Abstract
Type 1 diabetes is a chronic autoimmune disease in which the destruction of pancreatic β cells leads to hyperglycemia. The prevention of hyperglycemia is very important to avoid or at least postpone the development of micro- and macrovascular complications, also known as late complications. These include diabetic retinopathy, chronic renal failure, diabetic neuropathy, and cardiovascular diseases. The impact of long-term hyperglycemia has been shown to persist long after the normalization of blood glucose levels, a phenomenon known as metabolic memory. It is believed that epigenetic mechanisms such as DNA methylation, histone modifications, and microRNAs, play an important role in metabolic memory. The aim of this review is to address the impact of long-term hyperglycemia on epigenetic marks in late complications of type 1 diabetes.
Keywords: type 1 diabetes, late complications, metabolic memory, epigenetics, DNA methylation, histone modification, microRNA
1. Introduction
Type 1 diabetes (T1D) is one of the most common chronic diseases of childhood, however, it can be diagnosed at any age. T1D is an autoimmune disease characterized by the destruction of pancreatic β cells, leading to insulin deficiency [1,2]. The goal of the treatment is to reach average blood glucose levels below 8.6 mmol/L (154 mg/dL) and glycated hemoglobin (HbA1c) below 7% (53 mmol/L), and to spend more than 70% of the time in the glycemic range between 3.9 and 10 mmol/L (70 and 180 mg/dL) [3]. Keeping glycemic control within recommended targets is important in order to prevent or at least postpone the development of micro- and macrovascular complications, also known as late complications. These include diabetic retinopathy, chronic renal failure, diabetic neuropathy, cardiovascular diseases, and hearing impairment [2,4,5,6,7].
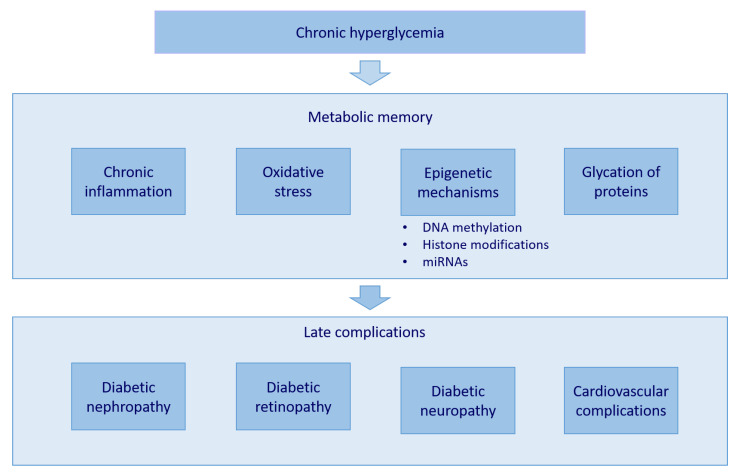
Several clinical and animal studies demonstrated that the early establishment of intensive blood-glucose control reduces the risk for the development of late complications [8]. For example, a Diabetes Control and Complication Trial (DCCT) was carried out, in which conventional and intensive therapy were compared. The study showed that participants in conventional therapy had higher glycated hemoglobin (HbA1c) values than those in intensive therapy. At the end of the study, the participants in conventional therapy also adopted intensive therapy. HbA1c levels lowered and no difference was observed between groups [9]. In the follow-up study Epidemiology of Diabetes Interventions and Complications (EDIC), researchers discovered that participants in intensive therapy during the DCCT study had a lower incidence of late complications [4]. This phenomenon was called metabolic memory. It is believed that chronic inflammation, oxidative stress, glycation of proteins, and epigenetic mechanisms contribute to metabolic memory (Figure 1) [10].
Figure 1.
The metabolic memory occurs as a consequence of long-term hyperglycemia. Chronic inflammation, oxidative stress, glycation of proteins, and epigenetic mechanisms—namely, DNA methylation, histone modifications, and microRNAs—all contribute to its features. The consequence is the emergence of late complications, such as diabetic nephropathy, diabetic retinopathy, diabetic neuropathy, and cardiovascular complications.
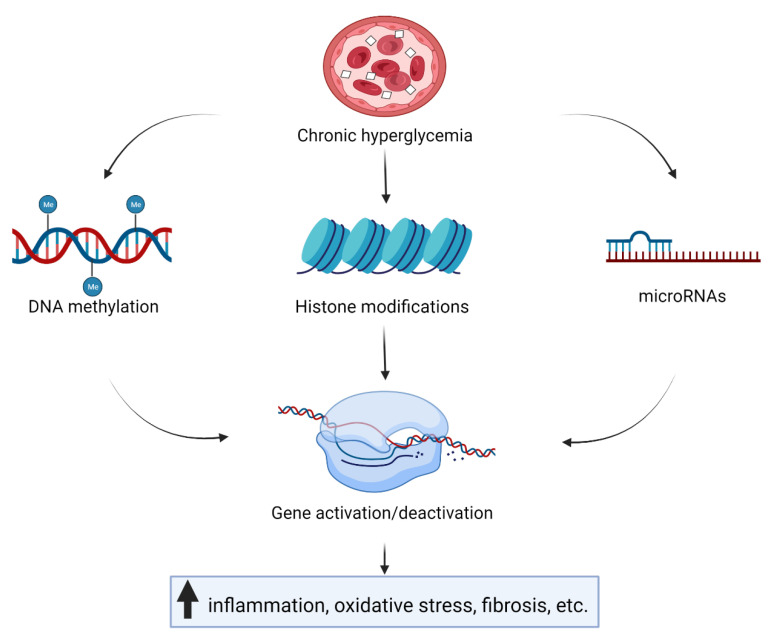
Epigenetic mechanisms—namely, DNA methylation, histone modifications, and microRNAs (miRNAs)—can influence transcription activity without changing the DNA sequence. They are essential for the regulation of all biological processes and are labile to environmental influences, such as nutrition, chemicals, stress, drugs, and infections [11,12]. Epigenetic changes have been associated with cancer [13], autoimmune and inflammatory diseases [14], cardiovascular diseases [15], obesity, and type 2 diabetes [16]. Furthermore, maternal nutrition during pregnancy was reported to influence fetal epigenetic modifications, which can cause a variety of long-term metabolic disorders in the offspring [17]. The aim of this review is to summarize discoveries of the impact of long-term hyperglycemia on epigenetic marks regarding late complications in T1D (Figure 2).
Figure 2.
Chronic hyperglycemia influences gene transcription through epigenetic mechanisms—namely, DNA methylation, histone modifications, and microRNAs. The consequence is an increase in inflammation, oxidative stress, fibrosis, etc.
2. DNA Methylation and Late Complications
DNA methylation is a covalent modification of DNA involving the transfer of a methyl group to the fifth carbon of a cytosine residue to form 5-methylcytosine by DNA methyltransferases. Most DNA methylation occurs in a CpG dinucleotide context [18]. Demethylation is mediated through the family of ten-eleven translocase enzymes [19]. DNA methylation is essential for normal development, and it plays a very important role in several processes, such as the regulation of tissue-specific gene expression, genomic imprinting, X-chromosome inactivation, and silencing of repetitive element transcription and transposition [20]. Aberrant DNA methylation changes have been detected in several diseases [21].
DNA methylation analysis on blood DNA samples from DCCT participants showed that long-term hyperglycemia influenced DNA methylation. The association between the mean HbA1c value during DCCT and 186 CpG sites were identified. The persistence of DNA methylation patterns in monocytes collected 16 or 17 years later during the EDIC indicated a metabolic memory [22]. HbA1c levels and T1D duration were positively correlated with PhenoAge, a DNA methylation age calculator [23].
Different methylation patterns were observed between individuals with T1D with end-stage kidney disease and those without kidney disease in blood-derived DNA [24,25,26] and in DNA extracted from saliva [27]. Hypermethylation of genes was reported in blood-derived DNA in individuals with chronic kidney disease [26]. Global hypermethylation was also reported in peripheral blood mononuclear cells isolated from type 2 diabetes individuals with albuminuria [28]. Genome-wide DNA methylation analysis in peripheral-blood-cell-derived DNA from T1D individuals with and without diabetic nephropathy identified 19 CpG sites to be associated with the risk for the development of diabetic nephropathy. Among them, one CpG was located upstream of the transcription start site of the UNC13B gene, previously linked to diabetic nephropathy. Methylation levels on this CpG were higher in the case group [29]. Higher values of DNA methylation calculators, PhenoAge, and GrimAge were associated with a decrease in renal function [23]. Quantitative DNA methylation profiling in renal cell models showed that short-term exposure to high glucose levels is insufficient to cause alterations in DNA methylation profiles [30].
Genome-wide DNA methylation analysis in blood samples identified 349 CpG sites, corresponding to 233 genes, to be differentially methylated between individuals with proliferative diabetic retinopathy and individuals with long-term diabetes with no or mild retinopathy. Differences were observed in genes with known function in the retina and eye development, inflammation, diabetic complications, and oxidative stress. The majority of CpG sites showed decreased methylation in individuals with proliferative diabetic retinopathy. However, no difference in global methylation between the groups was observed [31]. In the retina of streptozotocin (STZ)-induced diabetic rats exposed to high glucose, hypermethylation was observed at the promoter region of POLG1, a catalytic subunit of the mitochondrial DNA replication enzyme. Hypermethylation persisted after the reversal of high glucose levels to normal levels. Similar findings were observed in retinal endothelial cells [32].
In peripheral blood cells of T1D individuals with cardiac autonomic neuropathy, increased DNA methylation was observed in the first intron of the NINJ2 gene, coding a cell surface adhesion protein with a possible role in nerve regeneration. On the other hand, decreased methylation was reported in the first intron of the BRSK2 gene, involved in neuronal polarization, and in the 5′ untranslated region of the CLDN4 gene, coding a component of the tight junction [33]. A minor allele C of rs11085721 in the gene encoding DNMT1, a deoxyribonucleic acid methyltransferase, was associated with the risk for cardiac autonomic neuropathy but not for peripheral neuropathy in women with T1D [34]. The age calculator GrimAge was linked with the development of diabetic peripheral neuropathy and cardiovascular autonomic neuropathy [23].
Lower global DNA methylation was observed in diabetic foot ulcers fibroblasts and diabetic foot fibroblasts, compared with non-diabetic foot fibroblasts. DNA methylation patterns persisted even after multiple cell culture passages under normoglycemic conditions [35]. Furthermore, in animal models, global DNA hypomethylation induced by long-term hyperglycemia and its persistence in the normoglycemic environment were also identified in STZ-induced adult zebrafish [36] and STZ-induced rat bladder detrusor [37]. Genomic DNA hypomethylation was present in the liver but not in the kidney in diabetic rats [38]. In diabetic zebrafish, high glucose levels inducted the Parp family of enzymes, which led to stimulation of ten-eleven translocase enzymes, and the consequence was demethylation of high glucose-responsive loci, such as uhrf1, grtp1a, gcat, hnrnpa0, etc. [39].
3. Histone Modifications and Late Complications
Covalent posttranslational modifications of histones change histone–DNA interaction and thus influence gene transcription and other DNA processes, such as repair, replication, and recombination [40]. Different posttranslational modifications are known, such as histone acetylation, histone phosphorylation, histone methylation, and histone ubiquitination [41]. Histone acetylation and histone phosphorylation are normally associated with transcriptional activation. Histone acetylation occurs on the ε-amino group of lysine side chains. This causes the neutralization of the lysine’s positive charge, which weakens the interaction between histones and DNA. Histone phosphorylation also alters the charge of the histone protein. N-terminal histone tails of serine, threonine, and tyrosine are normally phosphorylated [42,43]. Histone methylation can lead to transcriptional activation or repression, depending on the site and level of methylation. Methylation occurs on the side chains of lysines, arginines, and histidines without affecting the histone charge. Lysines can be mono-, di-, or tri-methylated; arginines can be mono-, symmetrically, or asymmetrically di-methylated, whereas histidines can be monomethylated [44]. Histone monoubiquitination occurs on histone H2A at lysine 119 (H2AK119) and on histone H2B at lysine 120 (H2BK120). H2A monoubiquitination is linked to transcriptional repression, whereas H2B monoubiquitination is associated with transcriptional activation [45]. Aberrant histone modifications were linked to several different diseases and conditions [46,47,48].
In diabetic rat mesangial cells, the decrease in the H3 lysine 9 trimethylation 3 (H3K9me3) mark was found at promoter sites of pro-fibrotic genes such as COL11A1, CTGF, and SERPINE1, which contribute to the development of diabetic nephropathy [49]. The reduction in the repressive H3K27me3 mark was also identified at the promotor/enhancer sites of pro-fibrotic and inflammatory genes in diabetic rat renal mesangial cells [50]. Similar findings were observed in the kidneys of OVE6 mice and STZ-induced diabetic rats, together with increased levels of the activating epigenetic mark H3K4 demethylation [51]. In kidneys of STZ-induced diabetic rats treated with curcumin, increased acetylation of histone H3, together with increased dephosphorylation of histone H3, was reported [52]. High glucose levels influenced H2A and H2B monoubiquitination levels. In cultured glomerular mesangial cells, H2A ubiquitination was increased, and H2B ubiquitination was reduced [53]. However, H2AK119 and H2BK120 ubiquitination were increased in the whole kidney of diabetic animals, whereas in glomeruli of diabetic animals, their levels were reduced [54].
In vascular smooth muscle cells from diabetic db/db mice, decreased levels of H3K9me3 were identified at promoter sites of inflammatory genes—namely, IL-6, MCSF, and MCP-1. These aberrant chromatin changes persisted even after cells were cultured in vitro, indicating metabolic memory [55]. The persistence of the activating H3K4me1 mark was observed at the promotor site of the NF-κB subunit p65 in aortic endothelial cells even when the cells were removed from the hyperglycemic environment [56,57]. Genome-wide histone H3K9/K14 hyperacetylation analysis in primary human aortic endothelial cells exposed to high or low glucose showed different histone acetylation patterns. The hyperacetylation was observed in regions of genes annotated to diabetes, coronary artery disease, and other cardiovascular diseases as a consequence of hyperglycemia [58].
Chronically high glucose levels caused changes in histone H3K4 and K9 demethylation in human monocytes [59]. In DCCT/EDIC participants, HbA1c levels were associated with monocytes H3K9 acetylation (H3K9Ac). Monocytes isolated from participants with a history of higher HbA1c levels had more promotor regions enriched with H3K9Ac. These promoters corresponded to genes related to numerous diabetes and diabetes complication-related pathways, including the TNFR2 signaling and the NF-κB pathway [60]. Miao et al. observed similar findings [61,62]. An in vitro study on human monocytes exposed to high glucose levels showed suppressed pro-inflammatory gene expression after the treatment with curcumin by decreasing histone acetylation [63].
In the diabetic retina, contradictory findings were observed. Histone hyperacetylation was observed in the retinas of diabetic rats, together with increased expression of inflammatory proteins [64]. On the contrary, in the retina of STZ-induced rats exposed to high glucose levels, the global acetylation of histone H3 was decreased, which persisted even after the reversal of hyperglycemia [65].
High glucose levels caused aberrant expression of various histone-modifying enzymes. Increased levels of SET7/9, a methyltransferase, stimulated the expression of extracellular matrix genes in renal mesangial cells [49,66,67], inflammatory genes in monocytes [68], and cultured vascular smooth muscle cells [55]. In renal mesangial cells, downregulation of EZH2, an H3K27me3 methyltransferase, and upregulation of H3K27me3 demethylases KDM6B and KDM6A were identified [50]. Levels of SEV39H1 methyltransferase were decreased in diabetic conditions in vascular smooth muscle cells [55] and mesangial cells [69]. Single nucleotide polymorphism rs17353856 in the SUV39H2 gene, a methyltransferase, was associated with diabetic retinopathy in individuals with T1D [70]. In cultured human monocytes, high glucose caused upregulation of p300, histone acetyltransferase and coactivator of NF-κB, and downregulation of histone deacetylase activity 2. These were reversed after the treatment with curcumin [63].
4. miRNAs and Late Complications
miRNAs are small ~22-nucleotide-long non-coding RNAs and negative regulators of targeted genes expression by binding to the 3′ untranslated region of mRNA [71]. They are involved in many biological processes, including cell differentiation, proliferation, cell metabolism, and apoptosis, and are often perturbed in many disease states [72]. Since they can be detected in plasma, urine, cerebrospinal, and other extracellular fluids, they can serve as potential noninvasive diagnostic biomarkers for many different diseases and conditions [73].
Increased levels of miR-21 [74,75,76,77], miR-135a [78], miR-192 [79,80], miR-200b/c [81], miR-214 [82,83], and miR-377 [84] were observed in diabetic kidney. All of them contributed to renal hypertrophy and fibrosis. miR-21 was shown to promote fibrosis of the kidney by targeting several metabolic pathways [74,75]. Dey et al. showed that miR-21 enhanced high glucose-induced TORC1 activity, leading to renal cell hypertrophy and fibronectin expression [76]. Inhibition of SMAD7 and PTEN by miR-21 increased the expression of pro-fibrotic and extracellular matrix genes [77]. Decreased levels of PTEN were also associated with increased expression of miR-214 as a consequence of high glucose [82,83]. TGF-β-induction of miR-200b and miR-200c downregulated FOG2, an inhibitor of PI3K, thereby leading to activation of the PI3K–Akt pathway and glomerular mesangial hypertrophy [81]. Reduction of E-box repressors Zeb1/2 by miR-192 led to increased expression of collagen [79,80], and miR-200b/c [81]. miR-377 enhanced oxidative stress in mesangial cells and contributed to increased fibronectin protein production by targeting SOD1, SOD2, and PAK1 [84].
On the contrary, levels of several miRNAs were decreased in the diabetic kidney, indicating their protective role in diabetic nephropathy [85,86,87,88,89,90]. The reduction in miR-25 resulted in increased levels of its target NOX4, resulting in increased generation of oxidative stress in mesangial cells [85,86]. Loss of miR-29b was linked to progressive diabetic kidney injury with microalbuminuria, renal fibrosis, and inflammations. Its direct targets are ColI/III/IV, fibrillin, and elastin [87]. The decrease in miR-146a correlated with increased albuminuria and glomerular damage, making it a potential biomarker for disease progression [88]. The expression of miR-424 in renal tissue of T1D rats was lower, compared with normal controls. Rictor was found to be its direct target [90].
In the retina of Akita mice, upregulation of miR-200b was observed. By downregulating its target OXR1, miR-200b was demonstrated to play a protective role against oxidative stress [91]. However, contradictory findings were identified in the retina of STZ-induced diabetic rats, as miR-200b was downregulated. The transfection of endothelial cells with miR-200b lowered the expression of VEGF mRNA and protein [92]. miR-451a was also downregulated in the diabetic retina. In diabetic conditions, miR-451a was identified to have a protective effect on mitochondrial function [93].
Levels of miR-39c were increased in the trigeminal ganglion tissue of STZ-induced diabetic mice. Targeting Atg4B miR-39c negatively affected the growth of trigeminal sensory neurons and the diabetic corneal nerve regeneration [94]. Upregulation of miR-341 and downregulation of let-7i were found in sensory neurons in mice with diabetic polyneuropathy. Restoring of let-7i or knocking down of miR-341 improved diabetic polyneuropathy phenotype [95].
Single nucleotide polymorphisms in miRNAs were associated with late complications. An rs2910164 polymorphism in seed sequence within miR-146a, leading to a reduction in mature miR-146a, was associated with diabetic nephropathy, and retinopathy [89,96]. In the southern Brazilian population, an association between the A allele of miR-126 rs4636297 and protection against diabetic retinopathy was observed [96]. miR-449b rs10061133 polymorphism was significantly associated with a decrease in risk for proliferative diabetic retinopathy, with a minor G allele being protective against diabetic retinopathy by altering a Dicer cleavage site [97].
A case–control study identified different plasma levels of miRNA associated with diabetic nephropathy. Levels of miR-25, miR-27a, miR-126, miR-130b, miR-132, miR-152, miR-320, miR-326, miR-340, and miR-660 were elevated, and levels of miR-181a, miR-223, and miR-574-3p were lowered. After pancreas–kidney transplantation, levels of miR-25, miR-27a, miR-130b, miR-132, miR-152, miR-320, miR-326, miR-340, miR-574-3p, and miR-660 were normalized [98]. In the plasma of individuals with severe diabetic kidney disease, miR-21-3p and miR-378-3p were upregulated, whereas miR-16-5p and miR-29a-3p were downregulated [99]. Circulating let-7b-5p and miR-21-5p were associated with the increased risk for the development of end-stage renal disease. Conversely, let-7c-5p and miR-29a-3p were associated with protection against rapid progression [100]. miR-21 could be used as a potential biomarker in peripheral blood for early detection of nephropathy in T1D since its levels start to increase within the first 5 years from the onset of diabetes [74]. In extracellular vesicles extracted from individuals with diabetic retinopathy, miR-150-5p was found to be downregulated, whereas miR-30b-5p was upregulated. miR-21-3p was also upregulated in samples of individuals with and without diabetic retinopathy [101]. Circulating miR-320a and miR-27b were linked to the incidence and progression of retinopathy [102]. miR-518-3p and miR-618 isolated from serum were positively associated with multiple microvascular complications: diabetic nephropathy, diabetic retinopathy, peripheral neuropathy, and cardiovascular autonomic neuropathy [103].
Urinary miRNA profiling in diabetic nephropathy linked 18 miRNAs with the development of microalbuminuria [104]. A set of 27 miRNAs was found in matched urine samples from T1D individuals at different levels in different stages of nephropathy [105]. Ghai et al. identified several changes in miRNAs concentration in urine and urinary extracellular vesicles between individuals with T1D with various grades of diabetic nephropathy or microalbuminuria [106]. In children and adolescents with T1D, urinary miR-377 levels were higher in individuals with microalbuminuria and positively correlated with HbA1c, carotid intimal thickness, and urinary albumin creatinine ration, while levels of miR-216a were lower and negatively correlated with these variables [107].
5. Future Perspectives
Establishing and maintaining normoglycemia after the onset of T1D is very important for preventing late T1D complications. The international study of glycemic control in people with T1D showed that glycemic control is still suboptimal [108]. The use of continuous glucose monitoring was shown to reduce glucose variability [109]. However, the discovery of biomarkers for the early detection of late complications is still crucial. A growing body of evidence suggests the correlation between long-term hyperglycemia and epigenetic modifications. Their persistence after the normalization of blood glucose levels indicates their involvement in metabolic memory, making them a vital area of research.
Epigenetic marks are good candidates as biomarkers for the early detection of late complications since they represent a link between the environment and gene expression [12] and are stable in both tissue and body fluids [110]. As opposed to genetics, epigenetic marks are mostly reversible; therefore, they could also be potential targets for novel therapeutic approaches [11]. However, due to the complexity of epigenetic marks, such as diversity in cell types [110], further studies are still necessary to elucidate the exact mechanisms of long-term hyperglycemia’s influence on epigenetic marks.
The use of the latest nucleotide sequencing detection techniques could give us new insights. Long-read sequencing enables the study of a wide range of modifications in single-molecule sequencing on native DNA. It can surpass traditional bisulfite sequencing for studying DNA methylation. Bisulfite sequencing, a golden standard for studying DNA methylation, is a DNA destructive method, and mapping generated short-read data to repetitive or low-complexity regions is very difficult [111]. The single-cell analyses allow the study of cell-specific epigenetic marks, compared with traditional profiling methods that analyze the bulk population of cells. Although these approaches are still under development, they will provide a more comprehensive understanding of how hyperglycemia influences epigenetic marks in different cell types [112,113,114]. Hopefully, knowledge of epigenetic modifications could contribute to the early detection of late complications and the possible development of targeted therapies.
6. Conclusions
A growing body of evidence shows the relationship between late complications of T1D and epigenetic mechanisms. Long-term hyperglycemia causes aberrant epigenetic marks that persisted even after the normoglycemic environment was established and maintained, indicating the involvement of epigenetics in metabolic memory. These marks can be used as biomarkers for the early detection of micro- and macrovascular complications. They may also serve as good targets for novel therapeutic approaches since epigenetic modifications can be reversed.
Acknowledgments
Figure 2 was created with BioRender.com accessed on 11 April 2022.
Author Contributions
Conceptualization, B.Č.K., K.T.P. and N.B.; writing—original draft preparation, B.Č.K.; visualization, B.Č.K.; writing—review and editing, J.K., R.Š., B.J.B., T.T., M.D., K.T.P., T.B. and N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the financial support from the Slovenian Research Agency (research core funding P3-0343).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maahs D.M., West N.A., Lawrence J.M., Mayer-Davis E.J. Epidemiology of type 1 diabetes. Endocrinol. Metab. Clin. N. Am. 2010;39:481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383:1–223. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Glycemic targets: Standards of medical care in diabetes. Diabetes Care. 2022;45:S83–S96. doi: 10.2337/dc22-S006. [DOI] [PubMed] [Google Scholar]
- 4.Nathan D.M. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care. 2014;37:9–16. doi: 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind M., Svensson A.-M., Kosiborod M., Gudbjörnsdottir S., Pivodic A., Wedel H., Dahlqvist S., Clements M., Rosengren A. Glycemic Control and Excess Mortality in Type 1 Diabetes. N. Engl. J. Med. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 6.Schade D.S., Lorenzi G.M., Braffett B.H., Gao X., Bainbridge K.E., Barnie A., Cruickshanks K.J., Dalton D., Diminick L., Gubitosi-Klug R., et al. Hearing impairment and type 1 diabetes in the diabetes Control and complications trial/ epidemiology of diabetes interventions and complications (DCCT/EDIC) cohort. Diabetes Care. 2018;41:2495–2501. doi: 10.2337/dc18-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yau J.W.Y., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.J., Dekker J.M., Fletcher A., Grauslund J., et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venugopal S. Hyperglycemic memory and its long term effects in diabetes. Biomed. Res. 2016;2016:S354–S361. [Google Scholar]
- 9.Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J. Pediatr. 1994;125:177–188. doi: 10.1016/S0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 10.Testa R., Bonfigli A.R., Prattichizzo F., La Sala L., De Nigris V., Ceriello A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients. 2017;9:437. doi: 10.3390/nu9050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moosavi A., Ardekani A.M. Role of epigenetics in biology and human diseases. Iran. Biomed. J. 2016;20:246–258. doi: 10.22045/ibj.2016.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalli G., Heard E. Advances in epigenetics link genetics to the environment and disease. Nature. 2019;571:489–499. doi: 10.1038/s41586-019-1411-0. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S., Kelly T.K., Jones P.A. Epigenetics in cancer. Carcinogenesis. 2009;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surace A.E.A., Hedrich C.M. The role of epigenetics in autoimmune/inflammatory disease. Front. Immunol. 2019;10:1–16. doi: 10.3389/fimmu.2019.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasher D., Greenway S.C., Singh R.B. The impact of epigenetics on cardiovascular disease. Biochem. Cell Biol. 2020;98:12–22. doi: 10.1139/bcb-2019-0045. [DOI] [PubMed] [Google Scholar]
- 16.Ling C., Rönn T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019;29:1028–1044. doi: 10.1016/j.cmet.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franzago M., Fraticelli F., Stuppia L., Vitacolonna E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics. 2019;14:215–235. doi: 10.1080/15592294.2019.1582277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore L.D., Le T., Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melamed P., Yosefzon Y., David C., Tsukerman A., Pnueli L. Tet enzymes, variants, and differential effects on function. Front. Cell Dev. Biol. 2018;6:22. doi: 10.3389/fcell.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angeloni A., Bogdanovic O. Enhancer DNA methylation: Implications for gene regulation. Essays Biochem. 2019;63:707–715. doi: 10.1042/EBC20190030. [DOI] [PubMed] [Google Scholar]
- 21.Tost J. DNA methylation: An introduction to the biology and the disease-associated changes of a promising biomarker. Mol. Biotechnol. 2010;44:71–81. doi: 10.1007/s12033-009-9216-2. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z., Miao F., Braffett B.H., Lachin J.M., Zhang L., Wu X., Roshandel D., Carless M., Li X.A., Tompkins J.D., et al. DNA methylation mediates development of HbA1c-associated complications in type 1 diabetes. Nat. Metab. 2020;2:744–762. doi: 10.1038/s42255-020-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roshandel D., Chen Z., Canty A.J., Bull S.B., Natarajan R., Paterson A.D. DNA methylation age calculators reveal association with diabetic neuropathy in type 1 diabetes. Clin. Epigenetics. 2020;12:1–16. doi: 10.1186/s13148-020-00840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth L.J., Kilner J., Nair V., Liu H., Brennan E., Kerr K., Sandholm N., Cole J., Dahlström E., Syreeni A., et al. Assessment of differentially methylated loci in individuals with end-stage kidney disease attributed to diabetic kidney disease: An exploratory study. Clin. Epigenetics. 2021;13:1–19. doi: 10.1186/s13148-021-01081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swan E.J., Maxwell A.P., Mcknight A.J. Distinct methylation patterns in genes that affect mitochondrial function are associated with kidney disease in blood-derived DNA from individuals with Type 1 diabetes. Diabet. Med. 2015;32:1110–1115. doi: 10.1111/dme.12775. [DOI] [PubMed] [Google Scholar]
- 26.Smyth L.J., McKay G.J., Maxwell A.P., McKnight A.J. DNA hypermethylation and DNA hypomethylation is present at different loci in chronic kidney disease. Epigenetics. 2013;9:366–376. doi: 10.4161/epi.27161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapienza C., Lee J., Powell J., Erinle O., Yafai F., Reichert J., Siraj E.S., Madaio M. DNA methylation profling identifes epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics. 2011;6:20–28. doi: 10.4161/epi.6.1.13362. [DOI] [PubMed] [Google Scholar]
- 28.Maghbooli Z., Larijani B., Emamgholipour S., Amini M., Keshtkar A., Pasalar P. Aberrant DNA methylation patterns in diabetic nephropathy. J. Diabetes Metab. Disord. 2014;13:1–8. doi: 10.1186/2251-6581-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell C.G., Teschendorff A.E., Rakyan V.K., Maxwell A.P., Beck S., Savage D.A. Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med. Genom. 2010;3:33. doi: 10.1186/1755-8794-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan E.P., Ehrich M., Brazil D.P., Crean J.K., Murphy M., Sadlier D.M., Martin F., Godson C., van den Boom D., Maxwell A.P., et al. DNA methylation profiling in cell models of diabetic nephropathy. Epigenetics. 2010;5:396–401. doi: 10.4161/epi.5.5.12077. [DOI] [PubMed] [Google Scholar]
- 31.Agardh E., Lundstig A., Perfilyev A., Volkov P., Freiburghaus T., Lindholm E., Rönn T., Agardh C.D., Ling C. Genome-wide analysis of DNA methylation in subjects with type 1 diabetes identifies epigenetic modifications associated with proliferative diabetic retinopathy. BMC Med. 2015;13:1–9. doi: 10.1186/s12916-015-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tewari S., Zhong Q., Santos J.M., Kowluru R.A. Mitochondria DNA replication and DNA methylation in the metabolic memory associated with continued progression of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2012;53:4881–4888. doi: 10.1167/iovs.12-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gastoł J., Kapusta P., Polus A., Pitera E., Biela M., Wołkow P., Pawliński Ł., Kieć-Wilk B. Epigenetic mechanism in search for the pathomechanism of diabetic neuropathy development in diabetes mellitus type 1 (T1DM) Endocrine. 2020;68:235–240. doi: 10.1007/s12020-019-02172-9. [DOI] [PubMed] [Google Scholar]
- 34.Santos-Bezerra D.P., Admoni S.N., Mori R.C., Pelaes T.S., Perez R.V., Machado C.G., Monteiro M.B., Parisi M.C., Pavin E.J., Queiroz M.S., et al. Genetic variants in DNMT1 and the risk of cardiac autonomic neuropathy in women with type 1 diabetes. J. Diabetes Investig. 2019;10:985–989. doi: 10.1111/jdi.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park L.K., Maione A.G., Smith A., Gerami-Naini B., Iyer L.K., Mooney D.J., Veves A., Garlick J.A. Genome-wide DNA methylation analysis identifies a metabolic memory profile in patient-derived diabetic foot ulcer fibroblasts. Epigenetics. 2014;9:1339–1349. doi: 10.4161/15592294.2014.967584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen A.S., Sarras M.P., Leontovich A., Intine R.V. Heritable transmission of diabetic metabolic memory in zebrafish correlates with DNA hypomethylation and aberrant gene expression. Diabetes. 2012;61:485–491. doi: 10.2337/db11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Tar M.T., Davies K.P. Hyperglycemic memory in the rat bladder detrusor is associated with a persistent hypomethylated state. Physiol. Rep. 2020;8:1–10. doi: 10.14814/phy2.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams K.T., Garrow T.A., Schalinske K.L. Type I diabetes leads to tissue-specific DNA hypomethylation in male rats. J. Nutr. 2008;138:2064–2069. doi: 10.3945/jn.108.094144. [DOI] [PubMed] [Google Scholar]
- 39.Dhliwayo N., Sarras M.P., Luczkowski E., Mason S.M., Intine R.V. Parp inhibition prevents ten-eleven translocase enzyme activation and hyperglycemia-induced DNA demethylation. Diabetes. 2014;63:3069–3076. doi: 10.2337/db13-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tessarz P., Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014;15:703–708. doi: 10.1038/nrm3890. [DOI] [PubMed] [Google Scholar]
- 41.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes C.E., English D.M., Cowley S.M. Acetylation and Co: An expanding repertoire of histone acylations regulates chromatin and transcription. Essays Biochem. 2019;63:97–107. doi: 10.1042/EBC20180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossetto D., Avvakumov N., Côté J. Histone phosphorylation. Epigenetics. 2012;7:1098–1108. doi: 10.4161/epi.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greer E.L., Shi Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012;13:343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao J., Yan Q. Histone ubiquitination and deubiquitination in transcription, DNA damage response, and cancer. Front. Oncol. 2012;2:1–9. doi: 10.3389/fonc.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cobos S.N., Bennett S.A., Torrente M.P. The impact of histone post-translational modifications in neurodegenerative diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:1982–1991. doi: 10.1016/j.bbadis.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Yuan Q., Xie L. Histone Modifications in Aging: The Underlying Mechanisms and Implications. Curr. Stem Cell Res. Ther. 2018;13:125–135. doi: 10.2174/1574888X12666170817141921. [DOI] [PubMed] [Google Scholar]
- 48.Audia J.E., Campbell R.M. Histone modifications and cancer. Cold Spring Harb. Perspect. Biol. 2016;8:1–31. doi: 10.1101/cshperspect.a019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun G., Reddy M.A., Yuan H., Lanting L., Kato M., Natarajan R. Epigenetic histone methylation modulates fibrotic gene expression. J. Am. Soc. Nephrol. 2010;21:2069–2080. doi: 10.1681/ASN.2010060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia Y., Reddy M.A., Das S., Oh H.J., Abdollahi M., Yuan H., Zhang E., Lanting L., Wang M., Natarajan R. Dysregulation of histone H3 lysine 27 trimethylation in transforming growth factor-β1-induced gene expression in mesangial cells and diabetic kidney. J. Biol. Chem. 2019;294:12695–12707. doi: 10.1074/jbc.RA119.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komers R., Mar D., Denisenko O., Xu B., Oyama T.T., Bomsztyk K. Epigenetic changes in renal genes dysregulated in mouse and rat models of type 1 diabetes. Lab. Investig. 2013;93:543–552. doi: 10.1038/labinvest.2013.47. [DOI] [PubMed] [Google Scholar]
- 52.Tikoo K., Meena R.L., Kabra D.G., Gaikwad A.B. Change in post-translational modifications of histone H3, heat-shock protein-27 and MAP kinase p38 expression by curcumin in streptozotocin-induced type I diabetic nephropathy. Br. J. Pharmacol. 2008;153:1225–1231. doi: 10.1038/sj.bjp.0707666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao C., Chen G., Liu L., Li X., He J., Jiang L., Zhu J., Xu Y. Impact of high glucose and proteasome inhibitor MG132 on histone H2A and H2B ubiquitination in rat glomerular mesangial cells. J. Diabetes Res. 2013;2013:589474. doi: 10.1155/2013/589474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goru S.K., Kadakol A., Pandey A., Malek V., Sharma N., Gaikwad A.B. Histone H2AK119 and H2BK120 monoubiquitination modulate SET7/9 and SUV39H1 in type 1 diabetes-induced renal fibrosis. Biochem. J. 2016;473:3937–3949. doi: 10.1042/BCJ20160595. [DOI] [PubMed] [Google Scholar]
- 55.Villeneuve L.M., Reddy M.A., Lanting L.L., Wang M., Meng L., Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc. Natl. Acad. Sci. USA. 2008;105:9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brasacchio D., Okabe J., Tikellis C., Balcerczyk A., George P., Baker E.K., Calkin A.C., Brownlee M., Cooper M.E., El-Osta A. Hyperglycemia Induces a Dynamic Cooperativity of Histone Methylase and Demethylase Enzymes Associated With Gene-Activating Epigenetic Marks That Coexist on the Lysine Tail. Diabetes. 2009;58:1229–1236. doi: 10.2337/db08-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El-Osta A., Brasacchio D., Yao D., Pocai A., Jones P.L., Roeder R.G., Cooper M.E., Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pirola L., Balcerczyk A., Tothill R.W., Haviv I., Kaspi A., Lunke S., Ziemann M., Karagiannis T., Tonna S., Kowalczyk A., et al. Genome-wide analysis distinguishes hyperglycemia regulated epigenetic signatures of primary vascular cells. Genome Res. 2011;21:1601–1615. doi: 10.1101/gr.116095.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miao F., Wu X., Zhang L., Yuan Y.C., Riggs A.D., Natarajan R. Genome-wide analysis of histone lysine methylation variations caused by diabetic conditions in human monocytes. J. Biol. Chem. 2007;282:13854–13863. doi: 10.1074/jbc.M609446200. [DOI] [PubMed] [Google Scholar]
- 60.Miao F., Chen Z., Genuth S., Paterson A., Zhang L., Wu X., Li S.M., Cleary P., Riggs A., Harlan D.M., et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes. 2014;63:1748–1762. doi: 10.2337/db13-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miao F., Gonzalo I.G., Lanting L., Natarajan R. In Vivo Chromatin Remodeling Events Leading to Inflammatory Gene Transcription under Diabetic Conditions. J. Biol. Chem. 2004;279:18091–18097. doi: 10.1074/jbc.M311786200. [DOI] [PubMed] [Google Scholar]
- 62.Miao F., Chen Z., Zhang L., Wang J., Gao H., Wu X., Natarajan R. RNA-sequencing analysis of high glucose-treated monocytes reveals novel transcriptome signatures and associated epigenetic profiles. Physiol. Genom. 2013;45:287–299. doi: 10.1152/physiolgenomics.00001.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yun J.M., Jialal I., Devaraj S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2011;22:450–458. doi: 10.1016/j.jnutbio.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kadiyala C.S.R., Zheng L., Du Y., Yohannes E., Kao H.Y., Miyagi M., Kern T.S. Acetylation of retinal histones in diabetes increases inflammatory proteins: Effects of minocycline and manipulation of histone acetyltransferase (HAT) and histone deacetylase (HDAC) J. Biol. Chem. 2012;287:25869–25880. doi: 10.1074/jbc.M112.375204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong Q., Kowluru R.A. Role of histone acetylation in the development of diabetic retinopathy and the metabolic memory phenomenon. J. Cell. Biochem. 2010;110:1306–1313. doi: 10.1002/jcb.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X., Li C., Li X., Cui P., Li Q., Guo Q., Han H., Liu S., Sun G. Involvement of histone lysine methylation in p21 gene expression in rat kidney in vivo and rat mesangial cells in vitro under diabetic conditions. J. Diabetes Res. 2016;2016:3853242. doi: 10.1155/2016/3853242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen J., Guo Y., Zeng W., Huang L., Pang Q., Nie L., Mu J., Yuan F., Feng B. ER stress triggers MCP-1 expression through SET7 / 9-induced histone methylation in the kidneys of db / db mice. Am. J. Physiol. 2014;306:916–925. doi: 10.1152/ajprenal.00697.2012. [DOI] [PubMed] [Google Scholar]
- 68.Li Y., Reddy M.A., Miao F., Shanmugam N., Yee J.K., Hawkins D., Ren B., Natarajan R. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-κB-dependent inflammatory genes: Relevance to diabetes and inflammation. J. Biol. Chem. 2008;283:26771–26781. doi: 10.1074/jbc.M802800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin S.H., Ho W.T., Wang Y.T., Chuang C.T., Chuang L.Y., Guh J.Y. Histone methyltransferase Suv39h1 attenuates high glucose-induced fibronectin and p21WAF1 in mesangial cells. Int. J. Biochem. Cell Biol. 2016;78:96–105. doi: 10.1016/j.biocel.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 70.Syreeni A., El-Osta A., Forsblom C., Sandholm N., Parkkonen M., Tarnow L., Parving H.H., McKnight A.J., Maxwell A.P., Cooper M.E., et al. Genetic examination of SETD7 and SUV39H1/H2 methyltransferases and the risk of diabetes complications in patients with type 1 diabetes. Diabetes. 2011;60:3073–3080. doi: 10.2337/db11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biogenesis M. Methods in MicroRNA Biogenesis, Identification, Function and Decay. Methods. 2019;152:1–2. doi: 10.1016/j.ymeth.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 72.Saliminejad K., Khorshid H.R.K., Fard S.S., Ghaffari S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019;234:5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 73.Mohr A.M., Mott J.L. Overview of microRNA biology. Semin. Liver Dis. 2015;35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fouad M., Salem I., Elhefnawy K., Raafat N., Faisal A. microRNA-21 as an Early Marker of Nephropathy in Patients with Type 1 Diabetes. Indian J. Nephrol. 2020;30:21–25. doi: 10.4103/ijn.IJN_80_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chau B.N., Xin C., Hartner J., Ren S., Castano A.P., Li J., Tran P.T., Kaimal V., Huang X., Chang A.N., et al. MicroRNA 21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci. Transl. Med. 2012;4:121ra18. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dey N., Das F., Mariappan M.M., Mandal C.C., Ghosh-Choudhury N., Kasinath B.S., Choudhury G.G. MicroRNA-21 orchestrates high glucose-induced signals to TOR complex 1, resulting in renal cell pathology in diabetes. J. Biol. Chem. 2011;286:25586–25603. doi: 10.1074/jbc.M110.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McClelland A.D., Herman-Edelstein M., Komers R., Jha J.C., Winbanks C.E., Hagiwara S., Gregorevic P., Kantharidis P., Cooper M.E. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin. Sci. 2015;129:1237–1249. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 78.He F., Peng F., Xia X., Zhao C., Luo Q., Guan W., Li Z., Yu X., Huang F. MiR-135a promotes renal fibrosis in diabetic nephropathy by regulating TRPC1. Diabetologia. 2014;57:1726–1736. doi: 10.1007/s00125-014-3282-0. [DOI] [PubMed] [Google Scholar]
- 79.Kato M., Zhang J., Wang M., Lanting L., Yuan H., Rossi J.J., Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc. Natl. Acad. Sci. USA. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Putta S., Lanting L., Sun G., Lawson G., Kato M., Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J. Am. Soc. Nephrol. 2012;23:458–469. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park J.T., Kato M., Yuan H., Castro N., Lanting L., Wang M., Natarajan R. FOG2 protein down-regulation by transforming growth factor-β1-induced MicroRNA-200b/c leads to akt kinase activation and glomerular mesangial hypertrophy related to diabetic nephropathy. J. Biol. Chem. 2013;288:22469–22480. doi: 10.1074/jbc.M113.453043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bera A., Das F., Ghosh-Choudhury N., Mariappan M.M., Kasinath B.S., Choudhury G.G. Reciprocal regulation of miR-214 and PTEN by high glucose regulates renal glomerular mesangial and proximal tubular epithelial cell hypertrophy and matrix expansion. Am. J. Physiol. Cell Physiol. 2017;313:C430–C447. doi: 10.1152/ajpcell.00081.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X., Shen E., Wang Y., Li J., Cheng D., Chen Y., Gui D., Wang N. Cross talk between miR-214 and PTEN attenuates glomerular hypertrophy under diabetic conditions. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Q., Wang Y., Minto A.W., Wang J., Shi Q., Li X., Quigg R.J. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008;22:4126–4135. doi: 10.1096/fj.08-112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fu Y., Zhang Y., Wang Z., Wang L., Wei X., Zhang B., Wen Z., Fang H., Pang Q., Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 2010;32:581–589. doi: 10.1159/000322105. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y., Li H., Liu J., Han P., Li X., Bai H., Zhang C., Sun X., Teng Y., Zhang Y., et al. Variations in MicroRNA-25 expression influence the severity of diabetic kidney disease. J. Am. Soc. Nephrol. 2017;28:3627–3638. doi: 10.1681/ASN.2015091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen H.Y., Zhong X., Huang X.R., Meng X.M., You Y., Chung A.C.K., Lan H.Y. MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol. Ther. 2014;22:842–853. doi: 10.1038/mt.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee H.W., Khan S.Q., Khaliqdina S., Altintas M.M., Grahammer F., Zhao J.L., Koh K.H., Tardi N.J., Faridi M.H., Geraghty T., et al. Absence of miR-146a in Podocytes Increases Risk of Diabetic Glomerulopathy via Up-regulation of ErbB4 and Notch- 1. J. Biol. Chem. 2017;292:732–747. doi: 10.1074/jbc.M116.753822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaidonis G., Gillies M.C., Abhary S., Liu E., Essex R.W., Chang J.H., Pal B., Sivaprasad S., Pefkianaki M., Daniell M., et al. A single-nucleotide polymorphism in the MicroRNA-146a gene is associated with diabetic nephropathy and sight-threatening diabetic retinopathy in Caucasian patients. Acta Diabetol. 2016;53:643–650. doi: 10.1007/s00592-016-0850-4. [DOI] [PubMed] [Google Scholar]
- 90.Wang G., Yan Y., Xu N., Hui Y., Yin D. Upregulation of microRNA-424 relieved diabetic nephropathy by targeting Rictor through mTOR Complex2/Protein Kinase B signaling. J. Cell. Physiol. 2019;234:11646–11653. doi: 10.1002/jcp.27822. [DOI] [PubMed] [Google Scholar]
- 91.Murray A.R., Chen Q., Takahashi Y., Zhou K.K., Park K., Ma J.-X. MicroRNA-200b downregulates oxidation resistance 1 (Oxr1) expression in the retina of type 1 diabetes model. Investig. Ophthalmol. Vis. Sci. 2013;54:1689–1697. doi: 10.1167/iovs.12-10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McArthur K., Feng B., Wu Y., Chen S., Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao Y., Dong L.J., Takahashi Y., Chen J., Liu X., Chen Q., Ma J.X., Li X.R. MiRNA-451a regulates RPE function through promoting mitochondrial function in proliferative diabetic retinopathy. Am. J. Physiol. Endocrinol. Metab. 2019;316:E443–E452. doi: 10.1152/ajpendo.00360.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu J., Hu X., Kan T. MiR-34c participates in diabetic corneal neuropathy via regulation of autophagy. Investig. Ophthalmol. Vis. Sci. 2019;60:16–25. doi: 10.1167/iovs.18-24968. [DOI] [PubMed] [Google Scholar]
- 95.Cheng C., Kobayashi M., Martinez J.A., Ng H., Moser J.J., Wang X., Singh V., Fritzler M.J., Zochodne D.W. Evidence for Epigenetic Regulation of Gene Expression and Function in Chronic Experimental Diabetic Neuropathy. J. Neuropathol. Exp. Neurol. 2015;74:804–817. doi: 10.1097/NEN.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 96.Massignam E.T., Dieter C., Pellenz F.M., Assmann T.S., Crispim D. Involvement of miR-126 rs4636297 and miR-146a rs2910164 polymorphisms in the susceptibility for diabetic retinopathy: A case–control study in a type 1 diabetes population. Acta Ophthalmol. 2021;99:e461–e469. doi: 10.1111/aos.14638. [DOI] [PubMed] [Google Scholar]
- 97.Liu E., Kaidonis G., McComish B.J., Gillies M.C., Abhary S., Essex R.W., Chang J.H., Pal B., Daniell M., Lake S., et al. MicroRNA-related genetic variants are associated with diabetic retinopathy in type 1 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2019;60:3937–3942. doi: 10.1167/iovs.18-25570. [DOI] [PubMed] [Google Scholar]
- 98.Bijkerk R., Duijs J.M.G.J., Khairoun M., Ter Horst C.J.H., Van Der Pol P., Mallat M.J., Rotmans J.I., De Vries A.P.J., De Koning E.J., De Fijter J.W., et al. Circulating MicroRNAs associate with diabetic nephropathy and systemic microvascular damage and normalize after simultaneous pancreas-kidney transplantation. Am. J. Transplant. 2015;15:1081–1090. doi: 10.1111/ajt.13072. [DOI] [PubMed] [Google Scholar]
- 99.Assmann T.S., Recamonde-Mendoza M., Costa A.R., Puñales M., Tschiedel B., Canani L.H., Bauer A.C., Crispim D. Circulating miRNAs in diabetic kidney disease: Case–control study and in silico analyses. Acta Diabetol. 2019;56:55–65. doi: 10.1007/s00592-018-1216-x. [DOI] [PubMed] [Google Scholar]
- 100.Pezzolesi M.G., Satake E., McDonnell K.P., Major M., Smiles A.M., Krolewski A.S. Circulating TGF-β1-regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes. Diabetes. 2015;64:3285–3293. doi: 10.2337/db15-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mazzeo A., Beltramo E., Lopatina T., Gai C., Trento M., Porta M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp. Eye Res. 2018;176:69–77. doi: 10.1016/j.exer.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 102.Zampetaki A., Willeit P., Burr S., Yin X., Langley S.R., Kiechl S., Klein R., Rossing P., Chaturvedi N., Mayr M. Angiogenic microRNAs linked to incidence and progression of diabetic retinopathy in type 1 diabetes. Diabetes. 2016;65:216–227. doi: 10.2337/db15-0389. [DOI] [PubMed] [Google Scholar]
- 103.Santos-Bezerra D.P., Santos A.S., Guimarães G.C., Admoni S.N., Perez R.V., Machado C.G., Pelaes T.S., Passarelli M., Machado U.F., Queiroz M.S., et al. Micro-RNAs 518d-3p and 618 are upregulated in individuals with type 1 diabetes with multiple microvascular complications. Front. Endocrinol. 2019;10:385. doi: 10.3389/fendo.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Argyropoulos C., Wang K., Bernardo J., Ellis D., Orchard T., Galas D., Johnson J. Urinary MicroRNA Profiling Predicts the Development of Microalbuminuria in Patients with Type 1 Diabetes. J. Clin. Med. 2015;4:1498–1517. doi: 10.3390/jcm4071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Argyropoulos C., Wang K., McClarty S., Huang D., Bernardo J., Ellis D., Orchard T., Galas D., Johnson J. Urinary MicroRNA Profiling in the Nephropathy of Type 1 Diabetes. PLoS ONE. 2013;8:e54662. doi: 10.1371/annotation/37e647d5-1781-4edf-86a8-e3b533c32ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghai V., Wu X., Bheda-Malge A., Argyropoulos C.P., Bernardo J.F., Orchard T., Galas D., Wang K. Genome-wide Profiling of Urinary Extracellular Vesicle microRNAs Associated With Diabetic Nephropathy in Type 1 Diabetes. Kidney Int. Rep. 2018;3:555–572. doi: 10.1016/j.ekir.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.El-Samahy M.H., Adly A.A., Elhenawy Y.I., Ismail E.A., Pessar S.A., Mowafy M.E.S., Saad M.S., Mohammed H.H. Urinary miRNA-377 and miRNA-216a as biomarkers of nephropathy and subclinical atherosclerotic risk in pediatric patients with type 1 diabetes. J. Diabetes Its Complicat. 2018;32:185–192. doi: 10.1016/j.jdiacomp.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 108.Prigge R., McKnight J.A., Wild S.H., Haynes A., Jones T.W., Davis E.A., Rami-Merhar B., Fritsch M., Prchla C., Lavens A., et al. International comparison of glycaemic control in people with type 1 diabetes: An update and extension. Diabet. Med. 2021;39:e14766. doi: 10.1111/dme.14766. [DOI] [PubMed] [Google Scholar]
- 109.Dovc K., Cargnelutti K., Sturm A., Selb J., Bratina N., Battelino T. Continuous glucose monitoring use and glucose variability in pre-school children with type 1 diabetes. Diabetes Res. Clin. Pract. 2019;147:76–80. doi: 10.1016/j.diabres.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 110.Berdasco M., Esteller M. Clinical epigenetics: Seizing opportunities for translation. Nat. Rev. Genet. 2019;20:109–127. doi: 10.1038/s41576-018-0074-2. [DOI] [PubMed] [Google Scholar]
- 111.Gouil Q., Keniry A. Latest techniques to study DNA methylation. Essays Biochem. 2019;63:639–648. doi: 10.1042/EBC20190027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chakraborty C., Bhattacharya M., Agoramoorthy G. Single-cell sequencing of miRNAs: A modified technology. Cell Biol. Int. 2020;44:1773–1780. doi: 10.1002/cbin.11376. [DOI] [PubMed] [Google Scholar]
- 113.Ahn J., Heo S., Lee J., Bang D. Introduction to single-cell dna methylation profiling methods. Biomolecules. 2021;11:1013. doi: 10.3390/biom11071013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chanou A., Hamperl S. Single-Molecule Techniques to Study Chromatin. Front. Cell Dev. Biol. 2021;9:699771. doi: 10.3389/fcell.2021.699771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.