Abstract
Galactomannan and its degradation products have been gaining attention based on their possible means for improving the natural defense of the host through modulation of the bacterial population in the gut. Herein, incomplete degradation products of galactomannan (IDPG) was supplemented into the diet of aged laying hens to investigate the efficacy of IDPG on the gut microbiome. Four treatments with six replicates of twelve 68-wk-old laying hens (Hy-Line variety brown) each were fed a basal diet supplemented with 0%, 0.01%, 0.025%, and 0.05% IDPG for 8 wk. Results showed that the propionate concentration significantly increased in laying hens fed a diet supplemented with 0.025% or 0.05% IDPG relative to the control diet (P < 0.05). Moreover, the results of 16S rRNA gene sequencing revealed that there was a notable elevation of microbiome species diversity due to the addition of IDPG, with a noted enrichment to phyla Bacteroidetes at the expense of Firmicutes and Proteobacteria. Metabolic prediction of the cecal microbiome suggested significant improvements to carbohydrate and lipid metabolism and a significant depletion for energy metabolism and infectious diseases. More importantly, a strong positive correlation between levels of genera Bacteroides, Rikenellaceae_RC9_gut_group, and Prevotellaceae_UCG-001 with high production of propionate was found using multivariate analysis. Our study demonstrated that IDPG acted by mainly enriching the phyla Bacteroidetes in the cecum, increasing species diversity, and cecal propionate concentrations. It seems that IDPG can be used as feed additives in laying hen farming due to its capacity to positively modulate the cecal microbiome and aid improve overall health.
Keywords: Bacteroidetes, cecal, incomplete degradation products of galactomannan, laying hen, propionate
Lay Summary
The health and nutritional status of poultry are largely interconnected with the gut microbiome, which directly or indirectly affects gut morphology, nutrition, and immune responses. Dietary fiber is resistant to digestion in the small intestines of monogastric animals but is completely or partially fermented in the distal gut, thus it is understood that they could stimulate gut health. Incomplete degradation products of galactomannan (IDPG) is an important member of the dietary fiber family of molecules, however, there exists scant research on their beneficial effects on human or animal health. Our study demonstrated that IDPG acted by mainly enriching the phyla Bacteroidetes in the cecum, which are common bacteria in the gut that are involved in the fermentation of carbohydrates. Thereafter, the enriched phyla Bacteroidetes produced propionate and reduced the abundance of phyla Firmicutes and Proteobacteria by competitive inhibition. IDPG has also increased species diversity and enhanced the stability of intestinal flora, thereby exhibiting excellent prebiotic activity.
Herein, the study of incomplete degradation products of galactomannan (IDPG) on the gut microbiome of laying hens will lay a foundation for the application of IDPG on other animals and even as a health care product in the future.
Introduction
Microbial communities in the gastrointestinal tract greatly influence various biological functions of a given host. Examples of such functions include maintaining host health, improving performance, and ensuring food and animal products’ safety (Chung et al., 2012; Mohd Shaufi et al., 2015; Sekirov et al., 2018). In the gastrointestinal tract, there are more microbes in the cecum than in the small intestine and colon, although all three have similar roles in digestion and absorption of nutrients (Mohd Shaufi et al., 2015). Each family of microbes is functionally interdependent, with an example being how certain indigestible fibers pass un-degraded from the small intestine into the ceca those microorganisms produce short-chain fatty acids (SCFA: mainly acetic acid, propionic acid, and butyric acid) and ammonia. Each of these ceca fermentation products is then absorbed by the intestinal epithelium to the host, a mechanism similar to the rumen (Li et al., 2017). As a result, analysis of the cecal microbiome represents a pivotal area of poultry nutrition research, which will lead to a better understanding of cecal microbial biodiversity and their interactions with the host. To our knowledge, some additives, including insect (Borrelli et al., 2017), probiotic (Brzóska et al., 2012), and dietary fiber (Cetin et al., 2005), may have regulating effects on gut microbiomes of grown livestock. Among these examples, dietary fibers can beneficially affect the host by selectively stimulating the growth or activity of certain bacterial species inhabiting the digestive tract without being digested (Patterson and Burkholder, 2003). Therefore, they were thought to be ideal feed additives.
Recently, as an important member of dietary fiber, galactomannan (GM) has garnered interest not only due to its outstanding immuno-enhancing activity but also due to its ability to modify the gut microbiota population profile in a positive sense (Gamal-Eldeen et al., 2006; Hernandez et al., 2011; Shtriker et al., 2018). Based on the consensus that a well-balanced cecal microflora is important for physical health (Anderson et al., 2000), galactomannan maintains this balance by reducing the load of pathogenic bacteria and enhancing the growth of beneficial bacteria through different mechanisms. It has been reported that galactomannan reduces attachment of Gram-negative bacteria such as Escherichia coli with intestinal mucosa through a mechanism involving binding bacterial FimH of type-1 fimbriae. The cause of this binding was found to be a high bacterial affinity for mannose residues on intestinal mucosa (Fernandez et al., 2002; Hooge, 2004). Moreover, studies have shown that galactomannan enhances the number of beneficial bacteria, such as Lactobacillus and Bifidobacteria spp. (McLaughlin et al., 2015). Notably, galactomannan and its degradation products induced the abundance of one specific identified species, Bacteroides ovatus, a well-known mannan fermenter (Bagenholm et al., 2017; Park et al., 2018). More importantly, the increased production of SCFA caused by probiotics induced by galactomannan and its degradation products, in which SCFA are involved in cell proliferation in the intestinal mucosa, reduced the pH of the brush border microenvironment and block the adhesion of pathogens (Lan et al., 2004; Reichardt et al., 2018). Furthermore, Zartl et al. (2018) found that the low molecular weight galactomannan has a better effect on inducing probiotics growth than high molecular weight galactomannan. This suggests that it may be necessary to shorten long-chain galactomannan prior to the investigation of galactomannan effect on gut microbial composition.
In nature, galactomannan is diffusely found in many plants or plant derivatives, such as locust bean (Pinheiro et al., 2011), Sesbania species (Pollard et al., 2011), and coffee grounds (Gu et al., 2020). Herein, we put our attention on the galactomannan from Sesbania cannabina seed and obtained incomplete degradation products of galactomannan (IDPG) through β-endo-mannanase hydrolysis (Tao et al., 2020b). After hydrolysis, the average molecular weight of IDPG was approximately one-tenth of the original galactomannan (342 to 20,000 Da). Previous results showed that IDPG supplementation significantly increases egg production and decreases feed conversion ratio (P < 0.05; Tao et al., 2021). Hassanein and Soliman (2010) suggested that the establishment of useful bacterial colonies could improve the nutrient digestibility in the intestine, thereby decreasing the value of feed conversion ratio. Herein, we aimed to identify the shifts in microbiota composition as well as SCFA production induced by IDPG to make certain beneficial effects on gastrointestinal health. It is our hope to fill the gap in galactomannan and its degradation products in poultry farming and provide a practical basis for further research on the relationship between galactomannan and microbial community in other animals or humans.
Materials and Methods
The experimental protocols used in this experiment, including animal care and use, were reviewed and approved by the Animal Care and Use Ethics Committee of Nanjing Forestry University (Nanjing, China).
Preparation of IDPG
IDPG was prepared from enzymatic hydrolysis of S. cannabina seeds. The S. cannabina seeds of 50 kg used in this experiment were purchased from a local farm in Yancheng city, Jiangsu province of China in 2020. At first, ground (Mini plant shredder F2102, Taisite instrument Co., Ltd., Tianjin, China) S. cannabina seeds were suspended into distilled water to reach a galactomannan concentration of 40 g/L, the pH of the solution was adjusted to 4.8 with 0.05 M citric acid buffer and then treated with β-mannanase (20 U/g galactomannan, 72 h, 50 °C). Endo-β-mannanase (EC 3.2.1.78) was prepared from Trichoderma reesei Rut C-30 using avicel as a substrate. Enzymes were deactivated (10 min, 100 °C) after the incubation period, and then the suspensions were centrifuged (16,465 × g, 10 min) with the resultant supernatants being referred to as IDPG solution. Finally, solid IDPG was prepared using a spray dryer (BUCHI, Flawil, Switzerland). The content of IDPG in the solid was 45.96% determined by a sulfuric acid hydrolysis method and high-performance anion-exchange chromatography with pulsed amperometric detection using mannose and galactose as an external standard. The content of protein and ash was 12.05% and 6.72%, respectively. The protein content of GM was monitored using the Bio-Rad protein assay with bovine serum albumin as a standard (Bradford, 1976). The ash content of GM was analyzed according to AACC-Methods 08-01.01 (Anonymous, 2000). These compositional analysis methods of the solid were in line with the work of Tao et al. (2020a).
Animal care and experimental design
A total of 288 laying hens (68-wk-old, Hy-Line variety brown) were randomly distributed into 4 dietary treatments consisting of 6 replicates (cages) with 12 birds per replicate and fed a basal diet supplemented with 0% (control group), 0.01%, 0.025%, and 0.05% IDPG for 8 wk, respectively. Ingredient composition and nutrient content of the basal diet are given in Table 1. In an environmentally controlled house, birds were allowed free access to water and mash feed in 3-level cages (120 cm × 60 cm ×50 cm; 0.09 m2 per chick) with controlled ventilation and lighting (16L:8D). The laying hens were fed twice per day (6:00 a.m. and 3:00 p.m.) and feed was mixed in the trough to ensure total consumption by the laying hens. According to the feeding situation of the previous day, the feeding amount for each new day was appropriately adjusted to ensure that there was no remaining feed in the trough each night. After 2 wk of preliminary testing, a formal experiment was carried out over 8 wk.
Table 1.
Composition and nutrient levels of basal diets (as-fed basis)
| Ingredients | Content, % | Nutrient levels1 | |
|---|---|---|---|
| Corn | 63.50 | Apparent metabolizable energy, MJ/kg | 11.16 |
| Soybean meal | 18.80 | Crude protein, % | 15.37 |
| Fish meal | 1.50 | Calcium, % | 3.79 |
| Rapeseed meal | 2.00 | Total phosphorus, % | 0.64 |
| Corn gluten meal | 1.20 | ||
| Soybean phospholipid | 1.00 | ||
| Limestone | 9.17 | ||
| Dicalcium phosphate | 1.40 | ||
| dl-Methionine | 0.10 | ||
| Sodium chloride | 0.33 | ||
| 1% Premix2 | 1.00 | ||
| Total | 100 |
Nutrient levels were the calculated values.
1% Premix was provided by Huamu Institute of Animal Science and Technology, and provided per kilogram of diet: vitamin A (transretinyl acetate), 1.08 × 104 IU; vitamin D3 (cholecalciferol), 2.7 × 103 IU; vitamin E (all-rac-α-tocopherol), 27 mg; menadione, 0.84 mg; thiamin, 0.72 mg; riboflavin, 5.4 mg; nicotinamide, 9 mg; calcium pantothenate, 36 mg; pyridoxine·HCl, 2.7 mg; biotin, 0.09 mg; folic acid, 0.24 mg; vitamin B12 (cobalamin), 0.009 mg; Fe (from ferrous sulfate), 100 mg; Cu (from copper sulfate), 8.0 mg; Mn (from manganese sulfate), 100 mg; Zn (from zinc oxide), 100 mg; I (from calcium iodate), 0.9 mg; Se (from sodium selenite), 0.3 mg.
Sample collection
At 76 wk of age, a total of 24 birds from all treatments were randomly selected (1 bird per replicate) and euthanized by cervical dislocation and then necropsied. Their entire gastrointestinal tracts were rapidly removed and placed on a chilled stainless steel tray. Cecal samples were quickly removed aseptically, the content of which was collected and divided into two portions. Each portion was then rapidly frozen in liquid nitrogen and stored at −80 °C until further analysis.
Determination of the concentration of SCFA and lactate in the cecum
The concentration of SCFA and lactate in cecal content samples of laying hens was measured by high-performance liquid chromatography (HPLC). Sample (0.10 ± 0.01 g) was mixed with 2 mL deionized water by a vortex meter (Vortex 1, IKA-Werke GmbH & CO. KG, Staufen, Germany) for 10 min. The resultant mixture was centrifuged at 16,465 × g for 30 min, and the obtained supernatant was diluted to be in the range of calibration, filtered through a 0.22 μm nylon filter, and then 10 μL of which was injected into the HPLC. The HPLC was equipped with a Bio-Rad Aminex HPX-87H column (7.8 mm × 300 mm) in combination with a Bio-Rad (Cat. No. 125-0139) guard column. The mobile phase of 5 mmol/L H2SO4 ran at 0.6 mL/min under 55 °C. External standards such as lactic acid, acetic acid, propionic acid, and butyric acid used for instrument calibration were sourced from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Quantification data and chromatograms were collected and analyzed with Agilent analysis system. The concentrations of cecal SCFA were calculated as follows:
Total bacterial genomic DNA extraction and 16S rDNA sequencing
For 16S rRNA gene sequencing, the total genomic DNA of each sample was extracted using the HiPure Stool DNA Kits (Magen, Guangzhou, China) according to the manufacturer’s protocols. Thereafter, the 16S rDNA V3 to V4 hypervariable regions of ribosomal RNA gene were amplified using polymerase chain reaction (PCR) containing primers (341F: 5’-CCTACGGGNGGCWGCAG-3’; 806R: 5’-GGACTACHVGGGTATCTAAT-3’; Guo et al., 2017), where the barcode was an eight-base sequence unique to each sample. The applied reaction conditions were as follows: 95 °C (2 min), followed by 27 cycles of 98 °C (10 s), 62 °C (30 s), and 68 °C (30 s), with a final extension at 68 °C (10 min). PCR reactions were performed in triplicate 50 μL mixture containing 5 μL of 10 × KOD buffer, 5 μL of 2.5 mM dNTPs, 1.5 μL of each primer (5 μM), 1 μL of KOD polymerase, and 100 ng of template DNA.
PCR products were purified from 2% agarose gels using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions. The DNA amplicon concentration of each sample was quantified using the ABI StepOnePlus Real-Time PCR System (Life Technologies, Foster City, CA, USA). Finally, the purified amplicons were pooled in equimolar and paired-end sequenced (2 × 250) on an Illumina platform according to the standard protocols.
Tag and operational taxonomic unit assessment
To get high-quality and clean reads, FASTP was employed to further filter the raw reads according to the following rules removing reads containing more than 10% of unknown nucleotides (N) and less than 60% of bases with quality (Q-value)>20. Next, the paired-end clean reads were merged as raw tags using FLASH (Magoč and Salzberg, 2011; version 1.2.11) with a minimum overlap of 10 bp and mismatch error rates of 2%. Subsequently, noisy sequences of the obtained raw tags were filtered by Quantitative Insights Into Microbial Ecology (QIIME; version 1.9.1) pipeline under specific filtering conditions to obtain high-quality clean tags (Caporaso et al., 2010; Bokulich et al., 2013). Clean tags were next evaluated for the presence of chimeric amplification using the UCHIME algorithm, and the determined chimeric tags were removed to ultimately unlock effective tags. Effective tags were clustered into operational taxonomic units (OTUs) of ≥97% similarity using UPARSE (Edgar, 2013) pipeline. In addition, the tag sequences with the highest abundances were selected as representative sequences within each cluster. Based on that, between groups, Venn analysis was performed using R project (version 3.4.1) to identify unique and common OTUs. Moreover, the alpha-diversity index of Chao1, Simpson, and Shannon indices were calculated in QIIME, and the beta diversity was estimated using weighted and unweighted UniFrac distance matrix generated by GUniFrac package (version 1.0) in R project. Statistical analysis of ANOSIM test was calculated using R project.
Taxonomic abundance profiling
A naive Bayesian model was used to classify the representative sequences into organisms using Ribosomal Database Project classifier (Wang et al., 2007; version 2.2) based on SILVA database (Pruesse et al., 2007) with the confidence threshold values ranging from 0.8 to 1.
Molecular functional enrichment prediction
The Kyoto Encyclopedia of Genes and Genomes pathway analysis of the OTUs was inferred using Tax4Fun (Aßhauer et al., 2015; version 1.0). Heat maps for the differentiated pathways were generated.
Multivariate analysis
Multivariate analyses were conducted to study the correlation between host cecal SCFA and lactate concentrations and microbial composition at the genus level by canonical correspondence analysis (CCA) using CANOCO 4.5 software (ter Braak, 1988). A total of 4 environmental variables (lactate, acetate, propionate, and butyrate) and 24 OTUs corresponding to the genus level were included in the CCA. A heat map based on the Pearson correlation test was used to assess the eventual association between the amount of key bacterial species and SCFA levels.
Statistical analysis
Data were analyzed by ANOVA using SPSS (2008) statistical software (Ver.16.0 for windows, SPSS Inc., Chicago, IL, USA). Differences in means among treatment groups were separated using Tukey’s multiple range test. P-values less than 0.05 were considered indicative of statistical significance.
Results
Effect of IDPG on cecal SCFA and lactate contents
Until the end of the experiment, 288 chickens survived healthily, and then the cecal SCFA and lactate were measured. As seen from Table 2, acetate is the main component in the cecum of laying hens, followed by butyrate, and finally propionate. Laying hens fed diets containing IDPG showed lower cecal lactate and acetate contents than those fed no IDPG. However, this difference was not statistically significant. In contrast, compared with the control group, the chickens fed a diet supplemented with IDPG (0.025% and 0.05%) showed a significant increase in cecal propionate (P < 0.05). Nevertheless, there was no obvious difference in the cecal butyrate contents between the IDPG-supplemented group and the control group. Concerning total SCFA, there was no significant difference between the varied IDPG dosages (0.025% and 0.05%) and the control group.
Table 2.
Contents of cecal short-chain fatty acids and lactate in laying hens fed diets supplemented with different doses of incomplete degradation products of galactomannan
| Organic acid | IDPG level, % | SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.025 | 0.05 | |||
| Lactate, mg/g cecum | 2.56 | 0.68 | 2.10 | 1.26 | 0.40 | 0.114 |
| Acetate, mg/g cecum | 22.09 | 17.49 | 20.17 | 21.09 | 1.17 | 0.172 |
| Propionate, mg/g cecum | 1.92b | 2.18b | 3.59a | 3.99a | 0.43 | 0.001 |
| Butyrate, mg/g cecum | 6.46 | 6.20 | 6.91 | 7.75 | 0.36 | 0.276 |
| Total SCFA, mg/g cecum | 30.47a | 25.88b | 30.67a | 32.84a | 1.69 | 0.006 |
Different superscript letters in the same row differ significantly (P < 0.05).
Tag and OTU-based analysis
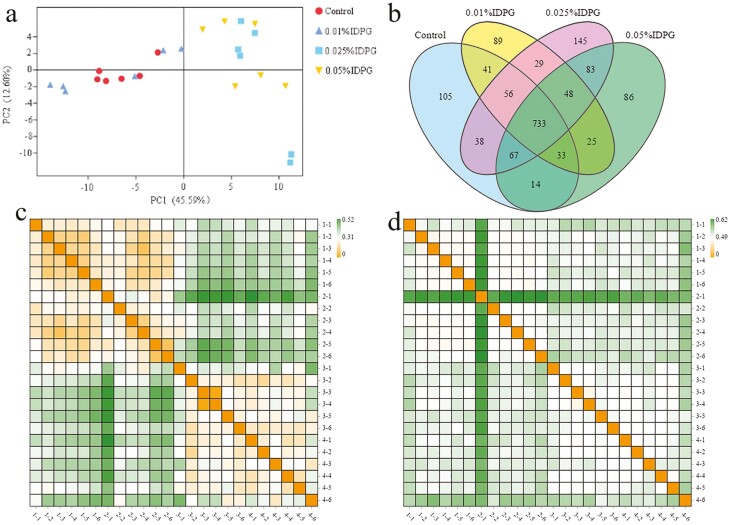
The cecal microbiota from groups supplemented with 0.025% and 0.05% IDPG were divided into two intersecting clusters that separated from the control group and group supplemented with 0.01% IDPG and occupied distinct positions in the principal component analysis (PCA) plot (Figure 1a). Furthermore, there did not appear to be much difference in microbial communities between the 0.025% IDPG-supplemented group and the 0.05% IDPG-supplemented group. In agreement with the PCA results, the numbers of OTUs in the groups supplemented with 0.025% and 0.05% IDPG increased significantly compared with the control group (Table 3). In addition, a Venn diagram was generated based on the OTUs distributed among four groups (Figure 1b). In total, 733 OTUs were common to four groups of cecal samples. More importantly, compared with the control group, 191, 305, and 242 OTUs were unique in the groups supplemented with 0.01%, 0.025%, and 0.05% IDPG, respectively, which was consistent with PCA results.
Figure 1.
Analysis of OTUs and diversity of cecal microbiome of four groups supplemented with different doses of incomplete degradation products of galactomannan (IDPG). PCA plot (a); Venn diagram of the OTUs in the four treatments (b); Heat maps based, respectively, on weighted and unweighted UniFrac distances of gut microbial communities of four treatments (c, d). Control group (1-1 to 1-6), 0.01% IDPG group (2-1 to 2-6), 0.025% IDPG group (3-1 to 3-6), and 0.05% IDPG group (4-1 to 4-6).
Table 3.
Alpha diversities and OTUs of the cecal samples in four groups supplemented with different doses of incomplete degradation products of galactomannan
| Items | IDPG level, % | SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 0.01 | 0.025 | 0.05 | |||
| OTUs | 1,585b | 1,562b | 1,884a | 1,798a | 34.09 | <0.001 |
| Chao1 | 2,516c | 2,568bc | 2,894a | 2,715b | 43.70 | <0.001 |
| Shannon | 6.63 | 6.45 | 6.75 | 6.61 | 0.06 | 0.465 |
| Simpson | 0.96 | 0.95 | 0.97 | 0.97 | 0.00 | 0.07 |
Different superscript letters in the same row differ significantly (P < 0.05).
To analyze alpha diversities of the samples, we calculated several indices including Chao1, Shannon, and Simpson values (Table 3). No difference in the Shannon index was found among treatments. However, the Chao1 index was higher (P < 0.05) in groups supplemented with 0.025% or 0.05% IDPG than in the control group. The Simpson index showed a slight increase in the IDPG-based group compared with the control group, yet the difference was not significant. Furthermore, the beta-diversity analysis showed a strong difference in the relative abundance of microbial species (weighted UniFrac) between IDPG-supplemented groups and the control group, but not in the type of microbe (unweighted UniFrac; Figure 1c and d). Moreover, R statistic ANOSIM computed on phylogenetic distances among samples revealed that IDPG administration of 0.025% or 0.05% promoted a shift in the bacterial community (Table 4), yet no difference was observed between the 0.01% IDPG-supplemented group and the control group.
Table 4.
ANOSIM analysis between the incomplete degradation products of galactomannan-supplemented group (0.01%, 0.025%, and 0.05%) and the control group
| Comparison | R value | P-value |
|---|---|---|
| Control vs. 0.01% IDPG | −0.04 | 0.641 |
| Control vs. 0.025% IDPG | 0.91 | 0.003 |
| Control vs. 0.05% IDPG | 0.92 | 0.001 |
Change of gut microbiota composition after IDPG-based diet
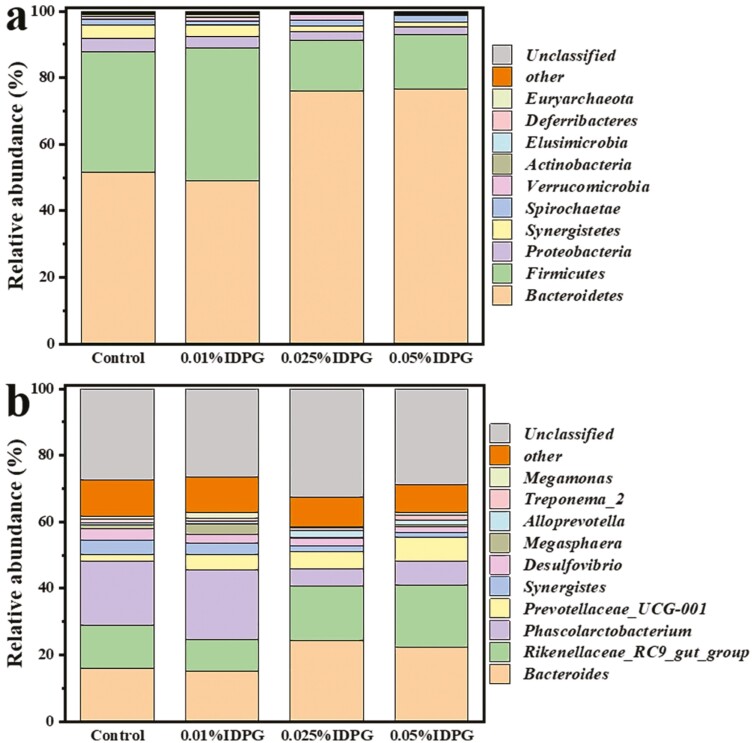
As shown in Figure 2, samples highlighted the predominance of Bacteroidetes followed by Firmicutes, Proteobacteria, and Synergistetes at the phylum level, together accounting for more than 95% of the total sequences in all groups. Moreover, a noticeable increase in relative abundance (P < 0.05) of phyla Bacteroidetes was seen in the IDPG treatment groups compared with control (Figure 2a). Specifically, the values increased from 51.71% in the control birds to 76.09% in the 0.025% IDPG-fed laying hens, and further to 76.71% in the 0.05% IDPG-fed laying hens of the total cecal microbiome. We also observed a drastic decrease in Firmicutes, Proteobacteria, and Synergistete with exposure to 0.025% or 0.05% IDPG compared with the control group (P < 0.05). At a finer level, the majority of classifiable sequences belonged to genera Bacteroides (16.06%), Rikenellaceae_RC9_gut_group (12.83%), Phascolarctobacterium (19.16%), Prevotellaceae_UCG-001 (2.19%), Synergistes (4.16%), and Desulfovibrio (3.48%) in the control group, whereas the values for the 0.025% IDPG-supplemented group were 24.24%, 16.34%, 5.31%, 5.27%, 1.66%, and 2.28%, respectively (Figure 2b). Within the phyla Bacteroidetes, the relative abundance of genera Bacteroides, Rikenellaceae_RC9_gut_group, Prevotellaceae_UCG-001, and Alloprevotella significantly increased in 0.025% and 0.05% IDPG-supplemented group (P < 0.05) relative to the control group. However, there was a decrease in the relative abundance of genera Phascolarctobacterium (19.16% to 5.31% and 7.04%) and Megasphaera (1.31% to 0.34% and 0.70%) within the phyla Firmicutes in 0.025% and 0.05% IDPG-supplemented group (P < 0.05) relative to the control group. We also found a noticeable reduction of genera Synergistes in 0.025% and 0.05% IDPG-supplemented group compared with the control group (P < 0.05). With respect to the genus Desulfovibrio, its relative abundance in the cecal microbiome of the 0.05% IDPG-supplemented group was significantly reduced (P < 0.05) compared with the control group, while that in the 0.025% IDPG group was reduced but not significantly.
Figure 2.
The cecal microbiome of laying hens fed diets supplemented with different doses of incomplete degradation products of galactomannan. Compositions of gut bacteria in at the phylum level (a). Composition of gut bacteria in ceca at the genus level (b).
Molecular functional enrichment predictions
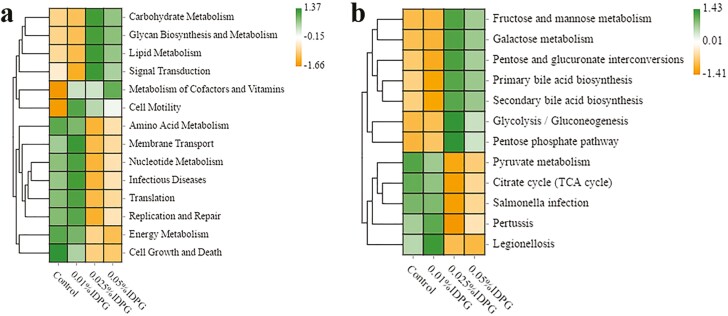
Metabolic functions, as predicted by Tax4Fun, showed that the IDPG-supplemented group had the highest number of enriched functions compared with the control group. Within carbohydrate metabolism, pathways such as fructose and mannose metabolism, galactose metabolism, pentose phosphate pathway, and glycolysis/glycogenesis were each specifically enriched in the (0.025% and 0.05%) IDPG-fed laying hens (P < 0.05; Figure 3b). However, the pathway of tricarboxylic acid (TCA) cycle within the carbohydrate metabolism depleted in the (0.025% and 0.05%) IDPG-supplemented groups relative to the control (P < 0.05). In addition, significant depletions on amino acid metabolism and energy metabolism accompanied by enrichment on the lipid metabolism were observed in the laying hens fed a diet supplemented with 0.025% or 0.05% IDPG (P < 0.05; Figure 3a). More importantly, on subclass level 3 metabolic prediction, Salmonella infections in the 0.025% or 0.05% IDPG-fed group were lower than that in the control group (P < 0.05).
Figure 3.
Heat maps of the comparison of cecal microbial functionality in the laying hens supplemented with different doses of incomplete degradation products of galactomannan. Tukey’s honestly significant difference showed significant differences between the groups (P < 0.05). Metabolic prediction based on subclass level 2 and 3, respectively (a, b).
Cecal SCFA and lactate concentration and correlation with key bacterial species
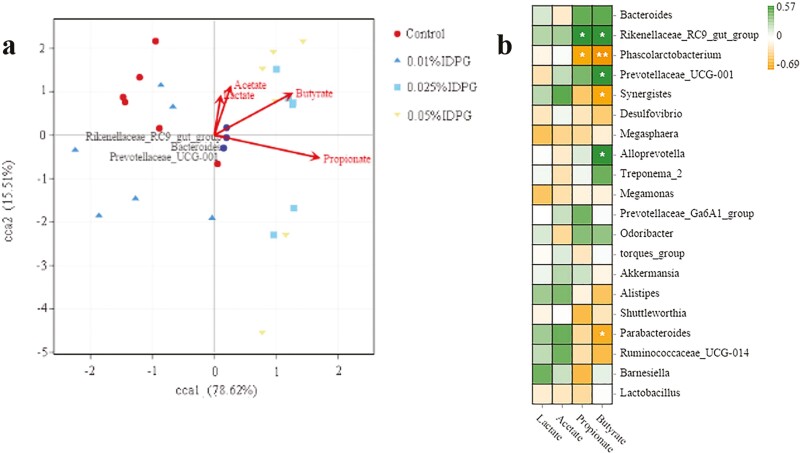
The CCA results showed that the concentration of butyrate, propionate, acetate, and lactate had a consistent correlation with the bacterial composition at the genus level. The effect of propionate was the most significant, followed by butyrate, acetate, and finally lactate. Moreover, high levels of Bacteroides, Rikenellaceae_RC9_gut_group, and Prevotellaceae_UCG-001 strongly correlated with elevated production of propionate. In contrast, Phascolarctobacterium and Synergistes correlated negatively with propionate (Figure 4b).
Figure 4.
The correlation between short-chain fatty acids concentration and key microbes at the genus level. Canonical correspondence analysis plot (a); Heat map (b). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In the present study, propionate concentration increased markedly in the cecum, with higher doses (0.025% and 0.05%) of IDPG driving the presence of more propionate. This result is consistent with a report that galactomannan serves as a strong catalyst for propionate production by intestinal flora (Reichardt et al., 2009). Moreover, 0.025% or 0.05% IDPG supplementation resulted in a slight increase in butyrate concentration. In addition, a decrease in lactate concentration was noted. Regarding both butyrate and lactate, these changes were not found to be statistically significant. This could be explained by the fact that lactate is an intermediate of the fermentation reactions that ultimately produce acetate, butyrate, and propionate. Therefore, decreased lactate may actually be utilized to produce propionate and butyrate (Seeliger et al., 2002; Duncan et al., 2004). Another interesting finding is the dramatic increase in propionate concentration because propionate is an inhibitor of cholesterol synthesis. This leads to a decrease in plasma cholesterol levels alongside increased cecal propionate, a finding which was revealed in previous studies (Demigné et al., 1995; Chen et al., 2013). In addition, propionate is an important microbial metabolite fermented from complex carbohydrates, which correlate to advantageous effects on host health (Bergman, 1990). Thereby, we have deduced that the elevated propionate could be essential for providing appropriate intestinal environments and alterations to the intestinal microbiota.
A wealth of data indicate that gut microbiota is strong determinant of host physiology and general health. In particular, gut microbiota play a critical role in maintaining normal physiological functions of the intestine (Wu et al., 2018). To investigate the effect of IDPG on the gut microbiome, we analyzed 16S rRNA sequences from the cecal contents of aged laying hens after 8 wk of feeding with IDPG. The PCA and a Venn diagram of the observed OTUs (Figure 1a and b) revealed a significant difference in the cecal microbial community between the 0.025% and 0.05% IDPG-supplemented group and the control group as well as the 0.01% IDPG-supplemented group. Moreover, IDPG supplementation significantly increased alpha-diversity levels compared with the control group, as determined by estimations such as Chao1 and Shannon index. Chao1 index reflects the species richness of the intestinal microorganisms and is sensitive to rare species. The elevated Chao1 indices herein imply the effect of IDPG as a driver of increased numbers of rare microorganism species. Interestingly, it has been reported that the level of diversity in each microbiome was significantly linked to the relative abundance of the Bacteroidetes, and microbiomes enriched for Firmicutes or Actinobacteria had a lower level of diversity (Turnbaugh et al., 2009). Therefore, the elevation of alpha diversity in the cecal microbiome of hens fed diets supplemented with IDPG suggests a shift to the proportions of Bacteroidetes, Firmicutes, and Actinobacteria. Additionally, the slight increase in the Simpson index induced by the IDPG addition reveals that the addition of IDPG makes the original dominant species more prominent. Similarly, beta-diversity analysis, based on weighted and unweighted UniFrac distance, indicates that IDPG mainly brought about a main change to the abundance of shared species, followed by species richness. Based on these results, this change in shared species abundance may have occurred mainly in the phyla Firmicutes, Bacteroidetes, and Actinobacteria. To confirm this, we then classified these representative phyla.
Bacteroidetes and Firmicutes are the two most abundant bacterial phyla in the cecum of laying hens, where Bacteroidetes account for more than 50% of the total cecal microbiome of laying hens over 26 wk of age (Videnska et al., 2014). Feeding an IDPG-based diet induced a significant increase in the phyla Bacteroidetes from 51.71% to a maximum level representing 76.71% of the total cecal microbiome. The increase in the abundance of the phyla Bacteroidetes could be explained by an increase in the genera Bacteroides, Rikenellaceae_RC9_gut_group, and Prevotellaceae_UCG-001. Other phyla, such as Firmicutes, Proteobacteria, and Synergistetes tended to correspondingly decrease in abundance. The decreased abundance of the phyla Firmicutes can be explained by the decrease in the genera Phascolarctobacterium and Megasphaera. Consistent with our findings, Hoving et al. (2018) demonstrated that manno-oligosaccharides (MOS) increased the abundance of Bacteroidetes and decreased the abundance of Firmicutes in the cecal microbiome of the female mice.
Bacteroidetes are common bacteria in the gut that are involved in many important metabolic activities, including fermentation of carbohydrates, synthesis of propionate via the succinate pathway, biotransformation of bile acids, and prevention of pathogen colonization (Flint et al., 2008). Comparative and functional genomic studies predict that the ability of gut Bacteroides species to utilize diverse glycans depends on a series of gene clusters that are referred to as polysaccharide utilization loci (PUL; Bjursell et al., 2006). Recently, a detailed genetic, biochemical, and enzyme structural characterization of a galactomannan-specific PUL from B. ovatus revealed an interplay of two mannan-specific surface glycan-binding proteins, two GH26 endo-β-mannanases and a GH36 exo-α-galactosidase in the deconstruction of this plant cell wall polysaccharide (Reddy et al., 2016; Bagenholm et al., 2017). Moreover, in the genera Bacteroides, enzymes involved in carbohydrate depolymerization are attached to the cell surface, which may be an advantage in nutrition uptake (Senoura et al., 2011). A new mannan catabolic pathway in genera Bacteroides was also revealed involving 1,4-β-mannanase, a mannobiose and/or sugar transporter, mannobiose 2-epimerase, and mannosylglucose phosphorylase before finally progressing to glycolysis (Senoura et al., 2011). Accordingly, the energy-efficient strategy for mannan metabolism, in which a unique mannosylglucose phosphorylase can directly produce phosphorylated mannose without ATP consumption, would provide intestinal anaerobic bacteria with evolutionary advantages. These outstanding characteristics constitute the major nutrient acquisition strategy deployed by Bacteroidetes bacteria and thus are intrinsically linked to the colonization of nutritional niches and the establishment of microbial ecosystems. Unlike Bacteroidetes, which are enriched for many carbohydrate metabolism pathways, Firmicutes are enriched for transport systems (Turnbaugh et al., 2009). In our present study, consumption of IDPG induced a relative increase in certain Bacteroidetes at the expense of Firmicutes (mainly Phascolarctobacterium and Megasphaera), which is similar to what was observed for mice-fed MOS-enriched diets (Hoving et al., 2018). Notably, the use of an IDPG-based diet resulted in lower Firmicutes to Bacteroidetes ratios. This result is important considering that an increased ratio of Firmicutes to Bacteroidetes has been shown to be associated with obesity in humans and mice due to the increased energy harvesting capacity of bacterial species in the phyla Firmicutes (Turnbaugh et al., 2006, Turnbaugh et al., 2009). The phylum Proteobacteria, known for containing numerous pathogenic bacteria such as Salmonella and Helicobacter, is related to dysbiosis and the progression of several diseases (Shin et al., 2015). The observed reduction of the abundance of phylum Proteobacteria therefore may be ascribed to the ability of mannose in IDPG to bind to mannose-binding lectins of Gram-negative bacteria expressing type 1 fimbriae and as a consequence reduced bacterial attachment to the intestinal epithelial cells (Ganner and Schatzmayr, 2012). In all, the reduction of the abundance of Proteobacteria in laying hens fed the diet supplemented with IDPG was shown to help with maintaining favorable intestinal environments and overall host health.
In response to the dramatic change in the cecal microbial community induced by IDPG, the metabolic function of the cecal microbiome was also altered. Metabolic prediction of the cecal microbiome revealed significant enrichment for a number of expected functional categories, including carbohydrate and lipid metabolism (Figure 3). Moreover, metabolic profile-based clustering indicated that the noted alteration of metabolic function was highly consistent with changes to species abundance. This results in changes to several pathways related to the metabolism of IDPG, specifically carbohydrate metabolism (e.g., fructose and mannose metabolism, galactose metabolism, pentose phosphate pathway, and glycolysis/glycogenesis). Several other pathways were markedly depleted alongside modified carbohydrate metabolism, including amino acid metabolism, energy metabolism, and infectious diseases. This dramatic shift of energy metabolism may be ascribed to the depletion of the TCA cycle at level 3 metabolic prediction and the unique energy-efficient pathway for IDPG metabolism in genera Bacteroides, as previously mentioned. Another point that cannot be ignored was that inclusion of IDPG in the diet of laying hens notably depleted Salmonella infection based on subclass level 3 metabolic prediction. This again serves as a strong indicator that IDPG supplementation can greatly assist a laying hen’s ability to maintain overall health.
Interestingly, we found a strong positive correlation between levels of genera Bacteroides, Rikenellaceae_RC9_gut_group, and Prevotellaceae_UCG-001 and production of propionate. In accordance with this result, the proportion of propionate that is present in total SCFA correlated with the relative abundance of phyla Bacteroidetes. This was congruent with a report on the impact of diet on intestinal microbiota composition and fermentation products in obese men as reported by Salonen et al. (2014). In addition, genera Phascolarctobacterium and Synergistes correlated negatively with propionate concentrations, as predicted based on the dramatic change in the microbiome community. As a consequence, we can gain valuable insight through gut microbe–metabolite associations that the significant enrichment of phyla Bacteroidetes induced by IDPG produce propionate and competitively restrain the proliferation of other phyla such as Firmicutes, Proteobacteria, and Synergistete.
In this study, changes in the gut microbiota as a result of implemented IDPG into the diets of laying hens were found to both be statistically significant and provide highly unique observations. Specifically, the phyla Bacteroidetes were shown to play a key role in the coordination of IDPG degradation and an ensuing increase to cecal propionate concentrations. However, the pathway by which gut microbiota exert their positive effects on the intestinal tract as well as throughout the rest of the body remains a critical and unanswered question. We believe that the beneficial effects on hens of IDPG dietary supplementation merit further investigation considering the current lack of knowledge regarding IDPG utilization and the growing interest in gastrointestinal health in both animals and humans.
Acknowledgments
This work was supported by the National promotion project of scientific and technological achievements of the State Forestry and Grassland Administration (2020133137), National Key R&D Program of China (2016YFD0600803), and the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
Glossary
Abbreviations
- CCA
canonical correspondence analysis
- HPLC
high-performance liquid chromatography
- IDPG
incomplete degradation products of galactomannan
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MOS
manno-oligosaccharides
- OTUs
operational taxonomic units
- PCA
principal component analysis
- PCR
polymerase chain reaction
- PUL
polysaccharide utilization loci
- QIIME
quantitative insights into microbial ecology
- RDP
ribosomal database project
- SCFA
short-chain fatty acids
- TCA cycle
tricarboxylic acid cycle
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Literature Cited
- Anderson, D. B., McCracken V. J., Aminov R. I., Simpson J. M., Mackie R. I., Verstegen M. W. A., and Gaskins H. R.. . 2000. Gut microbiology and growth-promoting antibiotics in swine. Nutr. Abstr. Rev. Ser. B Livest. Feed. Feed. 70:101–108. https://www.researchgate.net/publication/40194758. [Google Scholar]
- Anonymous. 2000. Approved Methods of the American Association of Cereal Chemists (10th edn). Method 08-01. Am. Assoc. Cereal Chem. [Google Scholar]
- Aßhauer, K. P., Wemheuer B., Daniel R., and Meinicke P.. . 2015. Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics. 31:2882–2884. doi: 10.1093/bioinformatics/btv287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagenholm, V., Reddy S. K., Bouraoui H., Morrill J., Kulcinskaja E., Bahr C. M., Aurelius O., Rogers T., Xiao Y., Logan D. T., . et al. 2017. Galactomannan catabolism conferred by a polysaccharide utilization locus of Bacteroides ovatus: enzyme synergy and crystal structure of a β-mannanase. J. Biol. Chem. 292:229–243. doi: 10.1074/jbc.M116.746438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman, E. N. 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70:567–590. doi: 10.1152/physrev.1990.70.2.567 [DOI] [PubMed] [Google Scholar]
- Bjursell, M. K., Martens E. C., and Gordon J. I.. . 2006. Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J. Biol. Chem. 281:36269–36279. doi: 10.1074/jbc.M606509200 [DOI] [PubMed] [Google Scholar]
- Bokulich, N. A., Subramanian S., Faith J. J., Gevers D., Gordon J. I., Knight R., Mills D. A., and Caporaso J. G.. . 2013. Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat. Methods 10:57–59. doi: 10.1038/nmeth.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli, L., Coretti L., Dipineto L., Bovera F., Menna F., Chiariotti L., Nizza A., Lembo F., and Fioretti A.. . 2017. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 7:1–11. doi: 10.1038/s41598-017-16560-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Braak, C. J. F. 1988. CANOCO-an extension of DECORANA to analyze species-environment relationships. Vegetatio. 75:159–160. doi: 10.1007/BF00045629 [DOI] [Google Scholar]
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Brzóska, F., Śliwiński B., and Stecka K.. . 2012. Effect of Lactococcus lactis vs. Lactobacillus spp. bacteria on chicken body weight, mortality, feed conversion and carcass quality. Ann. Anim. Sci. 12:549–559. doi: 10.2478/v10220-012-0046-y [DOI] [Google Scholar]
- Caporaso, J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Pẽa A. G., Goodrich J. K., Gordon J. I., . et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin, N., Goclo B. K., and Cetin E.. . 2005. The effects of probiotic and mannanoligosaccharide on some haematological and immunological parameters in turkeys. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 52:263–267. doi: 10.1111/j.1439-0442.2005.00f736.x [DOI] [PubMed] [Google Scholar]
- Chen, W. L., Anderson J. W., and Jennings D.. . 2013. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Exp. Biol. Med. 175:215–218. doi: 10.3181/00379727-175-41791 [DOI] [PubMed] [Google Scholar]
- Chung, H., Pamp S. J., Hill J. A., Surana N. K., Edelman S. M., Troy E. B., Reading N. C., Villablanca E. J., Wang S., Mora J. R., . et al. 2012. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 149:1578–1593. doi: 10.1016/j.cell.2012.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demigné, C., Morand C., Levrat M. A., Besson C., Moundras C., and Rémésy C.. . 1995. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br. J. Nutr. 74:209–219. doi: 10.1079/BJN19950124 [DOI] [PubMed] [Google Scholar]
- Duncan, S. H., Louis P., and Flint H. J.. . 2004. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, R. C. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10:996–998. doi: 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- Fernandez, F., Hinton M., and van Gils B.. . 2002. Dietary mannan-oligosaccharides and their effect on chicken caecal microflora in relation to salmonella enteritidis colonization. Avian Pathol. 31:49–58. doi: 10.1080/03079450120106000 [DOI] [PubMed] [Google Scholar]
- Flint, H. J., Bayer E. A., Rincon M. T., Lamed R., and White B. A.. . 2008. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat. Rev. Microbiol. 6:121–131. doi: 10.1038/nrmicro1817 [DOI] [PubMed] [Google Scholar]
- Gamal-Eldeen, A. M., Amer H., and Helmy W. A.. . 2006. Cancer chemopreventive and anti-inflammatory activities of chemically modified guar gum. Chem. Biol. Interact. 161:229–240. doi: 10.1016/j.cbi.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Ganner, A., and Schatzmayr G.. . 2012. Capability of yeast derivatives to adhere enteropathogenic bacteria and to modulate cells of the innate immune system. Appl. Microbiol. Biotechnol. 95:289–297. doi: 10.1007/s00253-012-4140-y [DOI] [PubMed] [Google Scholar]
- Gu, J., Pei W., Tang S., Yan F., Peng Z., Huang C., Yang J., and Yong Q.. . 2020. Procuring biologically active galactomannans from spent coffee ground (SCG) by autohydrolysis and enzymatic hydrolysis. Int. J. Biol. Macromol. 149:572–580. doi: 10.1016/j.ijbiomac.2020.01.281 [DOI] [PubMed] [Google Scholar]
- Guo, M., Wu F., Hao G., Qi Q., Li R., Li N., Wei L., and Chai T.. . 2017. Bacillus subtilis improves immunity and disease resistance in rabbits. Front. Immunol. 8:354. doi: 10.3389/fimmu.2017.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanein, S. M., and Soliman N. K.. . 2010. Effect of probiotic (Saccharomyces cerevisae) adding to diets on intestinal microflora and performance of hy-line layer hens. J. Am. Sci. 6:159–169. http://www.jofamericanscience.org/journals/am-sci/am0611/ 21_3117am0611_159_169.pdf. [Google Scholar]
- Hernandez, J. F., Pombo M., Aoki M., Moins-Teisserenc H., Santander S. P., Fiorentino S., and Mooney N.. . 2011. Galactomannan from Caesalpinia spinosa induces phenotypic and functional maturation of human dendritic cells. Int. Immunopharmacol. 11:652–660. doi: 10.1016/j.intimp.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Hooge, D. M. 2004. Meta-analysis of broiler chicken pen trials evaluating dietary mannan oligosaccharide, 1993–2003. Int. J. Poult. Sci. 3:163–174. doi: 10.3923/ijps.2004.163.174 [DOI] [Google Scholar]
- Hoving, L., Katiraei S. D., Heijink M., Pronk A., van der Wee-Pals L., Streefland T., Giera M., Willems van Dijk K., and van Harmelen V.. . 2018. Dietary mannan oligosaccharides modulate gut microbiota, increase fecal bile acid excretion, and decrease plasma cholesterol and atherosclerosis development. Mol. Nutr. Food Res. 62:1–29. doi: 10.1002/mnfr.201700942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, Y., Xun S., Tamminga S., Williams B. A., Verstegen M. W. A., and Erdit G.. . 2004. Real-time PCR detection of lactic acid bacteria in cecal contents of Eimeria tenella-infected broilers fed soybean oligosaccharides and soluble soybean polysaccharides. Poult. Sci. 83:1696–1702. doi: 10.1093/ps/83.10.1696 [DOI] [PubMed] [Google Scholar]
- Li, M., Zhou H., Pan X., Xu T., Zhang Z., Zi X., and Jiang Y.. . 2017. Cassava foliage affects the microbial diversity of Chinese indigenous geese caecum using 16S rRNA sequencing. Sci. Rep. 7:1–10. doi: 10.1038/srep45697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoč, T., and Salzberg S. L.. . 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 27:2957–2963. doi: 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, H. P., Motherway M. O. C., Lakshminarayanan B., Stanton C., Paul Ross R., Brulc J., Menon R., O’Toole P. W., and van Sinderen D.. . 2015. Carbohydrate catabolic diversity of bifidobacteria and lactobacilli of human origin. Int. J. Food Microbiol. 203:109–121. doi: 10.1016/j.ijfoodmicro.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Mohd Shaufi, M. A., Sieo C. C., Chong C. W., Gan H. M., and Ho Y. W.. . 2015. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 7:4. doi: 10.1186/s13099-015-0051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. H., Kim Y. M., and Kim I. H.. . 2018. Egg production, egg quality, blood profiles, cecal microflora, and excreta noxious gas emission in laying hens fed with fenugreek (Trigonella foenum-graecum L.) seed extract. J. Poult. Sci. 55:47–53. doi: 10.2141/jpsa.0170011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, J. A., and Burkholder K. M.. . 2003. Application of prebiotics and probiotics in poultry production. Poult. Sci. 82:627–631. doi: 10.1093/ps/82.4.627 [DOI] [PubMed] [Google Scholar]
- Pinheiro, A. C., Bourbon A. I., Rocha C., Ribeiro C., Maia J. M., Gonalves M. P., Teixeira J. A., and Vicente A. A.. . 2011. Rheological characterization of κ-carrageenan/galactomannan and xanthan/galactomannan gels: comparison of galactomannans from non-traditional sources with conventional galactomannans. Carbohydr. Polym. 83:392–399. doi: 10.1016/j.carbpol.2010.07.058 [DOI] [Google Scholar]
- Pollard, M. A., Fischer P., and Windhab E. J.. . 2011. Characterization of galactomannans derived from legume endosperms of genus Sesbania (Faboideae). Carbohydr. Polym. 84:550–559. doi: 10.1016/j.carbpol.2010.12.019 [DOI] [Google Scholar]
- Pruesse, E., Quast C., Knittel K., Fuchs B. M., Ludwig W., Peplies J., and Glöckner F. O.. . 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188–7196. doi: 10.1093/nar/gkm864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, S. K., Bågenholm V., Pudlo N. A., Bouraoui H., Koropatkin N. M., Martens E. C., and Stålbrand H.. . 2016. A β-mannan utilization locus in Bacteroides ovatus involves a GH36 α-galactosidase active on galactomannans. FEBS Lett. 590:2106–2118. doi: 10.1002/1873-3468.12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt, N., Gniechwitz D., Steinhart H., Bunzel M., and Blaut M.. . 2009. Characterization of high molecular weight coffee fractions and their fermentation by human intestinal microbiota. Mol. Nutr. Food Res. 53:287–299. doi: 10.1002/mnfr.200700509 [DOI] [PubMed] [Google Scholar]
- Reichardt, N., Vollmer M., Holtrop G., Farquharson F. M., Wefers D., Bunzel M., Duncan S. H., Drew J. E., Williams L. M., Milligan G., . et al. 2018. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 12:610–622. doi: 10.1038/ismej.2017.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen, A., Lahti L., Salojärvi J., Holtrop G., Korpela K., Duncan S. H., Date P., Farquharson F., Johnstone A. M., Lobley G. E., . et al. 2014. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 8:2218–2230. doi: 10.1038/ismej.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger, S., Janssen P. H., and Schink B.. . 2002. Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol. Lett. 211:65–70. doi: 10.1016/S0378-1097(02)00651-1 [DOI] [PubMed] [Google Scholar]
- Sekirov, I., Russell S. L., Antunes L. C. M., and Finlay B. B.. . 2018. Gut microbiota in health and disease. Physiol. Rev. 90:859–904. doi: 10.1159/000481627 [DOI] [PubMed] [Google Scholar]
- Senoura, T., Ito S., Taguchi H., Higa M., Hamada S., Matsui H., Ozawa T., Jin S., Watanabe J., Wasaki J., . et al. 2011. Biochemical and biophysical research communications new microbial mannan catabolic pathway that involves a novel mannosylglucose phosphorylase. Biochem. Biophys. Res. Commun. 408:701–706. doi: 10.1016/j.bbrc.2011.04.095 [DOI] [PubMed] [Google Scholar]
- Shin, N. R., Whon T. W., and Bae J. W.. . 2015. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33:496–503. doi: 10.1016/j.tibtech.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Shtriker, M. G., Hahn M., Taieb E., Nyska A., Moallem U., Tirosh O., and Madar Z.. . 2018. Fenugreek galactomannan and citrus pectin improve several parameters associated with glucose metabolism and modulate gut microbiota in mice. Nutrition. 46:134–142. doi: 10.1016/j.nut.2017.07.012 [DOI] [PubMed] [Google Scholar]
- Tao, Y., Huang C., Lai C., Huang C., and Yong Q.. . 2020a. Biomimetic galactomannan/bentonite/graphene oxide film with superior mechanical and fire retardant properties by borate cross-linking. Carbohydr. Polym. 245:116508. doi: 10.1016/j.carbpol.2020.116508 [DOI] [PubMed] [Google Scholar]
- Tao, Y., Wang T., Huang C., Lai C., Ling Z., Zhou Y., and Yong Q.. . 2021. Production performance, egg quality, plasma biochemical constituents and lipid metabolites of aged laying hens supplemented with incomplete degradation products of galactomannan. Poult. Sci. 100:101296. doi: 10.1016/j.psj.2021.101296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y., Yang L., Lai C., Huang C., Li X., and Yong Q.. . 2020b. A facile quantitative characterization method of incomplete degradation products of galactomannan by ethanol fractional precipitation. Carbohydr. Polym. 250:116951. doi: 10.1016/j.carbpol.2020.116951 [DOI] [PubMed] [Google Scholar]
- Turnbaugh, P. J., Hamady M., Yatsunenko T., Cantarel B. L., Duncan A., Ley R. E., Sogin M. L., Jones W. J., Roe B. A., Affourtit J. P., . et al. 2009. A core gut microbiome in obese and lean twins. Nature. 457:480–484. doi: 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh, P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., and Gordon J. I.. . 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 444:1027. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- Videnska, P., Sedlar K., Lukac M., Faldynova M., Gerzova L., Cejkova D., Sisak F., and Rychlik I.. . 2014. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS One. 9:1–14. doi: 10.1371/journal.pone.0115142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., Garrity G. M., Tiedje J. M., and Cole J. R.. . 2007. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261–5267. doi: 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, W., Xiao Z., An W., Dong Y., and Zhang B.. . 2018. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS One. 13:1–21. doi: 10.1371/journal.pone.0197762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zartl, B., Silberbauer K., Loeppert R., Viernstein H., Praznik W., and Mueller M.. . 2018. Fermentation of non-digestible raffinose family oligosaccharides and galactomannans by probiotics. Food Funct. 9:1638–1646. doi: 10.1039/c7fo01887h [DOI] [PubMed] [Google Scholar]