Abstract
The fluffy genes flbA–flbE are well-known players in the upstream developmental activation pathway that activates the key gene brlA of central developmental pathway (CDP) to initiate conidiation in Aspergillus nidulans. Here, we report insignificant roles of their orthologs in radial growth of Beauveria bassiana under normal culture conditions and different stresses although flbA and flbD were involved in respective responses to heat shock and H2O2. Aerial conidiation level was lowered in the deletion mutants of flbB and flbE (~15%) less than of flbA and flbC (~30%), in which the key CDP genes brlA and abaA were repressed consistently during normal incubation. The CDP-controlled blastospore production in submerged cultures mimicking insect hemolymph was abolished in the flbA mutant with brlA and abaA being sharply repressed, and decreased by 55% in the flbC mutant with only abaA being downregulated. The fungal virulence against a model insect was attenuated in the absence of flbA more than of flbC irrespective of normal cuticle infection or cuticle-bypassing infection (intrahemocoel injection). These findings unravel more important role of flbA than of flbC, but null roles of flbB/D/E, in B. bassiana’s insect–pathogenic lifecycle and a scenario distinctive from that in A.nidulans.
Keywords: entomopathogenic fungi, upstream developmental activators, gene expression and regulation, aerial conidiation, blastospore production, virulence, stress response
1. Introduction
The fluffy genes flbA–flbE encode signal transducers that are well-known players in the upstream developmental activation (UDA) pathway to activate the key activator gene brlA of central developmental pathway (CDP) required for initiation of conidiation in Aspergillus nidulans, as well reviewed [1,2,3]. The signal transducers are the G-protein signaling protein FlbA, the b-ZIP transcription factor (TF) FlbB, the TF FlbC with two C2H2 zinc finger DNA-binding domains, the c-Myb TF FlbD and the FlbB-interacting protein FlbE, respectively. The expression of brlA is activated through the cascades fluG-flbA, fluG-flbC and fluG-flbE/flbB/flbD, in which fluG acts as a core UDA regulator [4,5,6,7,8,9,10,11,12,13,14]. The activated brlA leads to sequential activation of the downstream CDP genes abaA and wetA and the velvety family gene vosA, which are collectively essential for conidial production and maturation [15,16,17,18]. The fluffy genes were found in the early analyses of repressive fluffy mutations, which led to functional loss of brlA and ‘fluffy’ (conidiation abolished) colony morphology [19,20,21,22]. Such fluffy genes have corresponding orthologs in many filamentous fungal genomes annotated in the past decade. The post-genomic era has witnessed increasing evidences of ascomycetous genome divergence, making it necessary to reconsider whether the genetic control principles on the asexual development of A. nidulans are applicable to Pezizomycotina [3,23,24,25]. The necessity is emphasized by the existence of an approximately one-fold molecular difference between small [437–534 amino acids (aa)] and large (860–914 aa) FluG homologs and of multiple FluG-like regulators similar to small FluG in different lineages of ascomycetes [13,26]. Even in aspergilli, fluG may not necessarily play the same regulatory role in asexual development as elucidated in A. nidulans. For example, conidial yield of fluG null mutant was moderately decreased in A. flavus [27] but not affected at all in A. niger [28].
The insect–pathogenic fungus Beauveria bassiana (Hypocreales: Cordycipitaceae) has the broadest host spectrum among all insect pathogens [29] and serves as a main source of fungal pesticides to combat against wide-spectrum arthropod pests [30]. Designing or improving large-scale production technology of high-quality conidia as active ingredients of fungal pesticides requires the knowledge of regulatory mechanisms underlying asexual developmental activation. In B. bassiana, three CDP genes (brlA, abaA and wetA) and downstream vosA regulate conidial production and maturation [31,32] as documented in A. nidulans [1,2,3], although the two fungi have distinctive conidiation modes, namely the formation of spore balls (clustered conidia) on tiny zigzag rachises (conidiophores) and of chained conidia on phialides. Previously, disruption of fluG in B. bassiana led to a very limited defect (~10% decrease) in aerial conidiation but a sharp increase in submerged blastospore production, accompanied by time-course-active transcription profiles of all CDP genes and flbA–flbE in both aerial and submerged cultures [26]. As the coding gene of regulator of G-protein signaling, Bbrgs1 orthologous to flbA in A. nidulans was reported to mediate conidiation and heat tolerance in B. bassiana but play no role in the fungal virulence [33]. Similar to aerial conidiation, blastospore production of B. bassiana is an asexual developmental process under the control of either brlA or abaA and crucial for the fungal proliferation by yeast-like budding in insect hemocoel to accelerate insect death from mummification [32]. Although null role of fluG in the fungal UDA pathway was shown in our previous study [26], it remains unknown whether and how most of those fluffy genes act as regulators of asexual developmental processes in B. bassiana. In this study, we generated and analyzed single-gene deletion and complementation mutants of flbA–flbE in order to elucidate their functions in the insect–pathogenic lifecycle of B. bassiana. An emphasis was placed upon a possible role of each target gene in conidiation, blastospore production in vitro andin vivo, and a possibility of its transcriptional link to brlA or abaA.
2. Materials and Methods
2.1. Bioinformatic Analysis of Fungal FlbA–FlbE Orthologs
The amino acid sequences of A. nidulans FlbA–FlbE (NCBI accession numbers: EAA58402, CBF79600, EAA64532, EAA66152 and EAA65198, respectively) were used as queries to search through the NCBI genome databases of B. bassiana [34] and some other ascomycetous fungi including entomopathogens and non-entomopathogens via BLASTp analysis (http://blast.ncbi.nlm.nih.gov/blast.cgi, accessed on 20 March 2022). Conserved domains and nuclear localization signal (NLS) motif were predicted from each query protein and its B. bassiana ortholog at http://smart.embl-heidelberg.de/ (accessed on 20 March 2022) and http://nls-mapper.iab.keio.ac.jp/ (accessed on 20 March 2022), respectively. The sequence identities of each B. bassianaFlb protein to those orthologues found in other fungal species were analyzed by sequence alignment with a program at http://www.bio-soft.net/format/DNAMAN.htm/ (accessed on 20 March 2022), followed by phylogenetic analysis with a maximum likelihood method in MEGA7 at http://www.megasoftware.net/ (accessed on 20 March 2022).
2.2. Subcellular Localization of FlbA–FlbE in B. bassiana
Green fluorescence-tagged fusion proteins of FlbA–FlbE were expressed in the wild-type strain B. bassiana ARSEF 2860 (designated WT) as described previously for expression of FluG-GFP fusion protein [26]. Briefly, the coding sequence of each target gene was amplified from the WT cDNA with paired primers (Table S1) and ligated to the N-terminus of gfp (GenBank accession U55763) in the vector pAN52-C-gfp-bar using a one-step cloning kit (Vazyme, Nanjin, China). In the vector, capital C denotes the cassette 5′-PmeI-SpeI-EcoRV-EcoRI-BamHI-3′ driven by the homologous promoter Ptef1 [35,36]. The resultant vector pAN52- x-gfp-bar (x = flbA, flbB, flbC, flbD or flbE) was integrated into the WT strain via Agrobacterium-mediated transformation. Putative transgenic strains were screened by the bar resistance to phosphinothricin (200 μg/mL). For each transformation, a transgenic strain showing strong green fluorescence signal was incubated for full conidiation on Sabouraud dextrose agar (4% glucose, 1% peptone and 1.5% agar) plus 1% yeast extract (SDAY). Conidia from the culture were suspended in SDBY (i.e., agar-free SDAY) and incubated at optimal 25 °C for 3 d in the light/dark (L:D) cycles of 0:24, 12:12 and 24:0 on a shaking bed (150 rpm). Hyphal samples from the cultures were stained with DAPI (4′,6′-diamidine-2′-phenylindole dihydrochloride; Sigma-Aldrich, Shanghai, China) and visualized with laser scanning confocal microscopy (LSCM). The green fluorescence intensity of a fixed circular area of cytoplasm or nucleus was quantified from each of 33–35 cells in the hyphae of the culture grown in each L:D cycle by means of the software ImageJ at https://imagej.nih.gov/ij/ (accessed on 20 March 2022) and used to compute the ratio of nuclear versus cytoplasmic green fluorescence intensity (N/C-GFI) as an index of relative accumulation level of each fusion protein in the nucleus of each cell.
2.3. Generation of Targeted Gene Mutants
The disruption strategy of fluG in our previous study [26] was adopted to generate null mutants of flbA–flbE by deleting full-length coding and partial flanking DNA sequences of each target gene from the WT genome through homologous recombination of its 5′ and 3′ flanking fragments separated by bar marker in the vector p0380-5′x-bar-3′x (Figure S1A–E). The constructed vectors were individually integrated into the WT strain as aforementioned. Further, the full-length coding sequence of each target gene with flanking regions was amplified from the WT DNA and ligated to the HindIII/XbaI sites in p0380-sur-gateway to exchange for the gateway fragment. The resultant p0380-sur-x was ectopically integrated into an identified Δflb mutant for targeted gene complementation in the same transformation system. Putative mutants were screened by the bar resistance to phosphinothricin (200 μg/mL) or the sur resistance to chlorimuron ethyl (10 μg/mL). Expected recombinant events in the colonies were identified through PCR (Figure S1F–J) and real-time quantitative PCR (qPCR) analyses (Figure S1K–O). Listed in Table S1 are pairs of primers used for amplification of DNA fragments and detection of targeted DNA and cDNA samples. The identified deletion mutant (DM) of each gene and its complementation mutant (CM) were evaluated in parallel with the parental WT strain in the following experiments of three independent replicates per strain unless specified otherwise.
2.4. Assays for Growth Rates under Normal Culture Conditions and Stresses
For all DM and control (WT and CM) strains, 1μL aliquots of a 106 conidia/mL suspension were spotted on the plates of rich medium SDAY, 1/4 SDAY (amended with 1/4 of each SDAY nutrient), minimal medium Czapek–Dox agar (CDA; 3% sucrose, 0.3% NaNO3, 0.1% K2HPO4, 0.05% KCl, 0.05% MgSO4 and 0.001% FeSO4 plus 1.5% agar) and CDAs amended with different carbon or nitrogen sources. After a 7-day incubation at the optimal regime of 25 °C in a light/dark (L:D) cycle of 12:12, the diameter of each colony was measured as a growth index using two measurements taken perpendicular to each other across the center. Typical colony images were also collected.
The same method was also used to initiate colony growth on CDA plates alone (control) or supplemented with oxidants, osmotic agents and cell wall perturbing agents as described previously [26]. Cellular response to heat shock was assayed by exposing normal 2 d-old SDAY colonies to 42 °C for 3 and 6 h, followed by 5-d growth recovery at 25 °C. Colony images and diameter values were collected as aforementioned. Relative growth inhibition (RGI) of each DM or control strain under a given stress was computed as an index of its sensitivity to the stress using the formula RGI = (dc–ds)/dc×100, where dc and ds denote the respective diameters of control and stressed colonies. For the ΔflbD mutant more sensitive to H2O2 than its control strains, gradient H2O2 concentrations (1.0–3.5 mM) were added to CDA plates for comparison and verification of their responses to the oxidative stress induced by H2O2.
2.5. Assays for Conidial Yield and Quality
The cultures for quantification of biomass and conidiation capacity were initiated by spreading 100 μL aliquots of a 107 conidia/mL suspension on SDAY plates (9 cm diameter) overlaid with or without cellophane and incubated for 9 d at the optimal regime of 25 °C and L:D 12:12. During the period of incubation, three samples were taken from each cellophane-free plate culture on days 5, 7 and 9 using a cork borer (5 mm diameter). Conidia in each sample were released into 1 mL of 0.02% Tween 80 via a 10-min supersonic vibration, followed by assessing the conidial concentration in the suspension with a hemocytometer and converting it to the number of conidia per square centimeter of plate culture. Biomass levels were assessed from three cellophane-overlaid SDAY cultures on days 3, 5 and 7, respectively. The conidial quality of each strain was assessed as the indices of median germination time (GT50, h) at 25 °C, median lethal time (LT50, min) for tolerance to a 45 °C wet–heat stress and median lethal dose (LD50, J/cm2) for resistance to UVB irradiation (weighted wavelength: 312 nm), as described elsewhere [26].
2.6. Insect Bioassays
The fifth-instar larvae of greater wax moth (Galleria mellonella) were assayed for the virulence of each DM or control strain in two infection modes. Briefly, normal cuticle infection (NCI) was initiated by immersing a group of ~35 larvae (three groups per strain) for 10 s in 40 mL of a 107 conidia/mL suspension. Cuticle-bypassing infection (CBI) was initiated by injecting 5 μL of a 105 conidia/mL suspension into the hemocoel of each larva in each of three groups. All groups of larvae inoculated for NCI or CBI were maintained at 25 °C. Their survival/mortality records were taken every 12 h (CBI) or 24 h (NCI). The time-mortality trend in each group was subjected to modeling analysis for the estimation of LT50 (d) as a virulence index via NCI or CBI.
2.7. Analyses of Virulence-Related Cellular Events
For the deletion mutants significantly compromised in virulence, several cellular events essential for NCI and hemocoel colonization were examined or analyzed in parallel with their control strains. To reveal the impact of a deleted gene on initiation of NCI, conidial adherence to insect cuticle was assessed on locust (Locusta migratoria manilensis) hind wings pre-treated in 37% H2O2 as described elsewhere [37]. Every 5 μL of a 107 conidia/mL suspension in sterile water was spotted on the center of each hind wing attached to 0.7% water agar, followed by an 8-h incubation at 25 °C. Counts of conidia were made immediately from three microscopic fields of each wing and repeated after 30 s washing in sterile water. The percent ratio of post-wash versus pre-wash counts was computed as an index of conidia adherence to the wing cuticle with respect to the WT strain. Due to a reliance of conidial adherence upon hydrophobicity [38,39,40,41,42], conidial hydrophobicity of each strain was assessed in an aqueous-organic system as described previously [43,44]. After hyphal invasion into insect body, hemocoel colonization relies upon the yeast-like budding proliferation of hyphal bodies (i.e., blastospores) formed by the hyphae through dimorphic transition under the control of the key CDP genes brlA and abaA [32] and is tightly linked to a speed of host death from mummification [26,41]. To reveal a status of proliferation in vivo, the abundance of hyphal bodies was microscopically examined in the hemolymph samples taken from surviving larvae 96 h post-NCI or 72 h post-CBI. The hemocytometer was used to assess the concentration of hyphal bodies from each of three samples per larva (three larvae per strain) taken 72–216 h post-NCI or 60–108 h post-CBI as described previously [26]. Further, 100 mL aliquots of a 106 conidia/mL suspension in trehalose-peptone broth (TPB), a medium amended from CDB (i.e., agar-free CDA) with 3% trehalose as sole carbon source and 0.3% peptone as sole nitrogen source to mimic insect hemolymph, were incubated for 5 d on the shaking bed at 25 °C. From day 2 onwards, blastospore concentration and biomass level (mg/mL) were measured daily from each culture to estimate dimorphic transition rate (no. blastospores/mg biomass) in vitro as a reference to the proliferation in vivo.
2.8. Transcriptional Profiling
The qPCR analysis was performed to verify expected recombinant events in the mutants and gain insight into their phenotypic changes. Briefly, cellophane-overlaid SDAY and submerged TPB cultures were initiated as aforementioned and incubated for 7 and 5 d at the optimal regime, respectively. From the end of a 48-h incubation onwards, total RNA was extracted daily from each of the SDAY or TPB cultures under the action of RNAiso Plus Kit (TaKaRa, Dalian, China), and reversely transcribed into cDNA under the action of PrimeScript RT reagent kit (TaKaRa). The cDNA samples derived from three independent cultures on each sampling occasion were used as templates in qPCR analysis to assess: (1) daily transcript levels of flbA–flbE in the WT cultures grown for 2–7 d on SDAY; (2) transcript levels of each flb gene in the 3 d-old SDAY and TPB cultures of its DM and control strains; (3) daily transcript levels of the key CDP genes brlA and abaA in the SDAY and TPB cultures of each DM compromised in asexual development and its control strains; and (4) transcript levels of 38 phenotype-related genes in the 3 d-old SDAY cultures of each DM compromised in a given phenotype and its control strains. The analyzed genes are well known in function, including the coding genes of five superoxide dismutases (Sod1–Sod5), six catalases (Cat1–Cat6), five hydrophobin or hydrophobin-like proteins (Hyd1–Hyd5), 11 subtilisin-like Pr1 family proteases and 11 heat-shock family proteins. The qPCR analysis with paired primers (Tables S1 and S2) was performed using the SYBR Premix ExTaq kit (TaKaRa). The transcript level of the fungal β-actin gene was used as an internal standard. A threshold-cycle (2−ΔΔCt) method was used to compute relative transcript levels for: (1) each flb gene in the daily SDAY cultures of the WT strain with respect to the standard at the end of 48 h incubation; (2) each flb gene in the 3 d-old SDAY or TPB cultures of related mutants with respect to the WT standard; (3) brlA and abaA in the daily SDAY and TPB cultures of related mutants with respect to the WT standard; and (4) phenotype-related genes in the 3 d-old SDAY cultures of related mutants with respect to the WT standard. One-fold transcript change was considered as a significant level of down- or upregulation for each of the analyzed genes.
2.9. Statistical Analysis
All experimental data were subjected to one-way analysis of variance and Tukey’s honestly significant difference (HSD) test for phenotypic differences between each DM and its control strains.
3. Results
3.1. Phylogenetic Linkages and Sequence Comparison of Fungal FlbA-FlbE Orthologs
BLASTp search with the queries of A. nidulans resulted in identification of FlbA, FlbB, FlbC, FlbD and FlbE orthologs in B. bassiana and selected ascomycetous fungi. The orthologs of each Flb protein were found in most, but not all, of the surveyed fungal genomes, and clustered in phylogeny to distinctive clades or subclades obviously associated with fungal lineages (Figure S2). FlbA (EJP68072), FlbB (EJP63983), FlbC (EJP70334), FlbD (EJP63935) and FlbE (EJP69751) in B. bassiana shared higher sequence identities with their orthologs in Hypocreales than in other orders. The ortholog of FlbD in Cordyceps militaris was exceptionally clustered to an orphan clade distant from the clades of its orthologs in Cordyciptaceae and other fungal lineages and shared a sequence identity of only 39% with the B. bassiana FlbD.
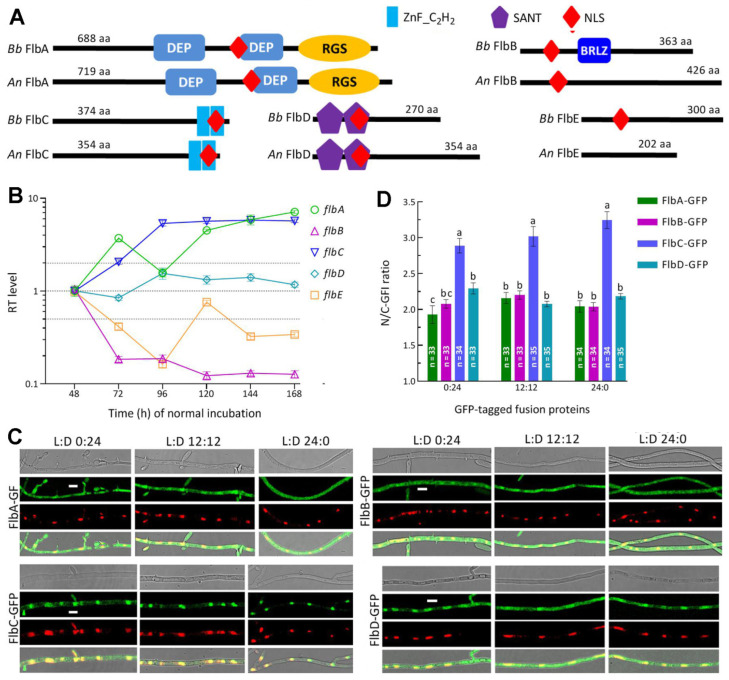
Conserved domain analysis revealed a structural similarity of each Flb protein between B. bassiana and A. nidulans (Figure 1A). FlbA features a C-terminal RGS (Regulator of G protein signaling) domain and two DEP domains that have been proposed to play a selective role in targeting DEP domain-containing proteins to specific subcellular membranous sites [45,46]. A BRLZ (basic region leucin zipper) domain usually present in eukaryotic bZIP domain-containing transcription factors [47] was predicted from the FlbB sequence of B. bassiana at an e-value of 0.0558 but not predictable from the counterpart of A. nidulans, as shown previously [48]. FlbC and FlbD have two C-terminal ZnF_C2H2 domains and two N-terminal SANT (SWI3, ADA2, N-CoR and TFIIIB) domains involved in DNA binding [49], respectively. The SANT domains of FlbD were also revealed in a previous analysis [3]. However, no distinguishable domain was predicted from the FlbE sequence of B. bassiana or A. nidulans, although previous sequence analysis of FlbE revealed a linkage of multiple regions (domains) with subcellular localization of FlbB in A. nidulans [14]. In addition, an NLS motif was predicted from the amino acid sequences of all Flb proteins in the two fungi but not predictable from the A. nidulans FlbE sequence.
Figure 1.
Sequence, transcription and subcellular features of FlbA–FlbE in B. bassiana. (A) Sequence comparison of FlbA–FlbE between B. bassiana (Bb) and A. nidulans (An). Domains and nuclear localization signal (NLS) were predicted from each protein sequence at http://smart.embl-heidelberg.de/ (accessed on 20 March 2022) and http://nls-mapper.iab.keio.ac.jp/ (accessed on 20 March 2022), respectively. (B) Relative transcript (RT) levels of flbA–flbE in the SDAY cultures of wild-type Bb strain (WT) during a 7-d incubation at the optimal regime of 25 °C in a light/dark (L:D) cycle of 12:12 with respect to the standard level on day 2. (C) LSCM images (scales: 5 μm) for subcellular localization of green fluorescence-tagged Flb fusion proteins expressed in the WT strain. Cell samples were taken from the 3 d-old SDBY cultures grown at 25 °C in the respective L:D cycles of 0:24, 12:12 and 24:0 and stained with the nuclear dye DAPI (shown in red). Each four-image panel show bright, expressed (green), stained (red nuclei) and overlapped (yellow nuclei) images of the same field, respectively. (D) Nuclear versus cytoplasmic green fluorescence intensity (N/C-GFI) ratios of the fusion proteins in the hyphal cells. Error bars denote standard deviations (SDs) of the means from three cDNA samples analyzed via qPCR (B) or 33 to 35 cells in the examined hyphae (D).
3.2. Transcription Profiles and Subcellular Localization of FlbA–FlbE in B. bassiana
During a period of 7-d incubation on SDAY at the optimal regime, five flb genes showed differential transcription profiles in the WT cultures with respect to the standard of each at the end of 48 h incubation (Figure 1B). The expression of flbB was repressed by more than 80% consistently during the period while flbE was downregulated significantly on most sampling occasions. In contrast, flbA and flbC were increasingly upregulated or expressed at high levels during the period, followed by transcript level of flbD fluctuating around the standard. These data demonstrated that, in the WT strain, flbA and flbC were far more active than flbD at transcriptional level while flbB and flbE were repressively expressed during the period of normal incubation
The nuclear localization of each Flb protein suggested by the predicted NLS motif was confirmed by the expression of green fluorescence-tagged fusion proteins in the WT strain. As shown in LSCM images (Figure 1C), the fusion proteins accumulated more in the nuclei than in the cytoplasm of hyphal cells stained with DAPI (shown in red) regardless of the hyphae from the cultures grown in an L:D cycle of 0:24, 12:12 or 24:0. The N/C-GFI ratios assessed in the hyphal cells from the three L:D cycles were, on average, 1.92, 2.16 and 2.04 for FlbA-GFP, 2.08, 2.20 and 2.04 for FlbB-GFP, 2.89, 3.04 and 3.25 for FlbC-GFP, and 2.29, 2.08 and 2.18 for FlbD-GFP, respectively (Figure 1D). These observations revealed that FlbC accumulated in the nuclei significantly more than did FlbA, FlbB and FlbD (Tukey’s HSD, p < 0.01) and that each Flb protein accumulated more in the nuclei than in the cytoplasm in a fashion independent of light. However, we failed to express FlbE-GFP in the WT strain in many attempts, leaving its subcellular localization unexplored.
3.3. Differential Roles of flbA–flbE in Radial Growth, Aerial Conidiation and Stress Tolerance
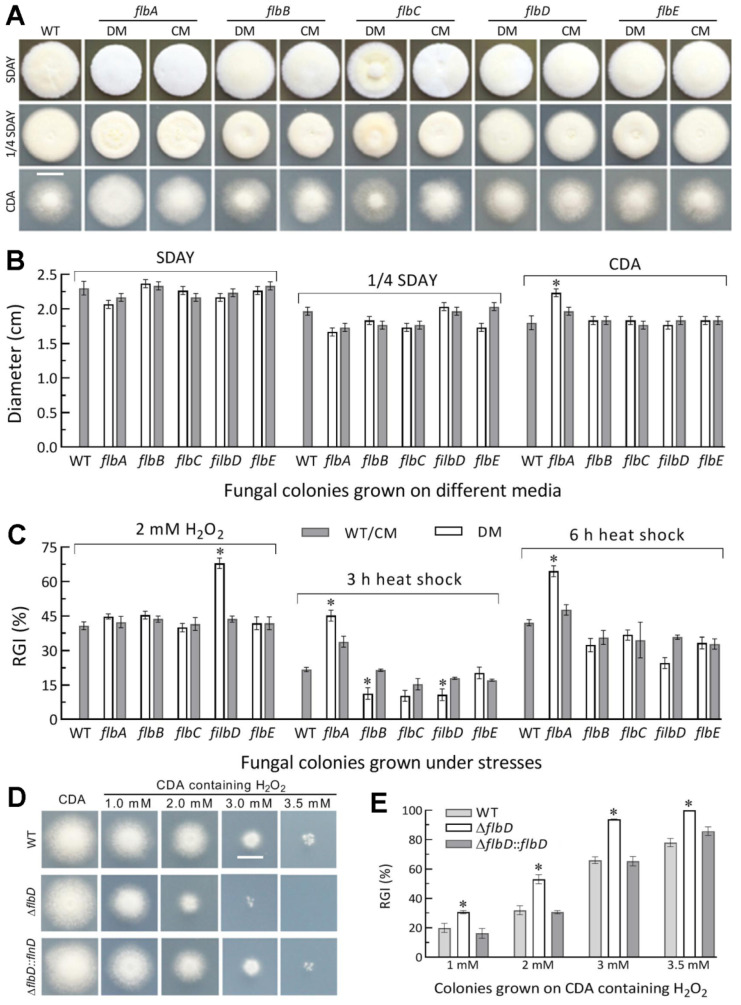
The deletion mutants of five flb genes grew as well as their control strains during a 7-d incubation after initiation of colony growth by spotting 103 conidia on the plates of rich medium SDAY, 1/4 SDAY and minimal medium CDA at the optimal regime. The colonies of all mutants did not show any fluffy phenotype (Figure 2A), and were similar to those of control strains in diameter (Figure 2B). As an exception, the ΔflbA mutant showed a moderate increase in colony size on CDA. Similar colony growth also occurred on CDAs amended with different carbon (glucose, trehalose, fructose, lactose, maltose, mannitol, glycerol, sodium acetate, olive oil and oleic acid) or nitrogen (NaNO2, NH4Cl and NH4NO3) sources (Figure S3). In the assays for cellular responses to stress cues, only the ΔflbA and ΔflbD mutants were significantly more sensitive than their control strains to a 3- or 6-h heat shock at 42 °C during normal growth and an oxidative stress induced with 2 mM H2O2, respectively (Figure 2C). Both of them exhibited null responses to the other oxidant menadione, three osmotic agents (NaCl, KCl and sorbitol) and two cell wall perturbing agents (Congo red and calcofluor white) as did the remaining deletion mutants (Figure S4). The elevation of the ΔflbD mutant’s sensitivity to H2O2 was further clarified by its responses to gradient concentrations of H2O2 (Figure 2D) and its growth inhibition percentage increased from 11% at 1.0 mM to 28% at 3.0 mM (Figure 2E) in comparison to the responses of its control strains.
Figure 2.
Radial growth rates of Δflb mutants (DM) and control (WT and CM) strains in B. bassiana. (A,B) Images (scale: 10 mm) and diameters of fungal colonies grown at the optimal regime of 25 °C and L:D 12:12 for 7 d on rich medium SDAY, 1/4 SDAY and minimal medium CDA after initiated with 103 conidia. (C) Relative growth inhibition (RGI) percentages of fungal colonies incubated at 25 °C for 7 d on CDA containing H2O2 and of SDAY colonies incubated at 25 °C for 5-d growth recovery after exposing 2 d-old colonies to a 42 °C heat shock for 3 or 6 h. (D,E) Images (scale: 10 mm) and relative growth inhibition of the ΔflbD and control strains incubated for 6.5 d on H2O2-containing CDA plates. * p < 0.05 (Tukey’s HSD). Error bars: SDs from three replicates.
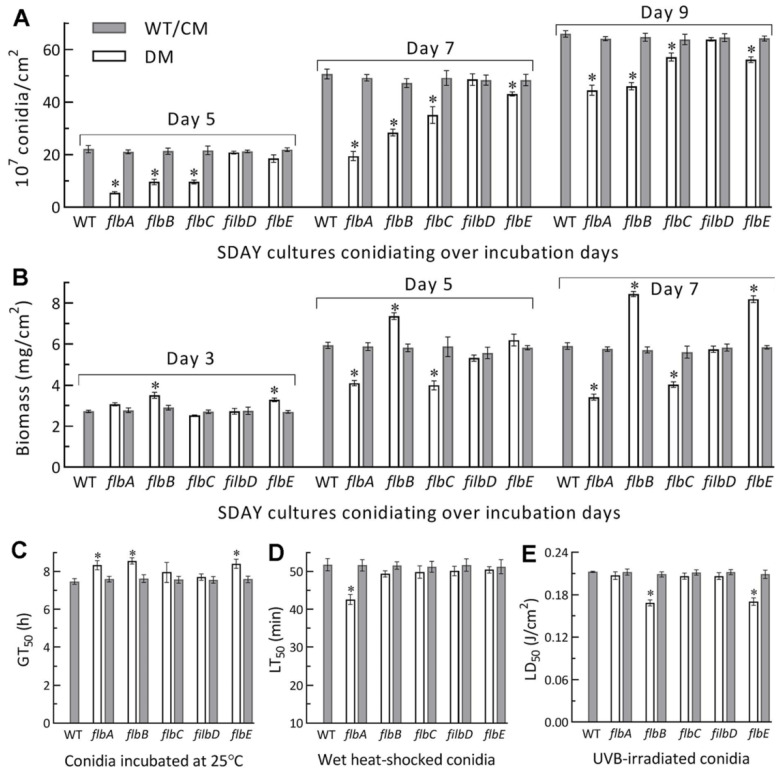
Next, SDAY cultures were initiated by spreading 100 μL aliquots of a 107 conidia/mL suspension to quantify conidial yields and accumulated biomass levels. During a 9-d incubation at the optimal regime, the conidial yields of ΔflbA, ΔflbB and ΔflbC were reduced by 75%, 65% and 65% on day 5, respectively, in comparison to the mean WT yield of 22.1 × 107 conidia/cm2 plate culture (Figure 3A). The yield reductions diminished to 62%, 43% and 31% on day 7 and to 33%, 30% and 14% on day 9, at the time of which the WT yield reached 66.0 × 107 conidia/cm2 plate culture. The ΔflbE mutant exhibited a significant yield decrease of ~15% on day 7 or 9. The accumulated biomass levels in the cellophane-overlaid SDAY cultures were increased by 28%, 24% and 43% in ΔflbB on days 3, 5 and 7, and 21% and 38% in ΔflbE on days 3 and 7, but decreased by 31% and 42% in ΔflbA and 33% and 32% in ΔflbC on days 5 and 7, respectively (Figure 3B). Neither conidial yield nor biomass level was affected in the ΔflbD cultures. Among the indices of conidial quality, moreover, GT50 as a viability index was moderately increased by 12–15% in ΔflbA, ΔflbB and ΔflbE compared to the WT strain (Figure 3C), followed by heat tolerance decreased by 18% in ΔflbA (Figure 3D) and UVB resistance lowered by ~20% in both ΔflbB and ΔflbE (Figure 3E).
Figure 3.
Aerial conidiation and conidial quality of Δflb mutants (DM) and their control (WT and CM) strains in B. bassiana. (A,B) Conidial yields and biomass levels measured from the SDAY cultures during a 9-d incubation at the optimal regime of 25 °C and L:D 12:12, respectively. The cultures were initiated by spreading 100 μL aliquots of a 107 conidia/mL suspension. (C–E) Median germination time GT50 (h) for conidial viability at 25 °C, LT50 (min) for conidial tolerance to a 45 °C wet–heat stress and LD50 (J/cm2) for conidial resistance to UVB irradiation, respectively. *p< 0.05 in Tukey’s HSD tests. Error bars: SDs of the means from three independent replicates.
All mentioned phenotypes were well restored to the WT levels by targeted gene complementation. The experimental data demonstrated dispensable roles for all of five flb genes in the radial growth of B. bassiana under normal culture conditions but significant roles for flbA and flbD in the fungal responses to heat shock and H2O2, respectively. The reduced conidial yields of the ΔflbA and ΔflbC mutants correlated with their lowered biomass accumulation levels. Such correlation was not seen in the ΔflbB and ΔflbE mutants, which were facilitated in biomass accumulation but less compromised in conidiation. The fungal conidiation capacity was eventually reduced by ~30% in the absence of flbA or flbC, ~15% in the absence of flbB or flbE, but not affected in the absence of flbD. These results indicated that fluffy phenotype was not caused by the deletion of each flb gene in B. bassiana as was documented in A. nidulans [1,2].
3.4. Differential Roles of flbA–flbE in Host Infection and Virulence-Related Cellular Events
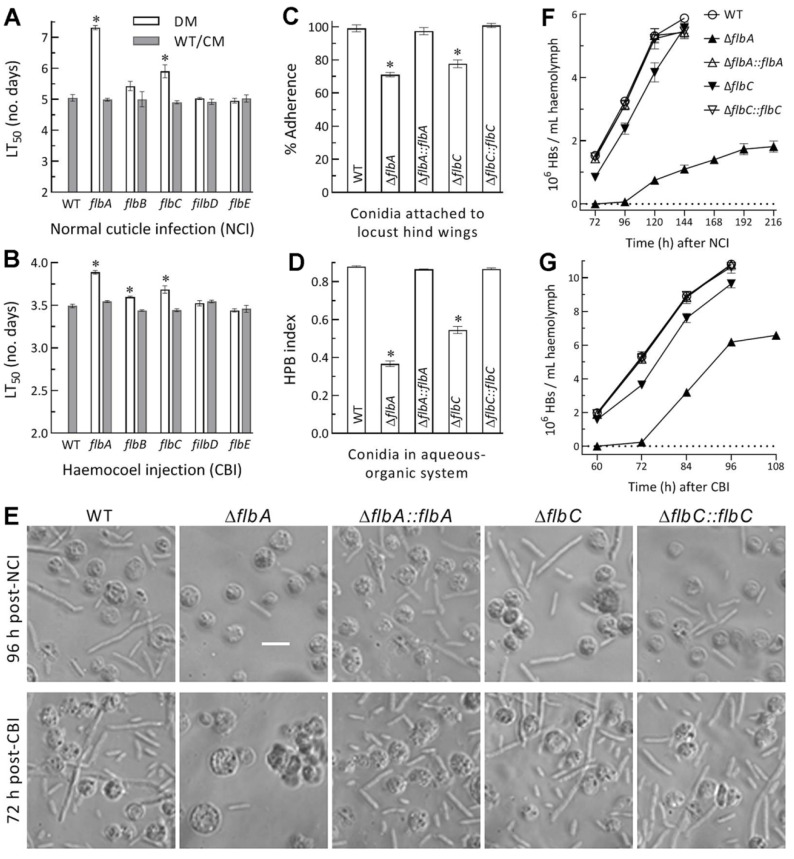
NCI and CBI in the standardized bioassays resulted in the mean LT50 values of 5.05 and 3.49 d for the WT strain against G. mellonella larvae, respectively. Compared to these mean values, the LT50s of ΔflbA and ΔflbC were prolonged significantly (Tukey’s HSD, p< 0.05) by 45% and 17% via NCI (Figure 4A) and 11% and 6% via CBI (Figure 4B), respectively. The ΔflbB LT50 was slightly prolonged via CBI but not via NCI. The remaining deletion mutants showed little virulence change via either infection mode in comparison to their control strains.
Figure 4.
Virulence and related cellular events of Δflb mutants (DM) and control (WT and CM) strains in B. bassiana. (A,B) LT50s (d) estimated by fitting the time-mortality trends of G. mellonella larvae after topical application (immersion) of a 107 conidia/mL suspension for normal cuticle infection (NCI) and intrahemocoel injection of ~500 conidia per larva for cuticle-bypassing infection (CBI), respectively. (C) Conidial adherence to locust hind wing cuticle assessed as percent ratios of post-wash versus pre-wash counts with respect to the WT standard. (D) Conidial hydrophobicity (HPB) index quantified in an aqueous-organic system. (E) Microscopic images (scale bar: 20 μm) for the status of hyphal bodies (HBs; slender cells) and host hemocytes (spherical or subspherical cells) in the hemolymph samples taken from surviving larvae 120 h post-NCI and 72 h post-CBI. (F,G) Concentrations of hyphal bodies in the hemolymph samples taken over the time after NCI and CBI, respectively. * p < 0.05 in Tukey’s HSD tests. Error bars: SDs of the means from three independent replicates.
For insight into significantly attenuated virulence of ΔflbA and ΔflbC via both NCI and CBI, cellular events critical for NCI and hemocoel colonization by proliferation in vivo were compared between the two mutants and their control strains. As a crucial trait for initiation of NCI, conidial adherence to locust wing cuticle was lowered by 29% and 23% for ΔflbA and ΔflbC relative to the WT strain, respectively (Figure 4C), accompanied by 52% and 33% reductions in conidial hydrophobicity (Figure 4D) determinant to the adherence [38,39,40,41,42]. A linear correlation was highly significant between the measurements of conidial hydrophobicity and adherence from the tested strains (r2 = 0.975, F1,19 = 116.7, p < 0.0017).
Next, a status of yeast-like budding proliferation in insect hemocoel to accelerate host death from mummification was examined under a microscope. Hyphal bodies formed by the WT strain were far more abundant than those formed by the ΔflbA mutant in the hemolymph samples taken from the larvae surviving 96 h post-NCI or 72 h post-CBI (Figure 4E). Consequently, the concentrations of hyphal bodies formed by the WT strain in the samples on average were 1.5 × 106, 3.3 × 106, 5.3 × 106 and 5.9 × 106 cells/mL at 72, 96, 120 and 144 h post-NCI (Figure 4F), and 2.0 × 106, 5.3 × 106, 8.9 × 106 and 10.8 × 106 cells/mL at 60, 72, 84 and 96 h post-CBI (Figure 4G), respectively. In contrast, the corresponding ΔflbA concentrations after NCI and CBI were not measurable on the first sampling occasion, and decreased, respectively, by 98% and 96%, 86% and 64%, and 81% and 43% on the following sampling occasions. The concentrations of hyphal bodies produced by the ΔflbC mutant after NCI and CBI decreased by 45% and 20% on the first sampling occasion, and the reductions diminished to only 5% and 11% on the last sampling occasion, respectively. These data demonstrated that the formation and proliferation in vivo of hyphal bodies were blocked in the absence of flbA much more than of flbC and highlighted more important role of flbA than of flbC in the adaptation of B. bassiana insect-pathogenic lifestyle.
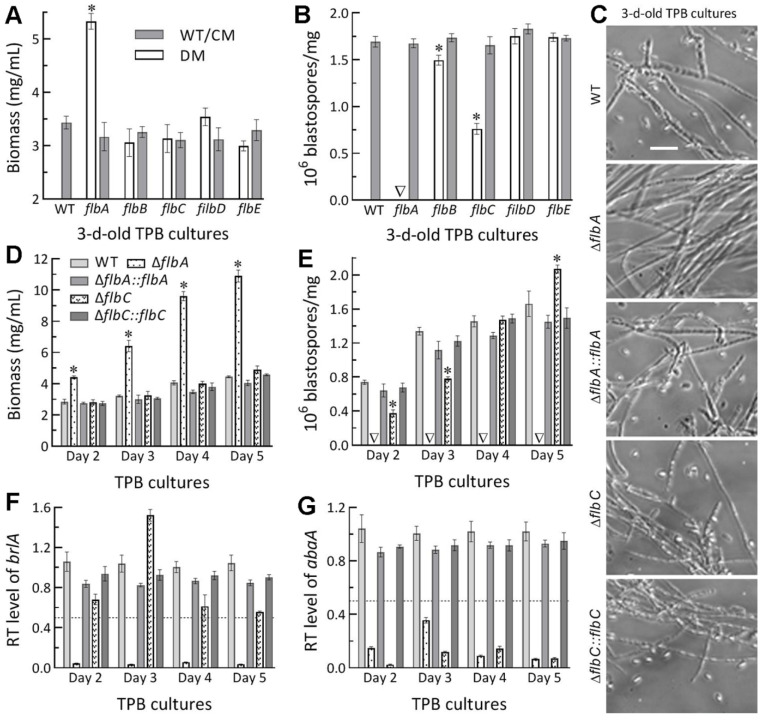
Submerged blastospore production in vitro serves as a reference to the status of dimorphic (hypha-blastospore) essential for the fungal proliferation in vivo. In the 3 d-old TPB cultures mimicking insect hemolymph, biomass level was markedly enhanced by 55% in ΔflbA among the tested mutants and control strains (Figure 5A). Intriguingly, blastospore production was abolished in ΔflbA and reduced by 55% in ΔflbC (Figure 5B). Microscopic examination of culture samples revealed no blastospore formation in the ΔflbA cultures (Figure 5C). Further time-course monitoring of the ΔflbA cultures demonstrated a biomass accumulation level increasingly enhanced by 54–146% (Figure 5D) and a dimorphic transition rate consistently abolished (Figure 5E) during the period of 2- to 5-d incubation. Despite insignificant changes in biomass production during the period, dimorphic transition rate in the ΔflbC cultures decreased by 49% on day 2, 43% on day 5, but increased significantly by 26% on day 5 in comparison to the measurements from the WT cultures. These results uncovered a true fluffy phenotype in the submerged TPB cultures of ΔflbA but different changes of blastospore production not associated with the biomass levels in the ΔflbC cultures.
Figure 5.
Submerged blastospore production of Δflb mutants (DM) and their control (WT and CM) strains in B. bassiana. (A,B) Biomass levels and dimorphic transition rates measured from the 3 d-old TPB cultures grown at 25 °C, respectively. (C) Microscopic images (scale: 20 μm) for a status of blastospore production in the 3 d-old cultures of a 106 conidia/mL TPB mimicking insect hemolymph. (D,E) Time-course biomass levels and dimorphic transition rates in the TPB cultures incubated at 25 °C for 2–5 d on a shaking bed. (F,G) Relative transcript (RT) levels of the key CDP genes brlA and abaA in the TPB cultures of mutants with respect to the WT standard during the 5-d incubation. The dashed line denotes a significant level of one-fold (50%) downregulation. * p < 0.05 (A,B,D,E) in Tukey’s HSD tests. Error bars: SDs of the means from three independent replicates.
In B. bassiana, both aerial conidiation and submerged blastospore production in vitro are asexual developmental processes genetically controlled by the key CDP gene brlA or abaA [32]. In the present study, the expression of brlA in the TPB cultures of the ΔflbA mutant versus the WT strain was repressed by 94–99% (nearly abolished) during the period of a 5-d incubation (Figure 5E), accompanied by sharp repression of abaA by 66–96% during the same period (Figure 5F). In contrast, the time-course transcript levels of abaA alone were reduced by 86–97% in the TBP cultures of the ΔflbC mutant.
The above results indicated more important role of flbA than of flbC, but dispensable roles of flbB, flbD and flbE, in B. bassiana’s host infection and hemocoel colonization. The more attenuated virulence of ΔflbA than of ΔflbC via either NCI or CBI was due to two reasons. Compared to flbC, flbA was more involved in the conidial hydrophobicity and adherence required for initiation of NCI. Second, flbA was more involved in the transcriptional activation of both brlA and abaA to mediate dimorphic transition, which is essential for the fungal blastospore production in vitro and proliferation in vivo by yeast-like budding to speed up host mummification to death [32].
3.5. Linkages of Altered Phenotypes with Transcriptional Changes of RelatedGenes
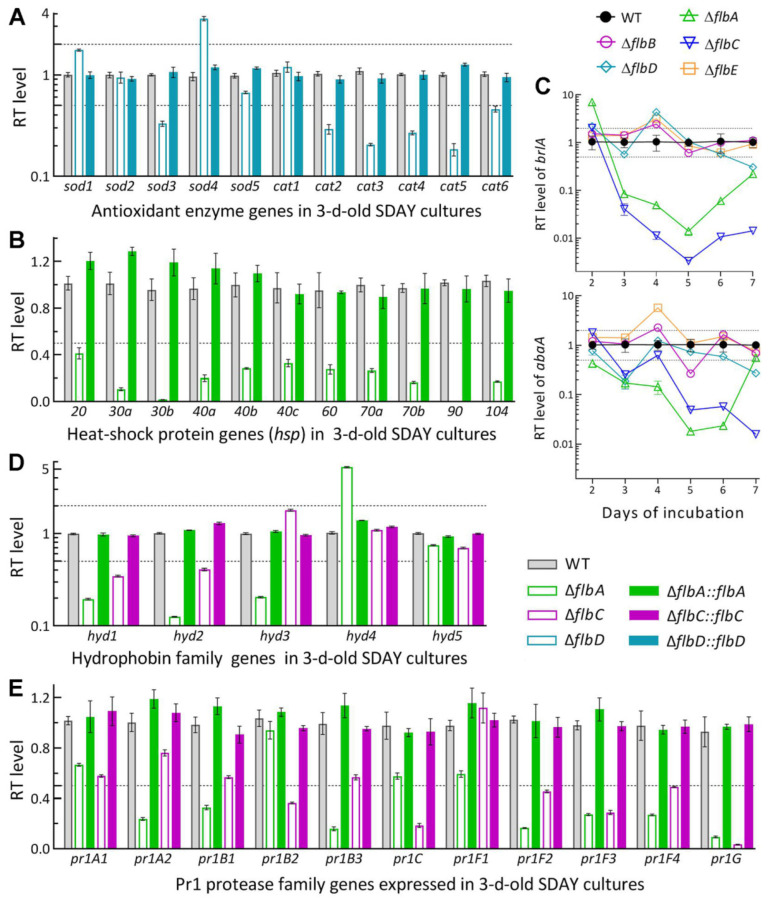
Transcript levels of some functionally characterized genes were assessed by qPCR analysis to gain an in-depth insight into the phenotypic defects of those Δflb mutants. Among the analyzed genes, five of six catalase genes were downregulated by 55–85% in the ΔflbD mutant relative to the WT strain (Figure 6A), including cat2/catB and cat5/catP as major contributors to total catalase activity required for decomposition of H2O2 in B. bassiana [50]. Expression levels of 11 heat shock protein genes [51,52,53] were all sharply repressed or even abolished in ΔflbA (Figure 6B). The repressed genes correlated well with an elevated sensitivity of ΔflbD to H2O2 and of ΔflbA to a 3- or 6-h heat shock at 42 °C during the normal growth at 25 °C.
Figure 6.
Relative transcript (RT) levels of clustered genes associated with phenotypes of the flb mutants with respect to the WT standard in B. bassiana. The tested cDNA samples were derived from the SDAY cultures initiated by spreading 100 μL aliquots of a 107 conidia/mL suspension and incubated for 3 d (A,B,D,E) or 2–7 d (C) at the optimal regime of 25 °C and L:D 12:12. The lower and/or upper dashed lines denote significant levels of one-fold down- and upregulation, respectively. Error bars: SDs of the means from three independent cDNA samples examined by qPCR analysis.
As key CDP activator genes, brlA and abaA were significantly downregulated in the SDAY cultures of ΔflbA on days 3–7 and 2–6, respectively, during the period of a 7-d incubation at the optimal regime (Figure 6C). The two CDP genes were also downregulated in the ΔflbC cultures in most samples, but differentially expressed in the remaining Δflb mutants at insignificant levels with respect to the WT standard. The transcript changes of brlA and abaA correlated with the conidiation defects observed in ΔflbA and ΔflbC but were not indicative of less suppressed conidiation in the ΔflbB and ΔflbE mutants.
Five hydrophobin genes (hyd1–hyd5) and 11 Pr1 protease genes were analyzed to reveal their impacts on the NCI process of the ΔflbA and ΔflbC mutants compromised in virulence. Among those, hyd1 and hyd2 are reported to mediate the biosynthesis of classes I and II hydrophobins and their assembly into an outermost rodlet-bundle layer of conidial coat determinant to conidial hydrophobicity and adherence to insect cuticle [38]. In this study, hyd1 and hyd2 were more downregulated in the 3 d-old SDAY cultures of ΔflbA (80% and 87%) than of ΔflbC (65% and 59%) relative to the WT strain, accompanied by two of three other function-unknown hyd genes differentially expressed in ΔflbA but not affected in ΔflbC (Figure 6D). Previously, five Pr1 genes (pr1A2, pr1B1, pr1B2, pr1C and pr1G) were confirmed as significant contributors to total activity of secreted Pr1 proteases and success of NCI while the remaining Pr1 genes were redundant in function [54]. In the present study, three of the five functional Pr1 genes were markedly downregulated in the 3 d-old SDAY cultures of ΔflbA (pr1A2, pr1B1 and pr1G) and ΔflbC (pr1B2, pr1C and pr1G), accompanied by four and three other Pr1 genes significantly repressed in the two mutants, respectively (Figure 6E). The transcript changes of these genes were well restored by targeted gene complementation, giving an explanation for blocked NCI and attenuated virulence in the absence of flbA or flbC.
4. Discussion
As presented above, the protein sequences encoded by five flb genes in B. bassiana were similar to the corresponding orthologs in A. nidulans and four of them were shown to accumulate more in nucleus than in cytoplasm irrespective of incubation under light or in full darkness. Their deletion mutants showed no fluffy phenotype in normal plate cultures. This is different from fluffy phenotypes caused by the loss-of-function mutations of flbA–flbE in A. nidulans [1,2]. Indeed, our Δflb mutants were not compromised in radial growth on rich and scant media under normal culture conditions and also under different types of stresses. Exceptionally, the ΔflbA growth was facilitated moderately on CDA and CDAs amended with some of tested carbon or nitrogen sources but suppressed significantly by heat shock. The defects of ΔflbA and ΔflbC in aerial conidiation were more conspicuous than those of ΔflbB and ΔflbE but mitigated to limited levels with increasing incubation time. The true fluffy phenotype was observed only in the submerged ΔflbA cultures mimicking insect hemolymph, resulting in blocked proliferation in vivo and reduced virulence. The ΔflbD mutant displayed insignificant changes in all examined phenotypes except its increased sensitivity to oxidative stress induced by H2O2 and its involvement in the expression of most catalase genes. The time-course transcription profiles of brlA and abaA in the SDAY cultures were correlated with the conidiation defects in ΔflbA and ΔflbC but not with those in ΔflbB and ΔflbE. These results uncover that five flb genes function in B. bassiana in a fashion distinctive from their orthologs in A. nidulans, as discussed below.
Our data demonstrated substantial roles of flbA and flbC in the early activation of key CDP genes to initiate aerial conidiation in B. bassiana [32] due to consistently repressed expression of either brlA or abaA in their plate cultures during normal incubation. Particularly, the role of flbA in asexual development was further clarified by abolished blastospore production and nearly abolished expression of both brlA and abaA in the submerged ΔflbA cultures. This is well in accordance with the in vitro blastospore production abolished in the absence of brlA or abaA [32] and suggests a transcriptional link of flbA to brlA in B. bassiana as elucidated previously in A. nidulans [4,20]. The transcriptional repression of abaA instead of brlA in the submerged ΔflbC cultures led to the blastospore production significantly reduced at the early stage of incubation but increased at the late stage, hinting at closer link of flbC to abaA than to brlA at transcriptional level. However, it remains elusive how flbA or flbC acts as an activator of brlA or abaA in the present study because fluG has been shown to play no role in the UDA pathway of B. bassiana [26] and hence differ from the regulatory role of its homolog characterized in the same pathway of A. nidulans [1,2,3]. We tried to explore a possible role for flbA or flbC in activating the expression of brlA or abaA through yeast one-hybrid assays, but failed in repeated attempts because strong automatic activities of the promoters PflbA, PflbC, PbrlA and PabaA ligated to the plasmid pAbAi led to an infeasibility to perform the yeast assays. Recently, AbaA was evidently bound to the promoter of the velvet protein gene veA in Metarhizium robertsii [55], suggesting a distinctive linkage between abaA and veA in the fungal insect pathogen.
Aside from greater role in asexual development, flbA was more important than flbC for the adaptation of B. bassiana to insect–pathogenic lifestyle and was involved in transcriptional mediation of multiple genes encoding heat-shock proteins. The importance is well presented by more attenuated virulence of ΔflbA than of ΔflbC via NCI or CBI. The virulence difference between the two mutants was likely due to differential repression of their key hyd genes, which determine conidial hydrophobicity and adherence required for initiation of NCI [37,38,39]. The difference also could be attributable to differentially delayed or blocked proliferation in vivo by yeast-like budding, which determines a speed of host mummification to death [26,40,41,42,56]. Importantly, the blocked proliferation in vivo of ΔflbA was evidenced by the abolition of blastospore production in vitro and the drastic repression of both brlA and abaA in the TPB cultures mimicking insect hemolymph. This highlights an essentiality of flbA for colonization of host hemocoel by B. bassiana. Notably, our ΔflbA mutant was much less compromised in aerial conidiation than was the deletion mutant of Bbrgs1 (flbA) constructed previously in the background of another B. bassiana strain (ARSEF 252) and also different from the previous mutant not compromised in virulence despite their similar defects in heat tolerance [33]. We speculate that the differential phenotypes of the two ΔflbA mutants could be largely attributed to a big (>10-fold) difference in conidiation capacity between the two WT strains. In our WT strain, opposite rhythms of two frequency (FRQ) proteins (Frq1 and Frq2) in nucleus can persistently activate the expression of CDP genes in a circadian day to orchestrate nonrhythmic conidiation for a high yield of ~55 × 107 conidia/cm2 achieved in 7 or 8 d-old plate cultures or on insect cadaver surfaces regardless of photoperiod change [57,58]. However, most genomes of several B. bassianas trains available in the NCBI databases contain a single FRQ rather than two FRQs, which exist only in the WT strain used in the present study.
In conclusion, our study unravels transcriptional links of flbA and flbC to brlA and/or abaA in B. bassiana and greater role of flbA than of flbC in fungal conidiation, blastospore production and insect–pathogenic lifecycle. However, flbB, flbD and flbE were not influential on the expression of brlA or abaA irrespective of limited or little contribution to conidiation. Neither were they involved in submerged blastospore production and cellular events associated with host infection, hemocoel colonization and virulence. In addition, flbA and flbD were involved in the fungal responses to heat shock and H2O2, respectively, and also in the expression of related stress-responsive genes. These findings offer a novel insight into the roles of five ‘fluffy’ genes that are distinctive from those characterized in A. nidulans.
Acknowledgments
She-Long Zhang (Equipment and Technology Service Platform, College of Life Sciences, Zhejiang University) is acknowledged for technical assistance with LSCM analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8040334/s1. Table S1: P Paired primers used for manipulation of flbA, flbB, flbC, flbD and flbE in B. bassoama; Table S2: Paired primers used in the qPCR analysis of phenotype-related genes in B. bassiana; Figure S1: Generation and identification of flbA–flbE mutants in B. bassiana.; Figure S2: Phylogenetic trees of FlbA–FlbE orthologs found in selected filamentous fungi; Figure S3: The diameters of fungal colonies grown at the optimal regime of 25 °C and L:D 12:12 for 7 d on the plates of minimal CDA and CDAs amended with different carbon or nitrogen sources; Figure S4: Images (scale: 10 mm) of fungal colonies incubated at 25 °C for 7 d on the plates of CDA alone (control) or supplemented with the indicated concentrations of menadione (MND), H2O2, KCl, NaCl, sorbitol (SBT), Congo red (CGR) and calcofluor white, respectively, and of SDAY colonies incubated at 25 °C for 5-d growth recovery after 2 d-old colonies were exposed to a 42 °C heat shock for 3 and 6 h.
Author Contributions
Conceptualization, C.-T.G. and M.-G.F.; methodology, C.-T.G. and S.-H.Y.; software, S.-H.Y. and M.-G.F.; validation, C.-T.G. and M.-G.F.; formal analysis, M.-G.F. and C.-T.G.; investigation, C.-T.G. and X.-C.L.; resources, M.-G.F. and S.-H.Y.; data curation, C.-T.G. and M.-G.F.; writing—original draft preparation, C.-T.G.; writing—review and editing, M.-G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 31772218) and Zhejiang Provincial R&D Program (Grant 2022C02058). The authors declare no conflict of interest.
Institutional Review Board Statement
Not available.
Informed Consent Statement
Not available.
Data Availability Statement
All data presented in this study are included in the paper and associated Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Etxebeste O., Garzia A., Espeso E.A., Ugalde U. Aspergillus nidulansasexual development: Making the most of cellular modules. Trends Microbiol. 2010;18:569–576. doi: 10.1016/j.tim.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Park H.S., Yu J.H. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012;15:669–677. doi: 10.1016/j.mib.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Ojeda-López M., Chen W., Eagle C.E., Gutiérrez G., Jia W.L., Swilaiman S.S., Huang Z., Park H.-S., Yu J.-H., Cánovas D., et al. Evolution of asexual and sexual reproduction in the aspergilli. Stud. Mycol. 2018;91:37–59. doi: 10.1016/j.simyco.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee B.N., Adams T.H. fluG and flbA function interdependently to initiate conidiophore development in Aspergillus nidulans through brlA beta activation. EMBO J. 1996;15:299–309. doi: 10.1002/j.1460-2075.1996.tb00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etxebeste O., Ni M., Garzia A., Kwon N.J., Fischer R., Yu J.H., Espeso E.A., Ugalde U. Basic-zipper-type transcription factor FlbB controls asexual development in Aspergillus nidulans. Eukaryot. Cell. 2008;7:38–48. doi: 10.1128/EC.00207-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etxebeste O., Herrero-García E., Araújo-Bazán L., Rodríguez-Urra A.B., Garzia A., Ugalde U., Espeso E.A. The bZIP-type transcription factor FlbB regulates distinct morphogenetic stages of colony formation in Aspergillus nidulans. Mol. Microbiol. 2009;73:775–789. doi: 10.1111/j.1365-2958.2009.06804.x. [DOI] [PubMed] [Google Scholar]
- 7.Garzia A., Etxebeste O., Herrero-Garcia E., Fischer R., Espeso E.A., Ugalde U. Aspergillus nidulans FlbE is an upstream developmental activator of conidiation functionally associated with the putative transcription factor FlbB. Mol. Microbiol. 2009;71:172–184. doi: 10.1111/j.1365-2958.2008.06520.x. [DOI] [PubMed] [Google Scholar]
- 8.Garzia A., Etxebeste O., Herrero-Garcia E., Ugalde U., Espeso E.A. The concerted action of bZip and cMyb transcription factors FlbB and FlbD induces brlA expression and asexual development in Aspergillus nidulans. Mol. Microbiol. 2010;75:1314–1324. doi: 10.1111/j.1365-2958.2010.07063.x. [DOI] [PubMed] [Google Scholar]
- 9.Kwon N.J., Garzia A., Espeso E.A., Ugalde U., Yu J.H. FlbC is a putative nuclear C2H2 transcription factor regulating development in Aspergillus nidulans. Mol. Microbiol. 2010;77:1203–1219. doi: 10.1111/j.1365-2958.2010.07282.x. [DOI] [PubMed] [Google Scholar]
- 10.Kwon N.J., Shin K.S., Yu J.H. Characterization of the developmental regulator FlbE in Aspergillus fumigatus and Aspergillus nidulans. Fungal Genet. Biol. 2010;47:981–993. doi: 10.1016/j.fgb.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Xiao P., Shin K.S., Wang T., Yu J.H. Aspergillus fumigatus flbB encodes two basic leucine zipper domain (bZIP) proteins required for proper asexual development and gliotoxin production. Eukaryot. Cell. 2010;9:1711–1723. doi: 10.1128/EC.00198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arratia-Quijada J., Sánchez O., Scazzocchio C., Aguirre J. FlbD, a Myb transcription factor of Aspergillus nidulans, is uniquely involved in both asexual and sexual differentiation. Eukaryot. Cell. 2012;11:1132–1142. doi: 10.1128/EC.00101-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iradi-Serrano M., Tola-Garcia L., Cortese M.S., Ugalde U. The early asexual development regulator fluG codes for a putative bifunctional enzyme. Front. Microbiol. 2019;10:778. doi: 10.3389/fmicb.2019.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otamendi A., Perez-de-Nanclares-Arregi E., Oiartzabal-Arano E., Cortese M.S., Espeso E.A., Etxebeste O. Developmental regulators FlbE/D orchestrate the polarity site-to-nucleus dynamics of the fungal bZIP transcription factor FlbB. Cell. Mol. Life Sci. 2019;76:4369–4390. doi: 10.1007/s00018-019-03121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams T.H., Boylan M.T., Timberlake W.E. brlA is necessary andsufficient to direct conidiophore development in Aspergillus nidulans. Cell. 1988;54:353–362. doi: 10.1016/0092-8674(88)90198-5. [DOI] [PubMed] [Google Scholar]
- 16.Sewall T.C., Mims C.W., Timberlake W.E. abaA controls phialide differentiation in Aspergillus nidulans. Plant Cell. 1990;2:731–739. doi: 10.1105/tpc.2.8.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirabito P.M., Adam T.H., Timberlake W.E. Interactions of three sequentially expressed genes control temporal and spatial specificity in Aspergillus development. Cell. 1989;57:859–868. doi: 10.1016/0092-8674(89)90800-3. [DOI] [PubMed] [Google Scholar]
- 18.Marshall M.A., Timberlake W.E. Aspergillus nidulans wetA activates spore-specific gene expression. Mol. Cell. Biol. 1991;11:55–62. doi: 10.1128/mcb.11.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams T.H., Hide W.A., Yager L.N., Lee B.N. Isolation of a gene required for programmed initiation of development by Aspergillus nidulans. Mol. Cell. Biol. 1992;12:3827–3833. doi: 10.1128/mcb.12.9.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee B.N., Adams T.H. The Aspergillus nidulansfluGgene is required for production of an extracellular developmental signal. Genes Dev. 1994;8:641–651. doi: 10.1101/gad.8.6.641. [DOI] [PubMed] [Google Scholar]
- 21.Lee B.N., Adams T.H. Overexpression of flbA, an early regulator of Aspergillus asexual sporulation leads to activation of brlA and premature initiation of development. Mol. Microbiol. 1994;14:323–334. doi: 10.1111/j.1365-2958.1994.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 22.Wieser J., Adams T.H. flbD encodes a myb-like DNA binding protein that controls initiation of Aspergillus nidulans conidiophore development. Genes Dev. 1995;9:491–502. doi: 10.1101/gad.9.4.491. [DOI] [PubMed] [Google Scholar]
- 23.de Vries R.P., Riley R., Wiebenga A., Aguilar-Osorio G., Amillis S., Uchima C.A., Anderluh G., Asadollahi M., Askin M., Barry K., et al. Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus. Genome Biol. 2017;18:28. doi: 10.1186/s13059-017-1151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etxebeste O., Otamendi A., Garzia A., Espeso E.A., Cortese M.S. Rewiring of transcriptional networks as a major event leading to the diversity of asexual multicellularity in fungi. Crit. Rev. Microbiol. 2019;45:548–563. doi: 10.1080/1040841X.2019.1630359. [DOI] [PubMed] [Google Scholar]
- 25.Mead M.E., Borowsky A.T., Joehnk B., Steenwyk J.L., Shen X.X., Sil A., Rokas A. Recurrent loss of abaA, a master regulator of asexual development in filamentous fungi, correlates with changes in genomic and morphological traits. Genome Biol. Evol. 2020;12:1119–1130. doi: 10.1093/gbe/evaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo C.T., Peng H., Tong S.M., Ying S.H., Feng M.G. Distinctive role of fluG in the adaptation of Beauveria bassiana to insect-pathogenic lifecycle and environmental stresses. Environ. Microbiol. 2021;23:5184–5199. doi: 10.1111/1462-2920.15500. [DOI] [PubMed] [Google Scholar]
- 27.Chang P.K., Scharfenstein L.L., Mack B., Ehrlich K.C. Deletion of the Aspergillus flavus orthologue of A. nidulans fluG reduces conidiation and promotes production of sclerotia but does not abolish aflatoxin biosynthesis. Appl. Environ. Microbiol. 2012;78:7557–7563. doi: 10.1128/AEM.01241-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F.F., Krijgsheld P., Hulsman M., de Bekker C., Muller W.H., Reinders M., de Vries R.P., Wösten H.A.B. FluG affects secretion in colonies of Aspergillus niger. Antonie van Leeuwenhoek. 2015;107:225–240. doi: 10.1007/s10482-014-0321-2. [DOI] [PubMed] [Google Scholar]
- 29.Feng M.G., Poprawski T.J., Khachatourians G.G. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: Current status. Biocontrol Sci. Technol. 1994;4:3–34. doi: 10.1080/09583159409355309. [DOI] [Google Scholar]
- 30.de Faria M., Wraight S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control. 2007;43:237–256. doi: 10.1016/j.biocontrol.2007.08.001. [DOI] [Google Scholar]
- 31.Li F., Shi H.Q., Ying S.H., Feng M.G. WetA and VosA are distinct regulators of conidiation capacity, conidial quality, and biological control potential of a fungal insect pathogen. Appl. Microbiol.Biotechnol. 2015;99:10069–10081. doi: 10.1007/s00253-015-6823-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhang A.X., Mouhoumed A.Z., Tong S.M., Ying S.H., Feng M.G. BrlA and AbaA govern virulence-required dimorphic switch, conidiation and pathogenicity in a fungal insect pathogen. mSystems. 2019;4:e00140-19. doi: 10.1128/mSystems.00140-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang W.G., Scully L.R., Zhang L., Pei Y., Bidochka M.J. Implication of a regulator of G protein signalling (BbRGS1) in conidiation and conidial thermotolerance of the insect pathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 2008;279:146–156. doi: 10.1111/j.1574-6968.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- 34.Xiao G.H., Ying S.H., Zheng P., Wang Z.L., Zhang S.W., Xie X.Q., Shang Y.F., Zheng H.J., Zhou Y., St Leger R.J., et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012;2:483. doi: 10.1038/srep00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D.Y., Tong S.M., Guan Y., Ying S.H., Feng M.G. The velvet protein VeA functions in asexual cycle, stress tolerance and transcriptional regulation of Beauveria bassiana. Fungal Genet. Biol. 2019;127:1–11. doi: 10.1016/j.fgb.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Mou Y.N., Gao B.J., Ren K., Tong S.M., Ying S.H., Feng M.G. P-type Na+/K+ ATPases essential and nonessential for cellular homeostasis and insect pathogenicity of Beauveria bassiana. Virulence. 2020;11:1415–1431. doi: 10.1080/21505594.2020.1836903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Q., Yu L., Ying S.H., Feng M.G. Comparative roles of three adhesin genes (adh1–3) in insect-pathogenic lifecycle of Beauveria bassiana. Appl. Microbiol. Biotechnol. 2021;105:5491–5502. doi: 10.1007/s00253-021-11420-w. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S.Z., Xia Y.X., Kim B., Keyhani N.O. Two hydrophobins are involved in fungal spore coat rodlet layer assembly and each playdistinct roles in surface interactions, development and pathogenesis in the entomopathogenic fungus, Beauveria bassiana. Mol. Microbiol. 2011;80:811–826. doi: 10.1111/j.1365-2958.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 39.Shao W., Cai Q., Tong S.M., Ying S.H., Feng M.G. Nuclear Ssr4 is required for the in vitro and in vivo asexual cycles and global gene activity of Beauveria bassiana. mSystems. 2020;5:e00677-19. doi: 10.1128/mSystems.00677-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren K., Mou Y.N., Tong S.M., Ying S.H., Feng M.G. DIM5/KMT1 controls fungal insect pathogenicity and genome stability by methylation of histone H3K4, H3K9 and H3K36. Virulence. 2021;12:1306–1322. doi: 10.1080/21505594.2021.1923232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren K., Mou Y.N., Yu L., Tong S.M., Ying S.H., Feng M.G. SET1/KMT2-governed histone H3K4 methylation coordinates the lifecycle in vivo and in vitro of the fungal insect pathogen Beauveria bassiana. Environ. Microbiol. 2021;23:5541–5554. doi: 10.1111/1462-2920.15701. [DOI] [PubMed] [Google Scholar]
- 42.Mou Y.N., Ren K., Tong S.M., Ying S.H., Feng M.G. Essential role of COP9 signalosome subunit 5 (Csn5) in insect pathogenicity and asexual development of Beauveria bassiana. J. Fungi. 2021;7:642. doi: 10.3390/jof7080642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holder D.J., Kirkland B.H., Lewis M.W., Keyhani N.O. Surface characteristics of the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology-UK. 2007;153:3448–3457. doi: 10.1099/mic.0.2007/008524-0. [DOI] [PubMed] [Google Scholar]
- 44.Wang J.J., Qiu L., Chu Z.J., Ying S.H., Feng M.G. The connection of protein O-mannosyltransferase family to the biocontrol potential of Beauveria bassiana, a fungal entomopathogen. Glycobiology. 2014;24:638–648. doi: 10.1093/glycob/cwu028. [DOI] [PubMed] [Google Scholar]
- 45.Burchett S.A. Regulators of G protein signaling: A bestiary of modular protein binding domains. J. Neurochem. 2000;75:1335–1351. doi: 10.1046/j.1471-4159.2000.0751335.x. [DOI] [PubMed] [Google Scholar]
- 46.Wong H.C., Mao J., Nguyen J.T., Srinivas S., Zhang W., Liu B., Li L., Wu D., Zheng J. Structural basis of the recognition of the dishevelled DEP domain in the Wnt signaling pathway. Nat. Struct. Biol. 2000;7:178–1184. doi: 10.1038/82047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurst H.C. Transcription factors 1: bZIP proteins. Protein Profile. 1995;2:101–168. [PubMed] [Google Scholar]
- 48.Cortese M.S., Etxebeste O., Garzia A., Espeso E.A., Ugalde U. Elucidation of functional markers from Aspergillus nidulans developmental regulator FlbB and their phylogenetic distribution. PLoS ONE. 2011;6:e17505. doi: 10.1371/journal.pone.0017505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klempnauer K.H., Sippel A.E. The highly conserved amino-terminal region of the protein encoded by the v-myb oncogene functions as a DNA-binding domain. EMBO J. 1987;6:2719–2725. doi: 10.1002/j.1460-2075.1987.tb02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z.L., Zhang L.B., Ying S.H., Feng M.G. Catalases play differentiated roles in the adaptation of a fungal entomopathogen to environmental stresses. Environ. Microbiol. 2013;15:409–418. doi: 10.1111/j.1462-2920.2012.02848.x. [DOI] [PubMed] [Google Scholar]
- 51.Wang J., Ying S.H., Hu Y., Feng M.G. Mas5, a homologue of bacterial DnaJ, is indispensable for the host infection and environmental adaptation of a filamentous fungal insect pathogen. Environ. Microbiol. 2016;18:1037–1047. doi: 10.1111/1462-2920.13197. [DOI] [PubMed] [Google Scholar]
- 52.Wang J., Ying S.H., Hu Y., Feng M.G. Vital role for the J-domain protein Mdj1 in asexual development, multiple stress tolerance and virulence of Beauveria bassiana. Appl. Microbiol. Biotechnol. 2017;101:185–195. doi: 10.1007/s00253-016-7757-4. [DOI] [PubMed] [Google Scholar]
- 53.Wang J., Chen J.W., Hu Y., Ying S.H., Feng M.G. Roles of six Hsp70 genes in virulence, cell wall integrity, antioxidant activity and multiple stress tolerance of Beauveria bassiana. Fungal Genet. Biol. 2020;144:103437. doi: 10.1016/j.fgb.2020.103437. [DOI] [PubMed] [Google Scholar]
- 54.Gao B.J., Mou Y.N., Tong S.M., Ying S.H., Feng M.G. Subtilisin-like Pr1 proteases marking evolution of pathogenicity in a wide-spectrum insect-pathogenic fungus. Virulence. 2020;11:365–380. doi: 10.1080/21505594.2020.1749487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu H., Tong Y.M., Zhou R., Wang Y.L., Wang Z.X., Ding T., Huang B. Mr-AbaA regulates conidiation by interacting with the promoter regions of both Mr-veA and Mr-wetA in Metarhiziumrobertsii. Microbiol. Spectr. 2021;9:e00823-21. doi: 10.1128/Spectrum.00823-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren K., Mou Y.N., Ying S.H., Feng M.G. Conserved and noncanonical activities of two histone H3K36 methyltransferases required for insect-pathogenic lifestyle of Beauveria bassiana. J. Fungi. 2021;7:956. doi: 10.3390/jof7110956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong S.M., Wang D.Y., Cai Q., Ying S.H., Feng M.G. Opposite nuclear dynamics of two FRH-dominated frequency proteins orchestrate non-rhythmic conidiation of Beauveria bassiana. Cells. 2020;9:626. doi: 10.3390/cells9030626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tong S.M., Gao B.J., Peng H., Feng M.G. Essential roles of two FRQ proteins (Frq1 and Frq2) in Beauveria bassiana’s virulence, infection cycle and calcofluor-specific signaling. Appl. Environ. Microbiol. 2021;87:e02545-20. doi: 10.1128/AEM.02545-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this study are included in the paper and associated Supplementary Materials.