Abstract
Despite rapidly evolving pathobiological mechanistic demystification, coupled with advances in diagnostic and therapeutic modalities, chronic obstructive pulmonary disease (COPD) remains a major healthcare and clinical challenge, globally. Further compounded by the dearth of available curative anti-COPD therapy, it is posited that this challenge may not be dissociated from the current lack of actionable COPD pathognomonic molecular biomarkers. There is accruing evidence of the involvement of protracted ‘smoldering’ inflammation, repeated lung injury, and accelerated lung aging in enhanced predisposition to or progression of COPD. The relatively novel uncharacterized human long noncoding RNA lnc-IL7R (otherwise called LOC100506406) is increasingly designated a negative modulator of inflammation and regulator of cellular stress responses; however, its role in pulmonary physiology and COPD pathogenesis remains largely unclear and underexplored. Our previous work suggested that upregulated lnc-IL7R expression attenuates inflammation following the activation of the toll-like receptor (TLR)-dependent innate immune system, and that the upregulated lnc-IL7R is anti-correlated with concomitant high PM2.5, PM10, and SO2 levels, which is pathognomonic for exacerbated/aggravated COPD in Taiwan. In the present study, our quantitative analysis of lnc-IL7R expression in our COPD cohort (n = 125) showed that the lnc-IL7R level was significantly correlated with physiological pulmonary function and exhibited COPD-based stratification implications (area under the curve, AUC = 0.86, p < 0.001). We found that the lnc-IL7R level correctly identified patients with COPD (sensitivity = 0.83, specificity = 0.83), precisely discriminated those without emphysematous phenotype (sensitivity = 0.48, specificity = 0.89), and its differential expression reflected disease course based on its correlation with the COPD GOLD stage (r = −0.59, p < 0.001), %LAA-950insp (r = −0.30, p = 0.002), total LAA (r = −0.35, p < 0.001), FEV1(%) (r = 0.52, p < 0.001), FVC (%) (r = 0.45, p < 0.001), and post-bronchodilator FEV1/FVC (r = 0.41, p < 0.001). Consistent with other data, our bioinformatics-aided dose–response plot showed that the probability of COPD decreased as lnc-IL7R expression increased, thus, corroborating our posited anti-COPD therapeutic potential of lnc-IL7R. In conclusion, reduced lnc-IL7R expression not only is associated with inflammation in the airway epithelial cells but is indicative of impaired pulmonary function, pathognomonic of COPD, and predictive of an exacerbated/ aggravated COPD phenotype. These data provide new mechanistic insights into the ailing lung and COPD progression, as well as suggest a novel actionable molecular factor that may be exploited as an efficacious therapeutic strategy in patients with COPD.
Keywords: pulmonary function, chronic obstructive pulmonary disease (COPD), lung inflammation, long noncoding RNA, lnc-IL7R, GOLD stage, %LAA-950insp, FEV1(%), FVC(%)
1. Clinical Relevance
-
(i)
Protracted ‘smoldering’ inflammation, repeated lung injury, and accelerated lung aging are implicated in the enhanced predisposition to or progression of COPD;
-
(ii)
Altered lnc-IL7R expression in the human airway epithelium and blood is clinically relevant in pulmonary pathophysiology;
-
(iii)
The lnc-IL7R level is significantly downregulated in patients with COPD and is correlated with disease progression;
-
(iv)
The correlation of circulating lnc-IL7R with improved pulmonary function, and anti-correlation with PM2.5, PM10, and SO2 provide some mechanistic insights into pulmonary dysfunction and COPD, as well as suggest potential actionable novel biotherapeutics/biologics for the treatment of COPD.
2. Introduction
Chronic obstructive pulmonary disease (COPD), a noncommunicable disease group characterized by progressive but preventable airflow obstruction and impaired respiration, including chronic bronchitis, refractory asthma, and emphysema, constitutes a major global health challenge [1,2]. Based on the World Health Organization’s Global Health Estimates, accounting for approximately 100,000,000 lost healthy life-years in 2019 alone, compared with 2000, COPD now ranks as the third leading cause of mortality globally [2].
As already demonstrated by our team and others, long-term exposure to air pollutants, including nitrogen oxides (NOx), ambient ozone (O3), emitted hydrocarbons (HC), and fine particulate matter <10 μm (PM10) or <2.5 μm (PM2.5) in aerodynamic diameter, is implicated in the progressive decline in pulmonary function in patients with COPD/emphysema and cannot be decoupled from reported reduction in lung function indices, including the forced vital capacity (FVC), forced expiratory volume in 1 sec (FEV1), maximum mid-expiratory flow (MMEF), and FEV1/FVC ratio elicited by the cumulative increase in ambient air pollution [3].
Primarily affecting the lungs, there is increasing evidence implicating chronic systemic inflammation in the progressive largely irreversible airflow obstruction that is characteristic of COPD [4,5]. This chronic inflammation is driven by a cascade of both nonspecific innate and specific acquired immune responses in the lungs, and though more predominant in the lung parenchyma and bronchial walls of the small airways [5], differences in the nature of such inflammation, and its location define the COPD phenotype, namely chronic bronchitis, refractory asthma, or emphysema, as well as influence clinical course and therapy response [5,6]. The flaring of this COPD-associated inflammation during acute exacerbation or aggravation of COPD is characterized by increased tissue pooling of alveolar macrophages, neutrophils, Tc1, Th1, and Th17 lymphocytes, as well as innate lymphoid cells (ILCs) from the circulation [6,7]. In concert with structural cells of the epithelium, endothelium, and fibroblasts, these pooled cells secrete various mediators of inflammation, including chemokines, cytokines, lipid mediators, and growth factors [7].
Systemic inflammation is increasingly implicated in accelerated lung aging and associated decrease in lung function [8]. Several studies indicate that one of every two patients with COPD exhibit accelerated decrease in pulmonary function [8], which, unlike ‘senile emphysema’ that is associated with the physiological aging of the lungs with resultant enlarged alveolar spaces and loss of lung elasticity in the elderly, is conversely defined by marked alveolar wall destruction and peripheral airway fibrosis [8,9]. This lung injury can result in permanent reduction in the gas exchange surface area and respiratory function of patients with COPD [10]. The loss of pulmonary elasticity due to the proteolytic destruction of lung parenchyma and alveolar walls plays a major role in COPD pathognomonic airway obstruction, and while the irreversibility of such lung damage regardless of pharmacologic therapy has been opined [8,9], we posit that with improved understanding of COPD pathobiology and further unraveling of its underlying mechanism, not only is reduced disease progression through inhibition of proinflammation and associated enzymatic signaling possible, but lung tissue regeneration and reversibility of obstructive defects, with consequent improved pulmonary function, is probable [10,11,12].
Aside from innate immune cells, the epitranscriptomic memory potential of lung epithelium has been suggested to drive immune responses associated with mucus hyperreactivity and remodeling of the airway related to COPD. While several susceptibility genes have been implicated in airway remodeling, the culpability of long noncoding RNAs (lncRNAs), such as lnc-IL7R is increasingly documented in the regulation of postexposure airway inflammation, COPD pathogenesis, and disease progression [3,13,14].
The present study, exploring for causative association between lnc-IL7R and COPD, provides evidence that the elevated profile of circulating lnc-IL7R reflects improved pulmonary function, and is anti-correlated with effectors of pulmonary dysfunction PM2.5, PM10, and SO2, suggesting the therapeutic feasibility of lnc-IL7R as a potential actionable novel biotherapeutic/biologic for the treatment of COPD.
3. Methods
In the present single-center, prospective, non-randomized study, we enrolled 125 patients with COPD, diagnosed consistent with international recommendations, as described in our previous publication [3]. Patients were clinically stable (without exacerbation, at least for one month), without significant comorbidities or history of systemic corticosteroids, diuretics, cytotoxic agents, or alcohol abuse (alcohol/day > 40 g/day). COPD severity was based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [15], and the percentages of low attenuation area below −950 Hounsfield units (%LAA-950insp) defined classification into no/mild emphysema (%LAA-950insp < 6), moderate emphysema (6 ≤ %LAA-950insp < 14), and severe emphysema (%LAA-950insp ≥ 14) [3,16]. Patients were either current smokers (n = 49), ex-smokers (n = 66), or never smokers (n = 10). Hematologic variables, including blood lnc-IL7R, were derived from routine blood workup. Other data retrieved included smoking history (status, pack-year), anthropometric (height, weight, body mass index), geospatial (location coordinates), ambient air pollution, spirometric (FEV1, FVC, FEV1/FVC), and computed tomography (%LAA-950insp, total LAA) variables. Oxygen saturation (SvO2, SpO2) levels were measured using a Sibelmed® Datospir micro C portable spirometer (Sibel, S.A.U., Barcelona, Spain). Bronchodilator response (BDR) was calculated using the formula:
where change in FEV1 ≥ 12% and 400 mL defined a positive BDR. Clinical sample preparation and quantitative reverse transcription PCR (RT-qPCR) for determination of the lnc-IL7R level in the whole blood of participants were strictly performed as earlier described [3].
Statistical Analysis
All data are expressed as mean ± standard deviation (SD) or percentages (%). Differences between categorical variables was compared using Pearson’s chi-squared (χ2) test. The paired t-test was used for comparing two continuous variables. The Student’s t-test was used to assess alterations in pulmonary function based on %LAA-950insp, FEV1, FVC, FEV1/FVC, and lnc-IL7R expression levels. Comparison of ≥3 categorical variables was performed using the Kruskal-Wallis test with post hoc Dunn’s multiple comparison test. Spearman’s rank correlation was used to determine the relationship between variables. p-value ≤ 0.05 defined statistical significance. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp. Released 2017, Armonk, NY, USA: IBM Corp). Geospatial visualization and analysis were performed using the ArcGIS server software version 10.8.1 (ESRI, Redlands, CA, USA).
4. Results
4.1. Baseline Characteristics of Our COPD Cohort
The baseline characteristics of our COPD cohort (n = 125 vs. calculated required sample size of 89) are presented in Table 1. According to the COPD GOLD guidelines (15), 14.4% of all COPD patients exhibited mild COPD (FEV1 = 85.4 ± 5.2%), while 46.4%, 30.4%, and 8.8% were moderate (FEV1 = 63.8 ± 8.6%), severe (FEV1 = 40.0 ± 5.7%), and very severe (FEV1 = 24.8 ± 4.0%) COPD cases, respectively (Table 1). Across all GOLD stage groups, the intergroup differences were statistically significant. All pulmonary function indices, namely FEV1(L), FEV1(%), and FEV1/FVC(%), were inversely correlated with smoking pack-years and COPD exacerbation in the previous year but positively correlated with lnc-IL7R expression (Table 1). Compared with patients with mild COPD (GOLD I), 29%, 59%, or 70% reduced median lnc-IL7R expression was observed in patients with moderate (GOLD II), severe (GOLD III), or very severe (GOLD IV) COPD, respectively (Table 1, also see Supplementary Table S1). Females were more predisposed to severe COPD (GOLD stage III/IV) than their male counterparts (69.2% female vs. 35.7% male) (Table 1).
Table 1.
Baseline characteristics of our COPD cohort (n = 125).
| Variables | Patients with COPD (GOLD Stage, n = 125) | |||
|---|---|---|---|---|
| I (n = 18) | II (n = 58) | III (n = 38) | IV (n = 11) | |
| Age (years) | ||||
| Median (IQR) | 68.00 (65.25–71.50) | 68.50 (62.25–73.00) | 70.50 (67.00–77.25) | 66.00 (63.00–69.00) |
| Sex, n (%) | ||||
| Male | 17 (94.44) | 55 (94.83) | 31 (81.58) | 9 (81.82) |
| Female | 1 (5.56) | 3 (5.17) | 7 (18.42) | 2 (18.18) |
| BMI, Kg/m2 | ||||
| Median (IQR) | 23.90 (21.63–26.29) | 24.14 (21.16–26.60) | 22.30 (20.00–24.50) | 20.60 (19.90–22.98) |
| Tobacco smoking, n (%) | ||||
| Current smoker | 5 (27.78) | 31 (53.44) | 11 (28.95) | 2 (18.18) |
| Ex-smoker | 13 (72.22) | 23 (39.66) | 22 (57.89) | 8 (72.73) |
| Never-smoker | 0 (0.00) | 4 (6.90) | 5 (13.16) | 1 (9.09) |
| Smoking pack-years | ||||
| Mean ± SD (Min-Max) | 48.89 ± 35.19 (5.00–150.00) | 49.02 ± 36.34 (0.00–180.00) | 49.30 ± 35.66 (0.00–156.00) | 56.73 ± 37.65 (0.00–123.00) |
| Median (IQR) | 42.50 (20.50–60.00) | 40.00 (23.00–60.00) | 40.00 (25.00–75.00) | 46.00 (35.00–85.00) |
| Pulmonary function indices | ||||
| FEV1 (L) Median (IQR) |
1.90 (1.74–2.11) | 1.61 b’ (1.38–1.90 | 0.99 a’b’c’d’ (0.74–1.12) | 0.58 a’b’c’d’ (0.52–0.66) |
| FEV1 % Median (IQR) |
84.55 (81.3–86.68) |
65.00 ab’c (57.38–72.00) | 39.05 a’b’c’d’ (35.00–45.00) | 25.00 a’b’c’d’ (22.10–27.95) |
| FEV1/FVC % Median (IQR) |
63.68 (61.25–66.87) |
59.25 a’b’ (54.12–65.50) | 46.50 a’b’c’d’ (42.11–55.25) | 41.41 a’b’c’d’ (30.93–45.67) |
| Emphysema severity | ||||
| Null/Mild (%) | 66.67 | 19.05 | 0.00 | 0.00 |
| Moderate (%) | 33.33 | 66.67 | 69.23 | 20.00 |
| Severe (%) | 0.00 | 14.28 | 30.77 | 80.00 |
| Lnc-IL7R expression | ||||
| Median (IQR) | 0.88 (0.79–0.94) | 0.59 (0.50–0.69) | 0.29 (0.19–0.41) | 0.18 (0.14–0.43) |
| COPD exacerbation in previous year | ||||
| 1.21 ± 1.03 | 1.09 ± 1.01 | 3.59 ± 2.10 | 3.74 ± 1.35 | |
COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; M, male; F, female; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; BMI, body mass index; IQR, interquartile range; %LAA-950insp, percentages of low attenuation area below—950 Hounsfield units. The values of FEV1/FVC % and FEV1 % were analyzed by Kruskal-Wallis test and Dunn’s multiple comparison test (a p < 0.05, a’ p < 0.01, compared with non-smoker; b’ p < 0.01, compared with smoker; c p < 0.05, c’ p < 0.01, compared with COPD patients with GOLD stage I; d’ p < 0.01, compared with COPD patients with GOLD stage II.
The mean FEV1 and FVC of our patients were 56.3 ± 19.2% and 79.80 ± 18.47%, respectively (Supplementary Table S1).
4.2. lnc-IL7R Expression Is Associated with Physiological Pulmonary Function and Exhibits Diagnostic Relevance for COPD-Based Patient Stratification
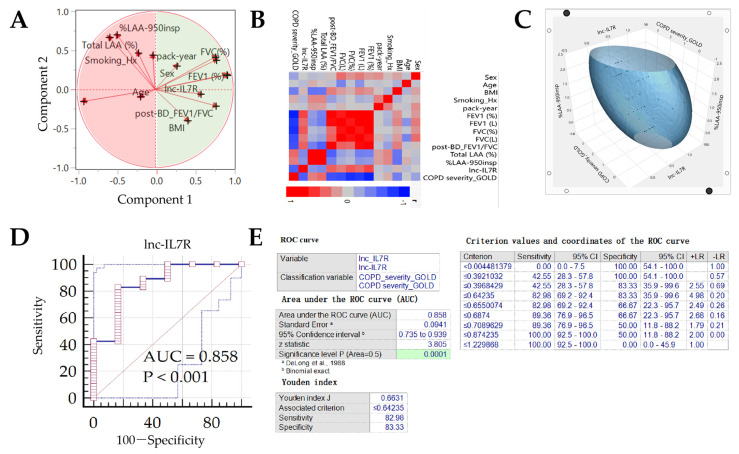
Corroborating the results above, principal component analyses showed that while smoking history, pack-year, age, total LAA, and %LAA-950insp (component 2) were anti-correlated with normal lung function, male sex, BMI, FEV1, FVC, FEV1/FVC, and lnc-IL7R (component 1) were positively correlated with same (Figure 1A). In concordance, component 1 factors were negatively correlated with GOLD COPD severity, while component 2 variables positively correlated with it (Figure 1B). Moreover, associative ellipsoid 3D visualization showed that increasing lnc-IL7R expression profile was associated with ameliorating COPD and %LAA-950insp-based emphysema severity (Figure 1C). Of great clinical relevance, lnc-IL7R expression exhibited excellent capability to discriminate between patients with no/mild COPD (GOLD I) and exacerbated/aggravated cases (GOLD II, III, IV) (area under the curve, AUC = 0.86, p < 0.001) (Figure 1D,E). A Youden’s J index of 0.66 (sensitivity = 83.0, specificity = 83.3) affirmed lnc-IL7R diagnostic relevance, with an optimal threshold value/cutoff point ≤ 0.64 defining exacerbated COPD (Figure 1E).
Figure 1.
lnc-IL7R positively correlates with physiological pulmonary function and exhibits diagnostic relevance for COPD-based patient stratification. (A) Visualization of principal component analysis based on factor analysis loading showing the associative predisposition of our panel of COPD-related variables and their stratification into components 1 and 2. (B) Correlative heat map of our panel of COPD-related variables. (C) Ellipsoid 3D image of the association between lnc-IL7R, GOLD-defined COPD severity, and %LAA-950insp-based Emphysema severity. (D) Graphical depiction of the ROC curve and AUC value of lnc-IL7R expression in our COPD cohort. (E) Statistical chart of the ROC curve of lnc-IL7R expression. ROC, receiver operating characteristic; AUC, area under curve.
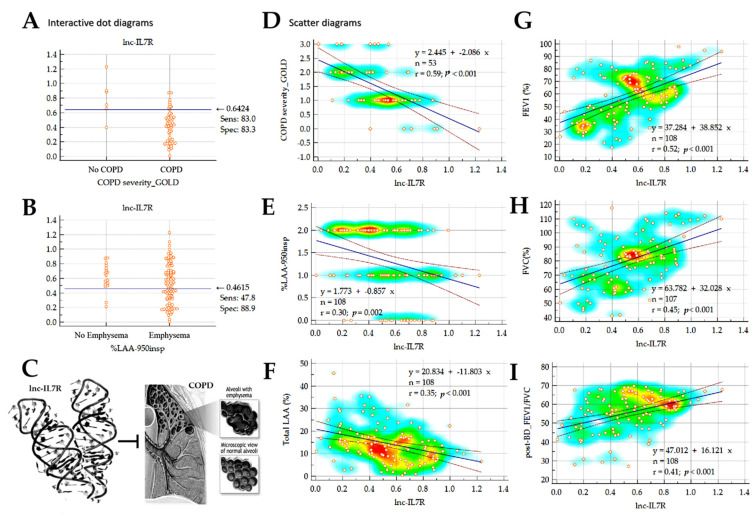
4.3. lnc-IL7R Level Correlates with COPD Status and Emphysematous Phenotype, and Its Differential Expression Reflects Disease Course
Consistent with Figure 1E, we observed that subjects with lnc-IL7R expression ≤0.64 were invariably COPD cases (Figure 2A), and cases with emphysematous phenotype were defined by an lnc-IL7R expression ≤0.46 (Figure 2B,C). Correlative analyses further demonstrated that lnc-IL7R expression was anti-correlated with COPD severity (r = 0.59, p < 0.001), emphysema severity (r = 0.30, p = 0.002), and emphysema status (r = 0.35, p < 0.001) (Figure 2D–F). Conversely, lnc-IL7R expression was positively correlated with pulmonary function indices, namely FEV1 (r = 0.52, p < 0.001), FVC (r = 0.45, p < 0.001), and the post-bronchodilator (post-BD) FEV1/FVC ratio (r = 0.41, p < 0.001).
Figure 2.
lnc-IL7R level correlates with COPD status and emphysematous phenotype, and its differential expression reflects disease course. Dot plots showing stratification of patients into (A) No-COPD or COPD, and (B) No-Emphysema or Emphysema groups, based on the expression of lnc-IL7R. (C) Pictorial depiction of the inhibitory role of lnc-IL7R expression on COPD with/or emphysematous phenotype. Scatter heat map plots depicting the correlation between lnc-IL7R expression, and (D) GOLD-defined COPD severity, (E) %LAA-950insp-based Emphysema severity, (F) total LAA, (G) FEV1, (H) FVC, or (I) post-BD FEV1/FVC. LAA, lung attenuation area; %LAA-950insp, percentage of lung attenuation area with values less than −950 Hounsfield Units on inspiratory CT scan; BD, bronchodilator.
4.4. lnc-IL7R, a Probable Biological Response Modifier, Exhibits Strong Anti-COPD Therapeutic Potential
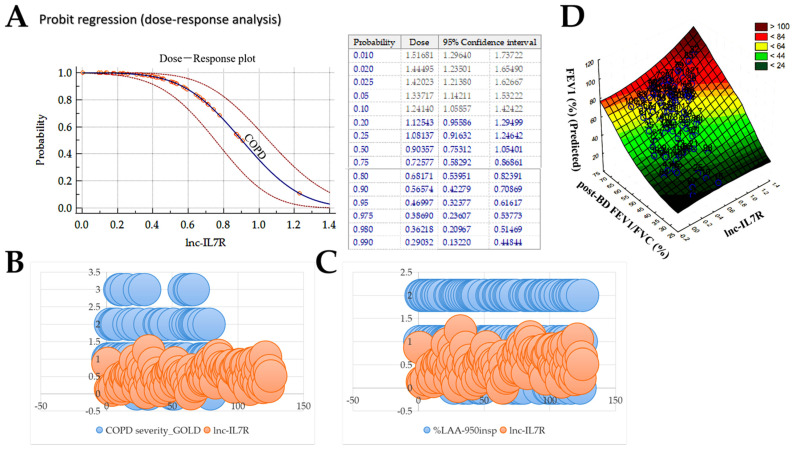
Furthermore, using bioinformatics-aided dose–response modeling we simulated the relationship between the internal dose metric, namely lnc-IL7R expression, and susceptibility to COPD. We observed that as the expression level of lnc-IL7R increased, the probability of COPD decreased with a threshold value of ~0.65 (Chi-squared, X2 = 11.95, p = 0.0005) (Figure 3A), which is reminiscent of a pharmacological effective dose (ED), where the ED is the concentration of lnc-IL7R that elicits a strong anti-COPD biological response. Consistent with this, after numeralization of the GOLD stage (0, GOLD 1; 1, GOLD II; 2, GOLD III; 3, GOLD IV), we showed that while high and moderate expression of lnc-IL7R is associated with no/mild COPD (GOLD I) and moderate COPD (GOLD II), patients with severe cases (GOLD III, IV) were lnc-IL7R negative (Figure 3B). Similarly, compared with the lnc-IL7R nonexpressors with severe emphysema, lnc-IL7R expressors, dependent on expression level, exhibited no, mild, or moderate emphysema (Figure 3C). Consistent with these results, we also found a statistically significant linear correlation between lnc-IL7R expression, post-BD FEV1/FVC ratio, and predictor FEV1 (%) (Figure 3D). In corroboration, understanding that bronchodilator response (BDR) reflects eosinophilic airway inflammation, bronchial atrophy and hyperactivity, lung function decline, and is a major criterion for the diagnosis of the asthma-COPD overlap syndrome, as well as a potential marker for delineating different types of COPD phenotypes [17,18], added to the statistically significant correlation between lnc-IL7R expression and post-BD FEV1/FVC ratio (r = 0.41, p < 0.001) (Supplementary Figure S1A), we observed higher lnc-IL7R levels in patients with negative BDR (FEV1 < 12% and 400 mL), compared with their BDR positive peers (1.5-fold, p < 0.001) (Supplementary Figure S1B). More so, the concomitant increase in lnc-IL7R levels and post-BD FEV1/FVC ratio was mostly associated with BDR negative status (coefficient of determination, R2 = 0.17; F-ratio = 17.62; p = 0.0001) (Supplementary Figure S1C,D). These data indicate that higher lnc-IL7R reflects an absence of airflow obstruction, is a probable biological response modifier, and exhibits strong anti-COPD therapeutic potential.
Figure 3.
lnc-IL7R exhibits strong anti-COPD therapeutic potential. (A) Probit regression-based dose–response plot (left panel) and table (right panel) of the effect of lnc-IL7R expression on COPD probability. Bubble plots show how lnc-IL7R expression stratifies patients according to (B) GOLD-defined COPD severity, and (C) %LAA-950insp-based emphysema severity. (D) Surface plot of the correlation between lnc-IL7R expression, post-BD FEV1/FVC, and predicted FEV1.
5. Discussion
Consistent with contemporary knowledge that only a dismal proportion (<1%) of all proposed biomarkers end up being translated to clinical utility [19,20], the identification of disease pathognomonic biomarkers that inform efficacious disease management or facilitate novel therapeutic approaches continues to lag behind the significant increase in our understanding of COPD pathophysiology and pathogenesis in the past decades. Against this background, and accentuating the unwritten consensus in pulmonary medicine that “no biomarker other than lung function has been shown to be useful, to date, for the diagnosis of COPD” [21], the present study, for the first time to the best of our knowledge, demonstrates that lnc-IL7R expression level (i) is associated with physiological pulmonary function, (ii) correlates with COPD status and emphysematous phenotype, and (iii) exhibits diagnostic relevance for COPD-based patient stratification. We also demonstrated that (iv) lnc-IL7R differential expression reflects disease course, and that (v) lnc-IL7R, a probable biological response modifier, exhibits strong anti-COPD therapeutic potential.
The strong positive correlation between lnc-IL7R expression levels and spirometric pulmonary function indices, exemplified by a 4.9-fold reduction in lnc-IL7R expression level eliciting marked decline in FEV1 (L) (3.3-fold, r = 0.37), FEV1 (%) (3.4-fold, r = 0.52), and post-bronchodilator FEV1/FVC ratio (1.5-fold, r = 0.41) is of clinical significance especially as the contemporary definition of COPD is based on a fixed FEV1/FVC ratio or on the lower limits of FEV1/FVC of a healthy reference population [22]. Furthermore, cognizant of an evolving understanding that the presence of reduced post-bronchodilator FEV1/FVC (usually <0.70 per GOLD criteria) may not necessarily be the strongest confirmation of the presence of persistent airflow limitation or predictor of COPD progression and eventual disease-specific mortality [22,23], the present study also provided clinical evidence that lnc-IL7R expression level was not only anti-correlated with spirometric GOLD-based COPD severity (r = −0.59) but also with computer tomography-based total LAA (%) (r = −0.35), %LAA-950insp (r = −0.30), and history of clinical COPD exacerbation in previous year. This is particularly interesting, as it demonstrates that unlike its spirometric predecessors, our proposed lnc-IL7R expression-based diagnosis and/or prognosis of COPD takes into account COPD-pathognomonic lung inflammation and tissue damage that are captured with CT imaging, thus connoting a broader definition of abnormal lung function, which is purportedly missed by the spirometric definition of COPD in ~40% of the population, even in the presence of smoking history and displayed COPD symptomatology [24].
Our present findings also lend some credence to the recently proposed dual axes pathophysiological basis of COPD, namely, airway-predominant and emphysema-predominant [24], especially with lnc-IL7R expression serving as a probable molecular bridge between the suppressed spirometric PFT indices (airway-predominant component) and increased CT imaging indices reflective of emphysema status and severity (emphysema-predominant). Consistent with the fundamental principle of GOLD that any clinically feasible COPD diagnostics should be simple and applicable worldwide [15], and in light of the seemingly impracticability of the COPDGene’s proposal, which is largely dependent on the relatively expensive CT imaging modality, in low-income countries [15,24], considering its ability to recapitulate CT evidence of airway inflammation and/or emphysema [3,14], the present study proposes the clinical utility of lnc-IL7R expression as a surrogate biomarker of COPD based on its pathognomonic attribution as demonstrated herein. In terms of early identification and initiation of appropriate therapy, we posit that the altered expression of lnc-IL7R may be diagnostically reliable in that group of patients who may fall outside the current spirometric GOLD COPD definition but are indeed at risk of COPD, actually at high risk of COPD-specific death, or exhibit COPD-like symptoms.
Our data, which for the first time, to the best of our knowledge, show that as the expression level of lnc-IL7R increased, the probability of COPD decreased profoundly, with associated statistically significant linear correlation between lnc-IL7R expression, post-BD FEV1/FVC ratio, and predicted FEV1 (%), is diagnostically relevant. The diagnosis of COPD, as per the GOLD guidelines [15,16], requires post-bronchodilator FEV1/FVC < 0.70 combined with FEV1 < 0.80 of the predicted value, thus, highlighting the clinical significance of the demonstrated concomitant increase in lnc-IL7R expression and the spirometric indicators of pulmonary function and/or health. In concert with other findings documented herein, we posit that altered lnc-IL7R expression may be a diagnostically valuable indicator of altered pulmonary function, and a clinically feasible, readily accessible, and relatively cheap surrogate biomarker of preventable and treatable COPD, which is usually insidious and associated with an aberrant inflammatory response of the lungs to noxious particulate matters or gases.
In conclusion, the present study provides some evidence that reduced lnc-IL7R expression is associated with inflammation in the airway epithelial cells, indicative of impaired pulmonary function, pathognomonic of COPD and predictive of an exacerbated/ aggravated COPD phenotype. These data provide new mechanistic insight into the ailing lung and COPD progression, as well as suggest a novel actionable molecular factor that may be exploited as an efficacious therapeutic strategy in patients with COPD.
Acknowledgments
The authors thank all collaborating physicians from the Shuang Ho Hospital, Taipei Medical University for their assistance with data retrieval and collation. We also appreciate the thought-provoking discussions with the medical staff of the Division of Pulmonary Medicine, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City.
Abbreviations
%LAA-950insp: percentage of lung attenuation area with values less than −950 Hounsfield Units on inspiratory CT scan; AQM: air quality monitoring; AUC: area under the curve; BDR: Bronchodilator response; BMI: body mass index; COPD: chronic obstructive pulmonary disease; CT: computed tomography; FEV1%: percent predicted forced expiratory volume in 1 s; FVC: predicted forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; lncRNA: long noncoding RNA; lnc-IL7R: long noncoding interleukin-7 receptor α-subunit gene.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines10040786/s1. Figure S1: Correlation between lnc-IL7R and bronchodilator response; Table S1: Association of lnc-IL7R, with GOLD COPD severity, and pulmonary function in our cohort (n = 125).
Author Contributions
O.A.B., S.-M.W., W.-L.S. and K.-Y.L.: study conception and design, collection and assembly of data, data analysis and interpretation. O.A.B.: manuscript writing. C.-W.L., P.-H.F., H.-C.C., S.-C.H., K.-Y.C., T.-T.C., W.-T.L. and C.-H.T.: data analysis and interpretation. O.A.B., S.-M.W., P.-H.F., W.-T.L. and K.-Y.L.: provision of resources and administrative oversight. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by grants from the Ministry of Science & Technology of Taiwan grants MOST108-2314-B-038-111-MY3, MOST108-2314-B-038-063-MY3, the Ministry of Education of the Republic of China, DP2-110-21121-01-T-01-01, and Taipei Medical University/Shuang Ho Hospital 108TMU-SHH-08, 110TMU-SHH-19.
Institutional Review Board Statement
The study was approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB Approval No.: N201512054, N201803059, N201902021), and was conducted compliant with guidelines from the Declaration of Helsinki regarding studies involving human subjects.
Informed Consent Statement
Written informed consent was obtained from all participants before sample collection.
Data Availability Statement
The data used in the current study are all contained in the manuscript and may be obtained upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Negewo N.A., Gibson P.G., McDonald V.M. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology. 2015;20:1160–1171. doi: 10.1111/resp.12642. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Global Health Estimates. 2020. [(accessed on 21 February 2022)]. Available online: https://www.who.int/data/global-health-estimates.
- 3.Wu S.M., Sun W.L., Lee K.Y., Lin C.W., Feng P.H., Chuang H.C., Ho S.C., Chen K.Y., Chen T.T., Liu W.T., et al. Determinants of Pulmonary Emphysema Severity in Taiwanese Patients with Chronic Obstructive Pulmonary Disease: An Integrated Epigenomic and Air Pollutant Analysis. Biomedicines. 2021;9:1833. doi: 10.3390/biomedicines9121833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinden N.J., Stockley R.A. Systemic inflammation and comorbidity in COPD: A result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax. 2010;65:930–936. doi: 10.1136/thx.2009.130260. [DOI] [PubMed] [Google Scholar]
- 5.King P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015;4:68. doi: 10.1186/s40169-015-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017;131:1541–1558. doi: 10.1042/CS20160487. [DOI] [PubMed] [Google Scholar]
- 7.Barnes P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Lange P., Celli B., Agustí A., Boje Jensen G., Divo M., Faner R., Guerra S., Marott J.L., Martinez F.D., Martinez-Camblor P., et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 9.Mercado N., Ito K., Barnes P.J. Accelerated ageing of the lung in COPD: New concepts. Thorax. 2015;70:482–489. doi: 10.1136/thoraxjnl-2014-206084. [DOI] [PubMed] [Google Scholar]
- 10.Penkala I.J., Liberti D.C., Pankin J., Sivakumar A., Kremp M.M., Jayachandran S., Katzen J., Leach J.P., Windmueller R., Stolz K., et al. Age-dependent alveolar epithelial plasticity orchestrates lung homeostasis and regeneration. Cell Stem Cell. 2021;28:1775–1789.e5. doi: 10.1016/j.stem.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zacharias W.J., Frank D.B., Zepp J.A., Morley M.P., Alkhaleel F.A., Kong J., Zhou S., Cantu E., Morrisey E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson J.D., Theurer W.M. A stepwise approach to the interpretation of pulmonary function tests. Am. Fam. Physician. 2014;89:359–366. [PubMed] [Google Scholar]
- 13.Devadoss D., Long C., Langley R.J., Manevski M., Nair M., Campos M.A., Borchert G., Rahman I., Chand H.S. Long Noncoding Transcriptome in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2019;61:678–688. doi: 10.1165/rcmb.2019-0184TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S.M., Feng P.H., Chuang H.C., Ho S.C., Fan Chung K., Chen K.Y., Wu G.S., Chen T.T., Tseng C.H., Liu W.T., et al. Impaired lnc-IL7R modulatory mechanism of Toll-like receptors is associated with an exacerbator phenotype of chronic obstructive pulmonary disease. FASEB J. 2020;34:13317–13332. doi: 10.1096/fj.202000632R. [DOI] [PubMed] [Google Scholar]
- 15.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Global Initiative for Chronic Obstructive Lung Disease. 2020. [(accessed on 22 October 2021)]. Available online: http://www.goldcopd.org/
- 16.Occhipinti M., Paoletti M., Bartholmai B.J., Rajagopalan S., Karwoski R.A., Nardi C., Inchingolo R., Larici A.R., Camiciottoli G., Lavorini F., et al. Spirometric assessment of emphysema presence and severity as measured by quantitative CT and CT-based radiomics in COPD. Respir. Res. 2019;20:101. doi: 10.1186/s12931-019-1049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastie A.T., Martinez F.J., Curtis J.L., Doerschuk C.M., Hansel N.N., Christenson S., Putcha N., Ortega V.E., Li X., Barr R.G., et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2017;5:956–967. doi: 10.1016/S2213-2600(17)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst P., Saad N., Suissa S. Inhaled corticosteroids in COPD: The clinical evidence. Eur. Respir. J. 2015;45:525–537. doi: 10.1183/09031936.00128914. [DOI] [PubMed] [Google Scholar]
- 19.Leung J.M., Obeidat M., Sadatsafavi M., Sin D.D. Introduction to precision medicine in COPD. Eur. Respir. J. 2019;53:1802460. doi: 10.1183/13993003.02460-2018. [DOI] [PubMed] [Google Scholar]
- 20.Drucker E., Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J. 2013;4:7. doi: 10.1186/1878-5085-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannino D.M. Biomarkers for chronic obstructive pulmonary disease diagnosis and progression: Insights, disappointments and promise. Curr. Opin. Pulm. Med. 2019;25:144–149. doi: 10.1097/MCP.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 22.Topalovic M., Exadaktylos V., Decramer M., Berckmans D., Troosters T., Janssens W. Using dynamics of forced expiration to identify COPD where conventional criteria for the FEV₁ /FVC ratio do not match. Respirology. 2015;20:925–931. doi: 10.1111/resp.12540. [DOI] [PubMed] [Google Scholar]
- 23.Lowe K.E., Regan E.A., Anzueto A., Austin E., Austin J.H.M., Beaty T.H., Benos P.V., Benway C.J., Bhatt S.P., Bleecker E.R., et al. COPDGene® 2019: Redefining the Diagnosis of Chronic Obstructive Pulmonary Disease. Chronic Obstr. Pulm. Dis. 2019;6:384–399. doi: 10.15326/jcopdf.6.5.2019.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young K.A., Strand M., Ragland M.F., Kinney G.L., Austin E.E., Regan E.A., Lowe K.E., Make B.J., Silverman E.K., Crapo J.D., et al. Pulmonary Subtypes Exhibit Differential Global Initiative for Chronic Obstructive Lung Disease Spirometry Stage Progression: The COPDGene® Study. Chronic Obstr. Pulm. Dis. 2019;6:414–429. doi: 10.15326/jcopdf.6.5.2019.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in the current study are all contained in the manuscript and may be obtained upon reasonable request from the corresponding author.