Abstract
Bacterial superantigens (BSAgs) cause massive stimulation of the immune system and are associated with various pathologies and diseases. To address the role of antibodies in protection against BSAgs, we screened the sera of 29 human volunteers for antibodies to the SAgs staphylococcal enterotoxin A (SEA), SEB, SEC1, and toxic shock syndrome toxin 1 (TSST-1). Although all volunteers had detectable levels of antibodies against SEB and SEC1, many (9 out of 29 volunteers) lacked detectable antibody to SEA or had minimal titers. Antibody titers to TSST-1 were well below those to SEB and SEC1, and three volunteers lacked detectable antibody to this BSAg. In addition, pooled immunoglobulin preparations obtained from different companies had antibody titers against SEs and TSST-1. There was a good correlation between antibody titers and inhibition of superantigenic effects of these toxins. Transfer of SEB-specific antibodies, obtained from pooled sera, suppressed in vitro T-cell proliferation and totally protected mice against SEB. These data suggest that the inhibitory activity of human sera was specific to antibodies directed against the toxins. Thus, it may be possible to counteract with specific antibodies BSAg-associated pathologies caused by stimulation of the immune system.
Bacterial superantigens (BSAgs), such as staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin 1 (TSST-1), are pyrogenic virulence factors produced by Staphylococcus aureus (9, 11, 13, 26). These microbial SAgs bind to both human major histocompatibility antigen class II molecules on the surface of antigen-presenting cells and germ line-encoded variable domain sequences of the specific T-cell receptor variable β chain on T lymphocytes (9, 11). Thus, BSAgs bypass the normal antigen-specific restrictions by creating a wedge between T-cell receptor and class II molecules and hence activate significantly greater numbers of T lymphocytes. The majority of stimulated T cells are programmed to acquire susceptibility to cell death by Fas- and Fas ligand-mediated apoptosis, or alternatively they enter into a state of specific nonresponsiveness (anergy), which may last for several months after the initial encounter with the BSAg. The activation of antigen-presenting cells and T cells results in production of pathological levels of proinflammatory cytokines that contribute to several serious pathologies and lethal toxic shock syndrome (11, 17, 22, 26).
Low serum antibody titers to BSAgs have been associated with the recurrence of toxic shock syndrome (10, 23, 28). Vaccination with nonsuperantigenic forms of BSAgs mitigates many of the symptoms of SE exposure (4, 14, 27). Vaccinated animals had high protective antibody titers against SEs and were fully protected against lethal challenge (4, 27). Thus, antibody responses may play a major role in protection against BSAgs. Here, we studied the prevalence of anti-SE and anti-TSST-1 antibodies in normal human volunteers and several pooled intravenous immunoglobulin (IVIG) products and examined if there is a correlation between antibody titers and suppression of T-cell responses to BSAgs. In addition, we evaluated the efficacy of SEB-specific antibodies obtained from pooled immunoglobulin against lethal doses of SEB in an in vivo model.
MATERIALS AND METHODS
Human sera and immunoglobulin.
Volunteers, recruited from the laboratory, clerical, and maintenance staffs, were all in good health and ranged from 18 to 59 years old. All gave written informed consent to participate in this study, which was approved by the institutional human use committee. Participation and results were coded for purposes of maintaining confidentiality. Blood was collected, and serum was separated by centrifugation and frozen at −70°C until tested.
Anti-SEB human hyperimmune globulin (SEBIGH) was obtained from Hyland Laboratories, Los Angeles, Calif. (lot 750A15; 150 mg/ml; cold ethanol fractionation; Cohn/Fraction 2). This preparation was obtained from serum collected by repeated plasmaphoresis from 10 volunteer donors with high titers of antibody to SEB. Pooled IVIG (Venoglobulin-S; 50 mg/ml; 99% immunoglobulin G [IgG]) was a gift from Alpha Therapeutic Corp. (Los Angeles, Calif.).
BSAgs and LPS.
SEA, SEB, SEC1, and TSST-1 were purchased from Toxin Technology (Sarasota, Fla.). Each toxin was judged to be greater than 95% pure by electrophoresis on sodium dodecyl sulfate–5 to 20% gradient polyacrylamide gels. The toxins were prepared in phosphate-buffered saline (PBS) (140 mM NaCl, 50 mM Na2H2PO3, pH 7.4). Escherichia coli 055:B5-derived lipopolysaccharide (LPS) was obtained from Difco Laboratories (Detroit, Mich.) and reconstituted with PBS. Aliquots were stored at −70°C for future use.
Antitoxin antibodies.
Serum antibody titers against the enterotoxins or TSST-1 were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (4). Serial dilutions of 1:4 or 1:8 (starting at a 1:100 dilution) of the each serum sample in triplicate were examined, and after addition of peroxidase-labeled mouse anti-human IgG, Fc-specific antibody (Accurate Chemical, Westbury, N.Y.), and the substrate 2,2′-azino-di(3-ethybenthiazoline sulfonate) (ABTS) (Kirkegaard and Perry Laboratories, Gaithersburg, Md.), absorbance was determined at 410 nm after 15 to 30 min in a microplate reader. Between each step, all wells were washed four times with PBS containing 0.2% Tween 20.
T-lymphocyte proliferation assay.
Peripheral blood mononuclear cells were isolated from heparinized blood of healthy humans by Ficoll gradient centrifugation. Isolated peripheral blood mononuclear cells were washed three times in RPMI 1640 medium. The cell pellet was resuspended in RPMI 1640 with 5% fetal bovine serum (FBS), and 100 μl of the cell suspension (105 cells) was added to triplicate wells of 96-well flat-bottom plates containing 50 μl of diluted human sera, affinity-purified anti-SEB antibody, or medium control. Fifty microliters of SEA, SEB, SEC1, or TSST-1 was added to each of triplicate wells. The cultures were incubated at 37°C in an atmosphere of 5% CO2–95% air for 3 days and with 1 μCi of [3H]thymidine (Amersham, Arlington Heights, Ill.) for 12 h before harvesting onto glass fiber filters. The amount of [3H]thymidine incorporation was measured with a liquid scintillation counter.
Affinity purification of human anti-SEB antibodies.
SEB was coupled to cyanogen bromide-activated Sepharose 4B (Sigma Chemical Co., St. Louis, Mo.) according to the manufacturer's directions. Affinity purification was performed on an EconoSystem (Bio-Rad, Melville, N.Y.). Absorbance was monitored at 280 nm. SEBIGH was diluted to 1 mg/ml with PBS and passed over the SEB column. The column was washed with PBS until the absorbance returned to baseline, and the bound antibody was eluted with 0.1 M glycine (pH 2.5). The antibodies were dialyzed extensively against PBS, and the amount of protein was measured. More than 99% of the specific antibodies to SEB were depleted from SEBIGH after several passages of the sera over the immunoaffinity column (data not shown).
Mice and passive protection assay.
Pathogen-free BALB/c mice, 10 to 12 weeks old, were obtained from Harlan Sprague-Dawley, Inc. (Frederick Cancer Research and Development Center, Frederick, Md.). Mice were maintained under pathogen-free conditions and fed laboratory chow and water ad libitum. For passive transfer studies, 10 (≈2.0 μg/mouse) or 100 (≈20 μg/mouse) 50% lethal doses (LD50) of SEB were incubated with unpurified sera (500 μl), 200 μl of affinity-purified anti-SEB antibody (150 μg), 400 μl of nonspecific antibody (150 μg), or 200 μl of PBS. Mice were injected intraperitoneally with the mixture and, 3 h later, with 70 μg of LPS, as previously described (4). Deaths were recorded after 4 days. Challenge controls were mice injected with either LPS or SEB (no death was observed).
Statistical methods.
For the T-cell proliferation assay, mean values and standard deviations were compared using Student's t test. Final lethality was statistically scored using Fisher exact tests.
RESULTS
Presence of anti-BSAg antibodies in human serum.
Tables 1 and 2 illustrate levels of binding to SEA, SEB, SEC1, and TSST-1 for serum samples obtained from volunteers and two pooled immunoglobulin products. All of the sera tested had moderate to high levels of antibodies against SEB, and SEC1. In sharp contrast to the case for SEB and SEC1, nine of the volunteers lacked detectable anti-SEA titers, and the majority of the remaining individuals had low titers for this SAg (Table 1). This observation is in total agreement with previous studies showing that pooled human sera reacted weakly with SEA (24). Although three individuals lacked responses to TSST-1, their overall titers were higher than those for SEA. Interestingly, pooled IVIG obtained from a commercial source showed results similar to those for sera obtained from volunteers (Table 2). For pooled IVIG, titers to SEB and SEC1 were 1:12,800, and titers against SEA and TSST-1 were lower (1:400 and 1:1,600, respectively). As expected, SEBIGH had the highest titers against SEB and lower titers against TSST-1, and anti-SEA antibodies were undetected in this preparation. This product also contained large amounts of anti-SEC1 antibodies. Although SEBIGH was obtained from individuals with high titers against SEB, we showed that antibodies against this SAg cross-reacted and possibly neutralized toxic effects of other BSAgs (6).
TABLE 1.
BSAg-specific serum IgG responses of volunteers measured by ELISA
| Titera | No. of volunteers with titer against:
|
|||
|---|---|---|---|---|
| SEA | SEB | SEC | TSST-1 | |
| NDb | 9 | 0 | 0 | 3 |
| 50 | 10 | 3 | 2 | 7 |
| 400 | 9 | 12 | 10 | 16 |
| 1,600 | 1 | 12 | 14 | 2 |
| 12,800 | 0 | 2 | 3 | 1 |
Serum titers measured by ELISA. Data are presented as the reciprocal serum dilution resulting in an absorbance reading two times higher than that for the negative control (ELISA wells containing either no BSAg or no serum).
ND, not detected (<50).
TABLE 2.
Reactivity of pooled human immunoglobulin preparations to different BSAgs
| Source | Titera against:
|
|||
|---|---|---|---|---|
| SEA | SEB | SEC1 | TSST-1 | |
| Pooled IVIG | 400 | 12,800 | 12,800 | 1,600 |
| SEBIGH | NDb | >12,800 | >12,800 | 400 |
Data are presented as the reciprocal dilution resulting in an absorbance reading two times higher than that for the negative control (ELISA wells containing either no BSAg or no serum).
ND, not detected (<100).
High anti-BSAg antibody titers neutralized T-cell responses.
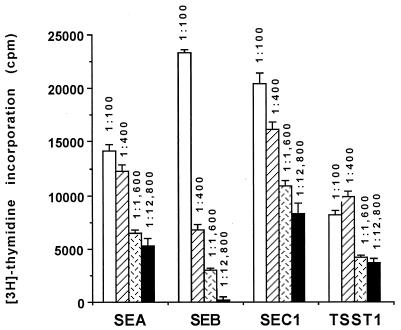
We next investigated if there is a positive correlation between antitoxin titers and inhibition of human T-cell responses to BSAgs. Sera from volunteers with titers of 1:100, 1:400, 1:1,600, or 1:12,800 were pooled and then tested for their ability to inhibit T-cell responses to SEA, SEB, SEC1, and TSST-1 (Fig. 1). Compared to FBS, which does not contain detectable antienterotoxin or anti-TSST-1 antibodies, all human volunteer sera tested suppressed BSAg-induced T-lymphocyte proliferation (5). Sera from different groups varied in their ability to neutralize BSAgs. For the SEA, SEB, and SEC1, there was a very good correlation between titer and inhibition of T-lymphocyte responses to the corresponding SAg (Fig. 1). Although pooled sera obtained from high-titer individuals had a much larger capacity to inhibit responses to TSST-1-induced T-lymphocyte proliferation than those from low-titer individuals, there was a lesser correlation between the ability of each group of sera to inhibit T-cell stimulation.
FIG. 1.
Inhibition of T-lymphocyte responses to BSAgs by pooled sera. Sera from individuals with the same titer were pooled and tested for inhibition of BSAg activities. Results are represented as mean counts per minute (± standard deviation) for triplicate wells incubated with 100 ng of SEA, SEB, or SEC1 per ml or 1 μg of TSST-1 per ml for 72 h and then pulsed-labeled for 12 h with [3H]thymidine. Control values (incorporation of [3H]thymidine for cultures that contained 10% FBS and BSAg) were 28,078 ± 2,658, 29,496 ± 1,702, 32,439 ± 5,405, and 19,961 ± 2,559 cpm for SEA-, SEB-, SEC1-, and TSST-1-treated cultures, respectively. The P value was <0.001 for all experiments except for the 1:100 dilution of anti-SEB and anti-SEC1 sera, compared to cultures that were stimulated with the corresponding BSAg in presence of FBS. The P value was <0.05 for the 1:100 dilution of anti-SEB and anti-SEC1 sera compared to cultures that were stimulated with SEB in presence of FBS.
In vivo protection against BSAg is mediated by specific antibody.
To remove the effect of anticytokine and other potential nonspecific neutralizing activities commonly found in pooled sera or IVIG products (1–3), we affinity purified anti-SEB antibodies. This product was then tested for its ability to neutralize the lethal effect of the toxin in a previously established animal model (22). Mice were given a lethal dose of SEB in addition to a potentiating dose of LPS and one of the following: unpurified SEBIGH, nonspecific antibodies (flowthrough), anti-SEB specific antibodies (eluate), or buffer (Table 3). As expected, the unpurified pooled sera contained some neutralizing antibodies against SEB. When antibodies were injected concomitantly with the toxin, both unpurified antibodies and the affinity-purified antibody preparation fully protected the mice against low doses of SEB (P < 0.001 versus nonspecific flowthrough IgG). Because of the limited amounts of protective antibodies in the unpurified fraction, less protection was afforded to mice that received higher dose of the toxin. Mice that received 100 LD50 of the toxin and unpurified antibodies had 70% survival (P = 0.02 versus nonspecific flowthrough).
TABLE 3.
Protection induced by transfer of anti-SEB antibodiesa
| Therapy | No. live/no. dead after challenge with:
|
|||||
|---|---|---|---|---|---|---|
| 10 LD50
|
100 LD50
|
|||||
| 0 h | 4 h | 10 h | 0 h | 4 h | 10 h | |
| Eluate (specific) | 10/0b | 10/0b | 5/5 | 10/0b | 10/0b | 2/8 |
| Unpurified SEBIGH | 10/0b | 9/1b | 1/9 | 7/3c | 4/6d | 0/10 |
| Flowthrough | 0/10 | 0/10 | NDe | 1/9 | 0/10 | ND |
| Buffer | 0/10 | 0/10 | ND | ND | ND | ND |
Mice (10 per group) were challenged with 10 or 100 LD50 of SEB at the same time as the therapy, or therapy was initiated 4 or 10 h after toxin injection. A potentiating dose of LPS was given to mice 3 h after SEB injection. Mice were observed for 96 h after the challenge. Controls included age-matched mice that received LPS or SEB alone (no lethality was observed).
P < 0.001 versus nonspecific flowthrough IgG.
P = 0.02 versus nonspecific flowthrough IgG.
P = 0.09 versus nonspecific flowthrough IgG.
ND, not determined.
To further understand the kinetics of protection, we passively transferred specific or nonspecific antibodies to mice at 4 and 10 h after SEB challenge and scored the lethality at 4 days. When treatment was delayed for 4 h, only specific antibody completely protected the animals, regardless of the challenge dose, perhaps because of the larger amounts of the specific antibodies. However, a 10-h delay in administration of therapy substantially decreased survival. In these groups, purified anti-SEB antibodies protected 50 and 20% of mice against 10 and 100 LD50, respectively. We observed 90% survival in mice that were challenged with 10 LD50 and given a rescuing dose of unpurified pooled immunoglobulin 4 h later. At the higher challenge dose, survival was reduced to 40% (P = 0.09 versus nonspecific flowthrough) when treatment with the protective antibodies was delayed by 4 h. If treatment with unpurified antibody was delayed by 10 h, little to no survival was observed. The flowthrough fraction that contained no detectable antibodies against SEB was not protective.
These data suggest that the protective effect of pooled IgG against BSAgs was localized within the specific antitoxin IgG fraction and indicate that there is a window of opportunity for therapy after BSAg exposure. In these experiments we also attempted to correlate the concentration of specific anti-SEB antibodies required for T-cell activation with the amount needed for protection against SEB-induced lethality. However, this was extremely difficult to examine because cross-reactive and possibly neutralizing heterologous anti-SE antibodies (such as anti-SEC1 to -3) that coeluted with anti-SEB also protect mice against SEB challenge (reference 6 and unpublished data).
DISCUSSION
Several published reports have documented therapeutic uses of IVIG against BSAg-induced toxic shock syndrome (12, 16, 20) and many other infectious agents (18, 19, 21). However, in many cases the exact mechanisms of immunoglobulin therapy remain unestablished. Previously, Takei and colleagues used in vitro methods to show that IVIG contains anti-SE antibodies that suppress SE-induced T-cell stimulation (24). These investigators suggested that inhibition of T-cell responses was due to specific suppression of binding or T-cell recognition of the BSAg. Other studies demonstrated that cytokine suppression was intimately linked to the ability of IVIG to neutralize the BSAgs (7, 8, 25). In one study, in vitro treatment of mononuclear cells with IVIG substantially inhibited the release of inflammatory cytokines (interleukin-6 and tumor necrosis factor alpha) induced by SEB (25). Interestingly, more recent studies showed that IVIG decreased in vitro SEB-induced T-cell responses without significant suppression of gamma interferon or tumor necrosis factor alpha release (7).
In these studies antibody titers against BSAgs in the IVIG preparations were not determined. This is extremely important because variations in titers against BSAgs among different lots of IVIG have been reported (15). Fluctuations in titers may have altered the outcome of the experiments and make it very difficult to compare the different studies (7, 8, 21, 23). Moreover, in previous studies the efficacy of IVIG preparations in vivo was not examined and in vivo protection studies were not performed.
Here, we showed that the majority of population tested had measurable amounts of anti-SE antibodies, and a good correlation between serum antibody titers and inhibition of T-cell induction by the BSAgs SEA, SEB SEC1, and TSST-1 was observed. Although the exact mechanism(s) by which IVIG inhibits BSAg actions is unknown, in this study we clearly demonstrated that only specific antibody against SEB obtained from pooled IgG protected mice from a high lethal dose of SEB. The nonspecific antibodies in the pooled sera showed no protection against SEB challenge. The specific antibodies, but not the unpurified SEBIGH, fully protected mice against a high dose of SEB for up to 4 h (Table 3). Interestingly, when rhesus monkeys were given anti-SEB antibodies 20 h after a lethal challenge of SEB, the antibody preparations fully rescued the monkeys (unpublished observation). In the LPS-potentiated mouse model, SEB-induced lethality is observed within the first 12 h (perhaps because of robust release of cytokines). Lethality in rhesus monkeys is observed at later times, and this may explain the differences in the therapeutic window for mice and rhesus monkeys (unpublished observations).
In conclusion, our data suggest that immunotherapy against BSAgs could be initiated a few hours following the exotoxin release. Furthermore, the experiments presented in this study identified a possible use of a mouse surrogate assay as a correlate of immunity and T-lymphocyte proliferation studies as biomarker and surrogate end points for assessing in vivo biological responses in humans and may be relevant to BSAg-associated clinical toxicity. These types of models potentially can facilitate transition and evaluation of therapeutic antibodies against BSAgs.
REFERENCES
- 1.Abe Y, Horiuchi A, Miyame M, Kimura S. Anti-cytokine nature of natural human immunoglobulin: one possible mechanism of the clinical effect of IV IgG therapy. Immunol Rev. 1994;139:5–19. doi: 10.1111/j.1600-065x.1994.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 2.Achiron A, Margalit R, Hershkoviz R, Markovits D, Reshef T, Melamed E, Cohen I, Lider O. Intravenous immunoglobulin treatment of experimental T cell-mediated autoimmune disease—upregulation of T cell proliferation and downregulation of tumor necrosis factor alpha secretion. J Clin Invest. 1994;93:600–605. doi: 10.1172/JCI117012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson U, Bjork L, Skansen-Saphir U, Andersson J. Pooled human IgG modulates cytokine production in lymphocytes and monocytes. Immunol Rev. 1994;139:21–42. doi: 10.1111/j.1600-065x.1994.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 4.Bavari S, Dayas B, Ulrich R G. Superantigen vaccines: a comparative study of genetically attenuated receptor binding mutants of staphylococcal enterotoxin A. J Infect Dis. 1996;174:338–345. doi: 10.1093/infdis/174.2.338. [DOI] [PubMed] [Google Scholar]
- 5.Bavari S, Hunt R E, Ulrich R U. Divergence of human and nonhuman primate lymphocyte responses to bacterial superantigens. Clin Immunol Immunopathol. 1995;76:248–254. doi: 10.1006/clin.1995.1123. [DOI] [PubMed] [Google Scholar]
- 6.Bavari S, Ulrich R G, LeClaire R D. Cross-reactive antibodies prevent the lethal effects of Staphylococcus aureus superantigens. J Infect Dis. 1999;180:1365–1369. doi: 10.1086/314977. [DOI] [PubMed] [Google Scholar]
- 7.Campbell D E, Georgiou G M, Kemp A. Pooled human immunoglobulin inhibits IL-4 but not IFN-gamma or TNF-alpha secretion following in vitro stimulation of mononuclear cells with staphylococcal superantigen. Cytokine. 1999;11:359–365. doi: 10.1006/cyto.1998.0435. [DOI] [PubMed] [Google Scholar]
- 8.Darville T, Milligan L B, Laffoon K K. Intravenous immunoglobulin inhibits staphylococcal toxin-induced human mononuclear phagocyte tumor necrosis factor alpha production. Infect Immun. 1997;65:366–372. doi: 10.1128/iai.65.2.366-372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser J D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989;339:221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- 10.Freeman J D, Beer D J. Expanding perspectives on the toxic shock syndrome. Adv Intern Med. 1991;36:363–385. [PubMed] [Google Scholar]
- 11.Herman A, Kappler J W, Marrack P, Pullen A M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 12.Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O'Rourke K, Talbot J, Low D E. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. Clin Infect Dis. 1999;28:800–807. doi: 10.1086/515199. [DOI] [PubMed] [Google Scholar]
- 13.Marrack P, Kappler J W. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson I M, Verdrengh M, Ulrich R G, Bavari S, Tarkowski A. Protection against Staphylococcus aureus sepsis by vaccination with recombinant enterotoxin A devoid of superantigenicity. J Infect Dis. 1999;180:1370–1373. doi: 10.1086/315023. [DOI] [PubMed] [Google Scholar]
- 15.Norrby-Teglund A, Basma H, Andersson J, McGeer A, Low D E, Kotb M. Varying titers of neutralizing antibodies to streptococcal superantigens in different preparations of normal polyspecific immunoglobulin G: implications for therapeutic efficacy. Clin Infect Dis. 1998;26:631–638. doi: 10.1086/514588. [DOI] [PubMed] [Google Scholar]
- 16.Perez C M, Kubak B M, Cryer H G, Salehmugodam S, Vespa P, Farmer D. Adjunctive treatment of streptococcal toxic shock syndrome using intravenous immunoglobulin: case report and review. Am J Med. 1997;102:111–113. [PubMed] [Google Scholar]
- 17.Rahimpour R, Mitchell G, Khandaker M H, Kong C, Singh B, Xu L, Ochi A, Feldman R D, Pickering J G, Gill B M, Kelvin D J. Bacterial superantigens induce down-modulation of CC chemokine responses in human monocytes via an alternative chemokine ligand-independent mechanism. J Immunol. 1999;162:2299–2307. [PubMed] [Google Scholar]
- 18.Siber G. Immune globulin to prevent nosocomial infections. N Engl J Med. 1992;327:269–271. doi: 10.1056/NEJM199207233270409. [DOI] [PubMed] [Google Scholar]
- 19.Siber G R, Leszczynski J, Pena-Cruz V, Ferren-Gardner C, Anderson R, Hemming V G, Walsh E E, Burns J, McIntosh K, Gonin R, Anderson L J. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J Infect Dis. 1992;165:456–463. doi: 10.1093/infdis/165.3.456. [DOI] [PubMed] [Google Scholar]
- 20.Stevens D L. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu Rev Med. 2000;51:271–288. doi: 10.1146/annurev.med.51.1.271. [DOI] [PubMed] [Google Scholar]
- 21.Stiehm E R. New uses for intravenous immune globulin. N Engl J Med. 1991;325:123–125. doi: 10.1056/NEJM199107113250209. [DOI] [PubMed] [Google Scholar]
- 22.Stiles B G, Bavari S, Krakauer T, Ulrich R G. Toxicity of staphylococcal enterotoxins potentiated by lipopolysaccharide: major histocompatibility complex class II molecule dependency and cytokine release. Infect Immun. 1993;61:5333–5338. doi: 10.1128/iai.61.12.5333-5338.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolz S J, Davis J P, Vergeront J M, Crass B A, Chesney P J, Wand P J, Bergdoll M S. Development of serum antibody to toxic shock toxin among individuals with toxic shock syndrome in Wisconsin. J Infect Dis. 1985;151:883–889. doi: 10.1093/infdis/151.5.883. [DOI] [PubMed] [Google Scholar]
- 24.Takei S, Arora Y K, Walker S M. Intravenous immunoglobulin contains specific antibodies inhibitory to activation of T cells by staphylococcal toxin superantigens. J Clin Invest. 1993;91:602–607. doi: 10.1172/JCI116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toungouz M, Denys C H, De Groote D, Dupont E. In vitro inhibition of tumour necrosis factor-alpha and interleukin-6 production by intravenous immunoglobulins. Br J Haematol. 1995;89:698–703. doi: 10.1111/j.1365-2141.1995.tb08404.x. [DOI] [PubMed] [Google Scholar]
- 26.Ulrich R G, Bavari S, Olson M A. Bacterial superantigens in human disease: structure, function and diversity. Trends Microbiol. 1995;3:463–468. doi: 10.1016/s0966-842x(00)89011-3. [DOI] [PubMed] [Google Scholar]
- 27.Ulrich R G, Olson M A, Bavari S. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine. 1998;16:1857–1864. doi: 10.1016/s0264-410x(98)00176-5. [DOI] [PubMed] [Google Scholar]
- 28.Vergeront J M, Stolz S J. Prevalence of serum antibody to staphylococcal enterotoxin F among Wisconsin residents: implication for toxic shock syndrome. J Infect Dis. 1983;148:692–698. doi: 10.1093/infdis/148.4.692. [DOI] [PubMed] [Google Scholar]