Abstract
The pharmacodynamic properties of the ketolides HMR 3647 (telithromycin) and HMR 3004 were studied against Helicobacter pylori. Both ketolides showed a pronounced concentration-dependent killing, a significant postantibiotic effect, a long postantibiotic sub-MIC effect, and a reduction of intracellular H. pylori.
Helicobacter pylori establishes persistent infection in the gastric mucosa of humans. Antimicrobial treatment of H. pylori is now widely recommended for patients with peptic ulcer who are carrying this bacterium. H. pylori has so far been considered to colonize exclusively extracellularly. There are, however, considerable data supporting an intracellular location of the bacterium in vivo (4, 8). Due to the increasing problems with macrolide resistance in H. pylori (5, 9), the development of new treatment regimens, including antibiotics with intracellular activity, is needed. The aim of our study was to evaluate the pharmacodynamic properties of the ketolides HMR 3647 (telithromycin) and HMR 3004 against extracellular and intracellular H. pylori.
(This work was presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September, 1998.)
H. pylori NCTC 11637 was grown on chocolate agar plates (Columbia II agar base BBL; Becton Dickinson and Co., Franklin Lake, N.J.) supplemented with 10% horse serum and 8.5% horse blood. For broth cultures we used brucella broth (pH 7.3) supplemented with 10% fetal calf serum. Plates and broth cultures were incubated at 37°C in a humid atmosphere under microaerophilic conditions (7% CO2 and 87% N2). Broth cultures, incubated for 3 days, were prepared prior to the experiments. The turbidity was measured at an optical density at 530 nm of 0.5, which corresponds to 108 CFU/ml. HMR 3647 and HMR 3004 with known potencies were kindly provided by Hoechst Marion Roussel, Romainville, France. A stock solution was made before each experiment by dissolving 10.2 mg of the drug in 10 ml of sterile distilled water supplemented with 20 μl of glacial acetic acid.
MICs were determined by the macrodilution technique with an inoculum of 5 × 105 to 1 × 106 CFU/ml, according to NCCLS standards (11). The MIC was defined as the lowest concentration inhibiting visible growth after 3 days. To investigate whether the killing pattern was concentration dependent, bacteria at a density of 106 CFU/ml were exposed to 2, 5, 10, and 50 times the MIC. A growth control was also included. Samples were withdrawn after 0, 1, 2, 3, 6, 9, 12, and 24 h, and appropriate dilutions were seeded on chocolate agar in volumes of 0.1 and 0.01 ml. The plates were incubated for 5 days before the colonies were counted. The sensitivity of the viable count is estimated at 10 to 50 CFU/ml.
Postantibiotic effect (PAE) and postantibiotic sub-MIC effect (PASME) determinations were performed with cultures of 107 CFU/ml exposed to 10 times the MIC for 2 h at 37°C. Unexposed controls were included. To eliminate the antibiotics, both cultures were washed twice with broth by centrifugation for 10 min at 1,400 × g and then diluted in fresh broth to obtain 104 CFU/ml. The bacterial culture in the postantibiotic phase was divided into three tubes, of which two tubes were reexposed to subinhibitory concentrations of 0.1 and 0.3 times the MIC. Regrowth was followed by viable count at the start, before and after the wash, and after 4, 6, 8, 11, 24, 48, and 72 h. The PAE and PASME were defined according to the following formula: TPA − C, where TPA is the time required for the count for previously exposed cultures to increase by 1 log10 unit above the count observed immediately after the wash. C is the corresponding time for the unexposed control. The experiments were done in triplicate.
Intracellular activity was studied in a human epithelial cell line, HEp-2 (ATCC CCL 23). The cell culture medium (pH 7.2) contained RPMI 1640 (Gibco BRL Life Technologies, Paisley, Scotland), 10% fetal calf serum, 20 mM HEPES, 2 mM glutamine, and 0.05% NaHCO3. The cells were treated with trypsin, seeded into cell culture inserts with pores measuring 0.45 μm (Becton Dickinson) at a density of 105 cells/ml, and incubated at 35°C with 5% CO2. Monolayers of HEp-2 cells were exposed to 106 CFU of H. pylori/ml suspended in cell medium and allowed to internalize for 9 h at 35°C with 5% CO2. Extracellular bacteria were eradicated by washing the wells five times with medium containing 50 mg of gentamicin (Roussel, Denham Uxbridge, United Kingdom) per liter, followed by incubation for 2 h in the same medium. To verify that the extracellular H. pylori had been eradicated, samples were plated onto chocolate agar. The cell culture inserts fit into a specially constructed in vitro kinetic apparatus (7). Briefly, it consisted of a glass chamber with two exits, which connects to a pump (C6-T; Alitea AB, Stockholm, Sweden). A metal rack for the inserts was placed in the chamber, and a magnet ensured homogenous mixing. Cell culture medium, supplemented with 0.03 M glucose and 0.0001% cycloheximide (Sigma, St. Louis, Mo.), and the antibiotics at a concentration of 10 times the MIC were added into the glass chamber and inserts. The flow rate was set to achieve a half-life inside the inserts of approximately 12 h, which corresponds to the kinetics in humans. The device was placed on a magnetic stirrer standing in a thermostat set at 35°C with 5% CO2. Controls without antibiotics were grown simultaneously in a companion tissue culture plate. After 0, 2, 4, 8, and 24 h, inserts were collected for viable counts and determination of antibiotic concentration. The cells were washed three times with phosphate-buffered saline, removed from the membranes by gentle scraping with a transfer pipette, and lysed with distilled water for 10 min. After 5 min of centrifugation at 10,000 × g, the pellets were dissolved in 1 ml of brucella broth and plated on chocolate agar as described above. The experiments were done in triplicate.
The antibiotic concentration was determined in the media from both the glass chamber and the inserts before cell lysis. A microbiological agar diffusion method was used with tryptone-glucose agar (pH 7.4). Plates were seeded with a standardized inoculum of Sarcina lutea. Antibiotic standards and samples were placed into agar wells at a volume of 0.03 ml. All assays were done in triplicate, and the plates were incubated overnight at 37°C. The limit of detection was 0.03 mg/liter, and the correlation coefficient for the standard curves was always >0.99.
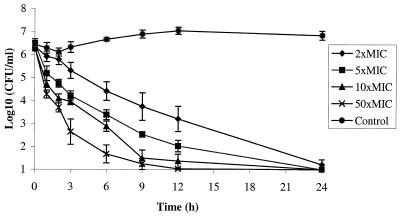
The MIC for H. pylori NCTC 11637 was 0.5 and 0.125 mg/liter for HMR 3647 and HMR 3004, respectively. A pronounced concentration-dependent killing was found with both ketolides. For HMR 3647, a bactericidal effect of 3 log10 was seen within 3 h for 50 times the MIC, within 6 h for 10 and 5 times the MIC, and within 12 h for 2 times the MIC (Fig. 1). For HMR 3004, the bactericidal effect was achieved at 6 h with 50 times the MIC and at 12 h with 10 times the MIC. At the lower concentrations of 5 and 2 times the MIC, the reduction was 2 log10 after 24 h. The PAEs for HMR 3647 and HMR 3004 were 13 h (range, 8 to 22 h) and 6 h (range, 5 to 33 h), respectively. Addition of a subinhibitory concentration of 0.1 times the MIC in the postantibiotic phase yielded long PASMEs ranging from 24 to >48 h for HMR 3647 and 12 h for HMR 3004. At the concentration of 0.3 times the MIC, no regrowth could be detected within 72 h for either of the ketolides.
FIG. 1.
Concentration-dependent killing with HMR 3647. Bars indicate standard deviations.
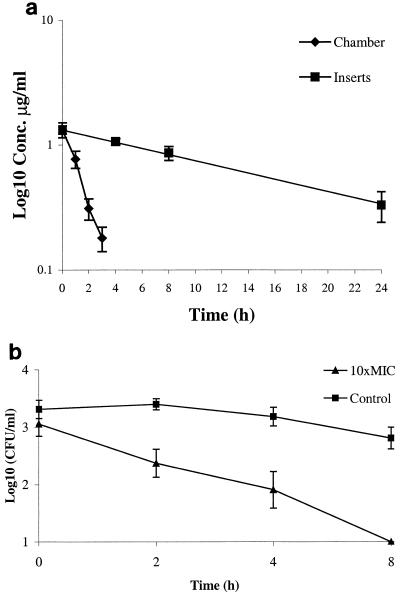
The antibiotic concentration within the culture inserts is presented in Fig. 2a. The mean half-life was determined to be 13 h (range, 6.5 to 16.6 h). The experiments were limited to 8 h since the number of viable bacteria in the controls then declined. Therefore, the effect of subinhibitory concentrations could not be studied in these experiments. The intracellular killing during 8 h was 2 log10 for HMR 3647 (Fig. 2b) and 1.8 log10 for HMR 3004.
FIG. 2.
(a) Concentration curve for HMR 3004. Bars indicate standard deviations. (b) Intracellular H. pylori exposed to 10 times the MIC of HMR 3647. Bars indicate standard deviations.
The ketolide HMR 3647 (telithromycin) belongs to a new class of macrolides and is presently in clinical development. Although earlier macrolides exhibit a long PAE, their antibacterial activity is not usually concentration dependent and the major determinant of killing is the duration of free drug concentrations above the MIC (2). The pharmacodynamics of the ketolides is less well known. However, a significant PAE and PASME have been demonstrated (1; I. Odenholt, E. Löwdin, and O. Cars, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-540, 1999), as has concentration-dependent killing (W. A. Craig and D. R. Andes, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2141, 2000). Our study showed a clear concentration-dependent killing of H. pylori. Sörberg et al. demonstrated a long PAE of approximately 15 h for H. pylori after exposure to 10 times the MIC of clarithromycin for 5 h (13). We experienced a wide range of PAEs between the experiments, from 8 to 22 h. Pharmacodynamic studies on H. pylori pose technical difficulties, and even with optimal technical conditions the growth rate sometimes alters. In broth cultures the bacteria turn into a coccoid form after a period of time (3). The coccoid form is metabolically inactive, and it is not yet known what triggers the transformation. The delayed regrowth in the PAE experiments thus may be due not only to the antibiotic exposure. However, with exposure to a sub-MIC concentration of 0.1 times the MIC after preexposure to 10 times the MIC for only 2 h, the reductions in bacteria were similar to those following the static exposure to 10 times the MIC for 24 h. The subinhibitory concentration was obviously sufficient to maintain a critical concentration of drug at the site of the ribosomes. This could be explained by the fact that ketolides form a strong complex with 23S rRNA (6). This pronounced effect of subinhibitory concentrations corresponds well with results from studies on macrolides and azithromycin, in which long PASMEs have been described (12). Pharmacodynamic studies are often performed under static conditions, but it is important to include kinetic models. We have previously developed a kinetic method for intracellular pathogens that was found to be useful in an initial screening phase of drug evaluation. HMR 3647 and HMR 3004 are rapidly accumulated in HEP-2 cells, with an intracellular/extracellular ratio of about 10 (14, 15; I. Garcia, A. Pascual, S. Ballesta, and E. J. Perrera, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-112, 1998). The theory of intracellular H. pylori is still controversial but it seems that the bacteria can hide within the epithelial cells of the mucosa. This has been revealed by electron microscopy and by time-lapse photography (4, 10). In the present study a fairly constant amount of intracellular bacteria was obtained in the controls for 8 h. During this period both HMR 3647 and HMR 3004 exhibited 1.8 to 2 log10 reductions of bacteria, and the effect on intracellular H. pylori was evident. This new group of ketolide antibiotics seems to be promising for treatment of extracellular and intracellular H. pylori.
Acknowledgments
This work was supported by grants from Hoechst Marion Roussel, Romainville, France.
We thank Tord Nyström for excellent technical assistance.
REFERENCES
- 1.Boswell F J, Andrews J M, Wise R. Pharmacodynamic properties of HMR 3647, a new ketolide, on respiratory pathogens, enterococci and Bacteroides fragilis demonstrated by studies of time-kill kinetics and postantibiotic effect. J Antimicrob Chemother. 1998;41:149–153. doi: 10.1093/jac/41.2.149. [DOI] [PubMed] [Google Scholar]
- 2.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 3.Enroth H, Wreiber K, Rigo R, Risberg D, Uribe A, Engstrand L. In vitro aging of Helicobacter pylori: changes in morphology, intracellular composition and surface properties. Helicobacter. 1999;4:7–16. doi: 10.1046/j.1523-5378.1999.09034.x. [DOI] [PubMed] [Google Scholar]
- 4.Evans D G, Evans D J, Jr, Graham D Y. Adherence and internalization of Helicobacter pylori by HEp-2 cells. Gastroenterology. 1992;102:1557–1567. doi: 10.1016/0016-5085(92)91714-f. [DOI] [PubMed] [Google Scholar]
- 5.Glupczynski Y, Burette A, Lamy V, Garrino M G. Evolution of H. pylori primary resistance to antimicrobial agents in Belgium between 1995 and 1998. Gut. 1998;43(Suppl. 2):A48. [Google Scholar]
- 6.Hansen L H, Mauvais P, Douthwaite S. The macrolide-ketolide antibiotic binding site is formed by structures in domains II and V of 23S ribosomal RNA. Mol Microbiol. 1999;31:623–631. doi: 10.1046/j.1365-2958.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 7.Hultén K, Rigo R, Gustafsson I, Engstrand L. New pharmacokinetic in vitro model for studies of antibiotic activity against intracellular microorganisms. Antimicrob Agents Chemother. 1996;40:2727–2731. doi: 10.1128/aac.40.12.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hultén K. Helicobacter pylori: transmission, survival and eradication. Ph.D. dissertation. Uppsala, Sweden: Uppsala University; 1997. [Google Scholar]
- 9.Hultén K, Jaup B, Stenquist B, Engstrand L. Combination treatment with ranitidine is highly efficient against Helicobacter pylori despite negative impact of macrolide resistance. Helicobacter. 1997;2:188–193. doi: 10.1111/j.1523-5378.1997.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 10.Löfman C, Rigo R, Block M, Hultén K, Enroth H, Engstrand L. Bacterium-host interactions monitored by time-lapse photography. Nat Med. 1997;3:930–931. doi: 10.1038/nm0897-930. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. Tentative guideline M100–S8. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 12.Odenholt-Tornqvist I, Löwdin E, Cars O. Postantibiotic effects and postantibiotic sub-MIC effects of roxithromycin, clarithromycin, and azithromycin on respiratory tract pathogens. Antimicrob Agents Chemother. 1995;39:221–226. doi: 10.1128/aac.39.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sörberg M, Hanberger H, Nilsson M, Nilsson L E. Pharmacodynamic effects of antibiotics and acid pump inhibitors on Helicobacter pylori. Antimicrob Agents Chemother. 1997;41:2218–2223. doi: 10.1128/aac.41.10.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vazifeh D, Abdelghaffar H, Labro M T. Cellular accumulation of the ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob Agents Chemother. 1997;41:2099–2107. doi: 10.1128/aac.41.10.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vazifeh D, Preira A, Bryskier A, Labro M T. Interactions between HMR 3647, a new ketolide, and human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 1998;42:1944–1951. doi: 10.1128/aac.42.8.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]