Tuberculosis (TB) is still one of the top 10 causes of death in low and lower-middle income countries [1]. TB's long and complex treatment, side-effects, and development of resistant bacteria compromise treatment success. To improve treatment outcomes, therapeutic drug monitoring (TDM) has been included in TB treatment guidelines [2–4] to be considered for specific situations in which there is documented or expected poor response to treatment, drug toxicity, or a lower drug concentration. Several strategies for implementation of TDM for programmatic use have been proposed to overcome barriers to widespread use of TDM [5, 6], including more accessible techniques such as dried blood spot analysis or saliva and urine testing [7], but uptake in programmatic care is still limited [8].
Short abstract
Survey responses indicate that there is concern surrounding cost-effectiveness and the resources available in different settings to implement therapeutic drug monitoring in TB. Robust research is needed to better inform of the potential long-term benefits. https://bit.ly/34PFSfd
To the Editor:
Tuberculosis (TB) is still one of the top 10 causes of death in low and lower-middle income countries [1]. TB's long and complex treatment, side-effects, and development of resistant bacteria compromise treatment success. To improve treatment outcomes, therapeutic drug monitoring (TDM) has been included in TB treatment guidelines [2–4] to be considered for specific situations in which there is documented or expected poor response to treatment, drug toxicity, or a lower drug concentration. Several strategies for implementation of TDM for programmatic use have been proposed to overcome barriers to widespread use of TDM [5, 6], including more accessible techniques such as dried blood spot analysis or saliva and urine testing [7], but uptake in programmatic care is still limited [8].
To understand current use and barriers and facilitators to implementing TDM in TB management, we performed an electronic survey, which can be found at this webpage: https://is.gd/TDMSurvey
The survey consisted of six sections asking participants if and how TDM is used in their setting. Several questions had multiple-choice answers. The survey was piloted by three of the co-authors (G.B. Migliori, D. Goletti and C.A. Peloquin) and it took on average 15 min to complete. The survey was built in RedCap Systems and distributed through the Global Tuberculosis Network (160 email addresses), the International Association for Therapeutic Drug Monitoring and Clinical Toxicology (638 email addresses), and the Global Drug-resistant TB Initiative members (400 email addresses).
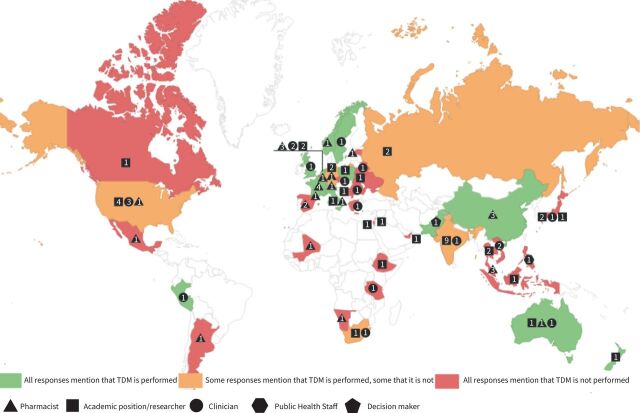
Responses were received from 86 participants, spanning 46 countries across all seven continents (figure 1).
FIGURE 1.
Map of experts’ responses. Numbers represent the number of replies for each category from a specific country. For participants with multiple roles, the first chosen is represented on the map.
Participants worked as clinicians (19; 22%), pharmacists (31; 36%), researchers (34; 40%) and public health staff (8; 9%). Half worked as academic professionals (43; 50%). By type of medical facility, most participants worked in a university hospital (36; 42%), followed by TB expert centre (22; 26%), general hospital (18; 21%), private hospital (9; 10%), infectious diseases centre (8; 9%) and outpatient clinic (6; 7%), with several working in more than one facility (12; 13.9%). A majority answered on behalf of their institution only (60; 70%), with 11 (13%) and 15 (17%) responding for region and country, respectively.
TDM was performed by 43 out of the total of 86 survey participants (50%), within a total of 18 countries. Those who undertook TDM indicated the following reasons for initiation: gastro-intestinal abnormalities were the most common indication (24/43; 56%), followed by chronic kidney disease, cirrhosis and HIV positivity (22/43; 51%), acute kidney injury (20/43; 47%), diabetes mellitus (19/43; 44%), pregnancy (18/43; 42%), malnutrition (17/43; 40%), and age <18 years (9/43; 21%). Two participants mentioned that all patients were eligible for TDM and one included “COVID-19” as a comorbidity.
Approximately half of all participants reported performing TDM for all TB patients irrespective of drug susceptibility (18/43; 42%), followed by performing TDM if resistant to rifampicin and/or other first-line agents (7/43; 16%), or if there was multidrug-resistant TB (3/43; 7%) or extensively drug-resistant TB (1/43; 2%), or applying additional criteria such as slow-response or rapid relapse (3/43; 7%) or other, undisclosed criteria (11/43; 26%).
Considering drugs for which TDM is performed, 12/43 (28%) performed TDM on all first-line drugs and 27/43 (63%) performed on at least rifampicin. The second most frequent drug class analysed was aminoglycosides (17; 40%), followed by linezolid (16/43; 37%), fluoroquinolones (12/43; 27%), carbapenems (11/43; 26%), bedaquiline and cycloserine (9/43; 21%).
TDM was most often performed in pharmacology laboratories (21/43; 49%), followed by university laboratories (7/43; 16%), microbiology laboratories (4/43; 9%); public health institutions (4/43; 9%), private laboratories (4/43; 9%) and chemistry laboratories (3/43; 7%). Sampling strategies varied, with 13 (30%) performing peak and trough concentrations, 12 sampling at different time points for each drug (28%), and some collecting a full pharmacokinetic curve (6/43; 14%). The samples most often utilised for TDM were plasma and serum (20/43; 47%), with a few participants reporting they performed TDM on dried blood spot samples (3/43; 7%), saliva or cerebro-spinal fluid (2/43; 5%). More than half of responses (24; 56%) indicated that drug assays were validated according to US Food and Drug Administration, Clinical and Laboratory Standards Institute or European Medicines Agency guidelines.
Participants indicated that TDM results were received in under 5 days (15/43; 35%), in 5–10 days (9/43; 21%), or more than 10 days (3/43; 7%) and that they would use TDM results as advice for dose adjustment (25/43; 58%), changing the drug (12/43; 28%), or change in route of administration (11/43; 26%).
Participants utilised a variety of guidelines to perform TDM: national guidelines (10/43; 23%), specific articles (9/43; 21%), international guidelines (8/43; 19%) and facility guidelines (5/43; 12%).
TDM was most often paid for by public healthcare or health insurance (21/43; 49%). Alternative sources of funding were TB-specific research grants (6/43; 14%), hospital funds (6/43; 14%) or the patients themselves (5/43; 12%).
The following responses arose from questions open to all 86 participants, regardless of whether or not they performed TDM. Concerning costs, approximately half the participants considered TDM to be cost-effective (39/86; 45%), with some (8/86; 9%) stating it depended on several factors, such as long-term dosing, length of hospital stay, and choosing the right patients for TDM. A minority considered TDM not cost-effective (8/86; 9%) and the rest responded that they do not know the answer to this question (31/86; 36%). Furthermore, participants would predominantly spend a maximum of EUR 20 (14/86; 16%) or EUR 10 (14/86; 16%) per assay, with some reporting they would go up to EUR 50 (5/86; 6%) or even EUR 100 (5/86; 6%).
Participants reported the main barriers to TDM usage to be a lack of knowledge among medical staff (32/86; 37%), followed by lack of funding and lack of guideline usage (30/86; 35%), no political/governmental will (17/86; 20%), TDM implementation costs being too high (11/86; 13%), and TDM implementation too complicated (6/86; 7%). One participant specifically mentioned that “many clinicians don't necessarily agree with TDM”, and another explained that the workload is very high as it is, without introducing a new technique.
Participants indicated training efforts should focus on clinical/laboratory staff (29/86; 34%), then educate stakeholders (26/86; 30%) and, finally, hospital administrators (18/86; 22%).
The present study is the first to investigate TDM use in TB through a worldwide survey.
Our survey showed that progress in TDM is being made, with half of the survey participants using TDM. Cheaper and easy to use assays [9–11], clinical guidelines [2–4] and laboratory quality control standards [12] are all increasing in availability. However, the main challenge is a lack of widespread information about TDM, with progress being recognised by participants who look towards guidelines and research when performing TDM.
Half of our responders work in an academic field, indicating that responses arrived from experts already using or interested in using the technique. One potential limiting factor is that even through choosing dissemination networks relevant to the field, we might not have captured a representative sample of the target population of potential TDM users.
Responses to our survey indicate that there is concern surrounding cost-effectiveness and the resources available in different settings to implement TDM. Robust research in diverse settings is needed through high-quality studies that investigate and can better inform stakeholders of the potential long-term cost-savings TDM can provide [13].
The electronic survey is open for further data collection and, if you would like to contribute towards further research in this field, you are invited to complete it: https://is.gd/TDMSurvey
Shareable PDF
Acknowledgements
This project is part of the scientific activities of the Global Tuberculosis Network and of the WHO Collaborating Centre for TB and Lung Diseases, Tradate, Italy.
Footnotes
Conflict of interest: The authors disclose no potential conflicts of interest.
Support statement: I. Margineanu is funded by a doctoral project from the European Union Horizon 2020 research and innovation programme, under the Marie-Skłodowska Curie grant agreement 713660. The funding source had no impact on any decision-making regarding this paper. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1. www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death World Health Organization. The Top 10 Causes of Death. Date last accessed: 15 May 2021. Date last updated: 9 December 2020.
- 2.Nahid P, Dorman SE, Alipanah N, et al. . Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63: e147–e195. doi: 10.1093/cid/ciw376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahid P, Mase SR, Migliori GB, et al. . Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med 2019; 200: e93–e142. doi: 10.1164/rccm.201909-1874ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO Operational Handbook on Tuberculosis: Module 4: Treatment: Drug-resistant Tuberculosis Treatment. Geneva, World Health Organization, 2020.
- 5.Ghimire S, Bolhuis MS, Sturkenboom MGG, et al. . Incorporating therapeutic drug monitoring into the World Health Organization hierarchy of tuberculosis diagnostics. Eur Respir J 2016; 47: 1867–1869. doi: 10.1183/13993003.00040-2016 [DOI] [PubMed] [Google Scholar]
- 6.van der Burgt EPM, Sturkenboom MG, Bolhuis MS, et al. . End TB with precision treatment! Eur Respir J 2016; 47: 680–682. doi: 10.1183/13993003.01285-2015 [DOI] [PubMed] [Google Scholar]
- 7.Alffenaar J-WC, Heysell SK, Mpagama SG. Therapeutic drug monitoring: the need for practical guidance. Clin Infect Dis 2019; 68: 1065–1066. doi: 10.1093/cid/ciy787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HY, Heysell SK, Mpagama S, et al. . Challenging the management of drug-resistant tuberculosis. Lancet 2020; 395: 783. doi: 10.1016/S0140-6736(20)30049-0 [DOI] [PubMed] [Google Scholar]
- 9.Kim HY, Ruiter E, Jongedijk EM, et al. . Saliva-based linezolid monitoring on a mobile UV spectrophotometer. J Antimicrob Chemother 2021; 76: 1786–1792. doi: 10.1093/jac/dkab075 [DOI] [PubMed] [Google Scholar]
- 10.Szipszky C, Van Aartsen D, Criddle S, et al. . Determination of rifampin concentrations by urine colorimetry and mobile phone readout for personalized dosing in tuberculosis treatment. J Pediatric Infect Dis Soc 2021; 10: 104–111. doi: 10.1093/jpids/piaa024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mave V, Kadam D, Gaikwad S, et al. . Measuring TB drug levels in the hair in adults and children to monitor drug exposure and outcomes. Int J Tuberc Lung Dis 2021; 25: 52–60. doi: 10.5588/ijtld.20.0574 [DOI] [PubMed] [Google Scholar]
- 12.Aarnoutse RE, Sturkenboom MGG, Robijns K, et al. . An interlaboratory quality control programme for the measurement of tuberculosis drugs. Eur Respir J 2015; 46: 268–271. doi: 10.1183/09031936.00177014 [DOI] [PubMed] [Google Scholar]
- 13.Kim HY, Ulbricht E, Ahn YK, et al. . Therapeutic drug monitoring practice in patients with active tuberculosis: assessment of opportunities. Eur Respir J 2021; 57: 2002349. doi: 10.1183/13993003.02349-2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02787-2021.Shareable (382.1KB, pdf)