Abstract
The Dietary Inflammatory Index (DII) was extensively used to examine the inflammatory potential of diet related to colorectal cancer (CRC). This meta-analysis aimed to update the evidence of the association between the DII and CRC across various culture-specific dietary patterns. Literature search was performed through online databases (Scopus, Web of Science, PubMed, and EBSCOHost). Observational studies exploring the association between the DII and CRC, published between 2017 and 2021, were included. The risk ratio (RR) and 95% confidence interval (CI) were separately computed for 12 studies comparing the highest and lowest DII scores and for 3 studies that presented continuous DII scores. A high DII score was associated with a higher risk of CRC (RR:1.16; 95% CI, 1.05–1.27). In the subgroup analysis, significant associations were seen in cohort design (RR: 1.24; 95% CI, 1.06–1.44), those lasting for 10 years or longer (RR: 2.95; 95% CI, 2.47–3.52), and in adjustment factor for physical activity (RR: 1.13; 95% CI, 1.07–1.20). An increase of one point in the DII score elevates the risk of CRC by 1.34 (95% CI: 1.15–1.55) times. The findings call for standardized measurement of the inflammatory potential of diet in future studies to enable the establishment of global guidelines for CRC prevention.
Keywords: Dietary Inflammatory Index, colorectal cancer, dietary pattern, modifiable risk factor
1. Introduction
Colorectal cancer (CRC) is currently the second leading cause of cancer-related mortality worldwide. In 2020 alone, approximately 1.9 million people were diagnosed with CRC, and 935,000 of them died within the same year [1]. The high burden of CRC in countries with a medium to high human development index (HDI) suggested the potential role of both sedentary lifestyles and dietary patterns in the development of CRC [2].
More than 60% of the overall CRC cases were reported as sporadic, occurring in people without genetic predisposition or family history of CRC [3]. Such a trend further points to the impact of modifiable risk factors in the CRC development. It is generally believed that long-term exposure to an unhealthy diet, physical inactivity, smoking, and alcohol consumption are all likely to trigger the chronic systemic inflammation, which eventually induces the proliferation of cancer cells [4,5]. Molecular studies also demonstrated that the production of pro-inflammatory cytokines and arrays of free radicals present at cellular levels were attributable to the consumption of an unhealthy diet [6,7,8].
Furthermore, numerous studies provided insight into the relationship between specific food items and CRC. For example, a high intake of red meats combined with a low intake of vegetables was shown to elevate the risk of CRC by 2.6 times [9]. In contrast, a high intake of fruits, cereals, nuts, and milk and dairy products lower the risk of CRC by 64% [10]. Another study also reported that the risk of CRC increased by 17% and 18%, respectively, as a result of consuming 100 g of red meats and 50 g of processed meat products daily [11]. Additionally, a recent study demonstrated that taking more than two servings of sugar-sweetened beverages per day heightened the risk of CRC by 16% [12].
There were various types of dietary assessment methods frequently used for epidemiological purposes, which includes the 24 h dietary recall, the dietary record, and the food frequency questionnaire (FFQ). However, they were unable to relate the specific dietary risk factor with levels of inflammatory markers well-established in CRC. The advent of the Dietary Inflammatory Index (DII) provides a quantitative means to study the relationship between the pro-inflammatory diet and CRC. It allows the assessment of the inflammatory potential of individual food items using an FFQ, by which a DII score can be calculated. A higher DII score suggests a stronger inflammatory potential of a food item [13]. Remarkably, DII has been utilized in substantial epidemiologic studies that include different ethnicities and various health outcomes. To date, the evidence on the usefulness of the DII in predicting the risk of CRC is established mainly focus on the Western diet with little input from other parts of the world based on the studies published before 2017 [14,15]. As CRC emerges as a major cancer type globally, this review was designed to update the evidence of the association between the DII and CRC based on the latest studies on a wide range of culture-specific dietary patterns.
2. Materials and Methods
The review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency [16]. Guided by the PRISMA, the authors performed the systematic literature search through formulation of related research questions. The systematic searching process consists of identification, screening, and eligibility stage, which were performed for each database. The authors independently appraised the quality of included studies using the Newcastle–Ottawa quality assessment scale. Following that, the authors read through all full-text articles for data extraction and analysis.
2.1. Formulation of the Research Question
The research question was formulated based on the PICO concept: a tool often used to assist authors in developing suitable research questions for systematic review. It consists of population or problem, interest, and context or outcome. Based on this concept, the authors have included the three main aspects in the review: adults (population), Dietary Inflammatory Index (interest), and CRC (outcome), which led the authors to the main research question, “How does the Dietary Inflammatory Index determine the risk of CRC in various adult populations?”.
2.2. Systematic Searching Strategies
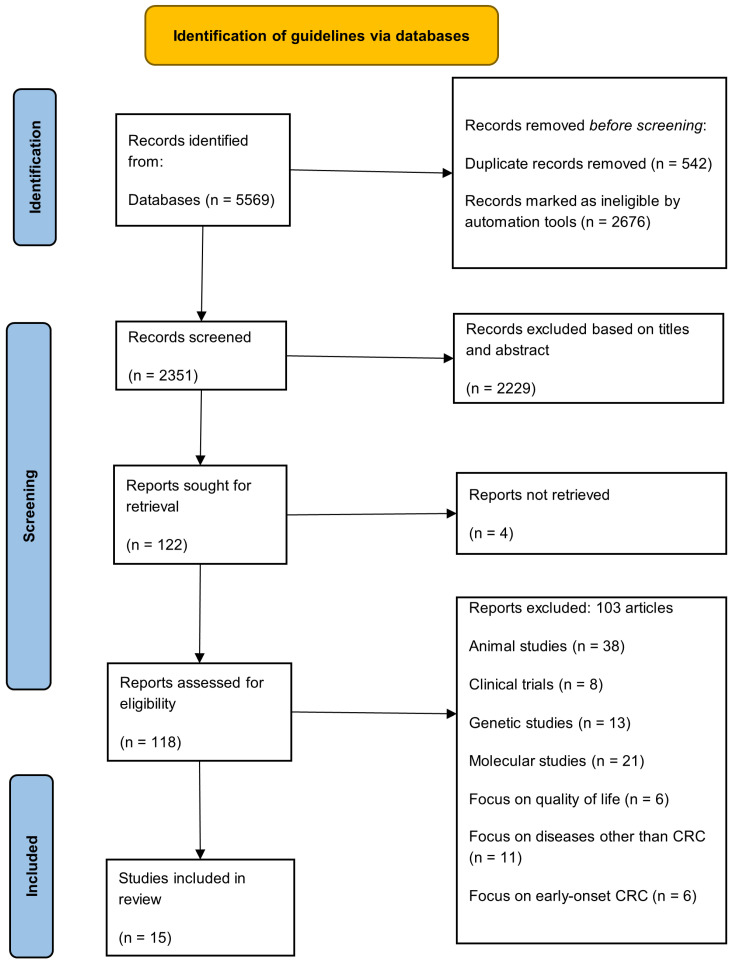
The systematic searching strategy was initiated with the identification, followed by screening and eligibility process (Figure 1).
Figure 1.
PRISMA flowchart.
2.3. Identification
A comprehensive literature search performed between 1 and 4 November 2021 through Scopus, Web of Science, PubMed, and EBSCOHost. These four online databases covered scientific publications in more than 30,000 journals [17,18]. The discoverability of articles was enhanced using synonyms of keywords and the medical subject headings, combined by using the Boolean operators (Table 1). A total of 5569 records were retrieved from the databases, and 542 duplicates were removed. The remaining records were then exported to a Microsoft Excel spreadsheet for screening.
Table 1.
Keyword search used in the identification process.
| Database | Search String |
|---|---|
| Scopus | TITLE-ABS-KEY ((“dietary inflammatory index” OR “dietary inflammatory score” OR “diet-related inflammation” OR “dietary inflammatory potential” OR “proinflammatory diet” OR “anti-inflammatory diet”) AND (“colorectal cancer *” OR “colorectal neoplas *” OR “colorectal tumo * r” OR “colorectal malignanc *”)) |
| Web of Science | TS = ((“dietary inflammatory index” OR “dietary inflammatory score” OR “diet-related inflammation” OR “dietary inflammatory potential” OR “proinflammatory diet” OR “anti-inflammatory diet”) AND (“colorectal cancer *” OR “colorectal neoplas *” OR “colorectal tumo * r” OR “colorectal malignanc *”)) |
| PubMed | ((“dietary inflammatory index” OR “dietary inflammatory score” OR “diet-related inflammation” OR “dietary inflammatory potential” OR “proinflammatory diet” OR “anti-inflammatory diet”) AND (“colorectal cancer” OR “colorectal neoplasm” OR “colorectal tumor” OR “colorectal tumour” OR “colorectal malignancy” OR “colorectal malignancies”)) |
| EBSCOHost | ((“dietary inflammatory index” OR “dietary inflammatory score” OR “diet-related inflammation” OR “dietary inflammatory potential” OR “proinflammatory diet” OR “anti-inflammatory diet”) AND (“colorectal cancer” OR “colorectal neoplasm” OR “colorectal tumor” OR “colorectal tumour” OR “colorectal malignancy” OR “colorectal malignancies”)) |
The symbol * is used in the search strategy as truncation and wildcard function for keywords variation purposes.
2.4. Screening
The titles and abstracts of all the records were screened for eligibility. An article was retained for the analysis only if (i) it was published between 2017 and 2021, (ii) a full article was available, (iii) it described an observational study exploring the association between the DII and CRC, and (iv) it was published in English. The duration of published articles screened was determined based on the recent development of DII in cancer. Despite the consistency of DII as an instrument in substantial evidence-based research, the additional food parameters and revised scoring algorithms enhanced the comparability between studies [19]. Considering the utilization of revised version DII in recently published studies concerning colorectal cancer, the selection of the latest five years duration is justified. Review articles, editorials, proceedings, commentary articles, and articles focusing on cancers other than CRC were excluded.
2.5. Eligibility
Of the 118 full-text articles screened for eligibility, 103 were excluded. They ranged from animal studies (n = 38), clinical trials (n = 8), genetic studies (n = 13), molecular studies (n = 21), and studies on diseases other than CRC (n = 11) to studies on early-onset CRC (n = 6). The remaining 15 articles (9 case-control and 6 cohort studies) were subjected to the quality appraisal.
2.6. Quality Appraisal
The quality of the selected studies was assessed using the Newcastle–Ottawa quality assessment scale [20]. This scale was designed for nonrandomized studies, focusing on the sample selection, the comparability of study groups, and the ascertainment of exposure or outcome of interest. The total number of stars rewarded to a study indicated its quality, either low (≤3 stars), moderate (4–6 stars) or high (≥7 stars). The quality assessment of the selected studies was independently performed by two authors. Any disagreements between them were resolved by consensus, and a third reviewer was consulted when necessary. The results for the quality assessment are presented in Table 2. The quality of the studies ranged from moderate to high, and all of them were included in the meta-analysis.
Table 2.
Quality appraisal of selected studies using Newcastle–Ottawa quality assessment scale for case control and cohort studies.
| Studies (Case-Control Studies) | Selection (Maximum ****) | Comparability (Maximum **) | Exposure (Maximum ***) | Total Scores (Maximum 9) | |||||
| Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls on the Basis of the Design or Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate | ||
| Abulimi et al., 2020 [21] | * | * | * | * | ** | - | * | - | 7 |
| Byrd et al., 2020 [22] | * | * | * | * | ** | * | * | - | 8 |
| Cho et al., 2019 [23] | - | * | - | * | ** | * | * | - | 6 |
| Niclis et al., 2018 [14] | * | - | * | * | * | - | - | * | 5 |
| Obon-Santacana, 2019 [24] | - | - | - | * | ** | - | * | - | 4 |
| Rafiee et al., 2019 [25] | * | * | * | * | * | * | * | - | 7 |
| Sharma et al., 2017 [26] | - | * | * | * | * | * | * | - | 6 |
| Shivappa et al., 2017 [27] | * | * | - | * | * | * | * | - | 6 |
| Yuan et al., 2021 [28] | * | * | - | * | * | * | * | - | 6 |
| Studies (cohort studies) | Selection (maximum ****) | Comparability (maximum **) | Outcome (maximum ***) | Total scores (maximum 9) | |||||
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Brouwer et al., 2017 [29] | * | - | * | * | * | * | * | * | 7 |
| Harmon et al., 2017 [30] | * | - | - | * | - | * | * | - | 4 |
| Ratjen et al., 2019 [31] | * | - | * | * | - | * | * | * | 6 |
| Tabung et al., 2017 [32] | * | * | * | * | * | * | * | * | 8 |
| Zheng et al., 2020 [33] | * | - | * | * | * | * | * | * | 7 |
| Wesselink et al., 2021 [34] | * | - | * | * | * | * | * | * | 7 |
* denotes the scoring of one star; ** represents the scoring of two stars that is also the maximum scoring for the comparability domain; *** represents the scoring of three stars and the maximum scoring for the outcome domain; **** represents the scoring of four stars and the maximum scoring for selection domain.
2.7. Data Abstraction and Analysis
The information extracted from each included study ranged from the author’s names, year of publication, study location, study design, study period, study instrument used, sample size, type of data (categorical or continuous), measure of association (odds ratio (OR) or hazard ratio (HR) with the corresponding 95% confidence interval (CI)), range of DII scores, and number of food parameters to adjusted factors (Table 3). The random-effect meta-analysis was performed separately for 12 studies that compared the two categories with the highest (pro-inflammatory) and lowest (anti-inflammatory) DII scores and three studies that presented continuous DII scores. The Review Manager 5.4 (RevMan) software was used to generate the summary risk ratios (RRs) and the corresponding 95% Cis, while I2 statistics was used to test the heterogeneity of the studies. Given the heterogeneity of the studies that categorized the DII scores, a series of stratified analyses was also conducted based on the study design, region, study period, and adjusted factors (family history of CRC, educational level, comorbidities, physical activity, and BMI).
Table 3.
Data extracted from the studies included for meta-analysis.
| Author, Year | Study Location | Study Design | Study Period | Study Instrument | Number of Food Parameters | Sample Size | Range of DII Scores | Type of Data and Comparison | Measures of Association | Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Abulimiti et al., 2020 [21] | China | Case control | 2010–2019 | 81-item FFQ 1 | 34 | 2502 cases | −5.96 to +6.01 | Categorical | OR = 1.40 (95% CI 1.16, 1.68) | Age, sex, marital status, residence, education level, occupation, income, BMI 2, smoking status, family history of CRC, comorbidities |
| 2538 controls | Quartile 4 vs. Quartile 1 | |||||||||
| Brouwer et al., 2017 [29] | Netherlands | Prospective cohort | 2006–2012 | 183-item FFQ | 28 | 457 | −11.7 to +8.4 | Categorical | HR = 1.37 (95% CI 0.80, 2.34; p > 0.05) | Age, smoking status, education level |
| Tertile 3 (0.3 to 8.4) vs. Tertile 1 (−11.7 to <−1.8) | ||||||||||
| Byrd et al., 2020 [22] | United States | Case control | 1991–2002 | 126-item FFQ | 19 | 765 cases | (controls): −0.7 ± 2.4 | Categorical | OR = 1.31 (95% CI 0.98, 1.75) | Age, sex, education, NSAIDs 3 use, hormone use, family history of CRC, smoking status, BMI, alcohol intake, physical activity |
| 1986 controls | (cases): −0.5 ± 2.4 | Quintile 5 vs. Quintile 1 | ||||||||
| Cho et al., 2019 [23] | Korea | Case control | 2010–2013 | 106-item FFQ | 35 | 632 cases | (controls): 0.94 ± 2.24 | Categorical | OR = 1.38 (95% CI 1.12, 1.71) | Age, sex, family history of CRC, education level, BMI, physical activity, smoking status, alcohol intake |
| 1295 controls | (cases): 1.77 ± 1.97 | High vs. Low | ||||||||
| Harmon et al., 2017 [30] | United States | Prospective cohort | 1993–2010 | 169-item FFQ | 28 | 190,963 | −6.64 to +4.95 | Categorical | HR = 1.21 (95% CI 1.11, 1.32) | Age, sex, race, comorbidities, smoking status, BMI, family history of CRC, education level, aspirin use, hormones use |
| Quartile 4 (−0.52 to 4.95) vs. Quartile 1 (−6.64 to −3.66) | ||||||||||
| Niclis et al., 2018 [14] | Argentina | Case control | 2008–2015 | 127-item FFQ | 22 | 144 cases | −3.15 to +3.77 | Categorical | OR = 1.56 (95% CI 1.20, 2.03) | Age, sex, BMI, smoking status, socioeconomic status, physical activity, NSAIDs use |
| 302 controls | Tertile II (0.6–1.86) vs. Tertile 1 (<0.65) | |||||||||
| Obon-Santacana et al., 2019 [24] | Spain | Case control | 2008–2013 | 140-item FFQ | 30 | 1852 cases | (men): −5.11 to 5.47 | Continuous DII (per one unit increase) | OR = 1.14 (95% CI 1.10, 1.18) | Sex, age, education level, study area, family history of CRC, smoking status, physical activity, BMI, NSAIDs use |
| 3447 controls | (women): −5.64 to 5.12 | |||||||||
| Rafiee et al., 2019 [25] | Iran | Case control | 2017–2018 | 148-items FFQ | 21 | 134 cases | −4.23 to +3.89 | Categorical | OR = 2.64 (95% CI 1.40, 4.99) | Age, sex, physical activity, salt intake, comorbidities, smoking, family history of CRC, cooking method, supplement intake |
| 240 controls | Tertile 3 (>0.04) vs. Tertile 1 (<−1.13) | |||||||||
| Ratjen et al., 2019 [31] | Germany | Prospective cohort | 2009–2011 | 112-item FFQ | 27 | 1404 | −3.99 to +4.11 | Continuous DII (per one unit increase) | HR = 1.08 (95% CI 0.97, 1.20) | Sex, age at diet assessment, BMI, physical activity, survival time, tumor location, metastasis, other type of cancers, therapy, smoking status, alcohol intake |
| Sharma et al., 2017 [26] | Canada | Case control | 1999–2003 | 169-item FFQ | 29 | 547 cases | −5.19 to +6.93 | Categorical | OR = 1.65 (95% CI 1.13, 2.42) | Age, sex, BMI, physical activity, comorbidities, family history of CRC, smoking status, alcohol intake, NSAIDs use |
| 685 controls | Quartile 4 (≥0.3582) vs. Quartile 1 (<−2.036) | |||||||||
| Wesselink et al., 2021 [34] | Netherlands | Prospective cohort | 2010–2017 | 204-item FFQ | 28 | 1478 | −12.2 to +8.5 | Categorical | HR = 0.98 (95% CI 0.94, 1.04; p > 0.05) | Age, sex, staging, BMI, smoking status, NSAIDs use, comorbidities |
| Tertile 3 (1.2 to <8.5) vs. Tertile 1 (−12.2 to <−1.0) | ||||||||||
| Shivappa et al., 2017 [27] | Jordan | Case control | 2010–2012 | 90-item FFQ | 18 | 153 cases | −2.25 to +2.86 | Continuous DII (per one unit increase) | OR = 1.45 (95% CI 1.13, 1.85) | Age, sex, education level, physical activity, BMI, smoking status, family history of CRC |
| 202 controls | ||||||||||
| Tabung et al., 2017 [32] | United States | Prospective cohort | 1993–2014 | 122-item FFQ | 32 | 87,042 | −6.62 to +5.39 | Categorical | HR = 1.06 (95% CI 0.90, 1.26) | Age, race, education level, smoking status, comorbidities, regular NSAIDs use, estrogen use, BMI, physical activity |
| Quintiles 5 vs. Quintiles 1 | ||||||||||
| Yuan et al., 2021 [28] | United States | Case control | 2005–2015 | 175-item FFQ | 34 | 587 cases | −5.9 to +4.6 | Continuous DII (per one unit increase) | OR = 1.07 (95% CI 0.97, 1.19) | Age, gender, race, BMI, education level, smoking status, comorbidities, NSAIDs use, family history of CRC, supplements use |
| 1313 controls | ||||||||||
| Zheng et al., 2020 [33] | United States | Prospective cohort | 1993–2015 | 122-item FQ | 32 | 161,808 | −6.80 to +3.25 | Categorical | HR = 0.72 (95% CI 0.46, 1.12) | Age, race, smoking status, income levels, cancer staging, education level, physical activity, BMI |
| Tertile 1 | ||||||||||
| (−5.96 to −2.25) vs. Tertile 3 (−0.18 to 3.82) |
1 FFQ, food frequency questionnaire; 2 BMI, body mass index; NSAIDs 3, non-steroidal anti-inflammatory drugs.
3. Results
The 15 studies included in the analysis covered ten countries from different regions, including the United States (n = 5) [22,28,30,32,33], the Netherlands (n = 2) [29,34], Argentina (n = 1) [14], Spain (n = 1) [24], Germany (n = 1) [31], Canada (n = 1) [26], Iran (n = 1) [25], Jordan (n = 1) [27], Korea (n = 1) [23], and China (n = 1) [21]. Geographically, most of the studies were from the regions of America (AMR) (n = 7) [14,22,26,28,30,32,33], followed by the European region (EUR) (n = 7) [24,29,31,34], the western Pacific region (WPR) (n = 2) [21,23] and the eastern Mediterranean region (EMR) (n = 3) [25,27]. The study period ranged from five years and less (n = 6) [23,24,25,26,27,31], six to ten years (n = 6) [14,21,28,29,30,34], and more than ten years (n = 3) [22,32,33].
The studies assessed 18 to 35 food parameters using the FFQ. The highest and lowest measures of association were observed in Iran (OR: 2.64; 95% CI: 1.40, 4.99) [25] and the United States (HR: 0.72; 95% CI: 0.46, 1.12) [33], respectively.
Association between DII and the Risk of CRC
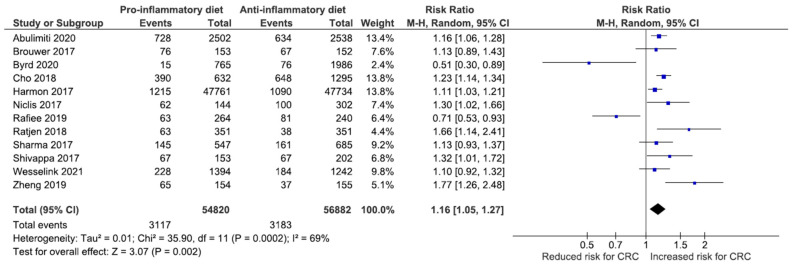
Despite the heterogeneity of the 12 studies that categorized DII scores (I2 = 69%, p = 0.0002), the pooled analysis on 111,702 individuals demonstrated a significant association between a high DII score and the risk of CRC (RR: 1.16; 95% CI, 1.05–1.27) (Figure 2).
Figure 2.
Forest plot for 12 studies comparing the risk of CRC between high (pro-inflammatory) and low (anti-inflammatory) scores.
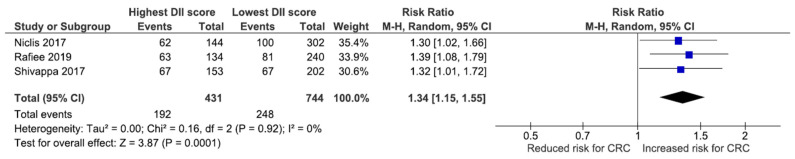
Another analysis on 1175 individuals from three studies also showed that an increase of one point in the DII score elevated the risk of CRC by 1.34 (95%CI: 1.15, 1.55) times. Data synthesis stratified by dichotomous outcome for DII score (Figure 3) showed that high DII score group had increased 34% risk of CRC (RR:1.34; 95% CI, 1.15–1.55) in a random effect model with no heterogeneity (I2 = 0%, p = 0.000).
Figure 3.
Forest plot for three studies relating the risk of CRC to continuous DII scores.
Due to the high heterogeneity result, several subgroup analyses on study design, groups for DII score, study settings based on WHO region, duration of study, and adjustment factors were conducted (Table 4). Stratified by the study design, positive association was found between DII score and risk for CRC in case control studies (RR: 1.14; 95% CI, 0.89–1.45) and cohort studies (RR: 1.24; 95% CI, 1.06–1.44) but with a high degree of heterogeneity. The summary RR indicated no heterogeneity for grouping of DII score either analysed in form of continuous data or categorical baseline. Notwithstanding that, the categorical form of data analysis presented significant increased risk for CRC by 61% (RR: 1.61; 95% CI, 1.26–2.05), much higher than that of continuous presentation of DII score (RR: 0.35; 95% CI, 0.28–0.41). Low heterogeneity (22%) was observed in studies conducted among the eastern Mediterranean region, which could possibly be due to sharing of culture with similar dietary pattern. In regions such as the Europe and Asia, high heterogeneity observed with 98% and 95% each, highlighted the influence of cultural blend within the study population that masked the dietary pattern. Subgroup analysis based on the study duration highlighted significant association with CRC risk when conducted for 10 years or more (RR: 2.95; 95% CI, 2.47–3.52). Positive relationship was seen in all studies with adjustment factors for family history of CRC, education level, comorbidities, physical activity, and BMI but with moderate to high heterogeneity. Low heterogeneity (55%) was seen in studies without adjustment for family history of CRC with RR: 1.31; 95% CI 1.10–1.56, and no heterogeneity in studies without adjustment for physical activity with RR: 1.13; 95% CI 1.07–1.20.
Table 4.
Subgroup analyses of studies reporting the risk for CRC between high (pro-inflammatory) and low (anti-inflammatory) scores.
| Subgroups | No. of Studies | RR (95% CI) | Heterogeneity | Significance Test | ||
|---|---|---|---|---|---|---|
| I2 (%) | p | Z | p | |||
| Study design | ||||||
| Case-control | 7 | 1.14 (0.89, 1.45) | 81% | 0.000 | 1.03 | 0.300 |
| Cohort | 4 | 1.24 (1.06, 1.44) | 63% | 0.030 | 2.74 | 0.006 |
| Groups | ||||||
| Continuous | 4 | 0.35 (0.28, 0.41) | 0% | 0.400 | 10.12 | 0.000 |
| Categorical | 3 | 1.61 (1.26, 2.05) | 0% | 0.900 | 3.80 | 0.000 |
| Region | ||||||
| AMR | 4 | 0.32 (0.24, 0,40) | 62% | 0.050 | 8.29 | 0.000 |
| EUR | 4 | 0.40 (0.33, 0.47) | 98% | 0.000 | 10.50 | 0.000 |
| Asia | 2 | 0.44 (0.34, 0.54) | 95% | 0.000 | 8.59 | 0.000 |
| EMR | 2 | 0.36(0.21, 0.52) | 22% | 0.260 | 4.61 | 0.000 |
| Study period | ||||||
| Less than 10 years | 11 | 1.12 (0.94, 1.35) | 97% | 0.000 | 1.27 | 0.200 |
| 10 years or more | 2 | 2.95 (2.47, 3.52) | 92% | 0.001 | 12.01 | 0.000 |
| Adjustment for family history of CRC | ||||||
| Yes | 8 | 1.01 (0.82, 1.24) | 97% | 0.000 | 0.06 | 0.950 |
| No | 5 | 1.31 (1.10, 1.56) | 55% | 0.060 | 3.01 | 0.003 |
| Adjustment for education level | ||||||
| Yes | 8 | 1.11 (0.89, 1.39) | 98% | 0.000 | 0.93 | 0.350 |
| No | 5 | 1.12 (0.90, 1.39) | 75% | 0.003 | 1.04 | 0.300 |
| Adjustment for comorbidities | ||||||
| Yes | 5 | 1.08 (0.97, 1.20) | 64% | 0.030 | 1.41 | 0.160 |
| No | 8 | 1.18 (0.92, 1.50) | 96% | 0.000 | 1.28 | 0.200 |
| Adjustment for physical activity | ||||||
| Yes | 9 | 1.11 (0.89, 1.39) | 95% | 0.000 | 0.93 | 0.350 |
| No | 4 | 1.13 (1.07,1.20) | 0% | 0.890 | 4.38 | 0.000 |
| Adjustment for BMI | ||||||
| Yes | 5 | 1.60 (1.54, 1.67) | 96% | 0.000 | 23.81 | 0.000 |
| No | 2 | 0.86 (0.78, 0.96) | 92% | 0.001 | 2.84 | 0.004 |
4. Discussion
This meta-analysis confirmed the association between high DII scores and the increased risk of CRC. It was grounded on the standardized quantification of inflammatory markers produced individual food parameters, which has long been used to predict the risk of chronic non-communicable diseases including cancer [35,36]. Instead of giving general recommendations on high-fat and low-fiber food intake, the DII enables an objective assessment of dietary patterns and the risk of cancer at an individual level [21,37,38]. While the Asian population contributed to more than half of the CRC cases [2,39], this study fills the gap by providing evidence on a wider range of culture-specific dietary patterns across the world based on the latest studies.
In the subgroup analysis, cohort studies showed a more significant association between high DII score and increased risk for CRC, as compared to case-control studies. As cohort studies allow a better control of confounding factors in nature, such finding implies a stronger impact of pro-inflammatory dietary patterns on the CRC development as compared with the study duration and other adjustment factors. It is also worth highlighting that a relatively low degree of heterogeneity in the studies from the EMR supported the notion that the Mediterranean diet had protective effect against risk for CRC, reflecting the role of specific dietary pattern that is possibly culturally driven.
Additionally, when presented in categorical form, DII score demonstrated significant increased risk for CRC rather than interpreted in continuous per-one-unit increment. The most commonly used classification in the selected studies was based on three categories and hence provides easy and convenient comparison. Nevertheless, future validation studies on standardization of DII score calculation are necessary to strengthen the interpretation against risk for CRC and act as a baseline for guideline development. Even though adjustment factors studied showed proportionate relationship with CRC risk, the high degree of heterogeneity obtained should be considered cautiously.
Although the pooled RR for CRC in the study was lower than that published by existing literatures, the findings corroborated the positive association between high DII score and increased risk for CRC as evidence in other studies. In a review comparing four studies among the Western population, more proinflammatory diet scores were linked with a 12–65% higher risk of CRC compared to the anti-inflammatory diets [40]. Similarly, an increase of one point in the DII score was suggestive of elevating the risk for CRC by 7% in a relatively more heterogenous population [41]. The range of risk difference indicate the sensitivity of culture-specific dietary patterns as incorporated in the current meta-analysis. While the Westernized diet has been frequently examined to exert pro-inflammatory effects that further enhances the risk for CRC, limited studies are available to support the role of dietary pattern in the non-Western population. Thus, more studies are warranted to explore the culture-specific dietary pattern particularly in the settings of diverse, multiracial Asian countries.
The most common approach used to measure the adherence to a routine healthy dietary intake is the Healthy Eating Index (HEI) or the Mediterranean diet since the indices were based on certain dietary recommendations [32]. Overall, the Mediterranean diet composed of rich, plant-based foods, including the fruits, vegetables, nuts, legumes, and whole grain products, combined with regular intake of seafood and low intake of red and processed meat [42]. Although many studies relate the practice of Mediterranean diet with less risk for CRC, in regions where plant-based foods serve as the food staple, the incidence of CRC continues to rise [43]. Future research works should consider the influencing factors concomitant to dietary pattern that could possibly contributed to the progression of CRC over time.
High intake of processed and red meat has been linked to increased risk of CRC. However, in countries like Korea where the CRC incidence rate is relatively high, with 44.5 cases per 100,000 persons per year [44], studies reported less than 20% of the population consumed processed meat products [45], indicating the presence of other factors that may influence the tumor progression. Numerous empirical studies proposed that the presence of mutagenic compounds, such as the heterocyclic amines (HCAs), polycyclic aromatic hydrocarbons (PAHs), and acrylamides formed during food preparation, raised the risk of CRC [24,36,46]. Understanding the cooking method and food processing involved provide beneficial value to explain the impact of food culture in the complex mechanism of CRC.
The intake of dietary fiber on daily basis has crucial role in the functionality of the guts to enhance transit time and stool formation. Disturbance of the physiologic system affected by the food solubility and fermentability causes tremendous effects towards the gut lining, thus potentially causing tumorigenesis. Notwithstanding that, a recently published longitudinal study revealed that consumption of dietary fiber intake improved the physical function and overall health of the CRC survivors upon completion of their treatment [47]. Changing to healthier diet pattern containing more anti-inflammatory foods had protective effect against cancer recurrence as well as prolonged survival [9,15,35,38]. Therefore, extensive health campaigns and awareness towards a healthy, balanced diet should target the average-risk groups and be initiated as early as possible.
Other important confounders considered in the studies included age and sex. When comparing the DII score across opposite gender, few studies suggested sex differentials pertaining to the risk of CRC [14,24]. A comparison study on Canadian populations showed that a 33% reduction in the risk of CRC in men [41] was attributable to the intake of anti-inflammatory diets, whereas no significant association was observed with similar dietary pattern among women. More studies are required to explain the role of sex differentials and dietary intake against the risk for CRC. Similarly, when accounted together with the physical activity factor, high DII score showed tendency to raise the CRC risk among individuals with sedentary lifestyle [22]. Collectively, this explained the importance of healthy lifestyle of an individual in a holistic manner whereby the modifiable risk factors are interdependent, leading to rapid progression towards CRC [48].
The interplay of modifiable risk factors, such as unhealthy dietary patterns and sedentary lifestyles, have partly contributed to the complexity of obesity to an extent [49]. Several epidemiological evidence have demonstrated that individuals with higher-than-normal BMI are likely to have a higher risk for CRC [8,49,50,51,52]. In a large, population-based cohort trial, obesity during early adulthood and a constantly increasing BMI throughout the lifespan were significantly associated with CRC [53]. Recent genetic studies suggested that for every one-unit increase in genetically predicted BMI, there is an increase in the odds ratios for CRC [54,55,56], indirectly implying the causality relationship between obesity and CRC. Although the current review reported statistically significant heterogeneity between studies with adjustment for BMI, future studies should consider the influence of residual confounding to delineate the true effect of BMI on CRC.
The review provides evidence on potential useful instrument for standardized comparison regarding the inflammatory potential of diet pertaining to CRC using an indexing approach. The score of DII can be interpreted both in continuous and categorical groups, contributing to the dynamic of its usability in the interpretation of future cancer-related research. Provided that the Asian region has the highest burden of CRC worldwide and that dietary intake is culturally specific, studies that explore the nutritional risk factors among multiracial Asian population are warranted. Moreover, the standardized calculation method of DII across all studies increased the comparability and thus also provides a valid description.
The limitation of the review includes the heterogenous nature of studies, including the study population characteristics, sample size, study design, and follow-up periods. The questionnaires used for food assessment were different and thus contributed to the recall bias. Nutritional assessment that excludes specific culture food items limits the ability to explore further regarding local influence on dietary pattern. Furthermore, the baseline calculation of DII score considered in the studies may not represent the true long-term dietary pattern, as adult diets vary over time. Despite substantial heterogeneity observed across the studies, subgroup analyses were performed to explore the source of heterogeneity. Grouping of DII score, study settings and adjustment factor for physical activity were likely related to the heterogeneity trend. Nonetheless, inevitable small sample size and confounding factors in each study contributed to the limitation potentially affecting the result.
5. Conclusions
Substantial evidence supported the association between inflammatory potential of diet and the development of CRC. However, future research involving multiethnic population, such as in Asian regions, is needed to explain the climbing CRC incidence and plan for preventive intervention strategies. Concerted efforts tailored to specific-culture dietary patterns call for multisectoral engagement in program planning to ensure effective outcome. Quantitative evidence as reported by the DII score and the impact towards CRC risk in the review helps to advocate to the public health authorities about the importance of tackling the underlying factors that shape the dietary pattern.
Acknowledgments
We would like to thank the team for their continuous commitment and efforts in the completion of the manuscript. We also thank the Dean of the Faculty of Medicine, Universiti Kebangsaan Malaysia, for his endless support and motivation.
Author Contributions
Conceptualization, S.S.S.S. and A.M.N.; methodology, R.H. and M.H.J.; validation, A.M.N., M.H.J. and Z.M.I.; formal analysis, S.S.S.S.; investigation, S.S.S.S.; resources, R.H.; data curation, S.S.S.S.; writing—original draft preparation, S.S.S.S. and H.-K.C.; writing—review and editing, H.-K.C. and M.R.A.H.; visualization, A.M.N.; supervision, M.R.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Kebangsaan Malaysia, grant number FF-2021-121.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IARC GLOBOCAN 2020-Cancer Today. [(accessed on 23 December 2021)]. Available online: https://gco.iarc.fr/today/home.
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Keum N.N., Giovannucci E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 4.Hofseth L.J., Hebert J.R., Chanda A., Chen H., Love B.L., Pena M.M., Murphy E.A., Sajish M., Sheth A., Buckhaults P.J., et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020;17:352–364. doi: 10.1038/s41575-019-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson D.C., Prochaska J.D., Yu X., Kaul S. An examination between census tract unhealthy food availability and colorectal cancer incidence. Epidemiol. Cancer. 2020;67:101761. doi: 10.1016/j.canep.2020.101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy N., Moreno V., Hughes D.J., Vodicka L., Vodicka P., Aglago E.K., Gunter M.J., Jenab M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Asp. Med. 2019;69:2–9. doi: 10.1016/j.mam.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Seiwert N., Heylmann D., Hasselwander S., Fahrer J. Mechanism of colorectal carcinogenesis triggered by heme iron from red meat. Biochim. et Biophys. Acta-Rev. Cancer. 2020;1873:188334. doi: 10.1016/j.bbcan.2019.188334. [DOI] [PubMed] [Google Scholar]
- 8.Atef N., Alieldin N., Sherif G., Loay I., Mahmoud A.M., Mohamed G. Microsatellite instability and life style factors in sporadic colorectal cancer. Asian Pac. J. Cancer Prev. 2020;21:1471–1480. doi: 10.31557/APJCP.2020.21.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seow A., Quah S.R., Nyam D., Straughan P.T., Chua T., Aw T.C. Food groups and the risk of colorectal carcinoma in an Asian population. Cancer. 2002;95:2390–2396. doi: 10.1002/cncr.10971. [DOI] [PubMed] [Google Scholar]
- 10.Park Y., Lee J., Oh J.H., Shin A., Kim J. Dietary patterns and colorectal cancer risk in a Korean population. Medicine. 2016;95:e3759. doi: 10.1097/MD.0000000000003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouvard V., Loomis D., Guyton K.Z., Grosse Y., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Mattock H., Straif K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 12.Hur J., Otegbeye E., Joh H.-K., Nimptsch K., Ng K., Ogino S., A Meyerhardt J., Chan A.T., Willett W.C., Wu K., et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut. 2021;70:2330–2336. doi: 10.1136/gutjnl-2020-323450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivappa N., Steck S.E., Hurley T.G., Hussey J.R., Hébert J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niclis C., Pou S.A., Shivappa N., Hébert J.R., Steck S.E., Díaz M.D.P. Proinflammatory dietary intake is associated with increased risk of colorectal cancer: Results of a case-control study in Argentina using a multilevel modeling approach. Nutr. Cancer. 2018;70:61–68. doi: 10.1080/01635581.2018.1397710. [DOI] [PubMed] [Google Scholar]
- 15.Fowler M.E., Akinyemiju T.F. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int. J. Cancer. 2017;141:2215–2227. doi: 10.1002/ijc.30922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;88:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkle C., Pendlebury D.A., Schnell J., Adams J. Web of Science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 2020;1:363–376. doi: 10.1162/qss_a_00018. [DOI] [Google Scholar]
- 18.Baas J., Schotten M., Plume A., Cote G., Karimi R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant. Sci. Stud. 2020;1:377–386. doi: 10.1162/qss_a_00019. [DOI] [Google Scholar]
- 19.Shivappa N., Wirth M.D., Hussey J.R., Hurley T.G. Perspective: The Dietary Inflammatory Index (DII)—Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019;10:185–195. doi: 10.1093/advances/nmy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Abulimiti A., Zhang X., Shivappa N., Hébert J.R., Fang Y.-J., Huang C.-Y., Feng X.-L., Chen Y.-M., Zhang C.-X. The dietary inflammatory index is positively associated with colorectal cancer risk in a Chinese case-control study. Nutrients. 2020;12:232. doi: 10.3390/nu12010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrd D.A., Judd S.E., Flanders W.D., Hartman T.J., Fedirko V., Agurs-Collins T., Bostick R.M. Associations of Novel Dietary and Lifestyle Inflammation Scores with Incident Colorectal Cancer in the NIH-AARP Diet and Health Study. JNCI Cancer Spectr. 2020;4:pkaa009. doi: 10.1093/jncics/pkaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho Y.A., Lee J., Oh J.H., Chang H.J., Sohn D.K., Shin A., Kim J. Genetic risk score, combined lifestyle factors and risk of colorectal cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2019;51:1033–1040. doi: 10.4143/crt.2018.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obón-Santacana M., Romaguera D., Gracia-Lavedan E., Molinuevo A., Molina-Montes E., Shivappa N., Hebert J.R., Tardón A., Castaño-Vinyals G., Moratalla F., et al. Dietary inflammatory index, dietary non-enzymatic antioxidant capacity, and colorectal and breast cancer risk (MCC-Spain study) Nutrients. 2019;11:1406. doi: 10.3390/nu11061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafiee P., Shivappa N., Hébert J.R., Nasab S.J., Bahrami A., Hekmatdoost A., Rashidkhani B., Sadeghi A., Houshyari M., Hejazi E. Dietary inflammatory index and odds of colorectal cancer and colorectal adenomatous polyps in a case-control study from Iran. Nutrients. 2019;11:1213. doi: 10.3390/nu11061213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma I., Zhu Y., Woodrow J.R., Mulay S., Parfrey P.S., Mclaughlin J.R., Hebert J.R., Shivappa N., Li Y., Zhou X., et al. Inflammatory diet and risk for colorectal cancer: A population-based case–control study in Newfoundland, Canada. Nutrition. 2017;42:69–74. doi: 10.1016/j.nut.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Shivappa N., Hébert J.R., Steck S.E., Hofseth L.J., Shehadah I., Bani-Hani K.E., Al-Jaberi T., Al-Nusairr M., Heath D., Tayyem R. Dietary Inflammatory Index and odds of colorectal cancer in a case-control study from Jordan. Appl. Physiol. Nutr. Metab. 2017;42:744–749. doi: 10.1139/apnm-2017-0035. [DOI] [PubMed] [Google Scholar]
- 28.Yuan F., Deng L., Sun X., Chen Z., Shivappa N., Sheth A.K., Cooper G.S., Hebert J.R., Li L. Dietary inflammatory index and risk of colorectal adenoma: Effect measure modification by race, nonsteroidal anti-inflammatory drugs, cigarette smoking and body mass index? Cancer Causes Control. 2021;32:837–847. doi: 10.1007/s10552-021-01436-y. [DOI] [PubMed] [Google Scholar]
- 29.Brouwer J.G.M., Makama M., van Woudenbergh G.J., Vasen H.F., Nagengast F.M., Kleibeuker J.H., Kampman E., Van Duijnhoven F.J. Inflammatory potential of the diet and colorectal tumor risk in persons with Lynch syndrome. Am. J. Clin. Nutr. 2017;106:1287–1294. doi: 10.3945/ajcn.117.152900. [DOI] [PubMed] [Google Scholar]
- 30.Harmon B.E., Wirth M.D., Boushey C.J., Wilkens L.R., Draluck E., Shivappa N., Steck S.E., Hofseth L., Haiman C.A., Marchand L.L. The Dietary Inflammatory Index is associated with colorectal cancer risk in the multiethnic cohort. J. Nutr. 2017;147:430–438. doi: 10.3945/jn.116.242529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratjen I., Shivappa N., Schafmayer C., Burmeister G., Nothlings U., Hampe J., Hebert J.R., Lieb W., Schlesinger S. Association between the Dietary Inflammatory Index and All-Cause Mortality in Colorectal Cancer Long-Term Survivors. Int. J. Cancer. 2019;144:1292–1301. doi: 10.1002/ijc.31919. [DOI] [PubMed] [Google Scholar]
- 32.Tabung F.K., Steck S.E., Ma Y., Liese A.D., Zhang J., Lane D.S., Ho G.Y.F., Hou L., Snetselaar L., Ockene J.K., et al. Changes in the inflammatory potential of diet over time and risk of colorectal cancer in postmenopausal women. Am. J. Epidemiol. 2017;186:514–523. doi: 10.1093/aje/kwx115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J., Tabung F.K., Zhang J., Murphy E.A., Shivappa N., Ockene J.K., Caan B., Kroenke C.H., Hébert J.R., Steck S.E. Post-cancer diagnosis dietary inflammatory potential is associated with survival among women diagnosed with colorectal cancer in the Women’s Health Initiative. Eur. J. Nutr. 2020;59:965–977. doi: 10.1007/s00394-019-01956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wesselink E., Staritsky L.E., van Zutphen M., Geijsen A.J., Kok D.E., Kruyt F., Veenstra R.P., Bilgen E.J.S., Kouwenhoven E.A., de Wilt J.H., et al. The association between the adapted dietary inflammatory index and colorectal cancer recurrence and all-cause mortality. Clin. Nutr. 2021;40:4436–4443. doi: 10.1016/j.clnu.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Liang Y., Jiao H., Qu L., Liu H. Positive association between dietary inflammatory index and gastric cancer risk: A systematic review and meta-analysis. Nutr. Cancer. 2020;72:1290–1296. doi: 10.1080/01635581.2019.1679197. [DOI] [PubMed] [Google Scholar]
- 36.Eckert-dreher R.G., Coelho D., Zibetti A.W., Felipe K.B., Wilhelm-filho D., Pedrosa R.C. Dietary Patterns and Empirical Dietary Inflammatory Index in Southern Brazil and Risk of Colorectal Cancer: A Case-Control Study. Food Nutr. Sci. 2020;11:281–300. doi: 10.4236/fns.2020.114021. [DOI] [Google Scholar]
- 37.Farazi M., Jayedi A., Shab-Bidar S. Dietary inflammatory index and the risk of non-communicable chronic disease and mortality: An umbrella review of meta-analyses of observational studies. Crit. Rev. Food Sci. Nutr. 2021:1–10. doi: 10.1080/10408398.2021.1943646. [DOI] [PubMed] [Google Scholar]
- 38.Namazi N., Larijani B., Azadbakht L. Association between the dietary inflammatory index and the incidence of cancer: A systematic review and meta-analysis of prospective studies. Public Health. 2018;164:148–156. doi: 10.1016/j.puhe.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Sun D., Cao M., Li H., He S., Chen W. Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin. J. Cancer Res. 2020;32:129–139. doi: 10.21147/j.issn.1000-9604.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steck S.E., Guinter M., Zheng J., Thomson C.A. Index-based dietary patterns and colorectal cancer risk: A systematic review. Adv. Nutr. 2015;6:763–773. doi: 10.3945/an.115.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivappa N., Godos J., Hébert J.R., Wirth M.D., Piuri G., Speciani A.F., Grosso G. Dietary inflammatory index and colorectal cancer risk—a meta-analysis. Nutrients. 2017;9:1043. doi: 10.3390/nu9091043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulpen M., van den Brandt P. A Mediterranean diet adherence and risk of colorectal cancer: The prospective Netherlands Cohort Study. Eur. J. Epidemiol. 2020;35:25–35. doi: 10.1007/s10654-019-00549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong M.C., Ding H., Wang J., Chan P.S., Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intest. Res. 2019;17:317–329. doi: 10.5217/ir.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khil H., Kim S.M., Hong S.E., Gil H.M., Cheon E., Lee D.H., Kim Y.A., Keum N. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-81877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hur S.J., Jo C., Yoon Y., Jeong J.Y., Lee K.T. Controversy on the correlation of red and processed meat consumption with colorectal cancer risk: An Asian perspective. Crit. Rev. Food Sci. Nutr. 2019;59:3526–3537. doi: 10.1080/10408398.2018.1495615. [DOI] [PubMed] [Google Scholar]
- 46.Bishehsari F., Mahdavinia M., Vacca M., Malekzadeh R., Mariani-Costantini R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J. Gastroenterol. 2014;20:6055–6072. doi: 10.3748/wjg.v20.i20.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kenkhuis M.F., van Roekel E.H., Breedveld-Peters J.J.L., Breukink S.O., Janssen-Heijnen M.L.G., Keulen E.T.P., VAN Duijnhoven F.J.B., Mols F., Weijenberg M.P., Bours M.J.L. Longitudinal Associations of Sedentary Behavior and Physical Activity with Quality of Life in Colorectal Cancer Survivors. Med. Sci. Sports Exerc. 2021;53:2298–2308. doi: 10.1249/MSS.0000000000002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soffian S.S.S., Nawi A.M., Hod R., Chan H.K., Hassan M.R.A. Area-level determinants in colorectal cancer spatial clustering studies: A systematic review. Int. J. Environ. Res. Public Health. 2021;18:10486. doi: 10.3390/ijerph181910486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sko F., Carlsson A.C., Schmidt P.T., Forsberg A.M. The prediction of colorectal cancer using anthropometric measures: A Swedish population-based cohort study with 22 years of follow-up. United Eur. Gastroenterol. J. 2019;7:1250–1260. doi: 10.1177/2050640619854278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carr P.R., Amitay E.L., Jansen L., Alwers E., Roth W., Herpel E., Kloor M., Schneider M., Bläker H., Chang-Claude J., et al. Association of BMI and major molecular pathological markers of colorectal cancer in men and women. Am. J. Clin. Nutr. 2020;111:562–569. doi: 10.1093/ajcn/nqz315. [DOI] [PubMed] [Google Scholar]
- 51.Li H., Boakye D., Chen X., Hoffmeister M., Brenner H. Association of Body Mass Index with Risk of Early-Onset Colorectal Cancer: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. Suppl. 2021;116:2173–2183. doi: 10.14309/ajg.0000000000001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J.-B., Luo S., Wong M.C.S., Li C., Feng L.-F., Peng J.-H., Li J.-H., Zhang X. Longitudinal associations between BMI change and the risks of colorectal cancer incidence, cancer-relate and all-cause mortality among 81,388 older adults. BMC Cancer. 2019;19:1082. doi: 10.1186/s12885-019-6299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng R., Du M., Zhang B., Xin J., Chu H., Ni M., Zhang Z., Gu D., Wang M. Body mass index (BMI) trajectories and risk of colorectal cancer in the PLCO cohort. Br. J. Cancer. 2018;119:130–132. doi: 10.1038/s41416-018-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.David J., Mitchell J.S., Law P., Palin K., Tuupanen S., Gylfe A., Hänninen U.A., Cajuso T., Tanskanen T., Kondelin J., et al. Mendelian randomisation analysis strongly implicates adiposity with risk of developing colorectal cancer. Br. J. Cancer. 2016;115:266–272. doi: 10.1038/bjc.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thrift A.P., Gong J., Peters U., Chang-Claude J., Rudolph A., Slattery M.L., Chan A.T., Locke A.E., Kahali B., Justice A.E., et al. Mendelian randomization study of body mass index and colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2015;24:1024–1031. doi: 10.1158/1055-9965.EPI-14-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki S., Goto A., Nakatochi M., Naritaet A., Yamaji T., Sawada N., Katagiri R., Iwagami M., Hanyuda A., Hachiya T., et al. Body mass index and colorectal cancer risk: A Mendelian randomization study. Cancer Sci. 2021;112:1579–1588. doi: 10.1111/cas.14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.