In the Beginning…
The concept of identifying and treating patients’ comorbidities before surgery initially appeared in research on cardiothoracic surgery, with a focus on reducing risk factors that increase cardiac strain such as anemia, hypothyroidism, and diuretic use [13]. The goal was to prepare patients preoperatively to reduce the risk of a high-risk surgical intervention and to allow for earlier detection of complications.
Over time, the concept of preoperative risk factor management has been extended to other procedures such as total joint arthroplasty (TJA), spine surgery, urologic surgery, and gastrointestinal surgery [17, 68, 78]. In orthopaedics, preoperative risk factor management in TJA has grown as an area of research, resulting in reduced readmissions, emergency department (ED) visits, length of stay (LOS), and overall costs as well as increased discharge to home after TJA [7, 19, 41].
Preoperative risk management programs in orthopaedics are evolving, and several models have been implemented to address risk factors before TJA. Some programs have focused on screening for risk factors preoperatively with recommended interventions when patients meet specific criteria, such as low hemoglobin levels, elevated hemoglobin A1c levels, and elevated BMI [7, 12, 19, 28, 41, 56]. Many of these programs also use dedicated “navigators”, who typically are nurses and social workers, to guide patients throughout the risk management process [7, 12, 19]. Other institutions simply implement preoperative surgical selection criteria without detailed guidance regarding the management of modifiable risk factors [50, 51].
The Argument
Patients identifying as Black or Hispanic are more likely to have a postoperative complication, a longer LOS, a lower likelihood of home discharge, a visit to the ED, and be readmitted after TJA [3, 18, 53, 59, 69]. Although patients identifying as Black and Hispanic have experienced some improvement in LOS after TJA, when compared with White patients, disparities in complication rates and hospital readmissions still persist [5, 70, 73]. Women are more likely to stay longer in the hospital and have a postoperative complication following TJA; they are also less likely to be discharged home [14]. Similar findings of increased LOS, ED visits, and readmissions have been discovered when investigating the association between patients with lower incomes versus patients with higher incomes as well as Medicaid insurance compared with private insurance after TJA [20, 60, 61]. Additionally, patients with lower incomes face an increased risk of mortality and revision after THA than those with higher incomes [22]. However, race, ethnicity, gender, and income do not exist in isolation and the intersection of these factors also is associated with increased LOS [35, 36]. Understanding the disparities that patients from racial and ethnic minority backgrounds, women, and patients with lower incomes face related to readmissions, ED visits, complications, and LOS after TJA is critical to eliminating these disparities. Our review provides an overview of disparities in TJA related to race, ethnicity, gender, income, and insurance.
Comprehensive preoperative risk management programs seek to reduce the frequency of readmissions and ED visits as well as to shorten LOS after TJA, and some have reported success in those areas [7, 19, 41]. However, we are concerned that these types of programs, by enforcing strict eligibility criteria without providing robust care management pathways to achieve cutoffs, may worsen access for TJA and may prevent participation in preoperative risk management programs for patients from racial and ethnic minority backgrounds, women, and patients with lower incomes [3, 74]. Additionally, comprehensive risk management programs decrease readmissions, ED vists, and LOS overall, but may not be providing those same benefits to patients from racial and ethnic minority backgrounds, women, and patients with lower incomes. Unconscious bias by orthopaedic surgeons may also result in surgeons being less likely to consider patients from racial and ethnic minority backgrounds, women compared with men, and patients with lower incomes as surgical candidates [23].
In addition, preoperative risk management programs sometimes rely on services provided by other specialties such as bariatric surgery, endocrinology, and cardiology to assist patients in reaching safer preoperative goals. As in orthopaedic surgery [1, 15, 40], insurance restrictions may impede patients’ access to care, and many of the same biases that have been observed in orthopaedic surgery in terms of access exist in those specialties, too [2, 31, 37, 71]. Some patients may also need additional assistance to prepare for surgery, such as guidance from social workers, nursing care coordinators, or substance use counselors. These services might not be offered by all orthopaedic practices and might not be accessible to all patients. Our review explores the use of preoperative cutoffs in preoperative risk management programs and the referral pathways for nonorthopaedic specialist care within these programs to determine how cutoffs and specialist referrals may be impacting patients from racial and ethnic minority backgrounds, women, patients with lower incomes, and patients with Medicaid.
The goal of comprehensive risk management programs is to reduce the risk of postoperative complications, readmissions, ED visits, and reduce LOS; consequently, these programs should be implemented for all patients regardless of race, ethnicity, gender, or income. Given that patients from racial and ethnic minority backgrounds, women, and patients with lower incomes historically have had more frequent readmissions and ED visits, longer LOS, as well as decreased discharges home after TJA [14, 69, 76], our review investigates how comprehensive risk management programs may be associated with readmissions, ED visits, LOS, discharge disposition, and postoperative complications for these patient populations.
Essential Elements
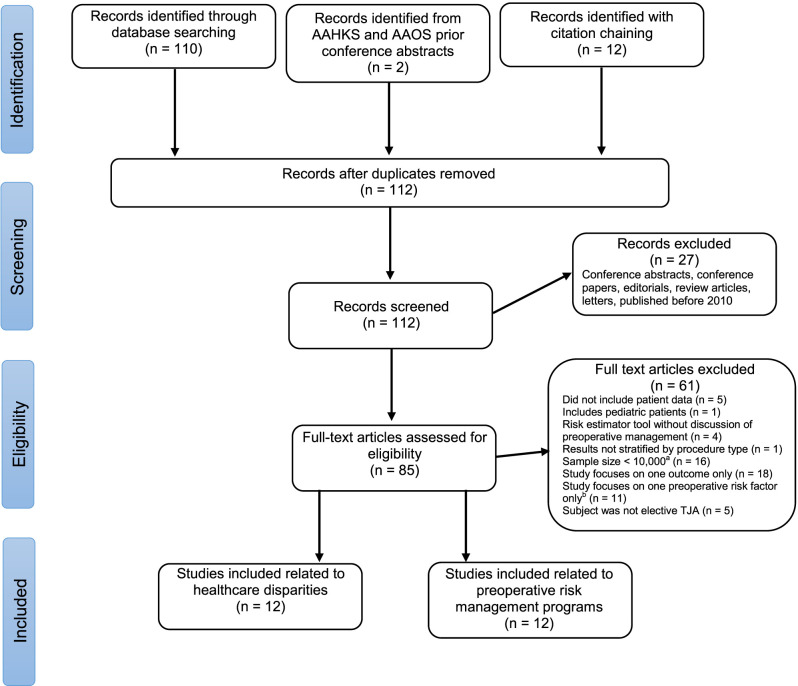
We searched the Ovid MEDLINE and Embase databases for articles that covered the concepts of “arthroplasty”, “preoperative risk management”, and “healthcare disparities” (Supplementary Fig. 1; http://links.lww.com/CORR/A753). Given the relatively small number of articles our search returned (n = 98), we also searched through abstracts presented at the American Association of Orthopaedic Surgeons conferences from 2020 and 2021 and abstracts presented at the American Association of Hip and Knee Surgeons from 2013 through 2021 and found two articles of interest. Additionally, we performed citation chaining, defined as examining reference lists for additional studies and identifying articles that cited relevant studies, on articles related to preoperative risk management and found 12 relevant studies.
We included studies if they were original research published after 2010, the operation of interest was elective TJA in adult patients. The included studies needed clinical data for the procedure of interest (THA, TKA, or both), and focused on more than one postoperative outcome such as LOS, readmissions, functional status, surgical site infection incidence, or ED visits. Specifically for studies describing associations between race, gender, insurance type, and income, we excluded studies with a sample size less than 10,000 patients. We could not apply this exclusion criterion for studies describing preoperative risk management programs because most of these studies were retrospective institutional studies. Regarding studies describing preoperative risk management programs, we only included programs that considered at least three patient factors such as anemia, obesity, and hemoglobin A1c level for potential modification and were not solely tools that investigated associations between specific risk factors and postoperative readmissions to estimate risk. We found 24 articles that met our inclusion criteria, 12 articles related to healthcare disparities and 12 related to preoperative risk management programs (Fig. 1).
Fig. 1.
Literature search strategy and study selection process. aCriterion does not apply to studies describing preoperative risk management programs. bCriterion does not apply to studies describing healthcare disparities; AAHKS = American Association of Hip and Knee Surgeons; AAOS = American Academy of Orthopaedic Surgeons; TJA = total joint arthroplasty.
Of the 12 articles related to disparities in care (Table 1), one study was related to preoperative risk factors and the time to TJA, number of patient comorbidities, in-hospital complications, and discharge disposition, specifically focusing on disparities in care for women [14]. Four studies investigated disparities in care for patients from ethnic and racial minority backgrounds: investigating the associations between race and ethnicity with procedure utilization, LOS, readmissions, and postoperative complications [18, 62, 70, 73]. Three studies investigated disparities in complications, LOS, discharge disposition, and readmissions for THA, and investigated race, gender, comorbidity burden, and insurance type [67, 75, 76]. In three studies, investigators explored the association between insurance type and disparities in mortality, complications, readmissions, and LOS after THA and TKA [47, 72, 77]. Finally, Holbert et al. [32] investigated associations between living in a medically underserved community and LOS, discharge disposition, readmissions, costs, and ED visits. We used the Methodological Index for Non-Randomized Studies (MINORS) criteria [65] for noncomparative studies to critically appraise these 12 studies (Supplementary Table 1; http://links.lww.com/CORR/A754). The ideal score for noncomparative studies using the MINORS criteria is 16, and the median (range) score of studies related to disparities in care was 8 (7 to 11)
Table 1.
Description of studies found in literature search related to disparities in care
| Study | Total number of patients | Procedure | Study type | Study purpose | Conclusions | Mentions healthcare disparities? | Mentions preoperative risk factor management? |
| Disparities in care for women | |||||||
| Cheah et al. [14] | 5,048,371 | THA and TKA | Retrospective study using the NIS | Provide a population-based epidemiologic assessment of preoperative risk factors and gender disparities among patients undergoing TJA | Women present later for TJA, have more comorbidities, more in-hospital complications, and are less likely to be discharged home compared with men. | Yes (gender) | No |
| Disparities in care for patients from racial and ethnic minority backgrounds | |||||||
| Cusano et al. [18] | 262,954 | TKA | Retrospective study using ACS-NSQIP | Investigated associations between race and ethnicity and procedure utilization, LOS, 30-day readmissions, and postoperative complications | Black race compared with White race was associated with more readmissions, longer LOS, and incidence of any postoperative complication. Hispanic/Latino ethnicity compared with non-Hispanic White patients was associated with longer LOS and incidence of any postoperative complication. | Yes (race and ethnicity) | No |
| Sheth et al. [62] | 11,574 | THA | Retrospective study using ACS-NSQIP | Investigated recent trends in procedure utilization, comorbidity profiles, hospital LOS, 30-day mortality, readmissions, and complications, and risk factors for AEs among patients identifying as Black | Between 2011 and 2017, there have been improvements in procedure utilization, comorbidity profiles, and LOS; however, no differences in 30-day mortality, readmissions, or complications. | Yes (race) | Yes, discussed that preoperative management of risk factors is a target for intervention |
| Trivedi et al. [70] | 19,496 | TKA | Retrospective study using ACS-NSQIP | Analyzed trends in mortality, LOS, postoperative surgical and medical complications, and readmissions after TKA and developed a preoperative risk stratification model for Black patients undergoing TKA | Overall, there have been improvements in annual trends in LOS, proportion of inpatient stays > 2 days, and postoperative surgical complications for Black patients after TKA. The study also developed a risk stratification nomogram for Black patients. | Yes (race) | Yes, discussed management of risk factors preoperatively such as anemia and tobacco use |
| Venugopal et al. [73] | 12,767 | TKA | Retrospective study using ACS-NSQIP | Analyzed trends in utilization, comorbidity profiles, hospital LOS, 30-day mortality, readmissions, and complications among patients identifying as Hispanic/Latino | Between 2011 and 2017, there have been improvements in procedure utilization, comorbidity profiles, and LOS, but the incidence of any postoperative event and inpatient stay > 2 days have increased. | Yes (race and ethnicity) | Yes, discussed that preoperative management of risk factors could be helpful for this patient population |
| Disparities in care based on race, gender, comorbidity burden, and insurance type | |||||||
| SooHoo et al. [67] | 138,399 | THA | Retrospective study using statewide California data | Studied patient and provider factors that are associated with complications after THA | The authors found that the incidence of 90-day complications after THA, including dislocations and revision surgeries, was associated with treatment at low-volume hospitals, Black race, and patients with more comorbidities. | Yes (race) | No |
| Weiner et al. [75] | 41,832 | THA | Retrospective study using Illinois COMPdata administrative database | Investigated patient characteristics associated with LOS and discharge disposition | The authors found that female gender, compared with male gender was associated with LOS > 3 days and nonhome discharge. Black race, compared with White race was associated with LOS > 5 days and nonhome discharge. Medicaid insurance was associated with LOS > 3 days, LOS > 5 days, and nonhome discharge. | Yes (gender, race, and insurance type) | Yes, mentioned that preoperative risk management programs may cause patients to be excluded |
| White et al. [76] | 274,851 | THA | Retrospective study using California, Florida, and New York SIDs | Investigated differences in readmission by insurance payer, race or ethnicity, and income | The authors found that Medicaid insurance compared with private insurance, Black race compared with White race, and lower incomes compared with higher incomes are associated with higher readmissions. | Yes (race, income, and insurance type) | Yes, discussion mentioned addressing modifiable risk factors preoperatively |
| Disparities in care based on insurance type | |||||||
| Maman et al. [47] | 922,819 | TKA | Retrospective study using New York, Florida, Maryland, Kentucky, and California SIDs | Investigated whether insurance type is associated with in-hospital mortality and morbidity after TKA | Medicaid insurance is associated with an increased odds of mortality and complications after TKA. | Yes (insurance type and race) | No |
| Veltre et al. [72] | 1,352,505 | TKA | Retrospective study using the NIS | Investigated the association between patient insurance type and in-hospital complications after TKA | The authors found that public insurance (Medicare or Medicaid) is associated with more medical complications and greater mortality; non-White race compared with White race is associated with complications after TKA. | Yes (insurance type and race) | Yes, discussion mentioned that preoperative risk factor management is important to reduce modifiable risk factors before surgery |
| Xu et al. [77] | 295,572 | THA | Retrospective study using California, Florida, and New York SIDs | Investigated the relationship between insurance type and in-hospital mortality, postoperative complications, readmissions, and LOS after THA | The authors found that Medicaid insurance compared with private insurance is associated with increased in-hospital mortality, cardiovascular and infectious complications, readmissions, and LOS | Yes (insurance type and race) | No |
| Disparities in care, other | |||||||
| Holbert et al. [32] | 11,451 | THA and TKA | Retrospective institutional study | Identified associations between living in a medically underserved area and LOS, discharge disposition, readmissions, costs, and ED visits | The authors found that living in a medically underserved area was associated with longer LOS, nonhome discharge, increased costs, and increased ED visits | Yes (medically underserved communities) | Yes, stated that patients were cared for in a coordinated Joint Replacement Center and received preoperative medical evaluations |
NIS = National Inpatient Sample; ACS-NSQIP = American College of Surgeons National Surgical Quality Improvement Program; AEs = adverse events; SID = State Inpatient Database; ED = emergency department.
We found an additional 12 studies that described the implementation of comprehensive preoperative risk management programs (Table 2) [7, 12, 19, 24, 25, 29, 41, 51, 54, 56-58]. We used the MINORS criteria [65] for comparative studies to critically appraise these 12 studies (Supplementary Table 2; http://links.lww.com/CORR/A755). The ideal score for comparative studies using the MINORS criteria is 24, and the median (range) score of studies related to preoperative risk management programs was 15 (13 to 17). As citation chaining is not as comprehensive as a structured literature search, it is possible that we missed some studies related to this topic, and we understand this may be a limitation of our study. However, given that we performed citation chaining in addition to our comprehensive literature search, we do not believe that we missed relevant studies that would have impacted the findings of our review.
Table 2.
Studies describing comprehensive preoperative risk management programs
| Study | Total number of patients | Procedure | Study type | Study purpose | Conclusions | Mentions eligibility criteria? | Includes citations for eligibility criteria? | Mentions healthcare disparities? |
| Bernstein et al. [7] | 665 | THA and TKA | Retrospective institutional study | Evaluated the impact of a preoperative risk factor management program on postoperative LOS, readmissions, discharge disposition, and cost | The authors found that the preoperative protocol decreased LOS and cost, and there was no difference in discharge location or 90-day readmissions. | Recommended BMI < 40 kg/m2 or between 35-40 kg/m2 if additional comorbidity and recommended HgbA1c < 7.0%; additional recommendations for other lab values including platelets, hemoglobin, and creatinine | No | No mention of race, gender, or income related to disparities in care; insurance type could not be acquired for participants |
| Bullock et al. [12] | 3114 | THA and TKA | Retrospective institutional study | Evaluated performance of a “bundle” to decrease PJI incidence after THA or TKA | Implementation of the bundle to modify risk factors was associated with a decreased incidence of PJI after TKA. | Recommended BMI < 40 kg/m2, HgbA1c < 7.0%, and smoking < 0.5 packs/day | No | No mention of race, gender, income, or insurance type related to disparities in care |
| Dlott et al. [19] | 463 | THA and TKA | Retrospective institutional study | Examined associations between implementation of a preoperative risk factor management protocol and LOS, ED visits, and readmissions following THA or TKA | Implementation of the protocol was associated with reduced LOS and ED visits. | Recommended BMI < 38 kg/m2 and HgbA1c < 8% | No | No mention of race, gender, income, or insurance type related to disparities in care |
| Featherall et al. [25] | 6090 | THA | Retrospective institutional study | Assessed association between implementation of a care pathway for THA with LOS, discharge disposition, 90-day complications, and cost | Implementation of the full protocol was associated with reduced LOS, an increase in home discharges, and decreased cost. Implementation of the full protocol was not associated with change in 90-day complications. | Recommended HgbA1c < 7%; no BMI cutoff specified | Yes | Male gender and White race were associated with reduced LOS and increased home discharges but did not discuss disparities in care; found that public insurance was associated with increased LOS, decreased home discharge, and increased 90-day complications but did not discuss disparities in care; no mention of income |
| Featherall et al. [24] | 6760 | TKA | Retrospective institutional study | Assessed association between implementation of a care pathway for TKA with LOS, discharge disposition, 90-day complications, and cost | Implementation of the full protocol was associated with decreased LOS, increased home discharges, and reduced cost. Implementation of the full protocol was not associated with change in 90-day complications. | Same as above study | N/A | Male gender and White race were associated with reduced LOS and increased home discharge but do not discuss disparities in care; nonWhite race was associated with increased 90-day complications; found that public insurance is associated with increased LOS, decreased home discharge, and increased 90-day complications; no mention of income |
| Gray et al. [29] | 1536 | THA and TKA | Retrospective institutional study | Evaluated association between implementation of CJR model and cost, discharge disposition, complications, readmissions, and LOS | Implementation of CJR model was associated with reduced costs, increased home discharges, decreased readmissions, complications, and LOS. | None | N/A | No mention of race, gender, income, or insurance type related to disparities in care |
| Kim et al. [41] | 1194 | THA and TKA | Retrospective institutional study | Assessed the association between a preoperative risk management program and postoperative readmissions, discharge location, LOS, and infection incidence | Implementation of this program was associated with lower readmission proportions and lower proportions of discharge to a postacute care facility. | Recommended BMI ≤ 40 kg/m2 and HgbA1c < 8% | No | No mention of race, gender, or insurance type related to disparities in care; Mentioned that SES was not evaluated though it can be associated with discharge disposition |
| Nussenbaum et al. [51] | 995 | THA and TKA | Retrospective institutional study | Determined whether implementation of preoperative screening criteria was associated with reduced complications and SSI incidence | Implementation of the preoperative screening criteria was associated with reduced total complications and SSI incidence. | Recommended BMI ≤ 35 kg/m2, HgbA1c 7%, hemoglobin ≥ 11 g/dL, and albumin 3.5 g/dL | No | No mention of race, gender, or income related to disparities in care. Only included patients treated at VA facility |
| Plate et al. [54] | 751 | THA | Retrospective institutional study | Evaluate implementation of the CJR model and its associations with surgery time, discharge disposition, LOS, and costs | Implementation of the CJR model was associated with reduced LOS and increased home discharges. It was not associated with changes in surgery time, 90-day readmissions, or costs. | Recommended BMI < 40 kg/m2, HgbA1c < 7.5%, and hemoglobin > 11 g/dL | No | Mentioned that increased scrutiny of patient selection may lead to disparities in access; no mention of disparities in care specific to race, gender, income, or insurance type |
| Ryan et al. [57] | 1248 | TKA | Retrospective institutional study | Evaluated implementation of CJR bundle and its association with surgery time, LOS, discharge disposition, and costs | Implementation of the CJR bundle was associated with decreased LOS and increased home discharges. It was not associated with surgery time or cost. | Recommended BMI < 40 kg/m2, HgbA1c < 7.5%, and hemoglobin > 11 g/dL | No | Mentioned that no difference in gender of patients receiving TKA before and after implementation of bundle; no mention of disparities in care related to race, gender, income, or insurance type |
| Ryan et al. [56] | 2308 | TKA | Retrospective multi-hospital study | Examined association of a preoperative checklist with LOS, discharge disposition, ED visits, and readmissions | Treatment at a CJR center was associated with reduced LOS and fewer discharges to SNFs. It was not associated with ED visits or readmissions. | Recommended BMI ≤ 40 kg/m2 and HgbA1c < 7.5%, hemoglobin > 11 g/dL, albumin 3 g/dL, and others related to smoking and platelets | No | No mention of race, gender, or income related to disparities in care; only included patients with Medicare insurance |
| Schultz et al. [58] | 216 | THA and TKA | Retrospective institutional study | Assessed the association between implementation of an accelerated recovery protocol and LOS, complications, discharge disposition, and cost | Implementation of the protocol was associated with reduced LOS, increased home discharges, reduced complications, and reduced costs. | Required HgbA1c < 8.0%; no hard cutoff for BMI | No | Mentioned that lower income is associated with disparities in access to TJA in introduction and that as a county hospital, their patient population often has lower incomes. There was no mention of race, gender, or insurance types related to disparities in care. |
HgbA1c = hemoglobin A1c; PJI = prosthetic joint infection; ED = emergency department; CJR = Comprehensive Care for Joint Replacement; SES = socioeconomic status; SSI = surgical site infection; VA = Veterans Affairs; SNF = skilled nursing facility.
What We (Think) We Know
We found that an increased risk of mortality, complications, readmissions, longer LOS, and less functional improvement are observed among patients from racial and ethnic minority backgrounds, women compared with men, and patients with lower incomes (Table 1) [14, 18, 47, 62, 67, 72, 73, 75-77]. Many potential reasons for these disparities have been offered in studies comparing patient functional status, LOS, readmissions, and discharge home after TJA, including poorer health status, delays in presentation, difficulty accessing care, and insurance type [34, 39, 52, 69, 76]. Although these factors may contribute to disparities in care, race, gender, income, and insurance type should not be seen as independent risk factors in TJA because they are not biological modifiers of LOS, readmissions, ED visits, functional status, and postoperative complication rates [44, 46]. Some studies have validated this and found that Black patients and women have clinically similar functional status and pain resolution compared with White patients and men after TJA, even when presenting with more pain, worse function, and more comorbidities preoperatively [42, 48]. Additional research has shown that race itself is not associated with clinically meaningful differences for most pain and functional status indicators after TKA, and that Black patients and White patients are equally appropriate to be considered for TJA, although these studies had much smaller sample sizes than those included in our systematic search [4, 55]. The intersection among Medicaid insurance, gender, and race or ethnicity may further complicate the trends in LOS, readmissions, and ED visits seen in these patients [75, 76].
One potential confounder in many of the studies found in our review is the presence of patient comorbidities. As previous research has shown, patients from racial and ethnic minority backgrounds and patients with Medicaid have been considered “high-risk” because they are more likely to present with multiple comorbidities [47, 72, 76, 77]. In fact, biased clinical decision making tools such as risk scores have been developed to predict the risk of 30-day readmission after THA or TKA that assign points to patients who identify as Black, patients with lower incomes, and patients insured by Medicaid [63, 64]. Bundled payment models were introduced in TJA to reduce costs and improve the quality of care for patients [21]; however, these quality incentive plans may be discouraging surgeons from operating on patients with a high number of comorbidities [75].
Preoperative risk management programs were implemented to intervene in modifiable risk factors that can be addressed preoperatively to reduce the likelihood of complications like surgical site infections and prosthetic joint infections, readmissions, long LOS, and reoperation as well as reduced costs [7, 12, 19, 24, 25, 29, 41, 51, 54, 56-58]. These programs focus on managing modifiable risk factors such as obesity, malnutrition, diabetes, anemia, smoking, and substance use and make recommendations or referrals for managing these factors preoperatively. When managing these risk factors, some programs rely on artificial cutoffs for BMI and hemoglobin A1c that may prevent patients from receiving surgery [45, 74]. Of the 12 programs we found describing preoperative risk management programs (Table 2), 11 specifically included recommended values for hemoglobin A1c [7, 12, 19, 24, 25, 41, 51, 54, 56-58] and nine included recommended values for BMI [7, 12, 19, 41, 51, 54, 56-58]. The variation in recommended values in these studies from below 6.5% to below 8% for hemoglobin A1c and below 35 kg/m2 to below 40 kg/m2 for BMI emphasizes the contrived nature of these values. Overall, preoperative risk management programs have been shown to reduce length of stay and readmissions, yet these programs have not performed analyses stratified by race to see their specific impact on patients from racial and ethnic minority backgrounds, women, and patients with lower incomes [7, 12, 19, 41]. The use of such programs seeks to reduce the risk of postoperative complications and, therefore, improve patient safety in the preoperative setting. These programs should also modify the perception that patients from racial and ethnic minority backgrounds, women, patients with lower incomes, and patients insured by Medicaid need additional perioperative services.
However, despite the use of preoperative risk management programs, patients from racial and ethnic minority backgrounds, patients with lower incomes, and patients who are insured by programs that provide low reimbursements for services often do not access specialty services such as dentists, weight management clinics, endocrinologists, smoking cessation clinics, and internal medicine providers [38]. For example, Ryan et al. [56] found that only 34% of patients for whom a referral for preoperative treatment was recommended for weight loss, anemia, smoking cessation, pain management, hematology, endocrinology, or cardiology received a referral to the appropriate specialist. The remaining 11 studies we found related to preoperative risk management programs (Table 2) did not attempt to measure the success of their referrals to outside specialists [7, 12, 19, 24, 25, 29, 41, 51, 54, 57, 58]. Without well-established referral pathways and nurse navigation, preoperative risk management becomes a barrier to TJA, and fails to provide the necessary resources for patients from racial or ethnic minority backgrounds and patients with lower incomes to reach preoperative guideline thresholds. Preoperative risk management programs will not achieve their stated purpose without appropriate support and standardized referral processes that will allow patients to receive treatment for their comorbidities.
Knowledge Gaps and Unsupported Practices
Because limited research has been conducted on how preoperative risk management programs influence postoperative LOS, readmissions, ED visits, and complications for patients from racial and ethnic minority backgrounds, women, patients with lower incomes, or patients insured by Medicaid, there are many gaps in our knowledge. Of the 12 studies we found detailing preoperative risk management programs (Table 2), none performed stratified analyses by race, gender, or income level to evaluate the effectiveness of preoperative risk management programs [7, 12, 19, 24, 25, 29, 41, 51, 54, 56-58]. Only one of the 12 studies, Plate et al. [54], mentions that preoperative risk management programs may incentivize physicians to restrict care to patients with lower incomes or who have a high number of comorbidities. Plate et al. [54] also analyzed the patient demographics before and after implementation of their Comprehensive Care for Joint Replacement model and found no differences before and after implementation, but they only looked at age, gender, and BMI. Kim et al. [41] recognized in the discussion section that patient income was not included but would be important to include in future studies. When considering patient insurance, one study only included Medicare patients [56], one study was conducted at a Veterans Affairs hospital [51], and another study used deidentified patient data and was unable to obtain patient insurance type [7]. The remaining nine studies describing preoperative risk management programs (Table 2) did not perform analyses considering patient insurance type.
Two studies by Featherall et al. [24, 25] found that men (compared with women) and White race (compared with races other than White) were associated with reduced LOS and increased home discharges, and public insurance was associated with increased LOS, decreased home discharges, and increased 90-day complications. However, these studies did not discuss how this relates to disparities in care and did not perform stratified analyses to further investigate these trends [24, 25]. Schultz et al. [58] discussed that as their institution is a county hospital, they may be more likely to have patients with lower incomes and that lower incomes can be associated with disparities in access, but again they do not perform stratified analyses to further investigate.
Many of these studies were conducted at an institutional level; thus, a further analysis stratified by race, gender, income, or insurance type may not have been possible because of sample size. Further research on this topic is necessary to ensure that these programs are not worsening healthcare disparities in readmissions, ED visits, and LOS after TJA for patients from racial and ethnic minority backgrounds, women, patients with lower incomes, and patients with Medicaid, who are often the most in need of TJA.
We found considerable heterogeneity in preoperative risk management programs. Of the nine studies that include recommendations for preoperative BMI and hemoglobin A1c, only one provides specific citations to studies to support the chosen values [25]. Additionally, only one of the 12 studies we found related to preoperative risk management programs mentions that these programs could restrict access to care for patients [54].
Preoperative risk management programs may place undue burdens on patients that preclude them from receiving TJA, such as absolute cutoffs for BMI and hemoglobin A1c levels. These binary restrictions negatively affect patients with lower incomes and patients from racial or ethnic minority backgrounds [74]. Enforcing strict cutoffs without taking a comprehensive view of the patient that looks beyond the biological aspects of health and includes an understanding of the psychosocial factors that affect patient care is an unsupported practice and research evaluating the impact of these cutoffs, especially within preoperative risk management programs, is necessary to confirm patients are receiving high-quality, equitable care.
Most importantly, we lack quantifiable metrics assessing the impact of the preoperative risk management pathway on patients from racial and ethnic minority backgrounds, women, patients with lower incomes, and patients with Medicaid insurance. For example, these programs do not report their perception of an acceptable risk level for the patient to proceed with surgery, the percentage of patients who have used risk-reduction strategies, or the length of time patients remained in the program before receiving surgery. Although preoperative risk management programs have demonstrated improved LOS, reduced admissions and ED visits, and reduced postoperative complications [7, 12, 19, 24, 25, 29, 41, 51, 54, 56-58], these analyses were not stratified by race, gender, income, or insurance type, and so we are unable to see if there any associations between race, gender, income, or insurance type and these improvements. Two studies found that after implementation of these programs, race, gender, and insurance type were associated with differences in LOS, discharge disposition, and complications although they did not discuss the potential implications of these findings or perform stratified analyses to further investigate [24, 25]. This may suggest that preoperative risk management programs primarily improve LOS, discharge disposition, readmissions, and postoperative complications for White patients with higher incomes, but do not provide benefits of a similar magnitude for patients from racial and ethnic minority backgrounds, women, patients with Medicaid, and patients with lower incomes.
Barriers and How to Overcome Them
Several barriers related to the implementation and monitoring of preoperative risk management programs may be hindering these programs from achieving their potential benefits in terms of equitable TJA care.
First, we believe that hard cutoffs for BMI, hemoglobin A1c, and smoking cessation should be eliminated. Instead, surgeons should engage with patients in shared decision-making and increased patient education regarding timing of TJA and individualized targeted goals for management of these risk factors [43]. Preoperative risk management programs should employ navigators to help patients throughout the management process to ensure that they are making progress on their goals and have the necessary support to meet them before proceeding with surgery.
An additional barrier regarding implementation of preoperative risk management programs is reimbursement from the Centers for Medicare & Medicaid Services (CMS). If quality incentive measures such as bundling only emphasize the importance of postoperative LOS, readmissions, and complications instead of the pathways to achieve improvements in these areas, meaningful and persistent changes in preoperative risk management will be more difficult. Advocacy efforts are necessary to ensure that reimbursement of preoperative risk management programs matches the resources needed for equitable care.
Barriers related to monitoring of preoperative risk management programs include the difficulty in recording sociodemographic variables including race, ethnicity, and income as well as obtaining the sample size necessary to provide stratified analyses with these same variables. Orthopaedic surgeons should ensure their practices are updating and maintaining race, ethnicity, and income information for patients who seek their expertise for management of hip and knee osteoarthritis. Given that many of the preoperative risk management programs we found were implemented relatively recently, we encourage these researchers to repeat their analyses with data collected since their implementation to reach sample sizes large enough to perform stratified analyses by race, ethnicity, gender, income, and insurance type. For any orthopaedic surgeons who want to implement preoperative risk management programs, we recommend initiating programs to track metrics related to their new program now so that they can more easily research the associations between race, ethnicity, gender, income, and insurance type and their programs (Table 3). We have developed a list of quantifiable metrics for two levels of analysis: Tier 1 and a more robust Tier 2 analysis for preoperative risk management programs (Table 3). The Tier 2 analysis includes everything from Tier 1 plus additional elements that are more difficult to capture. These recommendations are separated into four sections: patient characteristics, patient factors, program factors, and surgical factors. Because several of these metrics require a quantification of patient risk, we recommend evaluating the scoring systems detailed in a recent review [33]. This article discussed the pros and cons of 10 different risk stratification tools that can be used to predict readmission and discharge status after TJA. The tools included in the review are the Total Joint Replacement Risk Calculator [10, 11], the American College of Surgeons Risk Calculator [8], the Duke Readmission Calculator [27], the OrthoCincy Readmission Tool [30], the Readmission Risk Assessment Tool [9], the Iowa Discharge Disposition Score [26], the Cleveland Clinic Calculator [6], the Risk Assessment and Prediction Tool [66], the Penn Arthroplasty Risk Score [16], and the Outpatient Arthroplasty Risk Assessment [49, 79].
Table 3.
Recommendations for metrics to evaluate preoperative risk factor management programs
| Metrics | Tier 1 Analysis | Tier 2 Analysis |
| Patient characteristics | ||
| Specific stratified analyses for patient outcomes should be performed, examining: | ||
| Race | X | X |
| Ethnicity | X | X |
| Age | X | X |
| Gender | X | X |
| Insurance status | X | X |
| Income | X | |
| Patient factors | ||
| Institution’s acceptable patient risk level to proceed with surgery (that is, if scorea, maximum allowable score to proceed with surgery) | X | X |
| Comorbidities (such as BMI, DM, hypertension, heart disease, or depression) | X | X |
| Quantification of patient risk during the initial presentation (the initial risk scorea) | X | |
| Quantification of patient risk at POD 0 (the risk scorea at POD 0) | X | |
| Social support (lives alone, family support) | X | |
| Housing status | X | |
| Program factors | ||
| Institution hard-stop values for HgA1C, BMI, and albumin levels | X | X |
| Percentage of patients for whom conservative management is recommended over surgery based on high risk | X | |
| Percentage of patients participating in program who subsequently undergo TJA after spending 6 months in the risk management program | X | |
| Average time patients spend in program before undergoing TJA | X | |
| Total number of referrals to specialists per patient | X | |
| Total number of successfully completed referrals to specialist per patient | X | |
| Average wait time in days to see a specialist | X | |
| Specialists accept Medicaid, yes or no? (including bariatrics, nutrition or metabolic clinic, dental, endocrinology, infectious disease, cardiology, nephrology, psychiatry, and hematology departments) | X | |
| Partnerships established with social work programs that accept Medicaid or uninsured patients (alcohol use, substance use, psychology, smoking cessation, or social work) | X | |
| Surgical factors | ||
| Inpatient versus outpatient surgery | X | X |
| LOS | X | X |
| Postdischarge resources needed (inpatient rehabilitation, SNF, home PT or nursing, outpatient PT or telerehabilitation for postoperative PT) | X | X |
| 30- and 90-day readmissions and ED visits | X | X |
| Transfusion usage | X | X |
| Frequency of SSI | X | X |
| Patient-reported outcome measures | X | |
Refer to review by Howie et al. [33] for scoring tools; DM = diabetes mellitus; LOS = length of stay; POD = postoperative day; HbA1c = hemoglobin A1c; SNF = skilled nursing facility; PT = physical therapy; ED = emergency department; SSI = surgical site infection.
5-year Forecast
We believe that three main factors will make preoperative risk management programs more common in the next few years. First, the shift to bundled reimbursement models by CMS will incentivize a focus on preoperative management to reduce LOS, readmissions, ED visits, and postoperative complications. Second, as more TJAs are performed in the outpatient setting, these programs can help identify which patients are most likely to tolerate outpatient arthroplasty, and make the procedure safer for them. Third, we believe that reimbursement for preoperative risk management will improve, and because of this, more orthopaedic practices will incorporate preoperative risk factor mitigation into their care pathways.
Acknowledgments
We thank Mary I. O’Connor MD for her review and comments. We also thank Movement is Life, a group of healthcare professionals focused on the elimination of musculoskeletal disparities, for their assistance regarding this topic. Alexandria Brackett MLIS, AHIP aided with the search terms of the literature search.
Footnotes
Both authors certify that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
The opinions expressed are those of the writers, and do not reflect the opinion or policy of CORR® or The Association of Bone and Joint Surgeons®.
References
- 1.Almaguer AM, Hsu AR, Pearson JM, et al. Do geographic region, Medicaid status, and academic affiliation affect access to care among Medicaid and privately insured total hip arthroplasty patients? J Arthroplasty. 2019;34:2866-2871. [DOI] [PubMed] [Google Scholar]
- 2.Altieri MS, Yang J, Yin D, Talamini MA, Spaniolas K, Pryor AD. Patients insured by Medicare and Medicaid undergo lower rates of bariatric surgery. Surg Obes Relat Dis. 2019;15:2109-2114. [DOI] [PubMed] [Google Scholar]
- 3.Amen TB, Varady NH, Rajaee S, Chen AF. Persistent racial disparities in utilization rates and perioperative metrics in total joint arthroplasty in the US: a comprehensive analysis of trends from 2006 to 2015. J Bone Joint Surg Am. 2020;102:811-820. [DOI] [PubMed] [Google Scholar]
- 4.Ang DC, Tahir N, Hanif H, Tong Y, Ibrahim SA. African Americans and whites are equally appropriate to be considered for total joint arthroplasty. J Rheumatol. 2009;36:1971-1976. [DOI] [PubMed] [Google Scholar]
- 5.Aseltine RH, Jr., Wang W, Benthien RA, et al. Reductions in race and ethnic disparities in hospital readmissions following total joint arthroplasty from 2005 to 2015. J Bone Joint Surg Am. 2019;101:2044-2050. [DOI] [PubMed] [Google Scholar]
- 6.Barsoum WK, Murray TG, Klika AK, et al. Predicting patient discharge disposition after total joint arthroplasty in the United States. J Arthroplasty. 2010;25:885-892. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein DN, Liu TC, Winegar AL, et al. Evaluation of a preoperative optimization protocol for primary hip and knee arthroplasty patients. J Arthroplasty. 2018;33:3642-3648. [DOI] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boraiah S, Joo L, Inneh IA, et al. Management of modifiable risk factors prior to primary hip and knee arthroplasty: a readmission risk assessment tool. J Bone Joint Surg Am. 2015;97:1921-1928. [DOI] [PubMed] [Google Scholar]
- 10.Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in Medicare patients undergoing TKA. Clin Orthop Relat Res. 2012;470:130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471:574-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bullock MW, Brown ML, Bracey DN, Langfitt MK, Shields JS, Lang JE. A bundle protocol to reduce the incidence of periprosthetic joint infections after total joint arthroplasty: a single-center experience. J Arthroplasty. 2017;32:1067-1073. [DOI] [PubMed] [Google Scholar]
- 13.Burack B. The optimal time for cardiac surgery. Am J Cardiol. 1963;12:4-10. [DOI] [PubMed] [Google Scholar]
- 14.Cheah C, Hussein IH, El Othmani A, Rizvi SA, Sayeed Z, El-Othmani MM. Assessing preoperative risk factors with sex disparities in total joint arthroplasty patients and financial outcomes from the national inpatient sample database. J Am Acad Orthop Surg. 2020;28:e969-e976. [DOI] [PubMed] [Google Scholar]
- 15.Chun DS, Leonard AK, Enchill Z, Suleiman LI. Racial disparities in total joint arthroplasty. Curr Rev Musculoskelet Med. 2021;14:434-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courtney PM, Whitaker CM, Gutsche JT, Hume EL, Lee GC. Predictors of the need for critical care after total joint arthroplasty: an update of our institutional risk stratification model. J Arthroplasty. 2014;29:1350-1354. [DOI] [PubMed] [Google Scholar]
- 17.Cui HW, Turney BW, Griffiths J. The preoperative assessment and optimization of patients undergoing major urological surgery. Curr Urol Rep. 2017;18:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cusano A, Venugopal V, Gronbeck C, Harrington MA, Halawi MJ. Where do we stand today on racial and ethnic health inequities? Analysis of primary total knee arthroplasty from a 2011-2017 national database. J Racial Ethn Health Disparities. 2021;8:1178-1184. [DOI] [PubMed] [Google Scholar]
- 19.Dlott CC, Moore A, Nelson C, et al. Preoperative risk factor optimization lowers hospital length of stay and postoperative emergency department visits in primary total hip and knee arthroplasty patients. J Arthroplasty. 2020;35:1508-1515. [DOI] [PubMed] [Google Scholar]
- 20.Dlott CC, Pei X, Ittner JL, Lefar SL, O'Connor MI. Intersectionality of net worth and race relative to utilization of total hip and knee arthroplasty. J Arthroplasty. 2021;36:3060-3066. [DOI] [PubMed] [Google Scholar]
- 21.Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278. [DOI] [PubMed] [Google Scholar]
- 22.Edwards NM, Varnum C, Overgaard S, Pedersen AB. Impact of socioeconomic status on the 90- and 365-day rate of revision and mortality after primary total hip arthroplasty: a cohort study based on 103,901 patients with osteoarthritis from national databases in Denmark. Acta Orthop. 2021;92:581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etienne G, Pierce TP, Khlopas A, et al. Cultural biases in current medical practices with a specific attention to orthopedic surgery: a review. J Racial Ethn Health Disparities. 2018;5:563-569. [DOI] [PubMed] [Google Scholar]
- 24.Featherall J, Brigati DP, Arney AN, et al. Effects of a total knee arthroplasty care pathway on cost, quality, and patient experience: toward measuring the triple aim. J Arthroplasty. 2019;34:2561-2568. [DOI] [PubMed] [Google Scholar]
- 25.Featherall J, Brigati DP, Faour M, Messner W, Higuera CA. Implementation of a total hip arthroplasty care pathway at a high-volume health system: effect on length of stay, discharge disposition, and 90-day complications. J Arthroplasty. 2018;33:1675-1680. [DOI] [PubMed] [Google Scholar]
- 26.Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31:2705-2709. [DOI] [PubMed] [Google Scholar]
- 27.Goltz DE, Ryan SP, Howell CB, Attarian D, Bolognesi MP, Seyler TM. A weighted index of elixhauser comorbidities for predicting 90-day readmission after total joint arthroplasty. J Arthroplasty. 2019;34:857-864. [DOI] [PubMed] [Google Scholar]
- 28.Gottschalk MB, Johnson JP, Sadlack CK, Mitchell PM. Decreased infection rates following total joint arthroplasty in a large county run teaching hospital: a single surgeon's experience and possible solution. J Arthroplasty. 2014;29:1610-1616. [DOI] [PubMed] [Google Scholar]
- 29.Gray CF, Prieto HA, Duncan AT, Parvataneni HK. Arthroplasty care redesign related to the comprehensive care for joint replacement model: results at a tertiary academic medical center. Arthroplast Today. 2018;4:221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greiwe RM, Spanyer JM, Nolan JR, Rodgers RN, Hill MA, Harm RG. Improving orthopedic patient outcomes: a model to predict 30-day and 90-day readmission rates following total joint arthroplasty. J Arthroplasty. 2019;34:2544-2548. [DOI] [PubMed] [Google Scholar]
- 31.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44:258-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holbert SE, Cheema M, Brennan J, MacDonald J, King P, Turcotte J. Patients from medically underserved areas are at increased risk for non-home discharge and emergency department return after total joint arthroplasty. J Arthroplasty. Published online January 3, 2022. DOI: 10.1016/j.arth.2021.12.033.qddd. [DOI] [PubMed]
- 33.Howie CM, Mears SC, Barnes CL, Stambough JB. Readmission, complication, and disposition calculators in total joint arthroplasty: a systemic review. J Arthroplasty. 2021;36:1823-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsiang WR, Lukasiewicz A, Gentry M, et al. Medicaid patients have greater difficulty scheduling health care appointments compared with private insurance patients: a meta-analysis. Inquiry. 2019;56:46958019838118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inneh IA. The combined influence of sociodemographic, preoperative comorbid and intraoperative factors on longer length of stay after elective primary total knee arthroplasty. J Arthroplasty. 2015;30:1883-1886. [DOI] [PubMed] [Google Scholar]
- 36.Inneh IA, Iorio R, Slover JD, Bosco JA, 3rd. Role of sociodemographic, co-morbid and intraoperative factors in length of stay following primary total hip arthroplasty. J Arthroplasty. 2015;30:2092-2097. [DOI] [PubMed] [Google Scholar]
- 37.Jelani QU, Jhamnani S, Spatz ES, et al. Financial barriers in accessing medical care for peripheral artery disease are associated with delay of presentation and adverse health status outcomes in the United States. Vasc Med. 2020;25:13-24. [DOI] [PubMed] [Google Scholar]
- 38.Johns WL, Layon D, Golladay GJ, Kates SL, Scott M, Patel NK. Preoperative risk factor screening protocols in total joint arthroplasty: a systematic review. J Arthroplasty. 2020;35:3353-3363. [DOI] [PubMed] [Google Scholar]
- 39.Kamath AF, Horneff JG, Gaffney V, Israelite CL, Nelson CL. Ethnic and gender differences in the functional disparities after primary total knee arthroplasty. Clin Orthop Relat Res. 2010;468:3355-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the Affordable Care Act. J Arthroplasty. 2015;30:1498-1501. [DOI] [PubMed] [Google Scholar]
- 41.Kim KY, Anoushiravani AA, Chen KK, et al. Perioperative orthopedic surgical home: optimizing total joint arthroplasty candidates and preventing readmission. J Arthroplasty. 2019;34:S91-S96. [DOI] [PubMed] [Google Scholar]
- 42.Lavernia CJ, Villa JM. Does race affect outcomes in total joint arthroplasty? Clin Orthop Relat Res. 2015;473:3535-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leopold SS. Editor's Spotlight/Take 5: Eligibility criteria for lower-extremity joint replacement may worsen racial and socioeconomic disparities. Clin Orthop Relat Res. 2018;476:2297-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leopold SS. Editorial: Beware of studies claiming that social factors are “independently associated” with biological complications of surgery. Clin Orthop Relat Res. 2019;477:1967-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leopold SS. Editorial: The shortcomings and harms of using hard cutoffs for BMI, hemoglobin A1c, and smoking cessation as conditions for elective orthopaedic surgery. Clin Orthop Relat Res. 2019;477:2391-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leopold SS, Beadling L, Calabro AM, et al. Editorial: The complexity of reporting race and ethnicity in orthopaedic research. Clin Orthop Relat Res. 2018;476:917-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maman SR, Andreae MH, Gaber-Baylis LK, Turnbull ZA, White RS. Medicaid insurance status predicts postoperative mortality after total knee arthroplasty in state inpatient databases. J Comp Eff Res. 2019;8:1213-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mannion AF, Impellizzeri FM, Naal FD, Leunig M. Women demonstrate more pain and worse function before tha but comparable results 12 months after surgery. Clin Orthop Relat Res. 2015;473:3849-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meneghini RM, Ziemba-Davis M, Ishmael MK, Kuzma AL, Caccavallo P. Safe selection of outpatient joint arthroplasty patients with medical risk stratification: the “outpatient arthroplasty risk assessment score”. J Arthroplasty. 2017;32:2325-2331. [DOI] [PubMed] [Google Scholar]
- 50.Morrell AT, Golladay GJ, Kates SL. Surgical selection criteria compliance is associated with a lower risk of periprosthetic joint infection in total hip arthroplasty. Arthroplast Today. 2019;5:521-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nussenbaum FD, Rodriguez-Quintana D, Fish SM, Green DM, Cahill CW. Implementation of preoperative screening criteria lowers infection and complication rates following elective total hip arthroplasty and total knee arthroplasty in a veteran population. J Arthroplasty. 2018;33:10-13. [DOI] [PubMed] [Google Scholar]
- 52.O'Connor MI, Hooten EG. Breakout session: Gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469:1883-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okike K, Chan PH, Prentice HA, Navarro RA, Hinman AD, Paxton EW. Association of race and ethnicity with total hip arthroplasty outcomes in a universally insured population. J Bone Joint Surg Am. 2019;101:1160-1167. [DOI] [PubMed] [Google Scholar]
- 54.Plate JF, Ryan SP, Black CS, et al. No changes in patient selection and value-based metrics for total hip arthroplasty after comprehensive care for joint replacement bundle implementation at a single center. J Arthroplasty. 2019;34:1581-1584. [DOI] [PubMed] [Google Scholar]
- 55.Riddle DL, Slover J, Keefe FJ, Ang DC, Dumenci L, Perera RA. Racial differences in pain and function following knee arthroplasty: a secondary analysis from a multicenter randomized clinical trial. Arthritis Care Res (Hoboken). 2021;73:810-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryan SP, Howell CB, Wellman SS, et al. Preoperative optimization checklists within the comprehensive care for joint replacement bundle have not decreased hospital returns for total knee arthroplasty. J Arthroplasty. 2019;34:S108-S113. [DOI] [PubMed] [Google Scholar]
- 57.Ryan SP, Plate JF, Black CS, et al. Value-based care has not resulted in biased patient selection: analysis of a single center's experience in the care for joint replacement bundle. J Arthroplasty. 2019;34:1872-1875. [DOI] [PubMed] [Google Scholar]
- 58.Schultz BJ, Segovia N, Castillo TN. Successful implementation of an accelerated recovery and outpatient total joint arthroplasty program at a county hospital. J Am Acad Orthop Surg Glob Res Rev. 2019;3:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shahid H, Singh JA. Racial/ethnic disparity in rates and outcomes of total joint arthroplasty. Curr Rheumatol Rep. 2016;18:20. [DOI] [PubMed] [Google Scholar]
- 60.Shau D, Shenvi N, Easley K, Smith M, Guild G, 3rd. Medicaid is associated with increased readmission and resource utilization after primary total knee arthroplasty: a propensity score-matched analysis. Arthroplast Today. 2018;4:354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaw JH, Wesemann LD, Ayoola AS, Les CM, Charters MA, North WT. Comparison of area deprivation index, socioeconomic parameters, and preoperative demographics with postoperative emergency department visits after total knee arthroplasty. J Arthroplasty. 2021;36:2788-2794. [DOI] [PubMed] [Google Scholar]
- 62.Sheth M, Chambers M, Gronbeck C, Harrington MA, Halawi MJ. Total hip arthroplasty in Black/African American patients: an updated nationwide analysis. J Racial Ethn Health Disparities. 2021;8:698-703. [DOI] [PubMed] [Google Scholar]
- 63.Siracuse BL, Chamberlain RS. A preoperative scale for determining surgical readmission risk after total hip replacement. JAMA Surg. 2016;151:701-709. [DOI] [PubMed] [Google Scholar]
- 64.Siracuse BL, Ippolito JA, Gibson PD, Ohman-Strickland PA, Beebe KS. A preoperative scale for determining surgical readmission risk after total knee arthroplasty. J Bone Joint Surg Am. 2017;99:e112. [DOI] [PubMed] [Google Scholar]
- 65.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [DOI] [PubMed] [Google Scholar]
- 66.Slover J, Mullaly K, Karia R, et al. The use of the risk assessment and prediction tool in surgical patients in a bundled payment program. Int J Surg. 2017;38:119-122. [DOI] [PubMed] [Google Scholar]
- 67.SooHoo NF, Farng E, Lieberman JR, Chambers L, Zingmond DS. Factors that predict short-term complication rates after total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2363-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spina NT, Aleem IS, Nassr A, Lawrence BD. Surgical site infections in spine surgery: preoperative prevention strategies to minimize risk. Global Spine J. 2018;8:31S-36S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stone AH, MacDonald JH, Joshi MS, King PJ. Differences in perioperative outcomes and complications between African American and white patients after total joint arthroplasty. J Arthroplasty. 2019;34:656-662. [DOI] [PubMed] [Google Scholar]
- 70.Trivedi A, Ezomo OT, Gronbeck C, Harrington MA, Halawi MJ. Time trends and risk factors for 30-day adverse events in Black patients undergoing primary total knee arthroplasty. J Arthroplasty. 2020;35:3145-3149. [DOI] [PubMed] [Google Scholar]
- 71.Tsui ST, Yang J, Zhang X, et al. Health disparity in access to bariatric surgery. Surg Obes Relat Dis. 2021;17:249-255. [DOI] [PubMed] [Google Scholar]
- 72.Veltre DR, Yi PH, Sing DC, et al. Insurance status affects in-hospital complication rates after total knee arthroplasty. Orthopedics. 2018;41:e340-e347. [DOI] [PubMed] [Google Scholar]
- 73.Venugopal V, Gronbeck C, Harvey L, Patel AP, Harrington MA, Halawi MJ. Time trends in perioperative characteristics and health outcomes in Hispanic patients undergoing primary total knee arthroplasty. J Racial Ethn Health Disparities. 2021;8:1475-1481. [DOI] [PubMed] [Google Scholar]
- 74.Wang AY, Wong MS, Humbyrd CJ. Eligibility criteria for lower extremity joint replacement may worsen racial and socioeconomic disparities. Clin Orthop Relat Res. 2018;476:2301-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiner JA, Adhia AH, Feinglass JM, Suleiman LI. Disparities in hip arthroplasty outcomes: results of a statewide hospital registry from 2016 to 2018. J Arthroplasty. 2020;35:1776-1783. [DOI] [PubMed] [Google Scholar]
- 76.White RS, Sastow DL, Gaber-Baylis LK, Tangel V, Fisher AD, Turnbull ZA. Readmission rates and diagnoses following total hip replacement in relation to insurance payer status, race and ethnicity, and income status. J Racial Ethn Health Disparities. 2018;5:1202-1214. [DOI] [PubMed] [Google Scholar]
- 77.Xu HF, White RS, Sastow DL, Andreae MH, Gaber-Baylis LK, Turnbull ZA. Medicaid insurance as primary payer predicts increased mortality after total hip replacement in the state inpatient databases of California, Florida and New York. J Clin Anesth. 2017;43:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zangenberg MS, Horesh N, Kopylov U, El-Hussuna A. Preoperative optimization of patients with inflammatory bowel disease undergoing gastrointestinal surgery: a systematic review. Int J Colorectal Dis. 2017;32:1663-1676. [DOI] [PubMed] [Google Scholar]
- 79.Ziemba-Davis M, Caccavallo P, Meneghini RM. Outpatient joint arthroplasty-patient selection: update on the outpatient arthroplasty risk assessment score. J Arthroplasty. 2019;34:S40-S43. [DOI] [PubMed] [Google Scholar]