Abstract
Background
Early‐onset group B streptococcal disease (EOGBSD) is the most frequent cause of serious infection in the newborn period. Current strategies used to prevent EOGBSD are focused upon maternal antibiotic prophylaxis to reduce transmission of GBS to the infant. Observational studies have suggested that the administration of intramuscular penicillin to the newborn immediately following delivery may be an effective strategy to reduce the incidence of EOGBSD.
Objectives
To determine if the administration of intramuscular penicillin to newborns at birth is a safe and effective method to prevent morbidity and mortality from EOGBSD.
Search methods
The standard search strategy of the Neonatal Review Group was used. This included searches of electronic databases: Oxford Database of Perinatal Trials, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 1, 2004), and MEDLINE (1966 ‐ Dec 2003); and previous reviews including cross references, expert informants and journal hand searching in the English language as well as conference and symposia proceedings published in Pediatric Research.
Selection criteria
Randomised trials in which intramuscular penicillin was administered as prophylaxis for EOGBSD within four hours of birth.
Outcomes considered were EOGBSD, neonatal mortality, late‐onset GBSD, neonatal sepsis, and other secondary outcomes such as neurodevelopmental status and length of hospital stay.
Data collection and analysis
The search for and assessment of trials for inclusion, quality assessment and data extraction were undertaken independently by the reviewers. Meta‐analysis was not undertaken as data from only one trial is included in this review. Data were analysed using relative risk (RR) with 95% confidence intervals (CI).
Main results
One randomised controlled trial was included in this review. In this trial of 1187 infants of birthweight 501 to 2000 grams, there were no significant differences found for the outcomes of EOGBSD (RR 0.73; 95%CI 0.32, 1.62), or neonatal mortality (RR 0.78; 95% CI 0.55, 1.11). No other outcomes were able to be assessed.
Authors' conclusions
This review does not support the routine use of intramuscular penicillin to prevent EOGBSD in newborn infants. There is a discrepancy between this finding and the results of a number of larger non‐randomised trials. Explanations for this are proposed. There is a need for this intervention to be tested as a component of the existing prevention strategies in widespread use.
Plain language summary
Intramuscular penicillin for the prevention of early onset group B streptococcal infection in newborn infants
Not enough evidence to know whether giving antibiotics as a routine to new born babies reduces group B streptococcus infection in the first week of life. Group‐B Streptococcus (GBS) is a common bacteria which can be passed from the mother to the newborn and can lead to infection in the first week of life (neonatal Early Onset Group‐B Streptococcal Disease or EOGBSD). Although rare, (approximately one per thousand births) it is the most common cause of serious infection in newborn babies. Currently, usual management to reduce the risk of infection is to give antibiotics to women at increased risk in labour and to observe the newborn baby closely for signs of infection. Giving an injection of penicillin immediately after birth to newborn babies routinely has been proposed as another way of preventing infection. This review included only one trial and does not have enough data to show whether treating the newborn with intramuscular penicillin should be used to prevent infection in newborn babies. Good quality trials are needed.
Background
Early‐onset group B streptococcal disease (EOGBSD) is the most frequent cause of serious infection in the newborn period (Boyer 1988). In developed countries, the incidence of EOGBSD varies from 0.3 to 1 per 1000 live births (CDC 2002; Flenady 1998), although the incidence may be significantly higher in subpopulations within these countries, such as indigenous Australians and blacks in North America (ASG 1995; CDC 2002). The case fatality rate for EOGBSD is 2% to 8% in term infants and 25% to 30% in preterm infants, and adverse neurologic sequelae occur in 15% to 30% of survivors of group B streptococcal meningitis (Baker 1995).
The gastrointestinal tract and vagina are colonised with group B streptococcus (GBS) in 15% to 40% of women, with transmission to the newborn occurring before or during delivery. Intrapartum GBS colonisation occurs in 40% to 70% of infants of colonised mothers (Gotoff 1997; Baker 1995). In the absence of antibiotic therapy, EOGBSD occurs in 1% to 2% of the newborns of GBS colonised mothers (CDC 2002).
A number of perinatal risk factors that increase the likelihood of EOGBSD have been identified. These include prematurity, prolonged rupture of membranes, intrapartum maternal fever, GBS positive vaginal culture, GBS bacteriuria and previous infant with EOGBSD (Flenady 1998; CDC 2002; Boyer 1988). Strategies involving various combinations of maternal screening and intrapartum chemoprophylaxis have been proposed to reduce the transmission of GBS to the infant and therefore EOGBSD (Boyer 1985; Boyer 1988; Gotoff 1997; ACOG 1996). Active immunisation of pregnant women to prevent peripartum maternal and neonatal disease has been reported (Boyer 1988) but at present, there is no vaccine available for clinical use. Most research has been focused on chemoprophylaxis, aimed at either eradicating maternal colonisation, or preventing the subsequent colonisation of the newborn. Various strategies that provide maternal intrapartum chemoprophylaxis have been proposed (ACOG 1996; Gotoff 1997; AAP 1997; CDC 2002; ASID 2002). A systematic review has concluded that intrapartum antibiotic treatment of women colonised with GBS reduces infant colonisation and early‐onset neonatal infection (Smaill 2003). The widespread use of intrapartum ampicillin, however, may be responsible for an increase in the incidence of early‐onset infections with non‐GBS organisms such as E. coli which are resistant to ampicillin (Towers 1998; Joseph 1998).
The complete absence of EOGBSD was reported as an unexpected benefit of a policy of routine administration of intramuscular penicillin to prevent gonococcal ophthalmia (Steigman 1975; Steigman 1978). This serendipitous observation was supported by the report of a nine‐fold reduction in the incidence of EOGBSD in low birth weight infants who were given penicillin within two hours of birth compared to historical controls (Lloyd 1979). In response to the conclusions of these uncontrolled and retrospective studies, a large prospective controlled trial was undertaken. This study, in which infants were assigned to one of two prophylactic regimens according to alternating week of birth, concluded that a single dose of intramuscular penicillin significantly reduced EOGBSD (Siegel 1980). A significant increase in early‐onset disease due to penicillin‐resistant organisms during the first half of this study was reported. A subsequent extension of this trial was undertaken, and concluded that penicillin prophylaxis did not increase the incidence of disease caused by penicillin‐resistant pathogens (Siegel 1982). When the outcomes of infants delivered over a twenty‐two year period were reviewed, it was further concluded that there was no increase in the incidence of disease caused by penicillin‐resistant organisms, and that the incidence of late‐onset GBS disease was not affected (Siegel 1996).
A retrospective chart review of infants born over a three year period described a decreased incidence of clinical sepsis and positive blood culture for GBS in infants who received penicillin prophylaxis (Patel 1994). However, mortality from EOGBSD was not reduced and there was a non‐significant increase in negative blood cultures from infants who died. As GBS bacteraemia may be present at the time of delivery, the administration of intramuscular penicillin may result in only partial treatment, or suppression, of EOGBSD. Although the success of postnatal penicillin presumes maternal colonisation with GBS, the maternal colonisation status has not been documented in the above reports. This may be a critical factor in determining the role of postnatal chemoprophylaxis of the newborn as a part of an overall strategy to reduce EOGBSD (Boyer 1988).
In summary, evidence from uncontrolled, retrospective and non‐randomised controlled prospective studies supports the use of postnatal intramuscular penicillin in newborns to prevent EOGBSD (Steigman 1978; Lloyd 1979; Patel 1994; Siegel 1996). However, postnatal intramuscular penicillin may not adequately treat infection acquired in‐utero, and may increase mortality from penicillin‐resistant organisms (ACOG 1996; Boyer 1988). This systematic review will examine the effectiveness and safety of intramuscular penicillin in newborn infants by including data from randomised trials which report clinically meaningful outcomes such as death or disability due to EOGBSD, the incidence of EOGBSD and colonisation with GBS, as well as clinically significant outcomes related to early‐onset infection with other organisms.
Objectives
The primary objective of the review is to answer the question: Is the administration of intramuscular penicillin to newborns at birth a safe and effective method to prevent morbidity and mortality from EOGBSD?
A secondary objective is to undertake subgroup analyses to determine if the effects vary according to:
Patient population i. gestational age ii. birth‐weight iii. maternal colonisation status iv. any maternal risk factors for EOGBSD (preterm delivery <37 wks, colonisation with GBS, GBS bacteriuria, rupture of membranes > 18 hrs, intrapartum fever > 37.5, previous infant with EOGBSD) v. maternal intrapartum chemoprophylaxis
Method of treatment i. timing of administration (before or after one hour from delivery) ii. dose
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Newborn infants.
Types of interventions
Intramuscular penicillin administered as prophylaxis for EOGBSD within four hours of birth. Control intervention may be placebo, or no treatment.
Types of outcome measures
One or more of the following outcomes must be reported.
Primary:
Mortality (Neonatal and/or before discharge)
EOGBSD (< 48 hours, < 7 days)
Early‐onset sepsis (all organisms)
Neonatal sepsis (any sepsis within 28 days)
Late‐onset GBS disease (beyond first week of life)
Secondary:
Neonatal colonisation with GBS
Hypersensitivity reactions
Neurodevelopmental outcome (cerebral palsy, sensorineural hearing loss, visual impairment, developmental delay)
Length of hospital stay
Admission to Special Care Nursery
EOGBSD will be defined as isolation of GBS from a normally sterile site in an infant aged < 7 days (CDC 2002).
Search methods for identification of studies
The standard search strategy of the Neonatal Review Group was used.
Searches of the Oxford Database of Perinatal Trials, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 1, 2004), MEDLINE (1966 ‐ Dec 2003) were conducted using MeSH terms infant‐newborn, streptococcal infections and penicillin*, and text terms streptococcus agalactiae, and also previous reviews including cross references, expert informants and journal hand searching in the English language. Conference and symposia proceedings published in Pediatric Research were searched. There was no limitation according to publication language.
Data collection and analysis
Criteria and methods used to assess the methodological quality of the trials: standard method of the Cochrane Collaboration and its Neonatal Review Group were used. Two reviewers worked independently to search for and assess trials for inclusion and methodological quality. Studies were assessed using the following key criteria: blindness of randomisation, blindness of intervention, completeness of follow up and blinding of outcome measurement. Data were extracted independently by the reviewers. Differences were resolved by discussion and consensus of the reviewers. Where necessary, attempts were made to contact investigators for additional information or data. If further data become available, future updates will include these if appropriate.
As this review only includes data from one trial, meta‐analysis was not performed. For the results from the individual trial, relative risk and 95% confidence intervals are reported.
Results
Description of studies
The search strategy identified nine publications of reports of clinical trials. One trial was included in the review (Pyati 1983), for which there is an additional two publications (Pyati 1979, Ramamurthy 1979). Three trials were excluded (Gerard 1979; Patel 1999; Siegel 1982), for which one trial (Siegel 1982) has an additional publication (Siegel 1980). One trial is awaiting assessment (Schauf 1982), for which there is one additional publication (Gahey 1985). Attempts have been made to contact the authors of the study awaiting assessment (Schauf 1982) as more information is required in order to include the data in the review. To date, no additional data have been received.
This review will therefore include available patient outcome data from one randomised trial (Pyati 1983). In this trial conducted in a single institution between January 1977 and April 1981, infants weighing between 501 and 2000 grams were randomised (table of random numbers, opaque envelopes) to either Penicillin G 100000 units/kg I‐M within 60 to 90 minutes of birth or no treatment. Data were reported for the following outcomes: neonatal mortality; EOGBSD defined as GBS isolated from blood or cerebrospinal fluid within the first five days in an infant with signs consistent with the disease; case fatality rate; late‐onset GBSD; and colonisation rate. Data from only two of these outcomes were able to be included in this review. Further details of this included trial are shown in the table "Characteristics of included studies".
The remaining three trials were excluded from the review as quasi‐random methods of allocation were employed (Gerard 1979; Patel 1999; Siegel 1982). A brief description of these trials is presented here.
The first of these (Gerard 1979) reports a trial in which 67 infants born to mothers colonised with GBS were allocated according to odd or even date of birth to either intra‐muscular penicillin prophylaxis or treatment based upon postnatal culture results . There were no cases of systemic group B streptococcal disease (GBSD) or side effects in either group. The other two much larger trials were not restricted to infants of colonised mothers. The first of these (Siegel 1982) allocated 32058 infants to one of two established regimens (intra‐muscular penicillin or topical tetracycline ointment) used for the prevention of gonococcal ophthalmia in alternating blocks of one week. This trial reported a significantly lower incidence of GBSD in the penicillin group, and there were no observed hypersensitivity reactions. The other large study (Patel 1999) allocated infants in alternating blocks of two or three months over a three year study period to receive either intramuscular penicillin within one hour of birth or no treatment. Outcomes are reported for 10998 infants delivered during the study period. There were significant reductions in the incidence of both clinical and identified (culture‐positive) sepsis, GBSD and GBS blood culture, sepsis deaths and all deaths in the penicillin prophylaxis group. These trials are summarised in the additional table "Characteristics of quasi‐randomised trials" (Table 1).
1. Characteristics of quasi‐randomised trials.
| Study ID | Methods | Participants | Interventions | Results | Notes |
| Siegel 1982 | Allocated to one of two established regimens for prevention of gonococcal ophthalmia in alternating weeks during study period. | 32058 infants born during period Dec1977 to May 1981. No birth weight or gestational age restrictions noted. | Penicillin group (n = 16082) received penicillin G 50000 units I‐M if birth weight > 2000 g, or 25000 units I‐M if < 2000 g, within 1 hour of birth. Control group (n = 15967) received topical tetracycline ointment. | Lower incidence of EOGBSD (0.2 vs 1.5 cases per 1000 live births, p < 0.001) and GBSD in penicillin group (0.6 vs 1.7 cases per 1000 live births, p = 0.004), non‐significant increase in disease due to penicillin‐resistant pathogens (2.2 vs 1.6 cases per 1000 live births, p = 0.32) and combined mortality (1.1 vs 0.7 per 1000 live births, p = 0.27) in penicillin group | |
| Gerard 1979 | Infants born to mothers found to be GBS carriers allocated to 'immediate treatment' or 'late treatment' according to odd or even date of birth | Vaginal culture performed on 1115 women; 76 (6.8%) carried GBS. Study group comprised of 67 of the 76 last trimester GBS positive mothers. (Cultures taken from ear canal, gastric aspirate and fetal side of placenta immediately following birth.) | Immediate treatment (n = 29) received penicillin G 50000 to 100000 units/kg/day I‐M in two doses from delivery room, and continued for 7 days if any site positive. Late treatment (n = 38) received penicillin therapy only if cultures positive. | 13 (45%) of 29 assigned to immediate treatment and 16 (42%) of 38 assigned to late treatment found to be colonised. No cases of systemic GBS disease in either group. No cases of late onset GBSD or side effects attributable to antibiotic therapy observed. | |
| Patel 1999 | Allocated to receive penicillin prophylaxis or not in alternating blocks of 3 months for two years, then 2 month blocks for the third year. | 10998 live births during study period Oct 92 to Sept 95. (8134 term, 2864 preterm) | Penicillin prophylaxis group (n = 5389, 3989 term, 1400 preterm) received aqueous penicillin 50000 units I‐M within 1 hour of birth | Lower incidence of the following outcomes in penicillin group: clinical sepsis (1.7% vs 2.5%, p < 0.01); identified sepsis (0.7% vs 1.2%, p < 0.01); GBSD (0.4% vs 0.9%, p < 0.001); GBS blood culture (0.11% vs 0.37%, p < 0.01); sepsis deaths (0.1% vs 0.3%, p < 0.01); and all deaths (0.5% vs 1.0%, p < 0.01). No identified adverse reactions, systemic or local, to penicillin. No increase in nosocomial infection rate and sepsis related to other pathogens. | Maternal prophylaxis policy adopted four months into study period. |
As stated in the protocol, we did not plan to include the data from non‐randomised trials in the meta‐analysis. Furthermore, these three trials are cluster trials with the unit of allocation being a time period. In these trials, the time periods were either one day (Gerard 1979), one week (Siegel 1982), or two or three months (Patel 1999). There is no indication in the reports of these trials that cluster allocation was taken into account in the analysis of the outcome data. Analysis of treatment effects in these trials without taking into account the cluster allocation would likely lead to an overestimation of effect size. On the other hand, to apply the appropriate statistical methods to these data would require values for both cluster size, which was not controlled and was quite variable, and intraclass correlation coefficients which are not available. A more detailed discussion of issues further supporting the exclusion of the non‐randomised trials is to be found below.
Risk of bias in included studies
There was only one identified study which contributed data to this review (Pyati 1983). In this randomised controlled trial, assignment of eligible infants was made from opaque envelopes with the sequence from a published table of random numbers. The intervention was not blinded. Out of 1418 infants, 118 were not included due to lack of consent or death prior to transfer to the neonatal unit. There were 113 post‐randomisation exclusions (61 from intervention group, 52 from control group) due to failure to obtain blood culture or incorrect receipt of intervention. Subsequently, outcomes are available for 1187 infants. An intention‐to‐treat analysis was not presented. It is not clear if blinded assessment of outcomes was performed, although in an unblinded clinical trial such as this, it would be unlikely for most outcomes.
Effects of interventions
This review includes published outcomes from one trial involving 1187 newborn infants (Pyati 1983). No significant differences were found for the outcomes of EOGBSD (RR 0.73; 95%CI 0.32, 1.62), or neonatal mortality (RR 0.78; 95% CI 0.55, 1.11).
Late‐onset GBSD was diagnosed in nine infants in the penicillin group and three infants in the control group. Although it was stated that this difference was not significant (p = 0.07), these data were not able to be included in the review as it was not stated how many infants in each group were able to be assessed for this outcome. Colonisation rates were reported to be similar (67 per 1000 live births in penicillin group versus 65 per 1000 live births in control group), but actual numbers in each group were not reported.
There were no data available for this review's pre‐specified outcomes of sepsis (early‐onset or neonatal), hypersensitivity reactions, length of hospital stay, admission to special‐care nursery or neurodevelopmental outcome. Planned sub‐group analyses according to patient population (gestational age, birthweight or maternal factors such as colonisation status, risk factors or chemoprophylaxis) or method of administration (timing or dose) were not able to be performed as these were not reported.
Discussion
The results of this review which only included published data from one trial (Pyati 1983) do not support the routine use of intramuscular penicillin to newborn infants to prevent EOGBSD.
In this non‐blinded randomised trial, intramuscular penicillin was administered to infants weighing between 5001 and 2000 grams within 60 to 90 minutes of birth. The participants were not selected according to the presence of risk factors other than the low birthweight, and the rate of colonisation was not significantly different between the two allocation groups. The study population were at increased risk of EOGBSD by virtue of the birthweight criteria: the overall incidence of EOGBSD in the trial was 20 per 1000 live births (24 of 1187). The lower rate of EOGBSD in the penicillin group was not statistically significant (10 of 589 or 17.0 per 1000 live births versus 14 of 598 or 23.4 per 1000 live births). Twenty‐two of the 24 infected infants were symptomatic at or shortly after birth (within 4 hours), and bacteraemia was present in 21 of these infants. Therefore, the penicillin would have been administered to some infants already infected.
The two largest of the non‐randomised trials, which were not included in this review, found significant benefits from the administration of penicillin. One study (Siegel 1982) found significant reductions in the incidence of EOGBSD and all GBSD but non‐significant increases in disease due to penicillin‐resistant pathogens and combined mortality. The other study (Patel 1999) which used slightly different outcomes found significant reductions in clinical sepsis, GBSD, GBS positive blood culture, sepsis deaths and all deaths. In this latter study there was no increase in nosocomial sepsis or sepsis related to other pathogens. Both studies included infants of all gestations and birthweights. There was no selection according to maternal risk factors. One study (Patel 1999) adopted a policy of maternal chemoprophylaxis during the course of the trial. The third non‐randomised study (Gerard 1979) only included infants of mothers known to be GBS carriers during the last trimester. There were no identified cases of GBSD in either the penicillin or control group.
The failure of the single included randomised trial to demonstrate the significant benefit from the intervention of intramuscular penicillin which was clearly demonstrated in the non‐randomised time‐clustered trials may be explained by differences in study design, participants or, less probably, the intervention.
Study design: In the included trial which included 1187 infants, the direction of effect was consistent with the significant benefits shown in the larger excluded trials which involved in excess of forty thousand infants. A randomised trial of the same magnitude may have also demonstrated statistically significant differences. On the other hand, the cluster methodology used in the non‐randomised trials may have resulted in an overestimation of effect as it does not seem that this was taken into account in the statistical analysis of these trials. In addition, allocation concealment is not feasible in trials of this nature which may also produce an overestimation of effect (Schulz 1995).
Participants: Differences in the risk of either colonisation or EOGBSD in the included populations may explain the discrepancy between included and excluded trials. The infants included in the randomised trial were all between 501 and 2000 grams birthweight who were transferred to the neonatal intensive care unit. The baseline risk of colonisation and EOGBSD in this group was greater than in the participants of the other trials which had no birthweight or gestational age restrictions (For example, control group EOGBSD of 2.3% in Pyati 1983 versus 0.9% in Patel 1999). In these trials, the majority of participants were term infants. In preterm, low birthweight infants, infection may be more likely to be present at the time of delivery (prior to the administration of the penicillin), whereas in term infants, infection may not become established until after the colonisation which occurs during labour and delivery. An intervention delivered immediately after birth may be more likely to be of benefit in this clinical situation. In only one trial (Gerard 1979) was the GBS colonisation status of the mother was known. No subgroup analyses were possible according to clinically significant risk factors such as gestational age or duration of membrane rupture.
Intervention: In all trials, the interventions were comparable, although the intramuscular penicillin was continued for 72 hours in the included randomised trial (Pyati 1983) whereas it was administered as a single dose in the two larger excluded trials (Siegel 1982; Patel 1999). There were differences in the general management of all infants, however, between the included and excluded trials. In the included randomised trial, blood and surface cultures were obtained (prior to penicillin administration) in all infants. Both groups were observed by house staff and attending physicians for signs of sepsis. In the two excluded non‐randomised cluster trials, infants from neither group received cultures immediately following delivery, and did not receive other than routine surveillance. There may have been more scope for co‐interventions or contamination due to the time‐clustered designs, although all trials were not blinded and therefore at some risk of this.
This review provides no data related to adverse effects of the intervention or long term outcomes such as neurodevelopmental status. However, it was noted in the results of the included trial (Pyati 1983) that there were no systemic or local adverse reactions to the penicillin during the study period. It was also noted in one excluded trial (Gerard 1979) that no secondary ill effects could be attributed to the antibiotic therapy. In the largest excluded trial (Siegel 1982) it was stated that there was a non‐significant increase in diseases caused by penicillin‐resistant pathogens in the penicillin group compared to the control group.
The data in the single included trial did not allow any analyses according to pre‐specified subgroups. In particular, no reference was made to the presence of maternal risk factors (such as membrane rupture or fever) or antibiotic prophylaxis. One small excluded trial (Gerard 1979) recruited participants on the basis of maternal colonisation with GBS and allocated them to either early treatment with intramuscular penicillin (in delivery room) or treatment only if demonstrated neonatal colonisation. There were no cases of EOGBSD in either group. A policy of maternal antibiotic chemoprophylaxis to prevent GBS sepsis in the newborn was introduced following the commencement of one excluded trial (Patel 1999) and implemented gradually over the ensuing year. Prophylactic antibiotics were given to mothers with premature rupture of membranes < 34 wk, any preterm delivery ≤ 36 wk, membrane rupture >18 hours, or any mother with chorioamnionitis (AAP 1997). Overall, 14% of mothers received antibiotics, but the data do not allow analysis according to allocated group within this subgroup.
Protocols for the prevention of EOGBSD, based upon either screening or maternal risk factors, are now in widespread use (AAP 1997; CDC 2002). These protocols and guidelines have been largely concerned with management strategies involving the mother in the antepartum or peripartum period and are designed to reduce transmission of the organism to the newborn either before or during delivery. Recently, more attention has been paid to the investigation and management of the newborn with recommendations determined by the maternal risk factors, the adequacy of the intrapartum management and significantly the gestational age and symptomatology of the newborn (ASID 2002; PCPG 2003). The use of intramuscular penicillin to prevent EOGBSD in the newborn must be considered within this context and it must therefore be evaluated as a component of the overall prevention strategy. A randomised trial in which intramuscular ampicillin was administered to infants of GBS‐colonised women demonstrated reductions in infant colonisation and sepsis with GBS and neonatal deaths from infection (Boyer 1986). However, the infant management was a component of the intrapartum maternal chemoprophylaxis strategy and its effect cannot be evaluated in isolation. This trial has been included in the systematic review of intrapartum antibiotics for GBS colonisation (Smaill 2003).
A selective intervention delivered in the immediate postpartum period may be of most value as an adjunct to the currently employed prevention strategies of maternal antibiotic prophylaxis according to maternal risk factors or colonisation status. For example, if maternal antibiotics were indicated according to whichever strategy but not given, or given outside of the recommended time period, an intervention directed at the newborn immediately following delivery may be of most utility. At present, there is not any evidence to support or refute this.
Authors' conclusions
Implications for practice.
The results of this review do not support the routine use of intramuscular penicillin to the newborn immediately following birth to prevent EOGBSD. Until more high quality evidence becomes available, the primary strategy for the prevention of EOGBSD should continue to be based upon recommended policies of maternal antibiotic chemoprophylaxis (Smaill 2003; ASID 2002; CDC 2002; PCPG 2003) and assessment and management of the newborn that is appropriate for the gestational age and risk of disease (PCPG 2003).
Implications for research.
To evaluate the effectiveness of this intervention, which had demonstrated benefits in observational and other non‐randomised trials, a large randomised trial would be required. Particular attention would need to be given to the risk profile of the participants and the prevention strategy employed (risk factor vs screening approach). The trial would need to be of sufficient size to allow meaningful analyses of outcomes in subgroups such as according to maternal risk factors, use of intrapartum antibiotics and gestational age. Other outcomes such as late‐onset GBSD and neurodevelopmental status would need to be assessed. Furthermore, the effect of the more widespread use of penicillin on the emergence of resistant organisms in neonatal nurseries would need to be monitored.
What's new
| Date | Event | Description |
|---|---|---|
| 12 March 2012 | Amended | Additional table linked to text. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 22 October 2008 | Amended | Converted to new review format. |
| 23 January 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
None
Data and analyses
Comparison 1. IM penicillin versus no penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early onset group B streptococcal disease | 1 | 1187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.32, 1.62] |
| 2 Neonatal mortality | 1 | 1187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.55, 1.11] |
1.1. Analysis.
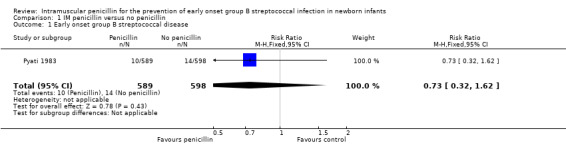
Comparison 1 IM penicillin versus no penicillin, Outcome 1 Early onset group B streptococcal disease.
1.2. Analysis.
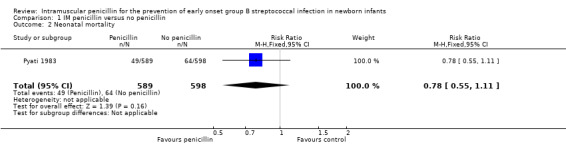
Comparison 1 IM penicillin versus no penicillin, Outcome 2 Neonatal mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Pyati 1983.
| Methods | RCT (table of random numbers, opaque envelopes) Blinding of randomisation: Yes Blinding of intervention: No Complete follow‐up: No Blinding of outcome: Can't tell | |
| Participants | 1418 infants weighing between 501 and 2000 g admitted to NICU, 118 not included (no consent or died), 113 excluded post‐randomisation (61 intervention, 52 control), 1187 infants in study population. | |
| Interventions | Crystalline Penicillin G 100,000 U/kg I‐M following blood and surface cultures within 60 to 90 minutes of birth, repeated every 1 hours for 72 hours (n = 589). Control ; no early treatment (n = 598). Antibiotics given to infants with suspected sepsis regardless of group assignment. | |
| Outcomes | Neonatal mortality. Early‐onset GBSD. Case fatality rate. Late‐onset GBSD. Colonisation rate. | |
| Notes | Early‐onset GBSD defined as GBS isolated from blood or CSF during first 5 days in neonate with signs of disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gerard 1979 | Quasi‐randomised trial. (Allocation alternating according to even or odd date of birth) |
| Patel 1999 | Quasi‐randomised trial. (Allocation according to time period of birth, in alternating two or three month blocks) |
| Siegel 1982 | Quasi‐randomised trial. (Allocation alternating according to week of birth) |
Contributions of authors
PW was primarily responsible for this review and wrote the background. All reviewers contributed to the development of the protocol and trial search. Using a trial‐inclusion checklist prepared by PW, all reviewers independently assessed identified trials according to the inclusion criteria stated in the protocol (study type, participants, intervention and outcomes). Differences were resolved by consensus. A data sheet was used for data extraction, which was performed by VF and PW. VF initially entered data which was checked by PW. PW and VF wrote the discussion and conclusions.
Sources of support
Internal sources
Centre for Clinical Studies ‐ Women's and Children's Health, Mater Mothers' Hospital, South Brisbane, Queensland, Australia.
J P Kelly Research Foundation, Mater Hospital, South Brisbane, Queensland, Australia.
External sources
Department of Health and Ageing, Commonwealth Government, Canberra ACT, Australia.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Pyati 1983 {published data only}
- Pyati SP, Pildes RS, Jacobs NM, Ramamurthy RS, Yeh TF, Raval DS, et al. Penicillin in infants weighing two kilograms or less with early‐onset group B streptococcal disease. The New England Journal of Medicine 1983;308:1383‐9. [DOI] [PubMed] [Google Scholar]
- Pyati SP, Ramamurthy RS, Amma P, Jacobs NM, Yeh TF, Pildes RS. Prospective evaluation of penicillin prophylaxis for neonatal group B streptococcal (GBS) infection. Pediatric Research 1979;13:503A. [Google Scholar]
- Ramamurthy RS, Pyati SP, Pildes RS. Penicillin prophylaxis for neonatal group‐B streptococcal infection. Lancet 1979;2:246‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gerard 1979 {published data only}
- Gerard P, Verghote‐D'Hulst M, Bachy A, Duhaut G. Group B streptococcal colonization of pregnant women and their neonates. Epidemiological study and controlled trial of prophylactic treatment of the newborn. Acta Paediatrica Scandinavica 1979;68:819‐23. [DOI] [PubMed] [Google Scholar]
Patel 1999 {published data only}
- Patel DM, Rhodes PG, LeBlanc MH, Graves GR, Glick C, Morrison J. Role of postnatal penicillin prophylaxis in prevention of neonatal group B streptococcus infection. Acta Paediatrica 1999;88:874‐9. [DOI] [PubMed] [Google Scholar]
Siegel 1982 {published data only}
- Siegel JD, McCracken GH Jr, Threkkeld N, Milvenan B, Rosenfield CR. Single‐dose penicillin prophylaxis against neonatal group B streptoccal infections: a controlled trial in 18,738 newborn infants. The New England Journal of Medicine 1980;87:769‐75. [DOI] [PubMed] [Google Scholar]
- Siegel JD, McCracken GH Jr, Threlkeld N, DePasse BM, Rosenfeld CR. Single‐dose penicillin prophylaxis of neonatal group‐B streptococcal disease: conclusion of a 41 month controlled trial. Lancet 1982;1:1426‐30. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Schauf 1982 {published data only}
- Ghaey K, Tolpin M, Schauf V, Chankinis C, Pyati S, Nelson K, Pildes R, Riff L. Penicillin prophylaxis and the neonatal microbial flora. The Journal of Infectious Diseases 1985;152:1070‐3. [DOI] [PubMed] [Google Scholar]
- Schauf V, Tolpin M, Ghaey K, et al. Penicillin prophylaxis against neonatal group B streptococcal infection ‐ is it safe?. Pediatric Research 1982;15:307A. [Google Scholar]
Additional references
AAP 1997
- American Academy of Pediatrics. Revised guidelines for prevention of early‐onset group B streptococcal (GBS) infection. Pediatrics 1997;99:489‐96. [DOI] [PubMed] [Google Scholar]
ACOG 1996
- American College of Obstetricians and Gynecologists. Prevention of early‐onset group B streptococcal disease in newborns. ACOG Committee Opinion. 1996; Vol. 173:1‐8.
ASG 1995
- Australasian Study Group for neonatal Infections. Early onset group B streptococcal infection in Aboriginal and non‐Aboriginal infants. The Medical Journal of Australia 1995;163:302‐6. [DOI] [PubMed] [Google Scholar]
ASID 2002
- Australasian Society for Infectious Diseases. Management of perinatal infections. Australasian Society for Infectious Diseases, 2002. [Google Scholar]
Baker 1995
- Baker CJ, Edwards MS. Group B streptococcal infections. In: Remington J, Klein JO, eds. Infectious diseases of the fetus and newborn infant. 4th Edition. Philadelphia: WB Saunders, 1995:980‐1054. [Google Scholar]
Boyer 1985
- Boyer KM, Gottof SP. Strategies for chemoprophylaxis of GBS early onset infections. Antibiotics and Chemotherapy 1985;35:267‐80. [DOI] [PubMed] [Google Scholar]
Boyer 1986
- Boyer KM, Gotoff SP. Prevention of early‐onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. The New England Journal of Medicine 1986;314:1665‐9. [DOI] [PubMed] [Google Scholar]
Boyer 1988
- Boyer KM, Gotoff SP. Antimicrobial prophylaxis of neonatal group B streptococcal sepsis. Clinics in Perinatology 1988;15:831‐50. [PubMed] [Google Scholar]
CDC 2002
- Centers for Disease Control and Prevention. Early‐onset group B streptococcal disease ‐ United States, 1998‐1999. MMWR. Morbidity and Mortality Weekly Report 2002; Vol. 49:793‐6.
Flenady 1998
- Flenady V, King J, Woodgate P, Cartwright D, Brown A. Early onset neonatal GBS sepsis ‐ a case control study. Proceedings of the 2nd Annual Congress of the Perinatal Society of Australia and New Zealand. 1998.
Gotoff 1997
- Gotoff SP, Boyer KM. Prevention of early‐onset neonatal group B streptococcal disease. Pediatrics 1997;99:866‐9. [DOI] [PubMed] [Google Scholar]
Joseph 1998
- Joseph TA, Pyati SP, Jacobs N. Neonatal early‐onsey escherichia coli disease. The effect of intrapartum ampicillin. Archives of Pediatrics and Adolescent Medicine 1998;152:35‐40. [DOI] [PubMed] [Google Scholar]
Lloyd 1979
- Lloyd DJ, Belgaumkar TK, Scott KE, Wort AJ, Aterman K, Krause VW. Prevention of group‐B beta‐haemolytic streptococcal septicaemia in low‐birth‐weight neonates by penicillin administered within two hours of birth. Lancet 1979;1:713‐15. [DOI] [PubMed] [Google Scholar]
Patel 1994
- Patel DM, LeBlanc MH, Morrison JC, Graves GR, Glick CG, Martin JN Jr, et al. Postnatal penicillin prophylaxis and the incidence of group B streptococcal sepsis in neonates. Southern Medical Journal 1994;87:1117‐20. [DOI] [PubMed] [Google Scholar]
PCPG 2003
- Perinatal Clinical Practice Guidelines Working Party. Clinical practice guidelines for the prevention of neonatal early onset group B streptococcal disease. http://qheps.health.qld.gov.au/cpg/guide/Southern_Zone/GBS.pdf 2003.
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Siegel 1996
- Siegel JD, Cushion NB. Prevention of early‐onset group B streptococcal disease: another look at single‐dose penicillin at birth. Obstetrics and Gynecology 1996;87:692‐8. [DOI] [PubMed] [Google Scholar]
Smaill 2003
- Smaill F. Intrapartum antibiotics for Group B streptococcal colonisation (Cochrane Review). Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000115] [DOI] [PubMed] [Google Scholar]
Steigman 1975
- Steigman AJ, Bottone EJ, Hanna BA. Does intramuscular penicillin at delivery prevent group B beta hemolytic streptococcal disease of the newborn infant? [letter]. Journal of Pediatrics 1975;87:496. [DOI] [PubMed] [Google Scholar]
Steigman 1978
- Steigman AJ, Bottone EJ, Hanna BA. Intramuscular penicillin administration at birth: prevention of early‐onset group B streptococcal disease. Pediatrics 1978;62:842‐4. [PubMed] [Google Scholar]
Towers 1998
- Towers CV, Carr MH, Padilla G, Asrat T. Potential consequences of widespread antepartal use of ampicillin. American Journal of Obstetrics and Gynecology 1998;179:879‐83. [DOI] [PubMed] [Google Scholar]


