Abstract
Over the years, a large number of sorption experiments using the aminopolycarboxylic acid (APCA)-functionalized adsorbents were carried out in batch conditions, but prospective research should also be directed towards column studies to check their industrial/commercial feasibility. In this context, sorption studies of five-component heavy metal ion (HMI) solutions containing Zn2+, Pb2+, Cd2+, Ni2+, and Co2+ in equimolar concentrations were assessed in fixed-bed columns using some APCA-functionalized chitosan-clinoptilolite (CS-CPL) cryogel sorbents in comparison to unmodified composite materials. The overall sorption tendency of the APCA-functionalized composite sorbents followed the sequence Co2+ < Zn2+ < Cd2+ ≤ Pb2+ < Ni2+, meaning that Co2+ ions had the lowest affinity for the sorbent’s functional groups, whereas the Ni2+ ions were strongly and preferentially adsorbed. To get more insights into the application of the composite microbeads into continuous flow set-up, the kinetic data were described by Thomas and Yoon–Nelson models. A maximum theoretical HMI sorption capacity of 145.55 mg/g and a 50% breakthrough time of 121.5 min were estimated for the column containing CSEDTA-CPL cryogel sorbents; both values were much higher than those obtained for the column filled with pristine CS-CPL sorbents. In addition, desorption of HMIs from the composite microbeads in dynamic conditions was successfully achieved using 0.1 M HCl aqueous solution. Moreover, a theoretical analysis of APCA structures attached to composite adsorbents and their spatial structures within the complex combinations with transition metals was systematically performed. Starting from the most stable conformer of EDTA, coordinative combinations with HMIs can be obtained with an energy consumption of only 1 kcal/mole, which is enough to shift the spatial structure into a favorable conformation for HMI chelation.
Keywords: aminopolycarboxylic acids, chelating agents, theoretical analysis, fixed-bed column studies, multicomponent heavy metal ions solution
1. Introduction
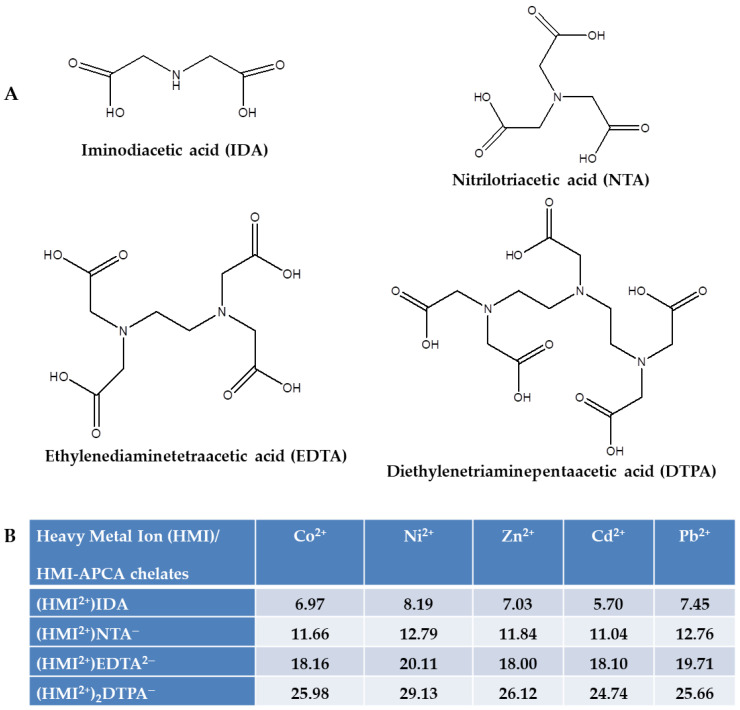
The presence of heavy metal ions (HMIs) in the environment generates huge problems due to their high toxicity and non-biodegradability, and, therefore, their removal by eco-friendly and low-cost sorbents is a “must-do” requirement for manufacturing or processing industrial activities [1,2]. Aminopolycarboxylic acids (APCAs) are well-known as an important group of chelating agents that form stable structures with HMIs [3,4]. They have several carboxylate groups attached to one or more nitrogen atoms capable of strongly binding HMIs. Iminodiacetic acid (IDA), nitrilotriacetic acid (NTA), ethylenediaminetetraacetic acid (EDTA), and diethylenetriaminepentaacetic acid (DTPA) are the most widely used APCAs (Figure 1A): (i) to remove HMIs from aqueous environments, (ii) to prevent the generation of HMIs precipitates, (iii) to impede catalysis mediated by HMIs, or (iv) to control HMIs solubility/availability in the aqueous medium [5]. The broad range of applications of APCAs is attributed to their structural characteristics and unique properties to form stable and water-soluble chelates with HMIs. The stability of the formed chelates is directly connected with the number of the carboxylate groups in the structure of APCAs: the higher their number the higher the stability of the HMIs-APCA chelates, i.e., the pK values (Figure 1B) [6].
Figure 1.
(A) Chemical structures of the most important aminopolycarboxylic acids (APCAs) used in heavy metal ions (HMIs) removal/recovery processes; (B) pK values for some HMIs-APCAs chelates, Adapted with permission from reference [6], 2013, Elsevier Ltd.
Consequently, the surface of a broad range of adsorbent materials has been successfully decorated with APCAs ligand groups, and their adsorption performance and selectivity towards various HMIs has been intensively studied [6,7,8,9,10,11,12,13]. For instance, high adsorption capacities towards Cu2+, Co2+, Ni2+, Pb2+, Zn2+, and Cd2+ ions were reported for acrylonitrile-divinylbenzene copolymers modified by IDA moieties [7,8]. Excellent sorption ability for Cr3+ ions of NTA-modified magnetic mesoporous microspheres was also demonstrated [13]. APCAs-modified chitosan (CS) derivatives were also prepared and investigated for removal and recovery of Cu2+ [14,15], Ni2+ [16,17], Co2+ [17], Pb2+ [18,19,20,21], or rare earths [22] from simulated aqueous solutions. Bio-derived trifunctional CS−EDTA-β−cyclodextrin sorbents were designed and tested for the simultaneous adsorption of various HMIs (Pb2+, Cd2+, Hg2+, Ni2+, and Cu2+) and organic pollutants (bisphenol-S, ciprofloxacin, procaine, imipramine, methylene blue, crystal violet, and safranin O) from wastewaters [23,24].
Recently, our group has systematically analyzed the sorption behavior of Cu2+ ions from mono-component aqueous solutions onto CS-EDTA cryobeads [15]. A fast kinetic rate, a remarkable reusability, and a sorption capacity of 168 mg Cu2+/g were demonstrated for the EDTA functionalized sorbents [15]. The Fe3+, Co2+, and Cu2+ ions sorption in binary and ternary systems by porous EDTA-CS and DTPA-CS composite derivatives was also explored [25]. It was found that the experimental sorption capacity for removal of Fe3+ ions from their mixture with Cu2+ ions increased with the number of carboxylate moieties attached to the CS support matrix, a sorption capacity of 161.60 mg/g, 189.61 mg/g, and 206.65 mg/g being found for CS, CS-EDTA and CS-DTPA, respectively. Thus, surface functionalization of sorbent materials with APCAs ligand groups has been found to provide them with better adsorptive properties.
So far, the sorption experiments using the APCAs-functionalized adsorbents were mainly carried out in batch conditions, but prospective research should also be directed towards column, pilot-plant, and full-scale studies to check their feasibility at the industrial/commercial level. Moreover, the stability of APCAs ligands attached to adsorbent materials should be regularly supervised during the HMIs sorption to ensure that the leaching of APCA moieties is not occurring in the treated wastewaters. Herein, as the real-life aqueous environments are composed of more complex HMIs mixtures, sorption studies towards five-component metal ion solutions were assessed in fixed-bed columns. Thereafter, to examine the feasibility of APCA-functionalized CS-clinoptilolite (CPL) composite cryogel sorbents for treatment of HMI-containing wastewaters, a theoretical analysis of EDTA and DTPA structures in complex combinations with transition metal ions was also performed.
2. Results and Discussion
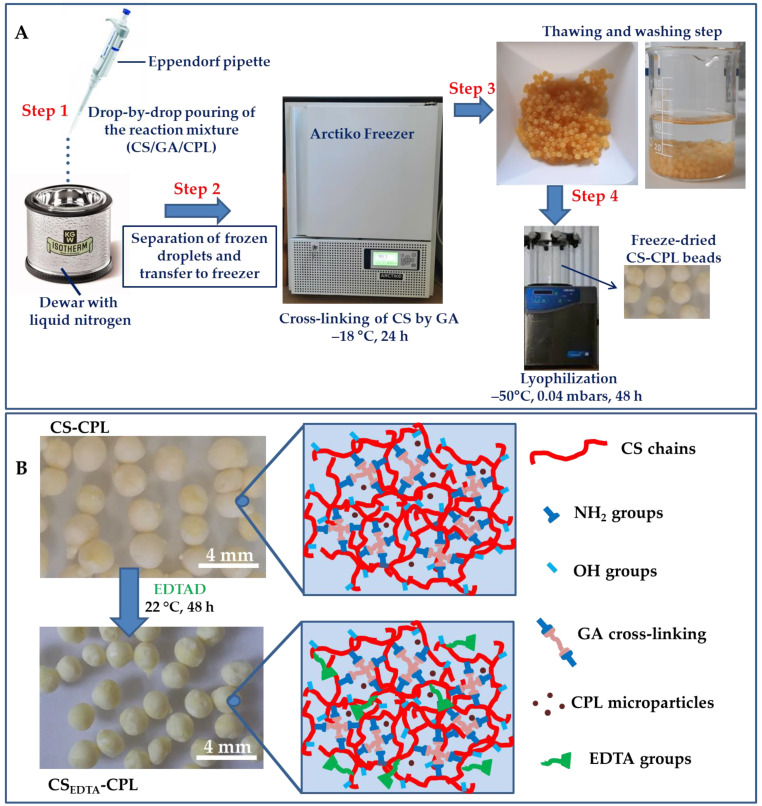
In this work, chemically cross-linked CS-CPL composite cryogel sorbents by glutharaldehyde (GA) with a high porosity and large pore sizes were prepared by a cryogelation process, according to a procedure previously reported [26]. Briefly, CS-CPL composite cryogels were formed as beads by pouring a homogeneous dispersion consisting of CS, CPL, and GA into liquid nitrogen using an Eppendorf pipette (Step 1, Figure 2A). The frozen droplets were then separated from liquid nitrogen and immediately transferred in an Arctiko Freezer at −18 °C to ensure the complete cross-linking of CS by GA (Step 2, Figure 2A). After 24 h, the CS-CPL beads were thawed at room temperature and washed with MilliQ water to remove unreacted compounds (Step 3, Figure 2A). Thereafter, the CS-CPL composite beads were freeze-dried in a LABCONCO FreeZone apparatus for 48 h, at –50 °C and 0.04 mbar (Step 4, Figure 2A). The CS-CPL sorbents were further modified with EDTA moieties to enhance the sorption performance of the pristine composites. The introduction of EDTA ligand moieties onto CS-CPL sorbents was achieved by reaction with 4,4′–ethylenebis(2,6– morpholinedione) (EDTAD) in a acetic acid-methanol (1:1 v/v) mixture at 22 °C for 48 h (Figure 2B). The energy dispersive X-ray analysis (EDX) on the surface of CS-CPL and CSEDTA-CPL microbeads (Table 1) proved the presence of C, N, O, Al, Si, Na, K, and Ca elements from CS and CPL, revealing the good stability of CPL microparticles within the CS matrix during the functionalization step. In addition, the higher atomic ratio percentages found for N in the case of CSEDTA-CPL microbeads is a further indication of the successful modification of CS-CPL sorbents with EDTA functional groups. The evaluation of porosity, pore sizes, and Brunauer–Emmett–Teller (BETH2O) surface area of composite sorbents (Table 2) pointed out the presence of a macroporous structure for both sorbents, which could provide a fast access of HMIs to a large number of chelating sites.
Figure 2.
(A) A schematic procedure of the preparation of CS-CPL sorbents. (B) Optical pictures showing freeze-dried CS-CPL microbeads before and after interaction with 4,4′–ethylenebis(2,6– morpholinedione) (EDTAD) and schematic illustration of –NH2, -OH, and EDTA functional groups available within CS-CPL and CSEDTA-CPL composite sorbents available for interaction with HMIs.
Table 1.
EDX elemental atomic composition of CS-CPL and CSEDTA-CPL microbeads surface.
| Sample | EDX Elements Atomic Ratio, % | |||||||
|---|---|---|---|---|---|---|---|---|
| C | N | O | Al | Si | Na | K | Ca | |
| CS-CPL | 44.03 | 8.93 | 40.00 | 0.50 | 2.13 | 0.27 | 0.10 | 3.13 |
| CSEDTA-CPL | 47.97 | 9.90 | 35.50 | 0.50 | 2.00 | 0.27 | 0.17 | 2.83 |
Table 2.
Some features of the CS-CPL and CSEDTA-CPL composite sorbents.
| Sample Code | CPL, wt.% | Porosity, 1 % |
Mean Pore Sizes, 2 μm | Water Uptake, 3 g/g |
BETH2O Data 4 | |
|---|---|---|---|---|---|---|
| Area, m2/g | Monolayer, g/g | |||||
| CS-CPL | 20 | 78.19 ± 2.04 | 21.49 ± 5.27 | 30.27 ± 3.48 | 431.16 ± 29.31 | 0.123 ± 0.084 |
| CSEDTA-CPL | 20 | 86.57 ± 1.31 | 30.16 ± 6.18 | 45.04 ± 4.82 | 450.34 ± 30.66 | 0.128 ± 0.067 |
1 Porosity was determined by liquid displacement method [27]; 2 mean pore sizes were evaluated from SEM micrographs using Image J 1.48v analyzing software; 3 water uptake was calculated with Equation (4) from Section 4. Materials and Methods; 4 BETH2O surface area of composite sorbents was evaluated based on dynamic water vapor sorption isotherms considering a relative humidity up to 40%.
2.1. Experimental Data on HMIs Sorption in Fixed-Bed Column
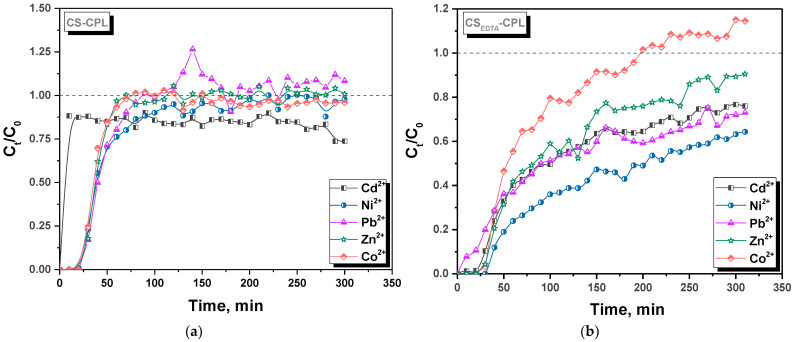
Analysis of sorbents efficiency in fixed-bed column sorption experiments is performed with the consideration of their upscaling towards real wastewater treatment applications [28,29,30,31]. The performance of fixed-bed columns is described by the breakthrough curves, which are representations of normalized effluent concentration as a function of flow time or bed volume. In this work, the performance of CS-CPL and CSEDTA-CPL microbeads in continuous flow set-ups was investigated, under competitive conditions, using a simulated aqueous solution containing Zn2+, Pb2+, Cd2+, Ni2+, and Co2+ in equimolar concentrations (C = 0.5 mmol/L). The breakthrough curves for each individual metal ion for the CS-CPL and CSEDTA-CPL supports are depicted in Figure 3.
Figure 3.
Experimental breakthrough curves for individual HMIs sorption from simulated multicomponent solutions by CS-CPL (a) and CSEDTA-CPL (b) microbeads in fixed-bed column experiments.
For both types of microbeads, a certain degree of chromatographic effect was observed, due to the competition of solute species for the available functional groups. The column filled with CS-CPL microbeads showed much steeper breakthrough profiles for all tested HMIs (especially for Cd2+) compared to the one containing CSEDTA-CPL microbeads. This is because the sorbent’s available active sites saturate more rapidly, i.e., it has a lower sorption capacity. For the column containing CSEDTA-CPL microbeads, a clear separation of the breakthrough profiles was observed, as a result of the sorbent’s different affinity for the HMIs. Thus, the interaction order followed the sequence Co2+ < Zn2+ < Cd2+ ≤ Pb2+ < Ni2+, meaning that Co2+ had the lowest affinity for the sorbent’s functional groups, whereas between Ni2+ and the CSEDTA-CPL microbeads, the strongest interactions were established. The obtained sequence for CSEDTA-CPL microbeads is well correlated to the sequence of the HMIs-EDTA chelates pK values listed in Figure 1B.
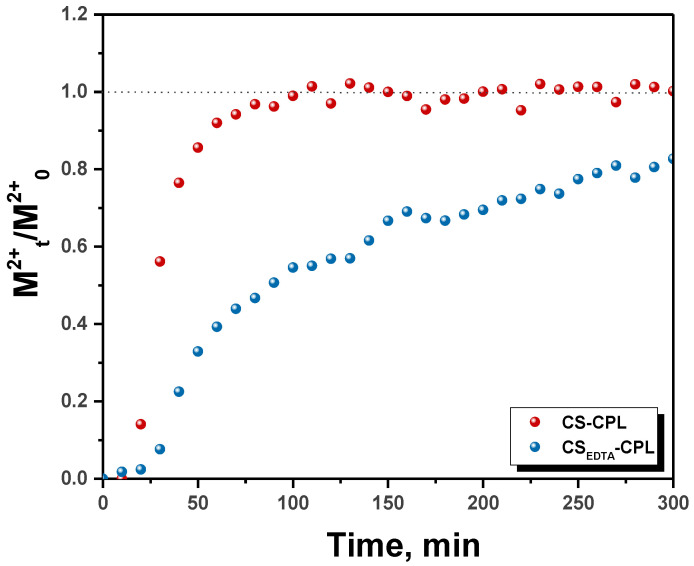
To be effective in column set-ups, a material should possess high adsorption capacity as well as fast sorption kinetics for the target solutes [32]. In order to gain more insight into the columns performance, the breakthrough curves for the two tested systems were represented for the sum of all HMIs in the solution (Figure 4).
Figure 4.
The CS-CPL and CSEDTA-CPL columns saturation with HMIs as a function of time.
As seen in Figure 4, the column with CS-CPL microbeads was exhausted quickly, after approximately 100 min, whereas for the one with CSEDTA-CPL microbeads only approximately 80% saturation was found after 300 min. This shows that CSEDTA-CPL microbeads have a much higher sorption capacity for HMIs, as already presented in our recent work [15,25] in batch sorption experiments.
In order to get more insights into the application of CS-CPL and CSEDTA-CPL beads in continuous flow set-up, the kinetic data obtained in the fixed-bed experiments were fitted with the Thomas and Yoon–Nelson models, and the fitting results are listed in Table 3. The Thomas model (Equation (1)) was derived from the Langmuir adsorption isotherm and the second-order reaction kinetics, under the assumption of zero longitudinal dispersion in the fixed bed [33]:
| (1) |
where Ct (mg/L) and C0 (mg/L) are the concentration of HMIs in effluent and influent, respectively, kTh is the Thomas rate constant (L/mg∙min), q0 (mg/g) is the maximum sorption capacity, m (mg) is the mass of adsorbent, and Q (mL/min) is the flow rate.
Table 3.
Fitting parameters of Thomas and Yoon–Nelson models for the fixed-bed column experiments.
| Sample | m 1 (mg) | Thomas | Yoon–Nelson | ||||
|---|---|---|---|---|---|---|---|
| kTH (L/min∙mg) | q0 (mg/g) | R2 | kYN (mL/min·mg) | τ (min) | R2 | ||
| CS-CPL | 109.9 | 5.38 × 10−4 | 68.72 | 0.985 | 0.133 | 30.64 | 0.985 |
| CSEDTA-CPL | 205.8 | 4.85 × 10−5 | 145.55 | 0.862 | 0.012 | 121.51 | 0.862 |
1 Sorbent mass loaded in the columns.
The Yoon–Nelson model (Equation (2)) is based on the assumption that the expected sorption decrease rate is related to the breakthrough of the adsorbent [34]:
| (2) |
where kYN (mL/min·mg) represents the Yoon–Nelson reaction constant, and τ (min) is the time at which 50% breakthrough is achieved.
The parameter q0 in the Thomas model provides an estimate for the maximum sorption capacity of a sorbent in dynamic conditions, whereas τ in the Yoon–Nelson model gives the time for 50% breakthrough. Thus, for the column containing CSEDTA-CPL microbeads, a maximum HMI sorption capacity of 145.55 mg/g and a 50% breakthrough time of 121.5 min were estimated, with both being much higher than for the column containing CS-CPL microbeads. In addition, the kinetic data obtained in the fixed-bed experiments for CSEDTA-CPL cryogel sorbents towards each HMI were further analyzed by the Thomas and Yoon–Nelson models. The maximum theoretical sorption capacities of the CSEDTA-CPL composite sorbent for Co2+, Zn2+, Cd2+, Pb2+, and Ni2+ ions were found to be 79.57, 130.03, 158.72, 166.78, and 249.53 mg/g, respectively (Table S1). A 50% breakthrough time of 66.43, 108.56, 132.52, 139.24, and 208.34 min was assessed for Co2+, Zn2+, Cd2+, Pb2+, and Ni2+ ions, respectively (Table S1). The efficiency of the composite sorbents prepared in this study was compared with that of other sorbents reported in the literature (Table S2). Albeit all sorbents included in Table S2 can be exploited for the removal of HMIs from multicomponent mixtures, our CSEDTA-CPL sorbent exhibited the highest sorption capacity. Overall, it is clearly demonstrated that the modification of CS based structures with APCA moieties is a powerful strategy to significantly improve their HMI chelation properties, the obtained materials being highly suitable for water purification in continuous flow conditions.
To ascertain the functional groups involvement in the sorption of HMIs by CS-CPL and CSEDTA-CPL microbeads, FTIR spectra of the microbeads before and after the contact with HMI multicomponent solution were recorded (Figures S1 and S2, Supporting Information). Thus, the FTIR spectrum of pristine CS-CPL microbeads presents characteristic bands for stretching vibrations of -OH and -NH groups (at 3435 cm−1), stretching vibrations of -CH and CH2 groups (at 2926 cm−1 and 2878 cm−1), C=O stretching (at 1651 cm−1), -NH2 bending (at 1564 cm−1), CH2 bending (at 1414 cm−1), CH2 deformations (at 1383 cm−1), C-O-C bridges deformation vibrations (at 1151 cm−1), and C-O stretching (1082 cm−1 and 1036 cm−1) that are attributed to the CS matrix [35,36]. The presence of CPL particles within the microbeads is supported by the characteristic bands at 463 cm−1, 609 cm−1, and 795 cm−1 [37]. However, the absence of the band characteristic to -NH2 bending vibrations, as well as the very broad bands at 1641 cm−1 and 1383 cm−1 (attributed to asymmetric and symmetric stretching vibrations of -COO- groups) in the spectrum of CSEDTA-CPL microbeads support the successful functionalization with EDTA moieties [15]. The sorption of HMIs by both microbeads led to several spectral changes. Thus, the disappearance of the -NH2 group bending vibrations from the spectrum of CS-CPL microbeads, after the interaction with the HMIs supports the involvement of these groups in the sorption process. In the spectrum of CSEDTA-CPL microbeads after interaction with HMIs, the band at 1589 cm−1 (blue-shifted as in the spectrum of corresponding pristine sample) indicates the involvement of carboxyl groups in the complexation of HMIs.
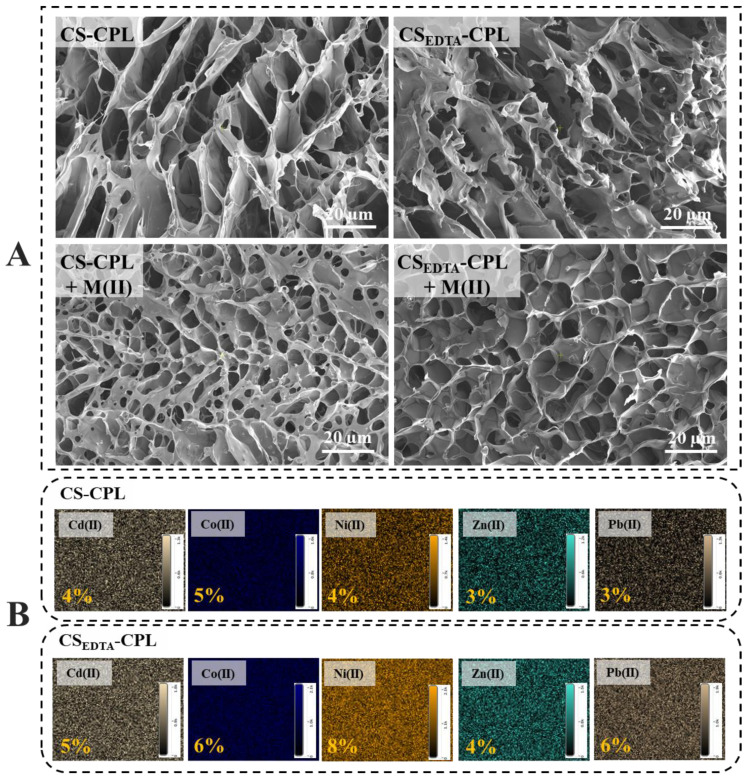
The internal structure of CS-CPL and CSEDTA-CPL microbeads before and after interaction with the multicomponent HMI solution was analyzed by SEM (Figure 5A). A heterogeneous porous morphology with interconnected pores could be observed for all samples. The mean pore sizes of the CS-CPL and CSEDTA-CPL before HMI sorption were approximately 21.49 ± 5.27 μm and 30.16 ± 6.18 μm, whereas after M(II) sorption their diameters drastically decreased to approximately 5.65 ± 1.42 μm and 5.87 ± 1.26 μm, respectively, which indicates a strong interaction between HMIs and functional groups of the support matrix. The EDX mapping of CS-CPL and CSEDTA-CPL microbeads surface after interaction with the HMI solution (Figure 5B) shows the uniform distribution of each HMI on the sorbent surface. The elemental analysis of CSEDTA-CPL after M(II) sorption further sustains the high affinity of this sorbent for Ni2+ ions (8% atomic ratio—Figure 5B).
Figure 5.
(A) SEM micrographs of CS-CPL and CSEDTA-CPL microbeads before and after interaction with the multicomponent HMIs solution; (B) EDX mapping and elemental analysis of CS-CPL and CSEDTA-CPL microbeads surface after interaction with the HMI solution.
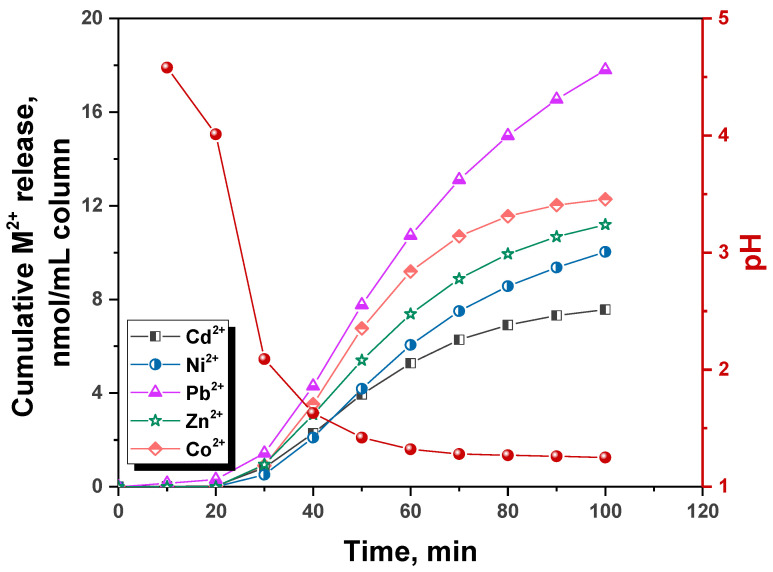
The desorption of HMIs from the CSEDTA-CPL microbeads was also investigated in dynamic conditions, using 0.1 M HCl aqueous solution as eluent. In Figure 6, the cumulative release amounts of all HMIs (in nmol/mL column), in tandem with the effluent pH evolution, as a function of elution time is depicted.
Figure 6.
Cumulative M2+ release (nmol/mL column) in dynamic conditions and effluent pH during desorption of HMIs with 0.1M HCl from the CSEDTA-CPL microbeads.
In acidic media, the amino and carboxyl groups from CS backbone and EDTA moieties, respectively, are protonated, the HMIs being thus desorbed from the sorbent. As it is seen, the pH of collected effluent quickly decreased in the first 30 min of the desorption experiment down to around pH 2, afterwards swiftly evolving towards an equilibrium value. Simultaneously, the release of HMIs was low in the first 30 min of the desorption experiment, but quickly increased afterwards. The cumulative HMI amounts determined after 100 min of running time were 17.8 nmoles of Pb2+, 7.56 nmoles of Cd2+, 12.28 nmoles of Co2+, 10.03 nmoles of Ni2+, and 11.19 nmoles of Zn2+ (calculated with respect to mL column). Overall, the dynamic desorption experiment highlights the possibility that the microbeads investigated in this work can be cleaned of the sorbed HMIs in continuous flow set-ups, with potential for regeneration and reuse in successive sorption/desorption cycles without the need to remove the materials from the column.
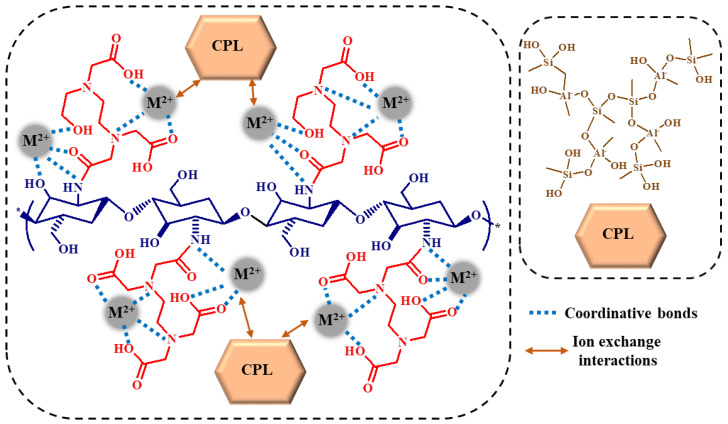
A schematic representation of the possible HMI sorption mechanism for CSEDTA-CPL cryogel sorbent is depicted in Figure 7. The coordinative bonds between the functional groups of modified CS network and HMIs and the ion exchange between the Na+, K+, and Ca2+ from CPL and Co2+, Zn2+, Cd2+, Pb2+, and Ni2+ ions are the most probable processes according to experimental and characterization results.
Figure 7.
Possible mechanism for the sorption of HMIs onto the CSEDTA-CPL cryogel sorbents.
2.2. Theoretical Analysis of EDTA and DTPA Structures Attached to CS-CPL Composite Adsorbents and Their Spatial Structures within the Complex Combinations with Transition Metals
Using the Gaussian and hybrid functional B3LYP program package [38,39,40] together with the 6–31 g orbital set, the structures of EDTA and DTPA were investigated to identify the most energetically stable conformer, the interaction with a CS unit, as well as their spatial structures within the complex combinations with transition metal ions. The theoretical investigations were performed in vacuum considering a single structural unit, without solvent influences or symmetry constraints. In addition, the polarization functions corresponding to elements that are in the periodic system at least in the second period were also considered. Under these conditions, five structures were identified for EDTA whose relative energy (kcal/mole), spatial distribution, and electric dipole moment are included in Table 4.
Table 4.
Conformers of EDTA.
| Conformer | Relative Energy (kcal/mole) | Spatial Structures 1 | Dipole Moment (Debye) |
|---|---|---|---|
| EDTA-1 | 0.00 |
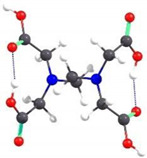
|
0.00 |
| EDTA-2 | 6.44 |
|
6.32 |
| EDTA-3 | 6.72 |
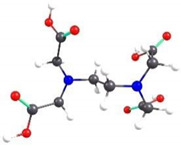
|
2.20 |
| EDTA-4 | 8.88 |
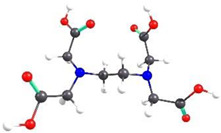
|
1.84 |
| EDTA-5 | 16.09 |
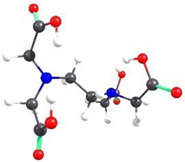
|
4.91 |
1 Legend colors: grey-C, red-O, blue-N, white-H.
The conformer, EDTA-1, has two hydrogen bonds in its structure, between the carboxyl groups found at the same N atom, which contribute to the stabilization of the spatial distribution, being thus the most energetically stable structure with a symmetry characterized by zero dipole moment (Table 4). The next conformer of the EDTA structure (EDTA-2) has a hydrogen bond between the hydroxyl group and the N atom to which the acidic moiety is bonded. The conformer of EDTA-2 displays a ‘zig-zag’ structure, which explains the high value of the dipole moment of over 6 Debye. In the structure of EDTA-3 and of the following conformers, no hydrogen bonding interaction contributing to the energy stabilization of the conformers is observed. In terms of energy difference from the previous conformer, the difference is not very large, less than one kcal/mole, but one can observe the difference in spatial distribution reflected by the dipole moment value, which is approximately 4 Debye. EDTA-4 is characterized by the existence of a symmetry plane perpendicular to the main chain, but also by the rotation of the carboxyl groups symmetric to the C-C bond within the substituent. The last conformer considered, EDTA-5, is characterized by a perpendicularity of the planes in which the substituent carboxyl structures are located to the same N atom.
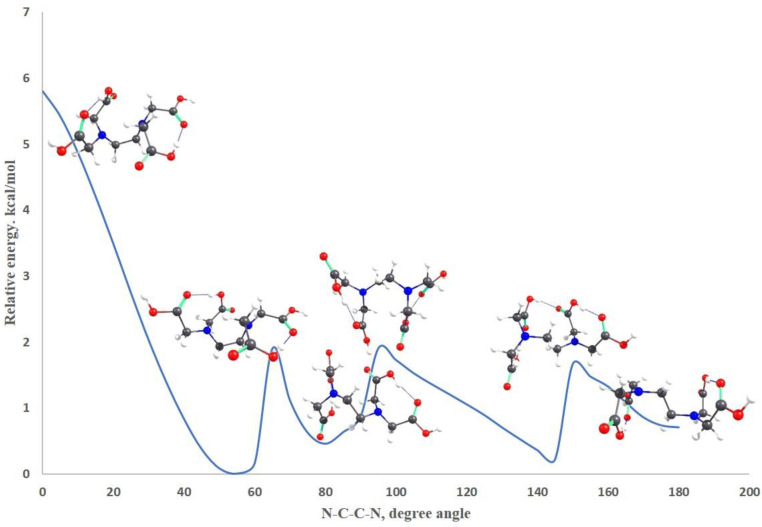
It should be pointed out that the EDTA structure characterized by a high energetic stability, EDTA-1, does not have a spatial distribution that favors the formation of complex combinations with HMIs. However, to ensure this, the spatial structure of EDTA-1 conformer has to undergo a rotation around the central C-C bond. A graphical representation of the relative energy variation registered by modifying the N-C-C-N dihedral angle from 180 degrees, as it is approximately in EDTA-1, to zero degrees is illustrated in Figure 8. It was found that a complete rotation requires a relative energy of about 6 kcal/mole (Figure 8). The changes in spatial structure during rotation shows the formation of a molecular framework favorable to the generation of coordinative structures with metal ions even from an angle of 145 degrees, which requires a rotational energy of 1 kcal/mole. Similar energy barriers, around 1 kcal/mole, also characterize the other hypothetic structures favorable to the formation of complex combinations with transition metal ions. As the dihedral angle decreases towards zero, the rotational energy increases to 6 kcal/mole. It can be concluded that starting from the most stable conformer of EDTA, coordinative combinations with metal ions can be obtained with an energy consumption of only 1 kcal/mole, which is enough to shift the spatial structure into a favorable conformation for metal ion chelation.
Figure 8.
The variation of the relative energy considering the values of the dihedral angle N-C-C-N.
Compared to EDTA, the structure of DTPA is more complex, having an additional aminocarboxylic fragment inserted into the main chain (Figure 1A). The conformer of DTPA with the lowest energy has been also determined by applying similar calculations as in the case of EDTA structure. In Table 5, the top five conformer structures of DTPA have been selected with respect to their relative energy.
Table 5.
Conformers of DTPA.
| Conformer | Relative Energy (kcal/mole) | Spatial Structures 1 | Dipole Moment (Debye) |
|---|---|---|---|
| DTPA-1 | 0.00 |
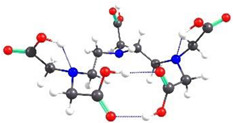
|
7.64 |
| DTPA-2 | 1.88 |
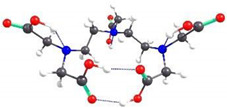
|
5.45 |
| DTPA-3 | 1.92 |
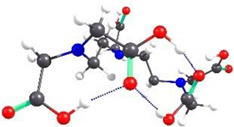
|
3.36 |
| DTPA-4 | 5.98 |
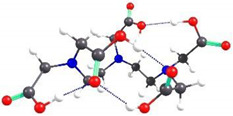
|
5.63 |
| DTPA-5 | 6.44 |
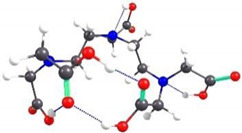
|
6.59 |
1 Legend colors: grey-C, red-O, blue-N, white-H.
The most energetically stable DTPA structure contains four interactions of hydrogen-bonding type (Table 5): (i) two between the hydrogen atom from hydroxyl groups that are part of the carboxyl residues attached to marginal N atoms of the main chain and the ketone O atoms of the other group, and (ii) two between the hydroxyl groups of the other acid functions of the marginal N atom. The first set of interactions is exerted at a distance of approximately 1.78 Å, whereas the second one is at approximately 2.0 Å. Analyzing the structures presented in Table 5, it can be seen that these intra-molecular interactions through hydrogen bonds characterize all the selected conformers. For EDTA, the most energetically stable conformer had an electric dipole moment of zero, whereas in the case of DTPA, we obtained a value higher than 7 Debye, which is larger than that of the other conformer structures listed in Table 5. The second conformer structure, DTPA-2, contains five hydrogen bonds; the additional one corresponds to the interaction between the hydroxyl group of the acid function located at the N atom in the center of the main chain and the corresponding N atom and is on the opposite side of the plane containing the main chain atoms. This spatial distribution of the molecule led to a decrease in the dipole moment value to approximately 5.45 Debye. In the DTPA-3 conformer, both carboxyl groups that form a hydrogen bond with the N atom to which they bind are on opposite sides of the molecular plane, leading to a further decrease in the dipole moment to 3.36 Debye due to the increased symmetry of the molecular system. If a hydrogen-bonding interaction is formed between two carboxyl groups attached to a marginal and central N atom on the same side of the molecular plane, the DTPA-4 conformer structure is obtained. The new interaction is established at a distance of approximately 1.8 Å between the H atom of the hydroxyl group of the acid group bound to the marginal N atom and the O in the hydroxyl group located at the central N atom, which is also involved in a similar interaction with that N atom. In the structure of the last conformer considered here, DTPA-5, there are five hydrogen bonds; one of the second types is on the opposite side of the molecular plane from the other two of the same type made at the others marginal and central N atoms. This spatial distribution of atoms can be achieved by reorienting the interaction from the previous conformer between the OH groups of two acid groups on the same side of the main chain plane towards the marginal N atom to which the functional group is bonded.
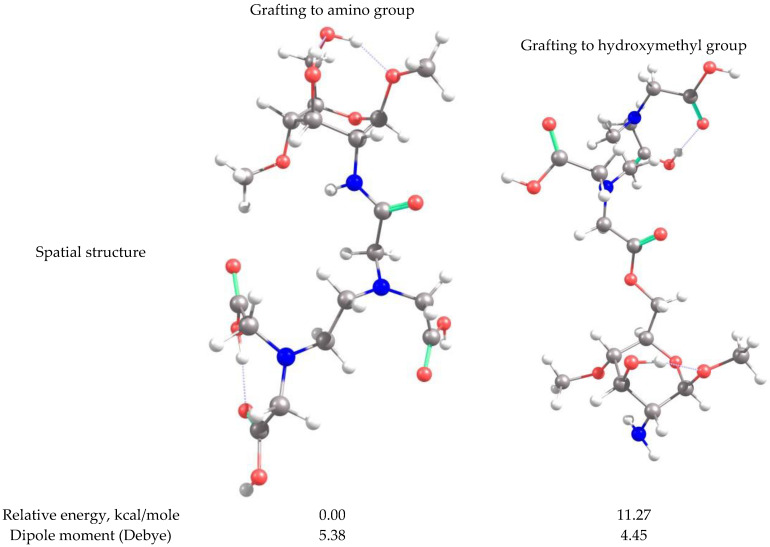
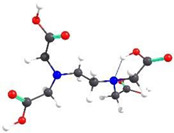
Because the structures of APCAs in the HMI sorption studies are not self-standing, but grafted on CS-CPL composite adsorbents, theoretical investigations were also carried out on the spatial conformation of these structures considering that grafting of APCAs can be accomplished either to the O atom of the hydroxymethyl group or to the amino N atom of the CS backbone. Considering the same functional analysis and set of basic orbitals, spatial structures of CSEDTA-CPL units were obtained for each grafting modality (Figure 9). It should be noted that only one structural unit was used from the CS chain, and methyl structures were linked to the valences of the marginal atoms. The structure obtained by grafting EDTA to the amino group is energetically more stable than the one resulting from attaching the EDTA residue to the hydroxymethyl group in the CS chain (Figure 9). It should be noted that between the carboxyl groups attached to the same N atom in EDTA, there is an interaction by hydrogen bonding. Analyzing the obtained structures, it can be concluded that in the case of complexation with transition metal ions, coordinative bonds can be formed between three carboxyl groups and metal ions, and also there is a possibility of formation of two dative bonds with N atoms in the EDTA structure.
Figure 9.
Spatial structures of EDTA grafted onto the CS unit and the corresponding values of the relative energy and dipole moment.
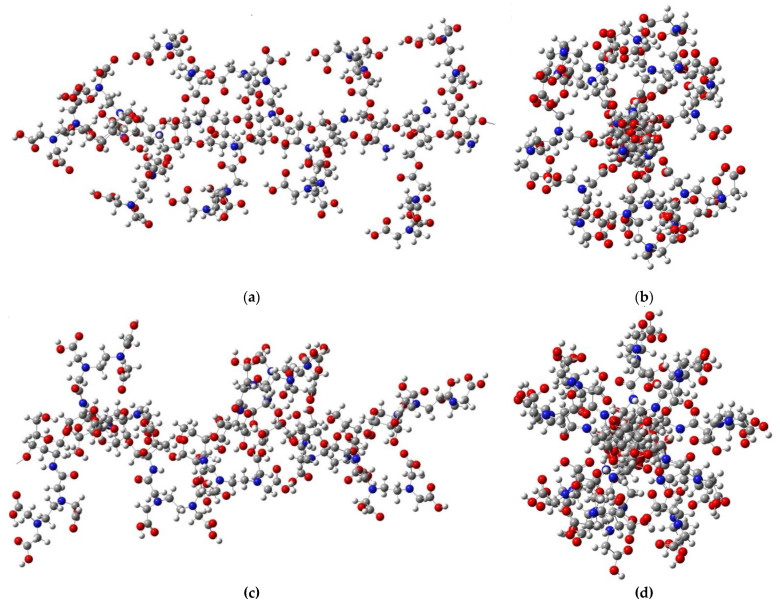
If the EDTA structures were grafted to each hydroxymethyl functional group within the CS units, the chain of ten structural units would be spatially distributed as shown in Figure 10a. However, from the perpendicular representation on the chain, it can be observed that the EDTA structures are distributed at a certain angle one to another, so that spatial constraints are minimal, resulting in a circular structure around the CS chain (Figure 10b). This distribution can also be associated with the helical spatial structure of the CS itself. Similar structures were also obtained when EDTA was grafted to the amino groups in the CS unit. The distribution of EDTA structures around the CS chain is slightly different from the previous case due to steric hindrances between the grafted structures (Figure 10c,d).
Figure 10.
Spatial representation of a structure consisting of ten CS units with EDTA grafted to the hydroxymethyl groups: (a) along the chain; (b) perpendicular to the chain. Spatial representation of a structure consisting of ten CS units with EDTA grafted to the N atom of the amino groups: (c) along the chain; (d) perpendicular to the chain.
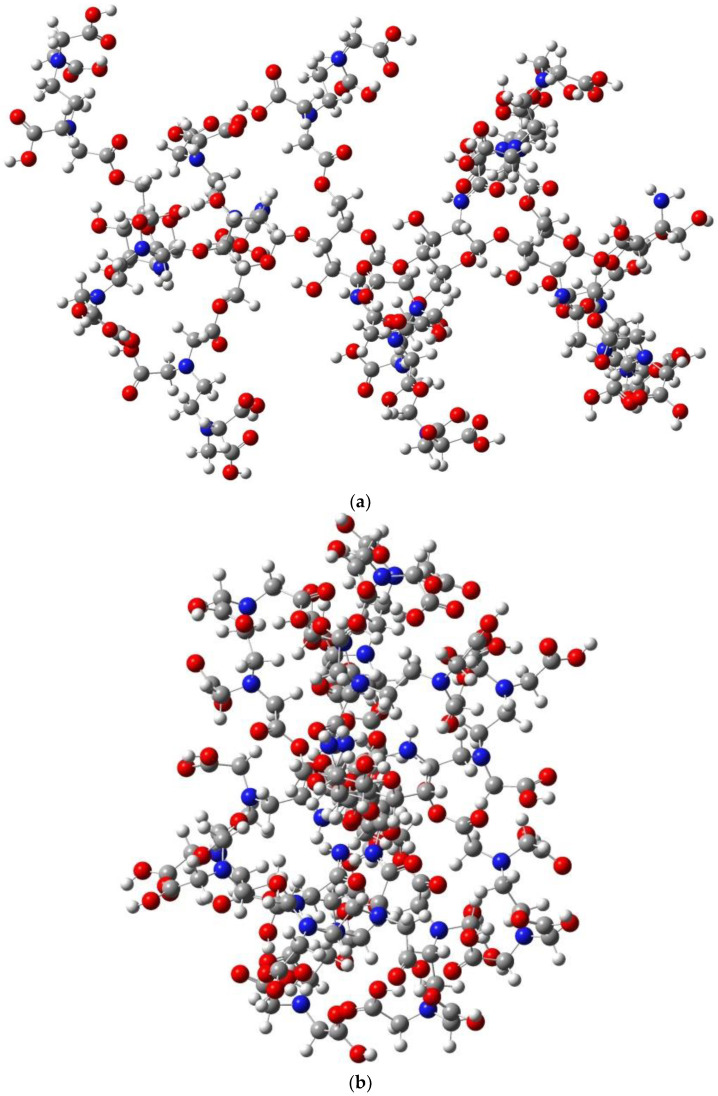
If EDTA grafting to the CS units is done alternately, with one unit grafted to the hydroxymethyl groups and the next to the amino groups, the spatial structure of a chain consisting of six CS units is shown in Figure 11. It can be seen the arrangement of EDTA structures on both sides and the rotation of the grafted structures around the main chain due to steric hindrances and to the helical structure of the CS chain.
Figure 11.
Spatial representation of a structure consisting of six CS units with EDTA grafted alternately to the N atom of the amino groups and the O atom of the hydroxymethyl groups: (a) along the chain; (b) perpendicular to the chain.
2.3. Theoretical Analysis on the Electronic Transitions of EDTA and DTPA Structures
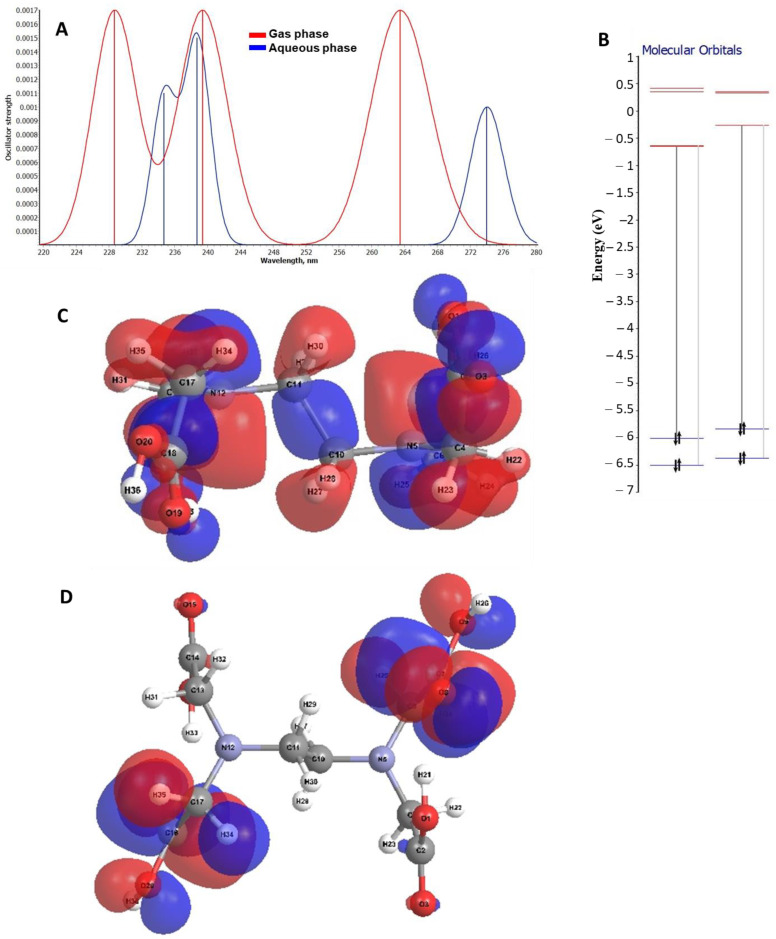
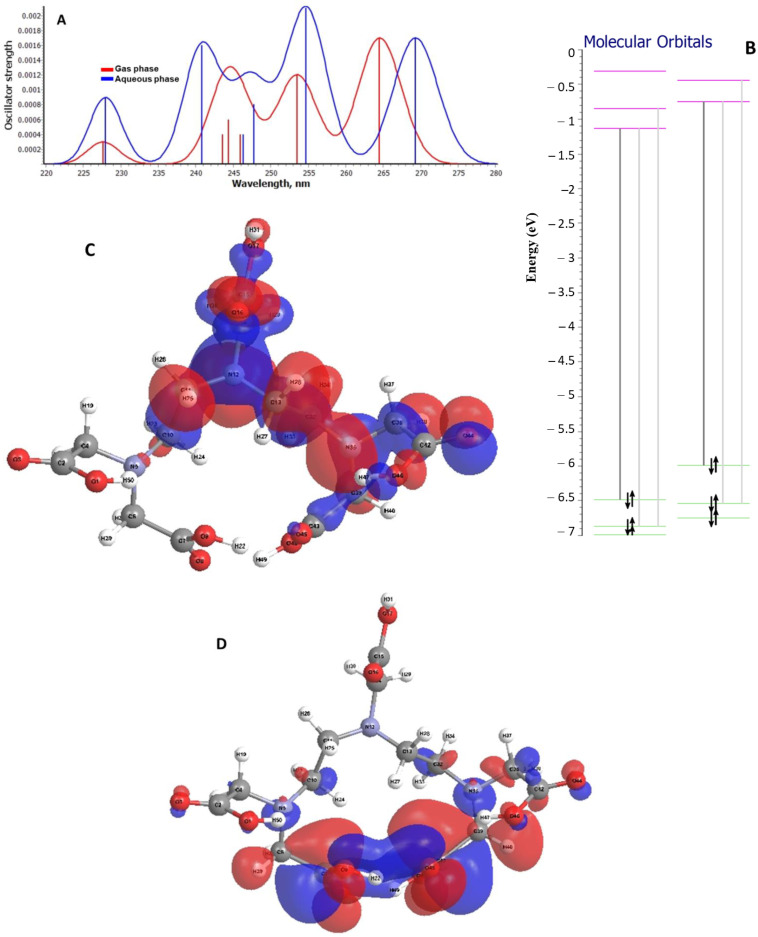
Theoretical investigations were also carried out on the electronic transitions in the EDTA and DTPA molecular structures. Theoretical electronic spectra were simulated both in the gas phase and in water. Thus, for the most stable EDTA structure, the spectral simulations were obtained using the TD-DFT procedure (Figure 12A). A shift of the electronic transitions towards lower wavelengths in solution than in the gas phase was observed. For example, HOMO → LUMO transitions are found in gas at 274 nm, whereas in solution they are found at 263.5 nm. This is due to the interaction of the studied structure with the solvent, which led to an increase in the energy of the LUMO orbital and thus the transition energy at this level. This aspect is also evident in the energetic diagram plotted to illustrate the electronic transitions between boundary levels (Figure 12B). Analyzing the composition of the boundary orbitals, it can be seen that the spectral transitions are determined by the jumping/dropping of electrons from the pz orbital located at N atoms to the px orbital of C atoms. This can also be seen from the spatial representation of the molecular boundary orbitals in Figure 12C,D.
Figure 12.
(A) Theoretical electronic spectrum for the most energetically stable EDTA conformer corresponding to both gas and aqueous phase. (B) Fragment from the energy diagram illustrating electronic transitions between boundary levels for EDTA structure: (a) gas phase; (b) aqueous phase. The spatial representation of the boundary orbitals for EDTA structure in gaseous phase (C) HOMO and (D) LUMO.
Regarding the study of electronic transitions within the DTPA molecule, it can be seen from Figure 13A that the HOMO → LUMO transition in the aqueous state is at a lower energy than that in the gas phase, the energy difference being almost 5 nm. In the aqueous phase it can be seen from the energy diagram (Figure 13B) that, following interactions with water molecules, the energy corresponding to the first vacant molecular level is lower than that in the gas phase, which also explains the lower energy of the electronic transition between the boundary levels in solution versus gas. The structure of the DTPA boundary orbitals is illustrated in Figure 13C,D. A transfer of electrons from the atomic orbitals of the atoms inside the chain to the orbitals of the marginal atoms involved in the formation of the hydrogen bond interaction can be observed (Figure 13C,D). Thus, the formation of hydrogen bonds is favored by the interaction with water molecules.
Figure 13.
(A) Simulation of the electronic spectra for the DTPA structure in both the gas and aqueous phase. (B) Fragment from the energy diagram of the molecular orbitals of the DTPA structure illustrating the first electronic transition: (a) gas phase; (b) aqueous phase. The spatial representation of boundary orbitals for DTPA structure in gaseous phase (C) HOMO and (D) LUMO.
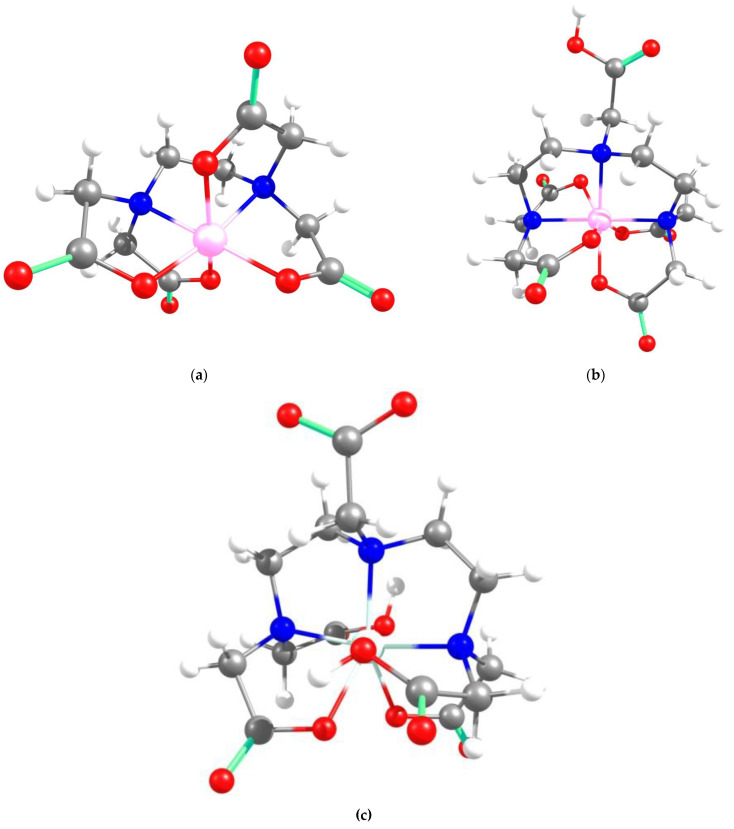
Concerning the complexation of EDTA and DTPA structures with metal ions, for the gaseous state, without taking into account that the ligand is grafted onto a CS structure, the spatial distributions of the investigated structures illustrated in Figure 14a,b were proposed. The metal ions form dative bonds with the N atom and coordinative bonds with the O atoms from the carboxyl groups at the end of the chain in the case of the DTPA structure. Depending on solution pH, some carboxyl groups may form dative bonds with the coordinated metal ions, as illustrated in Figure 14c, where a structure with DTPA as ligand and Cu as transition metal ion is represented.
Figure 14.
Proposals for the spatial representation of EDTA (a) and DTPA (b) structures investigated in coordination with transition metal ions. (c) The spatial structure for CuC14N3H19O10 with two dative bonds from two carboxyl groups.
3. Conclusions
The sorption performances in fixed-bed columns of APCA-functionalized chitosan-based composite cryogels, as microbeads, towards five-component HMI solutions containing Zn2+, Pb2+, Cd2+, Ni2+, and Co2+ was thoroughly investigated in this study in comparison to those of unmodified composite sorbents. The column filled with unmodified microbeads showed much steeper breakthrough profiles for all tested HMIs compared to the one containing EDTA-functionalized microbeads. This behavior was associated with the rapid saturation of composite sorbent’s available active sites. For the column containing CSEDTA-CPL microbeads, a clear separation of the breakthrough profiles was observed; the interaction order followed the sequence Co2+ < Zn2+ < Cd2+ ≤ Pb2+ < Ni2+, indicating that Ni2+ ions showed the highest affinity for the sorbent’s functional groups. The obtained sequence for CSEDTA-CPL microbeads has been well correlated to the sequence of the HMIs-EDTA chelates pK values.
In order to get information about the maximum sorption capacity of a sorbent in dynamic conditions and the time for 50% breakthrough, the kinetic data obtained in the fixed-bed experiments were fitted by Thomas and Yoon–Nelson models. Thus, for the column containing CSEDTA-CPL microbeads, a maximum HMIs sorption capacity of 145.55 mg/g and a 50% breakthrough time of 121.5 min were estimated, with both being much higher than for the column containing unmodified CS-CPL microbeads. In addition, the dynamic desorption experiment highlights the possibility that the microbeads investigated in this work can be cleaned of the sorbed HMIs in continuous flow set-ups, with potential for regeneration and reuse in successive sorption/desorption cycles without the need to remove the materials from the column.
Moreover, using the Gaussian and hybrid functional B3LYP program package together with the 6–31 g orbital set, the structures of EDTA and DTPA were investigated to identify the most energetically stable conformer, the interaction with a CS unit as well as their spatial structures within the complex combinations with transition metal ions. It was concluded that starting from the most stable conformer of EDTA, coordinative combinations with metal ions can be obtained with an energy consumption of only 1 kcal/mole, which is enough to shift the spatial structure into a favorable conformation for metal ion chelation. Theoretical investigations were also carried out on electronic transitions in the EDTA and DTPA molecular structures: their electronic spectra were simulated both in the gas phase and in water. Thus, for the most stable EDTA structure, a shift of the electronic transitions towards lower wavelengths in solution than in the gas phase was observed. For example, HOMO→LUMO transitions were found in gas at 274 nm, whereas in solution they were found at 263.5 nm. This is due to the interaction of the studied structure with the solvent, which led to an increase in the energy of the LUMO orbital and thus the transition energy at this level. Concerning the complexation of EDTA and DTPA structures with metal ions, for the gaseous state, it was shown that the metal ions can form dative bonds with the N atom and coordinative bonds with the O atoms from the carboxyl groups at the end of the chain. Overall, it was clearly demonstrated that the modification of CS based structures with APCA moieties is a powerful strategy to significantly improve their HMIs chelation properties, the obtained materials being highly suitable for water purification in continuous flow conditions.
4. Materials and Methods
4.1. Materials
CS-CPL and CSEDTA-CPL composites, as microbeads, were used as sorbents for removal of Zn2+, Pb2+, Cd2+, Ni2+, and Co2+ ions from multicomponent aqueous solution. The preparation of CS-CPL and CSEDTA-CPL composites has been previously published [15,25]. Briefly, CS-CPL composites chemically cross-linked by glutaraldehyde (GA), as microbeads, were prepared by a cryogenic process in three stages: (i) freezing of synthesis mixture as droplets at—196 °C using liquid nitrogen; (ii) storage in the frozen state at −18 °C for 24 h to complete CS cross-linking by GA; (iii) thawing at room temperature for 1 h and washing with MilliQ water to remove the unreacted compound; and (iv) lyophilization for 48 h, at –50 °C and 0.04 mbar. Next, a part of the CS-CPL composites was functionalized with EDTA moieties, according to a protocol already reported [25]. Pb(NO3)2 (≥99%); Zn(NO3)2·6H2O (≥99%); Ni(NO3)2·6H2O (≥97%); Cd(NO3)2·4H2O (≥99%); Co(NO3)2·6H2O (≥99%) were purchased from Fluka (Sigma-Aldrich, Steinheim, Germany) and were used as received.
4.2. Methods
4.2.1. SEM and EDX Analysis
Scanning electron microscopy (SEM) studies were performed using a Verios G4 UC (Thermo Scientific, Brno, Czech Republic) device equipped with energy dispersive X-ray (EDX) analyzer (Octane Elect Super SDD detector, Mahwah, NJ, USA). The CS-CPL and CSEDTA-CPL microbeads were coated with a 10 nm platinum layer using a Leica EM ACE200 Sputter to provide electrical conductivity and to prevent charge build-up during exposure to the electron beam. SEM analysis was carried out in high vacuum mode using a secondary electron detector (Everhart-Thornley Detector, Thermo Scientific, Brno, Czech Republic) at an accelerating voltage of 5 kV.
4.2.2. FTIR Analysis
FTIR spectra of composite microbeads, before and after HMI sorption, were recorded by a Bruker Vertex FT-IR spectrometer (Bruker, Ettlingen, Germany), at a resolution of 2 cm−1, in the range 4000–400 cm−1, by KBr pellet technique.
4.2.3. Porosity Evaluation
The porosity (P, %) of CS-CPL and CSEDTA-CPL cryogel sorbents was obtained by the liquid displacement method [27]. Thus, a certain amount of dried microbeads (0.01 g) was immersed in a fixed volume of isopropanol (V1) for 5 min. As isopropanol is a non-solvent for the CS network, it enters only into the network pores but does not interact with its components. The sorbent porosity was calculated using Equation (3):
| (3) |
where V2 is the total volume of isopropanol containing the immersed microbeads, and V3 is the volume of isopropanol after the sample removal.
4.2.4. Mean Pore Sizes Determination
The average pore sizes of all samples were obtained by Image J 1.48 v analyzing software [27]. In this regard, we measured at least 20 pores (voids) on each of three independent SEM micrographs taken for every sample.
4.2.5. Water Uptake
The water uptake was evaluated by immersing a certain amount of dried microbeads (0.01 g) in 10 mL MilliQ water over a time span of 60 min. The swollen microbeads were weighed after wiping the excess of MilliQ water using a filter paper. The water uptake (g/g) was calculated as [41]:
| (4) |
where Ww and Wd are the weights of swollen and freeze-dried microbeads, respectively.
4.2.6. BET Surface Area
The BET surface area was estimated from dynamic water vapor sorption isotherms considering a relative humidity up to 40%. The experimental data were recorded at 25 °C using an IGAsorp gravimetric analyzer (Hiden Analytical, Warrington, UK), following the protocol previously applied for xanthan-based cryogels [42].
4.2.7. Fixed-Bed Column Studies
Continuous fixed-bed sorption studies were performed using a multicomponent equimolar solution of Pb2+, Zn2+, Ni2+, Cd2+, and Co2+ (0.5 mmol/L of each metal ion) at pH 4.5 to investigate the efficacy of CS-CPL and CSEDTA-CPL beads in deep-cleaning of wastewaters in dynamic competitive conditions. The beads were packed (bed height = 5 cm) into a glass-column with the internal diameter of 1.45 cm that was connected to a Lambda Hiflow peristaltic pump (Lambda Laboratory Instruments, Brno, Czech Republic) at the top end to ensure a 1 mL/min constant flow rate of the multicomponent solution. Before passing the solution of heavy metal ions, the beads were equilibrated at pH 4.5 by passing an aqueous solution with the same pH through the column.
The effluent was automatically sampled in fractions of 10 mL using a Lambda OMNICOLL Fraction Collector (Lambda Laboratory Instruments, Brno, Czech Republic). The desorption of heavy metal ions was also studied in dynamic conditions by passing a 0.1 M HCl aqueous solution through the column at a flow rate of 1 mL/min.
The heavy metal ion concentrations in the collected samples were determined by Flame Atomic Absorption Spectrometry (FAAS) using a high-resolution ContrAA 300 Analytik Jena spectrometer (Analytik Jena, Jena, Germany) equipped with a Xenon lamp as a continuum radiation source. Measurements of each analyte were carried out in triplicate. The residual concentration of each metal ion was determined at the characteristic maximum wavelengths of 213.8 nm for Zn2+ ions, 217.0 nm for Pb2+, 228.8 nm for Cd2+, 232.0 nm for Ni2+ ions, and 240.7 nm for Co2+.
Acknowledgments
The authors gratefully thank Maria Marinela Lazar for recording the FTIR spectra and Maria Ignat for AAS measurements.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gels8040221/s1, Table S1. Parameters corresponding to Thomas and Yoon–Nelson models fitted for each HMI on CSEDTA-CPL column. Table S2 [43,44,45,46,47,48,49]. Sorption performance of various sorbents reported in the literature compared with that of our CSEDTA-CPL sorbents. Figure S1. FT-IR spectra of CS-CPL composites before and after interaction with HMIs. Figure S2. FT-IR spectra of CSEDTA-CPL composites before and after interaction with HMIs.
Author Contributions
Conceptualization, M.V.D.; methodology, M.V.D., I.H., C.A.G. and D.H.; validation, M.V.D., I.H., C.A.G. and D.H.; formal analysis, I.H., C.A.G. and D.H.; investigation, I.H., C.A.G. and D.H.; resources, M.V.D.; writing—original draft preparation, M.V.D. and C.A.G.; writing—review and editing, M.V.D.; project administration, M.V.D. and C.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
M.V.D. and C.A.G. thank the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI through the projects TE117/10.10.2018, PN-III-P2-2.1-PED-2019-1996, and PN-III-P4-ID-PCE-2020-0296.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qi X., Tong X., Pan W., Zeng Q., You S., Shen J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021;315:128221. doi: 10.1016/j.jclepro.2021.128221. [DOI] [Google Scholar]
- 2.Fan S., Liu Z., Wu Y., Zhang Y., Hu H., Huang Z., Qin Y., Liang J. 3D porous tubular network-structured chitosan-based beads with multifunctional groups: Highly efficient and selective removal of Cu2+ Int. J. Biol. Macromol. 2021;171:17–27. doi: 10.1016/j.ijbiomac.2020.12.184. [DOI] [PubMed] [Google Scholar]
- 3.Prete P., Fiorentino A., Rizzo L., Proto A., Cucciniello R. Review of aminopolycarboxylic acids–based metal complexes application to water and wastewater treatment by (photo-) Fenton process at neutral pH. Curr. Opin. Green Sustain. Chem. 2021;28:100451. doi: 10.1016/j.cogsc.2021.100451. [DOI] [Google Scholar]
- 4.Ridzwan M.H., Yaakob M.K., Zabidi Z.M., Hamzah A.S., Shaameri Z., Rashid F.N.A.A., Kassim K., Mohammat M.F., Pungot H.N., Hamali M.A.M., et al. Computational insight into the quantum chemistry, interaction and adsorption energy of aminopolycarboxylic acid chelating agents towards metal cations. Comput. Theor. Chem. 2022;1208:113579. doi: 10.1016/j.comptc.2021.113579. [DOI] [Google Scholar]
- 5.Bucheli-Witschel M., Egli T. Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol. Rev. 2001;25:69–106. doi: 10.1111/j.1574-6976.2001.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 6.Repo E., Warchoł J.K., Bhatnagar A., Mudhoo A., Sillanpää M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013;47:4812–4832. doi: 10.1016/j.watres.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Dinu M.V., Dragan E.S. Heavy metals adsorption on some iminodiacetate chelating resins as a function of the adsorption parameters. React. Funct. Polym. 2008;68:1346–1354. doi: 10.1016/j.reactfunctpolym.2008.06.011. [DOI] [Google Scholar]
- 8.Dinu M.V., Dragan E.S., Trochimczuk A.W. Sorption of Pb(II), Cd(II) and Zn(II) by iminodiacetate chelating resins in non-competitive and competitive conditions. Desalination. 2009;249:374–379. doi: 10.1016/j.desal.2009.03.016. [DOI] [Google Scholar]
- 9.Li Y., He J., Zhang K., Liu T., Hu Y., Chen X., Wang C., Huang X., Kong L., Liu J. Super rapid removal of copper, cadmium and lead ions from water by NTA-silica gel. RSC Adv. 2019;9:397–407. doi: 10.1039/C8RA08638A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z., Weng S., Li C., Jin M., Wan D. Dense and robust aminopolycarboxylic acid-decorated porous monoliths for eliminating trace Cu(II) or Zn(II) from water. Colloids Surf. A. 2020;586:124310. doi: 10.1016/j.colsurfa.2019.124310. [DOI] [Google Scholar]
- 11.Zhang K., Dai Z., Zhang W., Gao Q., Dai Y., Xia F., Zhang X. EDTA-based adsorbents for the removal of metal ions in wastewater. Coord. Chem. Rev. 2021;434:213809. doi: 10.1016/j.ccr.2021.213809. [DOI] [Google Scholar]
- 12.Badsha M.A.H., Khan M., Wu B., Kumar A., Lo I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021;408:124463. doi: 10.1016/j.jhazmat.2020.124463. [DOI] [PubMed] [Google Scholar]
- 13.Liang L., Wang J., Tong X., Zhang S. Enhanced adsorptive removal of Cr(III) from the complex solution by NTA-modified magnetic mesoporous microspheres. Environ. Sci. Pollut. Res. 2022 doi: 10.1007/s11356-022-19039-8. [DOI] [PubMed] [Google Scholar]
- 14.Fujita S., Sakairi N. Water soluble EDTA-linked chitosan as a zwitterionic flocculant for pH sensitive removal of Cu(II) ion. RSC Adv. 2016;6:10385–10392. doi: 10.1039/C5RA24175H. [DOI] [Google Scholar]
- 15.Lazar M.M., Dinu I.A., Dinu M.V. Synthesis of ethylenediminetetraacetic acid-functionalized chitosan cryogels as potential sorbents of heavy metal ions. Mater. Plast. 2021;58:155–166. doi: 10.37358/MP.21.2.5487. [DOI] [Google Scholar]
- 16.Nagib S., Inoue K., Yamaguchi T., Tamaru T. Recovery of Ni from a large excess of al generated from spent hydrodesulfurization catalyst using picolylamine type chelating resin and complexane types of chemically modified chitosan. Hydrometallurgy. 1999;51:73–85. doi: 10.1016/S0304-386X(98)00073-5. [DOI] [Google Scholar]
- 17.Repo E., Warchol J.K., Kurniawana T.A., Sillanpää M.E.T. Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA- modified chitosan: Kinetic and equilibrium modeling. Chem. Eng. J. 2010;161:73–82. doi: 10.1016/j.cej.2010.04.030. [DOI] [Google Scholar]
- 18.Inoue K., Ohto K., Yoshizuka K., Yamaguchi T., Tanaka T. Adsorption of lead(II) ion on complexane types of chemically modified chitosan. Bull. Chem. Soc. Jpn. 1997;70:2443–2447. doi: 10.1246/bcsj.70.2443. [DOI] [Google Scholar]
- 19.Ayati A., Tanhaei B., Sillanpaa M. Lead(II)-ion removal by ethylenediaminetetraacetic acid ligand functionalized magnetic chitosan–aluminum oxide–iron oxide nanoadsorbents and microadsorbents: Equilibrium, kinetics, and thermodynamics. J. Appl. Polym. Sci. 2017;134:44360. doi: 10.1002/app.44360. [DOI] [Google Scholar]
- 20.Khawar A., Aslam Z., Abbas S.J.A. Pb (II) biosorption using DAP/EDTA-modified biopolymer (Chitosan) Chem. Eng. Commun. 2018;205:1555–1567. doi: 10.1080/00986445.2018.1460598. [DOI] [Google Scholar]
- 21.Zheng L., Gao Y., Du J., Zhang W., Huang Y., Wang L., Zhao Q., Pan X. A novel, recyclable magnetic biochar modified by chitosan–EDTA for the effective removal of Pb(II) from aqueous solution. RSC Adv. 2020;10:40196. doi: 10.1039/D0RA07499C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roosen J., Binnemans K. Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers. J. Mater. Chem. A. 2014;2:1530–1540. doi: 10.1039/C3TA14622G. [DOI] [Google Scholar]
- 23.Zhao F., Repo E., Yin D., Chen L., Kalliola S., Tang J., Iakovleva E., Tam K.C., Sillanpää M. One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep. 2017;7:15811. doi: 10.1038/s41598-017-16222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma M., Lee I., Hong Y., Kumar V., Kim H. Multifunctional β-cyclodextrin- EDTA- chitosan polymer adsorbent synthesis for simultaneous removal of heavy metals and organic dyes from wastewater. Environ. Pollut. 2022;292:118447. doi: 10.1016/j.envpol.2021.118447. [DOI] [PubMed] [Google Scholar]
- 25.Dinu M.V., Humelnicu D., Lazar M.M. Analysis of copper(II), cobalt(II) and iron(III) sorption in binary and ternary systems by chitosan-based composite sponges obtained by ice-segregation approach. Gels. 2021;7:103. doi: 10.3390/gels7030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sáez P., Dinu I.A., Rodríguez A., Gómez J.M., Lazar M.M., Rossini D., Dinu M.V. Composite cryo-beads of chitosan reinforced with natural zeolites with remarkable elasticity and switching on/off selectivity for heavy metal ions. Int. J. Biol. Macromol. 2020;164:2432–2449. doi: 10.1016/j.ijbiomac.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Dinu M.V., Gradinaru A.C., Lazar M.M., Dinu I.A., Raschip I.E., Ciocarlan N., Aprotosoaie A.C. Physically cross-linked chitosan/dextrin cryogels entrapping Thymus vulgaris essential oil with enhanced mechanical, antioxidant and antifungal properties. Int. J. Biol. Macromol. 2021;184:898–908. doi: 10.1016/j.ijbiomac.2021.06.068. [DOI] [PubMed] [Google Scholar]
- 28.Fan Z., Zhang Q., Gao B., Li M., Liu C., Qiu Y. Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere. 2019;217:85–94. doi: 10.1016/j.chemosphere.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Roy D., Neogi S., De S. Adsorptive removal of heavy metals from battery industry effluent using MOF incorporated polymeric beads: A combined experimental and modeling approach. J. Hazard. Mater. 2021;403:123624. doi: 10.1016/j.jhazmat.2020.123624. [DOI] [PubMed] [Google Scholar]
- 30.Callura J.C., Shi Q., Dzombak D.A., Karamalidis A.K. Selective recovery of rare earth elements with ligand-functionalized polymers in fixed-bed adsorption columns. Sep. Purif. Technol. 2021;265:118472. doi: 10.1016/j.seppur.2021.118472. [DOI] [Google Scholar]
- 31.Pathirana C., Ziyath A.M., Egodawatta P., Bandara N.J.G.J.K., Jinadasa B.S.N., Bandala E.R., Wijesiri B., Goonetilleke A. Biosorption of heavy metals: Transferability between batch and column studies. Chemosphere. 2022;294:133659. doi: 10.1016/j.chemosphere.2022.133659. [DOI] [PubMed] [Google Scholar]
- 32.Wang W., Fan M., Ni J., Peng W., Cao Y., Li H., Huang Y., Fan G., Zhao Y., Song S. Efficient dye removal using fixed-bed process based on porous montmorillonite nanosheet/poly(acrylamide-co-acrylic acid)/sodium alginate hydrogel beads. Appl. Clay Sci. 2022;219:106443. doi: 10.1016/j.clay.2022.106443. [DOI] [Google Scholar]
- 33.Thomas H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944;66:1664–1666. doi: 10.1021/ja01238a017. [DOI] [Google Scholar]
- 34.Yoon Y.H., Nelson J.H. Appication of gas adsorption kinetics I. A theoretical model for respirator cartridge service life. Am. Ind. Hyg. Assoc. 1984;45:509–516. doi: 10.1080/15298668491400197. [DOI] [PubMed] [Google Scholar]
- 35.Queiroz M.F., Melo K.R.T., Sabry D.A., Sassaki G.L., Rocha H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs. 2015;13:141–158. doi: 10.3390/md13010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghiorghita C.-A., Dinu M.V., Dragan E.S. Burst-free and sustained release of diclofenac sodium from mesoporous silica/PEI microspheres coated with carboxymethyl cellulose/chitosan layer-by-layer films. Cellulose. 2022;29:395–412. doi: 10.1007/s10570-021-04282-y. [DOI] [Google Scholar]
- 37.Dragan E.S., Dinu M.V., Timpu D. Preparation and characterization of novel composites based on chitosan and clinoptilolite with enhanced adsorption properties for Cu2+ Biores. Technol. 2010;101:812–817. doi: 10.1016/j.biortech.2009.08.077. [DOI] [PubMed] [Google Scholar]
- 38.Becke A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98:5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- 39.Lee C., Yang W., Parr R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- 40.Stephens P.J., Devlin F.J., Chabalowski C.F., Frisch M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994;98:11623–11627. doi: 10.1021/j100096a001. [DOI] [Google Scholar]
- 41.Dinu M.V., Lazar M.M., Dragan E.S. Dual ionic cross-linked alginate/clinoptilolite composite microbeads with improved stability and enhanced sorption properties for methylene blue. React. Funct. Polym. 2017;116:31–40. doi: 10.1016/j.reactfunctpolym.2017.05.001. [DOI] [Google Scholar]
- 42.Raschip I.E., Dinu M.V., Fifere N., Darie-Nita R., Pamfil D., Popirda A., Logigan C. Thermal, mechanical and water sorption properties of xanthan-based composite cryogels. Cellul. Chem. Technol. 2020;54:915–924. doi: 10.35812/CelluloseChemTechnol.2020.54.88. [DOI] [Google Scholar]
- 43.Li Z., Wang L., Meng J., Liu X., Xu J., Wang F., Brooks P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018;344:1–11. doi: 10.1016/j.jhazmat.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen T.C., Loganathan P., Nguyen T.V., Vigneswaran S., Kandasamy J. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem. Eng. J. 2015;270:393–404. doi: 10.1016/j.cej.2015.02.047. [DOI] [Google Scholar]
- 45.Šabanović E., Memić M., Sulejmanović J., Selović A. Simultaneous adsorption of heavy metals from water by novel lemon-peel based biomaterial. Polish J. Chem. Technol. 2020;22:46–53. doi: 10.2478/pjct-2020-0007. [DOI] [Google Scholar]
- 46.Kemik Ö.F., Ngwabebhoh F.A., Yildiz U. A response surface modelling study for sorption of Cu2+, Ni2+, Zn2+ and Cd2+ using chemically modified poly(vinylpyrrolidone) and poly(vinylpyrrolidone-co-methylacrylate) hydrogels. Adsorp. Sci. Technol. 2017;3:263–283. doi: 10.1177/0263617416674950. [DOI] [Google Scholar]
- 47.Joseph I.V., Tosheva L., Doyle A.M. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020;8:103895. doi: 10.1016/j.jece.2020.103895. [DOI] [Google Scholar]
- 48.Fato P.F., Li D.W., Zhao L.J., Qiu K., Long Y.T. Simultaneous Removal of Multiple Heavy Metal Ions from River Water Using Ultrafine Mesoporous Magnetite Nanoparticles. ACS Omega. 2019;4:7543–7549. doi: 10.1021/acsomega.9b00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humelnicu D., Lazar M.M., Ignat M., Dinu I.A., Dragan E.S., Dinu M.V. Removal of heavy metal ions from multi-component aqueous solutions by eco-friendly and low-cost composite sorbents with anisotropic pores. J. Hazard. Mater. 2020;381:120980. doi: 10.1016/j.jhazmat.2019.120980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.