Abstract
Recent studies suggest that erythromycin can suppress the production of some cytokines and may be an effective treatment for asthma. Eosinophil chemotactic cytokines have been suggested to contribute to the pathogenesis of asthma by the recruitment of eosinophils. We hypothesized that erythromycin modulates eosinophil chemotactic cytokine production. To test the hypothesis, we evaluated the potential of erythromycin to modulate the release of eosinophil chemoattractants from the human lung fibroblast cell line HFL-1. HFL-1 released eotaxin, granulocyte-macrophage colony-stimulating factor, and regulated and normal T-cell expressed and presumably secreted (RANTES) in response to interleukin-1β or tumor necrosis factor alpha. Erythromycin attenuated the release of these cytokines and eosinophil chemotactic activity by the HFL-1. The suppressive effect on eotaxin was the most marked of these cytokines. Erythromycin therapy also suppressed eotaxin mRNA significantly. These results suggest a mechanism that may account for the apparent beneficial action of macrolide antibiotics in the treatment of allergic airway disorders.
Asthma is a chronic disease of the airways that are prone to constrict because of inflammation (21). The inflammatory infiltrate is predominantly composed of eosinophils, and current concepts suggest that eosinophils are major effector cells in this disease (7, 12). Eosinophils migrate in response to chemoattractants, such as leukotrienes and several chemokines (31, 32). The importance of cytokine mobilization in promoting airway eosinophil recruitment is demonstrated by the effectiveness of cytokine antagonists in blocking airway eosinophilia after allergen exposure (13, 29). Consistent with this concept, studies with genetically altered animals lacking the eosinophil chemotactic cytokine or transcription factors, which regulate the production of eosinophil chemoattractants, do not develop airway eosinophilia, lung damage, or airway hyperreactivity (2, 45).
Low-dose erythromycin (ERY) therapy has been accepted as an effective therapy for diffuse panbronchiolitis (26). The therapeutic effect of ERY has been extended to include other inflammatory diseases, including the eosinophilic airway disease, asthma (19, 25, 30). Although the precise mechanism(s) for the beneficial effect in asthma remains unclear, several studies have suggested that the beneficial effects are due to an anti-inflammatory mechanism. Macrolides have been shown to inhibit the proliferation of mononuclear cells (36), reduce the formation of superoxide by neutrophils (3, 28), and show suppressive effects upon cytokine production (24, 25, 40, 41).
Based on the above, we hypothesized that ERY might modulate eosinophil chemotactic activity to account for its beneficial effect in asthma. To test this hypothesis, we investigated the effect of ERY on the production of eosinophil chemotactic cytokines from a lung fibroblast cell line. We found that ERY significantly attenuated eosinophil chemotactic activity by the supernatant fluids from a lung fibroblast cell line and that eotaxin, granulocyte-macrophage colony-stimulating factor (GM-CSF), and regulated and normal T-cell expressed and presumably secreted (RANTES) production were suppressed by ERY. These effects may play a role in eosinophil recruitment and have relevance to ERY efficacy in bronchial asthma.
MATERIALS AND METHODS
Cell cultures.
Human fetal lung fibroblasts (HFL-1, lung, diploid, human, passage 14) were purchased from the American Type Culture Collection (Rockville, Md.) and used because of difficulty in obtaining sufficient quantities of primary human lung airway fibroblasts for these experiments. The cell line, HFL-1, was initiated from the lung tissue of a 16- to 18-week-old human fetus in 1975. Morphology of HFL-1 is fibroblast-like and retains features of normal lung fibroblasts including collagen and fibronectin production (8, 20). The HFL-1 were suspended at 1.0 × 106 cells/ml in Ham's F-12 supplemented with 10% heat-inactivated fetal bovine serum. Cell suspensions (3 ml) were added to 30-mm-diameter tissue culture dishes (Corning, Corning, N.Y.) and were cultured at 37°C in a 5% CO2 atmosphere. After 2 to 3 days in culture, the cells had reached confluence and the culture medium was replaced with 2 ml of medium supplemented as described above and incubated for one additional day.
ERY and stimulants.
The culture medium was removed from the cells by washing twice with serum-free medium, and the cells were incubated with ERY (1, 10, or 100 μg; Sigma, St. Louis, Mo.) at 37°C in a humidified 5% CO2 atmosphere. After 2 h tumor necrosis factor alpha (TNF-α; 10 ng/ml; Sigma) or interleukin-1β (IL-1β; 1 ng/ml; Sigma) was added for 48 h. In preliminary experiments, these concentrations of IL-1β or TNF-α produced the maximal cytokine stimulation of the doses tested. Controls included the same concentrations of penicillin or streptomycin (GIBCO, Grand Island, N.Y.). Cell injury was evaluated by microscopy (cell shape, detachment from tissue culture dish) and trypan blue exclusion. The supernatant fluids were then harvested and stored at −80°C until assayed.
Measurement of cytokines in the supernatant fluids.
The concentrations of eotaxin, GM-CSF, IL-5, RANTES, and LTB4 were measured in the cell supernatant fluids using a commercially available enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minn.) according to the manufacturer's instructions. The minimum concentrations detected by these methods were 15 pg/ml for eotaxin, GM-CSF, IL-5, RANTES and 200 pg/ml for LTB4.
Evaluation of mRNA expression.
Cytokine mRNA was analyzed by reverse transcriptase PCR (RT-PCR). HFL-1 were incubated with ERY for 14 h and cytokines for 12 h, and the total cellular RNA was extracted from adherent cells using a modification of the methods of Chomczynski and Sacchi (10). The RNA was reverse transcribed using a commercially available kit (Promega, Madison, Wis.). One microgram of the reverse-transcribed DNA was then mixed with Ready-to-Go PCR Beads (Pharmacia, Piscataway, N.J.) and the front and back primers (Table 1) added at 0.2 μM final concentration. PCR was performed in a Perkin-Elmer model 480 thermal cycler using at 94°C for 2 min and 35 cycles consisting of 94°C for 45 s, a primer annealing temperature as specified in Table 1 for 45 s, and 72°C for 2 min, followed by 72°C for an additional 7 min. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a “housekeeping gene” with the PCR. The DNA was subjected to agarose gel, and the intensity of the bands was quantitated by densitometry. The results were expressed as the ratio of intensity to the GAPDH.
TABLE 1.
PCR primers and conditions
| PCR product | Primer sequence (5′-3′)a | Cycle no. | Anneal temp (°C) |
|---|---|---|---|
| Eotaxin | fwd-5′ ACATGAAGGTCT CCGCAGCACTTC | 35 | 61 |
| rev-5′ TTGGCCAGGTTAAA GCAGCAGGTG | |||
| GM-CSF | fwd-5′ ATTGCGGCCCC GCCTGGAGCTGTACAAG | 35 | 55 |
| rev-5′ ATTCTCGAGAC TGGCTCCCAGCAGTCAAA | |||
| RANTES | fwd-5′ GGCACGCCTCG CTGTCATCCTCA | 35 | 65 |
| rev-5′ CTTGATGTGGG CACGGGGCAGTG | |||
| GAPDH | fwd-5′ ACCACAGTCCAT GCCATCA | 35 | 55 |
| rev-5′ TCCACCACCCTG TTGCTGTA |
fwd, forward; rev, reverse.
Effects of ERY on eosinophil chemotactic activity by HFL-1 supernatant fluids.
Eosinophils were isolated with a modified method of Hansel et al. (14) with a magnetic cell separation system (Becton Dickinson, Franklin Lakes, N.J.). Briefly, venous blood anticoagulated with 130 mM trisodium citrate was obtained from normal human volunteers and diluted with phosphate-buffered saline PBS in a 1:1 ratio. Diluted blood was overlaid on an isotonic Percoll solution (density, 1.082 g/ml; Sigma) and then centrifuged at 1,000 × g for 30 min at 4°C with a Beckman TJ-6 centrifuge. The supernatant and mononuclear cells at the interface were carefully removed, and red blood cells in the sediment were lysed with two cycles of hypotonic lysis (0.1% KHCO3 and 0.83% NH4Cl). Isolated granulocytes were washed two times with PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer (25 mM PIPES, 50 mM NaCl, 5 mM KCl, 25 mM NaOH, and 5.4 mM glucose; pH 7.4) containing 1% defined calf serum (DCS; HyClone Laboratories, Logan, Utah), and an approximately equal volume of anti-CD16 antibody conjugated with magnetic particles (Miltenyi Biotec, Bergisch Gladbach, Germany) was added to the cell pellet. After 60 min on ice, 5 ml of PIPES buffer with 1% DCS was added to the cell-antibody mixture. The resuspended cells were loaded onto the separation column positioned in the magnetic cell separation system with a strong magnetic field. The cells were eluted three times with 5 ml of PIPES buffer with 1% DCS. The purity of the eosinophils counted by Randolph's stain was >94%; the viability was >98%. The eosinophils were resuspended in Gey's solution at 2.0 × 106 cells/ml and used for the chemotaxis assay.
Eosinophil chemotactic activity (ECA) was assayed in 48-well microchemotaxis chambers (Neuroprobe, Inc., Cabin John, Md.) as previously described (15). The bottom wells of the chamber were filled with 25 μl of the supernatant fluids from HFL-1. A 10-μm-thick polyvinylpyrrolidone-free polycarbonate filter (pore size, 5 μm) was placed over the samples. The silicon gasket and the upper pieces of the chamber were applied, and 50 μl of the cell suspension was placed into the upper wells. The chambers were incubated in humidified air in 5% CO2 at 37°C for 180 min. Nonmigrated cells were wiped away from the filter. The filter was immersed in methanol for 5 min, stained with a modified Wright's stain, and mounted on a glass slide. Cells that had completely migrated through the filter were counted using light microscopy. The ECA was expressed as the mean number of migrated cells per high-power field (HPF) from duplicate wells.
Statistical analysis.
Data were analyzed by Dunnett's one-way analysis of variance with a Bonferroni correction. In all cases, a P value of <0.05 was considered significant. The data are expressed as the mean ± the standard error of the mean (SEM).
RESULTS
Effects of ERY on cytokine production from HFL-1.
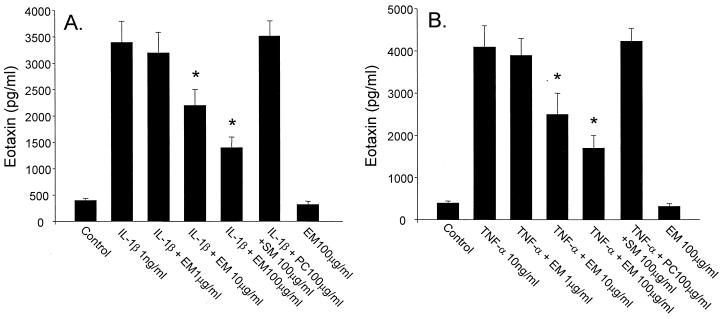
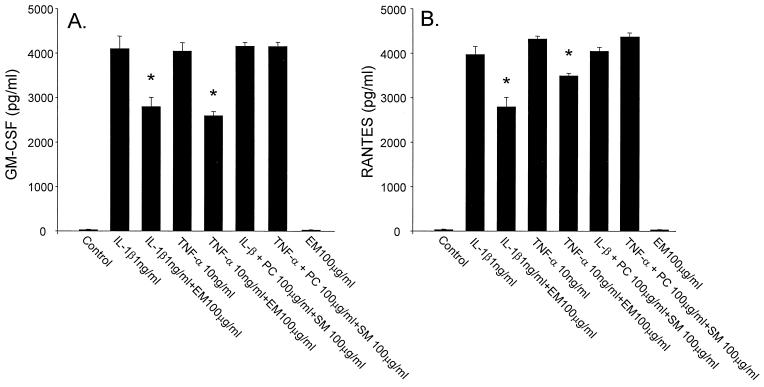
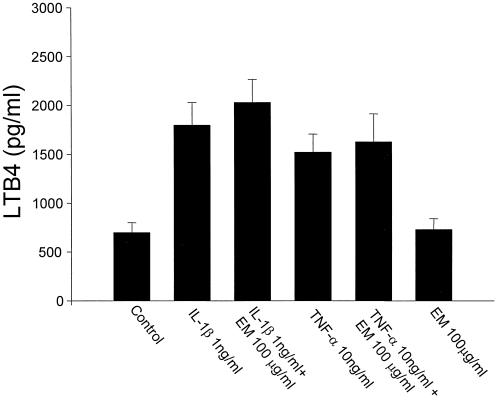
HFL-1 released eotaxin, GM-CSF, RANTES, and LTB4 spontaneously, and the inflammatory cytokines, IL-1β and TNF-α, stimulated the release of these cytokines from HFL-1. ERY inhibited eotaxin release dose dependently from HFL-1 stimulated with IL-1β (Fig. 1A) or TNF-α (Fig. 1B). ERY had no effect on the release of eotaxin from unstimulated HFL-1. ERY (100 μg/ml) also inhibited IL-1β- or TNF-α-stimulated GM-CSF (Fig. 2A) and RANTES (Fig. 2B). However, the inhibitory effects on these cytokines were less pronounced than on eotaxin, and ERY doses lower than 100 μg/ml had no significant effect on GM-CSF or RANTES. ERY had no significant effect on LTB4 release from unstimulated or stimulated HFL-1 supernatant fluids (Fig. 3). IL-5 was not detected in any supernatant fluids. Neither penicillin nor streptomycin modulated the cytokine production (data not shown).
FIG. 1.
Dose-dependent effects of ERY (EM) on eotaxin release from HFL-1 stimulated with IL-1β (1 ng/ml, panel A) or TNF-α (10 ng/ml, panel B) (n = 4). The eotaxin concentration is on the ordinate, and the ERY concentration is on the abscissa. SM, streptomycin; PC, penicillin. Values are expressed as the mean ± the SEM. ∗, P < 0.05 compared with supernatant fluids without ERY incubation.
FIG. 2.
Effects of ERY (EM) on GM-CSF (A) or RANTES (B) release from HFL-1 stimulated with IL-1β (1 ng/ml) or TNF-α (10 ng/ml) (n = 4). The eotaxin concentration is on the ordinate, and the various experimental groups are on the abscissa. SM, streptomycin; PC, penicillin. Values are expressed as the mean ± the SEM. ∗, P < 0.05 compared with supernatant fluids without ERY incubation.
FIG. 3.
Effects of ERY (EM) on LTB4 release from HFL-1 stimulated with IL-1β (1 ng/ml) or TNF-α (10 ng/ml) (n = 4). The eotaxin concentration is on the ordinate, and the experimental groups are on the abscissa. Values are expressed as the mean ± the SEM.
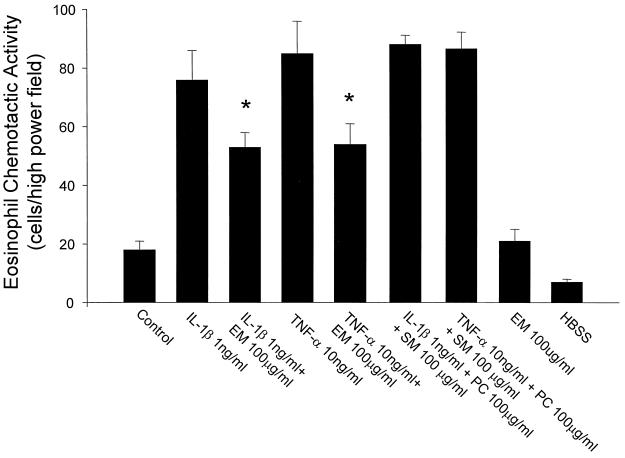
Effects of ERY on eosinophil chemotactic activity.
IL-1β or TNF-α stimulated eosinophil chemotactic activity from HFL-1. ERY significantly suppressed the eosinophil chemotactic activity from HFL-1 stimulated with IL-1β or TNF-α (Fig. 4).
FIG. 4.
Effects of ERY (EM) on eosinophil chemotactic activity from HFL-1 (n = 4). The eosinophil chemotactic activity is on the ordinate, and the experimental groups are on the abscissa. SM, streptomycin; PC, penicillin. Values are expressed as the mean ± the SEM. ∗, P < 0.05 compared with supernatant fluids without ERY incubation.
Effects of ERY on mRNA expression from HFL-1.
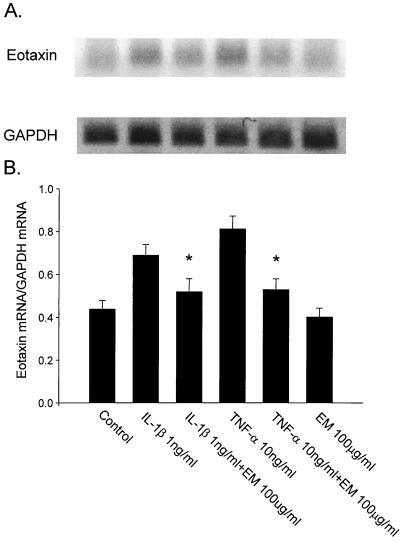
Semiquantitative RT-PCR was performed to evaluate the effect of ERY on cytokine mRNA expression in HFL-1. IL-1β- or TNF-α-induced eotaxin mRNA expression was suppressed by preincubation with ERY significantly (Fig. 5). However, ERY had no significant effect on GM-CSF and RANTES mRNA expression from HFL-1 (data not shown).
FIG. 5.
Effects of ERY (EM) on eotaxin mRNA expression from HFL-1 stimulated with IL-1β or TNF-α. Representative results with RT-PCR for eotaxin and GAPDH are presented. Results with RT-PCR for eotaxin, GAPDH (A), and densitometry data with eotaxin are expressed as a ratio of eotaxin mRNA to GAPDH mRNA (eotaxin mRNA/GAPDH mRNA; B). The ratio of eotaxin mRNA to GAPDH mRNA is on the ordinate, and the ERY concentration is on the abscissa (n = 3). ∗, P < 0.05 compared with supernatant fluids without ERY incubation.
DISCUSSION
The present study demonstrated that HFL-1 released eosinophil chemotactic activity or cytokines, including eotaxin, GM-CSF, and RANTES, in response to IL-1β or TNF-α. ERY attenuated the release of these cytokines and eosinophil chemotactic activity by the HFL-1 stimulated by IL-1β or TNF-α. ERY had no effect on cytokine production or eosinophil chemotactic activity by unstimulated HFL-1. Consistent with these results, ERY treatment of HFL-1 also showed the suppressive effect on the expression of eotaxin mRNA significantly. HFL-1 released LTB4 spontaneously and in response to IL-1β or TNF-α; however, ERY had no effects on LTB4 release. Other antimicrobials tested, including penicillin and streptomycin, did not alter cytokine production or eosinophil chemotactic activity.
Macrolide antibiotics have been shown to modulate cytokine production, including chemoattractants for neutrophils (33), monocytes (18), eosinophils (23), lymphocytes, and bronchial epithelial cells (22, 40). Roxithromycin, one of the macrolide antibiotics, has been reported to suppress sputum eosinophils and eosinophil cationic protein in asthmatic patients (38). These results are consistent with macrolide antibiotics having favorable effects in asthma by modulating eosinophil chemotactic cytokines.
The levels used in these studies are likely clinically relevant. Peak levels of erythromycin in serum vary between 1 and 10 μg/ml, depending on the dosage, route of administration, etc., but lung levels may be even higher with some macrolide antibiotics (34). Furthermore, other in vitro investigations have used similar concentrations of macrolides to investigate the effects of erythromycin on IL-8 release by bronchial epithelial cells (40) with apparent in vivo effects (26).
We investigated the effect of ERY on HFL-1 because lung fibroblasts constitute 35 to 40% of the cells in the interstitium of the lung and are activated to proliferate and synthesize various cytokines during inflammation (27). Moreover, studies of asthmatic biopsies have suggested the importance of fibroblast activation to eosinophil infiltration (35), and fibroblasts have been reported to produce large amounts of the eosinophil chemotactic cytokines, RANTES, GM-CSF, and eotaxin in response to various stimuli (39, 42). In the present study, IL-1β or TNF-α stimulated the release of these cytokines and an increase in eosinophil chemotactic activity. These observations are consistent with the concept that fibroblasts may be an important source of eosinophil chemoattractants in allergic airway disorders. However, a limitation of these studies was that they were done in vitro with a human fibroblast cell line. The effects of ERY on primary cultures of human airway fibroblasts are important issues for future research.
Both IL-1β and TNF-α are found at increased levels in lung lavage fluid from patients with asthma, and its spontaneous release is augmented in alveolar macrophages from adult patients with asthma and in wheezy infants (5, 6, 44). ERY could also affect cytokine release by the suppression of these cytokines. ERY has been shown to decrease TNF-α and IL-1β levels in the macrophage cell line J-774 (17). However, studies of bronchoalveolar lavage fluid from subjects treated for 3 days of azithromycin revealed no difference in bronchoalveolar lavage levels of TNF-α or IL-1β (4).
Although ERY attenuated eotaxin, GM-CSF, and RANTES release in this study, the suppressive effect of eotaxin was most marked with eotaxin. Considering the concentration and suppressive effects of these cytokines, the attenuation of eosinophil chemotactic activity by HFL-1 supernatant fluids is most likely due to eotaxin inhibition by ERY. Eotaxin is highly selective in eosinophil recruitment (11), and patients with asthma have high concentrations of eotaxin in the bronchoalveolar lavage fluid and an increased expression of eotaxin mRNA in the airways (9). Lung fibroblasts are reported to produce large amount of eotaxin compared to another cells, including bronchial epithelial cells, lymphocytes, and monocytes (42). Eotaxin attenuation of EM may be crucial for the inhibition of eosinophil infiltration by EM.
Recently, Abe et al. (1) reported that IL-8 gene repression by macrolide antibiotics was mediated mainly via the activator protein-1 (AP-1) but not by the nuclear factor (NF)-κB site in the bronchial epithelial cell line. Abe et al. showed that both AP-1 and NF-κB were important factors for TNF-α-induced IL-8 gene transcription and that macrolide antibiotics inhibited the TNF-α-induced binding of AP-1 to its sequence in the IL-8 gene but not the binding of NF-κB. It has been reported that the promoters of the eotaxin, GM-CSF, and RANTES genes also contain binding sites for the redox-responsive transcription factors AP-1 and NF-κB and that the responsive elements may differ according to the type of cells or stimulation (16, 37, 43). Differential activation and binding of inducible transcription factors to the promoter regions of chemokine genes may explain the different effects of ERY on these eosinophil chemotactic cytokines.
The present study suggests that lung fibroblasts are an important source of eosinophil chemotactic activity, and the inhibitory effects of ERY on eosinophil chemotactic cytokine release by lung fibroblasts may be one of the mechanisms of decreased airway hyper-responsiveness and the resulting amelioration of disease activity. These results demonstrate a mechanism of action of macrolide antibiotics altering eosinophil accumulation in vitro and suggest the potential usefulness of macrolides in the treatment of allergic airway disorders.
ACKNOWLEDGMENTS
This work was supported by a Merit Review grant from the Veterans' Administration and a grant from Rotary International.
REFERENCES
- 1.Abe S, Nakamura H, Inoue S, Takeda H, Saito H, Kato S, Mukaida N, Matsushima K, Tomoike H. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding site in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2000;22:51–60. doi: 10.1165/ajrcmb.22.1.3400. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto T, Numata F, Tamura M, Takata Y, Higashida N, Takashi T, Takeda K, Akira S. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J Exp Med. 1998;187:1537–1542. doi: 10.1084/jem.187.9.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson R. Erythromycin and roxithromycin potentiate human neutrophil locomotion in vitro by inhibition of leukoattractant-activated superoxide generation and autooxidation. J Infect Dis. 1989;159:966–973. doi: 10.1093/infdis/159.5.966. [DOI] [PubMed] [Google Scholar]
- 4.Aubert J D, Juillerat-Jeanneret L, Fioroni P, Dayer P, Plan P A, Leuenberger P. Function of human alveolar macrophages after a 3-day course of azithromycin in healthy volunteers. Pulm Pharmacol Ther. 1998;11:263–269. doi: 10.1006/pupt.1998.0123. [DOI] [PubMed] [Google Scholar]
- 5.Azevedo I, de Blic J, Dumarey C H, Scheinmann P, Vargaftig B B, Bachelet M. Increased spontaneous release of tumour necrosis factor-alpha by alveolar macrophages from wheezy infants. Eur Respir J. 1997;10:1767–1773. doi: 10.1183/09031936.97.10081767. [DOI] [PubMed] [Google Scholar]
- 6.Borish L, Mascali J J, Dishuck J, Beam W R, Martin R J, Rosenwasser L J. Detection of alveolar macrophage-derived IL-1β in asthma: inhibition with corticosteroids. J Immunol. 1992;149:3078–3082. [PubMed] [Google Scholar]
- 7.Bousquet J, Chanez P, Lacoste J Y, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel F-B. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 8.Breul S D, Bradley K H, Hance A J, Schafer M P, Berg R A, Crystal R G. Control of collagen production by human diploid lung fibroblasts. J Biol Chem. 1980;255:5250–5260. [PubMed] [Google Scholar]
- 9.Brown J R, Kleimberg J, Marini M, Sun G, Bellini A, Mattoli S. Kinetics of eotaxin expression and its relationship to eosinophil accumulation and activation in bronchial biopsies and bronchoalveolar lavage (BAL) of asthmatic patients after allergen inhalation. Clin Exp Immunol. 1998;114:137–146. doi: 10.1046/j.1365-2249.1998.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Zepeda E A, Rothenberg M E, Ownbey R T, Celestin J, Leder P, Luster A D. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 12.Gleich G J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990;85:422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- 13.Grimaldi J C, Yu N X, Grunig G, Seymour B W, Cottrez F, Robinson D S, Hosken N, Ferlin W G, Wu X, Soto H, O'Garra A, Howard M C, Coffman R L. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3) J Leukoc Biol. 1999;65:846–853. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]
- 14.Hansel T T, De Vries I J, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 15.Harvath L, Falk W, Leonard E J. Rapid quantification of neutrophil chemotaxis: use of polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37:39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- 16.Hein H, Schluter C, Kulke R, Christophers E, Schroder J M, Bartels J. Genomic organization, sequence, and transcriptional regulation of the human eotaxin gene. Biochem Biophys Res Commun. 1997;237:537–542. doi: 10.1006/bbrc.1997.7169. [DOI] [PubMed] [Google Scholar]
- 17.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, Iuvone T, D'Acquisto F, Di Rosa M. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292:156–163. [PubMed] [Google Scholar]
- 18.Iino Y, Toriyama M, Kudo K, Natori Y, Yuo A. Erythromycin inhibition of lipopolysaccharide-stimulated tumor necrosis factor alpha production by human monocytes in vitro. Ann Otol Rhinol Laryngol Suppl. 1992;157:16–20. doi: 10.1177/0003489492101s1005. [DOI] [PubMed] [Google Scholar]
- 19.Kamada A K, Hill M R, Ikle D N, Brenner A M, Szefler S J. Efficacy and safety of low-dose troleandomycin therapy in children with severe, steroid-requiring asthma. J Allergy Clin Immunol. 1993;91:873–882. doi: 10.1016/0091-6749(93)90345-g. [DOI] [PubMed] [Google Scholar]
- 20.Katayama K, Seyer J M, Raghow R, Kang A H. Regulation of extracellular matrix production by chemically synthesized subfragments of type I collagen carboxy propeptide. Biochemistry. 1991;30:7097–7104. doi: 10.1021/bi00243a009. [DOI] [PubMed] [Google Scholar]
- 21.Kay A B. Asthma and inflammation. J Allergy Clin Immunol. 1991;87:893–910. doi: 10.1016/0091-6749(91)90408-g. [DOI] [PubMed] [Google Scholar]
- 22.Khair O A, Devalia J L, Abdelaziz M M, Sapsford R J, Davies R J. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur Respir J. 1995;8:1451–1457. [PubMed] [Google Scholar]
- 23.Kohyama T, Takizawa H, Kawasaki S, Akiyama N, Sato M, Ito K. Fourteen-member macrolides inhibit interleukin-8 release by human eosinophils from atopic donors. Antimicrob Agents Chemother. 1999;43:907–911. doi: 10.1128/aac.43.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konno S, Adachi M, Asano K, Okamoto K, Takahashi T. Anti-allergic activity of roxithromycin: inhibition of interleukin-5 production from mouse T lymphocytes. Life Sci. 1993;52:L25–L30. doi: 10.1016/0024-3205(93)90154-u. [DOI] [PubMed] [Google Scholar]
- 25.Konno S, Asano K, Kurokawa M, Ikeda K, Okamoto K, Adachi M. Antiasthmatic activity of a macrolide antibiotic, roxithromycin: analysis of possible mechanisms in vitro and in vivo. Int Arch Allergy Immunol. 1994;105:308–316. doi: 10.1159/000236773. [DOI] [PubMed] [Google Scholar]
- 26.Koyama H, Geddes D M. Erythromycin and diffuse panbronchiolitis. Thorax. 1997;52:915–918. doi: 10.1136/thx.52.10.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama S, Sato E, Masubuchi T, Takamizawa A, Nomura H, Kubo K, Nagai S, Izumi T. Human lung fibroblasts release chemokinetic activity for monocytes constitutively. Am J Physiol. 1998;275:L223–L230. doi: 10.1152/ajplung.1998.275.2.L223. [DOI] [PubMed] [Google Scholar]
- 28.Labro M T, el Benna J, Babin-Chevaye C. Comparison of the in vitro effect of several macrolides on the oxidative burst of human neutrophils. J Antimicrob Chemother. 1989;24:561–572. doi: 10.1093/jac/24.4.561. [DOI] [PubMed] [Google Scholar]
- 29.Mauser P J, Pitman A, Witt A, Fernandez X, Zurcher J, Kung T, Jones H, Watnick A S, Egan R W, Kreutner W, et al. Inhibitory effect of the TRFK-5 anti-IL-5 antibody in a guinea pig model of asthma. Am Rev Respir Dis. 1993;148:1623–1627. doi: 10.1164/ajrccm/148.6_Pt_1.1623. [DOI] [PubMed] [Google Scholar]
- 30.Miyatake H, Taki F, Taniguchi H, Suzuki R, Takagi K, Satake T. Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma. Chest. 1991;99:670–673. doi: 10.1378/chest.99.3.670. [DOI] [PubMed] [Google Scholar]
- 31.Mould A W, Matthaei K I, Young I G, Foster P S. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Investig. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noso N, Sticherling M, Bartels J, Mallet A I, Christophers E, Schroder J M. Identification of an N-terminally truncated form of the chemokine RANTES and granulocyte-macrophage colony-stimulating factor as major eosinophil attractants released by cytokine-stimulated dermal fibroblasts. J Immunol. 1996;156:1946–1953. [PubMed] [Google Scholar]
- 33.Oishi K, Sonoda F, Kobayashi S, Iwagaki A, Nagatake T, Matsushima K, Matsumoto K. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun. 1994;62:4145–4152. doi: 10.1128/iai.62.10.4145-4152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel K B, Xuan D, Tessier P R, Russomanno J H, Quintiliani R, Nightingale C H. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob Agents Chemother. 1996;40:2375–2379. doi: 10.1128/aac.40.10.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roche W R, Beasley R, Williams J H, Holgate S T. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;i:520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 36.Roche Y, Gougerot-Pocidalo M A, Fay M, Forest N, Pocidalo J J. Macrolides and immunity: effects of erythromycin and spiramycin on human mononuclear cell proliferation. J Antimicrob Chemother. 1986;17:195–203. doi: 10.1093/jac/17.2.195. [DOI] [PubMed] [Google Scholar]
- 37.Roebuck K A, Carpenter L R, Lakshminarayanan V, Page S M, Moy J N, Thomas L L. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-κB. J Leukoc Biol. 1999;65:291–298. doi: 10.1002/jlb.65.3.291. [DOI] [PubMed] [Google Scholar]
- 38.Shoji T, Yoshida S, Sakamoto H, Hasegawa H, Nakagawa H, Amayasu H. Anti-inflammatory effect of roxithromycin in patients with aspirin-intolerant asthma. Clin Exp Allergy. 1999;29:950–956. doi: 10.1046/j.1365-2222.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 39.Takamizawa A, Koyama S, Sato E, Masubuchi T, Kubo K, Sekiguchi M, Nagai S, Izumi T. Bleomycin stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activity. J Immunol. 1999;162:6200–6208. [PubMed] [Google Scholar]
- 40.Takizawa H, Desaki M, Ohtoshi T, Kawasaki S, Kohyama T, Sato M, Tanaka M, Kasama T, Kobayashi K, Nakajima J, Ito K. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;156:266–271. doi: 10.1164/ajrccm.156.1.9612065. [DOI] [PubMed] [Google Scholar]
- 41.Takizawa H, Desaki M, Ohtoshi T, Kikutani T, Okazaki H, Sato M, Akiyama N, Shoji S, Hiramatsu K, Ito K. Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun. 1995;210:781–786. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 42.Teran L M, Mochizuki M, Bartels J, Valencia E L, Nakajima T, Hirai K, Schroder J M. Th1- and Th2-type cytokines regulate the expression and production of eotaxin and RANTES by human lung fibroblasts. Am J Respir Cell Mol Biol. 1999;20:777–786. doi: 10.1165/ajrcmb.20.4.3508. [DOI] [PubMed] [Google Scholar]
- 43.Thomas R S, Tymms M J, McKinlay L H, Shannon M F, Seth A, Kola I. ETS1, NFκB and AP1 synergistically transactivate the human GM-CSF promoter. Oncogene. 1997;14:2845–2855. doi: 10.1038/sj.onc.1201125. [DOI] [PubMed] [Google Scholar]
- 44.Virchow J C, Jr, Walker C, Hafner D, Kortsik C, Werner P, Matthys H, Kroegel C. T cells and cytokines in bronchoalveolar lavage fluid after segmental allergen provocation in atopic asthma. Am J Respir Crit Care Med. 1995;151:960–968. doi: 10.1164/ajrccm/151.4.960. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, Cohn L, Zhang D H, Homer R, Ray A, Ray P. Essential role of nuclear factor κB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]