Abstract
Cancer immunotherapy has achieved multiple clinical benefits and has become an indispensable component of cancer treatment. Targeting tumor-specific antigens, also known as neoantigens, plays a crucial role in cancer immunotherapy. T cells of adaptive immunity that recognize neoantigens, but do not induce unwanted off-target effects, have demonstrated high efficacy and low side effects in cancer immunotherapy. Tumor neoantigens derived from accumulated genetic instability can be characterized using emerging technologies, such as high-throughput sequencing, bioinformatics, predictive algorithms, mass-spectrometry analyses, and immunogenicity validation. Neoepitopes with a higher affinity for major histocompatibility complexes can be identified and further applied to the field of cancer vaccines. Therapeutic vaccines composed of tumor lysates or cells and DNA, mRNA, or peptides of neoantigens have revoked adaptive immunity to kill cancer cells in clinical trials. Broad clinical applicability of these therapeutic cancer vaccines has emerged. In this review, we discuss recent progress in neoantigen identification and applications for cancer vaccines and the results of ongoing trials.
Keywords: cancer therapy, neoantigens, immunotherapy, vaccine
1. Introduction
Cancers are driven by genetic instabilities that rapidly accumulate somatic mutations and eventually alter cell properties. Excellent progression has resulted from understanding the mechanisms of genetic mutations, immune recognition of tumor antigens, tumor-mediated immunosuppression, immune surveillance, and tumor escape. Genome sequencing has revealed the heterogeneity of cancer cells, as evidenced by the Cancer Genome Atlas [1,2,3,4]. Neoantigens are a group of tumor-specific antigens (TSAs) arising from genetic variations or retroelements and are considered one of the vital characteristics and derivations of cancers. They have aberrant residues caused by gene alterations that are only expressed on tumor cells and serve as ideal foreign targets for recognition by T cells with high-affinity T-cell receptors (TCRs) [3,5,6]. Theoretically, targeting neoantigens avoids unwanted off-target effects and can precisely guide effector cells to tumor cells. Neoantigen vaccination could be an active immunotherapy and provide immunogens to the immune system to elicit an antitumor immune response. Cancer vaccines have been rapidly developed as a practical method to boost target-specific humoral and cellular immunity and induce long-lasting immune protection [7]. Various vaccination approaches are under investigation and are broadly categorized based on their design methods, including tumor lysates, cell-based vaccines, gene-based vaccines, and peptide-based vaccines. This review summarizes the current knowledge, development, and challenges associated with immunotherapeutics targeting neoantigens by assessing current cancer clinical trials of vaccines to provide insights into the clinical development of personalized cancer immunotherapy.
2. Types of Cancer Antigens
Tumor cells have a wide range of protein-expression profiles that differ from those of normal cells. There are different types of tumor antigens: tumor-associated antigens (TAAs), TSAs, and unconventional antigens (UCAs) [8].
Compared to normal cells, TAAs are unmodified self-proteins that are abnormally expressed in cancerous cells due to oncogenic signaling processes. The upregulated expression of these wild-type proteins or glycoproteins enables TAAs to act as self-antigens on tumor cells. Most TAAs refer to overexpressed tumor antigens, for example, the receptor for advanced glycation endproducts-1 (RAGE-1), human telomerase reverse transcriptase (hTERT), human epidermal growth factor receptor 2 (HER2), mesothelin, and mucin 1 (MUC1) [9,10,11,12,13,14]. In addition, TAAs can be cell-lineage-differentiation antigens (e.g., prostate-specific antigen (PSA) and prostatic-acid phosphatase (PAP), which are typically not expressed in adult tissues [15,16], and cancer/germline antigens (also known as cancer/testis) (e.g., melanoma-associated antigen 1 (MAGE-A1), melanoma-associated antigen 3 (MAGE-A3), New York esophageal squamous-cell carcinoma 1 (NY-ESO-1), and preferentially expressed antigen of melanoma (PRAME), which are typically only expressed in immune-privileged germline cells [17,18,19,20,21]. These TAAs may represent universal targets for chimeric-antigen-receptor-T (CAR-T) therapy in patients with the same malignancy.
In comparison, TSAs are neoantigens expressed by cancer cells. The uniqueness of the mutant epitopes makes them more likely to be identified by the diverse TCRs of T cells, which are not depleted during clonal selection in the thymus. The degraded peptide fragments of mutant proteins become tumor antigens that play essential roles in T-cell-mediated immunity against cancer. Neoantigens could represent the differences between the peptide repertoires of the major-histocompatibility-complex (MHC) presentations of cancer cells and normal cells. Most TSAs arise from somatic mutations of non-synonymous single-nucleotide variants, frameshifts, infusion or deletion (INDEL) mutations, gene fusion, splice variants, and retroelements [22]. Unlike TAAs, which are self-antigens not recognized by T cells, TSAs are aberrant proteins absent in T-cell clonal selection during thymus education and are, therefore, more likely to escape the central tolerance mechanism.
Unconventional antigens (UCAs) originate from aberrant transcription, translation, or post-translational modifications in tumor cells. Some UCAs may be completely tumor specific, whereas others may also occur in normal cells.
3. Neoantigen-Induced Antitumor Immunity
Regarding the molecular mechanism, neoantigens are proposed to enhance their immunogenicity by modulating immune synapses in several ways: (1) Compared to wild epitopes, neoepitopes strengthen the TCR–MHC-I stability with higher levels of binding affinities and then result in a robust immune response [23]. (2) The absence of neoepitopes in MHC presentation during T-cell selection in thymus education improves TCR recognition [24]. (3) Flanking residues of neoepitopes interfere with and compete with endogenous peptides on the MHC binding groove [25].
In the tumor microenvironment (TME), abundant tumor antigens can be secreted via tumor-derived exosomes, which are further enhanced through tumor-cell death caused by immune modulation, radiotherapy, or chemotherapy [26,27,28]. Tumor-infiltrating antigen-presenting cells (APCs) capture tumor antigens and migrate to regional lymph nodes. The epitopes of the captured antigens presented on human-leukocyte-antigen (HLA) molecules of APCs can initiate the activation and differentiation of tumor-specific CD4+ and CD8+ T cells in the draining lymph node, resulting in the expansion of effector T cells in secondary lymphoid organs [29]. Many effector cells then travel through the bloodstream to the tumor site by involving various chemokines (e.g., C-C motif chemokine ligand 2 (CCL2), C-X-C motif chemokine ligand 2 (CXCL2), and C-X-C motif chemokine ligand 16 (CXCL16)) [30,31]. Activated CD8+ T cells can recognize the expressed neoepitope–HLA complexes on tumor cells and then kill cancer cells through the degranulation of cytotoxic proteins, such as perforin, granzyme, and granulysin. CD4+ T cells indirectly modulate antitumor cellular and humoral immune responses. Released tumor antigens prime more tumor-reactive immune cells into the TME and trigger adaptive-immune memory responses [29]. However, these immune reactions can be inhibited by an immunosuppressive microenvironment.
4. Neoantigen Identification
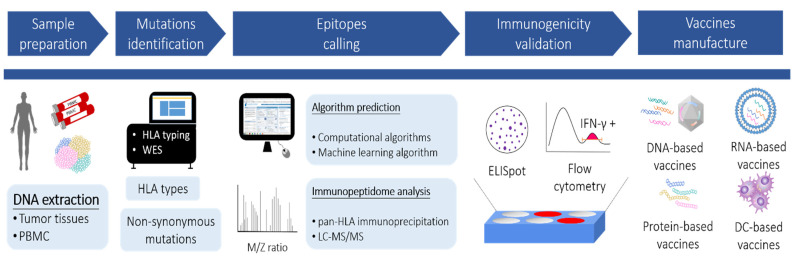
Approaches for identifying neoantigens and proceeding to vaccine manufacture are illustrated in Figure 1, which involve the discovery of the mutanome by next-generation sequencing (NGS), prediction of HLA epitopes by algorithms or mass spectrometry (MS), and functional validation by immunological assays.
Figure 1.
Schematic representation of neoantigen selection for therapeutic cancer vaccines. The DNA samples are extracted from cancer tissues and peripheral blood mononuclear cells (PBMC), respectively. Non-synonymous mutations and HLA types are obtained through whole-exome sequencing (WES) and HLA genotyping following bioinformatic analyses. The potential neoepitopes derived from the identified mutations are prioritized according to (1) algorithm prediction or (2) immunopeptidome analysis. Afterward, each prioritized neoepitope is examined by immunological assays (e.g., ELISpot or flow cytometry) to validate the immunogenicity. Vaccines encoding the selected neoepitopes are then generated in various formats, including DNA-based, RNA-based, peptide-based, and dendritic-cell (DC)-based vaccines.
4.1. Discovery of the Mutanome by Next-Generation Sequencing
Practitioners and researchers used to have technical restrictions until the advent of advanced high-throughput NGS technologies. Reliable sequencing data are generated at a lower price with greater accuracy to identify individual gene variations in tumor samples. Identifying the entirety of somatic cancer mutations in an individual tumor, referred to as variant calling of the mutanome, yields potential neoantigens. Typically, a small fraction of tumor biopsies is required for DNA and RNA sequencing to obtain the variation profile of the tumor. For SNVs, INDELs, and gene fusions, variants of the mutanome could be detected by comparing WES data from tumor tissue and healthy samples (e.g., PBMC) of the same individual to exclude germline variants [32]. Endogenous retroelement-derived antigens were identified from RNA-expression data. For splicing variants, tools such as SplAdder [33], SpliceGrapher [34], and ASGAL mainly compare the spliced alignments of RNA-seq data to genome references and then generate splicing graphs to predict alternative cleaving events [35]. In addition, these tools integrate proteomic databases to analyze cancer-specific germline and somatic mutations that are rapidly developing. A recently reported proteogenomic tool called QUILTS can be used to generate variants including SNVs, INDELs, fusions, and junctions from RNA-seq data [36].
4.2. HLA-Epitope Calling by Computational Algorithms
Only a small portion of expressed neoantigens can fit perfectly into the binding pockets of HLA molecules and possess adequate immunogenicity to elicit immune responses. Selecting neoepitopes with the highest probability of increasing tumor-specific immune responses is critical for designing neoantigen vaccines [6]. The prediction of neoepitopes using computational algorithms is a commonly applied methodology. These programs (e.g., NetMHCpan [37], MULTIPRED [38], IEDB [39], and EpitoolKit [40]) simulate the binding affinity of antigen epitopes with the MHC alleles of subjects and predict potential neoantigens.
The MHC-I-epitope-prediction algorithms have gained greater attention because hypotheses assume that CD8+ T-cell-mediated immune responses play a more vital role in antitumor immunity [41]. CD8+ T cells infiltrating the tumors has correlated with a better prognosis of the disease [42]. Furthermore, CD4+ T cells also play essential roles in cancer immunity. Kreiter et al. reported that mutant MHC-class-II epitopes could drive CD4+ T-cell-mediated therapeutic immune responses to cancer [43]. Trans et al. reported that the application of adoptive cell therapy (ACT) using neoantigen ERBB2 (HER2) interaction protein-specific CD4+ tumor-infiltrating lymphocytes (TILs) achieved tumor regression in a patient with metastatic cholangiocarcinoma [44]. Due to these clinical findings indicating that CD4+ T-cell-mediated antitumor immunity is indispensable for cancer immunotherapy, MHC-II-epitope predictors have been recently improved. For instance, NetMHCIIpan adapted the NN-align algorithms, which add the influence of the core structure of epitopes and the flanking-region characteristics, thereby substantially facilitating MHC-II-binding-prediction performance [45,46].
4.3. Identification of HLA Epitopes by Mass Spectrometry (MS)
Recent developments in MS-based sequencing technology have expanded the detection of peptide epitopes on MHC molecules [47,48]. For MS detection, HLA molecules from harvested cell lines or resected tumors can be isolated by pan-HLA immunoprecipitation (IP). After several washes to remove the unwanted mixture, binding epitopes of HLA molecules can be dissociated, purified, and subsequently analyzed by liquid chromatography–tandem MS (LC–MS/MS) [49]. Algorithms have been developed for immunogenic antigen discovery and the establishment of high-resolution, raw quantitative MS data for the patient-customized peptide repertoire, such as MaxQuant [50], SWATH-MS [51], Proteome discovery [52], and PEAKS studio [53]. MS-based sequencing enables researchers to directly identify clinically relevant neoepitopes in human cancer tissues. MS-based HLA-immunopeptidome profiling is also practical for spotting epitopes from post-translational modification [54]. For instance, a study revealed 11 epitopes from gene variants of over 90,000 immunopeptides identified from melanoma patients. Through MS analysis, phosphorylated immune epitopes were identified, and positions 4 and 6 of the 9–12-mer HLA-binding peptide were the major phosphorylation sites [55].
4.4. Prediction of HLA Epitopes by Machine Learning Algorithms
By combining it with experimental HLA-immunopeptidome profiling, machine learning in silicon algorithms was developed to provide a rapid and accurate prediction platform. Abelin et al. developed a neural-network prediction algorithm using an extensive dataset collected via MS profiling of HLA-associated peptidomes and found that it outperformed the experimentally measured epitope affinities [56]. GibbsCluster, another machine-learning model built on MS-analysis data integrated with in vitro binding-affinity results, showed an outstanding performance for predicting antigen-restricted epitopes [57,58]. In addition, Bulik-Sullivan et al. launched a computational model named EDGE for epitope prediction, which was trained using a dataset of HLA–MS neoantigen peptides and genomic data of 74 patients. EDGE validation showed a nine-fold-higher positive predictive value than that obtained from tumor test sets using binding-affinity data [59]. The in silico ligand-prediction algorithms ameliorated the previously high false-discovery rate of predicted ligands of specific HLA alleles. Nevertheless, considerable experimental data are required to train the algorithms, especially for the less prevalent HLA alleles for which there are not enough data on epitope affinity or MS results. The sensitivity of algorithms varied among different types of gene alterations and committed bioinformatics tools to optimize HLA-molecule-binding epitope prediction. The Human Immuno-Peptidome Project Consortium aims to establish a repertoire of peptides presented by HLA molecules to facilitate data collection [60]. With more disclosure of epitope sequences, a steadier immunopeptidome database will provide reliable and trustworthy predictions. Table 1 lists the methods and platforms that are commonly used to predict neoantigens.
Table 1.
Methods and platforms commonly used for predicting neoantigens.
| Method [Ref] | Principle | Year |
|---|---|---|
| NetMHCpan [37] |
Comparison of epitope sequences by artificial neural networks that provide peptide–MHC-I-affinity predictions | 2016 |
| NetMHCIIpan [61] |
Pan-specific predictor able to predict binding affinities for all HLA-class-II molecules based on neural networks | 2013 |
| MHCflurry [62] |
Neutral networks including mass-spectrometry datasets for predicting peptide–MHC-I affinities | 2018 |
| ConvMHC [63] |
peptide–MHC interactions encoded into image-like array data and analyzed by deep convolutional neural network | 2017 |
| PLAtEAU [64] |
Defines shared consensus epitopes arising from a series of eluted nested peptides and quantified by mass spectrometry | 2018 |
| MuPeXI [65] |
Integration of somatic mutation calls, list of HLA types, an optional gene-expression profile, and NetMHCpan 3.0 to provide immunogenicity score based on similarity to non-mutated wild-type peptide | 2017 |
| NeoPrepPipe [66] |
Predicts neoantigen burdens and provide insights into the tumor heterogeneity, somatic mutation calls, and patient HLA haplotypes | 2019 |
| EpitopeHunter [67] |
Integrates expression of RNA with artificial neutral networks of immunogenicity-prediction algorithm based on the hydrophobicity of the TCR contact residues | 2015 |
| Neopepsee [68] |
Integrates sequence and amino-acid-immunogenicity information, including antigen processing and presentation to reduce the false-discovery rate | 2018 |
5. Neoantigen-Derived Cancer Vaccines
5.1. Tumor Lysates and Allogeneic Tumor-Cell-Based Vaccine
Autologous tumor lysates or allogeneic tumor cells obtained from patients were the earliest developed cancer vaccines. By administering either inactivated resected tumor lysates or allogeneic tumor-cell lysates with additional components such as adjuvants and cytokines, these cancer vaccines could present epitopes of tumor antigens to activate both CD4+ and CD8+ T cells in the human body [69,70,71].
An autologous tumor-lysate vaccine from Vaccinogen Inc, OncoVax, which uses Bacillus Calmette-Guerin (BCG) as an adjuvant, was shown to extend the recurrence-free period and reduce the risk for recurrences in surgically resected patients with stage II colon cancer. Their phase Ⅲ trial (NTC02448173) evaluating further clinical benefits of OncoVax is ongoing [72]. GVAX (Cell Genesys, Inc., South San Francisco, CA, USA) is an allogeneic whole-tumor-cell vaccine that consists of two prostate-cancer cell lines, LNCaP and PC-3, transfected with a human granulocyte-macrophage-stimulating factor (GM-CSF) gene. Phase I/II studies demonstrated its safety and clinical activity; however, it failed to reach clinical efficacy in a phase III trial of advanced prostate cancer [73]. To improve the overall survival rate, GVAX was recently used with chemotherapy agents and ipilimumab to treat metastatic pancreatic cancer in the trial stage [74]. Other studies on tumor-cell vaccines include melacine (an allogenic melanoma tumor-cell-lysate vaccine) [75], canvaxin (an antigen-rich allogeneic whole-cell vaccine developed from three melanoma cell lines) [76], and TRIMELVax (a heat-shocked melanoma-cell-lysate vaccine) [77]. Although all epitopes are included in this type of vaccine, the contents of neoantigens are quite low, and most are wild-type endogenous peptides, which might dilute the expected immune responses and increase the risk of adverse reactions. Research on optimizing this approach, such as combination therapy and optimized carriers to transport the cells, might address the current limitations of tumor lysates or allogeneic tumor-cell-based vaccines.
5.2. DNA-Based Vaccines
DNA vaccines can be introduced into cells and tissues via non-viral or viral gene-delivery systems. After being introduced into the cytoplasm, DNA migrates to the nucleus and initiates the production of antigens. Physical forces mainly represent the non-viral methods of facilitating intracellular gene transfection by transiently loosening the cell-membrane structure. These systems include electroporation, microinjection, and a gene gun to transfect plasmid DNA into the tissue [78]. Although the physical delivery system offers highly efficient gene transfection, tissue damage resulting from the applied physical forces may cause low activity [79]. GNOS-PV02, a neoantigen-DNA vaccine with plasmid-encoded IL-12 administered by electroporation and intradermal injection, entered a phase I/II clinical study with the combination of pembrolizumab for the treatment of advanced hepatocellular carcinoma. The up-to-date result revealed that the objective response rate (ORR) was 25% without reported dose-limiting toxicities (DLTs). Post-vaccination TCR-repertoire analysis identified novel expanded T-cell clones in both peripheral blood and tumor tissue, which potentially mediated the observed regression of tumors [80].
DNA vaccines can also be delivered by viral carriers such as adenoviruses, modified vaccinia viruses, lentiviruses, and retroviruses. The adenovirus is a non-envelope, double-stranded DNA virus commonly used as a viral vector among these viruses. Adenoviral-vector vaccines replace genes that enable replication of transgenes or other genes of interest, making the vector unable to generate their genome copies after delivery. This property also provides the virus with a higher package capacity to incorporate large transgene sequences [81]. Compared to other virus-based vectors, adenoviral vectors have less potential genotoxicity and have been applied to infectious diseases such as COVID-19 [82], Ebola virus [83], and malaria [84]. Nous-209 is a virus-based cancer vaccine encoding 209 commonly shared frameshift mutations of microsatellite instability tumors and uses the Great Ape Adenoviruses vectors for priming and Modified Vaccinia Ankara vectors for boosting. The preliminary results of the phase I study combined with pembrolizumab showed no DLTs. Seven out of the twelve enrolled patients had confirmed partial responses (PRs), and two patients had stable disease (SD), suggesting that Nous-209 is safe and immunogenic and may contribute to early clinical outcomes [85]. PRGN-2009, a human papillomavirus (HPV) therapeutic vaccine encoding 35 non-HLA-restricted epitopes of HPV 16 and 18 by a novel gorilla adenoviral vector, increased the number of T cells targeting HPV 16 or HPV 18 after vaccination in all six recruited patients in a phase I study without observed DLTs [86]. However, pre-existing immunity against particular virus serotypes prevents the efficacy of virus-based vaccines [87]. This problem may be overcome using viral vectors derived from other species [88]. Nonetheless, it remains to be determined whether existing immunity will decrease the immunization potential for a repeated dose of vaccine constructed in the same or similar serotype virus.
In addition to viral vectors, microbes are also candidates for carrying target antigens. Lm-platform technology is an antigen delivery platform via Listeria monocytogenes developed by ADVAXIS. Attenuated Listeria monocytogenes carrying the bacterial vector expresses fusion proteins containing adjuvant parts and target antigens to T cells after phagocytosis. ADXS-503 is a phase I study of pembrolizumab plus the Lm vaccine targeting 11 common hotspot mutations and 11 TAAs of metastatic non-small-cell lung carcinoma (NSCLC). Antigen-specific T cells were found in all patients with a transient release of pro-inflammatory cytokines. Seven of the nine recruited patients also showed antigen spreading. The ORR was 11%, and the disease-control rate (DCR) was 44%, with one achieving a PR and three achieving SD. The vaccine was well-tolerated without reported immune-related adverse events (irAEs) [89]. Another phase I study, ADXS-NEO-2, targeted personalized neoantigens for each cancer patient. Preliminary findings included immune-cell proliferation, antigen-specific T-cell response, and antigen spreading in one patient at 108 colony-forming units (CFUs). However, two patients had manageable DLTs at an initial dose of 109 CFUs, and the current state of this trial remains unclear [90]. The neoantigen-DNA-vaccine trials currently in the active or completed stages are listed in Table 2.
Table 2.
Clinical trials of neoantigen-DNA vaccines.
| Trial No. (Brand Name) |
Target | Indication | Format/Route of Administration | Combination Therapy | Status |
|---|---|---|---|---|---|
| NCT03122106 | Personalized NeoAg + Mesothelin | Pancreatic Cancer | Plasmid DNA/Electroporation + IM injection | N/A | Phase 1, Active, Not Recruiting |
|
NCT04015700 (GNOS-PV01) |
Personalized NeoAg | Unmethylated Glioblastoma | Plasmid DNA/Electroporation + IM injection | Pembrolizumab, Plasmid encoded IL-12 (INO-9012) |
Phase 1, Recruiting |
|
NCT04251117 (GNOS-PV02) |
Personalized NeoAg + Mesothelin | HCC | Plasmid DNA/Electroporation + IM injection | Pembrolizumab, Plasmid encoded IL-12 (INO-9012) |
Phase 1/2a, Recruiting |
|
NCT04990479 (Nous-PEV) |
Personalized NeoAg | Melanoma, NSCLC |
Adenovirus vector + Vaccinia virus vector/IM injection | Pembrolizumab | Phase 1, Recruiting |
|
NCT04041310 (Nous-209) |
Personalized NeoAg | MSI-H CRC, gastric, G-E junction tumors |
Adenovirus vector + vaccinia virus vector/IM injection | Pembrolizumab | Phase 1/2, Active, Not Recruiting |
|
NCT05018273 (VB10.NEO) |
Personalized NeoAg | Solid Tumors | Plasmid DNA/IM injection | Atezolizumab | Phase 1b, Recruiting |
| NCT02348320 | Personalized NeoAg | Triple-Negative Breast Cancer | Plasmid DNA/Electroporation + IM injection | N/A | Phase 1, Completed |
|
NCT03953235 (SLATE) |
Shared Neoantigen |
Shared neoantigen positive tumors | Adenovirus vector + RNA vector/Not specific | Nivolumab, Ipilimumab |
Phase 1/2, Recruiting |
|
NCT03265080 (ADXS-NEO) |
Personalized NeoAg | Colon Cancer, Head & Neck Cancer, NSCLC, Urothelial Carcinoma, Melanoma |
Lm-based vector/I.V. infusion | Pembrolizumab (selectively) |
Phase 1, Active, Not Recruiting |
|
NCT03847519 (ADXS-503) |
Personalized NeoAg | NSCLC, Metastatic SCC, Metastatic NSCLC |
Lm-based vector/I.V. infusion | Pembrolizumab (selectively) |
Phase 1/2, Recruiting |
Abbreviations: CRC, colorectal cancer. HCC, hepatocellular carcinoma. I.V., intravascular infusion. I.M., intramuscular injection. Lm, Listeria monocytogenes. MSI-H, high microsatellite instability. NSCC, small-cell lung cancer. NSCLC, non-small-cell lung cancer.
5.3. mRNA-Based Vaccines
Additionally, mRNA vaccines have shown substantial potential against diseases during the COVID-19 pandemic [91]. Theoretically, mRNA vaccines are internalized in the cytoplasm, and antigens of interest can be translated without mutagenesis concerns. The magnitude and rate of mRNA translation are typically higher than those of DNA vaccines. Currently, mRNA can be rapidly produced using in vitro transcription (IVT) methods, making it feasible for scale-up manufacturing. These characteristics make mRNA vaccines powerful tools for responding to emergent needs.
The significant clinical breakthrough of the application of mRNA cancer vaccines was first published by Sahin et al. [92]. Thirteen patients with stage III and IV melanoma received at least eight doses of personalized neoantigen vaccines percutaneously into the inguinal lymph nodes. Each patient’s five–ten mutations were selected based on the predicted high-affinity binding to autologous HLA class I and HLA class II. Not only were de novo immune responses observed, but pre-existing immune responses against predicted neoantigens were also augmented in all patients. Eight patients remained recurrence-free during the follow-up period. One patient experienced a complete response of metastases, which contributed to neoantigen-vaccine monotherapy. Another patient had a rapid, complete response within two months with PD1-blockade combination therapy. These results translated into sustained progression-free survival (PFS) and significantly reduced the cumulative sum of metastatic events compared to those before vaccine treatment. Notably, immune escape was observed in one patient who initially had a PR but suffered from metastasis two months after 12 vaccinations and follow-up surgeries. Loss of β-2 microglobulin was observed in autologous tumor cells, leading to HLA-class-I dysfunction [92].
Additionally, mRNA-4157 is the neoantigen-mRNA-vaccine trial of Moderna and is currently under phase I evaluation for solid tumors. From the updated outcome, the vaccine’s safety was acceptable, with only mild-related adverse events reported [93]. Remarkably, the response rate was 50% for HPV-negative head and neck squamous-cell carcinoma combined with pembrolizumab, and the median PFS was compared favorably to pembrolizumab monotherapy. In addition, 14 of 16 patients with resected solid tumors receiving vaccine monotherapy remained disease free. The trial is ongoing for efficacy analysis [94]. However, the other trial of neoantigen-mRNA vaccines, mRNA-4650, did not proceed because no clinical response was observed. In this study, neoepitopes for each patient were selected by HLA-I prediction and validated by TIL–APC coculture, plus any mutations in the hot driver genes of Kirsten rat sarcoma virus (KRAS), tumor protein p53 (TP53), and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA). Despite the suboptimal clinical results, T-cell reactivity against several predicted neoepitopes was found in the post-vaccination PBMC of some patients. TCR analysis revealed neoantigen-specific clonotypes capable of recognizing designed neoantigens, suggesting that a combination of immune-checkpoint inhibitors (ICIs) or immune-cell therapy could have clinical benefits [95].
Naked RNA is vulnerable to extracellular RNAse and can undergo rapid degradation that limits the internalization of the vaccine. Improved mRNA-delivery systems facilitate vaccine protection, distribution, and release. For instance, ionizable lipid nanoparticles (LNPs) are self-assembled particles commonly used for RNA delivery. LNPs are stable at physiological pH, but the ionizable coated lipid can interact with the ionic endosomal membrane in an acidic endosomal microenvironment, thus promoting membrane fusion and RNA release. Moreover, mRNA has intrinsic immunogenicity, recognized mostly by toll-like receptor-7 and -8, and activates downstream interferon pathways and pro-inflammatory cytokine release. Although this might augment adaptive-immune responses, it could also dampen the antigen presentation. Unwanted double-stranded RNA (dsRNA) produced during IVT can activate RNA-dependent protein kinase, phosphorylate eukaryotic elongation factor-2, and block mRNA translation [96]. Several strategies have been investigated to overcome this limitation. Baiersdörfer et al. presented a dsRNA-removal method using cellulose in an ethanol-containing buffer. Up to 90% of dsRNA contaminants can be removed, resulting in better translation efficacy in vivo [97]. CureVax AG developed an RNA/protamine complex that serves as a toll-like receptor 7/8 (TLR7/8) adjuvant, increasing antitumor immunity after vaccination [98]. Luo et al. reported a formulation of synthetic polymeric nanoparticles with an intrinsic activating property for the stimulator of interferon genes (STING), leading to the inhibition of tumor progression in three types of cancer models [99]. In addition, BioNTech developed an RNA-lipoplex cancer-vaccine platform, Lipo-MERIX, which can precisely target dendritic cells (DC) in the lymphoid compartment by systematic administration (intravenous injection) to induce a potent immune response [100]. Several trials evaluating Lipo-MERIX carrying TAA or TSA for different types of solid tumors are ongoing. A relative trial targeting TAA for advanced melanoma, BNT-111, has recently received FDA fast-track designation [101]. Active and completed neoantigen-mRNA-vaccine trials are listed in Table 3.
Table 3.
Clinical trials of neoantigen RNA vaccines.
| Trial No. (Brand Name) |
Target | Indication | Format/Route of Administration | Combination Therapy | Status |
|---|---|---|---|---|---|
| RO7198457 | |||||
| NCT03289962 | Personalized NeoAg | Solid tumors | RNA-Lipoplex/I.V. | Atezolizumab | Phase 1a/1b, Recruiting |
| NCT03815058 | Personalized NeoAg | Advanced Melanoma | RNA-Lipoplex/I.V. | Pembrolizumab | Phase 2, Recruiting |
| NCT04486378 | Personalized NeoAg | Colorectal Cancer Stage II, III |
RNA-Lipoplex/I.V. | N/A | Phase 2, Recruiting |
| NCT04161755 | Personalized NeoAg | Pancreatic Cancer | RNA-Lipoplex/I.V. | Atezolizumab, mFOLFIRINOX |
Phase 1, Recruiting |
| IVAC mutanome | |||||
| NCT02035956 | Personalized NeoAg | Melanoma | Not specific/Intra-nodal | RBL001/RBL002 (TAA RNA Vaccine) |
Phase 1, Completed |
| NCT02316457 | Personalized NeoAg | Breast Cancer (TNBC) |
Nanoparticulate lipoplex RNA/I.V. |
IVAC_W_bre1_uID (TAA RNA vaccine) |
Phase 1, Active, Not Recruiting |
| mRNA-4157 | |||||
| NCT03897881 | Personalized NeoAg | Melanoma | lipid encapsulated RNA/I.M. | Pembrolizumab | Phase 2, Active, Not Recruiting |
| NCT03313778 | Personalized NeoAg | Solid tumors | lipid encapsulated RNA/I.M. | Pembrolizumab | Phase 1, Recruiting |
| mRNA-5671 | |||||
| NCT03948763 | KRAS common mutations | Solid Tumors | lipid encapsulated RNA/I.M. | Pembrolizumab (selectively) |
Phase 1, Recruiting |
Abbreviations: I.V., intravascular infusion. I.M., intramuscular injection. TAA, tumor-associated antigens. TNBC, triple-negative breast cancer.
5.4. Protein and Peptide Vaccines
Peptide-based vaccines use synthetic peptides to trigger peptide-specific immune responses against cancer. It is intuitive and cost-effective, and no intricate logistics are required for transport and restoration. As reviewed by Shemesh et al., neoantigen vaccines derived from peptides, along with mRNA, have undergone the most ongoing clinical trials [102]. The primary outcomes of peptide vaccines showed promising results in treating melanoma and brain malignancies in multiple trials [103,104].
Hilf et al. conducted the GAPVAC trial for glioblastoma by administering peptide vaccines containing the predicted neoantigens and glioma-related TAAs. Notably, Th1 cells were induced in 11 of 13 patients receiving the neoepitope vaccine. In one patient who had a complete response after vaccination but experienced recurrence two years afterward, high infiltration by T cells was found, with a favorable ratio of CD8+/FOXP3+ (forkhead box P3+) Treg cells from the re-resected tumor [105]. Similar results were reported by Keskin et al., who demonstrated that neoantigen-specific CD4+ and CD8+ T cells enriched in the memory phenotype were found after neoantigen-peptide administration. This study further proved that neoantigen-specific T cells triggered by the vaccine could migrate into intracranial glioblastoma tumors [103].
Recently, Platten et al. tested the safety and efficacy of a mutated isocitrate dehydrogenase 1 (IDH1) peptide vaccine in a phase I trial. Mutations in IDH1 are molecular characteristics of certain gliomas that contribute to the early stages of tumor development. Patients with the IDH1 R132H variant were recruited and treated with a 20-mer peptide containing a mutated spot. A mutant-specific T-cell response was found in over 90% of recruited patients with appropriate safety profiles [106]. In recent years, elongated CD8+ T-cell epitopes have been thought to enhance epitope-specific anticancer immunity. Unlike the predicted short epitopes, long peptides are believed to only be processed and presented by professional APC, leading to robust T-cell induction. In the mutant IDH1 trial, a single LSP (long synthetic peptide) was presented across various MHC alleles and, therefore, could be applied as an off-the-shelf product.
Moreover, the combination of neoantigen-peptide vaccines and ICIs has been validated in several trials. The NEO-PV-01 phase Ib clinical trial of a personalized peptide vaccine plus anti-PD1 (anti-programmed death-1) agent was evaluated for safety and efficacy in patients with advanced melanoma, NSCLC, and bladder cancer. Persistent cytotoxic T-cell responses were identified post-vaccination, without severe adverse reactions, in all three cancer cohorts. The median ORR and PFS were favorably compared with historical results for anti-PD-1 monotherapy but could not firmly attribute these outcomes to the vaccine because it was a single-arm investigation [107]. The comparison of neoantigen-peptide-vaccine monotherapy or in combination with ICIs was validated in an ongoing trial, GEN-009 [108].
NeoVax is a personalized long-peptide vaccine plus poly-ICLC (polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose) (i.e., a TLR-3 and MDA5 (melanoma differentiation-associated protein 5) agonist) [104,107]. A long-term follow-up study revealed that all patients with resected metastatic melanoma who had previous NeoVax treatment were still alive up to four years after treatment. Six of the eight patients had no evidence of disease. T cells with reactivity against certain vaccinated neoantigens persisted in the circulating blood of patients during the priming, boosting, and post-vaccination stages (up to 4.5 years). After the vaccination period, these functional T cells shifted to the less exhausted memory phenotype. Encouragingly, T cells able to target non-vaccinated TAAs or neoantigens were identified only in the post-vaccination sample, suggesting that the neoantigen-peptide vaccine could induce epitope spreading [108]. Epitope spreading has also been observed in several neoantigen-peptide-vaccine trials, including the NEO-PV-01, GEN-009, and glioblastoma trials [103,105,107,108]. In the NeoVax follow-up study, enhanced epitope spreading was observed in one patient experiencing recurrence in the post-vaccination period, but no evidence of disease after pembrolizumab therapy was shown, indicating that the combination of the neoantigen vaccine and ICIs could further improve clinical outcomes [108]. More neoantigen-peptide-vaccine trials in the active and completed stages are summarized in Table 4.
Table 4.
Clinical trials of neoantigen-peptide vaccines.
| Trial No. (Brand Name) | Target | Indication | Format/Route of Administration | Combination Therapy | Status |
| NCT04799431 | Personalized NeoAg | MMR-p Colon Cancer Pancreatic Ductal Cancer |
Peptide + poly-ICLC/subcutaneous | Retifanlimab | Phase 1, Not Yet Recruiting |
| NCT03956056 | Personalized NeoAg + Mesothelin | Pancreatic Cancer | Peptide + poly-ICLC/ subcutaneous | N/A | Phase 1, Recruiting |
| NCT04248569 | DNAJB1- PRKACA fusion |
Fibrolamellar Hepatocellular Carcinoma | Peptide + poly-ICLC | Nivolumab, Ipilimumab |
Phase 1, Recruiting |
| NCT04117087 | Common mutant KRAS | Colorectal Cancer Pancreatic Cancer |
Peptide + poly-ICLC | Nivolumab, Ipilimumab |
Phase 1, Recruiting |
| NCT04749641 | Histone H3.3-K27M mutant |
Diffuse Intrinsic Pontine Glioma | Peptide + poly-ICLC/subcutaneous | N/A | Phase 1, Recruiting |
|
NCT03715985 (NeoPepVac) |
Personalized NeoAg | Melanoma, NSCLC, Bladder, Urothelial Carcinoma, |
Peptide + CAF09b/I.P. + I.M. | N/A | Phase 1, Recruiting |
|
NCT03359239 (PGV-001) |
Personalized NeoAg | Urothelial/Bladder Cancer | Peptide + poly-ICLC | Atezolizumab | Phase 1, Recruiting |
|
NCT02149225 (GAPVAC) |
Personalized NeoAg | Glioblastoma | Peptide + poly-ICLC/not specific | TAA peptide vaccine, GM-CSF |
Phase 1, Completed |
| NeoVax | |||||
| NCT01970358 | Personalized NeoAg | Melanoma | Peptide + poly-ICLC/subcutaneous | N/A | Phase 1, Completed |
| NCT02950766 | Personalized NeoAg | Kidney cancer | Peptide + poly-ICLC/subcutaneous | Nivolumab, Ipilimumab |
Phase 1, Recruiting |
| NCT02287428 | Personalized NeoAg | Glioblastoma | Peptide + poly-ICLC | Pembrolizumab Temozolomide (Both selectively) |
Phase 1, Recruiting |
| NCT03929029 | Personalized NeoAg | Melanoma | Peptide + poly-ICLC + Montanide | Nivolumab Ipilimumab |
Phase 1b, Recruiting |
| NCT0402487 | Personalized NeoAg | Ovarian Cancer | Peptide + poly-ICLC | Nivolumab | Phase 1, Recruiting |
| NCT03219450 | Personalized NeoAg | Lymphocytic Leukemia | Peptide + poly-ICLC | Pembrolizumab Cyclophosphamide (both selectively) |
Phase 1, Recruiting |
| Neo-PV-01 | |||||
| NCT03380871 | Personalized NeoAg | Lung cancer | Peptide + poly-ICLC/subcutaneous | Pembrolizumab Carboplatin Pemetrexed |
Phase 1, Completed |
| NCT02897765 | Personalized NeoAg | Urinary Bladder Cancer Melanoma Lung Cancer |
Peptide + poly-ICLC/subcutaneous | Nivolumab | Phase 1, Completed |
Abbreviations: I.V., intravascular infusion. I.M., intramuscular injection. MMR-p, mismatch repair protein deficiency. NSCLC, non-small-cell lung cancer. poly-ICLC, polyinosinic-polycytidylic acid. TAA, tumor-associated antigens.
5.5. DC-Based Vaccines
The cell-based-vaccine approach exploits autologous DCs loaded with tumor antigens in various formats, including tumor lysates, DNA, mRNA, or peptides. Encouraging results, including Sipuleucel-T, an autologous DC vaccine targeting prostatic-acid phosphatase (PAP), a TAA, have demonstrated a significant improvement in overall survival for men with metastatic castration-resistant prostate cancer and was approved by the FDA [109]. For the neoantigen-pulsed DC vaccine, Carreno et al. conducted a trial applying an in vitro matured autologous DC vaccine stimulated by personalized neoantigen peptides in three patients with advanced melanoma. TCR-sequencing results indicated diverse neoantigen-specific clonotypes induced by personalized DC vaccines, and increased immunity was observed in all patients [110]. Moreover, a patient with metastatic pancreatic cancer experienced regression of multiple metastatic lesions 2.5 months after DC-based-vaccine treatment. In this case, the selected neoepitope was an HLA-A*0201–restricted KRAS-G12D epitope, and the patient received a vaccine containing a neoantigen plus DC and neoantigen-reactive CD8+CD137+ T cells [111]. Similar research on patients with heavily treated lung cancer by administering a neoantigen-peptide-loaded DC vaccine demonstrated a 25% ORR and 75% DCR. Although none of the recruited patients achieved CR, the results were auspicious considering the initially poor prognosis of the study population. In addition, they noticed that the neoantigen-loaded DC vaccine could re-induce objective responses to ICIs in patients who had a relapse after previous ICI treatment. This finding corresponds to that mentioned in the peptide-vaccine section, namely that the combination of cancer vaccines and ICMs could further provide synergetic therapeutic benefits [112].
6. Opinions and Future Perspectives
Therapeutic cancer vaccines have several promising clinical outcomes. However, all vaccines are still in the early stages of clinical trials (phases I and II). This may reflect difficulties in inducing a robust immune response to kill aggressive cancer cells in immunosuppressed patients. In addition, the variation in neoantigens in different individuals makes large-scale applications more challenging than targeting commonly shared antigens. Whether therapeutic vaccines can be applied and used in clinical practice depends on different factors, such as (1) the ability to yield sufficient numbers of T cells to overcome the suppressive TME, (2) augmented immune cells that can penetrate and infiltrate the tumors, (3) the use of adequate adjuvants and carriers, and (4) optimal selection of target antigens [113,114].
Moreover, T-cell exhaustion has been reported in numerous studies where vaccine-elicited T lymphocytes often express several inhibitory receptors [92,103,104,105]. A combination of ICIs or other immunotherapies is necessary to achieve synergistic efficacy. In addition to cytotoxic T cells, the importance of CD4+ T cells in cancer immunity has been well established. Notably, MHC-II-restricted tumor epitopes also play a crucial role in immunotherapy efficacy. Activated CD4+ cells could give rise to the induction of CD8+ T cells with less inhibitory profiles and strengthened effector functions. At the beginning of cancer-vaccine treatment, priming of the immunization determines the phenotype and magnitude of the vaccine-elicited immune response. Ideally, a subset of neoantigen-specific T cells with memory phenotypes is generated after antigen clearance. Continuous exposure to antigens can induce functional profiles of T cells, including memory T cells [115,116,117]. The expression of MHC-II epitopes by tumors can recruit more intratumoral T cells and inducible nitric-oxide-synthase-positive macrophages [118]. Including MHC-II epitopes and stimulants to activate CD4+ cells in cancer vaccines has been suggested to improve efficacy. Therefore, optimized priming and boosting regimens for vaccination should be carefully determined. Applying advanced technologies to identify TSAs and generate vaccines with potent adjuvants is the key to developing successful anticancer therapeutics.
Immunoengineering, the field that integrates nanotechnology, bioengineering, material sciences, drug delivery, and immunology, aims to elicit a robust antitumor immune response. In particular, nanoparticles provide better delivery efficiency and T-cell priming for gene-based and peptide-based vaccines. By loading or conjugating adjuvants, innate-immunity agonists, and target receptors to nanoparticles, co-delivery can enhance the magnitude of antitumor responses [119,120]. For instance, a "nanodiscs" mixing synthetic high-density lipoprotein, cysteine-modified antigens, and cholesterol-modified CpG adjuvant successfully promoted antigen presentation and eliminated established mouse tumors when combined with ICIs [121]. In addition, a biodegradable matrix loaded with small molecules and biologics implanted near the tumor or post-resection sites can reverse the immunosuppressive conditions. The matrix provides artificial immune niches that enable the in situ manipulation of cells [122]. Implantation of a biopolymer-based scaffold loaded with tumor-reactive T cells and agonists enhances antigen presentation and T-cell response to eradicate inoperable orthotropic tumors in mice [123,124]. Moreover, protein-based gels loaded with nanoparticles containing anti-CD47, an inhibitory ligand on cancerous cells, polarized macrophages to M1 phenotypes, and prolonged survival in mice with incomplete resection [125]. Further exploration using matrix-coated tumor neoantigens as cancer vaccines is required. These advanced methods aim to provide the best formulation and dosage of tumor antigens and adjuvants to induce the immune cells and improve the efficacy of therapeutic cancer vaccines.
The immune system is intricate and highly coordinated; the absence of specific cytokines or subsets of immune cells could substantially alter the subsequent cascade of responses, indicating that ex vivo immunostimulatory experiments may not precisely interpret the real circumstances in vivo. Emerging tools such as the three-dimensional modeling system and immune organ/tumor "on a chip" system could foster sophisticated examination of immune-organ function and immune-cell interaction [126]. For example, a microfluidic chip containing hepatocellular carcinoma cells was built to evaluate the time-dependent migration and cytotoxicity of TCR-engineered T cells. The device allowed the investigation of T-cell ability under different inflammatory conditions [127]. In addition, the microphysiological 3D cancer model used to test the efficacy of receptor-engineered cells was validated in lung-, breast-, and ovarian-cancer models [128,129].
Regarding the different types of formulations, mRNA vaccines have the advantage of a cost-effective and straightforward manufacturing procedure. On the other hand, favorable clinical outcomes were also observed in patients who received protein and peptide vaccines, such as NeoVax, Neo-PV-01, GAPVAC, and the IDH1 peptide vaccine for glioma. Targeting neoantigens through integrating immunotherapeutics, including vaccines, cell-based therapy, ICIs, and immunoengineering may provide opportunities to overcome the unmet needs of cancer immunotherapy.
7. Conclusions
The development of therapeutic cancer vaccines is a promising prospect for improving the safety and efficacy of the currently used immunotherapeutics. This is a ready-to-produce procedure with an extensive selection of formats. Targeting neoantigens and other TSAs enables immunogens to induce tumor-specific adaptive-immune responses. High-throughput sequencing, epitope-identified mass spectrometry, and predictive algorithms have enabled neoantigen epitopes to be disclosed and subsequently used to design vaccines. Two primary tactics for neoantigen vaccines are evolving. One harnesses personalized vaccines for personalized therapy, and the other utilizes shared neoantigens or viral oncoproteins as off-the-shelf therapeutics. The clinical results summarized in this review indicate encouraging progress in disease control and favorable immune responses. However, several hurdles remain, including on-target distribution, conversion of immunosuppressive environments, and antigen selection. By investigating adequate delivery systems, carriers, adjuvants, and new immunology research tools, these endeavors could gradually reach new heights. Numerous studies using various formats, therapeutic regimens, delivery systems, and combination therapies are still in progress. Targeting neoantigens could be a path to success for significant clinical improvement in cancer treatment.
Acknowledgments
We thank the support of members of the Cancer Vaccine and Immune Cell Therapy core lab, Chang Gung Memorial Hospital.
Author Contributions
Writing-original draft preparation, S.-C.P.; writing-review and editing, M.-T.C. and S.-I.H.; funding acquisition, S.-I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Chang Gung Memorial Hospital (CIRPG3I0041~43, CIRPG3I0021~23, CIRPG3I0031~33, CIRPG2I0011~13) and Ministry of Science and Technology of Taiwan (MOST 108-2320-B-182A-023 -MY3, MOST 109-2320-B-182A-008-MY3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence M.S., Stojanov P., Mermel C.H., Robinson J.T., Garraway L.A., Golub T.R., Meyerson M., Gabriel S.B., Lander E.S., Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence M.S., Stojanov P., Polak P., Kryukov G.V., Cibulskis K., Sivachenko A., Carter S.L., Stewart C., Mermel C.H., Roberts S.A., et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood L.D., Parsons D.W., Jones S., Lin J., Sjoblom T., Leary R.J., Shen D., Boca S.M., Barber T., Ptak J., et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 5.Ciriello G., Miller M.L., Aksoy B.A., Senbabaoglu Y., Schultz N., Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat. Genet. 2013;45:1127–1133. doi: 10.1038/ng.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran E., Robbins P.F., Rosenberg S.A. ‘Final common pathway’ of human cancer immunotherapy: Targeting random somatic mutations. Nat. Immunol. 2017;18:255–262. doi: 10.1038/ni.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollingsworth R.E., Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulie P.G., Van den Eynde B.J., van der Bruggen P., Boon T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 9.Van den Eynde B.J., van der Bruggen P. T cell defined tumor antigens. Curr. Opin. Immunol. 1997;9:684–693. doi: 10.1016/S0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 10.Oesterling J.E. Prostate specific antigen: A critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J. Urol. 1991;145:907–923. doi: 10.1016/S0022-5347(17)38491-4. [DOI] [PubMed] [Google Scholar]
- 11.Hollingsworth M.A., Swanson B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 12.Vonderheide R.H., Hahn W.C., Schultze J.L., Nadler L.M. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 13.Chang K., Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn O.J., Gantt K.R., Lepisto A.J., Pejawar-Gaddy S., Xue J., Beatty P.L. Importance of MUC1 and spontaneous mouse tumor models for understanding the immunobiology of human adenocarcinomas. Immunol. Res. 2011;50:261–268. doi: 10.1007/s12026-011-8214-1. [DOI] [PubMed] [Google Scholar]
- 15.Correale P., Walmsley K., Nieroda C., Zaremba S., Zhu M., Schlom J., Tsang K.Y. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J. Natl. Cancer Inst. 1997;89:293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 16.Muniyan S., Chaturvedi N.K., Dwyer J.G., LaGrange C.A., Chaney W.G., Lin M.-F. Human Prostatic Acid Phosphatase: Structure, Function and Regulation. Int. J. Mol. Sci. 2013;14:10438–10464. doi: 10.3390/ijms140510438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karbach J., Neumann A., Atmaca A., Wahle C., Brand K., von Boehmer L., Knuth A., Bender A., Ritter G., Old L.J., et al. Efficient in vivo priming by vaccination with recombinant NY-ESO-1 protein and CpG in antigen naive prostate cancer patients. Clin. Cancer Res. 2011;17:861–870. doi: 10.1158/1078-0432.CCR-10-1811. [DOI] [PubMed] [Google Scholar]
- 18.Simpson A.J., Caballero O.L., Jungbluth A., Chen Y.T., Old L.J. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann O., Caballero O.L., Stevenson B.J., Chen Y.T., Cohen T., Chua R., Maher C.A., Panji S., Schaefer U., Kruger A., et al. Genome-wide analysis of cancer/testis gene expression. Proc. Natl. Acad. Sci. USA. 2008;105:20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Smet C., Lurquin C., van der Bruggen P., De Plaen E., Brasseur F., Boon T. Sequence and expression pattern of the human MAGE2 gene. Immunogenetics. 1994;39:121–129. doi: 10.1007/BF00188615. [DOI] [PubMed] [Google Scholar]
- 21.Gnjatic S., Cao Y., Reichelt U., Yekebas E.F., Nölker C., Marx A.H., Erbersdobler A., Nishikawa H., Hildebrandt Y., Bartels K., et al. NY-CO-58/KIF2C is overexpressed in a variety of solid tumors and induces frequent T cell responses in patients with colorectal cancer. Int. J. Cancer. 2010;127:381–393. doi: 10.1002/ijc.25058. [DOI] [PubMed] [Google Scholar]
- 22.Smith C.C., Selitsky S.R., Chai S., Armistead P.M., Vincent B.G., Serody J.S. Alternative tumour-specific antigens. Nat. Rev. Cancer. 2019;19:465–478. doi: 10.1038/s41568-019-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritsch E.F., Rajasagi M., Ott P.A., Brusic V., Hacohen N., Wu C.J. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunol. Res. 2014;2:522–529. doi: 10.1158/2326-6066.CIR-13-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan F., Duitama J., Al Seesi S., Ayres C.M., Corcelli S.A., Pawashe A.P., Blanchard T., McMahon D., Sidney J., Sette A., et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J. Exp. Med. 2014;211:2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milicic A., Price D.A., Zimbwa P., Booth B.L., Brown H.L., Easterbrook P.J., Olsen K., Robinson N., Gileadi U., Sewell A.K., et al. CD8+ T cell epitope-flanking mutations disrupt proteasomal processing of HIV-1 Nef. J. Immunol. 2005;175:4618–4626. doi: 10.4049/jimmunol.175.7.4618. [DOI] [PubMed] [Google Scholar]
- 26.Wolfers J., Lozier A., Raposo G., Regnault A., Thery C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 27.Zitvogel L., Casares N., Pequignot M.O., Chaput N., Albert M.L., Kroemer G. Immune response against dying tumor cells. Adv. Immunol. 2004;84:131–179. doi: 10.1016/s0065-2776(04)84004-5. [DOI] [PubMed] [Google Scholar]
- 28.Green D.R., Ferguson T., Zitvogel L., Kroemer G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boon T., Cerottini J.C., Van den Eynde B., van der Bruggen P., Van Pel A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 30.Bacon K., Baggiolini M., Broxmeyer H., Horuk R., Lindley I., Mantovani A., Maysushima K., Murphy P., Nomiyama H., Oppenheim J., et al. Chemokine/chemokine receptor nomenclature. J. Interferon Cytokine Res. 2002;22:1067–1068. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- 31.Dubinett S.M., Lee J.M., Sharma S., Mule J.J. Chemokines: Can effector cells be redirected to the site of the tumor? Cancer J. 2010;16:325–335. doi: 10.1097/PPO.0b013e3181eb33bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tureci O., Vormehr M., Diken M., Kreiter S., Huber C., Sahin U. Targeting the Heterogeneity of Cancer with Individualized Neoepitope Vaccines. Clin. Cancer Res. 2016;22:1885–1896. doi: 10.1158/1078-0432.CCR-15-1509. [DOI] [PubMed] [Google Scholar]
- 33.Kahles A., Ong C.S., Zhong Y., Ratsch G. SplAdder: Identification, quantification and testing of alternative splicing events from RNA-Seq data. Bioinformatics. 2016;32:1840–1847. doi: 10.1093/bioinformatics/btw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers M.F., Thomas J., Reddy A.S., Ben-Hur A. SpliceGrapher: Detecting patterns of alternative splicing from RNA-Seq data in the context of gene models and EST data. Genome Biol. 2012;13:R4. doi: 10.1186/gb-2012-13-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denti L., Rizzi R., Beretta S., Vedova G.D., Previtali M., Bonizzoni P. ASGAL: Aligning RNA-Seq data to a splicing graph to detect novel alternative splicing events. BMC Bioinform. 2018;19:444. doi: 10.1186/s12859-018-2436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggles K.V., Tang Z., Wang X., Grover H., Askenazi M., Teubl J., Cao S., McLellan M.D., Clauser K.R., Tabb D.L., et al. An Analysis of the Sensitivity of Proteogenomic Mapping of Somatic Mutations and Novel Splicing Events in Cancer. Mol. Cell. Proteom. 2016;15:1060–1071. doi: 10.1074/mcp.M115.056226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurtz V., Paul S. NetMHCpan-4.0: Improved Peptide-MHC Class I Interaction Predictions Integrating Eluted Ligand and Peptide Binding Affinity Data. J. Immunol. 2017;199:3360–3368. doi: 10.4049/jimmunol.1700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang G.L., Khan A.M., Srinivasan K.N., August J.T., Brusic V. MULTIPRED: A computational system for prediction of promiscuous HLA binding peptides. Nucleic Acids Res. 2005;33:W172–W179. doi: 10.1093/nar/gki452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vita R., Overton J.A., Greenbaum J.A., Ponomarenko J., Clark J.D., Cantrell J.R., Wheeler D.K., Gabbard J.L., Hix D., Sette A., et al. The immune epitope database (IEDB) 3.0. Nucleic Acids Res. 2015;43:D405–D412. doi: 10.1093/nar/gku938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schubert B., Brachvogel H.P., Jurges C., Kohlbacher O. EpiToolKit–A web-based workbench for vaccine design. Bioinformatics. 2015;31:2211–2213. doi: 10.1093/bioinformatics/btv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridman W.H., Pagès F., Sautès-Fridman C., Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 42.Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., Tosolini M., Camus M., Berger A., Wind P., et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 43.Kreiter S., Vormehr M., van de Roemer N., Diken M., Lower M., Diekmann J., Boegel S., Schrors B., Vascotto F., Castle J.C., et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S., et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen M., Lund O. NN-align. An artificial neural network-based alignment algorithm for MHC class II peptide binding prediction. BMC Bioinform. 2009;10:296. doi: 10.1186/1471-2105-10-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andreatta M., Karosiene E., Rasmussen M., Stryhn A., Buus S., Nielsen M. Accurate pan-specific prediction of peptide-MHC class II binding affinity with improved binding core identification. Immunogenetics. 2015;67:641–650. doi: 10.1007/s00251-015-0873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt D.F., Henderson R.A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A.L., Appella E., Engelhard V.H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 48.Cravatt B.F., Simon G.M., Yates Iii J.R. The biological impact of mass-spectrometry-based proteomics. Nature. 2007;450:991–1000. doi: 10.1038/nature06525. [DOI] [PubMed] [Google Scholar]
- 49.Kasuga K. Comprehensive analysis of MHC ligands in clinical material by immunoaffinity-mass spectrometry. Methods Mol. Biol. 2013;1023:203–218. doi: 10.1007/978-1-4614-7209-4_14. [DOI] [PubMed] [Google Scholar]
- 50.Mommen G.P.M., Frese C.K., Meiring H.D., van Gaans-van den Brink J., de Jong A.P.J.M., van Els C.A.C.M., Heck A.J.R. Expanding the detectable HLA peptide repertoire using electron-transfer/higher-energy collision dissociation (EThcD) Proc. Natl. Acad. Sci. USA. 2014;111:4507–4512. doi: 10.1073/pnas.1321458111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberger G., Koh C.C., Guo T., Röst H.L., Kouvonen P., Collins B.C., Heusel M., Liu Y., Caron E., Vichalkovski A., et al. A repository of assays to quantify 10,000 human proteins by SWATH-MS. Sci. Data. 2014;1:140031. doi: 10.1038/sdata.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veit J., Sachsenberg T., Chernev A., Aicheler F., Urlaub H., Kohlbacher O. LFQProfiler and RNP(xl): Open-Source Tools for Label-Free Quantification and Protein-RNA Cross-Linking Integrated into Proteome Discoverer. J. Proteome Res. 2016;15:3441–3448. doi: 10.1021/acs.jproteome.6b00407. [DOI] [PubMed] [Google Scholar]
- 54.Bassani-Sternberg M., Bräunlein E., Klar R., Engleitner T., Sinitcyn P., Audehm S., Straub M., Weber J., Slotta-Huspenina J., Specht K., et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat. Commun. 2016;7:13404. doi: 10.1038/ncomms13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Regnier M., Gourbeyre P., Pinton P., Napper S., Laffite J., Cossalter A.M., Bailly J.D., Lippi Y., Bertrand-Michel J., Bracarense A., et al. Identification of Signaling Pathways Targeted by the Food Contaminant FB1: Transcriptome and Kinome Analysis of Samples from Pig Liver and Intestine. Mol. Nutr. Food Res. 2017;61:1700433. doi: 10.1002/mnfr.201700433. [DOI] [PubMed] [Google Scholar]
- 56.Abelin J.G., Keskin D.B., Sarkizova S., Hartigan C.R., Zhang W., Sidney J., Stevens J., Lane W., Zhang G.L., Eisenhaure T.M., et al. Mass Spectrometry Profiling of HLA-Associated Peptidomes in Mono-allelic Cells Enables More Accurate Epitope Prediction. Immunity. 2017;46:315–326. doi: 10.1016/j.immuni.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen M., Connelley T., Ternette N. Improved Prediction of Bovine Leucocyte Antigens (BoLA) Presented Ligands by Use of Mass-Spectrometry-Determined Ligand and in Vitro Binding Data. J. Proteome Res. 2018;17:559–567. doi: 10.1021/acs.jproteome.7b00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreatta M., Lund O., Nielsen M. Simultaneous alignment and clustering of peptide data using a Gibbs sampling approach. Bioinformatics. 2013;29:8–14. doi: 10.1093/bioinformatics/bts621. [DOI] [PubMed] [Google Scholar]
- 59.Bulik-Sullivan B., Busby J., Palmer C.D., Davis M.J., Murphy T., Clark A., Busby M., Duke F., Yang A., Young L., et al. Deep learning using tumor HLA peptide mass spectrometry datasets improves neoantigen identification. Nat. Biotechnol. 2019;37:55–63. doi: 10.1038/nbt.4313. [DOI] [PubMed] [Google Scholar]
- 60.Caron E., Aebersold R., Banaei-Esfahani A., Chong C., Bassani-Sternberg M. A Case for a Human Immuno-Peptidome Project Consortium. Immunity. 2017;47:203–208. doi: 10.1016/j.immuni.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Karosiene E., Rasmussen M., Blicher T., Lund O., Buus S., Nielsen M. NetMHCIIpan-3.0, a common pan-specific MHC class II prediction method including all three human MHC class II isotypes, HLA-DR, HLA-DP and HLA-DQ. Immunogenetics. 2013;65:711–724. doi: 10.1007/s00251-013-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Donnell T.J., Rubinsteyn A., Bonsack M., Riemer A.B., Laserson U., Hammerbacher J. MHCflurry: Open-Source Class I MHC Binding Affinity Prediction. Cell Syst. 2018;7:129–132.e4. doi: 10.1016/j.cels.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 63.Han Y., Kim D. Deep convolutional neural networks for pan-specific peptide-MHC class I binding prediction. BMC Bioinform. 2017;18:585. doi: 10.1186/s12859-017-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alvaro-Benito M., Morrison E., Abualrous E.T., Kuropka B., Freund C. Quantification of HLA-DM-Dependent Major Histocompatibility Complex of Class II Immunopeptidomes by the Peptide Landscape Antigenic Epitope Alignment Utility. Front. Immunol. 2018;9:872. doi: 10.3389/fimmu.2018.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bjerregaard A.M., Nielsen M., Hadrup S.R., Szallasi Z., Eklund A.C. MuPeXI: Prediction of neo-epitopes from tumor sequencing data. Cancer Immunol. Immunother. 2017;66:1123–1130. doi: 10.1007/s00262-017-2001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schenck R.O., Lakatos E., Gatenbee C., Graham T.A., Anderson A.R.A. NeoPredPipe: High-throughput neoantigen prediction and recognition potential pipeline. BMC Bioinform. 2019;20:264. doi: 10.1186/s12859-019-2876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chowell D., Krishna S., Becker P.D. TCR contact residue hydrophobicity is a hallmark of immunogenic CD8+ T cell epitopes. Proc. Natl. Acad. Sci. 2015;112:E1754–E1762. doi: 10.1073/pnas.1500973112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim S., Kim H.S., Kim E., Lee M.G., Shin E.C., Paik S., Kim S. Neopepsee: Accurate genome-level prediction of neoantigens by harnessing sequence and amino acid immunogenicity information. Ann. Oncol. 2018;29:1030–1036. doi: 10.1093/annonc/mdy022. [DOI] [PubMed] [Google Scholar]
- 69.Chan A.D., Morton D.L. Active immunotherapy with allogeneic tumor cell vaccines: Present status. Semin. Oncol. 1998;25:611–622. [PubMed] [Google Scholar]
- 70.Simons J.W., Mikhak B. Ex-vivo gene therapy using cytokine-transduced tumor vaccines: Molecular and clinical pharmacology. Semin. Oncol. 1998;25:661–676. [PubMed] [Google Scholar]
- 71.Phan V., Errington F., Cheong S.C., Kottke T., Gough M., Altmann S., Brandenburger A., Emery S., Strome S., Bateman A., et al. A new genetic method to generate and isolate small, short-lived but highly potent dendritic cell-tumor cell hybrid vaccines. Nat. Med. 2003;9:1215–1219. doi: 10.1038/nm923. [DOI] [PubMed] [Google Scholar]
- 72.Vermorken J.B., Claessen A.M., van Tinteren H., Gall H.E., Ezinga R., Meijer S., Scheper R.J., Meijer C.J., Bloemena E., Ransom J.H., et al. Active specific immunotherapy for stage II and stage III human colon cancer: A randomised trial. Lancet. 1999;353:345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 73.Arlen P.M., Mohebtash M., Madan R.A., Gulley J.L. Promising novel immunotherapies and combinations for prostate cancer. Future Oncol. 2009;5:187–196. doi: 10.2217/14796694.5.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu A.A., Bever K.M., Ho W.J., Fertig E.J., Niu N., Zheng L., Parkinson R.M., Durham J.N., Onners B., Ferguson A.K., et al. A Phase II Study of Allogeneic GM-CSF–Transfected Pancreatic Tumor Vaccine (GVAX) with Ipilimumab as Maintenance Treatment for Metastatic Pancreatic Cancer. Clin. Cancer Res. 2020;26:5129–5139. doi: 10.1158/1078-0432.CCR-20-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sondak V.K., Sosman J.A. Results of clinical trials with an allogenic melanoma tumor cell lysate vaccine: Melacine. Semin. Cancer Biol. 2003;13:409–415. doi: 10.1016/j.semcancer.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Hsueh E.C., Morton D.L. Antigen-based immunotherapy of melanoma: Canvaxin therapeutic polyvalent cancer vaccine. Semin. Cancer Biol. 2003;13:401–407. doi: 10.1016/j.semcancer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Gleisner M.A., Pereda C., Tittarelli A. A heat-shocked melanoma cell lysate vaccine enhances tumor infiltration by prototypic effector T cells inhibiting tumor growth. J. Immunother. Cancer. 2020;8:e000999. doi: 10.1136/jitc-2020-000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nayerossadat N., Maedeh T., Ali P. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiang S.D., Selomulya C., Ho J., Apostolopoulos V., Plebanski M. Delivery of DNA vaccines: An overview on the use of biodegradable polymeric and magnetic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010;2:205–218. doi: 10.1002/wnan.88. [DOI] [PubMed] [Google Scholar]
- 80.Yarchoan M., Gane E., Marron T., Rochestie S., Cooch N., Peters J., Csiki I., Perales-Puchalt A., Sardesai N. 453 Personalized DNA neoantigen vaccine (GNOS-PV02) in combination with plasmid IL-12 and pembrolizumab for the treatment of patients with advanced hepatocellular carcinoma. J. Immunother. Cancer. 2021;9:A481. doi: 10.1136/jitc-2021-SITC2021.453. [DOI] [Google Scholar]
- 81.He T.C., Zhou S., da Costa L.T., Yu J., Kinzler K.W., Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., Neuzil K.M., Hahn W., Hunt J., Mulligan M.J., et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tapia M.D., Sow S.O., Mbaye K.D., Thiongane A., Ndiaye B.P., Ndour C.T., Mboup S., Keshinro B., Kinge T.N., Vernet G., et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: A randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020;20:719–730. doi: 10.1016/S1473-3099(20)30019-0. [DOI] [PubMed] [Google Scholar]
- 84.Shiratsuchi T., Rai U., Kaneko I., Zhang M., Iwanaga S., Yuda M., Tsuji M. A potent malaria vaccine based on adenovirus with dual modifications at Hexon and pVII. Vaccine. 2017;35:6990–7000. doi: 10.1016/j.vaccine.2017.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Overman M., Fakih M., Le D., Shields A., Pedersen K., Shah M., Mukherjee S., Faivre T., Leoni G., D’Alise A.M., et al. 410 Phase I interim study results of Nous-209, an off-the-shelf immunotherapy, with pembrolizumab, for the treatment of tumors with a deficiency in mismatch repair/microsatellite instability (dMMR/MSI) J. Immunother. Cancer. 2021;9:A441. doi: 10.1136/jitc-2021-SITC2021.410. [DOI] [Google Scholar]
- 86.Floudas C., Strauss J., Allen C., Donahue R., Jochems C., Steinberg S., Cordes L., Brough D., Lankford A., McMahon S., et al. 483 Initial safety results and immune responses induced by a novel human papillomavirus (HPV)-specific gorilla adenovirus immunotherapy vaccine, PRGN-2009, in patients with advanced HPV-associated cancers. J. Immunother. Cancer. 2021;9:A513. doi: 10.1136/jitc-2021-SITC2021.483. [DOI] [Google Scholar]
- 87.Barouch D.H., Pau M.G., Custers J.H., Koudstaal W., Kostense S., Havenga M.J., Truitt D.M., Sumida S.M., Kishko M.G., Arthur J.C., et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 88.Guo J., Mondal M., Zhou D. Development of novel vaccine vectors: Chimpanzee adenoviral vectors. Hum. Vaccines Immunother. 2018;14:1679–1685. doi: 10.1080/21645515.2017.1419108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haigentz M., Ramalingam S.S., Gerstner G.J., Halmos B., Morganstein N., Vangala S., Parsi M., Kabala V., Simkhada D., Metran C., et al. A phase 1 study of an off-the shelf, multi-neoantigen vector (ADXS-503) in subjects with metastatic non-small cell lung cancer (NSCLC) progressing on pembrolizumab as last therapy. J. Clin. Oncol. 2021;39:2616. doi: 10.1200/JCO.2021.39.15_suppl.2616. [DOI] [Google Scholar]
- 90.Hecht J.R., Goldman J.W., Hayes S., Balli D., Princiotta M.F., Dennie J.G., Heyburn J., Sands T., Sheeri S., Petit R., et al. Abstract CT007: Safety and immunogenicity of a personalized neoantigen—Listeria vaccine in cancer patients. Cancer Res. 2019;79:CT007. doi: 10.1158/1538-7445.am2019-ct007. [DOI] [Google Scholar]
- 91.Pilishvili T., Gierke R., Fleming-Dutra K.E., Farrar J.L., Mohr N.M., Talan D.A., Krishnadasan A., Harland K.K., Smithline H.A., Hou P.C., et al. Effectiveness of mRNA COVID-19 Vaccine among U.S. Health Care Personnel. N. Engl. J. Med. 2021;385:e90. doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahin U., Derhovanessian E., Miller M., Kloke B.-P., Simon P., Löwer M., Bukur V., Tadmor A.D., Luxemburger U., Schrörs B., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 93.Burris H.A., Patel M.R., Cho D.C., Clarke J.M., Gutierrez M., Zaks T.Z., Frederick J., Hopson K., Mody K., Binanti-Berube A., et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J. Clin. Oncol. 2019;37:2523. doi: 10.1200/JCO.2019.37.15_suppl.2523. [DOI] [Google Scholar]
- 94.Bauman J., Burris H., Clarke J., Patel M., Cho D., Gutierrez M., Julian R., Scott A., Cohen P., Frederick J., et al. 798 Safety, tolerability, and immunogenicity of mRNA-4157 in combination with pembrolizumab in subjects with unresectable solid tumors (KEYNOTE-603): An update. J. Immunother. Cancer. 2020;8:A477. doi: 10.1136/jitc-2020-SITC2020.0798. [DOI] [Google Scholar]
- 95.Cafri G., Gartner J.J., Zaks T., Hopson K., Levin N., Paria B.C., Parkhurst M.R., Yossef R., Lowery F.J., Jafferji M.S., et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Inverstig. 2020;130:5976–5988. doi: 10.1172/JCI134915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 97.Baiersdörfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., Karikó K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Ther. Nucleic Acids. 2019;15:26–35. doi: 10.1016/j.omtn.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rauch S., Lutz J., Kowalczyk A., Schlake T., Heidenreich R. RNActive® Technology: Generation and Testing of Stable and Immunogenic mRNA Vaccines. Methods Mol. Biol. 2017;1499:89–107. doi: 10.1007/978-1-4939-6481-9_5. [DOI] [PubMed] [Google Scholar]
- 99.Luo M., Wang H., Wang Z., Cai H., Lu Z., Li Y., Du M., Huang G., Wang C., Chen X., et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 101.BioNTech Receives FDA Fast Track Designation for Its FixVac Candidate BNT111 in Advanced Melanoma. [(accessed on 19 November 2021)]. Available online: https://investors.biontech.de/news-releases/news-release-details/biontech-receives-fda-fast-track-designation-its-fixvac.
- 102.Shemesh C.S., Hsu J.C., Hosseini I., Shen B.Q., Rotte A., Twomey P., Girish S., Wu B. Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities. Mol. Ther. 2021;29:555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keskin D.B., Anandappa A.J., Sun J., Tirosh I., Mathewson N.D., Li S., Oliveira G., Giobbie-Hurder A., Felt K., Gjini E., et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., Zhang W., Luoma A., Giobbie-Hurder A., Peter L., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hilf N., Kuttruff-Coqui S., Frenzel K., Bukur V., Stevanović S., Gouttefangeas C., Platten M., Tabatabai G., Dutoit V., van der Burg S.H., et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 106.Platten M., Bunse L., Wick A., Bunse T., Le Cornet L., Harting I., Sahm F., Sanghvi K., Tan C.L., Poschke I., et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature. 2021;592:463–468. doi: 10.1038/s41586-021-03363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ott P.A., Hu-Lieskovan S., Chmielowski B., Govindan R., Naing A., Bhardwaj N., Margolin K., Awad M.M., Hellmann M.D., Lin J.J., et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell. 2020;183:347–362.e24. doi: 10.1016/j.cell.2020.08.053. [DOI] [PubMed] [Google Scholar]
- 108.Gillison M.L., Awad M.M., Twardowski P., Sukari A., Johnson M.L., Stein M.N., Hernandez R., Price J., Mancini K.J., Shainheit M., et al. Long term results from a phase 1 trial of GEN-009, a personalized neoantigen vaccine, combined with PD-1 inhibition in advanced solid tumors. J. Clin. Oncol. 2021;39:2613. doi: 10.1200/JCO.2021.39.15_suppl.2613. [DOI] [Google Scholar]
- 109.Nabhan C. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363:1966–1967. doi: 10.1056/NEJMc1009982. [DOI] [PubMed] [Google Scholar]
- 110.Carreno B.M., Magrini V., Becker-Hapak M., Kaabinejadian S., Hundal J., Petti A.A., Ly A., Lie W.-R., Hildebrand W.H., Mardis E.R., et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen F., Zou Z., Du J., Su S., Shao J., Meng F., Yang J., Xu Q., Ding N., Yang Y., et al. Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J. Clin. Inverstig. 2019;129:2056–2070. doi: 10.1172/JCI99538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ding Z., Li Q., Zhang R., Xie L., Shu Y., Gao S., Wang P., Su X., Qin Y., Wang Y., et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct. Target. Ther. 2021;6:26. doi: 10.1038/s41392-020-00448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tran T., Blanc C., Granier C., Saldmann A., Tanchot C., Tartour E. Therapeutic cancer vaccine: Building the future from lessons of the past. Semin. Immunopathol. 2019;41:69–85. doi: 10.1007/s00281-018-0691-z. [DOI] [PubMed] [Google Scholar]
- 114.van der Burg S.H. Correlates of immune and clinical activity of novel cancer vaccines. Semin. Immunol. 2018;39:119–136. doi: 10.1016/j.smim.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Masopust D., Ha S.J., Vezys V., Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: Implications for prime-boost vaccination. J. Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 116.Wirth T.C., Xue H.H., Rai D., Sabel J.T., Bair T., Harty J.T., Badovinac V.P. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fraser K.A., Schenkel J.M., Jameson S.C., Vezys V., Masopust D. Preexisting high frequencies of memory CD8+ T cells favor rapid memory differentiation and preservation of proliferative potential upon boosting. Immunity. 2013;39:171–183. doi: 10.1016/j.immuni.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alspach E., Lussier D.M., Miceli A.P., Kizhvatov I., DuPage M., Luoma A.M., Meng W., Lichti C.F., Esaulova E., Vomund A.N., et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nembrini C., Stano A., Dane K.Y., Ballester M., van der Vlies A.J., Marsland B.J., Swartz M.A., Hubbell J.A. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc. Natl. Acad. Sci. USA. 2011;108:E989–E997. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li A.V., Moon J.J., Abraham W., Suh H., Elkhader J., Seidman M.A., Yen M., Im E.J., Foley M.H., Barouch D.H., et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci. Transl. Med. 2013;5:204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spranger S., Gajewski T.F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer. 2018;18:139–147. doi: 10.1038/nrc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith T.T., Moffett H.F., Stephan S.B., Opel C.F., Dumigan A.G., Jiang X., Pillarisetty V.G., Pillai S.P.S., Wittrup K.D., Stephan M.T. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Invest. 2017;127:2176–2191. doi: 10.1172/JCI87624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stephan S.B., Taber A.M., Jileaeva I., Pegues E.P., Sentman C.L., Stephan M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015;33:97–101. doi: 10.1038/nbt.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen Q., Wang C., Zhang X., Chen G., Hu Q., Li H., Wang J., Wen D., Zhang Y., Lu Y., et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019;14:89–97. doi: 10.1038/s41565-018-0319-4. [DOI] [PubMed] [Google Scholar]
- 126.Gosselin E.A., Eppler H.B., Bromberg J.S., Jewell C.M. Designing natural and synthetic immune tissues. Nat. Mater. 2018;17:484–498. doi: 10.1038/s41563-018-0077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pavesi A., Tan A.T., Koh S., Chia A., Colombo M., Antonecchia E., Miccolis C., Ceccarello E., Adriani G., Raimondi M.T., et al. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight. 2017;2:e89762. doi: 10.1172/jci.insight.89762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ando Y., Siegler E.L., Ta H.P., Cinay G.E., Zhou H., Gorrell K.A., Au H., Jarvis B.M., Wang P., Shen K. Evaluating CAR-T Cell Therapy in a Hypoxic 3D Tumor Model. Adv. Healthc. Mater. 2019;8:e1900001. doi: 10.1002/adhm.201900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wallstabe L., Göttlich C., Nelke L.C., Kühnemundt J., Schwarz T., Nerreter T., Einsele H., Walles H., Dandekar G., Nietzer S.L., et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight. 2019;4:e126345. doi: 10.1172/jci.insight.126345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.