Abstract
Purpose
Effective treatment of dyslipidemia with lipid-lowering agents is pivotal in the management of metabolic-associated fatty liver disease (MAFLD) for preventing cardiovascular complications. We explored the associations between improvements in liver injuries indicated by changes in transaminases and a reduction in lipid levels in MAFLD patients with dyslipidemia and elevated transaminases during lipid-lowering therapies.
Methods
This prospective, cohort study enrolled consecutive MAFLD patients with hyperlipidemia and elevated transaminases. Patients were divided into a group receiving lipid-lowering agents and an age-, sex- and baseline lipid level-matched control group without receiving lipid-lowering agents. Clinical visits were performed at the 1st month and then every 3 months for 1 year.
Results
This study included 541 MAFLD patients (lipid-lowering group: 325 patients; control group: 216 patients). Compared with controls, there was a substantially greater reduction in alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), triglyceride (TG), total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c) in the lipid-lowering group after 12 months (all P < 0.05). The decrease in ALT was positively correlated with the decrease in TC (r = 0.332), TG (r = 0.180), LDL-c (r = 0.253) and apolipoprotein E (ApoE) (r = 0.119), while the decrease in AST was positively correlated with the decrease in TC (r = 0.228) and LDL-c (r = 0.192) (all P<0.05). The greater range of reduction in blood lipids (TC/TG/LDL-c), the higher the transaminase and GGT normalization rate (all P<0.05). Multivariate analysis confirmed that a TG decrease of over 50% remained an independent predictor of transaminase and GGT normalization (OR 2.07, 95% CI 1.12–3.84, P=0.020).
Conclusion
Lipid-lowering to target levels might be beneficial to liver injury improvements in MAFLD patients with dyslipidemia when receiving lipid-lowering agents.
Keywords: dyslipidemia, metabolic dysfunction-associated fatty liver disease, alanine aminotransferase, triglyceride, gamma glutamyl transpeptidase, statins
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide with a prevalence up to 25% in the general population.1 NAFLD was renamed metabolic dysfunction-associated fatty liver disease (MAFLD) by the international panel of experts in 2020. A recent meta-analysis including 116 studies shows that the global MAFLD prevalence in overweight/obese adults is estimated to be 50.7% (95% CI 46.9–54.4).2 The naming of MAFLD emphasizes the importance of metabolic abnormalities, thus emphasizing the importance of metabolic abnormalities in the management of MAFLD patients.3 Dyslipidemia is very common in NAFLD patients and is manifested by elevated triglyceride (TG) levels, elevated low-density lipoprotein cholesterol (LDL-c) levels and low high-density lipoprotein cholesterol (HDL-c) levels.4,5 A new study in Japan shows that the overall prevalence of hypertriglyceridemia was 64.1% in MAFLD patients.6 Moreover, this atherogenic dyslipidemia (elevated TG and LDL-c) has been proven as one of the most important established risk factors for cardiovascular disease (CVD), which is the most common cause of death in NAFLD patients.7 Therefore, treatment of dyslipidemia plays an important role in the long-term management of MAFLD patients.
The main hypolipidemic agents include statins and fenofibrate. The former inhibits 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase to effectively reduce LDL-c levels, and the latter acts as a peroxisome proliferator-activated receptor-α (PPARα) agonist that effectively decreases TG levels. Although there were previous concerns about the increased risk of hepatotoxicity from the main lipid-lowering treatments in NAFLD and most guidelines do not recommend them as specific therapy, statin treatment was proven safe in NAFLD patients with both dyslipidemia and elevated liver enzymes.8,9 Furthermore, several studies demonstrated the potential partially protective effect of statin treatment on NAFLD-associated inflammation and fibrosis through their pleiotropic properties.10–12 Data derived from 5 post hoc analyses of randomized control trials and 5 biopsy-based studies consistently (mainly those focused on statin treatment) demonstrated that the moderately abnormal levels of the liver enzymes containing alanine aminotransferase (ALT) and aspartate aminotransferase (AST) at baseline were ameliorated or achieved normalization in NASH.13 However, which factors determine liver enzyme level normalization under lipid-lowering treatments in MAFLD patients with dyslipidemia remain unclear.
Identification of the changes in the lipid profile and transaminases are commonly used blood biomarkers for MAFLD. Thus, it is of clinical importance to analyze the relationships between transaminase reduction and lipid changes. This may help to address whether specific treatments that target lipid management should be established to gain the beneficial effect of liver injury improvement in MAFLD patients. Therefore, we sought to explore the relationship between liver transaminase levels and lipid changes in MAFLD patients on lipid-lowering agents (eg, statins and fibrates) and to determine the predictive factors.
Materials and Methods
Study Patients and Design
In this prospective study, consecutive MAFLD outpatients in the fatty liver disease center of the First Affiliated Hospital of Sun Yat-sen University between October 2014 and January 2020 were enrolled. Diagnosis of MAFLD: 1) the presence of liver steatosis by abdominal ultrasonography; 2) one of the following three conditions: type 2 diabetes mellitus (T2DM), overweight/obesity (BMI ≥23 kg/m2 in Asians), and lean/normal weight with ≥2 metabolic risk abnormalities. Metabolic risk abnormalities are as follows: 1) blood pressure ≥130/85 mmHg or specific drug treatment; 2) waist circumference ≥90/80 cm in Asian men and women; 3) plasma TG ≥150 mg/dl or specific drug treatment; 4) prediabetes; 5) plasma HDL-c <40 mg/dl for men and <50 mg/dl for women or specific drug treatment; 6) plasma high-sensitivity C-reactive protein (hs-CRP) level >2 mg/L); 7) homeostasis model assessment of insulin resistance (HOMA-IR) score ≥2.5.3 Inclusion criteria: 1) elevated transaminase levels; 2) hyperlipidemia; 3) age 18 to 60 years old. Exclusion criteria: 1) pregnancy or lactation; 2) coinfection with viral hepatitis B and C; 3) presence of autoimmune liver disease-related antibodies; 4) alcohol consumption >10 g/day in females or >20 g/day in males; 5) cirrhosis; 6) history of inherited metabolic liver disease and malignancies; 7) a previous history of carotid or coronary artery stenting. The above exclusion criteria were made in order not to delay the treatment of liver damage caused by the above reasons and their treatment may affect the evaluation of the effect of lipid-lowering agents.
Allocation and Intervention
Controls were selected to match on the following variables: sex, age and baseline lipid level. All participants signed a written informed consent form. The study was approved by the local institute ethics committee [2014 No.112].
According to the intervention, patients were divided into two groups: a lipid-lowering group and a control group not receiving lipid-lowering agents. All patients received lifestyle interventions and were notified of the benefits and potential morbidities of the lipid-lowering agent options for MAFLD, and the final treatment was judged by the patients themselves. Lifestyle interventions included diet and exercise interventions. All patients received dietary recommendations from a professional nutritionist. Total daily calorie intake was calculated based on their own ideal body weight. Patients were instructed to perform aerobic exercise particular walking after excluding exercise contraindications. For lipid-lowering agents, moderate-intensity statins, such as rosuvastatin 10 mg or atorvastatin 20 mg, were prescribed, and fenofibrate 200 mg was provided to lower serum triglycerides. For those with combined hyperlipidemia, a combination of statins and fenofibrate was preferred if the TG level was over 5.7 mmol/L, while statins were given to the others.
Data Collection and Measurements
Structured questionnaires were applied to collect medical history, lifestyle habits, blood pressure and anthropometry parameters (weight, height, waist circumference [WC] and body mass index [BMI, kg/m2]). Laboratory tests were performed using serum samples acquired after at least 8 hours of fasting within one day of collection for serum markers including hs-CRP, AST, ALT, gamma glutamyl transpeptidase (GGT), lipid profiles [total cholesterol (TC, OSR6216), TG (OSR61118), LDL-c (OSR6283), HDL-c (OSR6287), apolipoprotein B (ApoB), apolipoprotein A-1 (ApoA-1) and apolipoprotein E (ApoE) were determined by the enzymatic colorimetric method with Beckman Coulter reagent test kits], insulin, and glucose, at both baseline and during treatment with the Abbott c8000 Automatic Biochemistry Analyzer (Abbott, Abbott Park, IL, USA).
The NAFLD Fibrosis Score (NFS) formula was −1.675+0.037 x age (years)+0.094 x BMI (kg/m2) +1.13 x impaired fasting glucose/diabetes (yes = 1, no = 0) +0.99 x AST/ALT ratio−0.013 x platelet count (x 109/L) −0.66 x albumin (g/dL).14 The fibrosis-4 index (FIB-4) index formula was (age [years] x AST [IU/L])/(platelet count [109/L] x (ALT [IU/L])1/2).15
The imaging evaluation of the liver included high-resolution B-mode abdominal ultrasonography in all patients. The liver stiffness measurement was conducted by 2D-SWE in most patients. Total liver fat content was detected by MRI-PDFF using a 3.0-Tesla scanner (Siemens 3.0T MAGNETOM Verio) in part of patients.
Treatment Response Assessment
Elevated transaminases were considered when ALT was elevated with or without elevated AST or GGT. Elevated ALT was defined as serum ALT > 19 U/L for women and >30 U/L for men.16 Elevated AST was defined as serum AST >37 U/L and elevated GGT was defined as serum GGT >50 U/L according to the reference range of our detector. Transaminase normalization was considered when both ALT and AST decreased to normal. Dyslipidemia, cardiovascular risk classes and LDL-c targets were defined in accordance with the Chinese guidelines for the management of dyslipidemia in adults.17 Dyslipidemia was defined as TC ≥5.2 mmol/L, LDL-c ≥3.4 mmol/L, TG ≥1.7 mmol/L, or HDL-c <1.0 mmol/L. Thus, patients were sorted as low, moderate, high, or very high risk for CVD, and the LDL-c treatment goals for each risk group were 3.4, 3.4, 2.6, and 1.8 mmol/L, respectively. Patients were treated to target when their LDL-c levels were lower than the treatment goal after 12 months of treatment.
Follow-Up
The treatment course was 12 months, and for both groups, the response of liver function tests to lifestyle intervention alone or in combination with lipid-lowering medications was evaluated at baseline, the first 4 weeks and every 12 weeks after treatment. The side effects of drugs, including symptoms, levels of transaminases and serum creatine kinase (CK) levels, were monitored during treatment.
Statistical Analysis
Data are expressed as means ± SD, numbers with percentages (%), or medians with interquartile ranges (IQRs). Comparisons of dichotomized variables between groups were analyzed by the chi-square test. Continuous variables were analyzed by Mann–Whitney U-tests and Student’s t-tests. The effects of lipid-lowering agents on parameters were investigated using repeated-measures analysis of variance (ANOVA). The correlation between the decrease in transaminase and other variables were analyzed by Spearman correlation coefficients. Predictors of transaminase normalization by lipid-lowering agents were analyzed by binary logistic regression analysis. A two-tailed P < 0.05 was considered significant. SPSS software (version 25.0, SPSS, Chicago, IL) was used for all analyses.
Results
Baseline Characteristics
At the beginning of the study, 562 MAFLD patients with baseline elevated transaminases and dyslipidemia were included (lipid-lowering group: 338 patients; control group: 224 patients). During the follow-up period, 9 patients were lost and 4 patients discontinued lipid-lowering agents because of elevated ALT or CK in the lipid-lowering group. Eight patients in the control group were lost during follow-up. Finally, 541 patients were included in the study (lipid-lowering group: 325 patients; control group: 216 patients). There was no statistically significant difference in transaminases, blood lipids, or other clinical indicators between these two groups at baseline (Table 1). In the lipid-lowering group, the most commonly prescribed lipid-lowering agent was atorvastatin (36.9%, 120/325, 20 mg daily), followed by rosuvastatin (29.5%, 96/325, 10 mg daily), fenofibrate (28.0%, 91/325, 200 mg daily), and atorvastatin combined with fenofibrate (5.5%, 18/325, atorvastatin 20 mg and fenofibrate 200 mg daily).
Table 1.
Comparison of Clinical and Laboratory Characteristics at Baseline and the 12th Month Between Lipid-Lowering Group and Control Group
| Characteristics | Lipid-Lowering Group (n=325) | Control Group (n=216) | Baseline Comparison | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 Months | P | Baseline | 12 Months | P | P | |
| Male, n (%) | 241 (74.2) | - | - | 167 (77.3) | - | - | 0.403 |
| Age, years | 41.9±12.8 | - | - | 41.4±12.6 | - | - | 0.677 |
| Weight, kg | 72.6±12.9 | 71.6±12.5 | 0.348 | 73.7±11.5 | 73.3±11.7 | 0.733 | 0.286 |
| BMI, kg/m2 | 26.0±3.5 | 25.5±3.4 | 0.100 | 26.0±3.1 | 26.0±3.3 | 0.892 | 0.273 |
| WC, cm | 88.4±9.3 | 87.4±8.8 | 0.248 | 89.3±8.2 | 89.3±8.8 | 0.962 | 0.841 |
| SBP, mmHg | 129±16 | 129±16 | 0.995 | 128±16 | 128±15 | 0.944 | 0.583 |
| DBP, mmHg | 84±13 | 84±13 | 0.928 | 83±13 | 83±12 | 0.912 | 0.582 |
| Hypertension, n (%) | 147 (45.2) | - | - | 98 (45.4) | - | - | 0.975 |
| T2DM, n (%) | 67 (20.6) | - | - | 41 (19.0) | - | - | 0.641 |
| ALT, U/L | 80±37 | 26±15 | <0.001 | 78±34 | 74±33 | 0.145 | 0.570 |
| AST, U/L | 52±31 | 25±13 | <0.001 | 53±33 | 49±22 | 0.100 | 0.686 |
| GGT, U/L | 89±61 | 40±19 | <0.001 | 89±58 | 82±41 | 0.090 | 0.920 |
| TC, mmol/L | 6.1±1.3 | 3.6±0.7 | <0.001 | 6.0±1.0 | 5.9±1.1 | 0.611 | 0.190 |
| TG, mmol/L | 3.1±1.9 | 1.4±0.7 | <0.001 | 2.9±1.7 | 2.7±1.4 | 0.090 | 0.317 |
| HDL-c, mmol/L | 1.2±0.5 | 1.2±0.4 | 0.453 | 1.2±0.4 | 1.2±0.4 | 0.827 | 0.307 |
| LDL-c, mmol/L | 4.0±0.9 | 2.5±0.8 | <0.001 | 3.9±0.8 | 3.8±0.7 | 0.175 | 0.656 |
| ApoA-1, g/L | 1.4±0.5 | 1.4±0.3 | 0.995 | 1.3±0.3 | 1.4±0.3 | 0.250 | 0.521 |
| ApoB, g/L | 1.1±0.3 | 1.0±0.3 | 0.001 | 1.1±0.2 | 1.0±0.2 | 0.097 | 0.112 |
| ApoE, mg/L | 57±28 | 47±25 | <0.001 | 54±22 | 57±40 | 0.451 | 0.276 |
| Lp-A, mg/L | 204±192 | 202±209 | 0.884 | 193±179 | 199±190 | 0.840 | 0.631 |
| UA, μmol/l | 416±104 | 393±103 | 0.015 | 415±106 | 407±116 | 0.700 | 0.966 |
| FFA, mmol/L | 572±220 | 418±118 | <0.001 | 543±177 | 509±162 | 0.264 | 0.353 |
| FBG, mmol/L | 5.6±1.2 | 5.4±1.0 | 0.006 | 5.5±1.3 | 5.3±1.2 | 0.213 | 0.453 |
| FINS, μU/mL | 12.1±9.6 | 11.3±8.6 | 0.328 | 11.5±6.0 | 11.5±7.9 | 0.956 | 0.552 |
| HOMA-IR | 3.1±3.2 | 3.0±2.8 | 0.655 | 2.8±1.7 | 2.8±1.9 | 0.813 | 0.336 |
| HbA1c, % | 6.1±1.1 | 5.9±1.1 | 0.735 | 6.1±1.2 | 5.8±1.1 | 0.219 | 0.981 |
| FIB-4 index | 1.27±0.95 | 1.23±0.80 | 0.756 | 1.25±0.77 | 1.19±0.67 | 0.643 | 0.904 |
| NFS | −1.36±1.62 | −1.70±2.03 | 0.366 | −1.48±1.82 | −2.12±2.35 | 0.325 | 0.728 |
| LSM, kPa | 6.5±2.4 | 6.4±2.3 | 0.700 | 6.1±1.6 | 6.6±2.0 | 0.239 | 0.277 |
Abbreviations: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; T2DM, type 2 diabetes; ALT, alanine aminotransferease; AST, aspartate aminotransferase; GGT, gamma glutamyltranspeptidase; TC, total cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; ApoA-1, apolipoprotein A-1; ApoB, apolipoprotein B; ApoE, apolipoprotein E; LP-A, lipoprotein-A; UA, uric acid; FFA, free fatty acid; FBG, fasting blood glucose; FINS, fasting insulin; HOMA-IR, homeostatic model assessment of insulin resistance; HbA1c, glycated hemoglobin; FIB-4, Fibrosis-4; NFS, NAFLD fibrosis score; LSM, liver stiffness measurement.
Effect of Lipid-Lowering Agents on Transaminases and Metabolic Indicators in MAFLD Patients with Dyslipidemia
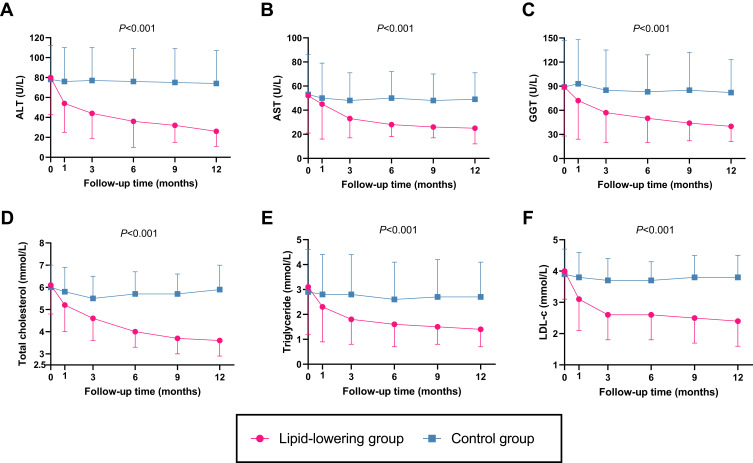
There was a substantial reduction in ALT, AST, GGT, TC, TG, LDL-c, ApoB, ApoE, free fatty acid (FFA) and fasting blood glucose after 12 months of treatment in the lipid-lowering group, whereas there were no significant changes in the laboratory indicators of the control group between baseline and 12 months (Table 1). Furthermore, the effects on the reduction in ALT, AST, GGT, TC, TG, LDL-c and ApoE were greater in the lipid-lowering group than in the control group during the study, as determined by using repeated-measures analysis of variance (ANOVA) (Figure 1 and Supplementary Table 1).
Figure 1.
Decrease trends of transaminases and blood lipids in the lipid-lowering group (n = 325) and control group (n = 216). Repeated-measures analysis of variance (ANOVA) for the comparison of effects on ALT (A), AST (B), GGT (C), total cholesterol (D), triglyceride (E), and LDL-c (F) between lipid-lowering group and control group. P values were for the ANOVA analysis between the two groups.
Abbreviations: ALT, alanine aminotransferease; AST, aspartate aminotransferase; GGT, gamma glutamyltranspeptidase; LDL-c, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
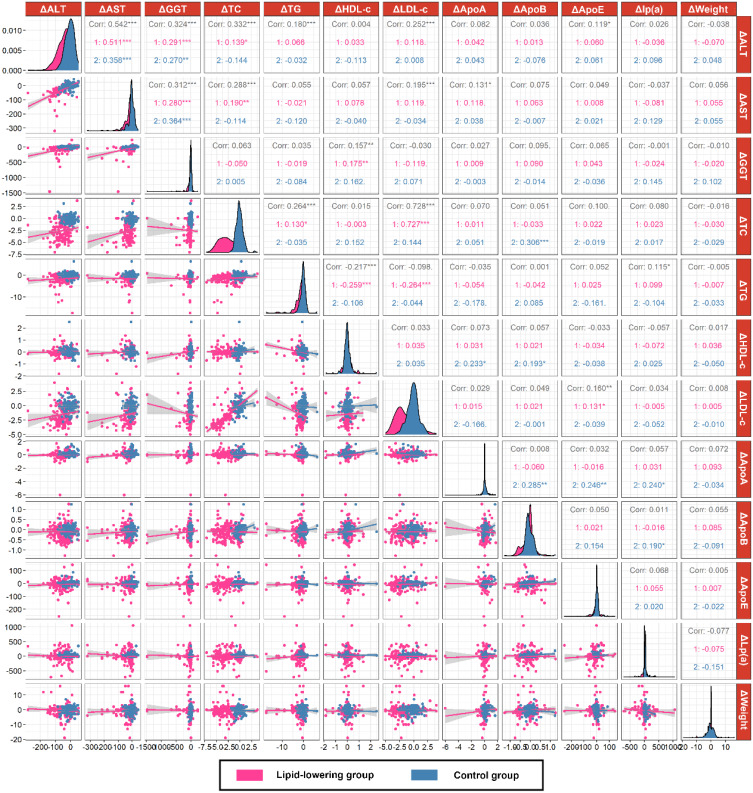
The Correlation Between Changes in Transaminases and Blood Lipids
In 541 MAFLD patients, the decrease in ALT was positively correlated with the decrease in TC (r = 0.332), TG (r = 0.180), LDL-c (r = 0.253) and ApoE (r = 0.119), while the decrease in AST positively correlated with the decrease in TC (r = 0.228) and LDL-c (r = 0.192) by spearman correlation coefficients (all P<0.05). In the lipid-lowering group, the decrease in ALT (r = 0.139, P<0.05) and AST (r = 0.190, P<0.01) was positively correlated with the decrease in TC, respectively. However, there was no correlation between the decrease in transaminase and blood lipids in the control group (Figure 2).
Figure 2.
Correlation analysis between changes in transaminase and blood lipids in the lipid-lowering group (n = 325) and control group (n = 216). ***P < 0.001, **P < 0.01. *P < 0.05.
Abbreviations: Δ, changes; ALT, alanine aminotransferease; AST, aspartate aminotransferase; GGT, gamma glutamyltranspeptidase; TC, total cholesterol; TG, triglyceride; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; ApoA-1, apolipoprotein A-1; ApoB, apolipoprotein B; ApoE, apolipoprotein E.
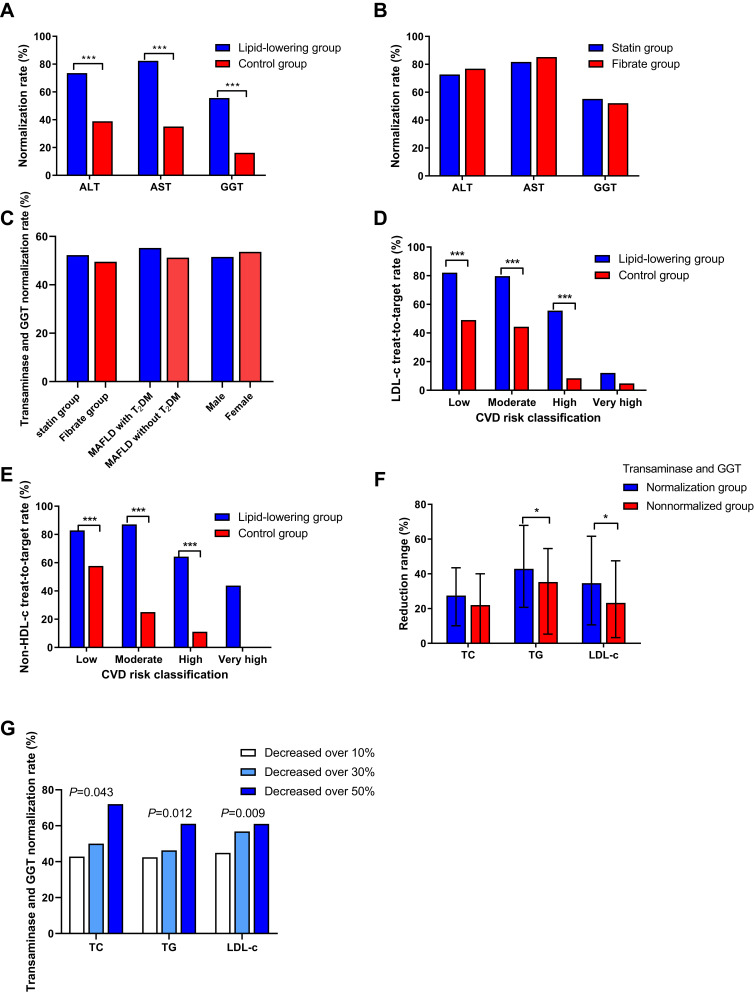
The Impact of Declining Blood Lipid Levels on Transaminase and GGT Normalization
The transaminase and GGT normalization rate in the lipid-lowering group (52.8%, 169/325) was significantly higher than that in the control group (29.6%, 64/216) after 12 months of follow-up (P<0.001). Additionally, the single ALT, AST or GGT normalization rate in the lipid-lowering group was higher than that in the control group (all P<0.001) (Figure 3A). Furthermore, when we divided patients receiving lipid-lowering agents into a statin group (n = 216) and a fibrate group (n = 91), the results showed that there was no difference in transaminase and GGT normalization rate between these two groups, and similar results were obtained in the MAFLD with T2DM group (n = 67) and without T2DM group (n = 258), as well as in the male group (n = 241) and female group (n = 84) (All P>0.5) (Figure 3B and C).
Figure 3.
The impact of declining blood lipid levels on transaminase and GGT normalization. Comparison of transaminase and GGT normalization rate between the lipid-lowering group (n = 325) and control group (n = 216) (A), statin group (n = 216) and fibrate group (n = 91) (B and C), MAFLD with T2DM group (n = 67) and without T2DM group (n = 258), the male group (n = 241) and female group (n = 84) (C), and groups of 3 different reduction ranges in blood lipids (G). Comparison of LDL-c (D) and non-HDL-c (E) treated to target rate between the lipid-lowering group and the control group. Comparison of reduction ranges in blood lipids between the transaminase and GGT normalization group (n = 169) and nonnormalized group (n = 156) (F). ***P < 0.001, *P < 0.05.
Abbreviations: ALT, alanine aminotransferease; AST, aspartate aminotransferase; GGT, gamma glutamyltranspeptidase; TC, total cholesterol; TG, triglyceride; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; CVD, cardiovascular disease; T2DM, type 2 diabetes.
In the lipid-lowering group, the proportions of patients who were at mild, moderate, high, and very high risk for CVD were 25.8% (84/325), 19.7% (64/325), 44.3% (144/325), and 10.2% (33/325), respectively, whereas in the control group, the proportions were 25.5% (55/216), 20.8% (45/216), 44.0% (95/216), and 9.7% (21/216), respectively. The proportion of patients with LDL-c treated to target was higher in the lipid-lowering group than in the control group (62.8% vs 25.9%, P<0.001), with a similar result for non-HDL-c (71.5% vs 24.5%, P<0.001) (Figure 3D and E).
The ranges of reduction in TG (P=0.047) and LDL-c (P=0.047) in the transaminase and GGT normalization group (n = 169) were higher than those in the transaminase and GGT nonnormalized group (n = 156) (Figure 3F). Additionally, according to the range of reduction in blood lipids (TC/TG/LDL-c), patients were divided into a decreased over 10% group, a decreased over 30% group and a decreased over 50% group, the results showed that the greater range of reduction in blood lipids (TC/TG/LDL-c), the higher the transaminase and GGT normalization rate (Figure 3G). There was no statistical difference in transaminase and GGT normalization rate between LDL-c treated to target group and LDL-c decreased over 50% group (53.7% vs 61.0%, P=0.263).
Independent Predictors of Transaminase Normalization by Lipid-Lowering Agents
Weight loss (OR 1.81, 95% CI 1.03–3.12, P=0.039), TC decreased over 50% (OR 1.84, 95% CI 0.98–3.40, P=0.049), TG decreased over 50% (OR 2.08, 95% CI 1.14–3.82, P=0.018) and LDL-c decreased over 50% (OR 1.85, 95% CI 1.03–3.32, P=0.04) were favorable predictors of transaminase and GGT normalization in the univariate analysis. Multivariate analysis confirmed that a TG decrease of over 50% remained an independent predictor of transaminase and GGT normalization (OR 2.07, 95% CI 1.12–3.84, P=0.020) (Table 2).
Table 2.
Univariate and Multivariate Analyses of Factors Associated with Transaminase and GGT Normalization by Lipid-Lowering Agents
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Female | 1.01 (0.55–1.85) | 0.985 | - | - |
| Age, years | 1.01 (0.99–1.03) | 0.479 | - | - |
| Baseline BMI ≥23kg/m2 | 0.74 (0.36–1.53) | 0.419 | - | - |
| Baseline TC ≥5.2mmol/L | 1.26 (0.62–2.53) | 0.523 | - | - |
| Baseline TG ≥1.7mmol/L | 1.25 (0.60–2.51) | 0.525 | - | - |
| Baseline LDL-c ≥3.4mmol/L | 1.23 (0.64–2.36) | 0.538 | - | - |
| Weight loss (%) | 1.81 (1.03–3.12) | 0.039 | 1.60 (0.87–2.95) | 0.132 |
| TC decreased over 50% | 1.84 (0.98–3.40) | 0.049 | 1.28 (0.61–2.65) | 0.514 |
| TG decreased over 50% | 2.08 (1.14–3.82) | 0.018 | 2.07 (1.12–3.84) | 0.020 |
| LDL-c decreased over 50% | 1.85 (1.03–3.32) | 0.040 | 1.64 (0.83–3.21) | 0.154 |
Abbreviations: GGT, gamma glutamyltranspeptidase; BMI, body mass index; TC, total cholesterol; TG, triglyceride; LDL-c, low-density lipoprotein cholesterol.
Effect of Lipid-Lowering Agents on Transaminases and Metabolic Indicators in MAFLD Patients with MRI-PDFF
There were 60 patients with liver fat content diagnosed by MRI-PDFF both before and after 12 months of treatment, including 48 males (80.0%) and 12 females (20.0%), with an average age of 45.4±12.7 years. The liver fat content decreased significantly after 12 months of treatment (P<0.05) (Supplementary Table 2). Further linear correlation analysis found that the decrease in liver fat content was positively correlated with the decrease in TC (r = 0.295) and LDL-c (r = 0.286) (all P<0.05) (Supplementary Figure 1).
Safety
Among the patients in the lipid-lowering group, 2 (0.6%) patients had asymptomatic elevated creatine kinase after taking rosuvastatin for 6 months (144 and 224 U/L, respectively). The other 2 (0.6%) patients had elevated ALT after taking rosuvastatin for 3 and 4 months, respectively. The ALT levels in one patient were 53 U/L at baseline and 126 U/L after 3 months, while in another patient 47 U/L at baseline and 179 U/L after 4 months. All four patients stopped taking rosuvastatin when increased CK or ALT was detected.
Discussion
Lipid-lowering agent-associated hepatic toxicity is often a concern in the treatment of individuals with MAFLD accompanied by both hypertriglyceridemia and liver injuries, and this may lead to the under-prescription or delayed initiation of statins.8,18 Previous studies support that statin treatment appears to be safe and might even improve abnormalities of hepatic enzymograms in NAFLD with concomitant hypertriglyceridemia and liver injuries.19–23 Our study confirmed the effectiveness and safety of lipid-lowering agents added to therapy compared to lifestyle monotherapy. More importantly, our study demonstrated that the extent to which lipid levels were lowered was correlated with the normalization of transaminase levels, which was independent of weight changes and the prescription of lipid-lowering agents. These important findings reveal that the liver benefits from LDL-c- or TG-lowering treatments.
The hallmark of NAFLD is the overaccumulation of intrahepatic lipids derived from an imbalance among lipid generation, efflux and degradation.24 In some NAFLD patients, increases in hepatic de novo synthesis of fatty acids and cholesterol are both involved in the pathogenesis of hepatocyte injury and death, and they subsequently cause progression to inflammation and subsequent fibrosis.25 Therefore, inhibition of intrahepatic lipogenesis might be a therapeutic target for MAFLD. Emerging population-based studies have showed that statin use may reduce the development and progression of MAFLD. A nationwide study from Korea that included 5,339,901 subjects showed that statins may reduce the risk of significant liver fibrosis.11 In addition to the cholesterol-lowering effects of statins, the results from animal model studies observed that statins decreased the levels of proinflammatory cytokines, such as TNF-α, TGF-β1, IL-1β and IL-6, and identified that small guanine triphosphate binding proteins (GTPases), proliferator-activated receptor α, and paraoxonase 1 are other potential key mediators that exert anti-inflammatory and anti-fibrosis effects on NAFLD.10,26 The fibrates have been shown to reduce hepatic steatosis, hepatic macrophage accumulation and inflammatory gene expression, and upregulate genes involved in beta oxidation in animal models.27 Fibrates could also reduce plasma ALT concentration of biopsy-confirmed NASH/NAFLD patients.28,29 Existing data suggest that the mechanism of statins or fibrates on MAFLD is multi-pathway. Our current study found that there was no difference in the transaminase reversion rate between the fibrate group and the statin group. Furthermore, the logistic regression model confirmed the independent effect of changes in TG, not statins or fibrates, on transaminases and GGT restorations. Taken together, these data support the hepatic benefits of either statins or fibrates treatment by transaminase and GGT reduction. However, whether statins and fibrates reduce transaminases and GGT in other ways in addition to cholesterol-lowering effect remains unclear.
A recent study has showed the association between ALT and AST reduction with TG reduction after treatment of saroglitazar in NAFLD patients with diabetic dyslipidemia.30 Statins, fibrates and saroglitazar have different mechanisms and targets on MAFLD patients. Statins inhibit HMG-CoA reductase to effectively reduce LDL-c levels, while fibrates acts as a PPARɑ agonist that effectively decreases TG levels.31–33 And saroglitazar is a dual PPAR ɑ/γ agonist.34 Therefore, whether the improvement of liver injuries in MAFLD patients receiving the aforementioned pharmaceutical treatment is directly associated with primary pharmacological property of the drugs or indirectly related to their lipid-lowering effect still deserves further investigation. Our research partly explained that the latter would be also potential mechanisms. Moreover, although a correlation between blood lipid reduction and transaminase reduction has been found, it is still unclear whether this correlation could be translated into useful predictor in the clinical practice. And our study firstly illustrated the predicting value of lipid-lowering effect could be reliable predictors of transaminase reduction.
Current researches have shown that there is still a high proportion of patients not treated to LDL-c target in patients with dyslipidemia. Two studies in the United States found that 37.5% and 57.4% of patients with dyslipidemia were not treated to achieve their LDL-c target, respectively.35,36 A recent Asian study also showed that up to 58.9% of NAFLD patients with dyslipidemia were not treated to their LDL-c target.37 Patients not treated to their LDL-c target become another important burden for the management of dyslipidemia in MAFLD patients. Our study also showed that there was a high proportion of patients not treated to their LDL-c target even though their transaminases and GGT returned to normal. Therefore, it would suggest continuing to use statins or even increasing the dose of statins to achieve their LDL-c target, which could ultimately reduce CVD risk in MAFLD patients.
The main strength of this study was that it was a prospective study with an age-, sex- and baseline lipid level-matched control group. However, this study had some limitations. First, our study was a single-center study. Second, there were not all patients receiving MRI-PDFF estimation, which affected the accuracy of assessing changes in liver fat content. Third, the overall weight loss of patients after treatment was not obvious, which may underestimate the efficacy of the lifestyle intervention when compared to lipid-lowering therapy. Furthermore, patients in our study were followed up for up to one year, and long-term observation of efficacy and safety is needed.
Conclusions
The present study shows that lipid-lowering agents can effectively reduce blood lipids, transaminases in MAFLD patients with both elevated liver enzymes and dyslipidemia. More importantly, our results demonstrate that the extent to which blood lipid levels were lowered was correlated with the treatment transaminase levels, which was independent of weight changes or the other effects of lipid-lowering agents. The more reduction of blood lipid levels, the more benefit of liver injury improvements indicated by liver enzymes. However, the study shows that the extent of blood lipid reduction and the rate of LDL-c treated to target are far from enough in MAFLD patients. These findings indicate that the use of enhanced lipid-lowering agents in these patients is necessary to simultaneously achieve the blood lipid treatment target and improve liver injury. However, long-term studies are required to further verify its long-term efficacy and safety in MAFLD patients.
Acknowledgments
We are grateful to Professor Aihua Lin in School of Public Health, Sun Yat-sen University for her assistance in statistical analysis of this study.
Funding Statement
This study is supported by National Natural Science Foundation of China (81870404, 81670518, 81170392), Guangdong Science and Technology Department (2014A020212118), Chinese Foundation for Hepatitis Prevention and Control (TQGB20140083), Guangdong Medical Research Foundation (A2019496) and China postdoctoral science foundation (2020M683128).
Abbreviations
MAFLD, metabolic-associated fatty liver disease; CVD, cardiovascular disease; NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; T2DM, type 2 diabetes; DBP, diastolic blood pressure; WC, waist circumference; SBP, systolic blood pressure; ALT, alanine aminotransferease; AST, aspartate aminotransferase; GGT, gamma glutamyltranspeptidase; TC, total cholesterol; LDL-c, low-density lipoprotein cholesterol; TG, triglyceride; ApoA-1, apolipoprotein A-1; HDL-c, high-density lipoprotein cholesterol; ApoB, apolipoprotein B; LP-A, lipoprotein-A; ApoE, apolipoprotein E; UA, uric acid; FFA, free fatty acid; FBG, fasting blood glucose; FINS, fasting insulin; HOMA-IR, homeostatic model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; HbA1c, glycated hemoglobin; FIB-4, Fibrosis-4; LSM, liver stiffness measurement; NFS, NAFLD fibrosis score; LFC, liver fat content.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval
The study protocol was approved by the local ethics committee (IEC for clinical research and animal trials of the First Affiliated Hospital of Sun Yat-sen University) [2014 No. 112], and was in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of nonalcoholic fatty liver disease. Metabolism. 2016;65:1017–1025. doi: 10.1016/j.metabol.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 2.Liu JY, Ayada I, Zhang XF, et al. Estimating global prevalence of metabolic dysfunction associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol. 2022;20(3):e573-e582. doi: 10.1016/j.cgh.2021.02.030 [DOI] [PubMed] [Google Scholar]
- 3.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 4.Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016;65(8):1109–1123. doi: 10.1016/j.metabol.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 5.Chatrath H, Vuppalanchi R, Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin Liver Dis. 2012;32(1):22–29. doi: 10.1055/s-0032-1306423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoneda M, Yamamoto T, Honda Y, et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol. 2021;56(11):1022–1032. doi: 10.1007/s00535-021-01828-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin YS, Gong XR, Li X, et al. Distinct cause of death profiles of hospitalized non-alcoholic fatty liver disease: a 10 years’ cross-sectional multicenter study in China. Front Med. 2021;7:584396. doi: 10.3389/fmed.2020.584396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderon RM, Cubeddu LX, Goldberg RB, Schiff ER. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: a therapeutic dilemma. Mayo Clin Proc. 2010;85:349e56. doi: 10.4065/mcp.2009.0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastori D, Polimeni L, Baratta F, Pani A, Ben MD, Angelico F. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2015;47:4–11. doi: 10.1016/j.dld.2014.07.170 [DOI] [PubMed] [Google Scholar]
- 10.Ahsan F, Oliveri F, Goud HK, et al. Pleiotropic effects of statins in the light of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cureus. 2020;12(9):e10446. doi: 10.7759/cureus.10446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JI, Lee HW, Lee KS, Lee HS, Park JY. Effects of statin use on the development and progression of nonalcoholic fatty liver disease: a nationwide nested case-control study. Am J Gastroenterol. 2021;116(1):116–124. doi: 10.14309/ajg.0000000000000845 [DOI] [PubMed] [Google Scholar]
- 12.Markova AA, Deterding K, Port K, et al. Liver stiffness across different chronic liver disease under therapy with statin in a real life cohort. Eur J Gastroenterol Hepatol. 2021;32(2):223–229. doi: 10.1097/MEG.0000000000001719 [DOI] [PubMed] [Google Scholar]
- 13.Athyros VG, Alexandrides TK, Bilianou H, et al. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement. Metabolism. 2017;71:17–32. doi: 10.1016/j.metabol.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 14.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a non-invasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496 [DOI] [PubMed] [Google Scholar]
- 15.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis patients with HIV/HCV co-infection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 16.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006 [DOI] [PubMed] [Google Scholar]
- 17.Joint committee issued Chinese guideline for the management of dyslipidemia in adults. 2016 Chinese guideline for the management of dyslipidemia in adults. Chin J Cardiovasc Dis. 2016;44(10):833–853. [DOI] [PubMed] [Google Scholar]
- 18.Rzouq FS, Volk ML, Hatoum HH, Talluri SK, Mummadi RR, Sood GK. Hepatotoxicity fears contribute to underutilization of statin medications by primary care physicians. Am J Med Sci. 2010;340:89–93. doi: 10.1097/MAJ.0b013e3181e15da8 [DOI] [PubMed] [Google Scholar]
- 19.Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376(9756):1916–1922. doi: 10.1016/S0140-6736(10)61272-X [DOI] [PubMed] [Google Scholar]
- 20.Athyros VG, Giouleme O, Ganotakis ES, et al. Safety and impact on cardiovascular events of longterm multifactorial treatment in patients with metabolic syndrome and abnormal liver function tests: a post hoc analysis of the randomized ATTEMPT study. Arch Med Sci. 2011;7(5):796–805. doi: 10.5114/aoms.2011.25554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tikkanen MJ, Fayyad R, Faergeman O, et al. Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild-to-moderate baseline elevations in alanine aminotransferase levels. Int J Cardiol. 2013;168:3846–3852. doi: 10.1016/j.ijcard.2013.06.024 [DOI] [PubMed] [Google Scholar]
- 22.Hyogo H, Ikegami T, Tokushige K, et al. Efficacy of pitavastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: an open-label, pilot study. Hepatol Res. 2011;41(11):1057–1065. doi: 10.1111/j.1872-034X.2011.00849.x [DOI] [PubMed] [Google Scholar]
- 23.Kargiotis K, Athyros VG, Giouleme O, et al. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J Gastroenterol. 2015;21(25):7860–7868. doi: 10.3748/wjg.v21.i25.7860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arguello G, Balboa E, Arrese M. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim Biophys Acta. 2015;1852(9):1765–1778. doi: 10.1016/j.bbadis.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 25.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi: 10.1053/j.gastro.2013.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islam MM, Poly TN, Walther BA, Yang HC, Jack L. Statin use and the risk of hepatocellular carcinoma: a meta-analysis of observational studies. Cancers. 2020;12(3):671. doi: 10.3390/cancers12030671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiri-Sverdlov R, Wouters K, van Gorp PJ, et al. Early diet-induced non-alcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J Hepatol. 2006;44:732–741. doi: 10.1016/j.jhep.2005.10.033 [DOI] [PubMed] [Google Scholar]
- 28.Laurin J, Lindor KD, Crippin JS, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–1467. doi: 10.1002/hep.510230624 [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Miranda C, Perez-Carreras M, Colina F, López-Alonso G, Vargas C, Solís-Herruzo JA. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40:200–205. doi: 10.1016/j.dld.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 30.Goyal O, Nohria S, Goyal P, et al. Saroglitazar in patients with non-alcoholic fatty liver disease and diabetic dyslipidemia: a prospective, observational, real world study. Sci Rep. 2020;10(1):21117. doi: 10.1038/s41598-020-78342-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–1164. doi: 10.1126/science.1059344 [DOI] [PubMed] [Google Scholar]
- 32.Prieur X, Coste H, Rodriguez JC. The human apolipoprotein AV gene is regulated by peroxisome proliferator-activated receptor-alpha and contains a novel farnesoid X-activated receptor response element. J Biol Chem. 2003;278(28):25468–25480. doi: 10.1074/jbc.M301302200 [DOI] [PubMed] [Google Scholar]
- 33.Vu-Dac N, Gervois P, Jakel H, et al. Apolipoprotein A5, a crucial determinant of plasma triglyceride levels, is highly responsive to peroxisome proliferator-activated receptor alpha activators. J Biol Chem. 2003;278(20):17982–17985. doi: 10.1074/jbc.M212191200 [DOI] [PubMed] [Google Scholar]
- 34.Jani RH, Kansagra K, Jain MR, et al. Pharmacokinetics, safety, and tolerability of saroglitazar (ZYH1), a predominantly PPARalpha agonist with moderate PPARgamma agonist activity in healthy human subjects. Clin Drug Investig. 2013;33:809–816. doi: 10.1007/s40261-013-0128-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong ND, Chuang J, Wong K, et al. Residual dyslipidemia among United States adults treated with lipid modifying therapy (data from National Health and Nutrition Examination Survey 2009–2010). Am J Cardiol. 2013;112:373–379. doi: 10.1016/j.amjcard.2013.03.041 [DOI] [PubMed] [Google Scholar]
- 36.Toth PP, Zarotsky V, Sullivan JM, Laitinen D. Dyslipidemia treatment of patients with diabetes mellitus in a US managed care plan: a retrospective database analysis. Cardiovasc Diabetol. 2009;8:26. doi: 10.1186/1475-2840-8-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoo S, Wong VW, Goh GB, et al. Suboptimal treatment of dyslipidemia in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35(2):320–325. doi: 10.1111/jgh.14794 [DOI] [PubMed] [Google Scholar]