formula versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Liuwei Dihuang Tang versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
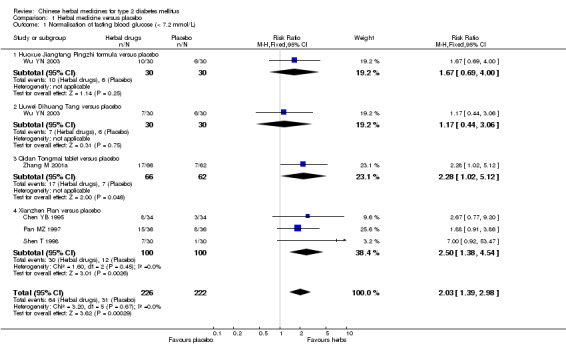
Comparison 1 Herbal medicine versus placebo, Outcome 1 Normalisation of fasting blood glucose (< 7.2 mmol/L).
1.2. Analysis.

Comparison 1 Herbal medicine versus placebo, Outcome 2 Fasting blood glucose levels (mmol/L).
1.3. Analysis.
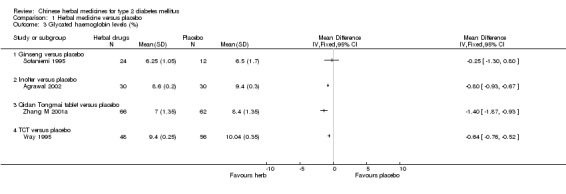
Comparison 1 Herbal medicine versus placebo, Outcome 3 Glycated haemoglobin levels (%).
1.4. Analysis.
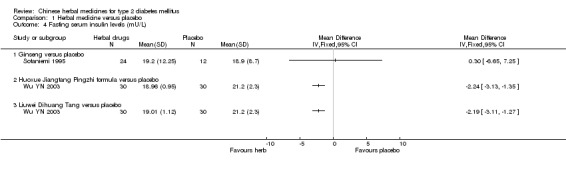
Comparison 1 Herbal medicine versus placebo, Outcome 4 Fasting serum insulin levels (mU/L).
Comparison 2. Herbal medicines versus hypoglycemic drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Normalisation of fasting blood glucose levels (< 7.2 mmol/L) | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Bushen Jiangtang Tang versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Composite Trichosanthis versus tolbutamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Jiangtang Kang granule versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Jiangtang No. 1‐3 capsule versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Jinqi Jiangtang capsule versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Kelening versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Ketangling versus gliclazide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Maziren Wan versus glibenclamide plus phenformin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Shenqi Jiangtang Yin versus tolbutamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Shenqi Yuxiao Tang versus gliclazide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Shengqing Jiangtang recipe versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.12 Shugan Huoxue recipe versus gliclazide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.13 Shuizhi Sanhuang Tang versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.14 Tangning Pian versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.15 Xiaoke Ling capsule versus gliclazide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.16 Xiaoke Tang versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.17 Xiaoke Yin versus glibenclamide plus Insoral | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.18 Yishen Huoxue Tiaogan versus gliclazide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.19 Yuquan Wan versus tolbutamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fasting blood glucose levels (mmol/L) | 21 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Bushen Jiangtang Tang versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Jianpi Jiangtang Tang versus gliclazide plus alginric sodium diester | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Jiangtang Kang granule versus glibenclamide (end of 60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Jiangtang Kang granule versus glibenclamide (end of 180 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Jiangtang No. 1‐3 capsule versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Jinqi Jiangtang capsule versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Kelening versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Maziren Wan versus glibenclamide plus phenformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Potentilla chinensis plus berberine versus metformin plus glipizide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Shenqi Jiangtang Yin versus tolbutamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Shenqi Yuxiao Tang versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Shengqing Jiangtang recipe versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.13 Shugan Huoxue recipe versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.14 Shuizhi Sanhuang Tang versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.15 Tangfu Kang versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.16 Tangning Pian versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.17 Tangzhi Xiao versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.18 Tianyuan Jiangtang Wan versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.19 Xiaoke Ling capsule versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.20 Xiaoyao San versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.21 Yiqi Yangyin Huayu Tang versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.22 Yiqi Yangyin Huoxue recipe versus glurenorm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.23 Yishen Huoxue Tiaogan versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Glycated haemoglobin levels (%) | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Jiangtang Kang granule versus glibenclamide (end of 60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Jiangtang Kang granule versus glibenclamide (end of 180 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Kelening versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Tangfu Kang versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Tangning Pian versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Tianyuan Jiangtang Wan versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Xiaoyao San versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fasting serum insulin levels (mU/L) | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Jiangtang Kang granule versus glibenclamide (end of 60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Jiangtang Kang granule versus glibenclamide (end of 180 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Kelening versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Tangfu Kang versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Tangzhi Xiao versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
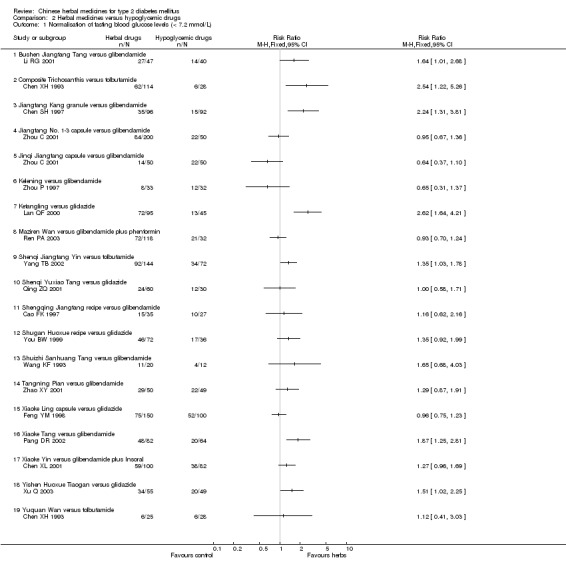
Comparison 2 Herbal medicines versus hypoglycemic drugs, Outcome 1 Normalisation of fasting blood glucose levels (< 7.2 mmol/L).
2.2. Analysis.
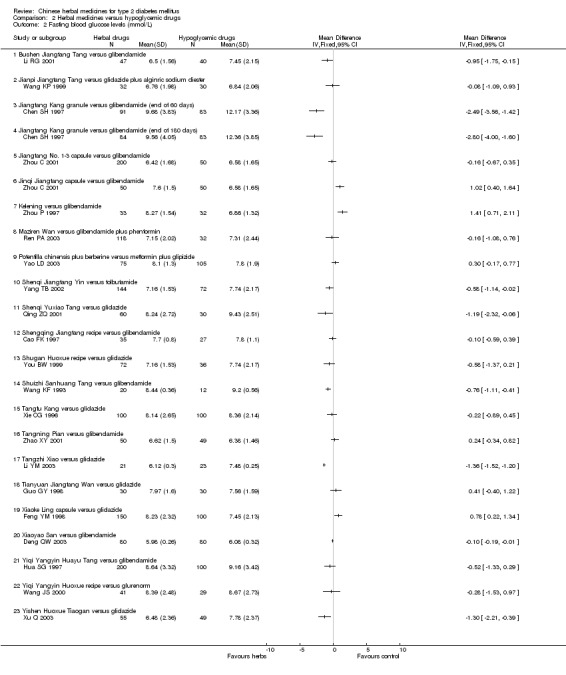
Comparison 2 Herbal medicines versus hypoglycemic drugs, Outcome 2 Fasting blood glucose levels (mmol/L).
2.3. Analysis.
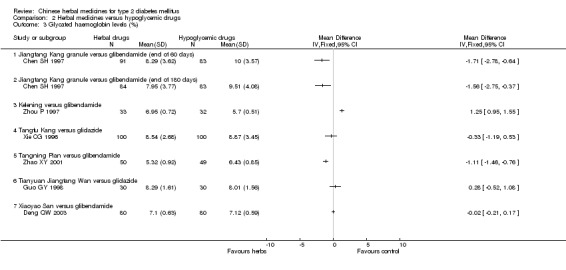
Comparison 2 Herbal medicines versus hypoglycemic drugs, Outcome 3 Glycated haemoglobin levels (%).
2.4. Analysis.
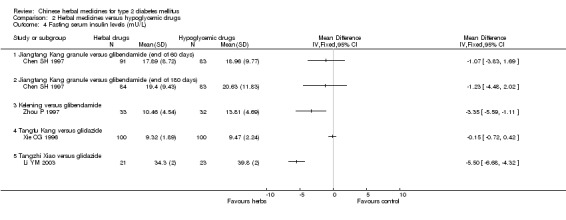
Comparison 2 Herbal medicines versus hypoglycemic drugs, Outcome 4 Fasting serum insulin levels (mU/L).
Comparison 3. Herbal medicine plus hypoglycaemic drug versus hypoglycaemic drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Normisation of fasting blood glucose levels (< 7.2 mmol/L) | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Danzhi Xiaoyao San plus hypoglycemic drug versus hypoglycemic drug | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Huatan Huoxue recipe plus glibenclamide and metformin versus glibenclamide and metformin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Jiangtang Fang plus glibenclamide versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Jiangtang Tiaozhi Tang plus glibenclamide versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Jianpi Huatan Huoxue plus metformin versus metformin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Jinli Da plus glibenclamide versusu glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Kelening plus glibenclamide versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Qimai Dahuang Tang plus gliclazide versus gliclazide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Qingre Huatan Huoxue Huayu plus glibenclamide versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Sanhuang Jiangtang recipe (partly plus hypoglycaemic drug) versus glipizide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Shengqi Huafen Tang plus glibenclamide and Insoral versus glibenclamide and Insoral | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.12 Shenqi Jiangtang Tang plus glibenclamide versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.13 Shugan Jianpi Huoxue Tang plus gliclazide and metformin versus gliclazide and metformin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.14 Sihuang Jiaonang plus glibenclamide versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.15 Tangniaobing No. 2 plus glipizide versus glipizide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.16 Xiaoke Fuzheng capsule plus glipizide or glibenclamide versus glipizide or glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.17 Xiaoke Wan (containing glibenclamide) versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.18 Xiaoyao San plus glibenclamide versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.19 Xuange Yin plus glipizide versus glipizide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.20 Yiqi Yangyin Bushen plus glibenclazide and metformin versus glibenclazide and metformin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.21 Yiqi Yangyin Huoxue Huayu recipe plus tolbutamide and persantine versus tolbutamide and persantine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.22 Yisheng Jiangtang Fang plus glibenclamide versus glibenclamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fasting blood glucose levels (mmol/L) | 25 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Astragalus plus gliclazide and metformin versus gliclazide and metformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Danzhi Xiaoyao San plus hypoglycemic drug versus hypoglycemic drug | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Herbal mixtures plus tolbutamide versus tolbutamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Jiangtang Fang plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Jiangtang Tiaozhi Tang plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Jianpi Huatan Huoxue plus metformin versus metformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Jinli Da plus glibenclamide versusu glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Kelening plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Potentilla chinensis plus metformin and glipizide versus metformin plus glipizide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Potentilla composite plus glipizide versus glipizide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Qimai Dahuang Tang plus gliclazide versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Qingre Huatan Huoxue Huayu plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.13 Sanhuang Jiangtang recipe (partly plus hypoglycaemic drug) versus glipizide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.14 Shengqi Huafen Tang plus glibenclamide and Insoral versus glibenclamide and Insoral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.15 Shenqi Jiangtang Tang plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.16 Shugan Jianpi Huoxue Tang plus gliclazide and metformin versus gliclazide and metformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.17 Tangniaobing No. 2 plus glipizide versus glipizide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.18 Tianyuan Jiangtang Wan plus hypoglycaemic drug versus hypoglycaemic drug | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.19 Xiaoke Wan (containing glibenclamide) versus glibenclamide (mild type) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.20 Xiaoke Wan (containing glibenclamide) versus glibenclamide (mediate type) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.21 Xiaoke Wan (containing glibenclamide) versus glibenclamide (severe type) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.22 Xiaotang Ling plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.23 Xiaoyao San plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.24 Xingyu Huashi recipe plus gliclazide versus gliclazide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.25 Xuange Yin plus glipizide versus glipizide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.26 Yisheng Jiangtang Fang plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.27 Zhonghui Chuanhuang Ye plus hypoglycemic drug versus hypoglycemic drug | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Glycated haemoglobin levels (%) | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Astragalus plus gliclazide and metformin versus gliclazide and metformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Danzhi Xiaoyao San plus hypoglycemic drug versus hypoglycemic drug alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Jiangtang Fang plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Kelening plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Tianyuan Jiangtang Wan plus hypoglycaemic drug versus hypoglycaemic drug | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Xiaotang Ling plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fasting serum insulin levels (mU/L) | 10 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Astragalus plus gliclazide and metformin versus gliclazide and metformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Buqi Zhiyin Huoxue Huayu plus glibenclamide and metformin versus glibenclamide and metformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Danzhi Xiaoyao San plus hypoglycemic drug versus hypoglycemic drug alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Jiangtang Fang plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Kelening plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Sanhuang Jiangtang recipe (partly plus hypoglycaemic drug) versus glipizide (pmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Shugan Jianpi Huoxue Tang plus gliclazide and metformin versus gliclazide and metformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Xiaotang Ling plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Xiaoyao San plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Yisheng Jiangtang Fang plus glibenclamide versus glibenclamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
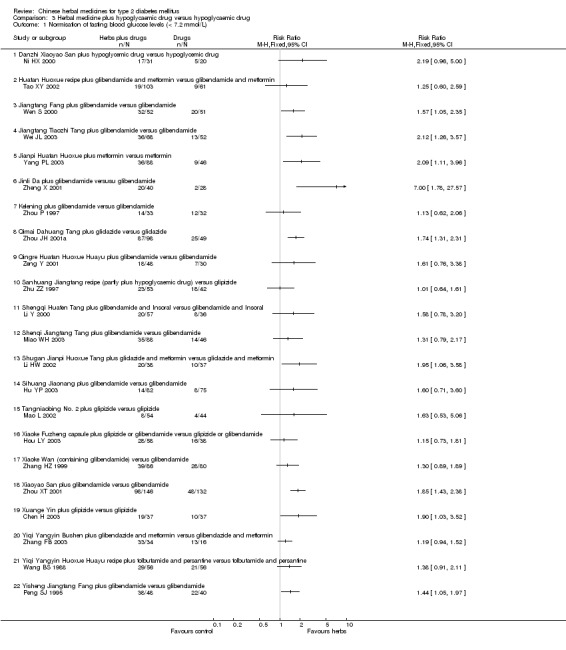
Comparison 3 Herbal medicine plus hypoglycaemic drug versus hypoglycaemic drug, Outcome 1 Normisation of fasting blood glucose levels (< 7.2 mmol/L).
3.2. Analysis.
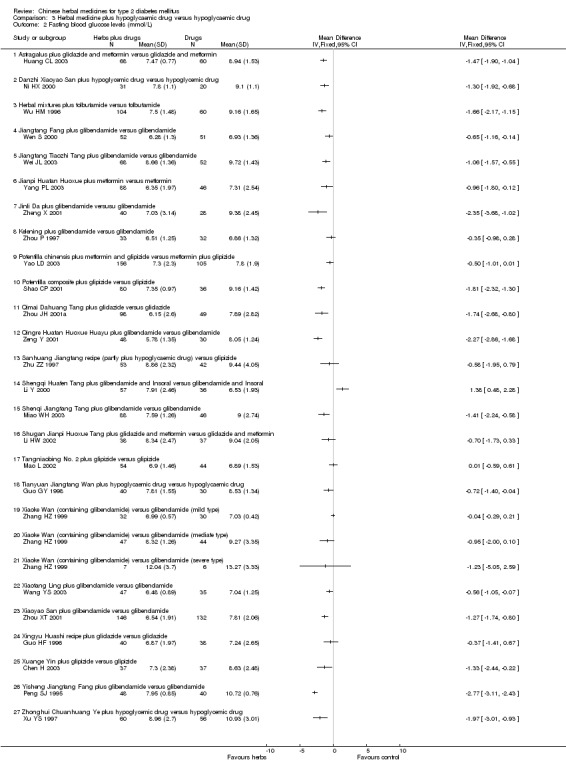
Comparison 3 Herbal medicine plus hypoglycaemic drug versus hypoglycaemic drug, Outcome 2 Fasting blood glucose levels (mmol/L).
3.3. Analysis.
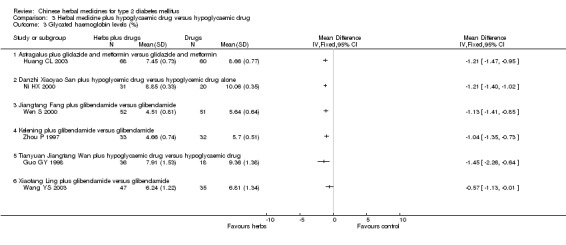
Comparison 3 Herbal medicine plus hypoglycaemic drug versus hypoglycaemic drug, Outcome 3 Glycated haemoglobin levels (%).
3.4. Analysis.
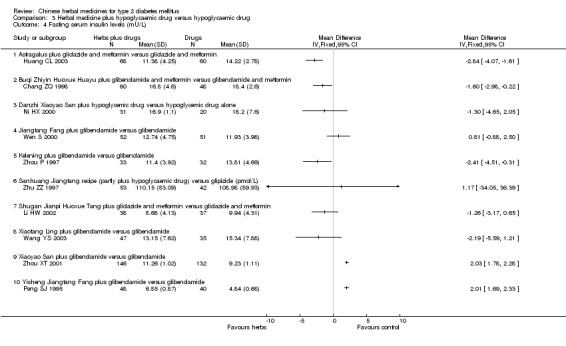
Comparison 3 Herbal medicine plus hypoglycaemic drug versus hypoglycaemic drug, Outcome 4 Fasting serum insulin levels (mU/L).
Comparison 4. Herbal medicine plus dietary and lifestyle modification versus dietary and lifestyle modification.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Normalisation of fasting blood glucose levels (< 7.2 mmol/L) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Qihuang capsule and Berberine plus diet and exercise versus diet and exercise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fasting blood glucose levels (mmol/L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Qihuang capsule and Berberine plus diet and exercise versus diet and exercise | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tibetan medicine plus diet and lifestyle versus diet and lifestyle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Glycated haemoglobin levels (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Qihuang capsule and Berberine plus diet and exercise versus diet and exercise | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fasting serum insulin levels (mU/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Qihuang capsule and Berberine plus diet and exercise versus diet and exercise | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.

Comparison 4 Herbal medicine plus dietary and lifestyle modification versus dietary and lifestyle modification, Outcome 1 Normalisation of fasting blood glucose levels (< 7.2 mmol/L).
4.2. Analysis.
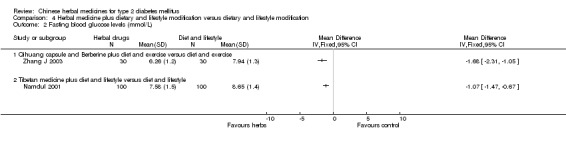
Comparison 4 Herbal medicine plus dietary and lifestyle modification versus dietary and lifestyle modification, Outcome 2 Fasting blood glucose levels (mmol/L).
4.3. Analysis.
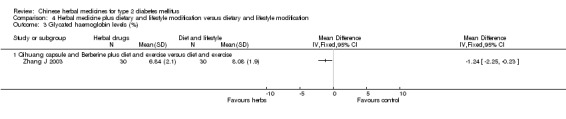
Comparison 4 Herbal medicine plus dietary and lifestyle modification versus dietary and lifestyle modification, Outcome 3 Glycated haemoglobin levels (%).
4.4. Analysis.
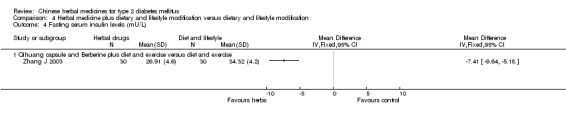
Comparison 4 Herbal medicine plus dietary and lifestyle modification versus dietary and lifestyle modification, Outcome 4 Fasting serum insulin levels (mU/L).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agrawal 1996.
| Methods | Crossover design and stratification based on age, fasting blood glucose, and body mass index. Generation of allocation sequence: not stated. Blinding: single blind, person doing measurements and analysis. Sample size estimation: no information. Withdrawal/drop‐out: no. | |
| Participants | Ethnic: Hindu; 40 patients (M/F 25/15; mean age 52.5 (41 ‐ 65 ) years; 20 in herb group; 20 in placebo group). Setting: community clinic. Inclusion criteria: non‐insulin‐dependent diabetes mellitus (NIDDM) diagnosed by WHO criteria of 1985. Exclusion criteria: patients took holy basil leaves; had blood urea, diarrhoea or dysentery, cancer, 2‐hour postprandial blood glucose > 350 mg/dl, did not like to eat the herb, and juvenile diabetes. | |
| Interventions | Experimental intervention:
Holy basil leaves (dried leaf powder in a sachet containing 2.5 g of fresh leaves), in 200 ml water, drink the mixture,daily, for two months. Control intervention: Placebo (spinach leaf powder in a sachet), in 200 ml water, drink daily, for two months. Before starting the interventions, patients were advised not to take any diabetic treatment at least seven days, then followed a run‐in period of 5‐day to wash out the remaining effect of hypoglycaemic drugs. |
|
| Outcomes | FBG, PBG, total cholesterol, body weight, and adverse effect. Only data from the first period of trial (before crossover) were used. |
|
| Notes | Data from body weight were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Agrawal 2002.
| Methods | Generation of allocation sequence: not stated. Blinding: double blind, decoded, identical active herbal product and placebo. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Hindu; 60 patients (30 in treatment group, M/F 22/8; mean age 45 years; 30 in placebo group, M/F 24/6, mean age 47 years). Setting: unstated. Inclusion criteria: newly diagnosed NIDDM patients (diagnostic criteria unstated). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Inolter (containing five herbs) capsule, no details for the usage, for three months. Control intervention: Placebo (identical to Inolter), no details usage, for three months. All patients were managed with diet and exercise regimen during the study period. |
|
| Outcomes | FBG, HbA1c, serum cholesterol, and adverse effect. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cao FK 1997.
| Methods | Generation of allocation sequence: not stated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 62 patients (27 in treatment group, M/F 12/15, mean age 56 years, mean disease duration 3.6 years; 35 in control group, M/F 15/20, mean age 55 years, mean disease duration 3.6 years). Setting: outpatients. Inclusion criteria: type 2 diabetes diagnosed by WHO criteria (1980), without severe heart, liver, kidney and brain diseases, and acute complications of diabetes. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Shengqing Jiangtang recipe (a basic herbal compound of 9 herbs) decoction, 400 ml/day, divided into two doses orally, for two months. Control intervention: glibenclamide orally, 5‐8 mg/day, b.i.d., for two months. Before the interventions, dietary and exercise therapies were applied to all patients for one week. |
|
| Outcomes | Symptoms, FBG, and adverse event. No follow‐up after the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chang ZQ 1998.
| Methods | Generation of allocation sequence: not stated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 126 patients (70 in treatment group, M/F 43/27, mean age 48.6 years, mean disease duration 1.5 years; including 10 cases of type 1 diabetics and 60 cases of type 2 diabetics; 56 in control group, M/F 32/24, mean age 46.7 years, mean disease duration 11 months; including 8 type 1 diabetics and 48 type 2 diabetics). Setting: inpatients. Inclusion criteria: diabetes diagnosed by WHO criteria (1980). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Herbal mixture (9‐12 herbs, prescribed based on TCM diagnosis) decoction, one dosage/day, divided into two times orally, for two months.
For type 2 diabetics, plus glibenclamide, 2.5‐5 mg, b.i.d., and metformin, 250 mg, t.i.d.; both for two months. Control intervention: glibenclamide, 2.5‐5 mg orally, b.i.d.; plus metformin, 250 mg, t.i.d., for two months. Co interventions in both groups were vitamin C, B1, B6, and dietary therapy. |
|
| Outcomes | FBG, HbA1c, and serum insulin. Data from fasting blood glucose and HbA1c could not be used due to inadequate report of mixed type 1 and type 2 diabetics in both groups. No follow‐up was reported after the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chen H 2003.
| Methods | Generation of allocation sequence: not stated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 74 patients (M/F 40/34; mean age 54.1 (40 ‐ 71) years; 37 in herb group; 37 in control group). Setting: hospital based. Inclusion criteria: non‐insulin‐dependent diabetes mellitus diagnosed by WHO criteria. Exclusion criteria: not stated. | |
| Interventions | Experimental intervention:
Xuange Yin (mixture of eight herbs) granule, one dosage/day, divided into two times orally, for two months.
Plus glipizide, 2.5‐5 mg, orally, t.i.d, and metformin, 0.25‐5 g, t.i.d; both for two months. Control intervention: glipizide, 2.5‐5 mg orally, t.i.d; plus metformin, 0.25‐5 g, t.i.d, for two months. |
|
| Outcomes | FBG and PBG. Outcome was measured at the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chen SH 1997.
| Methods | Generation of allocation sequence: not stated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: 5 patients lost at 60 days, 7 at 180 days in treatment group; 9 patients lost at 60 days and 180 days. The reason of loss was not stated. | |
| Participants | Ethnic: Chinese; 188 patients (96 in treatment group, M/F 55/41, mean age 51.2 years (35‐74), disease duration from 0.3‐15 years; 92 in control group, M/F 53/39, mean age 50.7 years (33‐77), disease duration from 0.1‐17 years). Setting: inpatients and outpatients. Inclusion criteria: type 2 diabetes diagnosed by WHO criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Jiangtang Kang (powder of herb Chrysanthemum), one bag (8g) orally, t.i.d, for two months. Control intervention: For 31 naive patients, glibenclamide, 2.5 mg orally, t.i.d., for two months; for previously treated patients, continued their original treatment without any changes, for two months. After two months' treatment, 85 patients from each group were randomly selected to continue their treatment for further four months. |
|
| Outcomes | FBG, HbA1c, and serum insulin. No follow‐up was reported after the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chen XH 1993.
| Methods | Generation of allocation sequence: not stated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 167 patients (114 in Trichosanthis group, M/F 31/83, age from 40‐78 years; 25 in Yuquan Wan group, M/F 12/13, age from 32‐69 years; 28 in D860 group, M/F 10/18, age from 20‐73 years). Setting: inpatients and outpatients. Inclusion criteria: type 2 diabetes diagnosed by WHO criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Composite radix trichosanthis (no details on the composition) tablet, 6 tablets (0.3 g/tab) orally, four times a day, for two months. Control intervention: ‐ Yuquan Wan (herbal pill), 9 g, four times a day orally, for two months; ‐ D860 (tolbutamide): 1 g, t.i.d., orally, for two months. |
|
| Outcomes | Symptoms, FBG, PBG, and adverse effects. No follow‐up was reported. |
|
| Notes | The skew randomisation with 1:2 of control to treatment was not explained for reasons. The control was then subdivided into Yuquan Wan group and D860 group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chen XL 2001.
| Methods | Generation of allocation sequence: random table. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 182 patients (100 in herb group, M/F 48/52, age from 22‐67 years; 82 in control group, M/F 40/42, age from 24‐68 years). Setting: unstated. Inclusion criteria: type 2 diabetes diagnosed according to textbook criteria, and all patients belonged to 'deficiency of Qi‐Yin and blood stasis' according to traditional Chinese medicine diagnosis. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Xiaoke Yin (mixture of 15 herbs), one dosage daily, decocted orally, b.i.d, for 90 days. Control intervention: glibenclamide, 2.5 mg orally, t.i.d., for 90 days; for those patients with unsatisfactory efficacy after 30 days, Insoral was added, 25 mg orally, t.i.d., for 60 days. |
|
| Outcomes | Symptoms and FBG. No follow‐up was performed. |
|
| Notes | The outcome of symptoms was not adequately reported and raw data could not be extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chen YB 1995.
| Methods | Generation of allocation sequence: not stated. Blinding: single blind, but not mentioned who was blinded. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 68 patients (M/F 32/36, mean age 54 years (39‐68), mean disease duration 6 years (1‐25 years). 34 in Bushen Huoxue group and 34 in placebo group). Setting: outpatients. Inclusion criteria: type 2 diabetes diagnosed by WHO criteria (WHO 1985), TCM diagnosis belonged to syndrome of 'kidney deficiency and blood stasis'; mean levels of fasting blood glucose > or = 6.05 mmol/L by two measurements prior the treatment; without ketoacidosis, recent infection, tuberculosis, and with normal functions of heart, liver, kidneys. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Bushen Huoxue No. I (composed of more than 8 herbs) tablet, 10 tablets (1 g/tab) orally, t.i.d., for two months. Control intervention: placebo (starch, named as Bushen Huoxue No. II), 3 tablets (0.75 g/tab), t.i.d., orally, for two months. All patients had a run‐in period of 4 weeks prior to the interventions, but kept their original treatments (12 continued dietary therapy, 56 continued oral hypoglycaemic drugs during the trial. |
|
| Outcomes | Symptoms, FBG, and adverse effects. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Deng QW 2003.
| Methods | Generation of allocation sequence: not stated. Blinding: not mentioned. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 160 patients (80 in treatment group, and 80 in control group, data of gender, age, and disease duration not reported). Setting: inpatients. Inclusion criteria: first time diagnosed type 2 diabetes (diagnostic criteria undefined). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Xiaoyao San (mixture of 12 herbs) decoction, one dosage daily; for six months. Control intervention: Glibenclamide, initiated from 2.5 mg orally, no details on regimen; for six months. |
|
| Outcomes | FBG, PBG, HbA1c, and adverse effects. Outcomes were measured at end of the treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Feng YM 1998.
| Methods | Generation of allocation sequence: alternative allocation according to the order of seeking medication. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 250 patients (150 in treatment group, M/F 95/55, mean age 43 years (32‐65), mean disease duration 15.6 years (1‐25 ). 100 in control group, M/F 58/42, mean age 45 years (35‐63), mean disease duration 16.8 years (1.3‐23)). Setting: unstated. Inclusion criteria: type 2 diabetes diagnosed by WHO criteria (WHO 1982), and diagnosed as syndrome of deficiency of both Qi and Yin with blood stasis by TCM diagnosis. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Xiaoke Ling (mixture of 12 herbs) capsule, 4‐6 capsules orally, t.i.d., for two months. Control intervention: gliclazide, 1‐2 tablet (80 mg/tab) orally, b.i.d., for two months. |
|
| Outcomes | Symptoms, and FBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Guo GY 1998.
| Methods | Stratified according to previous hypoglycaemic treatment (yes or no) before randomisation. Generation of allocation sequence: not stated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: 1 patient withdrawn due to allergic to the herbal intervention. | |
| Participants | Ethnic: Chinese; 130 patients (M/F 57/73, mean age 54.5 years (38‐72), mean disease duration 15.5 months (1 month to 12 years). For those who had previous hypoglycaemic treatment, divided into two groups: 40 in treatment group I, 30 in control group I; for those who were newly diagnosed, divided into two groups: 30 in treatment group II and 30 in control group II). Setting: unstated. Inclusion criteria: type 2 diabetes mellitus, diagnostic criteria not stated. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
‐ Group I: Tianyuan Jiangtang Wan ( mixture of 13 herbs) pill, 9 g orally, t.i.d., for two months; at the same time, continued previous oral hypoglycaemic drugs.
‐ Group II: Tianyuan Jiangtang Wan monotherapy, the same regimen as group I. Control intervention: ‐ Control group I: continued previous hypoglycaemic drugs, for two months. ‐ Control group II: gliclazide, 80‐160 mg/day, orally, for two months. |
|
| Outcomes | FBG, HbA1c, and adverse effects. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Guo HF 1996.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 78 patients (M/F 46/32, mean age 55 years (35‐81), mean disease duration 3.9 years (0.1‐37 years). 40 in treatment group and 38 in control group). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Xingyu Huashi recipe (mixture of 8 herbs) decoction, one dosage/day, divided into two times, orally, for two months; hypoglycaemic drugs: gliclazide plus captopril and alginric sodium diester, gliclazide 80‐240 mg/day, captopril 12.5‐75 mg/day, alginric sodium diester 150‐300 mg/day. Control intervention: gliclazide, 80‐240 mg/day orally, captopril 12.5‐75 mg/day, and alginric sodium diester 150‐300 mg/day orally, for two months. |
|
| Outcomes | FBG and PBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hou LY 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 96 patients (58 in herb group, M/F 39/19, mean age 49 years (44‐70), mean disease duration 7.8 years (2‐11). 38 in control group, M/F 25/13, mean age 52 years (47‐70), mean disease duration 8 years (2‐12)). Setting: inpatients. Inclusion criteria: type 2 diabetes diagnosed by WTO criteria (1998). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Xiaoke Fuzheng (mixture of more than 20 herbs) capsule, 4‐6 capsules, t.i.d., orally; plus glipizide or glibenclamide, for nine months. Control intervention: gliclazide, or glibenclamide, for nine months. |
|
| Outcomes | Symptoms and FBG. Outcomes were measured at end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hu YP 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 157 patients (82 in herb group, M/F 45/37, mean age 50.7 years (41‐70), mean disease duration 4.5 years (0.5‐9 years). 75 in control group, M/F 39/73, mean age 51.4 years (42‐73), mean disease duration 4.2 years (0.3‐10 years)). Setting: outpatients. Inclusion criteria: type 2 diabetes diagnosed by DAD criteria (DAD 1997). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Sihuang capsule (mixture of more than 17 herbs), 2 capsules, orally, t.i.d, for four months; plus glibenclamide, 2.5 mg orally, t.i.d. Control intervention: glibenclamide, 2.5 mg orally, t.i.d, for four months. |
|
| Outcomes | FBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hua SG 1997.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 300 patients (200 in treatment group, M/F 91/109, mean age 54 years (30‐79), mean disease duration 4.2 years (0.25‐11 years). 100 in control group, M/F 35/65, mean age 50 years (27‐72), mean disease duration 3.9 years (0.1‐12 years)). Setting: unstated. Inclusion criteria: type 2 diabetes mellitus diagnosed by WHO criteria, with basic normal function of heart, brain, kidney, and without severe complications and previous use of insulin. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Yiqi Yangyin Huayu Tang (mixture of 10 herbs) decoction, one dosage daily, decocted as 300 ml, two times orally, for three months. Control intervention: glibenclamide, 2.5‐5 mg orally, 2‐3 times daily, for three months. All patients were on diet control. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | A skew random allocation with 1:2 of control to treatment was not explained for reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Huang CL 2003.
| Methods | Generation of allocation sequence: random number table. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 128 patients (68 in treatment group, M/F 35/33, mean age 59 years (38‐70), mean disease duration 5.6 years (0.5‐15 years). 60 in control group, M/F 31/29, mean age 59 years (36‐71), mean disease duration 5 years (0.6‐14 years)). Setting: inpatients and outpatients. Inclusion criteria: type 2 diabetes mellitus diagnosed by WHO criteria (1999), without heart, liver or kidney disease. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Astragalus injection (herb extract), 20 ml in 250 ml of 0.9% saline, intravenously, daily for 30 days; then changed to Astragalus decoction, 40 g daily, for three months. During the treatment, patients took gliclazide and metformin. Control intervention: Gliclazide, 80 mg orally, b.i.d., plus metformin 0.5 g, t.i.d., orally; for four months. All patients were on dietary control during treatment. |
|
| Outcomes | Symptoms, FBG, PBG, FINS, INS, HbA1c, blood lipid, haemorrheology, blood pressure, and ISI. Outcomes were measured at end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lan QF 2000.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 140 patients (95 in herb group, M/F 70/25, mean age 49 years (30‐72); among them, 65 cases complicated with hyperlipidaemia, 26 with hypertension, 15 with coronary heart disease, 19 with peripheral neuropathy, 3 with diabetic feet. 45 in control group, age, gender, disease duration, and complications similar to herb group (no data reported)). Setting: unstated. Inclusion criteria: type 2 diabetes mellitus diagnosed by WHO criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Ketang Ling (mixture of eight herbs) decoction, 1 dosage daily, divided into three times orally, for two months. Control intervention: gliclazide, 80 mg orally, two times daily, for two months. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | A skew random allocation with 1:2 of control to treatment was not explained for reasons. The outcome of symptoms was not adequately reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Li HW 2002.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 75 patients (38 in herb group, M/F 23/15, mean age 54 years; mean disease duration 4.2 years; 37 in control group, M/F 20/17, mean age 51 years, mean disease duration 5 years). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus diagnosed by WHO criteria. Exclusion criteria: Patients with severe complications. | |
| Interventions | Experimental intervention:
Shugan Jianpi Huoxue Tang (mixture of 11 herbs) decoction, one dosage daily, orally; plus gliclazide 80‐160 mg, b.i.d., orally, and metformin 250‐500 mg, b.i.d., orally; for two months. Control intervention: gliclazide, 80‐160 mg orally, b.i.d., plus metformin 250‐500 mg, b.i.d., orally, for two months. Co‐interventions included dietary control and exercise. |
|
| Outcomes | FBG, FINS, and ISI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Li RG 2001.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 87 patients (47 in herb group, M/F 22/25, mean age 48 years (30‐70); mean disease duration 7 years; 40 in control group, M/F 18/22, mean age 48 years (31‐69), mean disease duration 7 years). Setting: unstated. Inclusion criteria: type 2 diabetes mellitus diagnosed by WHO criteria (1980). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Bushen Jiangtang Tang (mixture of 16 herbs) decoction, one dosage daily, two times orally; for two months. Control intervention: glibenclamide, 5 mg orally, t.i.d., for two months. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | The outcome of symptoms was not adequately reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Li Y 2000.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 93 patients (57 in herb group, M/F 35/22, mean age 52 years (35‐70); mean disease duration 6.2 years; 36 in control group, M/F 21/15, mean age 50 years (38‐69), mean disease duration 5.8 years). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus diagnosed by WHO criteria (1985). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Shengqi Huafen Tang (mixture of 12 herbs) decoction, one dosage daily, two times orally; plus glibenclamide and Insoral (regimen see below); for two months. Control intervention: glibenclamide, 7.5‐50 mg daily, or plus Insoral 25‐50 mg daily orally, t.i.d., for two months. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | The outcome of symptoms was not adequately reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Li YM 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: eight from herb group and six from control group lost follow‐up. | |
| Participants | Ethnic: Chinese; 58 patients (29 in herb group and 29 in control group, no data on gender and age). Setting: inpatients. Inclusion criteria: type 2 diabetes mellitus diagnosis criteria not defined. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Tangzhi Xiao (mixture of more than 8 herbs) capsule, 10 capsules, t.i.d., orally; for three months. Control intervention: gliclazide, 80 mg orally, b.i.d.; for three months. |
|
| Outcomes | Symptoms, FBG, FINS, ISI, blood pressure, SGPT, BUN, creatinine, and adverse reaction. Outcomes were measured at end of treatment. |
|
| Notes | Eight and six patients were excluded from herb and control groups respectively due to poor compliance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mao L 2002.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 98 patients (54 in herb group, M/F 23/31, no data on age and disease duration; 44 in control group, M/F 21/23, no data on age and disease duration). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus diagnosed by WHO criteria (1985). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Tangniaobing No. 2 formula (extracts from 17 herbs) decoction, 400 ml daily, two times orally; plus glipizide, 5‐30 mg/day orally, one time daily; for 90 days. Control intervention: glipizide, 5‐30 mg daily, orally, one time/day, for 90 days. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | The outcome of symptoms was not adequately reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Miao WH 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 134 patients (88 in herb group, M/F 42/46, age from 42‐71 years, and disease duration from 3 months to 15 years; 46 in control group, M/F 21/25, age from 38‐68 years, and disease duration from 1‐18 years). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus diagnosed by WHO criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Shenqi Jiangtang Tang (mixture of 12 herbs) decoction, one dosage daily, two times orally; plus glibenclamide, 2.5 mg orally, t.i.d.; for 60 days. Control intervention: glibenclamide, 2.5 mg orally, t.i.d, for 60 days. |
|
| Outcomes | Symptoms, FBG, and urine glucose. Outcomes were measured at end of the treatment. |
|
| Notes | A skew distribution of patients in two group (2:1) without explanation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Namdul 2001.
| Methods | Generation of allocation sequence: unstated. Blinding: not blinded. Sample size estimation: no information. Withdrawal/drop‐out: reported number but no reason explained. An intention‐to‐treat analysis was performed. | |
| Participants | Ethnic: Hindu; 200 patients (M/F 136/64; age from 30‐65 years; 100 in treatment group; 100 in control group). Setting: clinics. Inclusion criteria: newly diagnosed or untreated type 2 diabetes mellitus (diagnostic criteria unstated). Exclusion criteria: hypertension, heart disease, kidney failure, pregnancy, a period of lactation < 6 months, history of a blackout episode, or any complaint of vision loss. | |
| Interventions | Experimental intervention:
Tibetan medicines (four kinds, two of them were choosed), no details for usage; plus dietary and lifestyle modification, for six months. Control intervention: dietary and lifestyle modification alone, same as above, for six months. |
|
| Outcomes | FBG, PBG, HbA1c, and body weight. The outcomes were measured at three and six months. |
|
| Notes | Sent request on 19‐11‐2002. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ni HX 2000.
| Methods | Generation of allocation sequence: drawing method. Blinding: not blinded. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 51 patients (31 in treatment group, M/F 22/9, mean age 56 years (49‐67), mean disease duration 10.2 years (2‐18 years). 20 in control group, M/F 14/6, mean age 55 years (50‐69), mean disease duration 11.3 years (1‐18 years)). Setting: unstated. Inclusion criteria: type 2 diabetes mellitus diagnosed by WHO 1985 criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Danzhi Xiaoyao San (mixture of 13 herbs) decoction, one dosage daily, orally; maintaining hypoglycaemic drug (no details), for three months. Control intervention: hypoglycaemic drug alone, no details for regimen, for three months. All patients were treated by dietary therapy. |
|
| Outcomes | Symptoms, FBG, PBG, HbA1c, and FINS. No follow‐up was reported. |
|
| Notes | The outcome of symptoms was not adequately reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Pan MZ 1997.
| Methods | Generation of allocation sequence: unstated. Blinding: single blind, but not specified. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 72 patients (M/F 28/44, mean age 58 years (39‐70), mean disease duration 7.8 years (1‐26 years). 36 in treatment group and 36 in control group). Setting: outpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980), with syndromes of Qi‐Yin deficiency, and kidney deficiency complicated with blood stasis diagnosed by TCM criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Xianzhen Pian (mixture of more than 12 herbs) tablet, 10 tablets (1 g/tab) orally, t.i.d., for two months. Control intervention: placebo (starch), 3 tablets (0.75 g/tab), t.i.d., orally, for two months. All patients had a run‐in period of four weeks prior to the interventions, but maintained their original treatments (including diet control and oral hypoglycaemic drugs) during the trial. |
|
| Outcomes | Symptoms, FBG, PBG, and adverse effects. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pang DR 2002.
| Methods | Generation of allocation sequence: unstated. Blinding: not stated. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 146 patients (82 in herb group, M/F 56/26, mean age 51 years, mean disease duration 4.3 years; 64 in control group, M/F 36/28, mean age 49 years, mean disease duration 4.1 years). Setting: not stated. Inclusion criteria: type 2 diabetes mellitus, diagnosed by national textbook criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Xiaoke Tang (mixture of 12 herbs) decoction, one dosage daily, divided to two times orally, for three months. Control intervention: glibenclamide, 2.5‐5 mg, t.i.d., orally, for three months. |
|
| Outcomes | Symptoms and FBG. Outcomes were reported after three months' treatment but no follow‐up was reported. |
|
| Notes | The outcome of symptoms was not adequately reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Peng SJ 1995.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 88 patients (48 in treatment group, M/F 18/30, mean age 51 years (40‐72), mean disease duration 6.5 years (0.5‐28 years); 40 in control group, M/F 17/23, mean age 52 years (42‐65), mean disease duration 6.2 years (0.5‐27 years)). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980), with syndromes of Qi‐Yin deficiency, Yin deficiency and heat excess, Yin‐Yang deficiency, diagnosed by TCM criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Yishen Jiangtang Fang (mixture of 11 herbs) decoction, one dosage orally, every other day, for two months; plus
glibenclamide, 2.5 mg, b.i.d., orally, for two months. Control intervention: glibenclamide, 2.5 mg, b.i.d., orally, for two months. All patients had dietary control and exercise during the above treatments. |
|
| Outcomes | Symptoms, FBG and serum insulin. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Qing ZQ 2001.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 90 patients (60 in herb group, M/F 27/33, mean age 58 years (38‐72), mean disease duration 11 years (0.5‐19 years); 30 in control group, M/F 12/18, mean age 57 years (39‐73), mean disease duration 10 years (0.5‐18 years)). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1985), with syndromes of Qi‐Yin deficiency diagnosed by TCM criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Shenqi Yuxiao Tang (mixture of 11 herbs) decoction, one dosage daily, divided into two times orally, for three months. Control intervention: Diamicron (gliclazide), 80 mg, b.i.d., orally, for three months. All patients had dietary control and diabetes knowledge education before the above treatments. |
|
| Outcomes | Symptoms, FBG, and adverse effects. | |
| Notes | The outcome of symptoms was not adequately reported. The skew randomisation with 2:1 of treatment to control was not explained for reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ren PA 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 150 patients (118 in herb group, M/F 52/66, age from 28‐64 years, disease duration 0.5‐21 years; 32 in control group, no data on gender, age, and disease duration). Setting: outpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (1999). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Maziren Wan (mixture of 9 herbs) decoction, one dosage daily, divided into two times orally, for three months. Control intervention: glibenclamide, 2.5 mg, t.i.d., orally; plus phenformin hydrochloride, 25 mg, t.i.d., both for three months. |
|
| Outcomes | Symptoms, FBG, and body weight. Outcomes were measured at end of treatment. |
|
| Notes | A skew distribution of patients with 1:4 of control to treatment was not explained for reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Russo 1990.
| Methods | Randomised crossover trial. Generation of allocation sequence: unstated. Blinding: double blind, but no details on how the blinding was performed. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Brazilian; 34 patients (18 in Myrcia trial, M/F 5/13, median age 56 years (39‐71), median disease duration 5 years (2‐9 years); 16 in Bauhinia trial, M/F 2/14, median age 58 years (40‐67), median disease duration 5 years (1‐11 years)). Setting: unspecified. Inclusion criteria: type 2 diabetes mellitus, diagnosed according to the NDDG criteria (1979). Exclusion criteria: unstated. | |
| Interventions | For Myrcia uniflora trial:
Experimental intervention:
Myrcia uniflora (dried leaves) as herbal tea, one tea bag in boiling water daily, for eight weeks; then crossed to placebo for another eight weeks. Control intervention: placebo, tea package, one bag in boiling water daily, for eight weeks; then crossed to herbal product group for another eight weeks. For Bauhinia forficata trial: Experimental intervention: Bauhinian forficata (dried leaves) tea package, one tea bag in boiling water, daily, for eight weeks; then crossed to placebo for another eight weeks. Control intervention: placebo, tea package, one bag in boiling water daily, for eight weeks; then crossed to herbal product for another eight weeks. All patients had dietary control or oral hypoglycaemic drugs during the above treatments. |
|
| Outcomes | FBG, serum insulin, and adverse effects. Only data from the first period of studies are used. |
|
| Notes | This study was made of two trials testing two different herbal products. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shao CP 2001.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 116 patients (80 in herb group, M/F 51/29, mean age 55 years (39‐68), mean disease duration 6.4 years (1‐10 years); 36 in control group, M/F 19/17, mean age 54 years (38‐67), mean disease duration 6.3 years (1‐10 years)). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1985), without severe complication of liver, kidney, heart or brain. Exclusion criteria: <7.8 mmol/L after diet control and exercise; age below 18 years; pregnancy or breast feeding; incompliant with diet control or herbal medicine; severe heart, liver, or kidney complication; acidosis episode or infection within last one month; patients withdrawal or loss of follow‐up. | |
| Interventions | Experimental intervention:
Potentilla discolor (mixture of 12 herbs) oral liquid, 150 ml orally, t.i.d.; plus glipizide, 2 tablets orally, t.i.d., for two months. Control intervention: glipizide, 2 tablets orally, t.i.d., for two months. |
|
| Outcomes | FBG and PBG. No follow‐up was reported. |
|
| Notes | A skew distribution of patients with 1:2 of control to treatment was not explained for reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shen T 1998.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 60 patients (30 in treatment group, M/F 8/22, mean age 59 years (51‐68), mean disease duration 7.3 years (0.2‐13 years). 30 in control group, M/F 8/22, mean age 60.5 years (53‐70), mean disease duration 8 years (1‐22 years)). Setting: outpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980), with syndromes of Qi‐Yin deficiency, and kidney deficiency complicated with blood stasis diagnosed by TCM criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Xianzhen Pian (mixture of more than 12 herbs) tablet, 10 tablets (1 g/tab) orally, t.i.d., for eight weeks. Control intervention: placebo (starch), 3 tablets (0.75 g/tab), t.i.d., orally, for eight weeks. All patients had a run‐in period of four weeks prior to the interventions, but maintained their original treatments (including diet control and oral hypoglycaemic drugs) during the trial. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sotaniemi 1995.
| Methods | Multicentre trial. Generation of allocation sequence: unstated. Blinding: double blind, but did not stated how the blinding was performed. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Finn;
36 patients (all naive, 12 in Ginseng 100 group, M/F 4/8, mean age 59 years; 12 in Ginseng 200 group, M/F 5/7, mean age 57 years; 12 in placebo group, M/F 7/5, mean age 60 years).
Setting: unspecified.
Inclusion criteria: type 2 diabetes mellitus, newly diagnosed (no criteria stated), recruited from five health centres.
Exclusion criteria: unstated. There were an 8‐week run‐in period before randomisation. |
|
| Interventions | Experimental intervention:
‐ ginseng group I: 100 mg daily, for eight weeks. ‐ ginseng group II: 200 mg daily, for eight weeks. Control intervention: placebo (0 mg ginseng), daily, for eight weeks. Patients were instructed for diet recommendation and exercise. |
|
| Outcomes | FBG, HbA1c, serum insulin, and adverse effects. No follow‐up was reported. |
|
| Notes | Data from Ginseng group I and II were combined for analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tao XY 2002.
| Methods | Generation of allocation sequence: unstated. Blinding: not stated. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 164 patients (M/F 92/72, age from 28‐80 years, disease duration from less than one year to more than 10 years. 103 in treatment group and 61 in control group). Setting: 66 outpatients and 98 inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (not specified). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Huatan Huoxue recipe (mixture of 13 herbs) decoction, one dosage daily, divided into two times orally, for two months; plus
glibenclamide, 7.5‐15 mg, orally, or plus metformin 750 mg daily; for two months. Control intervention: glibenclamide, 7.5‐15 mg, orally, or plus metformin 750 mg; for two months. All patients had dietary control during the treatments. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | A skew distribution of patients with about 1:2 of control to treatment was not explained for reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tong J 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not stated. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 300 patients (200 in treatment group, M/F 86/114, mean age 51 years (33‐71), mean disease duration 4.7 years (1‐10 years). 50 in control group I, M/F 18/32, mean age 49 years (32‐73), mean disease duration 5 years (1‐10 years); 50 in control group II, M/F 19/31, mean age 50 years (30‐71), mean disease duration 4.5 years (1‐9)). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (1999). Exclusion criteria: patients complicated with ketoacidosis, hepatitis, tuberculosis, or severe infection. | |
| Interventions | Experimental intervention:
Jiangtang I, II, and III (three formulations of herbal compound) capsule, No. I, 4‐6 capsules to be taken in the morning, No. II, 4‐6 capsules to be taken at noon, No. III, 4‐6 capsules to be taken in the evening, for three months; Control group I: glibenclamide, 7.5‐15 mg daily, orally; for three months. Control group II: Jinqi Jiangtang capsule (herbal compound), 8 capsules, t.i.d., for three months. All patients were on dietary control during the above treatment. |
|
| Outcomes | Symptoms, FBG, PBG, urine glucose, and blood lipids. Outcomes were measured at end of treatment. |
|
| Notes | A skew distribution of patients with 1:4 of control to treatment was not explained for reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vray 1995.
| Methods | Multicentre, parallel group, stratified randomised trial. Generation of allocation sequence: unstated. Blinding: double blind, double placebo (tablets and capsules) were identical in appearance with the verum forms. Sample size estimation: yes. Withdrawal/drop‐out: yes, numbers and reasons were reported. 32 patients were wrongly included due to not meeting the inclusion criteria. 11 patients (5%) were lost to follow‐up due to administrative reasons. | |
| Participants | Ethnic: Chinese; 216 patients (56 in group A, M/F 20/36, mean age 57 years, mean disease duration 3.9 years; 56 in group B, M/F 20/36, mean age 56 years, mean disease duration 2.4 years; 50 in group C, M/F 25/25, mean age 56 years, mean disease duration 2.5 years; 54 in group D, M/F 19/35, mean age 53 years, mean disease duration 2.2 years). Setting: outpatients from 5 centres. Inclusion criteria: type 2 diabetes mellitus, age between 40 and 70 years, newly diagnosed or poorly controlled. Exclusion criteria: diabetes well controlled by diet alone, oral anti‐diabetic drug or insulin treatment had been prescribed during one month run‐in period, patients with renal insufficiency, advanced hepatic disease, intolerance to sulphonylureas, acute alcoholic intoxication or ketosis, etc. | |
| Interventions | Experimental intervention:
‐ Group C: traditional Chinese treatment (TCT, composed of three plants) capsule, 7 capsules, t.i.d., plus a placebo of glibenclamide, 1 tablet, t.i.d., both for three months. ‐ Group D: TCT, 7 capsules, t.i.d., plus glibenclamide, 1 tablet (2.5 mg), t.i.d., both for three months . Control intervention: ‐ Group A: placebo of TCT and placebo of glibenclamide, the same administration as above, for three months. ‐ Group B: glibenclamide plus placebo of TCT, the same administration as above, for three months. When patients developed hypoglycaemic symptoms, or if blood glucose level < 2.78 mmol/L was observed, glibenclamide (verum or placebo) dosage was reduced by half until the end of the study. |
|
| Outcomes | FBG, HbA1c, plasma insulin, weight, and adverse effects. The outcomes were measured at the three and six months follow‐up. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang BS 1988.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 112 patients (M/F 60/52, age from 44‐65 years, disease duration 2‐10 years; among them, 20 complicated with coronary heart disease, 34 with hypertension, 6 with cerebral vascular disease, 90 with retinal disease, 6 with vascular disease of low limbs. 56 in treatment group and 56 in control group). Setting: outpatients. Inclusion criteria: type 2 diabetes mellitus, unclear diagnostic criteria, majority of patients with symptoms of diabetes mellitus. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
‐ Yiqi Yangyin Huoxue Huayu (mixture of 12 herbs) decoction, 1 dosage decocted, into 2 times orally, for 3 months;
D860 (tolbutamide), plus persantine, no details for regimen, for 3 months. Control intervention: D860 and persantine, no details for regimen, for 3 months. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang JS 2000.
| Methods | Generation of allocation sequence: coin tossing. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: 10 patients in control group were dropped out and the reason was not stated. | |
| Participants | Ethnic: Chinese;
80 patients (41 in treatment group, M/F 19/22, mean age 57 years (44‐70); mean disease duration 5.8 years (1‐20 years); 39 in control group, 10 dropped out, M/F 14/15; mean age 55 years (45‐69); mean disease duration 5.4 years (1‐19 years)).
Setting: outpatients.
Inclusion criteria: type 2 diabetes mellitus, diagnosed according to the criteria of WHO 1985, differentiated as with deficiency of both qi‐yin and blood stasis syndrome by Chinese medical diagnosis.
Exclusion criteria: unstated. There was a run‐in period of four weeks in which diet and exercise therapy plus metformin were applied to all patients, but stopping any herbal medicines. After that, those with abnormal blood glucose were to be randomised. |
|
| Interventions | Experimental intervention:
Yiqi Yangyin Huoxue recipe (mixture of 13 herbs), decoction, one dosage per day, divided into two times orally; for 2‐3 months. Control intervention: glurenorm, 30 mg orally, t.i.d, for 2‐3 months. Both groups maintained diet and exercise therapy plus metformin treatment during trial. |
|
| Outcomes | Symptoms, FBG, and PBG. No follow‐up was reported. |
|
| Notes | Symptoms were scored based on Chinese diagnostic syndrome, and inadequately reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Wang KF 1993.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 32 patients (mean age 54 years (46‐70), mean disease duration 3.5 years (0.5‐8 years). Among them, 7 complicated with coronary heart disease, 8 with retinal disease, 4 with peripheral neuropathy, 2 with diabetic nephropathy. 20 in treatment group, M/F 8/12; 12 in control group, M/F 4/8). Setting: unstated. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Shuizhi Sanhuang Tang (mixture of 9 herbs) decoction, one dosage daily, for three months. Control intervention: glibenclamide, no details for regimen, for three months. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang KP 1999.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 62 patients (32 in treatment group, M/F 20/12, mean age 57 years (42‐71), mean disease duration 6.6 years (3‐15 years). 30 in control group, M/F 18/12; mean age 56 years (45‐73), mean disease duration 6.2 years (3.5‐12 years)). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Jianpi Jiangtang Tang (mixture of 14 herbs) decoction, one dosage decocted, into two times orally, for two months. Control intervention: gliclazide, 80 mg, b.i.d., plus alginric sodium diester, 50 mg, t.i.d., for two months. |
|
| Outcomes | Symptoms, FBG, and PBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang YS 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 82 patients (47 in treatment group, M/F 26/21, age from 34‐60 years, disease duration from 0.5‐10 years. 35 in control group, M/F 19/16; age from 33‐60 years, no data on disease duration). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (1985) without severe kidney, retina, nerve or heart complications. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Xiaotang Ling (mixture of herbs containing glibenclamide) capsule, 5 capsules daily orally, for three months. Control intervention: glibenclamide, from 2.5 mg, t.i.d., for three months. |
|
| Outcomes | FBG, PBG, HbA1c, urine glucose, and blood lipid, FINS, ISI, and BMI. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wei JL 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 120 patients (68 in herb group, M/F 38/30, mean age 53 years (38‐69), mean disease duration 7 years (2‐22 years). 52 in control group, M/F 29/23; mean age 50 years (38‐69), mean disease duration 6.3 years (2‐20)). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (1980), without ketosis, acidosis, infection, or severe heart, liver and kidney disease; with TC> or = 6 mmol/L or TG > or = 2.3 mmol/L. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Jiangtang Tiaozhi Tang (mixture of 12 herbs) decoction, one dosage (300 ml), orally, for two months. Patients with previous drugs, continued the drugs. Control intervention: glibenclamide, and/or metformin for two months. |
|
| Outcomes | Symptoms, FBG, PBG, blood lipid, and adverse effects. Outcomes were measured at end of the treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wen S 2000.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 103 patients (52 in treatment group, M/F 32/20, mean age 49 years (36‐60), mean disease duration 7 months (1‐17 months). 51 in control group, M/F 30/21; mean age 49 years (36‐59), mean disease duration 7 months (1.5‐18 months)). Setting: unspecified. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria, and without acute and chronic diabetic complications. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Jiangtang Fang (mixture of 9 herbs) decoction, one dosage daily, orally; plus glibenclamide, 2.5 mg daily, orally, both for three months. Control intervention: glibenclamide, 2.5 mg daily, orally, for three months. |
|
| Outcomes | FBG, PBG, HbA1c, and FINS. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wu HM 1996.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 164 patients (104 in treatment group, M/F 60/44, mean age 46 years (5‐75), disease duration from 0.5‐26 years; 60 in control group, no details on gender, age, and disease duration). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980), and classified into two syndromes by a diagnostic criteria of Chinese Medicine: deficiency of Qi‐Yin, and deficiency of Spleen and Kidney. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Two herbal mixtures: either Yiqi Yangyin Qingre recipe or Jianpi Zhuyun Yishen Huayu (use based on differentiation of the syndrome) decoction, one dosage daily, divided into two times orally; plus D860 (tolbutamide), 500 mg orally, t.i.d., both for two months. Control intervention: D860, 500 mg orally, t.i.d., for two months. The dosage of D860 would be adjusted based on the levels of fasting blood glucose to avoid hypoglycaemic event. |
|
| Outcomes | FBG and PBG. No follow‐up was reported. |
|
| Notes | There was a skew distribution numbers of participants in 2 groups. The author did not give reason for that. Letter sent to request (Aug. 2002). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wu YN 2003.
| Methods | Generation of allocation sequence: random number table. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 90 patients (30 in treatment group I, M/F 13/17, mean age 65 years, mean disease duration 19 years; 30 in treatment group II, M/F 12/18, mean age 64 years, mean disease duration 17 years; 30 in control group, M/F 16/14, mean age 66 years, mean disease duration 19 years). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria, and all patients complicated with hyperlipidaemia and not well controlled by metformin for two months. Exclusion criteria: occurrence of diabetic ketoacidosis or coma or severe infection within three months, complicated with acute cardiac infarction, again or stroke; retinopathy or diabetic nephropathy. | |
| Interventions | Experimental intervention I:
Huoxue Jiangtang Pingzhi formula (mixture of 12 herbs) decoction, one dosage into two times, daily; for 60 days. Experimental intervention II: Liuwei Dihuang Tang (mixture of 6 herbs) decoction, one dosage daily, divided into two times orally; for 60 days. Control intervention: placebo (water and vitamin B2), 200 ml daily divided into two times orally; for 60 days. All patients received dietary control and metformin during trial. |
|
| Outcomes | Symptoms, FBG, PBG, FINS, and blood lipids. Outcomes were measured at the end of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Xie CG 1996.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 200 patients (M/F 115/85. 100 in treatment group, mean age 55 years, mean disease duration 7.4 years; 100 in control group, mean age 57 years, mean disease duration 6.4 years). Setting: inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980), and classified into three syndromes by a diagnostic criteria of Chinese Medicine: Yin deficiency and heat excess, deficiency of Qi‐Yin, and deficiency of Yin‐Yang. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Tangfu Kang (mixture of more than 7 herbs) condensed pills, 6 g for mild, 8 g for mediate, 10 g for severe patients, t.i.d., orally; for two months. Control intervention: gliclazide, 80 mg orally, b.i.d., for mild; 80 mg orally, t.i.d., for mediate, 160 mg, b.i.d., for severe patients; all for two months. All patients received same dietary therapy during trial. |
|
| Outcomes | Symptoms, FBG, PBG, HbA1c, FINS, and adverse effects. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Xu Q 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 104 patients (55 in treatment group, M/F 27/28, mean age 59 years (44‐75), mean disease duration 5.4 years (0.6‐12); 49 in control group, M/F 25/24, mean age 58 years (42‐76), mean disease duration 5.5 years (0.5‐13)). Setting: unstated. Inclusion criteria: type 2 diabetes mellitus, diagnostic criteria not defined. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Yishen Huoxue Tiaogan (mixture of 14 herbs) decoction, one dosage daily; for two months. Control intervention: gliclazide, 80 mg orally, b.i.d., for two months. All patients were on dietary control during trial. |
|
| Outcomes | Symptoms, FBG, PBG, blood lipids, and haemorrheology. Outcomes were measured at end of the treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Xu YS 1997.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 116 patients (60 in treatment group, M/F 29/31, mean age 57 years, disease duration from 1‐15 years; 56 in control group, no details on gender, age, and disease duration). Setting: outpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Zhonghui Chuanhuang Ye (extracts from more than 10 herbs) liquid, 10 ml orally, t.i.d., for two months. During this intervention, patients maintained original hypoglycaemic and dietary treatments. Control intervention: patients maintaining their original hypoglycaemic and dietary treatments, for two months. |
|
| Outcomes | FBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yang PL 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 134 patients (88 in treatment group, M/F 42/46, age from 35‐68 years, disease duration from 3 months to 15 years; 46 in control group, M/F 22/24, age from 33‐70 years; disease duration from 4 months to 17 years). Setting: inpatients and outpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1985). Exclusion criteria: women with pregnancy or breastfeeding, poor compliance with dietary control, patients complicated with severe heart, liver or kidney diseases, or psychological disorder, patients with ketosis, acidosis, or infection within one month, and dropouts or withdrawals. | |
| Interventions | Experimental intervention:
Jianpi Huatan Huoxue recipe (mixture of 12 herbs) decoction, one dosage/day, divided into three times taking; plus metformin, 0.5 g, b.i.d, orally; both for three months. Control intervention: metformin, 0.5 g, orally, b.i.d., for three months. All patients received dietary control and exercise during the trial. |
|
| Outcomes | Symptoms, FBG, PBG, and urine glucose. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yang TB 2002.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 216 patients (144 in herb group, M/F 84/60, mean age 48 years (37‐63), disease duration from 1‐13 years; 72 in control group, M/F 44/28, mean age 49 years (38‐65), disease duration from 1‐14 years). Setting: not stated. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Shenqi Jiangtang Yin (mixture of 12 herbs) decoction, one dosage daily, divided into two times orally, for three months. Control intervention: tolbutamide, 1 g orally, t.i.d., for three months. All patients were advised to dietary control during treatment. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | The outcome of symptoms was not reported adequately. The skew randomisation with 2:1 of treatment to control was not explained for reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yao LD 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 336 patients (M/F 180/156, age from 39‐67 years. 156 in integrated therapy group; 105 in metformin group; and 75 in herb group). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (1999). Exclusion criteria: type 1 diabetes, drug‐induced, pregnant diabetes. | |
| Interventions | Experimental intervention:
Potentilla chinensis, 50 g daily , drinking as tea; plus Berberine (alkaloid from herb), 0.3 g, t.i.d., orally; for three months. Control intervention: metformin, 0.25‐0.5 g orally, t.i.d., plus glipizide, 5‐10 mg, t.i.d., for three months. Combined therapy group: Potentilla chinensis, plus metformin and glipizide, the same regimen as above. |
|
| Outcomes | FBG, PBG, and insulin release test. Outcomes were measured at 6 months of follow‐up. |
|
| Notes | There were skew distribution of numbers of patients among the three groups. The author did not give explanation for that. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
You BW 1999.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 108 patients (72 in treatment group, M/F 42/30, mean age 50 years (38‐64); 36 in control group, M/F 22/14, mean age 49 years (36‐63)). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria, without complications of heart, liver, and kidney. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Shugan Huoxue recipe (mixture of 12 herbs) decoction, one dosage daily, divided into two times orally, for three months. Control intervention: gliclazide, 80 mg orally, b.i.d., for three months. |
|
| Outcomes | FBG and PBG. No follow‐up was reported. |
|
| Notes | There was a skew distribution of numbers of participants in 2 groups. The author did not give reason for that. Letter sent to request (Aug. 2002). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zeng Y 2001.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 78 patients (48 in treatment group, M/F 28/20, mean age 49 years (38‐61), mean disease duration 3.2 years; 30 in control group, M/F 17/13, mean age 50 years (39‐63), mean disease duration 3.2 years). Setting: not stated. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (1985), without severe complications of heart, liver, and kidney. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Qingre Huatan Huoxue Huayu recipe (mixture of 9 herbs) decoction, one dosage daily, divided into two times orally; plus
glibenclamide, 5‐15 mg daily, b.i.d., or t.i.d., orally; for three months. Control intervention: glibenclamide, 5‐15 mg daily, b.i.d. or t.i.d., orally, for three months. |
|
| Outcomes | FBG and PBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhang FB 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 50 patients (34 in treatment group, mean age 62.6 years (37‐71), disease duration 6 months to 25 years; 16 in control group, mean age 63.2 years (37‐71), disease duration 6 months to 24 years). Gender was not reported. Setting: outpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by ADA criteria (1997). Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Yiqi Yangyin Bushen recipe (mixture of 10 herbs) decoction, one dosage daily, divided into two times orally;
glibenclazide, 10 mg daily, plus metformin, 0.5 mg t.i.d., orally; for 60 days. Control intervention: glibenclazide, 10 mg daily, plus metformin 0.5 mg, t.i.d., orally, for 60 days. |
|
| Outcomes | FBG, PBG, and HbA1c. No follow‐up was reported. |
|
| Notes | There was a skew distribution of numbers of patients in 2 groups. The author did not explain for this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhang HZ 1999.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 168 patients (86 in treatment group, M/F 35/51, mean age 48 years (30‐70), mean disease duration 7 years (1‐17 years); 80 in control group, M/F 31/49, mean age 46 years (28‐70), mean disease duration 7 years (1‐15 years)). Setting: unspecified. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1980), differentiated as Qi‐Yin deficiency by diagnostic criteria of Chinese medicine; among them 62 cases complicated with hyperlipaemia, 44 with hypertension, 17 with previous cerebral embolism, 38 with coronary heart disease, and 22 with peripheral neuropathy. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention: Xiaoke Wan (mixture of 7 herbs plus glibenclamide) pill, 5‐10 pills (containing 1.25‐2.5 mg glibenclamide) orally, 2‐3 times daily, for two months. Control intervention: glibenclamide, 1.25‐2.5 mg orally, 2‐3 times daily, for two months. | |
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhang J 2003.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 60 patients (30 in treatment group, M/F 12/18, mean age 50 years (34‐70), mean disease duration 10 months (0.5‐24); 30 in control group, M/F 14/16, mean age 50 years (33‐69), mean disease duration 11 months (0.6‐26)). Setting: outpatients and inpatients. Inclusion criteria: first‐time diagnosed type 2 diabetes mellitus, by WHO criteria (1999), without acute or severe complication. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Qihuang capsule (mixture of 8 herbs), 5 capsules, t.i.d.; plus strict dietary control and moderate exercise; for three months. Control intervention: strict dietary control and moderate exercise, daily, for three months. |
|
| Outcomes | Symptoms, FBG, HbA1c, blood lipids, and FINS. Outcomes were measured at end of the treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhang M 2001a.
| Methods | Generation of allocation sequence: random table method. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 128 patients (66 in treatment group, M/F 35/31, mean age 51 years, mean disease duration 7 years; 62 in control group, M/F 33/29, mean age 52 years, mean disease duration 6 years). Setting: unspecified. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1985); among them 64 cases complicated with hyperlipaemia, 59 with hypertension, 43 with previous cerebral embolism, 32 with coronary heart disease, and 96 with peripheral neuropathy. Exclusion criteria: severe diabetic renal disease, acidosis within three months, recent myocardial infarction or cerebral event. | |
| Interventions | Experimental intervention:
Qidan Tongmai (mixture of 10 herbs) tablet, 4 tablets, t.i.d., orally, maintained original hypoglycemic drugs; for two months. Control intervention: maintained original hypoglycaemic drug treatment, placebo (starch), 4 tablets orally, t.i.d., for two months. |
|
| Outcomes | FBG, PBG, HbA1c, and adverse effects. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhao XY 2001.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 99 patients (50 in treatment group, M/F 29/21, mean age 47 years (37‐60), mean disease duration 7.8 month; 49 in control group, M/F 22/27, mean age 46 years (35‐59), mean disease duration 7.6 months). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria. Exclusion criteria: not stated. | |
| Interventions | Experimental intervention:
Tangning Pian (mixture of more than 5 herbs) tablet, 0.3 g/tab, 5 tablets, b.i.d., orally; for three months. Control intervention: glibenclamide, 2.5 mg, daily, orally, for three months. All patients kept on dietary control and exercise. |
|
| Outcomes | FBG, PBG, and HbA1c. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zheng X 2001.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 68 patients (40 in treatment group, M/F 18/22, mean age 59 years (34‐72); 28 in control group, M/F 12/16, mean age 58 years (36‐70)). Setting: not stated. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1997). Exclusion criteria: not stated. | |
| Interventions | Experimental intervention:
Jinli Da condensed pill (mixture of more than 6 herbs), 9 g/bag, 1‐3 bags depending on levels of FBG, orally, daily; plus glibenclamide 2.5‐7.5 mg daily depending on levels of FBG; both for six months. Control intervention: glibenclamide, 2.5‐7.5 mg, daily, orally, depending on levels of FBG, for six months. All patients kept dietary control. |
|
| Outcomes | FBG and PBG. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhou C 2001.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 300 patients (200 in treatment group, M/F 87/113, mean age 49 years (32‐68), mean disease duration 4.7 years; 50 in control group I, M/F 18/32, mean age 47 years (31‐70), mean disease duration 5.1 years; 50 in control group II, M/F 19/31, mean age 48 years (29‐68), mean disease duration 4.5 years). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1999), without recently developed severe complications. Exclusion criteria: not stated. | |
| Interventions | Experimental intervention:
Jiangtang No. I, II, III (3 different herbal mixtures) capsule, 4‐6 capsules daily, No. I, II, III orally before breakfast, at lunch and dinner respectively; for 2‐4 months. Control interventions: ‐ Control group I: glibenclamide, 7.5‐15 mg daily, orally, t.i.d., for 2‐4 months. ‐ Control group II: Jinqi Jiangtang capsule (herbal medicine), 7‐10 capsules, t.i.d., orally, for 2‐4 months. All patients from three groups received education on diabetes, dietary control and suitable exercise. |
|
| Outcomes | Symptoms and FBG. No follow‐up was reported. |
|
| Notes | No explanation for the skew distribution of patients between groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhou JH 2001a.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 147 patients (98 in treatment group, M/F 57/41, mean age 54 years, mean disease duration 6 years; 49 in control group, M/F 29/20, mean age 53 years, mean disease duration 7 years). Setting: not stated. Inclusion criteria: type 2 diabetes mellitus, diagnosed by ADA criteria (ADA 1997), without recently developed severe complications. Exclusion criteria: not stated. | |
| Interventions | Experimental intervention: Qimai Dahuang Tang (mixture of 13 herbs) decoction, one dosage daily, divided in two times orally; plus gliclazide, 40 mg daily; for two months. Control intervention: gliclazide, 40‐160 mg, daily, orally, for two months. | |
| Outcomes | FBG and PBG. No follow‐up was reported. |
|
| Notes | The trial report stated using 2:1 to allocate patients, but no details on methods. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhou P 1997.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 98 patients (33 in combined treatment group, M/F 16/17, mean age 49 years (36‐59), mean disease duration 9 months (1‐17.5 months); 33 in treatment group, M/F 18/15, mean age 50 years (35‐59), mean disease duration 10 months (0.5‐16.5 months); 32 in control group, M/F 19/13, mean age 48 years (33‐60), mean disease duration 11 months (0.5‐18 months)). Setting: outpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria, and without any acute or chronic diabetic complications within three months prior to the study. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
‐ combined treatment: Kelening (mixture of 5 herbs) capsule, 12‐18 capsules daily, divided into three times orally; glibenclamide, dosage varied based on blood glucose levels,1‐2 times daily; both for three months. ‐ Herbal treatment alone: Kelening, 12‐18 capsules, divided into three times orally, for three months. Control intervention: glibenclamide, dosage varied based on blood glucose levels, 1‐2 times daily, orally, for three months. |
|
| Outcomes | Symptoms, FBG, PBG, HbA1c, FINS, and adverse effects. No follow‐up was reported. |
|
| Notes | One trial reported in two publications. Only data from primary one was extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhou XT 2001.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 278 patients (146 in treatment group, M/F 69/77, mean age 49.5 years (31‐70), mean disease duration 3.2 years; 132 in control group I, M/F 64/68, mean age 48.3 years (30‐68), mean disease duration 2.9 years). Setting: outpatients and inpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1995). Exclusion criteria: not stated. | |
| Interventions | Experimental intervention:
Xiaoyao San (mixture of 12 herbs) decoction, one dosage daily, divided in two times orally; plus glibenclamide, 2.5‐5 mg orally, t.i.d.; for three months. Control intervention: glibenclamide, 2.5‐5 mg orally, t.i.d., for three months. All patients received dietary control and moderate exercise. |
|
| Outcomes | Symptoms, FBG, and insulin. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhu ZZ 1997.
| Methods | Generation of allocation sequence: unstated. Blinding: not used. Sample size estimation: no information. Withdrawal/drop‐out: unstated. | |
| Participants | Ethnic: Chinese; 95 patients (53 in herbal treatment group, M/F 27/26, mean age 52 years (40‐70), mean disease duration 2.8 years (0.1‐5 years); 42 in control group, M/F 21/21, mean age 50 years (40‐70), mean disease duration 3 years (0.2‐5 years)). Setting: inpatients and outpatients. Inclusion criteria: type 2 diabetes mellitus, diagnosed by WHO criteria (WHO 1985), classified as deficiency of qi‐yin and deficiency of yin plus excess of heat. Exclusion criteria: unstated. | |
| Interventions | Experimental intervention:
Sanhuang Jiangtang recipe (mixture of 9 herbs) decoction, one dosage daily, divided into two times orally; plus tablet of the same herbal medicine, 8 tablets orally, t.i.d., for 4‐6 months. 12 out of 53 patients in this group maintained their hypoglycaemic drugs. Control intervention: glipizide, 5‐10 mg daily, 2‐3 times orally, adjusted based on blood glucose levels, the maximal dosage reached 30 mg daily; for 4‐6 months. Patients of the two groups were instructed for diet control and exercise. |
|
| Outcomes | Symptoms, FBG, and FINS. No follow‐up was reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
FBG: fasting blood glucose; PBG: postprandial blood glucose; FINS: fasting insulin; HbA1c: glycated haemoglobin levels; ISI: insulin sensitivity index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bhardwaj 1994 | Non‐randomised study testing a herbal powder in 30 patients with non‐insulin‐dependent diabetes mellitus. |
| Bhardwaj 1994b | Non‐randomised study testing a herbal powder in 30 patients with non‐insulin‐dependent diabetes mellitus. |
| Bian F 2000 | Randomised controlled trial testing integrated traditional Chinese and western medicine in patients with incipient diabetic nephropathy. The objective of the trial focused on treatment of the complication of diabetes (diabetic nephropathy). |
| Bian LE 2000 | Randomised controlled trial testing herbal medicine (Zhixiao Tongmai capsule) versus western drugs in patients with type 2 diabetes mellitus complicated with cerebral embolism. The trial objective is to assess efficacy of the herb on treatment of the complication of diabetes (diabetic cerebral embolism). |
| Blostein‐Fujii 1999 | Randomised trial comparing dietary components (flavonoids) versus placebo in 40 type 2 diabetic women. |
| Bu XC 2001 | Non‐randomised study comparing a herbal capsule (Zicui Tongmai) versus diamicron in 94 patients with diabetic hyperviscosity complicated with mild cerebral ischemia. |
| Cai JW 2001 | Randomised controlled trial comparing a self‐prescribed herbal mixture (Ziyin Huoxue Huayu therapy) versus a herbal patent medicine (Xiaoke Wan) in patients with type 2 diabetes mellitus with 'Yin deficiency and Blood stasis'. The control intervention did not fall into the category of the inclusion criteria. |
| Chen SL 2000 | Non‐randomised study comparing a herbal formula plus glipizide versus glipizide alone in 102 patients with type 2 diabetes mellitus without any complication. |
| Chen XY 2001 | Randomised controlled trial comparing a self‐prescribed herbal mixture containing worms versus a patent herbal medicine (Xiaoke Wan) in 375 patients with type 2 diabetes mellitus. The control intervention did not fall into the category of the inclusion criteria. |
| Cui YL 2003 | Randomised controlled trial comparing herbal mixture "Huatan Huoxue" formula versus glibenclamide and/or metformin in 103 patients with type 2 diabetes. The outcome was reported as global improvement including symptoms and laboratory tests. We could not extract data for each individual outcome measures due to inadequate reporting. |
| Deng C 2000 | Non‐randomised study comparing a herbal therapy versus glibenclamide in 114 patients with type 2 diabetes mellitus. |
| Dilawari 1987 | Randomised clinical trial comparing different dietary therapies in patients with diabetes mellitus. |
| Ding YL 1999 | Randomised clinical trial comparing herbal mixture (Huaqishen Tang) versus glibenclamide plus dietary therapy in 66 type 2 diabetic patients. The treatment duration lasted only one month (< 2 months). |
| Du X 2000 | Randomised controlled trial comparing Bushen Jiangtang capsule (herbal medicine) versus glibenclamide in 78 cases of type 2 diabetes mellitus. The duration of the interventions in both arms was variable depending on duration of disease from three weeks to three months. The outcomes were not reported separately. |
| Feng WH 2000 | Non‐randomised controlled study comparing combination of Chinese herbal medicine and western medicine versus western medicine alone in 40 patients with type 2 diabetes mellitus. |
| Frati 1991 | Non‐randomised study testing dietary therapy (nopal, Opuntia streptacantha) in both healthy subjects and type 2 diabetic patients. |
| Frati 1991b | Non‐randomised study testing dietary therapy (Opuntia streptacantha) in both healthy subjects and type 2 diabetic patients. |
| Frati 1992 | A crossover, single blinded study comparing nopal (dietary components) versus placebo in healthy subjects and diabetic patients. |
| Fuessl 1987 | Double‐blind, cross‐over trial comparing non‐absorbable carbohydrates (guar) versus placebo in patients with non‐insulin‐dependent diabetes mellitus. |
| Fujita 2001 | A double‐blind, placebo‐controlled randomised trial testing a food extract supplement. |
| Gao Y 1998 | Randomised controlled trial testing Tang Shen Ning (herbal mixture) in patients with diabetic nephropathy. The objective of the trial focused on treatment of the complication of diabetes (diabetic nephropathy). |
| Gao YM 2002 | Randomised controlled trial testing herbal medicine (Jiawei Meiqi Tang) versus gliclazide and metformin in 25 patients with type 2 diabetes mellitus with high blood pancreatic glucagon. The main objective of the trial is to assess efficacy of the herb on levels of blood glucagon in patients with type 2 diabetes. |
| Gui Y 2002 | Non‐randomised study comparing a herbal formula (self‐prescribed Xiaoke Ning) plus diamicron versus diamicron alone in 56 patients with type 2 diabetes mellitus. |
| Guo HX 2004 | Randomised controlled trial comparing Zhiyin Runzao Tang (herbal medicine) versus gliclazide in 80 cases of type 2 diabetes mellitus. The trial did not report treatment duration of the interventions. |
| Hale 1989 | A double‐blind, crossover, randomised trial comparing Xiaoke tea versus placebo (ordinary tea) in 12 patients with type 2 diabetes mellitus. The treatment duration was one month for each intervention (< 2 months). |
| Hosoda 2003 | A crossover, randomised trial comparing Oolong tea versus water in 20 patients with type 2 diabetes. The treatment duration was 30 days for each intervention (< 2 months). |
| Huang JY 2002 | Randomised controlled trial comparing combination therapy of Chinese herbal formula and glipizide plus metformin versus glipizide plus metformin alone in 96 patients with type 2 diabetes mellitus. The duration of treatment (40 days) was less than two months. |
| Huang Q 2003 | Randomised controlled trial testing Chinese herbal medicine in patients with type 2 diabetes mellitus. The reported outcomes did not fall into our categories. |
| Huang SM 1997 | Randomised controlled trial testing Chinese medicine (Tangxin Shen) and hypoglycaemic drug in patients with diabetic cardio‐vascular autonomous nephropathy. The objective of the trial focused on treatment of the complication of diabetes (diabetic nephropathy). |
| Jiang ZS 1997 | Randomised controlled trial comparing Chinese medicine (Salvia miltiorrhiza composita) plus hypoglycaemic drug(s) versus hypoglycaemic drug(s) alone in patients with type 2 diabetes mellitus. The treatment duration was 2 to 3 weeks (less than 2 months). |
| Jiao YX 2003 | Randomised controlled trial comparing Xihuang Jiangtang capsule (self‐prescribed herbal mixture) versus Xiao Ke Wan (another herbal medicine) in 87 patients with type 2 diabetes mellitus. The control intervention did not fall into the inclusion criteria. |
| Khan 1979 | Randomised controlled trial comparing Coccinia indica (a creeper growing in Bangladesh) versus placebo in 32 patients with undefined diabetes mellitus. The treatment duration was six weeks (less than 2 months). |
| Lan QF 2000b | Non‐randomised study comparing Shenji Ning (self‐prescribed herbal mixture) versus diamicron in 177 patients with type 2 diabetes mellitus. |
| Lang J 1998 | Randomised controlled trial comparing Panax notoginseng versus ticlid in patients with early diabetic nephropathy. The objective of the trial focused on treatment of the complication of diabetes (diabetic nephropathy). |
| Lei FY 2001 | Randomised controlled trial comparing a herbal mixture (Jianpi Huazhuo therapy) versus glibenclamide plus Insoral and liver protecting agent (Dongbao Gantai tablets) in 56 patients with type 2 diabetes mellitus complicated with fatty liver. The main objective of the intervention was to treat fatty liver. |
| Li M 1999 | Randomised controlled trial testing Tianma Duzhong capsule in patients with diabetic peripheral neuropathy. The objective of the trial focused on treatment of diabetic peripheral neuropathy. |
| Liang X 1999 | Randomised controlled trial comparing Jinmaitong composita versus Jinkui Shenqi in patients with diabetic peripheral neuropathy. The objective of the trial focused on treatment of diabetic peripheral neuropathy. |
| Liang XC 1989 | Randomised, placebo controlled trial testing herbal mixture "Yiqi Yangyin Huoxue" formula for diabetic patients, but outcome measures were not fallen into the category of this review. |
| Liang Z 2001 | Non‐randomised study comparing a self‐prescribed herbal powder (Jiangtang San) plus either metformin or glibenclamide versus metformin or glibenclamide in 50 patients with non‐insulin‐dependent diabetes mellitus. |
| Liu B 2002 | Randomised controlled trial comparing herbal medicine (Yiqi Yangyin Huoxue Jiangtang Tang) integrated with glibenclamide versus glibenclamide in 456 patients with diabetes mellitus including type 1 (three cases) and type 2 (453 cases). The trial was excluded due to confounding in both experimental and control groups. Another herbal medicine (Xiaoke Wan) was used for some of the patients in experimental group, while gliclazide and/or glipizide was used for some of the patients in control group. |
| Liu CH 2002 | Non‐randomised study comparing a self‐prescribed herbal decoction plus metformin versus metformin alone in 77 patients with type 2 diabetes mellitus. |
| Liu LL 2003 | Randomised controlled trial comparing Xiaotang San (self‐prescribed herbal mixture) plus glibenclamide versus Xiaoke Wan (herbal medicine containing glibenclamide) in 148 patients with type 2 diabetes mellitus. The control intervention did not fall into the inclusion criteria. |
| Liu QG 2000 | Randomised controlled trial comparing Xitang San (herbal medicine) plus glibenclamide versus glibenclamide alone in 60 cases of type 2 diabetes mellitus. The interventions in both arms were used for one month (less than two months). |
| Liu XL 2000 | Randomised controlled trial comparing Jiangtang Dan (herbal medicine) plus glibenclamide versus either Jiangtang Dan or glibenclamide alone in 225 cases of non‐insulin dependent diabetes mellitus in three arms. The interventions in all arms were used for one month (less than two months). |
| Lu GD 2002 | Randomised controlled trial comparing Rongshuan Ketang capsule (herbal medicine) versus metformin hydrochloride in 73 cases of type 2 diabetes mellitus. The interventions in both arms were used for one month (less than two months). |
| Luo J 1998 | Case series using medicinal plant Cryptolepis sanguinolenta for treatment of female patients with type 2 diabetes mellitus. |
| Ma Y 2002 | Non‐randomised study comparing a herbal tea (Potentilla discolor) plus metformin versus metformin alone in 90 patients with type 2 diabetes mellitus. |
| Meckes‐Lozyoa 1986 | Case report of using a herb (Opuntia streptacantha) in treatment of diabetes mellitus. |
| Moshi 2001 | Case series using medicinal plant Phyllanthus amarus for treatment of 21 patients with type 2 diabetes mellitus. |
| Niu ZY 2003 | Randomised controlled trial comparing Xuexi II capsule (self‐prescribed herbal mixture) versus dietary control and behaviour change in 80 participants including people with impaired glucose tolerance and with early stage type 2 diabetes mellitus. The results were not reported separately. |
| Pan XC 2002 | Randomised controlled trial comparing Xiaxiao Yin (self‐prescribed herbal remedy) versus Xiaoke Wan (another herbal medicine) in 89 patients with type 2 diabetes mellitus. The control intervention did not fall into the inclusion criteria. |
| Parikh 2001 | Randomised controlled trial testing Spirulina (functional nutrients of a therapeutic food) in 25 patients with type 2 diabetes. |
| Peng GR 2001 | Non‐randomised study comparing a combination therapy of Xiaoke Tang (herbal mixture) plus glibenclamide versus glibenclamide alone in 168 patients with type 2 diabetes mellitus. |
| Ren H 2000 | Randomised controlled trial testing Tangzhi Min capsules in patients with diabetic peripheral neuropathy. The objective of the trial focused on treatment of the complication of diabetic peripheral neuropathy. |
| Rodriguez‐Moran 1998 | Double‐blind, placebo‐controlled, randomised trial comparing dietary fiber (Plantago psyllium) versus placebo in patients with type 2 diabetes. |
| Ryan 2000 | Randomised, placebo‐controlled, single‐blind trial testing herbal tea (prepared using 2 plants: Populus tremuloides and Heracleum lanatum) in 40 patients with type 2 diabetes mellitus. The treatment duration was only 10 days. |
| Sang Y 1996 | Randomised controlled trial comparing Tangshen Kang capsules versus conventional drugs in patients with diabetic nephropathy. The objective of the trial focused on treatment of the complication of diabetic nephropathy. |
| Sharma 1990 | Randomised cross‐over trial comparing Fenugreek seeds (Trigonella foenum graecum) versus no intervention in 15 patients with non‐insulin dependent diabetes mellitus. The treatment duration was only 10 days. |
| Sitprija 1987 | Randomised, double blind, controlled trial comparing garlic (Allium sativum) versus placebo in 33 patients with type 2 diabetes mellitus. The treatment duration was four weeks (less than two months). |
| Song SY 2003 | Randomised controlled trial comparing Xiaoke Yin (herbal decoction) versus gliclazide plus another herbal medicine (Liuwei Dihuang Wan) in 202 patients with type 2 diabetes. The control intervention was confounded by the extra herbal medicine. |
| Sun YW 2002 | Non‐randomised controlled study comparing Chinese herbal medicine (self‐prescribed Ziyin Jiangtang capsule) versus diabetes education, dietary and exercise therapies in 90 cases of dormant diabetes. |
| Velussi 1997 | Randomised controlled trial comparing silymarin (herbal extract) versus no intervention in 60 diabetic patients with alcoholic liver cirrhosis. The participants did not fall into our category. |
| Vuksan 2000 | Ten type 2 diabetic patients were randomly administered 0 g (placebo), or 3, 6, 9 g ground American ginseng root in capsules at 120, 80, 40, or 0 minutes before a 25 g oral glucose challenge. The treatment duration was less than than two months. |
| Wang H 1997 | Randomised controlled trial comparing Chinese medicine (Sanqi Dan) versus Captopril in type 2 diabetic patients with microalbuminuria. The objective of the trial was to treat the complication of diabetic nephropathy. |
| Wang HL 2000 | Controlled clinical trial in 62 patients with type 2 diabetes who were allocated to receive either herbal medicine plus metformin or metformin alone according to the admission of medical care. |
| Wang ZL 1990 | Case series of 38 patients with type 2 diabetes mellitus were treated by Chinese herbal medicine (Ketang Ling). |
| Wu S 2000 | Randomised controlled trial testing modified recipe of Bai Fu Ling Wan in patients with incipient diabetic nephropathy. The objective of the trial focused on treatment of the complication of diabetic nephropathy. |
| Wu ST 2000 | Randomised controlled trial comparing herbal mixture "Jiawei Shenqi Wan" plus glibenclamide versus glibenclamide alone in type 2 diabetes. The outcome was global improvement including symptoms and laboratory tests. We could not extract original data for separate outcomes due to inadequate reporting. |
| Yan HH 2001 | Randomised controlled trial comparing a self‐prescribed herbal mixture (Yiqi Ziyin Huoxue Tang) versus a herbal medicine (Xiaoke Wan) in 90 patients with type 2 diabetes mellitus. The control intervention did not fall into the category of the inclusion criteria. |
| Yan ZY 2001 | Non‐randomised study comparing a herbal formula (Shenling Baishu San) plus glibenclamide versus glibenclamide alone in 56 patients with diabetes mellitus. |
| Yu H 2000 | Non‐randomised study comparing a herbal therapy (pungent‐moisturising) plus glibenclamide versus glibenclamide in 60 patients with diabetes mellitus. |
| Yu J 2001 | Randomised controlled trial comparing Gegen Su (herbal extract) combined with hypoglycaemic drugs versus hypoglycaemic drugs in 88 patients with type 2 diabetes. The drugs were not specified. |
| Yu JY 1995 | Randomised controlled trial comparing Abelmoschus manihot (alcohol extract) plus gliclazide and captopril versus gliclazide and captopril alone in patients with diabetic nephropathy. The objective of the trial was to treat the complication of diabetic nephropathy. |
| Zhang M 2001b | Randomised controlled trial comparing Huanglian Su (herbal extract berberine) versus metformin in 72 patients with type 2 diabetes. However, there was no data for the outcome measures and no response from the author. |
| Zhang XD 2002 | Randomised controlled trial comparing Jiangtang Wan (an herbal medicine) versus metformin in 210 patients with type 2 diabetes mellitus. The treatment duration was 20 days (less than two months). |
| Zhang XQ 2000 | Randomised controlled trial comparing Bushen Huoxue Tang (an herbal mixture) combined with glibenclamide versus glibenclamide alone in 78 patients with type 2 diabetes mellitus. The treatment duration was one month (less than two months). |
| Zhao QL 2003 | Randomised controlled trial comparing Tianqi Jiangtang capsule (herbal mixture) with another herbal medicine (Kelening) in 450 patients with type 2 diabetes mellitus. The control intervention did not fall into our categories. |
| Zhong SC 2000 | Non‐randomised study comparing a self‐prescribed herbal decoction (Jiangtang Tang) plus diamicron versus diamicron alone in 96 patients with type 2 diabetes mellitus. |
| Zhou JH 2001b | Non‐randomised study comparing a self‐prescribed herbal decoction (Qimai Jiangtang formula) plus metformin versus metformin alone in 100 patients with type 2 diabetes mellitus. |
| Zhu L 1992 | Randomised controlled trial comparing Tongyu Ling (herbal extract) plus D860 versus D860 alone or Tongyu Ling alone in three arms in patients with diabetic hyperlipaemia. The objective of the trial was to treat the complication of diabetic hyperlipaemia. |
| Zhu LQ 1999 | Randomised controlled trial comparing Chinese herbal mixture of 8 herbs plus glibenclamide versus glibenclamide alone in middle aged women with diabetes with kidney deficiency and bone metabolic disturbance. There was no information on type of the diabetic patients. |
| Zhu LZ 1991 | Randomised controlled trial testing herbal mixture "Tongyu Ling" tablet in diabetic patients (both type 1 and 2) with hyperlipaemia. The outcomes were not adequately reported, and data were not available. |
Contributions of authors
JIANPING LIU: Protocol development, third party for searching for trials, data analyses, development of final review, corresponding author.
SAMELINE GRIMSGAARD: Searching for trials, quality assessment, and revision of final review.
MEI ZHANG: Protocol development, searching for trials and quality assessment of trials, data extraction, and co development of final review.
WEIYA WANG: Searching for trials and quality assessment of trials, data extraction, and co development of final review.
Sources of support
Internal sources
National Research Centre in Complementary and Alternative Medicine, University of Tromso, Norway.
External sources
Pilot Fund for Oversea Chinese Scholars, Ministry of Education, China.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Agrawal 1996 {published data only}
- Agrawal P, Rai V, Singh RB. Randomized placebo‐controlled, single blind trial of holy basil leaves in patients with noninsulin‐dependent diabetes mellitus. International journal of clinical pharmacology and therapeutics 1996;34(9):406‐9. [PubMed] [Google Scholar]
Agrawal 2002 {published data only}
- Agrawal RP, Sharma A, Dua AS, Chandershekhar, Kochar DK, Kothari RP. A randomized placebo controlled trial of Inolter (herbal product) in the treatment of type 2 diabetes. Journal of the Association of Physicians of India 2002;50:391‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Cao FK 1997 {published data only}
- Cao FK, Qian J. [Clinical therapeutic observation of 20 cases of type 2 diabetes mellitus using Sheng Qing Jiang Tang therapy] [in Chinese]. Research of Traditional Chinese Medicine 1997;13(6):15‐6. [Google Scholar]
Chang ZQ 1998 {published data only}
- Chang ZQ, Chang XY. [Clinical observation of integrated traditional Chinese and western medicine in treatment of diabetes] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1998;18(11):674‐6. [PubMed] [Google Scholar]
Chen H 2003 {published data only}
- Chen HL. [Clinical study of Xuan Ge Yin granule for treatment of diabetes] [in Chinese]. Hubei Journal of Traditional Chinese Medicine 2003;25(2):22. [Google Scholar]
Chen SH 1997 {published data only}
- Chen SH, Sun YP, Chen XB, Cai PW, Chen KZ. [Effect of Jiangtangkang on blood glucose, sensitivity of insulin and blood viscosity in non‐insulin dependent diabetes mellitus] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1997;17(11):666‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Chen XH 1993 {published data only}
- Chen XH, Hu RD, Chang J. [Clinical study of composite tablet radix Trichosanthis for treatment of type 2 diabetes: curative effect of 167 cases] [in Chinese]. Journal of Chengdu University of Traditional Chinese Medicine 1993;16(4):20‐3. [Google Scholar]
Chen XL 2001 {published data only}
- Chen XL, Yao W. [Clinical study of self‐prescribed Xiao ke Yin for treatment of 100 cases of type 2 diabetes mellitus] [in Chinese]. Zhejiang Journal of Traditional Chinese Medicine 2001;36(7):290. [Google Scholar]
Chen YB 1995 {published data only}
- Chen YB, Zhang H. [Effect of Bushenhuoxue tablet on serum lipid peroxide, blood lipid and blood suger in type 2 diabetics] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1995;15(11):661‐3. [PubMed] [Google Scholar]
- Guo SS, Chen YB, Liang XC, Wang XD, Zhang H, Sun RY. [Effect of Xianzhen tablet on erythrocyte SOD, serum LOP, blood lipid and suger in patients with type 2 diabetes of deficiency of Kidney and blood stasis] [in Chinese]. Journal of Traditional Chinese Medicine 1995;36(5):291‐3. [Google Scholar]
Deng QW 2003 {published data only}
- Deng QW. [Clinical observation on Xiaoyaosan modified for treatment of type 2 diabetes mellitus] [in Chinese]. Hubei Journal of Traditional Chinese Medicine 2003;25(12):31. [Google Scholar]
Feng YM 1998 {published data only}
- Feng YM, Zhang XP, Wang J, Zhao M, Jiang SJ. [Clinical study of Xiaokeling capsule for treatment of type 2 diabetes with deficiency of both Qi and Yin complicated with stasis] [in Chinese]. Henan Traditional Chinese Medicine 1998;18(6):354‐5. [Google Scholar]
Guo GY 1998 {published data only}
- Guo GY, Yan Q, Wang ZC. [Clinical study of Tianyuan Jiangtang Pill for treatment of non‐insulin dependent diabetes mellitus] [in Chinese]. Hebei Journal of Traditional Chinese Medicine 1998;20(3):142‐3. [Google Scholar]
Guo HF 1996 {published data only}
- Guo HF, Deng Y. [Prescribed treatment based on dampness and stagnation for type 2 diabetes: clinical observation of 78 cases] [in Chinese]. Beijing Journal of Traditional Chnese Medicine 1996;15(4):15‐7. [Google Scholar]
Hou LY 2003 {published data only}
- Hou LY, Wu XY, Zhao YL. [Xiaoke Fuzheng capsule for treating 58 cases of type 2 diabetes mellitus] [in Chinese]. Chinese Journal of Traditional Medical Science and Technology 2003;10(3):173. [Google Scholar]
Hu YP 2003 {published data only}
- Hu YP. [Treatment of 82 cases of type 2 diabetes using integrated Chinese and western medicines] [in Chinese]. Hubei Journal of Traditional Chinese Medicine 2003;25(1):27. [Google Scholar]
Hua SG 1997 {published data only}
- Hua SG, Yi CL, Liu SM, Wan CY, Shou HW. [Clinical study of Yiqi Yangyin Huayu Tang in treatment of 200 cases of diabetes] [in Chinese]. The Practical Journal of Integrated Chinese with Modern Medicine 1997;10(15):1439‐40. [Google Scholar]
Huang CL 2003 {published data only}
- Huang CL, Lu YP. [Effect of Astragalus injection on insulin resistance in auxiliary treating patients with diabetes mellitus type 2] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 2003;23(10):779‐80. [PubMed] [Google Scholar]
Lan QF 2000 {published data only}
- Lan QF. [Clinical study of Sanjiao therapy for treatment of 95 cases of type 2 diabetes mellitus] [in Chinese]. Chinese Journal of Information on Traditional Chinese Medicine 2000;7(7):58. [Google Scholar]
Li HW 2002 {published data only}
- Li HW. [Effect of Shugan Jianpi Huaxue Tang on insulin sensitivity in patients with type 2 diabetes mellitus] [in Chinese]. New Journal of Traditional Chinese Medicine 2002;34(5):28‐9. [Google Scholar]
Li RG 2001 {published data only}
- Li RG. [47 cases of treating diabetes II with Kidney‐nourishing and antidiabetic decoction] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2001;7(4):175. [Google Scholar]
Li Y 2000 {published data only}
- Li Y. [Observation of 57 cases of type 2 diabetes treated by Chinese and western medicines] [in Chinese]. Journal of Practical Traditional Chinese Medicine 2000;16(4):20‐1. [Google Scholar]
Li YM 2003 {published data only}
- Li YM, Hou ZM, Li ZY, Zhou LL. [Study of Tangzhixiao on blood sugar and lipid content adjusting] [in Chinese]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2003;12(10):1011‐3. [Google Scholar]
Mao L 2002 {published data only}
- Mao L, Wang BS. [Clinical observation on Tangniaobing No. 2 formula plus glipizide for treatment of type 2 diabetes mellitus] [in Chinese]. New Journal of Traditional Chinese Medicine 2002;34(2):34‐5. [Google Scholar]
Miao WH 2003 {published data only}
- Miao WH. [Yiqi Huoxue recipe for treating 88 cases of type 2 diabetes mellitus] [in Chinese]. Journal of Sichuan of Traditional Chinese Medicine 2003;21(11):52‐3. [Google Scholar]
Namdul 2001 {published data only}
- Namdul T, Sood A, Ramakrishnan L, Pandey RM, Moorthy D. Efficacy of Tibetan medicine as an adjunct in the treatment of type 2 diabetes. Diabetes Care 2001;24(1):176‐7. [DOI] [PubMed] [Google Scholar]
Ni HX 2000 {published data only}
- Ni HX, Liu G, Luo SS, Wei JP, Yao DG. [Clinical study in treating type 2 diabetes mellitus according to liver in TCM] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 2000;20(8):577‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Pan MZ 1997 {published data only}
- Pan MZ, Guo SS, Liang XC, Chen XY, Xi PX, Wang XD, et al. [Effect of Xianzhen tablet on Na+‐K+‐ATPase, Ca+‐Mg2+‐ATPase, of red blood cell membrane in patients with non‐insulin dependent diabetes mellitus] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1997;17(1):13‐6. [PubMed] [Google Scholar]
Pang DR 2002 {published data only}
- Pang DR. [Clinical observation on Xiaoketang for treatment of 82 cases of type diabetes mellitus] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2002;8(6):337‐8. [Google Scholar]
Peng SJ 1995 {published data only}
- Peng SJ. [Clinical observation of diabetes treated by combination of Chinese traditional medicine and western medcine: comparison analysis of 88 cases] [in Chinese]. Guangxi Journal of Traditional Chinese Medicine 1995;18(2):1‐3. [Google Scholar]
Qing ZQ 2001 {published data only}
- Qing ZQ. [Observation on 60 cases of type 2 diabetes mellitus treated with Shenqi Yuxiao Tang ‐ compared with 30 cases of control treated with Diamicron] [in Chinese]. Zhejiang Journal of Traditional Chinese Medicine 2001;36(5):190‐1. [Google Scholar]
Ren PA 2003 {published data only}
- Ren PA. [Clinical therapeutic observation on Maziren Wan modiefied for treatment of type 2 diabetes mellitus] [in Chinese]. Modern Traditional Chinese Medicine 2003;4(1):29‐30. [Google Scholar]
Russo 1990 {published data only}
- Russo EMK, Reichelt AAJ, De‐Sa JR, Furlanetto RP, Moises RCS, Kasamatsu TS, et al. Clinical trial of Myrcia uniflora and Bauhinia forficata leaf extracts in normal and diabetic patients. Brazilian journal of medical and biological research 1990;23:11‐20. [PubMed] [Google Scholar]
Shao CP 2001 {published data only}
- Shao CP, Ying XB, Wu DH, Zhang XM, Xu YQ. [Clinical study on Potentilla discolor composita for treatment of type 2 diabetes mellitus] [in Chinese]. Shandong Journal of Traditional Chinese Medicine 2001;20(10):588‐9. [Google Scholar]
Shen T 1998 {published data only}
- Shen T, Guo SS, Liang XC, Wang Y, Tao LH. [Effects of Chinese herbs Xianzhen tablet on the deformability of erythrocyte in non‐insulin dependent diabetes mellitus patients with deficiency of both Qi and Yin and deficiency of Kidney with blood stasis] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1998;18(7):405‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Sotaniemi 1995 {published data only}
- Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non‐insulin‐dependent diabetic patients: effects on psychophysical performance, glucose homeostasis, serum lipids, serum aminoterminalpropeptide concentration, and body weight. Diabetes Care 1995;18(10):1373‐5. [DOI] [PubMed] [Google Scholar]
Tao XY 2002 {published data only}
- Tao XY, Li XL. [Clinical observation on Huatan Huoxue recipe for treatment of type 2 diabetes mellitus] [in Chinese]. Journal of Practical Chinese Internal Medicine [SHIYONG ZHONGYI NEIKE ZAZHI] 2002;16(1):23‐4. [Google Scholar]
Tong J 2003 {published data only}
- Tong J, Zhou C, Si YC. [Clinical observation on Jiangtang capsule for treatment of diabetes mellitus] [in Chinese]. China Journal of Traditional Chinese Medicine and Pharmacy 2003;18(2):124‐6. [Google Scholar]
Vray 1995 {published data only}
- Vray M, Attali JR. Randomized study of glibenclamide versus traditional Chinese treatment in type 2 diabetic patients. Diabete and Metabolisme (Paris) 1995;21(6):433‐9. [PubMed] [Google Scholar]
Wang BS 1988 {published data only}
- Wang BS, Song WX. [Clinical observation of Yiqi Yangyin Huoxue Huayu Tang plus D 860 for treatment of diabetes] [in Chinese]. Helongjiang Journal of Traditional Chinese Medicine 1988;24(4):21‐2. [Google Scholar]
Wang JS 2000 {published data only}
- Wang J, Zhao WJ, Xia J, Jin JS. [Effect of yiqi yangyin huoxue recipe on endothelin and nitric oxide of type 2 diabetic patients with deficiency of both Qi‐Yin and blood stasis syndrome] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 2000;20(8):571‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Wang KF 1993 {published data only}
- Wang KF. [Clinical study of Shuizhi Sanhuang Tang in treatment of 20 cases of diabetes] [in Chinese]. Hunan Journal of Traditional Chinese Medicine 1993;9(6):2‐4. [Google Scholar]
Wang KP 1999 {published data only}
- Wang KP, Ba XC. [Clinical observation of 32 cases of type 2 diabetes using Jianpi Jiangtang Tang] [in Chinese]. Jiangxi Journal of Traditional Chinese Medicine 1999;30(3):20. [Google Scholar]
Wang YS 2003 {published data only}
- Wang YS, Su XH, Li WD, Wang XY, Tian FS, Li YX, et al. [Clinical and experimental studies on Xiaotangling capsules in insulin resistance of type 2 diabetes mellitus] [in Chinese]. Chinese Journal of Information on Traditional Chinese Medicine 2003;10(3):17‐8, 79. [Google Scholar]
Wei JL 2003 {published data only}
- Wei JL, Shi HL. [Clinical observation of decoction for reducing blood sugar and regulating lipid in the treatment of diabetes innocens] [in Chinese]. Journal of Henan University of Chinese Medicine 2003;18(1):51‐2. [Google Scholar]
Wen S 2000 {published data only}
- Wen S, Tang ZY, Deng HM, Zhong QS. [Observation of curative effect on NIDDM treated with integrated TCM and western medicine] [in Chinese]. Guangxi Journal of Traditional Chinese Medicine 2000;23(2):1‐3. [Google Scholar]
Wu HM 1996 {published data only}
- Wu HM, Lin F. [Clinical study of 104 cases of elderly diabetes treated by integrated traditional Chinese and western medicine] [in Chinese]. Fujian Journal of Traditional Chinese Medicine 1996;27(6):8‐9. [Google Scholar]
Wu YN 2003 {published data only}
- Wu YN, Lu YZ. [A therapeutic observation on 30 cases of type 2 diabetes mellitus treated by Huoxue Jiangtang Pingzhi formula] [in Chinese]. Guangxi Journal of Traditional Chinese Medicine 2003;26(3):15‐8. [Google Scholar]
Xie CG 1996 {published data only}
- Xie CG, Zhang FR, Li MF, Wang Y, Zhang GH, Lan ZX. [Clinical therapeutic observation of Tang Fu Kang for treatmen of type 2 dabetes mellitus] [in Chinese]. Chinese Traditional Patent Medicine 1996;18(2):25‐7. [Google Scholar]
- Xie CG, Zhang FR, Li MF, Wang Y, Zhang GH, Sun HH, et al. [Clinical therapeutic observation of Tang Fu Kang for treatment of type 2 diabetes mellitus] [in Chinese]. Traditional Chinese Medicine Research 1997;10(2):23‐6. [Google Scholar]
Xu Q 2003 {published data only}
- Xu Q, Hao QG. [Yishen Huoxue Tiaogan therapy for treatment of 55 cases of type 2 diabetes mellitus] [in Chinese]. Shandong Journal of Traditional Chinese Medicine 2003;22(9):531‐2. [Google Scholar]
Xu YS 1997 {published data only}
- Xu YS, Cheng YC. [Effect of Zhonghui Chuan Huang Ye on blood suger, lipid, and hemorheology in type 2 diabetes] [in Chinese]. Chinese Traditional Patent Medicine 1997;19(8):23‐4. [Google Scholar]
Yang PL 2003 {published data only}
- Yang PL. [Treatment of 88 fat diabetes cases using TCM combined with WM] [in Chinese]. Journal of Yunnan College of Traditional Chinese Medicine 2003;26(1):38‐9. [Google Scholar]
Yang TB 2002 {published data only}
- Yang TB. [Shenqi Jiangtang Yin for treatment of 144 cases of type 2 diabetes mellitus] [in Chinese]. Hebei Journal of Traditional Chinese Medicine 2002;24(3):169‐70. [Google Scholar]
Yao LD 2003 {published data only}
- Yao LD, Liu MZ, Lu MX. [Study of thrifty genotype on non‐insulin dependent diabetes mellitus] [in Chinese]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2003;12(4):339‐40. [Google Scholar]
You BW 1999 {published data only}
- You BW, Su HZ. [Clinical observation of Sugan Huoxue treatment of non‐insulin dependent diabetes mellitus] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 1999;5(4):16‐7. [Google Scholar]
Zeng Y 2001 {published data only}
- Zeng Y, Liu SF. [Integrated Chinese and western medicine for treatment of 48 cases of type 2 diabetes mellitus] [in Chinese]. Journal of Traditional Chinese Medicine and Chinese Materia Medica of Jilin 2001;21(2):43. [Google Scholar]
Zhang FB 2003 {published data only}
- Zhang FB. [Integrated traditional Chinese medicine with western medicine for treating 34 cases of type 2 diabetes] [in Chinese]. Journal of Zhejiang College of Traditional Chinese Medicine 2003;27(3):36. [Google Scholar]
Zhang HZ 1999 {published data only}
- Zhang HZ. [Clinical observation of 86 cases of type 2 diabetes using treatment of Xiaokewan] [in Chinese]. Research of Traditional Chinese Medicine 1999;15(4):19‐20, 34. [Google Scholar]
Zhang J 2003 {published data only}
- Zhang J, Wang LQ, Yu JH. [Clinical observation on Qihuang capsule combined with Berberine hydrochloride for treatment of type 2 diabetes mellitus] [in Chinese]. Shandong Journal of Traditional Chinese Medicine 2003;22(10):598‐9. [Google Scholar]
Zhang M 2001a {published data only}
- Zhang M, Ma SP, Wang ZR, Xia T. [Effect of Qidan Tongmai tablet on glucose and lipid metabolism in patients with diabetes mellitus type 2] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 2001;21(11):825‐7. [PubMed] [Google Scholar]
Zhao XY 2001 {published data only}
- Zhao XY, Song XJ. [Therapeutic observation of Tang Ning Pian for treatment of type 2 diabetes mellitus] [in Chinese]. Chinese Traditional Patent Medicine 2001;23(11):850‐1. [Google Scholar]
Zheng X 2001 {published data only}
- Zheng X. [Observation on combination therapy of Chinese and western medicine for type 2 diabetes mellitus] [in Chinese]. Journal of Practical Traditional Chinese Medicine 2001;17(3):27. [Google Scholar]
Zhou C 2001 {published data only}
- Zhou C, Li XZ, Tong J, Xu JH, Hua XJ, Wang LP. [Clinical obsercation on phase differentiation of symptoms for treatment of 300 cases of type 2 diabetes mellitus] [in Chinese]. Chinese Journal of Information on Traditional Chinese Medicine 2001;8(4):57‐8. [Google Scholar]
Zhou JH 2001a {published data only}
- Zhou JH. [Clinical observation on 98 cases of type 2 diabetes mellitus treated mainly by Qimai Dahuang decoction] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2001;7(11):548‐9. [Google Scholar]
Zhou P 1997 {published data only}
- Zhou P. [Clinical study of Kelening treatment in non‐insulin dependent diabetes mellitus] [in Chinese]. The Practical Journal of Integrating Chinese with Modern Medicine 1997;10(19):1860‐1. [PubMed] [Google Scholar]
- Zhou P. [Clinical study of glibenclamide in combination with Kelening treatment in non‐insulin dependent diabetes mellitus] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1997;17(1):29‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Zhou XT 2001 {published data only}
- Zhou XT, Peng Y. [Curative effect observation of 146 cases with diabetes II treated with Xiaoyao Powder and antidiabetic agent] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2001;7(4):171‐2. [Google Scholar]
Zhu ZZ 1997 {published data only}
- Zhu ZZ, Xiong MQ, Lin AZ, Cai WJ, Chen ZX, Shen ST, et al. [Effect of Sanhuang Jiangtang Recipe on insulin peripheral resistance in type 2 diabetes] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1997;17(10):590‐3. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Bhardwaj 1994 {published data only}
- Bhardwaj PK, Dasgupta DJ, Prashar BS, Kaushal SS. Control of hyperglycaemia and hyperlipidaemia by plant product. Journal of the Association of Physicians of India 1994;42(1):33‐5. [PubMed] [Google Scholar]
Bhardwaj 1994b {published data only}
- Bhardwaj PK, Dasgupta DJ, Prashar BS, Kaushal SS. Effective reduction of LDL cholesterol by indigenous plant product. Journal of the Indian Medical Association 1994;92(3):80‐1. [PubMed] [Google Scholar]
Bian F 2000 {published data only}
- Bian F, Zhao P, Zhang Z. [Clinical study on treatment of incipient diabetic nephropathy by integrated traditional Chinese and Western medicine] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 2000;20(5):335‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Bian LE 2000 {published data only}
- Bian LE. [Clinical study of Zhixiao Tongmai capsule for treatment of type 2 diabetes mellitus complicated with cerebral embolism] [in Chinese]. Chinese Journal of Information on Traditional Chinese Medicine 2000;7(4):54‐6. [Google Scholar]
Blostein‐Fujii 1999 {published data only}
- Blostein‐Fujii A, Disilvestro RA, Frid D, Katz C. Short term citrus flavonoid supplementation of type 2 diabetic women: no effect on lipoprotein oxidation tendencies. Free radical research 1999;30:315‐20. [DOI] [PubMed] [Google Scholar]
Bu XC 2001 {published data only}
- Bu XC, Li YS, Qin YH. [A summary on 94 cases of mild cerebral ischemia caused by diabetic hyperviscosity syndrome treated by Zicui Tongmai capsule] [in Chinese]. Hunan Journal of Traditional Chinese Medicine 2001;17(4):17‐8. [Google Scholar]
Cai JW 2001 {published data only}
- Cai JW. [Observation of traditional Chinese medicine on the treatment of type 2 diabetes] [in Chinese]. Hebei Journal of Traditional Chinese Medicine 2001;23(6):419‐20. [Google Scholar]
Chen SL 2000 {published data only}
- Chen SL. [Therapeutic observation on 63 cases of type 2 diabetes using integrated traditional Chinese and western medicine] [in Chinese]. Hunan Journal of Traditional Chinese Medicine 2000;16(2):24. [Google Scholar]
Chen XY 2001 {published data only}
- Chen XY, Zhang Q, Ye M. [Clinical observation of treating 250 cases of NIDDM with Silkworm‐Pupa and Leech Decoction] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2001;7(6):297‐8. [Google Scholar]
Cui YL 2003 {published data only}
- Cui YL, Wang DJ. [Self‐prescribed herbal formula for treatment of type 2 diabetes] [in Chinese]. Jilin Journal of Traditional Chinese Medicine 2003;23(4):22. [Google Scholar]
Deng C 2000 {published data only}
- Deng C. [Treatment of 60 cases of type 2 diabetes according to theories of Liver diseases in traditional Chinese medicine] [in Chinese]. Journal of Practical Traditional Chinese Medicine 2000;16(11):19. [Google Scholar]
Dilawari 1987 {published data only}
- Dilawari JB, Ajit Kumar VK, Khurana S, Bhatnagar R, Dash RJ. Effect of legumes on blood suger in diabetes mellitus. The Indian journal of medical research 1987;85:184‐7. [PubMed] [Google Scholar]
Ding YL 1999 {published data only}
- Ding YL. [Clinical study of self‐prescribed Huaqishen Tang for treatment of type 2 diabetes mellitus] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 1999;5(4):28‐9. [Google Scholar]
Du X 2000 {published data only}
- Du X. [Clinical study of Bushen Jiangtang capsule for treatment of type 2 diabetes mellitus] [in Chinese]. Chinese Journal of Information on Traditional Chinese Medicine 2000;7(4):53‐4. [Google Scholar]
Feng WH 2000 {published data only}
- Feng WH. [Therapeutic study of integrated traditional Chinese and western medicine for treating type 2 diabetes mellitus] [in Chinese]. Chinese Journal of Information on Traditional Chinese Medicine 2000;7(4):52‐3. [Google Scholar]
Frati 1991 {published data only}
- Frati AC, Gordillo BE, Altamirano P, Ariza CR, Cortes‐Franco R, Chavez‐Negrete A, et al. Influence of nopal intake upon fasting glycemia in type 2 diabetics and healthy subjects. Archivos de investigacion medica 1991;22(1):51‐5. [PubMed] [Google Scholar]
Frati 1991b {published data only}
- Frati AC, Xilotldiaz N, Altamirano P, Ariza R, Lopez‐Ledesma R. The effect of two sequential doses of Opuntia streptacantha upon glycemia. Archivos de investigacion medica 1991;22(3‐4):333‐6. [PubMed] [Google Scholar]
Frati 1992 {published data only}
- Frati AC, Vera O, Ariza CR. Evaluation of nopal capsule in diabetes mellitus [Evaluacion de capsulas de nopal en diabetes mellitus]. Gaceta medica de Mexico 1992;128(4):431‐6. [PubMed] [Google Scholar]
Fuessl 1987 {published data only}
- Fuessl HS, Williams G, Adrian TE, Bloom SR. Guar sprinkled on food: effect on glycaemic control, plasma lipids and gut hormones in non‐insulin dependent diabetic patients. Diabetic Medicine 1987;4:463‐8. [DOI] [PubMed] [Google Scholar]
Fujita 2001 {published data only}
- Fujita H, Yamagami T, Ohshima K. Long‐term ingestion of a fermented soybean‐derived Touchi‐extract with alfa‐glucosidase inhibitory activity is safe and effective in humans with borderline and mild type‐2 diabetes. The Journal of Nutrition 2001;131:2105‐8. [DOI] [PubMed] [Google Scholar]
Gao Y 1998 {published data only}
- Gao Y, Lu R, Wang X, Geng J, Ren K, Wang Y, et al. A clinical trial of Tang Shen Ning for treatment of diabetic nephropathy. Journal of traditional Chinese medicine 1998;18(4):247‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Gao YM 2002 {published data only}
- Gao YM, Huang XY. [Curative effect of Jiawei Meiqi Tang for type 2 diabetes with high pancreatic glucagon] [in Chinese]. New Journal of Traditional Chinese Medicine 2002;34(4):36‐7. [Google Scholar]
Gui Y 2002 {published data only}
- Gui Y. [Combined TCM and Western medicine for treating 30 cases of type II diabetes mellitus] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2002;8(6):339. [Google Scholar]
Guo HX 2004 {published data only}
- Guo HX. [Clinical observation on Zhiyin Runzao Tang for treating 40 patients with type 2 diabetes] [in Chinese]. Jilin Journal of Traditional Chinese Medicine 2004;25(1):25. [Google Scholar]
Hale 1989 {published data only}
- Hale PJ, Horrocks PM, Wright AD, Fitzgerald MG, Nattrass M, Bailey CJ. Xiaoke Tea, a Chinese herbal treatment for diabets mellitus. Diabetic Medicine 1989;6:675‐6. [DOI] [PubMed] [Google Scholar]
Hosoda 2003 {published data only}
- Hosoda K, Wang MF, Liao ML, Chuang CK, Iha M, Clevidence B, et al. Antihyperglycemic effect of oolong tea in type 2 diabetes. Diabetes Care 2003;26(6):1714‐8. [DOI] [PubMed] [Google Scholar]
Huang JY 2002 {published data only}
- Huang JY, Huang L. [Treating 50 cases of type 2 diabetes mellitus by combined TCM and western medicine] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2002;8(8):481‐4. [Google Scholar]
Huang Q 2003 {published data only}
- Huang Q, Ni HX, Shao GM. [Effect of Chinese herbal medicine for activating blood circulation to remove stasis on CD11b/CD18 expression in patients with diabetes mellitus type 2] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 2003;23(6):430‐2. [PubMed] [Google Scholar]
Huang SM 1997 {published data only}
- Huang SM, Liao XY, Wu LF. [Clinical report of 60 cases of diabetic cardio‐vascular autonomous neuropathy by stasis removing treatment of combined traditional and western medicine] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1997;17(10):594‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Jiang ZS 1997 {published data only}
- Jiang ZS, Zhang SL, Cai XJ, Xing WJ, Wang SX. [Effect of Salvia miltiorrhiza composita on superoxide dismutase and malonyldialdehyde in treating patients with non‐insulin dependent diabetes mellitus (NIDDM)] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1997;17(1):32‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Jiao YX 2003 {published data only}
- Jiao YX, Dong XR, Jiang Q. [Clinical observation on Xihuang Jiangtang capsule for treating 87 cases of type 2 diabetes] [in Chinese]. Acta Chinese Medicine and Pharmacology 2003;31(4):41. [Google Scholar]
Khan 1979 {published data only}
- Azad Khan AK, Akhtar S, Mahtab H. Coccinia indica in the treatment of patients with diabetes mellitus. Bangladesh Medical Research Council Bulletin 1979;5(2):60‐6. [PubMed] [Google Scholar]
Lan QF 2000b {published data only}
- Lan QF. [Observation on therapeutic effect of self‐prescribed Shenjining Tang for treating type 2 diabetes mellitus] [in Chinese]. Guangxi Journal of Traditional Chinese Medicine 2000;23(4):25‐6. [Google Scholar]
Lang J 1998 {published data only}
- Lang J, Cao H, Wei A. [Comparative study on effect of Panax notoginseng and ticlid in treating early diabetic nephropathy] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1998;18(12):727‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Lei FY 2001 {published data only}
- Lei FY. [38 cases of type II diabetes complicated with fatty liver by treatment of 'strengthening the spleen and removing turbid pathogen'] [in Chinese]. Hubei Journal of Traditional Chinese Medicine 2001;23(6):18. [Google Scholar]
Li M 1999 {published data only}
- Li M, Wang X. Clinical observation on treatment of diabetic peripheral neuropathy with reinforced Tianma Duzhong capsule. Journal of Traditional Chinese Medicine 1999;19(3):182‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Liang X 1999 {published data only}
- Liang X, Cui L, Guo S. [Clinical study on jinmaitong composita on diabetic peripheral neuropathy] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1999;19(9):517‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Liang XC 1989 {published data only}
- Liang XC, Guo SS, Qian ZF, Tao LH, Wang Y. [Effect of "Yiqi Yangyin Huoxue" Prescription on hemorrheology of diabetes with symptoms of "Qi, Yin" deficiency and stasis of blood] [in Chinese]. Acta Academiae Medicinae Sinicae 1989;11(2):87‐91. [PubMed] [Google Scholar]
Liang Z 2001 {published data only}
- Liang Z. Treatment of 28 cases of type 2 diabetes by adding self‐prescribed herbal medicine Jiang Tang San [in Chinese]. Guangxi Journal of Traditional Chinese Medicine 2001;24(5):12‐3. [Google Scholar]
Liu B 2002 {published data only}
- Liu B, Ge SJ. [Clinical study on 235 cases of diabetes treated with integrated Chinese and western medicine] [in Chinese]. Forum on Traditional Chinese Medicine 2002;17(3):34‐5. [Google Scholar]
Liu CH 2002 {published data only}
- Liu CH, Zhao GW, Yi HY, Huang Y. [Observation on therapeutic effect of comprehensive therapy for treatment of 45 cases of type 2 diabetes] [in Chinese]. New Journal of Traditional Chinese Medicine 2002;34(5):30‐1. [Google Scholar]
Liu LL 2003 {published data only}
- Liu LL. [Clinical study on Xiaotangsan for treatment of type 2 diabetes mellitus] [in Chinese]. Shandong Journal of Traditional Chinese Medicine 2003;22(10):594‐6. [Google Scholar]
Liu QG 2000 {published data only}
- Liu QG, Chen XJ, Lu H, Liu J, Zhao HX, Cui F, et al. [Clinical study on effects of Jiangtang Dan on reducing blood glucose and activiating blood and removing stasis] [in Chinese]. Academic Periodical of Changchun College of Traditional Chinese Medicine 2000;16(4):12‐3. [Google Scholar]
Liu XL 2000 {published data only}
- Liu XL, Liang LJ, Liu XY. [Clinical study on diabetes treated by Xitang Powder] [in Chinese]. Hubei Journal of Traditional Chinese Medicine 2000;22(3):28. [Google Scholar]
Lu GD 2002 {published data only}
- Lu GD. [Observation on Rongshuan Ketang capsule for treatment of 43 patients with type 2 diabetes mellitus] [in Chinese]. Journal of Practical Traditional Chinese Medicine 2002;18(4):4‐5. [Google Scholar]
Luo J 1998 {published data only}
- Luo J, Fort DM, Carlson TJ, Noamesi BK, nii‐Amon‐Kotei D, King SR, et al. Cryptolepis sanguinolenta: an ethnobotanical approach to drug discovery and the isolation of a potentially useful new antihyperglycaemic agent. Diabetic Medicine 1998;15:367‐74. [DOI] [PubMed] [Google Scholar]
Ma Y 2002 {published data only}
- Ma Y, Wen SZ. [Study on therapeutic effect of herb Potentilla discolor for treatment of 50 cases of type 2 diabetes] [in Chinese]. Chinese Traditional and Herbal Drugs 2002;33(7):644. [Google Scholar]
Meckes‐Lozyoa 1986 {published data only}
- Meckes‐Lozyoa M, Roman‐Ramos R. Opuntia streptacantha: a coadjutor in the treatment of diabetes mellitus. The American journal of Chinese medicine 1986;14(3‐4):116‐8. [DOI] [PubMed] [Google Scholar]
Moshi 2001 {published data only}
- Moshi MJ, Lutale JJ, Rimoy GH, Abbas ZG, Josiah RM, Swai AB. The effect of Phyllanthus amarus aqueous extract on blood glucose in non‐insulin dependent diabetic patients. Phytotherapy Research 2001;15(7):577‐80. [DOI] [PubMed] [Google Scholar]
Niu ZY 2003 {published data only}
- Niu ZY. [Observation of the curative effect of Xuexi capsules II in the treatment of 50 cases of ICT and diabetes in initial stage] [in Chinese]. Henan Traditional Chinese Medicine 2003;23(9):30‐1. [Google Scholar]
Pan XC 2002 {published data only}
- Pan XC. [Study of self‐prescribed Xia Xiao Yin for treatment of 52 cases of type 2 diabetes mellitus] [in Chinese]. Guang Ming Journal Traditional Chinese Medicine 2002;17(3):36‐8. [Google Scholar]
Parikh 2001 {published data only}
- Parikh P, Mani U, Iyer U. Role of Spirulina in the control of glycemia and lipidemia in type 2 diabetes mellitus. Journal of Medicinal Food 2001;4(4):193‐9. [DOI] [PubMed] [Google Scholar]
Peng GR 2001 {published data only}
- Peng GR, Zhao B. [Clinical study on combination therapy of Xiaoketang and Glibenclamide for treatment of 92 cases of type 2 diabetes mellitus] [in Chinese]. Hunan Journal of Traditional Chinese Medicine 2001;17(2):17‐8. [Google Scholar]
Ren H 2000 {published data only}
- Ren H. A clinical study on treatment of diabetic peripheral neuropathy with Tang Zhi Min capsules. Journal of Traditional Chinese Medicine 2000;20(4):258‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Rodriguez‐Moran 1998 {published data only}
- Rodriguez‐Moran M, Guerrero‐Romero F, Lazcano‐Burciaga G. Lipid‐ and glucose‐lowering efficacy of Plantago psyllium in type 2 diabetes. Journal of diabetes and its complications 1998;12(5):273‐8. [DOI] [PubMed] [Google Scholar]
Ryan 2000 {published data only}
- Ryan EA, Imes S, Wallace C, Jones S. Herbal tea in the treatment of diabetes mellitus. Clinical and investigative medicine 2000;23(5):311‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Sang Y 1996 {published data only}
- Sang Y, Wang XB, Han Q. [Effects of tangshenkang capsule on diabetic nephropathy] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1996;16(7):398‐401. [PubMed] [Google Scholar]
Sharma 1990 {published data only}
- Sharma RD, Raghuram TC. Hypoglycaemic effect of Fenugreek seeds in non‐insulin dependent diabetic subjects. Nutrition Research 1990;10:731‐9. [Google Scholar]
Sitprija 1987 {published data only}
- Sitprija S, Plengvidhya C, Kangkaya V, Bhuvapanich S, Tunkayoon M. Garlic and diabetes mellitus phase II clinical trial. Journal of Medical Association of Thailand 1987;70(Suppl 2):223‐7. [PubMed] [Google Scholar]
Song SY 2003 {published data only}
- Song SY, Song FJ, Lei QF. [Clinical observation on Xiaokeyin for treating 108 cases of type 2 diabetes] [in Chinese]. Hebei Journal of Traditional Chinese Medicine 2003;25(8):577‐8. [Google Scholar]
Sun YW 2002 {published data only}
- Sun YW, Yuan L. [Therapeutic study on 60 cases of dormant diabetes treated by Ziyin Jiangtang capsule] [in Chinese]. Research of Traditional Chinese Medicine 2002;18(4):9. [Google Scholar]
Velussi 1997 {published data only}
- Velussi M, Cernigoi AM, Monte AD, Dapas F, Caffau C, Zilli M. Long‐term (12 months) treatment with an anti‐oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. Journal of Hepatology 1997;26:871‐9. [DOI] [PubMed] [Google Scholar]
Vuksan 2000 {published data only}
- Vuksan V, Leiter LA, Stavro MP, Josse RG, Sievenpiper JL, Xu Z, Beljan‐Zdravkovic U. Similar postprandial glycemic reductions with escalation of dose and administration time of American Ginseng in type 2 diabetes. Diabetes Care 2000;23(9):1221‐6. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Sievenpiper JL, Koo VYY, Francis T, Beljan‐Zdravkovic U, Xu Z, Vidgen E. American Ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Archives of internal medicine 2000;160:1009‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang H 1997 {published data only}
- Wang H. [Clinical study of Chinese medicine for treatment of diabetes complicated with minor albumin urine] [in Chinese]. Guangdong Medical Journal 1997;18(4):259‐60. [Google Scholar]
Wang HL 2000 {published data only}
- Wang HL. [Clinical study on 62 cases of type 2 diabetes mellitus treated with integrated Chinese and western medicine] [in Chinese]. Beijing Journal of Traditional Chinese Medicine 2000;19(4):35‐6. [Google Scholar]
- Wang HL. [Clinical study on 62 cases of type 2 diabetes mellitus treated with integrated Chinese and western medicine] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 2000;20(8):602. [PubMed] [Google Scholar]
Wang ZL 1990 {published data only}
- Wang ZL, Zhang Z, Gao SR, Huang JW, Wang CY, Wang BF, et al. [Effect of Ke‐Tang‐Ling administration on the function of islets cells in non‐insulin‐dependent diabetes mellitus] [in Chinese]. Chinese Journal of Modern Development in Traditional Medicine 1990;10(3):137‐40. [PubMed] [Google Scholar]
Wu S 2000 {published data only}
- Wu S, Han Y, Li J. Treatment of incipient diabetic nephropathy by clearing away the stomach‐heat, purging the heart fire, strengthening the spleen and tonifying the kidney. Journal of Traditional Chinese Medicine 2000;20(3):172‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Wu ST 2000 {published data only}
- Wu ST. [Study of Jiawei Shenqi Wan combined with glibenclamide for treatment of 58 cases of diabetes] [in Chinese]. Zhejiang Journal of Traditional Chinese Medicine 2000;35(5):194. [Google Scholar]
Yan HH 2001 {published data only}
- Yan HH, Zou BY. [Clinical observation on Yiqi Ziyin Huoxue Tang for treatment of 50 cases of type 2 diabetes mellitus] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2001;7(1):16‐7. [Google Scholar]
Yan ZY 2001 {published data only}
- Yan ZY. [35 cases of diabetes treated with Shenling Baishu San combined with glibenclamide] [in Chinese]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2001;7(6):301‐3. [Google Scholar]
Yu H 2000 {published data only}
- Yu H, Yu R, Lin S. [Study on therapeutic effect of Glibenclamide complemented with Pungent‐moisturising therapy for treatment of 30 cases of diabetes mellitus] [in Chinese]. Hunan Journal of Traditional Chinese Medicine 2000;16(1):9‐10. [Google Scholar]
Yu J 2001 {published data only}
- Yu J. [Clinical study on insulin resistence in patients with type 2 diabetes mellitus using Ge Gen Su] [in Chinese]. Shandong Journal of Traditional Chinese Medicine 2001;20(12):727‐9. [Google Scholar]
Yu JY 1995 {published data only}
- Yu JY, Xiong NN, Guo HF. [Clinical observation on diabetic nephropathy treated with alcohol of Abelmoschus manihot] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1995;15(5):263‐5. [PubMed] [Google Scholar]
Zhang M 2001b {published data only}
- Zhang M, Yu FH, Ding XP. [Observation on therapeutic effect of Huang Lian Su on type 2 diabetes] [in Chinese]. Hubei Journal of Traditional Chinese Medicine 2001;23(6):16. [Google Scholar]
Zhang XD 2002 {published data only}
- Zhang XD, Liu XQ. [Clinical study of Jiangtang Wan for treatment of type 2 diabetes mellitus] [in Chinese]. Henan Traditional Chinese Medicine 2002;22(3):40. [Google Scholar]
Zhang XQ 2000 {published data only}
- Zhang XQ. [Combination therapy of Bushen Huaxue Tang with glibenclamide for treatment of 40 cases of type diabetes mellitus] [in Chinese]. Journal of Anhui Traditional Chinese Medicine College 2000;19(4):27‐8. [Google Scholar]
Zhao QL 2003 {published data only}
- Zhao QL, Guo BR, Yang WJ. [Tianqi Jiangtang capsule for treating 300 cases of type 2 diabetes] [in Chinese]. Journal of Shandong University of Traditional Chinese Medicine 2003;27(3):191‐2. [Google Scholar]
Zhong SC 2000 {published data only}
- Zhong SC. [Clinical observation on 64 cases of diabetes II with therapy of Spleen‐Kidney treatment] [in Chinese]. Hunan Journal of Traditional Chinese Medicine 2000;16(6):13‐4. [Google Scholar]
Zhou JH 2001b {published data only}
- Zhou JH. [Treatment of 50 cases of type 2 diabetes using integrated traditional Chinese and western medicine] [in Chinese]. Hunan Journal of Traditional Chinese Medicine 2001;17(2):44. [Google Scholar]
Zhu L 1992 {published data only}
- Zhu L, Wang S, Xu R, Zhong J, Liu Y, Mao L. Clinical and experimental studies on tong yu ling in the treatment of diabetic hyperlipemia. Journal of Traditional Chinese Medicine 1992;12(3):163‐8. [PubMed] [Google Scholar]
Zhu LQ 1999 {published data only}
- Zhu LQ, Li HY, Liu YH. [Study on prevention and treatment of middle and aged women diabetes with kidney deficiency and bone metabolic disturbance] [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine 1999;19(4):215‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Zhu LZ 1991 {published data only}
- Zhu LZ, Wang SS, Xu RJ, Zhong JB, Liu YY, Mao L. Clinical and experimental studies on Tong Yu Ling in the treatment of diabetic hyperlipemia. Journal of Traditional Chinese Medicine 1992;12(3):163‐8. [PubMed] [Google Scholar]
- Zhu LZ, Wang SS, Xu RJ, Zhong JB, Liu YY, Mao L. [Clinical and experimental studies on Tong Yu Ling in the treatment of diabetic hyperlipemia] [in Chinese]. Journal of Traditional Chinese Medicine 1991;32(12):23‐5. [PubMed] [Google Scholar]
Additional references
ADA 1997
- American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183‐97. [DOI] [PubMed] [Google Scholar]
ADA 1999
- American Diabetes Association. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. American Diabetes Association: clinical practice recommendations 1999. Diabetes Care 1999;22(Suppl 1):S1‐114. [PubMed] [Google Scholar]
Bailey 1989
- Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care 1989;12(8):553. [DOI] [PubMed] [Google Scholar]
Chen 1991
- Chen MY, Kuang AF, Chen JL, Chen MD, Jin YQ. Therapeutic observation on efficacy of San Xiao capsule for type 2 diabetes mellitus. Journal of Chinese Patent Medicine 1991;13(1):21‐2. [Google Scholar]
Chen 1997
- Chen SH, Sun YP, Chen XS. Effect of Jiangtangkang on blood glucose, sensitivity of insulin and blood viscosity in non‐insulin dependent diabetes mellitus. Chinese Journal of Integrated Traditional and Western Medicine 1997;17(11):666‐8. [PubMed] [Google Scholar]
Cheng 2000
- Cheng JT. Review: Drug therapy in Chinese Traditional Medicine. Journal of clinical pharmacology 2000;40:445‐50. [DOI] [PubMed] [Google Scholar]
CMH 1999
- Chinese State Drug Administration. Material Compilation of new drugs approved between 1985 and 1998. http://www.sda.gov.cn (accessed by May 2002) 1999.
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 2003
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment 2003;7(1):1‐76. [PubMed] [Google Scholar]
Fleiss 1981
- Fleiss JL. Statistical Methods for Rates and Proportions. 2nd Edition. New York: Wiley, 1981:217‐34. [Google Scholar]
Fulder 1996
- Fulder S. The Handbook of Alternative and Complementary Medicine. Oxford: Oxford University Press, 1996. [Google Scholar]
Garrow 1988
- Garrow JS. Three limitations of body mass index. American Journal of Clinical Nutrition 1988;47(3):553. [DOI] [PubMed] [Google Scholar]
Gottieb 2000
- Gottieb S. Chinese herb may cause cancer (news). BMJ 2000;320:1623. [PMC free article] [PubMed] [Google Scholar]
Ishizaki 1996
- Ishizaki T, Sasaki F, Ameshima S, Shiozaki K, Takahashi H, Abe Y, et al. Pneumonitis during interferon and/or herbal drug therapy in patients with chronic active hepatitis. European Respiratory Journal 1996;9(12):2691‐6. [DOI] [PubMed] [Google Scholar]
Ivorra 1989
- Ivorra MD, Paya M, Villar A. A review of natural products and plants as potential antidiabetic drugs. Journal of Ethnopharmacology 1989;27:243. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. [DOI] [PubMed] [Google Scholar]
Liu 1991
- Liu CC, Chen XJ, Fang XW. Essentials of Traditional Chinese Medicine. In: Xu XC editor(s). The English‐Chinese Encyclopedia of Practical Traditional Chinese Medicine. Vol. 1, Beijing: Higher Education Press of China, 1991:1‐4. [Google Scholar]
Luo 1998
- Luo J, Fort DM, Carlson TJ, Noamesi BK, nii‐Amon‐Kotei D, King SR, et al. Cryptolepis sanguinolenta: an ethnobotanical approach to drug discovery and the isolation of a potentially useful new antihyperglycaemic agent. Diabetic Medicine 1998;15(5):367‐74. [DOI] [PubMed] [Google Scholar]
McGrady 1999
- McGrady A, Horner J. Complementary/Alternative therapies in general medicine: diabetes mellitus. In: Spencer JW, Jacobs JJ editor(s). Complementary/Alternative Medicine: An Evidence‐Based Approach. St. Louis: Mosby Inc., 1999:107‐13. [Google Scholar]
McKinlay 2000
- McKinlay J, Marceau L. US public health and the 21st century: diabetes mellitus. Lancet 2000;356:757‐61. [DOI] [PubMed] [Google Scholar]
Melchart 1999
- Melchart D, Linde K, Weidenhammer W, Hager S, Shaw D, Bayer R. Liver enzyme elevations in patients treated with traditional Chinese medicine. JAMA 1999;282(1):28‐9. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervetion efficacy reported in meta‐analyses. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Piaggio 2001
- Piaggio G, Pinol AP. Use of the equivalence approach in reproductive health clinical trials. Statistical Medicine 2001;20(23):3571‐7. [DOI] [PubMed] [Google Scholar]
Pocock 1991
- Pocock SJ. Clinical trials. A practical approach. 2. Chichester: John Wiley & Sons, 1991. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
SDA 2000
- Department of Essential Drug. The National Essential Drug List of China. The State Drug Administration 2000.
Shen 1997
- Shen ZF. Effect of Jinqi Jiangtang recipe on, insulin resistance and immunity in experimental animals. Chinese New Drug and Clinics 1997;8(1):23. [Google Scholar]
Tomlinson 2000
- Tomlinson B, Chan TY, Chan JC, Critchley JA, But PP. Toxicity of complementary therapies: an eastern perspective. Journal of Clinical Pharmacology 2000;40(5):451‐6. [DOI] [PubMed] [Google Scholar]
Vickers 1998
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials 1998;19(2):159‐66. [DOI] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second report. Technical Report Series 646. Geneva: World Health Organisation, 1980. [PubMed]
WHO 1985
- World Health Organisation. Diabetes Mellitus: Report of a WHO Study Group. Technical Report Series No. 727. Geneva: WHO, 1985. [PubMed]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
Yeh 2003
- Yeh GY, Kaptchuk TJ, Eisenberg DM, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 2003;26(4):1277‐94. [DOI] [PubMed] [Google Scholar]
Yuan 2000
- Yuan R, Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacology and Therapeutics 2000;86:191‐8. [DOI] [PubMed] [Google Scholar]
Zhou 1980
- Zhou AF. [Literature review on Traditional Chinese Medicines for treatment of diabetes mellitus] [in Chinese]. Hubei Journal of Traditional Chinese Medicine 1980;2(4):45‐8. [Google Scholar]
Zhu 1997
- Zhu ZZ, Xiong MQ, Lin AZ. Effect of Sanhuang Jiangtang recipe on insulin peripheral resistance in type II diabetics. Chinese Journal of Integrated Traditional and Western Medicine 1997;17(10):590‐3. [PubMed] [Google Scholar]
Zhu 1999
- Zhu LZ. Clinical observation of Tangzhi Pian for improving insulin resistance in type 2 diabetes mellitus. New Chinese Medicine 1999;31(4):13. [Google Scholar]


