Abstract
Probiotic bacteria, including the Enterococcus faecium strain, can improve intestinal mucosal health by several mechanisms, including modulation of the immune response, as well as by improving the protective function of the epithelial barrier. In this study, we tested the effect of Enterococcus faecium AL41 on the acute phase proteins response (blood), gene expression of selected molecules of mucosal immunity (immunoglobulin A, mucin-2, insulin-like growth factor 2) and mucus production (all parts of the small intestine) in broilers. Eighty broiler chicks were divided into two groups: a control and E. faecium AL41 (birds were inoculated with AL41 for 7 days) group. The whole experiment lasted 11 days. Our results revealed that the administration of E. faecium AL41 had no substantial effect on the concentrations of acute phase proteins, but we recorded a significant increase in β- and γ-globulin fractions at the end of the experiment, which may indicate an improvement in the immune status. A significant prolonged stimulatory effect of E. faecium AL41 on the relative expression of molecules (immunoglobulin A, mucin-2) as well as on the dynamic of mucus production in the chicken intestine was observed. In addition, AL41 significantly reduced the total number of enterococci in the cecum and faeces.
Keywords: Enterococcus faecium AL41, acute phase protein, probiotic bacteria, mucosal immune response, broiler chickens
1. Introduction
The mucosal immune system represents a separate part of the immune system that provides local immunity in the mucous membranes of the gastrointestinal as well as respiratory tracts. It essentially tolerates commensal microbes and at the same time responds quickly and effectively to pathogenic organisms [1]. The major gel-forming mucin (MUC-2) forms the primary barrier component of the mucus layers and represents the main site for secretory immunoglobulin A (IgA). The polymeric Ig receptor, which is expressed on the basolateral surface of epithelium, is used to transport polymeric IgA from the lamina propria to the luminal mucins to form the first lines of intestinal defence. IgA together with MUC-2 limit epithelial contact with pathogens and other potentially dangerous antigens and their penetration [2]. On the other hand, it selectively facilitates the adherent growth of normal intestinal microbiota [3]. Insulin-like growth factor 2 (IGF2) together with IGF-1 are known as intestinotropic factors mainly for the small intestinal epithelium [4].
Probiotics can improve intestinal mucosal health through several mechanisms, including the production of antimicrobials, short-chain fatty acids, modulation of the immune response, as well as competitive elimination of pathogenic bacteria, thereby enhancing epithelial barrier function [5]. Intestinal mucus layer is the first line of defence protecting epithelium against luminal threats including mechanical forces during the digestion process, enzymes and gut bacteria. The intestinal mucus also plays important roles in supporting the colonization with commensal bacteria, maintaining an appropriate environment for digestion and facilitating nutrient transport from the lumen to the underlying epithelium [6]. Intestinal morphological measurements, such as increased villus height, short crypt depth and higher villus height–crypt depth ratio indicate an increase in nutrient absorption by increasing the available surface area for nutrient absorption. The proliferation and differentiation of goblet cells affect the mucosal integrity and dynamic to maintain mucus thickness [6]. The amount of mucus production depends on the number of goblet cells in the intestinal villi and crypts, which is a health indicator of the intestine as these cells produce mucin and exclude harmful pathogens from adhesion to the intestinal epithelium [7].
Different probiotic strains’ (Lactobacillus casei, L. acidophilus, Bifidobacterium thermophilum, Bacillus subtilis, Enterococcus faecium) influence on the gut’s histomorphology changes has been studied. This result suggested that the addition of the mentioned probiotic strains can enhance the intestinal nutrient absorption and mucus production as well as intestinal architecture [8,9].
In addition, the demand for alternative feed additives for broilers such as probiotics, prebiotics, enzymes, organic acids, herbs and their extracts has increased in recent years due to their impact on productivity and animal health [10].
Enterococci are among the lactic acid bacteria (LABs), which include pathogenic and commensal microorganisms ubiquitous in the environment, even as intestinal symbionts of animals and humans. In addition, several Enterococcus strains are reported to produce antimicrobial compounds, including bacteriocins. Bacteriocin production is currently considered a probiotic property [11]. Currently, the enterococcal strains E. faecium and E. faecalis are the only enterococci used as probiotics or feed additives [12].
E. faecium AL41 is an enterocin M bacteriocin-producing probiotic strain belonging to the Firmicutes phylum, which fulfils EFSA rules [13,14].
The dietary supplementation with enterococcal probiotics may improve health and growth performances through the optimal utilization of nutrients and maintenance of intestinal integrity, and may reduce the death rate by regulating immune responses in broiler chickens [15]. One of the ways to monitor the health state, as well as assess metabolic alterations related to protein profile and immune responses, is the evaluation of acute phase reactants. Acute phase reactants are a group of proteins whose serum concentrations change in response to any injury, disturbances in homeostasis or stress as part of the non-specific innate immune response [16]. In addition to the determination of these specific proteins, serum protein electrophoresis could be of great diagnostic importance to describe the distribution of serum proteins and to assess the changes, especially in the gamma-globulin fraction caused by the overproduction of a single or a group of immunoglobulins [17]. Even though acute phase proteins may be relevant biomarkers of the health state, there are very few studies assessing the effect of probiotic supplementation on their synthesis.
In our previous experiments with broiler chicks, the administration of E. faecium AL41 strain resulted mainly in an immunomodulatory effect on cytokine expression during Salmonella and Campylobacter infections and increased the concentration of secretory IgA in the intestine flush [18,19]. Therefore, we decided to observe the effect of strain AL41 on the acute phase proteins response, distribution of blood serum proteins and important parameters of mucosal gut immunity in broiler chicks.
2. Materials and Methods
2.1. Experimental Scheme
Animals
The chickens were handled and killed in accordance with state regulations. The specific experiment was approved by the Ethics Committee of the Veterinary Medicine and Pharmacy in Košice followed by the Committee for Animal Welfare of the Ministry of Agriculture of the Slovak Republic (permit number 1184-3/2020-220).
Eighty 1-day-old COBB 500 male cock chicks were divided into two groups (n = 40). The following experimental groups were included in the study: the control group and the EF group, where birds were inoculated with E. faecium AL41 (CCM 8558). The experiment lasted 11 days. The chickens were placed in 4 hardwood pens with an area of 2.06 m2 (length 165 cm, width 125 cm, height 120 cm) covered with wood and fed a standard BR-1 compound feed (BR1, Čaňa, Košice, Slovakia) (Table 1) with access to water ad libitum. The broilers were kept at an ambient temperature of 30–32 °C with a relative humidity of 40–80% throughout the experiment, in a light/dark mode for 12 h. The control of the temperature and humidity in the room was performed 8 times a day (every 3 h) with a KlimaLogg Pro monitoring device with a signalling system. Environmental conditions were kept following the broiler breeding criteria [20]. Prior to the start of the experiment, faecal control samples were taken from the chickens for microbiological examination. The blood samples (zero day and 11th day of the experiment) and the samples from all individual parts of the small intestine (5th, 8th and 11th day of the experiment) were collected from 10 chickens from both groups in each sampling.
Table 1.
Composition of BR1 commercial diet.
| Ingredients g/kg | BR1 |
|---|---|
| Wheat | 290 |
| Maize | 300 |
| Soybean meal | 320 |
| Rapeseed oil | 40 |
| Fish meal | 20 |
| Limestone | 12 |
| Dicalcium phosphate | 10 |
| Sodium chloride | 2 |
| DL-methionine | 1 |
| Vitamin-mineral mix * | 5 |
| Composition by analysis (g/kg) | |
| dry matter | 899.9 |
| crude protein | 232.7 |
| fat | 64.5 |
| dietary fibre | 22.7 |
| ash | 53 |
| Ca (calcium) | 90.4 |
| P (phosphorus) total | 69.6 |
Vitamin and mineral premix *: vitamin A 12,500 IU/kg, vitamin D3 4000 IU/kg, vitamin E 80.00 mg/kg, Cu 15.00 mg/kg, vitamin D/25 cholecalciferol 1000 IU/kg, Jod 1.00 mg/kg, Mn 50.00 mg/kg, Zn 90.00 mg/kg, Fe 40.00 mg/kg, Se 30.00 mg/kg.
2.2. Preparing of Probiotic Strain
The probiotic strain of E. faecium AL41 was grown as previously detailed by Karaffová et al. [21]. A suspension of E. faecium in dose 109 CFU/0.2 mL was supplemented individually perorally to the chickens in the EF group daily, from the first to the seventh day of the experiment. To simulate the same manipulation stress, an equal volume of saline was applied to the control group with a Pasteur pipette.
2.3. Laboratory Analyses
The blood samples from chickens (on day 0 and at the end of the experiment—11th day) were taken into 1.1 mL serum gel separator tubes without additives and anticoagulants (Sarstedt, Nümbrecht, Germany). After letting the blood samples coagulate at room temperature, sera were separated by centrifugation at 3000× g for 15 min and then transferred into Eppendorf tubes. The serum samples were immediately processed and analysed, and aliquots were kept frozen at −20 °C for further laboratory analyses. The serum samples were analysed for the concentrations of total serum proteins (TP, g/L), the electrophoretic pattern of serum proteins and selected acute phase proteins. The biuret method was applied to measure the TP concentrations using commercially available diagnostic kits (Randox, Crumlin, UK) and the automated chemistry analyser Alizé (Lisabio, Pouilly en Auxois, France). The separation and distribution of serum protein fractions were performed by zone electrophoresis on agarose gel using an automated electrophoresis system Hydrasys with commercial diagnostic kits Hydragel 7 Proteine (Sebia Corporate, Lisses, Evry Cedex, France) [22].
The protein fractions were expressed as relative values (%) according to the optical density and their absolute concentrations (g/L) were quantified from the TP concentrations. Albumin–globulin ratios (A/G) were calculated as well. The concentrations of serum amyloid A (SAA, ng/mL) were quantified by double antibody sandwich enzyme-linked immunosorbent assay (ELISA) using a commercially available Chicken SAA ELISA kit (Immunology Consultants Laboratory, Inc., Portland, OR, USA). Haptoglobin (Hp, mg/mL) was measured spectrophotometrically using commercial colorimetric kits (Tridelta Development, Kildare, Ireland) in microplates.
2.3.1. Homogenization of Jejunal Samples and Isolation of Total RNA of IgA, MUC-2 and IGF-2 (Growth Factor) Gene
Samples of jejunum (20 mg weighted pieces) were immediately placed in RNA later solution (Qiagen, UK) and stored at −70 °C before RNA purification and reverse transcription as mentioned in Karaffová et al. [23].
2.3.2. Relative Expression of Genes in Quantitative Real-Time PCR (qRT-PCR)
The mRNA levels of IgA, MUC-2 and IGF-2 genes were determined. Additionally, mRNA relative expression of the reference gene, coding GAPDH (glyceraldehyde-3-phosphate dehydrogenase), was selected based on confirmed expression stability using the geNorm program. The primer sequences, annealing temperatures and times for each primer used for qRT-PCR are listed in Table 2. All primer sets allowed cDNA amplification efficiencies between 94% and 100%.
Table 2.
List of primers used for the chicken gene mRNA quantification.
| Primer | Sequence 5′–3′ | Annealing/Temperature Time | References |
|---|---|---|---|
| IgA Fw | GTCACCGTCACCTGGACTACA | 59 °C for 30 s | [24] |
| IgA Rev | ACCGATGGTCTCCTTCACATC | ||
| MUC-2 Fw | GCTGATTGTCACTCACGCCTT | 54 °C for 1 min | [25] |
| MUC-2 Rev | ATCTGCCTGAATCACAGGTGC | ||
| IGF-2 Fw | CTCTGCTGGAAACCTACTGT | 55 °C/30 s | [26] |
| IGF-2 Rev | GAGTACTTGGCATGAGATGG | ||
| GAPDH Fw | CCTGCATCTGCCCATTT | 59 °C/30 s | [27] |
| GAPDH Rev | GGCACGCCATCACTATC |
Amplification and detection of target products were performed using the CFX 96 RT system (Bio-Rad, Hercules, CA, USA) and Maxima SYBR Green qPCR Master Mix (Thermo Scientific, Waltham, MA, USA). Subsequent qRT-PCR to detect relative expression of mRNA of selected genes was performed for 36 cycles under the following conditions: initial denaturation at 95 °C for 2 min, subsequent denaturation at 95 °C for 15 s, annealing (Table 2) and final extension step for 2 min at 72 °C. A melting curve from 50 °C to 95 °C with readings at every 0.5 °C was generated for each individual qRT-PCR plate. All reactions were conducted in triplicate. We also confirmed that the efficiency of amplification for each selected gene was essentially 100% in the exponential phase of the reaction, where the quantification cycle (Cq) was calculated. The Cq values of the studied genes were normalised to an average Cq value of the reference gene (ΔCq), and the relative expression of each gene was calculated mathematically as 2–ΔCq.
2.4. Mucus Production
The duodenum, jejunum and ileum samples were obtained from randomly selected animals (n = 6). After exsanguinations from the small intestine, different segment samples were processed in duplicates for mucus determination according to Smirnov et al. [25] and modified by Faixová et al. [28]. The amount of produced mucus was determined by the ELISA assay technique (Apollo LB 913, Berthold Technologies, Bad Wildbad, Germany) at the wavelength 630 nm using the software PhotoRead version 2.2.2.1 (Apollo LB 913, Berthold Technologies, Bad Wildbad, Germany). The mucus production quantity results were expressed in grams (g ± standard deviation-SD).
2.5. Microbiology
In the experiment, the so-called rifampicin-labelled strain AL41 = CCM 8558 [13] was used to distinguish it from other enterococcal microbiota in the faeces (on 0, 8th and 11th day of the experiment) and in the cecum (8th and 11th day of the experiment). M-Enterococcus agar (M-Enterococcus agar, Difco) supplemented with rifampicin (100 μg) was used to capture the number of strain AL41 = CCM 8558. Total enterococcal counts were determined using M-Enterococcus agar (Difco, Sparks, MD, USA) and coliform bacteria were isolated on MacConkey agar (Difco, Sparks, MD, USA). All plates were cultured according to the genera at 37 °C for 24 h (in a partially anaerobic atmosphere). To eliminate the presence of Campylobacter and Salmonella, Rappaport-Vassiliadis Broth (Merck) was used to capture Salmonella sp., followed by seeding on Brilliant green agar (Becton and Dickinson, Cockeysville, MD, USA). CM0935 Campylobacter agar base (Karmali) supplemented with Campylobacter Selective Supplement (Karmali) SR0167 (Oxoid Ltd., Basingstoke, UK) was used to exclude the presence of Campylobacter sp. when cultured in an anaerobic box and at a temperature of 42 ℃. The faeces samples were processed by a standard microbiological method (ISO) and were grown on the media mentioned above. The bacterial counts were expressed in colony-forming units as CFU/g−1 ± sd.
All samples were free of Salmonella sp. and Campylobacter sp.
2.6. Statistical Analyses
All statistical analyses were carried out by the statistical software GraphPad Prism 8.3 (GraphPad Software Inc., San Diego, CA, USA). Kolmogorov–Smirnov test for normality was used to evaluate the distribution of the data. The statistical model of the unpaired T-test was applied to compare the means related to the two sample collections and to determine the significance of differences between the sample collection days in both groups. For other parameters, differences between the control and experimental groups were tested also by the unpaired t-test. The levels of statistical significance were expressed as p-value (p < 0.05, p < 0.01, p < 0.001). Values in figures are given as means resp. medians in the case of relative gene expression with standard deviations (±SD).
3. Results
3.1. Laboratory Analyses
As presented in Table 3, the analyses of the concentrations of Hp showed no significant differences between the days 0 and 11 in the control or in the experimental group of chickens. The concentrations of SAA were slightly but non-significantly higher on day 11 of the experiment in the control, as well as in the experimental chickens. The concentrations of total serum proteins were significantly higher on day 11 of the experiment when compared to day 0 in the control, as well as in the experimental group of chickens (p < 0.01 and p < 0.05, respectively). Serum protein electrophoresis identified six protein fractions in broiler chickens, including prealbumin, albumin, α1-, α2-, β- and γ-globulins. On day 11 of the evaluation, the relative concentrations of protein fractions showed a slightly non-significantly higher proportion of prealbumin in the experimental group compared to the control group. Albumin was the most prominent protein fraction and formed nearly 44% of total serum proteins in both groups of chickens. On day 11 of the evaluated period, its values were slightly lower in the experimental chickens than in the chickens of the control group. No significant differences in the relative concentrations of α1-globulins were found between the sample collections in the control or the experimental group. In the relative concentrations of α2-globulins, significant differences between the sample collections were obtained. Their values were significantly lower on day 11 of the experiment in both groups of animals as compared to day 0 (p < 0.05). While the mean relative concentrations of β-globulins in the control group were similar on days 0 and 11 of the experiment, their proportion in the experimental group was non-significantly higher on day 11. The relative values of γ-globulins were higher on day 11 of the experiment in both groups of chickens, and this difference was significant in the experimental group (p < 0.01). The A/G ratios on day 11 were non-significantly lower on day 11 than on day 0 in both groups of chickens.
Table 3.
Differences in the concentrations of evaluated acute phase proteins, total proteins (TP), serum protein fractions and albumin–globulin ratio (A/G) in control and experimental broiler chickens between the sample collections (mean ± SD).
| Parameter | Groups of Animals | ||||
|---|---|---|---|---|---|
| C (Control) | EF | ||||
| Day 0 | Day 11 | Day 0 | Day 11 | ||
| Hp (mg/mL) | 0.052 ± 0.071 | 0.025 ± 0.033 | 0.014 ± 0.013 | 0.022 ± 0.029 | |
| SAA (ng/mL) | 34.30 ± 11.38 | 48.62 ± 17.16 | 35.41 ± 12.30 | 44.22 ± 6.81 | |
| TP (g/L) | 26.7 ± 2.06 | 29.7 ± 1.46 b | 26.8 ± 2.19 | 29.1 ± 1.78 a | |
| prealb | % | 1.46 ± 0.30 | 1.36 ± 0.30 | 1.53 ± 0.29 | 1.77 ± 0.49 |
| g/L | 0.39 ±0.09 | 0.40 ± 0.08 | 0.41 ± 0.11 | 0.51 ± 0.13 | |
| alb | % | 43.9 ± 1.63 | 43.2 ± 2.49 | 43.7 ± 1.75 | 42.1 ± 2.72 |
| g/L | 11.7 ± 0.62 | 12.8 ± 0.76 b | 11.7 ± 1.23 | 12.3 ± 1.10 | |
| α1- | % | 4.6 ± 0.65 | 4.7 ± 0.67 | 4.2 ± 0.62 | 4.0 ± 0.52 |
| g/L | 1.2 ± 0.28 | 1.4 ± 0.17 | 1.1 ± 0.22 | 1.2 ± 0.14 | |
| α2- | % | 29.6 ± 0.90 | 27.9 ± 1.44 a | 29.5 ± 1.64 | 27.6 ± 1.11 a |
| g/L | 7.8 ± 0.73 | 8.3 ± 0.41 | 7.9 ± 0.53 | 8.0 ± 0.70 | |
| β- | % | 6.6 ± 0.50 | 6.6 ± 0.67 | 6.4 ± 1.14 | 7.3 ± 0.77 |
| g/L | 1.8 ± 0.11 | 2.0 ± 0.22 | 1.7 ± 0.30 | 2.1 ± 0.12 b | |
| γ- | % | 13.8 ± 0.98 | 16.2 ± 3.05 | 14.7 ± 1.26 | 17.2 ± 2.00 b |
| g/L | 3.7 ± 0.55 | 4.8 ± 1.12 a | 3.9 ± 0.41 | 5.0 ± 0.69 b | |
| A/G | 1.00 ± 0.08 | 0.94 ± 0.15 | 1.00 ± 0.10 | 0.94 ± 0.12 | |
Legend: a, b—superscripts in rows and groups of animals mean statistically significant differences between the sample collections (day 0 and day 11)—a p < 0.05, b -p < 0.01.
The absolute concentrations of prealbumin in the control group were approximately similar on days 0 and 11 of the evaluation; in the experimental group, the values obtained on day 11 were non-significantly higher. A trend of higher values on day 11 was also observed in the absolute concentrations of albumin; the differences in mean values between the sample collections were significant in the control group of chickens (p < 0.01). No significant differences were found in the absolute concentrations of α1- and α2-globulins between the sample collections of both groups of chickens. Differences between the sample collections occurred in the absolute concentrations of β-globulins, where the values obtained on day 11 in the experimental group of chickens were significantly higher than on day 0 (p < 0.01). The absolute concentrations of γ-globulins found in both groups of chickens on day 11 were significantly higher when compared to those on day 0. However, on day 11, a significantly higher mean γ-globulin value was recorded in the experimental group of chickens (p < 0.01).
3.2. Relative Expression of Genes in qRT-PCR
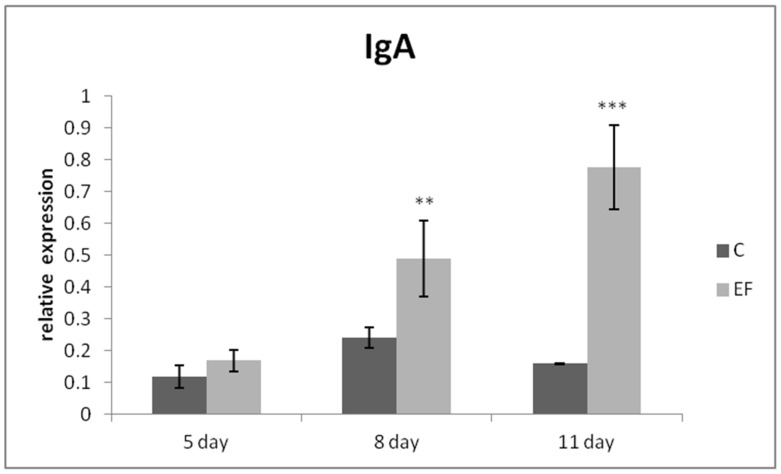
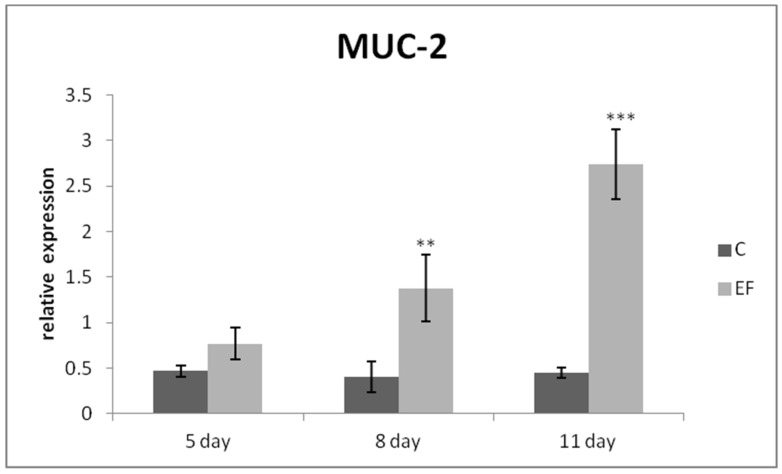
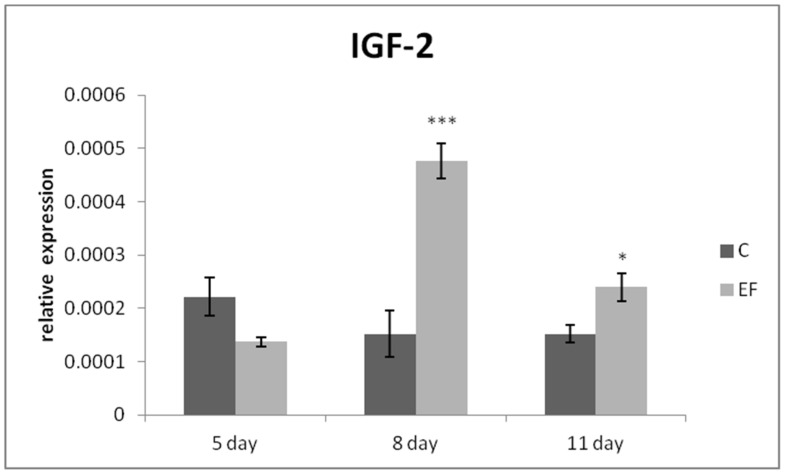
The relative expression of the IgA gene was markedly upregulated mainly on day 8 (p < 0.01), as well as day 11 in the EF group as compared to the control (p < 0.001) (Figure 1). The same tendency was recorded for MUC-2 gene expression, which was higher in the EF group on days 8 and 11 than in the control (p < 0.01; p < 0.001) (Figure 2). The relative expression of growth factor IGF-2 was upregulated in the experimental group in comparison with the control in the last two sampling days (p < 0.05; p < 0.001) (Figure 3).
Figure 1.
Relative expression of IgA gene in the jejunum of chickens treated with E. faecium AL41. Results at each time point are the median of 2–ΔCq. Means with different superscripts are significantly different ** p < 0.01; *** p < 0.001.
Figure 2.
Relative expression of MUC-2 gene in the jejunum of chickens treated with E. faecium AL41. Results at each time point are the median of 2–ΔCq. Means with different superscripts are significantly different ** p < 0.01; *** p < 0.001.
Figure 3.
Relative expression of IGF-2 gene in the jejunum of chickens treated with E. faecium AL41. Results at each time point are the median of 2–ΔCq. Means with different superscripts are significantly different * p < 0.05; *** p < 0.001.
3.3. Mucus Production
Mucus production was significantly increased in the duodenum (p < 0.05) as well as in the jejunum (p < 0.01) at the end of the experiment (day 11) in comparison with mucus production in the duodenum and ileum on day 8 of the experiment. The prolonged beneficial influence on mucus production quantity was observed at the end of the experiment in the duodenum (p < 0.001), jejunum (p < 0.001) and ileum (p < 0.001) in the experimental group compared to the relevant control group on day 11 of the experiment (Table 4).
Table 4.
The effect of peroral application of E. faecium AL41 on mucus quantity production in different segments of small intestine of chickens.
| Mucus Production (g ± SD) |
5th Day of Experiment | 8th Day of Experiment | 11th Day of Experiment |
|---|---|---|---|
| Duodenum | |||
| EF | 3.66 ± 1.22 | 3.21 ± 1.21 d | 4.44 ± 0.27 a,d |
| C | 2.37 ± 0.69 | 1.41 ± 0.26 | 1.72 ± 0.02 a |
| Jejunum | |||
| EF | 3.27 ± 1.65 | 2.99 ± 0.44 e | 3.91 ± 0.25 b,e |
| C | 2.82 ± 3.38 | 1.82 ± 0.17 | 1.66 ± 0.07 b |
| Ileum | |||
| EF | 3.70 ± 1.71 | 3.77 ± 1.37 | 4.11 ± 0.44 c |
| C | 1.12 ± 4.51 | 1.78 ± 0.18 | 1.33 ± 0.60 c |
Legend: The mucus quantity production in g ± SD (gram ± standard deviation); EF—experimental group with E. faecium AL41 (CCM 8558) application; C—control group; the same letters mean the significant difference in order: a,b,c at the level p < 0.001; d at the level p < 0.05; e at the level p < 0.01.
3.4. Microbial Screening
Strain AL41 = CCM 8558 alone colonized the digestive tract of chickens; when in the faeces it reached 3.0 log10 cfu/g in the experimental group (day 8), and on day 11 its numbers decreased only slightly. Enterococcal counts decreased significantly in the experimental group on day 8, as well as on day 11 of the experiment compared to the control (p < 0.05). We assume that the decrease in total enterococci in the experimental group was due to the action of E. faecium AL41 alone, through the production of bacteriocins, or by the production of lactic acid. Coliform counts in the faeces were not affected by the administration of E. faecium AL41 (Table 5).
Table 5.
The numbers of E. faecium AL41 = CCM8558, total enterococci and coliforms in faeces (log10 cfu/g) (n = 10). Means with different superscripts are significantly different * p < 0.05.
| Faeces | Control | EF |
|---|---|---|
| 0 day | ||
| EFAL41 | nt | nt |
| Enterococci | 6.46 (0.48) | 6.43 (0.47) |
| Coliform | 7.1 (0.0) | 6.92 (0.84) |
| 8 day | ||
| EFAL41 | nt | 2.60 (1.0) |
| Enterococci | 6.54 (0.81) | 5.29 (0.23) * |
| Coliform | 6.94 (0.74) | 7.1 (0.0) |
| 11 day | ||
| EFAL41 | nt | 2.17 (0.31) |
| Enterococci | 6.48 (0.81) | 5.09 (0.70) * |
| Coliform | 6.91 (0.84) | 6.98 (0.83) |
The numbers of strain E. faecium AL41 on day 8 were almost the same in the cecum and in the faeces. At the end of the experiment they decreased only slightly and did not differ between cecum and faeces (day 11 of the experiment). The same as in the faeces, the numbers of other enterococci markedly decreased in the experimental group compared to the control (p < 0.05) on day 8. The coliform counts in the cecum were also not affected by E. faecium AL41 (Table 6).
Table 6.
The numbers of E. faecium AL41 = CCM8558, total enterococci and coliforms in cecum (log10 cfu/mL) (n = 10). Means with different superscripts are significantly different * p < 0.05.
| Cecum | Control | EF |
|---|---|---|
| 8 day | ||
| EFAL41 | nt | 2.76 (0.43) |
| Enterococci | 6.40 (0.81) | 5.63 (0.75) * |
| Coliform | 6.79 (0.83) | 6.81 (0.83) |
| 11 day | ||
| EFAL41 | 2.16 (0.33) | |
| Enterococci | 4.61 (2.01) | 4.46 (1.37) |
| Coliform | 5.69 (0.75) | 5.87 (1.42) |
4. Discussion
The administration of probiotics in the feed of broiler chickens has been found to have a positive effect on the organism, manifested by improved health state and growth performances due to immunostimulation, the competitive exclusion of gut pathogens and a positive impact on the diversity and stability of intestinal microbiota [29,30]. However, the published data evaluating the effect of probiotics on the acute phase response are limited. The most important acute phase proteins in chickens are α1-acid glycoprotein, serum amyloid A, ceruloplasmin, transferrin, haptoglobin, fibrinogen and fibronectin [31]. The study conducted by Kefal and Toker [32] suggested that two commercial probiotic preparations—Broilact (enterococci, lactobacilli) and Bioplus 2b (Bacillus licheniformis, Bacillus subtilis) alone do not affect the serum concentrations of ceruloplasmin, transferrin and fibrinogen in broilers exposed to Salmonella typhimurium lipopolysaccharides. Similarly, the results obtained in our study showed no significant influence of the dietary administration of E. faecium AL41 on the concentrations of inflammatory markers in chickens, as no significant differences in the concentrations of haptoglobin and serum amyloid A were found between days 0 and 11 of the experiment. A significant increase in total protein values, as well as markedly higher concentrations of albumin on day 11, was recorded in both groups of animals. This might be associated with normal growth processes and feeding with protein-rich diets during the fattening period [33]. Furthermore, the increase in total serum proteins might be related to the redistribution of nutrients away from the immune response and acute phase protein synthesis, which resulted in the increased availability of nutrients for growth and development [34]. The increase in total serum proteins was accompanied by significantly higher proportions of β- and γ-globulin fractions mainly in the experimental chickens (EF group). A significant increase in γ-globulins was observed in control animals as well, but the increase was less evident in chickens fed without fodder recipes containing probiotics. Cetin et al. [35] reported that the supplementation of feed with probiotics resulted in elevated concentrations of immunoglobulin G and M in turkeys, which have been linked to better growth performance and disease resistance in the evaluated animals. Immunoglobulins are the main constituent of the γ-globulin fraction, but some immunoglobulin classes (IgM and IgG) may migrate into the β-globulin region [36]. Therefore, the more significant increase in β- and γ-globulin fractions in the experimental chickens could be a result of the increased synthesis of immunoglobulins due to the feed supplementation. Similarly, Stef et al. [37] presented higher serum gamma-globulin concentrations in broiler chickens supplemented with probiotics (Lactobacillus paracasei J.R., Lactobacillus rhamnosus 15b, Lactobacillus lactis y, Lactobacillus lactis FO) and amino acids, resulting in better immune statuses and growth performances. Likewise, Dev et al. [38] concluded that the administration of Lactobacillus acidophilus with mannan oligosaccharides led to higher serum globulin concentrations. However, they did not evaluate the distribution of globulin fractions. In our study, the administration of probiotics had no significant influence either on the absolute concentrations of α-globulins or on the albumin fraction. Although non-significant, the concentrations of prealbumin increased more markedly in the experimental group of chickens. As prealbumin is an important nutritional marker, its increase in experimental chickens may be related to adequate protein–calorie consumption and weight gain [39].
In the lumen of the gastrointestinal tract, digestion and absorption occur with the assistance of a broad spectrum of microbial species. The absorption takes place at the brush border, which involves epithelial surface extensions. This epithelial surface contains goblet cells, which secrete mucous fluids that cover the epithelial surface and protect it from harmful intraluminal components including pathogens. The bacterial population of the intestine influences the proliferation of mucosal cells. Mucins are major components in the cytoplasmic secretory granules of goblet cells. In addition, intestinal mucus also plays an important role in supporting the colonization by commensal bacteria, maintaining an appropriate environment for digestion and facilitating nutrient transport from the lumen to the underlying epithelium. [40]. Based on our results, we can state that a significant effect (p < 0.001) of E. faecium AL41 (CCM 8558) on the dynamics of mucus production in all parts of the small intestine (duodenum, jejunum, ileum) was recorded at the end of the experiment.
Hence, it is clear that probiotic supplementation in poultry production alters the microenvironment of the intestine and can induce alterations in mucin dynamics in the gastrointestinal tract of chickens [41]. In agreement with the previous statement, our results revealed the most significant upregulation of gene expression for MUC-2 and IgA on the last sampling (day 11), which may confirm the cumulative effect of the continuous administration of E. faecium AL41. Moreover, we observed the same trend in the quantification of mucin production in individual sections of the chickens’ small intestines, which was the highest on day 11 of the experiment in the EF group. These findings are very useful and relevant because intestinal bacterial homeostasis in chickens can be affected by mucin types, O-glycan composition (extent of mucin glycosylation and oligomerization) and mucus layer characteristics (inner and outer mucus thickness) [42]. In addition, MUC-2 is the predominant glycoprotein found in the small and large intestine mucus [43].
Similarly, in our previous study by Levkut et al. [44], we demonstrated that gene expression, as well as the concentration of MUC-2 and IgA in the intestinal flush from the jejunum, was markedly increased in the experimental broiler chicken group after 8 days of peroral application of synbioticum Lacto-Immuno-Vital (the product contains probiotic strains of Enterococcus faecium CECT 4515 and Bacillus amyloliquefaciens CECT 5940) compared to the control. Moreover, Aliakbarpour et al. [45] observed a significant increase in MUC-2 gene expression in broilers fed a diet supplemented with Bacillus subtilis.
Furthermore, we recorded the highest level of IGF-2 gene expression in the jejunum of the EF group, which is known to bind to intestinal epithelial cells and plays an important role in intestinal development [46]. In a recent study, Wu et al. [47] confirmed that supplementation with Enterococcus faecium NCIMB11181 in broiler feed had a notable effect on the IGF-2 gene expression as well as on other intestinal growth factors in the jejunum. These findings indicated that the addition of E. faecium AL41 to poultry feed strengthened the barrier function of the intestinal mucosa, as well as the parameters of the intestinal immune system.
The E. faecium strain AL41 sufficiently colonized the cecum of chickens in the experimental group, and at the same time reduced the numbers of total enterococci in both the cecum and the faeces in comparison to the control. The results in a study by Lauková et al. [13] showed that E. faecium AL41 (109 CFU ml−1) colonized the intestine of farm ostriches in an approximately similar number and was able to control their intestinal microbiota composition. In our case, it also reduced the total enterococcal counts in the intestines as well as in the faeces. We assume that the reduction in enterococci in the experimental group was caused by the activity of the produced enterocin M (bacteriocin), which has a proteinaceous character with inhibitory activity against other enterococci [14]. The enterococcal bacteriocins are now attractive to scientists as potential drug candidates for antibiotic replacement in the treatment of multidrug-resistant pathogens. In addition, based on the observed immunomodulatory effects of E. faecium AL41 in this experiment, we hypothesize that the preferentially undesirable strains of enterococci were reduced.
5. Conclusions
The results of the presented study suggest that the administration of E. faecium AL41 had only negligible effect on the concentrations of evaluated acute phase proteins. On the other hand, we observed a significant increase in β- and γ-globulin fractions on day 11 of the experiment, which might indicate an improvement in the immune status. Moreover, our results revealed a significant prolonged stimulatory effect of E. faecium AL41 on the relative expression of all selected molecules as well as on the dynamic of mucus production in the chicken intestine. In addition, our strain AL41 significantly reduced the total numbers of enterococci in the cecum and faeces of broiler chickens. We propose that E. faecium AL41 is a suitable candidate for preventive administration to feed in terms of improving the intestinal mucosal barrier, which may ultimately increase the chickens’ defence against particularly intestinal pathogens.
Acknowledgments
We would like to thank Lauková A. DVM CSc. for the preparation of bacterial strains.
Author Contributions
Conceptualization, V.K., C.T. and R.S.; methodology, V.K., C.T., A.L., Z.F. and R.S.; software, V.K., C.T., R.S. and A.L.; validation, M.L. (Mikuláš Levkut) and V.K.; formal analysis, M.L. (Martin Levkut); investigation, V.R. and V.K.; resources, Z.Š. and R.Ž.; data curation, V.R. and R.H.; writing—original draft preparation, V.K, C.T. and R.S.; writing—review and editing, V.K.; visualization, R.Ž.; supervision, O.N. and M.L. (Mikuláš Levkut); project administration, V.K, Z.F. and M.L. (Mikuláš Levkut); funding acquisition, C.T, R.S. and M.L. (Martin Levkut). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant agency for Science of Slovakia VEGA 1/0107/21, VEGA 1/0554/21, VEGA 1/0177/22 and VEGA 1/0314/20 from the Ministry of Education, Science, Research and Sport of the Slovak Republic.
Institutional Review Board Statement
The experiment was approved by the Ethics Committee of the University of Veterinary Medicine and Pharmacy and the Committee for Animal Welfare of the Ministry of Agriculture of the Slovak Republic (Č.k.Ro-1184-3/2020-220).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nochi T., Jansen C.A., Toyomizu M., van Eden W. The well-developed mucosal immune systems of birds and mammals allow for similar approaches of mucosal vaccination in both types of animals. Front. Nutr. 2018;5:60. doi: 10.3389/fnut.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q., Eicher S.D., Applegate T.J. Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult. Sci. 2015;94:172–180. doi: 10.3382/ps/peu064. [DOI] [PubMed] [Google Scholar]
- 3.Everett M.L., Palestrant D., Miller S.E., Bollinger R.R., Parker W. Immune exclusion and immune inclusion: A new model of host-bacterial interactions in the gut. Clin. Appl. Immunol. Rev. 2004;4:321–332. doi: 10.1016/j.cair.2004.03.001. [DOI] [Google Scholar]
- 4.Sun R.C., Choi P.M., Guo J., Erwin C.R., Warner B.W. Insulin-like growth factor 2 and its enterocyte receptor are not required for adaptation in response to massive small bowel resection. J. Pediatr. Surg. 2014;49:966–970. doi: 10.1016/j.jpedsurg.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz S., Suchodolski J. Understanding the canine intestinal microbiota and its modification by pro-, pre- and synbiotics—what is the evidence? Vet. Med. Sci. 2016;2:71–94. doi: 10.1002/vms3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duangnumsawang Y., Zentek J., Goodarzi Boroojeni F. Development and Functional Properties of Intestinal Mucus Layer in Poultry. Front. Immunol. 2021;12:745849. doi: 10.3389/fimmu.2021.745849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jha R., Das R., Oak S., Mishra P. Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals. 2020;10:1863. doi: 10.3390/ani10101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forte C., Acuti G., Manuali E., Casagrande Proietti P., Pavone S., Trabalza-Marinucci M., Moscati L., Onofri A., Lorenzetti C., Franciosini M.P. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult. Sci. 2016;95:2528–2535. doi: 10.3382/ps/pew164. [DOI] [PubMed] [Google Scholar]
- 9.He T., Long S., Mahfuz S., Wu D., Wang X., Wei X., Piao X. Effects of Probiotics as Antibiotics Substitutes on Growth Performance, Serum Biochemical Parameters, Intestinal Morphology, and Barrier Function of Broilers. Animals. 2019;9:985. doi: 10.3390/ani9110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pogány Simonová M., Chrastinová Ľ., Lauková A. Effect of Enterococcus faecium AL41 (CCM8558) and its enterocin m on the physicochemical properties and mineral content of rabbit meat. Agriculture. 2021;11:1045. doi: 10.3390/agriculture11111045. [DOI] [Google Scholar]
- 11.Hanchi H., Mottawea W., Sebei K., Hammami R. The Genus Enterococcus: Between probiotic potential and safety concerns-an update. Front. Microbiol. 2018;9:1791. doi: 10.3389/fmicb.2018.01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanifeh M., Spillmann T., Huhtinen M., Sclivagnotis Y.S., Grönthal T., Hynönen U. Ex-Vivo adhesion of Enterococcus faecalis and Eenterococcus faecium to the intestinal mucosa of healthy beagles. Animals. 2021;11:3283. doi: 10.3390/ani11113283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauková A., Kandričáková A., Ščerbová J. Use of bacteriocin-producing, probiotic strain Enterococcus faecium AL41 to control intestinal microbiota in farm ostriches. Lett. Appl. Microbiol. 2015;60:531–535. doi: 10.1111/lam.12409. [DOI] [PubMed] [Google Scholar]
- 14.Mareková M., Lauková A., Skaugen M., Nes I. Isolation and characterization of a new bacteriocin, termed enterocin M, produced by environmental isolate Enterococcus faecium AL41. J. Ind. Microbiol. Biotechnol. 2007;34:533–537. doi: 10.1007/s10295-007-0226-4. [DOI] [PubMed] [Google Scholar]
- 15.Huang L., Luo L., Zhang Y., Wang Z., Xia Z. Effects of the dietary probiotic, Enterococcus faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia coli O78-challenged broiler chickens. Probiotics Antimicrob. Proteins. 2019;11:946–956. doi: 10.1007/s12602-018-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nawaz S., Asif M., Bhutta Z.A., Kulyar M.F., Hussain R., Ramzan A., Shafeeq S., Shakir M.Z., Sarfaraz M.T., Li K. A comprehensive review on acute phase proteins in chicken. Eur. Poult. Sci. 2021;85:344. [Google Scholar]
- 17.Skeldon N. Interpreting protein electrophoresis in practice. Practice. 2018;40:183–193. doi: 10.1136/inp.k1923. [DOI] [Google Scholar]
- 18.Karaffová V., Bobíková K., Husáková E., Levkut M., Herich R., Revajová V., Levkutová M., Levkut M. Interaction of TGF-β4 and IL-17 with IgA secretion in the intestine of chickens fed with E. faecium AL41 and challenged with S. enteritidis. Res. Vet. Sci. 2015;100:75–79. doi: 10.1016/j.rvsc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Letnická A., Karaffová V., Levkut M., Revajová V., Herich R. Influence of oral application of Enterococcus faecium AL41 on TGF-β4 and IL-17 expression and immunocompetent cell distribution in chickens challenged with Campylobacter jejuni. Acta Vet. Hung. 2017;65:317–326. doi: 10.1556/004.2017.031. [DOI] [PubMed] [Google Scholar]
- 20.Cobb-Vantress Inc. Cobb500 Slow Feather Breeder Management Supplement. Cobb-Vantress; Siloam Springs, AR, USA: 2020. [Google Scholar]
- 21.Karaffová V., Marcinková E., Bobíková K., Herich R., Revajová V., Stašová D., Kavuľová A., Levkutová M., Levkut M., Jr., Lauková A., et al. TLR4 and TLR21 expression, MIF, IFN-β, MD-2, CD14 activation, and sIgA production in chickens administered with EFAL41 strain challenged with Campylobacter jejuni. Folia Microbiol. 2017;62:89–97. doi: 10.1007/s12223-016-0475-6. [DOI] [PubMed] [Google Scholar]
- 22.Nagy O., Tóthová C., Nagyová V., Kováč G. Comparison of serum protein electrophoretic pattern in cows and small ruminants. Acta Vet. Brno. 2015;84:187–195. doi: 10.2754/avb201584020187. [DOI] [Google Scholar]
- 23.Karaffová V., Bobíková K., Levkut M., Revajová V., Ševčíková Z., Levkut M. The influence of Farmatan® and Flimabend® on the mucosal immunity of broiler chicken. Poult. Sci. 2019;98:1161–1166. doi: 10.3382/ps/pey517. [DOI] [PubMed] [Google Scholar]
- 24.Lammers A., Wieland W.H., Kruijt L., Jansma A., Straetemans T., Schots A., den Hartog G., Parmentier H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010;34:1254–1262. doi: 10.1016/j.dci.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Smirnov A., Tako E., Ferket P.R., Uni Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006;85:669–673. doi: 10.1093/ps/85.4.669. [DOI] [PubMed] [Google Scholar]
- 26.Mudroňová D., Karaffová V., Košcová J., Bartkovský M., Marcincáková D., Popelka P., Klempová T., Certík M., Mačanga J., Marcincák S. Effect of fungal gamma-linolenic acid and beta-carotene containing prefermented feed on immunity and gut of broiler chicken. Poult. Sci. 2018;97:4211–4218. doi: 10.3382/ps/pey306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Boever S., Vangestel C., De Backer P., Croubels S., Sys S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008;122:312–317. doi: 10.1016/j.vetimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Faixová Z., Piešová E., Maková Z., Levkut M., Jr., Pistl J., Lauková A., Faix Š., Levkut M. Effect of dietary probiotic and plant extract supplementation on mucin dynamics in the chicken intestine and on performance of chickens. Folia Vet. 2012;56:15–16. [Google Scholar]
- 29.Alkhalf A., Alhaj M., Al-Homidan I. Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi J. Biol. Sci. 2010;17:219–225. doi: 10.1016/j.sjbs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K.W., Lee S.H., Lillehoj H.S., Li G.X., Jang S.I., Babu U.S., Park M.S., Kim D.K., Lillehoj E.P., Neumann A.P., et al. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult. Sci. 2010;89:203–216. doi: 10.3382/ps.2009-00418. [DOI] [PubMed] [Google Scholar]
- 31.Chamanza R., Toussaint J.M., Van Edeeren A.M., Van Veen L., Hulskamp-Koch C., Fabri T.H.F. Serum amyloid A and transferrin in chicken. A preliminary investigation of using acute phase variables to assess diseases in chickens. Vet. Q. 1999;21:158–162. doi: 10.1080/01652176.1999.9695012. [DOI] [PubMed] [Google Scholar]
- 32.Kefal S., Toker N.Y. Effects of probiotics on some acute phase proteins in broilers exposed to Salmonella typhimurium lipopolysaccharides. Arch. Geflügelk. 2006;70:270–277. [Google Scholar]
- 33.Szabó A., Mézes M., Horn P., Sütő Z., Bázár G.Y., Romvári R. Developmental dynamics of some blood biochemical parameters in the growing turkey (Meleagris gallopavo) Acta Vet. Hung. 2005;53:397–409. doi: 10.1556/AVet.53.2005.4.1. [DOI] [PubMed] [Google Scholar]
- 34.Zheng A., Luo J., Meng K., Li J., Bryden W.L., Chang W., Zhang S., Wang L.X.N., Liu G., Yao B. Probiotic (Enterococcus faecium) induced responses of the hepatic proteome improves metabolic efficiency of broiler chickens (Gallus gallus) BMC Genom. 2016;17:89. doi: 10.1186/s12864-016-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cetin N., Güclü B.K., Cetin E. The effects of probiotic and mannanoligosaccharide on some haematological and immunological parameters in turkeys. J. Vet. Med. 2005;52:263–267. doi: 10.1111/j.1439-0442.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 36.Capitelli R., Crosta L. Overview of psittacine blood analysis and comparative retrospective study of clinical diagnosis, hematology and blood chemistry in selected psittacine species. Vet. Clin. Exot. Anim. 2013;16:71–120. doi: 10.1016/j.cvex.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Stef L., Julean C., Cean A., Mot D., Stef D.S., Simiz E., Simiz F., Pet I., Marcu A., Corcionivoschi N. Evaluation of the administration effects of probiotics against Campylobacter jejuni on the immune system of broiler chickens. J. Anim. Sci. Biotechnol. 2016;49:16–21. [Google Scholar]
- 38.Dev K., Mir N.A., Biswas A., Kannoujja J., Begum J., Kant R., Mandal A. Dietary symbiotic supplementation improves the growth performance, body antioxidant pool, serum biochemistry, meat quality, and lipid oxidative stability in broiler chickens. Anim. Nutr. 2020;6:325–332. doi: 10.1016/j.aninu.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingenbleek Y., Young V. Transthyretin (prealbumin) in health and disease: Nutritional implications. Annu. Rev. Nutr. 1994;14:495–533. doi: 10.1146/annurev.nu.14.070194.002431. [DOI] [PubMed] [Google Scholar]
- 40.Bogusławska-Tryk M., Ziółkowska E., Sławinska A., Siwek M., Bogucka J. Modulation of intestinal histology by probiotics, prebiotics and synbiotics delivered in ovo in distinct chicken genotypes. Animals. 2021;11:3293. doi: 10.3390/ani11113293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Hack M.E.A., El-Saadony M.T., Shafi M.E., Qattan S.Y.A., Batiha G.E., Khafaga A.F., Abdel-Moneim A.E., Alagawany M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- 42.Sicard J.F., Le Bihan G., Vogeleer P., Jacques M., Harel J. Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell Infect. Microbiol. 2017;7:387. doi: 10.3389/fcimb.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohland C.L., MacNaughton W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 44.Levkut M., Jr., Karaffová V., Levkutová M., Seman V., Revajová V., Ševčíková Z., Herich R., Levkut M. Influence of Lacto-Immuno-Vital on growth performance and gene expression of IgA, MUC-2, and growth factor IGF-2 in the jejunum of broiler chickens. Poult. Sci. 2020;99:6569–6575. doi: 10.1016/j.psj.2020.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aliakbarpour H., Chamani M., Rahimi G., Sadeghi A., Qujeq D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Anim. Biosci. 2012;25:1285–1293. doi: 10.5713/ajas.2012.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freier S., Eran M., Reinus C., Ariel I., Faber J., Wilschanski M., Braverman D. Relative expression and localization of the insulin-like growth factor system components in the fetal, child and adult intestine. J. Pediatr. Gastroenterol. Nutr. 2005;40:202–209. doi: 10.1097/00005176-200502000-00023. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y., Zhen W., Geng Y., Wang Z., Guo Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci. Rep. 2019;9:10256. doi: 10.1038/s41598-019-46578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]