Abstract
Background:
Platelet-rich fibrin matrix (PRFM) has not been extensively studied as other platelet concentrates such as Choukron's platelet-rich fibrin (PRF). This randomized controlled trial aimed to evaluate PRFM regenerative ability in human periodontal intrabony defects.
Materials and Methods:
Fifteen patients (age: 30–55 years) having probing pocket depth (PPD) ≥6 mm, and radiographic evidence of bilateral vertical intrabony defects were recruited. A split-mouth design was used in each patient; one quadrant of the arch was treated with open flap debridement (OFD) alone (control group) and the other quadrant with OFD + PRFM (test group). The PRFM was prepared by dual-spin technology using a patented thixotropic separator gel. Outcome measures comprising plaque index, gingival index (GI), PPD, clinical attachment level (CAL), depth of the defect, defect fill (DF), and percentage of DF (PDF) were recorded at baseline, after 3 months and 6 months. The parameters were applicably analyzed using the Friedman test, Fisher's exact test, t-test, paired t-test, repeated measures ANOVA, and Post Hoc-Bonferroni correction.
Results:
The GI, a net reduction in CAL, and PPD of the test group were significantly better than the control group at 3 months and 6 months (P < 0.05), while DF and PDF showed significant results at 6 months (P < 0.05). A consistent early wound healing index of 1 at 1 week was displayed in the test group (66.7%) in comparison to the control group (33.7%).
Conclusion:
PRFM can be a clinically significant periodontal regenerative material in the treatment of vertical intraosseous defects.
Keywords: Guided tissue regeneration, periodontal, periodontal bone loss, periodontal pocket, platelet-rich fibrin
Introduction
Regeneration of periodontium is a challenge. The process requires an organized succession of biological events. The events range from cell migration to differentiation and incorporation of numerous cytokines and growth factors for its regulation.[1] Developing therapeutics that revolve around the tenet of endogenous regenerative technology (ERT) is crucial to speed up the translation of these events into clinical setup. Such therapeutics can equip the host's innate ability for regeneration by stimulating and initiating a latent auto-repair mechanism in patients. ERT in the periodontal field also addresses the patient's autogenous tools for regeneration, which are fibrin scaffolds and patient-derived growth factors (GFs), to provide an ideal position in the site of injury where the progenitor or stem cells from the adjacent tissues can be inducted for in situ periodontal regeneration.[2,3]
Platelets contain abundant vital growth factors entrapped in the alpha granules. These growth factors include fibroblast growth factor, transforming growth factor β1, insulin-like growth factor, epithelial growth factor, vascular endothelial growth factor, and platelet-derived growth factor.[3,4,5] Such growth factors promote the regeneration irrespective of being utilized alone or in combination.[3,4] Hence platelets play a crucial role in regeneration in addition to hemostasis. Various platelet-derived products have surfaced due to the technological advancements and varied protocols in the procurement of platelet concentrate. Each of them possessing diverse biology and application and are classified based on their leukocyte and fibrin content.[6]
Extensive work has been conducted on the regenerative properties of platelet-rich plasma (PRP) and platelet rich fibrin (PRF).[7,8,9] Previous studies have reported that PRF combined with open flap debridement (OFD) enhanced augmentation and regeneration of periodontal bone defects.[10,11,12,13] PRF matrix (PRFM) belongs to the second generation of platelet concentrates with better mechanical and biological properties than PRP and PRF.[5,6,14,15,16,17] PRFM varies from conventional PRP in its preparation and properties; it is prepared by two-step centrifugation of blood, yielding a thrombin-free concentrated PRFM and is synonymous with pure-PRF.[6] PRFM has been extensively used in orthopedics, facial plastic surgery, and recently in extraction sockets to enhance wound healing and bone formation.[15,16,18,19] PRFM has also been used during ridge preservation procedures and has been shown to stimulate a socket's quick osseous fill.[20]
There are limited studies on the regenerative capacity of PRFM in treating periodontal defects, such as horizontal defects,[21] but none on intrabony defects. Moreover, the PRFM used in the current study was prepared by dual-spin technology using a patented thixotropic separator gel (PRFM kit) to separate the blood cell layer (white and red cells). A faster and longer second spin yielded the formation of a cross-linked fibrin matrix, which is dense, with a concentrated number of viable platelets.[20] The cross-linking stabilizes the clot, prevents retraction, creates a consistency that resists displacement, maintains space, and thereby inhibiting the soft tissue invasion.[20] Also, the well-structured, organized fibrin matrix acts as a scaffold for migrating cells during tissue repair.[19] This study aimed to evaluate PRFM as a regenerative material compared to OFD in human periodontal intrabony defects. The null hypothesis was that there would not be a significant difference in the outcome measures between the therapies involving only OFD (control group) and OFD + PRFM (test group).
Materials and Methods
Study design
The Institutional Ethics Committee KLE Society's Institute of Dental Sciences, Bangalore (KLEDC/IEC/11-2012/04) approved the study and registered with the US National Institutes of Health Clinical Trials Registry (Register No – NCT03616925). The methods followed in the study were as per the Helsinki Declaration of 1975, amended in 2013.
G*Power software, Version 3.0.5, Heinrich-Heine-Universität Düsseldorf, was used for estimating the sample size. Parameters used to calculate the sample size were the mean difference in clinical attachment level (CAL) (0.67), SD of difference (0.93), α level set at 0.05, and the power of the study (1−β error probability) set at 0.9. The total sample size estimated was 24, i.e., n = 12 sites. An additional 25% of sites (3 sites) for each group were included, considering the possibility of participant attrition.
This prospective, single-center, randomized controlled clinical trial, based on the convenience sampling method, employed a split-mouth design and a follow-up period of 6 months. The surgical sites were randomized by a computer-generated tabulation method into the test group (PRFM + OFD) and control group (OFD alone). Surgeries were performed 2 weeks apart on the contralateral sites.
Study participants
The patients were enrolled by a single therapist (LS) from the outpatient section of the Department of Periodontics, KLE Society's Institute of Dental Sciences, Bangalore, Karnataka, India. Fifteen patients, aged between 30 and 55 years (m = 42 ± 13 years; 10 males and 5 females), were enrolled for the study [Figure 1]. Written informed consent for voluntary enrolment and publishing the acquired data was obtained from the patients. Healthy patients who were never smokers with a probing pocket depth (PPD) ≥6 mm and radiographic evidence of bilateral vertical intrabony defects were included. Patients who used medications affecting the number and function of platelets in the past 3 months or suffering from platelet related or immunologic diseases or with abnormal platelet counts or any other condition contraindicating periodontal surgery, history of periodontal therapy in the last 6 months and pregnant or lactating women were excluded.
Figure 1.
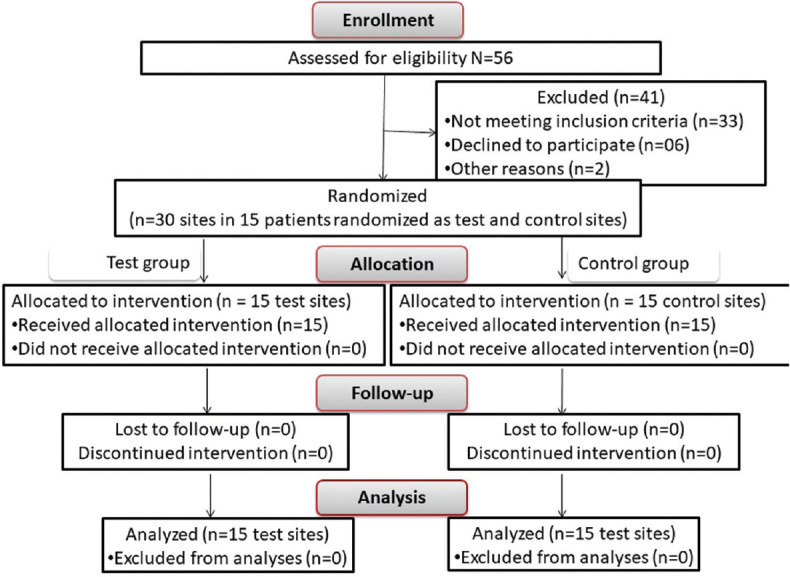
The CONSORT flowchart for patient enrolment, allocation, follow-up, and analysis
Presurgical procedure and baseline measurements
Following an initial examination and treatment planning, the selected patients were instructed for plaque control measures and subjected to Phase-I periodontal therapy, an occlusal adjustment, and a re-evaluation after four to 6 weeks.
Following random blood sugar level and complete hemogram, clinical parameters (plaque index [PI] by Silness and Loe 1964,[22] gingival index [GI]; Loe and Silness1963,[22] PPD and CAL) were recorded at baseline (BL) and followed up at 3 months, and 6 months postsurgery.
A customized acrylic stent with a reproducible fixed reference point (FRP) was fabricated on the patient's study cast to minimize the measurement error. The pre- and post-operative pocket depths were measured using a manual periodontal probe (No. 15 University of North Carolina) along the stent groove at a standardized entry point[23,24] [Figure 2a]. The distance from the FRP to the free gingival margin was recorded; CAL was determined by adding this distance to the PPD.[25]
Figure 2.
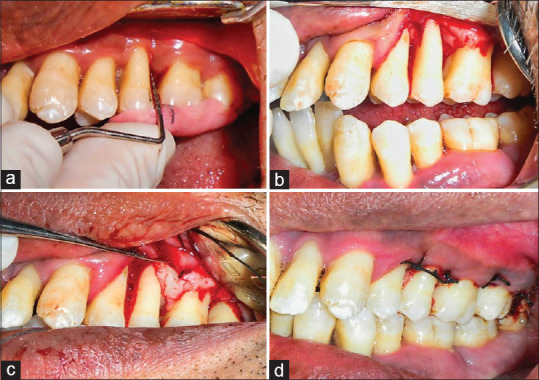
The clinical procedure of Platelet-rich fibrin matrix placement. (a) Preoperative clinical PPD. (b) Full-thickness flap reflected and debridement of the defect site. (c) Placement of platelet-rich fibrin matrix into the defect site. (d) Site sutured
Radiographic imaging of the surgical sites was performed using a long cone paralleling technique on an intraoral direct digital periapical radiovisiography (RVG-Suni Medical Imaging, Apteryx Inc., Acron, Ohio, USA). The exposures were made at 70 KVp, 7 mA for 0.2 s at a focus to film distance of 20 cm, using a film holder (Troll byte plus, Troll Dental, Trollhattan, Sweden). Linear measurements were made on the digitized images using analysis software (Image J software; Wayne Rasband, National Institute of Health-USA) to determine the intrabony defect depths.[26] The depth of defect (DD) was calculated by subtracting the distance between the cementoenamel junction (CEJ) to the base of the defect from CEJ to the alveolar crest.[27] The postoperative defect fill (DF) was calculated by subtracting the postoperative DD from DD at BL. The percentage of DF (PDF) was calculated using the below formula.[28]
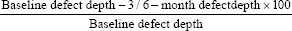
Surgical procedure
After achieving adequate anesthesia, a full-thickness mucoperiosteal flap was reflected. A thorough surgical degranulation followed by root planing and irrigation with normal saline was done [Figure 2b].
Control site
OFD was done, following which the elevated flaps were approximated with interrupted sutures, and a periodontal dressing was placed.
Test site
After thorough debridement, autologous PRFM was placed in situ, sutured, followed by a periodontal dressing [Figure 2c and d]. PRFM fabrication was done using a PRFM kit (Selphyl system Aesthetic factors, Wayne, New Jersey, U. S. A., Mesotherapy Worldwide, Dubai). A duel centrifuge technique consisting of an initial spin (REMI-8C, REMI, India) at 1100 g for 6 min to separate the blood cells from platelet-rich supernatant plasma followed-by supernatant spun at 1450 g for 15 min [Figure 3a-h].
Figure 3.
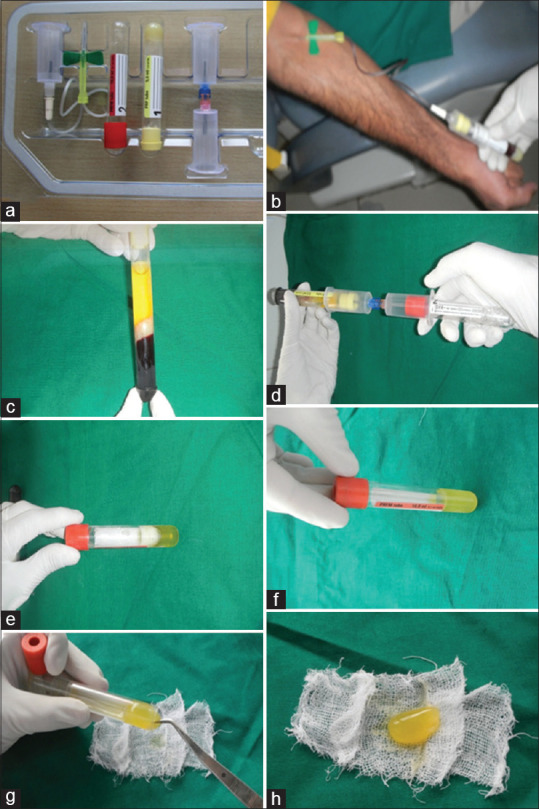
Kit and fabrication steps of platelet rich fibrin matrix. (a) Platelet-rich fibrin matrix kit. (b) Withdrawal of venous blood into a tube containing patented thixotropic separation gel. (c) Supernatant separated by centrifuge. (d) Transferring of supernatant to a second tube containing calcium chloride through a transferring device. (e) Transferred supernatant into the second tube for the second centrifuge. (f) Platelet-rich fibrin matrix procured. (g) Procured platelet-rich fibrin matrix gently being removed from the tube. (h) Final product platelet-rich fibrin matrix for application
A course of antibiotics, cap. Amoxicillin – LB (500 mg) thrice daily for 5 days and tablet diclofenac sodium (50 mg) thrice daily for 3 days and chlorhexidine digluconate (0.2%) mouth rinse (twice daily for 2 weeks) were prescribed postoperatively.[12] Every patient was given verbal and written postoperative instructions.
Postsurgical follow-up
One week following surgery, the periodontal dressing and sutures were removed, and the area was irrigated thoroughly with saline. Symptoms regarding discomfort, pain, and sensitivity were recorded, and the secondary outcome measure, early wound healing index (EHI),[29] was measured. The clinical and radiographic outcome measures were followed-up after 3 and 6 months.
Calibration of the study examiner and statistical analysis
A single therapist (KW) performed all the procedures, and the other examiner recorded all the clinical and radiographic measurements (SAB). Intra-examiner consistency assessed using the intra-class correlation coefficient was high (ICC = 0.9).
All the study parameters were analyzed using Statistical Package for the Social Sciences (I. B. M SPSSv. 10.5, Chicago, IL, USA); the significance of differences was set at α = 0.05. The intra-group comparison of PI and GI scores recorded at BL, 3 months, and 6 months were analyzed by the Friedman test, followed by Wilcoxon signed-rank test. PPD, CAL, and DD were analyzed using Repeated measures ANOVA, adjusted for lack of sphericity by Greenhouse–Geisser correction, followed by post hoc Bonferroni correction for pairwise comparison; the values of PDF at 3 months and 6 months postsurgery were analyzed using paired t-test. The intergroup comparison of PI and GI scores were analyzed using Fisher's exact test, while the values of PPD, CAL, DD, DF, and PDF were compared by using the Student's t-test.
Results
The evaluation was done in 30 intrabony sites in 15 patients. There were no dropouts in the number of individuals analyzed [Figure 1]. There were no complications reported during and after surgery among the patients from the control and test groups.
Intragroup comparisons of various time points
The site-specific changes in PI and GI during the BL and follow up time points were compared using the Freidman test. The Wilcoxon signed-rank test evaluated the differences in each pair of time points [Table 1]. Site-specific PI and GI scores in the control and test groups at different time points showed statistical improvement with time (P < 0.001). Except for 3 months versus 6 months of the test group's GI values (P = 0.08), all the pairs showed a significant improvement with time.
Table 1.
Intra-group comparison of clinical parameters, plaque index and gingival index at various time points
| Parameter | Group | Timepoint | Mean rank | Friedman test | Wilcoxon signed-rank test | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| χ 2 | P | Pairs | Z | P | ||||
| PI | Control | BL | 2.70 | 20.5 | <0.001* | BL versus 3M | 2.83 | 0.005* |
| 3M | 1.97 | BL versus 6M | 3.42 | 0.001* | ||||
| 6M | 1.33 | 3M versus 6M | 2.65 | 0.008* | ||||
| Test | BL | 2.93 | 26 | <0.001* | BL versus 3M | 3.61 | 0.001* | |
| 3M | 1.77 | BL versus 6M | 3.58 | 0.001* | ||||
| 6M | 1.3 | 3M versus 6M | 2.45 | 0.014* | ||||
| GI | Control | BL | 2.6 | 23.2 | <0.001* | BL versus 3M | 2.00 | 0.046* |
| 3M | 2.23 | BL versus 6M | 3.60 | 0.001* | ||||
| 6M | 1.17 | 3M versus 6M | 3.30 | 0.001* | ||||
| Test | BL | 3 | 28.5 | <0.001* | BL versus 3M | 3.51 | 0.001* | |
| 3M | 1.6 | BL versus 6M | 3.58 | 0.001* | ||||
| 6M | 1.4 | 3M versus 6M | 1.73 | 0.083 | ||||
*Significance set P<0.05. PI: PI (criteria of scoring according to Silness and Loe 1964); GI: GI (criteria of scoring according to Loe and Silness 1963); Control: Control group treated with open flap debridement only; Test: Test group treated with open flap debridement and Platelet-rich fibrin matrix. PI: Plaque index; GI: Gingival index; BL: Baseline; 3M: 3 months postsurgery; 6M: 6 months postsurgery
The intra-group comparison of PPD, CAL, and DD, was done using Repeated measures ANOVA, adjusted for lack of sphericity by Greenhouse–Geisser correction and followed by post hoc-Bonferroni correction test [Table 2]. The PPD and CAL values in both groups revealed a significant improvement with time (P < 0.05). The DD also displayed significant improvement with time; however, the post hoc test revealed no difference between the BL versus 3 months and 3 months versus 6 months in the control group.
Table 2.
Intra-group comparison of parameters, probing pocket depth, clinical attachment level and depth of the defect at various time points
| Parameter | Group | Time point | Mean±SD | ANOVA | Post hoc - Bonferroni correction | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| F | P | Pairs | MD (SE) | P | ||||
| PPD | Control | BL | 7.0±1.1 | 176.35 | <0.0005* | BL versus 3M | 1.40 (0.13) | 0.0005* |
| 3M | 5.6±1.1 | BL versus 6M | 2.33 (0.13) | 0.0005* | ||||
| 6M | 4.7±1.0 | 3M versus 6M | 0.93 (0.12) | 0.0005* | ||||
| Test | BL | 8.13±1.30 | 342.31 | <0.0005* | BL versus 3M | 2.73 (0.18) | 0.0005* | |
| 3M | 5.40±1.06 | BL versus 6M | 4.13 (0.17) | 0.0005* | ||||
| 6M | 4.00±0.93 | 3M versus 6M | 1.40 (0.13) | 0.0005* | ||||
| CAL | Control | BL | 6.27±1.28 | 65.28 | <0.0005* | BL versus 3M | 1.13 (0.13) | 0.0005* |
| 3M | 5.13±1.19 | BL versus 6M | 1.60 (0.16) | 0.0005* | ||||
| 6M | 4.67±1.05 | 3M versus 6M | 0.47 (0.13) | 0.011* | ||||
| Test | BL | 7.33±1.18 | 154.90 | <0.0005* | BL versus 3M | 1.80 (0.11) | 0.0005* | |
| 3M | 5.53±1.06 | BL versus 6M | 2.87 (0.19) | 0.0005* | ||||
| 6M | 4.47±0.74 | 3M versus 6M | 1.07 (0.18) | 0.0005* | ||||
| DD | Control | BL | 1.84±0.86 | 10.32 | 0.002* | BL versus 3M | 0.41 (0.16) | 0.059 |
| 3M | 1.42±0.58 | BL versus 6M | 0.74 (0.21) | 0.009* | ||||
| 6M | 1.10±0.42 | 3M versus 6M | 0.33 (0.11) | 0.031 | ||||
| Test | BL | 3.08±1.02 | 49.23 | <0.0005* | BL versus 3M | 0.59 (0.16) | 0.006* | |
| 3M | 2.49±1.02 | BL versus 6M | 1.72 (0.19) | 0.0005* | ||||
| 6M | 1.35±0.68 | 3M versus 6M | 1.14 (0.18) | 0.0005* | ||||
*Significance set at α=0.05. Control: Control group treated with open flap debridement only; Test: Test group treated with open flap debridement and Platelet-rich fibrin matrix; ANOVA: Repeated measures ANOVA, adjusted for lack of sphericity by Greenhouse-Geisser correction. PPD: Probing pocket depth; CAL: Clinical attachment level; DD: Depth of the defect; BL: Baseline; 3M: 3 months posturgery; 6M: 6 months postsurgery; SD: Standard deviation; MD: Difference of means; SE: Standard error
The intragroup comparison of PDF after 3 and 6 months was evaluated using paired t-test [Table 3]. Both the control and test groups showed a significant improvement in the PDF values with time (P < 0.05).
Table 3.
Intra-group comparison of mean percent defect fill after 3 and 6 months from baseline
| Group | Time line | Mean±SD | Paired difference, mean±SD | Paired t-test | |
|---|---|---|---|---|---|
|
| |||||
| t | P | ||||
| Control | 3M | 17.45±23.42 | 16.28±15.5 | 4.1 | 0.001* |
| 6M | 33.73±24.69 | ||||
| Test | 3M | 18.81±17.50 | 36.79±15.2 | 9.34 | 0.005* |
| 6M | 55.61±18.04 | ||||
*Significance set at α=0.05. Control: Control group treated with open flap debridement only; Test: Test group treated with open flap debridement and Platelet-rich fibrin matrix. 3M: 3 months postsurgery; 6M: 6 months postsurgery; SD: Standard deviation
Comparison of outcome measures between the two groups
The observed and expected PI, GI, and EHI frequencies between the two groups were analyzed using Fisher's exact test [Table 4]. There was no statistically significant difference between the groups regarding the PI scores at BL, 3 months, and 6 months (BL: P = 0.99; 3 months: P = 0.09; 6 months: 0.13). There was no statistically significant GI difference between the groups at BL, but the test group bettered the control group at 3 months and 6 months (P < 0.05). There was no difference between the groups regarding EHI after a week. However, the index score of 1 was found in a greater number of patients (10) in the test group compared to the control group.
Table 4.
Observed and expected frequencies of plaque, gingival, and early wound healing indices between the two groups according to Fisher’s exact test
| Parameter | Timeline | Scores | Control* | Test* | P † |
|---|---|---|---|---|---|
| PI | BL | 0 | 0 (0) | 0 (0) | 0.999 |
| 1 | 6 (40) | 7 (46.7) | |||
| 2 | 9 (60) | 8 (53.3) | |||
| 3M | 0 | 1 (6.7) | 5 (33.3) | 0.092 | |
| 1 | 12 (80) | 10 (66.7) | |||
| 2 | 2 (13.3) | 0 (0) | |||
| 6M | 0 | 6 (40) | 11 (73.3) | 0.139 | |
| 1 | 9 (60) | 4 (26.7) | |||
| 2 | 0 (0) | 0 (0) | |||
| GI | BL | 0 | 0 (0) | 0 (0) | 1.00 |
| 1 | 3 (20) | 3 (20) | |||
| 2 | 12 (80) | 12 (80) | |||
| 3M | 0 | 0 (0) | 11 (73.3) | 0.0005 | |
| 1 | 7 (46.7) | 4 (26.7) | |||
| 2 | 8 (53.3) | 0 (0) | |||
| 6M | 0 | 3 (20) | 14 (93.3) | 0.0005 | |
| 1 | 12 (80) | 1 (6.7) | |||
| 2 | 0 (0) | 0 (0) | |||
| EHI | 1W | 1 | 5 (33.3) | 10 (66.7) | 0.134 |
| 2 | 8 (53.3) | 5 (33.3) | |||
| 3 | 2 (13.3) | 0 (0) |
*Values formatted as observed (expected); †Significance set at α=0.05. Control: Control group treated with open flap debridement only; Test: Test group treated with open flap debridement and Platelet-rich fibrin matrix; Scores: Criteria of scoring for PI according to Silness and Loe (1964) and GI according to Loe and Silness (1963). BL: Baseline; 3M: 3 months; 6M: 6 months; 1W: 1 week; PI: Plaque index; GI: Gingival index; EHI: Early wound healing index
The outcome measures, PPD, a net reduction in PPD, CAL, a net reduction in CAL, DD, DF, and PDF were compared between the test and control groups using Student's t-test [Table 5]. The BL values of PPD, CAL, and DD in the test group were significantly more than that of the control group. However, after 3 months and 6 months, the PPD and CAL in the test group improved to yield marginally lesser than that of control.
Table 5.
Intergroup comparison of outcome measures, probing pocket depth, clinical attachment level, depth of the defect, and percentage of defect fill
| Parameter | Timeline | Control, mean±SD | Test group | t-test | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean±SD | MD | t | P | |||
| Periodontal pocket depth (mm) | BL | 7.00±1.07 | 8.13±1.30 | −1.13 | −2.61 | 0.02* |
| 3M | 5.60±1.06 | 5.40±1.06 | 0.20 | 0.52 | 0.61 | |
| 6M | 4.67±1.05 | 4.00±0.93 | 0.67 | 1.85 | 0.08 | |
| Periodontal pocket depth (reduction in mm) | 3M | 1.40±0.51 | 2.73±0.70 | −1.33 | −5.95 | 0.0005* |
| 6M | 2.33±0.49 | 4.13±0.64 | −1.80 | −8.66 | 0.0005* | |
| CAL (mm) | BL | 6.27±1.28 | 7.33±1.18 | −1.07 | −2.38 | 0.02* |
| 3M | 5.13±1.19 | 5.53±1.06 | −0.40 | −0.97 | 0.34 | |
| 6M | 4.67±1.05 | 4.47±0.74 | 0.20 | 0.60 | 0.55 | |
| CAL (reduction in mm) | 3M | 1.13±0.52 | 1.80±0.41 | −0.67 | −3.90 | 0.0005* |
| 6M | 1.60±0.63 | 2.87±0.74 | −1.27 | −5.03 | 0.0005* | |
| DD (mm) | BL | 1.84±0.86 | 3.08±1.02 | −1.24 | −3.59 | 0.0005* |
| 3M | 1.42±0.58 | 2.49±1.02 | −1.07 | −3.52 | 0.0005* | |
| 6M | 1.10±0.42 | 1.35±0.68 | −0.25 | −1.23 | 0.23 | |
| Defect fill (mm) | 3M | 0.29±0.57 | 0.35±0.26 | −0.05 | −0.33 | 0.74 |
| 6M | 0.61±0.67 | 1.50±0.60 | −0.89 | −3.84 | 0.0005* | |
| PDF (%) | 3M | 17.45±23.42 | 18.81±17.50 | −1.36 | −0.18 | 0.858 |
| 6M | 33.73±24.69 | 55.61±18.04 | −21.88 | −2.77 | 0.01* | |
P: P value set at significance, α=0.05; *Statistically significant. Control group: Treatment with open flap debridement only; Test group: Treatment with open flap debridement and platelet-rich fibrin matrix. BL: Baseline; 3M: 3 months; 6M: 6 months; SD: Standard deviation; MD: Difference in means; t: t-value; PDF: Percentage of defect fill; CAL: Clinical attachment level; DD: Depth of the defect; PDF: Percentage of defect fill
The net reductions in PPD, and CAL, and DF at both 3 months and 6 months were significantly better in the test group than in control (P < 0.05) [Figure 4]; the PDF was not statistically different at 3 months, but the mean values of the test group were significantly better than that of control at 6 months.
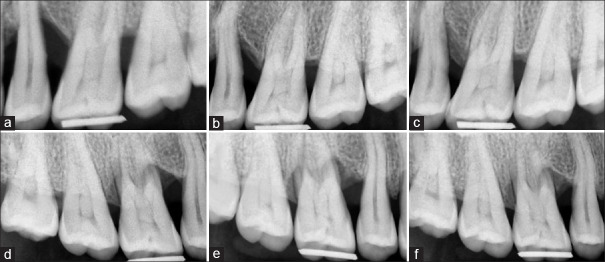
Figure 4.
Radiographic images of follow-up at baseline, 3 months, and 6 months intervals. (a) An intrabony defect from the test group at baseline. (b) An improvement in the intrabony defect from the test group at 3 months. (c) An improvement in the intrabony defect from the test group at 6 months. (d) An intrabony defect from the control group at baseline. (e) An improvement in the intrabony defect from the control group at 3 months. (f) An improvement in the intrabony defect from the control group at 6 months
Discussion
The present randomized controlled, double-blind clinical study was carried out to evaluate the efficacy of PRFM as a regenerative material in human 3-walled intraosseous periodontal defects. The periodontal tissue response to PRFM + OFD (test group) and only OFD (control) was evaluated by recording EHI, PI, GI, PPD, CAL, DD, DF, and PDF. The study found that the net reductions in PPD and CAL, and GI scores were significantly different in the groups at both 3 months and 6 months after surgery; the DF and PDF were different only after 6 months; The parameters, EHI, PI were not significantly different. Therefore, the null hypothesis was partly rejected.
The intergroup comparison of PPD and CAL at 3 months and 6 months and DD at 6 months did not produce significantly different values [Table 5]. However, it is noteworthy that the samples’ randomization conceded the BL values of PPD, CAL, and DD in the test group to be significantly higher. Moreover, the mean values of PPD, CAL, and DD in the test group at 6 months were approximately equal to that of the control, indicating better clinical results in the test group. The net reduction in PPD and CAL, DF, and PDF were also evaluated to tweak the interpretation of the results [Table 5]. The net reductions in PPD and CAL at both 3 months and 6 months were significantly higher in the test group than in control (P < 0.05); the DF and PDF were not statistically different at 3 months but showed significantly higher values after 6 months. There was no statistically significant difference in GI scores between the groups at BL, but the test group bettered the control group at 3 months and 6 months [Table 4]. These results confirmed significantly better clinical results in the test groups. A statistically significant net reduction in PPD and CAL and an increase in the DF and PDF in the test group implies that PRFM might have aided in the soft and hard tissue augmentation. A recent study conducted on horizontal bony defects, using intra-marrow penetration + OFD + PRFM reported similar results; there was no difference between test and control (OFD alone) in PI and GI scores at 9 months, while the test group improved significantly on PPD, CAL, DD, and PDF.[21] However, the PRFM in the previous study[21] was produced from a single spin technique, while the PRFM material in our study was prepared by using double spin and a patented thixotropic separator gel (Selphyl system Aesthetic factors). The biological properties of PRFM produced by dual-spin technology are superior to the single spin technology.[17] There are no research reports on evaluating PRFM efficacy on intraosseous defects; however, several studies supported the efficacy of PRF in periodontal regeneration.[11,30,31] A recent systematic literature review reported PRF to be an effective remedy in regenerating gingiva in patients with gingival recession.[32] In a human study using PRFM in extraction sockets, it was found that after 4 months, the sockets with PRFM showed rapid and enhanced osseous fill compared to barrier membranes and demineralized freeze-dried bone allografts (DFDBA).[33] The study concluded that the prolonged presence of growth factors in the PRFM scaffold at the healing sites could be the most significant factor that caused rapid osseous fill. The findings of the present study, supported by previous studies’ conclusions, allude to the potential of PRFM as a promising regenerative material.
The test group's favorable outcomes with significant osseous and soft tissue responses could be associated with the PRFM because there is sufficient evidence to suggest that PRFM can accelerate tissue regeneration by stimulating normal physiology.[14,17,20] It can be accredited to the platelets viable in PRFM, containing intrinsic growth factors and the fibrin matrix that acts as a scaffold for migrating endothelial cells, osteoblasts, and other cells required for tissue repair.[3,4,17] Furthermore, A study reported that the application of PRF in an intraosseous defect produced elevated levels of platelet-derived growth factor-BB in the crevicular fluid.[34]
No adverse events occurred during and after the surgery in the test group. Moreover, intra-group comparison of PI, GI, PPD, CAL, DD, and PDF at various time points in the test group indicated a consistent improvement with time, suggesting that PRFM is a safe and effective adjunctive for periodontal regeneration. The intragroup improvement in PPD reduction at 6 months compared to 3 months in both the groups [Table 2] could be attributed to the continued clinical attachment gain and bone fill as part of the regenerative process over time and not just to the initial tissue shrinkage.
There was no statistically significant difference between the groups regarding the PI scores at BL, 3 months, and 6 months. Also, there was no difference between the groups regarding EHI after a week [Table 4]. No difference in the PI scores between the test and control group suggested that the patient's ability to maintain the plaque control measures after grafting PRFM at the surgical site was similar to the conventional OFD. A similar EHI index suggested that the early healing of the soft tissue was also similar. According to the wound-healing index, uneventful healing is associated with minimal fibrin formation due to reduced surgical site trauma.[29] Results showed complete closure of flap with no fibrin line in the interproximal area of patients in the test group (66.7%) than the control group (33.7%). A canine study conducted a histological evaluation of extraction socket healing and found that PRFM produced rapid healing with higher osseous fill than non-viable materials (DFDBA).[33]
A split-mouth design was used in which 30 sites in 15 patients were selected randomly; this ensured the exclusion of the patient's specific characteristics and facilitated interpretation of the trial by reducing inter-patient variability.[35] Radiographic assessment is a painless, noninvasive method contrary to direct bone measurement; it is an effective method to measure the bone parameters to study regenerative procedures.[36] The study's limitations included the inability to assess the nature of regenerated periodontal tissues by histologic investigation due to ethical constraints and not considering the extent of possible gingival recession, which is a consequence of the employed surgical approach.
Conclusion
Considering the significant improvement in the clinical and radiographic parameters within this study's scope and limitations, PRFM can be recommended as a potential periodontal regenerative material in treating 3-walled intraosseous periodontal defects. Grafting PRFM in the intrabony defect after OFD can be considered safe and efficient. PRFM application may be a novel approach for periodontal regeneration; however, multicentre clinical trials with prospective, randomized controlled studies with larger sample sizes and long-term follow-up should be undertaken in the future to ascertain the current results. Furthermore, the periodontal regeneration capability of the PRFM graft needs to be confirmed by histomorphometry. Provided the limitations, this study's clinical relevance is that it evaluates and substantiates the utilization of this novel material with a novel strategy for procurement, ameliorate version of PRP amidst the plethora of platelet concentrates as a promising regenerative material in the field of periodontal regeneration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This clinical study was partially supported by a research grant from Medical Education Research Trust (MERT), (Project no: 2013-14/999). The authors thank Dr. Ashwin Prabhu for the technical assistance for this clinical study.
References
- 1.McCulloch CA. Basic considerations in periodontal wound healing to achieve regeneration. Periodontol 2000. 1993;1:16–25. [PubMed] [Google Scholar]
- 2.Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31:7892–927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Nevins M, Kao RT, McGuire MK, McClain PK, Hinrichs JE, McAllister BS, et al. Platelet-derived growth factor promotes periodontal regeneration in localized osseous defects: 36-month extension results from a randomized, controlled, double-masked clinical trial. J Periodontol. 2013;84:456–64. doi: 10.1902/jop.2012.120141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Leeman E, Carnes DC, Graves DT. Human osteoblasts synthesize and respond to platelet-derived growth factor. Am J Physiol. 1991;261:C348–54. doi: 10.1152/ajpcell.1991.261.2.C348. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee A, Debnath K. Comparative evaluation of growth factors from platelet concentrates: An in vitro study. J Indian Soc Periodontol. 2019;23:322–8. doi: 10.4103/jisp.jisp_678_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Hou X, Yuan J, Aisaiti A, Liu Y, Zhao J. The effect of platelet-rich plasma on clinical outcomes of the surgical treatment of periodontal intrabony defects: A systematic review and meta-analysis. BMC Oral Health. 2016;16:71. doi: 10.1186/s12903-016-0261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotsovilis S, Markou N, Pepelassi E, Nikolidakis D. The adjunctive use of platelet-rich plasma in the therapy of periodontal intraosseous defects: A systematic review. J Periodontal Res. 2010;45:428–43. doi: 10.1111/j.1600-0765.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- 9.Mohan SP, Jaishangar N, Devy S, Narayanan A, Cherian D, Madhavan SS. Platelet-rich plasma and platelet-rich fibrin in periodontal regeneration: A review. J Pharm Bioallied Sci. 2019;11:S126–S130. doi: 10.4103/JPBS.JPBS_41_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–34. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Chandradas ND, Ravindra S, Rangaraju VM, Jain S, Dasappa S. Efficacy of platelet rich fibrin in the treatment of human intrabony defects with or without bone graft: A randomized controlled trial. J Int Soc Prev Community Dent. 2016;6:S153–9. doi: 10.4103/2231-0762.189753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yajamanya SR, Chatterjee A, Hussain A, Coutinho A, Das S, Subbaiah S. Bioactive glass versus autologous platelet-rich fibrin for treating periodontal intrabony defects: A comparative clinical study. J Indian Soc Periodontol. 2017;21:32–6. doi: 10.4103/0972-124X.201628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel GK, Gaekwad SS, Gujjari SK, Veerendra Kumar SC. Platelet-rich fibrin in regeneration of intrabony defects: A randomized controlled trial. J Periodontol. 2017;88:1192–9. doi: 10.1902/jop.2017.130710. [DOI] [PubMed] [Google Scholar]
- 14.O’Connell S, Carroll R, Beavis A, Arnoczky S. Flow Cytometric Characterization of Cascade Platelet-Rich Fibrin Matrix (PRFM); The impact of Exogenous Thrombin on Platelet Concentrates (PC) In: Edison NJ, editor. Vol. 4. Musculoskeletal Transplant Foundation; 2006. p. 66. [Google Scholar]
- 15.O’Connell SM, Impeduglia T, Hessler K, Wang XJ, Carroll RJ, Dardik H. Autologous platelet-rich fibrin matrix as cell therapy in the healing of chronic lower-extremity ulcers. Wound Repair Regen. 2008;16:749–56. doi: 10.1111/j.1524-475X.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell SM, Hessler K, Dardik H. Cascade® autologous system platelet-rich fibrin matrix in the treatment of chronic leg ulcers. Adv Wound Care (New Rochelle) 2012;1:52–5. doi: 10.1089/wound.2011.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucarelli E, Beretta R, Dozza B, Tazzari PL, O’Connel SM, Ricci F, et al. A recently developed bifacial platelet-rich fibrin matrix. Eur Cell Mater. 2010;20:13–23. doi: 10.22203/ecm.v020a02. [DOI] [PubMed] [Google Scholar]
- 18.Sclafani AP. Safety, efficacy, and utility of platelet-rich fibrin matrix in facial plastic surgery. Arch Facial Plast Surg. 2011;13:247–51. doi: 10.1001/archfacial.2011.3. [DOI] [PubMed] [Google Scholar]
- 19.Mohammed SA, Alhijazi AY. Evaluation the effect of platelet rich fibrin matrix on bone healing. J Baghdad Coll Dent. 2011;23:65–70. [Google Scholar]
- 20.Simon BI, Gupta P, Tajbakhsh S. Quantitative evaluation of extraction socket healing following the use of autologous platelet-rich fibrin matrix in humans. Int J Periodontics Restorative Dent. 2011;31:285–95. [PubMed] [Google Scholar]
- 21.Debnath K, Chatterjee A. Treatment of horizontal defect with and without platelet-rich fibrin matrix: A randomized comparative clinical study. J Indian Soc Periodontol. 2018;22:406–13. doi: 10.4103/jisp.jisp_129_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(Suppl):610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 23.Rams TE, Slots J. Comparison of two pressure-sensitive periodontal probes and a manual periodontal probe in shallow and deep pockets. Int J Periodontics Restorative Dent. 1993;13:520–9. [PubMed] [Google Scholar]
- 24.Vandana KL, Gupta I. The location of cemento enamel junction for CAL measurement: A clinical crisis. J Indian Soc Periodontol. 2009;13:12–5. doi: 10.4103/0972-124X.51888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meadows CL, Gher ME, Quintero G, Lafferty TA. A comparison of polylactic acid granules and decalcified freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1993;64:103–9. doi: 10.1902/jop.1993.64.2.103. [DOI] [PubMed] [Google Scholar]
- 26.Talaiepour AR, Panjnoush M, Soleimanishayeste Y, Abesi F, Sahba S. A survey on the accuracy of radiovisiography in the assessment of interproximal intrabony defects. J. Dent. Tehran Univ. Med. Sci. 2005;2:29–32. [Google Scholar]
- 27.Trombelli L, Heitz-Mayfield LJ, Needleman I, Moles D, Scabbia A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J Clin Periodontol. 2002;29(Suppl 3):117–35. doi: 10.1034/j.1600-051x.29.s3.7.x. [DOI] [PubMed] [Google Scholar]
- 28.Eickholz P, Lenhard M, Benn DK, Staehle HJ. Periodontal surgery of vertical bony defects with or without synthetic bioabsorbable barriers.12-month results. J Periodontol. 1998;69:1210–7. doi: 10.1902/jop.1998.69.11.1210. [DOI] [PubMed] [Google Scholar]
- 29.Wachtel H, Schenk G, Böhm S, Weng D, Zuhr O, Hürzeler MB. Microsurgical access flap and enamel matrix derivative for the treatment of periodontal intrabony defects: A controlled clinical study. J Clin Periodontol. 2003;30:496–504. doi: 10.1034/j.1600-051x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 30.Ustaoğlu G, Uğur Aydin Z, Özelçi F. Comparison of GTR, T-PRF and open-flap debridement in the treatment of intrabony defects with endo-perio lesions: A randomized controlled trial. Med Oral Patol Oral Cir Bucal. 2020;25:e117–23. doi: 10.4317/medoral.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruthi PJ, Yadav N, Nawal RR, Talwar S, Lamba AK. Novel use of PRF and PDT in the management of trauma induced root resorption and infrabony defect. J Clin Diagn Res. 2015;9:ZD26–8. doi: 10.7860/JCDR/2015/12017.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miron RJ, Moraschini V, Del Fabbro M, Piattelli A, Fujioka-Kobayashi M, Zhang Y, et al. Use of platelet-rich fibrin for the treatment of gingival recessions: A systematic review and meta-analysis. Clin Oral Investig. 2020;24:2543–57. doi: 10.1007/s00784-020-03400-7. [DOI] [PubMed] [Google Scholar]
- 33.Simon B, Zatcoff A, Kong J, O’Connell S. Clinical and histological comparison of extraction socket healing following the use of autologous platelet-rich fibrin matrix (PRFM) to ridge preservation procedures employing demineralized freeze dried bone allograft material and membrane. Open Dent J. 2009;3:92. doi: 10.2174/1874210600903010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi AA, Padhye AM, Gupta HS. Platelet derived growth factor-BB levels in gingival crevicular fluid of localized intrabony defect sites treated with platelet rich fibrin membrane or collagen membrane containing recombinant human platelet derived growth factor-BB: A randomized clinical and biochemical study. J Periodontol. 2019;90:701–8. doi: 10.1002/JPER.18-0496. [DOI] [PubMed] [Google Scholar]
- 35.Hujoel P, DeRouen T. Validity issues in split-mouth trials. J Clin Periodontol. 1992;19:625–7. doi: 10.1111/j.1600-051x.1992.tb01709.x. [DOI] [PubMed] [Google Scholar]
- 36.Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human infrabony defects. II. Re-entry procedures and bone measures. J Periodontol. 1993;64:261–8. doi: 10.1902/jop.1993.64.4.261. [DOI] [PubMed] [Google Scholar]