Abstract
Objective.
Endothelial dysfunction in diabetes is generally attributed to oxidative stress, but this view is challenged by observations showing antioxidants do not eliminate diabetic vasculopathy. As an alternative to oxidative stress-induced dysfunction, we interrogated if impaired mitochondrial function in endothelial cells is central to endothelial dysfunction in the metabolic syndrome.
Approach and Results.
We observed reduced coronary arteriolar vasodilation to the endothelium-dependent dilator, acetylcholine (Ach), in Zucker Obese Fatty rats (ZOF, 34±15% [mean ± standard deviation] 10−3 M) compared to Zucker Lean rats (ZLN, 98±11%). This reduction in dilation occurred concomitantly with mitochondrial DNA (mtDNA) strand lesions and reduced mitochondrial complex activities in the endothelium of ZOF versus ZLN. To demonstrate endothelial dysfunction is linked to impaired mitochondrial function, administration of a cell-permeable, mitochondria-directed endonuclease (mt-tat-EndoIII), to repair oxidatively modified DNA in ZOF, restored mitochondrial function and vasodilation to Ach (94±13%). Conversely, administration of a cell-permeable, mitochondria-directed exonuclease (mt-tat-ExoIII) produced mtDNA strand breaks in ZLN, reduced mitochondrial complex activities and vasodilation to Ach in ZLN (42±16%). To demonstrate that mitochondrial function is central to endothelium-dependent vasodilation, we introduced (via electroporation) liver mitochondria (from ZLN) into the endothelium of a mesenteric vessel from ZOF and restored endothelium-dependent dilation to vasoactive intestinal peptide (VIP at 10−5 M, 4±3% vasodilation before mitochondrial transfer and 48±36% after transfer). Finally, to demonstrate mitochondrial function is key to endothelium-dependent dilation, we administered oligomycin (mitochondrial ATP synthase inhibitor) and observed a reduction in endothelium-dependent dilation.
Conclusions.
We conclude that mitochondrial function is critical for endothelium-dependent vasodilation.
Keywords: Endothelium, Endothelial Dysfunction, Mitochondria, Diabetes, Metabolic Syndrome
INTRODUCTION
Impaired endothelium-dependent vasodilation is a common denominator of many pathologies, e.g., diabetes, hypertension, atherosclerosis, ischemia-reperfusion [6, 20, 33, 54, 66] and is also associated with myriad cardiovascular risk factors including hyperlipidemia, hypercholesterolemia, reduced levels of tetrahydrobiopterin, elevated levels of inflammatory cytokines, obesity, and aging [1, 10, 12, 17, 18, 24, 28, 32, 35, 55, 61]. A unifying link among the pathologies and factors is oxidative stress, a manifestation of elevated production of reactive oxygen species (ROS) and/or impaired antioxidant defense mechanisms [29, 65]. In the vasculature, ROS production is most typically associated with activation of cytosolic NAD(P)H oxidases [13, 47]. Yet this cytosolic mechanism does not seem to account for all manifestations of ROS induced cell injury and oxidative stress; especially in the context of the many failed antioxidant trials that did not offer a benefit in the treatment of cardiovascular disease [30, 62]. Although numerous hypotheses have been offered to explain why antioxidant treatment did not ameliorate the consequences of vascular disease, it is important to note the chemicals used in the trials (vitamin E, vitamin C) would not preferentially target mitochondria. Importantly, several cardiovascular pathologies are characterized by abnormalities in mitochondrial structure and function [2, 49] and it is noteworthy to indicate that mitochondria are a major source of ROS [51, 58]. Mitochondrial oxidative stress can induce oxidative modification of mitochondrial DNA (mtDNA) [26], which can interfere with the transcription of key proteins and t-RNAs in the mitochondrial genome. An impairment in the expression of proteins from the mt-genome could negatively impact bioenergetic capacity of mitochondria in that the efficiency of electron transport may be attenuated with increased “numbers” of electrons leaking from the complexes and interacting with O2 to form •O2− [7, 8]. Based on the above, we hypothesized that injury to mtDNA, i.e., base lesions and DNA fragmentation, and impaired mitochondrial bioenergetics underscores endothelial dysfunction in an animal model of the metabolic syndrome (Zucker obese fatty rat [ZOF]) [22, 26].
METHODS
Animals and Anesthesia.
Zucker lean rats (ZLN, n=25) and Zucker Obese Fatty (ZOF, n=33) were purchased from Harlan Laboratories (USA). Mice (C57Bl/6J, n=6 [3 males and females]) were derived from our own colony. Animals were housed in the Comparative Medicine Facility at Northeast Ohio Medical University and maintained with a light-dark cycle of 12 hours, with free access to water and food ad libitum. Rats were studied when they were 7–10 months in age. The experimental use and care of the animals was approved by the Northeast Ohio Medical University Institutional Animal Use and Care Committee, following the Guidelines for the care and the use of laboratory animals by the National Institutes of Health (NIH publication no. 85–23). Rats were divided into 4 groups: ZLN controls (n=9); ZOF controls (n=17); ZLN treated with a recombinant protein containing a mitochondrial localization sequence, the tat protein from HIV to enable cell penetration, and exonuclease III to create abasic sites in mtDNA (ZLN+mt-tat-ExoIII, n=16); and ZOF treated with a recombinant protein containing a mitochondrial localization sequence, the tat protein from HIV to enable cell penetration, and endonuclease III to facilitate the repair of fragmented mtDNA (ZOF+mt-tat-EndoIII, n=16). Mice were used exclusively for the studies showing endothelial incorporation of mitochondria following electroporation.
Induction and Repair of mtDNA Damage.
Repair of fragmented mtDNA was achieved by i.v. or i.p. administration of mt-tat-EndoIII in ZOF [57]. Fragmentation of mtDNA was induced in lean rats (ZLN) by intravenous (i.v.) or intraperitoneal (i.p.) administration of mt-tat-ExoIII [57]. In preliminary studies, we found that either route of delivery produced an equivalent effect. For i.v. infusion, rats were anesthetized, and a catheter was placed in the right jugular vein and exteriorized through the nape of the neck. Rats were allowed to recover for 3 days, before initiating the treatments. ZLN were treated for 3 days with either 80 μg/day of mt-tat-ExoIII or heparinized saline in the control group (ZLN). ZOF were treated with 1 μg/g of mt-tat-EndoIII (ZOF+EndoIII) or heparinized saline. The heparinized saline treatments were considered as a vehicle controls for the recombinant protein.
Mitochondrial DNA Damage, Mitochondrial Function and Mitochondrial Reactive Oxygen Species (ROS) Production.
Mitochondrial DNA (mtDNA) was isolated, and the injury was measured using quantitative PCR [21], which is based on real time-PCR amplification of mitochondrial DNA fragments of different lengths. Comparisons of quantities of amplified products provide a quantitative assessment of mtDNA damage.
Thoracic aortae were excised from Zucker male rats and cleaned of periadventitial fat and connective tissues via gentle dissection. The aortae were opened to expose endothelial surface. Cells of endothelial surface were scrapped in SHE buffer (250mM sucrose, 10mM HEPES, 1mM EDTA, pH 7.2), and then digested with 2mg/ml of Type II collagenase (Worthington Biochemical 4176, 278u/mg). The mixtures were centrifuged at 2,000×g for 20-min using Sorvall RC5C plus high-speed centrifuge. The supernatants were collected, and protein concentrations were measured using the Lowry method prior to measurement of mitochondrial complex activities. An appropriate amount of supernatant was added to an assay mixture to measure the activities of mitochondrial electron transport chain. The electron transport activity (ETA) of complex I was determined by following the rotenone sensitive ubiquinone-1 (Q1)-stimulated NADH oxidation [14]. The ETA of complex II was assayed by thenoyltrifluoroacetone-sensitive ubiquinone-2 (Q2)- stimulated dichlorophenyl indophenol reduction [15]. The ETA of complex III was assayed by ubiquinol-2 (Q2H2)-stimulated cytochrome c reduction and verified by inhibition with antimycin A. The ETA of complex IV was assayed by measuring ferrocytochrome c oxidation, and further confirmed by inhibition with potassium cyanide [16]. Activities of the mitochondrial complexes were were normalized to the amount of protein.
Estimates of mitochondrial generation of ROS were performed by using MitoSOX™, which permeates live cells and preferentially targets mitochondria due to its lipophilicity and a positive charge. MitoSOX™ is rapidly oxidized by superoxide, but not by other ROS and reactive nitrogen species (RNS). The oxidized product fluoresces upon binding to the nucleic acids of mtDNA.
Assessment of Vasodilation in Isolated Arterioles.
Studies of isolated coronary arterioles were accomplished as described previously using isobaric conditions (60 cmH2O) [43, 60]. Microvessels were isolated from the hearts of ZLN and ZOF, and rats treated with the recombinant enzymes, e.g., ZLN with mt-tat-ExoIII and ZOF with mt-tat-EndoIII. Vasodilation to acetylcholine (Ach) and to sodium nitroprusside (SNP) was assessed to evaluate endothelium-dependent and –independent dilation, respectively.
Mesenteric arterioles were isolated in ZLN and ZOF rats with the purpose of establishing if mitochondrial function was critical for endothelium-dependent dilation. These vessels were prepared and studied in the manner of our studies of coronary microvessels [42, 60]. In this preparation, mitochondria were isolated from the liver of ZLN using a modification of the procedures we used for isolation of cardiac mitochondria (using 10 mM Tris-Cl buffer containing 0.25 M sucrose and 1 mM EDTA without nagarse treatment, three times of differential centrifugation at 750×g for 10 min and 10,000×g for 15 min) [36]. Mitochondria were then “labelled” with MitoTracker™ Red FM, which accumulates in mitochondria due to the negative membrane potential across the inner membrane. The labelled mitochondria were introduced in the perfusion circuit of the isolated vessel in a Ca++-free buffer, and by changing levels of the inflow and outflow reservoirs of the microvessel (raising the inflow and lowering the outflow to generate a pressure drop across the vessel and thus flow through the vessel) the mitochondrial suspension was perfused through the microvessel. Once the labeled mitochondria were visible in the vessel using fluorescence microscopy a series of electroporation pulses (Harvard Apparatus; Model LV) was administered to the vessel to facilitate transfer of mitochondria into the endothelial cells. The electroporation entailed a sequence of unipolar electrical pulses: Volts, 50 V; Pulse length, 50 μs; 10 pulses with an interval of 100 ms between each pulse. After 15 minutes, the mitochondrial suspension was cleared from the lumen using the standard physiological salt solution with normal electrolyte composition. Thirty to 45 min after flushing mitochondria from the lumen, fluorescence microscopy was used to determine the presence of fluorescent mitochondria in the endothelium (the plane of focus on the endothelium), and after acquisition of these images, dose-response curves to sodium nitroprusside (SNP) and vasoactive intestinal peptide (VIP) were completed. Before the mitochondrial transfer, initial responses to VIP and SNP were obtained, and vasoactive responses were re-assessed 30 minutes after transfer. VIP was used in these studies because its vasodilation in mesenteric vessels is completely inhibited by the arginine analogue, L-nitro-arginine methyl ester (L-NAME), indicating complete reliance on the production of nitric oxide (NO) for vasodilation by this agonist. To establish if mitochondrial production of ATP is required for endothelium-dependent dilation, we performed dose-response curves to Ach, VIP and SNP under control conditions in a vessel from ZLN and following administration of oligomycin (1 μM) for 3 hours.
To determine if the electroporation procedure enabled incorporation of mitochondria into endothelial cells we performed experiments using mouse mesenteric arteries. After anesthesia, the liver and mesentery were removed. The liver was used for isolation of mitochondria (described previously) and the mesentery was used to isolate the mesenteric artery. The vessel was cannulated, pressurized (60 cmH2O) and treated with fluorescein isothiocyanate-labelled acetylated low density lipoprotein (FITC-ac-LDL, administered via the pipettes intraluminally) at a concentration of 15 μg/ml for 4 hrs. Hepatic mitochondria (2mg/ml) were isolated and incubated with MitoTracker™ Red FM (200 nM) for 20 mins; then infused intraluminally in a Ca++-free buffer and subjected to an electroporation protocol as described above. After recovery of the vessel in physiological salt solution, the vessel was removed from the pipettes and imaged using fluorescence deconvolution using the FITC-ac-LDL signals to mark the endothelium and analyzing for red fluorescence (mitochondria) in the same plane.
Measurements of Nitric Oxide
To further demonstrate the dependence of NO generation on functional mitochondria, we created ρ0 endothelial cells, which are cells devoid of functional mitochondria through depletion of mtDNA. This was accomplished in rat aortic (RAEC, Cell Applications, Inc) or bovine aortic endothelial cells (BAEC, Cell Systems) with low doses of ethidium bromide (EtBr) that results in the loss of mitochondrial DNA and decreased production of ATP by the mitochondria [27]. RAEC (10 × 103) were added to 35 mm cell culture dishes in Rat EC medium (Cell Applications, Inc) containing 50 ng/ml of EtBr and 50 μg/ml of uridine The low dose of EtBr does not affect nuclear DNA, but prevents mtDNA replication. The addition of uridine is essential for ρ0 cell division. As the cells divide, they progressively lose their mtDNA, and after 14 divisions the number of mtDNA copies per cell is less than one [27]. Accordingly, these cells were allowed to proliferate for two weeks in culture. Fluorescence from MitoTracker Red (looking for punctate areas of fluorescence) was not detected, indicating there were no functional mitochondria generating an electrochemical gradient required to accumulate the fluorophore. The ρ0 endothelial cells were evaluated for the production of nitric oxide using electron paramagnetic resonance (EPR) or fluorescence analysis using a cell permeable compound that upon reacting with NO produces a highly fluorescent moiety. We analyzed fluorescence intensity using ImageJ in cells treated with the highest dose of Ach (Control, L-NAME treated, and ρ0 endothelial cells).
Spin-trapping measurements of NO from BAECs were performed with a Bruker EMX EPR spectrometer with Fe2+-(N-methyl-D-glucamine dithiocarbamate)2 [FeMGD] as a NO spin trap. The experiments were performed with ~5×106 control and ρ0 BAEC grown in 100-mm dishes. Cells were washed with physiological buffer solution (PBS); then 1.2 mL of PBS containing glucose (1 g/L), pyruvate (36 mg/L), CaCl2, MgCl2, the NO spin trap FeMGD (0.5 mM Fe2+ and 5 mM MGD); then either a calcium ionophore A23187 (20 μM) or Ach (10 μM) was added, and the cells were incubated for 20 min at 37o C in a humidified environment containing 5% CO2. After the incubation, the supernatant from each dish was collected and concentrated (freeze drying) under vacuum using a lyophilizer (Labconco Freezone, Kansas City, MO). The trapped NO in the supernatant was measured by EPR. The sample was scanned using the following parameters: center field, 3300 G; sweep width, 80 G; power, 6.33 mW; receiver gain, 1×105; modulation amplitude, 4 G; time of conversion: 20 ms; time constant, 81.92 msec; number of scan, 100. The spectral simulations were based on the hyperfine coupling constant of aN=12.69 G, and performed by WinSim program developed by National Institutes of Health, NIEHS. Spin quantification was based on double integration of the simulation spectrum of NO-FeMGD.
Fluorescence Assessment of NO production was achieved using the fluorophore, 4-Amino-5-Methylamino-2’,7’-Difluorofluorescein Diacetate (DAF), which produces a fluorescent benzotriazole when it reacts with NO. Fluorescence analysis (485 nm excitation/515 nm emission) was conducted under basal conditions and following administration of Ach (0.1 μM, 1 μM, 10 μM) to the RAEC. The concentration of Ach used in these experiments was less than in the EPR studies (10 μM) to avoid saturation of the fluorescent signal because the fluorescence analysis works best with low concentrations of the fluorochrome to minimize background [37]. At the highest dose of Ach, we administered L-NAME (500 μM) to block the production of NO and also qualitatively assess the reduction in the fluorescence signal to estimate specificity of DAF.
Data analysis.
Results were expressed as mean ± standard deviation (SD). A one-way ANOVA followed by the Bonferroni post hoc test was used for multiple comparisons where appropriate. A repeated-measures ANOVA was used to evaluate the dose-response relationships. For analysis of the mitochondrial function, samples were performed in pairs, e.g., ZOF and ZOF + mt-tat-EndoIII, so that t-tests were used to measure differences in function due to the intervention as multi-group comparisons were not made. A probability value of P < 0.05 was used to establish statistical significance. Two samples of mitochondrial complex activity were excluded as statistical outliers using the Grubb’s ESD (extreme studentized deviate) method. The two samples were calculated as outliers with a P < 0.05. Normal distribution of the data sets was evaluated using Shapiro-Wilk test with all groups showing a passing the test for normality.
RESULTS
Mitochondrial ROS, Mitochondrial DNA Base Lesions, and Mitochondrial Complex Activities
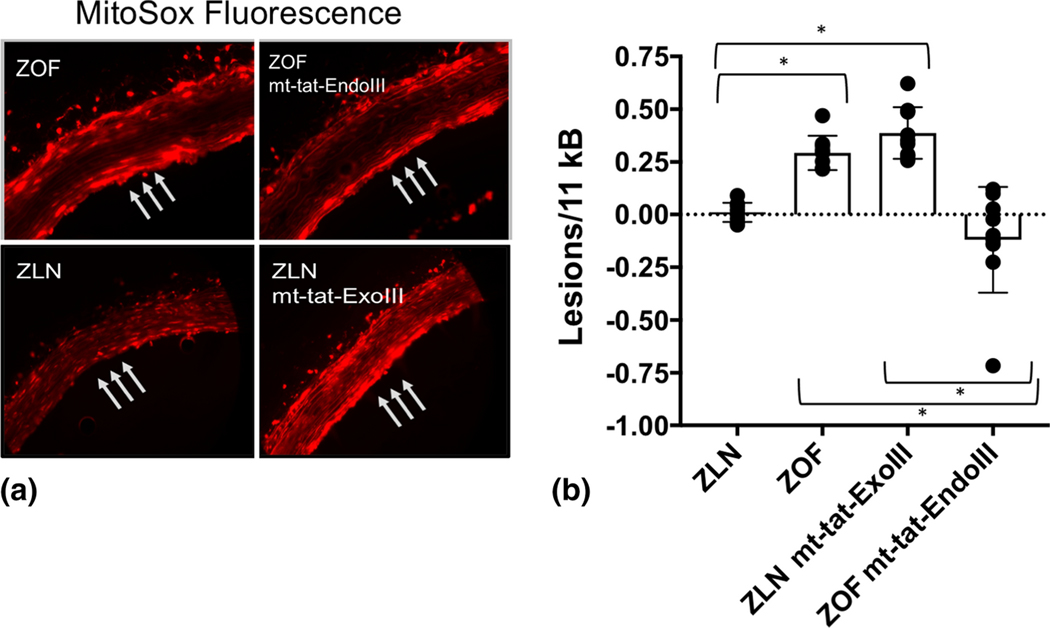
Figure 1 illustrates measurements of mitochondrial ROS (mtROS) production (MitoSOX fluorescence, Panel A) and mitochondrial base lesions in the 4 groups of rats (Panel B). Note, mtROS generation was higher in the ZOF rats compared to ZLN control (Figure 1A). When normalized to the levels of fluorescence in the ZLN rats, the increase was nearly increased 3-fold in vessels from ZOF rats. Note ROS generation (fluorescence) was marginally reduced in vessels from ZOF rats treated with mt-tat-EndoIII compared to untreated vessels. In this comparison, the fluorescence signals from vessels with mt-tat-EndoIII were normalized to vessels from untreated ZOF rats and the change was not significant (~15%). The ratiometric increase in MitoSOX fluorescence in rats treated with mito-tat-ExoIII (normalized to ZLN) was 60% higher, suggesting an increase mitochondrial ROS generation (P<0.05). Figure 1B also shows mitochondrial base lesions in the 4 groups of rats. Compared to the ZLN, the ZOF had significantly higher numbers of lesions per 10kB (P<0.05) and the base lesions in ZLN were not significantly different than zero. Treatment with mt-tat-ExoIII increased base lesions in the ZLN (P<0.05). Conversely, treatment with mt-tat-EndoIII significantly decreased base lesions in the ZOF (P<0.05). Although there are negative values after treatment with mt-tat-EndoIII, this is an artifact of the algorithm used to calculate base lesions and the value should be considered zero. Figure 2 illustrates activities of the mitochondrial complexes in the four groups. The activities of all complexes were decreased in ZOF rats compared to the ZLN controls (Panel A, P<0.05). Treatment of ZOF rats with mt-tat-EndoIII to restore mtDNA integrity increased activities of all complexes (Panel B, P<0.05); whereas, treatment of ZLN rats with mt-tat-ExoIII to induce mtDNA lesions reduced activity of the complexes (Panel C, P<0.05).
Figure 1.
Comparison of mitochondrial levels of ROS and mtDNA base lesions in ZLN, ZOF, ZOF+mt-tat-EndoIII, and ZLN+mt-tat-ExoIII groups (n=3–4 per group). A: When normalized to the MitoSox fluorescence signals were comparable in ZOF and ZOF+mt-tat-EndoIII, but were 1.6±0.1 fold higher in ZLN+mt-tat-ExoIII compared to ZLN (P<0.05). B: mtDNA lesion frequencies in all groups. In ZLN (n=4) lesion frequency was not different than zero, but was significantly elevated in ZOF rats (n=5, P<0.05). ZLN+mt-tat-ExoIII (n=5) had a higher base lesion frequency than ZLN or ZOF+mt-tat-EndoIII (P<0.05, n=7). ZOF+mt-tat-EndoIII did not have a lesion frequency different than zero, and the average was significantly less than ZOF (P<0.05). Values are mean ± standard deviation. *P<0.05
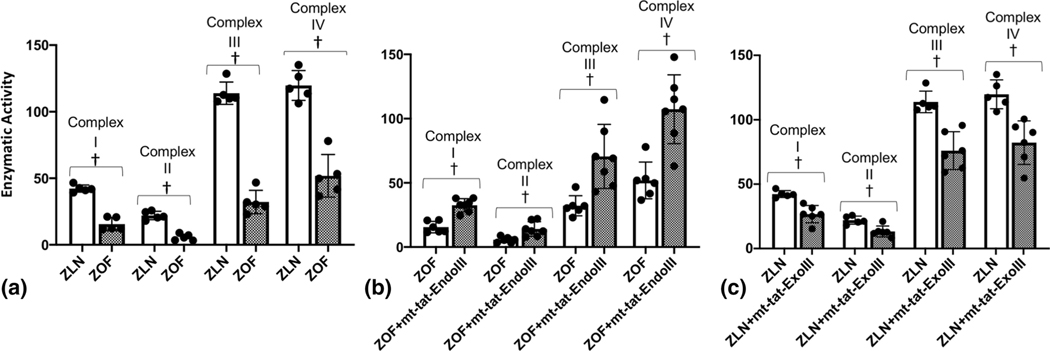
Figure 2.
Activities (nM substrate oxidized or reduced/min/mg protein) of mitochondrial complexes I, II, III, and IV in aortic endothelium from ZLN (n=5–6) and ZOF (n=5–7) rats. A. Activities of all mitochondrial complexes were lower in ZOF compared to ZLN rats (P<0.05). B. Activities of mitochondrial complexes were higher in ZOF+mt-tat-EndoIII compared to ZOF rats (P<0.05). C. Activities of mitochondrial complexes were lower in ZLN+mt-tat-ExoIII compared to ZLN rats (P<0.05). Values are mean ± standard deviation. † P<0.05
Microvascular Vasoactive Responses
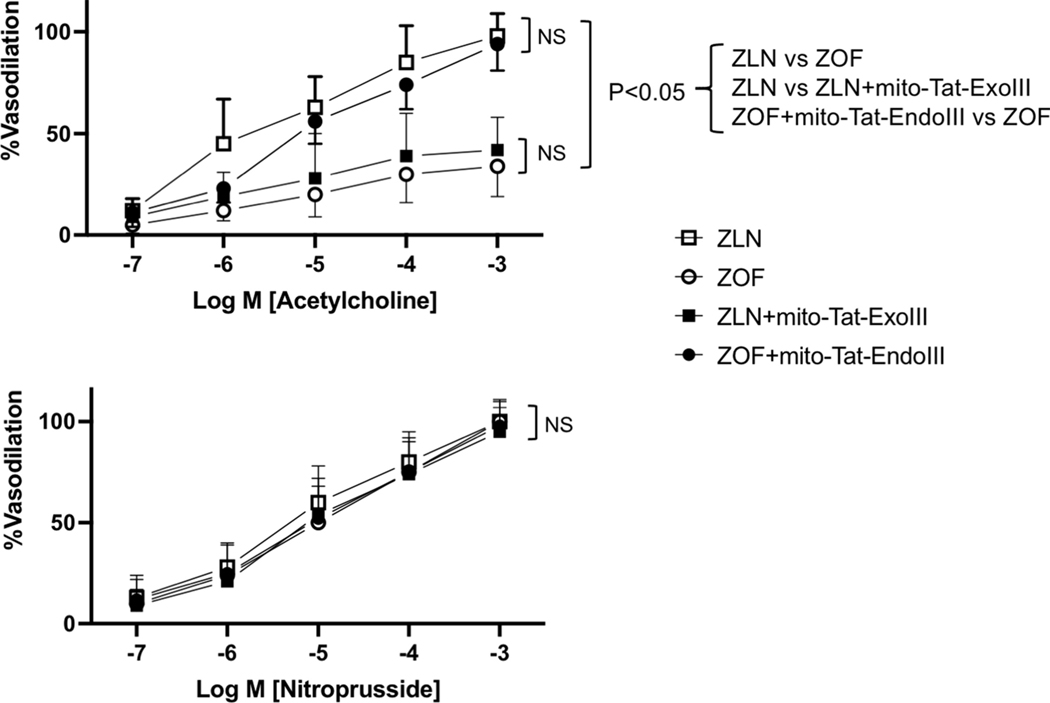
Figures 3, 4, and 5 show results of the isolated microvessel studies. Figure 3 summarizes results obtained from studies of isolated coronary arterioles from the treated and untreated ZOF and ZLN rats. Note the vasodilation to Ach was compromised in the ZOF rats compared to ZLN (P<0.05), and that treatment of the ZOF rats with mt-tat-Endo restored dilation to a level comparable to ZLN (Upper Panel). In contrast, administration of mt-tat-ExoIII to ZLN rats reduced Ach-induced dilation to the responses observed in the ZOF rats (Upper Panel, P<0.05). The Lower Panel reports vasodilation to the sodium nitroprusside in the isolated coronary arterioles. There were no differences observed among the 4 groups suggesting that vascular smooth muscle responses to NO are not compromised in ZOF rats or changed by any of the treatments.
Figure 3.
Dose-response relationships of coronary arterioles to acetylcholine (upper) and nitroprusside (lower) from ZLN (n=8), ZOF (n=8), ZOF+mt-tat-EndoIII (n=9), and ZLN+mt-tat-ExoIII (n=10). The dose-response relationships to Ach were lower in ZOF and mito-Tat-ExoIII compared to ZLN or ZOF+mito-Tat-EndoIII groups (P<0.05). Dose-response relationships to sodium nitroprusside (SNP) in all groups revealed no intergroup differences. Values are mean ± standard deviation.
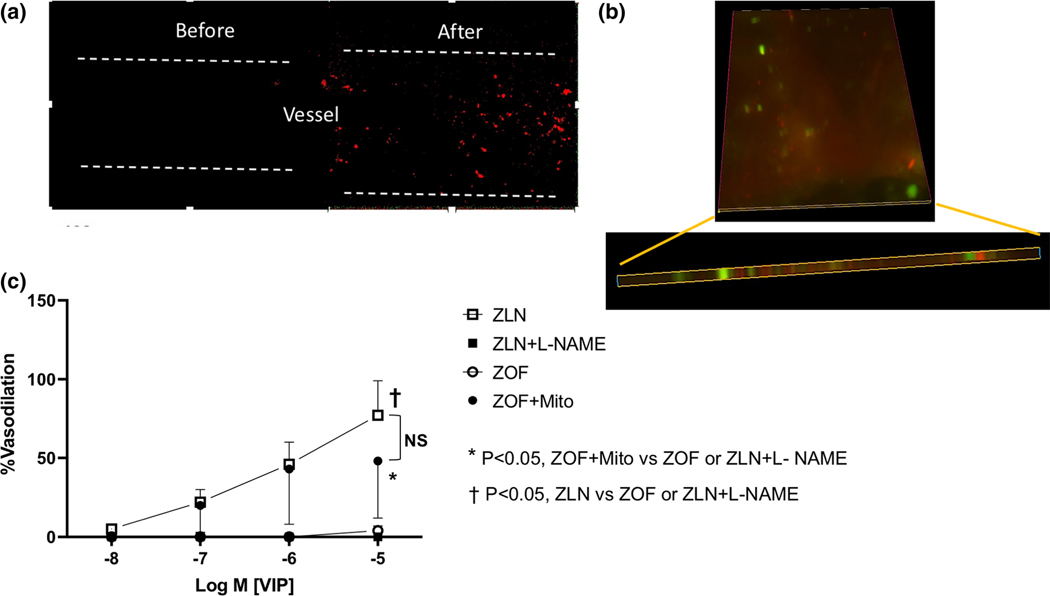
Figure 4.
Effects of mitochondrial transfer on endothelium-dependent vasodilation to VIP from mesenteric small arteries. Top Left, fluorescence image of the endothelium before and after mitochondrial transfer. Note the mitochondrial signals (MitoTracker™ Red FM) in the vessel after the transfer. Top Right, fluorescence signals from vessels treated with FITC-ac-LDL and then subjected to electroporation with mitochondria loaded with MitoTracker™ Red FM. Note the FITC-ac-LDL and MitoTracker signals were in the same plane. These signals were found nowhere else in the vessel or in the lumen. Bottom, vasodilation to VIP from ZLN rats (±L-NAME), and ZOF rats (before and after mitochondrial transfer [ZOF+Mito]). VIP produced potent dilation in ZLN vessels, which was completely abolished by L-NAME (n=6). VIP produced no dilation of ZOF vessels (n=4), but after mitochondrial transfer, dilation was restored (P<0.05 Mito-ZOF vs ZOF, n=4). Values are mean ± standard deviation.
Figure 5.
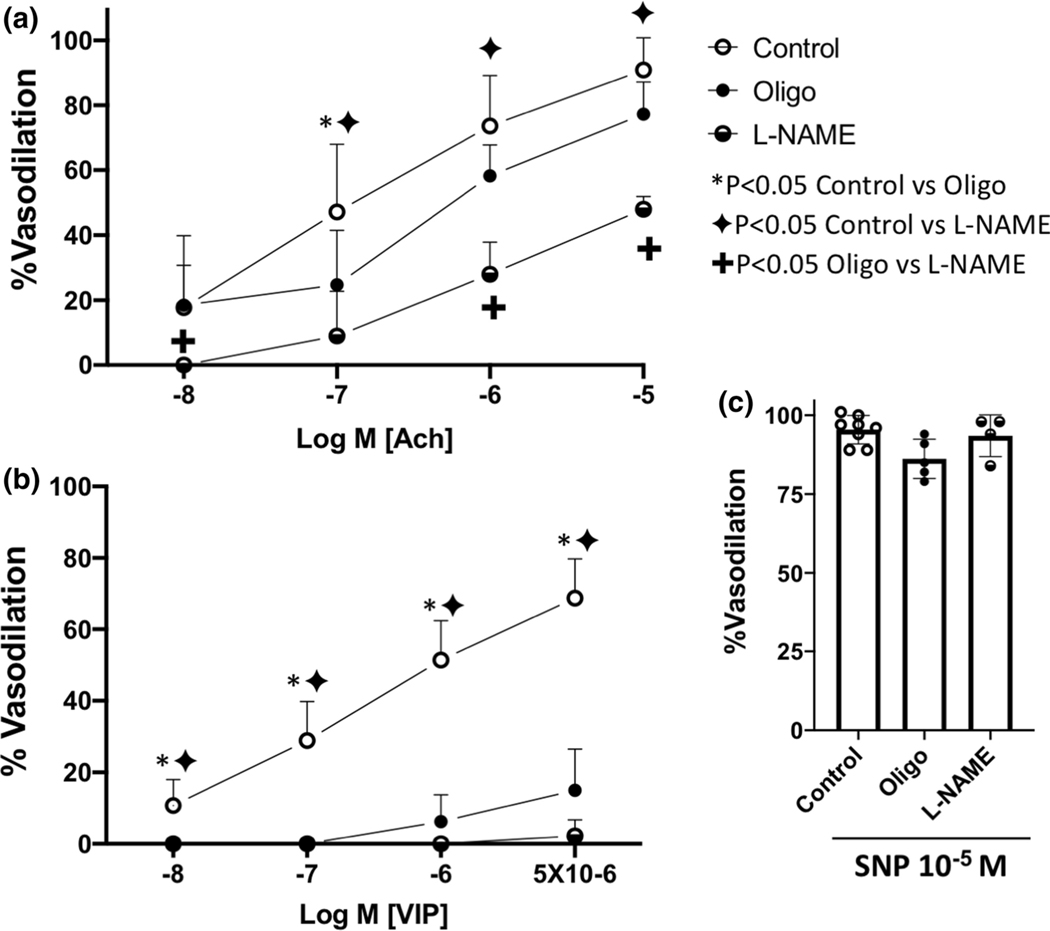
Effects of NOS inhibition (L-NAME) and ATP depletion (Oligomycin [Oligo]) on vasodilation of small mesenteric arteries from lean rats to Ach (Panel A), VIP (Panel B) and SNP (Panel C). Note, vasodilation to Ach was reduced by Oligo at only one dose, but responses to L-NAME were significantly attenuated compared to controls at most doses (P<0.05). Vasodilation to VIP was significantly attenuated by both L-NAME and oligo (P<0.05). Neither L-NAME or Oligo affected vasodilation to SNP. Values are mean ± standard deviation, n=4–9.
Effects of mitochondrial transfer, i.e., electroporation-mediated insertion of mitochondria from ZLN rats into mesenteric arterioles from ZOF rats are illustrated in Figure 4. The top left panel shows images of the endothelium of an isolated mesenteric microvessel (focused on the endothelium of the lumen) to determine the presence of the transferred mitochondria into the endothelium. The images show results before and after the mitochondrial transfer and the fluorescent signals indicate the presence of mitochondria in the endothelium. The images on the top right show results from mouse mesenteric arteries treated with FITC-ac-LDL and then subjected to electroporation. Note the FITC-ac-LDL and MitoTracker™ Red FM signals were in the same plane. These signals were found nowhere else in the vessel or in the lumen. The bottom panel shows the effects of mitochondrial transfer and inhibition of eNOS on dilation to VIP. Mesenteric arterioles dilated robustly to VIP, and this dilation was completely blocked by inhibition of eNOS using the arginine analogue, L-NAME. Dilation to VIP was also completely absent in arterioles isolated from ZOF rats. Note, that 30 min after the mitochondrial transfer (ZOF+Mito), dilation to VIP in the mesenteric vessels from the ZOF rats was similar (P=NS) to that of the ZLN rats. Two additional control experiments were completed. One control was the transfer of mitochondrial exposed to normal buffer (physiological levels of calcium are toxic to mitochondria) and these vessels showed no dilation to VIP. Second, performing the electroporation protocol in the absence of mitochondria did not rescue endothelium-dependent vasodilation (no vasodilation was observed in the ZOF rats in response to substance P. Thus, transfer of ZLN liver mitochondria into the endothelium of a blood vessel with endothelial dysfunction restores endothelial dependent dilation. We attempted to determine the amount of mtDNA incorporated into the endothelium, but were unable to make this determination. The reason we could not quantitatively determine this is because the endothelium has far fewer copies of mtDNA (normalized to μg of ribosomal RNA [rRNA]) than smooth muscle. Aortic endothelium averaged 0.25 × 106 copies of mtDNA/μg rRNA; whereas, aortic smooth muscle had 3.0 × 106 copies/μg rRNA, which is 12-fold higher than the endothelium. We could not isolate the endothelium from the small mesenteric artery, and decided that analysis of total mtDNA for the blood vessel would not be conclusive. Even if we doubled the endothelial mtDNA via mitochondrial transfer, it still would constitute a small fraction of the total and we would not be able to quantify the number of mitochondria transferred.
Because the results are consistent with mitochondrial function being critical to endothelium-dependent dilation, we evaluated if bioenergetics are the reason for this result. To demonstrate if ATP production plays a role in endothelium-dependent dilation, we treated isolated mesenteric arterioles of ZLN with oligomycin, to block mitochondrial ATP production or with L-NAME to inhibit the production of NO (Figure 5, panels A and B). Treatment with oligomycin modestly reduced endothelium-dependent vasodilation to Ach (Figure 5A), but had far more striking effects in blocking dilation to VIP (Figure 5B). L-NAME had a more potent effect on Ach-mediated vasodilation (Figure 5A) than oligomycin, but the inhibition of VIP-induced dilation was comparable for both L-NAME and oligomycin (Figure 5B). The dilation to VIP is nearly exclusively dependent on NO (100% inhibition of vasodilation at all but the highest dose of VIP), but a component Ach-induced endothelial dilation (~30–40% at the various doses) was largely independent of NO. These results support our concept that the production of NO is dependent on bioenergetics as dilation to the NO-dependent agonist, VIP, was largely blocked by oligomycin. Note, dilation to the NO donor, SNP (Figure 5C), was unaffected by either L-NAME or oligomycin suggesting that the diminished effects of VIP are not due to an effect on smooth muscle.
Nitric Oxide Production in Endothelial Cells
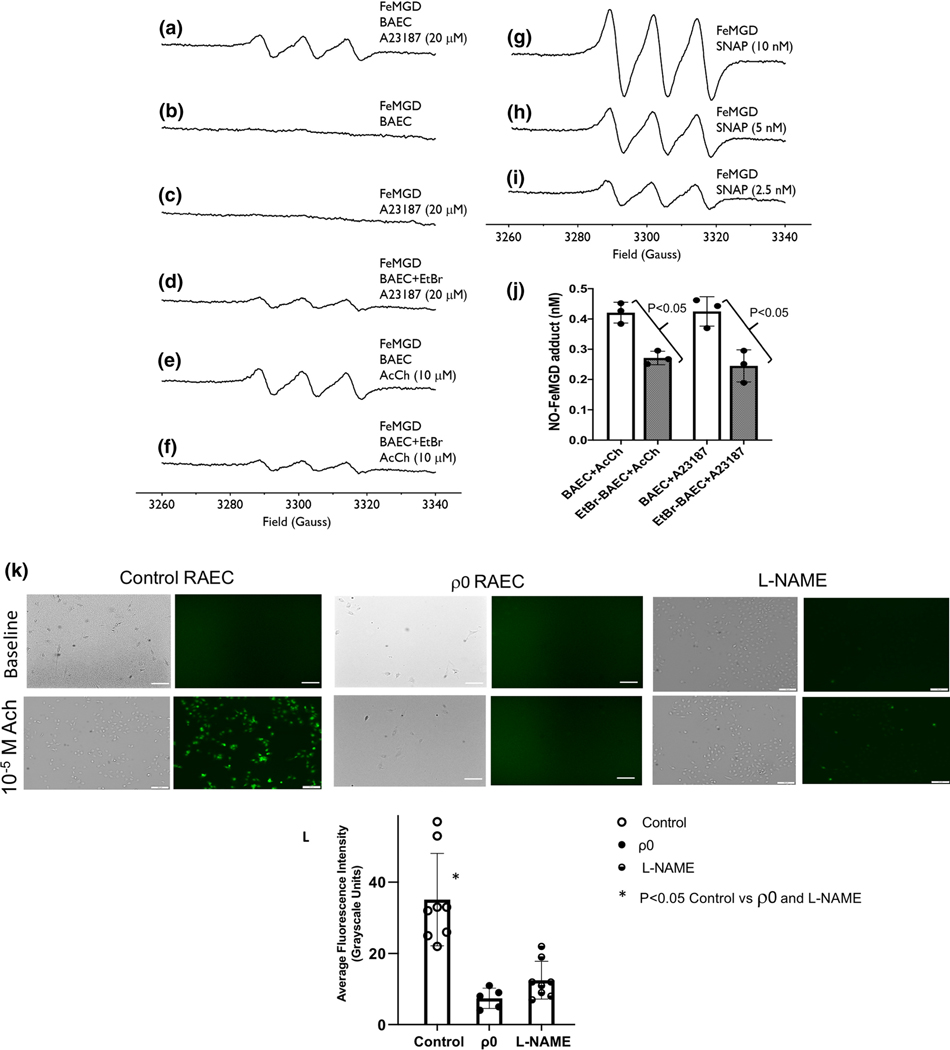
Figure 6 shows results of NO production in cultured (control and ρ0) BAEC and RAEC. BAEC were used for the EPR studies of NO production; whereas, RAEC were used for the imaging studies of DAF fluorescence. The top panel shows EPR signals obtained from using the NO spin-trap, FeMGD under basal conditions, stimulation with two agonists, and the use of NO donors. Traces A-D illustrate effects of the calcium ionophore A23187 on NO production (A), baseline NO production (B), measurements completed in a blank containing A23187 and FeMGD (C), and the effects of A23187 on NO production in the ρ0 BAEC (D). Note, in response to A23187, NO production is less in the ρ0 cells (D) than in the control BAEC treated with the agonist (A). Without stimulation, the basal production of NO in the endothelial cells is below levels of detection (B). Also, administration of A23187 and the spin trap do not chemically cause any production of NO (C). Traces E and F show NO production in response to Ach in control and ρ0 BAEC. Similar to the results obtained with A23187, NO production is decreased in the ρ0 cells. Specifically, in BAEC, administration of Ach results in the production of NO; however, in ρ0 cells, NO is not produced after administration of Ach. Traces G, H, and I show results obtained with varying amounts of the NO donor, SNAP. The middle right panel (J) summarizes the EPR results of NO production in the control and ρ0 BAEC. In response to either Ach or A23187, NO production was lower in the ρ0 BAEC compared to untreated controls. The panel K shows brightfield images and DAF fluorescence in control, ρ0, and L-NAME treated RAEC under basal conditions and following administration of Ach at a dose of 10−5 M. Supplemental Figure 1 shows images from additional doses of Ach. In the control RAEC (panel K), Ach increased DAF fluorescence from baseline, but no such changes were noted in either the ρ0 or L-NAME-treated endothelial cells. Panel L summarizes the aggregate results of fluorescence expressed as average grayscale intensity in control RAECs, ρ0 or L-NAME-treated RAEC in response to Ach. The level of fluorescence was significantly higher in the control RAECs compared to either the ρ0 or L-NAME treated cells. Inhibition of NO production attenuated the majority of the DAF signal, but a small fraction of fluorescence remained suggesting DAF is not completely specific for NO. Taken together, both the EPR results and the DAF fluorescence show the production of NO was decreased in the ρ0 endothelial cells (compared to controls).
Figure 6.
NO production in cultured bovine coronary artery endothelial cells (BAEC). A-I show EPR spectra obtained from using the NO spin-trap, FeMGD, following calcium ionophore A23187 in control BAEC (A), baseline BAEC without agonist (B), blank containing A23187 and FeMGD (C), A23187 in the ρ0 BAEC (D), Control BAEC after Ach (E), and ρ0 BAEC after Ach (F). G, H, and I show results obtained with varying amounts of the NO donor, SNAP. Panel J summarizes the EPR results of NO production in the control and ρ0 endothelial cells (values are mean ± standard deviation. n=3). Panel K shows brightfield and DAF fluorescence images in control and ρ0 RAEC under basal conditions and following administration of 10−5 M Ach. In the control HCAEC, Ach increased DAF fluorescence, which was substantially attenuated by L-NAME; whereas, Ach did not increase DAF fluorescence in the ρ0 HCAEC. In Panel L, the fluorescence signals at the highest dose of Ach were quantified (average grayscale intensity) revealing a substantial attenuation in fluorescence in the ρ0 (n=4) and L-NAME-treated (n=9) cells compared to control (n=12).
DISCUSSION
Our results support the concept that mitochondrial function, as suggested by activities of complexes I-IV, and mtDNA integrity are critical for endothelium-dependent vasodilation. Our results and interpretations are influenced by several issues pertaining to related literature on the role of the mitochondria on vascular function and our experimental design and approach.
Role of Mitochondria on Vascular Function
Although the concept we present—dependence of endothelial NO generation on normal mitochondrial function—is new, reports that mitochondrial function influences vascular and endothelial function have been the subject of previous investigations and reviews [31]. Recently, Zhou et al reported the importance of mitochondrial fission and suppression of mitophagy in microvascular injury following ischemia-reperfusion injury [68]. Another report revealed the effects of telomerase on endothelial dependent dilation [5]; however, this report did not explore if telomerase affected mitochondrial function or influenced mtDNA integrity. Although it is tempting to speculate that the actions of telomerase could mimic those of the mt-tat-EndoIII in the repair of mtDNA base lesions, mtDNA does not have the base sequence required for telomerase binding (TTAGGG); so, this possibility seems remote. Another report used a Seahorse extracellular flux analyzer to measure mitochondrial function in cerebral vessels from diabetic rats and reported a deterioration of function [40, 41]. Our observations extend this observation by showing that loss of mtDNA integrity was one of the reasons for the loss of mitochondrial function. Moreover, our results demonstrated the importance of mitochondrial function in the endothelium, not the entire microvessel. Importantly, we also established the connection between mitochondrial ROS and endothelial dysfunction as it related to mitochondrial function and mitochondrial DNA base lesions and oxidative modifications. Furthermore, our mitochondrial transfer experiments show the importance of normal mitochondrial function for endothelial production of nitric oxide and the consequential dilation.
The role of mitochondrial fission and fusion in endothelial function has also been implicated in pulmonary hypertension [59]. These authors reported that elevations in mitochondrial ROS led to mitochondrial fragmentation and endothelial dysfunction. This may have bearing on our work because if the number of mtDNA base lesions is sufficient, transcription of the mitochondrial genome could be impaired leading to fragmentation of mitochondria.
An enigma relates to the “chicken and egg” metaphor as to which comes first—increased production of mitochondrial ROS leading to mtDNA lesions, or increased mtDNA lesions, leading to increased mitochondrial production of ROS. Our data cannot distinguish between these two possibilities, although we did observe in the ZOF rats treated with mt-tat-EndoIII, restoration of mtDNA integrity, partial restoration of mitochondrial function and restoration of endothelium-dependent dilation, but not a significant decrease in mitochondrial superoxide. These observations suggest that the role of mitochondrial function and bioenergetics are perhaps more important than mitochondrial ROS generation.
Considerations of the Experimental Design and Approach
Our conclusions are critically dependent on some of the methodologies, protocols and model. We used measurements of mitochondrial complex activities instead of measurements of mitochondrial respiration as a surrogate for mitochondrial function. The reason for this was based on pragmatism—we could not harvest sufficient numbers of endothelial cells from the aorta to have sufficient numbers for measurements of respiration. Even with this caveat, enzymatic activity of mitochondrial complexes was previously reported to reflect mitochondrial function [50, 63]. Mitochondrial ROS generation was estimated from the fluorescent indicator, MitoSox, a positively charged, lipophilic probe that accumulates in mitochondria due to electrochemical forces, and preferentially measures mitochondrial ROS generation. Although this indicator has been used to indicate superoxide generation, a more conservative interpretation is that it indicates ROS; accordingly, our interpretations are based on mitochondrial ROS generation. Mitochondrial DNA base lesions were evaluated by PCR, in which different sets of primers were used to amplify different size portions of the mitochondrial genome. The rationale for this was the longer the segment, the higher the probability that the product would not be amplified correctly because the base lesion would stop the reverse transcription. Using an algorithm [21], we calculated the lesions per 11 kb of bases. This number corresponded reasonably well with results we previously published using a Southern analysis to analyze mtDNA lesions [26]. The negative numbers in Figure 2 represent an artifact of the algorithm and should be considered zero.
For the mitochondrial transfer studies, we elected to use mitochondria derived from the liver instead of vascular tissue or the heart. This decision was based on the ease of isolation in a “soft” tissue like the liver as opposed to tissues with larger amounts of connective tissue. Less stringent conditions for isolation translate to greater numbers of functional mitochondria. Although mitochondria in different organ systems have similar functions, e.g., aerobic metabolism, production of ATP, we realize that there may be organ-specific characteristics of mitochondria [39]. We do not believe such possibilities have affected the results, but this remains a possibility. Another consideration of the mitochondrial transfer experiments pertains to the numbers of functional mitochondria that are required to produce the beneficial effect. Unfortunately, we do not have an answer to this dilemma. We are confident that the mitochondria are functional as the accumulation of MitoTracker® is dependent on maintenance of a negative membrane potential because the positively charged, lipophilic fluorochrome moves in accordance with the electrochemical gradient. However, we do not know if there is a range of membrane potentials and a potential range in mitochondrial functions. Despite these limitations, we can state that the transfer of functional mitochondria into the endothelium of a mesenteric arteriole of ZOF rats restored endothelium-dependent vasodilation.
The experimental model we chose, the Zucker Obese Fatty Rat, has strengths and weaknesses as a model for Type 2 diabetes. Although females were not studied, female and male ZOF rats both demonstrate similar degrees of endothelial dysfunction as well as similar changes in blood glucose and triglycerides [9]. Although the ZOF model has been criticized for the mis-sense mutation in the leptin receptor that completely eliminates leptin signaling, there are data in the literature showing that the endothelium-dependent vasodilation is nearly normal in young ZOF rats [38].
Furthermore, interventions such as a low carbohydrate diet [22] or antibody neutralization of TNFα [54] restored endothelium-dependent vasodilation in ZOF rats suggesting that the absence of leptin signaling is not the primary mechanism for the dysfunction.
We would also like to mention that the recombinant proteins were administered systemically which could result in effects not specific to the endothelium. We previously reported that administration of mt-tat-EndoIII in ZOF rats, and mt-tat-ExoIII in ZLN rats did not affect blood glucose or circulating lipids [26]; thus, we conclude that the effects on endothelial function were not related to an effect on these factors. However, these recombinant proteins affect aspects of cardiac myocyte biology and appeared to affect the production of metabolic dilators by the working heart. However, our previous work did not address the role of mitochondrial function in endothelium-dependent dilation.
We would be remiss to not mention some aspects of our results using oligomycin. We used this ATPase inhibitor to block mitochondrial production of ATP as a means to understand the role of mitochondrial function in NO production. Our data with oligomycin are consistent with this view, although one consequence of administration of oligomycin would be activation of AMP-activated Kinase (AMPK) due to the reduction in cellular levels of ATP. There are disparate reports in the literature regarding the effects of AMPK activation on NO production. One observation is that AMPK phosphorylates eNOS on serine 1179 leading to activation [19, 44, 45, 64, 67]. However, one other group reported that AMPK phosphorylates eNOS on thr495 and exerts an inhibitory effect [69]. Although our study did not examine AMPK and phosphorylation of eNOS, our interpretations of the results obtained with oligomycin are potentially impacted by this issue. If AMPK has an inhibitory effect on eNOS activity, then the actions of oligomycin could be potentially explained by activation of this enzyme. However, the vast majority of the literature reports that AMPK activates eNOS, which would not confound our interpretation that proper mitochondrial function is essential for the production of NO.
We incorporated studies of both mesenteric and coronary small arteries in our study. One reason for doing this was to broaden the scope of the conclusions to more than one vascular bed as there are distinct organ-specific attributes in vascular control mechanisms. Although the effects of eNOS antagonism on Ach-induced vasodilation in mesenteric vessels was significant, responses to Ach after oligomycin were largely unaffected (Figure 5). This difference is difficult to reconcile through an action on NO production, but we speculate that the difference is due to a secondary effect of NOS inhibition. Specifically, as we and others previously reported, inhibition of NO production leads to an upregulation of other compensatory endothelium-dependent vasodilators [4, 46]. We speculate that upregulaton of these compensatory pathways was enhanced by the longer duration of the studies with oligomycin (3 hrs) compared to the shorter time course of NOS inhibition (10–15 minutes). This would explain why inhibition of NO production by oligomycin did not have the same effect as L-NAME in response to Ach.
The use of DAF for detection of NO has been criticized for a lack of specificity. Our imaging studies of the ρ negative endothelial cells show that the DAF signal was absent during Ach administration. However, we acknowledge that administration of L-NAME did not completely eliminate DAF fluorescence (Figure 6, panel K) suggesting some degree of non-specificity of the signal. However, the complete loss of the signal in the ρ negative endothelial cells along with the EPR results gives us confidence in the conclusion that NO production was decreased in cells without mitochondria.
Another issue that we will raise may be viewed as a limitation, but may actually be a strength of our study. In the experimental design, we included studies of cultured endothelial cells from different species: bovine and rat. We assessed endothelial-dependent dilation using two different agonists as well as measuring mitochondrial complex activities in endothelial cells freshly isolated from the aorta. We also documented mitochondrial transfer using electroporation in vessels from mice and rats. No matter, the species from which the cells were isolated, the organ system from which the vessels were isolated, whether the endothelial cells were cultured or “native” within a blood vessel, all results pointed in the same direction; that an impairment in endothelial cell mitochondrial function appears to be central to endothelial dysfunction in the metabolic syndrome.
There are two unresolved questions that bear on the interpretation of the mitochondrial transfer experiments. First, how many mitochondria were transferred into the vessel and second, what is the minimal number of functional mitochondria needed to restore endothelial dependent vasodilation? Unfortunately, we do not have an answer to these questions. The endothelium is a cell type that is not rich in mitochondria, with cytoplasmic volume fraction of 2–5% of most endothelial cells [48]. To provide perspective for this number, the cytoplasmic volume fraction of mitochondria in cardiac myocytes ranges from 22–37% depending on the species [3]. The question we cannot answer is how many functional mitochondria are needed to restore endothelial cell bioenergetics to a point where the NO can be produced. We can say, our technique allowed transfer of functional mitochondria into the endothelium of a small artery, but we cannot say how many were transferred.
Summary, Speculation, and Conclusions
We observed that ZOF rats, a model of type 2 diabetes with impaired endothelium-dependent vasodilation, had lower (compared to lean controls) enzymatic activities of the mitochondrial complexes I-IV, increased frequency of mtDNA base lesions, and increased mitochondrial ROS. To demonstrate a connection among mtDNA base lesions, mitochondrial function (complex activities) and endothelial dependent vasodilation, we used a cell permeable, mitochondrial-directed endonuclease to repair the base lesions in ZOF rats. This intervention restored endothelium-dependent dilation, improved mitochondrial complex activities, and eliminated mtDNA base lesions. We also observed that introduction of base lesions in mtDNA of ZLN rats using a cell permeable, mitochondrial-directed exonuclease impaired endothelium-dependent vasodilation and mitochondrial function while increasing mitochondrial ROS generation.
Further support for the connection between mitochondrial function and endothelium-dependent vasodilation was gained by showing that transfer of liver mitochondria from ZLN rats into the endothelium of isolated blood vessels from ZOF rats improved endothelium-dependent vasodilation; whereas, the mitochondrial ATPase inhibitor, oligomycin, inhibited dilation to endothelium dependent agonists in vessels from ZLN rats. Interestingly, vasodilation to an endothelium-independent agonist, sodium nitroprusside was comparable regardless of the condition or treatments suggesting vasodilation of smooth muscle was not as dependent on mitochondrial function.
A rhetorical question regarding the role of mitochondria in vascular function is, why is smooth muscle resistant to alterations in mitochondrial function? Despite compromised mitochondrial function in the ZOF and mt-tat-ExoIII treated ZLN rats, smooth muscle function (dilation to SNP) was not affected. In retrospect this result was not too surprising given what is known about smooth muscle biology. For example, earlier reports of histochemical profiles of coronary arterioles reveal these vessels show limited capacity for aerobic metabolism [11]. Furthermore, smooth muscle is very energetically efficient due to the latch-state in which tension can be maintained without continued cross-bridge recycling that requires the hydrolysis of ATP. As an example of this efficiency, the energy requirements of smooth muscle are 100- to 500-fold less than those of striated muscle [53]. With such efficiency is not surprising that vasoreactivity of smooth muscle appeared less dependent on mitochondrial function.
Another rhetorical question we pose, is how can endothelial NO production be so dependent on mitochondrial function? The signaling sequence for shear stress culminating in the production of NO provides insight into the answer. This sequence involves phosphorylation of focal adhesion kinase, src kinase, PI3 kinase, protein kinase B (Akt), and eNOS [34]. Importantly, each kinase uses ATP to phosphorylate its substrate. The point we are driving at is that the production of NO requires energy through the use of ATP to activate kinases involved in its production. Thus, there must be an energy requirement for the production of NO.
We speculate that many conditions associated with excessive generation or inadequate scavenging of ROS in the mitochondria may also have a component of injury associated with mtDNA base lesions. Perhaps it is not a coincidence that many such conditions, such as ischemia-reperfusion, hypertension, and pulmonary hypertension, are characterized by impaired endothelium-dependent vasodilation and increased levels of mitochondrial ROS [25, 52, 56, 59]. Freed et al reported that patients with coronary artery disease show impaired NO-mediated vasodilation and the principal endothelium-dependent dilator is H2O2 [23]. Although only about 50% of the patients with coronary disease were diabetic, they all showed evidence for excessive ROS production by the mitochondria. Such a finding could be explained by our results that the excessive ROS production has produced mtDNA base lesions and adversely affected mitochondrial function and NO production.
In conclusion, mitochondrial bioenergetics are critical for the endothelial generation of NO. In a model of the metabolic syndrome, impaired endothelium-dependent vasodilation appears to be induced by deficient mitochondrial bioenergetics, which are caused by mitochondrial DNA base lesions and mtDNA strand breaks. Enzymatic repair of the lesions restored mitochondrial bioenergetic capacity and endothelial function.
Supplementary Material
Acknowledgments
Sources of Funding
The authors acknowledge the following sources of funding: HL135024, HL135110 (WMC); HL135110, HL137008 (LY); HL142710 (VO). We also acknowledge support from the Schermer Family Trust and the Fibus Family Foundation.
Footnotes
Declarations
Disclosures
Dr. Wilson has an interest in Exscien, which supplied the recombinant proteins, mt-tat-EndoIII and mt-tat-ExoIII. There are no other disclosures or conflicts of interest.
Ethics Approvals
All animal experiments were approved by the Institutional Animal Care and Use Committee. The manuscript does not contain clinical studies or patient data.
Consent for Publication
All authors consent to publication and approve of this version of the paper.
Availability of Data
Data will be made available upon reasonable requests.
All authors contributed to the study with differing roles for material preparation (William M. Chilian, Glenn Wilson, Inna Shokolenko), and data collection and analysis (James P. Hardwick, Giacinta Guarini, Takahiko Kiyooka, Christopher Kolz, Joanna Peng, Yeong Renn Chen, Chwen Lih Chen, Patrick T. Kang, Donte Stevens, June Yun, Yuh Fen Pung, Danielle Janota, Vahagn Ohanyan, and Liya Yin. The first draft of the manuscript was written by William Chilian and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
REFERENCES
- 1.Alexander RW (1995) Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension 25:155–161 doi: 10.1161/01.hyp.25.2.155 [DOI] [PubMed] [Google Scholar]
- 2.Ballinger SW (2005) Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med 38:1278–1295 doi: 10.1016/j.freeradbiomed.2005.02.014 [DOI] [PubMed] [Google Scholar]
- 3.Barth E, Stammler G, Speiser B, Schaper J (1992) Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 24:669–681 doi: 10.1016/0022-2828(92)93381-s [DOI] [PubMed] [Google Scholar]
- 4.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R (1996) Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 94:3341–3347 [DOI] [PubMed] [Google Scholar]
- 5.Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD (2016) Critical Role for Telomerase in the Mechanism of Flow-Mediated Dilation in the Human Microcirculation. Circ Res 118:856–866 doi: 10.1161/CIRCRESAHA.115.307918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonetti PO, Lerman LO, Lerman A (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23:168–175 doi: 10.1161/01.atv.0000051384.43104.fc [DOI] [PubMed] [Google Scholar]
- 7.Brand MD (2005) The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans 33:897–904 doi: 10.1042/BST0330897 [DOI] [PubMed] [Google Scholar]
- 8.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N (2004) Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37:755–767 doi: 10.1016/j.freeradbiomed.2004.05.034 [DOI] [PubMed] [Google Scholar]
- 9.Brooks SD, Hileman SM, Chantler PD, Milde SA, Lemaster KA, Frisbee SJ, Shoemaker JK, Jackson DN, Frisbee JC (2018) Protection from chronic stress- and depressive symptom-induced vascular endothelial dysfunction in female rats is abolished by preexisting metabolic disease. Am J Physiol Heart Circ Physiol 314:H1085–H1097 doi: 10.1152/ajpheart.00648.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844 doi: 10.1161/01.res.87.10.840 [DOI] [PubMed] [Google Scholar]
- 11.Cannon MS, Jones CE, Peterson TV (1982) A histochemical evaluation of metabolism in the coronary vasculature of the primate. Blood Vessels 19:186–196 [PubMed] [Google Scholar]
- 12.Ceriello A, Motz E (2004) Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 24:816–823 doi: 10.1161/01.ATV.0000122852.22604.78 [DOI] [PubMed] [Google Scholar]
- 13.Chan SHH, Hsu K-S, Huang C-C, Wang L-L, Ou C-C, Chan JYH (2005) NADPH Oxidase-Derived Superoxide Anion Mediates Angiotensin II-Induced Pressor Effect via Activation of p38 Mitogen-Activated Protein Kinase in the Rostral Ventrolateral Medulla. Circ Res 97:772–780 doi: 10.1161/01.res.0000185804.79157.c0 [DOI] [PubMed] [Google Scholar]
- 14.Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KB, Zweier JL, Chen YR (2007) Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry 46:5754–5765 doi: 10.1021/bi602580c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YR, Chen CL, Pfeiffer DR, Zweier JL (2007) Mitochondrial Complex II in the Post-ischemic Heart: OXIDATIVE INJURY AND THE ROLE OF PROTEIN S-GLUTATHIONYLATION. J Biol Chem 282:32640–32654. Epub 32007 Sep 32611. [DOI] [PubMed] [Google Scholar]
- 16.Chen YR, Deterding LJ, Tomer KB, Mason RP (2000) Nature of the inhibition of horseradish peroxidase and mitochondrial cytochrome c oxidase by cyanyl radical. Biochemistry 39:4415–4422 doi: 10.1021/bi992652+ [DOI] [PubMed] [Google Scholar]
- 17.Cosentino F, Luscher TF (1998) Tetrahydrobiopterin and endothelial function. Eur Heart J 19 Suppl G:G3–8 [PubMed] [Google Scholar]
- 18.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G (2002) Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90:1159–1166 doi: 10.1161/01.res.0000020401.61826.ea [DOI] [PubMed] [Google Scholar]
- 19.Davis BJ, Xie Z, Viollet B, Zou MH (2006) Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55:496–505 doi: 10.2337/diabetes.55.02.06.db05-1064 [DOI] [PubMed] [Google Scholar]
- 20.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM (2000) Endothelial dysfunction in diabetes. Br J Pharmacol 130:963–974 doi: 10.1038/sj.bjp.0703393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Druzhyna NM, Musiyenko SI, Wilson GL, LeDoux SP (2005) Cytokines induce nitric oxide-mediated mtDNA damage and apoptosis in oligodendrocytes. Protective role of targeting 8-oxoguanine glycosylase to mitochondria. J Biol Chem 280:21673–21679 doi: 10.1074/jbc.M411531200 [DOI] [PubMed] [Google Scholar]
- 22.Focardi M, Dick GM, Picchi A, Zhang C, Chilian WM (2007) Restoration of coronary endothelial function in obese Zucker rats by a low-carbohydrate diet. Am J Physiol Heart Circ Physiol 292:H2093–2099 doi: 10.1152/ajpheart.01202.2006 [DOI] [PubMed] [Google Scholar]
- 23.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD (2014) Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 115:525–532 doi: 10.1161/CIRCRESAHA.115.303881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C (2007) Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation 115:245–254 doi: 10.1161/CIRCULATIONAHA.106.650671 [DOI] [PubMed] [Google Scholar]
- 25.Go KL, Lee S, Zendejas I, Behrns KE, Kim JS (2015) Mitochondrial Dysfunction and Autophagy in Hepatic Ischemia/Reperfusion Injury. Biomed Res Int 2015:183469 doi: 10.1155/2015/183469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guarini G, Kiyooka T, Ohanyan V, Pung YF, Marzilli M, Chen YR, Chen CL, Kang PT, Hardwick JP, Kolz CL, Yin L, Wilson GL, Shokolenko I, Dobson JG, Jr., Fenton R, Chilian WM (2016) Impaired coronary metabolic dilation in the metabolic syndrome is linked to mitochondrial dysfunction and mitochondrial DNA damage. Basic Res Cardiol 111:29 doi: 10.1007/s00395-016-0547-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashiguchi K, Zhang-Akiyama Q-M (2009) Establishment of Human Cell Lines Lacking Mitochondrial DNA. In: Stuart JA (ed) Mitochondrial DNA: Methods and Protocols. Humana Press, Totowa, NJ, p 383–391 [DOI] [PubMed] [Google Scholar]
- 28.Hatake K, Kakishita E, Wakabayashi I, Sakiyama N, Hishida S (1990) Effect of aging on endothelium-dependent vascular relaxation of isolated human basilar artery to thrombin and bradykinin. Stroke 21:1039–1043 doi: 10.1161/01.str.21.7.1039 [DOI] [PubMed] [Google Scholar]
- 29.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T (2001) Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104:2673–2678 doi: 10.1161/hc4601.099485 [DOI] [PubMed] [Google Scholar]
- 30.Jha P, Flather M, Lonn E, Farkouh M, Yusuf S (1995) The antioxidant vitamins and cardiovascular disease. A critical review of epidemiologic and clinical trial data. Ann Intern Med 123:860–872 doi: 10.7326/0003-4819-123-11-199512010-00009 [DOI] [PubMed] [Google Scholar]
- 31.Kadlec AO, Beyer AM, Ait-Aissa K, Gutterman DD (2016) Mitochondrial signaling in the vascular endothelium: beyond reactive oxygen species. Basic Res Cardiol 111:26 doi: 10.1007/s00395-016-0546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keaney JF Jr., Vita JA (1995) Atherosclerosis, oxidative stress, and antioxidant protection in endothelium-derived relaxing factor action. Prog Cardiovasc Dis 38:129–154 doi: 10.1016/s0033-0620(05)80003-9 [DOI] [PubMed] [Google Scholar]
- 33.Koltai MZ, Hadhazy P, Posa I, Kocsis E, Winkler G, Rosen P, Pogatsa G (1997) Characteristics of coronary endothelial dysfunction in experimental diabetes. Cardiovasc Res 34:157–163 doi: 10.1016/s0008-6363(97)00050-3 [DOI] [PubMed] [Google Scholar]
- 34.Koshida R, Rocic P, Saito S, Kiyooka T, Zhang C, Chilian WM (2005) Role of focal adhesion kinase in flow-induced dilation of coronary arterioles. Arterioscler Thromb Vasc Biol 25:2548–2553 doi: 10.1161/01.ATV.0000188511.84138.9b [DOI] [PubMed] [Google Scholar]
- 35.Kuo L, Davis MJ, Cannon MS, Chilian WM (1992) Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by L-arginine. Circ Res 70:465–476 doi: 10.1161/01.res.70.3.465 [DOI] [PubMed] [Google Scholar]
- 36.Lee HL, Chen CL, Yeh ST, Zweier JL, Chen YR (2012) Biphasic modulation of the mitochondrial electron transport chain in myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 302:H1410–1422 doi: 10.1152/ajpheart.00731.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leikert JF, Rathel TR, Muller C, Vollmar AM, Dirsch VM (2001) Reliable in vitro measurement of nitric oxide released from endothelial cells using low concentrations of the fluorescent probe 4,5-diaminofluorescein. FEBS Lett 506:131–134 doi: 10.1016/s0014-5793(01)02901-5 [DOI] [PubMed] [Google Scholar]
- 38.Lobato NS, Filgueira FP, Prakash R, Giachini FR, Ergul A, Carvalho MH, Webb RC, Tostes RC, Fortes ZB (2013) Reduced endothelium-dependent relaxation to anandamide in mesenteric arteries from young obese Zucker rats. PLoS One 8:e63449 doi: 10.1371/journal.pone.0063449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lotz C, Lin AJ, Black CM, Zhang J, Lau E, Deng N, Wang Y, Zong NC, Choi JH, Xu T, Liem DA, Korge P, Weiss JN, Hermjakob H, Yates JR 3rd, Apweiler R, Ping P (2014) Characterization, design, and function of the mitochondrial proteome: from organs to organisms. J Proteome Res 13:433–446 doi: 10.1021/pr400539j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merdzo I, Rutkai I, Sure VN, McNulty CA, Katakam PV, Busija DW (2017) Impaired Mitochondrial Respiration in Large Cerebral Arteries of Rats with Type 2 Diabetes. J Vasc Res 54:1–12 doi: 10.1159/000454812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merdzo I, Rutkai I, Tokes T, Sure VN, Katakam PV, Busija DW (2016) The mitochondrial function of the cerebral vasculature in insulin-resistant Zucker obese rats. Am J Physiol Heart Circ Physiol 310:H830–838 doi: 10.1152/ajpheart.00964.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merkus D, Brzezinska AK, Zhang C, Saito S, Chilian WM (2005) Cardiac myocytes control release of endothelin-1 in coronary vasculature. Am J Physiol Heart Circ Physiol 288:H2088–2092 doi: 10.1152/ajpheart.00522.2003 [DOI] [PubMed] [Google Scholar]
- 43.Merkus D, Duncker DJ, Chilian WM (2002) Metabolic regulation of coronary vascular tone: role of endothelin-1. Am J Physiol Heart Circ Physiol 283:H1915–1921 doi: 10.1152/ajpheart.00223.2002 [DOI] [PubMed] [Google Scholar]
- 44.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP (2003) Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem 278:31629–31639 doi: 10.1074/jbc.M212831200 [DOI] [PubMed] [Google Scholar]
- 45.Murakami H, Murakami R, Kambe F, Cao X, Takahashi R, Asai T, Hirai T, Numaguchi Y, Okumura K, Seo H, Murohara T (2006) Fenofibrate activates AMPK and increases eNOS phosphorylation in HUVEC. Biochem Biophys Res Commun 341:973–978 doi: 10.1016/j.bbrc.2006.01.052 [DOI] [PubMed] [Google Scholar]
- 46.Nishikawa Y, Stepp DW, Chilian WM (2000) Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol 279:H459–465 doi: 10.1152/ajpheart.2000.279.2.H459 [DOI] [PubMed] [Google Scholar]
- 47.Oelze M, Warnholtz A, Faulhaber J, Wenzel P, Kleschyov AL, Coldewey M, Hink U, Pongs O, Fleming I, Wassmann S, Meinertz T, Ehmke H, Daiber A, Munzel T (2006) NADPH oxidase accounts for enhanced superoxide production and impaired endothelium-dependent smooth muscle relaxation in BKbeta1−/− mice. Arterioscler Thromb Vasc Biol 26:1753–1759 doi: 10.1161/01.ATV.0000231511.26860.50 [DOI] [PubMed] [Google Scholar]
- 48.Oldendorf WH, Cornford ME, Brown WJ (1977) The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1:409–417 doi: 10.1002/ana.410010502 [DOI] [PubMed] [Google Scholar]
- 49.Opherk D, Zebe H, Weihe E, Mall G, Durr C, Gravert B, Mehmel HC, Schwarz F, Kubler W (1981) Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms. Circulation 63:817–825 [DOI] [PubMed] [Google Scholar]
- 50.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM (2004) Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res 94:53–59 doi: 10.1161/01.RES.0000109416.56608.64 [DOI] [PubMed] [Google Scholar]
- 51.Paraidathathu T, de Groot H, Kehrer JP (1992) Production of reactive oxygen by mitochondria from normoxic and hypoxic rat heart tissue. Free Radic Biol Med 13:289–297 doi: 10.1016/0891-5849(92)90176-h [DOI] [PubMed] [Google Scholar]
- 52.Patwari P, Lee RT (2007) Thioredoxins, mitochondria, and hypertension. Am J Pathol 170:805–808 doi: 10.2353/ajpath.2007.061243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul RJ (1990) Smooth muscle energetics and theories of cross-bridge regulation. Am J Physiol 258:C369–375 doi: 10.1152/ajpcell.1990.258.2.C369 [DOI] [PubMed] [Google Scholar]
- 54.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C (2006) Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res 99:69–77 doi: 10.1161/01.RES.0000229685.37402.80 [DOI] [PubMed] [Google Scholar]
- 55.Rubanyi GM (1991) Reversal of hypercholesterolemia-induced endothelial dysfunction by L-arginine. Circulaiton 83:1118–1120 [DOI] [PubMed] [Google Scholar]
- 56.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T (2008) Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10:1115–1126 doi: 10.1089/ars.2007.1989 [DOI] [PubMed] [Google Scholar]
- 57.Shokolenko IN, Alexeyev MF, LeDoux SP, Wilson GL (2005) TAT-mediated protein transduction and targeted delivery of fusion proteins into mitochondria of breast cancer cells. DNA Repair (Amst) 4:511–518 doi: 10.1016/j.dnarep.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 58.Sorescu D, Griendling KK (2002) Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail 8:132–140 doi: 10.1111/j.1527-5299.2002.00717.x [DOI] [PubMed] [Google Scholar]
- 59.Suresh K, Servinsky L, Jiang H, Bigham Z, Zaldumbide J, Huetsch JC, Kliment C, Acoba MG, Kirsch BJ, Claypool SM, Le A, Damarla M, Shimoda LA (2019) Regulation of mitochondrial fragmentation in microvascular endothelial cells isolated from the SU5416/hypoxia model of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 317:L639–L652 doi: 10.1152/ajplung.00396.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiefenbacher CP, DeFily DV, Chilian WM (1998) Requisite role of cardiac myocytes in coronary alpha1-adrenergic constriction. Circulation 98:9–12 doi: 10.1161/01.cir.98.1.9 [DOI] [PubMed] [Google Scholar]
- 61.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF (2000) Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192:1731–1744 doi: 10.1084/jem.192.12.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ (2003) Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 361:2017–2023 doi: 10.1016/S0140-6736(03)13637-9 [DOI] [PubMed] [Google Scholar]
- 63.Weinrich TW, Kam JH, Ferrara BT, Thompson EP, Mitrofanis J, Jeffery G (2019) A day in the life of mitochondria reveals shifting workloads. Sci Rep 9:13898 doi: 10.1038/s41598-019-48383-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Youn JY, Wang T, Cai H (2009) An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ Res 104:50–59 doi: 10.1161/CIRCRESAHA.108.178467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zalba G, San Jose G, Moreno MU, Fortuno MA, Fortuno A, Beaumont FJ, Diez J (2001) Oxidative stress in arterial hypertension: role of NAD(P)H oxidase. Hypertension 38:1395–1399 doi: 10.1161/hy1201.099611 [DOI] [PubMed] [Google Scholar]
- 66.Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM (2006) TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 26:475–480 doi: 10.1161/01.ATV.0000201932.32678.7e [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T Jr., JY (2006) AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol 26:1281–1287 doi: 10.1161/01.ATV.0000221230.08596.98 [DOI] [PubMed] [Google Scholar]
- 68.Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y (2018) NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol 113:23 doi: 10.1007/s00395-018-0682-1 [DOI] [PubMed] [Google Scholar]
- 69.Zippel N, Loot AE, Stingl H, Randriamboavonjy V, Fleming I, Fisslthaler B (2018) Endothelial AMP-Activated Kinase alpha1 Phosphorylates eNOS on Thr495 and Decreases Endothelial NO Formation. Int J Mol Sci 19 doi: 10.3390/ijms19092753 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.