Abstract
Context:
Rosuvastatin (RSV) is a new synthetic, hydrophilic statin with potent anti-inflammatory and osseodifferentiation actions. Autogenous bone graft (ABG) is still considered the gold standard in reconstructive bone surgery. Addition of platelet-rich fibrin (PRF) to ABG provides sustained release of various growth factors and facilitates survival of the graft.
Aims:
The study aims to clinically and radiographically compare the effectiveness of ABG and PRF with and without 1.2 mg RSV gel in the surgical treatment of intrabony defect in chronic Periodontitis patient.
Settings and Design:
This was a randomized controlled clinical trial.
Subjects and Methods:
Thirty-nine patients (one site per participant) with chronic periodontitis were randomly divided into three groups: Group 1 (open flap debridement [OFD] + placebo), Group 2 (OFD + ABG + PRF), and Group 3 (OFD + ABG + PRF + 1.2 mg RSV). Relative attachment level (RAL) and probing pocket depth (PPD) were recorded at baseline, 3, 6, and 9 months. Radiographic measurements such as defect height (A and B) and defect width (C) were calculated at baseline and 9 months.
Statistical Analysis Used:
Intergroup comparison was done using Kruskal–Wallis ANOVA. An intragroup comparison was done using Friedman test and Wilcoxon signed-rank test.
Results:
The mean PPD reduction and mean RAL gain were highly significant in Group 3 and Group 2 than Group 1. For Group 3, a significant reduction of defect height and width and a significant amount of bone fill were achieved than Group 2 and Group 1.
Conclusions:
Addition of 1.2 mg RSV gel, PRF, and ABG has synergistic effects, explaining their role as a regenerative material in the treatment of intrabony defects.
Keywords: Autogenous bone graft, intrabony defects, platelet-rich fibrin, regenerative periodontal surgery, rosuvastatin
Introduction
Chronic periodontitis is an inflammatory destructive disease of supporting periodontal tissues, leading to progressive attachment loss, bone loss, tooth mobility, and early tooth loss. Periodontal disease is characterized by the formation of osseous defects including furcation and intrabony defects.[1] Tissue destruction is mainly due to host inflammatory immune response to bacterial challenge characterized by the production of inflammatory cytokines and reactive oxygen species.[2] The severity of periodontal breakdown is directly related to increased pro-inflammatory cytokines and reduced anti-inflammatory cytokine levels. Intrabony defects are an anatomic sequel to the apical spread of plaque in the course of periodontitis. The goal of periodontal therapy includes not only the reduction of tissue inflammation but also the regeneration of lost periodontal tissues including bone defects. Regenerative therapies used should provide architectural support, vascular in growth, cellular recruitment, and clot stabilization. A combination of therapies may be required to achieve optimum results.
Statins which are commonly used hypolipidemic drugs act by inhibiting HMG coenzyme A reductase. Statins possess a wide range of pleiotropic effects such as antioxidant,[3] anti-inflammatory,[4] immunomodulatory,[5] and osteomodulatory effects.[6] Rosuvastatin (RSV) is a second-generation sulfur-containing, hydrophilic, synthetic statin. They are mainly used in reducing serum cholesterol and risk of cardiovascular disease through competitive inhibition of HMG-Co enzyme A reductase.[7] Due to their potent anti-inflammatory and osteostimulatory action, they have been widely applied in periodontal regenerative procedures. RSV inhibits p-selectin synthesis by endothelial cells via increased nitric oxide production. Reduced levels of C reactive protein in response to pro-inflammatory cytokines contribute to prevention and remission of periodontal disease.[8] Regenerative potential of RSV is mainly due to increased BMP-2 expression and enhanced alkaline phosphatase activity.[9] RSV is more potent with greater anti-inflammatory action and longer elimination half time in comparison to simvastatin and atorvastatin. RSV significantly reduced gingival inflammation, increased clinical attachment gain, and led to clinical and radiographic improvements in intrabony defects, when used as an adjunct in periodontal therapy.[10]
Autogenous bone grafts (ABGs) are still considered to be gold standard in periodontal regenerative procedures. They possess the most desirable properties for bone formation which includes osteogenesis, osteoconduction, and osteoinduction. Autogenous cortical bone graft can be obtained from a site adjacent to defect. It prevents the need for the second surgical site while leading to efficient regeneration in intraosseous periodontal defects.[11]
Platelet-rich fibrin (PRF), a second-generation platelet concentrate, was developed by Choukroun et al. (2006). It is an intimate assembly of slow polymerizing fibrin network. Slow polymerization during processing leads to incorporation of cytokines, glycan chain, and structural glycoproteins which facilitates healing.[12] The three-dimensional fibrin matrix can be used as a scaffold for various periodontal regenerative procedures. Addition of PRF to ABG binds the graft particles together, thus providing appropriate areas for new bone formation, especially during early healing. PRF also facilitates cellular migration and vascularization at the regenerative site leading to the survival of the graft. As fibrin matrix resorbs, cytokines (platelet-derived growth factor, transforming growth factor-beta 1, and insulin-like growth factor 1) are released gradually, thus providing a continual process of healing.[13] Due to the greater wound healing potential and wide range of applications, PRF has been considered a boon in field of periodontics. A greater improvement in clinical and radiographic parameters has been observed when PRF is used in the treatment of intrabony defects.[14] The present study aims to compare and evaluate the clinical and radiographic effectiveness of ABG and PRF with or without 1.2 mg RSV in the surgical treatment of intrabony defects in chronic periodontitis patients.
Subjects and Methods
Study Population: The present study is a 9-month, randomized, double matched, controlled clinical study. The study enrolled a total of 39 patients with chronic periodontitis. Written informed consent was obtained from each participant. Ethical clearance was obtained from the Institutional Ethical Committee.
Selection criteria
Inclusion criteria
Systemically healthy patients diagnosed with generalized chronic periodontitis having clinical attachment level ≥ 3 mm, probing depth ≥5 mm with vertical bone loss of ≥ 3 mm as measured from cementoenamel junction (CEJ) to the base of defect on at least one molar with two- or three-walled intrabony defect, presence of more than 20 natural teeth, sufficient platelet count (≥200,000/μL) for PRF preparation, and cooperative patients showing acceptable oral hygiene during presurgical (Phase 1) therapy with plaque index and gingival index scores <2.
Exclusion criteria
Patients with any systemic condition or medication that could alter periodontal status, substance/tobacco abuse, allergy to statin group, coagulation defects or on anticoagulant treatment, pregnant and lactating mother, and with lack of linguistic or psychiatric disorder or decline to sign informed consent.
Presurgical therapy
General assessments of participants were made through history, clinical examination, and routine investigations. All participants were treated with Phase 1 therapy including scaling and root planing and oral hygiene instructions with modified Bass method of toothbrushing. Following Phase 1 therapy, participants were evaluated after 6 weeks and those who met selection criteria were enrolled in the study. Clinical and radiographic parameters were recorded at baseline.
Patient grouping
Six weeks after Phase 1 therapy, selected sites (per site per patient) were randomly divided into 3 treatment groups (13 sites in each group). OFD of intrabony defect was done in all participants, followed by placement of placebo gel in Group 1 (OFD + placebo), mixture of ABG and PRF in Group 2 (OFD + ABG + PRF), and mixture of ABG and PRF with 1.2 mg RSV gel in Group 3 (OFD + ABG + PRF + RSV), respectively.
Platelet-rich fibrin preparation
PRF preparation followed Choukroun's protocol; 10 ml blood sample of patient was obtained by venipuncture method from median cubital vein of antecubital fossa without anticoagulant in a glass test tube. Test tube was centrifuged immediately at 3000 rpm for 10 min. The resultant product consists of 3 layers with PRF clot in the middle.
Rosuvastatin gel formulation
RSV preparation followed Thylin et al.'s protocol.[15] To a biocompatible solvent, preweighted methylcellulose was added and agitated at 50°C–60°C to obtain a clear solution. Preweighted quantity of RSV was added and dissolved to achieve a homogenous gel with a concentration of 1.2 mg.
Surgical procedure
0.12% chlorhexidine rinse was used for intraoral antisepsis. Extraoral antisepsis was achieved using iodine solution. After adequate local anesthesia, buccal and lingual incisions were made and mucoperiosteal flaps reflected. Meticulous defect and root debridement were carried out with ultrasonic instruments and area-specific curettes. No osseous recontouring was done.
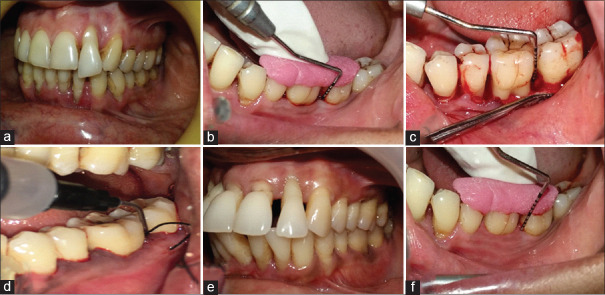
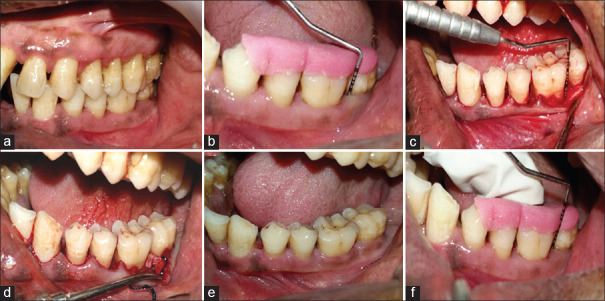
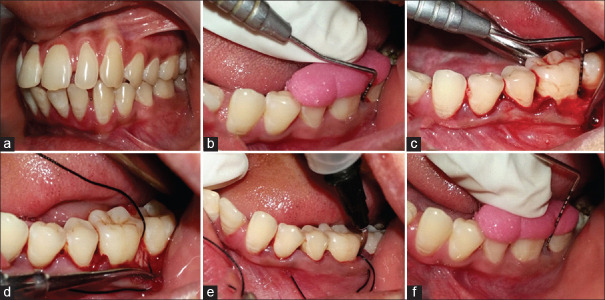
In Group 1, OFD of intrabony defect was performed with placement of placebo gel in the defect site [Figure 1]. In Group 2, ABG was obtained using an indigenously designed bone scraper, adjacent to the defect. ABG mixed with PRF at a proportion of 1:1 (v/v) was placed in the defect. PRF, compressed to form a membrane, was cut and adapted to the grafted defect [Figure 2]. In Group 3, 1.2 mg RSV in situ gel was combined with the mixture of ABG and PRF at a proportion of 1:1 (v/v) and delivered to the defect. PRF, compressed to form a membrane, was cut and adapted to the grafted defect [Figure 3]. The flap secured in position with 3-0 nonabsorbable silk suture (simple loop) and covered with a surgical dressing (Coe-pak). Patients were instructed with postoperative care and recalled after 2 weeks for suture removal.
Figure 1.
Clinical photographs of Group 1: (a) Intraoral preoperative; (b) Preoperative probing; (c) Intrabony defect; (d) Placebo gel delivery; (e) Postoperative at 1 month; (f) Postoperative probing at 9 months
Figure 2.
Clinical photographs of Group 2: (a) Intraoral preoperative; (b) Preoperative probing; (c) Intrabony defect; (d) Autogenous bone graft plus platelet-rich fibrin placed; (e) Postoperative at 1 month; (f) Postoperative probing at 9 months
Figure 3.
Clinical photographs of Group 3: (a) Intraoral preoperative; (b) Preoperative probing; (c) Intrabony defect; (d) Autogenous bone graft plus platelet-rich fibrin placed; (e) 1.2 mg rosuvastatin gel delivered; (f) Postoperative probing at 9 months
Clinical parameters such as probing pocket depth (PPD) and relative attachment level (RAL) were recorded using University of North Carolina-15 (UNC-15) probe and custom-made acrylic stent. Clinical parameters were evaluated at baseline (6 weeks after Phase 1 therapy), 3, 6, and 9 months postoperatively. Radiographic parameters were recorded using cone-beam computed tomography (CBCT) at baseline and 9 months. After a thorough evaluation of CBCT, CEJ adjacent to intrabony defect was determined. The following three parameters were recorded to assess the amount of bone fill: vertical distance or height from CEJ to the apex of the base of the bone defect (A), vertical distance or height from CEJ to the vertical level of crest of the bone defect (B), and horizontal width of bone defect from tooth to the crest of the bone defect (C) [Figure 4].
Figure 4.
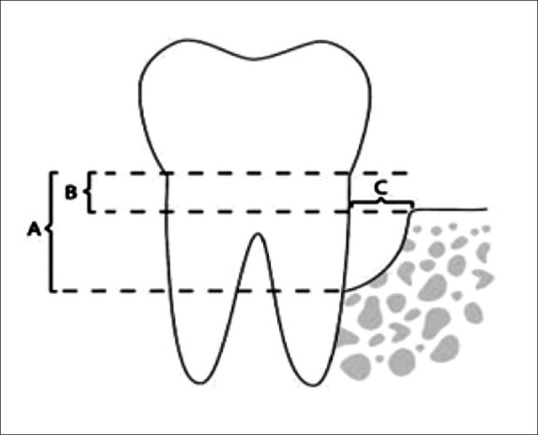
Radiographic parameters recorded using cone-beam computed tomography: (A) height from cementoenamel junction to base of bone defect; (B) height from cementoenamel junction to crest of bone defect; (C) width of bone defect
Data obtained were analyzed using the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, version 21.0. IBM Corp., Armonk, New York, USA). Intergroup comparison at all intervals was done using Kruskal–Wallis ANOVA. An intragroup comparison was done using Friedman test for more than two follow-ups and Wilcoxon signed-rank test for two follow-ups. Differences were considered to be statistically significant at P < 0.05 and statistically highly significant at P < 0.01.
Results
All 39 patients completed the study without any dropouts and with no reports of any adverse effects. Healing was uneventful without any postoperative complications. Each group had 13 participants with a mean age of 36.46, 37.08, and 39.08 for Group 1, Group 2, Group 3, respectively. Among the 39 participants, 16 were male and 23 were female. There was a statistically nonsignificant difference seen for the mean age and frequency of gender between the three groups (P > 0.05). Thus, age and gender can be considered to have no role as a confounder.
Intergroup comparison of PPD [Table 1] and RAL [Table 2] revealed a statistically nonsignificant difference seen for the values between the three groups (P > 0.05) at baseline. There was a statistically highly significant difference seen for the values between the three groups (P < 0.01) at 3 months, 6 months, and 9 months. Group 3 provided 4.46 ± 0.78 mm of PPD reduction compared with 3.38 ± 0.51 and 2.08 ± 0.64 in Group 2 and Group 1, respectively. RAL gain of 4.00 ± 0.71 mm was achieved in Group 3 compared with 2.77 ± 0.59 and 1.62 ± 0.51 in Group 2 and Group 1, respectively, both of which are highly significant between baseline and 9 months.
Table 1.
Intergroup comparison of probing pocket depth at baseline, 3, 6, and 9 months
| Groups | n | Mean | SD | SE | χ 2 | P value of Kruskal–Wallis ANOVA |
|---|---|---|---|---|---|---|
| PPD baseline | ||||||
| 1 | 13 | 6.77 | 0.832 | 0.231 | 1.052 | 0.591# |
| 2 | 13 | 7.00 | 0.913 | 0.253 | ||
| 3 | 13 | 7.15 | 0.987 | 0.274 | ||
| Total | 39 | 6.97 | 0.903 | 0.145 | ||
| PPD 3 months | ||||||
| 1 | 13 | 4.92 | 0.641 | 0.178 | 10.543 | 0.005** |
| 2 | 13 | 4.62 | 0.768 | 0.213 | ||
| 3 | 13 | 3.85 | 0.801 | 0.222 | ||
| Total | 39 | 4.46 | 0.854 | 0.137 | ||
| PPD 6 months | ||||||
| 1 | 13 | 4.77 | 0.599 | 0.166 | 18.192 | 0.000** |
| 2 | 13 | 3.85 | 0.801 | 0.222 | ||
| 3 | 13 | 3.08 | 0.862 | 0.239 | ||
| Total | 39 | 3.90 | 1.021 | 0.163 | ||
| PPD 9 months | ||||||
| 1 | 13 | 4.69 | 4.69 | 0.175 | 26.840 | 0.000** |
| 2 | 13 | 3.62 | 0.650 | 0.180 | ||
| 3 | 13 | 2.69 | 0.480 | 0.133 | ||
| Total | 39 | 3.67 | 1.009 | 0.162 |
Group 1: OFD + placebo; Group 2: OFD + ABG + PRF; Group 3: OFD + ABG + PRF + RSV. OFD: Open flap debridement; ABG: Autogenous bone graft; PRF: Platelet-rich fibrin; RSV: 1.2 mg rosuvastatin gel; n: Number of patients; PPD: Periodontal probing depth; SD: Standard deviation; SE: Standard error
Table 2.
Intergroup comparison of relative attachment level at baseline, 3, 6, and 9 months
| Groups | n | Mean | SD | SE | χ 2 | P value of Kruskal–Wallis ANOVA |
|---|---|---|---|---|---|---|
| RAL baseline | ||||||
| 1 | 13 | 10.38 | 0.870 | 0.241 | 0.749 | 0.687# |
| 2 | 13 | 10.46 | 0.519 | 0.144 | ||
| 3 | 13 | 10.62 | 0.768 | 0.213 | ||
| Total | 39 | 10.49 | 0.721 | 0.115 | ||
| RAL 3 months | ||||||
| 1 | 13 | 9.00 | 0.816 | 0.226 | 12.303 | 0.002** |
| 2 | 13 | 8.69 | 0.480 | 0.133 | ||
| 3 | 13 | 7.77 | 0.832 | 0.231 | ||
| Total | 39 | 8.49 | 0.885 | 0.142 | ||
| RAL 6 months | ||||||
| 1 | 13 | 8.77 | 0.725 | 0.201 | 18.721 | 0.000** |
| 2 | 13 | 7.92 | 0.760 | 0.211 | ||
| 3 | 13 | 7.00 | 0.816 | 0.226 | ||
| Total | 39 | 7.90 | 1.046 | 0.168 | ||
| RAL 9 months | ||||||
| 1 | 13 | 8.77 | 0.725 | 0.201 | 27.352 | 0.000** |
| 2 | 13 | 7.69 | 0.630 | 0.175 | ||
| 3 | 13 | 6.62 | 0.506 | 0.140 | ||
| Total | 39 | 7.69 | 1.080 | 0.173 |
Group 1: OFD + placebo; Group 2: OFD + ABG + PRF; Group 3: OFD + ABG + PRF + RSV. OFD: Open flap debridement; ABG: Autogenous bone graft; PRF: Platelet-rich fibrin; RSV: 1.2 mg rosuvastatin gel; n: Number of patients; RAL: Relative attachment level; SD: Standard deviation; SE: Standard error
Intergroup comparison of radiographic parameters revealed a statistically nonsignificant difference seen for the values between the three groups (P > 0.05) for A (CEJ to base of defect) at baseline, while a statistically highly significant difference seen for the values between the three groups (P < 0.01) at 9 months [Table 3]. Defect depth reduction of A for Group 3 was found 4.25 ± 0.29 mm which was highly significant compared to 2.90 ± 0.17 for Group 2 and 0.39 ± 0.15 for Group 1. For B (CEJ to bone crest adjacent to defect), a statistically nonsignificant difference was seen for the values between the three groups (P > 0.05) at baseline and 9 months. There was a statistically highly significant difference seen for the values between the three groups (P < 0.01) at 3 months and 6 months [Table 4]. For C (width of defect), a statistically nonsignificant difference was seen for the values between the three groups (P > 0.05) at baseline. There was a statistically highly significant difference seen for the values between the three groups (P < 0.01) from baseline to 9 months [Table 5]. Defect depth reduction of C for Group 3 was found 3.05 ± 0.46 mm which was highly significant in comparison of 0.21 ± 0.17 for Group 1 and significant in comparison of 2.66 ± 0.29 for Group 2.
Table 3.
Intergroup comparison of radiographic parameter “A” at baseline and 9 months
| Groups | n | Mean | SD | SE | χ 2 | P value of Kruskal–Wallis ANOVA |
|---|---|---|---|---|---|---|
| A baseline | ||||||
| 1 | 13 | 6.346 | 0.6186 | 0.1716 | 2.557 | 0.278# |
| 2 | 13 | 6.331 | 0.6434 | 0.1784 | ||
| 3 | 13 | 6.692 | 0.5499 | 0.1525 | ||
| Total | 39 | 6.456 | 0.6129 | 0.0981 | ||
| A 9 months | ||||||
| 1 | 13 | 5.953846 | 0.6172727 | 0.1712007 | 31.892 | 0.000** |
| 2 | 13 | 3.438462 | 0.4941971 | 0.1370656 | ||
| 3 | 13 | 2.446154 | 0.3777464 | 0.1047680 | ||
| Total | 39 | 3.946154 | 1.5746087 | 0.2521392 |
Group 1: OFD + placebo; Group 2: OFD + ABG + PRF; Group 3: OFD + ABG + PRF + RSV. OFD: Open flap debridement; ABG: Autogenous bone graft; PRF: Platelet-rich fibrin; RSV: 1.2 mg rosuvastatin gel; n: Number of patients, A: Radiographic height from cementoenamel junction to base of bone defect; SD: Standard deviation; SE: Standard error
Table 4.
Intergroup comparison of radiographic parameter “B” at baseline and 9 months
| Groups | n | Mean | SD | SE | χ 2 | P value of Kruskal–Wallis ANOVA |
|---|---|---|---|---|---|---|
| B baseline | ||||||
| 1 | 13 | 2.02 | 0.378 | 0.105 | 2.958 | 0.228# |
| 2 | 13 | 2.14 | 0.218 | 0.060 | ||
| 3 | 13 | 2.32 | 0.356 | 0.099 | ||
| Total | 39 | 2.16 | 0.340 | 0.054 | ||
| B 9 months | ||||||
| 1 | 13 | 1.88 | 0.400 | 0.111 | 0.267 | 0.875# |
| 2 | 13 | 1.97 | 0.206 | 0.057 | ||
| 3 | 13 | 2.02 | 0.423 | 0.117 | ||
| Total | 39 | 1.96 | 0.351 | 0.056 |
Group 1: OFD + placebo; Group 2: OFD + ABG + PRF; Group 3: OFD + ABG + PRF + RSV. OFD: Open flap debridement; ABG: Autogenous bone graft; PRF: Platelet-rich fibrin; RSV: 1.2 mg rosuvastatin gel; n: Number of patients; B: Radiographic height from cementoenamel junction to crest of bone defect; SD: Standard deviation; SE: Standard error
Table 5.
Intergroup comparison of radiographic parameter “C” at baseline and 9 months
| Groups | n | Mean | SD | SE | χ 2 | P value of Kruskal–Wallis ANOVA |
|---|---|---|---|---|---|---|
| C baseline | ||||||
| 1 | 13 | 3.84 | 0.482 | 0.134 | 0.424 | 0.809# |
| 2 | 13 | 3.78 | 0.318 | 0.088 | ||
| 3 | 13 | 3.82 | 0.462 | 0.128 | ||
| Total | 39 | 3.82 | 0.416 | 0.067 | ||
| C 9 months | ||||||
| 1 | 13 | 3.63 | 0.496 | 0.137 | 32.099 | 0.000** |
| 2 | 13 | 1.12 | 0.117 | 0.032 | ||
| 3 | 13 | 0.77 | 0.214 | 0.059 | ||
| Total | 39 | 1.84 | 1.327 | 0.213 |
Group 1: OFD + placebo; Group 2: OFD + ABG + PRF; Group 3: OFD + ABG + PRF + RSV. OFD: Open flap debridement; ABG: Autogenous bone graft; PRF: Platelet-rich fibrin; RSV: 1.2 mg rosuvastatin gel; n: Number of patients; C: Radiographic width of bone defect; SD: Standard deviation; SE: Standard error
On Intragroup comparison of Group 1, the mean PPD at baseline was 6.77 mm which reduced to 4.69 at 9 months. The mean RAL at baseline was 10.38 mm which reduced to 8.77 at 9 months. The mean of intrabony defect measurement “A” at baseline was 6.35 mm which reduced to 5.95 mm, whereas the mean value of B at baseline was 2.02 mm which reduced by 1.88 mm. The mean value of C at baseline was 3.84 mm which reduced by 3.63 mm [Figure 5]. There was a statistically highly significant difference seen for the values between all time intervals (P < 0.01).
Figure 5.
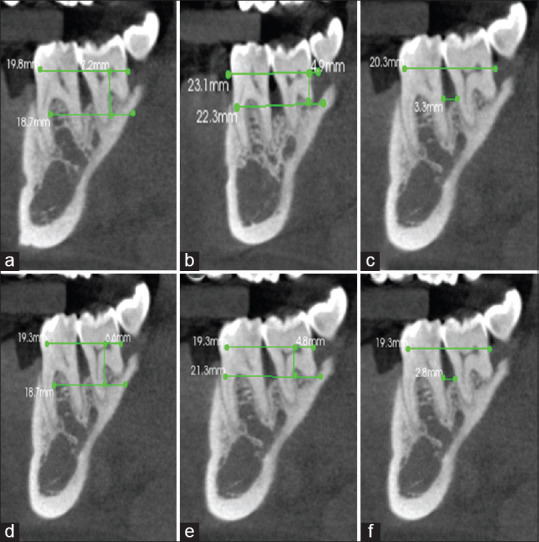
Measurement of radiographic parameters for Group 1 at baseline (preoperative) and 9 months (postoperative): (a) CBCT preoperative “A;” (b) CBCT preoperative “B;” (c) CBCT preoperative “C;” (d) CBCT postoperative “A;” (e) CBCT postoperative “B;” (f) CBCT postoperative “C”. A: Height from cementoenamel junction to base of bone defect, B: Height from cementoenamel junction to crest of bone defect, C: Width of bone defect, CBCT: Cone-beam computed tomography
For Group 2, the mean PPD at baseline was 7 mm which reduced to 3.62 at 9 months. The mean RAL at baseline was 10.46 mm which reduced to 7.69 at 9 months. The mean of intrabony defect measurement “A” at baseline was 6.33 mm which reduced to 2.85 mm, whereas the mean value of B at baseline was 2.14 mm which reduced by 1.97 mm. The mean value of C at baseline was 3.78 mm which reduced by 1.12 mm [Figure 6]. There was a statistically highly significant difference seen for the values between all time intervals (P < 0.01).
Figure 6.
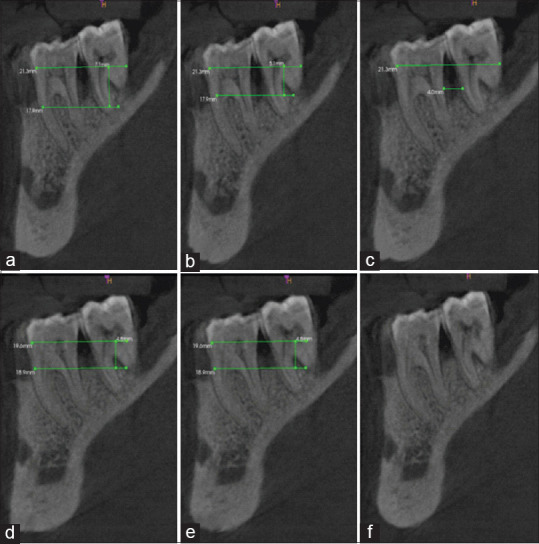
Measurement of radiographic parameters for Group 2 at baseline (preoperative) and 9 months (postoperative): (a) CBCT preoperative “A;” (b) CBCT preoperative “B;” (c) CBCT preoperative “C;” (d) CBCT postoperative “A;” (e) CBCT postoperative “B;” (f) CBCT postoperative “C”. A: Height from cementoenamel junction to base of bone defect, B: Height from cementoenamel junction to crest of bone defect, C: Width of bone defect, CBCT: Cone-beam computed tomography
For Group 3, the mean PPD at baseline was 7.15 mm which reduced to 2.69 at 9 months. The mean RAL at baseline was 10.62 mm which reduced to 6.62 at 9 months. The mean of intrabony defect measurement “A” at baseline was 6.56 mm which reduced to 2.48 mm, whereas the mean value of B at baseline was 2.32 mm which reduced by 2.02 mm. The mean value of C at baseline was 3.82 mm which reduced by 0.77 mm [Figure 7]. There was a statistically highly significant difference seen for the values between all time intervals (P < 0.01).
Figure 7.
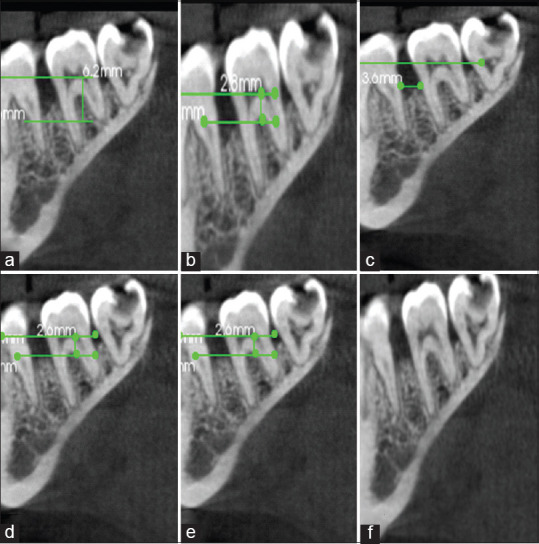
Measurement of radiographic parameters for Group 3 at baseline (preoperative) and 9 months (postoperative): (a) CBCT preoperative “A;” (b) CBCT preoperative “B;” (c) CBCT preoperative “C;” (d) CBCT postoperative “A;” (e) CBCT postoperative “B;” (f) CBCT postoperative “C”. A: Height from cementoenamel junction to base of bone defect, B: Height from cementoenamel junction to crest of bone defect, C: Width of bone defect, CBCT: Cone-beam computed tomography
Discussion
The ultimate goal of periodontal therapy is to prevent inflammatory disease progression and regeneration of lost periodontal tissues including bone. To date, various biomaterials have been used. Host-modulating properties of statins have led to their use in periodontal regenerative procedures. Statins combined with other regenerative biomaterials can be a potential approach in the future for the treatment of intrabony defects. In the present study, healing was uneventful in patients after regenerative therapy. There were no reports of any complication or adverse reactions with the use of locally delivered 1.2 mg RSV gel.
Reductions of PPD are of prime importance in evaluating the success of any periodontal therapy. Results suggest that PPD reduction was significantly higher in Group 3 in comparison to Group 1 and Group 2. Results of the present study are in accordance with the studies by Pradeep et al. at RSV-treated intrabony defects.[10,16] Gain in RAL is considered to be the primary clinical endpoint of regenerative therapies around natural teeth. Gain in attachment implies new attachment in previous periodontally diseased root surfaces. Gain in RAL was highly significant in Group 3 than in Group 2 and Group 1. The present study showed a significant gain in RAL which is in agreement with various studies at RSV-treated sites.[10,16,17] A greater clinical attachment gain has been observed with RSV local drug delivery than atorvastatin local drug delivery.[18]
New bone formation is frequently used as the primary outcome variable in the clinical controlled trial of regenerative therapy. In the present study, the amount of bone fill was assessed by CBCT which is a noninvasive painless alternative to direct measurement. Surgical re-entry was not followed due to ethical reasons. There was a statistically significant reduction of defect height (A and B) and width (C) for Group 2 than in Group 1 which confirms the beneficial effect of ABG and PRF with OFD than OFD alone. Lee et al. have demonstrated better regenerative outcomes when ABG was mixed with PRF than ABG alone.[19] In the present study, addition of PRF to ABG played an important role in maintaining and serving the graft material leading to effective bone regeneration and creating a continual process of healing. Since both are autologous preparations, risk of immunogenic reactions and disease transmission is minimal. Therapeutic autologous platelet concentrate (PRF) should present 1 million platelet/μL, considering that whole blood in humans contains 200,000 ± 75,000 platelet/μL. Therefore, a patient with a minimum platelet count of 200,000 platelet/μL was considered crucial for inclusion. Any patient with a platelet count below 200,000 platelet/μL was excluded from the study.[20] The present study effectively supports numerous studies that reported the treatment of periodontal intrabony defect using either PRF alone or in combination with various bone substitutes.[21,22,23]
For Group 3, a highly significant defect height (A and B) and width (C) reduction was seen compared to Group 1. Defect height (A and B) reduction was highly significant, whereas width (C) reduction was significant in comparison to Group 2. Results clearly showed the regeneration potential of 1.2 mg RSV with ABG and PRF. The results of the present study support a study by Saliem who found a significant amount of bone fill when RSV was used as an adjunct to SRP.[24] Studies using 1.2 mg RSV gel demonstrated a greater percentage of bone fill not only in furcation[17] but also in the treatment of intrabony defects.[25]
In the literature, better clinical and radiographic outcomes have been obtained for the treatment of intrabony defects with RSV than other statins.[26,27] RSV exhibits potent anti-inflammatory and osteostimulatory actions with a longer bioavailability. Actions of RSV also include antiplatelet aggregation, oxidative stress reduction, and restoring expression of Superoxide dismutase-1.[28] By regulating the expression of SCLO1A1 gene, RSV promotes osteoblast differentiation.[29] Indirectly RSV also inhibits osteoclast function by inhibiting interleukin-6 production by osteoblast and favoring bone formation. These salient features make RSV a more potential subject of research among statins.[30] Even with a low concentration, a significant amount of bone formation was evident either alone[31] or as an adjunct to both surgical and nonsurgical periodontal treatments.[32] The results of the present study imply that a combination of RSV, ABG, and PRF has a synergistic effect explaining their role as a regenerative biomaterial in the treatment of intrabony defects.
The limitations of the present study include that the allocation of patients into three groups makes the study a bit more complicated. Group 3 has a lot of combination which not only makes the procedure complex but also clinical application of PRF, ABG, and RSV time-consuming. The OFD plus placebo group could have been avoided as a vast amount of literature supports a better amount of bone fill with ABG compared to OFD alone. Further multicentered randomized clinical studies and longitudinal studies are required to warrant the additional benefits of using RSV either alone or in combination with various biomaterials in regenerative periodontal therapy.
To our best knowledge, there are no previous clinical studies using 1.2 mg RSV gel with PRF and ABG in the treatment of intrabony defects. Hence, no direct comparison can be made.
Conclusions
Addition of 1.2 mg RSV gel with PRF and ABG seems to have a favorable effect on RAL and PPD reduction. A significant reduction in height and width of intrabony defect was also observed. Thus, RSV, a second-generation, synthetic, hydrophilic statin, may provide new insight in periodontal regenerative techniques.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Offenbacher S. Periodontal diseases: Pathogenesis. Ann Periodontol. 1996;1:821–78. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 2.Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005;32(Suppl 6):108–29. doi: 10.1111/j.1600-051X.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 3.Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, Park YB, et al. Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci. 2001;69:2855–66. doi: 10.1016/s0024-3205(01)01363-7. [DOI] [PubMed] [Google Scholar]
- 4.Kavalipati N, Shah J, Ramakrishan A, Vasnawala H. Pleiotropic effects of statins. Indian J Endocrinol Metab. 2015;19:554–62. doi: 10.4103/2230-8210.163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow SC. Immunomodulation by statins: Mechanisms and potential impact on autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2009;57:243–51. doi: 10.1007/s00005-009-0038-5. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh-Choudhury N, Mandal CC, Choudhury GG. Statin-induced Ras activation integrates the phosphatidylinositol 3-kinase signal to Akt and MAPK for bone morphogenetic protein-2 expression in osteoblast differentiation. J Biol Chem. 2007;282:4983–93. doi: 10.1074/jbc.M606706200. [DOI] [PubMed] [Google Scholar]
- 7.Kones R. Rosuvastatin, inflammation, C-reactive protein, JUPITER, and primary prevention of cardiovascular disease--a perspective. Drug Des Devel Ther. 2010;4:383–413. doi: 10.2147/DDDT.S10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stalker TJ, Lefer AM, Scalia R. A new HMG-CoA reductase inhibitor, rosuvastatin, exerts anti-inflammatory effects on the microvascular endothelium: The role of mevalonic acid. Br J Pharmacol. 2001;133:406–12. doi: 10.1038/sj.bjp.0704070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda T, Matsunuma A, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun. 2001;280:874–7. doi: 10.1006/bbrc.2000.4232. [DOI] [PubMed] [Google Scholar]
- 10.Pradeep AR, Karvekar S, Nagpal K, Patnaik K, Guruprasad CN, Kumaraswamy KM. Efficacy of locally delivered 1.2% rosuvastatin gel to non-surgical treatment of patients with chronic periodontitis: A randomized, placebo-controlled clinical trial. J Periodontol. 2015;86:738–45. doi: 10.1902/jop.2015.140631. [DOI] [PubMed] [Google Scholar]
- 11.Pandit N, Pandit IK. Autogenous bone grafts in periodontal practice: A literature review. J Int Clin Dent Res Organ. 2016;8:27–33. [Google Scholar]
- 12.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Kökdere NN, Baykul T, Findik Y. The use of platelet-rich fibrin (PRF) and PRF-mixed particulated autogenous bone graft in the treatment of bone defects: An experimental and histomorphometrical study. Dent Res J (Isfahan) 2015;12:418–24. doi: 10.4103/1735-3327.166188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A, Pradeep AR. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: A randomized controlled clinical trial. J Periodontol. 2011;82:1705–12. doi: 10.1902/jop.2011.110075. [DOI] [PubMed] [Google Scholar]
- 15.Thylin MR, McConnell JC, Schmid MJ, Reckling RR, Ojha J, Bhattacharyya I, et al. Effects of simvastatin gels on murine calvarial bone. J Periodontol. 2002;73:1141–8. doi: 10.1902/jop.2002.73.10.1141. [DOI] [PubMed] [Google Scholar]
- 16.Pradeep AR, Garg V, Kanoriya D, Singhal S. Platelet-rich fibrin with 1.2% rosuvastatin for treatment of intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J Periodontol. 2016;87:1468–73. doi: 10.1902/jop.2016.160015. [DOI] [PubMed] [Google Scholar]
- 17.Pradeep AR, Karvekar S, Nagpal K, Patnaik K, Raju A, Singh P. Rosuvastatin 1.2 mg in situ gel combined with 1:1 mixture of autologous platelet-rich fibrin and porous hydroxyapatite bone graft in surgical treatment of mandibular Class II furcation defects: A randomized clinical control trial. J Periodontol. 2016;87:5–13. doi: 10.1902/jop.2015.150131. [DOI] [PubMed] [Google Scholar]
- 18.Pradeep AR, Garg V, Kanoriya D, Singhal S. 1.2% rosuvastatin versus 1.2% atorvastatin gel local drug delivery and redelivery in treatment of intrabony defects in chronic periodontitis: A randomized placebo-controlled clinical trial. J Periodontol. 2016;87:756–62. doi: 10.1902/jop.2016.150706. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Choi BH, Jung JH, Zhu SJ, Lee SH, Huh JY, et al. Maxillary sinus floor augmentation using autogenous bone grafts and platelet-enriched fibrin glue with simultaneous implant placement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:329–33. doi: 10.1016/j.tripleo.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Suchetha A, Lakshmi P, Bhat D, Mundinamane DB, Soorya KV, Bharwani GA. Platelet concentration in platelet concentrates and periodontal regeneration-unscrambling the ambiguity. Contemp Clin Dent. 2015;6:510–6. doi: 10.4103/0976-237X.169850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah M, Patel J, Dave D, Shah S. Comparative evaluation of platelet-rich fibrin with demineralized freeze-dried bone allograft in periodontal infrabony defects: A randomized controlled clinical study. J Indian Soc Periodontol. 2015;19:56–60. doi: 10.4103/0972-124X.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elgendy EA, Abo Shady TE. Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet-rich fibrin membrane in the treatment of periodontal intrabony defects. J Indian Soc Periodontol. 2015;19:61–5. doi: 10.4103/0972-124X.148639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathur A, Bains VK, Gupta V, Jhingran R, Singh GP. Evaluation of intrabony defects treated with platelet-rich fibrin or autogenous bone graft: A comparative analysis. Eur J Dent. 2015;9:100–8. doi: 10.4103/1305-7456.149653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saliem SS. Gel local drug delivery and redelivery of rosuvastatin in the treatment of class II furcation defects. IJABR. 2017;7:363–6. [Google Scholar]
- 25.Chatterjee D, Kapoor A, Vijay S, Sobti G, Kara D, Thanvi J. Efficacy of locally administered 1.2% rosuvastatin gel in patients with periodontitis: A randomized placebo controlled clinical trial. Eur J Dent. 2019;13:29–35. doi: 10.1055/s-0039-1688522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monjo M, Rubert M, Wohlfahrt JC, Rønold HJ, Ellingsen JE, Lyngstadaas SP. In vivo performance of absorbable collagen sponges with rosuvastatin in critical-size cortical bone defects. Acta Biomater. 2010;6:1405–12. doi: 10.1016/j.actbio.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Sinjab K, Zimmo N, Lin GH, Chung MP, Shaikh L, Wang HL. The effect of locally delivered statins on treating periodontal intrabony defects: A systematic review and meta-analysis. J Periodontol. 2017;88:357–67. doi: 10.1902/jop.2016.160384. [DOI] [PubMed] [Google Scholar]
- 28.Resch U, Tatzber F, Budinsky A, Sinzinger H. Reduction of oxidative stress and modulation of autoantibodies against modified low-density lipoprotein after rosuvastatin therapy. Br J Clin Pharmacol. 2006;61:262–74. doi: 10.1111/j.1365-2125.2005.02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monjo M, Rubert M, Ellingsen JE, Lyngstadaas SP. Rosuvastatin promotes osteoblast differentiation and regulates SLCO1A1 transporter gene expression in MC3T3-E1 cells. Cell Physiol Biochem. 2010;26:647–56. doi: 10.1159/000322332. [DOI] [PubMed] [Google Scholar]
- 30.Lazzerini PE, Capperucci C, Spreafico A, Capecchi PL, Niccolini S, Ferrata P, et al. Rosuvastatin inhibits spontaneous and IL-1 β-induced interleukin-6 production from human cultured osteoblastic cells. Joint Bone Spine. 2013;80:195–200. doi: 10.1016/j.jbspin.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Estanislau IM, Terceiro IR, Lisboa MR, Teles Pde B, Carvalho Rde S, Martins RS, et al. Pleiotropic effects of statins on the treatment of chronic periodontitis--A systematic review. Br J Clin Pharmacol. 2015;79:877–85. doi: 10.1111/bcp.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertl K, Parllaku A, Pandis N, Buhlin K, Klinge B, Stavropoulos A. The effect of local and systemic statin use as an adjunct to non-surgical and surgical periodontal therapy-A systematic review and meta-analysis. J Dent. 2017;67:18–28. doi: 10.1016/j.jdent.2017.08.011. [DOI] [PubMed] [Google Scholar]