Abstract
Daptomycin is an investigational lipopeptide antibiotic active against gram-positive organisms. The mechanism of action is unique, resulting in interference with cell membrane transport. The bactericidal activity of daptomycin was evaluated against glycopeptide-intermediate susceptible Staphylococcus aureus (GISA), vancomycin-resistant Enterococcus faecium (VREF), and methicillin-resistant S. aureus (MRSA) in an in vitro infection model with simulated endocardial vegetations. Simulated regimens of daptomycin at 6 mg/kg/day (D6) and 10 mg/kg/day (D10) were utilized. MICs and MBCs for daptomycin were determined in the absence and in the presence of albumin with the following results (MIC/MBC): for GISA-992, 0.5/1.0 and 16/16; for VREF-590, 2.0/2.0 and 32/32; and for MRSA-494, 0.25/0.25 and 1.0/4.0 μg/ml, respectively. During the first 8 h daptomycin significantly reduced the inoculum for all organisms. Daptomycin at 6 mg/kg/day and 10 mg/kg/day had log10 CFU/g reductions of 5 and 6, 3.4 and 5, and 6.4 and 6.5 by 8 h for GISA-992, VREF-590, and MRSA-494, respectively. Against both GISA-992 and VREF-590, the D10 regimen achieved the limit of detection at 72 h, with D6 regimens showing slight regrowth. A concentration-dependent killing effect was noted to occur, with daptomycin demonstrating a more rapid and greater kill from the D10 versus the D6 regimen. The results of this study suggest that daptomycin demonstrates significant (P < 0.05) activity against gram-positive organisms in a simulated sequestered infection site.
The members of multidrug-resistant enterococcal and staphylococcal infections, as well as of all other gram-positive infections, have been increasing at an alarming rate. The incidence of methicillin-resistant Staphylococcus aureus (MRSA), first reported in the United States in the 1960s, has led to a significant increase in the use of vancomycin over the last 40 years. Inappropriate use of antibiotics, including vancomycin, has led to the development of resistant organisms, with the appearance of vancomycin-resistant enterococci (VRE) and glycopeptide intermediate-sensitive S. aureus (GISA) (2, 9, 18). From 1989 to 1998, the Centers for Disease Control and Prevention reported that the number of VRE nosocomial infections increased from 0.3 to 21.2% and from 0.4 to 22.6% in nonintensive and intensive care units, respectively (5).
Since the appearance of vancomycin resistance among clinical isolates of enterococci, concern has been raised about the potential for transfer of the resistance genes to highly virulent strains of MRSA (16). However, the mechanism of vancomycin resistance in laboratory-derived glycopeptide-resistant S. aureus isolates is distinct from the mechanism found in enterococci (12).
The eight documented strains of S. aureus with reduced susceptibilities to vancomycin in vivo are MRSA strains which have developed decreased susceptibility with prolonged exposure to vancomycin (17). This potential emergence of new strains of GISA and the problematic treatments available for VRE increases the need for new therapeutic options.
One potential hopeful for the treatment of VRE and GISA is daptomycin, a novel lipopeptide antibiotic. Like vancomycin, it has broad activity against gram positives bacteria, but it has a different mechanism of action that results in interference with cell membrane transport. This drug was first investigated as an alternative to vancomycin in the late 1980s. Daptomycin was found to be efficacious in skin and soft tissue infections and bacteremia, but studies with daptomycin in endocarditis were stopped due to less-than-desired outcomes with earlier designed low-dose regimens of 2 mg/kg every 24 h (q24h) and 3 mg/kg q12h. In light of the increasing need for alternative treatments against resistant gram-positive bacteria, there is a renewed interest in daptomycin.
Therefore, we decided to evaluate two higher-dosing regimens of daptomycin, i.e., dosed once daily at a current newly designed dose of 6 mg/kg or a potential new regimen of 10 mg/kg versus vancomycin against three clinical strains, one each of GISA, VRE, and MRSA.
MATERIALS AND METHODS
Bacterial strains.
The three strains evaluated were GISA-992 (New Jersey strain, Centers for Disease Control, Atlanta, Ga.), VREF-590 (isolated from a patient with bacteremia), and MRSA-494 (isolated from a patient with endocarditis).
Antibiotics.
Daptomycin analytical powder (lot 44BY0; Cubist Pharmaceuticals, Inc., Cambridge, Mass.) was used. Vancomycin used was commercially purchased (lot 1NJ03M; Sigma Chemical Co., St. Louis, Mo.).
Medium.
All in vitro-simulated endocardial vegetation (SEV) models, except the daptomycin models, utilized Mueller-Hinton broth (Difco, Detroit, Mich.) supplemented with 25 mg of calcium and 12.5 mg of magnesium per liter (SMHB). Due to the dependency on calcium for activity, all daptomycin models utilized Mueller-Hinton broth supplemented with 75 mg of calcium and 12.5 mg of magnesium per liter (6, 10). Colony counts were determined using tryptic soy agar (TSA; Difco) plates.
Susceptibility testing.
The MICs and MBCs of the antibiotics were determined by broth microdilution in SMHB according to National Committee for Clinical Laboratory Standards guidelines (13). Daptomycin MICs and MBCs were determined in supplemented broth as described above. Daptomycin MICs and MBCs were also determined in the presence of 4 g of human albumin (American Red Cross, Detroit, Mich.) per dl. Five-microliter samples from clear wells were plated onto TSA plates for the determination of MBCs, and all samples were incubated at 35°C for 24 h.
KC experiments.
To determine the effect of the protein content of the SEV on daptomycin's bactericidal activity, a series of killing curve (KC) experiments were performed using GISA-992 as the test strain due to our previous experience with daptomycin's activity against this strain (1). The following conditions were compared: daptomycin simulated total concentration of 80 μg/ml in broth alone, broth plus 4 g of albumin per dl, broth and organisms embedded in an SEV, and broth with albumin and organisms embedded in an SEV. Briefly, three to five colonies from an overnight growth on TSA plates incubated at 35°C were added to normal saline and adjusted as necessary to produce a 2.0 McFarland standard suspension of organisms. This suspension was diluted appropriately with SMHB to achieve a final inoculum of 109 CFU/ml for killing curves without SEVs. For KC experiments with SEVs, the SEVs were prepared as described below and placed in test tubes containing SMHB. A stock solution of daptomycin at the concentrations listed above was then added to all KCs (with or without SEVs), and the test tubes were incubated at 35°C with constant shaking for 24 h. Samples of broth (0.1 ml) or SEVs were removed at 0, 8, and 24 h, homogenized (for SEVs) and diluted appropriately with cold 0.9% sodium chloride to avoid antibiotic carryover, plated onto TSA plates, and incubated for 24 h at 35°C. Growth controls without antibiotic were run in parallel to the antibiotic-containing test tubes. The limit of detection with this method is 2.0 log10 CFU/ml. All time-kill curve experiments were performed in duplicate.
Simulated vegetations.
Organism stocks were prepared by inoculating 5-ml test tubes of Mueller-Hinton broth with colonies harvested from fresh overnight growth on TSA. Test tubes were then incubated for 24 h on a rotator at 37°C. Test tubes were then centrifuged for 15 min at 3,500 × g, and the supernatant was removed. The remaining pellet of the organism was collected and resuspended to achieve a concentration of 1010 CFU/ml. Simulated vegetations were prepared by mixing 0.1 ml of organism suspension (final inoculum, 109 CFU/0.5 g), 0.4 ml of human cryoprecipitate from volunteer donors (American Red Cross), and 0.025 ml of platelet suspension (platelets mixed with normal saline, 250,000 to 500,000 platelets per clot in 1.5-ml siliconized Eppendorf tubes). Bovine thrombin (5,000 U/ml) at 0.05 ml was added to each tube after insertion of a sterile monofilament line into the mixture. The resultant simulated vegetations were then removed from the Eppendorf tubes with a sterile 21-gauge needle.
In vitro pharmacodynamic infection model.
An in vitro infection model consisting of a 250-ml one-compartment glass apparatus with sample ports incorporated, in which the SEVs were suspended and sealed with a rubber stopper, was utilized (9). The apparatus was prefilled with SMHB, and antibiotics were administered as boluses over a 72-h period into the central compartment via an injection port. The model apparatus was placed in a 37°C water bath throughout the procedure, and a magnetic stir bar was placed in the medium for thorough mixing of the drug in the model. Fresh medium (SMHB) was continuously supplied and removed from the compartment along with the drug via a peristaltic pump (Masterflex; Cole-Parmer Instrument Co., Chicago, Ill.) set to simulate the half-lives of the antibiotics. The pH was monitored throughout all experiments with daptomycin due to the possible effects on its activity (10). Daptomycin has been demonstrated to be approximately 93% protein bound (6, 11, 14; G. L. Brier, J. D. Wolny, H. R. Black. Abstr. 29th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1347, 1989). Since we determined that the SEVs contain an average total protein level of 6.8 to 7.4 g/dl and 3.0 to 3.5 g of albumin per dl (similar to human serum) and since the results of our KC experiments indicated that protein affected daptomycin's bactericidal activity but there were no differences between daptomycin's killing activity in broth plus albumin, broth plus SEVs, or broth plus albumin and SEVs (see Results), we therefore simulated total daptomycin concentrations in the in vitro model. Daptomycin was given at two dosages of 6 mg/kg or 10 mg/kg q24h for estimated human total Cmax/Cmin (maximum concentration of drug in serum/minimum concentration of drug in serum) levels of 80/10 and 130/16 μg/ml, respectively (Investigator Brochure [11 15 June 2000]). The pump rate was set at 0.4 ml/min to achieve an average half-life of 8 h. Vancomycin was administered to simulate 1 g q12h for a Cmax of 30 to 35 μg/ml and a Cmin of 5 to 10 μg/ml. The pump rate was set at 0.5 ml/min to achieve a half-life of 6 h. All infection model experiments were performed in duplicate to ensure reproducibility. In addition, models in the absence of antibiotics were performed over 72 h to assure adequate viability of the organisms in the model.
Pharmacodynamic analysis.
Three SEVs were removed from each model (total of six) at 0, 8, 24, 32, 48, and 72 h. The SEVs were homogenized and diluted in cold saline, and 20 μl in triplicate was plated onto TSA plates. Plates were then incubated at 35°C for 24 h, at which time colony counts were performed. The total reduction in log10 CFU/g over 72 h was determined by plotting time-kill curves based on the number of remaining organisms over the 72-h time period. The time to achieve a 99.9% bacterial load reduction was determined by linear regression (if r2 ≥ 0.9) or by visual inspection. Cmax to MIC ratios (Cmax/MIC), time above the MIC for 24 h (T > MIC24 h), and the area under the concentration time curve from 0 to 24 h (AUC0–24)/MIC ratio were determined for the different dosing regimens of daptomycin and vancomycin.
Pharmacokinetic analysis.
Samples were obtained from broth (0.5 ml from each infection model), through the injection port, at 0.5, 1, 2, 4, 8, 24, 32, 48, and 72 h for the determination of antibiotic concentrations. Samples were also taken from the SEVs obtained at 0, 8, 24, 32, 48, and 72 h for the determination of antibiotic concentrations within the SEV. Samples were stored at −70°C until ready for analysis. Vancomycin concentrations were determined by fluorescence polarization immunoassay (Abbott Diagnostics TDx). This assay has a limit of detection for vancomycin of 2.0 μg/ml with a percent coefficient of variation (CV) of ≤6%. The concentrations of daptomycin were determined by microbioassay utilizing Micrococcus luteus ATCC 9341. The SEV concentrations of daptomycin and vancomycin were also determined via microbioassay using the same indicator organism. Vancomycin concentrations in SEVs were determined by bioassay because the viscosity of the samples interfered with the fluorescence polarization assay. We have previously found these two assay techniques to be comparable (15). Briefly, blank 0.25-in. disks were spotted with 20 μl of the standards in broth or samples. Each standard was tested in triplicate by placing the disk on antibiotic assay medium 1 agar plates, which were preswabbed with a 0.5 McFarland suspension of the test organism. Plates were incubated for 18 to 24 h at 37°C, at which time the zone sizes were measured. Concentrations of 150, 100, and 10 μg/ml (1.25 μg/ml, lower limit of detection) were used as standards with an interday percent coefficient of variation (CV%) of ≤10%. Standards for the above assays were prepared in broth and in broth plus cryoprecipitate to correct for protein content. The antibiotic peak/trough values and half-lives were calculated from plots of the concentration-versus time plots. AUC, elimination half-lives, and Cmax/Cmin were determined by trapezoidal methods utilizing PKAnalyst (Micromath, Salt Lake City, Utah).
Resistance.
The 100-μl samples from each time point were plated directly onto TSA plates containing four- and eightfold the MIC of the respective antibiotic to assess the development of resistance. Plates were then examined for growth after 48 h of incubation at 35°C. Development of resistance was evaluated at the 24-, 32-, 48-, and 72-h time points. Any growth of organisms observed on the antibiotic-containing resistance plates after 48 h of incubation would be considered to be resistant. If resistance developed, further MIC and MBC testing on any of these organisms was performed to determine the level of resistance.
Statistical analysis.
Changes in the CFU per gram level at 48 and 72 h were compared by two-way analysis of variance with Tukey's Post-Hoc test. A P value of ≤0.05 was considered significant. All statistical analyses were performed using SPSS Statistical Software (release 6.1.3; SPSS, Inc., Chicago, Ill.).
RESULTS
Susceptibility testing.
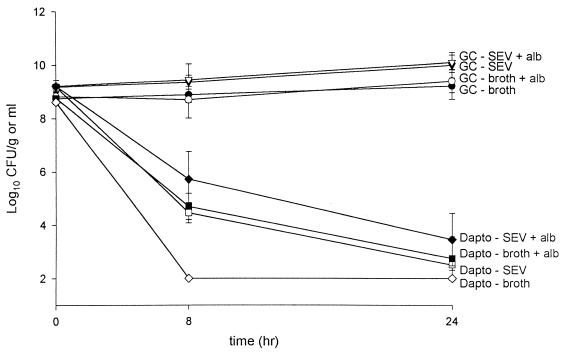
Microdilution MICs and MBCs of daptomycin with or without albumin and vancomycin for GISA-992, VREF-590, and MRSA-494 are listed in Table 1. KC results are displayed in Fig. 1. There were no noted differences in KC experiments in which daptomycin total concentrations were simulated in the presence of broth plus albumin, broth plus SEVs, or broth plus albumin and SEVs.
TABLE 1.
Susceptibility data for isolates tested
| Regimen | MIC/MBC (μg/ml) for strain:
|
||
|---|---|---|---|
| GISA-992 | VREF-590 | MRSA-494 | |
| Daptomycin without albumin | 0.5/1.0 | 2.0/2.0 | 0.25/0.25 |
| Daptomycin with albumin | 16/16 | 32/32 | 1.0/4.0 |
| Vancomycin | 8.0/8.0 | >64/>64 | 0.5/1.0 |
FIG. 1.
KC test tube experiments. Daptomycin simulated total concentrations of 80 μg/ml in broth (◊), in broth and albumin (■), in broth with SEV (□), and in broth with SEV and albumin (⧫) are indicated. Corresponding growth controls of these conditions are indicated by contrasting symbols (filled versus unfilled and unfilled versus filled).
Pharmacokinetics.
The pharmacokinetic parameters for daptomycin and vancomycin are listed in Table 2. The concentrations in the SEVs were similar to the levels obtained in the broth for daptomycin; however, vancomycin SEV concentrations were much lower than the concentration in broth.
TABLE 2.
Summary of pharmacokinetic data
| Antibiotic regimen and parameter | Mean result ± SD for strain:
|
||
|---|---|---|---|
| GISA-992 | VRE-590 | MRSA-494 | |
| Daptomycin, 10 mg/kg/day | |||
| Cmax (μg/ml) | 156.3 ± 12.4 | 129.8 ± 9.7 | 142.6 ± 5.2 |
| Trough (μg/ml) | 31.5 ± 8.6 | 20.6 ± 5.3 | 25.9 ± 4.7 |
| t1/2 (h) | 9.3 ± 0.6 | 9.0 ± 0.4 | 8.6 ± 0.9 |
| Cmax, SEV concn (μg/g) | 166.4 ± 12.3 | 118.7 ± 15.4 | 139.0 ± 8.8 |
| Daptomycin, 6 mg/kg/day | |||
| Cmax (μg/ml) | 104.2 ± 8.2 | 100.1 ± 5.9 | 113.1 ± 9.7 |
| Trough (μg/ml) | 9.9 ± 0.3 | 10.7 ± 0.07 | 9.4 ± 0.7 |
| t1/2 (h) | 7.4 ± 0.5 | 7.7 ± 0.6 | 6.7 ± 0.3 |
| Cmax, SEV concn (μg/g) | 121.4 ± 7.9 | 117.0 ± 10.6 | 96.1 ± 9.7 |
| Vancomycin, 1 g q12h | |||
| Cmax (μg/ml) | 34.8 ± 1.4 | 35.3 ± 0.3 | 39.2 ± 0.8 |
| Trough (μg/ml) | 12.1 ± 2.1 | 8.8 ± 1.6 | 12.7 ± 3.3 |
| t1/2 (h) | 7.7 ± 0.4 | 5.96 ± 0.09 | 7.4 ± 0.8 |
| Cmax, SEV concn (μg/g) | 4.2 ± 0.07 | 4.8 ± 1.1 | 6.2 ± 0.6 |
Pharmacodynamics.
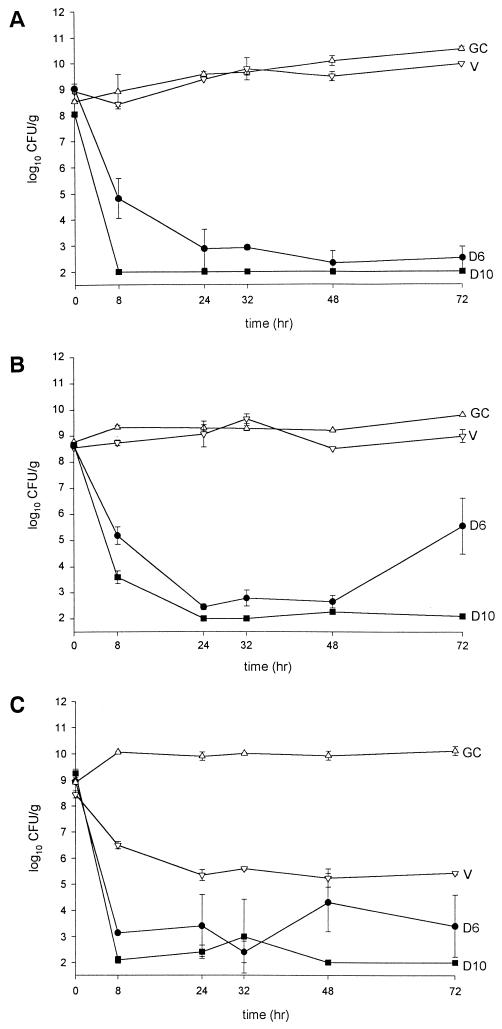
Pharmacodynamic results (changes in the log CFU/gram values over 72 h) are displayed in Table 3 and are graphically depicted in Fig. 2. Against GISA-992 daptomycin resulted in a 99.9% kill by 8 h for both regimens with 5- and 6-log10 CFU/g decreases for daptomycin at 6 mg/kg q24h (D6) and 10 mg/kg q24h (D10), respectively. The D10 regimen remained at the limit of detection throughout the 72 h, while with D6 slight regrowth was observed after 24 h. Against VREF-590 daptomycin resulted in time to 99.9% kill by 8 h for both regimens with 3.4- and 5-log10 CFU/g decreases in bacterial inocula, respectively. Concentration-dependent killing was observed with D10 achieving a 1.7-log greater kill than D6 at 8 h. At 72 hours D10 killed to detection limits, while some regrowth was noted for D6. For MRSA-494 daptomycin produced similar effects with an approximately 6.5-log10 CFU/g decrease in the inocula by 8 h for both regimens. As expected, vancomycin produced minimal killing during the first 24 h, with static activity during the remainder of the experimental period. Residual inoculum for the three organisms at 72 h is listed in Table 3.
TABLE 3.
Residual bacterial inoculum remaining after 72 h in the in vitro infection models
| Regimen | Residual inoculum (mean log10 CFU ± SD) of strain:
|
||
|---|---|---|---|
| GISA-992 | VREF-590 | MRSA-494 | |
| Growth control | 10.57 ± 0.09 | 9.85 ± 0.0 | 10.14 ± 0.17 |
| Daptomycin, 10 mg/kg/day | 2.0 ± 0.0 | 2.1 ± 0.08 | 2.0 ± 0.0 |
| Daptomycin, 6 mg/kg/day | 2.52 ± 0.43 | 5.57 ± 1.07 | 3.41 ± 1.19 |
| Vancomycin, 1 g q12h | 9.99 ± 0.0 | 9.02 ± 0.25 | 5.45 ± 0.07 |
FIG. 2.
In vitro model results with three multidrug-resistant strains. (A) GISA-992; (B) VREF-590. (C) MRSA-494. Key: GISA-992. D10, daptomycin at 10 mg/kg q24h (■); D6, daptomycin at 6 mg/kg q24h (●); V, vancomycin q12h (▿); and GC, growth control (▵).
Daptomycin's Cmax/MIC and AUC/MIC (in absence/presence of albumin) were as follows: GISA-992, 312.6/9.5 and 3,454/107.9; VREF-590, 64.9/4.0 and 710.2/44.4; and MRSA-494, 570.4/142.6 and 6,020.4/1,505.1 with D10 and GISA-992, 208.4/6.5 and 1,991.2/62.2; VREF-590, 50.0/3.1 and 496.8/31.1; and MRSA-494, 452.4/113.1 and 4,038.8/1,009.7 with D6, respectively. The Cmax/MIC and AUC/MIC values for vancomycin were 4.4 and 0.6, 78.4 and 63.2, and 7.1 and 1,135.2, respectively, for GISA-992, VREF-590, and MRSA-494.
The pH ranged from 6.95 to 7.19 for the 0-, 8-, 24-, 48-, and 72-h time points in all of the daptomycin experiments.
Resistance.
There was no evidence of daptomycin resistance observed at any of the samples at the 24-, 32-, 48-, and 72-h time points.
DISCUSSION
There is an increasing need for alternative agents in deep-seated infections, such as endocarditis and osteomyelitis, especially with the increasing incidence of resistant gram-positive organisms. Vancomycin, until the development of glycopeptide resistance, has been the agent employed against these organisms. Now, with the increasing incidence of VRE and the isolation of GISA, resistant organisms are becoming a concern, as viable treatment options become more limited.
Caron et. al. conducted an experimental endocarditis study, in which daptomycin was shown to consistently have homogeneous distribution throughout the vegetations (4). Their study showed that daptomycin had trough concentrations in the vegetations of approximately greater than or equal to half the trough concentrations that were obtained in serum. In an earlier experimental endocarditis study evaluating vancomycin against moderately penicillin and highly glycopeptide-resistant Enterococcus faecium, trough concentrations for vancomycin were shown to be substantially lower in the vegetations than in serum, a result which is similar to our findings (3).
In a rabbit endocarditis study evaluating daptomycin versus teicoplanin and vancomycin against S. aureus, daptomycin was found to be as efficacious as high-dose teicoplanin and was more efficacious, in some strains, than low-dose teicoplanin or vancomycin (9).
Daptomycin is approximately 93% protein bound (6, 11, 14; Brier et al., 29th ICAAC). Past in vivo experiments have led to less-than-desirable outcomes in deep-seated infections, including endocarditis. This was thought to be attributed to the high degree of protein binding and/or lower-than-optimal dosing, which resulted in lower-than-expected serum concentrations of daptomycin. In our in vitro infection model with SEVs, we dosed daptomycin using total drug concentrations. Our initial experiments in the SEV model using simulated free concentrations of daptomycin 6 mg/kg q24h and 3 mg/kg q12h for GISA-992 displayed no bactericidal activity. We suspected that the SEV protein content may have contributed to these observations. The material (cryoprecipitate) used to make the simulated vegetations was tested for albumin and total protein content. The cryoprecipitate was found to have an average of 3 to 3.5 g of albumin and 6.8 to 7.4 g of total protein per dl. The extensive KC experiments conducted in our laboratory demonstrated no difference in kill rates when daptomycin was added to albumin-SMHB, SEV-SMHB, or SEV-SMHB plus albumin. Therefore, the protein in our models mimics the protein found in humans, and this allowed for a better comparison between our results and in vivo studies.
Although our experiment was not designed to evaluate optimal pharmacodynamic predictors, a recent pharmacodynamic study determined that the outcome was predicted by the AUC/MIC ratio (A. Louie, P. Kaw, W. Liu, N. L. Jumbe, G. Vasudevan, M. H. Miller, and G. L. Drusano, 39th ICAAC, abstr. 173-A p. 1770, 1999). This study demonstrated, in an S. aureus strain with an MIC of 1 μg/ml, that an 80% effective dose was associated with an AUC of 114.8. In our experiments both regimens of daptomycin obtained significantly higher AUCs; even though the AUC/MIC values were significantly lower for GISA-992 and VREF-590, both the D10 and the D6 regimens resulted in significant killing against these organisms.
In our experiments we observed a concentration-dependent killing with daptomycin. In previous studies, it has been shown that, depending on the concentration of daptomycin, the presence of protein can significantly decrease the activity of daptomycin. These studies compared earlier dosing schemes of 2 mg/kg q24h and 3 mg/kg q12h. Daptomycin at 6 mg/kg administered as a q24h dose is currently being evaluated for the treatment of bacteremia. A study evaluating the effect of the postantibiotic effect (PAE) on Enterococcus faecalis and S. aureus found that dose-dependent effects were seen (6). These authors reported an approximately 1- to 7-h PAE when a daptomycin dose of 16 μg/ml was used. Daptomycin's concentration-dependent killing, long PAE, and long terminal half-life lends itself to once-daily dosing. While a 10-mg/kg/day dose has yet to be evaluated in animals or humans, we wanted to optimize the concentration-dependent effect of daptomycin so that we could more easily determine whether killing could be enhanced for organisms with higher MICs.
In conclusion, daptomycin at 6 or 10 mg/kg/day has significant activity (P < 0.05) against drug resistant gram-positive organisms. There appears to be a concentration-dependent killing noted with daptomycin. The kills achieved by both regimens demonstrated similar activity. However, if larger doses (e.g., 10 mg/kg/day) were needed, further studies would be necessary to evaluate the potential utility of this regimen.
ACKNOWLEDGMENTS
We thank Raymond Cha for his contribution with the KC experiments and for help in preparing the manuscript. We acknowledge Abbott Diagnostics for the use of the TDx analyzer for the assay of vancomycin.
ADDENDUM
When susceptibilities to daptomycin were retested in December 2000, they were found to be slightly different from those determined at the time of the experiment. The MICs and MBCs for GISA 992 were 0.5 and 1.0 μg/ml, respectively, in the absence of albumin and 4 and 8 μg/ml, respectively, in the presence of albumin; the MICs and MBCs for VREF-590 were both 4 μg/ml in the absence of albumin and 8 and 32 μg/ml, respectively, in the presence of albumin. No changes were observed for MRSA-494.
REFERENCES
- 1.Akins R L, Rybak M J. In vitro activities of daptomycin, arbekacin, vancomycin, and gentamicin alone and/or in combination against glycopeptide intermediate-resistant Staphylococcus aureus in an infection model. Antimicrob Agents Chemother. 2000;44:1925–1929. doi: 10.1128/aac.44.7.1925-1929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. Interim guidelines for prevention and control of staphylococcal infection associated with reduced susceptibility to vancomycin. Morb Mortal Wkly Rep. 1997;46:626–628. ,635. [PubMed] [Google Scholar]
- 3.Caron F, Carbon C, Gutmann L. Triple-combination penicillin-vancomycin-gentamicin for experimental endocarditis caused by a moderately penicillin- and highly glycopeptide-resistant isolate of Enterococcus faecium. J Infect Dis. 1991;164:888–893. doi: 10.1093/infdis/164.5.888. [DOI] [PubMed] [Google Scholar]
- 4.Caron F, Kitzis M D, Gutmann L, Cremieux A C, Maziere B, Vallois J M, Saleh-Mghir A, Lemeland J F, Carbon C. Daptomycin or teicoplanin in combination with gentamicin for treatment of experimental endocarditis due to a highly glycopeptide-resistant isolate of Enterococcus faecium. Antimicrob Agents Chemother. 1992;36:2611–2616. doi: 10.1128/aac.36.12.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Summary of notifiable diseases, United States. Morb Mortal Wkly Rep. 1998;47:19. [Google Scholar]
- 6.Hanberger H, Nilsson L E, Maller R, Isaksson B. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob Agents Chemother. 1991;35:1710–1716. doi: 10.1128/aac.35.9.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hospital Infection Control Practices Advisory Committee. Recommendations for preventing the spread of vancomycin resistance. Morb Mortal Wkly Rep. 1995;44:1–13. [PubMed] [Google Scholar]
- 8.Houlihan H H, Stokes D P, Rybak M J. Pharmacodynamics of vancomycin and ampicillin alone and in combination with gentamicin once-daily or thrice-daily against Enterococcus faecalis in an in vitro infection model. J Antimicrob Chemother. 2000;46:79–86. doi: 10.1093/jac/46.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Kaatz G W, Seo S M, Reddy V N, Bailey E M, Rybak M J. Daptomycin compared with teicoplanin and vancomycin for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1990;34:2081–2085. doi: 10.1128/aac.34.11.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamp K C, Rybak M J, Bailey E M, Kaatz G W. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob Agents Chemother. 1992;36:2709–2714. doi: 10.1128/aac.36.12.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee B L, Sachdeva M, Chambers H F. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob Agents Chemother. 1991;35:2505–2508. doi: 10.1128/aac.35.12.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira B, Bolye-Vavra S, de Jonge B L M, Daum R. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1788–1793. doi: 10.1128/aac.41.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7–A4. Villanova, Pa: NCCLS; 1997. [Google Scholar]
- 14.Rybak M J, Bailey E M, Lamp K C, Kaatz G W. Pharmacokinetics and bactericidal rates of daptomycin and vancomycin in intravenous drug abusers being treated for gram-positive endocarditis and bacteremia. Antimicrob Agents Chemother. 1992;36:1109–1114. doi: 10.1128/aac.36.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rybak M J, Bailey E M, Reddy V N. Clinical evaluation of teicoplanin fluorescence polarization immunoassay. Antimicrob Agents Chemother. 1991;35:1586–1590. doi: 10.1128/aac.35.8.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenover F C, Lancaster M V, Hill B C, Steward C D, Stocker S A, Hancock G A, O'Hara C M, Clark N C, Hiramatsu K. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenzel R P, Edmond M B. Vancomycin-resistant Staphylococcus aureus: infection control considerations. Clin Infect Dis. 1998;27:245–251. doi: 10.1086/514646. [DOI] [PubMed] [Google Scholar]