Abstract
Background
3D printing is a promising new technology for medicines' production. It employs additive manufacturing techniques, and is ideal for producing personalized medicines (e.g., patient-tailored dose, dosage form, drug release kinetics).
Objective
To investigate how 3D printing technologies can be implemented in a European pharmaceutical system, by suggesting different scenarios and assessing aspects that could affect its implementation.
Method
Qualitative, semi-structured interviews were conducted with key stakeholders (e.g., from ministry, authorities, research organizations, pharmacies) in the Netherlands to elicit perspectives on 3D printing of personalized medicines. The Netherlands were chosen since it has a strong tradition in compounding. Five general scenarios were investigated: placing the 3D printers in industry, community pharmacies, hospital pharmacies, compounding facilities, and in patients' homes. Content analysis was used, building on verbatim transcripts.
Results
Fifteen stakeholders were interviewed. Regulatory, economic, ethical and organizational challenges were identified to varying degrees in the different scenarios. The industry and home scenarios were associated with the most challenges, hospital pharmacies and compounding facilities with the least. Other important aspects identified were the role of community pharmacies, and who should design the tablets to be printed.
Conclusion
All potential scenarios for 3D printing of personalized medicines include challenges. These should be taken into account when pursuing the use of 3D printing of medicine.
Keywords: Personalized medicine, Compounding, Magistral medicines, 3D printing, Societal impact, Qualitative interviews, Extemporaneous preparation
1. Introduction
The increasing awareness about individual variation in drug response has prompted questioning of the pharmaceutical industry's “one-size-fits-all” approach, and a re-thinking of the current medicines' development and production model into that of personalized medicines.1,2 Personalized medicine is broadly defined as “Providing the right treatment to the right patient, at the right dose at the right time”.3 In this work, personalized medicines are discussed in terms of patient-tailored dose, dosage form and its design, and drug release kinetics regarding what would be the most beneficial and available for an individual on-demand, considering all relevant patient characteristics (age, weight, sex, co-morbidities, medicines intake, physiology, genetics, metabolism, lifestyle, routines and preferences, etc.).
Although one could question the feasibility of personalizing medicines on an individual level, individual medicines' production is already common practice. Compounding, also known as preparation of extemporaneous or magistral medicines, has always been an integral role of pharmacists within the pharmacy setting, although this role has been substantially reduced due to the increase of production by the pharmaceutical industry. Today, compounding in pharmacies is mostly regarded as a small-scale practice, reserved for special cases in which individual patients need a medicine that is not registered, not available, or that needs special altering in order to meet their specific needs. However, the rise of the personalized medicine concept places small-scale compounding into a new light.4
3D printing is a new technology for medicines production. It employs different additive manufacturing techniques that can produce tablets by adding layer upon layer, using a computer-aided design.2,5, 6, 7, 8, 9 While thus far, only one 3D printed drug has received FDA approval for large-scale industrial production and is now on the market,10 and one is on its way,11 it is important to note that there is a fundamental difference when discussing printing in the context of large-scale manufacturing in the industry setting or small-scale compounding in the pharmacy setting.
3D printing is a suitable technology for producing personalized medicines. It has the flexibility needed to adjust the dosage, the release profile and the physical appearance (e.g. size, shape, color, em(de)bossing) of drug delivery systems to the individual patient needs.12 A few studies have recently been conducted to showcase the feasibility of 3D printing in clinical settings as a (better) alternative to the existing compounding practice with regards to cost and safety and overall benefits to patients.13,14 Furthermore, 3D printed chewable medicines appeared to be more precise and easily customizable in terms of size (different doses) and organoleptic characteristics (color and taste) in a hospital setting.15 3D printing can also be used to create so-called ‘polypills’ that combine multiple active pharmaceutical ingredients (APIs) with unique release kinetics and possibility for physical isolation (e.g., in case of incompatibility) for each API in a single dosage unit,9,16 and enables the possibility of creating tablets with different tastes, which could further improve patient adherence.2,17 Researchers are currently developing the technology, i.e., the printers and software, and finding appropriate excipients to yield the optimal dose precision, release of APIs, stability and safety.7,15,18, 19, 20 While such technical developments are crucial for the efficacy of the technology, it is also important to take into consideration the societal aspects in order to fully comprehend its overall effectiveness.21 Societal aspects play an important role when integrating the new technology into the existing pharmaceutical system and assessing the feasibility of implementation in the real world. However, many of the legal, regulatory, ethical, organizational and social aspects regarding future 3D printing of personalized medicines remain unknown, for example, the locations where printing would take place, and the regulatory implications thereof.22
The Netherlands was chosen as a case study to explore future implications of 3D printing in society because of the current interest in compounding in this country. While it is a relatively small country, the impact of compounding on society, which is related to the proportion within the country, is high. About 18% (approximately 350/2000) of community pharmacies and an almost all (80) hospital pharmacies in the Netherlands are engaged in compounding, at least to some extent.23,24 In addition, there are approximately 20 compounding facilities (“doorleverende apotheken”) with a pharmacy status, which function as small industries and produce mostly officinal medicines in bulks, but also magistral preparations for named patients.25 Although the extent of compounding in community pharmacies and small hospital pharmacies in the Netherlands has declined over the past decades, mostly due to costs, compounded medicines still consist of approximately 5% of prescription medicines in the country,26 making it a relatively productive European country.27 More importantly, compounding has recently gained a renewed interest in the Netherlands as the cost of specialized medicines has skyrocketed and drug shortages have become more frequent.4 In this setting, common compounding practices and the trend towards personalized medicines on one hand, and the political discussion on costs and a sustainable healthcare system on the other hand, could provide a fruitful soil for a new technology such as 3D printing of medicines.
Another reason for choosing the Netherlands as a case study was due to the fact that some health authorities in the Netherlands have already started discussions around the implementation of 3D printing of pharmaceuticals. This discussion was initiated by the Netherlands Organization of Applied Scientific Research (TNO) that engaged stakeholders in the Netherlands with 3D printing in order to develop a case on 3D printers specifically designed for small-scale printing of medicines.28 3D printing of paediatric medicines has been recently initiated for treatment of heart diseases in clinical settings.29 The overall research aim of this study was to investigate how 3D printing technologies can be implemented in a European pharmaceutical system, by suggesting different scenarios and assessing several aspects that could affect implementation. Stakeholders in the Netherlands were interviewed about their perspectives on 3D printing of personalized medicines.
2. Methods
An exploratory, qualitative method was chosen to investigate this relatively unexplored field. Data were collected through semi-structured interviews with stakeholders currently engaged in or dealing with compounding in some way in the Netherlands. This included stakeholders involved in pharmaceutical regulation, policymaking, research and pharmaceutical practice. Relevant institutions were asked to propose a representative for interviewing. Additional informants were found through the snowball approach, i.e., asking interviewees to name relevant stakeholders to be included in the study. Interviewees were generally approached by e-mail and after indicating interest, an information letter and a consent form were sent. We continued recruiting respondents until data saturation had been reached, i.e. no new overall aspects were presented, and no new interviewees/organizations proposed.30,31
The interview guide was primarily informed by earlier studies7,16,17,22,32 but also by discussions in the researcher group. It was pilot tested, on persons with some knowledge on 3D printing, however not familiar with the Dutch context, with no subsequent changes. The main components of the semi-structured interview guide (see appendix A) remained constant for all interviews, while specific issues were highlighted according to the expertise of the interviewee. Interviewees were aquatinted with 3D printing, but a short overview of the technique was first presented to assure that there was a mutual understanding of the concept. Interviewees were then asked about current compounding practices in the Netherlands, advantages and disadvantages of 3D printing, and where and how they considered that 3D printers could be integrated in the current Dutch system. Then visual tools were used to present and explain 5 scenarios to the respondents (see figures in the result section), and they were asked to reflect on special considerations, challenges and opportunities, especially regarding legal, regulatory, safety, economic and organizational aspects in each scenario. The scenarios included placing the 3D printers in: industry; community pharmacies; hospital pharmacies; compounding facilities serving a) the extramural setting and b) the intramural setting; in the patients' home.
Most interviews were undertaken face-to-face (one through Skype), conducted in English by 2 researchers, whereof 1 was Dutch speaking. NB, with a background in global health, facilitated all interviews. IH, TLA and JH spoke Dutch and have a background in pharmacy; they were alternately present at the interviews. All interviewers had experience in qualitative research, including interviewing. Respondents did not receive any incentives. All interviews were audio-recorded and transcribed verbatim. The transcriptions were reviewed by one or more co-authors before being sent back to the respondent. The respondents reviewed and approved the transcriptions and responded to queries for further clarification. Finalized transcriptions, in total consisting of 121,249 words, were then imported into NVivo (version 12.6) and coded for analysis. Content analysis was used to systematically describe findings by structuring and classifying them into categories and themes.31,33 The analysis was primarily done by NB, while SKS reviewed the analysis and independently categorized some of the interviews. The identified categories were compared and discussed until consensus was reached. All authors, including the researchers who were present at the interviews, then validated the analysis, i.e., checked that the analysis was depicting the data and that no important perspectives were omitted.
The findings in this article focus on a subset of data relating to the settings and scenarios in which printing could take place and complementary findings that are related to the scenarios.
The processing of personal data in the study was approved by The Faculty of Health and Medical Sciences at University of Copenhagen (Ref. no.: 514–0342/19–3000) with no additional approval required for ethical clearance.34 Written informed consent was obtained from all participants prior to conducting the interviews, and participants' anonymity was safeguarded throughout the project. Each respondent was allocated a number as shown alongside any quotes in the results section.
Interviews were conducted from May to November 2019. The organizations/stakeholders represented can be seen in Table 1.
Table 1.
Organizations represented in interviews.
| Ministry of Health, Welfare and Sport (VWS) Medicines Evaluation Board (CBG) Two representatives from the Health and Youth Care Inspectorate (IGJ) National Health Care Institute (ZIN) National Institute for public health and environment (RIVM) Netherlands Organization for applied scientific research (TNO) Menzis (a health insurer) Royal Dutch Pharmacists Association (KNMP) International Pharmaceutical Federation (FIP) Lygature (a non-profit research enabler) Two hospital pharmacists One community pharmacist One pharmacist from a compounding pharmacy |
3. Results
In all 14 semi-structured interviews were conducted with 15 Dutch stakeholders (one interview with 2 respondents). Ten of the 15 respondents were pharmacists by training. Interviews lasted between 40 and 90 min.
Findings are presented in the following order:
-
•
Respondents' own suggestions for settings in which printing can be implemented
-
•
Reflections on the presented scenarios
-
•
Opinions on the importance of involving community pharmacies in the production and distribution process (relevant to the industry and home scenarios)
-
•
Reflections on where the tablet-design should be performed
3.1. Respondents own suggestions for settings
Before presenting the scenarios, respondents reflected upon how this new technology could fit into or change current pharmaceutical production and distribution practices. One respondent talked about the need to establish a “value chain” from supply of materials and printers to printing the tablets and distributing them to patients. When enquired about where printing could take place, respondents considered, among other things, the level of specialized knowledge needed to operate 3D printers, logistical issues, safety and liability, legislation and regulation, and economic feasibility.
Suggested settings in which printing could take place, included pharmacies in general, with respondents claiming that since compounding was already in their mandate, it was the most logical location for this activity. However, some respondents limited the pharmacies that should print to large hospital pharmacies and compounding facilities. They stated that many community pharmacies had stopped compounding, and therefore no longer had suitable staff nor facilities. Some respondents even suggested creating expert regional centers for 3D printing, as they considered 3D printing to be highly specialized and requiring extensive knowledge and expertise.
“Well, I think because it is personalized medicines it might not fit in the industry actually, so I can also not imagine it likely that every single pharmacy will have one. So it has to be something in between and then maybe the hospitals or compounding pharmacies. So yes, that level.” (Int 5).
Conversely, one respondent suggested having the printer at the doctor's office, and a few respondents mentioned the option of having the patients print their medication at home. Some respondents suggested that new types of pharmaceutical production would emerge, either industry would shift more into healthcare or pharmacies would specialize in 3D printing and become more like small pharmaceutical industries.
One respondent mentioned that the pharmaceutical industry might not be pleased with such changes, as it might weaken its position as the nearly sole medicinal product manufacturer.
“There is a lot of economic incentive from the pharmaceutical industry to keep this sector intact. So they also will push back when you try to implement this.” (Int 13).
3.2. Reflections on presented scenarios
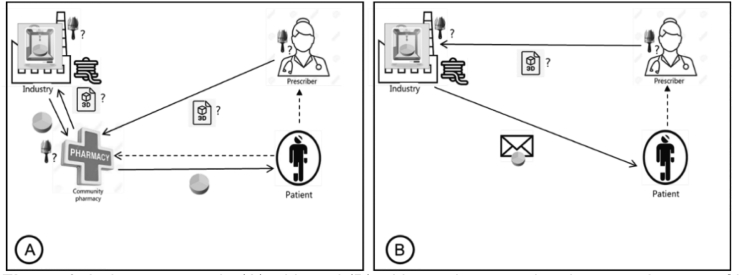
Regarding the industry scenarios (Fig. 1 A and B), some respondents believed that pharmaceutical companies would not be interested in printing personalized medicines as they are focused on mass production of large batches and would not want to deal with individual preparations. However, most respondents agreed that if 3D printing of personalized medicines was financially attractive and was to fit their business model, then they would likely be willing to engage in this new type of production. Some respondents thought that smaller and generic industries were more likely to be interested in personalized medicines than big pharmaceutical companies. One respondent stated that as the future of medicine shifts towards personalized medicines, industry will need to adapt to be able to meet such needs.
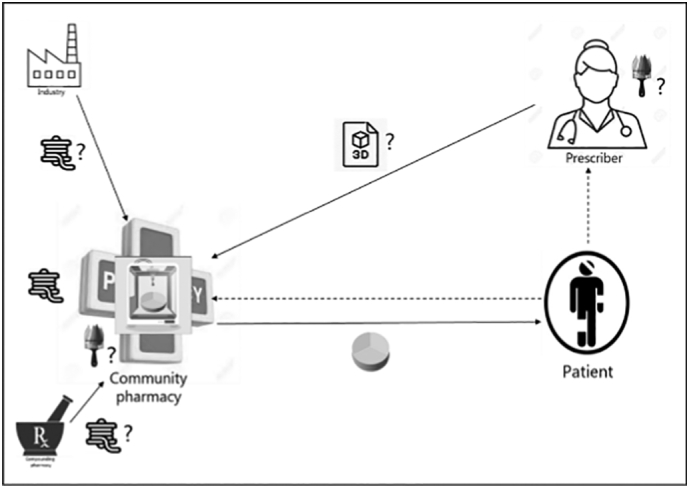
Fig. 1.
Industry scenario (A) with and (B) without pharmacy involvement. In case of hospitalization of the patient, the hospital pharmacy will be involved in scenario 1A.
Pictograms represent for all figures:  tablet design (i.e., the decisions on design, e.g., dose, physical appearance, release kinetics);
tablet design (i.e., the decisions on design, e.g., dose, physical appearance, release kinetics);  intermediate product (e.g., drug-loaded filament, prefilled cartridges, etc.);
intermediate product (e.g., drug-loaded filament, prefilled cartridges, etc.);  provision of the final digital file of the tablet design to be directly printed;
provision of the final digital file of the tablet design to be directly printed;  3D printed tablet;
3D printed tablet;  remote delivery to the patient;
remote delivery to the patient;  3D printing of tablets;? where the action could take place; ___ (solid line) transfer of something physical; --- (dashed line) not given anything physical.
3D printing of tablets;? where the action could take place; ___ (solid line) transfer of something physical; --- (dashed line) not given anything physical.
It was mentioned that a disadvantage of 3D printing in the industry is that the printing would take place remotely, which would create a lag time before patients receive their medicine.
“It all depends on how long it takes to deliver the medicine to the patient. If it takes a long time, for example longer than a week, I think then it is not feasible. But if industry works very efficient and flexible, that can be deliver medication within a few days to the patient. That could be a good alternative.” (Int 3).
Respondents mentioned that a regulatory requirement for the industry is to obtain a marketing authorization in order to be able to sell a product on the market, which requires providing specific details on the medicine's content and dose. Respondents thought it would therefore be extremely difficult for the industry to be flexible and adapt either content or dosage, while such tailoring is crucial to personalize medicines at an individual level.
“They need a marketing authorization for products that they deliver to the patient, and if you have a variation in how the pill is printed then there is a challenge in how you get your marketing authorization with a range of different products …. Now it's all fixed. You have a marketing authorization for the pill of 100 milligrams.” (Int 12).
Although this restriction exists, one respondent referred to article 40.3.c in the Dutch Medicines Act,35 where it is stated that in extraordinary exceptions, the pharmaceutical industry is, in principle, allowed to manufacture an unlicensed medicinal product adapted to an individual patient according to the specifications set by the physician and with a specific permit of the Inspectorate. Also, several respondents gave the example of advanced therapy medicinal products (ATMPs), such as CAR-T cell treatments approved by the European Medicines Agency (EMA) whereby the industry is providing personalized medicines at an individual level according to an authorized procedure.
“If you look at the very new treatments, the CAR-T cell treatments which is basically modifying genetic material tissue from a human and bring it back, is not done in a pharmacy, it is done by the industry and delivering to the pharmacy.” (Int 8).
Some respondents discussed other forms of flexibility in industry regulations; although they would not solve the problem of having to register each and every dosage, there was potential for adaptation of regulations in the future. “Bracketing” and “matrixing” were mentioned as examples of how within a dose range, not all dosages need to be tested, but it is sufficient to test the highest and lowest doses and infer on those in-between. Another concept that was mentioned was “quality by design”. This refers to the medicine's manufacturing process that allows some flexibility based on product knowledge and experience, applying ranges to certain manufacturing requirements.
Quality control challenges, such as the inability to properly test the final product when small amounts of medicine are prepared, would, according to respondents, likely have the strongest effect on the industry setting where regulation was more stringent. Extensive validation and compliance to good manufacturing practice (GMP) regulations of the printing process would need to be in place, including the 3D printers, software and cleaning procedures.
Compounding using 3D printers in a community pharmacy setting (Fig. 2) was considered an acceptable option for all respondents. However, the type of pharmacy where compounding could be done, differed.
Fig. 2.
Community pharmacy scenario.
Some respondents saw 3D printing as a logical step for community pharmacies. Since they already had direct contact with both patients and physicians, and had the legal authority to compound, it seemed only natural that pharmacies would take on this task. Pharmacists were identified as professionals who could successfully carry out the role of printing and also be responsible for the maintenance of the printers and ordering and handling of materials.
Some respondents mentioned that even pharmacists who do not currently compound, might still wish to do so in order to provide the best possible service to their patients. If 3D printing was to remove the economic constraints of compounding by lowering costs, respondents thought that many pharmacists might opt to start compounding again. 3D printing could also grant pharmacists a better position in the healthcare system, and strengthen their status as healthcare providers.
“…I think, especially when you are going to make something for personal fit, you want to have feedback from the person himself. So you can create this healthcare relationship between pharmacists and patient and strengthen it by making the personal fit and… get feedback on the therapy.” (Int 13).
However, many respondents were concerned about the costs of this new technology. Most respondents expected that the printers would be very expensive, and that their cost would be higher as their quality increased. Therefore, respondents thought that purchasing a printer in the pharmacy setting would be a large investment that would need to pay off in the long-run. At the same time, respondents expected the prices of printers to decrease over time, as often happens when a technology is scaled up. Purchasing pre-filled cartridges was considered as another high cost of the 3D printing system. Turnover was mentioned as a major factor that would determine the cost-effectiveness of the technology, and it would depend on the number of patients and the total amount of hours the printer would run. Turnover and the extent of printing would, according to some respondents, not only determine cost-effectiveness, but also affect the experience of pharmacists, as well as the acquisition and storage of starting materials.
“Where a facility will be located, it all depends on the costs of 3D printing the cost of ordering filaments and keeping those filaments because the filament has also a certain duration, and if you only use one filament, one drop off the filament every 2 years it will not be cost-effective… it all depends how cost-effective it is... It cannot be done in a community pharmacy. I think the turnover is too low and the skills (need) to be maintained.” (Int 3).
Some respondents expressed concerns that community pharmacists might not be sufficiently trained in compounding and therefore unable to grasp the printing technique.
“You must not be prepared to put them in a community pharmacy. I think it's too specialized and especially because you need knowledge [on] what you are doing.” (Int 9).
Respondents also emphasized the need for safeguards to minimize errors and increase the safety of printed medicines. These included creating traceable barcodes on cartridges to ensure the correct cartridge was being used, and error messages embedded in the software and printers to alert if any faults were detected.
Other concerns included lack of space. Respondents suggested that many pharmacies do not have enough room to stock medicines, therefore finding a place to set up the printer(s) and store pre-filled cartridges or raw materials would be difficult. In addition, the issue of cross-contamination was seen as especially relevant in the community pharmacy setting, as there would most likely be one machine to print different medicines for the patients. As a solution, disposable parts such as nozzles and building trays were suggested.
Liability was also raised by respondents, since the 3D printing technology is built from several components which would likely be developed and executed by different entities: the printer, the software, the materials (such as pre-filled cartridges), and those carrying out the printing. Thus, a question raised was who would be responsible and liable if something goes wrong with the printed medicine? Currently, within conventional compounding, the pharmacist takes responsibility for the medicines being compounded. One respondent suggested it would be best to try and make the entire printing system a “closed circuit” including the printer, software and cartridges, so that liability could be contained to one responsible entity.
Regarding the pharmacists' likelihood to accept this new technology, respondents expected that, as in any other technology, there would likely be pharmacists who are more open to innovation who would become the early adopters and front-runners, while others would perhaps be more reluctant to change. Yet respondents mentioned that pharmacists should adopt the technology, as compounding is part of the pharmacists' profession and knowledge, and it would be unfortunate if this 3D printing niche would be taken over by another profession.
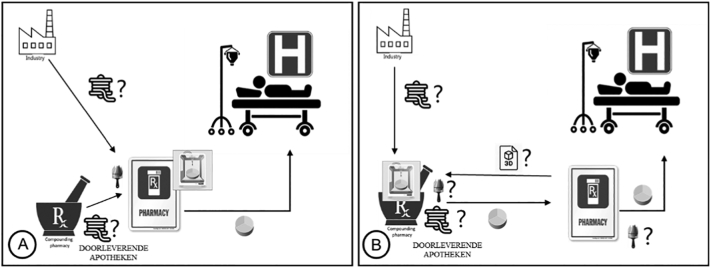
In the hospital scenario (Fig. 3A and B), respondents differentiated between large university hospitals and smaller community hospitals as pharmacies of smaller community hospitals are more comparable to community pharmacies with respect to their scale of compounding. Respondents believed that large university hospital pharmacies would be more likely to adopt 3D printing technology, as within this setting the technology would probably be more economically viable, as they have larger compounding facilities, more patients requiring specialized care, and are perceived as more open to innovation and better equipped to meet regulatory requirements. Some respondents added that smaller hospitals would be more likely to acquire printed medicines from either bigger hospitals or compounding facilities. Others claimed, however, that user-friendly printers should be developed for use even in smaller hospitals.
Fig. 3.
Hospital scenario, where 3D printing of dosage forms happens at (A) hospital pharmacy or (B) compounding facility. (Doorleverende apotheken = compounding facilities with a pharmacy status.)
Respondents also mentioned that hospital pharmacies in general would probably have more facilities for compounding. Also that hospital (clinical) pharmacists were viewed as better trained and skilled in compounding, as this is part of their specific education, than community pharmacists, and therefore more competent to compound with 3D printing technology.
“I think hospital pharmacists are reasonably skilled in compounding…. They use extra time on compounding in their education” (Int 6).
Respondents generally agreed that 3D printing in compounding facilities (Fig. 4) would be a good option. Many stated that since compounding facilities were already producing medicines in large quantities, there would be no major difference whether compounding would take place through 3D printing or conventional techniques. In this setting the turnover and economic viability were more favorable, as these facilities catered for more patients.
“If the community pharmacy doesn't have the facilities, I think this is a scenario that works well for other drugs, so why wouldn't it work for 3D printing?” (Int 2).
Fig. 4.
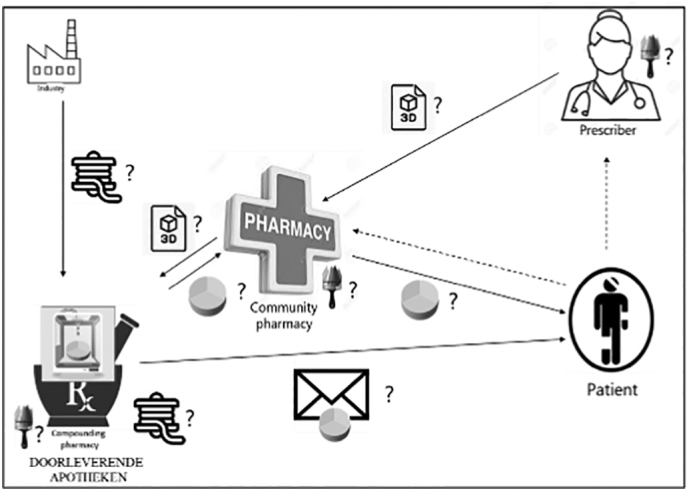
Compounding pharmacy scenario.
Although compounding facilities are registered as pharmacies, they have no counter where patients can receive their medication nor direct contact with patients, and it was mentioned that a community pharmacy (or hospital) would be required for dispensing the medicine.
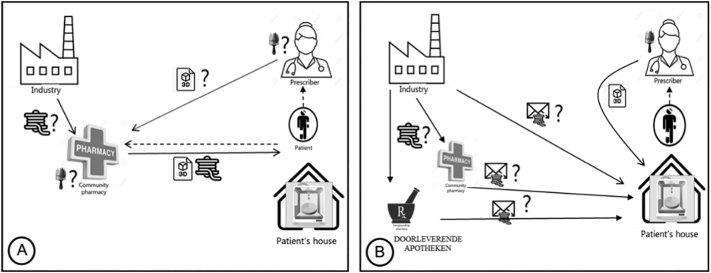
Although placing the 3D printers in the patient's home (Fig. 5A and B) was seen as a far-off futuristic scenario, most respondents did not dismiss it entirely, admitting it was an interesting “thinking outside the box” approach. For some it suited the trend of enabling patients to get more treatments at home and recognizing patient autonomy. Although most respondents did not consider it a feasible scenario in the near future, they envisioned this option in the long term:
Fig. 5.
Home scenarios (A) with and (B) potentially without pharmacy involvement.
“I think you need a skilled person in these things, and maybe in the end, well it's the same with other inventions, in time… people get accustomed to certain things so if people are accustomed to using 3D printing with all other things, maybe it's easier to adapt than now. But I think all regulations, they are not allowing this.” (Int 6).
The biggest concerns for respondents were regulatory and safety aspects, when printing at home. The first hurdle in the legal interpretation was that pharmacies are only allowed to dispense a registered, finished product to the patients, and thus providing patients with a pre-filled cartridge would not be possible. Secondly, the question was whether patients would be allowed to compound for their own use, given that compounding is only allowed in pharmacies and by pharmacists. One respondent suggested that in the context of home printing, the printer would need to be considered as a medical device.
“When it [the printer] is here [in the pharmacy], you can see it as a part of the machinery to make the product. When it's here [home] it's still the same product but it's not possible to see it as part of the machinery because this is not a pharmaceutical industry and it's also not a public pharmacy. So the only way to make this a legal option is to see it as a medical device… in combination with the cartridge, I think.” (Int 11).
Respondents thought that while some patients would be capable and responsible enough to print, others would be unable to do so. Some feared that those who need this technology the most will be the ones who are unable to print at home.
“We're heading towards…a division between the haves and the have-nots...I think it'll be a divide within healthcare again, between the tech-savvies and the non-tech-savvies.” (Int 8).
Some respondents felt patients could not print by themselves since they lacked knowledge about the printing process and would not know how to apply in-process and final product quality controls.
“You basically make the patient responsible for the quality check of the final product and I don't think we should do that. So I think from a patient safety point of view, and the responsibility you lay here on the patient, is too much.” (Int 5).
It was suggested that if 3D printing were to take place at home, very reliable control mechanisms would need to be put in place to prevent mistakes and printer misuse. These would include making the printing process as easy and safeguarded as possible, employing methods mentioned above under the community pharmacy scenario. Additionally, it was suggested that printers could be remotely controlled by professionals.
However, even if safeguards were in place, respondents thought that other factors should be taken into consideration. First, safe printing does not only depend on the actual printing, but also on other factors such as keeping the printer and working location clean, and handling maintenance. Other safety concerns included the presence of small children who might have access to the printed medicine, or accidently switching medication among members of the household. Another issue raised was intentional misuse and medicines' production from leftover cartridges or the access to production of addictive medicines such as opioids. Several respondents expressed concern about the potential misuse of this technology for production of recreational drugs with psychotropic effects, such as ecstasy.
For the reasons mentioned above, the need for a professional to be engaged in the home printing process was emphasized. It was suggested that the pharmacists should be closely involved in 3D printing at the patient's home, by programing the printers or having other means of controlling the printing.
“The pharmacist, he delivers the printing machine the recipe and the cartridges to the patient. Then he is responsible for the… filling of the cartridge and things like that…I think that's maybe a different situation.” (Int 6).
Here too, the issue of liability was raised, as respondents questioned who would be responsible for ensuring that the patient prints correctly, and who would be accountable if something went wrong. Continuity of care was also raised by one respondent who was concerned about solutions for patients who are suddenly unable to print either due to technical or other reasons. In these cases, they would have no alternative means of getting their medication in a timely manner, whereas if the medication had been printed in a pharmacy, pharmacists would be responsible for finding an alternative. Other logistical issues were also raised, such as who would be in charge of ordering cartridges, and who would adjust the file when dose adjustments were needed.
The economic burden was also more pronounced when 3D printing was meant only for one patient or one household. It was suggested by respondents that it would be viable only for very expensive medicines or for very specific patient groups with unmet needs. One respondent even suggested a scheme whereby 3D printers could be rented out for needed periods and then borrowed to other patients.
Some respondents questioned the benefit of home printing versus printing in the pharmacy setting. A few suggested that it might be useful in sparsely populated countries where there are large distances between the pharmacy and patients, but less relevant to the Netherlands. A few respondents suggested this scenario could be useful in situations where the formulation is not stable and must be printed in proximity to the time of administration.
Examples of patients undertaking some form of compounding at home included hormonal treatments whereby patients prepared injections using registered products. Syringe pumps and different IV medication such as Total Parenteral Nutrition (TPN) were also mentioned. However, none of the examples included a production step as is the case in 3D printing.
3.3. The role of the community pharmacy
In both the industry and home scenarios, the option of bypassing the pharmacy in delivering 3D-printed medicines was discussed. In the industry scenario, skipping the pharmacy was assessed both before industry printing, i.e. as a control between the physician and the industry, and after the printing, i.e. as a buffer between the industry and the patient. When discussing the possibility of the industry providing the medication directly to patients, one respondent mentioned that the industry would be very interested in taking on this role since they wish to have this direct contact with the patients.
“They [industry] will be super interested. Because the pharmaceutical industry is moving from a ‘business to business’ to a ‘business to consumer’ model. That's what they are trying desperately at all levels to do.” (Int 8).
However, another respondent thought it would be wise for industry to keep the existing distribution chain in place:
“Industry can do it, but they need to create a large infrastructure of the people who will get feedback from patients and questions from patients. I think that infrastructure is already existing in pharmacies. So the industry would be smart… I think they would keep the existing infrastructure.” (Int 13).
Regardless of the industry's wishes, most respondents were aware that direct contact between the pharmaceutical industry and patients was not allowed under current regulations. Respondents emphasized that there needs to be a pharmacy, or at least a pharmacist between the industry and the patient. It was mentioned that according to article 61.1 of the Dutch Medicines Act35 only pharmacists practicing their profession in pharmacies (or licensed general practitioners) can dispense prescription medication to patients, and they need to register to do so. Respondents also mentioned that handing over medication is not just a logistical task, but also includes providing information and guidance to the patients on how to use their medication. An additional issue was reimbursement from health insurance companies, as patients would not be able to be reimbursed because, as currently, the reimbursement for medicines is granted to pharmacies.
Having a pharmacy as a mediator between the physician and the medicine production was considered relevant in both the industry and the home scenarios. Here, the role of the pharmacy as an independent check and the importance of the pharmacist-patient relationship in the Dutch context, was emphasized by the respondents. This is as therapeutic monitoring and medication reviews are regularly performed at pharmacies, in order to increase patient safety and ensure the patient obtains the correct medication and dose. Also including checking for medicine interactions, contraindications, as well as the patient's past and current medication use.
Specifically to the industry setting, respondents argued that the industry should not make decisions for patients on their own. Additionally, concerns about industry having access to patient data, and patient privacy arose.
3.4. Where should the tablet-design be performed?
Most participants agreed that physicians would not be capable of designing 3D-printed tablets. They claimed that the physicians' responsibility should be limited to indicating the desired API and dose. After that, it should be the pharmacist who, from a more technical point of view, can provide input on issues such as combinations, compatibility and stability.
“That [tablet-design] is something that a doctor is not trained to do. Then again having intelligent software behind him, he might be able to do it. But I still believe we [pharmacists] do have a specific knowledge that a doctor does not.” (Int 8).
Another remark about design of medicines was that this could influence a medicine's characteristics, and therefore there should be limited designs, and each design should be checked and validated. Some respondents stressed the importance of collaboration between the physician and pharmacist in tablet-design. Patient involvement in choosing the external features and taste, was seen as important to improve adherence by some respondents, and it was mentioned that this could be done either through discussion with the pharmacist or individually, online. One respondent said it would be important to involve all three parties, physician, pharmacist and patient in the tablet-design.
“There should be an interaction between physician and patient and pharmacist. And that's very important for compounding. I think that's the key for compounding.” (Int 4).
For industry-based printing, it was suggested that the company would design the tablets. It was mentioned that there could be a regulatory problem if the design was done remotely, i.e., the design in the software done at one location and the actual printing at another:
“If it's remote [the tablet-design software from the printer], I see some regulatory problems… who is responsible for updating the computer with the printing software? So it's not a standalone operating machine.” (Int 12).
4. Discussion
In this article a number of scenarios in which 3D printing of personalized medicines can take place was proposed. The opportunities and challenges of 3D printing in the different scenarios and feasibility of implementation was explored. Of the scenarios presented, the scenario involving 3D printing in patients' homes was the one seen as most futuristic, whereas printing at facilities where other routine compounding is already taking place (compounding and large hospital pharmacies) were the ones seen as most realistic.
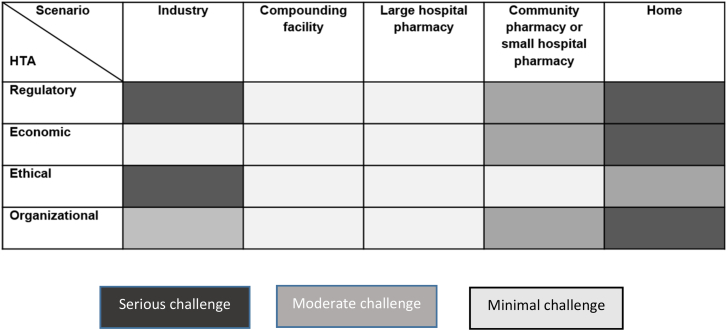
The main challenges that were raised for each scenario were regulatory, economical, ethical, and organizational factors (Fig. 6), which will be further discussed below. Based on the findings, a distinction has been made between large and small hospital pharmacies. As the small pharmacies face the same challenges as community pharmacies due to their scale of compounding, they have been placed together as one category.
Fig. 6.
Challenges with the different scenarios.
For all scenarios, current regulations were, to different degrees, seen as barriers for implementation of 3D printed medicine. Several specific regulatory challenges were brought forward which need to be taken into consideration when implementing 3D printed medicines in society. These challenges include: patient integrity (who should have access to patient health data?), and liability (who is responsible if something goes wrong when a complex logistic chain in combination with software, technical equipment and materials are working together to manufacture a medicinal product?). This study shows that the regulatory issues were seen as most problematic in the industry and home scenarios due to stringent regulation and lack of a regulatory framework, respectively. In the European Union, implementation of 3D printing for precision medicine in an industrial setting could benefit from the guidance of the EMA in order to obtain a harmonized approach within the EU legislative landscape. The EMA has gained ample experience with the EU marketing authorization of autologous advanced therapy medicinal products (ATMPs) for which small individualized batches also apply. As shown for ATMPs, marketing authorization of precision medicine for an individual patient is possible.
In contrast, for smaller pharmacies, regulatory issues concerned the “pre-filled cartridges” and “liability for the final product”. However, regulations change with technical developments, intended or unintended. The consequences of any regulatory changes, should be carefully considered.
Further, the economy of introducing a new and, at least initially, expensive technique is another area covered in this study. As pharmaceuticals are predominantly paid by third-party-payers (such as insurers or governments, i.e. tax payers), the benefits of personalized medicines manufacturing needs to be clear to them. On the other hand, manufacturers, be it industry, hospitals or pharmacies need to have their costs covered. Costs have to be weighed against benefits, so with potentially high costs those benefitting most from personalized medicines should be selected as first users. This could for example be children, as they often require other doses/dosages than those available from conventional manufacturing.36 Economic feasibility becomes more challenging as the setting becomes smaller, i.e. serving less patients. This implies debatable economic viability for 3D printed personalized medicines in society, especially in small pharmacies and home scenarios.
This study also shows that ethical issues are critical, in particular in the industry scenario, namely patient privacy and industry's direct involvement with patients, while in the home scenario it mainly concerns patient safety. Also, earlier studies have shown that safety issues are of concern.17,36 Potential safety issues include the quality of the product, but also the new responsibilities taken on by, for example, patients.
Finally, organizational adjustments would have to take place especially in the home setting, as well as in the industry and small pharmacy settings. As visible from Fig. 6, the compounding facility and large hospital pharmacy settings would require the least adaptation to enable 3D printing of personalized medicines. This is also suggested by Araújo et al. under the name ‘digital pharmacies’.37 Although on a smaller scale, 3D printing of personalized medicines in the hospital setting has been shown to be feasible,15 which indicates that the organizational aspect might be more easily dealt with than other factors.
Implementation of 3D printed medicine on a larger scale will change the organization, and hence the roles of stakeholders within the medicines' supply chain. This can lead to losses and wins for different stakeholders. For patients, they can gain more control over their medicines, not only within the home scenario. Although patients are currently not allowed to compound medicines, there is a trend for patients to become more responsible for their health, including pharmaceutical products (e.g. Hormones, total parenteral nutrition). The opportunity to be part of tablet design, (together with pharmacist and/or physician) taking into account the patient's individual preferences and/or needs, may positively affect the motivation to take the medicine, and potentially improve adherence.17 Earlier studies have shown rather good acceptance of the concept of 3D printed medicines among both pharmacists and patients.17,36 However, it has also been shown that patients differ with regard to whether they find designing tablets with individual features necessary or not.17 Even so, the respondents in this study had concerns about patients' competency.
The majority of respondents were pharmacists, and our results show that pharmacists consider their profession as crucial when implementing 3D printing of pharmaceutical products on a larger scale. They have the opportunity to expand their involvement in patient care, both by consulting patients (designing) but also in terms of quality assurance regarding combinations, compatibility and stability, i.e. using their pharmaceutical knowledge in new, patient-centered ways. It would therefore become pressing for the pharmacy profession to become involved early on. This includes educating pharmacists accordingly.
4.1. Limitations
This study has some methodological limitations. Interviews were conducted in the English language, which is not the native language of the respondents. To mitigate this, a Dutch co-author was normally present during the interviews to help with any language difficulties. Respondents were encouraged to use Dutch terms whenever they felt unable to express themselves in English.
Most interviewees were pharmacists by training and were therefore more likely in favor of a role in 3D printing for pharmacies and the pharmacist profession. This may have been especially pronounced in their opinions regarding tablet-design, where the vast majority doubted the physician's ability and considered pharmacists to be the most competent to design these tablets. On the other hand, respondents were identified by their respective organizations, and were hence most likely to be the most knowledgeable on the topic. The majority of authors are pharmacists - yet the first author and main interviewer as well as the two researchers responsible for the formal analysis are not. Further studies are needed to investigate additional perspectives such as those of the industry, other health care professionals and patients.
As to transferability: the Netherlands has a relatively strong compounding culture whereas other European countries might differ, having either an even stronger culture for compounding, or none at all, thereby limiting the transferability of results. However, as the Netherlands has come far in the discussions about implementation of 3D printing on a larger scale, other countries might be able to learn from this setting, for example when it comes to the challenges identified in this study.
5. Conclusions
In this study, the different challenges that society is likely going to be faced with when 3D printing technologies is implemented within pharmaceutical systems was identified and discussed, as illustrated by five scenarios. The challenges varied depending on the scenario. Specifically, regulatory, economic, ethical and organizational issues should be taken into account when pursuing the use of 3D printing of medicines. Regulatory, ethical and organizational issues were in general seen as the most challenging, and in particular within the industry and home scenarios. All in all, the final location(s) and organization of 3D printing for personalized medicines will be determined by which parties will, hopefully in collaboration, take the initiative to pursue 3D printing of personalized medicines on a larger scale.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. They received no financial support for the research, authorship, and/or publication of this article.
At the time of the study M.B. was an employee of the Copenhagen Centre for Regulatory Science (CORS). CORS is a cross-faculty university anchored institution involving various public (Danish Medicines Agency, Copenhagen University) and private stakeholders (Novo Nordisk A/S, Lundbeck A/S, Ferring pharmaceuticals A/S, LEO pharma A/S) as well as patient organizations (Rare Diseases Denmark). The center is purely devoted to the scientific aspects of the regulatory field and with a patient-oriented focus, and the research is not a company-specific product or directly company related. Currently, Marie Louise De Bruin is employed by Utrecht University conducting research under the umbrella of the Utrecht Centre for Pharmaceutical Policy and Regulation. This Centre receives no direct funding or donations from private parties, including the pharma industry. Research funding from public-private partnerships, e.g. IMI, The Escher Project (http://escher.lygature.org/), is accepted under the condition that no company-specific product or company-related study is conducted. The Centre has received unrestricted research funding from public sources, e.g., World Health Organization (WHO), Netherlands Organization for Health Research and Development (ZonMW), the Dutch National Health Care Institute (ZIN), EC Horizon 2020, the Dutch Medicines Evaluation Board (MEB), and the Dutch Ministry of Health.
N·B, S.K., N.G. and S.K.S. are or were employed as researchers at the University of Copenhagen. S.K.S. is also employed as researcher by Uppsala University. I.H., T.A. and J.H. are employed as researchers at the National Institute for Public Health and the Environment in the Netherlands.
Acknowledgments
The authors would like to thank all respondents for participating in this study. Johanna Lind was part of conceptualizing and designing the scenarios.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rcsop.2021.100073.
Contributor Information
Ingrid Hegger, Email: ingrid.hegger@rivm.nl.
Susanne Kaae, Email: susanne.kaae@sund.ku.dk.
Marie Louise De Bruin, Email: m.l.debruin@uu.nl.
Natalja Genina, Email: natalja.genina@sund.ku.dk.
Teresa Leonardo Alves, Email: teresa.leonardo.alves@rivm.nl.
Joelle Hoebert, Email: joelle.hoebert@rivm.nl.
Sofia Kälvemark Sporrong, Email: sofia.kalvemark-sporrong@farmaci.uu.se.
Appendix A. Supplementary data
Generic example of the interview guide
References
- 1.Schork N.J. Personalized medicine: time for one-person trials. Nature. 2015;520:609–611. doi: 10.1038/520609a. [DOI] [PubMed] [Google Scholar]
- 2.Norman J., Madurawe R.D., Moore C.M.V., Khan M.A., Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Abrahams E. Right drug—right patient—right time: personalized medicine coalition. Clin Transl Sci. 2008;1:11–12. doi: 10.1111/j.1752-8062.2008.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crommelin D.J.A., Bouwman-Boer Y. Pharmacy preparations: Back in the limelight? Pharmacists make up your mind! Int J Pharm. 2016;514:11–14. doi: 10.1016/j.ijpharm.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Jamróz W., Szafraniec J., Kurek M., Jachowicz R. 3D printing in pharmaceutical and medical applications – recent achievements and challenges. Pharm Res. 2018;35:176. doi: 10.1007/s11095-018-2454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivas L., Jaswitha M., Manikanta V., Bhavya B., Himavant B.D. 3D printing in pharmaceutical technology: a review. Int Res J Pharm. 2019;10:8–17. doi: 10.7897/2230-8407.100234. [DOI] [Google Scholar]
- 7.Alhnan M.A., Okwuosa T.C., Sadia M., Wan K.W., Ahmed W., Arafat B. Emergence of 3D printed dosage forms: opportunities and challenges. Pharm Res. 2016;33:1817–1832. doi: 10.1007/s11095-016-1933-1. [DOI] [PubMed] [Google Scholar]
- 8.Rahman Z., Barakh Ali S.F., Ozkan T., Charoo N.A., Reddy I.K., Khan M.A. Additive manufacturing with 3D printing: progress from bench to bedside. AAPS J. 2018;20 doi: 10.1208/s12248-018-0225-6. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh U., Ning S., Wang Y., Kong Y.L. Addressing unmet clinical needs with 3D printing technologies. Adv Healthc Mater. 2018;7:1800417. doi: 10.1002/adhm.201800417. [DOI] [PubMed] [Google Scholar]
- 10.Di Prima M., Coburn J., Hwang D., Kelly J., Khairuzzaman A., Ricles L. Additively manufactured medical products – the FDA perspective. 3D Print Med. 2015;2(1) doi: 10.1186/s41205-016-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett H. 3D Printing Industry. 2021. Triastek receives FDA IND clearance for 3D printed drug to treat rheumatoid arthritis.https://3dprintingindustry.com/news/triastek-receives-fda-ind-clearance-for-3d-printed-drug-to-treat-rheumatoidarthritis-184159/ [Google Scholar]
- 12.Raijada D, Wac K, Greisen E, Rantanen J, Geninga N. Integration of personalized drug delivery systems into digital health. Adv Drug Deliv Rev doi: 10.1016/j.addr.2021.113857(E-publication before print). [DOI] [PubMed]
- 13.Zheng Z., Lv J., Yang W., et al. Preparation and application of subdivided tablets using 3D printing for precise hospital dispensing. Eur J Pharm Sci. 2020;149:105293. doi: 10.1016/j.ejps.2020.105293. [DOI] [PubMed] [Google Scholar]
- 14.Öblom H., Sjöholm E., Rautamo M., Sandler N. Towards printed pediatric medicines in hospital pharmacies: comparison of 2D and 3D-printed orodispersible warfarin films with conventional oral powders in unit dose sachets. Pharmaceutics. 2019;11:334. doi: 10.3390/pharmaceutics11070334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyanes A., Madla C.M., Umerji A., et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: first single-Centre, prospective, crossover study in patients. Int J Pharm. 2019;567:118497. doi: 10.1016/J.IJPHARM.2019.118497. [DOI] [PubMed] [Google Scholar]
- 16.Khaled S.A., Burley J.C., Alexander M.R., Yang J., Roberts C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–314. doi: 10.1016/j.jconrel.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Fastø M.M., Genina N., Kaae S., Kälvemark Sporrong S. Perceptions, preferences and acceptability of patient designed 3D printed medicine by polypharmacy patients: a pilot study. Int J Clin Pharmacol. 2019;41:1290–1298. doi: 10.1007/s11096-019-00892-6. [DOI] [PubMed] [Google Scholar]
- 18.Cailleaux S., Sanchez-Ballester N.M., Gueche Y.A., Bataille B., Soulairol I. Fused deposition modeling (FDM), the new asset for the production of tailored medicines. J Control Release. 2021;330:821–841. doi: 10.1016/j.jconrel.2020.10.056. [DOI] [PubMed] [Google Scholar]
- 19.Xu X., Robles-Martinez P., Madla C.M., et al. Stereolithography (SLA) 3D printing of an antihypertensive polyprintlet: case study of an unexpected photopolymer-drug reaction. Addit Manuf. 2020;33:101071. doi: 10.1016/j.addma.2020.101071. [DOI] [Google Scholar]
- 20.Karavasili C., Gkaragkounis A., Moschakis T., Ritzoulis C., Fatouros D.G. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur J Pharm Sci. 2020;147:105291. doi: 10.1016/j.ejps.2020.105291. [DOI] [PubMed] [Google Scholar]
- 21.Banta D., Luce B. Oxford University Press; Oxford: 1993. Health Care Technology and Its Assessment: An International Perspective. [Google Scholar]
- 22.Lind J., Kälvemark Sporrong S., Kaae S., Rantanen J., Genina N. Social aspects in additive manufacturing of pharmaceutical products. Expert Opin Drug Deliv. 2017;14:927–936. doi: 10.1080/17425247.2017.1266336. [DOI] [PubMed] [Google Scholar]
- 23.KNMP . 2017. Input for the General Consultation on Medicines Policy (Permanent Commission Public Health of Parliament) 16 november. [Google Scholar]
- 24.Stichting Farmaceutische Kengetallen. Data en feiten. 2020. https://www.sfk.nl/publicaties/data-en-feiten/Dataenfeiten2020.pdf
- 25.Health and Youth Care Inspectorate, Netherlands. 2020. https://www.igj.nl/zorgsectoren/geneesmiddelen/geneesmiddelen-zonder-handelsvergunning/collegiaal-doorleveren Available online.
- 26.Apotheekbereiding — KNMP.nl Available online: https://www.knmp.nl/patientenzorg/geneesmiddelen/apotheekbereidingen/knmp-standpunt-bereiden. Accessed 05-11-20.
- 27.Vogler S., Arts D., Habl C. Austrian Health Institute (ÖBIG); 2006. Community Pharmacy in Europe. Lessons from Deregulation – Case Studies. [Google Scholar]
- 28.Medicijnen op Maat Maken Met een 3D-printer | TNO. 2020. https://www.tno.nl/nl/tno-insights/artikelen/medicijnen-op-maat-maken-met-3d-printer/ Available online.
- 29.Aulbers A. Enabling Tomorrow's Medicine by Means of 3D Printing. 3d Pharmaprinting Conference. 2021. https://www.3dpharmaprintingconference.com/speaker/enabling-tomorrows-medicine-by-means-of-3d-printing/
- 30.Saunders B., Sim J., Kingstone T., et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893–1907. doi: 10.1007/s11135-017-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graneheim U.H., Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24:105–112. doi: 10.1016/j.nedt.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Goyanes A., Scarpa M., Kamlow M., Gaisford S., Basit A.W., Orlu M. Patient acceptability of 3D printed medicines. Int J Pharm. 2017;530:71–78. doi: 10.1016/j.ijpharm.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh H.F., Shannon S.E. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 34.Act on Research Ethics Review of Health Research Projects | National Committee on Health Research Ethics – nvk.dk. 2018. https://en.nvk.dk/rules-and-guidelines/act-on-research-ethics-review-of-health-research-projects Available online.
- 35.Geneesmiddelenwet. https://wetten.overheid.nl/jci1.3:c:BWBR0021505&z=2021-07-01&g=2021-07-01
- 36.Rautamo M., Kvarnström K., Sivén M., Airaksinen M., Lahdenne P., Sandler N. Benefits and prerequisites associated with the adoption of oral 3D-printed medicines for pediatric patients: a focus group study among healthcare professionals. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araújo M., Sa-Barreto L., Gratieri T., Gelfuso G., Cunha-Filho M. The digital pharmacies era: how 3D printing technology using fused deposition modeling can become a reality. Pharmaceutics. 2019;11:128. doi: 10.3390/pharmaceutics11030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generic example of the interview guide