Abstract
Background
This is an updated version of the original Cochrane review published in Issue 1, 2008.
Narcolepsy is a disorder of the central nervous system, the main symptoms of which are excessive daytime sleepiness (EDS) and cataplexy (an abrupt and reversible decrease in or loss of muscle tone, affecting the limbs or trunk or both, elicited by emotional stimuli). Narcolepsy has an adverse impact on people's quality of life. Together with stimulant drugs (used to control EDS), antidepressants are usually recommended to counteract cataplexy. In addition, some antidepressants are also reported to improve EDS.
Objectives
To evaluate the effects of antidepressant drugs on EDS, cataplexy, quality of life, and their side effects in people with narcolepsy.
Search methods
We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library February issue, 2010), MEDLINE (1950 to March 2010), EMBASE (1980 to April 2010), PsycINFO (1872 to March 2010), and CINAHL (1981 to March 2010). Bibliographies of identified articles were reviewed to find additional references. Unpublished randomised trials were searched for by consulting governmental and non‐governmental clinical trial registers, disease‐specific websites, investigators and experts in the field, pharmaceutical companies/manufacturers.
Selection criteria
Parallel or cross‐over randomised or quasi‐randomised controlled trials testing the treatment of narcolepsy with any type of antidepressant drug versus no treatment, placebo, or another antidepressant drug.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data.
Main results
Three cross‐over and two parallel trials were included with a total of 246 participants. The methodological quality of all studies was unclear. As the trials tested different comparisons, or had a different design or dealt with different outcome measures, meta‐analysis was not performed. In one cross‐over trial (10 participants) femoxetine had no significant effect in eliminating or reducing EDS but significantly reduced cataplexy. Mild and transient side effects were reported in the femoxetine treatment period by two participants. In a second cross‐over trial (56 participants) viloxazine significantly reduced EDS and cataplexy. In a third cross‐over trial the authors inappropriately treated the trial design as a parallel study and no conclusions can be reached in favour of either drug. Two more trials with parallel design tested ritanserin versus placebo without finding differences of effectiveness in reducing EDS or cataplexy.
Authors' conclusions
Since the last version of this review no new studies were found.
There was no good quality evidence that antidepressants are effective for narcolepsy or improve quality of life. Despite the clinical consensus recommending antidepressants for cataplexy there is scarce evidence that antidepressants have a positive effect on this symptom. There is a clear need for well‐designed randomised controlled trials to assess the effect of antidepressants on narcolepsy.
Keywords: Humans, Antidepressive Agents, Antidepressive Agents/therapeutic use, Cataplexy, Cataplexy/drug therapy, Clomipramine, Clomipramine/therapeutic use, Fluvoxamine, Fluvoxamine/therapeutic use, Narcolepsy, Narcolepsy/drug therapy, Piperidines, Piperidines/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Antidepressant drugs for narcolepsy
Narcolepsy is a disorder of the central nervous system, the main symptoms of which are excessive daytime sleepiness (EDS) and cataplexy (an abrupt and reversible decrease in or loss of muscle tone, affecting the limbs and/or trunk, elicited by emotional stimuli). Narcolepsy has an adverse impact on people's quality of life. Together with stimulant drugs (used to control EDS), antidepressants are usually recommended to counteract cataplexy. In addition, some antidepressants are also reported to improve EDS. Five trials with 246 participants were included. There is no evidence that antidepressants have a beneficial effect on narcolepsy. Moreover, despite the clinical consensus recommending their use for cataplexy, there is scarce evidence to support the use of antidepressant drugs to treat this symptom.
Background
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews (Issue 1, 2008) on 'Antidepressant drugs for narcolepsy'.
Narcolepsy is a lifelong disorder of the central nervous system characterized by uncontrollable daytime sleepiness (also called "excessive daytime sleepiness") and intermittent abnormal manifestations of rapid eye movement (REM) sleep during wake or sleep‐wake transition, of which cataplexy is the most prominent. Cataplexy causes an abrupt and transient decrease or loss of muscle tone, affecting the limbs bilaterally, or the trunk or both, and is elicited by emotional stimuli (Guilleminault 2000). Estimates of the prevalence of narcolepsy range between the extremes of 0.23 and 590 cases per 100,000 (Israel and Japan respectively); in Caucasian ethnic groups there are about 20 to 60 cases per 100,000 (Partinen 2000).
Narcolepsy is a disorder of brain mechanisms governing the sleep‐wake cycle. The pathogenic cause is purported to be the dysfunction or destruction of a group of hypothalamic neurones transmitting the neurotransmitter hypocretin (also called orexin) (Mignot 2000). An autoimmune mechanism of damage has been hypothesized (Dauvilliers 2007; Mignot 2001).
The age at onset is between early childhood and mid adulthood; the peak is in the second decade (Guilleminault 2000; Silber 2002). The age at diagnosis has a mean delay of more than 10 years because of the frequent misdiagnosis with other neurological or psychiatric conditions (Dauvilliers 2001). The course is usually stable, sometimes with remissions or exacerbations. The main symptom, always present, is excessive daytime sleepiness, principally occurring as daily episodes of unwanted sleep. Other manifestations are the abnormal appearance of REM sleep features during the wake or sleep‐wake transition states. This includes cataplexy, sleep paralysis (inability to move during the transition from sleep to wakefulness), and hypnic hallucinations (hallucinations that occur during sleep‐wake transition). Disturbed nocturnal sleep and other sleep symptoms are also reported to affect people with narcolepsy.
Previous international diagnostic criteria (AASM 2001) deems the combination of excessive daytime sleepiness and cataplexy (narcolepsy with cataplexy subtype: about 80% of cases) as sufficient for a definite diagnosis. Current international diagnostic criteria (AASM 2005) states that together with the above reported two symptoms diagnosis "should be confirmed" by multiple sleep latency test (mean sleep latency less than or equal to eight minutes and two or more more sleep‐onset REM periods) or alternatively by hypocretin‐1 levels in cerebrospinal fluid (less than or equal to 110 pg/mL). When excessive daytime sleepiness is present without cataplexy (narcolepsy without cataplexy subtype) the diagnosis, according to previous international diagnostic criteria (AASM 2001), is confirmed if there are specific findings during nocturnal polysomnography (sleep latency less than 10 minutes and REM sleep latency less than 20 minutes) and multiple sleep latency test (mean sleep latency less than five minutes and two or more sleep‐onset REM periods). According to current international diagnostic criteria (AASM 2005) the diagnosis of narcolepsy without cataplexy is confirmed only by multiple sleep latency tests (mean sleep latency less than eight minutes and two or more sleep‐onset REM periods).
There is evidence that symptoms of narcolepsy may adversely affect people's quality of life, having a major impact upon education, recreation and occupation, with impaired learning, work performance, promotion and earnings (Beusterien 1999; Broughton 1981; Broughton 1983; Daniels 2001; Ervik 2006; Goswami 1998; Kales 1982; Teixeira 2004; Vignatelli 2004). Moreover, life‐threatening accidents may occur, for instance while driving (Findley 1995; George 1996). The most disabling symptom is excessive daytime sleepiness, whilst cataplexy is disabling for a subgroup of people with narcolepsy (Vignatelli 2004).
The goal of therapy for narcolepsy is to control the symptoms underlying the disability. For excessive daytime sleepiness The Standards of Practice Committee of the American Sleep Disorders Association (Littner 2001) recommend stimulant drugs such as amphetamine, dextroamphetamine, methamphetamine, methylphenidate, pemoline, which are indirect‐acting sympathomimetic drugs, or the use of stimulants with other modes of action which include modafinil, selegiline, caffeine and gamma‐hydroxybutyric acid (Guilleminault 2000; Mitler 1994; Nishino 2000). Their effect on cataplexy is reported to be absent or uncertain. The use of these drugs may be limited in some countries by legislation designed to counteract drug abuse (amphetamine and derivatives, gamma‐hydroxybutyric acid), as well as by drug licensing, availability and cost.
Recommended first‐line treatments for cataplexy include antidepressant drugs like tricyclic or serotonin selective re‐uptake inhibitors (Littner 2001), but other antidepressants (monoamine oxidase inhibitors type A (Hohagen 1993) or viloxazine (Mitler 1990) or reboxetine (Larrosa 2001)) have also been proposed as effective. Some of these drugs (Hohagen 1993; Larrosa 2001; Mitler 1990; Schrader 1986) are reported to be useful for excessive daytime sleepiness and some reports indicate that a significant minority of people with narcolepsy are treated with antidepressants alone (about 10% in the UK (Daniels 2001), and about 40% in Italy (Vignatelli 2004)).
Although the antidepressants have differing modes of action, the rationale for their use is that they all share an effect on the inhibition of uptake or the catabolism of monoaminergic neurotransmitters (Martindale 1996), thereby counteracting the imbalance of monoamines in the wake‐sleep cycle network of the brain, which is thought to be one of the neurochemical substrates of narcolepsy.
This review examines the effectiveness of antidepressant drugs in narcolepsy for reducing both excessive daytime sleepiness and cataplexy.
Objectives
Our objective was to determine the effectiveness and maintenance of effect of antidepressant drugs in the treatment of narcolepsy. The following hypotheses were tested. (1) Antidepressant drugs are more effective than no treatment or treatment with placebo in: (a) abolishing or reducing the occurrence of excessive daytime sleepiness; (b) abolishing or reducing the occurrence of cataplexy; (c) improving the quality of life.
(2) One class of antidepressants (non‐selective monoamine re‐uptake inhibitors versus serotonin selective re‐uptake inhibitors versus monoamine oxidase inhibitors type A versus other antidepressants) or one antidepressant drug is better than another. (3) The side effects do not exceed the beneficial effects.
Methods
Criteria for considering studies for this review
Types of studies
Parallel or cross‐over randomised or quasi‐randomised controlled trials testing the treatment of narcolepsy with any type of antidepressant drug versus no treatment, placebo, or another antidepressant drug.
Types of participants
Adults (18 years or more) with narcolepsy with or without cataplexy and with any other co‐morbidity or co‐treatment. Explicit diagnostic criteria of narcolepsy were not required for inclusion in the study; however, if found, trials referring to international standard criteria (International Classification of Sleep Disorders 1990 and following editions (AASM 2001; AASM 2005)) were to be distinguished from those without explicit standard diagnostic criteria by performing a sensitivity analysis.
Types of interventions
Therapy with antidepressant drugs (any dose, any regimen) was the intervention of interest. According to the international Anatomical Therapeutic Chemical (ATC) classification of drugs (www.whocc.no/atcddd/atcsystem.html), antidepressants are divided into the following chemical subgroups (fourth level): (1) non‐selective monoamine re‐uptake inhibitors (tricyclics); (2) serotonin selective re‐uptake inhibitors; (3) monoamine oxidase inhibitors type A; (4) other antidepressants.
Types of outcome measures
In order to evaluate the maintenance of the effect, if data were available, we planned to assess outcomes at the following time points post‐randomisation: (a) less than or equal to 30 days; (b) more than 30 days. Primary outcomes considered in this review were: (a) elimination of excessive daytime sleepiness according to subjective or objective indicators at long‐term follow up (see below); (b) elimination of cataplexy at long‐term follow up (see below).
All other possible outcomes were considered as secondary end points.
The outcomes were considered at the latest point of recording during the trial. If possible, the trial was classified according to outcomes: (a) short‐term outcome if recorded within 30 days of follow up; (b) long‐term outcome if recorded after 30 days of follow up; (c) both short and long‐term outcomes if data were available.
The pre‐specified outcomes belonged to the following domains:
(1) Excessive daytime sleepiness According to the recognized different domains of excessive daytime sleepiness, it was assessed, if present, by: (a) objective laboratory tests: (i) multiple sleep latency test (MSLT) (Thorpy 1992 ) measuring the mean time latency of falling asleep during four to five sessions trying to sleep; (ii) maintenance wakefulness test (MWT) (Doghramji 1997) measuring the mean time latency of falling asleep during four to five sessions trying to stay awake. (b) subjective validated scales, every one investigating chronic excessive daytime sleepiness during common life situations (Weaver 2001): (i) Epworth sleepiness scale (ESS) (Johns 1998); (ii) sleep‐wake activity inventory (SWAI) (Rosenthal 1993).
If present, the following outcomes were considered. (a) Elimination of excessive daytime sleepiness according to standard cut offs: (i) more than five minutes of mean time sleep latency for MSLT (Thorpy 1992); (ii) more than 11 minutes of mean time sleep latency for MWT (Doghramji 1997); (iii) fewer than 11 points for the ESS (Johns 1998); (iv) fewer than 11 points for the SWAI (Johnson 1999).
If available, the outcomes were measured and analysed as dichotomous variables (number of participants free from the symptom for each treatment group) at the longest common follow up. A separate analysis was considered for every objective and subjective test. (b) Reduction of excessive daytime sleepiness, measured as: (i) the comparison of mean values (in minutes) of laboratory tests between treatment groups at the longest common outcome follow up. A separate analysis was considered for MSLT and MWT; (ii) the comparison of mean ordinal scale values between treatment groups at the longest common outcome follow up. The standardized mean difference was considered for the two excessive daytime sleepiness scales.
(2) Cataplexy It was assessed by: (a) simple subjective reporting (only for abolition or more than 50% reduction, see below) ; (b) subjective validated scales or questionnaires (Stanford Cataplexy Questionnaire (Anic‐Labat 1999), the Postural Atonia Scale (Parkes 1998), the cataplexy domain of Ullanlinna Narcolepsy Scale (Hublin 1994)).
The following outcomes were considered: (i) Elimination of cataplexy measured and analysed as a dichotomous variable (number of participants free from the symptom for each treatment group) according to simple subjective reporting or scales at the latest common outcome follow up. (ii) Reduction of cataplexy by more than 50% with respect to baseline according to simple subjective reporting or scales, measured and analysed as a dichotomous variable (number of participants with reduction of the symptom by more than 50% for each treatment group), at the latest common outcome follow up. (iii) Reduction of cataplexy measured with the comparison of mean values of subjective ordinal scales between treatment groups at the latest common outcome follow up. The standardized mean difference was considered if more than one scale was found in the elected studies.
(3) Quality of life If assessed by validated general (i.e. Medical Outcome Study Short Form (SF36); Nottingham Health Profile (NHP); Sickness Impact Profile scale (SIP) etc) or sleep specific (Functional Outcome Sleep Questionnaire (Weaver 1997)) scales/questionnaires, the following outcome was considered: improvement of quality of life measured with the comparison of mean values of ordinal scales between treatment groups, at the latest common outcome follow up. A separate analysis was planned for each scale.
(4) Adverse events Number of adverse events, by type of side effect, collected by treatment allocation.
(5) Drop‐outs from treatment groups Number of drop‐outs measured as the number of participants suspending therapy: (a) for any reason, and (b) for adverse treatment events, and analysed as dichotomous variables.
Search methods for identification of studies
(1) We identified published randomised trials from the following sources: (a) Cochrane Central Register of Controlled Trials (CENTRAL); terms focused only on participants/condition (see Appendix 1 for details of this search). We performed our first search in The Cochrane Library Issue 3, 2003; a second one was updated in The Cochrane Library Issue 2, 2007. A further search was carried out using the February 2010 issue of The Cochrane Library.
(b) National Library of Medicine's MEDLINE database (from 1950): terms focused on participants/condition (the same as above) combined with the highly sensitive search strategy for identifying randomised controlled trials (Lefebvre 2001; Lefebvre 2009), without any other restriction. A first search was performed in 2003; a second one in April 2007. A further search was carried out on 10 March 2010 (details of the search strategy used on this occasion are given in Appendix 2).
(c) Elsevier's EMBASE (from 1980): terms focused on participants/condition (the same as above) combined with the highly sensitive search strategy for identifying randomised controlled trials (Lefebvre 2001), without any other restriction. A first search was performed in 2003; a second one in April 2007. A further search was carried out on 7 April 2010 (details of the search strategy used on this occasion are given in Appendix 4).
(d) PsycINFO (from 1872) and CINAHL databases (from 1981): terms focused on participants/condition only (the same as above). A first search was performed in 2003; a second one in April 2007. A further search was carried out on 10 March 2010 (see Appendix 3 for details of the search strategy used on that occasion).
(e) Checking reference lists: bibliographies of identified articles were reviewed to find additional references.
(f) 'Grey' literature including dissertations, conference proceedings and government reports were looked for by handsearching in specialized text books and sleep journals (Sleep; Journal of Sleep Research; Sleep Medicine Reviews; Sleep Medicine; Sleep and Hypnosis).
(2) We searched for unpublished randomised trials by: (a) consulting governmental and non‐governmental clinical trial registers; (b) consulting disease‐specific websites; (c) asking investigators and experts in the field; (d) asking pharmaceutical companies/manufacturers of antidepressants.
Data collection and analysis
Two review authors (RD, LV) independently reviewed all references with an abstract or full papers without an electronic abstract to assess potentially relevant randomised controlled trials and quasi‐randomised controlled trials. Disagreement was resolved by discussion. Subsequently from the full text, two review authors (LC, RD) independently assessed studies for inclusion. There was no blinding of authorship or results. Disagreement was resolved by discussion.
(1) Assessment of methodological quality We assessed study quality according section 6.7.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005) and Jüni 2001.
Selection bias The studies were assessed as to whether the generation of random sequence was: (a) adequate (computer‐generated random numbers; table of random‐numbers; drawing lots or envelopes; coin tossing; shuffling cards; throwing dice); (b) inadequate (case record number; date of birth; date of admission; alternation) or; (c) unclear (when generation was not clearly described).
The studies were assessed as to whether the allocation concealment was: (a) adequate (a priori numbered or coded drug containers prepared by an independent pharmacy; central randomisation; sequentially numbered sealed opaque envelopes); (b) inadequate (procedures different from the above; open allocation schedule; unsealed or non‐opaque envelopes) or; (c) unclear (when concealment sequences not clearly described). Performance bias The studies were assessed as to whether: (a) recipients, and (b) those providing care apart from the intervention were unaware of the assigned therapy; and, if declared, whether the procedure was adequate (if placebo explicitly described as 'indistinguishable', absence of revealing side‐effects), inadequate or unclear. Detection bias The studies were assessed as to whether those measuring outcomes were unaware of the assigned therapy (blinding); and, if declared, whether blinding was adequate, inadequate or unclear. In particular, in the case of the objective tests MSLT and MWT, blinding was considered adequate if assessment was made by an independent person or panel. To investigate the above criteria as sources of bias, a sensitivity analysis considering the category of 'adequate' versus 'inadequate or unclear' for each bias, was pre‐planned. Attrition bias Was documented as relative frequency of losses to follow up.
Use of diagnostic criteria The use or not of standard diagnostic criteria for the diagnosis narcolepsy was also used as a quality indicator and, if data were available would have been used in sensitivity analyses.
(2) Data extraction Two review authors (Luca Vignatelli and Roberto D'Alessandro) independently extracted the following data, when available, from included studies. Disagreement was resolved by discussion. (a) Data on participants at baseline (characteristics of the population that may affect the outcome): age, severity of excessive daytime sleepiness according to laboratory findings and to subjective scales, presence or absence of cataplexy and its severity according to scales, co‐morbidity (obstructive sleep apnea syndrome), co‐treatments (stimulant drugs). (b) Data on interventions: type of pharmaceutical antidepressant agent and class; dosage (mg per day); regimen; treatment length according to previously defined criteria. (c) Data on outcome measures following the definitions in the outcome section.
(3) Analysis plan Dichotomous data: the number of people who experienced the outcome in each group and the total number in each group were to be analysed using the Mantel‐Haenszel fixed‐effect model for risk ratios. Heterogeneity amongst the results of trials was to be assessed by calculating a test of heterogeneity (chi‐squared test). If heterogeneity was suggested, analyses using the DerSimonian and Laird random‐effects model were planned.
Continuous data: analyses using mean differences (for the comparison of mean values of MSLT, MWT, single cataplexy scales) or standardized mean differences (for the comparison of mean values of subjective excessive daytime sleepiness according to scales) were pre‐planned to estimate effect sizes using the inverse variance fixed‐effect model. Heterogeneity amongst the results of trials was to be assessed by using a test of heterogeneity (chi‐squared test). If heterogeneity was suggested, analyses using the DerSimonian & Laird random‐effects model were planned.
If cross‐over trials were found, we planned to analyse the data separately from parallel trials. In order to include data from cross‐over trials, we required data for all treatment periods and treatment groups regarding the number of allocated participants, exclusions and losses to follow up (Elbourne 2002). If a carry‐over effect as evaluated on a clinical basis was suspected and data reported by period, then the analysis was to be restricted to data from the first treatment period.
The analysis was going to be conducted separately for each outcome and stratified for each antidepressant class and single type of drug. Possible sources of heterogeneity between trials were going to be explored with the following subgroup analyses: co‐morbidity (participants with or without obstructive sleep apnea syndrome), co‐treatment with stimulant drugs, level of baseline severity of excessive daytime sleepiness or cataplexy or both.
Results
Description of studies
The first search strategy (2003) identified 153 reports, four of them selected by consensus (Luca Vignatelli and Roberto D'Alessandro) as potentially eligible for this review. After assessing the full text of articles (Luca Vignatelli and Roberto D'Alessandro), one trial (Mitler 1986) was excluded for the reasons summarised in the table 'Characteristics of excluded studies'. Information of one further trial (Mitler 1990) has been requested and obtained by two authors (Prof. Guilleminault and Prof. Mitler) for this updated version of the review. On the basis of published (Mitler 1990) and unpublished information this trial was included. However even if basic information was provided, many other data were reported to be lost.
The updated search (April 2007) identified, with possible duplications, 108 reports from CENTRAL, 68 from MEDLINE, 339 from EMBASE, 200 from PsycINFO, 87 from CINAHL. After selection, five studies were potentially eligible for this review. After assessing the full text of articles, three studies (Henry 1988; Smith 1996; Sonka 2006) were excluded for the reasons summarised in the table 'Characteristics of excluded studies'.
The updated search (March and April 2010) identified, with possible duplications, 16 reports from CENTRAL, 42 from MEDLINE, 387 from EMBASE, 295 from PsycINFO and CINAHL. After selection, 12 studies were potentially eligible for this review. After assessing the full text of articles, all studies (Anonymous 2007; Black 2006; Black 2009; Harsh 2006; Izzi 2009; Joo 2008; Lin 2008; Moller 2009; Ristanovic 2009; Saletu 2009; Weaver 2006; Xyrem 2005) were excluded for the reasons summarised in the table 'Characteristics of excluded studies'.
Therefore, a total of five trials (Lammers 1991; Mayer 2003; Mitler 1990; Schachter 1980; Schrader 1986) were eligible for this review.
One study (Schrader 1986) was a cross‐over trial and recruited 10 adults with narcolepsy, all of whom had cataplexy. The intervention tested was femoxetine, a serotonin selective re‐uptake inhibitor, at a dose of 300 mg twice daily, versus placebo. Each participant was randomly allocated first to one treatment then to the other. There was a run‐in period of seven days; each treatment period was of 28 days duration with a 14 day wash‐out period.
A second study (Mitler 1990) was a cross‐over trial that recruited 56 adults with narcolepsy, all of whom had cataplexy. The interventions tested were viloxazine, other antidepressants ATC system level group, at dose of 100 mg per day, versus placebo. From unpublished data, each participant was randomly allocated first to one treatment then to the other. There was a run‐in period of 15 days; each treatment period was of six weeks duration. It is not known if a washout period was applied.
A third study (Schachter 1980) was a cross‐over trial that recruited 18 adults with narcolepsy, all of whom had cataplexy. The interventions tested were fluvoxamine, a serotonin selective re‐uptake inhibitor, at doses between 25 mg and 200 mg per day, versus clomipramine, a non selective monoamine re‐uptake inhibitor, at doses between 25 mg and 200 mg per day. Each participant was randomly allocated first to one treatment then to the other. There was no run‐in period and each treatment period was of 21 days duration with a seven day wash‐out period. Two other trials (Lammers 1991; Mayer 2003) both tested ritanserin, a 5‐HT2 receptor blocker, previously used for some antidepressant action (Wiesbeck 2000). Even if this molecule is classified under no antidepressant category by ATC, we considered these studies eligible for this review as no other class clearly fit to this molecule.
The first study (Lammers 1991) was a parallel trial that recruited 28 adults with narcolepsy. The intervention drug was ritanserin at 2.5 mg bidaily versus placebo as add‐on treatment (psychostimulants and/or other antidepressants and/or gamma‐OH‐butyrate and/or other drugs). Each participant was randomly allocated to ritanserin (16 participants) or placebo (12 participants). After an initial baseline week without medication, the trial lasted 28 days.
The other study (Mayer 2003) was a parallel multi‐centre trial that recruited 134 adults with narcolepsy, with or without cataplexy. The interventions tested were ritanserin, 5 mg once daily (orally), or ritanserin, 10 mg once daily (orally), or placebo, as add‐on treatment (psychostimulants and/or other antidepressants and/or gamma‐OH‐butyrate and/or other drugs were permitted) or as first treatment. Forty‐six participants were randomly allocated to ritanserin 5 mg daily, 45 participants to ritanserin 10 mg daily, 43 participants to placebo. After two weeks of baseline without medication, the trial lasted 28 days.
Risk of bias in included studies
According to predefined criteria, the methodological quality of all studies was in general poor (Figure 1, Figure 2).
1.
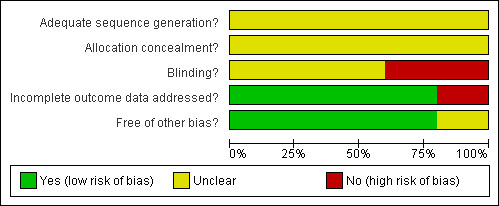
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
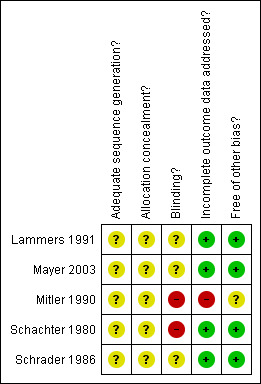
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
In the study of Schrader 1986, both the method of generation of the random list and the method of allocation concealment were unclear. The methods of blinding participants, carers and the procedure of assessment of the outcomes were unclear. All participants completed the two treatment periods. Diagnosis was made on presence of excessive daytime sleepiness and cataplexy without reference to international diagnostic criteria. Cataplexy was defined as "unequivocal attacks of emotionally precipitated cataplexy".
In the study of Mitler 1990, both the method of generation of the random list and the method of allocation concealment were unclear. The methods of blinding participants and the procedure of assessment of the outcomes were unclear. Carers were unblinded. It is not known how many participants completed the two treatment periods. Twenty participants were lost to follow up. Diagnosis was made on presence of history of excessive daytime sleepiness, presence of at least one of the REM sleep‐related symptoms (cataplexy, sleep paralysis, hypnagogic hallucinations), and two or more sleep‐onset REM periods at multiple sleep latency test, without reference to international diagnostic criteria.
In the study of Schachter 1980, the method of both the generation of the random list and the method of allocation concealment were unclear. The method of blinding participants to their assigned therapy was inadequate, and the method for blinding carers and the procedure of assessment of the outcomes were unclear. Six participants did not complete the fluvoxamine treatment period because of side effects. For one participant, data on cataplexy outcomes were missing. Reported analyses were per protocol rather than intention to treat. Diagnosis was made on presence of excessive daytime sleepiness and cataplexy without reference to international diagnostic criteria.
In the study of Lammers 1991, both the method of generation of the random list and of allocation concealment were unclear. The methods of blinding participants, carers and the procedure of assessment of the outcomes were unclear. All participants completed the study, but only 10 participants in the ritanserin group and 10 participants in the placebo group performed the MSLT. Diagnosis was made on the presence of "typical excessive daytime sleepiness with sleep attacks and at least one of the accessory symptoms (cataplexy, hypnagogic hallucinations or sleep paralysis)" without reference to international diagnostic criteria. Cataplexy was not defined.
In the study of Mayer 2003, both the methodology for generating the random list and for allocation concealment were unclear. The methods of blinding participants, carers and the procedure of assessment of the outcomes were unclear. Twelve out of 134 participants dropped out before trial completion: six from the ritanserin 5 mg group and six from the ritanserin 10 mg group; eight due to adverse events. All randomised participants were included in the analysis on an intention‐to‐treat basis. Diagnosis was made according to the 1990 International Classification of Sleep Disorders.
Effects of interventions
We did not perform the planned meta‐analysis as the trials had different designs or dealt with different outcome measures or different comparisons. We report the available data for each study according to our predefined outcomes. (1) Studies comparing antidepressants to placebo: The cross‐over study of Schrader 1986 tested femoxetine (used for a period of 28 days) versus placebo (another period of 28 days). The following outcomes were considered.
(a) Elimination of excessive daytime sleepiness according to objective laboratory tests at the short‐term follow up. Mean sleep latency from the MSLT remained at a pathological level, i.e. under five minutes, for all participants during both treatment periods.
(b) Reduction of cataplexy measured by comparison of mean values of subjective ordinal scales between treatment groups at the short‐term follow up. The number of cataplectic attacks per day was reduced (within‐patient differences in frequency: mean 1.29, standard deviation (SD) 1.59; paired t test 0.03; individual data from graph, according to the method of Elbourne 2002). The severity score per day was not reduced (within‐patient differences in score: mean 1.45, SD 1.70; paired t test 0.21; individual data from graph, according to the method of Elbourne 2002).
(c) Adverse events. Mild and transient nausea was reported in the femoxetine period by two participants. No problems with erection or ejaculation during sexual intercourse were reported by the six male participants.
(d) Drop‐outs from treatment groups. No participants suspended the therapy.
The cross‐over study of Mitler 1990 tested viloxazine (used for a period of six weeks) versus placebo (another period of six weeks). The following outcomes were considered.
(a) Reduction of excessive daytime sleepiness according to objective laboratory tests at the long‐term follow up. Mean sleep latency from the MWT was 8.39 minutes (standard deviation not reported) during the viloxazine period and 7.09 minutes (standard deviation not reported) during the placebo period. The difference, in favour of viloxazine, was reported to be statistically significant (P value = 0.0326). Mean sleep latency from the MSLT was 3.33 minutes (standard deviation not reported) during the viloxazine period and 2.88 minutes (standard deviation not reported) during the placebo period. The difference, in favour of viloxazine, was reported to be not statistically significant (P value = 0.0796).
(b) Reduction of cataplexy measured by comparison of mean values of subjective ordinal scales between treatment groups at the long‐term follow up. The frequency of cataplectic attacks, according to an ordinal scale (range of values 0‐9; 0 corresponds to never, 1 to <1 attack per week, 2 to 1‐3 attacks per week, 3 to 4‐6 attacks per week, 4 to 1 attack per day, 5 to 2‐3 attacks per day, 6 to 4‐6 attacks per day, 7 to 7‐10 attacks per day, 8 to 11‐15 attacks per day, 9 >15 attacks per day), was 1.77 (standard deviation not reported) during the viloxazine period and 2.94 (standard deviation not reported) during the placebo period. The difference, in favour of viloxazine, was reported to be statistically significant (P value = 0.0001).
The parallel study of Lammers 1991 tested ritanserin versus placebo, for a period of 28 days. The following outcomes were considered. (a) Reduction of excessive daytime sleepiness according to objective laboratory tests at the short‐term follow up. Mean sleep latency from the MSLT was reported to be not statistically different between the two groups at 28 days of follow‐up (data reported only in figure without numbers).
(b) Reduction of cataplexy measured by comparison of mean values of subjective ordinal scales between treatment groups at the short‐term follow up. The number of cataplectic attacks per day was reported to be not statistically different between the two groups at 28 days of follow‐up (data reported only in figure without numbers).
(c) Adverse events. Four participants in the ritanserin group and three participants in the placebo group were reported to have some kind of not specified adverse events.
(d) Drop‐outs from treatment groups. No participants suspended the therapy.
The parallel study of Mayer 2003 tested ritanserin 5 mg versus ritanserin 10 mg versus placebo, for a period of 28 days. The following outcomes were considered. (a) Reduction of cataplexy measured by comparison of mean values of subjective ordinal scales between treatment groups at the short‐term follow up. The number of cataplectic attacks per day was reported to be not statistically different between the two ritanserin groups and the placebo group at 28 days of follow‐up, comparing the ratios of the area under the curve at the end of follow up and at the baseline.
(b) Adverse events. Generic "disorders of the central nervous and gastrointestinal system and psychiatric disorders" were reported to occur, without specifying possible differences between groups.
(c) Drop‐outs from treatment groups. Six participants from the ritanserin 5 mg group and six participants from the ritanserin 10 mg group dropped out before trial completion, eight out of twelve because of adverse events (constipation, confusion, somnolence, bronchospasm, dry mouth, tongue paralysis).
(2) Studies comparing antidepressants The cross‐over study of Schachter 1980 tested fluvoxamine (used for a period of 21 days) versus clomipramine (for another period of 21 days). No objective laboratory test or subjective validated tools were used in the trial to evaluate excessive daytime sleepiness. The eligible outcomes were the following: elimination of cataplexy, reduction of cataplexy by more than 50%, adverse events, drop‐outs. However, no raw data were available in the study report on individual participants for these outcomes. Also, in the analysis the authors inappropriately used methods for a parallel rather than cross‐over design. Here we report only the published raw data for the cited outcomes.
(a) Elimination of cataplexy according to simple subjective report, at the short‐term follow up. Three participants out of 11 were free from attacks in the fluvoxamine period versus 4 participants out of 11 in the clomipramine period.
(b) Reduction of cataplexy by more than 50% according to simple subjective report, at the short‐term follow up. Five participants out of 11 had reduction of cataplexy in the fluvoxamine period versus 7 participants out of 11 in the clomipramine period.
(c) Adverse events. Number of adverse events, by type of event, are shown by treatment allocation in Table 1. (d) Drop‐outs from treatment groups. Six participants out of 18 suspended the treatment during the fluvoxamine period compared to zero participants out of 18 during the clomipramine period: five for severe gastrointestinal side‐effects (belching, nausea, abdominal discomfort) from day 2 to day 14, at 25 mg to 50 mg daily dosage; 1 for continuous headache on day 7, at 50 mg daily dosage.
1. Schachter 1980: adverse events.
| Adverse event | Fluvoxamine | Clomipramine |
| Nausea or "indigestion" | 6 participants | no participants |
| Weight gain | 1 participant | 1 participant |
| Headache | 2 participants | no participants |
| Dry mouth | no participants | 2 participants |
| Delayed ejaculation | no participants | 2 participants |
Discussion
There is still no reliable evidence to support the use of antidepressants to treat the main symptoms of narcolepsy (excessive daytime sleepiness and cataplexy). We found three cross‐over trials and two parallel trials. We did not combine the cross‐over trials for a meta‐analysis as they addressed different clinical questions or dealt with different outcome measures. Similarly we did not combine the two parallel trials as they dealt with different outcome measures.
In Schrader 1986 it was observed that the SSRI antidepressant femoxetine reduced the frequency of cataplexy; no effect was observed on excessive daytime sleepiness. In Mitler 1990 viloxazine was reported to improve both excessive daytime sleepiness and cataplexy. But the measure of effect seems to be of doubtful clinical significance. Moreover the result is burdened by a possible attrition bias. Schachter 1980, assuming 'a priori' the effectiveness of antidepressants in narcolepsy, compared a tricyclic antidepressant (clomipramine) to a SSRI antidepressant (fluvoxamine). The authors concluded that there was no difference in the effectiveness of the two drugs. However, they inappropriately treated the trial design as a parallel study and a calculation of the power of the study was not performed. As a result no conclusion can be reached either for equivalence of effectiveness or non‐effectiveness of the two drugs. In this updated version of the review we also included two studies that used ritanserin, a molecule not classified in a clear neuropharmacological category. Nevertheless, we chose to consider these trials as eligible because, among others, ritanserin was also reported to have an antidepressant action (Wiesbeck 2000). Neither of these trials found any effect on excessive daytime sleepiness or cataplexy with respect to the placebo. Moreover, some concerns about tolerability were raised by one of them.
All studies shared the same major weak points. The first was poor reporting of methodological procedures of the trials, with the consequent unclear methodological quality. So, we cannot exclude the possibility of strong influence by common biases. Second, in four out of five trials the duration of treatment was less than 30 days so, by our pre‐defined classification, all the outcomes were secondary. Third, given the pharmacological characteristics of antidepressant molecules, the absence (Schachter 1980) or brevity (seven days in Schrader 1986) of the run‐in period may introduce another confounder about the effectiveness or ineffectiveness of these drugs. Fourth, in Mitler 1990 a single blind design was applied, and there was a high attrition rate. Finally, in Schachter 1980, the tools used for the measurement of excessive daytime sleepiness were not validated, and the wash‐out duration of seven days was probably too short given the half‐life of the drugs.
According to the definition of the EC Regulation on Orphan Medical Products (EURORDIS 2007), narcolepsy is a "rare disease" that, together with other rare disorders, shares the peculiarity of being an "orphan disease" because it is an "orphan" of market interest. In this review we found some examples on how the antidepressant class shows this "orphan" feature, according to the definitions by the Ophanet group (Orpha 2007). First, we observed that because the hallmark anti‐cataplectic tryciclic antidepressants derive from a research process that cannot be patented, they were never tested against placebo with randomised controlled trials. Second, narcolepsy has such a small potential market that drug companies are not interested in embarking on randomised controlled trials testing selective serotonin reuptake inhibitors (SSRI) or atypical antidepressants. Referring to the latter class, the trial testing viloxazine versus placebo (Mitler 1990) enrolled 56 instead of the previously planned 60 participants and lost 20 of them to follow up because the drug company stopped funding (unpublished data, personal communication by one of the authors). Third, some promising molecules were withdrawn from the market. There are two examples in Italy (1) femoxetine which is no longer marketed everywhere in the world, or (2) viloxazine, one of the antidepressants widely used in Italy for narcolepsy up to 2002 (unpublished data from GINSEN ‐ Italian multicentre study on narcolepsy (Vignatelli 2004)), and withdrawn in 2002 from the Italian market by the pharmaceutical company because of the poor therapeutic effect on depression. Fourth, molecules with uncertain neuropharmacological action or doubtful efficacy for their principal indication were tested with low quality randomised controlled trials in narcolepsy to look for some other indication (see the above examples of femoxetine and ritanserin).
Therapy with antidepressants has been recommended to control cataplexy for about 40 years and established by clinical consensus (AASM 2001), basing the evidence mainly on case series (Chen 1995; Frey 1994; Godbout 1986; Guilleminault 1976; Hishikawa 1966; Hohagen 1993; Langdon 1986; Larrosa 2001; Mitler 1986; Mitler 1991; Parkes 1979; Passouant 1972; Roth 1974; Schmidt 1977; Shapiro 1974; Shapiro 1975; Smith 1996; Sonka 2006; Wyatt 1971). Nevertheless the key clinical questions on antidepressants for narcolepsy (class or molecule of choice, duration of the effect, the balance between benefits and harms) remain unresolved. Independent clinical researchers are the only candidates embarking on randomised controlled trials to give these answers, as drug companies will probably never be interested.
Authors' conclusions
Implications for practice.
Since the last version of this review no new studies were found.
The clinician considering the use of antidepressants for people with narcolepsy should bear in mind that: (1) there is no reliable evidence that antidepressant drugs reduce excessive daytime sleepiness; (2) there is scarce evidence to recommend the use of antidepressant drugs to treat cataplexy. However, if people with narcolepsy request a drug for cataplexy, antidepressants could be proposed making the following facts clear: (a) the individual drug or the drug class of choice are not known; (b) the duration of the effect, if any, is not known; (c) their use could be burdened by problems of tolerability.
Implications for research.
Since antidepressant drugs are still recommended for practice by clinical consensus and the available evidence is of poor methodological quality, there is a need for studies adequately exploring their effectiveness in narcolepsy.
In particular, further studies are required which address the following: (1) the effect of antidepressants on excessive daytime sleepiness; (2) the effect of antidepressants on cataplexy; (3) the effect of antidepressants on quality of life; (4) the long‐term effect on the above outcomes; (5) the effect on the above outcomes in comparison with side‐effects; (6) the use of validated objective and/or subjective tools to capture the above outcomes; (7) the application of methodological tools in the design of the trials to avoid more common biases; (8) reporting clearly the methodological procedures of trials; (9) comparing antidepressants of different classes on the above questions.
What's new
| Date | Event | Description |
|---|---|---|
| 28 April 2010 | New search has been performed | Searches updated March 2010; no new studies identified. New risk of bias tables and figures now included. |
| 26 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 14 November 2007 | New citation required but conclusions have not changed | Three new studies have been added. |
| 14 November 2007 | New search has been performed | We re‐ran our searches in April 2007; several new studies were identified as potentially relevant. As a result this review was substantively updated for issue 1/2008 ‐ three new trials were added. |
Acknowledgements
Fiorenzo Albani for advice on pharmacology; Anne Collins for editing the English; Piergiorgio Duca for advice on statistics (protocol phase); Tony Marson for advice on the design of the protocol and on the final draft of the first version of the review; Silvia Muzzi for a search of the references; Catrin Tudur Smith for advice on statistics (review phase); Professor Guilleminault and Professor Mitler for the information on the viloxazine trial.
Appendices
Appendix 1. CENTRAL search strategy
#1 (narcole*)
#2 (cataple*)
#3 (gelineau*)
#4 (anti‐cataple*)
#5 (anticataple*)
#6 MeSH descriptor Narcolepsy explode all trees
#7 MeSH descriptor Cataplexy explode all trees
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
Appendix 2. MEDLINE search strategy
This strategy (for the Ovid version of MEDLINE) was used for the March 2010 update of this review. It is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials published in Lefebvre 2009.
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ti.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. exp animals/ not humans.sh.
10. 8 not 9
11. narcole$.tw.
12. cataple$.tw.
13. gelineau$.tw.
14. anti‐cataple$.tw.
15. anticataple$.tw.
16. exp Narcolepsy/
17. exp Cataplexy/
18. or/11‐17
19. 10 and 18
Appendix 3. PsycINFO and CINAHL search strategy
For the March 2010 update of this review, a joint search was carried out using the EBSCOhost version of both databases.
(MM "Narcolepsy") OR (DE "Narcolepsy") OR (MM "Cataplexy") OR (narcolep* or cataple* or gelineau*) OR (anti‐cataple* or anticataple*)
Appendix 4. EMBASE search strategy
This strategy (for the Ovid version of EMBASE) was used for the April 2010 update of this review. It is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials published in Lefebvre 2001.
narcole* AND [embase]/lim OR (cataple* AND [embase]/lim) OR (gelineau* AND [embase]/lim) OR (anticataple* AND [embase]/lim) OR ('narcolepsy'/exp AND [embase]/lim) OR ('cataplexy'/exp AND [embase]/lim) OR (anti?cataple* AND [embase]/lim) AND (random* OR cross?over* OR factorial* OR placebo* OR volunteer* AND [embase]/lim OR (singl* OR doubl* OR trebl* OR tripl* AND (blind* OR mask*) AND [embase]/lim) OR ('randomized controlled trial'/exp AND [embase]/lim) OR ('randomization'/exp AND [embase]/lim) OR ('controlled study'/exp AND [embase]/lim) OR ('multicenter study'/exp AND [embase]/lim) OR ('phase 3 clinical trial'/exp AND [embase]/lim) OR ('phase 4 clinical trial'/exp AND [embase]/lim) OR ('double blind procedure'/exp AND [embase]/lim) OR ('single blind procedure'/exp AND [embase]/lim AND [2007‐2010]/py))
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lammers 1991.
| Methods | Double‐blind parallel randomised trial versus placebo: pre‐treatment period of 7 days; no run‐in period; treatment period of 28 days. | |
| Participants | 28 adult participants with narcolepsy (gender not known). Mean age 43 years (range 16‐67). Place of the study: The Netherlands (not specified ethnic composition of participants). Inclusion criteria: "typical EDS with sleep attacks and at least one of the accessory symptoms (cataplexy, hypnagogic hallucinations or sleep paralysis)". Exclusion criteria: not reported. Recruitment procedures: not reported. Not reported severity of the disease at inclusion. Pre‐trial treatment: 11 participants with no medication; 12 participants with psychostimulants; 6 participants with antidepressants; 5 participants with GHB; 3 participants with other drugs. | |
| Interventions | 16 participants allocated to ritanserin, dosage 2.5 mg bid. 12 participants allocated to placebo. | |
| Outcomes | Considered in the review and present in the study: (1) Reduction of EDS according to objective laboratory test at the short term follow‐up. (2) Reduction of cataplexy measured by mean values of subjective ordinal scales at the short‐term follow up. (3) Adverse events by treatment groups. (4) Drop‐outs by treatment groups. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants completed the trials; however 6 participants of the ritanserin group and 2 participants of the placebo group did not underwent the MSLT . |
| Free of other bias? | Low risk | Diagnosis was made on the presence of "typical excessive daytime sleepiness with sleep attacks and at least one of the accessory symptoms (cataplexy, hypnagogic hallucinations or sleep paralysis)" without reference to international diagnostic criteria. Cataplexy was not defined. |
Mayer 2003.
| Methods | Double‐blind parallel randomised trial versus placebo: pre‐treatment period of 14 days; no run‐in period; treatment period of 28 days. | |
| Participants | 134 adult participants with narcolepsy (50 women and 84 men). Mean age: 40.9 years (SD 14.2) in the placebo group; 43.2 years (SD 12.5) in the Ritanserin 5 mg group; 43.2 years (SD 15.0) in the Ritanserin 10 mg group. Place of the study: Belgium, France, Germany, Italy, Norway, Sweden, the Netherlands (not specified ethnic composition of participants). Inclusion criteria: 1990 International Classification of Sleep Disorders. Exclusion criteria: shift work, irregular sleep/wake habits, hepatic, cardiac, renal, mental disorders, substance or drug abuse, anti‐arrhythmic medication, potassium loss‐inducing diuretics, pregnancy or probability of pregnancy. Recruitment procedures: not reported. At baseline the main complaints were "sleep attacks" (40%), EDS (37%), cataplexy (12%). Pre‐trial treatment: 21 participants with no medication; 76 participants with psychostimulants; 66 participants with antidepressants (38 clomipramine and 8 viloxazine); 7 participants with benzodiazepines; 6 participants with GHB. "Medication intake between groups did not differ". | |
| Interventions | 46 participants allocated to ritanserin, dosage 5 mg once daily. 45 participants allocated to ritanserin, dosage 10 mg once daily. 43 participants allocated to placebo. | |
| Outcomes | Considered in the review and present in the study: (1) Reduction of cataplexy measured by mean values of subjective ordinal scales at the short‐term follow up. (2) Adverse events by treatment groups. (3) Drop‐outs by treatment groups. Not considered in the review (as picked‐up with not validated tools): elimination or reduction of EDS according to subjective report. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
| Incomplete outcome data addressed? All outcomes | Low risk | Twelve out of 134 participants dropped out before trial completion: six from the ritanserin 5 mg group and six from the ritanserin 10 mg group; eight due to adverse events. All randomised participants were included in the analysis on an intention‐to‐treat basis. |
| Free of other bias? | Low risk | Diagnosis was made according to the 1990 International Classification of Sleep Disorders. |
Mitler 1990.
| Methods | Single‐blind cross‐over versus placebo: run‐in period of 15 days; each treatment period of 6 weeks; not known if a wash‐out period was performed. | |
| Participants | 56 participants with narcolepsy. Mean and range of age not known. Place of the study: Canada and US (not specified ethnic composition of participants). Inclusion criteria: history of EDS, presence of at least one of the REM sleep‐related symptoms (cataplexy, sleep paralysis, hypnagogic hallucinations), and two or more sleep‐onset REM periods at MSLT. Exclusion criteria: not reported. Recruitment procedures: not reported. Mean duration of the disorder: not reported. Pre‐trial treatment: not reported. | |
| Interventions | Viloxazine: other antidepressants class (N06AX09); dosage 100 mg per day. Placebo. | |
| Outcomes | Considered in the review and present in the study: (1) Reduction of EDS according to objective laboratory test (MSLT and MWT) at the long‐term follow‐up. (2) Reduction of cataplexy measured with the comparison of mean values of a subjective ordinal scale between treatment groups at the long‐term follow up. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes | High risk | Procedures of generation and concealment sequences: unclear. Procedures to make recipients unaware of the assigned therapy: unclear. Procedures to make those providing care unaware of the assigned therapy: unblinding. Procedures of blinding (unawareness of the assigned therapy in the group of those measuring outcomes): undeclared. |
| Incomplete outcome data addressed? All outcomes | High risk | Possible attrition bias: 20 participants lost to follow up. |
| Free of other bias? | Unclear risk | It is not known if a washout period was applied. |
Schachter 1980.
| Methods | Double‐blind cross‐over versus placebo: pre‐treatment period, without any drugs, of 7 days; no run‐in period; first treatment period of 21 days; wash‐out of 7 days; second treatment period of 21 days. | |
| Participants | 18 adult participants with narcolepsy (7 women and 11 men). Mean age 48.8 years (range 31‐68). Place of the study: London, UK (not specified ethnic composition of participants). Inclusion criteria: "EDS + cataplexy". Exclusion criteria: not reported. Recruitment procedures: not reported. Mild cataplexy: 5 participants; moderate: 6; severe: 7. Mild "narcolepsy": 2 participants; moderate: 8; severe: 8. Pre‐trial treatment: 15 participants clomipramine (mean dosage 49 mg; of them: 4 only clomipramine, 5 also with maxindol, 5 also with dextro‐amphetamine, 1 also with efedrine); 2 participants with maxindol (4 and 6 mg in die); 1 participant with no therapy. | |
| Interventions | Fluvoxamine: serotonin selective re‐uptake inhibitors class (N06AB); dosage between 25 and 200 mg (average 76 mg). Clomipramine: tricyclics class (N06AA); dosage between 25 and 200 mg (average 60 mg). | |
| Outcomes | Considered in the review and present in the study: (1) Elimination of cataplexy according to simple subjective report, at the short‐term follow up. (2) Reduction of cataplexy by more than 50% with respect to baseline according to simple subjective report, at the short‐term follow up. (3) Adverse events by treatment groups. (4) Drop‐outs by treatment groups. Not considered in the review (as picked‐up with not validated tools): elimination or reduction of EDS according to subjective report. | |
| Notes | The authors inappropriately treat the trial design as a parallel study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes | High risk | Procedures to make recipients unaware of the assigned therapy: inadequate. Procedures to make those providing care unaware of the assigned therapy: unclear. Procedures of blinding (unawareness of the assigned therapy in the group of those measuring outcomes): unclear. |
| Incomplete outcome data addressed? All outcomes | Low risk | 6 participants did not complete the fluvoxamine treatment period because of side effects. One more participant data on cataplexy outcomes were missing. |
| Free of other bias? | Low risk | Each treatment period was of 21 days duration with a seven day wash‐out period. |
Schrader 1986.
| Methods | Double‐blind cross‐over versus placebo: pre‐treatment period, without any drugs, of 14 days; run‐in period of 7 days; first treatment period of 28 days; wash‐out of 14 days; second treatment period of 28 days. | |
| Participants | 10 participants with narcolepsy (4 women and 6 men). Mean age 50 years (range 36‐67). Place of the study: Oslo, Norway (not specified ethnic composition of participants). Inclusion criteria: "EDS + cataplexy". Exclusion criteria: not reported. Recruitment procedures: not reported. Mean duration of the disorder: 28 years. Pre‐trial treatment: 8 participants with clomipramine, previously dismissed for side effects. | |
| Interventions | Femoxetine: serotonin selective reuptake inhibitors drug, not reported in the ATC system; dosage 300 mg bid. Placebo. | |
| Outcomes | Considered in the review and present in the study: (1) Elimination of EDS according to objective laboratory tests (MSLT) at the short‐term follow up. (2) Reduction of cataplexy measured with the comparison of mean values of subjective ordinal scales between treatment groups at the short‐term follow up. (3) Adverse events by treatment groups. (4) Drop‐outs by treatment groups. Not considered in the review (as picked‐up with not validated tools): elimination or reduction of EDS according to subjective report. | |
| Notes | Full blood serotonin was assayed at the end of the run‐in period and one week after the start of both treatment periods to assure adequate intake of medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants completed the two treatment periods. |
| Free of other bias? | Low risk | Each treatment period was of 28 days duration with a 14 day wash‐out period. |
EDS: excessive daytime sleepiness GHB: gamma‐hyroxybutyric acid MSLT: multiple sleep latency test MWT: maintenance wakefulness test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 2007 | Narrative review. |
| Black 2006 | A randomised controlled trial testing drugs other than antidepressants (sodium oxybate). |
| Black 2009 | A randomised controlled trial testing drugs other than antidepressants (sodium oxybate). |
| Harsh 2006 | Narrative review of trials testing drugs other than antidepressants (armodafinil). |
| Henry 1988 | A controlled trial with a cross‐over design. Not known if randomised sequence. Vigilance assessed by psychometric test, not with clinical outcome measures. |
| Izzi 2009 | Not a randomised controlled trial (case report of three patients). |
| Joo 2008 | Not a randomised controlled trial. |
| Lin 2008 | Not a randomised controlled trial. |
| Mitler 1986 | Not a randomised controlled trial. Comparison of the ability to stay awake in a group of 4 people with narcolepsy before and after treatment with protriptyline (randomisation of 3 dose levels). |
| Moller 2009 | Not a randomised controlled trial. |
| Ristanovic 2009 | Post hoc analysis of data from a randomised controlled trial testing testing sodium oxybate, comparing patients discontinuing or not discontinuing antidepressant drugs before randomisation. |
| Saletu 2009 | A randomised controlled trial testing drugs other than antidepressants (modafinil). |
| Smith 1996 | Not a randomised controlled trial. Comparison of excessive daytime sleepiness (according to the Epworth sleepiness scale), cataplexy (number of attacks per week) and other sleep parameters before and after treatment with escitalopram in a group of 10 people with narcolepsy and cataplexy. |
| Sonka 2006 | Not a randomised controlled trial. Comparison of excessive daytime sleepiness and cataplexy before and after treatment with venlafaxine in a group of 4 people with narcolepsy and cataplexy. |
| Weaver 2006 | A randomised controlled trial testing drugs other than antidepressants (sodium oxybate). |
| Xyrem 2005 | A randomised controlled trial testing drugs other than antidepressants (sodium oxybate). |
Contributions of authors
Luca Vignatelli was primarily responsible for writing the review. Roberto D'Alessandro and Livia Candelise provided advice and guidance on the design of the protocol and writing the review.
Declarations of interest
Luca Vignatelli and Roberto D'Alessandro were the co‐ordinators of the Italian multicentre study for the validation of a questionnaire for the diagnosis of narcolepsy (GINSEN 2001). The study was funded by Dompé Biotec, a pharmaceutical company selling Provigil (modafinil), a stimulant drug for narcolepsy. No use of the drug was promoted in the study. Neither author was sponsored by Dompé nor has any direct or indirect interest in the sale of the drug.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Lammers 1991 {published data only}
- Lammers GJ, Arends J, Declerck AC, Kamphuisen HAC, Schouwink G, Troost J. Ritanserin, a 5‐ht2 receptor blocker, as add‐on treatment in narcolepsy. Sleep 1991;14:130‐2. [PubMed] [Google Scholar]
Mayer 2003 {published data only}
- Mayer G. Ritanserin improves sleep quality in narcolepsy. Pharmacopsychiatry 2003;36:150‐5. [DOI] [PubMed] [Google Scholar]
Mitler 1990 {published and unpublished data}
- Guilleminault C, Mancuso J, Salva MA, Hayes B, Mitler M, Poirier G, et al. Viloxazine hydrochloride in narcolepsy: a preliminary report. Sleep 1986;9:275‐9. [DOI] [PubMed] [Google Scholar]
- Mitler MM. Alerting drugs: do they really work?. Psychopharmacology Bulletin 1987;23:435‐9. [PubMed] [Google Scholar]
- Mitler MM, Hajdukovic R. Relative efficacy of drugs for the treatment of sleepiness in narcolepsy. Sleep 1991;14:218‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler MM, Hajdukovic R, Erman M, Koziol JA. Narcolepsy. Journal of Clinical Neurophysiology 1990;7:93‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schachter 1980 {published data only}
- Schachter M, Parkes JD. Fluvoxamine and clomipramine in the treatment of cataplexy. Journal of Neurology, Neurosurgery, and Psychiatry 1980;43:171‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schrader 1986 {published data only}
- Schrader H, Kayed K, Markset AC, Treidene HE. The treatment of accessory symptoms in narcolepsy: a double‐blind cross‐over study of a selective serotonin re‐uptake inhibitor (femoxetine) versus placebo. Acta Neurologica Scandinavica 1986;74:297‐303. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 2007 {published data only}
- Anonymous. Sodium oxybate: new drug. Fewer attacks of cataplexy in some patients. Prescrire International 2007; Vol. 16, issue 89:98‐101. [PubMed]
Black 2006 {published data only}
Black 2009 {published data only}
Harsh 2006 {published data only}
Henry 1988 {published data only}
- Henry GK, Hart RP, Kwentus JA, Sicola MJ. Effects of protriptyline on vigilance and information processing in narcolepsy. Psychopharmacology 1988;95:109‐112. [DOI] [PubMed] [Google Scholar]
Izzi 2009 {published data only}
Joo 2008 {published data only}
- Joo EY, Seo DW, Tae WS, Hong SB. Effect of modafinil on cerebral blood flow in narcolepsy patients. Sleep 2008; Vol. 31, issue 6:868‐73. [DOI] [PMC free article] [PubMed]
Lin 2008 {published data only}
Mitler 1986 {published data only}
- Mitler MM, Shafor R, Hajdukovich R, Timms RM, Browman CP. Treatment of narcolepsy: Objective studies on methylphenidate, pemoline, and protriptyline. Sleep 1986;9:260‐4. [DOI] [PubMed] [Google Scholar]
Moller 2009 {published data only}
- Moller LR, Ostergaard JR. Treatment with venlafaxine in six cases of children with narcolepsy and with cataplexy and hypnagogic hallucinations. Journal of Child and Adolescent Psychopharmacology 2009;19:197‐201. [DOI] [PubMed] [Google Scholar]
Ristanovic 2009 {published data only}
- Ristanovic RK, Liang H, Hornfeldt CS, Lai C. Exacerbation of cataplexy following gradual withdrawal of antidepressants: Manifestation of probable protracted rebound cataplexy. Sleep Medicine 2009;10:416‐21. [DOI] [PubMed] [Google Scholar]
Saletu 2009 {published data only}
- Saletu M, Anderer P, Saletu‐Zyhlarz GM, Mandl M, Saletu B, Zeitlhofer J. Modafinil improves information processing speed and increases energetic resources for orientation of attention in narcoleptics: double‐blind, placebo‐controlled ERP studies with low‐resolution brain electromagnetic tomography (LORETA). Sleep Medicine 2009; Vol. 10, issue 8:850‐8. [DOI] [PubMed]
Smith 1996 {published data only}
- Smith M, Parkes JD, Dahlitz M. Venlafaxine in the treatment of the narcoleptic syndrome. Journal of Sleep Research 1996;5(Supplement 1):217. [Google Scholar]
Sonka 2006 {published data only}
- Sonka K, Kemlink D, Pretl M. Cataplexy treated with escitalopram ‐ Clinical experience. Neuroendocrinology Letters 2006;27:174‐6. [PubMed] [Google Scholar]
Weaver 2006 {published data only}
Xyrem 2005 {published data only}
- Xyrem International Study Group. A double‐blind, placebo‐controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. Journal of Clinical Sleep Medicine 2005; Vol. 1, issue 4:391‐7. [PubMed]
Additional references
AASM 2001
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, revised: Diagnostic and Coding Manual. Rochester, Minnesota: American Academy of Sleep Medicine, 2001. [Google Scholar]
AASM 2005
- American Academy of Sleep Medicine. ICSD‐2 ‐ International Classification of Sleep Disorders. Diagnostic and Coding Manual. 2nd Edition. Westchester, Illinois: American Academy of Sleep Medicine, 2005. [Google Scholar]
Anic‐Labat 1999
- Anic‐Labat S, Guilleminault C, Kraemer HC, Meehan J, Arrigoni J, Mignot E. Validation of a cataplexy questionnaire in 983 sleep‐disorders patients. Sleep 1999;22:77‐87. [PubMed] [Google Scholar]
Beusterien 1999
- Beusterien KM, Rogers AE, Walsleben JA, Emsellem HA, Reblando JA, Wang L, et al. Health‐related quality of life effects of modafinil for treatment of narcolepsy. Sleep 1999;22:757‐65. [DOI] [PubMed] [Google Scholar]
Broughton 1981
- Broughton R, Ghanem Q, Hishikawa Y, Sugita Y, Nevsimalova S, Roth B. Life effects of narcolepsy in 180 patients from North America, Asia and Europe compared to matched controls. Canadian Journal of Neurological Science 1981;8:299‐304. [DOI] [PubMed] [Google Scholar]
Broughton 1983
- Broughton R, Ghanem Q, Hishikawa Y, Sugita Y, Nevsimalova S, Roth B. Life effects of narcolepsy: relationships to geographic origin (North American, Asian or European) and to other patient and illness variables. Canadian Journal of Neurological Science 1983;10:100‐4. [DOI] [PubMed] [Google Scholar]
Chen 1995
- Chen SY, Clift SJ, Dahlitz MJ, Dunn G, Parkes JD. Treatment in the narcoleptic syndrome: Self assessment of the action of dexamphetamine and clomipramine. Journal of Sleep Research 1995;4:113‐8. [DOI] [PubMed] [Google Scholar]
Daniels 2001
- Daniels E, King MA, Smith IE, Shneerson JM. Health‐related quality of life in narcolepsy. Journal of Sleep Research 2001;10:75‐81. [DOI] [PubMed] [Google Scholar]
Dauvilliers 2001
- Dauvilliers Y, Montplaisir J, Molinari N, Carlander B, Ondze B, Besset A, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001;57:2029‐33. [DOI] [PubMed] [Google Scholar]
Dauvilliers 2007
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007;369:499‐511. [DOI] [PubMed] [Google Scholar]
Doghramji 1997
- Doghramji K, Mitler MM, Sangal RB, Shapiro C, Taylor S, Walsleben J, et al. A normative study of the maintenance of wakefulness test (MWT). Electroencephalography and Clinical Neurophysiology 1997;103:554‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Ervik 2006
- Ervik S, Abdelnoor M, Heier MS, Ramberg M, Strand G. Health‐related quality of life in narcolepsy. Acta Neurologica Scandinavica 2006;114:198‐204. [DOI] [PubMed] [Google Scholar]
EURORDIS 2007
- EURORDIS. Rare diseases; understanding this public health priority. www.eurordis.org/article.php3?id_article=918, (accessed 13 June 2007).
Findley 1995
- Findley L, Unverzagt M, Guchu R, Fabrizio M, Buckner J, Suratt P. Vigilance and automobile accidents in patients with sleep apnea or narcolepsy. Chest 1995;108:619‐24. [DOI] [PubMed] [Google Scholar]
Frey 1994
- Frey J, Darbonne C. Fluoxetine suppresses human cataplexy: A pilot study. Neurology 1994;44(4):707‐9. [DOI] [PubMed] [Google Scholar]
George 1996
- George CFP, Boudreau AC, Smiley A. Comparison of simulated driving performance in narcolepsy and sleep apnea patients. Sleep 1996;19:711‐7. [DOI] [PubMed] [Google Scholar]
Godbout 1986
- Godbout R, Montplaisir J. The effect of zimelidine, a serotonin‐reuptake blocker, on cataplexy and daytime sleepiness of narcoleptic patients. Clinical Neuropharmacology 1986;9:46‐51. [DOI] [PubMed] [Google Scholar]
Goswami 1998
- Goswami M. The influence of clinical symptoms on quality of life in patients with narcolepsy. Neurology 1998;50(Suppl 1):31‐6. [DOI] [PubMed] [Google Scholar]
Guilleminault 1976
- Guilleminault C, Raynal D, Takahashi S, Carskadon M, Dement W. Evaluation of short‐term and long‐term treatment of the narcolepsy syndrome with clomipramine hydrochloride. Acta Neurologica Scandinavica 1976;54:71‐87. [DOI] [PubMed] [Google Scholar]
Guilleminault 2000
- Guilleminault C, Anagnos A. Narcolepsy. In: Kryger MH, Roth T, Dement WC editor(s). Principles and Practice of Sleep Medicine. 3. Philadelphia: WB Saunders Company, 2000:676‐86. [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. Cochrane Database of Systematic Reviews 2005, Issue 3. [Google Scholar]
Hishikawa 1966
- Hishikawa Y, Ida H, Nakai K, Kaneko Z. Treatment of narcolepsy with imipramine (tofranil) and desmethylimipramine (pertofran). Journal of the Neurological Sciences 1966;3:453‐61. [DOI] [PubMed] [Google Scholar]
Hohagen 1993
- Hohagen F, Mayer G, Menche A, Riemann D, Volk S, Meier‐Ewert KH, et al. Treatment of narcolepsy‐cataplexy syndrome with the new selective and reversible MAO‐A inhibitor brofaromine ‐ a pilot study. Journal of Sleep Research 1993;2:250‐6. [DOI] [PubMed] [Google Scholar]
Hublin 1994
- Hublin C, Kaprio J, Partinen M, Koskenvuo M, Heikkila K. The Ullanlinna Narcolepsy Scale: validation of a measure of symptoms in the narcoleptic syndrome. Journal of Sleep Research 1994;3:52‐9. [DOI] [PubMed] [Google Scholar]
Johns 1998
- Johns M. Rethinking the assessment of sleepiness. Sleep Medicine Reviews 1998;2:3‐15. [DOI] [PubMed] [Google Scholar]
Johnson 1999
- Johnson EO, Breslau N, Roth T, Roehrs T, Rosenthal L. Psychometric evaluation of daytime sleepiness and nocturnal sleep onset scales in a representative community sample. Biological Psychiatry 1999;45:764‐70. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of randomised controlled trials. In: Egger M, Smith GD, Altman DG editor(s). Systematic Reviews in Health Care: meta‐analysis in context. 2nd Edition. London: BMJ Publishing Group, 2001:87‐108. [Google Scholar]
Kales 1982
- Kales A, Soldatos CR, Bixler EO, Caldwell A, Cadieux RJ, Verrechio JM, et al. Narcolepsy‐cataplexy. II Psychosocial consequences and associated psychopathology. Archives of Neurology 1982;39:169‐71. [DOI] [PubMed] [Google Scholar]
Langdon 1986
- Langdon N, Shindler J, Parkes JD, Bandak S. Fluoxetine in the treatment of cataplexy. Sleep 1986;9:371‐3. [DOI] [PubMed] [Google Scholar]
Larrosa 2001
- Larrosa O, Llave Y, Barrio S, Granizo JJ, Garcia‐Borreguero D. Stimulant and anticataplectic effects of reboxetine in patients with narcolepsy: a pilot study. Sleep 2001;24:282‐5. [DOI] [PubMed] [Google Scholar]
Lefebvre 2001
- Lefebvre C, Clarke MJ. Identifying randomised trials. In: Egger M, Smith GD, Altman D editor(s). Systematic Reviews in Health Care: meta‐analysis in context. Second Edition. London: BMJ Publishing Group, 2001:69‐86. [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Littner 2001
- Littner M, Johnson SF, McCall WV, Anderson WM, Davila D, Hartse K, et al. Practice parameters for the treatment of narcolepsy: an update for 2000. Sleep 2001;24:451‐66. [PubMed] [Google Scholar]
Martindale 1996
- Reynolds JEF, editor. In: Reynolds JEF editor(s). Martindale, The Extra Pharmacopoeia. 1st Edition. London: Royal Pharmaceutical Society, 1996. [Google Scholar]
Mignot 2000
- Mignot E. Pathophysiology of Narcolepsy. In: Kryger MH, Roth T, Dement WC editor(s). Principles and Practice of Sleep Medicine. Philadelphia: WB Saunders Company, 2000:663‐75. [Google Scholar]
Mignot 2001
- Mignot E, Lin L, Rogers W, Honda Y, Qiu X, Lin X, et al. Complex HLA‐DR and ‐DQ interactions confer risk of narcolepsy‐cataplexy in three ethnic groups. American Journal of Human Genetics 2001;68:686‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitler 1987
- Mitler MM. Alerting drugs: do they really work?. Psychopharmacology Bulletin 1987;23:435‐9. [PubMed] [Google Scholar]
Mitler 1990
- Mitler MM, Hajdukovic R, Erman M, Koziol JA. Narcolepsy. Journal of Clinical Neurophysiology 1990;7:93‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitler 1991
- Mitler MM, Hajdukovic R. Relative efficacy of drugs for the treatment of sleepiness in narcolepsy. Sleep 1991;14:218‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitler 1994
- Mitler MM, Aldrich MS, Koob GF, Zarcone VP. Narcolepsy and its treatment with stimulants. ASDA Standards of Practice. Sleep 1994;17:352‐71. [PubMed] [Google Scholar]
Nishino 2000
- Nishino S, Okura M, Mignot E. Narcolepsy: genetic predisposition and neuropharmacological mechanisms. Sleep Medicine Reviews 2000;4:57‐99. [DOI] [PubMed] [Google Scholar]
Orpha 2007
- Orphanet. What is an orphan drug?. http://www.orpha.net/ (accessed 13 June 2007).
Parkes 1979
- Parkes JD, Schachter M. Clomipramine and clonazepam in cataplexy. Lancet 1979;2:1085‐6. [DOI] [PubMed] [Google Scholar]
Parkes 1998
- Parkes JD, Chen SY, Clift SJ, Dahlitz MJ, Dunn G. The clinical diagnosis of the narcoleptic syndrome. Journal of Sleep Research 1998;7:41‐52. [DOI] [PubMed] [Google Scholar]
Partinen 2000
- Partinen M, Hublin C. Epidemiology of Sleep Disorders. In: Kryger MH, Roth T, Dement WC editor(s). Principles and Practice of Sleep Medicine. Philadelphia: WB Saunders Company, 2000:558‐79. [Google Scholar]
Passouant 1972
- Passouant P, Cadilhac J, Ribstein M. Sleep privation with eye movements using antidepressive agents. Revue Neurologique 1972;127:173‐92. [PubMed] [Google Scholar]
Rosenthal 1993
- Rosenthal L, Roehrs TA, Roth T. The Sleep‐Wake Activity Inventory: a self‐report measure of daytime sleepiness. Biological Psychiatry 1993;34:810‐20. [DOI] [PubMed] [Google Scholar]
Roth 1974
- Roth B, Faber J, Nevsimalova S, Tosovsky J. The influence of imipramine, dexphenmetrazine and amphetamine‐sulphate on the composition of diurnal sleep of narcoleptics. Activitas Nervosa Superior (Praha) 1974;16:52‐3. [PubMed] [Google Scholar]
Schmidt 1977
- Schmidt HS, Clark RW, Hyman PR. Protriptyline: an effective agent in the treatment of the narcolepsy‐cataplexy syndrome and hypersomnia. American Journal of Psychiatry 1977;134:183‐5. [DOI] [PubMed] [Google Scholar]
Shapiro 1974
- Shapiro WR. Treatment of cataplexy with clomipramine. Transaction of the American Neurological Association 1974;99:99‐102. [PubMed] [Google Scholar]
Shapiro 1975
- Shapiro WR. Treatment of Cataplexy with Clomipramine. Archives of Neurology 1975;32:653‐6. [DOI] [PubMed] [Google Scholar]
Silber 2002
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population‐based study. Sleep 2002;25:197‐202. [DOI] [PubMed] [Google Scholar]
Teixeira 2004
- Teixeira VG, Faccenda JF, Douglas NJ. Functional status in patients with narcolepsy. Sleep Medicine 2004;5:477‐83. [DOI] [PubMed] [Google Scholar]
Thorpy 1992
- Thorpy MJ. The clinical use of the Multiple Sleep Latency Test. The Standards of Practice Committee of the American Sleep Disorders Association. Sleep 1992;15:268‐76. [DOI] [PubMed] [Google Scholar]
Vignatelli 2004
- Vignatelli L, D'Alessandro R, Mosconi P, Ferini‐Strambi L, Guidolin L, Vincentiis A, Plazzi G, on behalf of the GINSEN. Health‐related quality of life in Italian patients with narcolepsy: the Sf‐36 health survey. Sleep Medicine 2004;5:467‐75. [DOI] [PubMed] [Google Scholar]
Weaver 1997
- Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 1997;20:835‐43. [PubMed] [Google Scholar]
Weaver 2001
- Weaver TE. Outcome measurement in sleep medicine practice and research. Part 1: assessment of symptoms, subjective and objective daytime sleepiness, health‐related quality of life and functional status. Sleep Medicine Reviews 2001;5:103‐28. [DOI] [PubMed] [Google Scholar]
Wiesbeck 2000
- Wiesbeck GA, Weijers HG, Chick J, Boening J. The effects of ritanserin on mood, sleep, vigilance, clinical impression, and social functioning in alcohol‐dependent individuals. Alcohol 2000;35:384‐9. [DOI] [PubMed] [Google Scholar]
Wyatt 1971
- Wyatt RJ, Fram DH, Buchbinder R, Snyder F. Treatment of intractable narcolepsy with a monoamine oxidase inhibitor. New England Journal of Medicine 1971;285:987‐91. [DOI] [PubMed] [Google Scholar]


