Abstract
Cell therapy products have significant limitations, such as storage instability, difficulties with transportation, and toxicity issues such as tumorigenicity and immunogenicity. Extracellular vesicles (EVs) secreted from cells show potential for therapeutic agent development. EVs have not been widely examined as investigational drugs, and non-clinical studies for the clinical approval of EV therapeutic agents are challenging. EVs contain various materials, such as DNA, cellular RNA, cytokines, chemokines, and microRNAs, but do not proliferate or divide like cells, thus avoiding safety concerns related to tumorigenicity. However, the constituents of EVs may induce the proliferation of normal cells; therefore, the suitability of vesicles should be verified through non-clinical safety evaluations. In this review, the findings of non-clinical studies on EVs are summarized. We describe non-clinical toxicity studies of EVs, which should be useful for researchers who aim to develop these vesicles into therapeutic agents. A new method for evaluating the immunotoxicity and tumorigenicity of EVs should also be developed.
Keywords: immunogenicity, tumorigenicity, soft agar colony formation assay, biodistribution, drug delivery agents, microRNAs, cytokines
1. Introduction
Extracellular vesicles (EVs) are heterogeneous small membrane structures that originate from plasma membranes. Although most EVs have a diameter of 50–200 nm, larger ones are also observed. Generally, particles up to a diameter of 1000 nm are regarded as EVs [1,2]. They are typically isolated from the conditioned media of cultured cells. The contents of EVs include proteins, mRNA, microRNA (miRNA), and nucleic acids [3]. Each vesicle performs a specific function in transferring biological material(s) to induce biological processes, such as replication, growth, apoptosis, and necrosis [4,5,6]. They are also required for cell-to-cell communication to maintain a normal homeostatic state [7]. EVs can be used as cargo carriers in physiological or pathological conditions and are considered biomarkers representing altered normal physiological states [8]. Based on these characteristics, EVs can be used for diverse purposes, from cosmetic to therapeutic applications. The main advantage of EVs is their limited adverse effects when used for therapeutic or cosmetic purposes, because they are composed of cell-derived materials and because of their potential for targeted cell delivery [9]. In addition, compared to cells, they are easier to store and transport.
The first EV that was identified is involved in transferrin receptor elimination, which plays a role in the maturation of reticulocytes, as reported by Harding et al. in 1983 [10]. The authors demonstrated the release of multi-vascular endosomes from the plasma membrane by exocytosis in rat reticulocytes. EVs can be found in all types of body fluids, such as plasma [11], bile [12], breast milk [13], urine [14], ascites, and cerebrospinal fluid [15]. Thus, these vesicles show potential for revealing abnormal conditions in various organs. EVs from the blood can be used to detect inflammation or an aberrant immune system, whereas those from breast milk can be utilized to diagnose breast conditions [16]. Halvaei et al. reported that EVs can be used for the diagnosis of various cancers using cancer-specific miRNAs [17]. Therefore, the types of EVs with clinical potential, the cells from which they are derived and relevant preclinical studies are described below and summarized in Figure 1.
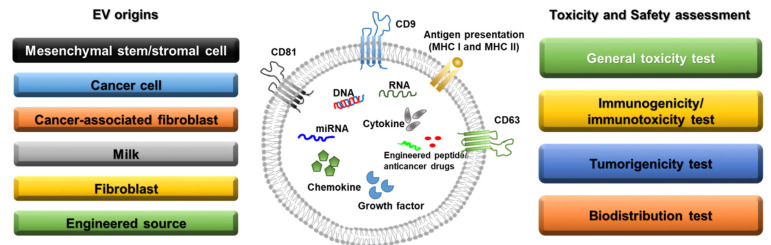
Figure 1.
Sources of extracellular vesicles and toxicity/safety assessments. EVs can originate from mesenchymal stem/stromal cells, cancer cells, cancer-associated fibroblasts, milk, normal fibroblasts, and engineered cells. EVs have a lipid bilayer and can contain transmembrane proteins, antigen presentation proteins, DNA, RNA, miRNA, cytokines, chemokines, growth factors, engineered peptides, and anticancer drugs. Before clinical studies of EVs, general toxicity, immunogenicity, tumorigenicity, and biodistribution tests should be performed in preclinical studies depending on the source of the EVs.
2. Categories and Characteristics of EVs
2.1. Mesenchymal Stem/Stromal Cell-Derived EVs
Mesenchymal stem cells (MSCs) have been widely investigated as therapeutic options for various diseases, including graft versus host disease [18] and cardiac [19], neurological [20], and orthopedic [21] disorders. MSCs mainly reduce inflammation, enhance progenitor cell proliferation, improve tissue repair, and decrease infection. According to the U.S. Food and Drug Administration, over 35,000 clinical trials have been conducted in the USA, France, and Canada on cell-based therapies [22]. However, despite the potency of MSCs, numerous side effects, such as tumorigenesis and immunogenicity, have been reported in preclinical and clinical trials [23]. In addition, there are some limitations to the generation and storage of MSCs intended for use as therapeutics [24]. To maintain the efficacy of MSCs and overcome these drawbacks, MSC-derived EVs have received attention as therapeutic agents that can be used for renal protection and to manage various disorders, including cardiac dysfunction, myocardial infarction, stroke, hepatic fibrosis, and vascular proliferative diseases [25,26,27,28,29,30]. In particular, MSC-derived EVs are composed of factors such as cytokines, growth factors, RNA, and miRNAs, which originate from MSCs and thus exert similar effects to those of MSCs [31].
The effects of MSC-derived EVs in cancer cell biology are controversial [32]. Many groups have reported that MSC-derived EVs increase cancer proliferation, invasion, and metastasis. Bone marrow MSC-derived EVs were reported to stimulate the hedgehog signaling pathway in the growth of osteosarcoma and gastric cancer [33], whereas adipocyte MSC-derived EVs promoted breast cancer cell growth via activation of the Hippo signaling pathway [34]. However, adipose MSC-derived EVs inhibited prostate cancer growth by delivering miR-145 [35].
MSC-derived EVs of different origins show different effects in various diseases with divergent mechanisms. Further information is provided in Table 1.
Table 1.
MSC-derived EVs of different origins with different effects in various diseases.
| EV Origin | Target Disease | Mechanisms & Characteristics | Animals Used | Ref. No. |
|---|---|---|---|---|
| Bone marrow-derived mesenchymal stem cells | Wound healing | Promoting M2 polarization of macrophages miR-223 wound healing by transferring EV-derived microRNA |
6–8 weeks old female C57BL/6 J mice | [28] |
| Mesenchymal stem cells | Alzheimer’s disease | Evaluating mouse cognitive deficits Stimulating neurogenesis in the subventricular zone Alleviating beta amyloid 1−42-induced cognitive impairment |
7–8-week-old C57BL/6 mice | [36] |
| Adipose tissue-derived mesenchymal stem/stromal cells | Cisplatin-induced acute kidney injury | Protection of animals from death due to cisplatin-induced acute kidney injury | 6-week-old male Sprague Dawley rats | [37] |
| Bone marrow-derived mesenchymal stem cells | Pilocarpine-induced status epilepticus | Neuroprotective and anti-inflammatory effects Increasing normal hippocampal neurogenesis and cognitive and memory function |
6–8-week-old C57BL/6 mice | [38] |
| Mesenchymal stromal cells | A newborn rat model of bronchopulmonary dysplasia (BPD) induced by 14 days of neonatal hyperoxia exposure (85% O2) | Protecting from apoptosis, inhibiting inflammation, and increasing angiogenesis Preventing the disruption of alveolar growth, increasing small blood vessel number, and inhibiting right heart hypertrophy at P14, P21, and P56 |
Newborn rats | [39] |
| Embryonic mesenchymal stem cells | Critical-sized osteochondral defects (1.5 mm diameter and 1.0 mm depth) | Complete restoration of cartilage and subchondral bone | 8-week-old female Sprague Dawley rats | [40] |
| Umbilical cord mesenchymal stem cells | Perinatal brain injury (hypoxic-ischemic and inflammatory with lipopolysaccharide) | Inhibiting the production of pro-inflammatory molecules and preventing microgliosis in rats with perinatal brain injury Decreasing TNF-α and IL-1β expression in injured brains |
2-day-old Wistar rat pups | [41] |
| Umbilical Cord mesenchymal stem cells | CCl4-induced liver injury | Suppressing the development of liver tumors Inhibiting oxidative stress in liver tumors Reducing oxidative stress and inhibiting apoptosis in liver fibrosis |
4–5-week-old female BALB/c mice | [42] |
| Mesenchymal stromal cells | Cavernous nerve injury (CNI) | Enhancing smooth muscle content and neuronal nitric oxide synthase (nNOS) in the corpus cavernosum Improving erectile function after CNI Increasing penile nNOS expression and alleviating cell apoptosis |
10-week-old male Sprague Dawley rats | [43] |
| Mesenchymal stem cells | Traumatic Brain Injury (TBI) with a 20 mm cylindrical impactor hemorrhaged over 12.5 min using a Masterflex pump |
Lowering Neurological Severity Score (NSS) (p < 0.05) during the first five days post-injury Faster full neurological recovery |
35–45 kg female Yorkshire swine | [44] |
| Mesenchymal stem cells | UV-irradiated skin | Attenuating UV-induced histological injury and inflammatory response in mouse skin Preventing cell proliferation and collagen deposition in UV-irradiated mouse skin Increasing antioxidant activity |
newborn and adult Kunming mice | [45] |
2.2. Cancer-Derived EVs
EVs from tumor cells can be produced and utilized to stimulate or inhibit tumor growth under various conditions, depending on whether they will or will not be used for cancer treatment. Cancer-derived EVs can be detected in all bodily fluids, such as the blood, saliva, urine, and bile [14,46,47]. Based on this characteristic, many scientists have attempted to develop cancer-derived EVs as noninvasive biomarkers for diagnosing cancer in early stages of disease [48]. Specifically, cancer-derived EVs contain various biomarkers, such as miR-17, miR-19a, miR-21, miR-126/miR-141, miR-146, and miR-409, which have a range of effects on tumor growth and can be used for cancer diagnosis and prognosis [49,50,51,52].
The extracellular matrix, cancer-associated fibroblasts, inflammatory immune cells, and tumor-associated vasculature are components of the tumor microenvironment, which can be a major source of tumor-derived EVs [53,54]. Cancer-associated fibroblasts are among the major sources of tumor EVs with different effects before and after chemotherapy [55]. In particular, following chemotherapies, EVs derived from cancer-associated fibroblasts were shown to promote the chemoresistance and proliferation of colorectal and breast cancers [56,57]. EVs from tumors under hypoxic conditions enhanced angiogenesis and metastasis by modulating the microenvironment [58]. Because tumor-derived EVs contain important components, including nucleic acids and oncogenic proteins, they can be used as biomarkers for diagnosis, prognosis, therapeutic response prediction, and targeted therapy [4].
2.3. EVs as Anticancer Drug Delivery Agents
Jang et al. reported that EV-delivered doxorubicin had a greater effect on reducing tumor size than administration of pure doxorubicin in a colon adenocarcinoma xenograft model [59]. Furthermore, the use of an αv integrin-specific iRGD peptide with EVs to deliver doxorubicin showed promising anticancer effects in an αv integrin-positive breast cancer model [60]. Following the investigation of paclitaxel using an EV delivery system in a tumor xenograft model, Kim et al. reported its anticancer effects in vitro and in vivo [61]. Another group reported that EV-encapsulated paclitaxel directly targeted cancer stem cells that exhibited anticancer drug resistance [62]. EVs loaded with the antitumor drugs withaferin A or celastrol were administered to a human lung cancer xenograft mouse model, in which they showed anticancer effects [63,64]. Engineered EVs with superparamagnetic-conjugated transferrin have been shown to target tumor cells and reduce tumor growth in vivo [65]. In addition, an engineered anti-epidermal growth factor receptor nanobody fused with the EV anchor signal peptide glycosylphosphatidylinositol showed direct activity against tumor cells positive for epidermal growth factor receptor-positive tumor cells [66].
Because of their stability in biological fluids, EVs can escape from lung clearance and cross the blood-brain barrier [67,68], thus easily reaching tumors in various organs such as the liver, brain, and breast. Based on these characteristics, EVs can be used for cancer-targeting therapies.
3. Toxicity and Safety Assessment of EVs
Below, we review toxicity studies involving diverse organ-derived EVs according to the type of examination, namely, general toxicity, immunogenicity, tumorigenesis, and biodistribution tests. In addition, safety evaluations related to EVs are summarized in Table 2.
Table 2.
EV toxicity and safety assessment.
| Types | Study Design | Results | Ref. |
|---|---|---|---|
| General toxicity | Intravenous injection of MSC-derived exosome to rats: analyzing hematological indexes | No side effects on hematology indexes | [69] |
| Intravenous/intraperitoneal injection of HEK293T-derived exosomes to C57BL/6 mice: Gross necropsy, histopathology, hematology analyses | No abnormal clinical signs, no abnormal body weight changes, no abnormal changes in blood chemistry, and no lesions found in tissues | [70] | |
| Intravenous injection of BJ fibroblast-derived exosomes to C57BL/6: Toxicology and necropsy analyses | Minimal to mild inflammation in liver and kidney, but mild immune activation of immune system | [71] | |
| Skin sensitization, photosensitization, eye and skin irritation, and acute oral toxicity with adipose stem cells (ASC)-derived exosomes in Sprague Dawley rats | No side effects and toxicity | [72] | |
| Intravenous injection of HEK Expi293F-derived exosomes to BALB/c mice: hematology analysis, pathological macroscopic analysis (brain, heart, lungs, liver, kidney, pancreas, spleen, skeletal muscle(hind leg), thymus, mesenteric lymph node, duodenum, caecum, tail vein) | No signs of toxicity and immune response | [73] | |
| Immunogenicity/ immunotoxicity |
Intravenous/intraperitoneal injection of HEK293T-derived EVs to C57BL/6 mice: Analyzing spleen immunophenotyping and rodent MAP | No signs of toxicity, minimal evidence of changes in immune markers | [70] |
| Exposure of leukocytes to MSC-derived or bovine milk-derived EVs: Leukocyte population assay | Both MSC-EV and BM-EV increased leukocyte proliferation by 1.8 to 2.5-fold in the presence of phytohemagglutinin | [74] | |
| Testing MSC-derived or bovine milk-derived EVs with plasma, HL-60 phagocytic cells, or RAW264.7 cells: complement activation assay, phagocytosis assay, or nitric oxide test | No complement activation elicited by MSC-EVs, while BM-EVs elicited 5-fold increase; neither BM-EVs nor MSC-EVs induced phagocytosis; no nitrite level changes with both EV types | [75] | |
| Systemic anaphylaxis of MSC-derived exosomes using guinea pigs | No systemic anaphylaxis response in guinea pigs | [69] | |
| Testing HEK293T-derived EVs with THP-1, U937 human monocytic cells: apoptosis/ necrosis assay, microsphere phagocytosis assay | Homeostatic level of apoptosis/necrosis maintained after EV exposure; lower EV dosage facilitated phagocytosis while no effect observed with higher EV dosage | [76] | |
| Testing HEK Expi293F-derived exosomes with human whole blood: human whole blood assay | Minimal cytotoxicity and pro-inflammatory cytokine response | [77] | |
| Testing fetal liver MSC-exosomes with NK differentiated from PBMCs: proliferation, cytotoxicity, intracellular phospho-Smad2/3 assay | Impaired natural killer cell function | [78] | |
| Gene toxicity | Exposure of lymphocytes to MSC-derived or bovine milk-derived EVs: alkaline comet assay | Neither MSC-EVs nor BM-EVs significantly increased comet tail length | [79] |
| Exposure of CHO-K1 Chinese hamster ovarian cells to MSC-derived or bovine milk-derived EVs: micronucleus assay | No increase in micronucleus-positive cells | [80] | |
| Testing TMZ-resistant exosomes with GBM: Alkaline comet assay | Chemoresistance to temozolomide in glioblastoma | [81] | |
| Tumorigenicity | Exposure of HGC-27 gastric cancer cells to MSC-derived exosomes: transwell migration, invasion, cell colony-forming, and soft agar assays | MSC-exosomes promoted migration and invasion of HGC-27 cells and while MSC-exosomes enhanced the colony formation of HGC-27 cells in serum-free conditions and cell sphere formation in soft agar | [81] |
| Subcutaneous injection of colorectal cancer stem cell (CRCSC)-exosomes to BALB/c mice: in vivo gene targeting, tumorigenicity assay, colony formation assay | Tumorigenesis and immunosuppressive tumor microenvironment in colorectal cancer | [82] | |
| Intravenous injection of melanoma-exosomes to B16F1 xenografted C57BL/6N mice: tumorigenicity test | Tumor progression | [83] | |
| Subcutaneous injection of MDA-MB-231-exosomes to SKOV3 and CoC1 xenografted BALB/c nude mice: tumorigenicity test | Tumor progression | [84] | |
| Intraperitoneal injection of both MDA-MB-231-exosomes and MDA-MB-231 to NOD/SCID nude mice: peritoneal carcinomatosis assay | Tumor progression | [85] |
3.1. General Toxicity Tests
According to several reports related to general toxicology following the administration of EVs, rare general toxicity was observed in rodent and non-rodent testing. Bagno et al. analyzed the hematology index of rats that were intravenously injected with MSC-derived EVs and reported no adverse effects [19]. Welton et al. also reported the absence of abnormal clinical signs, abnormal body weight changes, abnormal changes in blood chemistry, and lesions on/in the tissues of mice after intravenous or intraperitoneal injection with HEK293T-derived EVs [15]. Sun et al. evaluated the safety of EVs derived from human umbilical cord MSCs (hucMSCs) using rats [69]. They intravenously infused hucMSCs into rats after inducing acute myocardial infarction to test the safety and efficacy of the EVs. The body weights and blood chemistry of the rats were analyzed to detect liver and kidney functions. They reported that the hucMSCs protected against weight loss from acute myocardial infarction and had no adverse effects on hepatic or renal function. Furthermore, Mendt et al. reported the safety of MSC-derived engineered EVs, which contained exogenously loaded siRNA, following long-term administration in mice [71]. The EVs (109) were administered intraperitoneally every two days for four months into immunocompetent mice to evaluate potential toxicity. The researchers found no abnormalities following hematologic and chemical analyses of samples from the EV-treated groups compared with those from the vehicle control group. However, mild inflammation was observed in the liver, kidneys, lungs, brain, mesentery, and spleen of animals from both groups. In addition, minor toxicity was reported, with minimal to mild inflammation in the liver and kidneys after intravenous administration of BJ fibroblast (skin fibroblast)-derived EVs into C57BL/6 mice [23].
In summary, the efficacy of EVs is similar to that of the cells from which they were derived; however, there were fewer side effects for EVs than for the original cells [86]. Nonetheless, the preclinical toxicity test criteria for the filing of investigational new drug applications for these vesicles have not been clearly defined because of insufficient data. Whether conventional, general toxicity testing can detect the detailed toxicity due to EVs remains unclear. Therefore, detailed tools for evaluating the toxicity of EVs should be developed.
3.2. Immunogenicity/ Immunotoxicity Studies
Various preclinical studies have been conducted to evaluate safety based on the obvious efficacy of EVs, and rare notable immunogenicity has been reported [87,88,89]. Minor immune responses were reported by some researchers. For instance, Zhu et al. reported the immunogenicity of engineered HEK293T cell-derived EVs loaded with miRNA-199a-3p. To assess the EV-induced immune response, C57BL/6 mice were administered EVs intravenously and intraperitoneally for 3 weeks. At the end point, blood was harvested to examine hematology and immune markers, and spleen cells were collected to detect immunophenotypes. Minimal evidence of changes in immune markers was observed in the mice dosed with engineered EVs, but not with wild-type EVs [70]. Mendt et al. performed immunotyping of the spleen, bone marrow, and thymus in immunocompetent mice administered MSC-derived exosomes every 2 days via intraperitoneal injection for 3 weeks and found no significant changes in those mice compared to the non-treated mice [71]. Lu et al. reported that induced pluripotent stem cell (iPSC)-derived EVs induced less immunogenicity than iPSCs in non-human primates, such as rhesus macaques, which are similar to humans in terms of behavior, and immune system [90]. Moreover, because of the immunosuppressive characteristics of tumor-derived EVs, they were used for vaccination during tumor treatment. Specifically, dendritic cell vaccination using tumor-derived EVs has been shown to extend the survival time of WEHI3B-bearing mice [91]. Furthermore, tumor-derived EVs were shown to induce T-cell apoptosis, impairment of dendritic cell differentiation, and propagation of immunosuppressive myeloid suppressor cells, thus reducing natural killer cell activation [92,93]. Chalmin et al. showed that tumor-derived EVs had an immunosuppressive function in both mouse and human myeloid-derived suppressor cells to enhance the efficacy of cancer treatment [94].
In summary, the mechanism of action on the immune system differs depending on the origin and potential use of EVs. Therefore, it is necessary to clearly understand the characteristics of these vesicles on the immune system and perform toxicity tests. Additionally, EVs from most cell types contain major histocompatibility complex (MHC) molecules which are involved in antigen presentation [95]. EVs are not a single entity but contain multiple components that can induce immunogenicity or toxicity and interact with each other. In addition, because EVs are human-derived biopharmaceuticals, immunotoxicity/immunogenicity tests on animals as applied in small molecule investigations are not suitable. Therefore, to evaluate the immunogenicity and immunotoxicity of various cell-derived EVs, a powerful evaluation tool is necessary to predict their immunogenicity in the human body. We propose an evaluation method using human peripheral blood mononuclear cells (PBMCs) as a powerful tool to evaluate the immunogenicity of EVs.
3.3. Tumorigenicity Tests
Lu et al. studied iPSC-derived EVs rather than iPSCs because iPSCs can form teratomas after transplantation [90]. Their results showed that EVs posed no risk of teratoma formation even though their effects were similar to those of iPSCs in rhesus macaque monkeys following topical administration of a bolus dose of 50 μg EVs onto inflicted wounds. The results were evaluated using wound area analysis and histology after 14 days to detect the efficacy and formation of teratomas. Lee et al. revealed that EVs released by ectopic expression of EIF3C in human hepatocellular carcinoma promoted angiogenesis and tumorigenesis using a Huh7 xenograft model and a human umbilical vein endothelial cell tube formation model as in vivo and in vitro tests, respectively [96]. Vallabhaneni et al. reported that EVs derived from human MSCs accelerated tumor growth and metastasis with changes in the tumor microenvironment [97]. They used the MCF-7 xenograft model to test the effects of human MSC-derived EVs on the growth of breast tumors in an immunodeficient mouse model for 40 days. Larger tumors with increased angiogenesis were observed in the group administered MCF-7 cells together with EVs compared to those in mice administered MCF cells alone. In addition, larger tumors were observed in the group treated with MCF-7 cells and EVs than in mice treated with only MCF-4 cells, with increased angiogenesis as the main mechanism.
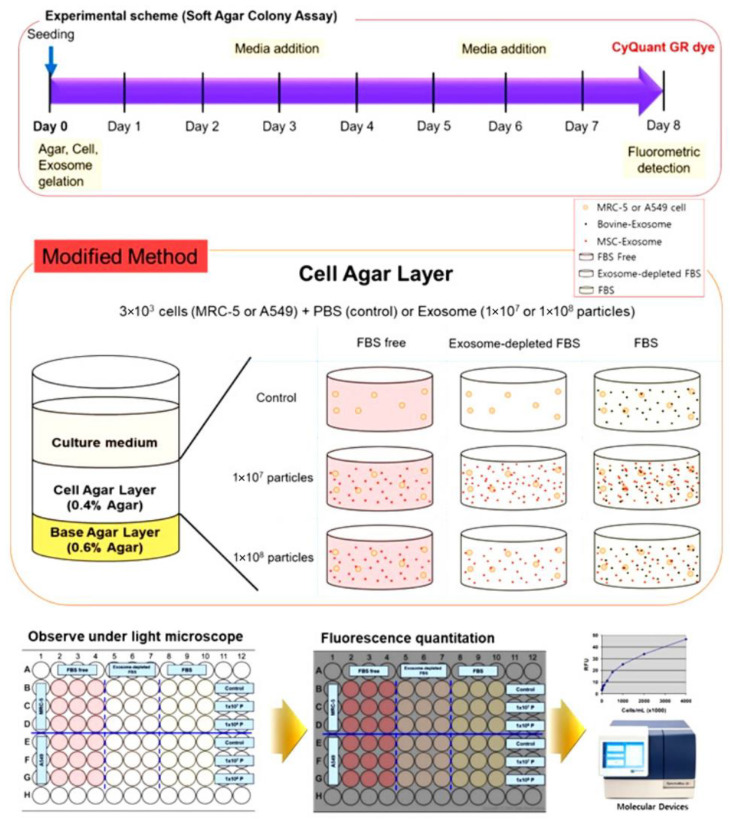
The effect of EVs on tumorigenesis varies depending on the state of the vesicle in terms of assembly, the source of cells from which the vesicles originate, and their contents. In addition, because the characteristics of EVs derived from different cell types show different effects in various cells, including cancer cells, existing techniques used in tumorigenicity tests of cell therapy products are not sufficient for evaluating EV-derived tumorigenicity. Therefore, an in vitro tumorigenicity test should be performed before in vivo tumorigenicity testing of EVs. The soft agar colony formation assay is an in vitro tumorigenicity assay that is useful for determining the effects of EVs on the tumorigenesis of tumor cells and normal cells (Figure 2). This test can be used to evaluate the growth of tumor cells and tumorigenesis of normal cells in an in vitro system.
Figure 2.
Experimental design of soft agar colony formation assay in vitro. For in vitro tumorigenicity test of EVs, MRC-5 and A549 cells (3 × 103 cells/well) are divided into three groups (FBS Free, exosome-depleted FBS, and normal FBS contained well) and each group is assigned a PBS-treated control group. The EVs are incubated with cells on the cell agar layer as shown in the diagram, and the medium is replaced every 3 days. On the 8th day of culture, the agar is solubilized to dissolve both agar and cells, and after treatment with CyQuant GR dye, fluorescence is measured at 485/520 nm to determine whether colony formation increased. FBS, fetal bovine serum; PBS, phosphate-buffered saline.
3.4. Biodistribution Tests
Biodistribution analysis is an important safety evaluation method for determining the residual amount, residual position, and clearance time of biopharmaceuticals such as cell, gene, and EV therapies [98,99,100]. To evaluate safety during biodistribution assessments of gene and cell therapies, globally established preclinical studies are performed [74]. Periodic evaluation of the biodistribution of EVs is necessary for preclinical drug development, such as for gene and cell therapies. However, the biodistribution testing of EVs is challenging because of the complexity of detection methods and lack of precedent. Nevertheless, various methods for detecting the distribution of EVs have been suggested. Wiklander et al. determined the in vivo biodistribution of EVs based on the cell source, administration route, and targeting [101]. To evaluate the biodistribution of EVs, the authors used enhanced green fluorescent protein-positive EVs and DiR-labeled EVs. Over 80% of the intravenously injected EVs were detected in the liver for over 48 h. However, different delivery routes such as intraperitoneal (i.p.) and subcutaneous (s.c.) administration influenced the distribution pattern, and EVs were observed in both the liver and gastrointestinal tract following the abovementioned routes of administration. In contrast, after intravenous injection, EVs were not detected in the liver, which is a different pattern from i.p and s.c. administrations. Smyth et al. demonstrated the biodistribution and delivery efficiency of different cancer-cell-derived EVs [102]. Fluorescently labeled and radiolabeled EVs were administered to nude mice to analyze the biodistribution of vesicles in vivo. PC3- and MCR-7-derived EVs showed similar distributions to major organs such as the liver, spleen, and kidneys. EV levels in the blood disappeared within 24 h after systemic exposure, including following intravenous administration.
As such, the biodistribution of EVs depends on the route of administration and the cells from which they were derived. Therefore, it is essential to verify their safety by clearly describing the in vivo distribution and clearance time through preclinical studies. Currently, the above methods are used, and new methods are being developed.
4. Conclusions and Future Perspectives
We have described the features of and preclinical studies performed on EVs. These vesicles show advantages similar to those of the cells of origin but exhibit lower toxicity, such as reduced immunogenicity and tumorigenicity. Thus, several groups worldwide have embarked on developing EVs as therapeutic agents and have performed preclinical testing and clinical trials of these vesicles. EVs can withstand mass processing, quality control, storage, and management more easily than cells. Thus, EVs show potential for clinical applications as chemical drugs.
However, as mentioned above, the toxicity of these vesicles may vary depending on the cell type and target disease; therefore, preclinical studies based on the characteristics of the specific vesicle system are needed. This review provides useful information for researchers performing preclinical studies of EVs. We hope to devise a more general and powerful assessment tool or model capable of detecting the general toxicity, immunogenicity/immunotoxicity, and tumorigenicity of various cell-derived EVs in the future.
Author Contributions
Conceptualization, M.H.Y. and A.-R.L.; methodology, M.H.Y. and A.-R.L.; validation, M.H.Y., A.-R.L. and K.-S.M.; formal analysis, M.H.Y.; investigation, M.H.Y., A.-R.L. and K.-S.M.; resources, M.H.Y. and A.-R.L.; data curation, M.H.Y.; writing—original draft preparation, M.H.Y. and A.-R.L.; writing—review and editing, M.H.Y.; visualization, M.H.Y. and A.-R.L.; supervision, M.H.Y.; funding acquisition, M.H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korean Health Technology R&D Project, Ministry of Health & Welfare (grant number HI20C0437 to Min Heui Yoo).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Povero D., Eguchi A., Li H., Johnson C.D., Papouchado B.G., Wree A., Messer K., Feldstein A.E. Extracellular vesicles (EVs) are heterogeneous small membrane structures that originate from plasma membranes. Although most EVs have a diameter of 50–200 nm, larger ones are also observed. PLoS ONE. 2014;9:e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Yuan X., Shi H., Wu L., Qian H., Xu W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015;8:83. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliot S., Schneider A., Simons M. Exosomes: Vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013;352:33–47. doi: 10.1007/s00441-012-1428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsaif M., Elliot S.A., MacKenzie M.L., Prado C.M., Field C.J., Haqq A.M. Energy Metabolism Profile in Individuals with Prader-Willi Syndrome and Implications for Clinical Management: A Systematic Review. Adv. Nutr. 2017;8:905–915. doi: 10.3945/an.117.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Verrilli M.A., Court F.A. Exosomes: Mediators of communication in eukaryotes. Biol. Res. 2013;46:5–11. doi: 10.4067/S0716-97602013000100001. [DOI] [PubMed] [Google Scholar]
- 8.He C., Zheng S., Luo Y., Wang B. Exosome Theranostics: Biology and Translational Medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caby M.P., Lankar D., Vincendeau-Scherrer C., Raposo G., Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 12.Masyuk A.I., Huang B.Q., Ward C.J., Gradilone S.A., Banales J.M., Masyuk T.V., Radtke B., Splinter P.L., LaRusso N.F. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G990–G999. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Admyre C., Johansson S.M., Qazi K.R., Filén J.-J., Lahesmaa R., Norman M., Neve E.P.A., Scheynius A., Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 14.Pisitkun T., Shen R.F., Knepper M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welton J.L., Loveless S., Stone T., von Ruhland C., Robertson N.P., Clayton A. Cerebrospinal fluid extracellular vesicle enrichment for protein biomarker discovery in neurological disease; multiple sclerosis. J. Extracell. Vesicles. 2017;6:1369805. doi: 10.1080/20013078.2017.1369805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirza A.H., Kaur S., Nielsen L.B., Størling J., Yarani R., Roursgaard M., Mathiesen E.R., Damm P., Svare J., Mortensen H.B., et al. Breast Milk-Derived Extracellular Vesicles Enriched in Exosomes From Mothers With Type 1 Diabetes Contain Aberrant Levels of microRNAs. Front. Immunol. 2019;10:2543. doi: 10.3389/fimmu.2019.02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halvaei S., Daryani S., Eslami S.Z., Samadi T., Jafarbeik-Iravani N., Bakhshayesh T.O., Majidzadeh A.K., Esmaeili R. Exosomes in Cancer Liquid Biopsy: A Focus on Breast Cancer. Mol. Ther. Nucleic Acids. 2018;10:131–141. doi: 10.1016/j.omtn.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amorin B., Alegretti A.P., Valim V., Pezzi A., Laureano A.M., Lima da Silva M.A., Wieck A., Silla L. Mesenchymal stem cell therapy and acute graft-versus-host disease: A review. Hum. Cell. 2014;27:137–150. doi: 10.1007/s13577-014-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagno L., Hatzistergos K.E., Balkan W., Hare J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018;26:1610–1623. doi: 10.1016/j.ymthe.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukai T., Tojo A., Nagamura-Inoue T. Mesenchymal stromal cells as a potential therapeutic for neurological disorders. Regen. Ther. 2018;9:32–37. doi: 10.1016/j.reth.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes T.L., Kimura H.A., Gomes Pinheiro C.C., Shimomura K., Nakamura N., Ferreira J.R., Gomoll A.H., Hernandez A.J., Franco Bueno D. Human Synovial Mesenchymal Stem Cells Good Manufacturing Practices for Articular Cartilage Regeneration. Tissue Eng. Part C Methods. 2018;24:709–716. doi: 10.1089/ten.tec.2018.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golchin A., Farahany T.Z. Biological Products: Cellular Therapy and FDA Approved Products. Stem Cell Rev. Rep. 2019;15:166–175. doi: 10.1007/s12015-018-9866-1. [DOI] [PubMed] [Google Scholar]
- 23.Lukomska B., Stanaszek L., Zuba-Surma E., Legosz P., Sarzynska S., Drela K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019;2019:9628536. doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin T.H., Lee S., Choi K.R., Lee D.Y., Kim Y., Paik M.J., Seo C., Kang S., Jin M.S., Yoo T.H., et al. Quality and freshness of human bone marrow-derived mesenchymal stem cells decrease over time after trypsinization and storage in phosphate-buffered saline. Sci. Rep. 2017;7:1106. doi: 10.1038/s41598-017-01315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai H.T., Sheu J.-J., Chiang J.Y., Shao P.-L., Wu S.-C., Chen Y.-L., Li Y.-C., Sung P.-H., Lee F.-Y., Yip H.-K. Early administration of cold water and adipose derived mesenchymal stem cell derived exosome effectively protects the heart from ischemia-reperfusion injury. Am. J. Transl. Res. 2019;11:5375–5389. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu B., Zhang L., Liang C., Liu B., Pan X., Wang Y., Zhang Y., Zhang Y., Xie W., Yan B., et al. Stem Cell-Derived Exosomes Prevent Aging-Induced Cardiac Dysfunction through a Novel Exosome/lncRNA MALAT1/NF-kappaB/TNF-alpha Signaling Pathway. Oxid. Med. Cell Longev. 2019;2019:9739258. doi: 10.1155/2019/9739258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan B., Zhang Y., Liang C., Liu B., Ding F., Wang Y., Zhu B., Zhao R., Yu X.-Y., Li Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics. 2020;10:6728–6742. doi: 10.7150/thno.42259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He X., Dong Z., Cao Y., Wang H., Liu S., Liao L., Jin Y., Yuan L., Li B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019;2019:7132708. doi: 10.1155/2019/7132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T., Yan Y., Wang B., Qian H., Zhang X., Shen L., Wang M., Zhou Y., Zhu W., Li W., et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shabbir A., Cox A., Rodriguez-Menocal L., Salgado M., Van Badiavas E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015;24:1635–1647. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park K.S., Bandeira E., Shelke G.V., Lässer C., Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019;10:288. doi: 10.1186/s13287-019-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vakhshiteh F., Atyabi F., Ostad S.N. Mesenchymal stem cell exosomes: A two-edged sword in cancer therapy. Int. J. Nanomedicine. 2019;14:2847–2859. doi: 10.2147/IJN.S200036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi J., Zhou Y., Jiao Z., Wang X., Zhao Y., Li Y., Chen H., Yang L., Zhu H., Li Y. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth Through Hedgehog Signaling Pathway. Cell Physiol. Biochem. 2017;42:2242–2254. doi: 10.1159/000479998. [DOI] [PubMed] [Google Scholar]
- 34.Wang S., Su X., Xu M., Xiao X., Li X., Li H., Keating A., Zhao R.C. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res. Ther. 2019;10:117. doi: 10.1186/s13287-019-1220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Che Y., Shi X., Shi Y., Jiang X., Ai Q., Shi Y., Gong F., Jiang W. Exosomes Derived from miR-143-Overexpressing MSCs Inhibit Cell Migration and Invasion in Human Prostate Cancer by Downregulating TFF3. Mol. Ther. Nucleic Acids. 2019;18:232–244. doi: 10.1016/j.omtn.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Reza-Zaldivar E.E., Hernández-Sapiéns M.A., Gutiérrez-Mercado Y.K., Sandoval-Ávila S., Gomez-Pinedo U., Márquez-Aguirre A.L., Vázquez-Méndez E., Padilla-Camberos E., Canales-Aguirre A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019;14:1626–1634. doi: 10.4103/1673-5374.255978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J.H., Ha D.H., Go H.K., Youn J., Kim H.K., Jin R.C., Miller R.B., Kim D.H., Cho B.S., Yi Y.W. Reproducible Large-Scale Isolation of Exosomes from Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Application in Acute Kidney Injury. Int. J. Mol. Sci. 2020;21:4774. doi: 10.3390/ijms21134774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long Q., Upadhya D., Hattiangady B., Kim D.K., An S.Y., Shuai B., Prockop D.J., Shetty A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA. 2017;114:E3536–E3545. doi: 10.1073/pnas.1703920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braun R.K., Chetty C., Balasubramaniam V., Centanni R., Haraldsdottir K., Hematti P., Eldridge M.W. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem. Biophys. Res. Commun. 2018;503:2653–2658. doi: 10.1016/j.bbrc.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 41.Thomi G., Surbek D., Haesler V., Joerger-Messerli M., Schoeberlein A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019;10:105. doi: 10.1186/s13287-019-1207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang W., Tan Y., Cai M., Zhao T., Mao F., Zhang X., Xu W., Yan Z., Qian H., Yan Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl4-Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018;2018:6079642. doi: 10.1155/2018/6079642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouyang X., Han X., Chen Z., Fang J., Huang X., Wei H. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res. Ther. 2018;9:246. doi: 10.1186/s13287-018-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams A.M., Dennahy I.S., Bhatti U.F., Halaweish I., Xiong Y., Chang P., Nikolian V.C., Chtraklin K., Brown J., Zhang Y., et al. Mesenchymal Stem Cell-Derived Exosomes Provide Neuroprotection and Improve Long-Term Neurologic Outcomes in a Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J. Neurotrauma. 2019;36:54–60. doi: 10.1089/neu.2018.5711. [DOI] [PubMed] [Google Scholar]
- 45.Wang T., Jian Z., Baskys A., Yang J., Li J., Guo H., Hei Y., Xian P., He Z., Li Z., et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials. 2020;257:120264. doi: 10.1016/j.biomaterials.2020.120264. [DOI] [PubMed] [Google Scholar]
- 46.Han Y., Jia L., Zheng Y., Li W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018;14:633–643. doi: 10.7150/ijbs.25018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lasser C., Alikhani V.S., Ekström K., Eldh M., Torregrosa Paredes P., Bossios A., Sjöstrand M., Gabrielsson S., Lötvall J., Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nonaka T., Wong D.T.W. Saliva-Exosomics in Cancer: Molecular Characterization of Cancer-Derived Exosomes in Saliva. Enzymes. 2017;42:125–151. doi: 10.1016/bs.enz.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeffer S.R., Grossmann K.F., Cassidy P.B., Yang C.H., Fan M., Kopelovich L., Leachman S.A., Pfeffer L.M. Detection of Exosomal miRNAs in the Plasma of Melanoma Patients. J. Clin. Med. 2015;4:2012–2027. doi: 10.3390/jcm4121957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z., Ma Y.Y., Wang J., Zeng X.F., Li R., Kang W., Hao X.K. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. Onco. Targets Ther. 2016;9:139–148. doi: 10.2147/OTT.S95565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J.C., Zhao J.T., Gundara J., Serpell J., Bach L.A., Sidhu S. Papillary thyroid cancer-derived exosomes contain miRNA-146b and miRNA-222. J. Surg. Res. 2015;196:39–48. doi: 10.1016/j.jss.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 52.Grimolizzi F., Monaco F., Leoni F., Bracci M., Staffolani S., Bersaglieri C., Gaetani S., Valentino M., Amati M., Rubini C., et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci. Rep. 2017;7:15277. doi: 10.1038/s41598-017-15475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luga V., Zhang L., Viloria-Petit A.M., Ogunjimi A.A., Inanlou M.R., Chiu E., Buchanan M., Hosein A.N., Basik M., Wrana J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Hu C., Chen M., Jiang R., Guo Y., Wu M., Zhang X. Exosome-related tumor microenvironment. J. Cancer. 2018;9:3084–3092. doi: 10.7150/jca.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X., Li Y., Zou L., Zhu Z. Role of Exosomes in Crosstalk Between Cancer-Associated Fibroblasts and Cancer Cells. Front. Oncol. 2019;9:356. doi: 10.3389/fonc.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richards K.E., Zeleniak A.E., Fishel M.L., Wu J., Littlepage L.E., Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fiori M.E., Di Franco S., Villanova L., Bianca P., Stassi G., De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol. Cancer. 2019;18:70. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng W., Hao Y., He C., Li L., Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol. Cancer. 2019;18:57. doi: 10.1186/s12943-019-0982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jang S.C., Kim O.Y., Yoon C.M., Choi D.S., Roh T.Y., Park J., Nilsson J., Lötvall J., Kim Y.K., Gho Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 60.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 61.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L., Inskoe E., Piroyan A., Sokolsky M., Okolie O., et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J., Zheng Y., Zhao M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2016;7:533. doi: 10.3389/fphar.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munagala R., Aqil F., Jeyabalan J., Agrawal A.K., Mudd A.M., Kyakulaga A.H., Singh I.P., Vadhanam M.V., Gupta R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017;393:94–102. doi: 10.1016/j.canlet.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aqil F., Kausar H., Agrawal A.K., Jeyabalan J., Kyakulaga A.H., Munagala R., Gupta R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016;101:12–21. doi: 10.1016/j.yexmp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Qi H., Liu C., Long L., Ren Y., Zhang S., Chang X., Qian X., Jia H., Zhao J., Sun J., et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano. 2016;10:3323–3333. doi: 10.1021/acsnano.5b06939. [DOI] [PubMed] [Google Scholar]
- 66.Kooijmans S.A., Aleza C.G., Roffler S.R., van Solinge W.W., Vader P., Schiffelers R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles. 2016;5:31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawikova I., Askenase P.W. Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res. 2015;1617:63–71. doi: 10.1016/j.brainres.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moyano A.L., Li G., Boullerne A.I., Feinstein D.L., Hartman E., Skias D., Balavanov R., van Breemen R.B., Bongarzone E.R., Månsson J.E., et al. Sulfatides in extracellular vesicles isolated from plasma of multiple sclerosis patients. J. Neurosci. Res. 2016;94:1579–1587. doi: 10.1002/jnr.23899. [DOI] [PubMed] [Google Scholar]
- 69.Sun L., Xu R., Sun X., Duan Y., Han Y., Zhao Y., Qian H., Zhu W., Xu W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy. 2016;18:413–422. doi: 10.1016/j.jcyt.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 70.Zhu X., Badawi M., Pomeroy S., Sutaria D.S., Xie Z., Baek A., Jiang J., Elgamal O.A., Mo X., Perle K., et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles. 2017;6:1324730. doi: 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mendt M., Kamerkar S., Sugimoto H., McAndrews K.M., Wu C.C., Gagea M., Yang S., Blanko E.V.R., Peng Q., Ma X., et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3:e99263. doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ha D.H., Kim S.D., Lee J., Kwon H.H., Park G.H., Yang S.H., Jung J.Y., Lee J.H., Park S.R., Youn J., et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul. Toxicol. Pharmacol. 2020;115:104686. doi: 10.1016/j.yrtph.2020.104686. [DOI] [PubMed] [Google Scholar]
- 73.Saleh A.F., Lázaro-Ibáñez E., Forsgard M.A., Shatnyeva O., Osteikoetxea X., Karlsson F., Heath N., Ingelsten M., Rose J., Harris J., et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 2019;11:6990–7001. doi: 10.1039/C8NR08720B. [DOI] [PubMed] [Google Scholar]
- 74.Nordgren T.M., Heires A.J., Zempleni J., Swanson B.J., Wichman C., Romberger D.J. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J. Nutr. Biochem. 2019;64:110–120. doi: 10.1016/j.jnutbio.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tong L., Hao H., Zhang Z., Lv Y., Liang X., Liu Q., Liu T., Gong P., Zhang L., Cao F., et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics. 2021;11:8570–8586. doi: 10.7150/thno.62046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosas L.E., Elgamal O.A., Mo X., Phelps M.A., Schmittgen T.D., Papenfuss T.L. In vitro immunotoxicity assessment of culture-derived extracellular vesicles in human monocytes. J. Immunotoxicol. 2016;13:652–665. doi: 10.3109/1547691X.2016.1148089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aslan C., Kiaie S.H., Zolbanin N.M., Lotfinejad P., Ramezani R., Kashanchi F., Jafari R. Exosomes for mRNA delivery: A novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol. 2021;21:20. doi: 10.1186/s12896-021-00683-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan Y., Herr F., Vernochet A., Mennesson B., Oberlin E., Durrbach A. Human Fetal Liver Mesenchymal Stem Cell-Derived Exosomes Impair Natural Killer Cell Function. Stem Cells Dev. 2019;28:44–55. doi: 10.1089/scd.2018.0015. [DOI] [PubMed] [Google Scholar]
- 79.Maji S., Yan I.K., Parasramka M., Mohankumar S., Matsuda A., Patel T. In vitro toxicology studies of extracellular vesicles. J. Appl. Toxicol. 2017;37:310–318. doi: 10.1002/jat.3362. [DOI] [PubMed] [Google Scholar]
- 80.Zeng A., Wei Z., Yan W., Yin J., Huang X., Zhou X., Li R., Shen F., Wu W., Wang X., et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018;436:10–21. doi: 10.1016/j.canlet.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Huang F., Yao Y., Wu J., Liu Q., Zhang J., Pu X., Zhang Q., Xia L. Curcumin inhibits gastric cancer-derived mesenchymal stem cells mediated angiogenesis by regulating NF-kappaB/VEGF signaling. Am. J. Transl. Res. 2017;9:5538–5547. [PMC free article] [PubMed] [Google Scholar]
- 82.Hwang W.L., Lan H.Y., Cheng W.C., Huang S.C., Yang M.H. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J. Hematol. Oncol. 2019;12:10. doi: 10.1186/s13045-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gyukity–Sebestyen E., Harmati M., Dobra G., Németh I.B., Mihály J., Zvara Á., Hunyadi-Gulyás É., Katona R., Nagy I., Horváth P., et al. Melanoma-Derived Exosomes Induce PD-1 Overexpression and Tumor Progression via Mesenchymal Stem Cell Oncogenic Reprogramming. Front. Immunol. 2019;10:2459. doi: 10.3389/fimmu.2019.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L., He M., Fu L., Jin Y. Exosomal release of microRNA-454 by breast cancer cells sustains biological properties of cancer stem cells via the PRRT2/Wnt axis in ovarian cancer. Life Sci. 2020;257:118024. doi: 10.1016/j.lfs.2020.118024. [DOI] [PubMed] [Google Scholar]
- 85.Piao Y.J., Kim H.S., Hwang E.H., Woo J., Zhang M., Moon W.K. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget. 2018;9:7398–7410. doi: 10.18632/oncotarget.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang C., Robbins P.D. Immunosuppressive exosomes: A new approach for treating arthritis. Int. J. Rheumatol. 2012;2012:573528. doi: 10.1155/2012/573528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Natasha G., Gundogan B., Tan A., Farhatnia Y., Wu W., Rajadas J., Seifalian A.M. Exosomes as immunotheranostic nanoparticles. Clin. Ther. 2014;36:820–829. doi: 10.1016/j.clinthera.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 88.Baglio S.R., Pegtel D.M., Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yeo R.W., Lai R.C., Zhang B., Tan S.S., Yin Y., The B.J., Lim S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 90.Lu M., Peng L., Ming X., Wang X., Cui A., Li Y., Wang X., Meng D., Sun N., Xiang M., et al. Enhanced wound healing promotion by immune response-free monkey autologous iPSCs and exosomes vs. their allogeneic counterparts. EBioMedicine. 2019;42:443–457. doi: 10.1016/j.ebiom.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gu X., Erb U., Büchler M.W., Zöller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int. J. Cancer. 2015;136:E74–E84. doi: 10.1002/ijc.29100. [DOI] [PubMed] [Google Scholar]
- 92.Xiang X., Poliakov A., Liu C., Liu Y., Deng Z.B., Wang J., Cheng Z., Shah S.V., Wang G.J., Zhang L., et al. Induction of mye;loid-derived suppressor cells by tumor exosomes. Int. J. Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tian X., Shen H., Li Z., Wang T., Wang S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J. Hematol. Oncol. 2019;12:84. doi: 10.1186/s13045-019-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chalmin F., Ladoire S., Mignot G., Vincent J., Bruchard M., Remy-Martin J.P., Boireau W., Rouleau A., Simon B., Lanneau D., et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 96.Lee H.Y., Chen C.K., Ho C.M., Lee S.S., Chang C.Y., Chen K.J., Jou Y.S. EIF3C-enhanced exosome secretion promotes angiogenesis and tumorigenesis of human hepatocellular carcinoma. Oncotarget. 2018;9:13193–13205. doi: 10.18632/oncotarget.24149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vallabhaneni K.C., Penfornis P., Dhule S., Guillonneau F., Adams K.V., Mo Y.Y., Xu R., Liu Y., Watabe K., Vemuri M.C., et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6:4953–4967. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonin P., Gaillard C. Gene transfer vector biodistribution: Pivotal safety studies in clinical gene therapy development. Gene Ther. 2004;11((Suppl. 1)):S98–S108. doi: 10.1038/sj.gt.3302378. [DOI] [PubMed] [Google Scholar]
- 99.Kamiyama Y., Naritomi Y., Moriya Y., Yamamoto S., Kitahashi T., Maekawa T., Yahata M., Hanada T., Uchiyama A., Noumaru A., et al. Biodistribution studies for cell therapy products: Current status and issues. Regen. Ther. 2021;18:202–216. doi: 10.1016/j.reth.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yi Y.W., Lee J.H., Kim S.Y., Pack C.G., Ha D.H., Park S.R., Youn J., Cho B.S. Advances in Analysis of Biodistribution of Exosomes by Molecular Imaging. Int. J. Mol. Sci. 2020;21:665. doi: 10.3390/ijms21020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wiklander O.P., Nordin J.Z., O’Loughlin A., Gustafsson Y., Corso G., Mäger I., Vader P., Lee Y., Sork H., Seow Y., et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smyth T., Kullberg M., Malik N., Smith-Jones P., Graner M.W., Anchordoquy T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.