OBJECTIVES:
Describe the effects of data literacy training and continuous performance reports on ICU staff compliance with the 6-element ICU quality bundle approach known as the ABCDEF (A–F) bundle and patient outcomes.
DESIGN:
Stepped-wedge cluster randomized trial conducted during an institutional A–F bundle implementation program.
SETTING:
Single-center study conducted in eight adult ICUs.
PATIENTS:
Adult patients admitted for at least 24 hours, not undergoing active withdrawal of life support or palliative care.
INTERVENTIONS:
Four ICUs in the intervention group received bundle-related staff education, data literacy training, and weekly bundle performance reports during the 12-month study period. The four ICUs in the nonintervention group received none of these interventions. Bundle compliance and patient outcomes were tracked, including ICU and hospital mortality, transfer and discharge, discharge disposition, mechanical ventilation, and ICU delirium.
MEASUREMENT AND RESULTS:
In the intervention group, staff education alone increased bundle compliance from 9% to 16% (p < 0.0001); data literacy training further increased compliance from 16% to 21% (p = 0.03). This improvement was sustained throughout the study period including the onset of the COVID-19 pandemic and was greater than improvement in the nonintervention group (p < 0.001). Full A–F bundle compliance was associated with a lower likelihood of next-day ICU and hospital mortality, discharge to a facility other than home, and was associated with a higher likelihood of next-day extubation in patients. Next-day ICU and hospital discharge likelihood decreased, and delirium frequency was not affected.
CONCLUSIONS:
This is the first study demonstrating that the combination of staff education, data literacy training, and access to performance data improves A–F bundle compliance, sustains performance, and improves ICU patient outcomes (ICU and hospital mortality, mechanical ventilation duration, and home discharge rates). In contrast to previous studies, increased bundle compliance did not hasten ICU or hospital discharges or reduce delirium frequency in patients.
Keywords: ABCDEF bundle compliance, bundle care, data literacy training, performance reports, protocol compliance
BACKGROUND AND SIGNIFICANCE
Implementation of evidence-based practices can significantly improve ICU patient care and outcomes. A multicomponent ICU care bundle, the ABCDEF (A–F) bundle, was developed by the Society of Critical Care Medicine (SCCM) and promoted through their ICU Liberation Campaign (1). The principal objective of the A–F bundle is to optimize the environment for ICU patients to heal, while liberating patients from pain, agitation, oversedation, delirium, mechanical ventilation, and immobility (2). The A–F bundle elements translate to the following processes of care: 1) A for the assessment of pain, 2) B for Spontaneous Awakening Trials and Spontaneous Breathing Trials, 3) C for choice of analgesia and sedation, 4) D for delirium, 5) E for early mobility and exercise, and 6) F for family and patient engagement (1, 2). In a recent 76-site multidisciplinary ICU Liberation Collaborative study, bundle implementation was associated with improvements in survival, mechanical ventilation use, coma and delirium, ICU readmission rates, and improved home discharge rates for ICU survivors (3). Despite over a decade of conclusive evidence supporting the A–F bundle and its positive impacts on ICU patient outcomes, the A–F bundle has not been consistently implemented (2–6).
Implementation of an evidence-based bundle in the ICU (e.g., central catheter–associated blood stream infection bundle) is more effective when bundle implementation, focused on a single issue, is paired with culture change using a multidisciplinary, team-based approach (7–9). However, even when a team-based approach is taken, efforts to implement more complex care bundles, such as the A–F bundle, have been less successful. Barriers include A–F bundle complexity and a lack of information technology support (5, 6). Specifically, the availability of bundle-related performance metrics, along with staff comprehension of these metrics, are important determinants to manage and course-correct bundle performance in real time (9–12).
Accessing and analyzing bundle performance metrics from existing electronic health record (EHR) systems is challenging for busy ICU clinicians (3, 11, 13, 14). The multicomponent complexity of the A–F bundle creates unique IT challenges due to the quantity of data collected, the inability to customize EHR dashboards around the bundle, missing data, and lack of clinical decision support (11, 13, 15). Despite these perceived IT barriers, hard evidence is lacking that increasing staff access to bundle-related data improves A–F bundle compliance and ICU patient outcomes (16).
We hypothesized that providing multiprofessional clinical education around the bundle, combined with data literacy (DL) training and continuous compliance reporting, would result in improved adherence to the A–F bundle. The specific aims of this study were: 1) to understand the effects of bundle-related ICU staff education, DL training, and bundle performance reports on staff compliance with the A–F bundle and 2) to measure the impact of increased bundle compliance on ICU patient outcomes.
MATERIALS AND METHODS
Study Design
This was a single-center study of the eight ICUs at a quaternary care hospital conducted between October 1, 2018, and October 31, 2019. The University of Southern California Institutional Review Board approved the protocol and waived informed consent requirements for patients and staff (HS-18-00750). We initially planned a stepped-wedge cluster randomized trial as the A–F bundle was being implemented across all eight ICUs as standard daily practice. However, only the first four randomized ICUs received the intervention as part of this study (the remaining ICUs received the interventions subsequently, outside the study timeline). This was due to a mid-study shift in timing and education needed to accommodate hospital A–F bundle implementation, clinical education needs, and support for other organizational priorities. The nonintervention ICUs thus effectively became concurrent control units (labeled nonintervention units hence forth). Intervention group units included cardiovascular surgery, general surgery, neurosciences, and general medical ICUs. Nonintervention units included cardiopulmonary and mechanical circulatory support, oncology, general surgical, and surgical specialty ICUs.
Interventions
Randomization of training order was performed by the study biostatistician at the onset of the study, with each ICU randomized to different crossover dates. Randomization was performed in multiple waves as outlined in Figure 1. Each wave consisted of one ICU in the intervention group undergoing the following: month-1 standard A–F bundle clinical education using usual and customary care (UCC) education standards of the institution, month-2 monitoring of bundle performance, month-3 DL training and introduction of weekly bundle performance reports (UCC+DL), and months 4–12 ongoing bundle performance monitoring and distribution of weekly bundle performance reports.
Figure 1.
Stepped wedge study schematic. M0: Baseline measurement. M1: The first intervention was usual customary care (UCC) that included standard clinical education over a 1-mo period. In this case, this was defined as standard clinical education and protocol roll out. M2: A–F bundle compliance measurement. M3: The second intervention was UCC plus the use of compliance reports to aid in A–F bundle compliance monitoring and feedback (UCC+ data literacy [DL]). Note UCC+DL = UCC plus the implementation of compliance reports to trigger and support action for A–F bundle elements. M4: A–F bundle compliance measurement. M5–M12: Continued A–F bundle measurement. *Units that did not receive the planned intervention.
In the absence of universally defined and accepted protocols for the A–F bundle, the critical care interprofessional team at our institution created a set of bundle protocols founded on evidence-based practice (17–27). Role-based training for the UCC intervention was created by the critical care interprofessional team for each clinical discipline caring for patients in the ICUs (i.e., physicians, nurse practitioners, nurses, pharmacists, respiratory, physical, and occupational therapists, case managers, and social workers). A lead in each clinical discipline developed the protocol and reviewed and aligned the education asset with the critical care interprofessional working group. Education included the impetus for bundled care, the expectations for each clinical role, and how the bundle would change their daily practice. The intervention approach is described in Supplemental Digital Content, Table 1 (http://links.lww.com/CCX/A972). Clinicians were instructed to apply the bundle daily to every ICU patient.
Data literacy training included the bundle compliance algorithm, how EHR documentation informs performance reports, and how to read, interpret, and act on data presented (Supplemental Digital Content, Table 2, http://links.lww.com/CCX/A972). During these 30-minute small group educational sessions, staff received a bundle documentation guide and sample compliance reports with examples of how to connect bundle compliance with action to improve bundle performance (Supplemental Digital Content, Figs. 1–3, http://links.lww.com/CCX/A971; legend, http://links.lww.com/CCX/A986).
Data Collection
Patient demographics, bundle element eligibility, staff bundle compliance, and clinical outcomes were collected daily on all eligible adult ICU patients (≥ 18 yr old) admitted to any of the eight ICUs over 13 months. Patients were excluded if they were not in an ICU for at least 24 hours, were undergoing life support withdrawal, or were receiving palliative care.
Clinical data were extracted from our Cerner EHR data warehouse and then deidentified and analyzed. Full bundle compliance was defined as being compliant with all six bundle elements. Failure to deliver any of the six elements was defined as bundle noncompliance. Patient outcomes were measured in all ICU patients who were eligible to receive the bundle and included: ICU and hospital transfer and discharge, discharge disposition, the continued use of mechanical ventilation, the daily frequency of ICU delirium measured using the Confusion Assessment Method for the ICU (CAM-ICU), and ICU and hospital mortality (truncated at 30 d). Other patient data collected are outlined in Table 1.
TABLE 1.
Demographics
| Group | Total Cohort | Four Intervention Units | Four Nonintervention Units | p | |||
|---|---|---|---|---|---|---|---|
| N a | N = 7,300 | N = 3,729 | N = 3,571 | ||||
| Statistical calculation | Mean | sd | Mean | sd | Mean | sd | |
| Age | 59.8 | 15.9 | 58.6 | 16.1 | 61 | 15.6 | < 0.0001 |
| BMI (kg/m2) | 28.8 | 16.5 | 29.1 | 19.9 | 28.4 | 12 | 0.053 |
| Case mix index | 5.0 | 4.5 | 5.3 | 4.4 | 4.7 | 4.5 | < 0.0001 |
| N | n | (%) | n | (%) | n | (%) | |
| Sex | 0.001 | ||||||
| Female | 3,179 | 43.55 | 1,696 | 45.48 | 1,483 | 41.53 | |
| Male | 4,121 | 56.45 | 2,033 | 54.52 | 2,088 | 58.47 | |
| ICU type intervention units | NA | ||||||
| Surgical ICU | 570 | 7.81 | 570 | 15.29 | 0 | 0 | |
| Neuro ICU | 1,494 | 20.47 | 1,494 | 40.06 | 0 | 0 | |
| Medical ICU | 619 | 8.48 | 619 | 16.6 | 0 | 0 | |
| Cardiac ICU | 1,046 | 14.33 | 1,046 | 28.05 | 0 | 0 | |
| ICU type nonintervention units | NA | ||||||
| Surgical ICU | 1,131 | 15.49 | 0 | 0 | 1,131 | 31.67 | |
| Surgical ICU | 1,167 | 15.99 | 0 | 0 | 1,167 | 32.68 | |
| Oncology ICU | 449 | 6.15 | 0 | 0 | 449 | 12.57 | |
| Cardiac ICU | 824 | 11.29 | 0 | 0 | 824 | 23.07 | |
NA = not applicable.
aAll numbers reflect ICU, encounter e.g., patient admission into the ICU.
Statistical Analysis
The sample size was determined by availability. Estimation based on historical data indicated 368 patients per month across eight ICUs. We could detect an 8% difference (10% to 18%) of full bundle compliance between month-2 and month-4. Effect size analysis was conducted using two proportions cluster-randomized design, two-sized z-test, with 46 per cluster (368 patients per month divided by eight ICUs), eight clusters, intracluster correlation of 0.01, with significance level of 0.05 and power of 0.8. The four intervention units were used for primary outcome analyses. Secondary patient outcome analyses used data from all eight ICUs but tested the intervention and nonintervention groups separately.
Patient demographics were reported by intervention and nonintervention groups. With an a priori α equals to 0.05 for the primary outcome (difference in bundle compliance between UCC vs UCC+DL), we focused secondary patient outcomes on estimating the intervention effect size in ICUs. Missing data were generally low (< 7%) (Table 1); analyses therefore used complete cases only, without imputation. The primary outcome compared bundle compliance post UCC+DL (month 4) with that of UCC only (month 2) to assess the impact of DL training and performance reports on bundle compliance.
Secondary bundle compliance measurements included compliance with individual bundle elements and sustainability of full bundle compliance (long-term effects over months 5–12). Long-term effects were modeled using the same nested statistical model, estimating treatment effects relative to month 4.
Two types of secondary patient outcome analyses were conducted, including prediction of next-day outcomes and sensitivity analysis. Prediction of next-day patient outcomes was used for ICU and hospital transfer, discharge, mortality, mechanical ventilation, and ICU delirium. For mechanical ventilation, only patients with at least 2 consecutive days of data who were receiving invasive mechanical ventilation on the first day were included. Similarly, only ICU patient-days without delirium were included. Discharge disposition was categorized as a binary outcome (discharge home vs other location). Both primary and secondary analyses used generalized estimating equations, so each patient’s daily bundle compliance was nested within the ICU visit and then within the ICU units. We allowed estimation of covariance across ICUs as we could not assume independence due to the presence of “float” staff who may have been exposed to the intervention in one unit before an intervention was applied in another unit. All models included patient case mix index (CMI), study time, days between study initiation and the A–F bundle collection date (account for secular trends) as covariates. For patient next day outcomes, daily full bundle compliance was also included. For discharge disposition, A–F bundle compliance was aggregated for each patient as overall full compliance (compliant or noncompliant) over the ICU period, as full bundle compliance for all days in the ICU unit. Sensitivity analyses were performed for clinical outcomes (ICU and hospital length of stay [LOS] and mortality) using Cox proportional hazards analyses with daily bundle compliance as time-varying independent variables to validate the primary analyses. Other covariates were the same as used in the primary analysis. All analyses used SAS (v9.4) (SAS Institute, Cary, NC) and Tableau Desktop (v2019.2; Professional Edition, Seattle, WA).
RESULTS
Patient Population
Of total eight eligible ICU units, all eight were randomly assigned to a date for intervention. However, four units were unable to participate according to the planned timeline before start of the intervention. A total of 40,651 24-hour records from 7,300 ICU encounters and 6,415 distinct ICU patients were analyzed, including 19,521 records, 3,925 encounters and 3,608 patients in the intervention group, and 21,130 records, 3,803 encounters, and 3,427 patients in the nonintervention group. Characteristics of patients in the nonintervention group differed when compared with the intervention group on primary diagnosis, CMI, ventilation hours, and discharge to home (Table 1) (Supplemental Digital Content, Table 3, http://links.lww.com/CCX/A972). Cardiac admissions accounted for the largest admission category but was lower in the nonintervention group. CMI, frequency of mechanical ventilation, and discharge to home were also lower, where duration of mechanical ventilation was higher in the nonintervention group.
Clinical Education
The interprofessional team received role-based education per the education plan (Supplemental Digital Content, Table 1, http://links.lww.com/CCX/A972). Of the nursing team in the intervention group, 20% of the nursing staff did not receive training on elements B, D, and E if they were not available for practice integration, on leave, or unable to dedicate the time during the clinical education month. All clinical staff across the clinical disciplines received the DL training.
Primary Outcome: Effects of Clinical Education and Data Literacy on Bundle Compliance
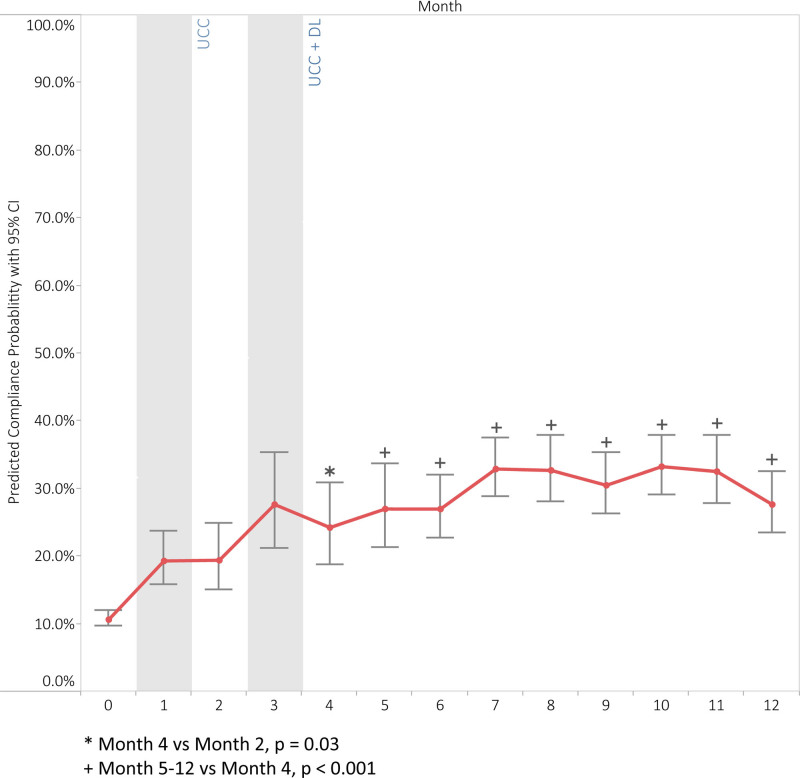
Figure 2 illustrates absolute bundle compliance rates over the study period, showing larger improvements in the intervention group (p < 0.001) and a smaller but significant improvement for the nonintervention group (p = 0.004). Figure 3 displays the results of the multivariable logistical regression analysis showing average predicted daily full bundle compliance probability by implementation month for the intervention group, after adjusting for covariates. From baseline to month-2, clinical education (UCC) alone was associated with a significant improvement in full A–F bundle compliance from 9% to 16% (adjusted odds ratio [AOR], 2.48; 95% CI, 1.79–3.43; p < 0.0001). The addition of DL training and weekly availability of performance reports from month-2 to month-4 (UCC+DL) further increased full bundle compliance from 16% to 21% (AOR, 1.52; 95% CI, 1.05–2.22; p = 0.03). The predicted compliance probability demonstrates a consistent and sustained increase from baseline through UCC, UCC through UCC+DL, and beyond (Supplemental Digital Content, Table 4, http://links.lww.com/CCX/A972). UCC and UCC+DL increased the compliance of each bundle element as well (Supplemental Digital Content, Figs. 4–9, http://links.lww.com/CCX/A971; legend, http://links.lww.com/CCX/A986). Bundle compliance decreased in both groups during the first 4 months of the COVID-19 pandemic, but the improvement in compliance compared with baseline remained significant only for the intervention units during this period (Fig. 2).
Figure 2.
Effect of interventions: A–F bundle compliance over time. Pre COVID: October 2018 to February 2020; pre-COVID line trends: intervention units p < 0.0001; nonintervention units p = 0.004. Post COVID: October 2018 to June 2020; post-COVID line trends: intervention units p < 0.0001; nonintervention units p = 0.469. UCC: Clinical education. UCC+DL: DL training and ongoing performance reports. M1: Month 1 of intervention period, UCC. M3: Month 3 of intervention period, UCC+DL. Aggregate A–F bundle compliance rates chronologically over the study period for all four intervention units compared with the four nonintervention units that did not receive the A–F bundle implementation. Data were gathered retrospectively past the study period to evaluate the impact of COVID-19. The initial 3 mo of the investigation were used as a baseline measurement for bundle compliance. The dotted line traversing the y-axis represents the intervention points in time for each unit and the presence of COVID-19. The p values presented were produced by the Tableau software linear regression trend line statistical function that measures the significance of the trend line produced. DL = data literacy, UCC = usual customary care.
Figure 3.
Effect of interventions: Predicted daily compliance probability. Predicted compliance probability across study time using marginal means estimated from multivariable logistic regression model. The model adjusts for study month, case mix index, days between study initiation and the A–F bundle collection date and aligns the intervention month across the four intervention units.
Secondary Outcome: Association of Full A–F Bundle Compliance With Patient Outcomes
Improvements in full bundle compliance over time were also associated with improved patient outcomes as supported by the next-day analysis (Table 2) and survival analysis (Supplemental Digital Content, Table 5, http://links.lww.com/CCX/A972). Full bundle compliance on a given day was associated with a lower likelihood of in-ICU mortality the next-day (0.8% compliant vs 1.2% noncompliant; AOR, 0.57 [0.36– 0.89]; p = 0.01) and next-day in-hospital mortality (0.8% compliant vs 1.6% noncompliant; AOR, 0.59 [0.40–0.87]; p = 0.01) in intervention groups. By contrast, full bundle compliance had no effect on ICU or hospital mortality in nonintervention units (p = 0.32 and p = 0.50, respectively). Full bundle compliance was also associated with an increased likelihood of extubation the next day, both for intervention (20.9% compliant vs 15% noncompliant; AOR, 1.56 [1.32–1.86]; p < 0.0001) and nonintervention units (15.1% compliant vs 8.8% noncompliant; AOR, 1.79 [1.39–2.31]; p < 0.0001). Full bundle compliance was not associated with an increased likelihood of being delirium-free the next-day. Full bundle compliance in intervention units was associated with a 16% lower likelihood of next-day ICU transfer to a general unit (AOR, 0.84 [0.73–0.96]; p = 0.01) and a 16% lower likelihood of next-day hospital discharge (AOR, 0.84 [0.73–0.96]; p = 0.01), but no significant effects were seen on ICU transfer and hospital discharge in nonintervention units. Full bundle compliance was associated with higher likelihood of ICU survivors being discharged to home both in intervention units (61.4% compliant vs 47.5% noncompliant; nonhome discharge AOR, 0.68 [0.48–0.97]; p = 0.03) and nonintervention units (66% compliant vs 41.8% noncompliant; nonhome discharge AOR, 0.40 [0.26–0.62]; p < 0.0001). Finally, sensitivity analyses demonstrated a similar conclusion to our primary analysis using predicted next-day outcomes (Supplemental Digital Content, Table 5, http://links.lww.com/CCX/A972).
TABLE 2.
Association of Full Bundle Compliance with Patient Outcomes
| Unit Cohort | Four Interventions Units | Four Nonintervention Units | ||||
|---|---|---|---|---|---|---|
| Outcomes | n | AOR (95% CI) | p | n | AOR (95% CI) | p |
| ICU mortality, next-daya | 15,193 | 0.57 (0.36–0.89) | 0.01 | 16,208 | 0.78 (0.48–1.27) | 0.32 |
| Hospital mortality, next-daya | 15,299 | 0.59 (0.40–0.87) | 0.01 | 16,434 | 0.86 (0.56–1.32) | 0.50 |
| ICU transfer, next-day (exclude expired patients)a | 13,045 | 0.84 (0.73–0.96) | 0.01 | 12,518 | 1.16 (0.98–1.38) | 0.09 |
| Hospital discharge, next-day (exclude expired patients)a | 12,650 | 0.84 (0.73–0.96) | 0.01 | 12,081 | 1.13 (0.95–1.34) | 0.18 |
| Off ventilationa (only patients on ventilator) | 6,209 | 1.56 (1.32–1.86) | < 0.0001 | 7,154 | 1.79 (1.39–2.31) | < 0.0001 |
| Delirium free next-day, CAM-ICU negative (only patients presenting with delirium)a | 2,034 | 1.01 (0.81–1.24) | 0.96 | 1,426 | 0.86 (0.64–1.16) | 0.32 |
| Discharge disposition, other than homeb | 3,273 | 0.68 (0.48–0.97) | 0.03 | 2,917 | 0.40 (0.26–0.62) | < 0.0001 |
AOR = adjusted odds ratio.
aCompliance association with outcomes of the following day per patient record. Logistic regression using generalized estimation equation. Analysis nested within patient’s ICU visit and then nested within ICU units. Covariates include daily A–F bundle compliance, case mix index (CMI), study month, days between study initiation and the A–F bundle collection date.
bLogistic regression was used to test association between the A–F bundle and nonhome discharge. Bundle compliance was aggregated as compliance percentage over the ICU period per patient and categorized as binary variable: full compliance for all ICU admission days vs all other. Analysis nested within ICU units, covariates include aggregated compliance, CMI, study month, days between study initiation and the A–F bundle collection date.
Two-sided significance level was set to 0.05 for all analyses.
DISCUSSION
We hypothesized that clinical education combined with DL training of ICU staff and ongoing bundle compliance reporting would improve adherence to the A–F bundle and patient outcomes. This study demonstrated that clinical education alone significantly increased bundle compliance from 9% to 16% (78% increase) and that subsequent DL training and sharing of weekly bundle performance reports with ICU staff further increased bundle compliance to 21% (230% increase). Moreover, this improvement in compliance was sustained over time, despite the extreme challenges created by the COVID-19 pandemic. Nonintervention ICUs were unable to sustain their improvement in compliance over time and during the pandemic, ending the study at their baseline compliance rate. These results aligned well with the previous studies that used bundle compliance reports to assess and improve bundle performance (3, 11, 13, 14). To our knowledge however, this is the first study to quantify the relative impact of providing DL training and bundle performance data on both bundle compliance and patient outcomes.
All ICUs began this study at the same level of full A–F bundle compliance and initially improved their compliance over time; however, significantly smaller improvements were seen in the nonintervention group. The improvement seen in the nonintervention ICUs may be explained by the success of SCCM’s ICU Liberation Campaign in educating its membership about the A–F bundle and/or the spillover effect of sharing ICU staff between units in intervention and nonintervention groups (e.g., “float pool nurses” and respiratory therapists, etc.). Additionally, Figure 2 demonstrates that the nonintervention group A–F bundle compliance decreased significantly during the onset of the pandemic. Conversely, significant gains in bundle performance in the intervention group remained throughout this period. We believe the ongoing presence of weekly performance reports in the intervention grou p kept performance of the A–F bundle at the forefront of the clinician’s mind.
In both intervention and nonintervention ICUs, improved bundle compliance was associated with significant improvements in duration of mechanical ventilation and discharge status of ICU survivors. Improvements were greater in the intervention groups with higher bundle compliance, supporting the findings in earlier studies that improved bundle compliance has a positive, dose-response effect on ICU patient outcomes (3, 13, 28). These differences in patient outcomes were observed in the face of statistically significant differentiating patient characteristics that included primary diagnosis, CMI, mobility restriction at admission, presence of mechanical ventilation, and age. A difference in primary diagnosis was expected given the differing specialty focus of each of the eight ICUs. Importantly, CMI, mobility restriction at admission, presence of mechanical ventilation, and age were higher in the intervention group versus the nonintervention group, suggest a robust effect of the interventions. Full bundle compliance in the intervention group was also associated with a lower likelihood of ICU and hospital mortality, a benefit which was not seen in the nonintervention group despite their lower average CMI. This suggests that to realize a mortality benefit with the bundle, ICUs must achieve a certain minimum level of compliance with the A–F bundle which was not reached by the nonintervention units in our study, regardless of differing patient characteristics across ICUs.
In this study, increased bundle compliance in the intervention group was associated with longer rather than shorter ICU and hospital LOS and did not impact the frequency of ICU delirium. This contrasts with previous studies showing significant reductions in ICU and hospital LOS with increased bundle compliance (3, 5, 6, 13, 28–30). The decreased likelihood of next-day ICU transfer or hospital discharge seen in the intervention group may be explained by the reductions in mortality in the intervention group (i.e., patients who might otherwise have died survived to stay longer in the ICU and hospital) and/or due to other factors influencing overall hospital throughput independent of the bundle. This is consistent with our finding that increased bundle compliance in the nonintervention group where there was no mortality benefit was not associated with an increased ICU or hospital LOS in these units. The delirium finding might be explained by clinical education which increased the use of the CAM-ICU tool in the intervention group, leading to an increased frequency of delirium detection in these ICUs (31, 32).
There are several limitations to this study. First, this was a real-world study, where operational challenges prevented maintaining the fidelity of the study design using a sample size calculation that was based on all eight ICUs. Our findings remained significant even though only four ICUs received the intervention. Second, there was variability in the clinical education received by staff both within and across disciplines. For example, nursing clinical education was spread across month 1 for each of the four intervention units, but approximately 20% of nurses working in the intervention units did not complete formal bundle training due to scheduling conflicts. Third, to support continuous improvement, we iterated compliance definitions to reflect revisions in policy during the study, for example, defining intervention frequencies associated with mobility level. Fourth, there was a lack of a true randomized concurrent control group to test the impact of the interventions; patient populations across the eight ICUs differed significantly and were not strictly comparable from a clinical care perspective. Fifth is the use of CMI as a proxy measure for patient acuity. CMI was retrospectively included in the analysis and may have been influenced by the bundle (i.e., CMI may have decreased over time with the benefits of bundle care delivery since it is calculated for patients at the end of each hospitalization, unlike other acuity measures that are calculated at admission). Finally, a comparison of the effects of full versus partial bundle compliance was not performed since each individual bundle element was implemented concurrently as part of the complete bundle, so it was difficult to assess individual or combined element impact on any single patient outcome. When tested for significance, each of the six individual elements appeared to have an impact on outcomes, but it was not possible to disentangle these individual effects. Thus, we focused primarily on whether the entire bundle was implemented compared with partial bundle compliance. Finally, a significant challenge to measuring bundle performance was our Cerner EHR version which did not facilitate bundle documentation, viewing of bundle elements, or retrieval and display of aggregated data to create bundle performance reports.
CONCLUSIONS
This study demonstrated the impact of performance measurement, continuous feedback, and DL training on increasing and sustaining A–F bundle compliance, which translated to significant improvements in ICU patient outcomes. The availability of data to reflect on performance and provide effective feedback is central to improving the quality of care (33). Based on these findings, we recommend that EHR manufacturers incorporate standard A–F bundle metrics created by the SCCM (1) and make bundle performance reports accessible to users in real time (3, 5, 15). EHR data need to be available in a format that supports clinical care, decision-making, and interprofessional collaboration around the A–F bundle in real time, as well as providing aggregated data to support meaningful use and continuous quality improvement efforts (3, 34–36). Furthermore, implementation of the A–F bundle should include education and training to increase DL for sustained bundle adherence.
ACKNOWLEDGMENTS
We thank Keck Medicine of USC Critical Care Institute and Critical Care Working Group for their support and participation to make this study successful.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
This work was performed at Keck Medicine of USC, Stanford, VA.
Dr. Ding is supported by grant UL1TR001855 from the National Center for Advancing Translational Science of the U.S. National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Society of Critical Care Medicine: ICU Liberation Collaborative. 2018. Available at: https://www.sccm.org/Clinical-Resources/ICULiberation-Home/Get-Started. Accessed July 1, 2020
- 2.Ely EW: The ABCDEF bundle: Science and philosophy of how ICU liberation serves patients and families. Crit Care Med 2017; 45:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pun BT, Balas MC, Barnes-Daly MA, et al. : Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019; 47:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehm LM, Vasilevskis EE, Mion LC: Interprofessional perspectives on ABCDE bundle implementation: A focus group study. Dimens Crit Care Nurs 2016; 35:339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masica A, Collinsworth A, Kouznetsova M, et al. : Implementation of the ABCDE bundle: Results from a real-world, pragmatic study design. Implement Sci 2015; 10:A3 [Google Scholar]
- 6.Miller MA, Govindan S, Watson SR, et al. : ABCDE, but in that order? A cross-sectional survey of Michigan intensive care unit sedation, delirium, and early mobility practices. Ann Am Thorac Soc 2015; 12:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pronovost P, Needham D, Berenholtz S, et al. : An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006; 355:2725–2732 [DOI] [PubMed] [Google Scholar]
- 8.Resar R, Griffin FA, Haraden C, et al. : Using Care Bundles to Improve Health Care Quality. IHI Innovation Series White Paper. Cambridge, MA, Institute for Healthcare Improvement, 2012. Available at: www.IHI.org. Accessed July 2020 [Google Scholar]
- 9.Trogrlić Z, van der Jagt M, Bakker J, et al. : A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care 2015; 19:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal G, Peden CJ, Mohammed MA, et al. ; Emergency Laparotomy Collaborative: Evaluation of the collaborative use of an evidence-based care bundle in emergency laparotomy. JAMA Surg 2019; 154:e190145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassett R, Adams KM, Danesh V, et al. : Rethinking critical care: Decreasing sedation, increasing delirium monitoring, and increasing patient mobility. Jt Comm J Qual Patient Saf 2015; 41:62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMellow J, Kim TY: Technology-enabled performance monitoring in intensive care: An integrative literature review. Intensive Crit Care Nurs 2018; 48:42–51 [DOI] [PubMed] [Google Scholar]
- 13.Barnes-Daly MA, Pun BT, Harmon LA, et al. : Improving health care for critically ill patients using an evidence-based collaborative approach to ABCDEF bundle dissemination and implementation. Worldviews Evid Based Nurs 2018; 15:206–216 [DOI] [PubMed] [Google Scholar]
- 14.Proctor E, Luke D, Calhoun A, et al. : Sustainability of evidence-based healthcare: Research agenda, methodological advances, and infrastructure support. Implement Sci 2015; 10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collinsworth AW, Masica AL, Priest EL, et al. : Modifying the electronic health record to facilitate the implementation and evaluation of a bundled care program for intensive care unit delirium. EGEMS (Wash DC) 2014; 2:1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavallée JF, Gray TA, Dumville J, et al. : The effects of care bundles on patient outcomes: A systematic review and meta-analysis. Implement Sci 2017; 12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanques G, Viel E, Constantin JM, et al. : The measurement of pain in intensive care unit: Comparison of 5 self-report intensity scales. Pain 2010; 151:711–721 [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Inouye SK, Bernard GR, et al. : Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286:2703–2710 [DOI] [PubMed] [Google Scholar]
- 19.Devlin JW, Skrobik Y, Gélinas C, et al. : Executive summary: Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46:1532–1548 [DOI] [PubMed] [Google Scholar]
- 20.Klompas M, Anderson D, Trick W, et al. ; CDC Prevention Epicenters: The preventability of ventilator-associated events. The CDC Prevention Epicenters Wake Up and Breathe Collaborative. Am J Respir Crit Care Med 2015; 191:292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kress JP, Pohlman AS, O’Connor MF, et al. : Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000; 342:1471–1477 [DOI] [PubMed] [Google Scholar]
- 22.Girard TD, Kress JP, Fuchs BD, et al. : Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): A randomised controlled trial. Lancet 2008; 371:126–134 [DOI] [PubMed] [Google Scholar]
- 23.Hermes C, Acevedo-Nuevo M, Berry A, et al. : Gaps in pain, agitation and delirium management in intensive care: Outputs from a nurse workshop. Intensive Crit Care Nurs 2018; 48:52–60 [DOI] [PubMed] [Google Scholar]
- 24.Marra A, Ely EW, Pandharipande PP, et al. : The ABCDEF bundle in critical care. Crit Care Clin 2017; 33:225–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris PE, Goad A, Thompson C, et al. : Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 2008; 36:2238–2243 [DOI] [PubMed] [Google Scholar]
- 26.Schweickert WD, Pohlman MC, Pohlman AS, et al. : Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009; 373:1874–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sessler CN, Gosnell MS, Grap MJ, et al. : The Richmond agitation-sedation scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166:1338–1344 [DOI] [PubMed] [Google Scholar]
- 28.Barnes-Daly MA, Phillips G, Ely EW: Improving hospital survival and reducing brain dysfunction at seven California community hospitals: Implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med 2017; 45:171–178 [DOI] [PubMed] [Google Scholar]
- 29.Bounds M, Kram S, Speroni KG, et al. : Effect of ABCDE bundle implementation on prevalence of delirium in intensive care unit patients. Am J Crit Care 2016; 25:535–544 [DOI] [PubMed] [Google Scholar]
- 30.Kram SL, DiBartolo MC, Hinderer K, et al. : Implementation of the ABCDE bundle to improve patient outcomes in the intensive care unit in a rural community hospital. Dimens Crit Care Nurs 2015; 34:250–258 [DOI] [PubMed] [Google Scholar]
- 31.Spronk PE, Riekerk B, Hofhuis J, et al. : Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med 2009; 35:1276–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Eijk MM, van Marum RJ, Klijn IA, et al. : Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med 2009; 37:1881–1885 [DOI] [PubMed] [Google Scholar]
- 33.Kaye AD, Okanlawon OJ, Urman RD: Clinical performance feedback and quality improvement opportunities for perioperative physicians. Adv Med Educ Pract 2014; 5:115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ervin JN, Kahn JM, Cohen TR, et al. : Teamwork in the intensive care unit. Am Psychol 2018; 73:468–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumenthal D, Tavenner M: The “meaningful use” regulation for electronic health records. N Engl J Med 2010; 363:501–504 [DOI] [PubMed] [Google Scholar]
- 36.Jha AK: Meaningful use of electronic health records: The road ahead. JAMA 2010; 304:1709–1710 [DOI] [PubMed] [Google Scholar]