Abstract
Pivotal trials showed the effectiveness of the monoclonal antibody ocrelizumab in relapsing and progressive multiple sclerosis (MS). However, data on everyday practice in MS patients and markers of treatment effectiveness are scarce. We aimed to collect real-world data from ocrelizumab-treated MS patients, relapsing-remitting (RR) and progressive MS patients (PMS), including active secondary progressive MS (aSPMS) and primary progressive MS (PPMS) patients, and to explore potential prognostic factors of clinical outcome. Patients were enrolled at MS centres in the Campania region, Italy. We collected clinic-demographic features retrospectively one year before ocrelizumab start (T−1), at ocrelizumab start (T0), and after one year from ocrelizumab start (T1). We explored possible clinical markers of treatment effectiveness in those patients receiving ocrelizumab treatment for at least one year using multilevel-mixed models. We included a total of 383 MS patients (89 RRMS and 294 PMS; 205 females, mean age: 45.8 ± 11.2, disease duration: 12.7 ± 11.6 years). Patients had a mean follow-up of 12.4 ± 8.2 months, and 217 patients completed one-year ocrelizumab treatment. Overall, EDSS increased from T−1 to T0 (coeff. = 0.30, 95% coefficient interval [CI] = 0.19–0.41, p < 0.001) without a further change between T0 and T1 (p = 0.61). RRMS patients did not show an EDSS change between T−1 and T0 nor between T0 and T1. Conversely, PMS patients showed EDSS increase from T−1 to T0 (coeff. = 0.34, 95% CI = 0.22–0.45, p < 0.001) without a further change between T0 and T1 (p = 0.21). PMS patients with a time from conversion shorter than 2 years showed increased EDSS from T−1 to T0 (coeff. = 0.63, 95% CI = 0.18–1.08, p = 0.006) without a further change between T0 and T1 (p = 0.94), whereas PMS patients with a time from conversion longer than 2 years showed increased EDSS from T0 to T1 (coeff. = 0.30, 95% CI = 0.11–0.49, p = 0.002). Naïve patients showed an EDSS decrease between T0 and T1 (coeff. = −0.30, 95% CI = −0.50–−0.09, p = 0.004). In conclusion, our study highlighted that early ocrelizumab treatment is effective in modifying the disability accrual in MS patients.
Keywords: progression, multiple sclerosis, ocrelizumab, disease-modifying treatment, real-world
1. Introduction
Ocrelizumab (OCR) is a humanized monoclonal antibody that acts by depleting CD20+ B cells while preserving innate immunity. OCR was reported to be effective on disease inflammatory activity and progression for both relapsing-remitting (RR), active progressive and primary progressive multiple sclerosis (MS) patients in the OPERA I/II [1,2] and ORATORIO [3] randomised clinical trials (RCTs) without major safety concerns. Similarly, OCR was reported to prevent disability accrual in both RRMS and primary progressive MS patients and hence, it may also be useful in those patients with progressive disease course with or without relapses following the clear-cut RR disease stage (i.e., active secondary progressive MS) [4,5,6].
While RCTs are extremely useful to assess treatment safety and efficacy, the population included in these studies do not necessarily reflect the heterogeneity of the population in clinical settings. Hence, real-world data are always warranted to confirm treatment efficacy and safety in clinical settings [7]. Since its approval, OCR has been widely used in clinical practice, and few real-world studies have confirmed its safety and efficacy profile in either monocentric [4,8,9,10,11] or multicentric [5,12,13] settings. The aforementioned studies and previous post-hoc analyses from RCTs also sought to investigate possible predictors of OCR efficacy with conflicting results. Specifically, post-hoc analyses from RCTs showed that OCR was equally effective in all patients independently of their age, sex, previous disease-modifying treatment and baseline EDSS [6,14].
In contrast, in a monocentric real-world setting, naïve patients and patients with a lower EDSS benefited the most from the OCR effect in terms of disability accrual [4]. Studies evaluating the predictors of OCR effectiveness in a real-world, multicentric framework are still lacking. In addition, neither the post-hoc analysis from RCTs nor the data from real-world studies explored the association between the OCR treatment start in relation to time from confirmed progressive clinical phenotype diagnosis to OCR effectiveness. In line with previously published data [15,16], we hypothesized that OCR would show an effectiveness profile overlapping with data from RCTs and that OCR may be more effective in preventing disability accrual when introduced upon the appearance of the course of the progressive disease.
2. Methods
2.1. Procedures and Participants
This retrospective study included MS patients starting treatment with OCR according to clinical practice between January 2018 and December 2019 at nine MS centres in the Campania region of Italy. We performed a descriptive analysis for the whole study sample and an analysis of effectiveness, including those patients with at least 1 year of follow-up. For each patient, we collected the following information: demographic data (i.e., gender and age), history of MS (i.e., MS onset date, MS course (RRMS or active secondary progressive and primary progressive following Lublin’s criteria [17]), annual relapse rate (ARR) in the 2 years before OCR start, time from conversion to progressive MS and EDSS 1 year before OCR start when available), date of OCR start, previous disease-modifying treatment (DMTs (DMTs were classified as a first-line treatment for interferon, glatiramer acetate, teriflunomide, dimethyl fumarate or as a second-line treatment for natalizumab, rituximab, siponimod, fingolimod and alemtuzumab)), EDSS at OCR start, and clinical outcomes (i.e., EDSS 1 year after OCR start and number of MS relapses). The time from conversion to secondary progressive MS was retrospectively collected. Clinicians made the SPMS diagnosis at each participating site following the Lublin criteria [17] based on a ‘steadily increasing, objectively documented neurological dysfunction/disability without recovery’. Patients were classified as naïve if they did not receive any DMTs before OCR started.
The present study was conducted in accordance with specific national laws and the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Given its retrospective design, this study did not interfere with the care received by patients. In addition, specific ethical approval was not required due to the retrospective design and since all clinical assessments were part of the clinical practice in a university- or hospital-based specialized centre setting. However, as per Italian regulations [18], the principal investigator site notified the local ethics committee ‘Carlo Romano’ about this retrospective study. Patients provided their informed consent to collect data for clinical purposes.
2.2. Statistical Analyses
Demographic and clinical features for the study population are presented using mean standard deviation (SD), median and range as appropriate. Statistical analysis was performed by AC, who was blind to patients’ identities and did not contribute to data collection. The effectiveness of OCR was evaluated by analysing (i) occurrence of relapse, (ii) EDSS at 1 year after OCR start. To properly evaluate the effect of OCR in modifying the trajectory of disability accrual, we evaluated changes in EDSS 1 year before OCR start (T−1), EDSS at OCR start (T0), and EDSS 1 year after OCR start (T1) using multivariable mixed models including time points as factor of interest, EDSS as the dependent variable, age, gender and centre as covariates, and subject ID as a random factor. To further explore possible demographic and clinical features affecting the effectiveness of OCR in modifying disability accrual trajectories, following the previous post-hoc analysis from RCTs [6], we performed the same analysis by dividing patients according to age (less or more than 40 years), gender, clinical disease course (relapsing vs. progressive MS), progressive MS course (active secondary progressive MS vs. primary progressive), DMTs status (naïve vs. non-naïve) and EDSS (<4 vs. ≥4).
Furthermore, we also performed the same analysis by dividing patients according to disease duration (≤10 years vs. >10 years) and time from secondary progressive MS diagnosis following Lublin’s phenotypic classification [17] (≤2 years vs. >2 years). Cut-offs were selected considering that 10 years is the time from relapsing-remitting to progressive disease course in terms of natural MS history [19,20]. In comparison, 2 years is the transitioning period where uncertainty exists for clinicians in defining the transition from relapsing to progressive disease course [21].
3. Results
3.1. Clinico-Demographic Features
We included 383 OCR-treated patients (205 females, mean age 45.8 ± 11.2 years). Eighty-nine patients (23%) were RRMS, whereas 294 patients (77%) were progressive MS (PMS) patients. Specifically, 165 patients (43.1%) were active secondary progressive patients and 129 (33.7%) were primary progressive MS patients. Median EDSS was 5.5 (1–8.5) with a median disease duration of 11 years (0–41). Seventy-four patients (19.3%) were naïve to any DMTs, 154 patients (40.2%) switched to OCR from first-line DMTs, and 155 patients (40.5%) switched to OCR from second-line DMTs. Overall, patients were followed up for 12.4 ± 8.2 months, with 217 (57%) patients receiving follow-up for more than 12 months. Demographic and clinical features for the total sample and for the sample included in the effectiveness analysis are reported in Table 1.
Table 1.
Demographic and clinical features for the whole sample and for patients included in the effectiveness analysis.
| Total Population | Patients with at Least 1 Year Follow-Up |
p-Value | |
|---|---|---|---|
| Number of subjects | 383 | 217 | |
| Sex | |||
| Male, N (%) | 178 (46.5) | 115 (53) | 0.13 |
| Female, N (%) | 205 (53.5) | 102 (47) | |
| Age, mean (SD) (years) | 45.8 (11.2) | 46.6 (10.6) | 0.37 |
| ARR pre-OCR start, mean (SD) | 0.29 (0.3) | 0.28 (0.33) | 0.72 |
| EDSS 1 year pre-OCR start, median (Range) | 5 (0–8) | 5.25 (1–8) | 0.39 |
| EDSS at OCR start, median (Range) | 5.5 (1–8.5) | 5.5 (1–8.5) | 0.27 |
| Disease duration, median (Range) (years) | 11 (0–41) | 11 (1–41) | 0.76 |
| MS course | |||
| Relapsing-remitting, N (%) | 89 (23) | 32 (15) | 0.04 * |
| Active secondary progressive, N (%) | 165 (43) | 100 (46) | |
| Primary progressive, N (%) | 129 (34) | 85 (39) | |
| Previous Therapy | |||
| First-line, N (%) | 154 (40.2) | 96 (44) | 0.78 |
| Second-line, N (%) | 155 (40.5) | 80 (37) | |
| Naïve, N (%) | 74 (19.3) | 41 (19) | |
| Ocrelizumab courses, median (Range) | 2 (1–5) | 4 (2–5) | <0.001 * |
Abbreviations: N = number; SD = standard deviation; MS = multiple sclerosis; ARR = annualised relapse rate; OCR = ocrelizumab, EDSS = expanded disability status scale. * whole sample vs. sample for the effectiveness analysis, p < 0.05.
3.2. Effectiveness Analysis
Patients included in the effectiveness analysis did not differ in terms age, sex, ARR, disease duration, EDSS at T−1 and T0 and treatment status compared with the whole sample. Conversely, in the effectiveness analysis, there was a higher prevalence of both active secondary progressive MS patients (43% vs. 46%, p = 0.04) and primary progressive MS patients compared with the whole sample (34% vs. 39%, p = 0.04) (Table 1). For those patients who received follow-up for at least 1 year, two out of 185 (1%) patients experienced a relapse over the follow-up time period with a mean time from T0 of 5 months.
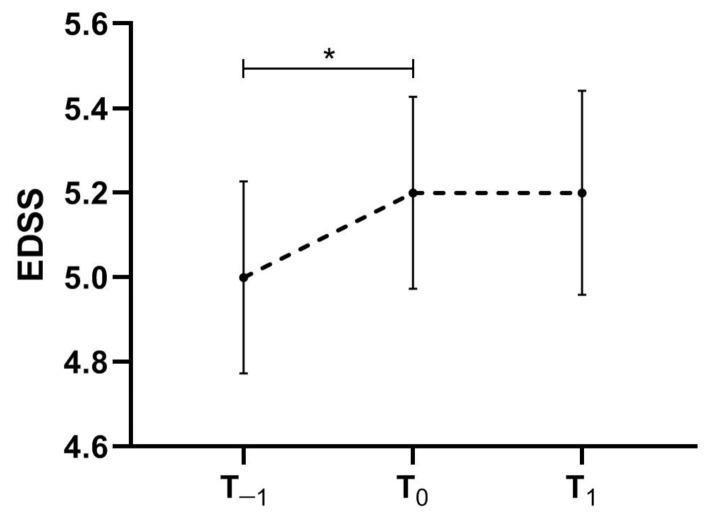
Overall, EDSS increased from T−1 to T0 (coeff. = 0.30, 95% coefficient interval [CI] = 0.19–0.41, p < 0.001) without a further change between T0 and T1 (p = 0.61) (Figure 1).
Figure 1.
EDSS trajectory between T−1, T0 and T1 for the whole multiple sclerosis sample included in the effectiveness analysis. * p < 0.05 at multivariable mixed models including time-points as factor of interest, EDSS as the dependent variable, age, gender and centre as covariates and subject ID as a random factor. Dash line represents EDSS trajectory.
3.3. Post-Hoc Effectiveness Analysis
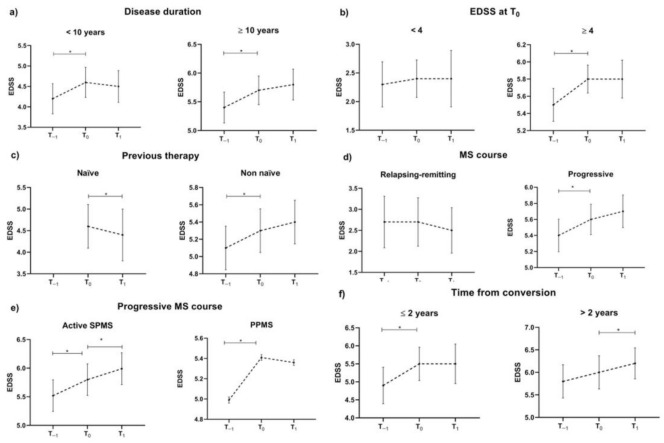
The results of the post-hoc analysis are summarised in Table 2 and Figure 2. Age and gender did not affect OCR effectiveness.
Table 2.
Post-hoc effectiveness analysis.
| Features | Number | EDSS (Mean [SD]) | ||||
|---|---|---|---|---|---|---|
| T−1 | T0 | T1 | p-Value (T0 vs. T−1) | p-Value (T1 vs. T0) | ||
| Sex | ||||||
| Male | 115 | 4.9 (1.8) | 5.1 (1.7) | 5.2 (1.8) | <0.001 * | 0.08 |
| Female | 102 | 5.1 (1.7) | 5.3 (1.6) | 5.2 (1.8) | <0.001 * | 0.26 |
| Age | ||||||
| <40 years | 63 | 4.0 (2.0) | 4.3 (2.0) | 4.5 (2.2) | <0.001 * | 0.10 |
| ≥40 years | 154 | 5.3 (1.4) | 5.5 (1.4) | 5.5 (1.5) | <0.001 * | 0.78 |
| Disease duration | ||||||
| <10 years | 94 | 4.2 (1.8) | 4.6 (1.8) | 4.5 (1.9) | <0.001 * | 0.23 |
| ≥10 years | 123 | 5.4 (1.5) | 5.7 (1.4) | 5.8 (1.5) | 0.002 * | 0.05 |
| EDSS at T0 | ||||||
| <4 | 38 | 2.3 (1.2) | 2.4 (1.0) | 2.4 (1.5) | 0.96 | 0.88 |
| ≥4 | 179 | 5.5 (1.3) | 5.8 (1.1) | 5.8 (1.5) | <0.001 * | 0.65 |
| Previous Therapy | ||||||
| Naïve | 41 | - | 4.6 (1.6) | 4.4 (1.9) | - | 0.004 * |
| Non-naïve | 176 | 5.1 (1.7) | 5.3 (1.7) | 5.4 (1.7) | <0.001 * | 0.09 |
| MS course | ||||||
| Relapsing-remitting | 32 | 2.7 (1.7) | 2.7 (1.6) | 2.5 (1.5) | 0.60 | 0.08 |
| Progressive | 185 | 5.4 (1.4) | 5.6 (1.3) | 5.7 (1.4) | <0.001 * | 0.21 |
| Progressive MS course | ||||||
| Active secondary progressive MS | 100 | 5.5 (1.4) | 5.8 (1.4) | 6.0 (1.4) | <0.001* | 0.009* |
| Primary progressive MS | 85 | 5.0 (0.1) | 5.4 (0.1) | 5.4 (0.1) | <0.001* | 0.87 |
| Time from conversion | ||||||
| ≤2 years | 24 | 4.9 (1.2) | 5.5 (1.1) | 5.5 (1.3) | 0.006 * | 0.94 |
| >2 years | 66 | 5.8 (1.5) | 6.0 (1.5) | 6.2 (1.4) | 0.10 | 0.002 * |
Abbreviations: MS = multiple sclerosis; EDSS = expanded disability status scale. * multivariable mixed models including time-points as a factor of interest, EDSS as the dependent variable, age, gender and centre as covariates and Subject ID as a random factor.
Figure 2.
Post-hoc effectiveness analysis. EDSS trajectory between T−1, T0 and T1 according to disease duration (a), EDSS at T0 (b), treatment status (c), MS disease course (d,e) and time from conversion (f). * p < 0.05 at multivariable mixed models including time-points as a factor of interest, EDSS as the dependent variable, age, gender and centre as covariates and subject ID as a random factor. Dash lines represent EDSS trajectory.
Patients with a disease duration shorter than 10 years showed increased EDSS from T−1 to T0 (coeff. = 0.43, 95% CI = 0.24–0.63, p < 0.001) without a further change between T0 and T1 (p = 0.23). Conversely, patients with disease duration longer than 10 years showed increased EDSS from T−1 to T0 (coeff. = 0.21, 95% CI = 0.08–0.34, p = 0.002) with a trend toward a further EDSS increase from T0 and T1 (coeff. = 0.13, 95% CI = 0.00–0.27, p = 0.05).
Patients with EDSS at T0 lower than 4 showed no EDSS change between T−1, T0 and T1, while patients with EDSS at T0 higher than 4 showed increased EDSS from T−1 to T0 (coeff. = 0.36, 95% CI = 0.25–0.47, p < 0.001) without a further change between T0 and T1 (p = 0.65).
Non-naïve patients showed increased EDSS from T−1 to T0 (coeff. = 0.28, 95%CI = 0.17–0.40, p < 0.001) without a further change between T0 and T1 (p = 0.10). Naïve patients showed EDSS decrease between T0 and T1 (coeff. = −0.30, 95%CI = −0.50–−0.09, p = 0.004).
RRMS patients did not show EDSS change between T−1 and T0 nor between T0 and T1. Conversely, PMS patients showed EDSS increase from T−1 to T0 (coeff. = 0.34, 95%CI = 0.22–0.45, p < 0.001) without a further change between T0 and T1 (p = 0.21). In detail, active secondary progressive MS patients showed EDSS increase from T−1 to T0 (coeff. = 0.23, 95%CI = 0.12–0.35, p < 0.001) and between T0 and T1 (coeff. = 0.19, 95%CI = 0.05–0.33, p = 0.009) while primary progressive MS patients showed EDSS increase from T−1 to T0 (coeff. = 0.34, 95%CI = 0.21–0.46, p < 0.001) without a further change between T0 and T1 (p = 0.87). When grouping secondary progressive MS patients according to time from conversion (shorter or longer than 2 years), only patients with a shorter time from conversion showed stable EDSS during therapy (shorter than 2 years: coeff. = 0.02, 95%CI = −0.42–0.45, p = 0.94; longer than 2 years: coeff. = 0.30, 95%CI = 0.11–0.49, p = 0.002)
4. Discussion
Previous studies have shown that DMTs are generally more effective in RRMS if used earlier in the disease course than later [16]. OCR was also reported to be more effective in preventing the reaching of the milestone EDSS 6.5 when started early over the disease course in RRMS [15]. Similarly, it may be hypothesized that also in progressive clinical phenotypes, early OCR treatment start might show a higher efficacy in mitigating disease activity and disability accrual. In light of this background, in this study, we aimed to investigate the efficacy of OCR treatment in both RRMS and PMS patients in a real-world, multicentric Italian setting and to explore the potential impact of early OCR introduction as a prognostic factor for treatment success in reducing disability accrual.
The clinical effectiveness data in our cohort are generally in line with those reported in the pivotal phase 3 clinical trials and in the few real-world studies conducted so far [2,3,8,9,10,11,12].
With patients who received follow up for at least 1 year, OCR showed a strong effect on relapse occurrence, with only two patients experiencing relapses with a mean time from T0 of 5 months. Compared with the OCR phase 3 trials OPERA I and II, our RRMS cohort at the time of OCR initiation was older (37 vs. 46 years), had a longer disease duration (6.7 vs. 11 years) and patients were less frequently treatment-naïve (74% vs. 19%) [2]. The mean number of relapses in the year prior to OCR initiation was remarkably lower than in the OPERA trials (mean ± SD, 0.28 ± 0.33 vs. 1.3 ± 0.6) [2]. This may be explained by the older age, longer disease duration, lower proportion of treatment-naïve patients in our cohort and by the requirement of relapse activity prior to screening as a key eligibility criterion in the OPERA trials [2]. Despite these differences, OCR efficacy on clinical disease activity was remarkable.
Overall, in our study, EDSS did not change with OCR treatment despite a previously increasing trend from T−1 to T0, in line with OCR efficacy on EDSS progression shown both in RR and primary progressive MS patients in OPERA and ORATORIO studies [2,3]. According to the OPERA and ORATORIO results, we also reported OCR efficacy in slowing disability accrual in the RRMS and primary progressive MS patients at the subgroup analysis in our real-world setting. This would suggest that OCR could not exert any effect on disability accrual on secondary progressive MS. However, taking a closer look at our post-hoc effectiveness analysis revealed that patients with time from conversion to secondary progressive MS ≤ 2 years benefited the most from OCR treatment in terms of disability accrual after 1 year of follow-up. In addition, DMT-naïve patients showed an EDSS decrease between T0 and T1, thus suggesting that, independently of disability level and disease duration, OCR is more able to exert an effect on an immune system, which has not been previously targeted with other medications [22]. Therefore, despite the short follow-up period, our results yield evidence of a precocious positive action of OCR on disability accrual trajectory also in secondary progressive MS patients, since the trend was of EDSS increase over the previous year.
These are relevant data helping MS experts in unravelling therapeutic decisions since we now have evidence of outcome prognostic factors.
The Limitations of our study include the retrospective design, which does not consider all possible confounding factors. Also, our population is from a well-defined geographical region, and its generalizability cannot be fully addressed. In addition, the follow-up is limited to 12.4 ± 8.2 months. This framework may hamper the proper evaluation of OCR efficacy for slowing down EDSS progression, and hence, real-world studies with periods of longer follow-up should be performed to confirm our results. In addition, the high variability in the sample clinical features (i.e., large disease duration and EDSS span) may also impact the significance of our results. Unfortunately, real-world studies are burdened with a large variability of demographic and clinical features for subjects involved in the study, especially in a multicentric framework. While this could be regarded as a limitation, such variability reflects the clinical settings and hence, represents the way clinicians may choose among different treatments to efficaciously treat their patients. Finally, we based our study on clinical outcomes. MRI outcomes are of utmost importance in multiple sclerosis. However, these data could not have been retrieved in a standardised manner in our multicentric study; hence, we could not draw any conclusion about OCR efficacy in terms of MRI activity in MS.
5. Conclusions
Our study confirmed in a real-world setting that OCR is an effective treatment for both RRMS and PMS patients. Furthermore, OCR is more effective in patients at the early stages during the disease course (naïve patients and patients recently converting to progressive phenotypes). Our study highlighted that early OCR treatment is effective in modifying the disability accrual in MS patients and, hence, OCR should be administered timely to catch the therapeutic window.
Author Contributions
R.L., A.C. and V.B.M., design and supervision of the study; A.C., data analysis; R.L., A.C. and V.B.M., manuscript drafting; R.L., E.S., R.I., G.M. (Giuseppina Miele), A.B., G.T.M. (Giorgia Maniscalco), L.S., F.R., M.D.G., L.L., F.T., M.M., M.F., N.C., R.D., M.P. (Maria Petracca), A.L.S., A.G., M.P. (Martina Petruzzo), M.D.A., S.B., G.L., G.T. and V.B.M., data acquisition and manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. Given its observational design, this study did not interfere with the clinical care received. Due to the retrospective design and since all clinical assessments were part of the clinical practice in a university- or hospital-based specialized centre setting, specific ethical approval was not required. Patients provided their informed consent to collect data for clinical purposes.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon reasonable request to the corresponding author.
Conflicts of Interest
Antonio Carotenuto received research grants from ALMIRALL, and honoraria from Novartis, Merck, and Biogen. Roberta Lanzillo received personal compensation for speaking or consultancy from Biogen, Teva, Genzyme, Merck, Novartis, and Almirall. Vincenzo Brescia Morra received personal compensation for speaking or consultancy from Biogen, Teva, Genzyme, Merck, Novartis, and Almirall. Elisabetta Signoriello received speaker honoraria and/or consultancy fees from Biogen, Teva, Genzyme, Merck, Novartis, Almirall, and Roche. Marcello Moccia has received research grants from MAGNIMS-ECTRIMS, UK MS Society, and Merck; and honoraria from Ipsen, Merck, Roche, and Sanofi-Genzyme. Giacomo Lus received personal compensation for speaking or consultancy from Biogen, Teva, Genzyme, Merck, Novartis, Almirall, Merz, and Ipsen. Simona Bonavita received speaker honoraria and/or advisory board from Biogen-Idec, Sanofi-Genzyme, Bristol-Meyers, Merck-Serono, Novartis and Viatris. Leonardo Sinisi received advisory board honoraria and congress grants from Almirall, Biogen, Sanofi-Genzyme, Bristol-Meyers, Merck, Novartis, Viatris. Maria Petracca discloses travel/meeting expenses from Novartis, Roche and Merck, speaking honoraria from HEALTH&LIFE S.r.l., honoraria for consulting services from Biogen, and research grants from the Italian MS Foundation. Antonio Gallo received personal compensation for speaking and consultancy from Biogen, Bristol Myers Squibb, Merck-Serono, Mylan, Novartis, Roche, Sanofi-Genzyme, and Teva. Maria Di Gregorio received grants for traveling and congress participation by Biogen, Merck, Novartis, Teva, Roche and Sanofi-Genzyme. She also served as an advisory board member for Biogen, Merck, Novartis, and Bristol Myers Squibb.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kappos L., Li D., Calabresi P.A., O’Connor P., Bar-Or A., Barkhof F., Yin M., Leppert D., Glanzman R., Tinbergen J., et al. Ocrelizumab in relapsing-remitting multiple sclerosis: A phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 2.Hauser S.L., Bar-Or A., Comi G., Giovannoni G., Hartung H.P., Hemmer B., Lublin F., Montalban X., Rammohan K.W., Selmaj K., et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 3.Montalban X., Hauser S.L., Kappos L., Arnold D.L., Bar-Or A., Comi G., de Seze J., Giovannoni G., Hartung H.P., Hemmer B., et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 4.Cellerino M., Boffa G., Lapucci C., Tazza F., Sbragia E., Mancuso E., Bruschi N., Minguzzi S., Ivaldi F., Poire I., et al. Predictors of Ocrelizumab Effectiveness in Patients with Multiple Sclerosis. Neurotherapeutics. 2021;18:2579–2588. doi: 10.1007/s13311-021-01104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rojas J.I., Patrucco L., Fruns M., Hornung G., Flores J., Carnero Contentti E., Lopez P.A., Pettinicchi J.P., Caride A., Galleguillos L., et al. Real-world experience of ocrelizumab in multiple sclerosis patients in Latin America. Arq. Neuro-Psiquiatr. 2021;79:305–309. doi: 10.1590/0004-282x-anp-2020-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner B., Cree B.A.C., Kappos L., Montalban X., Papeix C., Wolinsky J.S., Buffels R., Fiore D., Garren H., Han J., et al. Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis. J. Neurol. 2019;266:1182–1193. doi: 10.1007/s00415-019-09248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalincik T., Kuhle J., Pucci E., Rojas J.I., Tsolaki M., Sirbu C.A., Slee M., Butzkueven H., Group M.S.S.L., Group M.S.S. Data quality evaluation for observational multiple sclerosis registries. Mult. Scler. 2017;23:647–655. doi: 10.1177/1352458516662728. [DOI] [PubMed] [Google Scholar]
- 8.Daniels K., van der Nat P.B., Frequin S., van der Wees P.J., Biesma D.H., Hoogervorst E.L.J., van de Garde E.M.W. Real-World Results of Ocrelizumab Treatment for Primary Progressive Multiple Sclerosis. Mult. Scler. Int. 2020;2020:5463451. doi: 10.1155/2020/5463451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prockl V., Nickel F.T., Utz K.S., Frohlich K., Engelhorn T., Hilz M.J., Lee D.H., Linker R.A., Huhn K. Real world application of ocrelizumab in multiple sclerosis: Single-center experience of 128 patients. J. Neurol. Sci. 2020;415:116973. doi: 10.1016/j.jns.2020.116973. [DOI] [PubMed] [Google Scholar]
- 10.Sempere A.P., Berenguer-Ruiz L., Borrego-Soriano I., Burgos-San Jose A., Concepcion-Aramendia L., Volar L., Aragones M., Palazon-Bru A. Ocrelizumab in Multiple Sclerosis: A Real-World Study From Spain. Front. Neurol. 2020;11:592304. doi: 10.3389/fneur.2020.592304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coban H., Germaine S., Dimaandal I., Haberli N., Padam C., Creed M.A., Imitola J. Real-world experience of ocrelizumab initiation in a diverse multiple sclerosis population. Mult. Scler. Relat. Disord. 2021;53:103021. doi: 10.1016/j.msard.2021.103021. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Diaz E., Perez-Vicente J.A., Villaverde-Gonzalez R., Berenguer-Ruiz L., Candeliere Merlicco A., Martinez-Navarro M.L., Gracia Gil J., Romero-Sanchez C.M., Alfaro-Saez A., Diaz I., et al. Real-world experience of ocrelizumab in multiple sclerosis in a Spanish population. Ann. Clin. Transl. Neurol. 2021;8:385–394. doi: 10.1002/acn3.51282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pontieri L., Blinkenberg M., Bramow S., Papp V., Rasmussen P.V., Kant M., Schafer J., Mathiesen H.K., Jensen M.B., Sirakov G., et al. Ocrelizumab treatment in multiple sclerosis: A Danish population-based cohort study. Eur. J. Neurol. 2022;29:496–504. doi: 10.1111/ene.15142. [DOI] [PubMed] [Google Scholar]
- 14.Wolinsky J.S., Engmann N.J., Pei J., Pradhan A., Markowitz C., Fox E.J. An exploratory analysis of the efficacy of ocrelizumab in patients with multiple sclerosis with increased disability. Mult. Scler. J. Exp. Transl. Clin. 2020;6:1–7. doi: 10.1177/2055217320911939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovannoni G., Kappos L., de Seze J., Hauser S.L., Overell J., Koendgen H., Manfrini M., Wang Q., Wolinsky J.S. Risk of requiring a walking aid after 6.5 years of ocrelizumab treatment in patients with relapsing multiple sclerosis: Data from the OPERA I and OPERA II trials. Eur. J. Neurol. 2021;29:1238–1242. doi: 10.1111/ene.14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalmer T.A., Baggesen L.M., Norgaard M., Koch-Henriksen N., Magyari M., Sorensen P.S., Danish Multiple Sclerosis G. Early versus later treatment start in multiple sclerosis: A register-based cohort study. Eur. J. Neurol. 2018;25:1262-e1110. doi: 10.1111/ene.13692. [DOI] [PubMed] [Google Scholar]
- 17.Lublin F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014;72((Suppl. S1)):1–5. doi: 10.1159/000367614. [DOI] [PubMed] [Google Scholar]
- 18.Agenzia Italiana del Farmaco (AIFA) Determinazione dell’Agenzia Italiana del Farmaco, 20 Marzo 2008. [(accessed on 2 April 2022)];Gazzetta Ufficiale della Repubblica Italiana. 2008 76:67–74. Available online: http://www.omceoge.org/newsdoc/AIFA_20_3_2008.pdf. [Google Scholar]
- 19.Weinshenker B.G., Bass B., Rice G.P., Noseworthy J., Carriere W., Baskerville J., Ebers G.C. The natural history of multiple sclerosis: A geographically based study. I. Clinical course and disability. Pt 1Brain. 1989;112:133–146. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- 20.Runmarker B., Andersen O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Pt 1Brain. 1993;116:117–134. doi: 10.1093/brain/116.1.117. [DOI] [PubMed] [Google Scholar]
- 21.Carotenuto A., Signoriello E., Lanzillo R., Vaia Y., Moccia M., Bonavita S., Lus G., Brescia Morra V. Unraveling diagnostic uncertainty in transition phase from relapsing-remitting to secondary progressive multiple sclerosis. Mult. Scler. Relat. Disord. 2020;43:102211. doi: 10.1016/j.msard.2020.102211. [DOI] [PubMed] [Google Scholar]
- 22.Capasso N., Nozzolillo A., Scalia G., Lanzillo R., Carotenuto A., De Angelis M., Petruzzo M., Sacca F., Russo C.V., Brescia Morra V., et al. Ocrelizumab depletes T-lymphocytes more than rituximab in multiple sclerosis. Mult. Scler. Relat. Disord. 2021;49:102802. doi: 10.1016/j.msard.2021.102802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request to the corresponding author.