Abstract
Background
Although multiple medications are often utilized to achieve optimal treatment outcomes, polypharmacy (use of five or more medications) among older population is associated with several detrimental effects. Trajectories of polypharmacy among older population over time has not been described.
Objective
This study estimated polypharmacy prevalence and clusters of individuals with similar patterns of change in polypharmacy among a cohort of older Australian women with and without dementia.
Method
Longitudinal prospective cohort data from the oldest birth cohort (1921–1926) of the Australian Longitudinal Study on women's Health (ALSWH) were analysed. Survey data were linked with Pharmaceutical Benefit Schemes (PBS) data to obtain information about the type and number of prescription medications for each year 2003–2015. Group based trajectory modelling was used to identify distinct trajectory groups, based on the presence of polypharmacy for each year of observation. Trajectories were named based on distinctive and meaningful subgroups that followed approximately the same developmental course and probability assignment rule. Generalized estimating equation was used to identify factors associated with polypharmacy.
Results
A total of 10,372 women were eligible for the inclusion in the study. Prevalence of polypharmacy increased over time and reached as high as 71.19% and 71.29% in 2014 for women with and without dementia, respectively. Four distinct polypharmacy trajectories were identified: ‘Consistent Polypharmacy’ (55.88%);‘Low Polypharmacy’ (24.52%); ‘Rapid Increasing Polypharmacy’ (12.50%); and ‘Moderate Polypharmacy’ (7.12%). Dementia, Residential Aged Care (RAC), frailty and comorbid condition were the key drivers of polypharmacy in this cohort.
Conclusion
The prevalence of polypharmacy among older women increased over time, with most women have a pattern of consistent polypharmacy or rapidly increasing polypharmacy. Appropriate, sustainable, and effective strategies for reducing medication use should be implemented for women as they age, and particularly for those with dementia and those in residential care.
Keywords: Polypharmacy, Dementia, Trajectory analysis, Aged care, Frailty
1. Introduction
Older people tend to use a large number of medications as they live with several chronic conditions, requiring multiple medication therapies to cure, slow the progression or reduce the symptoms of each disease.1 When several medications are used concurrently, it is referred to as polypharmacy, which is often linked to inappropriate prescribing, adverse effects (AEs),2 preventable and unplanned hospitalisation,3 frailty and impaired cognition.4,5 These risks also increase with the age-associated decline in changes in health status and physiological function. Frailty also overlaps with comorbidity and is associated with increased risk of AEs and poor health outcomes.6,7 However, it should be noted that adverse outcomes of polypharmacy do not depend solely upon exposure to the number of medications but also to the pharmacokinetic and pharmacodynamic profile of medications. This includes the mechanism of action (drug class), half-life, dose response, maximal effect and drug–drug interactions; these are variable according to age, sex and other individual characteristics.8 Given these multiple considerations, it is debated that the number of medications prescribed to older people in and of itself is not the problem as long as the right combination of medications is prescribed.9 Polypharmacy can therefore be classed as being of two types: appropriate polypharmacy (‘many drugs’) and inappropriate polypharmacy (‘too many drugs’).10
There is no single agreed definition of polypharmacy11; however, use of five or more medications has been supported as a definition of polypharmacy to estimate the risk of medication-related adverse effects such as frailty, disability, mortality and falls.12,13 Polypharmacy has also been defined as five or more drugs, excluding topical dermatological, ophthalmological, vitamin and mineral supplements14; other studies have defined concurrent use of five to nine drugs as polypharmacy, and ten or more drugs as hyperpolypharmacy.15,16
In Australia, the prevalence of polypharmacy increased from 33.2% in 2006 to 36.2% in 2017 among the general older population aged 70 or more,12 and is set to rise.17 People with dementia have even higher rates of polypharmacy due to multiple comorbidities requiring several medications, and sometimes due to addition of drugs to manage dementia or related symptoms.18 A cross-sectional study of the Danish population (age > 65) showed that polypharmacy is more likely to occur among people with dementia compared to people without dementia (62.6% Vs 35.1%).19 Prevalence of polypharmacy is also higher among older people residing in long-term care facilities, many of whom have dementia.20 A study conducted in Canada reported that residents of long-term care facilities aged 66 years and above received nine or more concurrent medications, with more medications for those with multiple chronic conditions.20
Previous longitudinal studies on polypharmacy in Australia12 and internationally5 have estimated overall prevalence of polypharmacy over time12 and associations between polypharmacy and outcomes such as frailty.5 However, these studies did not assess within-individual changes in polypharmacy over time. Investigating variations in polypharmacy pattern (or trajectories) can be useful in identifying the vulnerable groups and designing necessary interventions.
Group Based Trajectory Modelling (GBTM) reports the course of an outcome over age or time and is utilized to identify clusters of individuals and trajectory groups, who have followed a similar developmental trajectory on an outcome of interest. This method determines the form and number of groups that best fit the data and provides a metric for assuring the precision of group assignments. Prediction of the trajectory of each group estimates the probability of membership for each individual of a group and assigns them to the group for which they demonstrate the highest probability.21 In this study, Group Based Trajectory Model (GBTM), was used to identify clusters of individuals with similar patterns of change in polypharmacy among women in the 1921–26 birth cohort of the Australian Longitudinal Study on Women's Health (ALSWH). Potential health and non-health-related predictors of polypharmacy among these women were also investigated.
2. Method
Data from the 1921–26 birth cohort of the ALSWH, a longitudinal prospective cohort study, were analysed. Participants were randomly sampled through Medicare Australia, the national health insurer's database. They have been surveyed every three years from 1996 until 2011, and six-monthly thereafter. Women in the survey were found to be broadly representative of women of a similar age in the Australian population.22 The ALSWH has also gained approval to access national and state-based administrative datasets such as the Medicare Benefits Schedule (MBS), Pharmaceutical Benefits Scheme (PBS), National Death Index (NDI), Aged-Care Datasets, Cancer Registry and Admitted Patient Data Collection (APDC).23 Ethics approval was granted by the University of Newcastle and the University of Queensland for ALSWH and data linkage has been approved by the Australian Institute of Health and Welfare (AIHW) and relevant state-based ethics committees.
Women with dementia were identified from multiple data sources (ALSWH survey, Aged Care, APDC, and NDI data) following methods described by Waller et al.24 Given that our study focused on pharmaceutical use by women with dementia, PBS data were not used to identify dementia. However, very few cases were identified through PBS alone, and more than 60% of women with dementia were identified by more than one data source. Aged-care data were the most common source of ascertainment in combination with other sources (>70%) or as the sole source (15%). Fewer than 1% of cases were solely ascertained from self-reported survey data. To address potential sources of bias in ascertaining dementia cases (e.g., under-reporting through survey only), multiple data sources were used to identify women with dementia. Eligibility for this study required women to be alive until 2003 and provided consent for data linkage. Participating women's ages were 77 to 82 years in 2003 and 89 to 94 years in 2015.
2.1. Pharmaceutical use
Generic drug names, supply date and year and ATC codes were obtained from the PBS dataset.25 The PBS includes fully subsidised drugs since 2002 and all prescriptions since 2012. However, most prescriptions would be fully subsidised and captured in the data, as around 98% of the women had some form of concession status for health care. For this study, data from the oldest birth cohort (1921–1926) of the ALSWH were linked with PBS data to obtain information about the type and number of prescription medications for each year 2003–2015.
2.2. Polypharmacy
Polypharmacy was defined as prescriptions for five or more unique medications for each observation period. The definition was consistent with previous literature,14 and is also one of the most commonly reported definitions for polypharmacy.11 Antidementia medications were excluded from the polypharmacy count to have comparable analyses between women with and without dementia. Antibiotics, medications for topical applications, vitamins, herbal medications and dietary supplements were excluded as these are not often considered in traditional methods of assessing prescribing quality and are inconsistently included in polypharmacy measures.26 In addition, the PBS dataset does not capture over-the-counter medications records. Anatomical Therapeutic Chemical (ATC) codes were counted for each individual for each year from the year 2003 to 2015.
2.3. Sociodemographic predictors/variables
Participants' educational qualification status was obtained from ALSWH Survey 1 (1996) and categorised into three groups: Higher (university degree/high university degree), Trade Certificate/diploma, and High School or below.
Other variables were created for each year, taken from the most recent ALSWH survey for the period 2003 to 2015. These included:
Area of residence: Major city, inner regional, outer regional/rural/remote.
Marital status: Married and de facto (in a relationship) were considered ‘Partnered’ whereas separated, divorced, widowed, and never married were grouped under ‘Non-partnered’.
The number of chronic diseases (0 or 1, 2 or more) was based on responses to questions ‘Have you ever been told by a doctor that you have…’ and ‘Have you ever been diagnosed or treated for…’ with the response options diabetes, heart disease, hypertension, osteoporosis, cancer, arthritis for both questions.
General Practitioner (GP) visits were categorised for each year as ‘Low’ (less than five) and ‘High’ (five or more).
Residential Aged Care (RAC) status: was obtained from age care data where start date and end date to RAC were used to assess whether they were in RAC for the particular year.
Medication review: Women utilising any of the services of the MBS item numbers (900, 903, 245, 249, 132, 133) at least once in a particular year were considered as having medication review for that year.
Frailty (Scores: 0, 1, 2, 3, 4, and 5) was calculated from the survey closest to the date of the first dementia indicator (2005, 2008 or 2011) based on deficits in five domains. The domains were fatigue (feeling worn out), resistance (ability to climb a single flight of stairs), ambulation (ability to walk 100 m), illnesses (>5), and loss of weight of more than 5%.27 This score was used to reflect the phenotype definition of frailty.28,29 Frailty scores >2 were considered frail.27 Information about baseline demographic characteristics (year 2003) were obtained from Survey 3 of the ALSWH (2002; age 76–81). Missing values were filled with information from preceding surveys (Survey 2 or 1) where appropriate. However, for the GEE analysis, variables like frailty, RAC status, dementia, chronic diseases, medication review, GP visits were obtained from the closest survey for the years from 2003 to 2015. A list of variables and relevant datasets can be found in Supplementary Table S1.
2.4. Statistical analysis
Descriptive analyses using frequencies and medians were performed wherever appropriate. Comparisons of sample characteristics were performed using chi-square and Wilcoxon signed-rank test. A dichotomised yearly indicator for polypharmacy was used in the logistic GBTM. The model allows simultaneous use of several multinomial logistic regression equations to estimate the probability of membership in each group and the probability of indicating polypharmacy as a function of time.30 Groups of individuals who followed similar patterns arising from their probability of polypharmacy from 2003 to 2015 were identified. Individuals were assigned to mutually exclusive groups based on their patterns of polypharmacy over time using the maximum-probability assignment rule; they were assigned to the group for which their probability of membership was highest. The Bayesian Information Criterion (BIC), group size and predictability of group membership was used to select the appropriate number of groups.31 In addition, the effect of the addition of groups on the BIC value was assessed incrementally. Trajectory shapes were adjusted using higher- or lower-order terms (linear, quadratic, or cubic), depending on the significance of those terms.
Details on the SAS procedure for group-based trajectory analysis have been described elsewhere.32 Multicollinearity among independent variables was assessed using Variance inflation factor (VIC). All statistical analyses were performed using SAS version 9.4, at an a priori significance level of 0.05.
A set of polypharmacy patterns (or trajectories) were selected and named based on distinctive and meaningful subgroups that followed approximately the same developmental course and probability assignment rule. The group membership based on their pre-existing or baseline characteristics were then explored. The demographic variables were baseline age, area, level of education and marital status.
2.5. Supplementary analysis
A series of longitudinal Generalized Estimating Equation (GEE) models were applied to estimate the associations between the aforementioned variables and polypharmacy, taking into account time. All variables were entered individually as main effects into separate models to produce unadjusted effect estimates. Then a final multivariate model was run which included all variables as main effects. In all models, polypharmacy status was the binary outcome, a logit link function was used, time (year) was included as a main effect and observations were clustered based on participant ID. Odds ratios and 95% confidence intervals were calculated for all effect estimates. Variable selections was carried out based on clinical importance identified through extensive examination of the existing literature.5,8,12,18 A p-value of 5% was applied to determine statistical significance.
3. Results
The cohort contained 12,342 women enrolled in the ALSWH in 1996. The final cohort, after excluding those deceased before 2003 (n = 1349) and those who denied consent for PBS data linkage (n = 711), comprised 10,372 women (See Supplementary Fig. S1). The participating women's mean baseline age was 79 (SD = 1.49) ranging from 77 to 82. The proportion of the cohort included in this analysis that had deceased during the observation period ranged from 3.11% in 2004 to 55.33% in 2015. Baseline demographic and clinical characteristics are shown in Table 2.
Table 2.
Baseline demographics and clinical characteristics of the overall cohort and by trajectory, Year 2003 (N = 10,372).
| Variables |
Total N = 10,372 |
Group 1 (Low Polypharmacy) N = 2542 (24.52%) |
Group 2 (Moderate Polypharmacy) N = 730 (7.11%) |
Group 3 (Consistent Polypharmacy) N = 5796 (55.88%) |
Group 4 (Rapid Increasing Polypharmacy) N = 1304 (12.50%) |
*P-Value |
|---|---|---|---|---|---|---|
| Baseline Age (Years) | <0.001 | |||||
| Age ≤ 79 | 5061 (48.79) | 1242 (48.86) 1240.4 |
331 (45.34) 356.2 |
2909 (50.19) 2828.1 |
579 (44.40) 636.28 |
** |
| Age ≥ 80 | 5311 (51.21) | 1300 (51.14) 1301.6 |
399 (54.66) 373.8 |
2887 (49.81) 2967.9 |
725 (55.60) 667.72 |
** |
| Area of Residence | ||||||
| Major cities | 4469 (43.09) | 1016 (39.97) | 333 (45.62) | 2571 (44.36) | 549 (42.10) | 0.006 |
| Inner regional | 3940 (37.99) | 1017 (40.01) | 251 (34.38) | 2159 (37.25) | 513 (39.34) | |
| Outer region, remote and very remote areas | 1963 (18.93) | 509 (20.02) | 146 (20.00) | 1066 (18.39) | 242 (18.56) | |
| Qualification (Survey 1) | <0.001 | |||||
| Tertiary Education | 1485 (15.12) | 431 (17.87) 364.75 |
120 (17.52) 103.59 |
702 (12.83) 827.18 |
232 (18.52) 189.48 |
** |
| Secondary Education or below | 8335 (84.88) | 1981 (82.13) 2047.3 |
565 (82.48) 581.41 |
4768 (87.17) 4642.8 |
1021 (81.48) 1063.5 |
** |
| Missing | 552 | |||||
| Marital Status (Survey 3) | 0.2347 | |||||
| Partnered (married and de facto relationship) | 4720 (45.60) | 1166 (45.94) | 361 (49.05) | 2603 (45.02) | 590 (45.52) | |
| Non-partnered (widowed, separated, divorced, and never married) | 5632 (54.40) | 1372 (54.06) | 375 (50.95) | 3179 (54.98) | 706 (54.48) | |
| Missing | 20 | |||||
| Dementia | <0.001 | |||||
| Yes | 423 (4.08) | 125 (4.92) 103.67 |
11 (1.51) 29.77 |
269 (4.64) 236.38 |
18 (1.38) 53.18 |
** |
| No | 9949 (95.92) | 2417 (95.08) 2438.30 |
719 (98.49) 700.23 |
5527 (95.36) 5559.6 |
1286 (98.62) 1250.80 |
** |
| GP Visits (Survey 3) | ||||||
| Low (<5 visits/year) | 4079 (39.46) | 1653 (65.31) 998.34 |
279 (37.86) 288.06 |
1471 (25.48) 2278 |
676 (52.16) 514.56 |
<0.001 ** |
| High (≥5 visits/year) | 6258 (60.54) | 878 (34.69) 1531.7 |
458 (62.14) 441.94 |
4302 (74.52) 3495 |
620 (47.84) 789.44 |
** |
| Missing | 35 | |||||
| Medication Review | <0.001 | |||||
| Yes (at least once in a year) | 107 (1.04) | 2 (0.08) 26.21 |
8 (1.10) 7.52 |
92 (1.60) 59.80 |
5 (0.39) 13.45 |
** |
| No | 10,202 (98.96) | 2525 (99.92) 2499.80 |
717 (98.90) 717.48 |
5670 (98.40) 5702.20 |
1283 (99.61) 12.82.50 |
** |
| Missing | 63 | |||||
| Chronic Diseases | <0.001 | |||||
| No or one disease | 4325 (41.70) | 1758 (69.13) 1060 |
280 (37.99) 304.4 |
1666 (28.74) 2416.9 |
621 (47.92) 543.75 |
** |
| Two or more diseases | 6047 (58.30) | 785 (30.87) 1482 |
457 (62.01) 425.60 |
4130 (71.26) 3379.10 |
675 (52.08) 760.25 |
** |
| Frailty | <0.001 | |||||
| Yes (Frailty Score ≥ 3) | 2605 (25.12) | 295 (11.61) 638.44 |
152 (20.82) 183.34 |
1968 (33.95) 1455.70 |
190 (14.57) 14.57 |
** |
| No (Frailty score ≤ 2) | 7767 (74.88) | 2248 (88.39) 1903.60 |
578 (79.18) 546.66 |
3828 (66.05) 4340.30 |
1114 (85.43) 976.49 |
** |
| Residential Aged Care (RAC) Status | <0.001 | |||||
| In RAC | 603 (5.81) | 117 (4.60) 147.78 |
5 (0.68) 42.44 |
474 (8.18) 336.96 |
7 (0.54) 75.81 |
** |
| Not in RAC | 9769 (94.19) | 2425 (95.40) 2394.20 |
725 (99.32) 687.56 |
5322 (91.82) 5459 |
1297 (99.46) 1228.20 |
** |
* P value represents results from chi-square test. ** Expected cell counts for significant p value
3.1. Prevalence of polypharmacy
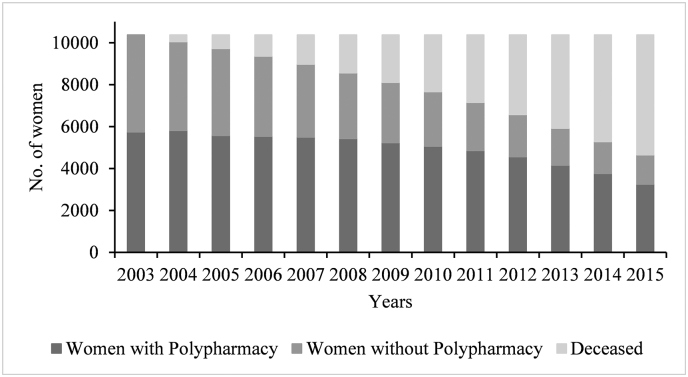
Prevalence of polypharmacy at baseline was 57.68% for women with dementia and 55.24% for women without dementia; however, 4.08% of women with dementia at baseline were also on antidementia drugs. Prevalence of polypharmacy increased consistently throughout the observation period and reached as high as 71% in 2014 before slightly dipping in 2015 (67%) for women with dementia. Fig. 1 and Table 1 illustrate the progression of polypharmacy from 2003 (ages 77 to 82) to 2015 (ages 89 to 94) and accounting for attrition due to death.
Fig. 1.
Progression of polypharmacy with attrition by to death among women in ALSWH's old birth cohort, N = 10,372, 2003–2015. Women were aged 77–82 in 2003 and 89–94 in 2015. The figure also includes attrition due to death.
Table 1.
Progression of polypharmacy among women with and without dementia, 2003–2015 (N = 10,372).
| Year | Number with dementia | Polypharmacy (%) | Number without dementia | Polypharmacy (%) | Dead (%) |
|---|---|---|---|---|---|
| 2003 | 423 | 244 (57.68) | 9949 | 5496 (55.24) | 0 |
| 2004 | 549 | 307 (55.91) | 9502 | 5501 (57.89) | 321 (3.09) |
| 2005 | 697 | 404 (57.96) | 9026 | 5168 (57.25) | 649 (6.25) |
| 2006 | 798 | 462 (57.89) | 8562 | 5075 (59.27) | 1012 (9.75) |
| 2007 | 927 | 555 (59.87) | 8044 | 4940 (61.41) | 1401 (13.50) |
| 2008 | 1165 | 781 (67.03) | 7390 | 4646 (62.86) | 1817 (17.51) |
| 2009 | 1243 | 851 (68.46) | 6867 | 4378 (63.75) | 2262 (21.80) |
| 2010 | 1351 | 939 (69.50) | 6308 | 4121 (65.32) | 2713 (26.15) |
| 2011 | 1486 | 1055 (70.99) | 5660 | 3804 (67.20) | 3226 (31.11) |
| 2012 | 1455 | 1032 (70.92) | 5115 | 3528 (68.97) | 3802 (36.65) |
| 2013 | 1367 | 965 (70.59) | 4555 | 3186 (69.94) | 4450 (42.90) |
| 2014 | 1184 | 843 (71.19) | 4090 | 2916 (71.29) | 5098 (49.15) |
| 2015 | 970 | 654 (67.42) | 3681 | 2595 (70.49) | 5721 (55.15) |
3.2. Polypharmacy trajectory
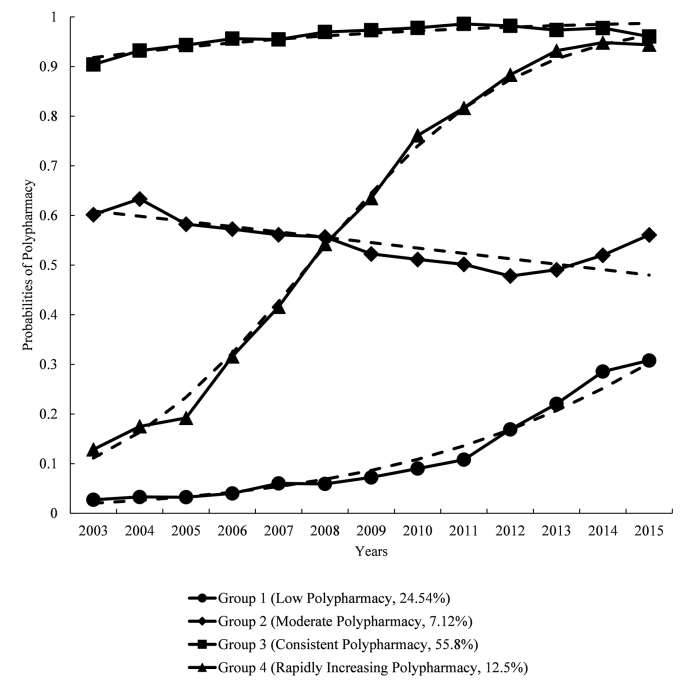
Based on the BIC and the expected patterns from real-world practice and literature, the model with four polypharmacy groups was used for further analysis to identify the predictors of polypharmacy.21,31 When four polypharmacy trajectories were considered (see.
Fig. 2), the groups included Group 1 with ‘low polypharmacy’ (24.52%); Group 2 with ‘moderate polypharmacy’ (7.12%); Group 3, that had ‘consistent polypharmacy’ (55.88%) and Group 4, that had ‘rapidly increasing polypharmacy’ (12.5%). The best-fitting model had an observed BIC of −32,655.82.
Fig. 2.
Polypharmacy groupings in the trajectory model using four groups. Dotted line indicates expected probabilities and the solid line indicates actual probabilities. Women were aged 77–82 in 2003 and 89–94 in 2015.
The low polypharmacy group began with a 2% probability of polypharmacy in 2003 (ages 77–82), which remained under 5% until 2008, then gradually increased thereafter to a maximum of 30% in 2015 (ages 89–94). The moderate polypharmacy group began with a 60% probability of polypharmacy, which negligibly decreased over time, reaching a 48% probability at the end of the observation period. The consistent polypharmacy group maintained the highest probability of polypharmacy across the observation period, with around 50–60% of the women having polypharmacy in any year. The rapidly increasing polypharmacy group began with a 11% probability of polypharmacy in 2003, which skyrocketed to 74% in 2010 and 96% in 2015.
The baseline characteristics of the patients in each trajectory group are shown in Table 2. There were significant differences in the demographic characteristics of the study population across the four groups except for marital status. Dementia was significantly associated with group membership, however this represents baseline dementia and does not consider dementia as a time varying covariate and this also applied to residential aged care status. Subsequent supplementary analysis using GEE accounts for incident dementia and aged care admissions.
3.3. Results for supplementary analysis
GEE analysis showed that polypharmacy increased with time. Table 3 shows the unadjusted and adjusted odds ratios for the relationship between polypharmacy and the associated predictor variables. Women with dementia were more likely to have polypharmacy (OR 1.12; 95% CI 1.00–1.26), not including the antidementia drugs, when compared to women without dementia. Women with frailty were less likely to have polypharmacy (OR 0.81; 95% CI 0.75–0.87) when compared to women without frailty. Polypharmacy occurred more often in women with two or more chronic diseases (OR 1.52; 95% CI: 1.36–1.69) when compared to women with one or no chronic disease. Women residing in RAC were three times more likely to have polypharmacy (OR 3.39; 95% CI: 3.08–3.74) when compared to women who were not in RAC. Women without medication reviews were more likely to have polypharmacy (OR 1.70; 95%CI: 1.60–1.81) when compared to women utilising medication review services in that year. High GP visits were associated with a higher likelihood of polypharmacy (OR 1.42; 95% CI 1.31–1.53) when compared to low GP visits. The unadjusted model showed that lower level of education was more likely to experience polypharmacy (OR 1.04; 95% CI 1.28–1.54) when compared to women with higher level of education.
Table 3.
Crude and adjusted longitudinal GEE estimates of the odds of polypharmacy among various predictors, 2003–2015 (N = 10,372).
|
Odds Ratio (95%CI) |
||
|---|---|---|
| Predictors | Unadjusted | Adjusted |
| Baseline Age ≥ 85 (Ref = Age ≤ 84) | 1.05 (0.99–1.09) | 1.06 (0.94–1.19) |
| Dementia: Yes (Ref = No) | 1.12 (1.04–1.21) | 1.12 (1.00–1.26) |
| Chronic Diseases Count: Two or more (Ref = One or no disease) | 2.30 (2.19–2.45) | 1.52 (1.36–1.69) |
| Frailty: Yes (Ref = No) | 1.48 (1.42–1.54 | 1.22 (1.38–1.32) |
| GP Visits: High (Ref = Low) | 1.81 (1.72–1.89) | 1.42 (1.31–1.53) |
| Medication Review: No (Ref = Yes) | 1.64 (1.56–1.73) | 1.70 (1.60–1.81) |
| Marital Status: Non-partnered (Ref = partnered) | 0.92 (0.88–0.97) | 0.91 (0.84–0.99) |
| RAC: In RAC (Ref = Not in RAC) | 2.90 (1.76–2.04) | 3.38 (3.07–3.73) |
| Qualification: Secondary Education (Ref = Tertiary Education) | 1.04 (1.28–1.54) | 0.97 (0.87–1.07) |
| Area: Inner Regional (Ref = Major cities) | 0.92 (0.86–0.98) | 0.74 (0.68–0.80) |
| Area: Outer regional/Remote (Ref = Major cities) | 0.90 (0.83–0.98) | 0.61 (0.55–0.68) |
Note. Non-frail = Frailty score ≤ 2; Frail = Frailty Score ≥ 3; RAC = Residential Aged Care; GP = General Practitioner (Low = GP visits <5 /year, High = GP visits ≥5/year); Tertiary education = University and higher degrees or trade certificate/diploma; Secondary education = High school or below. Outcome variable: Polypharmacy
4. Discussion
The study applied GBTM to report patterns of polypharmacy over the long term. In this large and highly representative cohort, four distinct groups of polypharmacy emerged. The cohort was observed for more than a decade. More than half of the total respondents fitted in one the ‘Consistent Polypharmacy’ group. Only one-quarter of women were categorised with ‘Low polypharmacy’, and even these women had a pattern of increasing polypharmacy in their later years. Factors associated with polypharmacy trajectories included dementia, RAC status, chronic diseases and frailty. Equally these factors were also significant in GEE models which included incident cases of dementia, other disease and RAC admission.
The findings showed a considerable increase in the prevalence of polypharmacy among older women with and without dementia when compared to previous studies conducted in Australia and overseas. One of the longitudinal studies in Australia conducted among older people aged 70 and above reported only modest increases in the rate of polypharmacy among older Australians, from 32.2% in 2007 to 36.2% in 2017. However, the study population was randomly selected from PBS dataset.12 Other estimates of polypharmacy in Australia ranged between 36% to 43%. A prospective observational study conducted in Western Australia among community-dwelling older men to examine prevalence of potentially suboptimal medications reported the polypharmacy prevalence of 35.8% whereas a cross-sectional study conducted among Australians of 50 years and above reported the polypharmacy prevalence of 43.5%. Both studies utilized self-reported medications.33,34 However, huge increases in polypharmacy over time have been reported in international studies. A study in the USA reported an increase of polypharmacy from 24% in 1992 to 39% in 2012 among people over 65 years of age.35 Likewise, a study conducted in the UK among people over 70 years old, showed a rise in polypharmacy from 11.4% in 1995 to 22.3% in 2010.36 Despite having similar criteria for measuring polypharmacy (i.e., ≥ 5 unique medications as polypharmacy), there were noticeable differences in the prevalence of polypharmacy in different studies, which could be due to differences in the settings and age ranges of participants. In addition to reporting a higher prevalence of polypharmacy, this study has also suggested that medications were being used at higher rates in Australia for a time. Polypharmacy is associated with adverse outcomes.2 Despite the debate around the number of concurrent medications prescribed, the number of medications itself may not be problematic as long as they are prescribed rationally.9,37 It is recommend that future studies identify and evaluate both appropriate and problematic polypharmacy.38 This may be particularly important for women with dementia who may also be on antidementia medications, and where medications for other conditions may have a role in mitigating or exacerbating dementia progression or effects.
The findings suggest that respondents have unique patterns of polypharmacy trajectories over time. Of the total 10,372 respondents in the sample, 55.88% of women were placed into the consistent polypharmacy group, which means these women were consistently using a higher number of medications throughout the study period. This group might have greatly contributed to the increase in polypharmacy percentage over time, as mentioned above. This emphasises the need for close monitoring of medications being prescribed to this population. While there are arguments about polypharmacy being supportive of longer life expectancy,39 there is evidence reporting severe adverse outcomes of long-term polypharmacy use,5 especially, concerning antipsychotics.40 Several approaches like the ‘geriatric-palliative approach’41 and ‘comprehensive geriatric assessment’39 have been proposed to combat polypharmacy. The meaningful and simultaneous discontinuation of overprescribed medications has shown a number of benefits like reduced mortality rates, reduced medical problems and improved quality of life.41 Of the total population in this study, 12.5% of women belonged to the ‘rapidly increasing polypharmacy’ group. A UK study showed that with the increased age of the older population, patterns of acquiring morbidity grow and thus the number of prescriptions an individual receives also multiply.38 Not all medications are needed to be used lifelong. Periodic review and management of medications is essential to optimise and improve medication use as well as to respond to the challenges posed by polypharmacy.38,42
This study reported a significant association between polypharmacy and frailty. A study conducted in Australia to investigate the relationship between polypharmacy and frailty among men aged 70 years and over showed an increase in frailty incident for the participants with polypharmacy.43 Likewise, a longitudinal study conducted in North America reported that people taking 4–6 medications had a 55% higher risk of frailty. However, the frailty index used in that study was different from ours.5 Additionally, a cohort study in Spain involving participants aged 70 years or more found that people with both frailty and polypharmacy had a higher risk of mortality and hospitalisation when compared to those without frailty and polypharmacy.44 Older people with frailty are more susceptible to ADEs due to multiple morbidities and age-related changes in pharmacokinetics and pharmacodynamics events.45 The prescriber, therefore, needs to be alert to ‘prescribing inertia’ (the continuation of medication even when the original indication is no longer present).46 Because some medications are more harmful than others, focusing on a limited number of medications—for example, psychotropics—could be a more effective and efficient approach to enhance quality use of medications among the older population.
Among the respondents, women with dementia were more likely to experience polypharmacy compared to women without dementia, even before accounting for antidementia drugs. Likewise, RAC status was also a significant predictor of polypharmacy. In this study, women living in RAC facilities were three times more likely to be exposed to polypharmacy when compared to women who were living in the community. Previous studies in Australia support this finding.47,48 In particular, one Australian study conducted among RAC residents living in Queensland reported that 91% of the residents were consuming five or more medications daily; however, the prevalence of potentially inappropriate high-risk medications was reported to be moderate.49 International studies have also reported a higher prevalence of polypharmacy among older people with dementia19 and long-term care users.20 The study also revealed higher odds of polypharmacy among women who did not have a medication review compared to those who had at least one medication review per year. In Australia and overseas, there is ample evidence suggesting beneficial outcomes of medication reviews in improving the quality use of medications in the older population.50,51 Moreover, the Australian Commission on Safety and Quality in Health Care has strongly urged the implementation of routine medication management reviews among the older population to combat excess chemical restraint, especially of those with dementia and residing in RAC.52 Multiple medications may be required for the management of Behavioural and Psychological Symptoms of Dementia (BPSD) and comorbid conditions.53 Other important reasons for over-prescription could be a lack of periodic medication review and the exclusion of the vulnerable population from the clinical trials and guidelines, which might have limited the development of evidence-based prescribing.54,55
In this study, women with secondary level of education were more likely to experience polypharmacy. Association between education level and polypharmacy have been generally been found inconsistent. A study conducted in Sweden among older people aged 75 to 89 reported that older people with low educational attainment had greater likelihood of polypharmacy,56 while a study conducted among the older population in Turkey reported no significant difference in number of drugs distributed among education levels.57 It is important to note that education levels were categorised differently in both studies. As older people are often prescribed with multiple medications for the management of comorbidities,58 higher level of education and health literacy could be advantageous for people for understanding their medications and side effects.59 In this study, women with high GP visits were more likely to have polypharmacy. GPs are ideally involved in optimizing medication management among older population.60 A case vignette study conducted in 31 European countries among older patients to investigate GPs deprescribing decision reported that most of the GPs were willing to deprescribe, especially for patients with multimorbidity.61 Higher likelihood of polypharmacy among women with higher GP visits in our study could be indicative of a need for effective implementation of interprofessional collaboration of GPs, pharmacists, and nurses to promote deprescribing and enable non-pharmacological supports.62
4.1. Strengths and limitations
This study is the first to investigate the polypharmacy trajectory among an older Australian population, enriching the limited knowledge in this field. The major strength of this study is that nationally representative longitudinal data and reliable PBS prescribing data for older Australians have been utilized. Comprehensive assessment of prescribed medications was established using the linkage with the national medications system; however, it should be noted that the PBS data covers dispensation of medications, which does not guarantee that those medications were consumed. A conservative method has been used to estimate polypharmacy by excluding topical, dermatological and ophthalmological treatments as well as vitamin and mineral supplements. Further, data linkage between the surveys and nationwide administrative datasets has allowed exploration of associations between health and non-health-related factors with polypharmacy. This study has some limitations. The membership probability of polypharmacy within each trajectory group may have been biased by deaths and/or loss to follow-up. Although non-death attrition from the PBS data is minimal, there is a risk that not all drugs are covered by the PBS. Specifically, PBS does not include over the counter medicines. The results are specific to women, hence may not necessarily be generalizable to other genders. There are studies reporting gender differences and the prevalence of polypharmacy12 as well as drug utilisation patterns and RAC status.26 There was oversampling from rural/remote areas in the ALSWH at twice the rate of women in urban areas to show the heterogeneity of health experiences of women residing outside metropolitan areas (for more detail visit https://www.alswh.org.au/). Although non-death attrition could be a greater source of bias in longitudinal studies, overall attrition has only minimal effects on the generalisability of ALSWH findings for surviving women in Australia who were born between 1921 and 26.63
5. Conclusion
The prevalence of polypharmacy among older women with and without dementia was as high as 70% in 2014. More than half of the respondents were part of the group that demonstrated consistent polypharmacy throughout the study duration. Understanding the characteristics of each trajectory would provide more insight and support individualised interventions for the most vulnerable. Frailty, RAC status, dementia and comorbid condition were the main factors to drive polypharmacy in this cohort. Appropriate, sustainable and effective strategies for reducing inappropriate medication use should be implemented. Future studies are required to identify the type of polypharmacy in terms of its appropriateness and evaluate the risks and benefits to the health outcomes of the older population.
Funding
The study was supported by Australian Government, Department of Health.
Authors' contributions
All authors had access to the data used in the study and take responsibility for the integrity of data and accuracy of the results.
Study concept and design: Kailash Thapaliya, Melissa L Harris, and Julie E Byles;
Acquisition of data: Julie E Byles, Kailash Thapaliya, and Melissa L Harris, via data access procedure for the Australian Longitudinal Study on Women's Health.
Analysis and interpretation of data: Kailash Thapaliya.
Preparation of Manuscript: Kailash Thapaliya.
Critical revision of the manuscript: Julie E Byles and Melissa L Harris.
Sponsor's Role: The institutions listed had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The research on which this study is based was conducted as part of the Australian Longitudinal Study on Women's Health that is managed by the University of Queensland and the University of Newcastle. We are grateful to the Australian Government Department of Health for funding this research and to the women who provided the survey data.
The authors acknowledge the assistance of the Data Linkage Unit at the Australian Institute of Health and Welfare (AIHW) for undertaking the data linkage to the National Death Index (NDI).
The authors also acknowledge the following:
-
•
Australian Government Departments of Health and Veterans' Affairs for providing Aged Care and MBS/PBS data and the Australian Institute of Health and Welfare (AIHW) as the integrating Authority.
-
•
Centre for Health Record Linkage (CHeReL), NSW Ministry of Health and ACT Health for the NSW Admitted Patients Data Collection and the ACT Admitted Patient Care Data Collection.
-
•
Queensland Health, including the Statistical Services Branch, for the Qld Hospital Admitted Patient Data Collection.
-
•
Department of Health Western Australia, including the Data Linkage Branch, and the WA Hospital Morbidity Data Collection.
-
•
SA NT Datalink and SA Health for the SA Public Hospital Separations Data Collection.
This project is supported by the Population Health Research Network, which is a capability of the Australian Government National Collaborative Research Infrastructure Strategy and Education Investment Fund Super Science Initiative.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rcsop.2021.100053.
Appendix A. Supplementary data
Supplementary Material
References
- 1.Bushardt R.L., Massey E.B., Simpson T.W., Ariail J.C., Simpson K.N. Polypharmacy: misleading, but manageable. Clin. Interv. Aging. 2008;3:383–389. doi: 10.2147/cia.s2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourgeois F.T., Shannon M.W., Valim C., Mandl K.D. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol. Drug Saf. 2010;19:901–910. doi: 10.1002/pds.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne R.A., Abel G.A., Avery A.J., Mercer S.W., Roland M.O. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br. J. Clin. Pharmacol. 2014;77:1073–1082. doi: 10.1111/bcp.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maher R.L., Hanlon J., Hajjar E.R. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 2014;13:57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veronese N., Stubbs B., Noale M., et al. Polypharmacy is associated with higher frailty risk in older people: an 8-year longitudinal cohort study. J. Am. Med. Dir. Assoc. 2017;18:624–628. doi: 10.1016/j.jamda.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulop T., Larbi A., Witkowski J.M., et al. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- 8.Hilmer S.N., Gnjidic D. The effects of polypharmacy in older adults. Clin. Pharmacol. Ther. 2009;85:86–88. doi: 10.1038/clpt.2008.224. [DOI] [PubMed] [Google Scholar]
- 9.Fried T.R., Mecca M.C. Medication appropriateness in vulnerable older adults: healthy skepticism of appropriate polypharmacy. J. Am. Geriatr. Soc. 2019;67:1123–1127. doi: 10.1111/jgs.15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronson J.K. In defence of polypharmacy. Br. J. Clin. Pharmacol. 2004;57:119–120. doi: 10.1111/j.1365-2125.2004.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page A.T., Falster M.O., Litchfield M., et al. Polypharmacy among older Australians, 2006–2017: a population-based study. Med. J. Aust. 2019;211:71–75. doi: 10.5694/mja2.50244. [DOI] [PubMed] [Google Scholar]
- 13.Gnjidic D., Hilmer S.N., Blyth F.M., et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 2012;65:989–995. doi: 10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Best O., Gnjidic D., Hilmer S.N., Naganathan V., McLachlan A.J. Investigating polypharmacy and drug burden index in hospitalised older people. Intern. Med. J. 2013;43:912–918. doi: 10.1111/imj.12203. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard R.E., Peel N.M., Scott I.A., et al. Polypharmacy among inpatients aged 70 years or older in Australia. Med. J. Aust. 2015;202:373–377. doi: 10.5694/mja13.00172. [DOI] [PubMed] [Google Scholar]
- 16.Dolder C.R., McKinsey J. Antipsychotic polypharmacy among patients admitted to a geriatric psychiatry unit. J. Psychiatr. Pract. 2011;17:368–374. doi: 10.1097/01.pra.0000405368.20538.cd. [DOI] [PubMed] [Google Scholar]
- 17.Woodward M.C. Deprescribing: achieving better health outcomes for older people through reducing medications. J. Pharm. Pract. Res. 2003;33:323–328. doi: 10.1002/jppr2003334323. [DOI] [Google Scholar]
- 18.Lau D.T., Mercaldo N.D., Harris A.T., Trittschuh E., Shega J., Weintraub S. Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis. Assoc. Disord. 2010;24:56–63. doi: 10.1097/WAD.0b013e31819d6ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristensen R.U., Nørgaard A., Jensen-Dahm C., Gasse C., Wimberley T., Waldemar G. Polypharmacy and potentially inappropriate medication in people with dementia: a nationwide study. J. Alzheimers Dis. 2018;63:383–394. doi: 10.3233/JAD-170905. [DOI] [PubMed] [Google Scholar]
- 20.Bronskill SE, Gill SS, Paterson JM, Bell CM, Anderson GM, Rochon PA. Exploring variation in rates of polypharmacy across long term care homes. J Am Med Dir Assoc. 2012;13:309.e15–21. DOI: 10.1016/j.jamda.2011.07.001. [DOI] [PubMed]
- 21.Nagin D.S., Odgers C.L. Group-based trajectory modelling in clinical research. Annu. Rev. Clin. Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 22.Gomersall S.R., Dobson A.J., Brown W.J. Weight gain, overweight and obesity: determinants and health outcomes from the Australian longitudinal study on Women’s health. Curr. Obes. Rep. 2014;3:46–53. doi: 10.1007/s13679-013-0077-4. [DOI] [PubMed] [Google Scholar]
- 23.Powers J., Ball J., Adamson L., Dobson A. Effectiveness of the National Death Index for establishing the vital status of older women in the Australian longitudinal study on Women’s health. Aust. N. Z. J. Public Health. 2000;24:526–528. doi: 10.1111/j.1467-842x.2000.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 24.Waller M., Mishra G.D., Dobson A.J. Estimating the prevalence of dementia using multiple linked administrative health records and capture–recapture methodology. Emerg Themes Epidemiol. 2017;14:1–9. doi: 10.1186/s12982-017-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paige E., Kemp-Casey A., Korda R., Banks E. Using Australian Pharmaceutical Benefits Scheme data for pharmacoepidemiological research: challenges and approaches. Public Health Res Pract. 2015;25 doi: 10.17061/phrp2541546. e2541546. [DOI] [PubMed] [Google Scholar]
- 26.Steinman M.A., Landefeld C.S., Rosenthal G.E., Berthenthal D., Sen S., Kaboli P.J. Polypharmacy and prescribing quality in older people. J. Am. Geriatr. Soc. 2006;54:1516–1523. doi: 10.1111/j.1532-5415.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 27.Lopez D., Flicker L., Dobson A. Validation of the frail scale in a cohort of older Australian women. J. Am. Geriatr. Soc. 2012;60:171–173. doi: 10.1111/j.1532-5415.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 28.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 29.Abellan van Kan G., Rolland Y.M., Morley J.E., Vellas B. Frailty: toward a clinical definition. J. Am. Med. Dir. Assoc. 2008;9:71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Franklin J.M., Shrank W.H., Pakes J., et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med. Care. 2013;51:789–796. doi: 10.1097/MLR.0b013e3182984c1f. [DOI] [PubMed] [Google Scholar]
- 31.Jones B.L., Nagin D.S., Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol. Methods Res. 2001;29(3):374–393. [Google Scholar]
- 32.Baumgartner S.E., Leydesdorff L. Group-based trajectory modeling (GBTM) of citations in scholarly literature: dynamic qualities of “transient” and “sticky knowledge claims”. J. Assoc. Inf. Sci. Technol. 2014;65:797–811. doi: 10.1002/asi.23009. [DOI] [Google Scholar]
- 33.Beer C., Hyde Z., Almeida O.P., et al. Quality use of medicines and health outcomes among a cohort of community dwelling older men: an observational study. Br. J. Clin. Pharmacol. 2011;71:592–599. doi: 10.1111/j.1365-2125.2010.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan T.K., Williamson M., Pirotta M., Stewart K., Myers S.P., Barnes J. A national census of medicines use: a 24-hour snapshot of Australians aged 50 years and older. Med. J. Aust. 2012;196:50–53. doi: 10.5694/mja11.10698. [DOI] [PubMed] [Google Scholar]
- 35.Kantor E.D., Rehm C.D., Haas J.S., Chan A.T., Giovannucci E.L. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818–1830. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guthrie B., Makubate B., Hernandez-Santiago V., Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med. 2015;13:74. doi: 10.1186/s12916-015-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon S.K., Okyere B.A., Strasser D. Polypharmacy and Rational Prescribing: Changing the Culture of Medicine One Patient at a Time. Curr Phys Med Rehabil Rep. 2019;7:141–158. doi: 10.1007/s40141-019-00220-z. [DOI] [Google Scholar]
- 38.Duerden M., Avery T., Payne R. The King’s Fund; London: 2013. Polypharmacy and Medicines Optimisation: Making it Safe and Sound. [Google Scholar]
- 39.Sergi G., De Rui M., Sarti S., Manzato E. Polypharmacy in the elderly: can comprehensive geriatric assessment reduce inappropriate medication use? Drugs Aging. 2011;28:509–518. doi: 10.2165/11592010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Suokas J.T., Suvisaari J.M., Haukka J., Korhonen P., Tiihonen J. Description of long-term polypharmacy among schizophrenia outpatients. Soc. Psychiatry Psychiatr. Epidemiol. 2013;48:631–638. doi: 10.1007/s00127-012-0586-6. [DOI] [PubMed] [Google Scholar]
- 41.Garfinkel D., Zur-Gil S., Ben-Israel H. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Isr. Med. Assoc. J. 2007;9:430–434. [PubMed] [Google Scholar]
- 42.Walsh E.K., Cussen K. “Take ten minutes”: a dedicated ten minute medication review reduces polypharmacy in the elderly. Ir. Med. J. 2010;103:236–238. [PubMed] [Google Scholar]
- 43.Gnjidic D., Hilmer S.N., Blyth F.M., et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin. Pharmacol. Ther. 2012;91:521–528. doi: 10.1038/clpt.2011.258. [DOI] [PubMed] [Google Scholar]
- 44.Bonaga B., Sánchez-Jurado P.M., Martínez-Reig M., et al. Frailty, polypharmacy and health outcomes in older adults: the frailty and dependence in Albacete study. J. Am. Med. Dir. Assoc. 2018;19:46–52. doi: 10.1016/j.jamda.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Koponen M.P.H., Bell J.S., Karttunen N.M., Nykänen I.A., Desplenter F.A.M., Hartikainen S.A. Analgesic use and frailty among community-dwelling older people: a population-based study. Drugs Aging. 2013;30:129–136. doi: 10.1007/s40266-012-0046-8. [DOI] [PubMed] [Google Scholar]
- 46.Gurwitz J.H. The physics of geriatric pharmacotherapy: overcoming therapeutic inertia and momentum. Am. J. Med. 2012;125:523–534. doi: 10.1016/j.amjmed.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Somers M., Rose E., Simmonds D., Whitelaw C., Calver J., Beer C. Quality use of medicines in residential aged care. Aust. Fam. Physician. 2010;39:413–416. [PubMed] [Google Scholar]
- 48.Bosboom P.R., Alfonso H., Almeida O.P., Beer C. Use of potentially harmful medications and health-related quality of life among people with dementia living in residential aged care facilities. Dement Geriatr Cogn Dis Extra. 2012;2:361–371. doi: 10.1159/000342172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poudel A., Peel N.M., Mitchell C.A., Gray L.C., Nissen L.M., Hubbard R.E. Geriatrician interventions on medication prescribing for frail older people in residential aged care facilities. Clin. Interv. Aging. 2015;10:1043–1051. doi: 10.2147/CIA.S84402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maidment I.D., Aston L., Hilton A., Iqbal N., Child A., Shaw R. Role of community pharmacists in the use of antipsychotics for behavioural and psychological symptoms of dementia (BPSD): a qualitative study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jokanovic N., Tan E.C.K., van den Bosch D., Kirkpatrick C.M., Dooley M.J., Bell J.S. Clinical medication review in Australia: a systematic review. Res. Soc. Adm. Pharm. 2016;12:384–418. doi: 10.1016/j.sapharm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Australian Commission on Safety and Quality in Health Care Medication Review. https://www.safetyandquality.gov.au/standards/national-safety-and-quality-health-service-nsqhs-standards/medication-safety-standard/continuity-medication-management/action-410; Accessed 23.11.2020.
- 53.Melesie G., Dinsa H. A literature review on: pathogenesis and management of dementia due to Alzheimer disease. Bio-Genetics J. 2013;1:18–31. [Google Scholar]
- 54.Brauner D.J., Muir J.C., Sachs G.A. Treating nondementia illnesses in patients with dementia. JAMA. 2000;283:3230–3235. doi: 10.1001/jama.283.24.3230. [DOI] [PubMed] [Google Scholar]
- 55.Marengoni A., Onder G. Guidelines, polypharmacy and drug-drug interactions in patients with multimorbidity. BMJ. 2015;350:h1059. doi: 10.1136/bmj.h1059. [DOI] [PubMed] [Google Scholar]
- 56.Haider S.I., Johnell K., Weitoft G.R., Thorslund M., Fastbom J. The influence of educational level on polypharmacy and inappropriate drug use: a register-based study of more than 600,000 older people. J. Am. Geriatr. Soc. 2009;571:62–69. doi: 10.1111/j.1532-5415.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 57.Gökçe Kutsal Y., Barak A., Atalay A., Baydar T., Kucukoglu S., Tuncer T. Polypharmacy in the elderly: a multicenter study. J. Am. Med. Dir. Assoc. 2009;10:486–490. doi: 10.1016/j.jamda.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 58.Wise J. Polypharmacy: a necessary evil. BMJ. 2013;28:347. doi: 10.1136/bmj.f7033. [DOI] [PubMed] [Google Scholar]
- 59.Alkatheri A.M., Albekairy A.M. Does the patients’ educational level and previous counseling affect their medication knowledge? Ann Torac Med. 2013;8:105–108. doi: 10.4103/1817-1737.109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bosch-Lenders D., Jansen J., Stoffers H.E., et al. The effect of a comprehensive, Interdisciplinary Medication Review on Quality of Life and Medication Use in Community Dwelling Older People with Polypharmacy. J. Clin. Med. 2021;10:600. doi: 10.3390/jcm10040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jungo K.T., Mantelli S., Rozsnyai Z., et al. General practitioners’ deprescribing decisions in older adults with polypharmacy: a case vignette study in 31 countries. BMC Geriatr. 2021;21:19. doi: 10.1186/s12877-020-01953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foley R.A., Hurard L.L., Cateau D., Koutaissoff D., Bugnon O., Niquille A. Physicians’, Nurses’ and Pharmacists’ Perceptions of Determinants to Deprescribing in Nursing Homes Considering Three Levels of Action: A Qualitative Study. Pharmacy (Basel) 2020;8:17. doi: 10.3390/pharmacy8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brilleman S.L., Pachana N.A., Dobson A.J. The impact of attrition on the representativeness of cohort studies of older people. BMC Med. Res. Methodol. 2010;10:71. doi: 10.1136/jech.2011.142976d.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material