Abstract
Obesity has become a worldwide epidemic; 340 million of children and adolescents were overweight or obese in 2016, and this number continues to grow at a rapid rate. Epidemiological research has suggested that air pollution affects childhood obesity and weight status, but the current evidence remains inconsistent. Therefore, the aim of this meta-analysis was to estimate the effects of childhood exposure to air pollutants on weight. A total of four databases (PubMed, Web of Science, Embase, and Cochrane Library) were searched for publications up to December 31, 2021, and finally 15 studies met the inclusion criteria for meta-analysis. Merged odds ratios (ORs), coefficients (β), and 95% confidence intervals (95% CIs) that were related to air pollutants were estimated using a random-effects model. The meta-analysis indicated that air pollutants were correlated with childhood obesity and weight gain. For obesity, the association was considerable for PM10 (OR = 1.12, 95% CI: 1.06, 1.18), PM2.5 (OR = 1.28, 95% CI: 1.13, 1.45), PM1 (OR = 1.41, 95% CI: 1.30, 1.53), and NO2 (OR = 1.11, 95% CI: 1.06, 1.18). Similarly, BMI status increased by 0.08 (0.03–0.12), 0.11 (0.05–0.17), and 0.03 (0.01–0.04) kg/m2 with 10 μg/m3 increment in exposure to PM10, PM2.5, and NO2. In summary, air pollution can be regarded as a probable risk factor for the weight status of children and adolescents. The next step is to conduct longer-term and large-scale studies on different population subgroups, exposure concentrations, and pollutant combinations to provide detailed evidence. Meanwhile, integrated management of air pollution is essential.
Keywords: childhood, air pollution, obesity, BMI, meta-analysis
1. Introduction
Obesity has become a worldwide epidemic and urgent health issue [1]. The prevalence of overweight and obesity has increased considerably in the last few decades and nearly tripled since 1975. With regard to children and adolescents, 340 million of them were overweight or obese in 2016, and this number continues to grow at a rapid rate [2,3]. Childhood obesity has been associated with obesity and increased risks for chronic disease in adulthood [4,5], and these adverse effects may last the whole lifetime [6]. Research has identified multiple factors that can lead to childhood obesity and it has been shown that childhood obesity can be attributed to genetic, dietary, and behavioral factors [7]. Despite genetic and metabolic predispositions, the rising epidemic of obesity indicates environmental factors may play a role in accelerating the progression of childhood obesity [8].
The Global Burden of Disease study revealed that air pollution can be the most adverse environmental health hazard for disease and mortality worldwide [9,10]. Well over 80% of urban dwellers suffer from air pollution, and the most seriously affected individuals were low-income residents [11]. In recent years, mounting evidence suggests that air pollution can be an obesogenic factor [12]. It is mainly through the biochemical and behavioral pathways that air pollution affects body weight. Metabolic disorders [13], inflammatory reactions [14], reduced sleep duration and quality [15] that are caused by air pollution all contribute to the accumulation of adipose tissue and weight gain, as has been demonstrated in animal trials. Additionally, the decline in air quality reduces a person’s willingness to engage in outdoor activities [16], which in turn increases indoor time, in order to reduce the impact of pollution on the human body [17].
Although there are numerous original studies on air pollution and obesity, the effects remained inconsistent and differed among the populations, pollutant types, and pollutant concentrations [12]. We found that previous studies were more concentrated on exposure during pregnancy [18,19], whereas recent studies have increasingly examined the direct effects of children’s exposure to air pollution on obesity [20,21]. However, the findings seem to be inconsistent even in studies of children and adolescents only [22,23,24]. For instance, Fioravanti et al. suggested that the evidence that air pollution causes obesity was limited [23]. In contrast, some studies indicated that long-term exposure to air pollutants might be correlated with weight gain and the development of obesity [20,21,25,26]. Among the available review articles, the existing studies have been mostly concentrated on adults or whole populations, and quantitative synthesis of the contribution of air pollution to children and adolescents remains scarce [12,27,28]. A meta-analysis by Parasin et al. examined the relationship between air pollution and childhood obesity, but did not distinguish between exposure during pregnancy and individual exposure, and also did not standardize when combining the effects of pollutants across studies [29]. Therefore, we believe that the topic still has potential for further research. As such, that this study aimed to systematically review and quantitatively analyze the scientific evidence on the influence of exposure to air pollution on weight gain and obesity in childhood.
2. Methods
2.1. Search Strategy
The systematic review and meta-analysis were based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [30]. A literature search was conducted through PubMed, Web of Science, Embase, and Cochrane Library to examine the relationship between childhood exposure to air pollutants and weight gain. The keywords included a combination of three main aspects, which were used to represent exposure (air pollutants), outcome (body weight status), and population (children and adolescents). The search strategies are presented in the Supplementary Materials. When searched in PubMed and Embase, the “[All fields]” tag was used. The search function “TS = Topic” was applied in Web of Science, represents topic term search limited to the fields of title, abstract, keyword, and Keyword Plus [31]. No restrictions were placed on the start time in the window of the search, for the period up to and including 31 December 2021, but the language was limited to articles in English. We also conducted a backward reference search and forward reference search based on the full-text articles meeting the study selection criteria in the search strategy while no additional studies were found.
2.2. Eligibility Criteria and Study Selection
The inclusion criteria were based on the following principles: (1) population: conducted on children and adolescents (≤18 years old); (2) exposure: short-term (<3 months) or long-term (≥3 months) exposure to ambient air pollution (PM, NOx, SOx, CO, O3); (3) outcome: overweight or obesity status measured by body mass index (BMI), waist circumference (WC), waist-to-height ratio (WHtR), skinfold thickness, or body fat; (4) article type: original research; and (5) article language: written in English. For articles to be include in the meta-analysis, the outcome indicators of interest were further restricted to provide the relative risks (RRs)/odd ratios (ORs)/hazard ratios (HRs)/coefficients (β), and corresponding 95% confidence intervals (CIs). Studies were excluded from the review if they met any of the criteria below: (1) studies conducted in adults only; (2) prenatal exposure; (3) body weight status includes birth weight only; (4) animal experimental studies; (5) studies on the effects of passive smoking, wood smoke, and (environmental) endocrine-disrupting chemicals; and (6) letters, editorials, protocols, or review articles.
2.3. Data Extraction and Quality Assessment
For the eligible studies, the extracted data included: basic information of studies (authors, publication year, country, study database, study design, study period, sample size, age, gender proportion), and exposure and outcome indicators (exposure type, exposure assessment, statistical model, adjusted covariates, effect estimates).
The National Institutes of Health’s Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [32] was used to assess the quality of the included research in the meta-analysis. The scale assessed each study in terms of 14 criteria, covering several aspects of study objectives, sample selection, exposure and outcome measurement, and statistical analysis. The total score ranged from 0 to 14 and was calculated by adding up the scores for each criterion. A quality assessment of studies was used to assist in measuring the strength of scientific evidence, but not for determining the inclusion and exclusion of studies.
The selection of studies, data extraction, and quality assessment were carried out independently by two reviewers, with disagreements resolved by a third reviewer.
2.4. Data Synthesis and Statistical Analysis
Weight and height were measured by professionals according to clinical standard protocols. Overweight and obesity were then defined according to different regional standards. Long-term exposure means exposure for longer than 3 months [28].
In this review, a random-effects model was used to assess the combined effects and the 95% CIs by incorporating RRs/ORs/HRs for binary outcome (obesity) and β for continuous outcome (BMI) from initial studies [28]. Since the definition of pollutant concentration increments varied across studies, we defined 10 μg/m3 as the standard increment; other reported units were converted (formulas: NO2: 1 ppb = 46/22.4 μg/m3; NOx: 1 ppb = 46/22.4 μg/m3; O3: 1 ppb = 48/22.4 μg/m3). Based on the assumption of a linear relationship between air pollution and obesity or BMI, the following equations were used to standardize the estimates of effects across studies [28,33]:
| OR(standardized) = OR(original) Increment(10)/Increment(original) | (1) |
| β(standardized) = β(original) × Increment(10)/Increment(original) | (2) |
In addition, when multiple models were available in the study or estimates from sensitivity analyses were reported, we used the full-adjusted model only, or the main model that was indicated by the researchers. For studies with different groups (such as gender, age group) for which overall effects were not accessible, we treated them as a separate research based on their respective sample sizes [21,22,34].
In order to examine the heterogeneity of the included studies, we used Chi-squared test and I2 statistics, either I2 > 50% or p-value of Chi-squared test < 0.10 was considered as statistically significant heterogeneity [35]. Publication bias was evaluated using Egger’s test and funnel plot [36]. We also conducted subgroup analyses to explore sources of heterogeneity according to study design (cohort or cross-sectional), country (China or others), and study quality (<13 or ≥13). To ascertain whether a particular study had an undue influence upon the overall results, a sensitivity analysis was carried out using the leave-one-out method. All statistical analyses were conducted using STATA (version 13.1). Statistically significant differences were determined as two-tailed p-value < 0.05.
3. Results
3.1. Study Selection
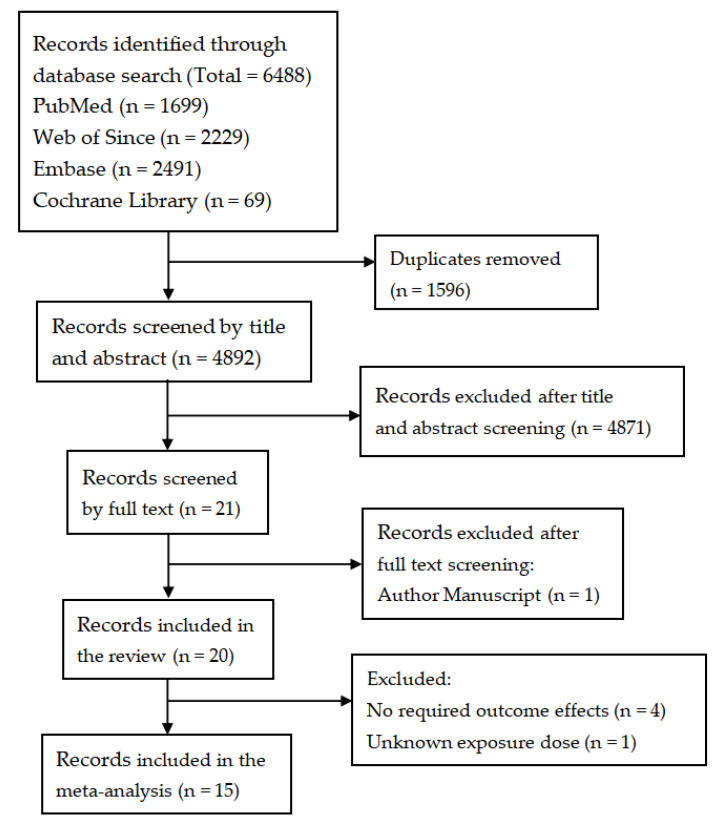
Figure 1 shows the flowchart of study selection. The initial keyword search identified 6488 records, including 1699 from PubMed, 2229 from Web of Since, 2491 from Embase, and 69 from Cochrane Library. After the removal of duplicate records, 4892 unique titles and abstracts were assessed, and 4871 records were further excluded. The full texts of the remaining 21 articles were reviewed, 20 met the inclusion criteria (one author manuscript excluded). Among the 20 studies that met the review inclusion criteria, 5 studies were further excluded due to inconsistency with the intended required outcome effects [24,37,38,39], and unavailability exposure dose [8]. A total of 15 studies were finally included in the meta-analysis [20,21,22,23,26,34,40,41,42,43,44,45,46,47,48].
Figure 1.
Flowchart of study selection.
3.2. Study Characteristics
The characteristics of the included studies are presented in Table 1. These studies were carried out in seven countries: six in China, three in Spain, two in the United States, and one in each of Italy, Mexico, the Netherlands, and the United Kingdom. Overall, the data reported on 683,081 participants; all the subjects were children and adolescents with two studies only for children under the age of five. As for study design, eight were cross-sectional studies while seven were cohort. All studies were long-term (≥3 months) exposures. In terms of research quality assessment, eight achieved a score of 13 and were considered as good quality (Supplementary Table S1).
Table 1.
Characteristics of the 15 included studies on the association between air pollution and childhood obesity.
| Study ID | Author (Year) | Country | Study Design | Study Period | Sample Size (Boy %) | Age | Quality a |
|---|---|---|---|---|---|---|---|
| 1 | Zheng et al. (2021) | China | Cross-sectional | 2019 | 36,456 (52.1) | 9–17 | 13 |
| 2 | Zhang et al. (2021a) | China | Cross-sectional | 2013–2014 | 44,718 (50.5) | 7–18 | 13 |
| 3 | Zhang et al. (2021b) | China | Cross-sectional | 2013–2014 | 9897 (50.3) | 10–18 | 13 |
| 4 | Tamayo et al. (2021) | Mexico | Cross-sectional | 2006 and 2012 | 4306 (51.5) | 2–18 | 11 |
| 5 | Bont et al. (2021) | Spain | Cohort | 2006–2018 | 416,955 (51.4) | 2–15 | 12 |
| 6 | Vrijheid et al. (2020) | UK | Cross-sectional | 2013–2016 | 1301 (54.7) | 6–11 | 11 |
| 7 | Guo et al. (2020) | China | Cross-sectional | 2013–2014 | 40,953 (48.3) | 6–17 | 13 |
| 8 | Bont et al. (2020) | Spain | Cohort | 2011–2016 | 79,992 (51.0) | 0–5 | 13 |
| 9 | Chen et al. (2020) | China | Cohort | 2012–2014 | 5752 (52.5) | 0–2 | 12 |
| 10 | Bont et al. (2019) | Spain | Cross-sectional | 2012 | 2660 (51.1) | 7–10 | 13 |
| 11 | Bloemsma et al. (2019) | Netherlands | Cohort | 1996–2014 | 3680 (51.9) | 3–17 | 12 |
| 12 | Kim et al. (2018) | US | Cohort | 2002–2003 | 2318 (50.6) | 6.5 ± 0.7 | 13 |
| 13 | Fioravanti et al. (2018) | Italy | Cohort | 2003–2004 | 719 (50.6) | 4–8 | 12 |
| 14 | McConnell et al. (2015) | US | Cohort | 2003–2014 | 3318 (49.6) | 10.1 ± 0.59 | 13 |
| 15 | Dong et al. (2014) | China | Cross-sectional | 2009 | 30,056 (50.4) | 2–14 | 11 |
Abbreviations: US, United States of America; UK, United Kingdom of Great Britain and Northern Ireland. a National Institutes of Health’s Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (NIH-QAT)
Table 2 demonstrates the exposure, outcome information, and association assessment methods. In order to assess the exposure to air pollutants, thirteen studies used model estimation (satellite-based spatial-temporal model, hybrid spatio-temporal model, land use regression model, machine-learning model, California line-source dispersion model) while two used monitoring station data directly. Weight status and obesity were mainly based on BMI and its related indicators (except for one which used waist circumference), meanwhile the reference standard for eight studies was based on international standards and seven on national standards.
Table 2.
Exposure, outcome, and statistical information of the 15 included studies on the association between air pollution and childhood obesity.
| Study ID | Author (Year) | Exposure | Duration | Exposure Assessment | Outcome Definition | Statistical Model | Adjusted Covariates |
|---|---|---|---|---|---|---|---|
| 1 | Zheng et al. (2021) | PM10, PM2.5, O3, NO2 | Long-term | Monitoring stations | Age-and-sex specific BMI cut-offs (Chinese national standard) | Multivariate regression model | Sex, age, paternal, sugar-sweetened beverage consumption, sweetened food consumption, frequency of having breakfast, fried food consumption, physical activity duration |
| 2 | Zhang et al. (2021a) | PM10, PM2.5, PM1, NO2 | Long-term | Satellite-based spatial-temporal model | Age-and-sex specific BMI cut-offs (Chinese national standard) | Mixed-effects linear and logistic regression models | Age, physical activity, fruit & vegetable intake, parental smoking, parental education, north or south, urban residency, regional GDP per capita |
| 3 | Zhang et al. (2021b) | PM10, PM2.5,PM1, NO2 | Long-term | Satellite-based spatial-temporal model | Waist circumference (Chinese national standard) | Generalized linear mixed-effects models | Age, sex, weight status, temperature, relative humidity, parental education level achieved, parental smoking status, parental alcohol consumption, family history of type 2 diabetes, hypertension, obesity, or cerebrovascular disease, outdoor physical activity time, diet of high fat, SSBs intake. |
| 4 | Tamayo et al. (2021) | PM2.5 | Long-term | Hybrid spatio-temporal model | Age-specific BMI (WHO standard) | Logistic regression models | Age, sex, SES, and smoking status |
| 5 | Bont et al. (2021) | PM10, PM2.5, NO2 | Long-term | Land use regression model | Age-and-sex specific BMI (WHO standard) | Cox proportional hazards models | Sex, deprivation index, nationality, deprivation index, and had age (1-year categories) in the strata statement. |
| 6 | Vrijheid et al. (2020) | NO2 | Long-term | Land use regression model | Age-and-sex specific BMI (WHO standard) | Linear regression models, and logistic regression models | Sex, maternal BMI, maternal education, maternal age at conception, parity, parental country of origin, breastfeeding, and birth weight |
| 7 | Guo et al. (2020) | PM2.5 | Long-term | Machine-learning model | Age-and-sex specific BMI cut-offs (Chinese national standard) | Logistic regression models | Sex, age, urbanity, boarding school or not, economic level, maternal occupation, maternal education, vegetable intake, fruit intake, beverages intake, activity times, ventilation, cooking fuel type, household heating fuel type, school heating fuel type, and secondhand smoke duration |
| 8 | Bont et al. (2020) | PM10, PM2.5, NO2 | Long-term | Land use regression model | BMI z-scores (WHO standard) | Linear spline multilevel model | Sex, age, deprivation index, nationality |
| 9 | Chen et al. (2020) | NO2 | Long-term | Land use regression model | Age- and sex-specific z scores for BMI (WHO standard) | Generalized estimating equation models, Distributed lag nonlinear models | Maternal age, maternal education, annual household income and residence area |
| 10 | Bont et al. (2019) | PM10, PM2.5, NO2 | Long-term | Land use regression model | Age- and sex-specific z scores for BMI (WHO standard) | Multilevel mixed linear and ordered logistic models | Maternal and paternal education, maternal and paternal country of birth, paternal employment status, number of siblings, household status and maternal smoking during pregnancy |
| 11 | Bloemsma et al. (2019) | PM10, PM2.5, NO2 | Long-term | Land use regression model | Age-and-sex specific BMI (International Obesity Task Force cut-offs) | Generalized linear mixed models | Age, sex maternal level of education, paternal level of education, maternal smoking during pregnancy, parental smoking in child’s home and neighborhood socioeconomic status and region |
| 12 | Kim et al. (2018) | NOx | Long-term | California line-source dispersion model | BMI (US CDC criteria) | Linear mixed effects models | Age, sex, race/ethnicity, parental education, and Spanish baseline questionnaire |
| 13 | Fioravanti et al. (2018) | PM10, PM2.5, NO2 | Long-term | Land use regression model | Age- and sex-specific z scores for BMI (WHO standard) | Logistic regression models, Generalized Estimating Equation models and linear regression models | Maternal and paternal education, maternal pre-pregnancy BMI, maternal smoking during pregnancy, gestational diabetes, maternal age at delivery, gestational age, childbirth weight, breastfeeding duration, age at weaning and inversely weighted for the probability of participation at baseline and at the two follow-ups, respectively |
| 14 | McConnell et al. (2015) | NOx | Long-term | California line-source dispersion model | Age-and-sex specific BMI (US CDC criteria) | Multilevel linear model | Sex, ethnicity, community, year of enrollment, and age |
| 15 | Dong et al. (2014) | PM10, NO2, SO2, O3 | Long-term | Monitoring stations | Age-and-sex specific BMI standards (Chinese CDC criteria) | Logistic regression | Age, gender, parental education, breastfeeding, low birth weight, area of residence per person, house decorations, home coal use, ventilation device in kitchen, air exchange in winter, passive smoking exposure, and districts |
Abbreviations: PM10, particulate matter with the diameter ≤ 10 mm; PM2.5, particulate matter with diameter ≤ 2.5 mm; PM1, particulate matter with the diameter ≤ 1 mm; NO2, nitrogen dioxide; NOx, nitrogen oxides; SO2, sulfur dioxide; O3, ozone; BMI, body mass index; WHO, World Health Organization; US, The United States; CDC, Center for Disease Control and Prevention.
3.3. Air Pollution on Obesity and BMI in Children and Adolescents
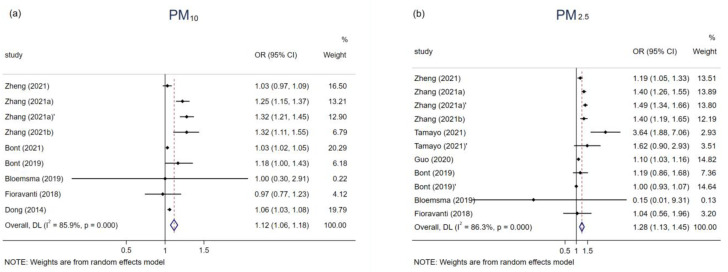
The association of childhood obesity and air pollutants was estimated using a pooled ORs, respectively 9, 11, 3, 2, and 11 studies investigated the effects of obesity in relation to PM10, PM2.5, PM1, O3, and NO2 exposure (Table 3). The meta-analysis results (Supplementary Table S2) showed that long-term exposure to air pollution could increase the risk of childhood obesity (Figure 2), the only pollutant that exhibited no significant correlation was O3 (OR = 1.08, 95% CI: 0.99,1.18), while the association was considerable for PM10 (OR = 1.12, 95% CI: 1.06,1.18), PM2.5 (OR = 1.28, 95% CI: 1.13,1.45), PM1 (OR = 1.41, 95% CI: 1.30,1.53), and NO2 (OR = 1.11, 95% CI: 1.06,1.18).
Table 3.
Summary effects and 95% confidence intervals of each pollutant on obesity and BMI in children and adolescents.
| Pollution Type | Author (Year) | Group | Sample Size | Incremental Scale | Original Effect | Transformed OR/β |
|---|---|---|---|---|---|---|
| obesity | ||||||
| PM10 | Zheng (2021) | Total | 36,456 | 10 μg/m3 | 1.03 (0.97, 1.09) | - |
| Zhang (2021a) | Boy | 22,573 | 10 μg/m3 | 1.25 (1.15, 1.37) | - | |
| Zhang (2021a)’ | Girl | 22,145 | 10 μg/m3 | 1.32 (1.21, 1.45) | - | |
| Zhang (2021b) | Total | 44,718 | 10 μg/m3 | 1.32 (1.11, 1.55) | - | |
| Bont (2021) | Total | 416,955 | 6.4 μg/m3 | 1.02 (1.02, 1.03) | 1.03 (1.02, 1.05) | |
| Bont (2019) | Home | 2660 | 5.6 μg/m3 | 1.10 (1.00, 1.22) | 1.18 (1.00, 1.43) | |
| Bloemsma (2019) | Total | 3680 | 1.06 μg/m3 | 1.00 (0.88, 1.12) | 1.00 (0.30, 2.91) | |
| Fioravanti (2018) | Total | 719 | 10 μg/m3 | 0.97 (0.77, 1.23) | - | |
| Dong (2014) | Total | 30,056 | 31 µg/m3 | 1.19 (1.11, 1.26) | 1.06 (1.03, 1.08) | |
| PM2.5 | Zheng (2021) | Total | 36,456 | 10 μg/m3 | 1.19 (1.05, 1.33) | - |
| Zhang (2021a) | Boy | 22,573 | 10 μg/m3 | 1.40 (1.26, 1.55) | - | |
| Zhang (2021a)’ | Girl | 22,145 | 10 μg/m3 | 1.49 (1.34, 1.66) | - | |
| Zhang (2021b) | Total | 44,718 | 10 μg/m3 | 1.40 (1.19, 1.65) | - | |
| Tamayo (2021) | Children | 1370 | 10 μg/m3 | 3.64 (1.88, 7.06) | - | |
| Tamayo (2021)’ | Adolescence | 1519 | 10 μg/m3 | 1.62 (0.90, 2.93) | - | |
| Guo (2020) | Total | 40,953 | 10 μg/m3 | 1.10 (1.03, 1.16) | - | |
| Bont (2019) | Home | 2660 | 2.7 μg/m3 | 1.05 (0.96, 1.15) | 1.19 (0.86, 1.68) | |
| Bont (2019)’ | School | 2660 | 10.7 μg/m3 | 1.00 (0.93, 1.08) | 1.00 (0.93, 1.07) | |
| Bloemsma (2019) | Total | 3680 | 1.17 μg/m3 | 0.80 (0.59 1.09) | 0.15 (0.01, 9.31) | |
| Fioravanti (2018) | Total | 719 | 5 μg/m3 | 1.02 (0.75, 1.40) | 1.04 (0.56, 1.96) | |
| PM1 | Zhang (2021a) | Boy | 22,573 | 10 μg/m3 | 1.38 (1.21, 1.57) | - |
| Zhang (2021a)’ | Girl | 22,145 | 10 μg/m3 | 1.44 (1.25, 1.67) | - | |
| Zhang (2021b) | Total | 44,718 | 10 μg/m3 | 1.42 (1.23, 1.64) | - | |
| O3 | Zheng (2021) | Total | 36,456 | 10 μg/m3 | 1.04 (1.00, 1.08) | - |
| Dong (2014) | Total | 30,056 | 11.3 ppb | 1.14 (1.04, 1.24) | 1.06 (1.02, 1.09) | |
| NO2 | Zheng (2021) | Total | 36,456 | 10 μg/m3 | 1.13 (1.04, 1.22) | - |
| Zhang (2021a) | Boy | 22,573 | 10 μg/m3 | 1.14 (1.04, 1.24) | - | |
| Zhang (2021a)’ | Girl | 22,145 | 10 μg/m3 | 1.21 (1.10, 1.34) | - | |
| Zhang (2021b) | Total | 44,718 | 10 μg/m3 | 1.44 (1.22, 1.71) | - | |
| Bont (2021) | Total | 416,955 | 21.8 μg/m3 | 1.03 (1.02, 1.04) | 1.01 (1.00, 1.02) | |
| Chen (2020) | Total | 5752 | 10 μg/m3 | 1.11 (1.00, 1.22) | - | |
| Bont (2019) | Home | 2660 | 13.7 μg/m3 | 1.05 (0.97, 1.13) | 1.04 (0.98, 1.09) | |
| Bont (2019)’ | School | 2660 | 22.3 μg/m3 | 1.09 (0.92, 1.28) | 1.04 (0.96, 1.12) | |
| Bloemsma (2019) | Total | 3680 | 8.9 μg/m3 | 1.40 (1.12, 1.74) | 1.46 (1.14, 1.86) | |
| Fioravanti (2018) | Total | 719 | 10 μg/m3 | 0.99 (0.86, 1.12) | - | |
| Dong (2014) | Total | 300,56 | 5.3 ppb | 1.13 (1.04, 1.22) | 1.13 (1.04, 1.21) | |
| BMI | ||||||
| PM10 | Zhang (2021a) | Boy | 22,573 | 10 μg/m3 | 0.11 (0.07, 0.14) | - |
| Zhang (2021a)’ | Girl | 22,145 | 10 μg/m3 | 0.09 (0.06, 0.12) | - | |
| Bont (2020) | Total | 79,992 | 6.3 μg/m3 | 0.02 (0.01, 0.03) | 0.04 (0.02, 0.05) | |
| PM2.5 | Zhang (2021a) | Boy | 22,573 | 10 μg/m3 | 0.15 (0.11, 0.19) | - |
| Zhang (2021a)’ | Girl | 22,145 | 10 μg/m3 | 0.13 (0.09, 0.17) | - | |
| Bont (2020) | Total | 79,992 | 1.5 μg/m3 | 0.01 (0.00, 0.01) | 0.05 (0.00, 0.09) | |
| NO2 | Zhang (2021a) | Boy | 22,573 | 10 μg/m3 | 0.05 (0.01, 0.09) | - |
| Zhang (2021a)’ | Girl | 22,145 | 10 μg/m3 | 0.04 (0.01, 0.08) | - | |
| Vrijheid (2020) | Total | 1301 | 92.8 μg/m3 | 0.15 (0.01, 0.28) | 0.02 (0.00, 0.30) | |
| Bont (2020) | Total | 79,992 | 21.3 μg/m3 | 0.02 (0.01, 0.03) | 0.01 (0.00, 0.02) | |
| Chen (2020) | Total | 5752 | 10 μg/m3 | 0.03 (0.01, 0.05) | - | |
| NOx | Kim (2018) | Total | 2318 | 9.4 ppb | 0.10 (0.03, 0.20) | 0.05 (0.02, 0.10) |
| McConnell (2015) | Total | 2994 | 16.8 ppb | 1.13 (0.61, 1.65) | 0.33 (0.18, 0.50) | |
Abbreviations: PM10, particulate matter with the diameter ≤ 10 mm; PM2.5, particulate matter with diameter ≤ 2.5 mm; PM1, particulate matter with the diameter ≤ 1 mm; NO2, nitrogen dioxide; NOx, nitrogen oxides; O3, ozone; BMI, body mass index; OR, odds ratio; β, regression coefficient.
Figure 2.
Associations of PM10 (a), PM2.5 (b), PM1 (c), O3 (d), and NO2 (e) with obesity in children and adolescents.
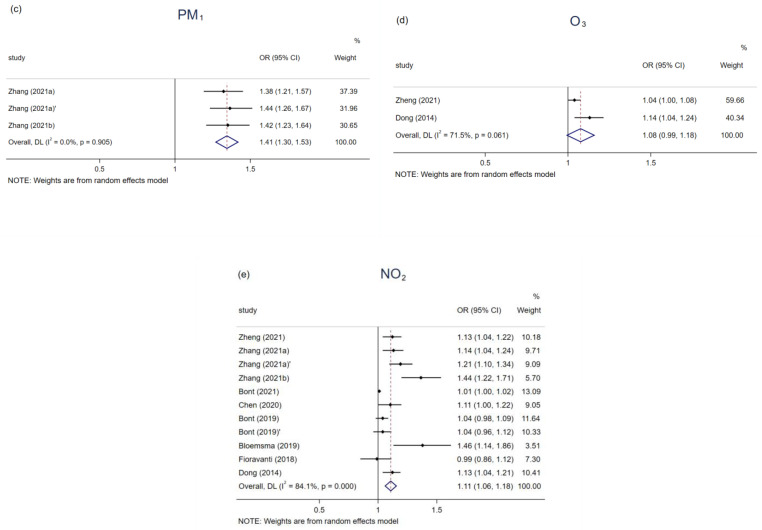
The relationships between PM10, PM2.5, NO2, and NOx and childhood BMI were reported by 3, 3, 5, and 2 studies (Table 3). The BMI status increased by 0.08 (0.03–0.12), 0.11 (0.05–0.17), and 0.03 (0.01–0.04) kg/m2 with 10 μg/m3 increment in exposure to PM10, PM2.5, and NO2, respectively. Exposure to NOx, however, was not significantly associated with BMI growth (Figure 3).
Figure 3.
Associations of PM10 (a), PM2.5 (b), NO2 (c), and NOx (d) with BMI in children and adolescents.
3.4. Heterogeneity, Publication Bias, and Sensitivity Analysis
In studies with obesity as an outcome, heterogeneity existed in the analysis of air pollutants (PM10: I2 = 85.9, p < 0.001; PM2.5: I2= 86.3, p < 0.001; O3: I2 = 71.5, p = 0.061; NO2: I2 = 84.1, p < 0.001), except for PM1 (I2 = 0, p = 0.905). Funnel plots (Supplementary Figure S1) and an Egger’s test showed potential publication bias that was only identified in NO2 (PM10: p = 0.076; PM2.5: p = 0.238; PM1: p = 0.324; NO2: p = 0.001; O3: N/A).
For the outcome of BMI status, heterogeneity was found among all the pollutants (PM10: I2 = 89.1, p < 0.001; PM2.5: I2 = 82.6, p = 0.003; NO2: I2 = 48.6, p = 0.0100; NOx: I2 = 91.0, p < 0.001), Funnel plots (Supplementary Figure S2) and an Egger’s test showed publication bias only existed in research that related to PM10 (PM10: p = 0.018; PM2.5: p = 0.131; NO2: p = 0.156; NOX: N/A).
Due to the limitation of the number of included studies, we only performed a subgroup analysis of the effects of PM10, PM2.5, and NO2 on obesity. The results of the subgroup analysis indicated that the effects of PM10, PM2.5, and NO2 on obesity in children and adolescents remained significant. However, the sources of heterogeneity were not well explained in terms of the study design, study region, and study quality (Supplementary Table S3).
The associations between exposure to PM10, PM2.5, PM1, and NO2 on childhood obesity in sensitivity analysis were generally similar and significant with the original findings (Supplementary Figure S3). Meanwhile, exposure to PM10, PM2.5, and NO2 had relatively robust effects on childhood BMI growth (Supplementary Figure S4).
4. Discussion
The objective of this study was to comprehensively assess the relationship between childhood exposure to air pollutants with obesity and weight status among children and adolescents. We conducted a systematic review and meta-analysis of 15 studies from 7 countries. The results showed that long-term exposure to particulate matter and NO2 was significantly correlated with the risk of childhood obesity, while BMI also showed similar elevated results. Although O3 and NOx also had a positive effect on the increase in weight status, none reached significant levels. Notably, as the aerodynamic diameter of particulate matter decreases, the fattening effect on children increases, and researchers have begun to concentrate on the smaller particle size pollutants (PM1).
Of the 15 studies that were included in the analysis, around 73% were published in the last three years, and most were conducted in developing countries. We compared a meta-analysis of the association between air pollutants and obesity in adults [28], where half of the included studies were published after 2019, with the difference that the study areas were predominantly developed countries. An identifiable trend is that concerns about air pollution and childhood obesity are rising rapidly in developing countries. One plausible explanation is that although developed countries still maintain high rates of childhood obesity [49,50], the effect of air pollutants on body weight has been limited because air pollution levels have declined significantly in these countries [28]. Developing countries, in contrast, appear confronted with a double crisis, with increasing prevalence and growth rates of childhood obesity on the one hand [51], and deteriorating air quality resulting from urbanization and industrialization on the other [52,53]. Thus, numerous original studies were conducted in developing countries in recent years, and the effects tend to be more significant, further providing foundations for the analysis of our research.
As the main air pollutants of concern in the present study, particulate matter significantly influenced childhood obesity and BMI growth. Our findings were consistent with the previous hypothesis that with smaller aerodynamic diameters of respirable particulate matter, the more toxic compounds would be adsorbed and also more easily inhaled deep into the lungs, therefore are more harmful to health [54,55]. In contrast to children, long-term exposure to PM10 and PM2.5 showed insignificant effects on adult obesity according to a meta-analysis that was conducted on adults [28]. Similar results were found for NO2 and O3, both pollutants were positively associated with the development of obesity. As can be seen, the effects of different pollutants on people can be diverse and complex, even for the same pollutant, the impact can vary depending on the characteristics of the population (i.e., age, gender, region). These specific mechanisms require further investigation and validation.
While the mechanisms linking exposure to air pollution and obesity are not completely understood, biochemical mechanisms have been commonly mentioned and accepted as the main obesity-causing mechanisms in relation to air pollutants [12,25,56]. Firstly, from the perspective of human metabolism, air pollutants entering the body from the respiratory tract may increase oxidative stress in tissues and systems [57]. Take PM2.5, as an example, it can affect gene expression in mitochondria in brown adipose tissue, resulting in increased production of reactive oxygen species in brown fat stores, which lead to metabolic dysfunction [13], and susceptibility to lipid metabolism and glucose metabolism [58]. Secondly, the inflammatory response that is triggered by air pollutants can lead to vascular damage as well as insulin resistance, can also have an impact on body weight [14]. Studies also found that the occurrence of sleep-disordered breathing (SDB) was related to exposure to air pollutants [59]. Those who lived in regions with high NO2 and PM2.5 levels were much more likely to suffer from SDB, which in turn caused mental and physical health disparities [60]. Sleep deprivation correlated with decreased levels of leptin secretion, lower thyroid stimulating hormone secretion, and lower glucose tolerance, all of which may increase BMI status [15]. Finally, behavioral mechanisms can be explained in another direction [12]. For example, air pollution can reduce people’s willingness to participate in outdoor activities [16]. In addition, it can also improve the consumption of trans fats and fast food [61], which may contribute to obesity. However, attention should be drawn to the fact that while the obesogenic mechanisms of air pollution have been validated in animal models, uncertainties remain for humans or for different pollutants, and more research should be conducted to elucidate such pathways.
The strength of the present study is that it comprehensively and quantitatively assesses the relationship between long-term exposure to air pollutants and childhood obesity. Previous studies focused on the whole population [12] or adults [28], with limited studies on children and adolescents [25,62]. Meanwhile, the majority of the original studies that were included in this study were published in the past three years, and the results are relatively new. Thirdly, the exposure doses in the original studies were mostly different, thus it is difficult to compare the effects, and we converted the data in a standardized way to improve the comparability of the data. Finally, analyses and collations were performed according to the standard methods that are required by the PRISMA checklist.
However, this meta-analysis still has many limitations that need to be noted. Firstly, although we systematically searched the currently that is available epidemiological evidence, the amount of research was still limited and the potential sources of heterogeneity remain to be explored. When we integrated the results across studies, the quality of the articles varied, exposure was not measured and estimated in a standardized way, analytical methods were imperfect, and the certainty of the evidence in the articles was generally poor, so caution should be exercised in interpreting the results as well. Secondly, the findings were based on numerous cross-sectional studies; therefore, causality was difficult to be determined accurately. According to GRADE system, the level of evidence for observational studies was still low. Thirdly, due to the complexity of growth patterns, the BMI that was applied to measure obesity in adults may not be applicable to a certain extent to children and adolescents. Fourth, for younger children (before two years of age), early obesity may be associated with the subsequent catch-up growth of low birth weight due to maternal exposure to air pollution [63], and the mechanisms for infants need to be further probed. Finally, obesity is a disease with complex causes, the magnitude of the direct role that is contributed by air pollution was unclear, and residual bias (i.e., socio-economic conditions, physical activity) may still influence the outcomes. For example, high-income families (or parents with higher education levels) tend to live in more privileged residential areas with relatively lower levels of air pollution, better green spaces and have a more structured diet, while low-income families tend to be the opposite. Likewise, parents who live in more polluted areas may restrict their children’s outdoor activities to reduce the exposure to air pollutants. These potentially confounding variables were not comprehensively captured and reasonably explained in studies
The results of the study further reveal the risk of air pollution on childhood obesity. The implications of air pollutants are direct and significant, not only for human health but also for the climate. Therefore, policy-makers can also benefit from these findings that economic development and urbanization can create a number of problems, especially in developing countries, and require reflection on how to develop appropriate policies to balance economic development and environmental pollution. A synergistic approach to air pollution and climate change management that is based on global cooperation is essential. Important sectors such as transportation, energy, and manufacturing are the main focus of high emissions of PM, SO2, NOx, and GHG. It is imperative to accelerate the transformation of the energy mix and use technology to drive low-carbon production. At the same time, it is essential to establish an integrated system of atmospheric monitoring, emissions supervision, and pollution remediation.
The present review also provides insights for future studies. Firstly, long-term cohort studies with large samples of children and adolescents across age, gender, and ethnicity are required in order to provide more representative and convincing results. Secondly, the diagnosis of childhood obesity requires more diverse anthropometric measures such as waist circumference, waist-to-hip ratio, subcutaneous fat, and total and high-density lipoprotein cholesterol. In addition, the health effects of ultrafine particulate matter still need to be clarified. Finally, interactions between multiple pollutants and their effects on humans also require estimation.
5. Conclusions
In summary, air pollutants can be considered as a probable risk factor for the weight status of children and adolescents. Although studies are still limited, our study provides some indication. The next step is to conduct longer-term and large-scale studies on different population subgroups, exposure concentrations, and pollutant combinations to provide detailed evidence on the impacts of air pollution on human health. Measures should also be taken by the government to regulate and control the emission of air pollutants to provide a pleasant living environment for residents.
Acknowledgments
We sincerely thank all support from all authors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19084491/s1, Table S1: Study quality assessment of the 15 studies that were included in the meta-analysis; Table S2: The summary results of all meta-analyses of air pollution on childhood obesity and BMI; Table S3: The summary results of subgroup analysis; Figure S1: Funnel study in the meta-analysis on the association between PM10 (a), PM2.5 (b), PM1 (c), and NO2 (d) exposure and childhood obesity; Figure S2: Funnel study in the meta-analysis on the association between PM10 (a), PM2.5 (b), and NO2 (c) exposure and childhood BMI status; Figure S3: Sensitivity analysis of PM10, PM2.5, PM1, and NO2 on childhood obesity; Figure S4: Sensitivity analysis of PM10, PM2.5, and NO2 on BMI of children and adolescents.
Author Contributions
C.H. and C.L. developed the idea of the study, participated in its design and coordination, and helped to draft the manuscript. F.Z. and J.Z. contributed to the acquisition and interpretation of data. S.W. and G.S. provided critical review and substantially revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Nutrition Research Foundation of Chinese Nutrition Society—Research Fund of Feihe Physical Nutrition and Health (Grant No: CNS-Feihe2018B01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ezzati M., Bentham J., Di Cesare M., Bilano V., Bixby H., Zhou B., Stevens G.A., Riley L.M., Taddei C., Hajifathalian K., et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Obesity and Overweight. World Health Organizaiton; Geneva, Switzerland: 2020. [Google Scholar]
- 3.Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., Lee A., Marczak L., Mokdad A.H., Moradi-Lakeh M., Naghavi M., et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wormser D., Kaptoge S., Di Angelantonio E., Wood A.M., Pennells L., Thompson A., Sarwar N., Kizer J.R., Lawlor D.A., Nordestgaard B.G., et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh G.M., Danaei G., Farzadfar F., Stevens G.A., Woodward M., Wormser D., Kaptoge S., Whitlock G., Qiao Q., Lewington S., et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmonds M., Llewellyn A., Owen C.G., Woolacott N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016;17:95–107. doi: 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- 7.Han J.C., Lawlor D.A., Kimm S.Y. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jerrett M., McConnell R., Wolch J., Chang R., Lam C., Dunton G., Gilliland F., Lurmann F., Islam T., Berhane K. Traffic-related air pollution and obesity formation in children: A longitudinal, multilevel analysis. Environ. Health. 2014;13:49. doi: 10.1186/1476-069X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K., Balakrishnan K., Brunekreef B., Dandona L., Dandona R., et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States Environmental Protection Agency Criteria Air Pollutants. [(accessed on 1 March 2022)];2017 EPA. Available online: https://www.epa.gov/criteria-air-pollutants.
- 11.WHO . Global Urban Ambient Air Pollution Database. WHO; Geneva, Switzerland: 2016. [Google Scholar]
- 12.An R., Ji M., Yan H., Guan C. Impact of ambient air pollution on obesity: A systematic review. Int. J. Obes. 2018;42:1112–1126. doi: 10.1038/s41366-018-0089-y. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z., Xu X., Zhong M., Hotchkiss I.P., Lewandowski R.P., Wagner J.G., Bramble L.A., Yang Y., Wang A., Harkema J.R., et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part. Fibre Toxicol. 2011;8:20. doi: 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Q., Yue P., Deiuliis J.A., Lumeng C.N., Kampfrath T., Mikolaj M.B., Cai Y., Ostrowski M.C., Lu B., Parthasarathy S., et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keith S.W., Redden D.T., Katzmarzyk P.T., Boggiano M.M., Hanlon E.C., Benca R.M., Ruden D., Pietrobelli A., Barger J.L., Fontaine K.R., et al. Putative contributors to the secular increase in obesity: Exploring the roads less traveled. Int. J. Obes. 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 16.Hu L., Zhu L., Xu Y., Lyu J., Imm K., Yang L. Relationship Between Air Quality and Outdoor Exercise Behavior in China: A Novel Mobile-Based Study. Int. J. Behav. Med. 2017;24:520–527. doi: 10.1007/s12529-017-9647-2. [DOI] [PubMed] [Google Scholar]
- 17.Neidell M. Information, Avoidance Behavior, and Health The Effect of Ozone on Asthma Hospitalizations. J. Hum. Resour. 2009;44:450–478. doi: 10.1353/jhr.2009.0018. [DOI] [Google Scholar]
- 18.Alderete T.L., Habre R., Toledo-Corral C.M., Berhane K., Chen Z., Lurmann F.W., Weigensberg M.J., Goran M.I., Gilliland F.D. Longitudinal Associations Between Ambient Air Pollution With Insulin Sensitivity, β-Cell Function, and Adiposity in Los Angeles Latino Children. Diabetes. 2017;66:1789–1796. doi: 10.2337/db16-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.C., Chen P.C., Hsieh W.S., Portnov B.A., Chen Y.A., Lee Y.L. Environmental factors associated with overweight and obesity in taiwanese children. Paediatr. Perinat. Epidemiol. 2012;26:561–571. doi: 10.1111/ppe.12001. [DOI] [PubMed] [Google Scholar]
- 20.Zheng H., Xu Z., Wang Q., Ding Z., Zhou L., Xu Y., Su H., Li X., Zhang F., Cheng J. Long-term exposure to ambient air pollution and obesity in school-aged children and adolescents in Jiangsu province of China. Environ. Res. 2021;195:110804. doi: 10.1016/j.envres.2021.110804. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z., Dong B., Chen G., Song Y., Li S., Yang Z., Dong Y., Wang Z., Ma J., Guo Y. Ambient air pollution and obesity in school-aged children and adolescents: A multicenter study in China. Sci. Total Environ. 2021;771:144583. doi: 10.1016/j.scitotenv.2020.144583. [DOI] [PubMed] [Google Scholar]
- 22.de Bont J., Casas M., Barrera-Gomez J., Cirach M., Rivas I., Valvi D., Alvarez M., Dadvand P., Sunyer J., Vrijheid M. Ambient air pollution and overweight and obesity in school-aged children in Barcelona, Spain. Environ. Int. 2019;125:58–64. doi: 10.1016/j.envint.2019.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fioravanti S., Cesaroni G., Badaloni C., Michelozzi P., Forastiere F., Porta D. Traffic-related air pollution and childhood obesity in an Italian birth cohort. Environ. Res. 2018;160:479–486. doi: 10.1016/j.envres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Huang J.V., Leung G.M., Schooling C.M. The association of air pollution with body mass index: Evidence from Hong Kong’s “Children of 1997” birth cohort. Int. J. Obes. 2018;43:62–72. doi: 10.1038/s41366-018-0070-9. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z., Zhao L., Huang Q., Hong A., Yu C., Xiao Q., Zou B., Ji S., Zhang L., Zou K., et al. Traffic-related environmental factors and childhood obesity: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021;22:e12995. doi: 10.1111/obr.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J.S., Gui Z.H., Zou Z.Y., Yang B.Y., Ma J., Jing J., Wang H.J., Luo J.Y., Zhang X., Luo C.Y., et al. Long-term exposure to ambient air pollution and metabolic syndrome in children and adolescents: A national cross-sectional study in China. Environ. Int. 2021;148:106383. doi: 10.1016/j.envint.2021.106383. [DOI] [PubMed] [Google Scholar]
- 27.Seo M.Y., Kim S.-H., Park M.J. Air pollution and childhood obesity. Clin. Exp. Pediatrics. 2020;63:382–388. doi: 10.3345/cep.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang S., Zhang X., Huang J., Lu X., Liu F., Gu D. Ambient air pollution and body weight status in adults: A systematic review and meta-analysis. Environ. Pollut. 2020;265:114999. doi: 10.1016/j.envpol.2020.114999. [DOI] [PubMed] [Google Scholar]
- 29.Parasin N., Amnuaylojaroen T., Saokaew S. Effect of Air Pollution on Obesity in Children: A Systematic Review and Meta-Analysis. Children. 2021;8:327. doi: 10.3390/children8050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Web of Science, Web of Science® Help. 2009. [(accessed on 1 March 2022)]. Available online: https://images.webofknowledge.com/WOK50B6/help/WOS/h_advanced_fieldtags.html.
- 32.National Heart, Lung, and Blood Institute, Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. [(accessed on 1 March 2022)];2014 Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tool.
- 33.Yang B.Y., Qian Z., Howard S.W., Vaughn M.G., Fan S.J., Liu K.K., Dong G.H. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ. Pollut. 2018;235:576–588. doi: 10.1016/j.envpol.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Tamayo-Ortiz M., Téllez-Rojo M.M., Rothenberg S.J., Gutiérrez-Avila I., Just A.C., Kloog I., Texcalac-Sangrador J.L., Romero-Martinez M., Bautista-Arredondo L.F., Schwartz J., et al. Exposure to pm2.5 and obesity prevalence in the greater mexico city area. Int. J. Environ. Res. Public Health. 2021;18:2301. doi: 10.3390/ijerph18052301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 36.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong H., Holstius D., Li Y., Seto E., Wang M. Air pollution and child obesity: Assessing the feasibility of measuring personal PM2.5 exposures and behaviours related to BMI in preschool-aged children in China. Obes. Med. 2019;16:100149. doi: 10.1016/j.obmed.2019.100149. [DOI] [Google Scholar]
- 38.Nikolic M., Stankovic A., Jovic S., Kocic B., Bogdanovic D. Effects of air pollution on growth in schoolchildren. Coll. Antropol. 2014;38:493–497. [PubMed] [Google Scholar]
- 39.Kim E., Park H., Park E.A., Hong Y.-C., Ha M., Kim H.-C., Ha E.-H. Particulate matter and early childhood body weight. Environ. Int. 2016;94:591–599. doi: 10.1016/j.envint.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 40.de Bont J., Hughes R., Tilling K., Diaz Y., de Castro M., Cirach M., Fossati S., Nieuwenhuijsen M., Duarte-Salles T., Vrijheid M. Early life exposure to air pollution, green spaces and built environment, and body mass index growth trajectories during the first 5 years of life: A large longitudinal study. Environ. Pollut. 2020;266:115266. doi: 10.1016/j.envpol.2020.115266. [DOI] [PubMed] [Google Scholar]
- 41.Chen X., Liao J., Xu S., Zhang B., Wang W., Cao Z., Mahai G., Yang X., Zhang Y., Liang S., et al. Associations of exposure to nitrogen dioxide and major roadways with growth trajectories and obesity at 2 years old: A prospective cohort study. Atmos. Environ. 2020;232:117574. doi: 10.1016/j.atmosenv.2020.117574. [DOI] [Google Scholar]
- 42.Bloemsma L.D., Wijga A.H., Klompmaker J.O., Janssen N.A.H., Smit H.A., Koppelman G.H., Brunekreef B., Lebret E., Hoek G., Gehring U. The associations of air pollution, traffic noise and green space with overweight throughout childhood: The PIAMA birth cohort study. Environ. Res. 2019;169:348–356. doi: 10.1016/j.envres.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 43.de Bont J., Díaz Y., de Castro M., Cirach M., Basagaña X., Nieuwenhuijsen M., Duarte-Salles T., Vrijheid M. Ambient air pollution and the development of overweight and obesity in children: A large longitudinal study. Int. J. Obes. 2021;45:1124–1132. doi: 10.1038/s41366-021-00783-9. [DOI] [PubMed] [Google Scholar]
- 44.Dong G.-H., Qian Z., Liu M.-M., Wang D., Ren W.-H., Flick L.H., Fu J., Wang J., Chen W., Simckes M., et al. Ambient Air Pollution and the Prevalence of Obesity in Chinese Children: The Seven Northeastern Cities Study. Obesity. 2014;22:795–800. doi: 10.1002/oby.20198. [DOI] [Google Scholar]
- 45.Guo Q., Xue T., Jia C., Wang B., Cao S., Zhao X., Zhang Q., Zhao L., Zhang J., Duan X. Association between exposure to fine particulate matter and obesity in children: A national representative cross-sectional study in China. Environ. Int. 2020;143:105950. doi: 10.1016/j.envint.2020.105950. [DOI] [PubMed] [Google Scholar]
- 46.Kim J.S., Alderete T.L., Chen Z., Lurmann F., Rappaport E., Habre R., Berhane K., Gilliland F.D. Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index. Environ. Health. 2018;17:64. doi: 10.1186/s12940-018-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McConnell R., Shen E., Gilliland F.D., Jerrett M., Wolch J., Chang C.-C., Lurmann F., Berhane K. A Longitudinal Cohort Study of Body Mass Index and Childhood Exposure to Secondhand Tobacco Smoke and Air Pollution: The Southern California Children’s Health Study. Environ. Health Perspect. 2015;123:360–366. doi: 10.1289/ehp.1307031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vrijheid M., Fossati S., Maitre L., Marquez S., Roumeliotaki T., Agier L., Andrusaityte S., Cadiou S., Casas M., de Castro M., et al. Early—Life Environmental Exposures and Childhood Obesity: An Exposome-Wide Approach. Environ. Health Perspect. 2020;128:067009. doi: 10.1289/EHP5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skinner A.C., Ravanbakht S.N., Skelton J.A., Perrin E.M., Armstrong S.C. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. 2018;141:3. doi: 10.1542/peds.2017-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spinelli A., Buoncristiano M., Kovacs V.A., Yngve A., Spiroski I., Obreja G., Starc G., Pérez N., Rito A.I., Kunešová M., et al. Prevalence of Severe Obesity among Primary School Children in 21 European Countries. Obes. Facts. 2019;12:244–258. doi: 10.1159/000500436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aiello A.M., Marques de Mello L., Souza Nunes M., Soares da Silva A., Nunes A. Prevalence of Obesity in Children and Adolescents in Brazil: A Meta-analysis of Cross-sectional Studies. Curr. Pediatr. Rev. 2015;11:36–42. doi: 10.2174/1573396311666150501003250. [DOI] [PubMed] [Google Scholar]
- 52.Greenstone M., Hanna R. Environmental Regulations, Air and Water Pollution, and Infant Mortality in India. Am. Econ. Rev. 2014;104:3038–3072. doi: 10.1257/aer.104.10.3038. [DOI] [Google Scholar]
- 53.North C.M., Rice M.B., Ferkol T., Gozal D., Hui C., Jung S.H., Kuribayashi K., McCormack M.C., Mishima M., Morimoto Y., et al. Air pollution in the Asia-Pacific Region: A Joint Asian Pacific Society of Respirology/American Thoracic Society perspective (Republication) Respirology. 2019;24:484–491. doi: 10.1111/resp.13531. [DOI] [PubMed] [Google Scholar]
- 54.Klompmaker J.O., Hoek G., Bloemsma L.D., Wijga A.H., van den Brink C., Brunekreef B., Lebret E., Gehring U., Janssen N.A.H. Associations of combined exposures to surrounding green, air pollution and traffic noise on mental health. Environ. Int. 2019;129:525–537. doi: 10.1016/j.envint.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 55.Li N., Georas S., Alexis N., Fritz P., Xia T., Williams M.A., Horner E., Nel A. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J. Allergy Clin. Immunol. 2016;138:386–396. doi: 10.1016/j.jaci.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ustulin M., Keum C., Woo J., Woo J.T., Rhee S.Y. Effects of climatic variables on weight loss: A global analysis. Sci. Rep. 2017;7:40708. doi: 10.1038/srep40708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei Y., Zhang J.J., Li Z., Gow A., Chung K.F., Hu M., Sun Z., Zeng L., Zhu T., Jia G., et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: Findings from a natural experiment in Beijing. FASEB J. 2016;30:2115–2122. doi: 10.1096/fj.201500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toledo-Corral C.M., Alderete T.L., Habre R., Berhane K., Lurmann F.W., Weigensberg M.J., Goran M.I., Gilliland F.D. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr. Obes. 2018;13:54–62. doi: 10.1111/ijpo.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen Y.L., Liu W.T., Lee K.Y., Chuang H.C., Chen H.W., Chuang K.J., Billings M.E., Gold D., Szpiro A., Aaron C.P., et al. Association of PM(2.5) with sleep-disordered breathing from a population-based study in Northern Taiwan urban areas. Environ. Pollut. 2018;233:109–113. doi: 10.1016/j.envpol.2017.10.052. [DOI] [PubMed] [Google Scholar]
- 60.Billings M.E., Gold D., Szpiro A., Aaron C.P., Jorgensen N., Gassett A., Leary P.J., Kaufman J.D., Redline S.R. The Association of Ambient Air Pollution with Sleep Apnea: The Multi-Ethnic Study of Atherosclerosis. Ann. Am. Thorac. Soc. 2019;16:363–370. doi: 10.1513/AnnalsATS.201804-248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Z., Herting M.M., Chatzi L., Belcher B.R., Alderete T.L., McConnell R., Gilliland F.D. Regional and traffic-related air pollutants are associated with higher consumption of fast food and trans fat among adolescents. Am. J. Clin. Nutr. 2019;109:99–108. doi: 10.1093/ajcn/nqy232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qureshi R., Jadotte Y., Zha P., Porter S.A., Holly C., Salmond S., Watkins E.A. The association between prenatal exposure to environmental tobacco smoke and childhood obesity: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2018;16:1643–1662. doi: 10.11124/JBISRIR-2017-003558. [DOI] [PubMed] [Google Scholar]
- 63.Reilly J.J., Armstrong J., Dorosty A.R., Emmett P.M., Ness A., Rogers I., Steer C., Sherriff A. Early life risk factors for obesity in childhood: Cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.