Abstract
Nanoparticles are often engineered as a scaffolding system to combine targeting, imaging, and/or therapeutic moieties into a unitary agent. However, mostly overlooked, the nanomaterial itself interacts with biological systems exclusive of application-specific particle functionalization. This nanoparticle biointerface has been found to elicit specific biological effects, which we have termed “ancillary effects.” In this review, we describe the current state of knowledge of nanobiology gleaned from existing studies of ancillary effects with the objectives of: describing the potential of nanoparticles to modulate biological effects independently of any engineered function; evaluating how these effects might be relevant for nanomedicine design and functional considerations, particularly how they might be useful to inform clinical decision-making; identifying potential clinical harm arising from adverse nanoparticle interactions with biology; and finally, highlighting the current lack of knowledge in this area as both a barrier as well as an incentive to the further development of nanomedicine.
Section 1. Introduction.
From a medical perspective, nanotechnology (in the form of nanomaterials) represents a wide frontier of novel technologies that are potentially applicable to advanced medical treatments, both for diagnostics and for therapy. Conversely, nanomaterials also are involved in a vast and (thus far) poorly mapped network of interactions with their biological environment. Nanoparticles (NPs, defined as particles 100 nm or less in size) used in biomedical research are highly diverse in structure, chemical composition, size, morphology, electrostatic charge, hydrophobicity, surface chemistry, and other properties. A direct consequence of this diversity is an even more diverse web of biological interactions within living systems, which is rarely investigated in detail and can result in unexpected ancillary “Ying and Yang” biological effects of nanoparticles – for better or worse. Thus far, the body of knowledge that has been assembled on this subject is far from complete or consistent. Most research into NPs has been and remains goal-directed; i.e., studied NPs were specifically designed to solve a problem, either as a research tool, or as an investigational therapeutic or diagnostic agent. Consequently, the NP is studied primarily in terms of the problem it was intended to solve, without significant evaluation of possible ancillary interactions of the NP within its biological environment. Flexibility as molecular “scaffolds” lend NP to functionalization with targeting moieties, imaging agents and drugs intended to elicit a particular biological effect within a specific and assumed well-defined context. However, the non-payload portion of NPs, frequently comprising the vast majority of the particle, is not merely inert material; it, too, interacts with surrounding biology in both overt and subtle ways. There has been comparatively little exploration of how payload-independent composition of NPs affects and influences the biological systems with which it comes into contact, especially if effects fall outside of the scope of the NPs intended purpose. Yet, without a thorough understanding of possible interactions between NPs and biology sufficient knowledge to predict a nanomedicine’s safety and total effects is lacking. More extensive knowledge of nanobiology will enable us to design better NPs to overcome the limitations and problems inherent to existing NP technologies. Furthermore, as we will show, some of the unanticipated effects of NPs in biological systems could even be exploited for novel therapeutic paradigms in nanomedicine.
Direct NP-induced toxicities (e.g., cell death, oxidative stress, and inflammatory responses) are the most thoroughly studied effects associated with NP exposure. However, nanomaterials may provoke more subtle effects, such as cell signalling cascades, gene expression, differentiation, and cell migration, among others. The long-term implications of such effects are not known and are a potentially significant barrier to widespread translation of nanomedicines. Conversely, these biological effects, if understood, could also be exploited for medical purposes.
This review will focus on the most relevant effects induced by clinical or translationally relevant NPs in the context of nanotherapy to inform future NP use and design, and also to provide a framework to address the core issues that are holding the field back. As in vitro data from NP studies have not been found to robustly correlate with in vivo observations, we will focus primarily on literature describing ancillary effects with supporting results obtained in vivo. We will also interpret the potential significance of these findings for the future of medicine and public health and underscore the need to improve our understanding of the non-directed biological effects of nanomaterials.
Section 2. The structure of nanoparticles and biological “identity”.
2.1. Nanoparticle classification and “pristine identity”.
A particular type of NP is distinguished from others by its unique physical and chemical characteristics. The most obvious method for categorizing a nanoparticle is based upon the physicochemical composition of the core material; this is the primary component of NP identity. However, while the core constitutes a major part of the particle, first contact with the particle’s environment is made not by the core but through the NP’s outer coating. Therefore, nanoparticles are often defined secondarily by the outward-facing surface of the particle, which frequently is of a different material than the central portion. This classification method works well for uniform or “core-shell” type nanostructures, prevalent in biomedical research, where the core can be either organic or inorganic, while the outer shell or coating is mostly organic for greater biocompatibility and solubility. There are, of course, highly complex nanostructures, which defy classification under this simplistic methodology. NP are sometimes also descriptively defined by other physicochemical traits: shape or geometry (tube, rod, sphere, star, etc.), surface charge (neutral, anionic, cationic), surface chemistry functional groups (e.g., amine, carboxyl, thiol), porosity, hydrophobicity, rigidity, and so on. These physicochemical traits define the foundational “pristine” identity of the particle and drive its interactions with biology via its affinity to various types of biomolecules.
2.2. Nanoparticle corona and “biological identity”
Before direct interaction with the cellular surface or internal cellular machinery of particular organ systems, NPs will typically come into contact with extracellular fluids (ECF) such as blood, lymph, and interstitial fluid, which contain a broad range of (bio)molecules. Due to the typically high surface-to-mass ratio of nanoscale structures, extensive interaction with these biomolecules occurs1, forming an affiliated biomolecule shell, or NP “corona” that includes mineral ions, lipids, sugars, carbohydrates, and especially proteins2. The composition of these coronas is determined by the biological environment and the pristine identity of the particle. NP coronas are complex, heterogeneous, and dynamic, and are formed almost instantaneously by a process that is spontaneous, stochastic, and irreversible3. Furthermore, the composition of the corona is temporally dynamic and contextually dependent; corona composition shortly after initial ECF exposure may differ from the corona of a NP which has been immersed in that same ECF for hours4. The type of ECF in which the NP is dispersed, variations in ECF content due to biological differences (e.g. species, sex, age), initial ECF composition at the route of NP entry, tissue/organ context5, disease states6, and NP metabolism/decay (among many other conditions) can all result in differing corona compositions. Abundant molecules at the initial entry side (usually the blood, i.e., serum) will typically constitute most of the corona.
The corona appears to serve a dual purpose: it naturally functions to mitigate NP toxicity by “walling off” the foreign material from delicate biological structures with a protective barrier of biomolecules7, and simultaneously facilitates clearance of the NP by scavenging immune cells. However, the biomolecules that associate with nanomaterial are important mediators of many complex biological processes whose functions could conceivably be impacted by interaction with NPs. The primary interface of NPs with complex living systems is typically indirect, via engagement with NP-associated corona components, rather than direct interaction with the nanomaterial8. For this reason, the corona makeup and assembly dynamics are a critical factor in directing nanoparticle-biological interactions2,8. The corona thus constitutes a bioactive shell which can bind to and activate cell surface receptors and other biological mechanisms based upon corona composition and nanoparticle localization3,9-11. It has been shown that immunoglobulins and lipoproteins found in serum can engage their respective receptors when adsorbed into silica NP coronas; the receptor binding of the protein epitopes remain accessible even when bound to the NP12. The bioactivity of coronas can both drive nanoparticle uptake by binding to endocytic receptors and activate other signalling cascades; it can also interact with the extracellular matrix or the cell membrane. The pristine identity of the nanoparticle may also redefine corona component functionality, by altering the secondary, tertiary, or quaternary structure of adsorbed corona proteins through particle-protein interaction3,13,14. Corona effects are likely not limited to interactions with extracellular or cell surface features, as coronas have been shown to persist after uptake, and therefore could continue to mediate interface with intracellular biology even after endocytosis, leading to various intracellular effects7.
The coronal association of biomolecules, driven by environmental context and NP pristine identity, could impart unanticipated biological activity on the particle—defining an NP’s “biological identity” on top of its pristine identity and driving ancillary effects through interactions. Importantly, the corona is not the sole determinant of NP-mediated biological effects, and a corona does not necessarily abrogate other effects which may arise from interactions with the NP core material or other non-coronal components15,16. As with much of our understanding of NP-biological interactions, our understanding of NP coronas (and the resulting impacts on surrounding biology) is fragmented and incomplete. Understanding of the relationship between pristine identity and biological identity, and the interaction of particle biological identity with biological systems, is crucial for further development of nanomedicines.
Section 3. Ancillary effects of nanomedicines: a double-edged sword.
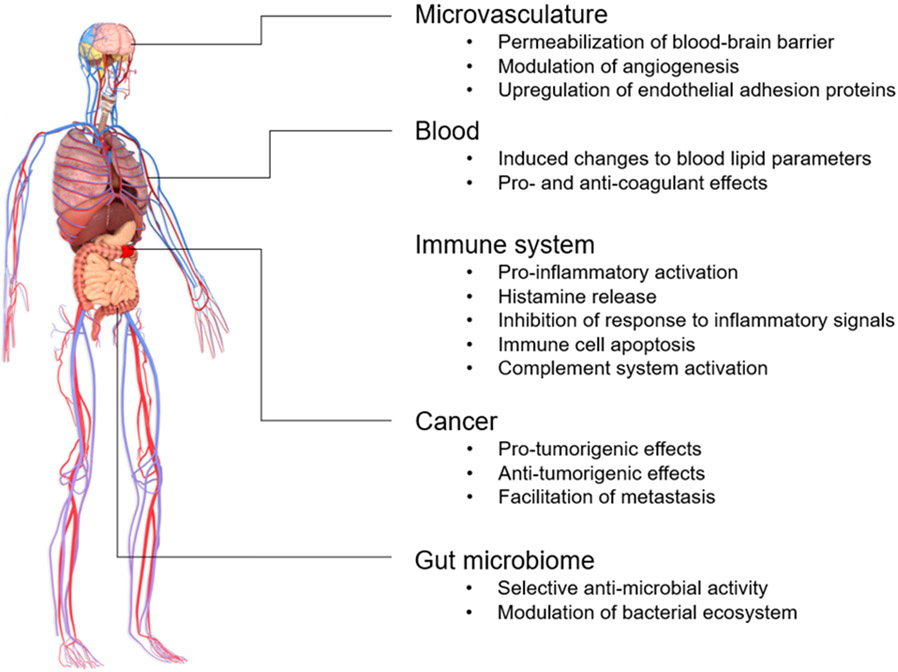
Traditionally, NP were viewed as excipients of drugs. However, the diverse assortment of payload-independent nanoparticle effects represents a potential untapped avenue of biological mechanisms which, in an appropriate context, might be leveraged for medicinal benefit. I.e., rather than merely functioning as a passive excipient, a NP might itself exhibit drug-like effects. These ancillary effects could conceivably be exploited either independently as a standalone nanomedicine or coupled with a conventional small molecule or biologic drug to improve and enhance therapeutic efficacy. But properties of nanomaterials can also induce potentially harmful effects at the cellular, tissue, and organ system levels with detrimental consequences for patients receiving the nanomedicine (Figure 1). The potential of certain nanomaterials to trigger inflammation and cytotoxicity is well-known1; however, other ancillary effects can be more subtle and might have pernicious effects. This review will consider selected relevant findings of potential medical usages for clinically relevant NP (or preclinical NP with translational potential), as well as some notable health and safety concerns arising from the use of NP as a medical tool, based on intrinsic effects arising from NP-biological interaction – the “Ying and Yang” of nanomedicine, interconnected intimately.
Figure 1.
Summary of select biological effects associated with translationally relevant nanomaterial.
3.1. Effects on vasculature and biological barriers.
The usefulness of drugs is highly dependent upon their potential to get to the site of intended action. For example, a drug which is highly effective in vitro when directly applied to cells is therapeutically useless if it cannot reach those cells in vivo. Thus, biological barriers effectively function as tissue and organ gatekeepers, and dictate which tissues are accessible to which drugs. The foremost barrier affecting pharmaceuticals is the blood vasculature, as most agents must diffuse through it to reach the site of intended action. In particular, the blood-brain barrier (BBB) is a major challenge for drug development, as it is less permissive and more selective to drugs than other vessels, allowing almost no paracellular diffusion17. It is known that nanomaterial characteristics, particularly particle size and shape, can influence the degree to which NP can pass through vascular barriers18. However, certain translationally relevant NP (notably gold, titanium dioxide, and silica) exhibit ancillary effects that modulate the permeability vascular endothelial barriers. NP could therefore be utilized as agents to facilitate or improve drug delivery at sites by modulating the permissiveness of the barrier to those tissues, which are otherwise problematic to reach for biologics or small molecule therapies. This permeabilization, however, could on the other hand present unforeseen problems by dysregulating the protective functions of the BBB and other vasculature, potentially exposing the brain or other vulnerable organs to toxins, pathogens, or metastatic cells that would otherwise be excluded by an intact barrier.
The integrity of microvasculature is sustained by cell-cell adhesion between vascular endothelial cells, forming a tight “seal” which prevents uncontrolled extravasation. Various types of NPs have been shown to affect the integrity of blood vasculature in several organs, including the BBB, in ways that can even drive metastasis. Certain NPs can induce the formation of micron-scale pores in vascular endothelium, creating leaks through which dysregulated paracellular diffusion can occur. For example, in microvasculature, adherens junctions initiate and maintain cell-cell adhesion in vascular endothelium using cell-cell adhesion proteins called cadherins and catenins. The interactions between the domains of cadherins in neighbouring cells initiates the formation of an adherens junction. Titanium dioxide NPs have been shown to bind to vascular endothelial cadherin (VE-cadherin) and disrupt the interactions between the VE-cadherin molecules that form the adherens junctions, increasing the bidirectional permeability of the vascular endothelium in subcutaneous blood vessels and in healthy lung vasculature 19,20. Disruption of inter-endothelial vascular junctions has also been observed with citrate-coated silver NPs, which triggers VE-cadherin downregulation. Gold NP were found to increase degradation rates of tight junction proteins in the BBB 21. Therefore, NP ancillary effects on vasculature could both compromise the integrity of the vasculature or create opportunities to deliver drugs beyond otherwise highly selective barriers.
In addition to ancillary effects on vascular integrity, NP might also influence the biological processes regulating neovascularization. Zinc oxide NP have been found to enhance development of capillary formation both in vitro and in vivo. The metallic component of the NP catalyses the formation of intracellular ROS in vascular endothelial cells, including hydrogen peroxide, a redox-active second messenger in the angiogenic signalling process. This subsequently drives sprouting and formation of new blood vessels from existing microvasculature 22. Such an NP may function to rapidly improve circulation at an injury site, facilitating further tissue growth and repair. Therefore, stimulation of neovascularization by a NP could be beneficial in enhancing healing. NP-based tissue engineering approaches are particularly interesting for the frontiers of regenerative medicine, including bone tissue regeneration, treatment of myocardial infarct injuries, scar tissue repair and cosmetic surgery, and organ transplantation. It is plausible that these emerging medical technologies could benefit by incorporating NP to harness ancillary effects for improved efficacy and effects otherwise potentially difficult to achieve.
3.2. Haemostatic regulation and alteration of blood parameters by NP.
NPs can modulate mechanisms regulating the delicate balance behind haemostasis and haemostatic regulation, in both pro-coagulant and anti-coagulant directions. Blood clotting is a complex process, typically initiated by adhesion of platelets at a site of vascular injury, followed by an activation cascade of soluble clotting factors, which generate an insoluble network of cross-linked fibrin proteins. Some types of NP have been shown to affect platelet adhesion, the initial step. Silver NP, either uncoated23 or PEGylated24, reduce platelet adhesion by altering the conformation of the adhesion protein integrin αIIbβ3, which had anti-coagulant effects in mice by ultimately reducing downstream generation of thrombin and fibrin. Similarly-sized uncoated gold NP had no comparable anti-platelet effects24. Effects on haemostatic regulation can also be mediated by NP interaction with secreted clotting factors. In this context, cationic poly(amidoamine) (PAMAM) dendrimers acted as a pro-coagulant by promoting the formation of fibrinogen aggregates via a thrombin-independent mechanism, wherein the cationic dendrimer interacts directly with fibrinogen25. The clot-forming potential of erythrocytes can be affected by NP as well, as titanium dioxide NP upregulate phosphatidylserine exposure on erythrocytes, which facilitated thrombin formation and erythrocyte aggregation in rats26. Furthermore, NP might modulate endothelial cell adhesiveness: silica NP were observed to upregulate endothelial PECAM expression and increase platelet adhesion to the vascular wall, which could contribute to thrombus formation27. [34046558] It is unlikely that nanotherapies would function more effectively than existing drugs for systemic haemostatic regulation; however, these effects could be exploited for localized haemostatic effects consistent with another therapeutic objective, e.g., by enhancing uptake of NP into tumours by inhibiting intratumorally platelet activation28. However, these effects are potentially also undesirable off-target effects and could contribute to risks such as brain ischemia or cardiac infarction. NP therapies thus might be contraindicated in patients receiving anticoagulants.
NPs can potentially affect the formation and pathology of atherosclerotic plaques. In atherosclerosis, fatty lesions develop along the wall of blood vessels, impairing blood flow and ultimately increasing risk of ischemia and acute infarct or stoke. These lesions are typically inflamed, due to infiltration by macrophages which become laden with phagocytosed lipids such as LDL and promoting disease progression. Long-term inhalation exposure to titanium dioxide29 and zinc oxide30 NP, which are commonly found in many consumer goods, has been shown to induce blood dyslipidaemia in rodents by an unknown mechanism. These changes, including elevation of LDL and total cholesterol, elevation of serum triglycerides with subsequent increased risk of atherosclerosis and plaque formation. Further, risks could arise from NP meddling with inflammatory functions as above in atherosclerotic plaques, both possibly enhancing their formation or slowing progress.
3.3. Microbiotic regulation.
There are relatively few identified therapeutic applications for orally administered nanomedicines. However, NP are already abundant in consumer products, including sunscreens, toothpaste, cosmetics, topical medications, and in food additives. The commonality of nanomaterial in these products means that exposure via the oral and inhalation routes is unavoidable and largely unknown. The microbial ecosystem of the GI tract, the gut microbiome, is a highly complex and dynamic biological milieu; current research is revealing an ever-expanding effect of the gut microbiome on multiple systems. Resident microbiota can be beneficial, commensal, or pathogenic to the host; thus, upsetting the delicate balance of this ecosystem might impair digestive efficiency or leave the host more vulnerable to opportunistic infections. The gut microbiome has recently been implicated in modulation or manipulation of an unexpectedly wide array of host biological processes, including metabolism, immunoregulation, and even behaviour, though a mechanistic understanding of the specific interactions between microbes and host remain poorly understood NP may also interact with bacteria or other microorganisms in the microbiome, and thus potentially impact the poorly understood interactions between host and microorganism 31. From a product safety and public health perspective, the effects of internalized NP on the microbiota of these organs, and their broader health consequences, is an unresolved question.
Silver and silver composition NP possess broad antimicrobial activity and are frequently incorporated into topical medical treatments for burns as an anti-infective agent. However, different species and strains of bacteria exhibit varying degrees of sensitivity to silver toxicity32. Thus, silver NP affect certain constituents of the bacterial species commonly found in the GI tract more than others: oral intake of silver NP alters microbiome homeostasis in mice and rats33-36. Gut biome perturbations are not exclusive to silver NP; gold nanoclusters37, titanium dioxide NP36, and zinc oxide NP38 exposure have also demonstrated alteration of gut homeostasis in mice. In addition to bacteria, bacteriophages are an integral component of the microbiome, representing a phage virome that interacts with and modulates gut bacterial ecology. The phage virome composition might also be impacted by NP exposure; for example, long-term intake of silver NP by rhesus monkeys has been shown to reduce the gut population of bacteriophages known to prey on pathogenic bacteria39.
The gut microbiome is also only one of many identified microbiomes in the human body. Though poorly studied, the lung microbiome is believed to have roles in the regulation of host immunity and homeostasis similarly to the gut microbiome and is also easily accessible to inhaled external nanomaterial. Microbicidal silver NPs disrupt the composition of the mouse lung microbiome in a coating-dependent manner, with citrate-coated silver NPs being the most disruptive40. To make matters even more complex, NP also could exert effects on tumour microbiomes, the relevance of which to cancer has only recently come into focus41-44.
The current limited understanding of the relationship between microbiome and host biology constrains our ability to interpret the significance of these NP-mediated perturbations of microbial populations, or to determine whether the induced changes might be beneficial, detrimental, or inconsequential. However, some ancillary effects on the host have been observed in a few of the aforementioned studies. For example, rats fed with silver NP exhibited changes in behavioural tests and histopathological alterations in the brain, though it is unclear whether the NP-induced microbiome disruption was a causative factor 34. In another rat study, silver NP-induced microbiome changes were accompanied by a decrease in expression of immunomodulatory genes in intestinal tissue, suggesting modulation of host immune response33.
3.4. Immunomodulation.
NP exhibit a wide range of effects on the immune system, even in translationally relevant forms. NP-mediated immunomodulation can be pro- or anti-inflammatory and may affect downstream other activities not directly related to inflammation, such as tissue remodelling or scavenging functions, as well as immune cell differentiation, maturation, and senescence. We will highlight notable studies which illustrate the divergent immunomodulatory effects of NP on unwanted systemic immunomodulation, to emphasize the variability of ancillary effects arising from NP-biological interaction. The elicited ancillary effects are highly dependent upon the physicochemical properties of the NP, the immune cell subset interacting with NP, and the nature of the surrounding environment.
Immunostimulation and pro-inflammatory effects.
The potential of many nanoscale materials to induce inflammation in organs and tissues is probably one of the earliest observed effects of nano-bio interaction. It is highly likely that ancillary mechanisms are integral to NP-mediated inflammatory responses. NP-triggered inflammatory response mechanisms are highly diverse and complex, but generally can be categorized into one of three mechanistic schemes: the intracellular route, wherein NP are taken up by sentinel immunocytes or other cells and provoke inflammation through various intracellular mechanisms; the Fc-mediated immune response route, wherein humoral immunogenicity of nanomaterial results in extensive opsonization with NP-specific antibodies and subsequent FcR activation; and the complement system activation route, caused by extracellular interaction of complement immunity factors with nanomaterial. With respect to the intracellular pathway of inflammation activation, there are any number of mechanisms by which cellular binding or uptake of nanomaterial could provoke inflammation. Such possibilities are too complex and varied to discuss in detail in this review, and have been extensively covered elsewhere1; however, such complexity is illustrative of the diversity of NP-mediated effects in biology. Generally, an inflammatory response will be driven by the release of inflammatory mediators (TNF, interleukins, interferons, ATP, etc.) from cells exposed to nanomaterial. Such a response could be provoked in any number of known (or unknown) ways: e.g., NP uptake leads to endosomal build-up and leakage followed by NP contact with cellular machinery, causing varied responses including protein unfolding and interference with cell signalling cascades, disruption of cellular metabolism and increased oxidative stress, activation of damage-associated molecular pattern receptors and autophagic pathways. These effects and others all ultimately lead to release of inflammatory mediators by secretion or by cell lysis, triggering recruitment and activation of the surrounding immune system and paracrine inflammatory responses in neighbouring cells. Importantly, the nature and of magnitude such effects are highly dependent on NP identity (both pristine and biological) and biological context. The activation of the complement system by NP is another important ancillary effect that is also a recurring clinical problem with approved nanodrugs in current use, and therefore will be described in greater detail further on in this section.
NP-induced immune activation, generally considered an unwanted side effect, could be medically useful certain circumstances: for example, to combat infection, to provoke a host defence response against cancer, or to stimulate antigenicity of vaccines. The pro-inflammatory nature of many nanomaterials may therefore be useful as a supplemental adjuvant for vaccines. Indeed, microparticle formulations of aluminium compounds (e.g, Alhydrogel) have been in clinical use in adjuvanted vaccines for decades (and gave rise to false and unwarranted but ongoing controversies). Investigations of nanoscale formulations of aluminium have shown improved adjuvant activity compared to microscale forms as well as reduced induction of local inflammation45. This improvement has been attributed to the increased potency of nanoalum in NLRP3 inflammasome activation in antigen-presenting cells46. Conventional microscale aluminium-based adjuvants are known to elicit a strong Th2-type immune response by CD4+ helper T cells. While efficient in provoking a strong and durable humoral immune response to antigens by B cells, it lacks a strong induction of a Th1-type helper T cell immune response and thus is not particularly effective at stimulating persistent cell-mediated immunity by activation of CD8+ T cells. Thus, its utility is limited in vaccines against diseases for which antibody responses are not particularly effective (e.g., HIV). However, nanoscale aluminium particles may be able to circumvent this deficiency. A polyacrylic acid-stabilized nanoscale formulation of Alhydrogel elicited a potent Th1 immune response in a murine model47, suggesting that nanoparticle adjuvant formulations could be used to appropriately tune the adaptive immune response in vaccinations where a stronger cell-mediated immunity is required. Additionally, other nanomaterials have been found to exhibit strong adjuvant activity with minimal local inflammation, such as biocompatible lipid nanoparticle formulations48-50, which could be a promising adjuvant alternative to irritation-producing aluminium salts or TLR agonist adjuvants and have been used in COVID-19 vaccines most recently. Immunostimulatory ancillary effects could also conceivably be useful in cancer; such effects and applications are discussed in section 3.5.
Complement activation.
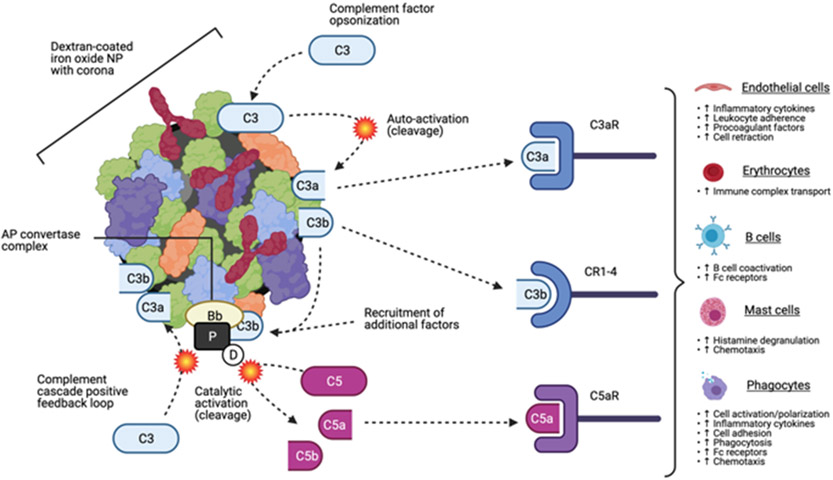
In addition to leukocytes, the complement system is affected by NP as well. Complement is a biochemical defence comprised of secreted factors, which act in parallel to and in conjunction with the other arms of immune system. The complement system is driven by interaction of complement factors with biochemically foreign surfaces and has three main functions: direct lysis of foreign cells (the “classical pathway”), opsonization of foreign cells and debris to facilitate clearance (the “alternative pathway”), and induction of inflammation to attract phagocytes (the “lectin pathway”). Complement immunity factors are often found within NP coronas; interaction with the NP surface or with other corona biomolecules can trigger activation of complement factors, potentially activating any of the three complement pathways51. However, activation of the alternative pathway appears to be the most prevalent and best-characterized effect of NP interaction with this system52,53. Alternative pathway activation by dextran-coated iron oxide NP has been closely studied. The initial step in this process appears to be the adsorption of complement factor C3 onto the NP corona. The resultant molecular interaction between C3 and other molecules on the NP surface can induce spontaneous hydrolysis of C3 into two subunits, C3a and C3b. C3b within the NP corona can further drive complement activation by forming an enzyme complex on the NP surface with other complement factors; this complex (AP convertase) catalyses cleavage of intact C3 into C3a and C3b, as well as cleavage of the complement factor C5 into C5a and C5b. The cleavage products of C3 and C5 can activate complement factor receptors in many tissues, potentially inducing a cascade of biological responses (Figure 2). C3a and C5a are also known anaphylatoxins which elevate risk of severe immune reactions. The potential of iron oxide NP to activate the alternative pathway appears to be independent of dextran structure, functionalization, and dextran/iron molar ratio, suggesting that the corona is the primary driver of C3 cleavage54.
Figure 2.
Activation of the alternative pathway of the complement immune system by dextran-coated iron oxide nanoparticles. Complement factor C3 is absorbed onto the particle surface, where interactions with nanomaterial may trigger self-cleavage into factors C3a and C3b. Factor C3b can facilitate formation of the alternative pathway (AP) convertase complex, which enzymatically cleaves complement factor C5 into factors C5a and C5b and creates positive feedback activation of additional factor C3. Cleaved complement factors can function as anaphylatoxins, opsonins, and activate complement factor receptors in a wide variety of cell types.
Complement activation is especially problematic for nanomedical purposes, as it can contribute to suboptimal NP pharmacokinetics and biodistribution, inflammation, allergy, and potentially a serious anaphylaxis-like condition, CARPA (Complement Activation-Related Pseudoallergy) which has been observed with several clinical nanomedicines. The complement system can be activated even by highly biocompatible NP, such as PLGA55, carbohydrate-coated iron oxide NP56, or liposomes57; with respect to the latter, increased activation was associated with high negative surface charge and morphological deviation from sphericity. CARPA-like patient reactions have occurred with several clinical nanomedicines, including Doxil (PEG-liposomal doxorubicin)58 and Feraheme (ferumoxytol)59, which received an FDA black box warning as a result.
Though generally considered undesirable, NP complement activation could conceivably be leveraged for therapeutic benefit as well. When co-administered with a model antigen, rod-shaped PEG NPs promoted the alternative pathway of complement activation, providing and adjuvant-like boost to the immune response and improving humoral immunogenicity60. Chitosan NP have also exhibit adjuvanticity via activation of complement and have been investigated extensively61-63. Such particles could form the basis of vaccine nanoadjuvants free of metals and endotoxin derivatives.
Immunomodulation, immunosuppression and anti-inflammatory effects.
We now understand that NP can be immunoregulatory or immunosuppressive in addition to being inducers of inflammation64. Immunosuppressive characteristics of NP have been observed in interactions with multiple immune cell classes. Such effects can include immune cell cytotoxicity or senescence, decreased antigen presentation, diminished or altered cytokine release profiles, and attenuated reactivity to pathogen-associated molecular signals.
Nanomaterial immunosuppression/anti-inflammation has potential applications, especially in the context of injurious immune overactivation. Clinical immunomodulatory agents, such as corticosteroids and mTOR inhibitors, are often used to mitigate localized pathological inflammatory responses; however, these drugs have broad off-site and off-target systemic effects, resulting in a complicated side effect profile. Even without targeting, NP are frequently preferentially incorporated into monocytes, macrophages, and other myeloid-derived cells with immunoregulatory functions, and thus represent a potential passive targeting tactic for nanomaterials with immunomodulatory properties. For example: negatively-charged particles composed of biodegradable poly(lactic-co-glycolic acid) (PLGA) copolymer, a nanomaterial, have shown the capability to exhibit immunomodulatory effects in inflammatory monocytes. In particular, PLGA particles exhibited therapeutically beneficial anti-inflammatory and immunosuppressive effects in multiple disease models. PLGA NP passively target inflammatory monocytes expressing the scavenger receptor MARCO, promoting splenic sequestration and apoptosis, resulting in a depletion of these cells from distal sites of inflammation. PLGA particles were shown to act therapeutically as an anti-inflammatory agent in murine models of West Nile virus-induced encephalitis, experimental autoimmune encephalomyelitis, cardiac infarction, kidney reperfusion injury, and inflammatory bowel disease65. These effects were achieved without apparent systemic immunodeficiency, a common and potentially severe side effect of anti-inflammatory drugs.
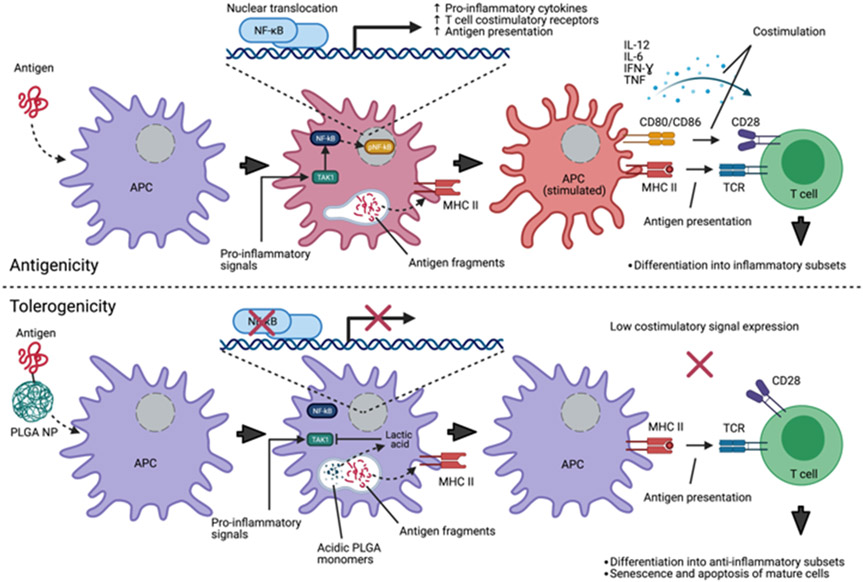
Additionally, internalized PLGA nanomaterial can suppress costimulatory signals from antigen-presenting cells such as macrophages and dendritic cells. The mechanism behind these PLGA-mediated ancillary effects in the immune system is not well understood but appears to be dependent on acidification caused by degradation of the particle into lactic acid and glycolic acid; therefore it is likely a product of the pristine, rather than the biologic, identity of the nanoparticle66. A highly promising application for NP with immunosuppressive properties (and perhaps one of the most promising for nanomedicines to date) is the development of tolerogenic NP for treatment of allergy and autoimmune disease, and in minimizing rejection in human organ transplantation without systemic immune suppression. By exposing an antigen-presenting cell to an antigen under immunosuppressive conditions, it is possible to teach the adaptive immune system to “forget” a pathological adaptive immune response, as the antigen is presented to T cells in the absence of a costimulatory signal, resulting in ablation of antigen-reactive T cells67 (Figure 3). This requires coupling an antigen to an immunosuppressive delivery NP; the delivery of the antigen to an antigen-presenting cell, (with activation suppressed by the internalized NP), can cause the reactivity to a known antigen to be diminished or eliminated. Separate administration of the inhibitory agent has not been found to be effective; co-delivery of both the antigen and immunosuppressive agent as a unified entity appears to be required. Although the co-packaging of an mTOR inhibitor or other immunosuppressive drug payload is probably the most clinically practical configuration for a tolerogenic NP, induction of tolerance has been demonstrated using only NP with immunosuppressive qualities. Antigen-conjugated PLGA NP have been shown to tolerize allergic airway inflammation in murine models68-70. Furthermore, PLGA NP decorated with donor peptides reduced allograft rejection in several murine transplant models, including skin71 and pancreatic islet cell72 transplants, a strategy which could be an alternative to broadly immunosuppressive anti-rejection drugs such as sirolimus or tacrolimus.
Figure 3.
Nanoparticle-mediated immunomodulatory effects contribute to antigen tolerance. Top, uptake of a foreign antigen by antigen-presenting cells (APC) in conjunction with extrinsic pro-inflammatory signal activation. This costimulation contributes to education of naïve T cells and activation/expansion of mature antigen-reactive T cells by increased antigen fragment presentation via major histocompatibility complexes, juxtacrine costimulatory receptor expression, and secretion of pro-inflammatory cytokines. Bottom, co-uptake of antigen with PLGA nanomaterial. Degradation of PLGA into acidic monomers results in inhibition of TAK1 by lactic acid, reducing phosphorylation and nuclear translocation of nF-kB, blunting responsiveness to pro-inflammatory signals which signal through TAK1. Antigen fragment presentation to T cells in the absence of significant costimulation results in non-activation of naïve cells and senescence/apoptosis of mature antigen-reactive T cells.
3.5. Ancillary effects in cancer and cancer therapy.
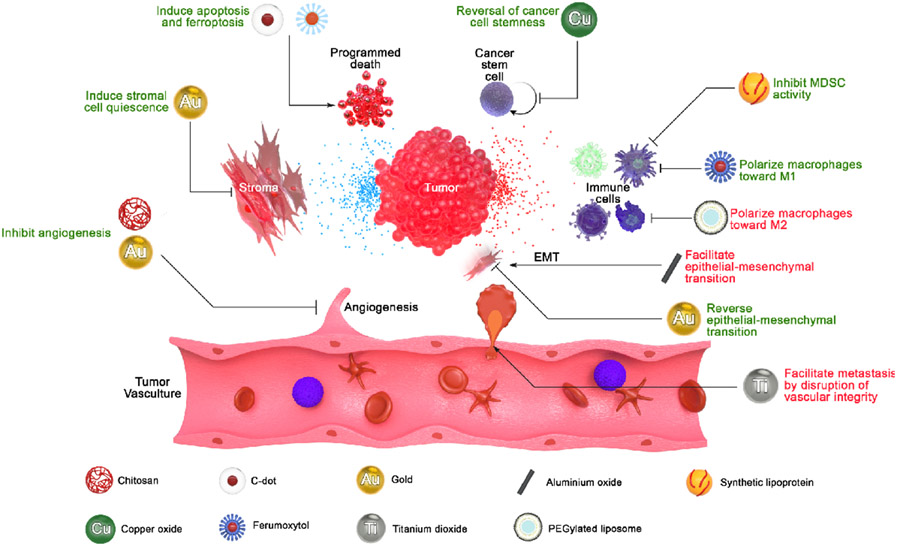
NP effects are especially interesting in the context of cancer, because prototypic nanomedicines are frequently designed as potential cancer therapeutics (usually as a drug delivery system and often coupled with targeting and/or imaging modalities). However, the ancillary effects of the NP carrier itself on cancer biology are often not considered. The biology of cancer is atypical, dysregulated, and extremely heterogeneous, and therefore presents a unique environment for NP-mediated ancillary effects. Furthermore, uptake of NPs into tumours is often amplified due to a poorly-defined vascular phenomenon described as the enhanced permeation and retention (EPR) effect, wherein biodistribution of administered NP is increased in tumors73. Thus, biomedical NP infused into to a patient are likely to be deposited into the tumour microenvironment at elevated concentration (though the magnitude of this effect is highly variable and often less pronounced in humans)74. Therefore, we must consider the interaction of the NP with biology in a cancer setting. Here, we highlight instances where ancillary effects were observed which may have translational relevance in cancer therapies, or instances where they may facilitate cancer growth and progression (Figure 4).
Figure 4.
Summary of nanoparticle effects in cancer environments. Various nanoparticles can directly affect cancer cells or tumor stroma, modulate responses of tumor-infiltrating immune cells, and facilitate or inhibit metastasis and tumor angiogenesis.
Notably, some NPs may exhibit carcinogenicity by induction of cellular changes which promote cancer development in normal tissues. This is a well-known property of nanomaterials with limited clinical relevance, such as carbon nanotubes, which have been shown to persist in the lungs of rodents after inhalation, eventually causing mesothelioma or mesothelioma-like lung injury 75-78. However, studies indicate potential mutagenicity or genotoxicity from commonly used NPs, such as titanium dioxide NP, which are nearly ubiquitous in consumer products. Although there is no conclusive evidence of a direct causal effect in carcinogenesis via typical exposure routes and at reasonable exposure levels and durations, titanium dioxide (inclusive of nanoscale forms) has recently been classified as a Group 2B substance by the International Agency for Research on Cancer, recognizing its possible carcinogenicity 79.
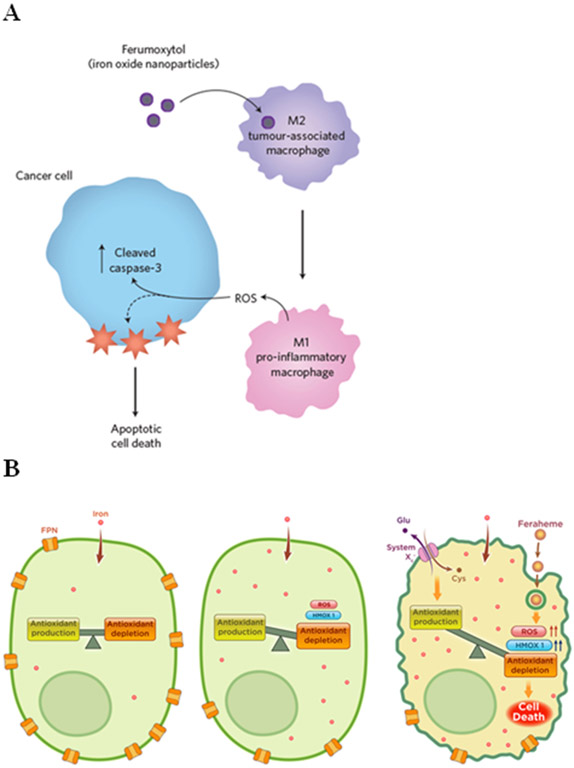
The immune cell compartment of tumours plays a significant role in cancer development, as many cancer types can effectively “reprogramme” or suppress infiltrating leukocytes to facilitate growth and metastasis. However, modulation of immune cell responses is also an ancillary NP effect, which could have interplay with tumour-mediated immunomodulation, and opsonized NP are readily scavenged by the macrophages, neutrophils, dendritic cells, and monocytes typically present in the tumour microenvironment. Consequential NP ancillary effects on the immune cell compartment within a tumour, and subsequent effects on the tumour itself, have so far been mostly overlooked, but there is increasing evidence NP-mediated ancillary effects can indeed impact the surrounding cancer biology. Feraheme, a clinical carboxymethyldextran-coated iron oxide nanoparticle, has shown promise as an agent to activate macrophages and counteract tumour-mediated manipulation of tumour-associated macrophages (TAM) phenotype. High-dose Feraheme repolarized TAM and liver Kupffer cells to an M1-like pro-inflammatory, cancer-inhibiting phenotype in a murine breast cancer/liver metastasis model; this slowed tumour growth and shielded the liver from metastatic seeding80. TAM repolarization is believed to occur through cellular iron enrichment. Abundant intracellular iron acts as a Fenton chemistry catalyst, generating superoxide radicals and driving adoption of the M1 phenotype (Figure 5A). Thus, this effect is primarily elicited by the composition of the particle (pristine identity), whereas the passive targeting of the particle to TAM is driven largely by the biomolecule corona of Feraheme (biological identity).
Figure 5.
A) Ferumoxytol iron oxide nanoparticles modulate macrophage polarization in tumors, driving M1 polarization and inducing killing of cancer cells via extracellular ROS generation. [Reprint figure adapted from PMID 27668797] B) Ferumoxytol uptake by iron-retaining leukemic cells activates the ferroptotic pathway, triggering cell death. [Reprint figure from PMID 30911166] C) Titanium dioxide nanoparticles modulate endothelial cell permissiveness in microvasculature and facilitate cancer metastasis. [Reprint figure from PMID 30692675] D) C-dots accumulate iron content in vivo, triggering ferroptotic cell death in cancer cells. [Reprint figure adapted from PMID 27668797]
Myeloid-derived suppressor cells (MDSC), an immunosuppressive type of immature monocyte often residing within tumours, are activated by gold NP functionalized with a biomimetic high-density lipoprotein (HDL)-like surface, via binding and activation of scavenger receptor type B-1 (SCARB1), pushing myeloid cells out of the pathological MDSC phenotypic niche. This shift reduced tumour growth in a lung cancer model, and both metastasis and growth in a melanoma model. Reduction in tumour MDSC burden also resulted in an increase in active tumour-infiltrating cytotoxic T lymphocytes, presumably increasing immune surveillance of the tumors81. These effects are interesting notably because lipoproteins are a common constituent of nanoparticle coronas. HDL apolipoproteins do not directly bind SCARB1; however, cholesteryl esters contained within HDL will specifically bind and activate the receptor, raising the possibility that other nanoparticles could elicit this effect as well. As this gold NP was not synthesized with cholesteryl esters, the SCARB1-activating biological activity of the particle is likely derived from sterol esters which were incorporated into the corona from serum.
On the opposing end of the immune response spectrum, NP may also modulate tumour immune cells in ways that aid cancer progression. Studies of empty liposomes lacking a drug payload are uncommon; in one rare study, it was shown that the liposome structure itself might have an adverse effect on cancer through interactions with immune cells. PEGylated liposomes without drug cargo elicited a tumour-promoting M2-like phenotype in TAM in a papillomavirus-induced tumour model, resulting in higher secretion of Th2 cytokines and local immunosuppression in the tumour microenvironment. Treatment with unloaded liposomes consequently increased tumour growth rate82. Thus, consideration must be given to NP composition for drug delivery nanophores intended for cancer therapy, as the NPs themselves might provoke responses that are counterproductive to the therapeutic mechanism.
Outside of the immune cell compartment, other types of tumoral stroma cells are susceptible to anti-tumorigenic effects mediated by nanobiological interaction, if indirectly. In the absence of any other drug payload or concomitant therapy, 20 nm gold NP reduce growth and metastasis of pancreatic ductal adenocarcinoma in mice. Though the effect mechanism is not immediately clear, analysis of the secretome of pancreatic cancer cells demonstrated a marked downregulation of many pro-growth factors and cytokines. Correspondingly, there was a downstream reduction in ECM secretion by stromal pancreatic stellate cells, both of which reduce cancer aggressiveness83. Gold NP monotherapy has also shown efficacy in rodent models of ovarian cancer, reducing both tumour proliferation and metastasis by interruption of the epithelial-to-mesenchymal transition of cancer cells, possibly by inhibition of MAP kinase signalling through an unknown mechanism84. In both models, gold NP were not cytotoxic to non-cancerous cells, suggesting that the effect was due to a specific interaction of the NP or NP-associated biomolecules with the dysregulated biology of cancer cells.
Furthermore, NP may directly contribute to programmed cell death pathway activation in cancer cells. Recently, our group has shown that iron-containing NP can modulate an iron-dependent cell death pathway called ferroptosis (Figure 5B). Overload of ferroportin-deficient AML cells with iron via Feraheme uptake generates large amounts of ROS by Fenton chemistry, which causes a buildup of oxidized membrane lipids, triggering ferroptotic cell death in the context of low ferroportin expression15. Similarly, fluorescent silica quantum carbon C-dots functionalized with a melanoma targeting peptide also induce ferroptosis in melanoma under cysteine-deficient conditions (Figure 5D). Remarkably, pristine C-dots contain no iron; however, C-dots apparently accumulate iron from the biological environment, perhaps incorporating metallic ions into the outer silica layer or the corona16. Therefore, pro-ferroptotic NPs might be useful as a novel therapeutic for cancers with cellular iron export defects or be used in combination with another inducer of oxidative stress.
Perhaps more significantly, vascular integrity perturbation by NPs might actually facilitate metastasis. The effects described by Peng et al. wherein titanium dioxide NP disrupted VE-cadherin binding and induced leakage in lung vasculature by (described in section 3.1) were also applicable to tumour vasculature; the increase in vascular permeability was shown to facilitate both extravasation of tumour cells from the primary tumour and to promote “seeding” of metastatic sites by increased permissiveness of circulating tumour cells in normal vasculature (Figure 5C). Further evidence suggests that this effect is not unique to titanium dioxide, and could occur with gold, silver, and silica NPs as well 19. An additional study determined that PEGylated gold NP of various sizes are predominantly taken up into tumours via the transcellular route through active transport, and not via the passive paracellular diffusion process commonly attributed to EPR; these internalized NP conceivably could exert ancillary effects on endothelial cell biology from within85. Both of these findings are potentially concerning for cancer nanotherapy development, as the EPR effect may not be a completely passive process; diffusing NPs might actually influence or alter the vascular endothelium through which they pass. On the other hand, this effect might be therapeutically exploitable to facilitate uptake of other NP or drugs out of the blood compartment but must be carefully balanced against possible increased metastasis. It remains to be seen which NP characteristics mediate this effect, and whether this effect is recapitulated by NP of other classes.
Section 4. Strategies for mitigating nanoparticle adversities.
Much effort has been focused on creating translational NP forms which are amenable to medical applications. The focus of these efforts has largely been to ameliorate obvious adverse NP effects such as cytotoxicity, particle aggregation in ECF, inflammation, immune cell sequestration, undesirable organ targeting and/or overwhelming of the mononuclear phagocytic system1. Progress has been made in this area by gaining a better understanding the relationship between NP characteristics and biological response in vivo, and we are learning to better manage these effects by modulating particle properties. However, we must further identify strategies to mitigate more subtle adverse events which arise from ancillary effects. The variations in NP structure and composition, coupled with the intricate complexity of biological systems, make prediction and control of ancillary effects challenging. NP development could follow several strategies that would lead to better scientific understanding of nanoparticle interactions in vivo, allowing the development of novel therapeutic approaches.
4.1. Rapid degradation/elimination strategies.
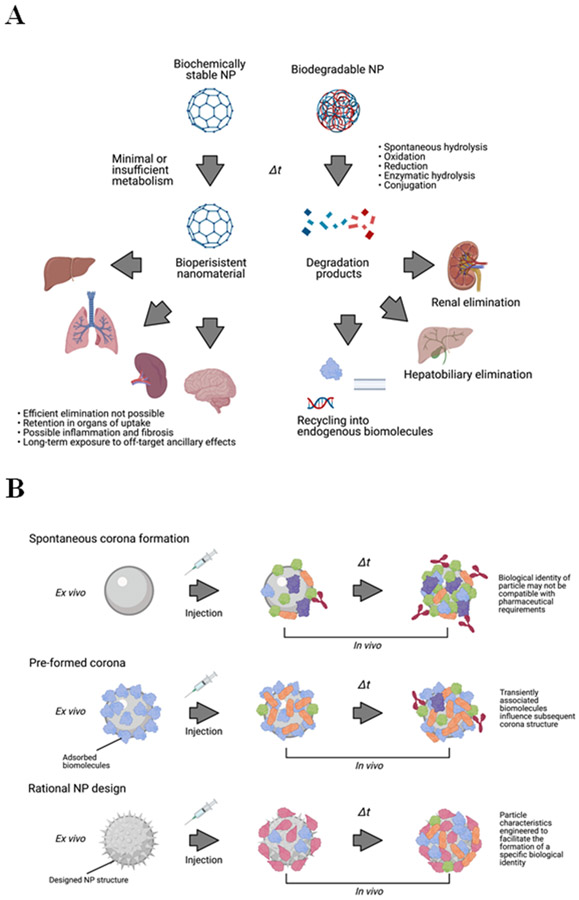
A significant obstacle for NP as medicines is the vastly different routes of metabolism that separate them from small molecule drugs or biologics. While the pharmacokinetic behavior of these conventional drugs is well understood and established, NP typically behave quite differently for reasons that are probably nested in their macromolecular scale. Foremost, a major roadblock to translation of many nanomaterials is biopersistence. Whereas most small organic molecules are predictably and efficiently eliminated by cytochrome P450s and other metabolic pathways, many nanomaterials defy rapid and efficient clearance from the body, because human biology lacks metabolic mechanisms to process many nanomaterials or nanoscale structures. Also problematic is the propensity of many NP to target certain organs, particularly the liver and spleen; disproportionately high local concentrations nanomaterial there makes elicitation of (potentially harmful) ancillary effects more likely, especially when biopersistent material lingers long after administration1. These characteristics could cause protracted off-target, off-site ancillary effects.
Constructing biomedical NP from biodegradable nanomaterials is a reasonable and practical approach to reduce ancillary effects arising from biopersistence. Biodegradable material can be broken down under physiological conditions into products that are non-toxic and/or can be eliminated readily by metabolic or elimination pathways (Figure 6A). For example, PLGA copolymer degrades into its monomers, lactic acid and glycolic acid, both of which are natural metabolites of human physiology (although rapid degradation of PLGA does produce transient local acidification). In addition to PLGA, which is well-studied and has been in medical use for decades, several other biodegradable nanomaterials are in widespread use today, including polylactic acid, PAMAM, and chitosan86. These polymer systems are highly flexible and adaptable, and can be used to construct NP of different sizes and shapes. Surface functionalization can also be employed to modulate degradation rates, or to make degradation selective to a particular condition (e.g., pH). However, it should be noted that biodegradable nanomaterials are not necessarily biocompatible, and nanoscale materials are prone to decay in vivo by burst degradation rather than gradual surface erosion, which could be problematic for biodegradable NP in medical applications1.
Figure 6.
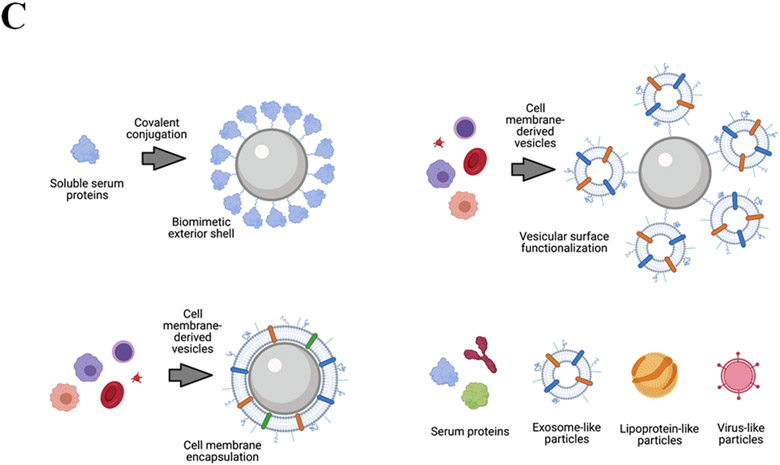
A) Left, persistent nanoparticles resist degradation and accumulate in target organs, resulting in long-term biological effects. Right, biodegradation strategy enables rapid elimination of nanomaterial or metabolism into bioidentical building blocks. B) Top, nanoparticles designed without consideration of corona composition may have undesirable biological interactions, extensive opsonization and rapid clearance, and/or accumulation in off-site organs and cell types. Middle, pre-addition of a defined corona prior to administration provides a foundation to influence further corona formation in vivo. Bottom, rational particle design strategy uses in silico modeling strategies to predict corona structure and composition in vivo, enabling control of the particle’s subsequent biological identity. C) Nanoparticles in excess of ~10 nm are too large to pass through glomerular fenestrations within the kidney, precluding efficient renal clearance of nanomaterial. Very small nanoparticles pass through fenestrations and can be eliminated in urine. D) Strategies to define the biological identity of particles by mimicking host biology. Upper left, nanoparticles surfaces are densely functionalized with serum proteins or other native biomolecules. Upper right, nanoparticles are conjugated to host cell surfaces or cell membrane-derived vesicles. Lower left, cell membrane derived from host cells is used to completely envelop nanoparticles within an extracellular vesicle-like structure. Lower right, biomimetic nanoparticles utilize bioidentical materials for particle construction.
An alternative elimination strategy for intravenously-administered NP involves producing ultra-small nanoparticles that are below approximately 10 nm in size with zwitterionic or positive surface charges87. Ultra-small NP are rapidly filtered through the kidney glomerulus and eliminated from the body in urine. Given the short duration of NP in the system, unwanted ancillary effects can be minimized, and clinical translation of otherwise biopersistent nanoparticles could be implemented with less concern for long-term retention in the body88. This strategy has been clinically investigated for inorganic silica-coated carbon-quantum C-dots, which are approximately 7 nm in diameter and are cleared renally89,90. A limitation of ultrasmall particles is extremely rapid renal clearance from the blood; the short half-life pharmacokinetics of such a small particle may not be compatible with the therapeutic purpose of a particular nanomedicine. However, this approach could also be facilitated with biodegradable nanomaterial, e.g., a larger polymeric NP that degrades or dissociates in circulation into sub-10 nm fragments which are able to undergo renal elimination.
4.2. Harnessing the corona architecture.
The corona is one of the primary determinants of an NP’s interactions with host biology. To date, corona formation has mostly been regarded as undesirable “biofouling” that should be minimized, e.g., via low-adhesion NP coatings such as PEG or other “stealth” materials to cloak the particle from immune recognition and minimize biological engagement. However, this strategy affects the biological fate of the NP, and it is unlikely that corona formation can ever be completely abolished. A more forward-thinking approach to nanotherapeutics is to view the NP-corona complex from a holistic perspective. I.e., the corona should be seen as an extension of the NP, rather than a hindrance, and the corona formation and composition (and how it will interface with biological systems) should be considered with respect to the particle’s intended function. Essentially, we could adapt the pristine identity of the NP as the foundation upon which to build the biological identity. Adjustment of corona architecture could therefore be exploited to mitigate problems with nanotherapeutics, including cytotoxicity, immunogenicity, pharmacokinetics, biodistribution, cell-specific uptake and intracellular compartmentalization—and even for regulation of ancillary effects.
Corona pre-formation is a basic strategy wherein NP are pre-incubated ex vivo in a defined medium of preferred corona components prior to administration (Figure 6B). Corona pre-formation allows some limited control over the corona composition, and is applicable to any existing NP type. This technique has been studied in animal models, though mostly using only albumin as the sole constituent of the preformed corona; even with this relatively simple and unsophisticated method, it has shown the potential to reduce binding of opsonins and complement immunity factors, as well as to improve NP stability, pharmacokinetics, and cellular uptake, and decrease particle cytotoxicity91-94. With greater knowledge of nano-bio interactions, more sophisticated predefined coronas could be designed with more advanced purposes, such as facilitating nanoparticle targeting or eliciting specific ancillary effects to synergize with a therapeutic function or minimizing off-target ancillary effects associated with the NP nanobiology. However, this method also has limitations, as the NP corona is not static, but rather is a dynamically evolving shell5,95. A pre-formed corona could be influential in setting a NP’s initial biological identity, but it is still heterogeneously transient and subject to modification by the biological environments within the host. The host biology is likely to “rewrite” the particle identity away from the initially defined one, though the kinetics of biomolecule exchange may still allow for practical modification of NP behaviour.
Another more advanced strategy for harnessing the corona is applying in silico modelling strategy for rational particle design to predict and direct corona formation in vivo. Molecular dynamics and structure-activity relationship modelling software has been used to predict corona assembly and NP-cell association96-98, and further modelling could analyse how corona-bearing NP would interact with biological barriers, cells, tissues, and organs, and predict biological consequences. These strategies could be integrated into rational particle design, i.e., a NP’s physicochemical characteristics, morphology, and functionalization would be purposefully manufactured to recruit a corona of a desired composition that is compatible with the biomedical function of the NP (Figure 6C). In effect, the pristine identity of the nanoparticle would be defined to facilitate the adoption of a specific biological identity in vivo. A few simple proof-of-concept studies have been performed with this aim. For example, polysaccharide chain structure on the exterior of a dextran NP can be manipulated to activate specific complement immunity pathways99. The NP surface can be functionalized to recruit specific factors into the corona; an in silico strategy was used to design a gold NP functionalization motif that would incorporate transferrin from blood serum into the corona. This particle exhibited high transferrin receptor binding100. NP surface chemistry has further shown the capability to influence the conformation of bound corona components: a recent study observed that polymer NP with different surface functional groups could stabilize or denature corona albumin proteins; such conformational differences were associated with differential binding to various types of macrophage scavenger receptors, and consequently allowed de facto targeting of specific macrophage subsets101.
Such studies demonstrate the possibility of “tuning” the NP corona to avoid unwanted ancillary effects. However, practical obstacles to this strategy are significant; in addition to our lack of knowledge about the nano-bio interface, differences in patient physiology and dysregulated conditions in disease states could complicate efforts to model corona formation. Both the pre-formed corona strategy and the rational NP design strategy will require vastly more sophisticated knowledge of corona dynamics and biological interfaces in order to be utilized effectively.
4.3. Biocompatible and biomimetic nanoparticles.
The biocompatibility of a particle describes its ability to avoid triggering an adverse response from the surrounding host biology; as described, many of these adversities can arise from ancillary effects caused by unwanted interactions with nanomaterial. For any clinical application, a high degree of biocompatibility is required. However, widely-utilized clinical NP materials such as PEG may not be as biocompatible as previously believed; for example, multiple studies have found high prevalence of anti-PEG antibodies in patient sera102,103, suggesting that PEG nanomaterial is actually fairly immunogenic. Therefore, it is important that we improve upon existing nanomaterials. Basic aspects of pristine identity (physicochemistry, morphology, geometry, functional groups and density, etc.) have a profound impact on NP biocompatibility, and thus can be adapted to help modulate interactions with biology. For example, deformable disc-shaped polymeric particles have shown favourable biocompatibility over rigid and spherical particles, perhaps because they mimic the size, shape, and elasticity of erythrocytes104 .
Beyond using artificial nanomaterials foreign to the host biology, another approach to biocompatibility is to incorporate biomaterials into NP construction; this could be highly promising to mitigate adverse ancillary effects arising from NPs, as endogenous materials are far less likely to provoke harmful responses. Biomimetic strategies for NP can generally take one of two approaches. First, a NP can be “disguised” with a biomimetic exterior, using natural biomolecules to cloak the foreign particle in seemingly native material (Figure 7D). There are several plausible ways to implement this. A simple version of this strategy is similar to the corona pre-adsorption method described in section 4.2, except that the desired corona components would be covalently conjugated to the core NP, rather than adsorbed; in effect, this would allow the synthesis of a permanent biomimetic “hard” corona that would not desorb in vivo. Another method involves surface conjugation of NP to cell-derived vesicles, platelets, or even whole cells, to effectively “hitchhike” the NP and conceal them within the biological presence of associated cell membrane, for example with NP conjugated to erythrocytes105-107, although this might itself cause unintended NP-induced effects on the carrier erythrocytes and thus requires NP refinement108,109. Yet another adaptation of this strategy involves encapsulation of a NP inside a cell membrane-derived vesicles created from the host’s own harvested cells (e.g., erythrocytes110,111 or platelets112). From the outside, the NP appears to be indistinguishable from any other cell. In this case, the NP is covered by a cellular “ghost”, which would eventually degrade after uptake into the targeted tissue, freeing the core NP.
For the second avenue of biomimetic approach, a core NP can be constructed entirely from bioidentical molecules that are natively present in the host biology. The chemotherapeutic drug nab-paclitaxel (Abraxane) is an example of this biomimetic strategy, wherein the “nanoparticle” is simply a human serum albumin protein used as a drug nanophore for paclitaxel delivery. This method could use any number of nanoscale biomaterials: lipoprotein-like particles, cell membrane-derived vesicles, virus-like particles, etc. By using material identical to the host’s own biology, such approaches could further reduce unpredictable results arising from ancillary effects of foreign nanomaterial, or hypersensitivity reactions.
Section 5. Conclusions and perspectives.
Viewed through the prism of medicine, our overview of current literature finds that the biological effects of nanoparticles, the nanobiology, are not only eclectic but also potentially a double-edged sword, which could contribute both to adverse effects and to novel mechanisms of therapeutic action. There are numerous pressing reasons to elucidate nanobiology.
Novel indications for NPs as drugs.
Nanomedicine promises the development of new therapeutic modalities that address a deficiency in the current state of medical care that small molecule drugs or other therapies simply cannot. NP have largely been regarded as little more than a means to an end: a passive carrier meant only to unify a collection of functional modalities into a single macromolecule, created with a specific intended purpose and function. This applied science approach has resulted in a “tunnel vision” of sorts, wherein only the biological effects elicited by the functional components of the NP are considered. This is a deficiency in the state of current research: as this review has shown, NPs alone can exhibit an eclectic array of ancillary effects, some of which may have therapeutic potential as nanomedicines while others are detrimental or even potentially dangerous. Perhaps some of these effects could be exploited clinically as new treatments or could be exploited in rational design approaches to facilitate or synergize with a payload drug. At the very least, NP should be re-evaluated as less-than-passive drug excipients which have effects independent of payload. This is particularly important when evaluating the effect of a drug carried by nanoparticles—how much of the effect is due to the particle versus the drug versus the combination of both? Often this is not considered appropriately.
Clinical suitability of NP as drugs.
Just as we lack adequate information to determine the best NP for a specific indication, we also lack sufficient information about nanobiology to determine when not to use a specific NP as a drug or vehicle. For example, many patient cohorts have special medical considerations: medication use, liver or kidney disease, pregnancy, etc. There is scant information to tell us what effects (if any) NPs might have in these populations; nanomedicine contraindications are another knowledge gap which must be addressed. Furthermore, a more complete understanding of nanobiology will help to avoid unnecessary errors of design, such as coupling a therapeutic payload to a NP carrier that actually elicits an antithetical ancillary effect and diminishes the therapeutic response.
Beyond nanomedicine: uncontrolled exposure.
NPs are not merely relevant only in the context of research or medicine. Some NP exposure is incidental and unavoidable, due to the presence of nanomaterials in the environment. We know very little about the significance of naturally-occurring NPs and how they have shaped and affected life on Earth. In addition, the prevalence of environmental NPs is ever increasing due to human industrial activity. NPs are widely used in consumer and industrial products and are inadvertently generated from basic human activities, such as the formation of fluorescent nanoparticles in pizza after heating113 or the emission of carbon nanoparticles in vehicle exhaust114. The ecological impact of engineered nanoparticles on natural flora and fauna is a concern especially because of industrial-scale release of biopersistent NPs; for example, photosynthetic inhibition in aquatic microalgae and phytoplankton exposed to titanium dioxide nanoparticles originating from topical sunscreens in seawater115, or contamination of seafood with marine microplastics116. NP contaminants raise concerns for both environmental conservation and for human health and welfare. It underscores the urgent scientific imperative to describe the continuum of interactions between NPs and biological systems at all levels of complexity.
Closing the knowledge gap.
Even basic knowledge of nanoparticles and biology is in flux: for example, the EPR effect, long believed to be a passive process arising from aberrant vasculature, may in fact be an active transport process, and therefore an effect of the nano-bio interface85. It is important to consider the reasons for our limited understanding of ancillary effects. Prior nanobiology studies are scattershot, limited, and of questionable relevance: for example, many effects have been observed only in vitro, while the majority of in vivo studies have been performed in animal models, rather than humans. Experimental NP dosages often are unattainable or not physiologically relevant. Furthermore, study findings might be applicable only to the very specific formulation of NP used: many non-commercial NPs used probably were not synthesized under strict and standardized conditions; thus, even the true identity of the NP being investigated might be questionable. In this scenario, biological effects associated with the NP might actually be attributable to factors other than the NP itself, such as the presence of residual synthesis by-products due to inadequate purification117 the use of chemical dispersants to facilitate NP solubility118, or contamination with bacterial endotoxins119. Unfortunately, consistent systematic study of nanoparticle-biological interactions have been not practical to date, due to experimental and model system heterogeneity, lack of standards for information reporting in scientific publication, and variations in NP manufacture, coupled with the rapid pace of innovation in this field. As a potential first step, Faria et al. have proposed a minimum standard for information reporting in nanobiological studies 120. Additional research standards along these lines would be helpful for making nanobiological research more methodical and systematic.
In addition to efforts to address these obstacles, it is crucial that we further our understanding with more comprehensive studies. Most NP studies do not consider ancillary biological effects at all, a short-sighted approach which should be rectified. The promise of nanomedicine cannot be fully realized if we do not understand how the engineered nanomaterials we create interact with the systems they are intended to regulate. This knowledge gap is the most significant barrier to the emergence of nanomedicine.
Acknowlegements.
We thank the Ella Maru studio for assistance in graphic design of figures in this paper and acknowledge financial support from NIH (5R01CA215700-05, 5R01CA218615-04, 5R01CA212379-04, 5R01CA183953-05).
Footnotes
The authors declare no competing interests.
References.
- 1.Parhiz H et al. Unintended effects of drug carriers: Big issues of small particles. Adv Drug Deliv Rev 130, 90–112, doi: 10.1016/j.addr.2018.06.023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monopoli MP, Aberg C, Salvati A & Dawson KA Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol 7, 779–786, doi: 10.1038/nnano.2012.207 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Kelly PM et al. Mapping protein binding sites on the biomolecular corona of nanoparticles. Nat Nanotechnol 10, 472–479, doi: 10.1038/nnano.2015.47 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Nguyen VH & Lee BJ Protein corona: a new approach for nanomedicine design. Int J Nanomedicine 12, 3137–3151, doi: 10.2147/IJN.S129300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox A et al. Evolution of Nanoparticle Protein Corona across the Blood-Brain Barrier. ACS Nano 12, 7292–7300, doi: 10.1021/acsnano.8b03500 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Del Pilar Chantada-Vazquez M et al. Proteomic investigation on bio-corona of Au, Ag and Fe nanoparticles for the discovery of triple negative breast cancer serum protein biomarkers. J Proteomics 212, 103581, doi: 10.1016/j.jprot.2019.103581 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Wang F et al. The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomedicine 9, 1159–1168, doi: 10.1016/j.nano.2013.04.010 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Tenzer S et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol 8, 772–781, doi: 10.1038/nnano.2013.181 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Foit L & Thaxton CS Synthetic high-density lipoprotein-like nanoparticles potently inhibit cell signaling and production of inflammatory mediators induced by lipopolysaccharide binding Toll-like receptor 4. Biomaterials 100, 67–75, doi: 10.1016/j.biomaterials.2016.05.021 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Wan S et al. The "sweet" side of the protein corona: effects of glycosylation on nanoparticle-cell interactions. ACS Nano 9, 2157–2166, doi: 10.1021/nn506060q (2015). [DOI] [PubMed] [Google Scholar]
- 11.O'Connell DJ et al. Characterization of the bionano interface and mapping extrinsic interactions of the corona of nanomaterials. Nanoscale 7, 15268–15276, doi: 10.1039/c5nr01970b (2015). [DOI] [PubMed] [Google Scholar]
- 12.Lara S et al. Identification of Receptor Binding to the Biomolecular Corona of Nanoparticles. ACS Nano 11, 1884–1893, doi: 10.1021/acsnano.6b07933 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Gagner JE, Lopez MD, Dordick JS & Siegel RW Effect of gold nanoparticle morphology on adsorbed protein structure and function. Biomaterials 32, 7241–7252, doi: 10.1016/j.biomaterials.2011.05.091 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary A, Khan S, Gupta A & Nandi CK Effect of surface chemistry and morphology of gold nanoparticle on the structure and activity of common blood proteins. New J Chem 40, 4879–4883, doi: 10.1039/c5nj03720d (2016). [DOI] [Google Scholar]
- 15.Trujillo-Alonso V et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat Nanotechnol 14, 616–622, doi: 10.1038/s41565-019-0406-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SE et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol 11, 977–985, doi: 10.1038/nnano.2016.164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daneman R & Prat A The blood-brain barrier. Cold Spring Harb Perspect Biol 7, a020412, doi: 10.1101/cshperspect.a020412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan C, Shuvaev VV, Bailey M, Muzykantov VR & Dziubla TD The Role of Carrier Geometry in Overcoming Biological Barriers to Drug Delivery. Curr Pharm Des 22, 1259–1273, doi: 10.2174/1381612822666151216151856 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Peng F et al. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat Nanotechnol 14, 279–286, doi: 10.1038/s41565-018-0356-z (2019). [DOI] [PubMed] [Google Scholar]
- 20.Setyawati MI et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat Commun 4, 1673, doi: 10.1038/ncomms2655 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Li CH et al. Gold Nanoparticles Increase Endothelial Paracellular Permeability by Altering Components of Endothelial Tight Junctions, and Increase Blood-Brain Barrier Permeability in Mice. Toxicol Sci 148, 192–203, doi: 10.1093/toxsci/kfv176 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Barui AK et al. Zinc oxide nanoflowers make new blood vessels. Nanoscale 4, 7861–7869, doi: 10.1039/c2nr32369a (2012). [DOI] [PubMed] [Google Scholar]
- 23.Shrivastava S et al. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano 3, 1357–1364, doi: 10.1021/nn900277t (2009). [DOI] [PubMed] [Google Scholar]
- 24.Ragaseema VM, Unnikrishnan S, Kalliyana Krishnan V & Krishnan LK The antithrombotic and antimicrobial properties of PEG-protected silver nanoparticle coated surfaces. Biomaterials 33, 3083–3092, doi: 10.1016/j.biomaterials.2012.01.005 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Jones CF et al. Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS Nano 6, 9900–9910, doi: 10.1021/nn303472r (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bian Y et al. Titanium dioxide nanoparticles enhance thrombosis through triggering the phosphatidylserine exposure and procoagulant activation of red blood cells. Part Fibre Toxicol 18, 28, doi: 10.1186/s12989-021-00422-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saikia J et al. Silica Nanoparticle-Endothelial Interaction: Uptake and Effect on Platelet Adhesion under Flow Conditions. ACS Appl Bio Mater 1, 1620–1627, doi: 10.1021/acsabm.8b00466 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J et al. 'Adhesion and release' nanoparticle-mediated efficient inhibition of platelet activation disrupts endothelial barriers for enhanced drug delivery in tumors. Biomaterials 269, 120620, doi: 10.1016/j.biomaterials.2020.120620 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Yu X et al. Changes of serum parameters of TiO(2) nanoparticle-induced atherosclerosis in mice. J Hazard Mater 280, 364–371, doi: 10.1016/j.jhazmat.2014.08.015 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Yan Z et al. Zinc oxide nanoparticle-induced atherosclerotic alterations in vitro and in vivo. Int J Nanomedicine 12, 4433–4442, doi: 10.2147/IJN.S134897 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert JA et al. Current understanding of the human microbiome. Nat Med 24, 392–400, doi: 10.1038/nm.4517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruparelia JP, Chatterjee AK, Duttagupta SP & Mukherji S Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4, 707–716, doi: 10.1016/j.actbio.2007.11.006 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Williams K et al. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology 9, 279–289, doi: 10.3109/17435390.2014.921346 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Javurek AB et al. Gut Dysbiosis and Neurobehavioral Alterations in Rats Exposed to Silver Nanoparticles. Sci Rep 7, 2822, doi: 10.1038/s41598-017-02880-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Brule S et al. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part Fibre Toxicol 13, 38, doi: 10.1186/s12989-016-0149-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J et al. Oral administration of rutile and anatase TiO2 nanoparticles shifts mouse gut microbiota structure. Nanoscale 10, 7736–7745, doi: 10.1039/c8nr00386f (2018). [DOI] [PubMed] [Google Scholar]
- 37.Wang L et al. Impact of Short-Term Exposure of AuNCs on the Gut Microbiota of BALB/c Mice. J Biomed Nanotechnol 15, 779–789, doi: 10.1166/jbn.2019.2731 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Li J et al. ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci Rep 7, 43126, doi: 10.1038/srep43126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gokulan K, Bekele AZ, Drake KL & Khare S Responses of intestinal virome to silver nanoparticles: safety assessment by classical virology, whole-genome sequencing and bioinformatics approaches. Int J Nanomedicine 13, 2857–2867, doi: 10.2147/IJN.S161379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alessandrini F et al. Pro-Inflammatory versus Immunomodulatory Effects of Silver Nanoparticles in the Lung: The Critical Role of Dose, Size and Surface Modification. Nanomaterials (Basel) 7, doi: 10.3390/nano7100300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pushalkar S et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov 8, 403–416, doi: 10.1158/2159-8290.CD-17-1134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riquelme E et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 178, 795–806 e712, doi: 10.1016/j.cell.2019.07.008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balachandran VP et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516, doi: 10.1038/nature24462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aykut B et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267, doi: 10.1038/s41586-019-1608-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Aldayel AM & Cui Z Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Control Release 173, 148–157, doi: 10.1016/j.jconrel.2013.10.032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruwona TB et al. Toward understanding the mechanism underlying the strong adjuvant activity of aluminum salt nanoparticles. Vaccine 34, 3059–3067, doi: 10.1016/j.vaccine.2016.04.081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khandhar AP et al. Physicochemical structure of a polyacrylic acid stabilized nanoparticle alum (nanoalum) adjuvant governs TH1 differentiation of CD4+ T cells. Nanoscale 12, 2515–2523, doi: 10.1039/c9nr09936k (2020). [DOI] [PubMed] [Google Scholar]
- 48.Swaminathan G et al. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine 34, 110–119, doi: 10.1016/j.vaccine.2015.10.132 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Thoryk EA et al. Co-Administration of Lipid Nanoparticles and Sub-Unit Vaccine Antigens Is Required for Increase in Antigen-Specific Immune Responses in Mice. Vaccines (Basel) 4, doi: 10.3390/vaccines4040047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirai S et al. Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses. Vaccines (Basel) 8, doi: 10.3390/vaccines8030433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.La-Beck NM, Islam MR & Markiewski MM Nanoparticle-Induced Complement Activation: Implications for Cancer Nanomedicine. Front Immunol 11, 603039, doi: 10.3389/fimmu.2020.603039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen F et al. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol 12, 387–393, doi: 10.1038/nnano.2016.269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banda NK et al. Mechanisms of complement activation by dextran-coated superparamagnetic iron oxide (SPIO) nanoworms in mouse versus human serum. Part Fibre Toxicol 11, 64, doi: 10.1186/s12989-014-0064-2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang G et al. Activation of Human Complement System by Dextran-Coated Iron Oxide Nanoparticles Is Not Affected by Dextran/Fe Ratio, Hydroxyl Modifications, and Crosslinking. Front Immunol 7, 418, doi: 10.3389/fimmu.2016.00418 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fornaguera C et al. Interactions of PLGA nanoparticles with blood components: protein adsorption, coagulation, activation of the complement system and hemolysis studies. Nanoscale 7, 6045–6058, doi: 10.1039/c5nr00733j (2015). [DOI] [PubMed] [Google Scholar]
- 56.Inturi S et al. Modulatory Role of Surface Coating of Superparamagnetic Iron Oxide Nanoworms in Complement Opsonization and Leukocyte Uptake. ACS Nano 9, 10758–10768, doi: 10.1021/acsnano.5b05061 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szebeni J et al. Liposome-induced complement activation and related cardiopulmonary distress in pigs: factors promoting reactogenicity of Doxil and AmBisome. Nanomedicine 8, 176–184, doi: 10.1016/j.nano.2011.06.003 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Neun BW, Barenholz Y, Szebeni J & Dobrovolskaia MA Understanding the Role of Anti-PEG Antibodies in the Complement Activation by Doxil in Vitro. Molecules 23, doi: 10.3390/molecules23071700 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hempel JC et al. Distinct in vitro Complement Activation by Various Intravenous Iron Preparations. Am J Nephrol 45, 49–59, doi: 10.1159/000451060 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Mueller SN, Tian S & DeSimone JM Rapid and Persistent Delivery of Antigen by Lymph Node Targeting PRINT Nanoparticle Vaccine Carrier To Promote Humoral Immunity. Mol Pharm 12, 1356–1365, doi: 10.1021/mp500589c (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y et al. Engineering biomaterial-associated complement activation to improve vaccine efficacy. Biomacromolecules 14, 3321–3328, doi: 10.1021/bm400930k (2013). [DOI] [PubMed] [Google Scholar]
- 62.Ghendon Y, Markushin S, Akopova I, Koptiaeva I & Krivtsov G Chitosan as an adjuvant for poliovaccine. J Med Virol 83, 847–852, doi: 10.1002/jmv.22030 (2011). [DOI] [PubMed] [Google Scholar]
- 63.AbdelAllah NH et al. Alginate-coated chitosan nanoparticles act as effective adjuvant for hepatitis A vaccine in mice. Int J Biol Macromol 152, 904–912, doi: 10.1016/j.ijbiomac.2020.02.287 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Huaux F Emerging Role of Immunosuppression in Diseases Induced by Micro- and Nano-Particles: Time to Revisit the Exclusive Inflammatory Scenario. Front Immunol 9, 2364, doi: 10.3389/fimmu.2018.02364 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Getts DR et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med 6, 219ra217, doi: 10.1126/scitranslmed.3007563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen RP, Bolandparvaz A, Ma JA, Manickam VA & Lewis JS Latent, Immunosuppressive Nature of Poly(lactic-co-glycolic acid) Microparticles. ACS Biomater Sci Eng 4, 900–918, doi: 10.1021/acsbiomaterials.7b00831 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cifuentes-Rius A, Desai A, Yuen D, Johnston APR & Voelcker NH Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat Nanotechnol 16, 37–46, doi: 10.1038/s41565-020-00810-2 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Smarr CB et al. Biodegradable antigen-associated PLG nanoparticles tolerize Th2-mediated allergic airway inflammation pre- and postsensitization. Proc Natl Acad Sci U S A 113, 5059–5064, doi: 10.1073/pnas.1505782113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Q et al. Use of Polymeric Nanoparticle Platform Targeting the Liver To Induce Treg-Mediated Antigen-Specific Immune Tolerance in a Pulmonary Allergen Sensitization Model. ACS Nano 13, 4778–4794, doi: 10.1021/acsnano.9b01444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Q et al. Antigen- and Epitope-Delivering Nanoparticles Targeting Liver Induce Comparable Immunotolerance in Allergic Airway Disease and Anaphylaxis as Nanoparticle-Delivering Pharmaceuticals. ACS Nano 15, 1608–1626, doi: 10.1021/acsnano.0c09206 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shah S et al. Optimizing PLG nanoparticle-peptide delivery platforms for transplantation tolerance using an allogeneic skin transplant model. Biomaterials 210, 70–82, doi: 10.1016/j.biomaterials.2019.04.030 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bryant J et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials 35, 8887–8894, doi: 10.1016/j.biomaterials.2014.06.044 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong AD, Ye M, Ulmschneider MB & Searson PC Quantitative Analysis of the Enhanced Permeation and Retention (EPR) Effect. PLoS One 10, e0123461, doi: 10.1371/journal.pone.0123461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramanathan RK et al. Correlation between Ferumoxytol Uptake in Tumor Lesions by MRI and Response to Nanoliposomal Irinotecan in Patients with Advanced Solid Tumors: A Pilot Study. Clin Cancer Res 23, 3638–3648, doi: 10.1158/1078-0432.CCR-16-1990 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Xu J et al. Multi-walled carbon nanotubes translocate into the pleural cavity and induce visceral mesothelial proliferation in rats. Cancer Sci 103, 2045–2050, doi: 10.1111/cas.12005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzui M et al. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Sci 107, 924–935, doi: 10.1111/cas.12954 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poland CA et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 3, 423–428, doi: 10.1038/nnano.2008.111 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Ryman-Rasmussen JP et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol 4, 747–751, doi: 10.1038/nnano.2009.305 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Humans I. W. G. o. t. E. o. C. R. t. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum 93, 1–413 (2010). [PMC free article] [PubMed] [Google Scholar]
- 80.Zanganeh S et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol 11, 986–994, doi: 10.1038/nnano.2016.168 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plebanek MP, Bhaumik D, Bryce PJ & Thaxton CS Scavenger Receptor Type B1 and Lipoprotein Nanoparticle Inhibit Myeloid-Derived Suppressor Cells. Mol Cancer Ther 17, 686–697, doi: 10.1158/1535-7163.MCT-17-0981 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajan R et al. Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate. J Control Release 271, 139–148, doi: 10.1016/j.jconrel.2017.12.023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saha S et al. Gold Nanoparticle Reprograms Pancreatic Tumor Microenvironment and Inhibits Tumor Growth. ACS Nano 10, 10636–10651, doi: 10.1021/acsnano.6b02231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arvizo RR et al. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc Natl Acad Sci U S A 110, 6700–6705, doi: 10.1073/pnas.1214547110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sindhwani S et al. The entry of nanoparticles into solid tumours. Nat Mater 19, 566–575, doi: 10.1038/s41563-019-0566-2 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Su S & Kang PM Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials (Basel) 10, doi: 10.3390/nano10040656 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Longmire M, Choyke PL & Kobayashi H Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine-Uk 3, 703–717, doi: 10.2217/17435889.3.5.703 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du BJ, Yu MX & Zheng J Transport and interactions of nanoparticles in the kidneys. Nature Reviews Materials 3, 358–374, doi: 10.1038/s41578-018-0038-3 (2018). [DOI] [Google Scholar]
- 89.Benezra M et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest 121, 2768–2780, doi: 10.1172/JCI45600 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phillips E et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci Transl Med 6, 260ra149, doi: 10.1126/scitranslmed.3009524 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Z et al. Insight into the preformed albumin corona on in vitro and in vivo performances of albumin-selective nanoparticles. Asian J Pharm Sci 14, 52–62, doi: 10.1016/j.ajps.2018.07.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng Q et al. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials 34, 8521–8530, doi: 10.1016/j.biomaterials.2013.07.102 (2013). [DOI] [PubMed] [Google Scholar]
- 93.Peng Q et al. Enhanced biostability of nanoparticle-based drug delivery systems by albumin corona. Nanomedicine (Lond) 10, 205–214, doi: 10.2217/nnm.14.86 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Cao H et al. Albumin Biomimetic Nanocorona Improves Tumor Targeting and Penetration for Synergistic Therapy of Metastatic Breast Cancer. Advanced Functional Materials 27, 1605679, doi: 10.1002/adfm.201605679 (2017). [DOI] [Google Scholar]
- 95.Bargheer D et al. The fate of a designed protein corona on nanoparticles in vitro and in vivo. Beilstein J Nanotechnol 6, 36–46, doi: 10.3762/bjnano.6.5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ban Z et al. Machine learning predicts the functional composition of the protein corona and the cellular recognition of nanoparticles. Proc Natl Acad Sci U S A 117, 10492–10499, doi: 10.1073/pnas.1919755117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walkey CD et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 8, 2439–2455, doi: 10.1021/nn406018q (2014). [DOI] [PubMed] [Google Scholar]
- 98.Palchetti S et al. Protein Corona Fingerprints of Liposomes: New Opportunities for Targeted Drug Delivery and Early Detection in Pancreatic Cancer. Pharmaceutics 11, doi: 10.3390/pharmaceutics11010031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coty JB, Eleamen Oliveira E & Vauthier C Tuning complement activation and pathway through controlled molecular architecture of dextran chains in nanoparticle corona. Int J Pharm 532, 769–778, doi: 10.1016/j.ijpharm.2017.04.048 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Santi M et al. Rational Design of a Transferrin-Binding Peptide Sequence Tailored to Targeted Nanoparticle Internalization. Bioconjug Chem 28, 471–480, doi: 10.1021/acs.bioconjchem.6b00611 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Vincent MP et al. Surface chemistry-mediated modulation of adsorbed albumin folding state specifies nanocarrier clearance by distinct macrophage subsets. Nat Commun 12, 648, doi: 10.1038/s41467-020-20886-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Q et al. Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population. Anal Chem 88, 11804–11812, doi: 10.1021/acs.analchem.6b03437 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen BM et al. Measurement of Pre-Existing IgG and IgM Antibodies against Polyethylene Glycol in Healthy Individuals. Anal Chem 88, 10661–10666, doi: 10.1021/acs.analchem.6b03109 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Palange AL, Palomba R, Rizzuti IF, Ferreira M & Decuzzi P Deformable Discoidal Polymeric Nanoconstructs for the Precise Delivery of Therapeutic and Imaging Agents. Mol Ther 25, 1514–1521, doi: 10.1016/j.ymthe.2017.02.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wibroe PP et al. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat Nanotechnol 12, 589–594, doi: 10.1038/nnano.2017.47 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Brenner JS et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun 9, 2684, doi: 10.1038/s41467-018-05079-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao Z, Ukidve A, Gao Y, Kim J & Mitragotri S Erythrocyte leveraged chemotherapy (ELeCt): Nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci Adv 5, eaax9250, doi: 10.1126/sciadv.aax9250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pan DC et al. Nanoparticle Properties Modulate Their Attachment and Effect on Carrier Red Blood Cells. Sci Rep 8, 1615, doi: 10.1038/s41598-018-19897-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan D et al. The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells. Plos One 11, e0152074, doi: 10.1371/journal.pone.0152074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piao JG et al. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano 8, 10414–10425, doi: 10.1021/nn503779d (2014). [DOI] [PubMed] [Google Scholar]
- 111.Hu CMJ et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proceedings of the National Academy of Sciences of the United States of America 108, 10980–10985, doi: 10.1073/pnas.1106634108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu CM et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121, doi: 10.1038/nature15373 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cong S et al. Fluorescent nanoparticles in the popular pizza: properties, biodistribution and cytotoxicity. Food Funct 10, 2408–2416, doi: 10.1039/c8fo01944d (2019). [DOI] [PubMed] [Google Scholar]
- 114.Holmen BA & Ayala A Ultrafine PM emissions from natural gas, oxidation-catalyst diesel, and particle-trap diesel heavy-duty transit buses. Environ Sci Technol 36, 5041–5050 (2002). [DOI] [PubMed] [Google Scholar]
- 115.Sendra M et al. Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment. Environ Int 98, 62–68, doi: 10.1016/j.envint.2016.09.024 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Smith M, Love DC, Rochman CM & Neff RA Microplastics in Seafood and the Implications for Human Health. Curr Environ Health Rep 5, 375–386, doi: 10.1007/s40572-018-0206-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Henry TB, Petersen EJ & Compton RN Aqueous fullerene aggregates (nC60) generate minimal reactive oxygen species and are of low toxicity in fish: a revision of previous reports. Curr Opin Biotechnol 22, 533–537, doi: 10.1016/j.copbio.2011.05.511 (2011). [DOI] [PubMed] [Google Scholar]
- 118.Jo MR et al. in Molecular & Cellular Toxicology Vol. 12(3) (Springer, Berlin, 2016). [Google Scholar]
- 119.Li Y & Boraschi D Endotoxin contamination: a key element in the interpretation of nanosafety studies. Nanomedicine (Lond) 11, 269–287, doi: 10.2217/nnm.15.196 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Faria M et al. Minimum information reporting in bio-nano experimental literature. Nat Nanotechnol 13, 777–785, doi: 10.1038/s41565-018-0246-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]